Abstract
Objectives: Anticipatory processes prepare the organism for upcoming experiences. The aim of this study was to investigate neural responses related to anticipation and processing of painful stimuli occurring with different levels of uncertainty. Experimental design: Twenty‐five participants (13 females) took part in an electroencephalography and functional magnetic resonance imaging (fMRI) experiment at separate times. A visual cue announced the occurrence of an electrical painful or nonpainful stimulus, delivered with certainty or uncertainty (50% chance), at some point during the following 15 s. Principal observations: During the first 2 s of the anticipation phase, a strong effect of uncertainty was reflected in a pronounced frontal stimulus‐preceding negativity (SPN) and increased fMRI activation in higher visual processing areas. In the last 2 s before stimulus delivery, we observed stimulus‐specific preparatory processes indicated by a centroparietal SPN and posterior insula activation that was most pronounced for the certain pain condition. Uncertain anticipation was associated with attentional control processes. During stimulation, the results revealed that unexpected painful stimuli produced the strongest activation in the affective pain processing network and a more pronounced offset‐P2. Conclusions: Our results reflect that during early anticipation uncertainty is strongly associated with affective mechanisms and seems to be a more salient event compared to certain anticipation. During the last 2 s before stimulation, attentional control mechanisms are initiated related to the increased salience of uncertainty. Furthermore, stimulus‐specific preparatory mechanisms during certain anticipation also shaped the response to stimulation, underlining the adaptive value of stimulus‐targeted preparatory activity which is less likely when facing an uncertain event. Hum Brain Mapp 36:744–755, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: predictability, expectancy, neuroimaging, event‐related potentials, stimulus‐preceding negativity, insula, functional magnetic resonance imaging, electroencephalography
INTRODUCTION
In everyday life, we often anticipate situations without knowing what will happen and when it will happen. In this study, we investigated anticipatory neural responses to visual cues predicting the occurrence of a painful stimulus with different levels of uncertainty, and how these cues modulate actual pain processing. Functional magnetic resonance imaging (fMRI) allows assessing neural activation with high spatial resolution whereas electroencephalography (EEG) measures neural processes with millisecond accuracy. To take advantage of both, we ran two separately performed fMRI and EEG experiments.
The so‐called stimulus‐preceding negativity (SPN) is a slow cortical EEG component, which is primarily observed during anticipation of stimuli with high emotional salience [van Boxtel and Boecker, 2004], but there is limited evidence on SPN and pain anticipation. During a threat‐of‐shock condition, a fear‐induced SPN with a frontocentral maximum has been observed from 500 to 1000 ms after cue presentation [Baas et al., 2002; Boecker et al., 2001]. Dipole modeling suggests generators in midline frontal areas such as the ACC. Analogously to the early and late phases of the contingent‐negative variation (CNV; [Gomez et al., 2003], one study differentiated between early (500 to 1000 ms after the cue) and late anticipation (500 ms before the stimulation) [Brown et al., 2008]. The early SPN showed a broad frontocentral distribution but did not differ between certain or uncertain cues. The late SPN had a more central distribution. A more mid‐central and posterior parietal SPN has also been reported during the last 1500 ms before actual painful stimulation [Hellwig et al., 2008]. In addition, there is evidence that longer anticipation duration and unpredictability in stimulus timing increases the amplitude of the stimulation‐related P2 component [Clark et al., 2008; Hauck et al., 2007].
Previous fMRI studies consistently showed neural activation in nociceptive and emotional processing areas already during the anticipation of pain, including dorsomedial and ventrolateral prefrontal cortex (PFC), anterior insula, ACC, orbitofrontal cortex (OFC), and periaqueductal gray [e.g., Drabant et al., 2011; Koyama et al., 2005; Kumari et al., 2007; Ploghaus, 1999; Ploner et al., 2010; Schunck et al., 2008; Wiech et al., 2010]. There is also evidence for cue‐based modulation of stimulation‐responses: Uncertain expectation of a painful stimulus enhances brain responses to a nonpainful stimulus [Sawamoto et al., 2000], whereas neural activation decreased when participants expected less pain than they actually received [Koyama et al., 2005]. Also, predictability seems to reduce activation in sensory‐discriminative processing areas [Carlsson et al., 2006].
Although most previous fMRI studies did not deliver painful stimuli in every trial [e.g., Drabant et al., 2011; Schunck et al., 2008], they did not compare anticipatory activation during certain versus uncertain trials. The only EEG study [Brown et al., 2008] trying to differentiate this could not find any differences. Based on these findings, the aim of this project was to differentiate between certain and uncertain conditions during anticipation as well as during the delivery of electrical pain. It is also unclear whether early versus late SPN components rely on the same underlying mechanisms. Regarding fMRI, no previous study differentiated between early and late anticipatory processes. We, therefore, also explored differences between early and late anticipatory processes in EEG and fMRI. From a cognitive psychology perspective, one would expect early anticipation to be associated with processing of cue‐information and basic attentional orienting processes, but not yet pain‐related anticipatory processes. During late anticipation, we expected pain‐specific preparatory mechanisms in the certain pain condition, which would accord with previous findings. During uncertain anticipation cue‐based expectancy mechanisms may be involved. Here, a dorsal fronto‐parietal system (cognitive control) and a limbic circuit (affective processing) have been postulated [Atlas et al., 2010]. Therefore, we expected unspecific attempts of emotion regulation or cognitive control to deal with uncertainty.
METHODS AND MATERIALS
Participants
Twenty‐five healthy participants (13 females) aged 19–36 years (mean age 24.12 years, SD = 4.24) without history of neurological or psychiatric disorders (confirmed with the Structural Clinical Interview for DSM‐IV, SCID) participated in a large scale research project applying additional paradigms, which are outside the scope of this article. Parts of these data have been published elsewhere [Hahn et al., 2013]. The pain paradigm reported in this article was the first task for all subjects. For technical reasons (scanner failure), the majority of participants (n = 21) completed the EEG session first. The two sessions were separated by around 50 days on average. All participants were right handed as assessed by the Edinburgh Handedness Inventory [Oldfield, 1971] and had normal or corrected‐to‐normal vision. Further exclusion criteria were past or present substance abuse, intake of psychopharmacological medication within the last 3 months and pregnancy (both assessed by urine tests). Participants were recruited via advertisements posted at the Medical University of Vienna and the University of Vienna, Austria. Written informed consent was obtained. The study was approved by the local Institutional Review Board and participants were treated according to the Declaration of Helsinki (1964). All participants were reimbursed for their participation.
Task
For both fMRI and EEG, the paradigm consisted of three different anticipation conditions: certain pain, certain no pain, and uncertain (50:50 chance of pain or no pain) anticipation and four different stimulation conditions: certain pain (a painful stimulus that had been announced with 100% certainty), certain no pain (a nonpainful stimulus that had been announced with 100% certainty), uncertain pain (a painful stimulus that had not been announced with certainty; probability of stimulation 50%), and uncertain no pain (a nonpainful stimulus that had not been announced with certainty; probability of stimulation 50%). A visual cue (see Fig. 1A) indicated the occurrence of a painful or nonpainful stimulus of 500 ms duration at some point during a 15 s anticipation interval. The cue was presented for the whole duration of the anticipation interval and during the delivery of the stimulus. Temporal uncertainty was induced in all three conditions as participants were not informed at which time point the stimulus would appear in the 15 s interval. Temporal uncertainty was introduced in all conditions to ensure attentional engagement during the whole experiment. Unbeknownst to the participant, duration of the anticipation phase was on average 10.43 s (SD = 2.71) for all conditions. To intensify temporal uncertainty, stimulation occurred once after 5 and once after 15 s for each condition. The paradigm consisted of four runs (duration 5.5 min each) with 15 trials each (five certain pain, five certain no pain and five uncertain trials, with randomized trial order). For illustration see Figure 1A.
Figure 1.
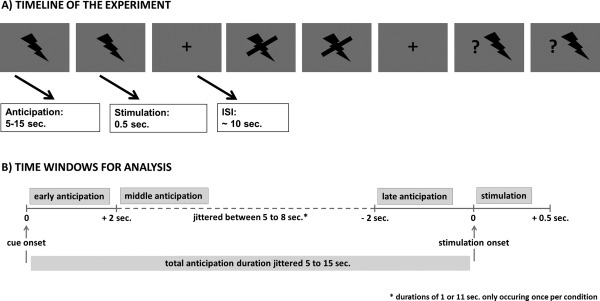
Task illustration. (A) Visualization of the pain anticipation paradigm showing three consecutive trials. The flash represented the certain pain anticipation cue, the crossed‐out flash represented the certain no pain cue and the flash with the question mark signified an uncertain trial with a 50% chance of painful stimulation. (B) Visualization of the three different time windows for data analysis (early and late anticipation, stimulation).
A DS5 Isolated Bipolar Constant Current Stimulator (Digitimer, London, UK) was used for delivery of painful and nonpainful electrical stimulation via a surface electrode with 7 mm diameter and a platinum pin (WASP electrode, Specialty Developments) attached to the dorsum of the left hand [Katsarava et al., 2006; Kaube et al., 2000; Lefaucheur et al., 2012]. The skin was sanitized before attaching the electrode with tape. We did not apply any gel. Impedance was kept low by stable attachment of the electrode to the skin. The control voltage for the electrical stimulator (DS5) was generated by a custom‐built electronic device using a PIC18F2455 microcontroller (Microchip Technology Inc., http://www.microchip.com) and a LTC1257 digital/analog converter (Linear Technology Corporation, http://www.linear.com). This electronic device was powered by a 9V AC/DC adapter (Ansmann AG, http://www.ansmann.de) and connected to the stimulus‐PC via a USB isolation cable (IF tools, http://iftools.com) ‐ both certified for medical applications. The custom‐built microcontroller as well as stimulus presentation were controlled via MATLAB using the stimulus presentation toolbox Cogent 2000 v1.32, developed by the Cogent 2000 team at FIL and ICN and Cogent Graphics developed by John Romaya at the LON at the Wellcome Department of Imaging Neuroscience.
Before each experimental session, participants underwent a standardized calibration procedure to individually determine a “nonpainful but detectable” and a “painful but tolerable” electrical stimulus. The first threshold was a clearly detectable sensation. To experience this sensation, participants received a series of ascending single‐pulses (pulse width 500 ms) starting from 0.05 mA in increasing increments of 0.05 mA until participants reported a tingly feeling. This corresponded to a 1 on a subjective rating scale ranging from 1 (detectable sensation) to 10 (unbearable pain). The second threshold was an above medium pain sensation, which corresponded to a 6 on the same scale. This threshold was determined by a step‐wise increase of the stimulus intensity until the subject responded with a 7 on the scale of 1 to 10. For each subject, we determined the painful stimulus for the experiment by averaging all calibration stimuli that the particular subject perceived as intensity 6. Stimulation intensities were ranging between 0.01 and 1.9 mA (no pain: mean EEG: 0.09 mA, mean fMRI: 0.10 mA, Min = 0.01 mA, Max = 0.4 mA; pain: mean EEG: 0.50 mA, mean fMRI: 0.50 mA, Min = 0.1 mA, Max = 1.9 mA). None of our subjects showed any signs of tissue damage or reported genuinely adverse experiences. Before each experiment participants completed five training trials to familiarize them with the task, using the calibrated stimulation values (1 and 6).
EEG Acquisition and Statistical Analysis
A 64‐channel EEG system (NEURO PRAX EEG System, NeuroConn, Germany) was used for EEG recordings. All signals were recorded within a frequency range of DC to 250 Hz and sampled at 500 Hz for digital storage. Vertical and horizontal electro‐oculograms were recorded to allow off‐line eye‐movement correction. Electrode impedances were controlled by a skin‐scratching procedure at each electrode site prior to recordings. All electrode impedances were kept below 2 kΩ, as checked with an impedance meter.
Data analysis was carried out using EEGLAB 6.03b [Delorme and Makeig, 2004], implemented in MATLAB 7.10.0 (The MathWorks, Natick, MA). EEG data were low‐pass filtered with a cut‐off frequency at 30 Hz (roll‐off 6 dB/octave) and re‐referenced to linked mastoids. Eye‐movement artifacts were corrected by an extended infomax independent component analysis [Bell and Sejnowski, 1995; Lee et al., 1999] applied to the single‐subject data. After discarding artifact‐afflicted independent components, data were segmented for the early anticipation phase locked to the cue indicating the upcoming stimulation condition (interval −1000–5000 ms). For the late anticipation phase, data were segmented locked to the onset of the electrical stimulation (−5000–1000 ms) for the different conditions. The mean of the last 500 ms prior to cue onset served as baseline interval for the anticipation phase. For the stimulation phase, the different conditions were segmented locked to stimulation onset (−200–1000 ms). The mean of the 200 ms prior to stimulation served as baseline interval. For an illustration of the time windows for the analysis, see Figure 1B. The MATLAB function lindetrend was applied to these data segments to control for slow drifts. Subsequently, further artifacts were removed after inspection with a ±75 µV criterion. On average, we rejected around 22% of trials per condition (certain pain: 21%, certain no pain: 23.8%, uncertain: 19.98%). For ERP, data analysis artifact‐free trials were averaged per participant and per condition. Subsequently, mean amplitudes were calculated for early (time interval 1000–2000 ms after cue‐onset) and late (time interval 1000–2000 ms before stimulus onset) SPN amplitudes during the anticipation phase. For the P2 component at stimulus delivery, a time window of 260–360 ms post stimulus onset and for the P2 at stimulus offset (stimulus duration was 500 ms) a time window of 230–330 ms post stimulus offset was chosen based on visual inspection. The anticipatory components, that is, early and late SPN mean amplitudes, were analyzed using two‐way repeated‐measures ANOVAs with the within‐subject factors electrode site (Fz, Cz, Pz) and condition (certain pain, certain no pain, and uncertain). Stimulation associated components, that is, P2 mean amplitudes, were analyzed using three way repeated‐measures ANOVAs with the within‐subject factors electrode site (Fz, Cz, Pz), certainty (certain, uncertain), and stimulation (pain, no pain). In case of multiple testing, we applied a Bonferroni–Holm correction.
fMRI Acquisition and Statistical Analysis
Functional MR images were acquired on a 3T whole‐body scanner (32‐channel head coil) using a gradient‐recalled EPI‐sequence with distortion correction (TR = 1.8 s, TE = 38 ms, FA = 60°, voxel size 1.5 × 1.5 × 3 mm, 23 slices, GRAPPA2). Data preprocessing was carried out in SPM8 (Wellcome Trust Centre for Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm) using standard algorithms and parameters unless specified differently. This included slice timing correction (reference = middle slice), motion correction, spatial normalization to MNI (Montreal Neurological Institute) stereotactic space using an in‐house scanner‐specific EPI template, and spatial smoothing (8 mm Gaussian kernel). We applied a 2 mm threshold for excessive head movement. Single subject analysis was performed based on the General Linear Model. We setup two separate models—one for analyzing effects of anticipation, and one for analyzing the effects of stimulus delivery. The anticipation model contained 13 regressors: for each condition (painful, nonpainful, uncertain), we modeled the first 2 s of the anticipation period, the last 2 s of the anticipation period, the middle anticipation period (variable time window between first and last 2 s), and the stimulation period (painful, nonpainful, uncertain painful, uncertain nonpainful), and convolved them with SPM8's standard canonical hemodynamic response function. In this model, the late anticipation period and stimulus delivery are highly correlated due to their temporal contingency. We, therefore, orthogonalized the late anticipation regressors with regard to the stimulation regressors to analyze late anticipation effects independently of stimulation effects. Moreover, we setup a second GLM, which modeled the actual duration of the anticipation period for each condition and the stimulation and, therefore, contained seven regressors to analyze the stimulation period. For an illustration of the time windows for the analysis, see Figure 1B. Additional nuisance regressors included realignment parameters and potentially confounding signals from white matter and ventricles. Group statistics were calculated using second‐level random effects' analyses in SPM8. Results are presented and interpreted at a cluster‐level corrected threshold of 0.05 (voxel threshold P = 0.001) using cluster‐level correction based on the random Gaussian field approach as implemented in SPM 8. To determine how the between‐subject variance in EEG components might relate to between‐subject variance in fMRI signal, we performed multiple regression analyses in SPM8 correlating each subject's mean EEG amplitude of interest with whole brain fMRI signal in the respective condition.
RESULTS
Early Anticipation Phase
EEG data
The 3 (condition) by 3 (electrode) repeated‐measures ANOVA showed a significant main effect of condition (F(2,48) = 6.313, P = 0.004, partial‐eta2 = 0.208). For visualization, see Figure 2A. Post hoc multiple comparisons revealed that the early SPN between 1000 and 2000 ms was more negative after the uncertain cue compared to the certain pain (P = 0.001) as well as certain no pain (P = 0.008) cue. Responses to the certain pain versus certain no pain cue did not differ (P = 0.684). We also observed a significant main effect of electrode (F(2,48) = 7.729, P = 0.001, partial‐eta2 = 0.244). Post hoc multiple comparisons showed that the early SPN was most pronounced at Fz (P = 0.006) and Cz (P = 0.010) compared to Pz. However, there was only a trend for a difference between Fz and Cz (P = 0.093). There was no significant interaction of condition and electrode (P = 0.615).
Figure 2.
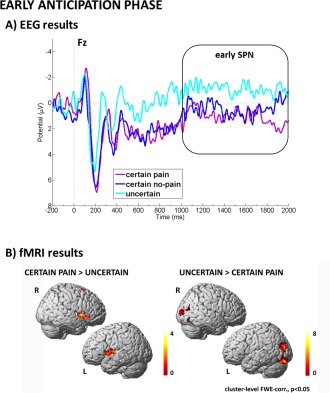
Visualization of results related to early anticipation. Panel A (EEG results) shows the modulation of the early SPN component by uncertainty. Panel B (fMRI results) shows the modulation of visual processing areas by uncertainty as well as specific pain‐related preparatory activity in anterior insula. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
fMRI data
Contrasting pain anticipation and uncertain anticipation during the first 2 s after cue presentation showed that certain pain anticipation was more associated with neural activation in regions related to affect processing and motor preparation, such as bilateral anterior insula as well as SMA. On the contrary, anticipation of nonpainful stimulation compared to uncertain anticipation did not reveal any significant clusters. The reverse contrasts (uncertain > pain, uncertain > no pain) revealed higher‐order visual areas, such as BA 17 and 18 as well as superior occipital gyrus as being more active during uncertain anticipation. For visualization, see Figure 2B. Comparing anticipation of painful stimulation to anticipation of nonpainful stimulation we observed no significant clusters. The reverse contrast revealed increased activation with anticipation of nonpainful stimulation in visual areas, such as BA 17 and 18. Full details on cluster size, coordinates, and statistical values are given in Table 1.
Table 1.
Significant brain activation clusters during early anticipation are given including MNI coordinates, cluster size (k), t‐value, and P‐value (all P‐values are cluster‐level FWE‐corrected, P < 0.05)
| Contrast | X | Y | Z | t‐value | k | P‐value | Region |
|---|---|---|---|---|---|---|---|
| Certain pain > uncertain | −52 | 6 | 2 | 4.3 | 308 | 0.002 | L. anterior Insula |
| 48 | 4 | 2 | 4.25 | 326 | 0.002 | R. anterior Insula | |
| −6 | 6 | 60 | 4.03 | 298 | 0.003 | bil. SMA | |
| Uncertain > certain pain | −12 | −88 | −12 | 8.57 | 1286 | <0.001 | L. BA 17, 18 |
| 16 | −92 | 4 | 6.79 | 527 | <0.001 | R. BA 17, 18 | |
| −28 | −78 | 20 | 4.76 | 682 | <0.001 | L. superior occipital Gyrus | |
| Uncertain > certain no pain | 16 | −88 | 0 | 5.51 | 231 | 0.01 | R. BA 17, 18 |
| −12 | −90 | −4 | 5.46 | 276 | 0.004 | L. BA 17, 18 | |
| Certain no pain > certain pain | −8 | −88 | −16 | 6.94 | 818 | <0.001 | L. lingual gyrus, BA 18 |
| 18 | −96 | 6 | 5.78 | 239 | 0.009 | R. superior occipital Gyrus |
Only the highest peak is included in the case of several confluent peaks.
Correlation analysis
During uncertain anticipation, we observed a significantly negative association of the early SPN at electrode Fz and fMRI signal in left visual processing areas (P = 0.007, k = 255, cluster‐level FWE‐corr., MNI peak‐coord. −8, −90, −18; note that as SPN has a negative amplitude value the negative correlation implies that higher SPN amplitudes are associated with higher BOLD activation).
Late Anticipation Phase
EEG data
The 3 (condition) by 3 (electrode) repeated‐measures ANOVA showed a significant main effect of condition (F(2,48) = 52.854, P < 0.001, partial‐eta2 = 0.688). For visualization, see Figure 3A. Post hoc multiple comparisons revealed that the late SPN during the last second of anticipation was more negative during certain pain anticipation compared to uncertain (P = 0.001) as well as certain no pain (P < 0.001) anticipation. Also, the uncertain condition yielded more negative SPN compared to the no pain condition (P < 0.001). We also observed a significant main effect of electrode (F(2,48) = 26.413, P < 0.001, partial‐eta2 = 0.524). Post hoc multiple comparisons showed that the late SPN was more negative at Pz compared to Fz (P < 0.001), and more negative at Cz compared to Fz (P < 0.001) while Pz and Cz did not differ (P = 0.911). The significant condition by electrode interaction (F(4,96) = 2.561, P = 0.043, partial‐eta2 = 0.096) was driven by the main effect of electrode, such that Pz and Cz did not differ for all conditions (all P‐values = 1), but that Fz was different from both Pz and Cz for all conditions (all P‐values < 0.005).
Figure 3.
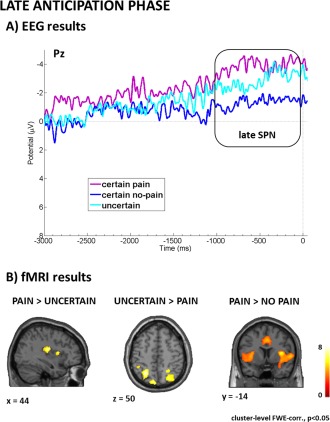
Visualization of results related to late anticipation. Panel A (EEG results) shows stimulus‐specific preparatory processes reflected in the late SPN component most pronounced for certain pain. Panel B (fMRI results) shows that certain pain anticipation was associated with pain‐related somatosensory preparatory mechanisms whereas uncertain anticipation recruited more attentional control processes. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
fMRI data
Contrasting certain pain anticipation and uncertain anticipation during the last 2 s before actual stimulation showed that pain anticipation was associated with stronger activation in the right posterior and middle insula. The reverse contrast revealed stronger activation of the left superior parietal, the right angular gyrus, the left cerebellum, and the medial OFC during uncertain anticipation. Comparing uncertain anticipation and anticipation of certain nonpainful stimulation showed stronger activation of two bilateral insula clusters ranging from anterior to posterior parts and a small cluster in BA 17. For visualization, see Figure 3B. The comparison between the anticipation of painful stimulation and the anticipation of nonpainful stimulation showed increased neural activation in two huge bilateral insula clusters ranging from anterior to posterior parts, bilateral middle anterior cingulate, right thalamus, left cerebellum, and right dorsolateral PFC. The comparisons of certain nonpainful with certain painful as well as uncertain anticipation did not reveal any significant clusters. Full details on cluster size, coordinates, and statistical values are given in Table 2.
Table 2.
Significant brain activation clusters during late anticipation are given including MNI coordinates, cluster size (k), t‐value, and P‐value (all P‐values are cluster‐level FWE‐corrected, P < 0.05)
| Contrast | X | Y | Z | t‐value | k | P‐value | Region |
|---|---|---|---|---|---|---|---|
| Certain pain > uncertain | 44 | −18 | 18 | 6.1 | 465 | <0.001 | R. posterior Insula |
| 52 | 0 | 4 | 4.9 | 277 | 0.005 | R. middle Insula | |
| Uncertain > certain pain | −26 | −58 | 50 | 5.55 | 1258 | <0.001 | L. superior parietal Cortex |
| 34 | −64 | 42 | 4.81 | 667 | <0.001 | R. inferior parietal Cortex | |
| −6 | −86 | −22 | 5.11 | 294 | 0.003 | L. Cerebellum | |
| 14 | 46 | −24 | 5.37 | 227 | 0.013 | R. medial OFC | |
| Certain pain > certain no pain | 40 | −14 | 18 | 10 | 5794 | <0.001 | R. Insula |
| −36 | −16 | 14 | 7.21 | 3603 | <0.001 | L. Insula | |
| 6 | 20 | 30 | 6.39 | 2009 | <0.001 | Bilateral middle Cingulate | |
| −4 | −58 | −18 | 5.04 | 1085 | <0.001 | Cerebellum | |
| 18 | −24 | −2 | 5.06 | 633 | <0.001 | R. Thalamus | |
| 30 | 56 | 30 | 4.09 | 225 | 0.013 | R. dorsolateral PFC | |
| Uncertain > certain no pain | 38 | −14 | 16 | 5.21 | 670 | <0.001 | R. posterior Insula |
| 44 | 18 | −14 | 4.66 | 607 | <0.001 | R. anterior Insula | |
| −36 | −16 | 14 | 4.85 | 302 | 0.003 | L. posterior Insula | |
| −50 | 4 | −6 | 4.09 | 265 | 0.006 | L. middle Insula | |
| −32 | 4 | 12 | 4.37 | 230 | 0.012 | L. anterior, middle Insula | |
| 2 | −76 | 8 | 3.83 | 206 | 0.02 | BA 17 |
Only the highest peak is included in the case of several confluent peaks.
Correlation analysis
During late anticipation there was a significantly positive correlation between late SPN at electrode Pz and fMRI signal in dorsal ACC (P = 0.032, k = 190, cluster‐level FWE‐corr., MNI peak‐coord. −6, 42, 16).
Actual Stimulation
EEG data
P2 stimulation onset
The 2 (certainty) by 2 (stimulation) by 3 (electrode) ANOVA with repeated measures showed a significant main effect of stimulation (F(1,24) = 160.125, P < 0.001, partial‐eta2 = 0.870) such that P2 was most positive for painful stimulation compared to nonpainful stimulation. We also observed a significant effect of electrode (F(2,48) = 18.968, P < 0.001, partial‐eta2 = 0.870). Post hoc multiple comparisons showed that P2 was most positive at Cz compared to Fz (P < 0.001) and Pz (P = 0.022) and at Pz compared to Fz (P = 0.003). The significant stimulation by electrode interaction (F(2,48) = 24.687, P < 0.001, partial‐eta2 = 0.506) was driven by the main effect of stimulation as the three electrodes showed similar differences for all conditions. All other main effects or interactions did not reveal significant results (all P‐values > 0.218).
P2 stimulation offset
The 2 (certainty) by 2 (stimulation) by 3 (electrode) ANOVA with repeated measures showed a significant main effect of certainty (F(1,23) = 4.874, P = 0.037, partial‐eta2 = 0.169), such that the P2 was more positive for uncertain stimulation than for certain stimulation. For visualization, see Figure 4A. We also observed a significant effect of stimulation (F(1,24) = 47.004, P < 0.001, partial‐eta2 = 0.662), such that the P2 was more positive for painful stimulation than for nonpainful stimulation. Furthermore, we observed a significant effect of electrode (F(2,48) = 21.868, P < 0.001, partial‐eta2 = 0.477. Post hoc multiple comparisons showed that P2 was most positive at Cz compared to Fz (P < 0.001) and Pz (P = 0.011) and at Pz compared to Fz (P = 0.001). There was no significant interaction effect (all P‐values > 0.110).
Figure 4.
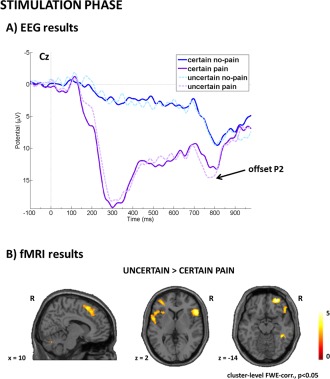
Visualization of results related to actual stimulation. Panel A (EEG results) shows the cue‐based modulation of the P2 offset potential with stronger P2 responses to the offset of uncertain painful stimulation compared to certain painful stimulation. Panel B (fMRI results) shows the cue‐based modulation of the affective pain‐network with stronger responses to unexpected painful stimulation. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
fMRI data
Contrasting uncertain painful stimulation with expected, that is, certain painful stimulation, showed stronger activation in a large right dorsomedial PFC cluster including SMA, bilateral lateral OFC, bilateral anterior insula, left cerebellum as well as right middle temporal gyrus during unexpected painful stimulation. However, contrasting certain painful with uncertain painful stimulation did not reveal any significant clusters. For visualization, see Figure 4B. Comparing actual pain processing during certain pain trials versus certain no pain trials showed increased activation in two large bilateral insula/rolandic operculum clusters, a right ACC and MCC cluster, right inferior frontal gyrus, left calcarine gyrus, and bilateral cerebellum. The reverse contrast showed activation in medial OFC associated with no pain processing. Comparing uncertain nonpainful stimulation versus certain nonpainful stimulation did not reveal any significant activation clusters. Full details on cluster size, coordinates, and statistical values are given in Table 3.
Table 3.
Significant brain activation clusters during stimulation are given including MNI coordinates, cluster size (k), t‐value, and P‐value (all P‐values are cluster‐level FWE‐corrected, P < 0.05).
| Contrast | X | Y | Z | t‐value | k | P‐value | Region |
|---|---|---|---|---|---|---|---|
| Uncertain pain > certain pain | 28 | 52 | −14 | 5.92 | 270 | 0.009 | R. lateral OFC |
| 56 | 20 | 2 | 5.37 | 967 | <0.001 | R. anterior Insula | |
| 64 | −34 | −6 | 5.29 | 479 | <0.001 | R. middle temporal Gyrus | |
| −10 | −80 | −26 | 4.96 | 188 | 0.04 | L. Cerebellum | |
| 10 | 18 | 56 | 4.89 | 1027 | <0.001 | R. PFC including SMA | |
| −50 | 20 | 0 | 4.67 | 726 | <0.001 | L. anterior insula | |
| −34 | 46 | 4 | 4.23 | 223 | 0.021 | L. lateral OFC | |
| Certain pain > certain no pain | 52 | 2 | 4 | 9.06 | 6684 | <0.001 | R. Insula, Operculum |
| −36 | −16 | 16 | 7.5 | 3200 | <0.001 | L. Insula, Operculum | |
| 4 | 24 | 28 | 6.43 | 1360 | <0.001 | R. ACC, MCC | |
| 26 | −70 | −28 | 5.85 | 140 | 0.105 | R. Cerebellum | |
| −6 | −62 | −16 | 4.9 | 709 | <0.001 | L. Cerebellum | |
| 36 | 34 | 6 | 4.44 | 228 | 0.019 | R. inferior frontal Gyrus | |
| −10 | −76 | 6 | 4.15 | 307 | 0.005 | bil. BA 17, 18 | |
| Certain no pain > certain pain | 6 | 32 | −12 | 5.31 | 235 | 0.017 | Medial OFC |
Only the highest peak is included in the case of several confluent peaks.
Correlation analysis
We did not observe any significant correlation between fMRI signal and EEG components during stimulation.
DISCUSSION
This study investigated the impact of uncertainty on early and late pain anticipation and pain processing using EEG and fMRI. We applied a cue‐based anticipation paradigm with a certain pain and no pain cue as well as an uncertain cue signaling the occurrence of either painful or nonpainful stimulation with 50% chance. Our data revealed three main findings. First, during the first 2 s of pain anticipation, we observed a strong effect of uncertain versus certain anticipation, which was reflected in a modulation of the frontal SPN and increased fMRI activation in visual processing areas. This suggests that the uncertain cue was associated with stronger affective anticipation processes and capturing more visual attention. Second, during the last 2 s before actual stimulation, our data showed stimulus‐specific somatosensory preparation processes as reflected in a centroparietal SPN and posterior insula activation that was most pronounced for certain pain trials. Uncertain anticipation was more associated with attentional control processes, as indicated by fMRI activation in parietal areas. Third, we found a cue‐based modulation of neural processes during actual stimulation. Unexpected painful stimuli produced the strongest activation in the affective pain processing network, which underlines the adaptive value of stimulus‐targeted preparatory activity that is less likely when facing an uncertain event. In the following, we provide an in‐depth discussion of these findings.
During early anticipation EEG data showed a modulation of the SPN by uncertainty with a frontal maximum possibly reflecting affective anticipatory processes [Boecker et al., 2001]. To our knowledge, all previous EEG studies on pain anticipation used uncertain anticipation conditions and compared them with safe, that is, no pain, conditions. No previous EEG study used a certain pain condition with 100% predictability of painful stimulation. Some studies [Baas et al., 2002; Boecker et al., 2001] compared a highly uncertain pain condition (only 6% of all trials followed by a shock) with a safe condition. Others [Hellwig et al., 2008] applied an uncertain condition (50% of trials) that was comparable to our study and contrasted it with a safe condition. Others [Brown et al., 2008] used a 50% predictability of intensity in their certain condition, the uncertain condition introduced uncertainty about the intensity of the laser stimulus. Our results suggest that early SPN variation reported in previous studies was driven by uncertainty rather than pain anticipation per se. We observed that this component is most pronounced for uncertain pain, whereas certain pain and certain no pain did not elicit amplitude differences. Previous dipole source modeling studies [Baas et al., 2002; Boecker et al., 2001] associated this early SPN component with ACC activity and hence affective anticipatory processes. Adding to previous data, our result of larger early SPN amplitudes for uncertain compared to certain anticipation in general, show that the previously observed modulation of early SPN is not due to dealing with threat of pain but more associated with dealing with the aversive situation of uncertainty about the upcoming stimulus.
The current fMRI results provide additional new insights into the neural mechanisms associated with early anticipatory processes. We observed an uncertainty effect in high‐level visual areas, suggesting increased visual processing in the first 2 s of the anticipation phase. This may reflect that the uncertain cue represented a more ambiguous stimulus with higher motivational relevance, and therefore captured more visual attention [e.g., Kastner et al., 1999]. This interpretation is in line with our correlative finding of the association of early SPN and fMRI signal in visual areas overlapping with the visual activation cluster in the contrast uncertain > certain pain anticipation. Subjects with increased affective anticipatory processes also show stronger visual responses to the uncertain cue, which we interpreted as an indicator of increased motivational relevance. Future studies may want to include salience measures to more thoroughly test these assumptions. Previous work by Legrain et al. [2011] on salience and neural responses in the pain matrix might be relevant here.
During certain pain anticipation, we observed increased stimulus‐specific preparatory activity in pain‐related brain regions, such as anterior insula and motor preparation regions, which is in line with previous evidence [e.g., Drabant et al., 2011; Schunck et al., 2008]. This is the first fMRI study to differentiate between certain and uncertain pain anticipation; most studies used uncertain pain anticipation conditions and compared those to safe conditions [Drabant et al., 2011; Schunck et al., 2008]. Our data revealed specific pain‐related preparatory activity only during anticipation of a certain painful stimulus, but not during uncertain anticipation or at least to a lesser extent.
During late anticipation the SPN component was most pronounced for certain pain trials followed by uncertain trials with a centroparietal maximum [Brown et al., 2008]. This suggests specific somatosensory anticipatory processes in preparation for the upcoming painful stimulus involving supplementary motor, posterior parietal, and somatosensory areas summing up over the vertex and parietal electrodes [Brunia and van Boxtel, 2004]. Interestingly, these preparatory processes are reduced with reduced probability of painful stimulation, as shown by a less negative potential shift during uncertain anticipation. This less negative potential shift was further correlated with increased dorsal ACC activation in fMRI data, which suggests that participants who perceived stronger “conflict” [Bush et al., 2000] during uncertain anticipation showed less pain‐specific preparatory activity.
Accordingly, fMRI data showed that preparation for a certain painful stimulus is associated with specific somatosensory pain‐related neural processes, whereas during anticipation of an uncertain stimulus we observed parietal activation. The latter can be associated with the dorsal attentional as well as fronto‐parietal control systems [Corbetta and Shulman, 2002; Vincent et al., 2008], which have previously been implicated in cue‐based expectancy modulation of pain [Atlas et al., 2010]. Together with the increased visual activation during the early anticipation phase these attentional processes may be a response to the higher motivational relevance of the uncertain cue. This interpretation is also bolstered by the increased mOFC activation during late anticipation in the uncertain condition. Previous research has implicated the mOFC in implicit motivational salience and stimulus‐related arousal even in the absence of a required response [Rothkirch et al., 2012]. Third, we observed a cue‐based modulation of fMRI activity during actual stimulation, such that unexpected compared to expected painful stimulation showed stronger activation in parts of the affective pain system, such as lateral OFC, anterior insula, and dorsomedial PFC. This suggests that unexpected painful stimulation is associated with stronger affective pain‐related neural processes, which is in accordance with previous evidence [Carlsson et al., 2006]. The authors speculated that predictability may reduce the aversiveness of the painful stimulus by inducing feelings of control in comparison to an uncontrollable highly aversive condition during uncertain trials. This further underlines the adaptive value of specific stimulus‐related preparatory mechanisms during the anticipation phase, which we observed only in the certain condition. EEG data did not reveal any cue‐associated modulation of the P2 component at stimulation onset. However, there was a modulation of the stimulation offset potential, such that unexpected pain produced a stronger P2 than expected pain, which accords with the fMRI data and pioneering EEG evidence [Clark et al., 2008]. In this study, we chose not to record subjective ratings of perceived pain intensity because we wanted to focus on rather automatic neural processes. Implementing ratings of subjective intensity would add a cognitive component, which may influence stimulus processing. We are aware that this could still be considered a limitation of our study. There is previous evidence on the impact of expectation on subjective pain experience, such that unpredictable stimuli are rated more painful [Carlsson et al., 2006], expectations can decrease or increase pain [Brown et al., 2008; Koyama et al., 2005; Wiech et al., 2010] or longer duration of anticipation intervals can change subjective ratings [Clark et al., 2008; Hauck et al., 2007]. Furthermore, there is a debate on possible limitations of electrical stimulation to study nociception [Lefaucheur et al., 2012; Perchet et al., 2012]. This debate highlights that while the subjective experience of pain can be consistently evoked and investigated using similar setups as the one used here, it remains unclear which nociceptive fibers are activated by electrical in comparison to thermal pain. The pinprick sensation that is observed with electrical stimulation using concentric electrodes (as in the present study) has been mostly associated with Aδ fibers, although [e.g., Katsarava et al., 2006]. Moreover, electrical pain has some practical advantages in comparison to thermal pain, one of them being the absence of skin lesions. Future studies should nevertheless extend our findings to the use of other types of pain stimulation. Another important drawback that needs to be considered when interpreting our results is the nonsimultaneous acquisition of the EEG and fMRI data. We chose an independent recording approach to look at the same mental processes using two complimentary methods instead of a fusion approach revealing insights on the relation of EEG and fMRI signals. A comprehensive review [Huster et al., 2012] discusses the advantages and disadvantages of fMRI‐informed EEG analysis and EEG‐informed fMRI analysis. The authors conclude that multivariate fusion models of simultaneous data are the least biased way but should be carefully selected based on the specific research question. Furthermore, fMRI results regarding late anticipation should be considered preliminary and be interpreted with caution. Due to the variable anticipation interval that has been applied in this study, late anticipation is more heterogeneous than early anticipation, which applies for both EEG and fMRI data. Furthermore, the temporal proximity of late anticipation and actual stimulation is a critical issue regarding fMRI data analysis as late anticipation may be confounded with actual stimulation to some extent. We tried to account for these issues by orthogonalizing the regressors of late anticipation and stimulation to increase specificity of the former. In EEG, temporal proximity is not an issue. Future studies with simultaneous EEG‐fMRI acquisition as well as experimental designs tailored to separate temporally proximate events could, therefore, reveal important insights regarding late anticipation processes. In addition, for logistic reasons, the majority of participants had completed the EEG session first, which may have caused order or learning effects confounding comparability of the results, Unfortunately, due to the unbalanced distribution, we cannot explicitly test for the existence of such confounds. However, since the time between the two measurements was at least more than 4 weeks, since the order of the conditions was randomized within each test session and each run, strong effects of order or learning on our results are considered as rather unlikely. Note also that such effects would mainly affect comparability of EEG and fMRI results, and that if anything they would result in false negatives, rendering the present results more conservative rather than too liberal.
Notwithstanding these limitations, our results provide new insights into early and late anticipation processes when facing certain and uncertain painful stimulation. Understanding the neural basis of pain anticipation and cue‐based modulation of pain processing in normally functioning individuals is of particular importance when trying to understand mental states where anticipatory or pain‐related processing is disrupted. We are convinced that our paradigm holds great potential for studying, for instance, the neural correlates of negative expectancy processes in depression or anxiety disorders. Furthermore, it may be useful for clarifying the impact of early versus late anticipatory processes related to hyper‐vigilance in chronic pain states such as fibromyalgia [e.g., Boersma and Linton, 2006; Crombez et al., 2005].
ACKNOWLEDGMENTS
We acknowledge clinical support by Anna Höflich, Dietmar Winkler, and Siegfried Kasper, as well as technical support by Sebastian Ganger and Jacqueline Atanelov. We further thank two anonymous reviewers for their valuable comments which greatly improved our manuscript. R. Lanzenberger received travel grants and conference speaker honoraria from AstraZeneca, Roche and Lundbeck A/S.
REFERENCES
- Atlas LY, Bolger N, Lindquist MA, Wager TD (2010): Brain mediators of predictive cue effects on perceived pain. J Neurosci 30:12964–12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas JMP, Kenemans JL, Boecker KBE, Verbaten MN (2002): Threat‐induced cortical processing and startle potentiation. Neuroreport 13:133–137. [DOI] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ (1995): An information‐maximization approach to blind separation and blind deconvolution. Neural Comput 7:1129–1159. [DOI] [PubMed] [Google Scholar]
- Boecker KBE, Baas JMP, Kenemans JL, Verbaten MN (2001): Stimulus‐preceding negativity induced by fear: A manifestation of affective anticipation. Int J Psychophysiol 43:77–90. [DOI] [PubMed] [Google Scholar]
- Boersma K, Linton SJ (2006): Expectancy, fear and pain in the prediction of chronic pain and disability: A prospective analysis. Eur J Pain 10:551–557. [DOI] [PubMed] [Google Scholar]
- Brown CA, Seymour B, Boyle Y, El‐Deredy W, Jones AK (2008): Modulation of pain ratings by expectation and uncertainty: Behavioral characteristics and anticipatory neural correlates. Pain 135:240–250. [DOI] [PubMed] [Google Scholar]
- Brunia CH, van Boxtel GJ (2004): Anticipatory attention to verbal and non‐verbal stimuli is reflected in a modality‐specific SPN. Exp Brain Res 156:231–239. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI (2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4:215–222. [DOI] [PubMed] [Google Scholar]
- Carlsson K, Andersson J, Petrovic P, Petersson KM, Ohman A, Ingvar M (2006): Predictability modulates the affective and sensory‐discriminative neural processing of pain. Neuroimage 32:1804–1814. [DOI] [PubMed] [Google Scholar]
- Clark JA, Brown CA, Jones AK, El‐Deredy W (2008): Dissociating nociceptive modulation by the duration of pain anticipation from unpredictability in the timing of pain. Clin Neurophysiol 119:2870–2878. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL (2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3:201–215. [DOI] [PubMed] [Google Scholar]
- Crombez G, Van Damme S, Eccleston C (2005): Hypervigilance to pain: An experimental and clinical analysis. Pain 116:4–7. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S (2004): EEGLAB: an open source toolbox for analysis of single‐trial EEG dynamics including independent component analysis. J Neurosci Methods 134:9–21. [DOI] [PubMed] [Google Scholar]
- Drabant EM, Kuo JR, Ramel W, Blechert J, Edge MD, Cooper JR, Goldin PR, Hariri AR, Gross JJ (2011): Experiential, autonomic, and neural responses during threat anticipation vary as a function of threat intensity and neuroticism. Neuroimage 55:401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez CM, Marco J, Grau C (2003): Preparatory visuo‐motor cortical network of the contingent negative variation estimated by current density. Neuroimage 20:216–224. [DOI] [PubMed] [Google Scholar]
- Hahn A, Kranz GS, Seidel EM, Sladky R, Kraus C, Kublbock M, Pfabigan DM, Hummer A, Grahl A, Ganger S, Windischberger C, Lamm C, Lanzenberger R (2013): Comparing neural response to painful electrical stimulation with functional MRI at 3 and 7T. Neuroimage 82C:336–343. [DOI] [PubMed] [Google Scholar]
- Hauck M, Lorenz J, Zimmermann R, Debener S, Scharein E, Engel AK (2007): Duration of the cue‐to‐pain delay increases pain intensity: A combined EEG and MEG study. Exp Brain Res 180:205–215. [DOI] [PubMed] [Google Scholar]
- Hellwig S, Weisbrod M, Jochum V, Rentrop M, Unger J, Walther S, Haefner K, Roth A, Fiedler P, Bender S (2008): Slow cortical potentials in human aversive trace conditioning. Int J Psychophysiol 69:41–51. [DOI] [PubMed] [Google Scholar]
- Huster RJ, Debener S, Eichele T, Herrmann CS (2012): Methods for simultaneous EEG‐fMRI: An introductory review. J Neurosci 32:6053–6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG (1999): Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron 22:751–761. [DOI] [PubMed] [Google Scholar]
- Katsarava Z, Ayzenberg I, Sack F, Limmroth V, Diener HC, Kaube H (2006): A novel method of eliciting pain‐related potentials by transcutaneous electrical stimulation. Headache 46:1511–1517. [DOI] [PubMed] [Google Scholar]
- Kaube H, Katsarava Z, Kaeufer T, Diener HC, Ellrich J (2000): A new method to increase nociception specificity of the human blink reflex. Clin Neurophysiol 111:413–416. [DOI] [PubMed] [Google Scholar]
- Koyama T, McHaffie JG, Laurienti PJ, Coghill RC (2005): The subjective experience of pain: Where expectations become reality. Proc Natl Acad Sci USA 102:12950–12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, ffytche DH, Das M, Wilson GD, Goswami S, Sharma T (2007): Neuroticism and brain responses to anticipatory fear. Behav Neurosci 121:643–652. [DOI] [PubMed] [Google Scholar]
- Lee TW, Girolami M, Sejnowski TJ (1999): Independent component analysis using an extended infomax algorithm for mixed subgaussian and supergaussian sources. Neural Comput 11:417–441. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Ahdab R, Ayache SS, Lefaucheur‐Menard I, Rouie D, Tebbal D, Neves DO, Ciampi de Andrade D (2012): Pain‐related evoked potentials: A comparative study between electrical stimulation using a concentric planar electrode and laser stimulation using a CO2 laser. Clin Neurophysiol 42:199–206. [DOI] [PubMed] [Google Scholar]
- Legrain V, Iannetti GD, Plaghki L, Mouraux A (2011): The pain matrix reloaded: A salience detection system for the body. Prog Neurobiol 93:111–124. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologica 9:97–113. [DOI] [PubMed] [Google Scholar]
- Perchet C, Frot M, Charmarty A, Flores C, Mazza S, Magnin M, Garcia‐Larrea L (2012): Do we activate specifically somatosensory thin fibres with the concentric planar electrode? A scalp and intracranial EEG study. Pain 153:1244–1252. [DOI] [PubMed] [Google Scholar]
- Ploghaus A (1999): Dissociating pain from its anticipation in the human brain. Science 284:1979–1981. [DOI] [PubMed] [Google Scholar]
- Ploner M, Lee MC, Wiech K, Bingel U, Tracey I (2010): Prestimulus functional connectivity determines pain perception in humans. Proc Natl Acad Sci USA 107:355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothkirch M, Schmack K, Schlagenhauf F, Sterzer P (2012): Implicit motivational value and salience are processed in distinct areas of orbitofrontal cortex. Neuroimage 62:1717–1725. [DOI] [PubMed] [Google Scholar]
- Sawamoto N, Honda M, Okada T, Hanakawa T, Kanda M, Fukuyama H, Konishi J, Shibasaki H (2000): Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal operculum/posterior insula: An event‐related functional magnetic resonance imaging study. J Neurosci 20:7438–7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schunck T, Erb G, Mathis A, Jacob N, Gilles C, Namer IJ, Meier D, Luthringer R (2008): Test‐retest reliability of a functional MRI anticipatory anxiety paradigm in healthy volunteers. J Magn Reson Imaging 27:459–468. [DOI] [PubMed] [Google Scholar]
- van Boxtel GJ, Boecker KBE (2004): Cortical measures of anticipation. J Psychophysiol 18:61–76. [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL (2008): Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol 100:3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiech K, Lin CS, Brodersen KH, Bingel U, Ploner M, Tracey I (2010): Anterior insula integrates information about salience into perceptual decisions about pain. J Neurosci 30:16324–16331. [DOI] [PMC free article] [PubMed] [Google Scholar]


