Abstract
Ego‐disturbances have been a topic in schizophrenia research since the earliest clinical descriptions of the disorder. Manifesting as a feeling that one's “self,” “ego,” or “I” is disintegrating or that the border between one's self and the external world is dissolving, “ego‐disintegration” or “dissolution” is also an important feature of the psychedelic experience, such as is produced by psilocybin (a compound found in “magic mushrooms”). Fifteen healthy subjects took part in this placebo‐controlled study. Twelve‐minute functional MRI scans were acquired on two occasions: subjects received an intravenous infusion of saline on one occasion (placebo) and 2 mg psilocybin on the other. Twenty‐two visual analogue scale ratings were completed soon after scanning and the first principal component of these, dominated by items referring to “ego‐dissolution”, was used as a primary measure of interest in subsequent analyses. Employing methods of connectivity analysis and graph theory, an association was found between psilocybin‐induced ego‐dissolution and decreased functional connectivity between the medial temporal lobe and high‐level cortical regions. Ego‐dissolution was also associated with a “disintegration” of the salience network and reduced interhemispheric communication. Addressing baseline brain dynamics as a predictor of drug‐response, individuals with lower diversity of executive network nodes were more likely to experience ego‐dissolution under psilocybin. These results implicate MTL‐cortical decoupling, decreased salience network integrity, and reduced inter‐hemispheric communication in psilocybin‐induced ego disturbance and suggest that the maintenance of “self”or “ego,” as a perceptual phenomenon, may rest on the normal functioning of these systems. Hum Brain Mapp 36:3137–3153, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: consciousness, psychedelics, sense of self, ego‐disturbances, ichstörungen, fMRI, connectivity, graph theory, medial temporal lobe, psychosis
INTRODUCTION
The concept of the self has been a topic of debate in philosophy and science since ancient times. It is at the center of some of the most influential theories on the nature of the human mind [Freud, 1924], including theories on the origins of modern human consciousness [Jaynes, 1976].The self is a pivotal feature of normal waking human consciousness, as demonstrated by the prevalence of personal and possessive pronounssuch as “I,” “me,” and “mine” in everyday speech. Indeed, the ubiquity of “self” in waking consciousness invites the assumption that it is a permanent feature of that consciousness. Certain altered states of consciousness reveal the self to be vulnerable however. Examples of states in which the “dissolution” of the self can be observed include: acute psychosis [Bleuler, 1911], temporal lobe epilepsy auras [Schenk and Bear, 1981], and putatively nonpathological states such as spiritual or mystical‐type experiences [Hood, 1975]. Importantly, self‐ or ego‐dissolution can also be experimentally induced by the neurobiological action of so‐called “psychedelic” drugs, such as psilocybin [Griffiths et al., 2011], LSD [Goodman, 2002; Lyvers and Meester, 2012], and dimethyltryptamines or “DMT” [Trichter et al., 2009].
“The self” is perhaps best viewed as an “umbrella” [Fleming et al., 2012] or “constellation” [Seth, 2013] construct that subsumes a broad range of mental phenomena, including: self‐awareness, self‐monitoring, self‐recognition, self‐identity, self‐control, sense of agency and ownership, theory‐of‐mind, subject‐object differentiation, reality‐testing, and even focused attention or goal‐directed cognition [Carhart‐Harris and Friston, 2010]. Indeed, that the self is such a broad construct explains why it is so difficult to define. The term “the self,” in its broadest sense, is synonymous with “the ego” as traditionally described in psychology [Freud, 1924].
Studying a broad range of states associated with altered sense of self (later called “ego‐disturbances” [Schneider, 1959]), Karl Jaspers [Jaspers, 1913] distinguished several domains of ego‐consciousness, which may be useful for understanding the phenomenon that is the focus of the present article. These domains include: (i) ego‐vitality (“awareness of existence”), (ii) ego‐activity (“awareness of one's own performance” or “sense of self‐agency”), (iii) ego‐consistency (“multimodal phenomena being perceived as integrated experience”), (iv) ego‐demarcation (“differentiation of the self from the outside world”), and (v) ego‐identity (“Me” as a narrative or a story that I can tell about myself). These five domains, being incorporated into the ego pathology inventory [Scharfetter, 1981], have been successfully used in studies of psychedelic [Vollenweider et al., 1997] and psychotic [Rohricht and Priebe, 2004] states.
It is also worth mentioning that recent work has made an effort to further conceptualize classic psychodynamic ideas [Freud, 1924] in the context of Bayesian brain and free‐energy minimization principle, describing the self/ego not just as a sensation, but also as a system with functions, such as reality‐testing, subserved by hierarchically higher brain circuits that function to suppress the free‐energy (“prediction error”) of the lower ones [Carhart‐Harris and Friston, 2010]. Thus, an alternative approach to studying the ego might be to focus on specific functions, such as reality‐testing, and to use corresponding behavioral measures [Carhart‐Harris et al., 2014a]. In the present study, however, we have chosen to examine the ego primarily from the phenomenological standpoint, as a complex sensory experience of being “me,” as being coherent in mental acts and distinct from the outside world.
Psilocybin is the prodrug of psilocin, a classic psychedelic substance and an indolealkylamine. Indolealkylamines are relatives of 5‐hydroxytryptamine (5‐HT, also known as serotonin), an endogenous neuromodulator that plays a crucial role in the regulation of mood, sleep and cognition [Canli and Lesch, 2007; Cowen and Sherwood, 2013; Monti et al., 2008]. Most psychedelics are serotonin receptor agonists and although their pharmacology is nonselective, their characteristic psychoactive effects appear to depend on stimulation of the serotonin 2A receptors. Psychedelics can produce experiences that are similar in some respects to those seen in acute psychotic states [Vollenweider et al., 1998; Vollenweider et al., 1999] and their effects are blocked by atypical antipsychotics and 5HT‐2A antagonist, ketanserin [Carter et al., 2007; Vollenweider et al., 1998]. These observations put psilocybin forward as a tool for studying certain aspects of psychotic states [Carhart‐Harris et al., 2013; Vollenweider et al., 1999]. Conceptually, one can think of drug‐induced psychedelic states as “psychotic experiences”, since they often feature severe distortion of perception and ego‐functions. Indeed, serotoninergic psychedelics have been found to alter a broad range of cognitive functions related to the self, including goal‐directed behavior [Kometer et al., 2012], reality‐testing [Carhart‐Harris et al., 2014a], and time perception [Wittmann et al., 2007].
There is very limited knowledge of the neural correlates of the self and its disturbances but there are reasons to think that medial temporal lobe (MTL) circuitry is important. Electrical stimulation of MTL regions has been found to produce visual hallucinatory phenomena similar to those reported under psychedelics [Rangarajan et al., 2014], as well as so‐called “dreamy states” (i.e. sensations of dreaming, déjà vu, and depersonalization‐like experiences) [Bancaud et al., 1994; Bartolomei et al., 2012; Lee et al., 2013]. Exploratory depth recordings in patients administered psychedelics in the mid‐20th century found abnormal activity that was most conspicuous in limbic regions including the MTLs [Monroe and Heath, 1961; Schwarz et al., 1956]. These findings have recently been supported by fMRI work demonstrating increased signal variance/amplitude in the MTLs [Tagliazucchi et al., 2014] and decreased MTL to default‐mode network (DMN) functional connectivity under psilocybin [Carhart‐Harris et al., 2014b]. Interestingly, the former result correlated positively with ratings of the “dreamlike” quality of the experience [Carhart‐Harris and Nutt, 2014]. Rodent fMRI work with a DMT‐related drug found an “activating” effect on the MTLs in the context of a generalized “deactivating” effect on the cortex [Riga et al., 2014]. Depth electrophysiological [Heath and Mickle, 1960; Sem‐Jacobsen et al., 1955; Sherwood, 1962] and later neuroimaging work [Friston et al., 1992; Gordon et al., 1994; Liddle et al., 1992] have also implicated the MTLs in psychotic states, with converging evidence supporting the involvement of parahippocampal regions in schizophrenia and schizophrenia‐like psychotic states [Acioly et al., 2010; Allen and McGuire, 2014; Bodnar et al., 2011; Friston et al., 1992; Prasad et al., 2004]. This has also been supported by in silico simulations demonstrating that disruption of parahippocampal cortex from other MTL regions results in memory deficits that mimic those seen in schizophrenia [Talamini et al., 2005]. Finally, depersonalization (a state of altered self‐awareness at the psychotic level equivalent to ego‐disturbances [Burgy, 2011; Sass et al., 2013]) has been linked to abnormal function of the MTL regions [Lambert et al., 2002; Lemche et al., 2013].
One of the key MTL regions, the parahippocampal cortex (PHC) is a multimodal hub that plays an important role in contextual processing, familiarity detection, memory retrieval, and associative memory [Aminoff et al., 2013]. It is also known as the “gateway to hippocampus” [Watrous et al., 2013] mediating its connectivity with major brain network hubs subserving higher cognition, such as the posterior cingulate (default mode network, DMN), superior parietal (attention/control network), and dorsomedial prefrontal (salience network) areas [Jones and Witter, 2007; Kondo et al., 2005; Ward et al., 2014; Watrous et al., 2013]. Recent work has revealed an anterior‐posterior functional segregation within the PHC, with the anterior part (aPHC) more involved in higher cognition and the posterior part contributing to spatial processing [Aminoff et al., 2013; Baldassano et al., 2013; Weniger and Irle, 2006]. Interestingly, binding of the 5‐HT2A receptors is reduced in this area in patients with schizophrenia [Burnet et al., 1996]. Finally, it is specifically the a PHC that is most closely associated with “dreamy” state experiences [Bartolomei et al., 2012; Guedj et al., 2010; Illman et al., 2012; Spatt, 2002; Takeda et al., 2011; Vignal et al., 2007]. Based on these findings, we predicted that decoupling of the PHC from the neocortex would correlate positively with ego‐dissolution [Carhart‐Harris, 2007; Carhart‐Harris and Friston, 2010; Carhart‐Harris et al., 2014b], consistent with the loss of a contextual “feed” into the ongoing stream of cognition.
Previous attempts to identify neural correlates of the self or ego have tended to focus primarily on the DMN [Carhart‐Harris and Friston, 2010; Qin and Northoff, 2011]. Several studies have found increased activation in the DMN during self‐referential processing [Buckner et al., 2008] and other high‐level cognitive functions related to the construct of “the self,” such as theory‐of‐mind [Li et al., 2014], mental time travel [Ostby et al., 2012], and moral decision‐making [Reniers et al., 2012], clearly implicating the DMN in “ego‐identity” (see above) or the “narrative self.” Our previous fMRI and MEG studies have found decreased cerebral blood flow, functional connectivity, and oscillatory power within the DMN [Carhart‐Harris et al., 2012; Carhart‐Harris et al., 2014b; Muthukumaraswamy et al., 2013] following administration of psilocybin. In addition, alpha desynchronisation in the posterior cingulate cortex (PCC) node of the DMN correlated positively with ratings of psilocybin‐induced ego‐disintegration [Carhart‐Harris et al., 2014b]. Based on these studies, we predicted that ego‐dissolution would correlate positively with altered DMN connectivity (e.g. reduced DMN integrity) and regions belonging to it (e.g. altered PCC diversity) under psilocybin.
The salience network and its component nodes have also been a focus of research of neural correlates of the self [Craig, 2009; Seth, 2013]. Interestingly, this network is very rich in the von Economo neurons that are hypothesized to contribute to the brain mechanisms of self‐awareness, higher cognition, and are specific for socially complex animals like great apes and macaques [Allman et al., 2010; Allman et al., 2011; Cauda et al., 2013; Evrard et al., 2012]. Compromised function of the salience network has previously been linked with empathy deficits in frontotemporal dementia [Seeley, 2008; Seeley, 2010], impaired self‐awareness in patients with traumatic brain injury [Ham et al., 2014] and has also been implicated in psychosis and the aberrant salience model of positive psychotic symptoms [Krishnadas et al., 2014; Manoliu et al., 2014; Palaniyappan and Liddle, 2012; Palaniyappan et al., 2013; Pu et al., 2012; White et al., 2010]. It is worth noting that the functions most strongly associated with the salience network fall under the construct of the “minimal self” comprising ego‐vitality, activity, consistency, and demarcation (see Jaspers’ domains of ego‐consciousness, above), but not under the “narrative” aspect of the self (ego‐identity) which are perhaps more related to the DMN and its interactions with the MTLs.
It has previously been proposed that the psychedelic state offers ideal experimental means of perturbing the self or ego so that it can be studied scientifically [Carhart‐Harris et al., 2014b], as well as to aid studies of some psychotic states [Carhart‐Harris et al., 2013]. Ego‐disturbances have been a central focus of schizophrenia research and its diagnostic criteria since the earliest clinical descriptions of the disorder [Bleuler, 1911; Feinberg, 2011; Fuentenebro and Berrios, 1995; Kircher and David, 2003; Nelson et al., 2012; Sass et al., 2013; Schneider, 1959] and their severity at admission have been demonstrated to substantially influence treatment outcome [Rohricht et al., 2009; Rohricht and Priebe, 2004]. All of the above supports the relevance of psychedelic research for understanding brain dynamics underlying a very broad range of altered states of consciousness, including both drug‐induced and endogenously occurring psychotic states.
In the present article, methods of large‐scale network analysis were employed to test our hypotheses. These methods have previously been used to inform our understanding of brain dynamics underlying higher cognitive functions [Sporns, 2010]; however, to the best of our knowledge, they have never been used to investigate the neural correlates of drug‐induced ego‐dissolution.
METHODS
Design and Participants
This was a within‐subjects, counterbalanced‐order, placebo‐controlled design. The study was approved by an NHS research ethics committee. Fifteen volunteers (13 males, 2 females, aged 32 [±8.9] years) with previous psychedelic experience were scanned on two occasions: (1) receiving saline injection (“placebo,” PCB‐session), 12 min task‐free fMRI scan, eyes closed, and (2) 2 mg psilocybin infusion (“psilocybin,” PSI‐session), midway through 12 min fMRI scan. All subjects underwent health screens prior to enrolment. Inclusion criteria were: age >21 years, no personal or immediate family history of a major psychiatric disorder, drug dependence, cardiovascular disease, and no history of a significant adverse response to a hallucinogen. All of the subjects had used psilocybin at least once before (mean number of uses per subject = 16.4, SD = 27.2) but not within 6 weeks before the study started. A standardized physical examination, including electrocardiogram, blood tests, urine test for drugs of abuse and pregnancy were carried out. Experienced clinicians at Bristol Royal Infirmary conducted a psychiatric assessment, during which the participants disclosed their drug use histories, and medical record was taken. The participants also completed the State Trait Anxiety Inventory and the Beck Depression Inventory.
Subjective Behavioral Measures
Twenty‐two visual analogue scale (VAS) items were completed by the volunteers shortly after they exited the scanner. The complete list of items can be found in the Table 1. All items had a bottom anchor of “no more than usually” (referring to normal waking consciousness) and a top anchor of “much more than usually,” consistent with the format of Dittrich's altered states of consciousness questionnaire [Dittrich, 1998]. Ratings ranged from zero to 100. In order to reduce the dimensionality of these data, Principal Component Analysis (PCA) was performed, yielding a first principal component (i.e. the item “I lost all sense of ego”) which was then used as a subsequent measure of interest (see Table 1 and Fig. 2). For the present analysis, VAS ratings completed at the end of the psilocybin session were used. Pearson correlation coefficients were computed between relevant biological measures and principal component scores. This was done after performing a Shapiro‐Wilk test for normality.
Table 1.
Two‐factor structure of the psychedelic experience
| Subjective ratings | PC1 (“ego‐loss”) | PC2 (“affective valence”) |
|---|---|---|
| I lost all sense of ego | 0.842 | 0.014 |
| My imagination was extremely vivid | 0.841 | −0.282 |
| The experience had a spiritual or mystical quality | 0.811 | 0.161 |
| I experienced a loss of separation from my environment | 0.756 | −0.129 |
| I saw geometric patterns | 0.725 | 0.240 |
| It felt like I was floating | 0.725 | −0.250 |
| I felt like I was merging with my surroundings | 0.717 | 0.526 |
| The experience had a supernatural quality | 0.693 | 0.649 |
| I felt afraid | 0.675 | −0.271 |
| I feared loosing control of my mind | 0.665 | 0.074 |
| My thoughts wandered freely | 0.661 | 0.470 |
| I saw events from my past | 0.637 | 0.049 |
| The experience had dreamlike quality | 0.589 | −0.251 |
| Things looked strange | 0.533 | −0.503 |
| I felt a profound inner peace | 0.463 | 0.476 |
| Sounds influenced things I saw | 0.406 | 0.244 |
| I felt unusual bodily sensations | 0.402 | −0.728 |
| My sense of time was distorted | 0.276 | −0.183 |
| My thinking was muddled | 0.247 | −0.705 |
| My sense of size and space was distorted | 0.189 | −0.193 |
| I felt suspicious and paranoid | 0.186 | −0.346 |
| I felt completely normal | 0.055 | 0.338 |
| Intensity of the experience | 0.74 | −0.189 |
| % Of variance explained (cumulative) | 36.37% | 50.33% |
Values are Pearson correlation coefficients (r) representing associations between first two principal components and corresponding subjective ratings.
36.37% 13.97% of the variance (50.33% cumulative)
Figure 2.
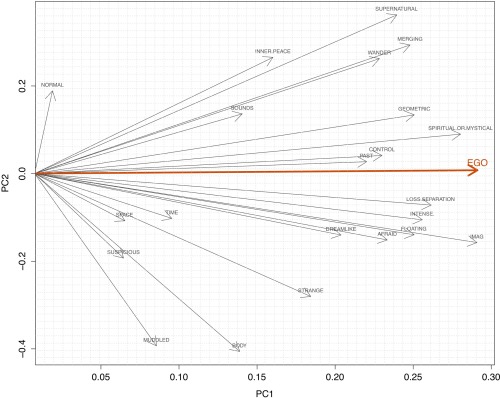
First two principal components extracted from the raw subjective measures. PC 1 (1st Principal Component): ego disintegration phenomena; PC2 (2nd Principal Component): perhaps bestrelates to the “emotional valence” of the experience; Component scores were normalized at the range. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Ethics
The study was approved by a National Health Service research ethics committee. All subjects gave consent to participate in the study after the procedures had been explained in details.
MRI
Image acquisition
MR images were acquired in Cardiff University's Brain Research Imaging Centre on a 3T Siemens Trio Tim MRI scanner (Siemens Healthcare, Erlangen, Germany) using a 32‐channel head coil. Anatomical reference images were acquired using the ADNI‐GO MPRAGE protocol: 1 mm isotropic voxels, TR = 2300 ms, TE = 2.98 ms, 160 sagittal slices, 256 × 256 in‐plane resolution, flip angle = 9 degrees, bandwidth = 240 Hz/pixel, GRAPPA acceleration = 2. Two hundred and forty (240) T2*‐weighted echo planar images were acquired during each session using a standardized sequence: 3 mm isotropic voxels, TR = 3000 ms, TE = 35 ms, field‐of‐view = 192 mm, flip angle = 90°, 53 axial slices per one TR, parallel acceleration factor = 2, 64 × 64 acquisition matrix.
Image preprocessing
For the present analysis, a standardized pipeline combining functions from SPM‐8 (http://www.fil.ion.ucl.ac.uk/spm) and FSL‐5.0 was implemented, largely based on the Data Processing Assistant for Resting‐State fMRI: Advanced Edition (DPARSFA, version 3.0) [Chao‐Gan and Yu‐Feng, 2010], installed on the MATLAB environment [MATLAB, 2013]. The raw EPI images subsequently underwent steps for spatial realignment, slice‐timing correction, linear [Jenkinson et al., 2002], and nonlinear [Andersson et al., 2007] registrations to high‐resolution native and standardized MNI spaces, correspondingly. Spurious variance was reduced by regressing‐out signal from the white matter and cerebrospinal fluid together with Friston's extended 24‐parameter motion correction model that includes current and past position of each of 6 head motion parameters along with their squares [Friston et al., 1996]. Next, the images were band‐pass filtered to eliminate biologically nonrelevant signals [Lowe et al., 1998] and smoothed with 6 mm Gaussian kernel. The resulting low‐frequency fluctuations, extracted using standardized parcellation schemes (see below), were used in the subsequent network analysis [Rubinov and Sporns, 2010].
Parcellation schemes
For the graph analysis, we used Craddock's atlas [Craddock et al., 2012] covering 200 regions‐of‐interest (ROIs). Based on prior hypothesis, 4 ROIs including the left and right anterior parahippocampal (aPHC, MNI coordinates: L [−31 −3 −33], R [36 −11 −27]), posterior cingulate (PCC, MNI coordinates: [1 −37 31]), and retrosplenial cortex (RSC, MNI coordinates: [1 −51 14]) were used to assess changes in diversity coefficients related to ego‐dissolution at the first step of the analysis (hypothesis‐driven part). The results were further replicated employing the extended 600‐ROI version of this atlas. Finally, for the analysis of within‐network integrity, Craddock's 200‐ROI parcellation was masked by each of the 7 networks from the Yeo's scheme [Yeo et al., 2011] in order to calculate within‐network mean clustering coefficients (one per each network).
After the preprocessing had been finished, the “psilocybin” data was split into 2 subsets: 5‐min prepsilocybin and 5‐min postpsilocybin.
Outlier detection
Outlier detection was done by a visual assessment of the first two principal component scores extracted from the subjective measures and imaging data (functional connectivity matrices) separately. No outliers were found.
Analysis of head motion
In order to further rule‐out possible contribution of motion‐related artifacts to our results, we also performed analysis of head movements (6‐parameter model), comparing averaged standard deviation and first principal component scores extracted from the motion time‐series: 1–prepsilocybin vs postpsilocybin, 2–placebo vs post‐psilocybin, as well as their associations with our measure of interest: “ego‐loss” composite score: first principal component extracted from the subjective measures (see Results). Among all comparisons, only one was significant: mean SD of head motion was slightly higher in the postpsilocybin as compared to prepsilocybin state (puncorr = 0.015). Head motion (SD, first PC) did not demonstrate significant impact on any of the network measures extracted from the motion‐corrected data. Likewise, “ego‐dissolution” scores did not correlate with motion or motion differences.
Assessment of temporal signal‐to‐noise ratio (tSNR)
To rule out the possibility that tSNR could account for the differences between conditions, a corresponding set analyses was performed measuring mean BOLD signal from a ROI divided by its standard deviation. Critically, neither of the SNR measures (for PCB, prepsilocybin, postpsilocybin conditions), nor their differences, correlated with ego‐dissolution phenomena. For graph metrics measured, only changes in tSNR and diversity coefficients of the RSC demonstrated strong negative relationship (r = −0.71, P < 0.005) (for the filtered data only), meaning that tSNR reductions (presumably associated with reduced activity) in RSC were associated with increases in the diversity of its connections.
Measure preparation
Adjacency matrices were constructed using Pearson correlation that represented functional connectivity (edges) between 200 ROI (nodes) as defined in the Craddock's parcellation scheme [Craddock et al., 2012].
Extraction of graph measures was carried out using Brain Connectivity Toolbox (BCT, http://www.brain-connectivity-toolbox.net) [Rubinov and Sporns, 2010].
Community structure and diversity coefficients
At the first stage, community structure was estimated using placebo data employing fine‐tune modularity algorithm [Rubinov and Sporns, 2011]. Five communities were estimated from the placebo data and labeled according to their localization and known functions: 1–Visual, 2–Somatosensory+Salience, 3–“Higher Cognition” community, 4–Control Network, 5–Subcortical/Temporal (See Fig. 1). Then, diversity coefficient was calculated for all 200 ROIs for the pre‐ and postpsilocybin‐infusion datasets as well as the entire placebo dataset. Diversity coefficient is a Shannon entropy‐based measure, defining how a particular ROI is connected to different communities (how “diverse” a particular region is in its connections). When the diversity coefficient is high, it implies more diffuse connectivity and when it is low it implies more restricted connectivity. For more detailed explanations and formal definition, see Supporting Information. The relationship between the diversity coefficients for the selected ROIs (i.e. the aPHC and PCC) and “ego‐dissolution” were analyzed first, followed by an exploratory analyses using all 200 ROIs (with correction for multiple tests using the false discovery rate, FDR). Diversity coefficients for “post‐psilocybin”, “pre‐psilocybin” vs. “post‐psilocybin” (difference between two states) and “pre‐psilocybin” were calculated. In addition, the last two parts of the analyses were repeated using placebo data instead of pre‐psilocybin in order to test the robustness of the findings.
Figure 1.
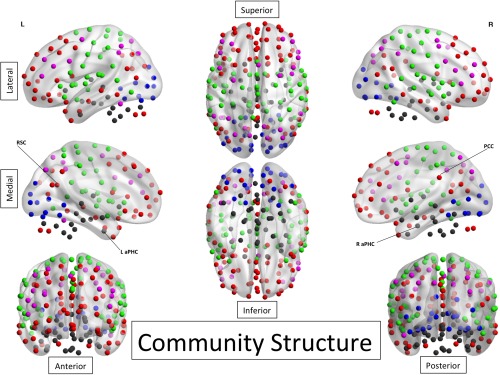
Communities (estimated for 12‐min placebo scan). L/R aPHC–Left/Right anterior Parahippocampal Cortex; PCC–Posterior Cingulate Cortex (midline region); RSC–Retrosplenial Cortex (midline region); Colors (communities): Blue–Visual. Green–Somatosensory+Salience. Red–DMN + Prefrontal [“Higher Cognition”]. Purple–Control Dark Gray–Subcortical/MTL. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Within‐network clustering coefficient
Within‐network integrity was assessed by calculating mean clustering coefficient for each of 7 networks from the Yeo's atlas. Next, intensity of the ego‐dissolution phenomena was analyzed as a function of mean clustering coefficient for each network in the same order as for other analyses (1–post‐psilocybin, 2–pre‐psilocybin vs. post‐psilocybin, 3–post‐psilocybin).
Hypothesis‐free analysis of the between‐ROI functional connectivity
For this part, the analysis of ego‐dissolution phenomena was conducted by looking at bivariate correlations between 200 ROIs, as implemented in the Network Based Statistics toolbox (NBS: https://sites.google.com/site/bctnet/comparison/nbs). This method employs nonparametric framework (permutation testing for each graph component clustered in a topological space) to control for the family‐wise error (FWE) rate.
Statistical analysis
Data analysis was conducted using R programming language, version 3.1(13) [R Core Team, 2014] and NBS toolbox [Zalesky et al., 2010] with linear modeling and correction for multiple comparisons (FDR and FWE, correspondingly).
Visualization
Results visualization was carried out using BrainNetviewer [Xia et al., 2013], version 1.43.
RESULTS
Subjective Behavioral Measures
The first principal component explained 36.37% of the data variance and was strongly associated with ego‐dissolution phenomena (see Table 1 and Fig. 2) measured by a VAS that read: “I lost all sense of ego” (r = 0.82). The first principal component also showed associations with other VAS items related to ego‐dissolution, such as: “I experienced a loss of separation from my surroundings” (r = 0.76) and “It felt like I was merging with my environment” (r = 0.72) and, interestingly, with “the experience had a spiritual/mystical quality” (r = 0.81) and “the experience had a supernatural quality” (r = 0.69) (see Table 1 and Fig. 2). Values for the first component were scaled and became our primary measure of interest.
The second component explained 13.97% of the variance (50.33% cumulative) and may be related to the emotional valence of the psychedelic experience. Higher scores in the second component were positively associated with “the experience had a spiritual/mystical quality” (0.65) which can be construed as being emotionally positive, whereas negative scores were associated with “my thinking was muddled” (r = −0.7), which relates to the psychotic symptom of thought‐disorder and “I experienced unusual bodily sensations” (r = −0.73).
Diversity Coefficients
As described above, this analysis included a hypothesis‐driven and whole‐brain, exploratory part. The hypothesis‐driven analysis revealed a strong negative correlation between the intensity of the ego‐dissolution phenomena and the diversity of the aPHC connections (See Table 2 and Fig. 3A), meaning that experiencing ego‐dissolution was associated with reductions in functional connectivity or “communication” between the aPHC and the (mostly) cortical communities under the drug. To test the robustness of this result, we also looked at the ROI‐based connectivity between the hippocampal formation and major brain networks (see Supporting Information) and an association between ego‐loss and reduced MTL‐cortical connectivity was confirmed using this approach.
Table 2.
Ego‐dissolution phenomena and diversity coefficients
| Region (ROI #) | postPSI | prePSI –postPSI | prePSI | PCB | postPSI‐PCB |
|---|---|---|---|---|---|
| R aPHC (87) | −0.76 (<0.001)**** | 0.53 (0.04)* | −0.47 (0.08)‘ | 0.01 (0.96) | 0.6 (0.017)* |
| L aPHC (27) | −0.69 (0.004)*** | 0.62 (0.01)** | −0.21 (0.44) | 0 (1) | 0.64 (0.01)** |
| RSC (58) | 0.28 (0.31) | −0.41 (0.13) | −0.04 (0.88) | 0 (1) | −0.4 (0.14) |
| PCC (46) | −0.17 (0.53) | −0.2 (0.47) | −0.34 (0.21) | −0.17 (0.54) | −0.04 (0.89) |
Pearson r (P‐value)
‘P < 0.1; *–P < 0.05; **–P < 0.01; ***P < 0.005; ****– P < 0.001
R/L aPHC–Right/Left Anterior Parahippocampal Cortex
RSC–Retrosplenial Cortex
PCC–Posterior Cingulate Cortex
prePSI–prepsilocybin; postPSI–postpsilocybin; PCB–placebo
Figure 3.
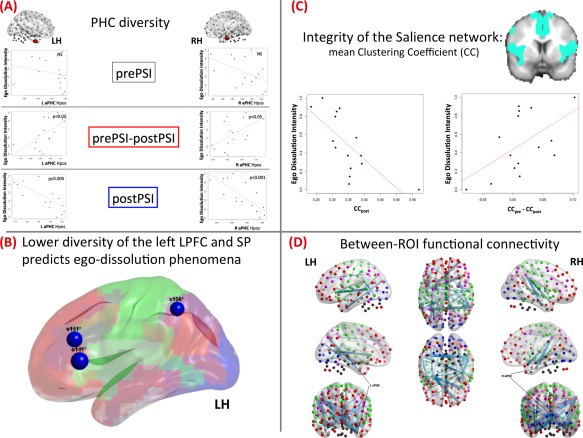
Neural correlates of psilocybin‐induced ego‐dissolution. (A) Ego‐dissolution correlates with decreased diversity of the anterior parahippocampal (aPHC) connections under psilocybin; (B) Lower diversity of the Left Dorsolateral Prefrontal (regions 141 and 151 in Craddock's atlas) and superior parietal (region 156) regions at baseline predicted ego‐disintegration phenomena under the drug. (C) Decreased integrity of the salience network correlates with ego‐dissolution: scatter plots (LEFT: after the psilocybin administration, RIGHT: difference between baseline and psilocybin‐induced states); results were significant at P < 0.05 (FDR‐corrected); brain template is color‐coded according to the baseline community structure (see Methods). (D) Ego‐dissolution is associated with psilocybin‐induced connectivity reductions between specific nodes, e.g. those crossing the two hemispheres and particularly those including medial temporal areas. Results were significant at P < 0.05 (FWE‐corrected); nodes are color‐coded according to the community structure (see Methods). Blue–Visual; Green–Somatosensory + Salience; Red–DMN + Prefrontal; Pink–Control; Dark Gray–Subcortical/MTL. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
It was somewhat surprising not to find any associations between the posteriomedial cortex (i.e. the PCC and the RSC) and ego‐dissolution, given that previous work has supported this. However, there was a strong association between the second principal component that related to emotional valence of the experience (see Table 1 and Fig. 2) and posteriomedial (retrosplenial) cortex diversity (See Table 3), for both conditions: before and after the psilocybin administration. Specifically, larger diversity of the retrosplenial area was associated with an increased likelihood of rather unpleasant experiences like muddled thinking, unusual bodily sensations and paranoid ideas.
Table 3.
Second principal component (relating to emotional valence of the experience) and diversity coefficients
| Region (ROI #) | postPSI | prePSI‐postPSI | prePSI | PCB | PCB‐postPSI |
|---|---|---|---|---|---|
| RSC (58) | −0.7 (0.003)*** | −0.2 (0.46) | −0.78 (<0.001)**** | −0.72 (0.002)*** | 0.22 (0.43) |
| PCC (46) | −0.3 (0.29) | −0.18 (0.51) | −0.43 (0.11) | −0.61 (0.02)* | −0.45 (0.09)a |
Pearson r (P‐value)
‘–P < 0.1; *–P < 0.05; **–P < 0.01; ***P < 0.005; ****– P < 0.001
RSC–Retrosplenial Cortex
PCC–Posterior Cingulate Cortex
prePSI–prepsilocybin; postPSI–post‐psilocybin; PCB–placebo
Diversity Coefficients: Hypothesis‐Free Part
In the hypothesis‐free diversity coefficient analyses, only the baseline state (prepsilocybin) revealed regions where there were significant negative associations with ego‐dissolution that survived adjustment for multiple tests (see Fig. 3B): left inferior frontal gyrus (belonging to the “higher cognition” community), left middle frontal gyrus and superior parietal lobule (“control network”). This means that lower diversity of the left frontoparietal regions (at baseline) was predictive of a higher likelihood of experiencing ego‐dissolution under the psilocybin.
Within‐Network Integrity
Analysis of mean clustering coefficient revealed that ego‐dissolution was strongly associated with disintegration of the salience network (See Table 4 and Fig. 3C). This finding was also supported when assessing differences between the placebo and postpsilocybin states.
Table 4.
Ego‐dissolution phenomena and within‐network integrity (mean clustering coefficients)
| r (pfdr) | |||||
|---|---|---|---|---|---|
| NWK | postPSI | prePSI‐postPSI | prePSI | PCB | PCB‐postPSI |
| VIS: | −0.27 (ns) | −0.23 (ns) | – 0.45 (ns) | −0.23 (ns) | 0.1 (ns) |
| SM: | −0.52 (0.09)a | 0.11 (ns) | −0.33 (ns) | 0.04 (ns) | 0.56 (0.1)a |
| FP: | −0.54 (0.09)a | 0.14 (ns) | −0.45 (ns) | −0.31 (ns) | 0.4 (ns) |
| SAL: | −0.72 (<0.05)a | 0.55 (0.23)a | −0.55 (0.16) | −0.21 (ns) | 0.77 (<0.01)a |
| OFC: | −0.14 (ns) | 0.12 (ns) | −0.07 (ns) | −0.39 (ns) | −0.17 (ns) |
| DLPFC: | −0.56 (0.09)a | 0.3 (ns) | −0.52 (0.16)a | −0.5 (ns) | 0.46 (ns) |
| DMN: | −0.45 (ns) | 0.36 (ns) | −0.18 (ns) | −0.39 (ns) | 0.28 (ns) |
Pearson r (P‐value)
VIS–Visual; SM–Sensorimotor; FP–Frontoparietal; SAL–Salience; OFC–Orbitofrontal;
DLPFC–Dorsolateral Prefrontal; DMN–Default Mode;
*– P < 0.05; **– P < 0.01 [FDR‐corrected]; °– P corr > 0.05 & P uncorr < 0.05
ns–nonsignificant
prePSI–pre‐psilocybin; postPSI–post‐psilocybin; PCB–placebo
Functional Connectivity: Whole‐Brain Analysis
Analyzing functional connectivity in a whole‐brain, hypothesis‐free manner, we found that experiencing ego‐dissolution was associated with reduced interhemispheric interplay and disconnection of the MTL areas from parietal lobes (See Fig. 3D). This analysis was repeated after excluding negative edges, and the resulting pattern was still the same.
DISCUSSION
The principal aim of the study was to identify changes in brain network properties related to psilocybin‐induced ego‐dissolution. As predicted, results revealed that this experience was associated with a disrupted interplay between the MTL and the neocortex. Analysis of within‐network integrity revealed an association between decreased salience network integrity and ego‐loss phenomena.
The brain network most often associated with self‐consciousness is the DMN [Qin and Northoff, 2011], which is known to be engaged in a broad range of functions that fall under the construct of the ego/self (see introduction) [Buckner et al., 2008; Li et al., 2014; Ostby et al., 2012; Reniers et al., 2012]. The PHC is particularly thought to mediate cross‐talk between the DMN and the hippocampus [Ward et al., 2014], and the disconnection between this region and the major brain networks seen in our study is broadly consistent with the previously hypothesized association between MTL‐DMN decoupling and ego‐dissolution [Carhart‐Harris, 2007; Carhart‐Harris and Friston, 2010; Carhart‐Harris et al., 2014b].
However, we did not find disintegration of the DMN to be associated with ego‐dissolution in the present analysis. Rather, the relationship between MTL‐cortex decoupling and ego‐dissolution was more generalized than predicted. In the present study, higher diversity of the posterior DMN nodes (both at baseline and under the drug) was associated with increased likelihood of unpleasant, psychosis‐like experiences like muddled thinking, unusual bodily sensations, and paranoid ideas. Reconciling the present results with previous ones, it is possible that the DMN is more related to the “narrative self” (ego‐identity), whereas the dynamics of the salience network may promote other aspects of self‐consciousness falling under construct of the “minimal” [Limanowski and Blankenburg, 2013] or “embodied self” [Seth, 2013] contributing to active inference and reality testing. Indeed, one of the most intriguing results of the present set of analyses was the finding that disintegration of the salience network related to ego‐dissolution.
The salience network [Seeley et al., 2007] and its component regions (e.g. the anterior insula and dorsal anterior cingulate cortex) have previously been linked with reasoning under uncertainty [Donoso et al., 2014; Singer et al., 2009], mental effort [Engstrom et al., 2013], self‐agency [Farrer and Frith, 2002], time perception [Craig, 2009], and salience evaluation [Menon and Uddin, 2010]. Atrophy in regions of the salience network such as is seen in frontotemporal dementia, has been linked to reduced empathy, self‐awareness, and self‐control [Seeley, 2008; Seeley, 2010]. Electrical stimulation of the dorsal anterior cingulate region has recently been found to induce feelings of an impending challenge that must be overcome, described as a “will to persevere”[Parvizi et al., 2013]. Notably, the salience network and its components have also been put forward as potential neural correlates of self‐consciousness and the “embodied self” in particular [Craig, 2009; Seth, 2013]. Compromised connectivity within this network has previously been linked with impaired self‐awareness in patients with traumatic brain injury [Ham et al., 2014]. The salience network and its components receive a heavy dopaminergic innervations [Haber, 2011; Tian et al., 2013] and are believed to play a role in modulating the switching between the default‐mode and executive networks [Uddin, 2014]. In Parkinson's disease, patients on dopaminergic medication showed an enhanced action‐effect binding (a measure of agency) relative to their own performance when off medication [Moore et al., 2010]. Furthermore, some pro‐dopaminergic stimulants are known to counteract effort‐induced depletion of regulatory control (also known as “ego depletion”) [Sripada et al., 2014], which is generally consistent with the notion that stimulants provide an “ego‐reinforcing” effect. The salience network has also been linked to perseverative behaviors including addiction [Goldstein and Volkow, 2011; Goldstein et al., 2010; Sutherland et al., 2012]. Damage to the insula has been associated with reduced craving [Naqvi et al., 2007] and fMRI studies have implicated impaired functioning of the salience network in addiction both at rest [Lerman et al., 2014] and during the performance of cognitively demanding tasks [Goldstein and Volkow, 2011; Goldstein et al., 2010]. Pro‐dopaminergic medication has been found to normalize these abnormalities and to reduce impulsivity [Goldstein and Volkow, 2011]. Interestingly, there is historical and renascent support for the use of psychedelic drugs in the treatment of addiction [Krebs and Johansen, 2012] with high‐dose sessions intended to promote ego‐dissolution being the model of choice in early studies [Grof et al., 1977]. Future research may focus on the relationship between ego‐dissolution and psychedelics’ putative therapeutic action. Thus, it has already been suggested that ego‐dissolution, as an important aspect of mystical experience [James, 1902; Maclean et al., 2012], is specifically relevant for positive therapeutic outcomes [Grof et al., 1977; Grof et al., 2008; MacLean et al., 2011].
Another intriguing aspect of the present results was the association between reduced interhemispheric connectivity and ego‐dissolution. Coherent functioning between the hemispheres has previously been proposed to contribute to the maintenance of a sense of self, particularly highlighting its contribution to the sense of agency and awareness of the body parts [Uddin, 2011]. “Split‐brain” patients show largely preserved self‐awareness; however, “ownership” and agency are sometimes compromised in these individuals [Uddin, 2011]. An fMRI study of a patient who had undergone a complete commissurotomy found relatively well preserved interhemispheric connectivity postoperation, presumably due to preserved subcortical‐cortical connections [Uddin et al., 2008]. It is noteworthy that many of the connections found to relate to ego‐dissolution in the present analysis included a MTL region. We might infer from this that interhemispheric connections involving the MTLs are particularly important for the maintenance of a coherent sense of self.
The mind‐altering effects of psychedelic drugs are known to vary considerably between individuals and efforts to determine predictors of drug‐response have, to our knowledge, been limited to psychological measures [Carhart‐Harris et al., 2014a; Studerus et al., 2012]. The present analyses are novel therefore, since they examined potential neurobiological predictors of response by looking at network metrics in the baseline data (placebo and pre‐psilocybin states). In a previous behavioral study, sensitivity to the suggestibility‐enhancing effects of LSD was best predicted by baseline “conscientiousness” [Carhart‐Harris et al., 2014a], that is, higher baseline conscientiousness predicted higher suggestibility under the drug. This finding was interpreted as implying that those who are normally most resistant to suggestion (i.e. highly conscientious individuals) are more sensitive to the (suggestibility‐enhancing) effects of psychedelics. The present results may be interpreted in a similar way, since individuals who displayed a low diversity of connections in executive regions (consistent with efficient network segregation–which is known to be reversed by psilocybin [Carhart‐Harris et al., 2013; Roseman et al., 2014], were the most likely to report ego‐dissolution under the drug. These results are broadly supportive of the idea that efficient network segregation is related to firm ego‐boundaries and that those who possess especially firm boundaries at baseline are more sensitive to the (ego‐dissolving) effects of psychedelics.
The present results can be interpreted from a Bayesian [Doya et al., 2011] and free‐energy minimization [Carhart‐Harris and Friston, 2010; Friston, 2010] perspectives. According to this view, the brain is seen as an inference machine making predictions about the world [von Helmholtz, 1866 2005] and updating them based on experience and more specifically “surprise” [Friston, 2010; Friston et al., 2013]. From this perspective, any mental act, including perception, is analogous to a scientific experiment: an inference is made based on prior knowledge and then modified or updated based on what is experienced [Fletcher and Frith, 2009]. According to our observations, psychedelics alter these core functions of the brain with the effect of diminishing confidence or “certainty” about perceived phenomena. It is intriguing to speculate about the mechanics underlying this phenomenon and the present results implicate a role for decreased PHC‐neocortex communication and/or decreased salience network integrity. Since the Bayesian model of brain function rests on hierarchical processing, further work is required to understand the relationships between the networks implicated in the present results, particularly focusing on the hierarchical relations between the default mode, salience and control networks and their roles in higher cognition [Buckner, 2013; Seth, 2013].
IMPLICATIONS FOR PSYCHOSIS RESEARCH
Similarities between early acute psychotic and psychedelic states have been highlighted by many authors [Bercel et al., 1956; Carhart‐Harris et al., 2013; Grof et al., 2008; Spitzer et al., 1996; Vollenweider and Geyer, 2001; Vollenweider et al., 1998] and psychedelics offer a useful means of studying brain mechanisms underlying various psychotic phenomena including ego‐disturbances. Broadly speaking, many phenomena occurring during psychedelic states can be considered “psychotic” in some sense and it is logical to infer that the changes in brain dynamics observed here with psilocybin may also arise spontaneously in nondrug‐induced psychoses, especially in those states where “ego‐disturbances” are a core feature [Bleuler, 1911; Bleuler, 1934; Jaspers, 1913].
In the present study, the first principal component yielded from the subjective measures of psychedelic experience was strongly associated with ego‐dissolution phenomena. This component also favored “productive” features of the psychedelic experience and was relatively independent from the emotional valence of the experience. This structure of psilocybin‐induced psychedelic experience observed in our study replicates results from previous exploratory factor analysis of two independent samples [Maclean et al., 2012]. Similarly, in a meta‐analysis conducted by Studerus et al. [Studerus et al., 2010], items related to ego‐dissolution (such as “feeling of unity,” “burring of boundaries between the self and surroundings,” “disappearance of time”) were strongly correlated with each other and, at the same time, were relatively independent from negative affect and psychosis‐like features of the experience (like catatonic symptoms, paranoia, ideas of “alien control,” etc.). The authors particularly conclude that under certain assumptions, altered states of consciousness can be conventionally differentiated based on whether they describe pleasant or unpleasant phenomena related to ego‐disturbances.
Remarkably, a similar structure of psychotic experience in schizophrenia has been reported previously with a “loss‐of‐boundary” component being related to the majority of productive phenomena, and persecutory (“paranoid”) negatively charged delusions being relatively independent from other symptoms [Bakhshaie et al., 2011; Minas et al., 1994]. As noted by Studerus et al., the appropriate factor structure describing altered states of consciousness particularly depends on its specific use [Studerus et al., 2010]. It is argued here that a two‐factor model may be of interest for psychiatric research, particularly when drawing parallels between drug‐induced and endogenously occurring ego‐disturbances.
It is important to highlight that higher scores on the first component were not explicitly associated with the presence of frightening aspects of the experience (more often, but not always, seen in acute endogenous psychotic states). Rather, it was negative loadings on the second component that were associated with negative aspects of the psychedelic experience: specifically, this component was associated with low scores on “muddled thinking”, “suspiciousness”, “strangeness” and “unusual bodily sensations”, and high scores on items pertaining to positive “spiritual” aspects of the experience (see Table 1 and Fig. 2). This suggests that ego‐dissolution per se is not an intrinsically unpleasant and/or pathological experience, and may in fact even be therapeutically useful [MacLean et al., 2011]. This, however, does not prevent us from drawing parallels with endogenous psychotic states, which may also manifest with positively charged and even mystical/spiritual content of the experiences [Buckley, 1981; Copolov et al., 2004]. This matter touches on a contentious and long‐running debate (whether or not spiritual experiences should be pathologised) that is beyond the remit of the present paper. Of note, unlike psychedelic states, acute endogenous psychosis has been demonstrated to manifest with increased activity and connectivity of the key DMN nodes, which, in turn, is associated with positive symptoms of schizophrenia [Buckner, 2013]. One of the principal distinguishing features between drug‐induced ego‐dissolution and endogenously occurring ego‐disturbances may be that the former is anticipated and even welcomed, whereas the latter is not. When ego‐dissolution is expected it is less likely to be met with distress and resistance. Related to this, since all of the participants in the present study had previous experience with psychedelics, further research directly comparing phenomenology of psychedelic states in experienced and inexperienced subjects is needed.
In the present study, we did not find effects of ego‐loss on the diversity of the posteriomedial cortex. Instead, it was the second component (“emotional valence” of the experience) that demonstrated strong associations, so that unpleasant “content” of the experience was associated with higher functional diversity of the posterior DMN node, RSC. Our results therefore support the idea that ego‐disturbances might be the sine qua non of a broad range of drug‐induced and endogenously occurring psychotic states arising from disintegration of the salience network and decoupling of the hippocampal formation from the major neocortical communities. Taking a Bayesian perspective, disturbance of ego‐functions (like reality‐testing) results in impaired inference about the world. This may result from a breakdown in the brain's normal hierarchical organization, perhaps forcing perception to rely more heavily on priors, which, in turn, may have “negative” or “positive” affective valence. This idea is also nicely in line with the so‐called “set and setting” concept [Zinberg, 1984], according to which previous experiences and personality of a patient taking psychedelic drug, together with the environment of the session, are two critical factors influencing the content of the psychedelic experience. Further work is required to determine mechanisms underlying these effects; however, based on the present results, it is reasonable to speculate that interplay between the DMN (incl., MTL regions) and the salience network may be involved.
LIMITATIONS
Several methodological aspects of the present work have to be addressed. One important limitation is the relatively small sample size. Therefore, caution is advised when generalizing our findings to broader populations, especially to people without previous psychedelic experience and psychosis‐prone individuals.
Unlike most of the previous studies analyzing direct effects of psychedelics on brain dynamics, the present analyses were specifically focused on those aspects of the drug experience that were the most variable among subjects. To do this, we extracted a composite score based on PCA of the 22 VAS items. PCA is a useful data‐reduction technique that identifies main components (within a multivariate dataset) that account for the largest proportion of the variance within the data. The first principal component typically identifies a subset of variables that are correlated with each other but also contain high between‐subject variance. Items related to ego‐dissolution fitted these criteria better than any of the others. It should be made clear that the results of the PCA did not imply that ego‐dissolution is the most marked or reliable effect of the drug; rather, it told us that it is the most variable among subjects and is closely related to many “productive” features of the experience. One interpretation of an item or variable that shows high between‐subject variance is that it is “noisy,” and when the variable is subjective, this might be because different individuals interpret it differently. This interpretation of the present PCA result is contradicted however by the high correlations between three items pertaining to ego‐dissolution. This suggests that the ego‐dissolution construct has convergent validity and so is measuring a meaningful aspect of the subjective experience.
It must be conceded however, that the construct of ego‐dissolution requires much more work to develop its validity. Moreover, characterizing various aspects of Ich‐störungen was not possible with the measures employed in the present study and the extracted composite score may describe several phenomenologically and physiologically related processes (e.g. disturbances of both the “narrative” and the “embodied” self). The use of multiple measures (e.g. subjective and behavioral), evaluating different domains and aspects of ego‐disturbances in the same studies is therefore advised. Thus, efforts should be made to demonstrate the convergent and divergent validity of the ego‐dissolution construct in various endogenously occurring and drug‐induced altered states of consciousness. Assessments tools like the Ego Pathology Inventory assessing different aspects of ego‐consciousness could be useful in this respect [Scharfetter, 1981].
CONCLUSIONS
The present results implicate MTL‐cortical decoupling, decreased salience network integrity, and reduced inter‐hemispheric communication in psilocybin‐induced ego disturbance and suggest that the maintenance of “self” or “ego,” as a perceptual phenomenon, may rest on the normal functioning of these systems. Future work is required to standardize evaluation of ego dissolution and ego‐disturbances more generally in order to investigate their similarities and differences among different altered states of consciousness. In this way, we also hope to develop our understanding of the “self” or “ego”, its neural correlates, and how it can be compromised in different conditions.
Supporting information
Supporting Information
Conflict of Interest: The authors have no conflicts of interest, which may influence the results.
REFERENCES
- Acioly MA, Carvalho CH, Tatagiba M, Gharabaghi A (2010): The parahippocampal gyrus as a multimodal association area in psychosis. J Clin Neurosci 17:1603–1605. [DOI] [PubMed] [Google Scholar]
- Allen P, McGuire PK (2014): Parahippocampal hypoactivation and vulnerability to schizophrenia. JAMA Psychiatry 71:1300–1301. [DOI] [PubMed] [Google Scholar]
- Allman JM, Tetreault NA, Hakeem AY, Manaye KF, Semendeferi K, Erwin JM, Park S, Goubert V, Hof PR (2010): The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Struct Funct 214:495–517. [DOI] [PubMed] [Google Scholar]
- Allman JM, Tetreault NA, Hakeem AY, Manaye KF, Semendeferi K, Erwin JM, Park S, Goubert V, Hof PR (2011): The von Economo neurons in the frontoinsular and anterior cingulate cortex. Ann N Y Acad Sci 1225:59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminoff EM, Kveraga K, Bar M (2013): The role of the parahippocampal cortex in cognition. Trends Cogn Sci 17:379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith. SM (2007): Non‐linear registration, aka Spatial normalisation. FMRIB Technical Report TR07JA2. Oxford, UK: FMRIB Centre.
- Bakhshaie J, Sharifi V, Amini J (2011): Exploratory factor analysis of SCL90‐R symptoms relevant to psychosis. Iran J Psychiatr 6:128–132. [PMC free article] [PubMed] [Google Scholar]
- Baldassano C, Beck DM, Fei‐Fei L (2013): Differential connectivity within the Parahippocampal Place Area. Neuroimage 75:228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancaud J, Brunet‐Bourgin F, Chauvel P, Halgren E (1994): Anatomical origin of deja vu and vivid 'memories' in human temporal lobe epilepsy. Brain 117 (Pt 1):71–90. [DOI] [PubMed] [Google Scholar]
- Bartolomei F, Barbeau EJ, Nguyen T, McGonigal A, Regis J, Chauvel P, Wendling F (2012): Rhinal‐hippocampal interactions during deja vu. Clin Neurophysiol 123:489–495. [DOI] [PubMed] [Google Scholar]
- Bercel NA, Travis LE, Olinger LB, Dreikurs E (1956): Model psychoses induced by LSD‐25 in normals. II. Rorschach test findings. AMA Arch Neurol Psychiatry 75:612–618. [DOI] [PubMed] [Google Scholar]
- Bleuler E (1911). Dementia Praecox or the Group of Schizophrenias. Intl Universities Pr Inc, 1968. 548 p. [Google Scholar]
- Bleuler E (1934). Textbook of Psychiatry: English Translation by A.A. Brill. Literary Licensing, LLC; (July 20, 2013). [Google Scholar]
- Bodnar M, Harvey PO, Malla AK, Joober R, Lepage M (2011): The parahippocampal gyrus as a neural marker of early remission in first‐episode psychosis: A voxel‐based morphometry study. Clin Schizophr Relat Psychoses 4:217–228. [DOI] [PubMed] [Google Scholar]
- Buckley P (1981): Mystical experience and schizophrenia. Schizophr Bull 7:516–521. [DOI] [PubMed] [Google Scholar]
- Buckner RL (2013): The brain's default network: Origins and implications for the study of psychosis. Dialogues Clin Neurosci 15:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL (2008): The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Burgy M (2011): Ego disturbances in the sense of Kurt Schneider: Historical and phenomenological aspects. Psychopathology 44:320–328. [DOI] [PubMed] [Google Scholar]
- Burnet PW, Eastwood SL, Harrison PJ (1996): 5‐HT1A and 5‐HT2A receptor mRNAs and binding site densities are differentially altered in schizophrenia. Neuropsychopharmacology 15:442–455. [DOI] [PubMed] [Google Scholar]
- Canli T, Lesch KP (2007): Long story short: The serotonin transporter in emotion regulation and social cognition. Nat Neurosci 10:1103–1109. [DOI] [PubMed] [Google Scholar]
- Carhart‐Harris R (2007): Waves of the unconscious: The neurophysiology of dreamlike phenomena and its implications for the psychodynamic model of the mind. Neuro‐Psychoanalysis 9:183–211. [Google Scholar]
- Carhart‐Harris RL, Friston KJ (2010): The default‐mode, ego‐functions and free‐energy: A neurobiological account of Freudian ideas. Brain 133(Pt 4):1265–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart‐Harris R, Nutt D (2014): Was it a vision or a waking dream? Front Psychol 5:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart‐Harris RL, Erritzoe D, Williams T, Stone JM, Reed LJ, Colasanti A, Tyacke RJ, Leech R, Malizia AL, Murphy K, Hobden P, Evans J, Feilding A, Wise RG, Nutt DJ (2012): Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci USA 109:2138–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart‐Harris RL, Leech R, Erritzoe D, Williams TM, Stone JM, Evans J, Sharp DJ, Feilding A, Wise RG, Nutt DJ (2013): Functional connectivity measures after psilocybin inform a novel hypothesis of early psychosis. Schizophr Bull 39:1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart‐Harris RL, Kaelen M, Whalley MG, Bolstridge M, Feilding A, Nutt DJ (2014a): LSD enhances suggestibility in healthy volunteers. Psychopharmacology (Berl) 232:785–794. [DOI] [PubMed] [Google Scholar]
- Carhart‐Harris RL, Leech R, Hellyer PJ, Shanahan M, Feilding A, Tagliazucchi E, Chialvo DR, Nutt D (2014b): The entropic brain: A theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Hum Neurosci 8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter OL, Hasler F, Pettigrew JD, Wallis GM, Liu GB, Vollenweider FX (2007): Psilocybin links binocular rivalry switch rate to attention and subjective arousal levels in humans. Psychopharmacology (Berl) 195:415–424. [DOI] [PubMed] [Google Scholar]
- Cauda F, Torta DM, Sacco K, D'Agata F, Geda E, Duca S, Geminiani G, Vercelli A (2013): Functional anatomy of cortical areas characterized by Von Economo neurons. Brain Struct Funct 218:1–20. [DOI] [PubMed] [Google Scholar]
- Chao‐Gan Y, Yu‐Feng Z (2010): DPARSF: A MATLAB toolbox for “Pipeline” data analysis of resting‐state fMRI. Front Syst Neurosci 4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copolov DL, Mackinnon A, Trauer T (2004): Correlates of the affective impact of auditory hallucinations in psychotic disorders. Schizophr Bull 30:163–171. [DOI] [PubMed] [Google Scholar]
- Cowen P, Sherwood AC (2013): The role of serotonin in cognitive function: Evidence from recent studies and implications for understanding depression. J Psychopharmacol 27:575–583. [DOI] [PubMed] [Google Scholar]
- Craddock RC, James GA, Holtzheimer PE, III , Hu XP, Mayberg HS (2012): A whole brain fMRI atlas generated via spatially constrained spectral clustering. Hum Brain Mapp 33:1914–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD (2009): How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci 10:59–70. [DOI] [PubMed] [Google Scholar]
- Dittrich A (1998): The standardized psychometric assessment of altered states of consciousness (ASCs) in humans. Pharmacopsychiatry 31:80–84. [DOI] [PubMed] [Google Scholar]
- Donoso M, Collins AG, Koechlin E (2014): Human cognition. Foundations of human reasoning in the prefrontal cortex. Science 344:1481–1486. [DOI] [PubMed] [Google Scholar]
- Doya K, Ishii S, Pouget A, Rao RPN (2011). Bayesian brain: Probabilistic approaches to neural coding. Cambridge, Massachusetts: The MIT Press; 344 p. [Google Scholar]
- Engstrom M, Landtblom AM, Karlsson T (2013): Brain and effort: Brain activation and effort‐related working memory in healthy participants and patients with working memory deficits. Front Hum Neurosci 7:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard HC, Forro T, Logothetis NK (2012): Von Economo neurons in the anterior insula of the macaque monkey. Neuron 74:482–489. [DOI] [PubMed] [Google Scholar]
- Farrer C, Frith CD (2002): Experiencing oneself vs another person as being the cause of an action: The neural correlates of the experience of agency. Neuroimage 15:596–603. [DOI] [PubMed] [Google Scholar]
- Feinberg I (2011): Corollary discharge, hallucinations, and dreaming. Schizophr Bull 37:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Dolan RJ, Frith CD (2012): Metacognition: computation, biology and function. Philos Trans R Soc Lond B Biol Sci 367:1280–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD (2009): Perceiving is believing: A Bayesian approach to explaining the positive symptoms of schizophrenia. Nat Rev Neurosci 10:48–58. [DOI] [PubMed] [Google Scholar]
- Freud S (1924): The Ego and the Id: CreateSpace Independent. North Charleston, USA: Publishing Platform, 2010.
- Friston K (2010): The free‐energy principle: A unified brain theory? Nat Rev Neurosci 11:127–138. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Liddle PF, Frith CD, Hirsch SR, Frackowiak RS (1992): The left medial temporal region and schizophrenia. A PET study. Brain 115 (Pt 2):367–382. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R (1996): Movement‐related effects in fMRI time‐series. Magn Reson Med 35:346–355. [DOI] [PubMed] [Google Scholar]
- Friston K, Schwartenbeck P, Fitzgerald T, Moutoussis M, Behrens T, Dolan RJ (2013): The anatomy of choice: Active inference and agency. Front Hum Neurosci 7:598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentenebro F, Berrios GE (1995): The predelusional state: A conceptual history. Compr Psychiatry 36:251–259. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND (2011): Oral methylphenidate normalizes cingulate activity and decreases impulsivity in cocaine addiction during an emotionally salient cognitive task. Neuropsychopharmacology 36:366–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Woicik PA, Maloney T, Tomasi D, Alia‐Klein N, Shan J, Honorio J, Samaras D, Wang R, Telang F, Wang GJ, Volkow ND (2010): Oral methylphenidate normalizes cingulate activity in cocaine addiction during a salient cognitive task. Proc Natl Acad Sci USA 107:16667–16672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman N (2002): The serotonergic system and mysticism: Could LSD and the nondrug‐induced mystical experience share common neural mechanisms? J Psychoactive Drugs 34:263–272. [DOI] [PubMed] [Google Scholar]
- Gordon E, Barry RJ, Anderson J, Fawdry R, Yong C, Grunewald S, Meares RA (1994): Single photon emission computed tomography (SPECT) measures of brain function in schizophrenia. Aust N Z J Psychiatry 28:446–452. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Richards WA, Richards BD, McCann U, Jesse R (2011): Psilocybin occasioned mystical‐type experiences: Immediate and persisting dose‐related effects. Psychopharmacology (Berl) 218:649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grof S, Halifax J, Kubler‐Ross E (1977): The Human Encounter with Death, 1st ed. E. P. Dutton; 240 p. [Google Scholar]
- Grof S, Hofmann A, Weil A (2008): LSD Psychotherapy (The Healing Potential Potential of Psychedelic Medicine): MAPS.org. [Google Scholar]
- Guedj E, Aubert S, McGonigal A, Mundler O, Bartolomei F (2010): Deja‐vu in temporal lobe epilepsy: Metabolic pattern of cortical involvement in patients with normal brain MRI. Neuropsychologia 48:2174–2181. [DOI] [PubMed] [Google Scholar]
- Haber SN (2011): Chapter 11. Neuroanatomy of Reward: A View from the Ventral Striatum In: Gottfried JA, editor. Neurobiology of Sensation and Reward. Boca Raton, Florida: CRC Press, 2011. [PubMed] [Google Scholar]
- Ham TE, Bonnelle V, Hellyer P, Jilka S, Robertson IH, Leech R, Sharp DJ (2014): The neural basis of impaired self‐awareness after traumatic brain injury. Brain 137(Pt 2):586–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RG, Mickle WA (1960): Evaluation of seven years experience with depth electrode studies in human patients In: Ramey ER, O'Doherty DS, editors. Electrical Studies of the Unanethetized Brain. New York: Harper and Brothers; p 214–47. [Google Scholar]
- Hood RW (1975): The construction and preliminary validation of a measure of reported mystical experience. J Sci Study Relig 14:29–41. [Google Scholar]
- Illman NA, Butler CR, Souchay C, Moulin CJ (2012): Deja experiences in temporal lobe epilepsy. Epilepsy Res Treat 2012:539–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W (1902): The Varieties Of Religious Experience: A Study In Human Nature CreateSpace Independent Publishing Platform (November 9, 2009). 284 p.
- Jaspers K (1913): Allgemeine Psychopathologie: English translation of the 7th edition: General Psychopathology. Baltimore: Johns Hopkins University Press; 1997. [Google Scholar]
- Jaynes J (1976): The origin of consciousness in the breakdown of the bicameral mind: Mariner Books, 2000. 512 p. [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S (2002): Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841. [DOI] [PubMed] [Google Scholar]
- Jones BF, Witter MP (2007): Cingulate cortex projections to the parahippocampal region and hippocampal formation in the rat. Hippocampus 17:957–976. [DOI] [PubMed] [Google Scholar]
- Kircher T, David A. (2003): The Self in Neuroscience and Psychiatry. Cambridge, United Kingdom: Cambridge University Press. [Google Scholar]
- Kometer M, Schmidt A, Bachmann R, Studerus E, Seifritz E, Vollenweider FX (2012): Psilocybin biases facial recognition, goal‐directed behavior, and mood state toward positive relative to negative emotions through different serotonergic subreceptors. Biol Psychiatry 72:898–906. [DOI] [PubMed] [Google Scholar]
- Kondo H, Saleem KS, Price JL (2005): Differential connections of the perirhinal and parahippocampal cortex with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol 493:479–509. [DOI] [PubMed] [Google Scholar]
- Krebs TS, Johansen PO (2012): Lysergic acid diethylamide (LSD) for alcoholism: Meta‐analysis of randomized controlled trials. J Psychopharmacol 26:994–1002. [DOI] [PubMed] [Google Scholar]
- Krishnadas R, Ryali S, Chen T, Uddin L, Supekar K, Palaniyappan L, Menon V (2014): Resting state functional hyperconnectivity within a triple network model in paranoid schizophrenia. The Lancet 383:S65. [Google Scholar]
- Lambert MV, Sierra M, Phillips ML, David AS (2002): The spectrum of organic depersonalization: a review plus four new cases. J Neuropsychiatry Clin Neurosci 14:141–154. [DOI] [PubMed] [Google Scholar]
- Lee H, Fell J, Axmacher N (2013): Electrical engram: How deep brain stimulation affects memory. Trends Cogn Sci 17:574–584. [DOI] [PubMed] [Google Scholar]
- Lemche E, Surguladze SA, Brammer MJ, Phillips ML, Sierra M, David AS, Williams SC, Giampietro VP (2013): Dissociable brain correlates for depression, anxiety, dissociation, and somatization in depersonalization‐derealization disorder. CNS Spectr 1–8. [DOI] [PubMed] [Google Scholar]
- Lerman C, Gu H, Loughead J, Ruparel K, Yang Y, Stein EA (2014): Large‐scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry 71:523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Mai X, Liu C (2014): The default mode network and social understanding of others: What do brain connectivity studies tell us. Front Hum Neurosci 8:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle PF, Friston KJ, Frith CD, Frackowiak RS (1992): Cerebral blood flow and mental processes in schizophrenia. J R Soc Med 85:224–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limanowski J, Blankenburg F (2013): Minimal self‐models and the free energy principle. Front Hum Neurosci 7:547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA (1998): Functional connectivity in single and multislice echoplanar imaging using resting‐state fluctuations. Neuroimage 7:119–132. [DOI] [PubMed] [Google Scholar]
- Lyvers M, Meester M (2012): Illicit use of LSD or psilocybin, but not MDMA or nonpsychedelic drugs, is associated with mystical experiences in a dose‐dependent manner. J Psychoactive Drugs 44:410–417. [DOI] [PubMed] [Google Scholar]
- MATLAB version 8.1. Natick, Massachusetts: The MathWorks Inc, 2013. [Google Scholar]
- MacLean KA, Johnson MW, Griffiths RR (2011): Mystical experiences occasioned by the hallucinogen psilocybin lead to increases in the personality domain of openness. J Psychopharmacol 25:1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean KA, Leoutsakos JM, Johnson MW, Griffiths RR (2012): Factor analysis of the mystical experience questionnaire: A study of experiences occasioned by the hallucinogen psilocybin. J Sci Study Relig 51:721–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoliu A, Riedl V, Zherdin A, Muhlau M, Schwerthoffer D, Scherr M, Peters H, Zimmer C, Forstl H, Bauml J, Wohlschlager AM, Sorg C (2014): Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr Bull 40:428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ (2010): Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct 214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minas IH, Klimidis S, Stuart GW, Copolov DL, Singh BS (1994): Positive and negative symptoms in the psychoses: Principal components analysis of items from the scale for the assessment of positive symptoms and the scale for the assessment of negative symptoms. Compr Psychiatry 35:135–144. [DOI] [PubMed] [Google Scholar]
- Monroe RR, Heath RG (1961): Effects of lysergic acid and various derivatives on depth and cortical electrograms. J Neuropsychiatr 3:75–82. [PubMed] [Google Scholar]
- Monti JM, Pandi‐Perumal SR, Jacobs BL, Nutt DJ (2008). Serotonin and Sleep: Molecular, Functional and Clinical Aspects. New York: Springer (eBook). [Google Scholar]
- Moore JW, Schneider SA, Schwingenschuh P, Moretto G, Bhatia KP, Haggard P (2010): Dopaminergic medication boosts action‐effect binding in Parkinson's disease. Neuropsychologia 48:1125–1132. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Carhart‐Harris RL, Moran RJ, Brookes MJ, Williams TM, Errtizoe D, Sessa B, Papadopoulos A, Bolstridge M, Singh KD, et al. (2013): Broadband cortical desynchronization underlies the human psychedelic state. J Neurosci 33:15171–15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A (2007): Damage to the insula disrupts addiction to cigarette smoking. Science 315:531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B, Thompson A, Yung AR (2012): Basic self‐disturbance predicts psychosis onset in the ultra high risk for psychosis “prodromal” population. Schizophr Bull 38:1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostby Y, Walhovd KB, Tamnes CK, Grydeland H, Westlye LT, Fjell AM (2012): Mental time travel and default‐mode network functional connectivity in the developing brain. Proc Natl Acad Sci USA 109:16800–16804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyappan L, Liddle PF (2012): Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J Psychiatry Neurosci 37:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyappan L, Simmonite M, White TP, Liddle EB, Liddle PF (2013): Neural primacy of the salience processing system in schizophrenia. Neuron 79:814–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvizi J, Rangarajan V, Shirer WR, Desai N, Greicius MD (2013): The will to persevere induced by electrical stimulation of the human cingulate gyrus. Neuron 80:1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad KM, Rohm BR, Keshavan MS (2004): Parahippocampal gyrus in first episode psychotic disorders: A structural magnetic resonance imaging study. Prog Neuropsychopharmacol Biol Psychiatry 28:651–658. [DOI] [PubMed] [Google Scholar]
- Pu W, Li L, Zhang H, Ouyang X, Liu H, Zhao J, Li L, Xue Z, Xu K, Tang H, et al. (2012): Morphological and functional abnormalities of salience network in the early‐stage of paranoid schizophrenia. Schizophr Res 141:15–21. [DOI] [PubMed] [Google Scholar]
- Qin P, Northoff G (2011): How is our self related to midline regions and the default‐mode network? Neuroimage 57:1221–1233. [DOI] [PubMed] [Google Scholar]
- R Core Team (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: ISBN: 3‐900051‐07‐0. Available at: http://www.R-project.org/. [Google Scholar]
- Rangarajan V, Hermes D, Foster BL, Weiner KS, Jacques C, Grill‐Spector K, Parvizi J (2014): Electrical stimulation of the left and right human fusiform gyrus causes different effects in conscious face perception. J Neurosci 34:12828–12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reniers RL, Corcoran R, Vollm BA, Mashru A, Howard R, Liddle PF (2012): Moral decision‐making, ToM, empathy and the default mode network. Biol Psychol 90:202–210. [DOI] [PubMed] [Google Scholar]
- Riga MS, Soria G, Tudela R, Artigas F, Celada P (2014): The natural hallucinogen 5‐MeO‐DMT, component of Ayahuasca, disrupts cortical function in rats: Reversal by antipsychotic drugs. Int J Neuropsychopharmacol 17:1269–1282. [DOI] [PubMed] [Google Scholar]
- Rohricht F, Priebe S (2004): Ego‐pathology and common symptom factors in schizophrenia. J Nerv Ment Dis 192:446–449. [DOI] [PubMed] [Google Scholar]
- Rohricht F, Papadopoulos N, Suzuki I, Priebe S (2009): Ego‐pathology, body experience, and body psychotherapy in chronic schizophrenia. Psychol Psychother 82(Pt 1):19–30. [DOI] [PubMed] [Google Scholar]
- Roseman L, Leech R, Feilding A, Nutt DJ, Carhart‐Harris RL (2014): The effects of psilocybin and MDMA on between‐network resting state functional connectivity in healthy volunteers. Front Hum Neurosci 8:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O (2010): Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 52:1059–1069. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O (2011): Weight‐conserving characterization of complex functional brain networks. Neuroimage 56:2068–2079. [DOI] [PubMed] [Google Scholar]
- Sass L, Pienkos E, Nelson B, Medford N (2013): Anomalous self‐experience in depersonalization and schizophrenia: A comparative investigation. Conscious Cogn 22:430–441. [DOI] [PubMed] [Google Scholar]
- Scharfetter C (1981): Ego‐psychopathology: The concept and its empirical evaluation. Psychol Med 11:273–280. [DOI] [PubMed] [Google Scholar]
- Schenk L, Bear D (1981): Multiple personality and related dissociative phenomena in patients with temporal lobe epilepsy. Am J Psychiatry 138:1311–1316. [DOI] [PubMed] [Google Scholar]
- Schneider K (1959). Clinical Psychopathology: The Classics of Psychiatry & Behavioral Sciences Library; Special ed edition, 1993. 173 p.
- Schwarz BE, Sem‐Jacobsen CW, Petersen MC (1956): Effects of mescaline, LSD‐25, and adrenochrome on depth electrograms in man. AMA Arch Neurol Psychiatry 75:579–587. [DOI] [PubMed] [Google Scholar]
- Seeley WW (2008): Selective functional, regional, and neuronal vulnerability in frontotemporal dementia. Curr Opin Neurol 21:701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW (2010): Anterior insula degeneration in frontotemporal dementia. Brain Struct Funct 214:465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sem‐Jacobsen CW, Petersen MC, Lazarte JA, Dodge HW, Jr. , Holman CB (1955): Intracerebral electrographic recordings from psychotic patients during hallucinations and agitation. Am J Psychiatry 112:278–288. [DOI] [PubMed] [Google Scholar]
- Seth AK (2013): Interoceptive inference, emotion, and the embodied self. Trends Cogn Sci 17:565–573. [DOI] [PubMed] [Google Scholar]
- Sherwood SL (1962): Electrographic depth recordings from the brains of psychotics. Ann N Y Acad Sci 96:375–385. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K (2009): A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci 13:334–340. [DOI] [PubMed] [Google Scholar]
- Spatt J (2002): Deja vu: Possible parahippocampal mechanisms. J Neuropsychiatry Clin Neurosci 14:6–10. [DOI] [PubMed] [Google Scholar]
- Spitzer M, Thimm M, Hermle L, Holzmann P, Kovar KA, Heimann H, Gouzoulis‐Mayfrank E, Kischka U, Schneider F (1996): Increased activation of indirect semantic associations under psilocybin. Biol Psychiatry 39:1055–1057. [DOI] [PubMed] [Google Scholar]
- Sporns O (2010): Networks of the Brain., 1st ed Cambridge, Massachusetts: The MIT Press; 424 p. [Google Scholar]
- Sripada C, Kessler D, Jonides J (2014): Methylphenidate blocks effort‐induced depletion of regulatory control in healthy volunteers. Psychol Sci 25:1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studerus E, Gamma A, Vollenweider FX (2010): Psychometric evaluation of the altered states of consciousness rating scale (OAV). PLoS One 5:e12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studerus E, Gamma A, Kometer M, Vollenweider FX (2012): Prediction of psilocybin response in healthy volunteers. PLoS One 7:e30800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA (2012): Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage 62:2281–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E, Carhart‐Harris R, Leech R, Nutt D, Chialvo DR (2014): Enhanced repertoire of brain dynamical states during the psychedelic experience. Hum Brain Mapp 35:5442–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y, Kurita T, Sakurai K, Shiga T, Tamaki N, Koyama T (2011): Persistent deja vu associated with hyperperfusion in the entorhinal cortex. Epilepsy Behav 21:196–199. [DOI] [PubMed] [Google Scholar]
- Talamini LM, Meeter M, Elvevag B, Murre JM, Goldberg TE (2005): Reduced parahippocampal connectivity produces schizophrenia‐like memory deficits in simulated neural circuits with reduced parahippocampal connectivity. Arch Gen Psychiatry 62:485–493. [DOI] [PubMed] [Google Scholar]
- Tian T, Qin W, Liu B, Jiang T, Yu C (2013): Functional connectivity in healthy subjects is nonlinearly modulated by the COMT and DRD2 polymorphisms in a functional system‐dependent manner. J Neurosci 33:17519–17526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trichter S, Klimo J, Krippner S (2009): Changes in spirituality among ayahuasca ceremony novice participants. J Psychoactive Drugs 41:121–134. [DOI] [PubMed] [Google Scholar]
- Uddin LQ (2011): Brain connectivity and the self: The case of cerebral disconnection. Conscious Cogn 20:94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ (2014): Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci 16:55–61. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Mooshagian E, Zaidel E, Scheres A, Margulies DS, Kelly AM, Shehzad Z, Adelstein JS, Castellanos FX, Biswal BB, Milham MP (2008): Residual functional connectivity in the split‐brain revealed with resting‐state functional MRI. Neuroreport 19:703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignal JP, Maillard L, McGonigal A, Chauvel P (2007): The dreamy state: Hallucinations of autobiographic memory evoked by temporal lobe stimulations and seizures. Brain 130(Pt 1):88–99. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Geyer MA (2001): A systems model of altered consciousness: Integrating natural and drug‐induced psychoses. Brain Res Bull 56:495–507. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Leenders KL, Scharfetter C, Maguire P, Stadelmann O, Angst J (1997): Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology 16:357–372. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider‐Scherpenhuyzen MF, Babler A, Vogel H, Hell D (1998): Psilocybin induces schizophrenia‐like psychosis in humans via a serotonin‐2 agonist action. Neuroreport 9:3897–3902. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vontobel P, Hell D, Leenders KL (1999): 5‐HT modulation of dopamine release in basal ganglia in psilocybin‐induced psychosis in man–a PET study with [11C]raclopride. Neuropsychopharmacology 20:424–433. [DOI] [PubMed] [Google Scholar]
- Von Helmholtz H (1866): Handbuch der Physiologischen Optik (Treatise on physiological optics). English translation by J.P.C. Southall‐Dover: Rochester, New York, 2005.
- Ward AM, Schultz AP, Huijbers W, Van Dijk KR, Hedden T, Sperling RA (2014): The parahippocampal gyrus links the default‐mode cortical network with the medial temporal lobe memory system. Hum Brain Mapp 35:1061–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watrous AJ, Tandon N, Conner CR, Pieters T, Ekstrom AD. (2013): Frequency‐specific network connectivity increases underlie accurate spatiotemporal memory retrieval. Nat Neurosci 16:349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weniger G, Irle E (2006): Posterior parahippocampal gyrus lesions in the human impair egocentric learning in a virtual environment. Eur J Neurosci 24:2406–2414. [DOI] [PubMed] [Google Scholar]
- White TP, Joseph V, Francis ST, Liddle PF (2010): Aberrant salience network (bilateral insula and anterior cingulate cortex) connectivity during information processing in schizophrenia. Schizophr Res 123:105–115. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Carter O, Hasler F, Cahn BR, Grimberg U, Spring P, Hell D, Flohr H, Vollenweider FX (2007): Effects of psilocybin on time perception and temporal control of behaviour in humans. J Psychopharmacol 21:50–64. [DOI] [PubMed] [Google Scholar]
- Xia M, Wang J, He Y (2013): BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS One 8:e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL (2011): The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Bullmore ET (2010): Network‐based statistic: Identifying differences in brain networks. Neuroimage 53:1197–1207. [DOI] [PubMed] [Google Scholar]
- Zinberg NE (1984). Drug, Set, And Setting: The Basis for Controlled Intoxicant Use. New Haven: Yale University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


