Abstract
Background
Carpinus tschonoskii (CT) has been previously studied for various activities in the improvement of skin diseases. In the present study, we examined the in vitro anti-acne vulgaris (AV) effect of CT leaves (CTL) and tellimagrandin I (TI), one of the main ellagitannins from CT, including skin barrier improvement and 5α-reductase inhibitory activity.
Methods
To test the anti-AV activities of CTL and TI, firstly, anti-oxidative and anti-inflammatory activities including DPPH radical scavenging activity, nitric oxide (NO) inhibitory activity, and cytokines [interleukin (IL)-6 and IL-8] were tested. Skin barrier improvement experiments were tested using developing cornified envelope (CE) formation, and filaggrin mRNA expression level was determined by RT-PCR. The 5α-reductase inhibitory activity was determined by measuring the testosterone levels in rat liver microsomes.
Results
CTL and TI showed potent anti-oxidative activity and anti-inflammatory activities. Especially, the cytokine production inhibitory activities of TI were found to be similar to the positive control, epigallocatechin gallate (EGCG). CTL and TI enhanced the CE formation and filaggrin mRNA expression levels and showed potent activities compared to that in the positive control, 1.5 mM Ca2+. In additionally, CTL and TI showed 5α-reductase inhibitory activities in a dose-dependent manner.
Conclusion
The results showed that CTL and TI inhibit AV endogenous factors such as 5α-reductase and inflammatory cytokines and affect exogenous factors such as developing skin barrier function (CE and filaggrin levels). Therefore, CTL and TI may be plant-derived agent, promising in the treatment of acne vulgaris.
Keywords: Carpinus tschonoskii, 5α-reductase inhibition activity, Anti-acne vulgaris, Skin barrier, Anti-inflammation, Anti-oxidant activity
Background
The skin confers protection from external stimuli including physical and chemical stresses such as air pollution, ultraviolet radiation, and wounds. Differentiation of keratinocytes is essential to maintain the barrier function of skin [1, 2]. Keratinocytes have a role in the skin barrier function and proliferate in the basal layer, being increasingly differentiated from the spinous layer to corneocytes. In the terminal differentiation process, increase of intercellular Ca2+ levels induces expression of periplakin, involucrin, loricrin, filament aggregating protein (filaggrin), and other factors that promote differentiation. In particular, filaggrin plays an important role in the formation of a cornified envelope (CE), because it binds to and is responsible for aggregation of keratin filaments. These skin barrier factors crosslink keratin filaments and ceramide in the plasma membrane, resulting in the formation of a CE, which is a strong physical barrier and permeation barrier comprising 10 nm thick layers of insoluble proteins [3–6].
When keratinocytes are exposed to external stimuli, they produce inflammation-induced factors including free radicals, cytokines, and nitric oxide (NO), resulting in inflammatory reactions. These inflammatory reactions impair the skin barrier [4, 6–8]. Keratinocytes produce a variety of cytokines that regulate cellular communication. Deregulated cytokine expression can thus contribute to a dysfunctional skin barrier as observed in chronic inflammation disease [8–10]. The cytoplasm of keratinocytes in all epidermal layers contains the pro-inflammatory cytokines, interleukin-6 (IL-6), IL-8, and tumor necrosis factor α (TNF-α) Especially, IL-6 is reported to downregulate filaggrin [8, 10–17]. Additionally, free radicals including reactive oxygen species (ROS) mediate oxidative stress and produce inflammatory cytokines such as IL and TNF contribute to the pathogenesis of many inflammatory skin diseases [8, 10, 18].
Acne vulgaris (AV) is a chronic inflammatory disease of the pilosebaceous units resulting from androgen-induced sebum production and inflammation [19–22]. Among these causes, androgen-induced sebum production is associated with 5α-reductase reactions [23–25]. The enzyme 5α-reductase metabolizes testosterone to dihydrotestosterone (DHT). However, imbalance between testosterone and DHT causes acne and benign prostatic hyperplasia [26, 27]. In AV, sebum secretion, dehydration of the corneum, and transepidermal water loss (TEWL) are increased. In clinical research, TEWL is an important factor for skin barrier function. A marked increase in TEWL was observed in patients with AV of moderate severity compared to that in patients with AV of mild severity and in normal controls. These results indicate that the degree of corneum permeability barrier impairment directly matches the severity of AV. In addition, the follicular epithelium also contributes to skin barrier functions. Impairment of the skin barrier occurs when the intensity of inflammation increases and weakens the follicular wall [28, 29].
Carpinus tschonoskii (CT), a deciduous broad-leaved arboreous tree, is a member of the genus Carpinus and Betulaceae family, native to Korea, Japan, and China [30, 31]. The genus Carpinus has been widely and traditionally used to treat bladder infection, osteoporosis, and anxiety disorders [32]. According to the “Coloured flora of Korea,” leaves of CT are oval shaped and have a doubly serrate margin [33]. The bark of CT has been used as a material for furniture and bed logs [34]. In a previous study, CT has been confirmed to show biological activities including cytoprotective activities, suppression of tyrosinase expression, whitening activities, anti-wrinkle, anti-allergic activities, and neuroprotective activities [31, 34–36].
Despite many studies on skin diseases, there have been few experiments demonstrating the skin improvement effects of CT in AV. The purpose of this study was conducted to assess the skin improvement effects of CT leaf (CTL) extract and tellimagrandin I (TI), which was isolated from CTL, on AV.
Methods
Plant materials
The leaves of Carpinus tschonoskii were obtained from the Yeoju Eco Park, Yeoju, Republic of Korea, in July 2018. Plant materials were distinguished by Kim Sungsik, Ph.D. (Korea National Arboretum, Pocheon). Voucher specimens were placed at the herbarium of the College of Pharmacy, Chung-Ang University (CTLYZ-1806).
General experimental procedures
The column chromatography isolation was performed on a Sephadex LH-20 column (10–25 m; GE Healthcare Bio-Science AB, Uppsala, Sweden). Structural identification was by one-dimensional nuclear magnetic resonance (1D-NMR) including 1H-NMR (600 MHz) and 13C-NMR (125 MHz) (JEOL, Tokyo, Japan) at Chung-Ang University.
Extraction, isolation and structure elucidation
Leaves (1.2 kg) of CT were extracted for 72 h at room temperature (25 °C) with 70% prethanol A (ethyl alcohol), after removing the solution under vacuum, the CTL extract (252 g) was obtained. The CTL extract (142 g) was dissolved in water, the water layer was filtered through Celite 545 (Duksan Pure Chemicals Co. Ltd., Seoul, Korea). Filtrate was concentrated and applied to Sephadex LH-20 column (25–100 μm; Pharmacia, Uppsala, Sweden) and eluted with water (H2O)-methanol (MeOH) gradient system, eleven fractions were obtained. Repeated column chromatography of fraction 10 (11.64 g) on Sephadex LH-20 with water-methanol gradient system to obtained tellimagrandin I (TI, 1.98 g). The structure of TI was identified by analysis of 1H-NMR and 13C-NMR spectra and comparison with reference [31].
Chemical and reagents
Dulbecco’s Modified Eagle Medium (DMEM), trypsin, and fetal bovine serum (FBS) were purchased from Welgene (Gyeongsan, Republic of Korea). Streptomycin–penicillin was purchased from Gibco (NY, USA). Calcium-free DMEM, superscript™ IV first-strand synthesis system, and Dream taq Green PCR Mix were purchased from Thermo Fisher Scientific (MA, USA). TRIzol reagent was purchased from Invitrogen (CA, USA). Sodium dodecyl sulfate (SDS), dithiothreitol (DTT), agarose, lipopolysaccharide (LPS), ethyl ether, 1,1-diphenyl-2-picrylhydrazyl (DPPH), Griess reagent, NG-Methyl-l-arginine acetate salt (L-NMMA), and thiazolyl blue tetrazolium bromide (MTT) were obtained from Sigma Aldrich (St. Louis, USA). Reagent set B, cytokine IL-6 and IL-8 ELISA sets used for immunoassay were purchased from BD Biosciences (NJ, USA). TI was acquired in a previous study [31].
Anti-oxidative activity
Measurement of DPPH radical scavenging activity
To evaluate the radical scavenging activities of CTL extract and TI, DPPH assay was conducted. DPPH is bound with the hydrogen of anti-oxidants, because nitrogen in hydrazyl on DPPH has an unstable radical. DPPH radical scavenging activities were assessed by confirming the color change in DPPH accompanied with the reaction to anti-oxidants [37]. To assess anti-oxidant activities, samples dissolved in anhydrous ethyl alcohol were added (20 μL) into a 96-well plate, followed by addition of 0.2 mM DPPH (180 μL). No sample adding, 0.2 mM DPPH 200 μL was made as the negative control. After gentle shaking for 15 min at room temperature, optical density (OD) was measured at 517 nm using an ELISA reader (TECAN, Salzburg, Austria). The OD was used for calculation as follows: the rate of inhibition (%) = [1 - (sample OD/negative control OD)] × 100. IC50 values were defined as the concentration needed to scavenge 50% free radicals. Ascorbic acid served as the positive control [38].
Cell culture
RAW 264.7 cells were purchased from the Korean Cell Line Bank. HaCaT cells (spontaneously immortalized keratinocyte cell line) were purchased from the Dermatology department of Chung-Ang University hospital. These cells were incubated at 37 °C in a humidified atmosphere (5% CO2) in DMEM containing 10% FBS, 100 IU mL− 1 penicillin G and 100 mg mL− 1 streptomycin.
Cytotoxicity
To evaluate the cytotoxicity of the CTL extract and TI, MTT assay was performed, and absorbance resulting from the mitochondrial conversion of MTT to formazan was measured. MTT is the colorimetric assay used to measure cell proliferation, viability or cytotoxicity. Cells were inoculated into a 96-well plate and placed in the incubator for 6 h. The cells were then treated with samples at various concentrations (12.5, 25, 50, and 100 μg mL− 1 or μM) and incubated for an additional 24 h. Afterward, the medium was replaced with 100 μL of phosphate buffered saline (PBS) containing 0.5 mg mL− 1 MTT and incubated for a further 4 h at 37 °C. MTT-PBS was then removed, and the formazan was dissolved in 100 μL dimethyl sulfoxide. The extent of MTT reduction to formazan within the cells was quantified by measuring the absorbance at 540 nm by using an ELISA reader. The cytotoxicity was calculated as cell viability (%) = sample OD/blank OD × 100.
Anti-inflammatory activity
Measurement of inhibitory activity on NO production
RAW 264.7 cells were inoculated into a 96-well plate and incubated for 6 h at 37 °C in 5% CO2. The cells were then incubated in a medium containing 1 μg mL− 1 LPS and the samples. After incubating for an additional 24 h with samples (100, 50, 25 μg mL− 1 for extract level; 100, 50, 25 μM for compound level), the NO contents were evaluated by Griess assay. Griess reagent was added to each of the supernatants from the cells. L-NMMA was used as a positive control. Inhibition of NO synthesis was calculated as inhibition rate (%) = [1 - (sample OD - blank OD)/(control OD - blank OD)] × 100, and the IC50 values were defined as concentrations that inhibit 50% of NO production [38].
Measurement of inhibitory activity on cytokine production
Differentiated HaCaT cells were inoculated into a 96-well plate and placed for 6 h at 37 °C in 5% CO2. A medium containing 1 μg mL− 1 LPS and samples were added to the wells and incubated for an additional 24 h. Afterward, the supernatant from the cells was stored in a 1.5 mL tube [39]. Concentrations of cytokines IL-6 and IL-8 were analyzed in the culture supernatants by ELISA following the manufacturer’s protocol. Cytokine production was measuring using the ELISA reader at 450 nm. The results were calculated using a standard calibration curve [40].
5α-reductase inhibitory activity
Liver microsomes containing 5α-reductase from 7-week-old male Sprague-Dawley (SD) rats were prepared. (Han Lym Lab. Animal Co., Hwaseong, Korea) Mature male SD rats were anesthetized using ethyl ether. Using a strong pair of dissecting scissors, the chest cavity of SD rats was opened and the hepatic portal vein was nicked. A syringe was used to inject cold STM buffer (270 mM sucrose, 10 mM Tris-HCl, pH 7.5, and 1 mM MgCl2, G-BIOSCIENCES, MO, USA) into the left ventricle of the heart. STM buffer was thus perfused to the liver and the color of the liver changed from a dark red to a light pink. The liver was then quickly dissected and minced in a beaker by using a pair of scissors. The minced tissue was then homogenized in 3-tissue volumes of STM buffer [41]. The homogenates were centrifuged at 10,000×g for 10 min. The pellet was washed with 2-pellet volumes of STM buffer. The supernatant from the centrifugation contained microsomes suspended in STM buffer; these were homogenized using a syringe with 18 G, 23 G, and 25 G needles [42]. The microsomes were divided into aliquots and stored in a deep freezer (− 80 °C); before use, the microsomes were diluted to 1/5 concentration.
To evaluate the inhibitory activity on 5α-reductase, each group was treated with phosphate buffer (pH 6.5), testosterone (500 μg mL− 1, 100 μL), microsomes (700 μL), nicotinamide adenine dinucleotide phosphate (NADPH, 770 μg mL− 1, 350 μL), and the sample or finasteride (600 μL) except the intact group and normal group. Finasteride was used as a positive control, which led to a decrease in DHT concentration. The intact group was the enzyme blank group, and the normal group had the full activity of 5α-reductase reaction without interference. The reaction was carried out in the incubator for 30 min at 37 °C. To complete the reaction, 2 mL of dichloromethane was added to every group [43]. All groups were stored at − 25 °C and concentrated to obtain an unfrozen organic solvent layer. The concentrates were dissolved in 500 μL of 50% methanol. Testosterone was quantified using HPLC instead of radioimmunoassay (RIA), because the RIA method has hazards from radioactive compounds and requires complex equipment. The analyte was eluted at a flow rate of 1 mL min− 1 using the binary gradient H2O (A) and ACN (B). The quantification wavelength of these chromatograms was set at 242 nm, which was optimized for testosterone. The injection volume was 10 μL. The data were analyzed using the Autochro-Win 3.0 plus software system. The following equation was used for calculation: 5α-reductase inhibitory rate (%) = [1 - (area of sample – area of intact)/(area of normal – area of intact)] × 100, and the IC50 values were defined as the concentrations that inhibit 50% of the 5α-reductase reaction.
mRNA expression of filaggrin
HaCaT cells were incubated at 37 °C in 5% CO2 in Ca2+ free DMEM containing 10% FBS, 100 IU mL− 1 penicillin G, 1.5 mM CaCl2, 1 M HEPES, and Glutamax. HaCaT cells were inoculated into 6-well plates at 3 × 105 cells mL− 1 and incubated in a 5% CO2 incubator at 37 °C for 24 h. The medium was then removed, and the cells were treated with appropriately diluted CTL extract and TI along with 20 ng mL− 1 TNF-α. The treated cells were incubated for about 1 day [17, 44].
Total RNA was extracted using TRIzol reagent. Next, 0.5 μg of total RNA was reverse-transcribed to cDNA using a Superscript™ IV first-strand synthesis system. The cDNA was used as a template for PCR amplification with 22 cycles using a DNA engine gradient cycler (MJ Research, Inc., MA, USA). The cycles consisted of the following steps: denaturation for 30 s at 95 °C, annealing at 56 °C for 30 s, and extension at 72 °C for 1 min [4]. β-Actin was used as the internal control. The PCR primers used were as follows: filaggrin, sense, AAGCTTCATGGTGATGCGAC, antisense, TCAAGCAGAAGAGGAAGGCA; and β-actin, sense, ACACTGTGCCCATCTACGAGGGG; antisense, ATGATGGAGTTGAAGGTAGTTTCGTGGAT.
The bands were visualized using a chemiluminescence imaging system (Fusion Sl, Collégien, France). Expression of filaggrin mRNA was normalized to that of β-actin by densitometry, and the EC50 values were defined as concentrations that caused a 50% effect.
Cornified envelope formation
CE assay was performed as an experimental method to measure keratinocyte differentiation ability. HaCaT cells were inoculated into 6-well plates at 3 × 105 cells mL− 1 and cultured in a 5% CO2 incubator at 37 °C for 24 h. Following the culture, the medium was removed and the cells were treated with required dilutions of CTL extract and TI in a medium containing 0.05 mM CaCl2. The normal control was treated with medium containing 0.05 mM CaCl2 and the positive control was treated with medium containing 1.5 mM CaCl2. After treatment with the samples, the cells were incubated for about 5 days.
The cells were then harvested and 1 mL of 10 mM Tris-HCl (pH 7.4) containing 2% SDS and 20 mM DTT was added to the pellet containing the cells. After this, the supernatant was sonicated for 3 min and boiled at 100 °C for 10 min. The samples were centrifuged (1200 rpm, 30 min, 4 °C), the precipitates were suspended in PBS, and the absorbance was measured at 340 nm. The following equation was used for calculation: CE formation rate (%) = [1 - (sample OD - normal OD)/(control OD – normal OD)] × 100, and EC50 values were defined as the concentrations that cause a 50% effect on CE formation [44].
Statistical analysis
Analysis of variance was performed using SPSS software (SPSS, Inc., Chicago, IL, USA). All data are expressed as the mean ± SD of triplicate experiments, and statistically significant differences were analyzed by one-way analysis of variance (p < 0.05), and t-test (***, p < 0.001; **, p < 0.01; *, p < 0.05).
Results
Anti-oxidative activity
DPPH radical scavenging activity
To assess the anti-oxidative activities of CTL extract and TI, DPPH radical scavenging activities were measured. CTL extract (IC50 = 31.42 ± 0.73 μg mL− 1) showed potent DPPH radical scavenging activities compared with the positive control, ascorbic acid (IC50 = 14.67 ± 2.90 μg mL− 1). TI (IC50 = 12.88 ± 0.30 μM) also showed potent DPPH radical scavenging activities compared with ascorbic acid (IC50 = 9.13 ± 0.49 μM) (Table 1).
Table 1.
IC50 values of Carpinus tschonoskii leaf (CTL) extract and tellimagrandin I (TI) on each bioactivity, including free radical scavenging activity, inhibitory activity of NO production, IL-6, IL-8 and 5α-reductase
| Samples | IC50 | |||||
|---|---|---|---|---|---|---|
| radical scavenging activity | inhibitory activity of NO | inhibitory activity of IL-6 | inhibitory activity of IL-8 | inhibitory activity on 5α-reductase | ||
| (μg mL−1) | CTL | 31.42 ± 0.73a | 5.98 ± 0.33a | 14.20 ± 7.07a | 7.46 ± 2.60a | 197.81 ± 1.92a |
| Positive Control | 14.67 ± 2.90b | 1.48 ± 0.98b | 2.98 ± 1.47b | 0.74 ± 0.09b | 40.65 ± 9.56b | |
| Compound (μM) | TI | 12.88 ± 0.30a | 5.44 ± 1.84a, b | 6.82 ± 2.37a | 0.56 ± 0.56a | 331.75 ± 14.62a |
| Positive Control | 9.13 ± 0.49b | 2.35 ± 1.14b | 6.68 ± 1.86a | 0.56 ± 0.52a | 45.98 ± 15.40b | |
The results were expressed as mean ± standard deviation (SD) of triplicate experiments. (n = 3) a-bin the same column are significantly different, p < 0.05. Positive control of free radical scavenging experiment is ascorbic acid, positive control of inhibitor activity of NO production is L-NMMA, positive control of inhibitory activity of IL-6 and IL-8 is EGCG, positive control of inhibitory activity of 5α-reductase is Finasteride
Cytotoxic activity
Before evaluating the improvement effects on skin barrier and AV, MTT assay was conducted to determine the cytotoxicity of CTL extract and TI on RAW 264.7 cells and HaCaT cells. No cytotoxicity of these compounds was observed (data not shown).
Anti-inflammatory activity
Inhibitory activity on NO production
In order to assess the anti-inflammatory activities of CTL extract and TI, NO inhibitory activities were measured in RAW 246.7 cells. As shown in Table 1, CTL (IC50 = 5.98 ± 0.33 μg mL− 1) extract showed potent anti-inflammatory activity, and TI (IC50 = 5.44 ± 1.84 μM) showed potent inhibitory effects against NO production compared with the positive control, L-NMMA (IC50 = 2.35 ± 1.14 μM).
Inhibitory activity on cytokine production
To assess the inhibitory effects on IL-6 of production, cells were treated with LPS (1 μg mL− 1) to induce inflammation. After LPS exposure, the inhibitory effects of CTL extract and TI on the cytokine levels was measured. IL-6 concentration was decreased in the sample-treated groups. As shown in Table 1, CTL extract (IC50 = 14.20 ± 7.07 μg mL− 1) showed potent anti-inflammatory activity. TI (IC50 = 6.82 ± 2.37 μM) showed a similar inhibitory activity compared with the positive control, EGCG (IC50 = 6.68 ± 1.86 μM) against IL-6 production.
These inflammatory reactions result in impairment of the skin barrier. As shown in Table 1, CTL extract (IC50 = 7.46 ± 2.60 μg mL− 1) showed moderate IL-8 production inhibitory activity. TI (IC50 = 0.56 ± 0.56 μM) also showed an equal inhibitory activity compared with the positive control, EGCG (IC50 = 0.56 ± 0.52 μM) against IL-8 production.
Cornified envelope formation activity
Relative CE formation levels were evaluated based on the normal control, the 0.05 mM Ca2+ treated group. CE formation levels showed potent increases with the CTL extract compared with the positive control (Table 2). At the compound level, the CE formation level showed potent increases with TI compared with the positive control, with 100 μM of TI showing strong CE formation.
Table 2.
EC50 values of Carpinus tschonoskii leaf (CTL) extract and tellimagrandin I (TI) for effective activity on cornified envelope (CE) formation
| Extract | IC50 (μg mL−1) | Compounds | IC50 (μM) |
|---|---|---|---|
| CTL | 30.42 ± 4.79 | TI | 27.15 ± 3.24 |
The results are expressed as mean ± standard deviation (SD) of triplicate experiments. (n = 3)
mRNA expression of filaggrin
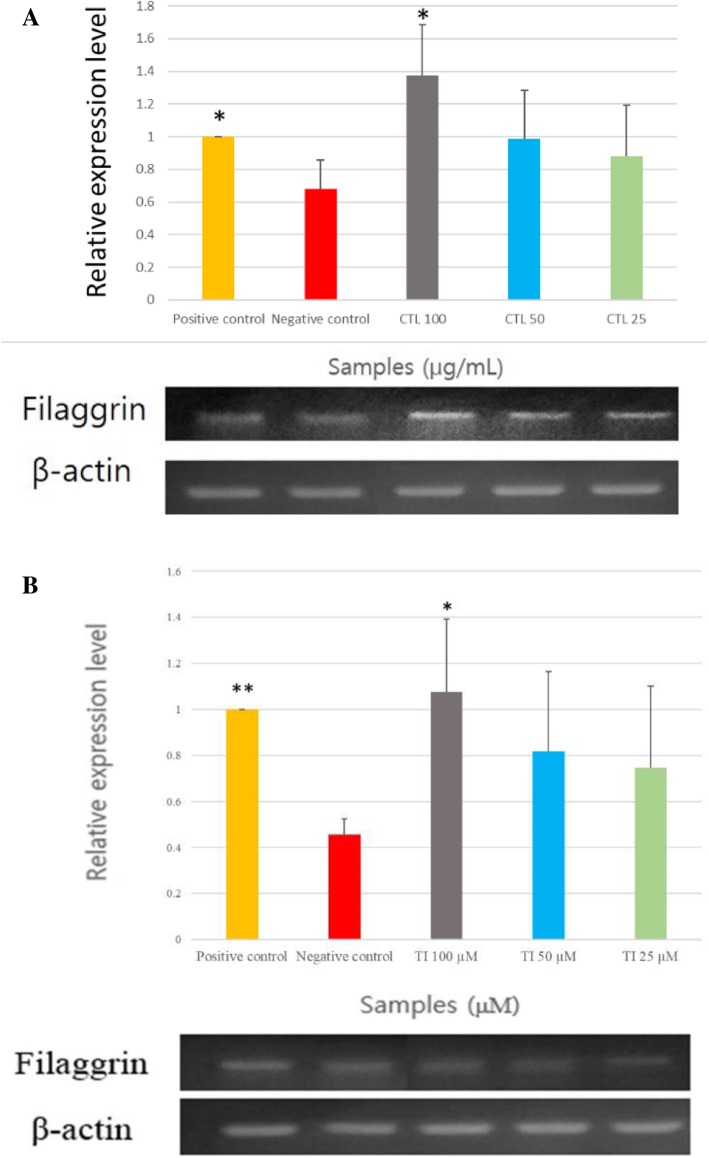
Relative filaggrin mRNA expression levels were evaluated based on the positive control, 1.5 mM Ca2+ treated group. Filaggrin mRNA expression levels appeared to be strongly induced upon treatment with the CTL extract compared with the positive control (Fig. 1). The filaggrin mRNA expression levels with CTL extracts were greater than those obtained with the positive control. At the compound level, filaggrin mRNA expression level showed potent increases with TI treatment compared with the positive control. The filaggrin mRNA expression level normalized to β-actin with 100 μM of IT was found to be similar to the positive control and greater than negative control.
Fig. 1.
Relative filaggrin mRNA expression level upon treatment with (a): Carpinus tschonoskii leaf (CTL) extract and (b): tellimagrandin I (TI) normalized to β-actin. The results are expressed as mean ± standard deviation (SD) of triplicate experiments. (n = 3) **, p < 0.01; *, p < 0.05, comparison with negative control group
5α-reductase inhibitory activity
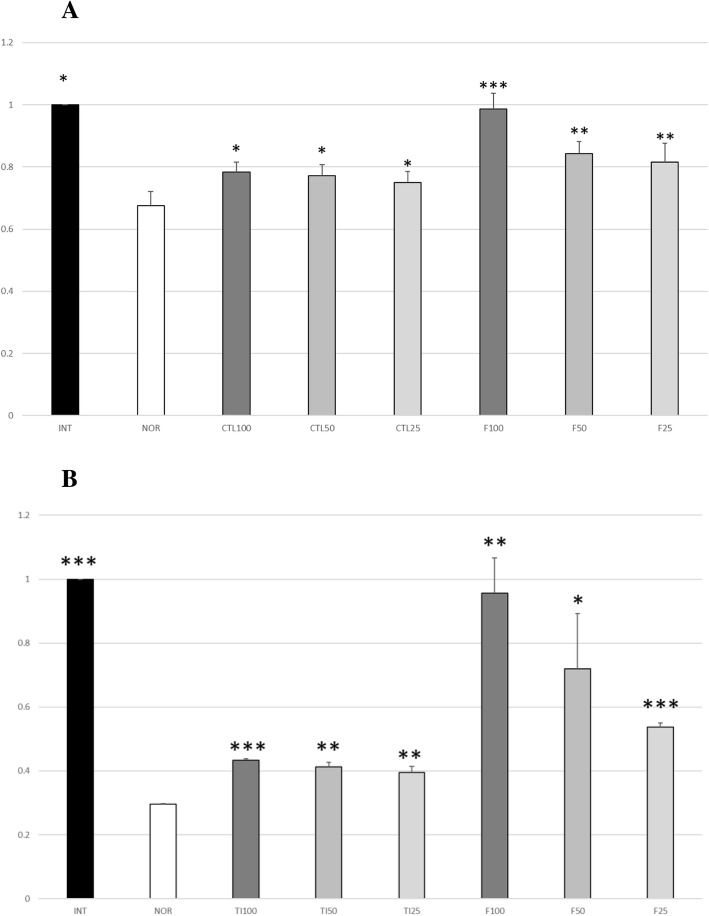
To evaluate the 5α-reductase inhibitory activity, the content of testosterone was determined through HPLC. The retention time of testosterone was 11.36 ± 0.1 min and was separated without interfering with other substances. Compared with the normal control, the sample-treated groups showed less decreased testosterone contents. Thus, CTL extract and TI suppressed the reaction of 5α-reductase with testosterone in a dose-dependent manner. As shown in Table 1 and Fig. 2, the CTL extract and TI showed 5α- reductase inhibitory activity.
Fig. 2.
Relative testosterone level upon treatment with (a): Carpinus tschonoskii leaf (CTL) extract and (b): tellimagrandin I (TI). The results are expressed as mean ± standard deviation (SD) of triplicate experiments. (n = 3) ***, p < 0.001; **, p < 0.01; *, p < 0.05, comparison with normal control group
Discussion
ROS and NO plays an important role in the adjustment of the skin’s response to stimuli such as UV, heat, and infection. NO synthase 2 (NOS2) that generates NO is found to be expressed in inflammatory skin barrier diseases such as psoriasis [45, 46]. Ellagitannins possess hexahydroxydiphenyl (HHDP), which is the reason for the anti-oxidative activity of TI [47–49].
IL-6 remarkably upregulates the expression of MAP 17, which reduces filaggrin expression and downregulates CE formation, and activated IL-6 is an immune regulator produced by human keratinocytes [15, 16, 50–52]. IL-8 induces ROS, which causes damage to keratinocytes and stimulates production of inflammatory cytokines and lipid chemoattractants such as leukotriene B4 [11, 53]. In this experiments, CTL and TI showed potent anti-oxidative, anti-inflammatory and cytokine production inhibitory activities suggested that CTL and TI are good agents to protect skin and regulate the immune system. Comparing with other monomeric ellagitannins from other natural plants, TI showed potent anti-oxidative, anti-inflammatory and cytokine production, similar to the positive control, EGCG [47, 48].
CE is one of the most important components of barrier function and is a criterion to distinguish the late differentiation of keratinocytes. Correct formation of CE is important for skin barrier functions [5, 16]. Filaggrin plays an important role in the formation of CE. Filaggrin aggregates keratin filaments in corneocytes. It plays a role in the mechanical strength of the CE, and thus, is the essential factor of barrier function [54]. In addition, filaggrin is degraded to amino acids such as histidine and glutamine, which are respectively converted to urocanic acid and 2-pyrrolidone-5-carboxylic acid, which are important moisturizing factors [55]. Previously, it was reported that Eucommia ulmoides oliver (EU) showed CE and filaggrin enhance effect and CTL showed potent CE and filaggrin enhance effect comparing with 50 μg/mL of EU [44]. Therefore, CTL and TI increased CE and filaggrin protein level is a good way to develop skin barrier.
Androgen is reported to be a key factor in acne pathogenesis; especially, DHT in adulthood could induce balding, prostate growth, and sebaceous gland activity [56]. Furthermore, the activated sebaceous gland increases the production of sebum leading to blockage of the sebaceous gland duct, causing acne. The enzyme 5α-reductase metabolizes testosterone to DHT, but abnormally high 5α-reductase activity results in excessive DHT production [26]. .It is reported that caffeoyl derivatives from Adina rubella showed good 5α-reductase inhibitory activity [57]. In this experiment, CTL and TI inhibit androgen-induced sebum production by inhibiting the 5α-reductase reaction that produces DHT.
In conclusion, CTL and TI showed high activity as anti-AV endogenous factors, such as anti-oxidative and anti-inflammatory activities, cytokine inhibition, 5α-reductase inhibition, and exogenous factor activities, such as CE formation and increased filaggrin mRNA expression, to improve the skin barrier. Although TI which was isolated from CTL seemed to be potential for the application as new drug CTL extract also seem more economic value than TI for the extract contains other ellagitannins.
Conclusion
CTL and TI showed potent anti-oxidative and anti-inflammatory activities with improved CE formation, increased filaggrin mRNA expression, and 5α-reductase inhibitory activity. These results suggest that CTL and TI might be plant-derived agent, promising in the treatment of acne vulgaris.
Acknowledgements
This study was carried out with the support of ´R&D Program for Forest Science Technology (Project No. 2017037A00-1919-BA01)´ provided by Korea Forest Service (Korea Forestry Promotion Institute).
Abbreviations
- AV
Acne vulgaris
- CE
Cornified envelope
- CT
CARPINUS tschonoskii
- CTL
CT leaves
- DHT
Dihydrotestosterone
- DMEM
Dulbecco’s Modified Eagle Medium
- DPPH
1,1-diphenyl-2-picrylhydrazyl
- DTT
Dithiothreitol
- DTT
Thiazolyl blue tetrazolium bromide
- EGCG
Epigallocatechin gallate
- FBS
Fetal bovine serum
- HHDP
Hexahydroxydiphenyl
- IL-6
Interleukin-6
- IL-8
Interleukin-8
- L-NMMA
NG-Methyl-l-arginine acetate salt
- LPS
Lipopolysaccharide
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NO
Nitric oxide
- NOS2
NO synthase 2
- OD
Optical density
- PBS
Phosphate buffered saline
- RIA
Radioimmunoassay
- ROS
Reactive oxygen species
- SD
Sprague-Dawley
- SDS
Sodium dodecyl sulfate
- TEWL
Transepidermal water loss
- TI
Tellimagrandin I
- TNF- α
Tumor necrosis factor α
Authors’ contributions
Conception and design: MWL and JY. Analysis and interpretation: IHH and JY. Experiments: IHH, JY and DHP. Drafting the manuscript for important intellectual content: IHH and MK. Final approval of the manuscript: MWL. All authors have read and approved the final manuscript.
Funding
This research was supported by Korea Forestry Promotion Institute. The funding body had no role in the study design, performance, data collection and analysis, decision to publish, or writing of the manuscript.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
Not applicable because the research did not involve human participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jun Yin, Email: yinjun89@naver.com.
In Hyoek Hwang, Email: grampus92@naver.com.
Min Won Lee, Phone: 82-2-820-5602, Email: mwlee@cau.ac.kr.
References
- 1.Rawlings A, Harding C. Moisturization and skin barrier function. Dermatol Ther. 2004;17:43–48. doi: 10.1111/j.1396-0296.2004.04S1005.x. [DOI] [PubMed] [Google Scholar]
- 2.Willis C, Shaw S, De Lacharriere O, Baverel M, Reiche L, Jourdain R, Bastien P, Wilkinson J. Sensitive skin: an epidemiological study. Br J Dermatol. 2001;145(2):258–263. doi: 10.1046/j.1365-2133.2001.04343.x. [DOI] [PubMed] [Google Scholar]
- 3.Kalinin A, Marekov LN, Steinert PM. Assembly of the epidermal cornified cell envelope. J Cell Sci. 2001;114(17):3069–3070. doi: 10.1242/jcs.114.17.3069. [DOI] [PubMed] [Google Scholar]
- 4.Yu HY, Yang IJ, Lincha V, Park IS, Lee D-U, Shin HM. The effects of the fruits of Foeniculum vulgare on skin barrier function and hyaluronic acid production in HaCaT keratinocytes. J Life Sci. 2015;25(8):880–888. doi: 10.5352/JLS.2015.25.8.880. [DOI] [Google Scholar]
- 5.Egawa G, Kabashima K. Barrier dysfunction in the skin allergy. Allergol Int. 2018;67(1):3–11. doi: 10.1016/j.alit.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Kezic S, Jakasa I. Filaggrin and skin barrier function. Curr Probl Dermatol. 2016;49:1–7. doi: 10.1159/000441539. [DOI] [PubMed] [Google Scholar]
- 7.Cho S-A, An S-S, Kim H-S, Kim H-K, Lee T-R. Evaluation of synergic anti-irritant effect exerted by combination of cosmetic anti-irritant ingredients against retinoic acid-induced irritation using HaCaT cell line. J Altern Anim Exp. 2009;3(2):5–10. [Google Scholar]
- 8.Floudas A, Saunders SP, Moran T, Schwartz C, Hams E, Fitzgerald DC, Johnston JA, Ogg GS, McKenzie AN, Walsh PT, et al. IL-17 receptor a maintains and protects the skin barrier to prevent allergic skin inflammation. J Immunol. 2017;199(2):707–717. doi: 10.4049/jimmunol.1602185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howell MD, Fairchild HR, Kim BE, Bin L, Boguniewicz M, Redzic JS, Hansen KC, Leung DY. Th2 cytokines act on S100/A11 to downregulate keratinocyte differentiation. J Invest Dermatol. 2008;128(9):2248–2258. doi: 10.1038/jid.2008.74. [DOI] [PubMed] [Google Scholar]
- 10.Bassoy EY, Towne JE, Gabay C. Regulation and function of interleukin-36 cytokines. Immunol Rev. 2018;281(1):169–178. doi: 10.1111/imr.12610. [DOI] [PubMed] [Google Scholar]
- 11.Wilmer J, Luster M. Chemical induction of interleukin-8, a proinflammatory chemokine, in human epidermal keratinocyte cultures and its relation to cytogenetic toxicity. Cell Biol Toxicol. 1995;11(1):37–50. doi: 10.1007/BF00769991. [DOI] [PubMed] [Google Scholar]
- 12.Kim C, Ji J, Ho Baek S, Lee JH, Ha IJ, Lim SS, Yoon HJ, Je Nam Y, Ahn KS. Fermented dried Citrus unshiu peel extracts exert anti-inflammatory activities in LPS-induced RAW264.7 macrophages and improve skin moisturizing efficacy in immortalized human HaCaT keratinocytes. Pharm Biol. 2019;57(1):392–402. doi: 10.1080/13880209.2019.1621353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin Z-M, Ma M, Li H, Qi Q, Liu Y-T, Yan Y-X, Shen Y-F, Yang X-Q, Zhu F-H, He S-J, et al. Topical administration of reversible SAHH inhibitor ameliorates imiquimod-induced psoriasis-like skin lesions in mice via suppression of TNF-α/IFN-γ-induced inflammatory response in keratinocytes and T cell-derived IL-17. Pharmacol Res. 2018;129:443–452. doi: 10.1016/j.phrs.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Ohsaki A, Tanuma SI, Tsukimoto M. TRPV4 channel-regulated ATP release contributes to γ-irradiation-induced production of IL-6 and IL-8 in epidermal keratinocytes. Biol Pharm Bull. 2018;41(10):1620–1626. doi: 10.1248/bpb.b18-00361. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Yu X, Wu C, Jin H. IL-36gamma inhibits differentiation and induces inflammation of keratinocyte via Wnt signaling pathway in psoriasis. Int J Med Sci. 2017;14(10):1002–1007. doi: 10.7150/ijms.20809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hänel K, Cornelissen C, Lüscher B, Baron J. Cytokines and the skin barrier. Int J Mol Sci. 2013;14(4):6720–6745. doi: 10.3390/ijms14046720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim BE, Howell MD, Guttman E, Gilleaudeau PM, Cardinale IR, Boguniewicz M, Krueger JG, Leung DY. TNF-α downregulates filaggrin and loricrin through c-Jun N-terminal kinase: role for TNF-α antagonists to improve skin barrier. J Invest Dermatol. 2011;131(6):1272–1279. doi: 10.1038/jid.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Q, Mrowietz U, Rostami-Yazdi M. Oxidative stress in the pathogenesis of psoriasis. Free Radic Biol Med. 2009;47(7):891–905. doi: 10.1016/j.freeradbiomed.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 19.Williams HC, Dellavalle RP, Garner S. Acne vulgaris. Lancet. 2012;379(9813):361–372. doi: 10.1016/S0140-6736(11)60321-8. [DOI] [PubMed] [Google Scholar]
- 20.Barros B, Thiboutot D. Hormonal therapies for acne. Clin Dermatol. 2017;35(2):168–172. doi: 10.1016/j.clindermatol.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Harris VR, Cooper AJ. Modern management of acne. Med J Aust. 2017;206(1):41–45. doi: 10.5694/mja16.00516. [DOI] [PubMed] [Google Scholar]
- 22.Ju Q, Tao T, Hu T, Karadağ AS, Al-Khuzaei S, Chen W. Sex hormones and acne. Clin Dermatol. 2017;35(2):130–137. doi: 10.1016/j.clindermatol.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Kurokawa I, Danby FW, Ju Q, Wang X, Xiang LF, Xia L, Chen W, Nagy I, Picardo M, Suh DH. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18(10):821–832. doi: 10.1111/j.1600-0625.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- 24.Drobnis EZ, Nangia AK. 5alpha-Reductase inhibitors (5ARIs) and male reproduction. Adv Exp Med Biol. 2017;1034:59–61. doi: 10.1007/978-3-319-69535-8_7. [DOI] [PubMed] [Google Scholar]
- 25.Traish AM. Negative impact of testosterone deficiency and 5alpha-Reductase inhibitors therapy on metabolic and sexual function in men. Adv Exp Med Biol. 2017;1043:473–526. doi: 10.1007/978-3-319-70178-3_22. [DOI] [PubMed] [Google Scholar]
- 26.Kumar T, Chaiyasut C, Rungseevijitprapa W, Suttajit M. Screening of steroid 5α-reductase inhibitory activity and total phenolic content of Thai plants. J Med Plant Res. 2011;5(7):1265–1271. [Google Scholar]
- 27.Kim EH, Brockman JA, Andriole GL. The use of 5-alpha reductase inhibitors in the treatment of benign prostatic hyperplasia. Asian J Urol. 2018;5(1):28–32. doi: 10.1016/j.ajur.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thiboutot D, Del Rosso JQ. Acne vulgaris and the epidermal barrier: is acne vulgaris associated with inherent epidermal abnormalities that cause impairment of barrier functions? Do any topical acne therapies alter the structural and/or functional integrity of the epidermal barrier? J Clin Aesthet Dermatol. 2013;6(2):18–24. [PMC free article] [PubMed] [Google Scholar]
- 29.Rocha MA, Bagatin E. Skin barrier and microbiome in acne. Arch Dermatol Res. 2018;310(3):181–185. doi: 10.1007/s00403-017-1795-3. [DOI] [PubMed] [Google Scholar]
- 30.Furuno T. Bark structure of deciduous broad-leaved trees grown in the San'in region, Japan. IAWA Journal. 1990;11(3):239–254. doi: 10.1163/22941932-90001181. [DOI] [Google Scholar]
- 31.Yin Jun, Ahn Hye Shin, Ha Seong Yi, Hwang In Hyeok, Yoon Kee Dong, Chin Young Won, Lee Min Won. Anti-skin ageing effects of phenolic compounds from Carpinus tschonoskii. Natural Product Research. 2018;33(22):3317–3320. doi: 10.1080/14786419.2018.1497026. [DOI] [PubMed] [Google Scholar]
- 32.Solmoe . Medicinal herbs: Medicinal plants in korea that are easy to find and use in nature. Seoul: NwxuBooks; 2010. [Google Scholar]
- 33.Lee TB. Coloured Flora of Korea. Korea: Hyang-munsa; 2003. [Google Scholar]
- 34.Zhang R, Kang KA, Piao MJ, Park JW, Shin TK, Yoo BS, Yang YT, Hyun JW. Cytoprotective activity of Carpinus tschonoskii against H2O2 induced oxidative stress. Nat Prod Sci. 2007;13(2):118–122. [Google Scholar]
- 35.Kim SC, Kang HK, Kim MK, Kang JH, Hyun JW, Yoo ES, Kang JI, Park DB, Boo HJ, Koh YS. Neuroprotective effects of Carpinus tschonoskii MAX on 6-hydroxydopamine-induced death of PC12 cells. Biomol Ther (Seoul) 2010;18(4):454–462. doi: 10.4062/biomolther.2010.18.4.454. [DOI] [Google Scholar]
- 36.Yamada P, Ono T, Shigemori H, Han J, Isoda H. Inhibitory effect of tannins from galls of Carpinus tschonoskii on the degranulation of RBL-2H3 cells. Cytotechnology. 2012;64(3):349–356. doi: 10.1007/s10616-012-9457-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hatano T, YASUHARA T, FUKUDA T, NORO T, OKUDA T. Phenolic constituents of licorice. II.: structures of Licopyranocoumarin, Licoarylcoumarin and Glisoflavone, and inhibitory effects of licorice Phenolics on xanthine oxidase. Chem Pharm Bull. 1989;37(11):3005–3009. doi: 10.1248/cpb.37.3005. [DOI] [PubMed] [Google Scholar]
- 38.Le T, Yin J, Lee M. Anti-inflammatory and anti-oxidative activities of phenolic compounds from Alnus sibirica stems fermented by lactobacillus plantarum subsp. argentoratensis. Molecules. 2017;22(9):1566–1574. doi: 10.3390/molecules22091566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chanput W, Mes J, Vreeburg RA, Savelkoul HF, Wichers HJ. Transcription profiles of LPS-stimulated THP-1 monocytes and macrophages: a tool to study inflammation modulating effects of food-derived compounds. Food Funct. 2010;1(3):254–261. doi: 10.1039/c0fo00113a. [DOI] [PubMed] [Google Scholar]
- 40.Zbytek B, Mysliwski A, Slominski A, Wortsman J, Wei ET, Mysliwska J. Corticotropin-releasing hormone affects cytokine production in human HaCaT keratinocytes. Life Sci. 2002;70(9):1013–1021. doi: 10.1016/S0024-3205(01)01476-X. [DOI] [PubMed] [Google Scholar]
- 41.Pryor PR. Isolating lysosomes from rat liver. Cold Spring Harb Protoc. 2016;2016(4):333–337. doi: 10.1101/pdb.prot084814. [DOI] [PubMed] [Google Scholar]
- 42.Shimizu K, Fukuda M, Kondo R, Sakai K. The 5α-reductase inhibitory components from heartwood of Artocarpus incisus: structure-activity investigations. Planta Med. 2000;66(01):16–19. doi: 10.1055/s-2000-11114. [DOI] [PubMed] [Google Scholar]
- 43.Kim JY, Lee JY, Yoon H-G, Kim Y, Jun W, Hwang KT, Cha MS, Lee Y-H. Inhibitory effect of Curcuma longa L. extracts on 5-alpha Reductase II activity. J Korean Soc Food Sci Nutr. 2014;43(2):318–322. doi: 10.3746/jkfn.2014.43.2.318. [DOI] [Google Scholar]
- 44.Hong IK, Kim EJ, Seok JH, Kim BH, Jang JD, Joe GJ, Choi SW. Effects of Eucommia ulmoides Oliver extract on inhibition of β-hexosaminidase and keratinocyte differentiation. J Soc Cosmet Sci Korea. 2014;40(1):21–28. [Google Scholar]
- 45.Cals-Grierson M-M, Ormerod A. Nitric oxide function in the skin. Nitric Oxide. 2004;10(4):179–193. doi: 10.1016/j.niox.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 46.Mohania D, Chandel S, Kumar P, Verma V, Digvijay K, Tripathi D, Choudhury K, Mitten SK, Shah D. Ultraviolet radiations: skin defense-damage mechanism. Adv Exp Med Biol. 2017;996:71–87. doi: 10.1007/978-3-319-56017-5_7. [DOI] [PubMed] [Google Scholar]
- 47.Park KH, Yin J, Yoon KH, Hwang YJ, Lee MW. Antiproliferative effects of new Dimeric Ellagitannin from Cornus alba in prostate Cancer cells including apoptosis-related S-phase arrest. Molecules. 2016;21(2):137–147. doi: 10.3390/molecules21020137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yin J, Wang H, Lee M. Anti-inflammatory activity of <i>Corylopsis coreana</i> uyeki extracts and isolated compounds by inhibiting nitric oxide and cytokine production. Pharmacogn Mag. 2019;15(61):317–321. doi: 10.4103/pm.pm_144_18. [DOI] [Google Scholar]
- 49.Okuda T. Systematics and health effects of chemically distinct tannins in medicinal plants. Phytochemistry. 2005;66(17):2012–2031. doi: 10.1016/j.phytochem.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 50.Kupper TS, Min K, Sehgal P, Mizutani H, Birchall N, Ray A, May L. Production of IL-6 by keratinocytes. Ann N Y Acad Sci. 1989;557(1):454–465. doi: 10.1111/j.1749-6632.1989.tb24038.x. [DOI] [PubMed] [Google Scholar]
- 51.Kalailingam P, Tan HB, Jain N, Sng MK, Chan JSK, Tan NS, Thanabalu T. Conditional knock out of N-WASP in keratinocytes causes skin barrier defects and atopic dermatitis-like inflammation. Sci Rep. 2017;7(1):7311–7325. doi: 10.1038/s41598-017-07125-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Varma SR, TO S, Arumugam I, Dilip N, Raghuraman M, Pavan KB, Rafiq M, Paramesh R. In vitro anti-inflammatory and skin protective properties of virgin coconut oil. J Tradit Complement Med. 2019;9(1):5–14. doi: 10.1016/j.jtcme.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang SC, Liao PY, Hung SJ, Ge JS, Chen SM, Lai JC, Hsiao YP, Yang JH. Topical application of glycolic acid suppresses the UVB induced IL-6, IL-8, MCP-1 and COX-2 inflammation by modulating NF-kappaB signaling pathway in keratinocytes and mice skin. J Dermatol Sci. 2017;86(3):238–248. doi: 10.1016/j.jdermsci.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 54.Gutowska-Owsiak D, de La Serna JB, Fritzsche M, Naeem A, Podobas EI, Leeming M, Colin-York H, O’Shaughnessy R, Eggeling C, Ogg GS. Orchestrated control of filaggrin–actin scaffolds underpins cornification. Cell Death Dis. 2018;9(4):412–429. doi: 10.1038/s41419-018-0407-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kezic S, Kammeyer A, Calkoen F, Fluhr J, Bos J. Natural moisturizing factor components in the stratum corneum as biomarkers of filaggrin genotype: evaluation of minimally invasive methods. Br J Dermatol. 2009;161(5):1098–1104. doi: 10.1111/j.1365-2133.2009.09342.x. [DOI] [PubMed] [Google Scholar]
- 56.Imperato-McGinley J, Gautier T, Cai L, Yee B, Epstein J, Pochi P. The androgen control of sebum production. Studies of subjects with dihydrotestosterone deficiency and complete androgen insensitivity. J Clin Endocrinol Metab. 1993;76(2):524–528. doi: 10.1210/jcem.76.2.8381804. [DOI] [PubMed] [Google Scholar]
- 57.Yin J, Heo JH, Hwang YJ, Le TT, Lee MW. Inhibitory activities of phenolic compounds isolated from Adina rubella leaves against 5alpha-Reductase associated with benign prostatic hypertrophy. Molecules. 2016;21(7):887–897. doi: 10.3390/molecules21070887. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.