Abstract
Self‐regulation of brain activation using real‐time functional magnetic resonance imaging has been used to train subjects to modulate activation in various brain areas and has been associated with behavioral changes such as altered pain perception. The aim of this study was to assess the comparability of upregulation versus downregulation of activation in the rostral anterior cingulate cortex (rACC) and left posterior insula (pInsL) and its effect on pain intensity and unpleasantness. In a first study, we trained 10 healthy subjects to separately upregulate and downregulate the blood oxygenation level‐dependent response in the rACC or pInsL (six trials on 4 days) in response to painful electrical stimulation. The participants learned to significantly downregulate activation in pInsL and rACC and upregulate pInsL but not rACC. Success in the modulation of one region and direction of the modulation was not significantly correlated with success in another condition, indicating that the ability to control pain‐related brain activation is site‐specific. Less covariation between the areas in response to the nociceptive stimulus was positively correlated with learning success. Upregulation or downregulation of either region was unrelated to pain intensity or unpleasantness; however, our subjects did not learn rACC upregulation, which might be important for pain control. A significant increase in pain unpleasantness was found during upregulation of pInsL when covariation with the rACC was low. These initial results suggest that the state of the network involved in the processing of pain needs to be considered in the modulation of pain‐evoked activation and its behavioral effects. Hum Brain Mapp 35:5784–5798, 2014. © 2014 Wiley Periodicals, Inc.
Keywords: real‐time fMRI, pain, rostral anterior cingulate cortex, posterior insula, neuromodulation
INTRODUCTION
The use of real time functional magnetic resonance imaging (rt‐fMRI) for neurofeedback of local brain regions has been well established over the past 10 years [Weiskopf, 2012; Weiskopf et al., 2003]. The blood oxygenation level‐dependent (BOLD) response of a number of brain regions had been modified by providing mainly visual feedback to the person and it has been shown that control of brain activation in many regions is possible and that this control is often associated with behavioral changes [Birbaumer et al., 2013]. Behavioral changes associated with rt‐fMRI include, for example, the altered appraisal and recognition of emotional materials related to anterior insula regulation in both healthy humans and schizophrenics [Caria et al., 2010; Ruiz et al., 2013], reduced cue craving in smoking cessation [Li et al., 2013] related to the control of anterior cingulate cortex activation, and improved linguistic processing when the right inferior frontal gyrus was regulated [Rota et al., 2009]. In some studies, however, no effect on behavioral measures was found [Johnston et al., 2011; Weiskopf et al., 2003].
In this context, rt‐fMRI might be beneficial in the treatment of chronic pain. Previous work has shown pain‐evoked activity in a large number of brain regions with a focus of the activation in the primary and secondary somatosensory cortex, the insula, the anterior cingulate cortex, thalamus, and the prefrontal cortex. Additional regions that were found to be involved in the processing of painful stimulation included the primary and supplementary motor cortices, posterior parietal cortex, posterior cingulate cortex, basal ganglia, hypothalamus, amygdala, parabrachial nuclei, and the periaqueductal gray [Apkarian et al., 2005]. The rostral anterior cingulate cortex (rACC) was found to be involved in the affective processing of pain, [Rainville et al., 1997] and is also an important relay station in descending inhibitory control of pain, [Bingel et al., 2006]. Sensory processing aspects of pain have been connected with the somatosensory cortex and the posterior insula [Frot et al., 2007; Hofbauer et al., 2001; Rainville et al., 1997]. Recent developments in pattern classification made it possible to identify a network of active regions in response to painful heat stimulation that can be used in later trials to differentiate between the neurological signatures of different levels of painful heat and innocuous warmth [Wager et al., 2013]. The identified regions included the above mentioned regions. In an initial study, deCharms et al. [2005] used rt‐fMRI feedback of the rACC to regulate pain perception in healthy humans and pain patients. Both groups learned to increase and decrease activity in the rACC in response to a painful heat stimulus in the case of healthy controls and to their ongoing clinical pain in case of the patients with chronic pain. In that study, rt‐fMRI feedback was combined with cognitive instructions similar to those used in cognitive‐behavioral treatment (focusing of attention, control of the stimulus, focus on sensory vs. affective aspects, or high vs. low stimulus intensity). The visual feedback used video images involving virtual fire images. For the training in the healthy group, several control groups were used that controlled either for the cognitive instructions, used another feedback site (posterior cingulate), or bogus feedback and one control group received behavioral training on how to cope with pain. For the patients, an autonomic feedback (skin conductance, heart rate, respiration) control and control instructions were used. In the acute painful stimulation group, both pain intensity and unpleasantness ratings corresponded to the changes in rACC activity with high differentiation of the rACC activation being accompanied by high changes in pain ratings without a clear description of the association of the direction of the brain change with the changes in pain perception. Further, the magnitude of the change in brain activation correlated with the change in the pain ratings. There was a steady increase in regulation ability over the course of the four feedback training sessions. Among all groups, only those with veridical rt‐fMRI feedback learned to control ACC activation and, thus, pain modulation. In the patients with chronic pain, the group receiving rt‐fMRI had a 44% decrease in pain ratings compared to the control group, who had a significantly smaller change. Although control groups were used, it is not clear to what extent the cognitive strategies or the visual image video feedback may have had an effect on the brain regions that were trained and may have interacted with the rt‐fMRI. There was no group that only received rt‐fMRI without cognitive instructions or visual images.
The relationship between pain and self‐regulation of brain activity has not been successfully addressed since this initial study as discussed in a recent review [Chapin et al., 2012]. The reasons for this lack of progress are not clear. It could be related to the complex design of the original study as described earlier. It is possible that the additional cognitive and visual strategies provided in the original study may themselves have influenced brain activation in the rACC and have interacted with the rt‐fMRI to alter pain perception. Finally, it is not clear from the original study to what extent only rACC or additional brain networks were active, since the specificity of the activation was not controlled. It is also important to note that pain involves multiple brain areas, which are also active in the processing of other salient information [Legrain et al., 2011].
In this study we aimed to identify and compare the effect of the self‐regulation of the activity of two core regions involved in the processing of pain using rt‐fMRI in an effort to determine the specificity of these regions for the modulation of pain and to elucidate differential effects of this modulation on pain intensity and unpleasantness. We chose the rostral part of the ACC because it exhibited stable activation in the presence of a painful electric stimulus. This region was more rostral to the site chosen by deCharms et al. [2005] and we added rt‐fMRI feedback of the posterior insula, because both are involved in different aspects of pain processing as noted above. We assumed that sensory pain aspects might be more related to activation of the posterior insula and affective aspects more related to activation of the anterior cingulate cortex. We chose posterior insula over somatosensory cortex, because a more reliable activation pattern across all subjects could be obtained from this region, similar to rACC, which was also very reliably activated [Apkarian et al., 2005]. Instead of providing feedback of the activation status of the entire dynamic network, we were interested in the differential contribution of these two regions to both pain intensity and unpleasantness ratings. We further compared the rt‐fMRI feedback‐based controllability of the regions in the two directions (upregulation and downregulation of the BOLD effect) and the relation to perceived control and the used cognitive strategy. Since both regions are active in response to painful stimulation and have been shown to be amendable to rt‐fMRI feedback training but only one region was fed back at a time, we also examined the specificity of the activation by comparing the activation of the critical region with both the other target region and a parietal region that was not involved in the feedback at all. In addition, we did not provide a cognitive strategy and used a feedback condition that did not involve virtual fire video images that might also have an effect on pain control. Finally, we used intracutaneous electrical stimulation as the nociceptive stimulus to assure that only nociceptive fibers would be stimulated.
MATERIALS AND METHODS
Participants
We examined 10 healthy adults, all right‐handed as assessed by the Edinburgh Handedness Inventory [Oldfield, 1971], with a mean age of 29 years (SD = 6.48, range: 20–41), four females (M = 27.0, SD = 3.92), and six males (M = 30.3, SD = 7.81). Subjects were recruited by announcements at the local university. Exclusion criteria were cardiovascular or neurological disorders, brain injury, acute pain, current analgesic medication, pregnancy, lifetime and current substance abuse or dependence, any mental disorder, and metallic implants. All subjects received a reimbursement of 60 €. The study adhered to the Declaration of Helsinki and was approved by the Ethics Committee of the Medical Faculty Mannheim, Heidelberg University, Germany. All subjects gave written informed consent after a detailed description of the complete study.
Stimulation Protocol
Painful monopolar transcutaneous electrical stimuli (Digitimer, DS7A, Welwyn Garden City, UK) were applied to the lateral inward facing skin of the first segment of the fourth digit of the right hand using a stainless steel concentric bipolar needle electrode (Nihon Kohden, Tokyo, Japan). This electrode has a small needle tip protruding 0.1 mm from an outer ring 1.2 mm in diameter serving as cathode, therefore, allowing the stimulation of Aδ nociceptive fibers located in the epidermis [Inui et al., 2002; Yoshino et al., 2010]. Pulses were given with a frequency of 2 Hz lasting 1 ms each. Stimulation with this electrode elicits a very unpleasant and painful feeling of a needle piercing the skin. Individual detection and pain thresholds were determined by the method of limits, averaging over the last two of three ascending and descending stimulation sequences. Pain tolerance was averaged over the last two of three ascending stimulation sequences. Stimulation strength was set at 70% between pain threshold and pain tolerance and adjusted to be rated between 6 and 7 on an 11 point verbal rating scale (ranging from 0 = no pain to 10 = strongest imaginable pain), allowing for a possible increase or decrease of perceived pain strength. The individually adjusted mean stimulation strength was 2.27 mA (SD = 1.76), the prebaseline intensity of this stimulus was rated as 6.40 (SD = 0.61) and the unpleasantness was assessed on a verbal rating scale (ranging from 0 = not unpleasant to 10 = extremely unpleasant) amounting to 6.70 (SD = 1.32). The postbaseline stimulus intensity was rated 6.10 (SD = 1.68) and the pain unpleasantness 7.25 (SD = 1.51).
Rt‐fMRI Feedback Protocol
The neurofeedback protocol consisted of a baseline run, 24 training trials spread over the course of four consecutive days, and an anatomical scan. On the first day the anatomical scan was taken, subjects were familiarized with the experimental setup and protocol, and the baseline run was recorded. Each training day consisted of six successive training trials; each trial was composed of six regulation phases lasting 45.0 s and six nonregulation phases lasting 22.5 s evenly distributed across each session. During the regulation phases painful electrical stimuli were given along with the real‐time feedback during the entire 45‐second phase (see Fig. 1a for the configuration of each trial). The baseline run was similar to the training trial but no feedback screen was shown.
Figure 1.
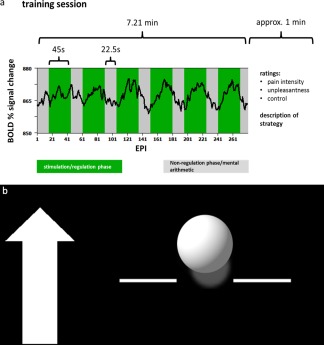
a: Course of one training trial. Over the course of one training trial (290 echo planar imaging sequences) the participants attempted either upregulation or downregulation of the activation in the target area during six regulation phases each lasting 45 s (green) while receiving painful electrical stimulation. Between the regulation phases, that is, in the nonregulation phases (gray) each lasting 22.5 s, the subjects performed mental arithmetic. An exemplary blood oxygenation level‐dependent (BOLD) time course is superimposed in black. b: Exemplary feedback screen. The arrow on the left indicated the direction in which the ball should be moved. The amplitude of the ball was computed as the difference between the percent signal change in the target region of interest and an unrelated region. The ball color was either blue or yellow for the rostral anterior cingulate cortex (rACC) and the posterior insula (pInsL) conditions. The color assignment to the target regions was randomized.
Feedback
Feedback was presented as a moving blue or yellow ball in front of a black background. During the regulation phases of the training trials a stationary white arrow appeared next to the ball on the left side of the screen indicating the vertical direction in which the ball should be moved (see Fig. 1b). Movements of the ball corresponded to changes in the computed BOLD signal of the regions of interest (ROI) of one of the targets (rACC or left posterior insula [pInsL]) and a baseline ROI that was to be unrelated to the activation caused by the stimulation and to pain processing [Caria et al., 2007; Haller et al., 2010]. For each training session, the feedback was updated every 1.5 s according to the repetition time (TR) of the fMRI protocol and the magnitude of the feedback was scaled using the visualizing program's built‐in auto‐scale function. To avoid the problem of an absolute scale in the positioning of the feedback symbol that might not be adequate for all subjects and lead to either ceiling effects, that is, an immobile symbol, or barely visible changes, the ROI signals of the regulation phase were averaged and nonregulation phase corrected. The feedback (vertical change of the ball position) was calculated as the difference of the percent signal change in the particular target ROI and a control region located in the parietal lobe, bordering the occipital lobe at the height of the pInsL, comprising in part Brodman area 39. Both the positions of the target ROIs and the control region were determined in an offline analysis of the baseline run. Individual functional datasets were transformed into Talairach space where the exact positions of the chosen ROIs were determined to verify their correct positions in the designated areas. The positions of the target regions were determined online guided by predetermined ROI positions from the baseline run. As the positioning of the ROIs was crucial it was monitored throughout the trials. The criteria for the target ROIs was (a) a position over the most significant cluster active during the stimulation phase and not active during the nonregulation phase and (b) being at the respective areas in the rACC and pInsL region, as these regions have been found to be most reliably active during the stimulation. As localizing reference for those cases where two foci of online activation in the rACC and pInsL were present, we used the location of the target ROIs of the baseline run. The positioning of the control region was guided by the offline analysis of the baseline run, and was also monitored online during the training trials to not exhibit significant activation or deactivation either in the presence of the nociceptive stimulus or during the nonregulation phase. The two different ROIs and two different directions in which the ball could be moved amounted to four conditions, which were counterbalanced over the four days: rACC up, rACC down, pInsL up, and pInsL down. The first and the last three trials of a training day were training trials for one of the ROIs (rACC or pInsL). The trial sequence of the conditions was counterbalanced (see Supporting Information Table SI) to avoid sequential learning of the conditions. The target region was discernible by the color of the moving ball. The color assignment was counterbalanced across subjects. During the baseline session, subjects were presented a nonchanging feedback screen showing a stationary white ball.
Instructions
The subjects were told that they could learn to control their brain activity and were informed that the vertical change of the blue or yellow ball was an indicator of their own brain activity in previously defined brain regions and that they would be able to observe the changes with a delay of a few seconds. They were informed that the two colors represented feedback of different brain activations. Subjects were not informed that the brain regions chosen were involved in the processing of pain and it was not suggested that a change in the activation could be related to a change in the perception of the painful stimulus. It was explained that the goal of the training was to assess if and to what extent it was possible to learn to alter brain activation in different brain areas during painful stimulation. The subjects were allowed to use any kind of strategy that would not involve body movement (e.g., muscle tension and relaxation). During the nonregulation phases, that is, in the absence of visual feedback, subjects were told to perform simple mental arithmetic for the purpose of stopping regulation attempts and ensuring comparability across subjects. For the mental arithmetic subjects were given a number between 800 and 1,100 from which two numbers between 3 and 7 should be subtracted repeatedly. After each trial the subjects had to rate their perceived control over the ball movement on an 11 point verbal rating scale (ranging from 0 = no control to 10 = absolute control), the average perceived pain intensity and unpleasantness of the nociceptive stimuli given in the regulation phases. After each training trial the subjects were asked to verbally report their individual regulation strategies.
Rt‐fMRI Data Acquisition
The fMRI data were acquired on a 3 T MAGNETOM Trio whole body MR scanner using a standard 12‐channel head coil (Siemens Medical Solutions, Erlangen, Germany). Shimming of the scanner was done manually to account for maximum magnetic field homogeneity. A standard gradient field map was recorded at the beginning of each measurement. For acquisition of fMRI data, a gradient‐echo echo planar imaging (EPI) sequence (TR = 1500 ms, echo time TE = 22 ms, matrix size = 96 × 96, flip angle α = 90°, bandwidth (BW) = 1270 Hz/px, parallel acquisition technique GRAPPA acceleration factor 2) was used. The EPI sequence was modified by inserting a real‐time data export functor into the EPI post processing pipeline immediately before the MosaicDecorator functor. The complete volume was converted into ANALYZE format accessible to a separate computer connected by local area network (LAN). Twenty‐four AC/PC aligned slices were acquired (voxel size = 2.2 × 2.2 × 3.5 mm3, gap = 0.5 mm). To avoid partial volume effects, a high in‐plane resolution was chosen. To compensate for the reduction in signal‐to‐noise ratio resulting from this a nonisotropic voxel size was necessary. A three‐dimensional (3D) fast low angle shot high‐resolution T1‐weighted anatomical scan was recorded from each subject (TR = 23 ms, TE = 5.02 ms, matrix size = 448 × 448, α = 25°, BW = 190 Hz/px, voxel size = 0.5 × 0.5 × 1.0 mm3) as anatomical reference. To reduce movement of the head, foam pegs (Siemens Medical Solutions, Erlangen, Germany) were used to immobilize the subject's head during the scanning periods. All steps of the training, scanning, and feedback procedures were always performed by the same person to ensure maximum comparability between all scans.
Online Analysis of the fMRI Data
Online data analysis was performed with Turbo BrainVoyager Version 1.1 (Brain Innovation, Maastricht, The Netherlands) as described in Weiskopf et al. [2003]. Online preprocessing of the functional data included incremental linear detrending, 3D motion detection and correction and drift removal. A recursive least squares regression algorithm was used for correlation analyses, for the linear detrending a linear predictor was added as a confound to the General Linear Model (GLM). The feedback signal reflects the unrelated region corrected percent signal change of the BOLD signal in the ROI. The software computes statistical maps from scan to scan based on the GLM. Feedback computation and visualization was performed with in‐house written scripts based on Presentation® Version 13.0 Build 01.23.09 (Neurobehavioral Systems, Albany, CA) on another computer connected with Turbo BrainVoyager via LAN.
Offline Analyses of the fMRI Data
Offline data preprocessing of the fMRI scans was performed using BrainVoyager QX 2.3 (Brain Innovation, Maastricht, The Netherlands). Gradient field map correction using the BrainVoyager plugin was performed on the EPI images to reduce geometric distortions and improve spatial accuracy to maximize fMRI sensitivity [Fellner et al., 2009]. Offline preprocessing comprised 3D motion correction (linear interpolation for motion detection and windowed sinc interpolation for motion correction). The datasets were then spatially smoothed with a Gaussian kernel with a full width half maximum of 8 mm3. Linear detrending was performed before high frequency artifacts were removed applying a highpass filter (0.006 Hz, approximately 2.5 times the duration of a combined regulation and nonregulation phase). A response function was estimated by convoluting a condition box‐car timecourse with a standard hemodynamic response function (two‐gamma HRF).
All functional datasets were coregistered with their respective anatomical dataset. The anatomical datasets were transformed into standard Talairach space using BrainVoyager. The anatomical transformation functions were then applied to the EPI datasets. All coregistration and transformation procedures were conducted manually to ensure maximum fit and overlap of the datasets. A group analysis based on the increase in BOLD percent signal change during the regulation phase with respect to the nonregulation phase was performed using a GLM. All results were Bonferroni corrected for multiple testing.
Offline time course data analysis of the feedback was automated with MATLAB R2011b (The MathWorks, Natick, MA), statistical analyses were performed with IBM SPSS Statistics Desktop (IBM, Armonk, NY) for windows, version 21.0 using analysis of variance (ANOVA), correlations and the Friedman Test, when ordinal data were used.
The positions of the target and unrelated regions were saved for each trial with every ROI having the same standard dimension of 12 × 12 × 1 voxels selected on the transversal slice. For each subject and each condition time courses from the target ROIs were extracted for all training trials. Feedback time courses were corrected for overall brain activity by subtracting the time course of a control ROI unrelated to the task as was done online in each training trial. This procedure further eliminated individual differences in signal amplitude. All further analyses were then based on this unrelated region corrected feedback signal, the procedure being identical to the online feedback calculation.
Only those ROI positions were saved that were used in the feedback calculation, that is, the target ROIs. The nontarget ROI position for the offline analyses, that is, the rACC in the insula down and the insula up condition, and pInsL in the rACC down and rACC up condition, was determined by averaging the ROI positions per subject from the two conditions where the nontarget ROI was the target and the position was saved. The signal change was extracted.
Both the target and unrelated ROIs serving as reference for later offline analyses were determined by averaging the ROI coordinates per condition, thus optimizing signal change comparability for each condition. Group target ROI activations in the baseline run are shown in the Supporting Information Table SII. The unrelated region corrected feedback time courses were averaged according to regulation and nonregulation phases for each trial of each condition, leaving six single means per subject and condition for regulation and nonregulation phases. The averaged differences of these six single means were averaged into a resulting mean per subject and condition. Later analyses were one‐tailed when a specific a priori hypothesis was present and two‐tailed if we had no assumptions about the direction of an association.
The training effect was defined as the difference of the modulation effect in the first and the last training trial of a condition. The modulation effect was determined by computing the difference of the unrelated region corrected signal change between the regulation and nonregulation phases of the trials. In the baseline, no regulation was attempted. The difference between the stimulation phases and the arithmetic phases in the baseline, thus, reflects the stimulation effect. To determine the effect of training on the ability to regulate brain activation, we compared the modulation effect in both Trial 1 and Trial 6 (the first and last trial of each condition) for all four conditions. One‐tailed paired samples t‐tests were used to assess changes from the stimulation effect in the baseline to the modulation effect in Trial 1 and in Trial 6 testing for both stability of activation in response to the painful stimulus and for a change due to training. A successful training effect was seen in the change of the modulation effect from Trial 1 to Trial 6.
RESULTS
Active Regions
A whole brain random effect analysis of the baseline run confirmed that the nociceptive stimulation activated the core brain regions involved in pain processing including the rACC, the left and right posterior insula, the bilateral secondary somatosensory cortex, the posterior cingulate gyrus, and the left middle frontal gyrus. Significant active clusters suitable for feedback were identified in the target regions, the pInsL and the rACC (see Fig. 2a,b and Supporting Information Table SII). Except for the right posterior insula in the pInsL up condition, all regions were significantly active in Trial 1 (the first neurofeedback training session) of all four (rACC down, rACC up, pInsL down, pInsL up) conditions. All regions with the exception of the right posterior insula were significantly active in Trial 6 (the last neurofeedback training session) of all four conditions (see Supporting Information Table SIII).
Figure 2.
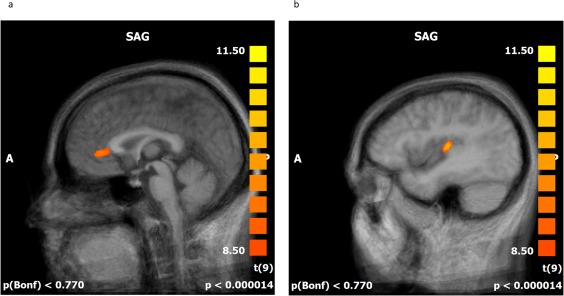
a,b: Offline statistical parametric mapping of the baseline run. Significant activations during the stimulation phases are superimposed over an averaged brain consisting of the 10 T1‐weighted anatomical scans of the subjects, sagittal view (SAG). Red to yellow indicates significant activation in the regulation phases. Individual anatomical and functional scans were transformed into Talairach space. Average activation (a) of the rACC (b) the pInsL.
Training Effects
In the rACC down condition, the modulation effect decreased significantly from Trial 1 to Trial 6 (t(9) = 1.966; P < 0.05) indicating that the subjects learned to decrease rACC activation in response to the stimulus. The height of the stimulation effect in the baseline and the modulation effect in Trial 1 were not significantly different, indicating that the subjects had not yet learned regulation in the first trial. The modulation effect in Trial 6 was also significantly different from the baseline run (t(9) = 2.054; P < 0.05) with the modulation effect being lower in Trial 6 indicating that the subjects had successfully learned to downregulate activation in the rACC also in comparison to the stimulation effect in the baseline.
A different pattern emerged for the rACC up condition. Here, the modulation effect in Trial 6 was not significantly different from that in Trial 1, or the stimulation effect in the baseline, nor were the stimulation effect and the Trial 1 modulation effect significantly different, indicating that there was no significant upregulation of rACC activity.
In the pInsL down condition, the modulation effect in Trial 6 was significantly lower than in Trial 1 (t(9) = 2.798; P < 0.05) indicating successful training. The stimulation effect in the baseline and the modulation effect in Trial 1 did not significantly differ, indicating that the subjects had not yet learned to downregulate insula activation. The modulation effect in Trial 6 was significantly different from the stimulation effect in the baseline run (t(9) = 2.116; P < 0.05) with the modulation effect being lower in Trial 6 indicating that the subjects had successfully learned to downregulate activation in the pInsL.
In the pInsL up condition, the modulation effect in Trial 6 was significantly higher than in Trial 1 (t(9) = −2.312; P < 0.05) indicating a successful training effect, whereas the stimulation effect in the baseline run did not differ significantly from the modulation effect in Trial 1, suggesting that the subjects had not yet learned to regulate activation. The stimulation effect in the baseline run did not differ significantly from the modulation effect in Trial 6.
To determine the response characteristics of one specific region, that is, the direction‐dependent differences in the regulation potential, we compared the modulation effects of the upregulation and downregulation conditions of the regions. One‐tailed paired samples t‐tests between the pInsL up and down conditions revealed that subjects were not consistently successful in upregulating and downregulating pInsL activation but managed to differentially regulate activity for the first time on Trial 3 (t(9) = −1.891; P < 0.05) and again on Trial 6 (t(9) = −2.846; P = 0.01). Paired samples t‐tests between the rACC up and down conditions revealed that the subjects were able to differentially regulate activation in the rACC only on Trial 5 (t(9) = 1.926; P < 0.05) as shown in Supporting Information Figure S1.
Comparison of the Controllability of Regions
The training effects of the rACC up, rACC down, pInsL up, and pInsL down conditions were not significantly correlated with each other, neither between the two directions in the same region, nor between the same directions in different regions (r(8): −0.22–0.31; P > 0.38, two‐tailed).
To assess the controllability of the regions, that is, to determine how well subjects learned to regulate the two regions, we compared the modulation effects of Trial 1 and Trial 6 of the two regions and the two directions. An ANOVA revealed a significant effect of regulation direction, that is, upregulation or downregulation, (F(7.89, 1.37) = 5.770, P < 0.05) but no significant effect of the region or an interaction between the region and regulation direction. One‐tailed paired samples t‐tests between the rACC up and rACC down condition revealed no significant differences in accordance with the nonsignificant training effect in the rACC up condition. For the pInsL up and pInsL down condition there was a significant difference (t(9) = −4.370; P < 0.001), indicating that the pain‐evoked activation in the pInsL was successfully regulated. The direction‐independent magnitude of the learning effect did not differ between the four conditions, emphasizing that the magnitude of the activation change after the six training trials was similar in both regions and directions (see Fig. 4).
Figure 4.
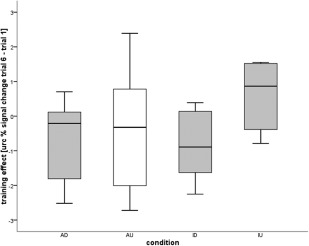
Magnitude of the regulation effect calculated from the unrelated region corrected BOLD signal change in the rACC upregulation (AU) and downregulation (AD) and pInsL upregulation (IU) and downregulation (ID) condition. The diagram shows the conditions in which control of the activation was achieved (gray) and the AU condition (white) where the group as a whole did not achieve successful regulation. The regulation effect was defined as the difference between regulation of the BOLD effect in the rACC or pInsL in Trial 6 and Trial 1. The absolute magnitude of the learning effect did not significantly differ between the conditions.
To determine whether subjects were able to upregulate and downregulate the activation of single regions, the modulation effects over all six trials per condition were calculated. If the average feedback signal of the region was correct, and the received feedback signal was correct in at least four out of six training trials, and if the magnitude of the feedback signal increased or decreased in the right direction for the specific condition from Trial 1 to Trial 6, a subject was considered a learner for the condition. The other subjects were categorized as nonlearners. Of the four conditions, the rACC down, pInsL down, and pInsL up conditions had seven learners and three nonlearners, the rACC up condition had four learners and six nonlearners, indicating that in both regions activation in both directions can be achieved. For the distribution of learners and nonlearners see Supporting Information Table SIV. There were no significant correlations between the training effect and person‐specific variables such as gender, age, handedness, stimulation strength, and pain intensity and unpleasantness ratings.
Covariation Between Target and Nontarget Region
To investigate a possible effect of the training of the regulation of one region on the response of the other region, we compared the absolute difference of the height of the unrelated region corrected signal change in the target region and the nontarget region in Trial 1 and Trial 6 using a two‐tailed paired samples t‐test. The difference in signal height (dissociation) between the target and the nontarget region increased from Trial 1 to Trial 6 only in the rACC up condition (t(9) = −2.261; P < 0.05) where the group training effect was not significant, that is, the group did not learn upregulation of the rACC. The dissociation of the activation levels did not change with the learning that took place in the rACC down, pInsL up, or pInsL down conditions.
To determine the relation between the magnitude of the covariation of regions and the magnitude of the training effect, we performed a two‐tailed correlation analysis between the mean absolute difference of the activation levels between the regions and the difference of the signal change between Trial 6 and Trial 1 (learning effect: Trial 6–Trial 1). A negative correlation in the downregulation conditions and a positive correlation in the upregulation conditions indicated that greater dissociation between the regions was related to a larger training effect. In the rACC down condition, there was a significant negative correlation between the magnitude of the training effect and the mean difference in the activation levels of the two regions (r(8)= −0.75; P < 0.05). In the rACC up condition there was a significant positive correlation between the magnitude of the training effect and the mean difference in the activation levels of the two regions (r(8) = 0.68; P < 0.05). In the pInsL down condition, the training effect did not correlate with the mean difference in the activation levels of pInsL and rACC. In the pInsL up condition there was a significant positive correlation between the magnitude of the training effect and the mean difference in the activation levels (r(8) = 0.85; P < 0.01). For a detailed depiction of the relationship between the learning effect and the difference in the activation levels between the target and nontarget ROI see Supporting Information Figure S2.
Pain Intensity and Unpleasantness Ratings
To test for the influence of upregulation or downregulation of the target regions on the evaluation of pain intensity and unpleasantness of the stimulus, a two‐tailed correlation analysis was performed. The baseline corrected modulation effects (i.e., the modulation effects corrected for the stimulation effect of the baseline in the respective region, indicating the magnitude of regulation success per trial) for every trial per condition and the corresponding pain and unpleasantness ratings were correlated. There was no significant correlation between the modulation effects of the trials and pain or unpleasantness ratings. Figure 3 shows the pain intensity and unpleasantness ratings of the baseline as well as Trials 1 and 6 in relation to feedback success. Neither pain intensity nor unpleasantness ratings changed significantly over the course of the six training trials (for an overview see Supporting Information Table SV)
Figure 3.
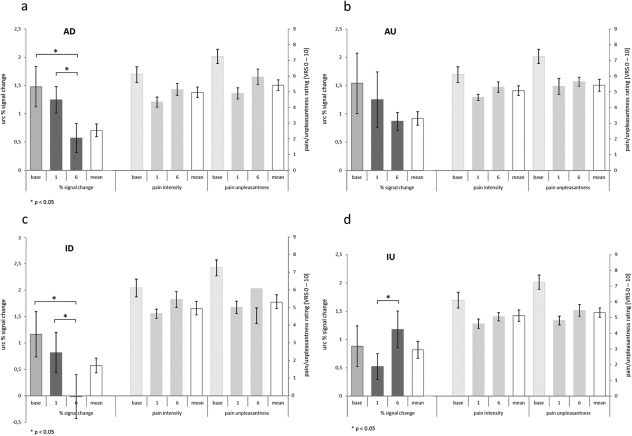
a–d: Unrelated region corrected (urc) brain activity in the respective target areas of rACC and pInsL, and pain intensity and unpleasantness ratings in the four conditions (a–d): (AD/AU: rACC down/up; ID/IU: pInsL down/up), during the baseline (base) run, the first and the last trial (1; 6), and the mean across all six trials as reference (white bar). The percent signal change was computed by averaging over the differences in the urc blood oxygenation level‐dependent signal of the regulation and nonregulation phases for each participant and averaging over all participants. This computation was identical to the feedback the subjects received during the training trials. A positive training effect is seen in the significant differences from Trial 1 to Trial 6 in the right direction as seen in the conditions. The pain intensity and pain unpleasantness ratings relate to a verbal rating scale (VRS) with pain intensity ranging from 0 = no pain to 10 = strongest imaginable pain and pain unpleasantness ranging from 0 = not unpleasant to 10 = extremely unpleasant.
To test for the maximum effect of regulation on pain and unpleasantness ratings, two‐tailed paired samples t‐tests of pain intensity and unpleasantness ratings in the first and the last trial of the upregulation and downregulation conditions were performed. In the first trial, there were no significant differences for either pain or unpleasantness ratings between the rACC down/up and the pInsL down/up conditions. The same was true for the last trial; there were no significant differences between the rACC up/down and the pInsL up/down conditions, indicating that increasing or decreasing activation in the rACC or the pInsL did not have a significant effect on pain intensity or unpleasantness (t (9) > −0.92 and < 0.84); P > 0.38).
To examine the relationship between the rt‐fMRI training specificity and the evaluation of pain intensity and unpleasantness in more detail, a two‐tailed correlation analysis was performed between the training effects (difference in modulation effect of Trials 1 and 6) of the four conditions, the mean absolute difference in activation levels between the target ROI and nontarget ROI, that is, the difference in the activation change in response to the stimulus in rACC and pInsL, and the change in pain intensity and unpleasantness ratings from Trial 1 to Trial 6. There was a significant positive correlation in the pInsL up condition of the mean difference in activation levels of the rACC and pInsL and the mean unpleasantness ratings, indicating that the low coactivation of rACC and pInsL while activation in the pInsL was increased, was associated with higher unpleasantness ratings (r(8) = 0.72; P < 0.05) as shown in Figure 5.
Figure 5.
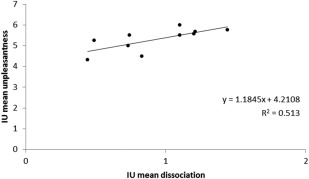
Relationship between the mean difference in activation levels of the target and nontarget ROI and the mean perceived unpleasantness in the pInsL upregulation (IU) condition. Greater difference in activation levels was associated with higher unpleasantness ratings.
Strategies
All strategies used by the subjects were recorded, reviewed, and later subsumed under four categories. Category 1 comprised strategies of either picturing some kind of ball in motion or silent verbal instructions to get the ball to move. Category 2 included distraction strategies such as focusing on another body part, continuing with the mental arithmetic, imagining a scene that was not described as being particularly emotional (like walking down the aisles in a grocery store), or relaxing. Category 3 consisted of strategies utilizing either positive or negative emotional memories. Category 4 comprised strategies involving the painful stimulus, imagining it to be more or less painful, change modality, or location. A few subjects reported using two strategies during one training trial. In these cases both were recorded and categorized.
A Friedman test was conducted to evaluate differences in the frequency of the use of strategies between the conditions, showing that strategy use was not significantly different in any of the conditions (χ 2 (3,10) = 12.132; P = 0.28). For the distribution of used strategies per trial see Supporting Information Figure S3. Due to the small sample size in relation to the number of strategy categories, strategy use of single conditions or conditions comprising one region or direction was not compared.
DISCUSSION
Active Regions During Painful Stimulation and rt‐fMRI Feedback
The off‐line analyses of the brain activity related to the nociceptive stimulation revealed all target regions identified individually during the baseline run to be active during both the first and the last trial of all four conditions (rACC up, rACC down, pInsL up, pInsL down). During the online feedback, activation in the target ROI was monitored and was present on every single feedback trial thus providing an adequate basis of the feedback signal. This ensured that regions active in response to the painful stimulation as found on the baseline run were also active during the feedback training. Both the target and the nontarget region were significantly active irrespective of whether subjects tried to upregulate or downregulate the BOLD response. Additional regions found to be significantly active in the baseline run were the right posterior insula (pInsR), bilaterally the secondary somatosensory cortex, the posterior cingulate cortex, and the left midfrontal cortex. These regions have frequently been described to be active during noxious stimulation and pain perception in general, [Apkarian et al., 2005; Iannetti et al., 2005]. Those regions were also active during the first training trials of all four conditions, suggesting that initial attempts of activity regulation did not influence the response of these regions to the painful stimulation, that is, the activation pattern was similar to the one seen when no regulation was attempted. With the exception of the pInsR, ipsilateral to the stimulation side, the same pattern of activation was also found in the last training trial of all four conditions. In Trial 6, the pInsR was deactivated in all conditions. Deactivation of this region during painful stimulation was seen previously in the presence of a cognitive task or distraction corresponding with a reduction of pain perception [Bantick et al., 2002]. A decrease in posterior insula activation has also been observed in connection with increased task demand. Harrison et al. [2011] found deactivation in the default mode network in response to distinct cognitive tasks that involved different cognitive modalities, an emotional face matching task and a cognitive interference task (Stroop). Both the face matching task and a modified Stroop color‐word interference task were presented in a low and high task demand condition. For the emotional face matching task, the low task demand consisted of 30 s of shape matching where subjects were to match a probe to a target (circular or oval shape). In the high task demand version in the same setup subjects were to match emotional faces. The Stroop task was differentiated in low and high task demand as subjects performed congruent and incongruent color‐word matching trials. The authors showed a deactivation of parts of the default mode network when subjects were performing a cognitive task. Moreover, in the high task demand condition, a deactivation of the posterior insula was observed, a region not previously identified as part of the default mode network. On the basis of this deactivation, it was possible to discriminate high from low performing subjects in the Stroop task; thus, the observed deactivation was indicative of performance. Taken together these findings suggest that the observed deactivation in the pInsR might be due to increased cognitive load of the regulation efforts. Interestingly, the contralateral posterior insula (pInsL) did not show deactivation even in the two conditions where the insula was not part of the feedback, that is, learning, and thus not the direct target of regulation efforts. This differential change in ipsilateral and contralateral posterior insula suggests that the regions play a different role within the pain processing network. Overall, with the exception of the contralateral posterior insula, the network found to be active in response to the painful stimulation alone (baseline), was still active in Trial 1 and in Trial 6.
Training and Controllability
Most rt‐fMRI neurofeedback studies used either three or four training trials on only one day [Caria et al., 2007; Caria et al., 2010; deCharms et al., 2005; Rota et al., 2009; Yoo et al., 2008], producing significant training effects. In this study, we increased the amount of training trials to six, giving subjects more time to train since two brain regions had to be upregulated and downregulated. We used four distinct conditions over the course of four days, thus increasing the difficulty of the regulation task by having the subjects switch between upregulation and downregulation and two different regions. Moreover, the tasks were counterbalanced over the four days thus switching both the order of regions and the order of upregulation and downregulation. The balancing protocol ensured that the first trial of each condition always took place on the first training day while the last trial of each condition was always on the fourth training day. By comparing the first and the last training trial of each condition we saw the full effect of the six training trials per condition in the course of four consecutive days of training. We found a positive training effect in three out of the four conditions. Only in the rACC upregulation condition, the mean BOLD percent signal change in Trial 6 was not significantly different from that in Trial 1 or the baseline. deCharms et al. [2005] had subjects upregulate and downregulate rACC activation in the presence of a painful thermal stimulus within the same trial. They observed an increase in the difference between the phases over the course of three training trials. The contribution of each phase to the increasing difference was not reported making it difficult to assess the subjects' ability to selectively upregulate and downregulate rACC activation. Weiskopf et al. [2003] had one subject learn to increase ACC activation, showing that an upregulation was possible, although in the absence of painful stimulation. When the difference between the regulation and nonregulation phases was examined, some subjects in the rACC up condition did learn to upregulate activation in the rACC. Furthermore, the variation of the training effect in the rACC upregulation condition was wider than in the other conditions, suggesting that some subjects were able to increase their regulation ability from Trial 1 to Trial 6 while others had higher activation in Trial 1, failing to achieve upregulation on the last trial. This wider distribution of the training effects might be due to the fact that this part of the ACC is active during different states and tasks. In the course of the painful stimulation, activation was already increased. The rostral region of the ACC we chose as target region is functionally connected with the periaqueductal gray (PAG), which plays an important role in pain modulation. Bingel et al. [2006] found the connectivity between the rostral ACC and the PAG to be increased during a placebo analgesia condition. In patients with chronic pain, the connectivity between these areas is reduced [Cifre et al., 2012]. From this, a reduction in pain perception would be accompanied by increased activation in these areas. However, since the rostral ACC has also been implicated in the generation of affective states [Critchley, 2005] and negative emotion increases rACC activity [Hamilton et al., 2007], there might have been a motivational component preventing some subjects from successfully upregulating rACC activation. This might have rendered rACC activation more difficult to modulate since the rACC may have been simultaneously involved in many processes beyond pain perception. We cannot rule out a ceiling effect for rACC upregulation. Finally, we are aware that likely significant effects of rACC upregulation might not have been detected due to power limitations related to our sample size of 10.
Controllability of the regions was not the same. While the subjects learned control over the pInsL, we found a directional bias in the rACC modulation, rendering upregulation more difficult. Comparing the controllability of the regions and directions of regulation, we found no significant differences among the successful conditions rACC down and pInsL up and down. Though not significant on a group level, some subjects did learn to upregulate rACC. These findings indicate that the rACC and the pInsL can be regulated in all directions, with rACC upregulation being seemingly more difficult.
Changes in Pain Intensity and Unpleasantness
Several studies have shown that control over specific brain areas is mirrored by changes in behavior [Caria et al., 2010; Haller et al., 2010; Hamilton et al., 2011; Rota et al., 2009; Sitaram et al., 2011]. Despite a wide base of evidence for the connection between regulation of local brain activity and behavior, the influence on pain perception and unpleasantness has not been replicated. Indeed some studies found that regulation of regional brain activity can be achieved without changes in behavior and cognition [Johnston et al., 2011; Weiskopf et al., 2003]. In this study, we found no significant connection between successful upregulation and downregulation of the rACC or the pInsL and pain and unpleasantness ratings. However, in contrast to the previous study by deCharms et al. [2005] rACC upregulation was not successful in our paradigm. Both the rACC and posterior part of the insula are part of a wider network involved in the processing of pain but also a variety of cognitive and behavioral functions such as emotional processing or somatosensory integration [Baumgartner et al., 2010; Bush et al., 2000; Johnston et al., 2011; Li et al., 2013]. It is possible that neuromodulation of areas that are involved in different functions may not yield obvious specific behavioral or cognitive changes [McCaig et al., 2011]. However, Caria et al. [2010] had subjects learn to upregulate activation in the left anterior insula, another region involved in multiple functions, and thus alter their subjective emotional response to aversive and neutral pictures. The subjects in the present study were involved in a demanding cognitive task while they received painful stimulation. In the particular situation of receiving painful stimuli and receiving feedback of their brain activity, while focusing attention on regulation efforts and evaluating success, it seems likely that pain perception is not the only source of activation in the pain‐related network. It is therefore possible that ‘simple regulation’ of single multifunctional nodes of a wider pain network does not lead to a significant change in the perception of pain intensity and unpleasantness [Cauda et al., 2012]. deCharms et al. [2005] used a combination of cognitive strategies, virtual image video feedback and rt‐fMRI feedback, which together might have had a stronger effect than pure rt‐fMRI feedback. Another difference between the two studies is the use of thermal stimulation, which activated Aδ and C‐fibers in the former and electric stimulation, which activated only Aδ fibers, in the present study. Although we did not find an effect of the change in the individual brain regions, we observed a significant effect when more than one region was involved. Unpleasantness ratings were higher when activation in the insula was increased but at the same time was disengaged from activation in the rACC. This points towards the importance of the state of connectivity of the pain‐related network instead of the response of single regions. This is in accordance with rt‐fMRI feedback approaches that use activated networks instead of single regions, using, for example, pattern classification [Lee et al., 2011; Sitaram et al., 2011]. Identifying patterns of brain activation corresponding with a specific task or a specific stimulus makes it possible to use rt‐fMRI neurofeedback as a means of altering behavior or function not clearly linked with one specific brain area. With this approach patterns of brain activation in response to painful and nonpainful thermal stimulation were distinguished, permitting the identification of pain perception from multiple networks [Brodersen et al., 2012] without relying on communication with the subject [Brown et al., 2011]. Given the complexity of the feedback task and our relatively small sample size, it is possible that with more subjects and more training trials, as regulation becomes easier and the cognitive load decreases, a possible effect of the regulation of the brain regions on the perception of pain intensity and unpleasantness could be detected.
Controllability
Previous rt‐fMRI studies reported successful learning of the control of the region involved in the feedback procedure but did usually not include more than one region. We found that the conditions, that is, areas and directions of regulation, were not controllable to the same degree by all subjects. Subjects who were successful in one condition were not necessarily successful in another. This implicates that not all subjects can learn to regulate brain activation related to painful stimulation in a specific area in a given time, not all regions can be regulated equally well, and there is no general ability to regulate pain‐related brain activation. Although the network involved in the processing of pain has been described quite consistently, there might be differences between subjects, making it necessary to further individualize feedback. A method of identifying network states can serve as a basis of such a feedback and has been described by Wager et al. [2013].
Upregulation and Downregulation
In previous studies, where both upregulation and downregulation were required, this was learnt within the same session [deCharms et al., 2005; Hamilton et al., 2007]. In our study we used separate training trials for the directions, thus making it possible to compare the course of controllability, as well as to monitor systematic changes that might occur independently from regulation efforts such as an overall decrease (habituation) or increase (sensitization) in the response of the area. Most studies found a significant training effect after three to four trials, either in regulating in one direction or with upregulation and downregulation combined. Since in our study one of the conditions was not learned by the group, comparison of upregulation and downregulation of the rACC was difficult. Comparing the single training trials, we found the only significant difference between upregulation and downregulation to be on the fifth training trial. For the pInsL conditions, there were significant differences on the third and on the sixth training (Supporting Information Fig. S1). These findings are surprising because in all previous studies the effect of learning appeared to be linear, increasing from trial to trial, whereas in our study learning did not follow a clear pattern. This might have several reasons. Most studies provided strategies [Caria et al., 2010; deCharms et al., 2005; Hamilton et al., 2007; Yoo et al., 2008; Zotev et al., 2011], only some gave none [Weiskopf et al., 2003]. Our protocol presented a more complex task since there was more than one region to regulate and the direction and region varied between the trials, which might explain why the subjects took longer to learn regulation. In addition, the participants did not know the functional context of the region to be controlled. Our results point out that learning does not necessarily occur in a linear fashion and might depend on the context information provided. Moreover, some subjects may need more time to practice than others and initial success might not be an indicator of a stable ability to regulate brain activation.
Covariation Between rACC and pInsL During Feedback
The target regions we chose are part of a network of regions involved in the processing of pain. There is evidence that perceived pain intensity and unpleasantness are related to the magnitude of activation of specific parts of this network [Rainville, 2002]. Although pain intensity and unpleasantness are correlated and the regions might therefore change in similar directions, dissociation of the activation levels of the regions may occur, for example, if one considers that the rACC is also involved in the descending inhibitory pain control network and on the background of increases in posterior insula activation with successful behavioral training [Diers et al., 2013]. In any given training trial, subjects only had information about activity of one of the target regions and thus could not be influenced by feedback of the activity in the other region. We found that in the three conditions in which the group as a whole learned successful regulation, dissociation levels, that is, the difference of the height of the BOLD percent signal change in the regulation phases between the target regions, did not change. This suggests that the difference in the pInsL and rACC activation did not increase or decrease with learning. Thus improvement of the regulation ability in one region was not limited to this region, but included the functionally connected region. Other studies showed that learning control over a specific brain area, even if this region is multimodal, does not necessarily involve other regions that belong to the same network [McCaig et al., 2011]. Dissociation between the activation levels did, however, increase in the rACC upregulation condition where the group did not learn regulation. Interestingly, there was an effect of the magnitude of the covariation between the target regions and learning. In three out of the four conditions (rACC upregulation and downregulation, pInsL upregulation), a higher mean difference in the activation correlated with the ability to learn regulation of activity in one region. This dissociation was also related to less pain unpleasantness and suggests that connectivity in pain‐related networks needs to be further investigated as noted above.
Strategies
Together with the reinforcement provided by the feedback, strategies used during the task may affect the result. During the learning process subjects learn to control processes they are not consciously aware of, with the search for and generation of successful strategies being the representation and realization of the regulation attempt [Roberts et al., 1989]. The role of strategies in neurofeedback regulation, however, is not clear. They could provide information about the function of the brain area in some cases. In others they are linked to a specific feedback task and the same strategy can be used to successfully achieve opposing effects [Siniatchkin et al., 2000]. The participants of our study were not provided with information about the function of the region or strategies that might work. The strategies described by our subjects after each training trial comprised four categories involving the feedback symbol and imagined commands, distraction, emotional involvement, or focus on the painful stimulus itself. We found no strategy to be predominant in any of the conditions and trials (Supporting Information Fig. S3). To draw definite conclusions about strategy preferences in the conditions from, for example, the first to the last training trial, a higher number of subjects would be necessary to statistically detect differences between the strategy categories. However, our results do show that successful regulation can be learned independent of a specific strategy, and that the activation of the target region in response to a painful stimulus can be regulated without using pain‐related strategies even in the presence of a painful stimulus.
CONCLUSIONS
We compared the controllability and the behavioral effects of rt‐fMRI feedback of two brain areas involved in the processing of painful stimulation. While the controllability of the rACC was limited to downregulation, subjects learned full regulation of the pInsL. Despite success in the two downregulation and one upregulation condition, the responses of the brain regions to the painful stimulus were not abolished, showing significant activation throughout the six trials suggesting modulation but not abolishment of the pain‐related brain response. The modulation effects achieved in the upregulation and downregulation conditions of the rACC and pInsL conditions were not correlated, suggesting that learning the modulation of one region is not related to learning the modulation of another region. We did find that lower covariation between the two regions correlated positively with the training effect and thus learning, in three out of four conditions. The lack of a significant correlation of rt‐fMRI self‐regulation with behavioral measures such as pain intensity and unpleasantness in the upregulation and downregulation of rACC and pInsL suggests that the pain‐related network can be influenced without changes in the perception of pain intensity and unpleasantness. We did not find an effect of the individual controllability of the two brain regions on pain intensity and unpleasantness. One reason for this might be the nature of the pain‐related network itself [Cauda et al., 2012], which is connected to other networks involved in emotional and cognitive processing [Cauda et al., 2012; Iannetti and Mouraux, 2010; Legrain et al., 2011]. Regulation of activation in these regions, even in the presence of a painful stimulus, will thus not inevitably lead to a change of pain perception, especially since pain perception is highly context‐dependent [Iannetti et al., 2008; Rhudy and Meagher, 2000; Wang et al., 2010; Wang and Mitchell, 2011]. Moreover, during the regulation efforts the target regions are involved in multiple tasks including attention, pain processing, and cognitive and emotional processes such as regulation and evaluation of success. In contrast to deCharms et al. [2005], we used rt‐fMRI feedback independent from cognitive strategies and image‐related feedback, which might explain why we did not see significant effects on pain intensity and unpleasantness, however, the failure to upregulate rACC in our subjects might also have contributed. In addition, we used a number of methods to ensure that only the target brain region was involved in the feedback regulation, this was also different compared to previous research. When the insula was upregulated and interaction with the rACC activation was low, we found a significant relationship with lower unpleasantness ratings, suggesting that the interrelationship between the involved brain regions might be more important than individual activation peaks. Latest developments in rt‐fMRI feedback are already based on connectivity or real‐time pattern classification [Esposito et al., 2003; LaConte et al., 2007; Ruiz et al., 2013; Sitaram et al., 2011]. In the context of pain, involving the state of the involved network in the calculation of the feedback might thus be of advantage in the control of pain‐related activation and could also help to develop this method as a treatment tool for patients with chronic pain, since learning modulation of single regions alone does not lead to a change in pain perception.
Supporting information
Supplementary Information
ACKNOWLEDGMENTS
The authors would like to thank Dr. C. Christmann from the Central Institute of Mental Health, Mannheim, Germany, for help with the setup of the experimental hardware and establishing the experimental procedure. We would also like to thank Professor K. Inui from National Institute for Physiological Sciences, Okazaki, Japan for providing the electrodes.
REFERENCES
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK (2005): Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9:463–484. [DOI] [PubMed] [Google Scholar]
- Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I (2002): Imaging how attention modulates pain in humans using functional MRI. Brain 125(Pt 2):310–319. [DOI] [PubMed] [Google Scholar]
- Baumgartner U, Iannetti GD, Zambreanu L, Stoeter P, Treede RD, Tracey I (2010): Multiple somatotopic representations of heat and mechanical pain in the operculo‐insular cortex: A high‐resolution fMRI study. J Neurophysiol 104:2863–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C (2006): Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain 120:8–15. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Ruiz S, Sitaram R (2013): Learned regulation of brain metabolism. Trends Cogn Sci 17:295–302. [DOI] [PubMed] [Google Scholar]
- Brodersen KH, Wiech K, Lomakina EI, Lin CS, Buhmann JM, Bingel U, Ploner M, Stephan KE, Tracey I (2012): Decoding the perception of pain from fMRI using multivariate pattern analysis. Neuroimage 63:1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JE, Chatterjee N, Younger J, Mackey S (2011): Towards a physiology‐based measure of pain: Patterns of human brain activity distinguish painful from non‐painful thermal stimulation. PLoS One 6:e24124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI (2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4:215–222. [DOI] [PubMed] [Google Scholar]
- Caria A, Veit R, Sitaram R, Lotze M, Weiskopf N, Grodd W, Birbaumer N (2007): Regulation of anterior insular cortex activity using real‐time fMRI. Neuroimage 35:1238–1246. [DOI] [PubMed] [Google Scholar]
- Caria A, Sitaram R, Veit R, Begliomini C, Birbaumer N (2010): Volitional control of anterior insula activity modulates the response to aversive stimuli. A real‐time functional magnetic resonance imaging study. Biol Psychiatry 68:425–432. [DOI] [PubMed] [Google Scholar]
- Cauda F, Torta DM, Sacco K, Geda E, D'Agata F, Costa T, Duca S, Geminiani G, Amanzio M (2012): Shared "core" areas between the pain and other task‐related networks. PLoS One 7:e41929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin H, Bagarinao E, Mackey S (2012): Real‐time fMRI applied to pain management. Neurosci Lett 520:174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifre I, Sitges C, Fraiman D, Munoz MA, Balenzuela P, Gonzalez‐Roldan A, Martinez‐Jauand M, Birbaumer N, Chialvo DR, Montoya P (2012): Disrupted functional connectivity of the pain network in fibromyalgia. Psychosom Med 74:55–62. [DOI] [PubMed] [Google Scholar]
- Critchley HD (2005): Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol 493:154–166. [DOI] [PubMed] [Google Scholar]
- deCharms RC, Maeda F, Glover GH, Ludlow D, Pauly JM, Soneji D, Gabrieli JD, Mackey SC (2005): Control over brain activation and pain learned by using real‐time functional MRI. Proc Natl Acad Sci USA 102:18626–18631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diers M, Zieglgansberger W, Trojan J, Drevensek AM, Erhardt‐Raum G, Flor H (2013): Site‐specific visual feedback reduces pain perception. Pain 154:890–896. [DOI] [PubMed] [Google Scholar]
- Esposito F, Seifritz E, Formisano E, Morrone R, Scarabino T, Tedeschi G, Cirillo S, Goebel R, Di Salle F (2003): Real‐time independent component analysis of fMRI time‐series. Neuroimage 20:2209–2224. [DOI] [PubMed] [Google Scholar]
- Fellner C, Doenitz C, Finkenzeller T, Jung EM, Rennert J, Schlaier J (2009): Improving the spatial accuracy in functional magnetic resonance imaging (fMRI) based on the blood oxygenation level dependent (BOLD) effect: Benefits from parallel imaging and a 32‐channel head array coil at 1.5 Tesla. Clin Hemorheol Microcirc 43:71–82. [DOI] [PubMed] [Google Scholar]
- Frot M, Magnin M, Mauguiere F, Garcia‐Larrea L (2007): Human SII and posterior insula differently encode thermal laser stimuli. Cereb Cortex 17:610–620. [DOI] [PubMed] [Google Scholar]
- Haller S, Birbaumer N, Veit R (2010): Real‐time fMRI feedback training may improve chronic tinnitus. Eur Radiol 20:696–703. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Glover GH, Gotlib IH (2007): Healthy individuals can use real‐time fMRI neurofeedback to modulate activity in the subgenual anterior cingulate cortex. Biol Psychiatry 61:30S–30S. [Google Scholar]
- Hamilton JP, Glover GH, Hsu JJ, Johnson RF, Gotlib IH (2011): Modulation of subgenual anterior cingulate cortex activity with real‐time neurofeedback. Hum Brain Mapp 32:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Contreras‐Rodriguez O, Soriano‐Mas C, Lopez‐Sola M, Deus J, Ortiz H, Blanco‐Hinojo L, Alonso P, Hernandez‐Ribas R, Cardoner N, Menchón JM (2011): Task‐induced deactivation from rest extends beyond the default mode brain network. PLoS One 6(7):e22964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer RK, Rainville P, Duncan GH, Bushnell MC (2001): Cortical representation of the sensory dimension of pain. J Neurophysiol 86:402–411. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Hughes NP, Lee MC, Mouraux A (2008): Determinants of laser‐evoked EEG responses: Pain perception or stimulus saliency? J Neurophysiol 100:815–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannetti GD, Mouraux A (2010): From the neuromatrix to the pain matrix (and back). Exp Brain Res 205:1–12. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Zambreanu L, Cruccu G, Tracey I (2005): Operculoinsular cortex encodes pain intensity at the earliest stages of cortical processing as indicated by amplitude of laser‐evoked potentials in humans. Neuroscience 131:199–208. [DOI] [PubMed] [Google Scholar]
- Inui K, Tran TD, Hoshiyama M, Kakigi R (2002): Preferential stimulation of Adelta fibers by intra‐epidermal needle electrode in humans. Pain 96:247–252. [DOI] [PubMed] [Google Scholar]
- Johnston S, Linden DE, Healy D, Goebel R, Habes I, Boehm SG (2011): Upregulation of emotion areas through neurofeedback with a focus on positive mood. Cogn Affect Behav Neurosci 11:44–51. [DOI] [PubMed] [Google Scholar]
- LaConte SM, Peltier SJ, Hu XP (2007): Real‐time fMRI using brain‐state classification. Hum Brain Mapp 28:1033–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Ruiz S, Caria A, Veit R, Birbaumer N, Sitaram R (2011): Detection of cerebral reorganization induced by real‐time fMRI feedback training of insula activation: A multivariate investigation. Neurorehabil Neural Repair 25:259–267. [DOI] [PubMed] [Google Scholar]
- Legrain V, Iannetti GD, Plaghki L, Mouraux A (2011): The pain matrix reloaded: A salience detection system for the body. Prog Neurobiol 93:111–124. [DOI] [PubMed] [Google Scholar]
- Li X, Hartwell KJ, Borckardt J, Prisciandaro JJ, Saladin ME, Morgan PS, Johnson KA, Lematty T, Brady KT, George MS (2013): Volitional reduction of anterior cingulate cortex activity produces decreased cue craving in smoking cessation: A preliminary real‐time fMRI study. Addict Biol 18:739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaig RG, Dixon M, Keramatian K, Liu I, Christoff K (2011): Improved modulation of rostrolateral prefrontal cortex using real‐time fMRI training and meta‐cognitive awareness. Neuroimage 55:1298–1305. [DOI] [PubMed] [Google Scholar]
- RC Oldfield. (1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Rainville P (2002): Brain mechanisms of pain affect and pain modulation. Curr Opin Neurobiol 12:195–204. [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC (1997): Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 277:968–971. [DOI] [PubMed] [Google Scholar]
- Rhudy JL, Meagher MW (2000): Fear and anxiety: Divergent effects on human pain thresholds. Pain 84:65–75. [DOI] [PubMed] [Google Scholar]
- Roberts LE, Birbaumer N, Rockstroh B, Lutzenberger W, Elbert T (1989): Self‐report during feedback regulation of slow cortical potentials. Psychophysiology 26:392–403. [DOI] [PubMed] [Google Scholar]
- Rota G, Sitaram R, Veit R, Erb M, Weiskopf N, Dogil G, Birbaumer N (2009): Self‐regulation of regional cortical activity using real‐time fMRI: The right inferior frontal gyrus and linguistic processing. Hum Brain Mapp 30:1605–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz S, Lee S, Soekadar SR, Caria A, Veit R, Kircher T, Birbaumer N, Sitaram R (2013): Acquired self‐control of insula cortex modulates emotion recognition and brain network connectivity in schizophrenia. Hum Brain Mapp 34:200–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siniatchkin M, Kropp P, Gerber WD (2000): Neurofeedback—the significance of reinforcement and the search for an appropriate strategy for the success of self‐regulation. Appl Psychophysiol Biofeedback 25:167–175. [DOI] [PubMed] [Google Scholar]
- Sitaram R, Lee S, Ruiz S, Rana M, Veit R, Birbaumer N (2011): Real‐time support vector classification and feedback of multiple emotional brain states. Neuroimage 56:753–765. [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E (2013): An fMRI‐based neurologic signature of physical pain. N Engl J Med 368:1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AL, Mouraux A, Liang M, Iannetti GD (2010): Stimulus novelty, and not neural refractoriness, explains the repetition suppression of laser‐evoked potentials. J Neurophysiol 104:2116–2124. [DOI] [PubMed] [Google Scholar]
- Wang T, Mitchell CJ (2011): Attention and relative novelty in human perceptual learning. J Exp Psychol Anim Behav Process 37:436–445. [DOI] [PubMed] [Google Scholar]
- Weiskopf N (2012): Real‐time fMRI and its application to neurofeedback. Neuroimage 62:682–692. [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Veit R, Erb M, Mathiak K, Grodd W, Goebel R, Birbaumer N (2003): Physiological self‐regulation of regional brain activity using real‐time functional magnetic resonance imaging (fMRI): Methodology and exemplary data. Neuroimage 19:577–586. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Lee JH, O'Leary H, Panych LP, Jolesz FA (2008): Neurofeedback fMRI‐mediated learning and consolidation of regional brain activation during motor imagery. Int J Imaging Syst Technol 18:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino A, Okamoto Y, Onoda K, Yoshimura S, Kunisato Y, Demoto Y, Okada G, Yamawaki S (2010): Sadness enhances the experience of pain via neural activation in the anterior cingulate cortex and amygdala: An fMRI study. Neuroimage 50:1194–1201. [DOI] [PubMed] [Google Scholar]
- Zotev V, Krueger F, Phillips R, Alvarez RP, Simmons WK, Bellgowan P, Drevets WC, Bodurka J (2011): Self‐regulation of amygdala activation using real‐time FMRI neurofeedback. PLoS One 6:e24522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


