Abstract
Recent functional magnetic resonance spectroscopy (fMRS) studies have shown changes in glutamate/glutamine (Glx) concentrations between resting‐state and active‐task conditions. However, the types of task used have been limited to sensory paradigms, and the regions from which Glx concentrations have been measured limited to sensory ones. This leaves open the question as to whether the same effect can be seen in higher‐order brain regions during cognitive tasks. Cortical midline structures, especially the medial prefrontal cortex (MPFC), have been suggested to be involved in various such cognitive tasks. We, therefore set out to use fMRS to investigate the dynamics of Glx concentrations in the MPFC between resting‐state and mental imagery task conditions. The auditory cortex was used as a control region. In addition, functional magnetic resonance imaging was used to explore task‐related neural activity changes. The mental imagery task consisted of imagining swimming and was applied to a large sample of healthy participants (n = 46). The participants were all competitive swimmers, ensuring proficiency in mental‐swimming. Glx concentrations in the MPFC increased during the imagery task, as compared to resting‐state periods preceding and following the task. These increases mirror BOLD activity changes in the same region during the task. No changes in either Glx concentrations or BOLD activity were seen in the auditory cortex. These findings contribute to our understanding of the biochemical basis of generating or manipulating mental representations and the MPFC's role in this. Hum Brain Mapp 36:3204–3212, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: medial prefrontal cortex, glutamate, glutamine, magnetic resonance spectroscopy, functional magnetic resonance imaging, mental imagery
INTRODUCTION
Functional magnetic resonance spectroscopy (fMRS) has been used recently to investigate changes in biochemical concentrations in particular brain regions when a task is being performed. These have shown that levels of glutamate/glutamine (Glx) increase during task performance, as compared to resting conditions [Gussew et al., 2010; Lally et al., 2014; Lin et al., 2012; Mangia et al., 2006; Schaller et al., 2014a, 2014b]. To date the tasks used have focused on sensory stimulation—either visual or tactile—and metabolite changes in the respective sensory regions of the brain. This leaves open the question as to whether equivalent metabolite changes occur in so‐called higher‐order regions during non‐sensory cognitive tasks.
Cortical midline structures (CMS), and especially the medial prefrontal cortex (MPFC), have been suggested to be involved in, among other things, various such cognitive tasks. These include self‐reference [Northoff and Bermpohl, 2004; Qin and Northoff, 2011], mind wandering [Mason et al., 2007], and consciousness [Huang et al., 2014a; Qin et al., 2010; Raichle et al., 2001]. Within the MPFC, task‐related activity has been shown to be associated with concentrations of Glx and GABA, as measured using MRS [Duncan et al., 2011, 2013, 2014a, 2014b; Enzi et al., 2012; Hu et al., 2013; Kapogiannis et al., 2013; Northoff et al., 2007]. Interestingly, MRS measures of Glx are altered in schizophrenia and depression, both of which are typified by cognitive defects [Alcaro et al., 2010; Poels et al., 2014; Sanacora et al., 2012; Walter et al., 2009]. Taken together, these prior findings from the MPFC suggest that there will be changes in metabolite concentrations in that region during cognitive tasks, but this remains to be tested.
We therefore aimed to investigate potential changes in Glx concentrations in the MPFC, as induced by a mental imagery task. The specific task used involved the imagination of swimming, as has been used previously [Macintyre et al., 2013]. This task requires the generation and manipulation of mental representations in the absence of sensory stimulation. A large sample of healthy participants was used (n = 46); all of whom were trained competitive swimmers to ensure proficiency in the task [Cumming and Hall, 2002; Martin et al., 1999; Short et al., 2005; Weinberga et al., 2003].
Glx concentrations in the MPFC were measured with MRS using a block design. An initial rest period was followed by the task and then a second period of rest. In addition, functional magnetic resonance imaging (fMRI) was used to measure task‐related activity changes. Building on previous resting‐state MRS studies of the MPFC [Duncan et al., 2011, 2013, 2014a, 2014b; Enzi et al., 2012; Northoff et al., 2007; Wiebking et al., 2014], we hypothesized that an increase in MPFC Glx would be accompanied by a reduction in fMRI activity during the task periods (as compared to rest).
MATERIALS AND METHODS
Participants
The study group consisted of 46 trained swimmers (right‐handed; 26 females; 18–29 years): 17 Canadian Swim Team members; 14 who had previously competed at the same level; and 10 triathletes who had competed below the national or international level. Swimmers often use mental imagery to rehearse their swimming and to enhance their motor abilities. Such training through mental imagery is common practice in high‐level athletes across a range of disciplines involving complex motor performance [Cumming and Hall, 2002; Martin et al., 1999; Short et al., 2005; Weinberga et al., 2003]. With this experience of mental imagery, we reasoned that these athletes were particularly suitable participants for this study.
Participants had no history of major psychiatric or neurological disorders, and written informed consent was obtained from each prior to the experiment. The study was approved by the ethics committees at both the University of Ottawa and the University of British Columbia. All the subjects were scanned using MRS and fMRI during two sessions on the same day. The data from five subjects were excluded due to anxiety and excessive movements during scanning.
fMRS Data Acquisition
Prior to scanning, participants were familiarized with the task and completed a practice run. MR scanning was performed on a Philips Achieva 3.0T MR scanner equipped with an 8‐channel phased array head coil. High resolution T1‐weighted anatomical images (FOV = 256 × 256 mm2; spatial resolution = 1 × 1 × 1 mm3; TE = 3.39 ms; TR = 2530 ms; flip angle = 8°) were first acquired to locate volumes of interest (VOIs), including the MPFC and right primary auditory cortex (RPAC) as a control VOI (Fig. 1A) [see also Duncan et al., 2013, 2014a, 2014b]. The purpose of choosing the auditory cortex as a control region was twofold: (1) this region was assumed to be a task‐irrelevant region during mental‐swimming [Macintyre et al., 2013], and so could be used to detect potential global Glx changes; and (2) given the fact that we included auditory cues (“swim” and “rest”) to instruct the participants to begin and stop mental‐swimming (see below for details), these auditory instructions were considered as confounds. Therefore, to control these task‐irrelevant effects, the auditory cortex was chosen.
Figure 1.
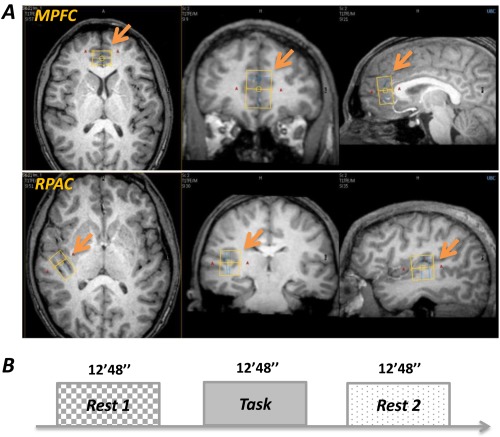
(A) The VOIs for single voxel proton MR spectroscopy (examples from one subject). The MPFC VOI was placed anterior to the genu of the corpus callosum, parallel to the AC‐PC plane; the RPAC VOI was placed parallel to Heschl's gyrus on the axial slice. (B) The fMRS paradigm consisted of three sessions for each VOI. Each session lasted 12 min and 48 s. The Rest1 and Rest2 were acquired as pre‐ and post‐ task baseline, and the participants were instructed to perform mental‐swim during the Task session. VOI: volumes of interest, MPFC: medial prefrontal cortex, RPAC: right primary auditory cortex. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
To achieve consistent VOIs positioning, placement was done by the same investigator for all subjects according to easily identifiable anatomical landmarks: the MPFC VOIs (20 × 15 × 30 mm3) were placed anterior to the genu of the corpus callosum, parallel to the AC‐PC plane; the RPAC VOIs (15 × 26 × 24 mm3) were placed parallel to Heschl's gyrus on axial slice. Single voxel proton MR spectroscopy was then performed using PRESS with the following parameters: single channel transmit/receive head coil, TR/TE = 3000 ms/24 ms, spectral width = 2000 Hz, data points = 2048, total number of signals averaged = 128, VAPOR water suppression. Spectra without water suppression were also acquired for use in the MRS analysis.
fMRS Paradigm
The fMRS paradigm (Fig. 1B) consisted of three sessions for each VOI: Rest1 (pre‐task baseline); Task (mental‐swim); and Rest2 (post‐task baseline). Each session lasted for 12 min and 48 s, yielding two 6 min and 24 s MRS datasets, one of which was used to acquire metabolite signals—water signal suppressed—and the other as a reference scan—water signal not suppressed—for absolute metabolite quantification. Participants were instructed to keep their eyes closed during the entire examination. Eye‐tracking during fMRI was not available; however, off‐line post‐scan recordings ensured that subjects did comply with this instruction. The scanning order of the two VOIs and the order of two MRS datasets (first and second half) were balanced across subjects.
The task session consisted of 16 mental‐swimming blocks interleaved by 8 s of rest. At the beginning of each block, an auditory cue “swim” instructed the subjects to begin mental‐swimming for either 30 or 50 s. The second cue, “take a rest”, instructed them to stop mental‐swimming and to rest for the next 8 s; rest was applied to avoid possible adaptation due to prolonged stimulation [Lin et al., 2012]. Eight 30 s‐blocks and eight 50 s‐blocks were assigned pseudo‐randomly during this task session.
To confirm that the subjects participated well during the task, two auditory‐based ratings were required between the task and rest scans. First, an effort rating “How much effort?” was presented. On this scale, a 5 indicates a great deal of mental effort for doing the mental swim, and a 1 indicates minimum effort. Subjects heard the spoken numbers 1–5 and pressed a button on a response box, using their right index finger, to indicate their choices. The second rated question was “How much feeling of flow?” [Bakker et al., 2011; Schüler and Brunner, 2009], with a 5 indicating maximal mental involvement and being absorbed in the mental‐swimming (rated as above). All stimuli were programmed using E‐Prime (Psychology Software Tools, Pittsburgh, PA) and delivered via an auditory stimulus presentation system designed for an MRI environment. The volume of the headphones was adjusted to the comfort level for each subject.
fMRS Data Analysis
Raw spectral data were automatically averaged, eddy‐current corrected by the scanner, and then exported for further analysis by LCModel 6.2‐1A. Using a simulated basis set optimized for the scanner (provided by the software producer) which included an experimentally measured metabolite‐nulled macromolecular spectrum, spectral fitting was performed over the range of 0.5–4.0 ppm, with absolute metabolite quantification achieved using the tissue water signal from the unsuppressed water spectrum as the internal reference. No other pre‐processing—baseline correction, zero‐filling, apodization functions—were applied prior to LCModel analysis.
Only outputs from metabolite concentrations with Cramer–Rao lower bounds (CRLB) below 20% were included for statistical analysis. Outputs with a CRLB higher than 20% were considered unreliable and discarded (See Supporting Information Figure S1 for a representative MR spectra and the LCModel fits). Combined values (Glx) of glutamate (Glu) and glutamine (Gln) were analyzed using both absolute concentrations and their ratio to Cr+PCr concentrations. The MPFC was the target region for the study and the RPAC was used as a regional specificity control.
For each region, a one‐way repeated measure analysis of covariance (ANCOVA) was used to test the task effect, taking subject age, gender, and the proportion of gray matter (GM) volume within the given MRS region as covariates. Individual GM volumes were calculated by applying the MRS region to the segmented GM maps created using the FSL program FAST [Duncan et al., 2013]. For regions showing a significant main effect, post hoc Tukey HSD tests were used to compare Rest1 versus Task, Rest2 versus Task, and Rest1 versus Rest2.
To examine the potential difference between the MPFC and RPAC in terms of task‐evoked changes in Glx, we first calculate an index, namely “Task‐evoked Glx,” to quantify the relative change of Glx during the task compared to both the pre‐task and post‐task baseline periods: Task − (Rest1 + Rest2)/2. In the next step, one‐sample t‐tests against zero were performed for the Task‐evoked Glx both in the MPFC and RPAC to confirm the above analysis by ANCOVA. Finally, a paired sample t‐test was used to examine the difference between the MPFC and RPAC in Task‐evoked Glx (and thus regional specificity).
Functional Magnetic Resonance Imaging
The same scanner was used to acquire gradient‐echo EPI images of the whole brain (TR, 1.0 s; TE, 30 ms; 21 slices; slice thickness = 6 mm; spacing = 0; field of view = 210 mm; flip angle = 76°; image matrix: 64 × 64). As in fMRS, a mental‐swim task with a block design was acquired in fMRI after the MRS session. Eighteen mental‐swim blocks were assigned pseudo‐randomly into six fMRI scans. Each block began with an auditory cue informing subjects to swim for 30 or 50 s. The subjects were instructed to press a button on a response box using their right index finger at the end of blocks. Right after pressing the button, subjects were instructed to “take a rest” and then a rest period was jittered from 32 to 36 s between blocks. As in fMRS, subjects were instructed to relax, stay awake and keep their eyes closed. Time‐locked cardiac and respiratory signals were recorded. Finally, high‐resolution anatomical images were acquired at the end of the experiment.
Pre‐processing steps were implemented in AFNI [Cox, 1996] (http://afni.nimh.nih.gov/afni). The first four frames of each fMRI run were discarded. Physiological noise correction consisted of removal of time‐locked cardiac and respiratory artifacts using RETROICOR [Glover et al., 2000]. The rest steps included slice timing correction, realignment, co‐registration, detrending, and removal of the effect of head motion by linear regression. This data then underwent normalization into Talaraich stereotactic space [Talairach and Tournoux, 1988], was resampled to 3 × 3 × 3 mm3 voxels, and spatially smoothed with 6 mm full‐width at half‐maximum isotropic Gaussian kernel.
To estimate the BOLD response for the mental‐swim task, we first expressed each signal value as a percentage change from its block onset value, and then calculated a mean percentage change within each block and averaged across all blocks [Garrett et al., 2010, 2011]. Next we performed the same analysis for the rest period (32–36 s) to obtain a baseline for the mental‐swim. Third, the mean BOLD response of the mental‐swimming target blocks was subtracted by the rest period control blocks to obtain a relative BOLD percentage signal change for mental swim. This subtraction approach controls for the auditory instruction, arousal, and/or attentional effects at the beginning of each block. Finally, the relative percentage signal change of mental‐swimming was extracted from the two MRS regions for each subject and entered into one sample t‐tests (against zero) at the group level. In addition, a paired sample t‐test was used to examine the difference in BOLD signal changes between the MPFC and RPAC.
RESULTS
Behavioral Data
By recruiting well trained swimmers and examining the performance from post‐scan self‐evaluations we ensured that all subjects performed well during the mental‐swim task in the MRS, as both the mean effort and feeling of flow were above medium level (2.5) for the whole group (Supporting Information Figure S2). Moreover, we confirmed that there was no significant difference between the MPFC and RPAC in either effort or feeling of flow evaluations during MRS acquisitions for mental‐swim (Supporting Information Figure S2).
fMRS Data
Next, we sought to detect the Glx changes in the MPFC during mental‐swim in the MRS. As expected, we observed a significant main effect in the MPFC by one‐way repeated measure ANCOVA (P = 0.001; Fig. 2A). Post hoc Tukey tests showed a significant difference for Rest1 versus Task and Rest2 versus Task, while no difference was seen for Rest1 versus Rest2. In contrast, no significant change in Glx was observed in the RPAC control region (Fig. 2A and Table 1). In addition, the Task‐evoked Glx, indexed by Task − (Rest1 + Rest2)/2, was significantly above zero for the MPFC (t (40) = 3.81, P < 0.001) but not the RPAC (t (40) = 1.11, P = 0.276), confirming the above observations. Furthermore, a significant difference in Task‐evoked Glx was seen between the MPFC and RPAC (t (40) = 2.10, P = 0.042), suggesting the Glx change was specific to the MPFC (Fig. 2B). Similar results were observed for the Glx ratios referenced to Cr + PCr (Supporting Information Figure S3).
Figure 2.
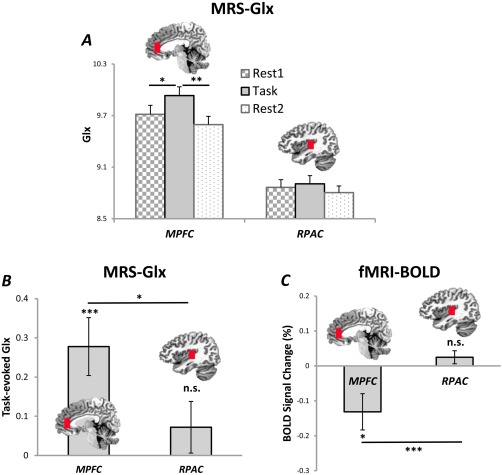
(A) Significant main effect (P = 0.001) was observed for the MPFC, but not for the RPAC, by one‐way repeated measure ANCOVA. Post hoc Tukey tests showed significant difference for Rest1 versus Task and Rest2 versus Task, while no difference for Rest1 versus Rest2 in the MPFC. (B) The Task‐evoked Glx, indexed by Task − (Rest1 + Rest2)/2, was significant above zero for the MPFC (P < 0.001) but not the RPAC (P = 0.276). A significant difference of Task‐evoked Glx was seen between the MPFC and RPAC (P = 0.042). (C) The MPFC, but not the RPAC, showed significant deactivation (P = 0.014) of the fMRI‐BOLD during mental‐swim. A significant difference of BOLD signal change was seen between the MPFC and RPAC (P = 0.002). *denotes P < 0.05; **denotes P < 0.01; ***denotes P < 0.005. MPFC: medial prefrontal cortex, RPAC: right primary auditory cortex, ANCOVA: analysis of covariance. Error bars indicate ± SEM. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 1.
ANCOVA between Rest1, Task, and Rest 2 for Glx in the MPFC and RPAC
| Glx | Rest1 (mean ± SD) | Task (mean ± SD) | Rest2 (mean ± SD) | ANCOVA | Rest1 versus Task | Rest2 versus Task | Rest1 versus Rest2 |
|---|---|---|---|---|---|---|---|
| MPFC | 9.72 ± 0.66 | 9.93 ± 0.65 | 9.60 ± 0.61 | F = 7.41 P = 0.001 | P < 0.05 | P < 0.01 | Non‐significant |
| RPAC | 8.86 ± 0.57 | 8.91 ± 0.60 | 8.80 ± 0.49 | F = 0.99 P = 0.367 | N/A | N/A | N/A |
Abbreviations: Glx, Combined value of Glutamate and Glutamine; MPFC, medial prefrontal cortex; RPAC, right primary auditory cortex; ANCOVA, analysis of covariance.
fMRI Data
Based on our MRS regions of interest, we focused fMRI analysis on the MPFC and RPAC. The MPFC showed significant deactivation (t (40) = −2.57, P = 0.014) during mental‐swimming in the fMRI while no significant (t (40) = 1.34, P = 0.186) signal change was observed in the RPAC. A significant difference in BOLD signal change was seen between the MPFC and RPAC (t (40) = −3.39 P = 0.002) (Fig. 2C). These findings demonstrate that the mental‐swim task recruited neural activity changes in the MPFC specifically, when compared to the RPAC.
DISCUSSION
For the first time, we found that an increase of glutamate/glutamine (Glx) in the MPFC, as distinguished from sensory cortex, could be driven by a simple mental imagery task. This finding demonstrates that the generation or manipulation of mental representations, in the absence of sensory stimulation, is sufficient to induce changes in Glx concentration. Parallel fMRI‐BOLD changes during mental‐swimming were found in the MPFC, along with the dynamic Glx increases. This was not the case in the auditory cortex. This finding bears major implications for understanding the biochemical basis of generating or manipulating mental representations in the MPFC, as well as various cognitive symptoms in mental disorders such as schizophrenia and depression.
Glx Increases in the MPFC
Previous functional MRS glutamate studies have focused on the visual [Lally et al., 2014; Lin et al., 2012; Mangia et al., 2007; Schaller et al., 2014a] and motor [Schaller et al., 2014b] cortices. They observed an increase in the level of Glx during visual or motor tasks, when compared to the preceding and following resting‐state periods [Gussew et al., 2010; Lally et al., 2014]. Our data complements and extends these results by showing a task‐related Glx increase in a “higher‐order” region, the MPFC. Furthermore, it suggests that Glx modulates not only resting‐state activity [Hu et al., 2013; Kapogiannis et al., 2013] but is also sensitive to functional changes in cognitive task demands. Although we did not observe a direct relationship between Glx and BOLD signal in the MPFC, one may argue that the MPFC Glx increase reflects a general alteration in neural activity, which is supported by our fMRI data. In contrast, we did not observe a Glx increase in the auditory cortex; our fMRI findings in this region were the same. This supports the regional specificity of a Glx increase in the MPFC during a mental task but not in the auditory cortex.
What are the physiological mechanisms underlying the observed task‐related increase in Glx? The possibilities are twofold: (1) The task‐related demands are of energetic‐metabolic origin; they may increase the oxidative metabolism, and accordingly the glutamate‐glutamine cycling, to make available a higher concentration of glutamate at the synaptic cleft [Hyder et al., 2006; Lin et al., 2012; Mangia et al., 2007; Schaller et al., 2014a, 2014b]; or (2) A task‐related glutamate increase may be of neuronal origin, and related to an increase in synaptic glutamate release [Gussew et al., 2010; Lally et al., 2014].
Our paradigm was carried out in blocks. The mean from an averaging across blocks was termed the Glx value. This approach is compatible with the first mechanism, an increase in oxidative metabolism and glutamate‐glutamine cycling. This mechanism has an approximate duration of 1 h [Hyder et al., 2006]. In contrast, the structure of our design—long blocks rather than single trials—makes it less likely for the Glx increase to result from increased synaptic release, as the latter occurs within the millisecond range. Accordingly, one would expect a true event‐related fMRS design to predominantly reflect synaptic release, while a block design, as ours is, would likely be more based on glutamate‐glutamine cycling. Future studies testing the same type of stimuli or task in event‐related and block‐based experiments would be necessary to decide this issue.
Finally, the MRS signal measures a combination of vesicular, as well as intrasynaptic/intracellular and extraintra synaptic/extracellular, Glx [Stagg, 2014; Stagg et al., 2011]. Our data remains, therefore, unable to determine the exact origin of task‐related Glx: is the origin intracellular or extracellular, or does the task‐related Glx come from vesicular sources? Future studies, possibly with 13‐C MRS that directly measures the glutamate‐glutamine cycling, are needed to determine the exact origin of the Glx increase.
Glx Increase in Response to Mental Task Demands
The fMRS studies to date have applied sensory and motor stimuli as they focus on the visual and motor cortex [Lin et al., 2012; Mangia et al., 2007; Schaller et al., 2014a, 2014b]. This left open the investigation of cognitive task induced Glx release in the absence of physical stimuli. To accomplish this, we applied a mental‐swimming task without the presentation of a stimulus. The behavioral data show that participants performed the requested mental‐swimming task. Furthermore, as the subjects were competitive swimmers, it could be assumed that they performed well during the task, as was documented in our subjective measures. The special group of participants in our study, however, might make it difficult to generalize the conclusion to the other populations, and so future investigations of other groups are warranted.
Interestingly, our data shows a task‐evoked increase in Glx in the MPFC but not in the auditory cortex. This suggests that a purely mental task is sufficient to modulate changes in glutamate, but only for the MPFC and not the task‐irrelevant RPAC (in accordance with our fMRI data). The MPFC has been associated with various mental functions, ranging from self‐reference [Qin and Northoff, 2011], mind wandering [Christoff et al., 2009; Mason et al., 2007], consciousness [Huang et al., 2014a, 2014b; Qin et al., 2010], and autobiographical memory [Spreng and Grady, 2010; Spreng et al., 2009]. One may now assume that some of these aforementioned mental processes were implicated in our mental‐swimming task. Due to the lack of a control task (e.g., self‐referential processing), however, we are unable to determine the exact mental component that induced Glx increase in the MPFC. Moreover, we did not find an association between either the task‐evoked Glx or BOLD signal change with our behavioral measures. We, therefore, remain unable to conclude that the effects we observed were specific to a mental‐swimming task. Nevertheless, the focus of our study was more on whether a mental task, using the mental‐swimming task as an example, would elicit Glx changes, rather than determining the exact mental or psychological component that is related to Glx increase. Future studies with more refined cognitive task designs are, therefore, necessary to associate MPFC Glx increase with a specific mental task component (if it is indeed so).
Our findings are highly relevant for psychiatric disorders such as depression and schizophrenia. They show changes in exactly the above described mental task components, self‐reference, and inner time consciousness for example [Northoff et al., 2007; Northoff, 2014a, 2014b], which have been generally associated with the CMS and the MPFC in particular. Specifically, our study may provide some background to the biochemical basis of these disorders relative to their various mental and behavioral symptoms [Egerton and Stone, 2012; Sanacora and Banasr, 2013; Stone, 2009]. The design and data presented here provide the groundwork for future applications of fMRS in psychiatric patients. It may be possible, for example, to use a mental task—for self‐reference or emotions in particular—to induce a change in Glx levels in the MPFC in depression [Northoff et al., 2007].
CONCLUSION
We demonstrate here for the first time a change in neural activity and a concomitant glutamate/glutamine (Glx) increase in the MPFC in response to a mental task. In contrast to the MPFC, we did not observe a Glx increase or neural activity changes in the auditory cortex, suggesting regional specificity of Glx in response to a mental task. Our data show a mentally induced increase in Glx levels in the MPFC, pointing to new understandings of neural‐biochemical relationships regarding the MPFC and cognitive symptoms. These findings are important in the research of psychiatric disorders, and may also benefit the area of sports psychology in its development of novel training strategies.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
Georg Northoff acknowledges the support of EJLB‐Michael Smith Foundation, Canada Institute of Health Research (CIHR), and Hope of Depression Foundation (HDRF). Henry Davis IV acknowledges the gracious support of two private donors, whose contributions to Swim Canada made this study possible. The authors are indebted to the athletes and coaches who made themselves available for the study. The authors declare no conflict of interest.
REFERENCES
- Alcaro A, Panksepp J, Witczak J, Hayes DJ, Northoff G (2010): Is subcortical‐cortical midline activity in depression mediated by glutamate and GABA? A cross‐species translational approach. Neurosci Biobehav Rev 34:592–605. [DOI] [PubMed] [Google Scholar]
- Bakker AB, Oerlemans W, Demerouti E, Slot BB, Ali DK (2011): Flow and performance: A study among talented dutch soccer players. Psychol Sport Exerc 12:442–450. [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW (2009): Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci USA 106:8719–8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW (1996): AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173. [DOI] [PubMed] [Google Scholar]
- Cumming J, Hall C (2002): Deliberate imagery practice: The development of imagery skills in competitive athletes. J Sports Sci 20:137–145. [DOI] [PubMed] [Google Scholar]
- Duncan NW, Enzi B, Wiebking C, Northoff G (2011): Involvement of glutamate in rest‐stimulus interaction between perigenual and supragenual anterior cingulate cortex: A combined fMRI‐MRS study. Hum Brain Mapp 32:2172–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan NW, Wiebking C, Tiret B, Marjańska M, Hayes DJ, Lyttleton O, Doyon J, Northoff G (2013): Glutamate concentration in the medial prefrontal cortex predicts resting‐state cortical‐subcortical functional connectivity in humans. PLoS One 8:e60312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NW Duncan, C Wiebking, Z Muñoz‐Torres, G Northoff (2014a): How to investigate neuro‐biochemical relationships on a regional level in humans? Methodological considerations for combining functional with biochemical imaging. J Neurosci Methods 221:183–188. [PubMed] [Google Scholar]
- Duncan NW, Wiebking C, Northoff G (2014b): Associations of regional GABA and glutamate with intrinsic and extrinsic neural activity in humans—A review of multimodal imaging studies. Neurosci Biobehav Rev 47:36–52. [DOI] [PubMed] [Google Scholar]
- Egerton A, Stone JM (2012): The glutamate hypothesis of schizophrenia: Neuroimaging and drug development. Curr Pharm Biotechnol 13:1500–1512. [DOI] [PubMed] [Google Scholar]
- Enzi B, Duncan NW, Kaufmann J, Tempelmann C, Wiebking C, Northoff G (2012): Glutamate modulates resting state activity in the perigenual anterior cingulate cortex—A combined fMRI‐MRS study. Neuroscience 227:102–109. [DOI] [PubMed] [Google Scholar]
- Garrett DD, Kovacevic N, McIntosh AR, Grady CL (2010): Blood oxygen level‐dependent signal variability is more than just noise. J Neurosci 30:4914–4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett DD, Kovacevic N, McIntosh AR, Grady CL (2011): The importance of being variable. J Neurosci 31:4496–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D (2000): Image‐based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med 44:162–167. [DOI] [PubMed] [Google Scholar]
- Gussew A, Rzanny R, Erdtel M, Scholle HC, Kaiser WA, Mentzel HJ, Reichenbach JR (2010): Time‐resolved functional 1H MR spectroscopic detection of glutamate concentration changes in the brain during acute heat pain stimulation. Neuroimage 49:1895–1902. [DOI] [PubMed] [Google Scholar]
- Hu Y, Chen X, Gu H, Yang Y (2013): Resting‐state glutamate and GABA concentrations predict task‐induced deactivation in the default mode network. J Neurosci 33:18566–18573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Dai R, Wu X, Yang Z, Liu D, Hu J, Gao L, Tang W, Mao Y, Jin Y, Wu X, Liu B, Zhang Y, Lu L, Laureys S, Weng X, Northoff G (2014a): The self and its resting state in consciousness: An investigation of the vegetative state. Hum Brain Mapp 35:1997–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Wang Z, Zhang J, Dai R, Wu J, Li Y, Liang W, Mao Y, Yang Z, Holland G, Zhang J, Northoff G (2014b): Altered temporal variance and neural synchronization of spontaneous brain activity in anesthesia. Hum Brain Mapp 35:5368–5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG (2006): Neuronal‐glial glucose oxidation and glutamatergic‐GABAergic function. J Cereb Blood Flow Metab 26:865–877. [DOI] [PubMed] [Google Scholar]
- Kapogiannis D, Reiter DA, Willette AA, Mattson MP (2013): Posteromedial cortex glutamate and GABA predict intrinsic functional connectivity of the default mode network. Neuroimage 64:112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally N, Mullins PG, Roberts MV, Price D, Gruber T, Haenschel C (2014): Glutamatergic correlates of gamma‐band oscillatory activity during cognition: A concurrent ER‐MRS and EEG study. Neuroimage 85:823–833. [DOI] [PubMed] [Google Scholar]
- Lin Y, Stephenson MC, Xin L, Napolitano A, Morris PG (2012): Investigating the metabolic changes due to visual stimulation using functional proton magnetic resonance spectroscopy at 7 T. J Cereb Blood Flow Metab 32:1484–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre TE, Moran AP, Collet C, Guillot A (2013): An emerging paradigm: A strength‐based approach to exploring mental imagery. Front Hum Neurosci 7:104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangia S, Tkác I, Gruetter R, Van De Moortele PF, Giove F, Maraviglia B, Uğurbil K (2006): Sensitivity of single‐voxel 1H‐MRS in investigating the metabolism of the activated human visual cortex at 7 T. Magn Reson Imaging 24:343–348. [DOI] [PubMed] [Google Scholar]
- Mangia S, Tkác I, Gruetter R, Van de Moortele PF, Maraviglia B, Uğurbil K (2007): Sustained neuronal activation raises oxidative metabolism to a new steady‐state level: Evidence from 1H NMR spectroscopy in the human visual cortex. J Cereb Blood Flow Metab 27:1055–1063. [DOI] [PubMed] [Google Scholar]
- Martin KA, Moritz SE, Hall CR (1999): Imagery use in sport: A literature review and applied model. Sport Psychol 13:245–268. [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN (2007): Wandering minds: The default network and stimulus‐independent thought. Science 315:393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G (2014a): Unlocking the Brain, Vol. I: Coding. New York: Oxford University Press. [Google Scholar]
- Northoff G (2014b): Unlocking the Brain, Vol. II: Consciousness. New York: Oxford University Press. [Google Scholar]
- Northoff G, Bermpohl F (2004): Cortical midline structures and the self. Trends Cogn Sci 8:102–107. [DOI] [PubMed] [Google Scholar]
- Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, Boeker H, Grimm S, Boesiger P (2007): GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat Neurosci 10:1515–1517. [DOI] [PubMed] [Google Scholar]
- Poels EM, Kegeles LS, Kantrowitz JT, Javitt DC, Lieberman JA, Abi‐Dargham A, Girgis RR (2014): Glutamatergic abnormalities in schizophrenia: A review of proton MRS findings. Schizophr Res 152:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Northoff G (2011): How is our self related to midline regions and the default‐mode network? Neuroimage 57:1221–1233. [DOI] [PubMed] [Google Scholar]
- Qin P, Di H, Liu Y, Yu S, Gong Q, Duncan N, Weng X, Laureys S, Northoff G (2010): Anterior cingulate activity and the self in disorders of consciousness. Hum Brain Mapp 31:1993–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci USA 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Banasr M (2013): From pathophysiology to novel antidepressant drugs: Glial contributions to the pathology and treatment of mood disorders. Biol Psychiatry 73:1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Treccani G, Popoli M (2012): Towards a glutamate hypothesis of depression: An emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 62:63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller B, Xin L, Gruetter R (2014a): Is the macromolecule signal tissue‐specific in healthy human brain? A 1 H MRS study at 7 tesla in the occipital lobe. Magn Reson Med 72:934–940. [DOI] [PubMed] [Google Scholar]
- Schaller B, Xin L, O'Brien K, Magill AW, Gruetter R (2014b): Are glutamate and lactate increases ubiquitous to physiological activation? A (1)H functional MR spectroscopy study during motor activation in human brain at 7Tesla. Neuroimage 93:138–145. [DOI] [PubMed] [Google Scholar]
- Schüler J, Brunner S (2009): The rewarding effect of flow experience on performance in a marathon race. Psychol Sport Exerc 10:168–174. [Google Scholar]
- Short SE, Tenute A, Feltz DL (2005): Imagery use in sport: Mediational effects for efficacy. J Sports Sci 23:951–960. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Grady CL (2010): Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J Cogn Neurosci 22:1112–1123. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS (2009): The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta‐analysis. J Cogn Neurosci 21:489–510. [DOI] [PubMed] [Google Scholar]
- Stagg CJ (2014): Magnetic resonance spectroscopy as a tool to study the role of GABA in motor‐cortical plasticity. Neuroimage 86:19–27. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V, Johansen‐Berg H (2011): What are we measuring with GABA magnetic resonance spectroscopy? Commun Integr Biol 4:573–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JM (2009): Imaging the glutamate system in humans: Relevance to drug discovery for schizophrenia. Curr Pharm Des 15:2594–2602. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. 1988. Co‐Planar Stereotaxic Atlas of the Human Brain. New York: G. Thieme; Thieme Medical Publishers, Stuttgart. [Google Scholar]
- Walter M, Henning A, Grimm S, Schulte RF, Beck J, Dydak U, Schnepf B, Boeker H, Boesiger P, Northoff G (2009): The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch Gen Psychiatry 66:478–486. [DOI] [PubMed] [Google Scholar]
- Weinberga R, Butta J, Knighta B, Burkeb KL, Jacksonc A (2003): The relationship between the use and effectiveness of imagery: An exploratory investigation. J Appl Sport Psychol 15:26–40. [Google Scholar]
- Wiebking C, Duncan NW, Tiret B, Hayes DJ, Marjaǹska M, Doyon J, Bajbouj M, Northoff G (2014): GABA in the insula—A predictor of the neural response to interoceptive awareness. Neuroimage 86:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


