Abstract
A hypoglossal–facial transfer is a common surgical strategy for reanimating the face after persistent total hemifacial palsy. We were interested in how motor recovery is associated with cortical reorganization of lip and tongue representation in the primary sensorimotor cortex after the transfer. Therefore, we used functional magnetic resonance imaging (fMRI) in 13 patients who underwent a hypoglossal–facial transfer after unilateral peripheral facial palsy. To identify primary motor and somatosensory tongue and lip representation sites, we measured repetitive tongue and lip movements during fMRI. Electromyography (EMG) of the perioral muscles during tongue and lip movements and standardized evaluation of lip elevation served as outcome parameters. We found an association of cortical representation sites in the pre‐ and postcentral gyrus (decreased distance of lip and tongue representation) with symmetry of recovered lip movements (lip elevation) and coactivation of the lip during voluntary tongue movements (EMG‐activity of the lip during tongue movements). Overall, our study shows that hypoglossal–facial transfer resulted in an outcome‐dependent cortical reorganization with activation of the cortical tongue area for restituded movement of the lip. Hum Brain Mapp 35:638–645, 2014. © 2012 Wiley‐Periodicals, Inc.
Keywords: facial palsy, functional magnetic resonance imaging (fMRI), hypoglossal–facial transfer, primary motor cortex (M1), primary somatosensory cortex (S1), cortical reorganization
INTRODUCTION
Peripheral facial palsy is the most common disorder of the cranial nerves with an incidence ranging from 20 to 30 cases per 100,000 people per year. The appointed causes are viral infections, trauma, iatrogenic injury, inflammation, metabolic diseases, and tumors affecting the facial nerve [Volk et al., 2010]. Besides problems with lip and eye closure and articulation difficulties, patients have severe problems with social interactions due to impaired emotional facial expression and asymmetric facial features [Neely and Neufeld, 1996].
Only a minority of these patients needs surgical treatment but patients who experience a persistent hemifacial lesion after tumor surgery are more often subject to a hypoglossal–facial transfer. This approach has demonstrated promising results [Guntinas‐Lichius et al., 2006; Pellat et al., 1997]. However, little is known about changes in the cortical organization after this transfer in humans.
In an animal model, an invasion of neighboring representation sites in the primary somatosensory cortex (S1) has been described after complete peripheral nerve lesions of the median nerve [Merzenich et al., 1984]. These lesions are accompanied by deficient motor and somatosensory function of the hand — a complete deafferentation of the cortical representation area together with motor impairment. Therefore, changes in the motor representation, such as enlarged motor representation areas and decreased cortical thresholds for areas proximal to the stump, have also been reported [Sanes et al., 1990].
In humans, comparable cortical processes in the somatosensory cortex have been observed after complete amputation of the upper limb using magnetoencephalography (MEG) [Elbert et al., 1994]: the neighboring lip area invades the former hand area. Additional changes have also been described for the motor cortex using transcranial magnetic stimulation (TMS) [Cohen et al., 1991]. TMS around the deafferented representation site evokes larger motor‐evoked potentials (MEPs) and recruits a larger percentage of the motoneuron pool.
In contrast to a complete denervation of a limb, patients with lesions of the facial nerve show motor impairments but no somatosensory disturbances because somatosensory representations of the face are transduced via the trigeminal nerve. Thus, for these patients, motor representation changes have been investigated. TMS after peripheral hemifacial palsy revealed that these patients also show increased MEPs in perioral muscles. Comparable to the observations after peripheral nerve lesions of a limb, an enlargement of the neighboring hand representation area contralateral to the facial palsy was observed, extending in a lateral direction into the site of the presumed face area (positron emission tomography and TMS [Rijntjes et al., 1997]). An additional TMS study provided strong evidence for bilateral changes in the primary motor representation (M1) of the tongue after facial palsy, with an invasion of the facial motor area by the tongue motor representation [Rodel et al., 2004].
Functional magnetic resonance imaging (fMRI) is capable of differentiating between tongue and lip representation sites in the pre‐ and postcentral gyrus in a spatially more precise way as possible with TMS [Hesselmann et al., 2004; Lotze et al., 2000a]. Therefore, it has been proposed that this method might also enable reorganization mapping after hypoglossal–facial transfer [Hesselmann et al., 2004]. Plastic processes underlying recovery after hypoglossal–facial transfer are most probably located in the primary motor cortex and not in other areas [Bitter et al., 2011]. Bitter et al. (2011) investigated three patients after hypoglossal–facial transfer with fMRI and they ascertained that lip movements were associated with activation in the glossal motor cortex. However, the small group of patients investigated did not allow for a correlation analysis of clinical features which is highly interesting for detecting associations of successful recovery induced by hypoglossal–facial transfer. Therefore, we used fMRI, electromyography (EMG) of labial movements and assessment of symmetry of labial movements in a group of patients who underwent hypoglossal–facial transfer in the late chronic state after peripheral unilateral facial palsy. We were especially interested in the associations between reorganization of M1 (posterior border of the precentral gyrus) but also S1 (postcentral gyrus) lip and tongue representation and clinical parameters such as recovery of lip elevation.
In detail we tested the following hypotheses:
Is it possible to verify a decrease of distance between center of gravity (COG) of representation sites of lip pursing and tongue elevation which might be caused by an extension of neighboring areas (for our study tongue) into the deafferented facial area [Rijntjes et al., 1997]?
Is there an increase of activation magnitude of representation sites around the deafferented area? In patients with deafferentiation, TMS around the deafferented representation site evokes larger MEPs and recruits a larger percentage of the motoneuron pool [Cohen et al., 1991]. It has been shown that MEPs positively correlate with fMRI magnitude [Lotze et al., 2003].
Are the M1 and S1 representations of lip and tongue between the affected and the nonaffected hemisphere asymmetric such as reported after unilateral deafferentation due to limb amputation (e.g. Lotze et al., 1999)?
Is there a correlation of associated representation of tongue and lip with clinical outcome (lip elevation symmetry, coactivation (EMG) of lip muscle during tongue movements)?
METHODS
Participants
We examined 13 patients (four males, nine females, average age 61 years, range 40–75 years), who suffered from a unilateral peripheral facial palsy (seven left, six right) after a surgical removal of a tumor (twelve vestibular tumors, one cholesteatoma). Denervation in these patients was verified electrophysiologically (lacking EMG during movement instruction on the affected side) and by photo documentation (subjects were photographed for the following tasks: eyelid closure, wrinkling the forehead, pursing the lips, sticking out of the tongue to the left and right side, elevation of the tongue against the palate and lip elevation). The denervation time after onset of facial palsy was 11 months on average (range 2–45 months). These patients underwent a hypoglossal–facial transfer at the Department of Neurosurgery of the University of Greifswald. We examined the participants after a postoperative time of 146 months on average (about 12 years; range 19–201 months) after the hypoglossal–facial transfer (Table 1). After the hypoglossal–facial transfer, patients were asked to do the regular exercises in front of a mirror (lip pursing, smiling, and sticking out the tongue).
Table 1.
Patient characteristics and affected side of the facial palsy
| Patient number | Sex | Age (years) | Side affected | Denervation time (months) | Time after transfer (months) |
|---|---|---|---|---|---|
| 1 | Male | 48 | Right | 6 | 176 |
| 2 | Female | 70 | Right | 11 | 102 |
| 3 | Male | 70 | Left | 2 | 173 |
| 4 | Male | 53 | Left | 9 | 172 |
| 5 | Female | 66 | Right | 3 | 201 |
| 6 | Male | 62 | Left | 10 | 19 |
| 7 | Female | 65 | Left | 7 | 161 |
| 8 | Female | 70 | Right | 9 | 116 |
| 9 | Female | 68 | Left | 6 | 94 |
| 10 | Female | 65 | Right | 14 | 200 |
| 11 | Female | 69 | Left | 16 | 191 |
| 12 | Female | 45 | Right | 4 | 180 |
| 13 | Female | 60 | Left | 45 | 117 |
The Ethics Committee of the Medical Faculty of the University of Greifswald approved this study. All subjects gave written informed consent according to the guidelines of the Declaration of Helsinki.
Clinical Tests
We used neurological tests of the motor and somatosensory function of the face, photos of facial movements and an EMG of the orbicularis oris muscle to document the clinical outcome. A precise neurological investigation was performed to differentiate deficits associated with damage of other cranial nerves (corneal and jaw reflex, von Frey Hair test of the facial skin of each side [trigeminal], function of the masseter muscle [trigeminal], eye contraction and wrinkle forehead, purse lips and show teeth [for testing different branches of the facial nerve], vowel recitation [glossopharyngeal and accessorial nerves], uvular deviation [glossopharyngeal nerve], gag reflex [vagal nerve], isocorie, direct and indirect light reaction, reaction of convergence and the eye movement [oculomotor, trochlear, and abducens nerves]). Furthermore, the House–Brackmann scale was used to evaluate facial nerve function after the hypoglossal–facial transfer [House and Brackmann, 1985].
Subjects were photographed before scanning for measuring the performance of lip elevation (Fig. 1, left). For this reason, we performed difference measurements, defined as the distance between the top of the upper lip being in a vertical line with the nasolabial fold, between the affected compared to the nonaffected side. Photos were taken in a standardized angle and distance. The evaluation of lip symmetry was rated by five independent judges. Furthermore, we tested the observers reliability by calculating Kendall's coefficient of concordance (Kendalls‐W) using the Statistical Package for the Social Sciences (SPSS 19.0). The grade of accordance among five raters for the photo documented lip elevation was high (Kendall‐W = 0.79; P < 0.0001). The resulting value was negatively expressed (multiplicated with −1) to ease a comparison with other outcome parameters (EMG).
Figure 1.
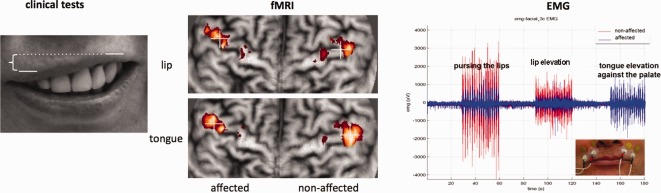
Illustration of tests and investigations performed. Left: Clinical test: Schematic image of the condition “lip elevation.” The patient shows right‐sided facial palsy. Comparison of distance difference between the superior border of the upper lip between facial sides. Middle: Demonstration of the flattening method for the same patient. The left cortical hemisphere is the affected (deafferented) side (anterior is superior). Crosses illustrate the COG of the activation cluster within the precentral gyrus. Right: EMG‐recordings of the superior orbicularis oris of the affected (blue) and the nonaffected (red) side during “pursing the lips,” “lip elevation” and “tongue elevation against the palate.” Only the affected side shows high coactivation during tongue elevation movement although it is less active during both other movements. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
For the assessment of facial–hypoglossal coactivation, we recorded a double‐sided surface myography (EMG) of the orbicularis oris muscle during the resting and activation phases. Two pairs of surface electrodes filled with conductive paste were attached with adhesive rings to both sides of the orbicularis oris muscle. The first electrode was attached to the muscle on the lateral side next to the philtrum, the second lateral to the nasolabial fold (Fig. 1, right). The electrodes were connected to a VARIOPORT‐B Biosignal‐Recorder (BECKER MEDITEC, Karlsruhe, Germany). EMG measurements were carried out immediately after the fMRI evaluation in a lying position, outside the MR environment to avoid artifacts since no MR‐compatible EMG system was available. We used blocks of 30‐s alternating rest and activation. The three different activation phases consisted of pursing the lips, lip elevation and elevating the tongue against the palate. EMG data were sampled with 100 Hz and amplified (300 times). Postprocessing of EMG data was performed using custom MATLAB software (MathWorks, Natick, MA, USA). Pairs of consecutive maxima and minima exceeding the noise threshold were detected for each activation block of the affected and nonaffected EMG data. Their difference was calculated and the resulting EMG amplitudes were averaged for each activation block. The mean EMG amplitudes for each condition were compared between the affected and nonaffected sides for all participants by using paired t‐tests in SPSS (IBM SPSS Statistics, Version 19).
Imaging
MRI was performed using a 3 T Scanner (Magnetom Verio, Siemens, Erlangen, Germany) equipped with a 32‐channel head coil. For each scanning, session field homogeneity was optimized by a shimming sequence and a GRE field map was acquired for the unwarping procedure. Echo planar imaging (EPI; TE, 30 ms; TR, 2,000 ms) was measured with 34 axially oriented slices of 3‐mm slice thickness with 1‐mm gap. We used a 64 × 64 matrix with a coronal tilt parallel to the central sulcus to maximize the resolution in z‐direction. EPIs were located in an anatomical scout (10 transversal T2‐weighted slices; 128 matrix, 5‐mm slice thickness) to cover the upper and lower areas of the pre‐ and postcentral gyrus. Additionally, an anatomical T1‐weighted three‐dimensional Magnetization Prepared Rapid Gradient Echo (MPRage) image was acquired for each participant. The total number of sagittal high‐resolution anatomical slices amounted to 176 (TR = 1,900 ms, TE = 2.52 ms, α = 90°, voxel size = 1 × 1 × 1 mm3).
Design and Tasks Performed in the Scanner
Ninety volumes were measured per task (units of four measurements during each task‐based movement phase alternating with five during rest phase indicated by a green [movement] or blue [rest] background with balanced brightness). To balance visual input between conditions, the frequency of the movement was presented with a 0.50Hz pulsation of a white circle, which was also present during rest. Visual signals were presented triggered by the scanner with the presentation software (Neurobehavioral Systems, Albany, NY, USA) and projected by a video projector on a screen, which could be observed via a double mirror system attached to the head coil. Participants were lying supine in the scanner and head movements were minimized by a rubber restraint. The following conditions were investigated in randomized sequence: “pursing of the lips” and “tongue elevation against the palate.” Each condition was trained before scanning to ensure correct performance and was monitored visually during fMRI measurement.
Data Reduction and Statistical Analysis
The fMRI was evaluated with the Statistical Parametric Mapping Program (SPM 8, Wellcome Institute for Imaging Neuroscience, London, UK) running on MATLAB (Version 7.4; MathWorks, Natick, MA,USA). Unwarping of geometrically distorted EPI was performed in the phase encoding direction with the help of a calculated field map. The scans of each individual were realigned to each other to correct for interscan movement artifacts. All sessions showed movement artifacts with a translation <1.5 mm and a rotation of <1.5°. The EPI of each subject was coregistered to the T1 anatomical data sets and resliced with 1 × 1 × 1 mm. EPIs were smoothed with a 6‐mm full‐width at half‐maximum isotropic Gaussian kernel to increase signal‐to‐noise ratio. We used this moderate smoothing for allowing high spatial accuracy. Furthermore, we performed an individual evaluation for representation maxima between hemispheres and were therefore not forced to correct for intersubject variation in brain anatomy for the normalization process. First‐level statistics were calculated with the hemodynamic response function and low‐frequency components were filtered with a cutoff of 128 s. Statistical maps were written in images, thresholded with P < 0.05, forced discovery rate (corrected) [Genovese et al., 2002], and used for overlay with the coregistered high‐resolution anatomic images in the 2D‐flattening method (for methodological background, see Lotze et al., 2000b).
We intended to evaluate the differences in the lip and tongue representation on the cortical surface between the affected and the nonaffected side. For this purpose, we directly measured distances between COG of cortical representation sites of the lip activation during the task “pursing of the lips” and of the tongue activation during “tongue elevation against the palate” within the pre‐ and postcentral gyrus (Fig. 1, middle). Additionally, we measured distances between the COG of functional representation within the pre‐ and postcentral gyrus in relation to the crossing of interhemispheric fissure and central sulcus, Cz. Using this structurally well‐defined location has been shown to be optimal for the evaluation of lip and tongue representation sites [Lotze et al., 2000b] and highly important for possible reorganization of hypoglossal innervation for facial motor function. Additionally, previous investigations using this method in healthy subjects revealed a highly symmetrical location of lip representations between hemispheres [Lotze et al., 2000a]. For all comparisons, the affected side (aff) was compared with the nonaffected (non) side. Furthermore, the coefficient of laterality (lateralization) was used to describe the quotient of the task “tongue elevation against the palate” between both hemispheres. For this purpose, we calculated the laterality index LI with ([D aff − D non]/[D aff + D non]) × 100 (%) [Nagata et al., 2001].
We performed calculations between fMRI values (laterality index of lip an tongue representation in M1 and S1; distance between the tongue elevation and the lip pursing representation sites in the affected hemisphere) with clinical outcome measures (symmetry of the lip during lip elevation as documented with a photo) and coactivation of lip–EMG during tongue elevation on the affected side.
RESULTS
Clinical Evaluation
All patients showed a persistent facial palsy on the affected side with a House–Brackmann Scale grade III (before surgical intervention the HBS grade was VI). Photo documentation of the maximal lip elevation demonstrated 5 ± 0.55 mm (standard deviation) less elevation on the affected side. The average EMG amplitude of the orbicularis oris muscle during “pursing the lips” was lower for the affected than for the nonaffected side t(12)= 3.42, P < 0.005).
For the lip elevation condition, EMG data of the affected side were associated with less symmetry detected via photo documentation (r = 0.60, P < 0.05). EMG amplitude over the affected side for lip elevation correlated positively with the time after hypoglossal–facial transfer (r = 0.56, P < 0.05). EMG during tongue elevation movement was also positively correlated with the photo‐documented outcome of lip elevation (r = 0.70, P < 0.01).
Cortical Representation and Correlations with Clinical Parameters
Lateralization of absolute distance between CZ and lip and tongue representation (anatomic site and COG of activation)
The lateralization of the distance between the Cz and the COG of the tongue and lip area between affected and nonaffected hemisphere in S1 correlated negatively with the clinical outcome as demonstrated in the photo documentation (tongue: r = −0.72, P < 0.01; lip: r = −0.67, P < 0.05 [significant for one‐sided comparison]). Consequently, the higher the lip symmetry during lip elevation, the greater the distance of Cz to COG of both the tongue and the lip in the affected side in comparison to the nonaffected side in S1. In M1, this effect did not reach significance level but showed a trend towards the same direction (tongue: r = −0.47; P = 0.076; lip: r = −0.51; P = 0.064).
Comparison of relative distance of the lip and tongue representation (COG) between hemispheres
fMRI evaluation showed a reduced distance in M1 between the COG of the tongue elevation and the lip pursing representation on the affected side (8.14 ± 6.14 mm) compared to the nonaffected side (10.30 ± 7.23 mm; t[12] = 2.23, P < 0.05; Fig. 2A). There was a trend for an association of decreased distance of representation areas between tongue and lip with coactivation of the lip and the tongue in the EMG‐measurements: The closer the representation site of the lip (pursing movement) was to that of the tongue (elevation movement) in the affected hemisphere, the higher the lip coactivation (orbicular EMG) during tongue elevation movements (M1: r = −0.53, P = 0.058; Fig. 2B, left; S1: r = −0.64, P < 0.01). Furthermore, the smaller the tongue–lip distance was in the affected hemisphere, the smaller the lateralization of lip elevation (the higher the lip symmetry) in the photo (M1: r = 0.64; P < 0.01; Fig. 2B, left; S1: r = 0.55; P < 0.05).
Figure 2.
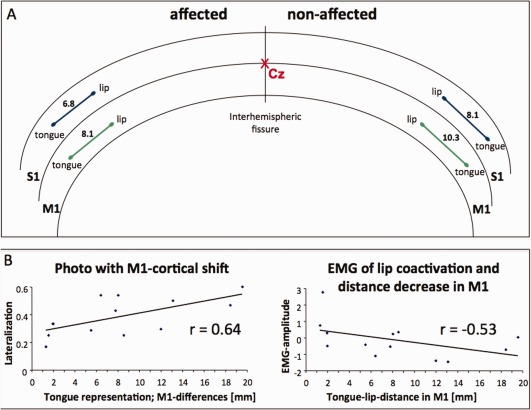
A: Schematic overview of the averaged results of the fMRI evaluation of representation sites (COG within S1 and M1) of the lip and the tongue in the nonaffected and the affected hemisphere. The tongue–lip distance was smaller in the affected hemisphere than in the nonaffected hemisphere (S1 = 6.8 < 8.1mm; M1 = 8.1 < 10.3 mm). The crossing of the interhemispheric fissure and the central sulcus is indicated with Cz. B: Correlation plots between fMRI representation in the precentral gyrus and clinical data (photo documentation of labial symmetry during lip elevation and labial EMG amplitude during tongue movements). Left: The more symmetrical the lip elevation in comparison with the affected and nonaffected side (less lateralization), the nearer the representation of the tongue to the lip. Right: The closer the representation site of the lip to that of the tongue in the precentral gyrus of the affected hemisphere, the higher the lip coactivation (orbicular EMG) during tongue movements. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Magnitude of BOLD effect
Activation magnitude in M1 was slightly increased for the affected side during tongue elevation movement (t[12] = 1.97; P < 0.05 [only significant for one‐sided comparison]).
DISCUSSION
The present study shows that long‐term functional recovery processes of lip movements after hypoglossal–facial transfer were associated with decreased distances between representation sites of the lip and tongue in the pre‐ and postcentral gyrus. A cortical reorganization of the tongue representation in the affected hemisphere did not only correlate with clinical outcome (photo‐documented lip elevation), but also with a higher coactivation of labial muscles (EMG) during tongue elevation movements. Those patients who successfully coactivated the tongue area during lip movements were those who showed better long‐term clinical outcome, but were unable to selectively recruit the original target muscles of the hypoglossal nerve without moving the associated muscles (labial muscles) simultaneously.
Even after a very long time period after the transfer, our patients showed a significant decrease in orbicularis–EMG amplitudes during lip elevation when compared to the nonaffected side. Therefore, surgical intervention and time were not effective for a complete motor restitution with respect to orbicular activity and lip elevation. Overall, the HBS was not capable of differentiating small changes observed during recovery of motor function contrary to other parameters used. All of our participants had an HBS score of III after a postoperative time of 12 months without further clinical improvement. Using the EMG and photo measurements, we were able to verify an increase in symmetry and activity of labial movements.
The fact that representation shifts in the postcentral gyrus correlated higher with the clinical outcome than those in the precentral gyrus might be caused by a generally more circumscribed and therefore more precisely definable somatosensory representation in response to a repetitive movement production. Although the representation in the postcentral gyrus is associated with the somatosensory feedback processing of the movement (e.g., tip of the tongue contact to teeth), the processes in the primary motor cortex are coding the whole movement pattern including stabilization, coding for movement direction and coordination of different muscle groups.
The extremely long‐lasting recovery process after hypoglossal–facial transfer is a matter of debate. Our patients were asked to do a regular training procedure in front of a mirror after surgery, a situation typical of these patients. We did not systematically evaluate how these instructions were implemented. However, it is known that repetitive training strategies after denervation have been shown to accelerate cortical plastic processes [Pereira et al., 2011] and recovery. Initially, after facial palsy without a reinnervation of the branches, motor training is not possible. After hypoglossal–facial transfer, additional time is needed until neural information can be transmitted via the suture line due to basal mechanisms of nerve regeneration (e.g., maximal nerve growth velocity 1 mm per day in man) [Sneddon, 1973].
The longer a complete denervation lasts, the more maladaptive processes might take place, overtaking functions of neighboring cortical areas (see, for instance, Lotze et al., 2006). These processes might be, for instance, a shift of neighboring areas into the deafferented area, as it has been described for severe facial palsy by Rijntjes et al. (1997) using TMS and positron emission tomography. These authors additionally demonstrated that reorganization is dependent on severity of denervation: patients with HBS grades III and IV showed a lateralization of the abductor pollicis brevis muscle associated centers of gravity contralateral to the facial palsy, whereas this could not be found in patients with lower degrees of facial palsy. In rats, deafferentation of the facial nerve induced extended disinhibition in the primary motor cortex area of both hemispheres [Farkas et al., 2000]. A delayed facial nerve repair in a crossface paradigm in rats resulted in accelerated and enhanced muscle reinnervation with reduced collateral axonal sprouting during a definite denervation period [Guntinas‐Lichius et al., 2000]. Comparable mechanisms exist in humans and therefore, short time periods of facial palsy and early intervention should be of great importance [Yetiser and Karapinar, 2007].
The combination of deafferentation (loss of sensory input) with deefferentation (loss of motor output) of large cortical areas results in massive cortical reorganization processes, including both motor and somatosensory reorganization [Cohen et al., 1991; Elbert et al., 1994]. Facial palsy, however, is more circumscribed and only affects the motor response (loss of motor output; deefferentation). Overall, during facial palsy, the displacement of the tongue area into the lip area is usually very small (on the cortical surface, approximately within the range of 2–3 mm) [Rijntjes et al., 1997; Rodel et al., 2004]. Furthermore, plastic changes also comprise an increase in facilitation in the affected somatotopic representation area. We could not demonstrate a significant shift of the tongue representation into the lip area, but we did observe a smaller distance of the COG between the lip and the tongue representation in the affected hemisphere. This closer representation of tongue elevation movement and lip pursing might be caused by hypoglossal branches, which formerly supplied tongue motor function and now represent partial facial function.
Other authors already pointed to the unique situation in patients suffering from facial palsy, where only motor function is impaired [Klingner et al., 2011; Rijntjes et al., 1999]. In these patients, a mismatch between the intact somatosensory response from the face and the denervated face muscles might result in specific cortical reorganization processes. For this special situation, together with the small cortical area affected, only mild shifts of neighboring representation sites into the lip area can be expected.
Bitter et al. (2011) postulated that, changes in somatotopic representation of the face, lip and tongue should be present only in the motor cortex but not in the somatosensory cortex as the facial denervation affects only the motor output and not the somatosensory input. With this investigation, we demonstrated that both reorganization processes are associated. Interestingly, some correlations between reorganization in S1, such as the decreased distance of COG of the lip and tongue and EMG‐coactivation, were more expressed. This emphasizes the close connection of motor and somatosensory cortex in active motor paradigms even when only one system is disconnected. It might be of interest to investigate the somatosensory representation in response to tactile stimulation without active movement performance in these patients.
Although time after surgical intervention was extremely long, the hypoglossal–facial transfer did not result in a specific recruitment of the facial branch to target the labial muscle. We did not record EMGs of the tongue during labial movements, which should be a topic of future studies, but we detected that a movement of the tongue coactivated lip elevation muscles in those patients who successfully recovered. Furthermore, these patients showed the largest shift of representation of lip movements into the area of the tongue in the affected hemisphere.
Overall, the plastic processes seem to be very unspecific, and it is extremely interesting how specific training procedures, such as EMG‐feedback training with selective recruitment of labial muscles but not tongue muscles, might help.
Absolute shifts of representation sites within a spatial resolution used in conventional fMRI experiments with a 3 × 3 mm spatial resolution are not the best way to detect these small shifts of plastic changes. A voxel‐by‐voxel group analysis is based on normalization procedures that additionally decrease spatial resolution due to smoothing (usually two to three times of the voxel size). Furthermore, the calculation of Euclidian distances seems to be less precise than flattening procedures [Lotze et al., 2000b]. In contrast, comparing the relative distances of representation sites between the COGs of lip and tongue representation between the affected and the nonaffected hemisphere seems to be better suited. These measures have been successfully applied for previous investigations on dipole shifts of somatosensory representation in MEG‐studies (e.g., Braun et al., 2001; Flor et al., 1995).
REFERENCES
- Bitter T, Sorger B, Hesselmann V, Krug B, Lackner K, Guntinas‐Lichius O. (2011): Cortical representation sites of mimic movements after facial nerve reconstruction: A functional magnetic resonance imaging study. Laryngoscope 121:699–706. [DOI] [PubMed] [Google Scholar]
- Braun C, Heinz U, Schweizer R, Wiech K, Birbaumer N, Topka H. (2001): Dynamic organization of the somatosensory cortex induced by motor activity. Brain 124:2259–2267. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Bandinelli S, Findley TW, Hallett M. (1991): Motor reorganization after upper limb amputation in man. A study with focal magnetic stimulation. Brain 114:615–627. [DOI] [PubMed] [Google Scholar]
- Elbert T, Flor H, Birbaumer N, Knecht S, Hampson S, Larbig W, Taub E. (1994): Extensive reorganization of the somatosensory cortex in adult humans after nervous system injury. Neuroreport 5:2593–2597. [DOI] [PubMed] [Google Scholar]
- Farkas T, Perge J, Kis Z, Wolff JR, Toldi J. (2000): Facial nerve injury‐induced disinhibition in the primary motor cortices of both hemispheres. Eur J Neurosci 12:2190–2194. [DOI] [PubMed] [Google Scholar]
- Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, Larbig W, Taub E. (1995): Phantom‐limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature 375:482–484. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. (2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15:870–878. [DOI] [PubMed] [Google Scholar]
- Guntinas‐Lichius O, Effenberger K, Angelov DN, Klein J, Streppel M, Stennert E, Neiss WF. (2000): Delayed rat facial nerve repair leads to accelerated and enhanced muscle reinnervation with reduced collateral axonal sprouting during a definite denervation period using a cross‐anastomosis paradigm. Exp Neurol 162:98–111. [DOI] [PubMed] [Google Scholar]
- Guntinas‐Lichius O, Streppel M, Stennert E. (2006): Postoperative functional evaluation of different reanimation techniques for facial nerve repair. Am J Surg 191:61–67. [DOI] [PubMed] [Google Scholar]
- Hesselmann V, Sorger B, Lasek K, Guntinas‐Lichius O, Krug B, Sturm V, Goebel R, Lackner K. (2004): Discriminating the cortical representation sites of tongue and up movement by functional MRI. Brain Topogr 16:159–167. [DOI] [PubMed] [Google Scholar]
- House JW, Brackmann DE. (1985): Facial nerve grading system. Otolaryngol Head Neck Surg 93:146–147. [DOI] [PubMed] [Google Scholar]
- Klingner CM, Volk GF, Maertin A, Brodoehl S, Burmeister HP, Guntinas‐Lichius O, Witte OW. (2011): Cortical reorganization in Bell's palsy. Restor Neurol Neurosci 29:203–214. [DOI] [PubMed] [Google Scholar]
- Lotze M, Grodd W, Birbaumer N, Erb M, Huse E, Flor H. (1999): Does the use of a myoelectric prosthesis prevent cortical reorganization and phantom limb pain? Nat Neurosci 2:501–502. [DOI] [PubMed] [Google Scholar]
- Lotze M, Erb M, Flor H, Huelsmann E, Godde B, Grodd W. (2000a): fMRI evaluation of somatotopic representation in human primary motor cortex. Neuroimage 11:473–481. [DOI] [PubMed] [Google Scholar]
- Lotze M, Seggewies G, Erb M, Grodd W, Birbaumer N. (2000b): The representation of articulation in the primary sensorimotor cortex. Neuroreport 11:2985–2989. [DOI] [PubMed] [Google Scholar]
- Lotze M, Käthner RJ, Erb M, Cohen LG, Grodd W, Topka H (2003): Comparison of representational maps using fMRI and TMS. Clin Neurophysiol 114:306–312. [DOI] [PubMed] [Google Scholar]
- Lotze M, Laubis‐Herrmann U, Topka H. (2006): Combination of TMS and fMRI reveals a specific pattern of reorganization in M1 in patients after complete spinal cord injury. Restor Neurol Neurosci 24:97–107. [PubMed] [Google Scholar]
- Merzenich MM, Nelson RJ, Stryker MP, Cynader MS, Schoppmann A, Zook JM. (1984): Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol 224:591–605. [DOI] [PubMed] [Google Scholar]
- Nagata SI, Uchimura K, Hirakawa W, Kuratsu JI. (2001): Method for quantitatively evaluating the lateralization of linguistic function using functional MR imaging. AJNR Am J Neuroradiol 22:985–991. [PMC free article] [PubMed] [Google Scholar]
- Neely JG, Neufeld PS. (1996): Defining functional limitation, disability, and societal limitations in patients with facial paresis: Initial pilot questionnaire. Am J Otol 17:340–342. [PubMed] [Google Scholar]
- Pellat JL, Bonnefille E, Zanaret M, Cannoni M. (1997): Hypoglossal‐facial anastomosis. A report of 60 cases. Ann Chir Plast Esthet 42:37–43. [PubMed] [Google Scholar]
- Pereira LM, Obara K, Dias JM, Menacho MO, Lavado EL, Cardoso JR. (2011): Facial exercise therapy for facial palsy: Systematic review and meta‐analysis. Clin Rehabil 25:649–658. [DOI] [PubMed] [Google Scholar]
- Rijntjes M, Tegenthoff M, Liepert J, Leonhardt G, Kotterba S, Muller S, Kiebel S, Malin JP, Diener HC, Weiller C. (1997): Cortical reorganization in patients with facial palsy. Ann Neurol 41:621–630. [DOI] [PubMed] [Google Scholar]
- Rijntjes M, Dettmers C, Buchel C, Kiebel S, Frackowiak RS, Weiller C. (1999): A blueprint for movement: Functional and anatomical representations in the human motor system. J Neurosci 19:8043–8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodel RM, Tergau F, Markus H, Laskawi R. (2004): Bilateral changes in cortical motor representation of the tongue after unilateral peripheral facial paralysis: Evidence from transcranial magnetic stimulation. Ann Otol Rhinol Laryngol 113:951–955. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Suner S, Donoghue JP. (1990): Dynamic organization of primary motor cortex output to target muscles in adult rats. I. Long‐term patterns of reorganization following motor or mixed peripheral nerve lesions. Exp Brain Res 79:479–491. [DOI] [PubMed] [Google Scholar]
- Sneddon HJ. (1973): Surgical disorders of the peripheral nerves.Baltimore:Williams & Wilkins; p267. [Google Scholar]
- Volk GF, Pantel M, Guntinas‐Lichius O. (2010): Modern concepts in facial nerve reconstruction. Head Face Med 6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetiser S, Karapinar U. (2007): Hypoglossal‐facial nerve anastomosis: A meta‐analytic study. Ann Otol Rhinol Laryngol 116:542–549. [DOI] [PubMed] [Google Scholar]


