Abstract
This study measured effective connectivity within the core face network in young children using a paediatric magnetoencephalograph (MEG). Dynamic casual modeling (DCM) of brain responses was performed in a group of adults (N = 14) and a group of young children aged from 3 to 6 years (N = 15). Three candidate DCM models were tested, and the fits of the MEG data to the three models were compared at both individual and group levels. The results show that the connectivity structure of the core face network differs significantly between adults and children. Further, the relative strengths of face network connections were differentially modulated by experimental conditions in the two groups. These results support the interpretation that the core face network undergoes significant structural configuration and functional specialization between four years of age and adulthood. Hum Brain Mapp 36:2161–2173, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: magnetoencephalography, face processing, childhood, dynamic causal modeling, cortical network
INTRODUCTION
Human adults exhibit a remarkable proficiency in perceiving faces in daily life. Contemporary brain research has proposed that a set of bilateral cortical regions is involved in the visual analysis of faces, including the inferior occipital cortex (i.e., occipital face area, OFA), an extrastriate region that mediates the early representation of physical features in faces [Liu et al., 2010; Rotshtein et al., 2005]; the middle fusiform gyrus (i.e., fusiform face area, FFA), a region that shows the greatest specificity for holistic face perception including structural and static components of facial identity and expressions [Grill‐Spector et al., 2004; Kanwisher and Yovel, 2006; Kanwisher et al., 1997]; and the superior temporal sulcus (STS), where the recognition of the dynamic properties of eye‐gaze and facial expressions are processed [Hoffman and Haxby, 2000; Pelphrey et al., 2005; Puce et al., 1998, 2003]. These three groups of brain regions connect to each other hierarchically to form the well‐known ‘core face network', a paradigmatic example of cortical specialization of function in the adult brain. The representations in the core face network are largely task nonspecific but can flexibly interact with other brain regions in an ‘extended’ set of functional brain subsystems for further processing of the meaning of information extracted from faces under different experimental contexts [Fairhall and Ishai, 2007; Gobbini and Haxby, 2007; Haxby et al., 2000, 2002; Ishai et al., 2005].
In contrast to the rich adult literature on the core face network, relatively little is known about the functional organization of this system in children, especially in early childhood. Three previous functional magnetic resonance imaging (fMRI) studies have examined functional connectivity for face processing mainly in mid‐to‐late childhood (7–17 years). Using a graph theoretical approach, Joseph et al. [2012] compared functional connectivity patterns of the core and extended face networks in children aged 5–12 years to that in adults. They found two face‐preferential developmental changes: a decrease in connections of the posterior visual network during childhood and significant reorganizations of the connections in the right OFA. A second fMRI study [Haist et al., 2013] also found that the activation of brain regions in the extended face network was markedly reduced in children aged 6–12 years compared to adults. But instead of OFA, these authors observed face‐specific developmental changes mainly in the right FFA and STS. In a third fMRI study, Cohen Kadosh et al. [2013] investigated differential processing of facial identification, expression and gaze direction in brain areas within the core face network among children (7–11 years), adolescents (12–17 years) and adults. Across three tasks, they found age‐related changes in bilateral OFA activation; however, the authors did not directly investigate functional patterns of connectivity between regions in the core network. Finally, a recent diffusion tensor imaging (DTI)‐fMRI study [Scherf et al., 2014] examined the structure–function relations in the face network of participants aged 6–23 years. Age‐related increases were found in the volume, fractional anisotropy and mean and radial, but not axial, diffusivities of the inferior longitudinal fasciculus. Critically, the structural differences observed in the inferior longitudinal fasciculus were tightly and specifically associated with the age‐related increase in the size of the FFA.
Taken together, the aforementioned studies indicate that there is substantial developmental fine‐tuning of structural and functional connections within the core face network. However, neither structural nor functional analyses permit any explicit inferences concerning the directionality of information flow within this network. An alternative analytic approach, effective connectivity, ‘refers explicitly to the influence that one neural system exerts over another, either at synaptic or population level’ [Friston, 2011], and therefore, permits inferences concerning the dynamic (activity‐dependent) and causal (task‐dependent) features in brain networks.
Dynamic casual modeling (DCM) is a recent advance in neuroimaging analyses that allows us to examine effective connectivity within the core face network, that is, the influence that component face‐sensitive regions directly exert on each other in response to external inputs [Daunizeau et al., 2011; Friston, 2011; Friston et al., 2003]. To date, there has been a single investigation of effective connectivity within the developing core face network using DCM [Cohen Kadosh et al., 2011]. In that study, three different aspects of face processing (identity, expression and gaze‐direction; termed ‘context‐specific effects’ for the DCM analysis) were analysed in children. Context‐specific changes in effective connectivity within the core face network [previously characterised in adults by Fairhall and Ishai, 2007] were compared among children (7–8 years), adolescents (10–11 years) and adults. The results showed that the basic structure of the core face network was present in children; however, the immature network failed to show the task‐dependent modulations of effective connectivity that were prominent in the adult network. The authors posited that the functional specialization and fine‐tuning of the component regions within the core face network continues well beyond the establishment of the basic layout of its structure at 11 years of age.
This study investigated effective connectivity during face processing in preschool children (3‐ to 6‐years‐old). Instead of fMRI, we obtained brain responses using a custom‐sized paediatric magnetoencephalography (MEG) system. The highly resolved temporal dynamics of inter‐regional coupling detected by MEG can extend the recent fMRI data in this area by delineating the directionality and causality of connectivity changes based on axonal conduction within the network [Fairhall and Ishai, 2007; Friston et al., 2003; Stephan et al., 2007].
Previously, using the same dataset, we have demonstrated that a face‐sensitive M170 brain response can be elicited in preschool children [He et al., 2015]. This finding was in broad agreement with a recent event‐related potential study [Kuefner et al., 2010], but contradicted previous MEG studies, which failed to find an adult‐like M170 in children [Kylliäinen et al., 2006; Taylor et al., 2010]. The negative results of these earlier MEG studies may be due to the fact that they tested children using conventional MEG systems, which are configured for use with adults and are thus less sensitive to neural activity from the smaller heads of children [He et al., 2014; see also Johnson et al., 2010; Irimia et al., 2014].
In this study, we used a hypothesis‐driven approach [Garrido et al., 2007a,b, 2009] to build and test models of effective connectivity during the perception of faces and scrambled faces in children and adults. First, we predefined six brain regions of interest (ROIs)—three in each hemisphere—as a bilaterally organised core face network (Fig. 1). ROI locations were originally derived from the fMRI literature [Henson et al., 2003] and have been tested in other EEG/MEG studies [Chen et al., 2009]. Based on previous DCM investigations of face processing [Chen et al., 2009; David et al., 2006; Fairhall and Ishai, 2007], three different models of network structure were evaluated for model structure (the model that is optimised between accuracy and complexity) and model parameters (the effect of experimental modulations on the connections within the best model).
Figure 1.
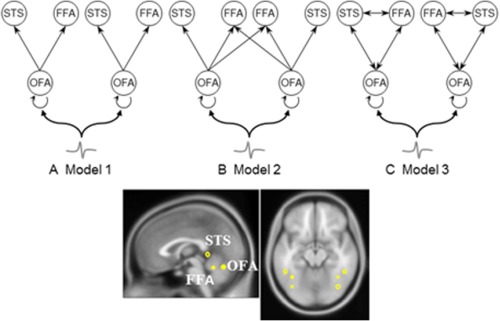
Model construction. The sources comprising the ‘core face network’ are connected with forward/backward connections as shown. OFA, occipital face area (inferior occipital gyrus); FFA, fusiform face area (fusiform gyrus); STS, superior temporal sulcus. Three different models were constructed within this neural architecture. (A) Model 1 has only intra‐hemispheric forward‐connections projecting from bilateral OFA to FFA and STS; (B) Model 2 shares the same intra‐hemispheric structures with Model 1, but has extra interhemispherical forward‐connections from OFA to contralateral FFA; (C) Model 3 has fully forward‐ and backward‐connected sources at the intrahemispheric level. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
We aimed to test two possibilities concerning the developmental changes in effective connectivity of the core face network: First, given the protracted development of cortical inter‐regional connectivity at lower levels of the human visual system [Khundrakpam et al., 2013; Klaver et al., 2011], we predicted that the architecture of the core face network (model structure) in children would differ from that of adults. We anticipated that connections from the right OFA are most likely to be involved in subsequent reorganization over development, as suggested by several previous developmental imaging [Golarai et al., 2007; Joseph et al., 2011; Scherf et al., 2007] and functional connectivity [Joseph et al., 2012] studies. Second, drawing from recent DCM evidence showing that even in adolescents the specific functional patterns of the inter‐regional connectives in the core face network are not yet established [Cohen Kadosh et al., 2011], we predicted that strengths of the connection weightings (model parameters) between regions during the dynamic processing of face‐specific information would also be different between young children and adults. This should be most evident in the effective connectivity between OFA and FFA [Cohen Kadosh et al., 2010, 2013; Gathers et al., 2004; Golarai et al., 2007; Haist et al., 2013; Joseph et al., 2011; Peelen et al., 2009; Scherf et al., 2007, 2014].
MATERIALS AND METHODS
Participants
Data reported in this study came from 15 children (7 females, 3‐ to 6‐years old, averaged age = 4.5 years, standard deviation [SD] = 0.9 years, all right handed) and 14 adults (7 females, averaged age = 26.8 years, SD= 5.7 years, 1 left handed). Handedness was determined by self‐report/parent‐report. All had normal or corrected‐to‐normal visual acuity and no history of neurological disorders. An additional seven children and four adults were tested in the same experiment (a total of 40 participants) but were excluded from DCM analysis because of: (1) excessive noise or artefacts (three children and two adults); (2) technical problems such as photo‐detector/eye‐tracker failure (four children and two adults).
The study was approved by Human Participants Ethics Committee at Macquarie University. Written informed consent was obtained from the adult participants and from the parents/guardians of the children prior to testing.
Visual Stimuli and Task
The four experimental stimulus categories (two sets of 43 coloured pictures each) were adapted from an earlier event‐related potential (ERP) study by Kuefner et al. [2010]. They consisted of: (1) upright faces (faces), (2) front‐on cars (cars), (3) phase‐scrambled faces (scrambled faces) and (4) phase‐scrambled cars (scrambled cars). Scrambled images were images that had undergone a Fourier phase randomization procedure. The low‐level visual properties of the scrambled faces/cars (e.g. special frequency spectrum, luminance, colour and contrast) were identical to that of the intact face/car pictures. A set of 41 colour pictures of cartoon alien faces were embedded pseudo‐randomly into the stimulus stream to serve as ‘catch’ trials which required a button press response to ensure that participants were actively attending to the stimuli in the experiment. All colour photographs were presented in a light grey frame that defined the boundary of the fixation box for the eye tracking system.
Experimental Procedure
MEG measurements were carried out with participants in a supine position in a magnetically shielded room (Fujihara Co., Tokyo, Japan). Images were projected onto a screen located 1 m above the participants' heads. For the adult MEG system, the projector was an InFocus Model IN5108 (InFocus, Portland). For the child MEG system, the projector was a Sharp Notevision Model PG10S (Sharp Electronics, Osaka, Japan). A fibre‐optic photo‐detector placed on the projection screen was used to measure the actual onsets of visual images and all MEG stimulus trigger latencies sent from the presentation control computer were subsequently adjusted accordingly.
Gaze position of the right eye was monitored by an SR Research Eyelink 1000 eye‐tracking system at a sampling rate of 1,000 Hz (http://www.sr-research.com/EL_1000.html). The visual angle of all experimental stimuli in both systems was within the parafoveal region (adult system: 3.10° × 4.58°; child system: 2.64° × 3.90°) [the visual angle of critical object recognition in picture processing is less than 4.5° on average, see Henderson et al., 1999, 2003].
At the beginning of each trial, a fixation point (a small star) appeared at the centre of the screen for 200 ms. Each image was presented only when eye fixations were maintained in the proximity of the fixation point for 500 ms. Catch trials (see below) were presented for 2,000 ms regardless of the fixation point or the button press [He et al., 2014].
The experiment was divided into six blocks. Each block lasted approximately 2 min with a short break between blocks. The total recording time for this experiment ranged from 15 to 20 min. Experimental stimuli were viewed passively without requiring a response; only the alien catch trial stimuli required a button press response. Catch trials were intended to ensure the maintenance of vigilance and to assess the compliance of participants with the experimental instructions, and therefore, were excluded from the DCM analysis. All trials were displayed in a pseudo‐randomised order, with experimental pictures being shown twice each, nonconsecutively.
For the child participants, child‐friendly data acquisition techniques were used to convey instructions, facilitate engagement in the experiment and to minimize movement artefacts during MEG recordings [Tesan et al., 2012]. All participants were trained with one block of four trials prior to the beginning of the MEG experiment.
MEG Data Acquisition
Prior to initiation of MEG measurements, five head position indicators (HPI) were attached to a tightly fitting elastic cap. Fiducial locations (bilateral preauricular points and the nasion) and the participant's head shape were recorded with a pen digitizer (PolhemusFastrack, Colchester, VT). Head movement was calculated by subtracting the positions of HPIs before and after each acquisition session and the tolerance level was set at a maximum of 5 mm for any single session.
MEG measurements were carried out with two KIT whole‐head MEG systems located in the same magnetically shielded room. The adult system (Model PQ1160R‐N2, KIT, Kanazawa, Japan) consisted of 160 coaxial first‐order gradiometers with a 50 mm baseline [Kado et al. 1999]. The child system (Model PQ1064R‐N2m, KIT, Kanazawa, Japan) had 64 first‐order axial gradiometers with a 50 mm baseline [Johnson et al., 2010]. MEG data were acquired using a sampling rate of 1,000 Hz and a filter bandpass of 0.03–200 Hz. Shim pads were placed in the child helmet for the child session to reduce head movement.
For the child participants, an experimenter remained in the magnetically shielded room during MEG recording sessions to make sure the child was comfortable and engaged during the experiment.
MEG Preprocessing
MEG data were preprocessed using SPM8 software [Litvak et al., 2011; http://www.fil.ion.ucl.ac.uk/spm/software/] running under Matlab 7.13 (The Math‐Works, Inc.). All sensor data were down‐sampled to 250 Hz, band‐pass‐filtered between 1.6 and 30 Hz, and epoched with a peristimulus window of −100 to 400 ms. Artefacts including blinks, eye‐movements and magnetocardiographic activity were rejected from each trial and channel with the Fieldtrip visual artefact rejection method implemented in SPM8 [Oostenveld et al., 2011]. Approximately 98% of trials in adults and 97% of trials in children survived the rejection procedure. Robust averaging was used to average across trials within the four categories of visual stimuli, that is, faces, cars, scrambled faces and scrambled cars, for each participant, condition and sensor [Litvak et al., 2011]. Outliers are down‐weighted by this method using an iterative robust general linear model [Wager et al., 2005], which calculates and reassigns weights to each sample of each trial according to how far it is from the mean response. To remove any high frequency noise introduced by the robust averaging step, the averaged epoched data were low‐pass filtered at 30 Hz.
Dynamic Causal Modeling
All DCM analyses were performed using DCM8 (a suite of SPM8), which estimates two sets of quantities within the framework: (1) the probability of the model (m) given the data (y), also known as the model evidence p(y | m), which can be used to compare model fittings between different model structures and; (2) the posterior distribution over model parameters (θ), which can be used to make inferences on model connectivity. All parameters were estimated using Bayesian methods by means of the expectation maximization algorithm [Friston et al., 2003]. A detailed description of the DCM framework for EEG/MEG data can be found in David et al. [2006] and Kiebel et al. [2009].
The DCM model inversion was restricted to the face‐sensitive effect (encoded by the B matrix) with scrambled faces as baseline condition coded as 0 and faces as modulations of the connections coded as 1 over the peristimulus interval of 0–200 ms (time‐window of the M170/N170‐a face‐sensitive event‐related magnetic fields (ERF)/ERP component showing stronger and faster response to faces than nonface objects (Kylliäinen et al. [2006], Kuefner et al. [2010], He et al. [2014, 2015]; please see Supporting Information for the identification of M170 peristimulus interval) across all participants.
DCM–model construction
The anatomical structure of the three DCM models was motivated by recent neurophysiological studies looking at the sources underlying the M170/N170 [see review by Rossion, 2014], neuroimaging studies revealing regions showing face‐sensitive haemodynamic brain responses [Kanwisher, 2010], and a theoretical framework of the neural systems for face processing [Haxby et al., 2000]. Three bilateral cortical sources, including inferior occipital gyrus (OFA), fusiform gyrus (FFA) , and STS, comprising the ‘core face network', were modelled as equivalent current dipoles given the MNI coordinates (converted from Talairach space using the algorithm in http://bioimagesuite.yale.edu/mni2tal/index.aspx): OFA, 42,−81, −15 (right)/ −39, −81, −15 (left); FFA, 42, −45, −27 (right)/ −39, −51, −24 (left); STS, 48, −42, 12 (right)/ −48, −42, 12 (left) [Chen et al., 2009; Henson et al., 2003].
Figure 1 illustrates the model architectures specified in this study. We note that the real and complete functional and structural connectivity of the core face network is still unsolved. Here we carefully defined our models based on the results of previous imaging studies using relevant methodology and technology. The connectivities of Models 1 and 2 were based on the core structure proposed by Fairhall and Ishai [2007] with only forward connections involved in the communication between OFA, FFA and STS (Model 1). The second model included an extra interhemispheric forward connection from OFA to contralateral FFA. This connection was added because of a report of a patient with acquired prosopagnosia with lesions of the left FFA and the right OFA, whose right FFA still exhibited normal face selective activation [Rossion et al., 2003]. A similar interhemispheric structure was used in the only previous DCM analysis of (adult) MEG face responses [Chen et al., 2009]. The third model was derived from a DCM analysis of EEG data, where the three regions were fully connected with both forward and backward pathways [David et al., 2006]. In this study, we specified the same experimental modulation—a contrast between faces and scrambled faces—used by Chen et al. [2009].
DCM–Bayesian model selection
Random‐effects procedure for bayesian model selection (BMS) was performed both at an individual level and at a group level based on the assumption that various cognitive strategies may be performed in dealing with cognitive tasks [Stephan et al., 2010]. This method for performing BMS has proven more robust and accurate compared to fixed‐effect analysis because outliers are largely suppressed during estimation [dRigoux et al., 2014]. After selecting the most likely cortical network engaged in the face perception task, we directly tested connectivity parameters in the winning model.
DCM–Bayesian parameter averaging
In this step, Bayesian parameter averaging (BPA) was used to estimate the modulatory effects of the experimental input over model parameters across participants in each group. Models with the largest model evidence contribute most to the single posterior density for the entire group. This step allows direct Bayesian inference at the group level.
A nonparametric bootstrapping test was used to evaluate whether the contrast‐dependent (i.e., the experimental modulation of connectivity) effects in BPA parameters were statistically significant. For each connection, the distribution of the log gain was reconstructed by generating 10,000 random samples from a Gaussian distribution based on the posterior mean (mEps) and SD (sEps) calculated in the BPA process. If the two‐tailed test had a >95% probability to be greater or smaller than unity (a posterior mean of zero), the connection was taken as significantly enhanced or weakened for the experimental context (faces vs. scrambled faces). Connections which satisfied this criterion are reported as P < 0.05.
RESULTS
Event‐related Magnetic Fields
MEG responses to faces showed distinct M100 and M170 peaks in adults but the M170 response was largely masked by the prominent preceding M100 in children. However, by subtracting brain responses to faces from that to scrambled faces, we were able to effectively separate the face‐sensitive M170 response in both groups. The resulting M170 response latencies were approximately 180 ms in children and 148 ms in adults (Fig. 2).
Figure 2.
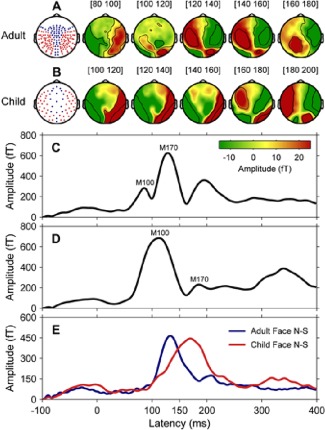
Event‐related magnetic fields in adults (N = 14) and children (N = 15). (A) Schematic diagram of sensor layout for 160‐channel adult MEG system and surface topography in adults for the difference response at 20 ms stepwise from 80 to 180 ms, encompassing the temporal window of M100 (83 ms) and M170 (148 ms). (B) Schematic diagram of sensor layout for 64‐channel child MEG system and surface topography in children for the difference response at 20 ms stepwise from 100 to 200 ms, encompassing the temporal window of M100 (110 ms) and M170 (180 ms). In both MEG sensor layouts, red dots indicate the temporal–occipital regions where the M170 response was maximal. Note here the child system does not have frontal region coverage. (C) Global field power of grand mean ERFs to faces in adults. (D) Global field power of grand mean ERFs to faces in children. (E) Global field power of grand mean ERFs to faces minus ERFs to scrambled faces (Face N–S) for adults (blue line) and children (red line). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DCM–Bayesian Model Selection
Three different DCM models were inverted for each participant. In random‐effects BMS, model exceedance probability was presented for one model being more likely than any other model given the observed data at both the individual level and group level. The model with the largest exceedance probability compared to other models represents an optimal balance between model accuracy (i.e., fit) and model complexity at both levels.
Figure 3A,C shows the BMS comparison results at the individual level, and Figure 3B,D shows the model exceedance probability of the three models at the group level.
Figure 3.
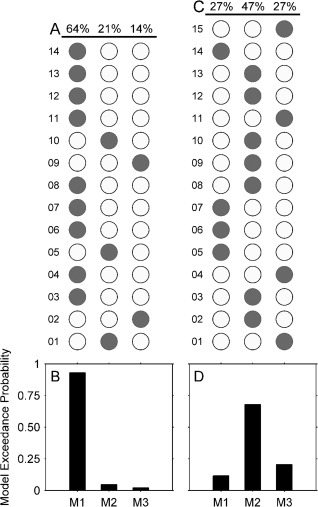
Bayesian model selection for the three models. (A) Model selection results over all adult participants (N = 14); (B) Model exceedance probability at the adult group level; (C) Model selection results over all child participants (N = 15); (D) Relative exceedance probability at the child group level. Grey dots in A and B indicate the winning models in individual participants.
Model 1—with only forward connections among OFA, FFA and STS in each hemisphere—was decisively the winning model in adults, showing a strong exceedance probability (>0.9) over the other two models across most adult participants (64%) and at the group level. In contrast, Model 2—with an extra interhemispherical forward connection from OFA to contralateral FFA—revealed the best fit of data at the group level in children (exceedance probability > 0.6). At the individual child level, Model 2 was preferred over the other two models in 47% of individuals, while Models 1 and 3 were both preferred in 23% of individuals.
Thus, for both adults and children, the winning models at the group level had the largest proportion of highest exceedance probability identification at the individual level. Neither group had outliers with especially strong exceedance probability of model fitting that might have driven the group model evidence. In addition, both the winning child and adult models that best explained the face‐sensitive effect returned an excellent fit to the data (Figs. S2 and S3 in the Supporting Information).
DCM–Bayesian Parameter Averaging
The results of the BPA for the effective connection‐to‐connection modulation as a function of the contrast‐dependent effects of face processing in the time‐window of the M170 are shown in Figure 4 (connection modulations above the significance criterion of P < 0.05). DCM posterior means and SDs for intrinsic, direct inputs and modulatory connections, weighted by posterior model probability of three models over all participants in each group are summarised in Table 1 (adults) and Table 2 (children).
Figure 4.
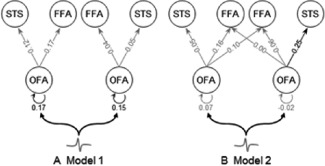
The modulatory effect of faces derived from the group optimal model identified by Bayesian model selection. (A) In adults (N = 14), the reciprocal connections of bilateral OFA are significantly enhanced by faces. (B) In children (N = 15), the connection from the right OFA to STS is significantly enhanced by faces. The exceedance probabilities for posterior means of these modulatory effects on connections are of 95%. Grey lines indicate posterior probability of modulatory effects smaller than 95%.
Table 1.
Bayesian model average on posterior means and standard deviations (mean ± SD) of intrinsic, input and modulation estimates across all connections for all adult participants (N = 14). Bold: P < 0.05
| Effective connectivity parameters | Right | Left |
|---|---|---|
| Intrinsic connectivity | ||
| OFA‐FFA | −0.04 ± 0.06 | 0.07 ± 0.06 |
| OFA‐STS | 0.03 ± 0.06 | −0.05 ± 0.06 |
| Extrinsic input | ||
| OFA | 0.01 ± 0.04 | 0.01 ± 0.04 |
| Modulation of connectivity | ||
| OFA‐FFA | −0.04 ± 0.04 | 0.17 ± 0.04 |
| OFA‐STS | 0.05 ± 0.03 | 0.12± 0.04 |
| OFA‐OFA (reciprocal) | 0.15 ± 0.01 | 0.17 ± 0.02 |
Table 2.
Bayesian model average on posterior means and standard deviations (mean ± SD) of intrinsic, input and modulation estimates across all connections for all child participants (N = 15). Bold: P < 0.05
| Effective connectivity parameters | Right | Left |
|---|---|---|
| Intrinsic connectivity | ||
| OFA‐FFA | −0.00 ± 0.06 | 0.02 ± 0.06 |
| OFA‐STS | −0.05 ± 0.05 | −0.00 ± 0.06 |
| OFA‐FFA (interhemisphere) | −0.01 ± 0.06 | −0.01 ± 0.06 |
| Extrinsic input | ||
| OFA | −0.07 ± 0.04 | −0.01 ± 0.04 |
| Modulation of connectivity | ||
| OFA‐FFA | −0.06 ± 0.06 | 0.16 ± 0.06 |
| OFA‐STS | 0.25 ± 0.03 | 0.05 ± 0.03 |
| OFA‐FFA (interhemisphere) | 0.00 ± 0.06 | 0.01 ± 0.05 |
| OFA‐OFA (reciprocal) | −0.02 ± 0.01 | 0.07± 0.01 |
The intrinsic connectivity of the ‘core face network’ was significant for the connection between right OFA and STS in children (strength = − 0.05, posterior > 0.95, P < 0.05). With regard to the face modulation effect, that is, faces versus scrambled faces, the right OFA to STS connection was significantly enhanced in children (strength = 0.25, posterior > 0.95, P < 0.05), and the bilateral reciprocal connections of OFA were significantly enhanced in adults (strength = 0.15 (right)/0.17(left), posterior > 0.95, P < 0.05).
DISCUSSION
The advent of custom‐sized paediatric MEG systems opens a new window into developmental brain function, particularly in the preschool (3‐ to 6‐years) age range, which has been largely excluded from neuroimaging research [Poldrack, 2010]. In this study, we examined the functional organisation of the core face network by analysing MEG data from children aged 3–6 years. Our child cohort was a relatively large sample with equal gender and age distributions. We addressed the following questions: (1) are there age‐related differences in the basic functional architecture (model structure) of the core face network between young children and adults? Alternatively, are adult patterns of connection present by 4 years of age? (2) Are there age‐related differences in the functional specificity (model parameters) of the network? That is, do the components of the network change their interactions with each other in a comparable fashion when encoding faces as opposed to nonface stimuli between young children and adults?
Developmental Differences in Model Structure of the Core Face Network
Our MEG results for adult participants showed the same network architecture that has been reported in previous fMRI studies [Cohen Kadosh et al., 2011; Fairhall and Ishai, 2007]: intrahemispheric forward‐connections projecting from bilateral OFA to FFA and STS. When adults are attentively viewing faces, the OFA exerts an intrinsic influence on both activations in the FFA and STS along two separate feed‐forward pathways. Fairhall and Ishai [2007] argued that the absence of feedback and/or lateral connections does not suggest there are no such anatomical connections, but rather reflects a lack of dynamic influences between regions with regard to the experimental modulatory effects [also see Friston et al., 2003; Penny et al., 2004].
In our child participants, the network structure yielded an extra interhemispheric connection between the OFA and the contralateral FFA on top of the adult core face network. This suggests a developmental reorganization of connectivity between these two regions (especially in the right hemisphere) resulting in the functional separation of featural versus configural/holistic face processing in the mature brain. Interestingly, an interhemispheric connection between OFA and FFA is preserved in severely prosopagnosic adults [Fox et al., 2008; Rossion et al., 2003]. Taken together with the present findings, this indicates that the OFA to contralateral FFA connectivity pattern reflects a developmental pruning of the core face network. This connection is later eliminated in normal development but fails to be eliminated or is reactivated in abnormal network development. The extra OFA to FFA connection in the immature brain also speaks to a Selectivism framework for the emerging cognitive functions in the brain [Changeux and Danchin, 1976], which predicts that brain development is based on gradually down‐weighting redundant neural connections between functionally connected brain regions.
There were larger individual differences in the organization of the core face network in children than adults. One explanation for this finding could be that the network structure in children might allow for more idiosyncratic connectivity between brain regions [Haist et al., 2013]. Over the course of development, this network becomes more regularised (expertized) through an interplay between genetics and experience [Gauthier and Nelson, 2001; Johnson, 2010].
Developmental Differences in Model Parameters of the Core Face Network
In adults, the modulatory effects of face inputs upon inter‐regional connections within the winning model were reflected in an increase of self‐connection strength between the bilateral OFAs (in contrast to scrambled face inputs). This result is consistent with previous evidence for the key role of the OFA in face processing in the adult brain [Pitcher et al., 2007, 2011; Rossion et al., 2003]. For example, transcranial magnetic stimulation of the OFA has been shown to interfere with the processing of different facial properties and subsequently affects the later integrative analysis of facial identification and expression [Cohen Kadosh et al., 2010].
We found no significant model parameter changes in the forward connections from OFA to FFA or to STS in our adults, in contrast to results reported in several previous DCM connectivity studies [Chen et al., 2009; Cohen Kadosh et al., 2011; Fairhall and Ishai, 2007; Nguyen et al., 2014]. These differences merit further investigation but are not incompatible with our findings for two main reasons. First, DCM parameters are sensitive to the peristimulus window of evoked responses [Garrido et al., 2007a] and the significant forward coupling between OFA and FFA reported in Chen et al.'s [2009] MEG study was computed from a much longer time‐window (−100 to 600 ms, for time‐frequency analysis) than ours (0–200 ms, for analysis of the face‐sensitive M170 response). Second, the two fMRI studies [Cohen Kadosh et al., 2011; Fairhall and Ishai, 2007] and the EEG–fMRI study of Nguyen et al. [2014] used more cognitively demanding active discrimination tasks. In contrast, our passive viewing experiment was not as effortful and may not, therefore, have modulated the forward connections through FFA and STS for further detailed holistic/configural face analysis.
Our results show that differences between the age groups were centered on the connections of the bilateral OFA during face processing. As noted above, this region has long been considered a key player in face processing and has also been shown to exhibit the largest face‐specific functional reorganisations in childhood [Golarai et al., 2007; Joseph et al., 2011, 2012; Scherf et al., 2007; but see Haist et al., 2013]. In contrast to adults, children did not show an increase in self‐connection strengths of the OFA. Joseph et al. [2011] reported a greater increase of face specialised activation in the OFA compared to that in the FFA from 6 years of age to adulthood. The shift of activations in the FFA to OFA with age when viewing faces attentively is consistent with the prediction of the Interactive specialization (IS) account in that the activity patterns of FFA and OFA become restricted during development [Johnson, 2011] such that, in adults, the feature‐related facial information is processed more elaborately in the OFA before passing on to the FFA.
In children, the only model parameter modulated by face versus nonface stimuli was an enhancement of the forward connection of the right OFA to the STS. Haist et al. [2013] failed to find any evidence for developmental differences in face processing in the OFA, but did report an age‐related association with activity within the STS (especially in the right hemisphere), a region that was more strongly activated in children than adults. Their findings disagree with several earlier observations that showed no developmental trend in face‐preferential intensity or volume of STS activation [Cohen Kadosh et al., 2011; Golarai et al., 2007]. Developmental changes in the STS specific to faces was also reported in two other studies, although it was less reliable than for corresponding changes in the FFA and OFA [Scherf et al., 2007]. Scherf et al. [2007] reported the size of the STS producing face‐specific activation increased significantly across development, and Joseph et al. [2011] reported increasing activation intensity with age within the STS. However, there was no evidence concerning the connectivity changes with age between the STS and OFA regions and no findings in young children regarding this connection that would enable direct differences between young childhood and adulthood to be discerned.
A possible interpretation of the enhanced connection between the right OFA to the STS is that it reflects children's preference for the eye‐regions of the face during the early processing of faces, since the STS is known to be specifically sensitive to eyes and eye movements [Hoffman and Haxby, 2000; Puce et al., 2003]. This interpretation is supported by findings from several studies that show the eye detection process (some term the N170 an ‘eye component/detector’ [Taylor et al., 2001]) has a more rapid maturational course [Taylor et al., 1999] than the global processing of faces on the basis of metric relationships between their constituent features [‘configural processing'; Mondloch et al., 2002], or on the basis of unitary facial features [‘holistic processing'; de Heering et al., 2007; Mondloch et al., 2007]. This interpretation is also consistent with findings from another functional connectivity study of the core face network [Cohen Kadosh et al., 2013], where children aged 10 were found to activate OFA for predominantly featural processing strategies, compared to adults who exhibited a more strategy‐flexible functional pattern of activation in the core face network.
The same study [Cohen Kadosh et al., 2013] reported different functional patterns in the bilateral OFA for children, adolescents and adults, therefore, suggesting that the functional profile of core face‐selective regions continues to develop throughout the first two decades of life. The transition of inter‐regional connections for face representations in the core network throughout early childhood might also be related to the perceptual challenges associated with the acquisition of expertise and the representational demands of face processing. These challenges increase significantly during 3–6 years old period of development, which consequently requires increased specialisation and restriction of neural activity in component regions, especially the FFA, in presenting faces in a more efficient, for example, holistic way [Gauthier et al., 2000; Haxby et al., 2002; Kanwisher, 2010].
From the small amount of work on cognitive brain development that has been done to date, a consensus has been reached that the establishment of functional brain networks undergoes a ‘local to distributed’ organising principle [Fair et al., 2009]. Generally, adult brains are more efficient because of a strengthening of long‐range brain connections during the ‘rewiring’ of local connections during development [Watts and Strongatz, 1998]. Therefore, we speculate that the different activity pattern of effective connectivity between component regions in the core face network in young children might reflect a more general development of functional brain networks across early childhood [functional‐connectivity‐MRI: Fair et al., 2009; Power et al., 2010; EEG: Bathelt et al., 2013]. This idea resonates with the IS framework, which posits that specialised brain regions are connected at birth due to anatomic restrictions; however they are ‘broadly tuned’ and much less selective than in adulthood[Johnson, 2000b,b, 2001, 2011; Johnson and Munakata, 2005]. Therefore, extensive experience with faces over development might shape functional regions and promote myelination in a way that specialised brain regions pose restrictions on the activity patterns of each other as well as on the white matter fibre tracts that connect them (or vice versa), to refine the network to use the most efficient nodes and pathways available.
There are several potential limitations to our interpretation of the current data. First, since we used adult faces as stimuli for both age groups one might argue that our age‐group differences may be related to an ‘own‐age bias’ in adults. We do not think this possibility is likely here because recent evidence shows that all 3‐year olds are adult face experts [Macchi Cassia et al., 2012]. In addition, an own‐age bias in face processing only clearly supports better active recognition in adults [Fulton and Bartlett 1991; Wright and Stroud, 2002] for the efficacy of encoding of individual faces within their peer age group, but this effect is quite mixed in adolescents and children [Anastasi and Rhodes, 2005]. Moreover, recent fMRI studies found similar face‐selective activation in the FFA in children and adults when viewing faces of familiar and unfamiliar adults and peers [Pierce and Redcay, 2008; Golarai et al., 2010]. Another potential limitation is that adults and children may differ in how they visually scan faces and other objects. However, we think this explanation is unlikely for two reasons. First, the existing literature suggests that the visual scan patterns of faces in children at 5‐years old [or even younger; Maurer and Salapatek, 1976; Maurer et al., 2007] are similar to adults [Want et al., 2003]. In addition, we used eye‐tracking to ensure that both groups fixated on centre of the visual stimuli, and the brief presentation (500 ms) left little possibility for group differences in ocular behaviour. Finally, age‐related differences in general cognitive abilities, such as memory and attention [Betts et al., 2006; Crookes and McKone, 2009; McKone et al., 2012] may have influenced our MEG measurements of the core face network. However, we used a child‐friendly task to make sure that both groups were performing at ceiling, achieving about 95% correct responses on catch trials.
A final note here is that the Bayesian selection approach can only ever inform us which model is most appropriate from those put forward for evaluation. We chose to constrain our model space to three candidate network structures that were strongly motivated by previous studies of adults and thus provided clear interpretability of results. We cannot, however, exclude the possibility that children's brain responses to faces are in fact captured best by a model other than one of those considered here, and it may be useful to revisit the current dataset in the light of potential future studies of face processing development.
CONCLUSIONS
The present findings provide the first characterisations of developmental changes in effective connectivity patterns of core face network in preschool aged children. To our knowledge, the current findings also represent the first step in monitoring the dynamics of emergent functional relations in the developing brain at millisecond scale using MEG. The novel findings here are that, unlike older children and adolescents who have already established the overall mature structural of the core network [Cohen Kadosh et al., 2011], young children aged between 3 and 6 years have an extra connection between OFA and contralateral FFA in the network—a redundant neural connection between OFA and FFA that might be gradually pruned during early childhood but could be preserved in neuropsychological lesion populations, such as prosopagnosia patients [Fox et al., 2008; Rossion et al., 2003]. Moreover, the adult network exhibited enhanced bilateral interconnections in the OFA in response to task demands, while in the child network the same task enhanced intrahemispheric connectivity between OFA and STS in the right hemisphere. These developmental changes in the OFA and its connectivity among other core face network regions (particularly in the right hemisphere) suggest that the face‐specific changes in the OFA play an important role in the maturation of the core face network during early childhood. Furthermore, our observations provide strong tests of hypotheses derived from theoretical frameworks such as Selectivism [Changeux and Danchin, 1976] and IS theories [Berl et al., 2006; Bunge and Wright, 2007; Grill‐Spector et al., 2008; Johnson, 2011].
Supporting information
Supplementary Informationarticle.
ACKNOWLEDGMENTS
The authors thank Romina Palermo and Douglas Cheyne for helpful comments during the design of the experiment and Bruno Rossion for providing the picture stimuli. The authors gratefully acknowledge the collaboration of Kanazawa Institute of Technology in establishing the KIT‐Macquarie MEG laboratory. Conflict of Interest: None declared.
Footnotes
Please note here that the use of the term OFA and FFA was to demonstrate the functional overlap in the pre‐defined inferior occipital gyrus and fusiform gyrus regions with more traditionally defined FFA and OFA regions.
REFERENCES
- Anastasi JS, Rhodes MG (2005): An own‐age bias in face recognition for children and older adults. Psychon Bull Rev 12:1043–1047. [DOI] [PubMed] [Google Scholar]
- Bathelt J, O'Reilly H, Clayden JD, Cross JH, de Haan M (2013): Functional brain network organisation of children between 2 and 5 years derived from reconstructed activity of cortical sources of high‐density EEG recordings. Neuroimage 82:595–604. [DOI] [PubMed] [Google Scholar]
- Berl MM, Vaidya CJ, Gaillard WD (2006): Functional imaging of developmental and adaptive changes in neurocognition. Neuroimage 30:679–691. [DOI] [PubMed] [Google Scholar]
- Betts J, Mckay J, Maruff P, Anderson V (2006): The development of sustained attention in children: The effect of age and task load. Child Neuropsychol 12:205–221. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wright SB (2007): Neurodevelopmental changes in working memory and cognitive control. Curr Opin Neurobiol 17:243–250. [DOI] [PubMed] [Google Scholar]
- Changeux JP, Danchin A (1976): Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature 264:705–712. [DOI] [PubMed] [Google Scholar]
- Chen CC, Henson RN, Stephan KE, Kilner JM, Friston KJ (2009): Forward and backward connections in the brain: A DCM study of functional asymmetries. Neuroimage 45:453–462. [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh K, Walsh V, Cohen Kadosh R (2010): Investigating face‐property specific processing in the right OFA. Soc Cogn Affect Neurosci 6:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh K, Cohen Kadosh R, Dick F, Johnson MH (2011): Developmental changes in effective connectivity in the emerging core face network. Cereb Cortex 21:1389–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh K, Johnson MH, Henson RN, Dick F, Blakemore SJ (2013): Differential face‐network adaptation in children, adolescents and adults. Neuroimage 69:11–20. [DOI] [PubMed] [Google Scholar]
- Crookes K, McKone E (2009): Early maturity of face recognition: No childhood development of holistic processing, novel face encoding, or face‐space. Cognition 111:219–247. [DOI] [PubMed] [Google Scholar]
- Daunizeau J, David O, Stephan KE (2011): Dynamic causal modelling: A critical review of the biophysical and statistical foundations. Neuroimage 58:312–322. [DOI] [PubMed] [Google Scholar]
- David O, Kiebel SJ, Harrison LM, Mattout J, Kilner JM, Friston KJ (2006): Dynamic causal modeling of evoked responses in EEG and MEG. Neuroimage 30:1255–1272. [DOI] [PubMed] [Google Scholar]
- de Heering A, Houthuys S, Rossion B (2007): Holistic face processing is mature at 4 years of age: Evidence from the composite face effect. J Exp Child Psychol 96:57–70. [DOI] [PubMed] [Google Scholar]
- dRigoux L, Stephan KE, Friston KJ, Daunizeau J (2014): Bayesian model selection for group studies—Revisited. Neuroimage 84:971–985. [DOI] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE (2009): Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol 5:e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairhall SL, Ishai A (2007): Effective connectivity within the distributed cortical network for face perception. Cereb Cortex 17:2400–2406. [DOI] [PubMed] [Google Scholar]
- Fox CJ, Iaria G, Barton JJ (2008): Disconnection in prosopagnosia and face processing. Cortex 44:996–1009. [DOI] [PubMed] [Google Scholar]
- Friston KJ (2011): Functional and effective connectivity: A review. Brain Connectivity 1:13–36. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W (2003): Dynamic causal modelling. Neuroimage 19:1273–1302. [DOI] [PubMed] [Google Scholar]
- Fulton A, Bartlett JC (1991): Young and old faces in young and old heads: The factor of age in face recognition. Psychol Aging 6:623–630. [DOI] [PubMed] [Google Scholar]
- Garrido MI, Kilner JM, Kiebel SJ, Friston KJ (2007a): Evoked brain responses are generated by feedback loops. Proc Natl Acad Sci USA 104:20961–20966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido MI, Kilner JM, Kiebel SJ, Stephan KE, Friston KJ (2007b): Dynamic causal modelling of evoked potentials: A reproducibility study. Neuroimage 36:571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido MI, Kilner JM, Kiebel SJ, Stephan KE, Baldeweg T, Friston KJ (2009): Repetition suppression and plasticity in the human brain. Neuroimage 48:269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathers AD, Bhatt R, Corbly CR, Farley AB, Joseph JE (2004): Developmental shifts in cortical loci for face and object recognition. NeuroReport 15:1549–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier I, Nelson CA (2001): The development of face expertise. Curr Opin Neurobiol 11:219–224. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Moylan J, Skudlarski P, Gore JC, Anderson AW (2000): The fusiform "face area" is part of a network that processes faces at the individual level. J Cogn Neurosci 12:495–504. [DOI] [PubMed] [Google Scholar]
- Gobbini MI, Haxby JV (2007): Neural systems for recognition of familiar faces. Neuropsychologia 45:32–41. [DOI] [PubMed] [Google Scholar]
- Golarai G, Ghahremani DG, Whitfield‐Gabrieli S, Reiss A, Eberhardt JL, Gabrieli JD, Grill‐Spector K (2007): Differential development of high‐level visual cortex correlates with category‐specific recognition memory. Nat Neurosci 10:512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golarai G, Liberman A, Yoon JM, Grill‐Spector K (2010): Differential development of the ventral visual cortex extends through adolescence. Front Hum Neurosci 3:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill‐Spector K, Knouf N, Kanwisher N (2004): The fusiform face area subserves face perception, not generic within‐category identification. Nat Neurosci 7:555–562. [DOI] [PubMed] [Google Scholar]
- Grill‐Spector K, Golarai G, Gabrieli J (2008): Developmental neuroimaging of the human ventral visual cortex. Trends Cogn Sci 12:152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haist F, Adamo M, Han Wazny J, Lee K, Stiles J (2013): The functional architecture for face‐processing expertise: FMRI evidence of the developmental trajectory of the core and the extended face systems. Neuropsychologia 51:2893–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI (2000): The distributed human neural system for face perception. Trends Cogn Sci 4:223–233. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI (2002): Human neural systems for face recognition and social communication. Biol Psychiatry 51:59–67. [DOI] [PubMed] [Google Scholar]
- He W, Brock J, Johnson BW (2014): Face‐sensitive brain responses measured from a four‐year‐old child with a custom‐sized child MEG system. J Neurosci Methods 222:213–217. [DOI] [PubMed] [Google Scholar]
- He W, Brock J, Johnson BW (2015): Face processing in the brains of pre‐school aged children measured with MEG. NeuroImage 106:317–327. [DOI] [PubMed] [Google Scholar]
- Henderson JM, Weeks PA, Hollingworth A (1999): The effects of semantic consistency on eye movements during complex scene viewing. J Exp Psychol Human 25:210–228. [Google Scholar]
- Henderson JM, Williams CC, Castelhano MS, Falk RJ (2003): Eye movements and picture processing during recognition. Percept Psychophys 65:725–734. [DOI] [PubMed] [Google Scholar]
- Henson RN, Goshen‐Gottstein Y, Ganel T, Otten LJ, Quayle A, Rugg MD (2003): Electrophysiological and haemodynamic correlates of face perception, recognition and priming. Cereb Cortex 13:793–805. [DOI] [PubMed] [Google Scholar]
- Hoffman EA, Haxby JV (2000): Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nat Neurosci 3:80–84. [DOI] [PubMed] [Google Scholar]
- Irimia A, Erhart MJ, Brown TT (2014): Variability of magnetoencephalographic sensor sensitivity measures as a function of age, brain volume and cortical area. Clin Neurophysiol 125:1973–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai A, Schmidt CF, Boesiger P (2005): Face perception is mediated by a distributed cortical network. Brain Res Bull 67:87–93. [DOI] [PubMed] [Google Scholar]
- Johnson BW, Crain S, Thornton R, Tesan G, Reid M (2010): Measurement of brain function in pre‐school children using a custom sized whole‐head MEG sensor array. Clin Neurophysiol 121:340–349. [DOI] [PubMed] [Google Scholar]
- Johnson MH (2000a): Cortical specialization for higher cognitive functions: Beyond the maturational model. Brain Cogn 42:124–127. [DOI] [PubMed] [Google Scholar]
- Johnson MH (2000b): Functional brain development in infants: Elements of an interactive specialization framework. Child Dev 71:75–81. [DOI] [PubMed] [Google Scholar]
- Johnson MH (2001): Functional brain development in humans. Nat Rev Neurosci 2:475–483. [DOI] [PubMed] [Google Scholar]
- Johnson MH (2010): Face perception: A developmental perspective In: Calder AJ, Rhodes G, Johnson MA, Haxby JV, editors. The Oxford Handbook of Face Perception. New York: Oxford University Press; pp 3–14. [Google Scholar]
- Johnson MH (2011): Interactive specialization: A domain‐general framework for human functional brain development? Dev Cogn Neurosci 1:7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH, Munakata Y (2005): Processes of change in brain and cognitive development. Trends Cogn Sci 9:152–158. [DOI] [PubMed] [Google Scholar]
- Joseph JE, Gathers AD, Bhatt RS (2011): Progressive and regressive developmental changes in neural substrates for face processing: Testing specific predictions of the Interactive Specialization account. Dev Sci 14:227–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JE, Swearingen JE, Clark JD, Benca CE, Collins HR, Corbly CR, Gathers AD, Bhatt RS (2012): The changing landscape of functional brain networks for face processing in typical development. Neuroimage 63:1223–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- H Kado, M Higuchi, M Shimogawara, Y Haruta, Y Adachi, J Kawai, H Ogata, G Uehara (1999): Magnetoencephalogram systems developed at KIT. IEEE Trans Appl Supercond 9:4057–4062. [Google Scholar]
- Kanwisher N (2010): Functional specificity in the human brain: A window into the functional architecture of the mind. Proc Natl Acad Sci USA 107:11163–11170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, Yovel G (2006): The fusiform face area: A cortical region specialized for the perception of faces. Philos Trans R Soc Lond B Biol Sci 361:2109–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM (1997): The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci 17:4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khundrakpam BS, Reid A, Brauer J, Carbonell F, Lewis J, Ameis S, Karama S, Lee J, Chen Z, Das S, Evans AC, Brain Development Cooperative G (2013): Developmental changes in organization of structural brain networks. Cereb Cortex 23:2072–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebel SJ, Garrido MI, Moran R, Chen CC, Friston KJ (2009): Dynamic causal modeling for EEG and MEG. Hum Brain Mapp 30:1866–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaver P, Marcar V, Martin E (2011): Neurodevelopment of the visual system in typically developing children. Prog Brain Res 189:113–136. [DOI] [PubMed] [Google Scholar]
- Kuefner D, de Heering A, Jacques C, Palmero‐Soler E, Rossion B (2010): Early visually evoked electrophysiological responses over the human brain (P1, N170) show stable patterns of face‐sensitivity from 4 years to adulthood. Front Hum Neurosci 3:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kylliäinen A, Braeutigam S, Hietanen JK, Swithenby SJ, Bailey AJ (2006): Face‐ and gaze‐sensitive neural responses in children with autism: A magnetoencephalographic study. Eur J Neurosci 24:2679–2690. [DOI] [PubMed] [Google Scholar]
- Litvak V, Mattout J, Kiebel S, Phillips C, Henson R, Kilner J, Barnes G, Oostenveld R, Daunizeau J, Flandin G, Penny W, Friston K (2011): EEG and MEG data analysis in SPM8. Comput Intell Neurosci 2011:852961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Harris A, Kanwisher N (2010): Perception of face parts and face configurations: An FMRI study. J Cogn Neurosci 22:203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchi Cassia V, Pisacane A, Gava L (2012): No own‐age bias in 3‐year‐old children: More evidence for the role of early experience in building face‐processing biases. J Exp Child Psychol 113:372–382. [DOI] [PubMed] [Google Scholar]
- Maurer D, Salapatek P (1976): Developmental changes in the scanning of faces by young infants. Child Dev 47:523–527. [PubMed] [Google Scholar]
- Maurer D, O'Craven KM, Le Grand R, Mondloch CJ, Springer MV, Lewis TL, Grady CL (2007): Neural correlates of processing facial identity based on features versus their spacing. Neuropsychologia 45:1438–1451. [DOI] [PubMed] [Google Scholar]
- McKone E, Crookes K, Jeffery L, Dilks DD (2012): A critical review of the development of face recognition: Experience is less important than previously believed. Cogn Neuropsychol 29:174–212. [DOI] [PubMed] [Google Scholar]
- Mondloch CJ, Le Grand R, Maurer D (2002): Configural face processing develops more slowly than featural face processing. Perception 31:553–566. [DOI] [PubMed] [Google Scholar]
- Mondloch CJ, Pathman T, Maurer D, Le Grand R, de Schonen S (2007): The composite face effect in six‐year‐old children: Evidence of adult‐like holistic face processing. Vis Cogn 15:564–577. [Google Scholar]
- Nguyen VT, Breakspear M, Cunnington R (2014): Fusing concurrent EEG‐fMRI with dynamic causal modeling: Application to effective connectivity during face perception. Neuroimage 102:60–70. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM (2011): FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011:156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelen MV, Glaser B, Vuilleumier P, Eliez S (2009): Differential development of selectivity for faces and bodies in the fusiform gyrus. Dev Sci 12:F16–F25. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, Michelich CR, Allison T, McCarthy G (2005): Functional anatomy of biological motion perception in posterior temporal cortex: An FMRI study of eye, mouth and hand movements. Cereb Cortex 15:1866–1876. [DOI] [PubMed] [Google Scholar]
- Penny WD, Stephan KE, Mechelli A, Friston KJ (2004): Modelling functional integration: A comparison of structural equation and dynamic causal models. Neuroimage 23 Suppl 1:S264–S274. [DOI] [PubMed] [Google Scholar]
- Pierce K, Redcay E (2008): Fusiform function in children with an autism spectrum disorder is a matter of “who”. Biol Psychiatry 64:552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher D, Walsh V, Yovel G, Duchaine B (2007): TMS evidence for the involvement of the right occipital face area in early face processing. Curr Biol 17:1568–1573. [DOI] [PubMed] [Google Scholar]
- Pitcher D, Walsh V, Duchaine B (2011): The role of the occipital face area in the cortical face perception network. Exp Brain Res 209:481–493. [DOI] [PubMed] [Google Scholar]
- Poldrack RA (2010): Interpreting developmental changes in neuroimaging signals. Hum Brain Mapp 31:872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Fair DA, Schlaggar BL, Petersen SE (2010): The development of human functional brain networks. Neuron 67:735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce A, Allison T, Bentin S, Gore JC, McCarthy G (1998): Temporal cortex activation in humans viewing eye and mouth movements. J Neurosci 18:2188–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce A, Syngeniotis A, Thompson JC, Abbott DF, Wheaton KJ, Castiello U (2003): The human temporal lobe integrates facial form and motion: Evidence from fMRI and ERP studies. Neuroimage 19:861–869. [DOI] [PubMed] [Google Scholar]
- Rossion B (2014): Understanding face perception by means of human electrophysiology. Trends Cogn Sci 18:310–318. [DOI] [PubMed] [Google Scholar]
- Rossion B, Caldara R, Seghier M, Schuller AM, Lazeyras F, Mayer E (2003): A network of occipito‐temporal face‐sensitive areas besides the right middle fusiform gyrus is necessary for normal face processing. Brain 126:2381–2395. [DOI] [PubMed] [Google Scholar]
- Rotshtein P, Henson RN, Treves A, Driver J, Dolan RJ (2005): Morphing Marilyn into Maggie dissociates physical and identity face representations in the brain. Nat Neurosci 8:107–113. [DOI] [PubMed] [Google Scholar]
- Scherf KS, Behrmann M, Humphreys K, Luna B (2007): Visual category‐selectivity for faces, places and objects emerges along different developmental trajectories. Dev Sci 10:F15–F30. [DOI] [PubMed] [Google Scholar]
- Scherf KS, Thomas C, Doyle J, Behrmann M (2014): Emerging structure‐function relations in the developing face processing system. Cereb Cortex 24:2964–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Harrison LM, Kiebel SJ, David O, Penny WD, Friston KJ (2007): Dynamic causal models of neural system dynamics:current state and future extensions. J Biosci 32:129–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Penny WD, Moran RJ, den Ouden HE, Daunizeau J, Friston KJ (2010). Ten simple rules for dynamic causal modeling. Neuroimage 49:3099–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MJ, McCarthy G, Saliba E, Degiovanni E (1999): ERP evidence of developmental changes in processing of faces. Clin Neurophysiol 110:910–915. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Edmonds GE, McCarthy G, Allison T (2001): Eyes first! Eye processing develops before face processing in children. NeuroReport 12:1671–1676. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Mills T, Zhang L, Pang EW (2010): Face Processing in Children: Novel MEG Findings. 17th International Conference on Biomagnetism Advances in Biomagnetism – Biomag 2010. In: Supek S, Sušac A, editors. Berlin: Springer. pp 314–317.
- Tesan G, Johnson BW, Crain S (2012): How the brain responds to any: An MEG study. Brain Lang 120:66–72. [DOI] [PubMed] [Google Scholar]
- Wager TD, Keller MC, Lacey SC, Jonides J (2005): Increased sensitivity in neuroimaging analyses using robust regression. Neuroimage 26:99–113. [DOI] [PubMed] [Google Scholar]
- Want SC, Pascalis O, Coleman M, Blades M (2003): Face facts: Is the development of face recognition in early and middle childhood really so special? In: Pascalis O, Slater A, editors. The Development of Face Processing in Infancy and Early Childhood. New York: Nova Science Publishers; pp 207–221. [Google Scholar]
- Watts DJ, Strogatz SH (1998): Collective dynamics of ‘small‐world’ networks. Nature 393:440–442. [DOI] [PubMed] [Google Scholar]
- Wright DB, Stroud JN (2002): Age differences in lineup identification accuracy: People are better with their own age. Law Hum Behav 26:641–654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Informationarticle.


