Abstract
Most of the previous task functional magnetic resonance imaging (fMRI) studies found abnormalities in distributed brain regions in mild cognitive impairment (MCI) and Alzheimer's disease (AD), and few studies investigated the brain network dysfunction from the system level. In this meta‐analysis, we aimed to examine brain network dysfunction in MCI and AD. We systematically searched task‐based fMRI studies in MCI and AD published between January 1990 and January 2014. Activation likelihood estimation meta‐analyses were conducted to compare the significant group differences in brain activation, the significant voxels were overlaid onto seven referenced neuronal cortical networks derived from the resting‐state fMRI data of 1,000 healthy participants. Thirty‐nine task‐based fMRI studies (697 MCI patients and 628 healthy controls) were included in MCI‐related meta‐analysis while 36 task‐based fMRI studies (421 AD patients and 512 healthy controls) were included in AD‐related meta‐analysis. The meta‐analytic results revealed that MCI and AD showed abnormal regional brain activation as well as large‐scale brain networks. MCI patients showed hypoactivation in default, frontoparietal, and visual networks relative to healthy controls, whereas AD‐related hypoactivation mainly located in visual, default, and ventral attention networks relative to healthy controls. Both MCI‐related and AD‐related hyperactivation fell in frontoparietal, ventral attention, default, and somatomotor networks relative to healthy controls. MCI and AD presented different pathological while shared similar compensatory large‐scale networks in fulfilling the cognitive tasks. These system‐level findings are helpful to link the fundamental declines of cognitive tasks to brain networks in MCI and AD. Hum Brain Mapp 36:1217–1232, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: mild cognitive impairment, Alzheimer's disease, default mode, neuronal network, fMRI
INTRODUCTION
Alzheimer's disease (AD) is a progressive neurodegenerative disease that is characterized by the presence of amyloid deposition, neurofibrillary tangles, and widespread functional disturbances in the brain [Terry et al., 1991; Touchon and Ritchie, 1999]. AD patients gradually lose capacity in memory, executive function, and other cognitive abilities and ultimately are unable to conduct activities of daily living [Husain and Garrett, 2005]. Mild cognitive impairment (MCI) is considered as a transitional stage between normal aging and AD [Petersen et al., 2001; Petersen et al., 1999]. Previous studies have reported that persons with MCI exhibited impairments in memory and many other cognitive functions [Arnaiz and Almkvist, 2003].
Three meta‐analyses have explored the brain activation with task‐based functional magnetic resonance imaging (fMRI) in MCI and AD. In the meta‐analysis of Schwindt and Black [2009], they included 14 fMRI and positron emission tomography (PET) studies of episodic memory in AD; the results revealed that healthy elderly showed greater activity in medial temporal lobe and frontal pole in both encoding and retrieval processing while AD showed increased activation in ventral lateral prefrontal cortex, superior temporal gyrus, and a number of other regions. Including 16 fMRI studies and 144 foci totally, Browndyke et al. [2013] found MCI and AD patients showed decreased activation in medial temporal lobe while increased activation in default mode and prefrontal gyrus in comparison with healthy controls during memory encoding. Jacobs et al. [2013] investigated the brain activity in people at risk of AD, MCI, and AD in 53 fMRI studies totally; they found the medial parietal regions and subcortical areas were differentially affected with disease progression; they concluded that AD patients might present neural network disruptions before cognitive deficits.
With increasing fMRI studies in AD, some cortical regions were found to be activated in a variety of tasks, Dickerson and Sperling [2009] proposed that AD might be a disease with multiple dysfunctional large‐scale neuronal networks instead of alterations in single brain regions. During associative memory task, MCI and AD patients were found to demonstrate memory network disruptions [Celone et al., 2006]. Default network activity exhibits high sensitivity and specificity in differentiating AD from healthy older adults [Greicius et al., 2004; Koch et al., 2012]. Dysfunctional activation of the default network was considered to play a critical role in understanding the cognitive decline observed in AD [Andrews‐Hanna et al., 2007; Chhatwal and Sperling, 2012]. The aforementioned studies provided a new promising large‐scale network approach to investigate the brain functional deficits in MCI and AD. Although resting‐state fMRI studies have provided preliminary findings about the large‐scale functional brain networks in MCI and AD, these insights have less been incorporated into task‐based fMRI studies. The difficulty of application large‐scale brain networks into task‐fMRI studies is the appropriate and reliable brain network parcellation. Based on the resting‐state fMRI data from 1,000 healthy participants and a data‐driven clustering approach, researchers have derived seven cortical neuronal networks: the visual, somatomotor, dorsal attention, ventral attention, limbic, frontoparietal, and default mode networks [Yeo et al., 2011]. This group further explored the functional projections from the cerebellum and striatum to these seven networks and parcellated the cerebellum and striatum into seven networks according their functional connectivity findings [Buckner et al., 2011; Choi et al., 2012]. Cortese et al. [2012] applied the idea of the seven reference neuronal systems into an ADHD meta‐analysis, and extended the previous findings that the dysfunctions in ADHD were not only involved in higher‐level cognitive functions but also in sensorimotor and default networks.
The seven neuronal networks functional parcellation can be integrated into an activation likelihood estimation (ALE) task‐based meta‐analysis to seek a feasible solution to determine network variation in MCI and AD and to understand their progresses in brain pathological aging at a system level. To date, although three meta‐analyses have been conducted to explore the fMRI findings in MCI and AD [Browndyke et al., 2013; Jacobs et al., 2013; Schwindt and Black, 2009], however, limited by the analytic strategies, no study has been conducted to systematically incorporate the brain functional parcellation into MCI and AD. In this study, we aimed to conduct a comprehensive review of the extant task‐based fMRI studies of MCI and AD from a systems neuroscience perspective and attempt to reveal neuronal dysfunction of large‐scale brain circuits in pathological aging progresses.
METHODS
Literature Search
To identify pertinent articles, an online search of the PubMed, EBSCOHost (PsycINFO, PsycARTICLES), ISI Web of Knowledge, and NeuroSynth databases were performed for articles published between January 1990 and January 2014. “In press” articles were also included. Because we intended to make two main comparisons (MCI patients vs. healthy controls, AD patients vs. healthy controls, respectively), we conducted literature searches separately for these two meta‐analyses.
The search terms related to MCI were “mild cognitive impairment, MCI, age‐associated memory impairment, cognitive decline, cognitive impairment no dementia, preclinical, subclinical, prodromal, prediagnostic, prediagnostic, presymptomatic, presymptomatic, early stages, early symptoms, early diagnosis, and early detection.” The search terms related to AD were “Alzheimer's disease, Alzheimer disease, Alzheimer's, Alzheimer, or AD, and dementia.”
The search terms related to fMRI were “functional magnetic resonance imaging, functional MRI, fMRI, imaging, neuroimaging, magnetic resonance imaging, MRI, functional imaging.” Search terms regarding MCI and AD were combined with different fMRI‐related terms. We conducted an additional literature search using the reference lists of the included studies and several relevant review articles [Browndyke et al., 2013; Chhatwal and Sperling, 2012; He et al., 2009; Jacobs et al., 2013; Schwindt and Black, 2009; Woodard and Sugarman, 2012] to identify as many potential studies as possible. To provide additional evidence in functional activity and neuronal networks, we also searched resting‐state fMRI studies in MCI and AD, details could be found in Supporting Information.
Study Selection
Inclusion criteria
For fMRI studies, two separate group comparisons were made in this meta‐analysis. For the MCI‐related meta‐analysis, studies must include both a group of MCI patients [Petersen 2004; Petersen et al., 2001; Petersen et al., 1999; Winblad et al., 2004] and a group of healthy controls. For the AD‐related meta‐analysis, studies should include a clinical sample of AD patients (diagnosed according to the NINCDS‐ADRDA, DSM‐IV, or ICD‐10 criteria) and a group of healthy controls. Furthermore, for task‐based fMRI meta‐analyses, the studies must focus on a certain cognitive task and reported three‐dimensional (3D) Talairach or Montreal Neurologic Institute (MNI) coordinates of between‐group comparisons. For resting‐state fMRI meta‐analyses, the studies should include a resting‐state fMRI scan and reported 3D Talairach or MNI coordinates of between‐group comparisons.
Exclusion criteria
Studies were excluded if they: (1) used any other neuroimaging methods such as PET, single photon emission computed tomography (SPECT), and other non‐fMRI procedures because we only included fMRI studies to exclude the variability across different neuroimaging findings; (2) reported only within‐group contrasts; (3) conducted a priori regions of interest (ROI) analysis; (4) assessed the effect of medication and took the fMRI results as outcomes while without reporting fMRI data at baseline. Moreover, two studies were excluded because they provided only the coordinates of neural deactivation [Gould et al., 2006; Rombouts et al., 2005].
Data Extraction
Two of the authors (HJL and XHH) determined whether the studies identified by our literature search should be included. Additionally, these authors independently extracted the demographic information, 3D coordinates, tasks, and contrasts of the included studies.
Deactivation coordinates were excluded during the data extraction procedure because they may have reflected different signal changes as activation coordinates (Ginger ALE forum, http://www.brainmap.org/forum/viewtopic.php?f=3&t=88). When studies included more than one group of MCI patients [Celone et al., 2006; Clément and Belleville, 2010; Clément and Belleville, 2012; Clément et al., 2013; Machulda et al., 2009; Vannini et al., 2007], the different groups were averaged into one pooled group, and the coordinates were also pooled. Two studies exploring therapeutic cholinesterase inhibitors [McGeown et al., 2008; Thiyagesh et al., 2010] were also included because these studies provided baseline fMRI activation coordinates of group comparisons.
Quantitative Meta‐Analysis Procedures
All of the Talairach coordinates were first transformed into the corresponding MNI locations [Lancaster et al., 2007]. All of the MNI coordinates were then input into a text file, which was subsequently loaded into a Java‐based version of GingerALE 2.3.1 (http://www.brainmap.org). ALE identifies the reported foci as centers of 3D Gaussian probability distributions around the specified coordinates. ALE models aim to assess the spatial uncertainty of coordinates within a study and detect convergence across studies [Laird et al., 2005; Turkeltaub et al., 2002]. Statistical significance was determined via a permutation test using randomly distributed foci. We computed 5,000 permutations using subject‐based FWHM values and the same number of foci were used to compute ALE values [Turkeltaub et al., 2012]. The thresholds of the final ALE maps were set at P < 0.05, and the maps were corrected for multiple comparisons using the false discovery rate (FDR pN) method. Minimal clusters were required to exceed 200 mm3 in volume. The Talairach Daemon identified the anatomical labels of the maximum of ALE values and the weighted centers of their coordinates [Lancaster et al., 2007].
We conducted several separate meta‐analyses: (1) comparisons between MCI patients and healthy controls across all of the task‐based fMRI studies; (2) comparisons between AD patients and healthy controls across all of the task‐based fMRI studies. We also investigated the functional activation and neuronal networks in specific cognitive tasks in MCI and AD, respectively. These tasks included memory encoding, memory retrieval, executive function and working memory, attention and visuospatial, language processing, and emotional processing. The MCI‐related and AD‐related resting‐state fMRI meta‐analyses were also investigated as a supplement of this review.
Relationship with Neuronal Networks
According to the ALE results, we separately calculated the number of significant voxels that overlapped the masks generated for the seven large‐scale neural networks [Buckner et al., 2011; Choi et al., 2012; Yeo et al., 2011] in MCI‐related and AD‐related changes. The results calculated from the cortical, cerebellar, and striatal networks were then merged. Chi‐square analyses were finally performed to compare the proportions of voxels exhibiting increased and decreased brain activity in the seven neural networks.
RESULTS
Search Results
The results of the initial reference search and study exclusion for task‐based fMRI meta‐analyses are presented in Figure 1. There were 39 and 36 studies reporting contrast coordinates of MCI patients and healthy controls, and AD patients and healthy controls, respectively. The MCI‐related meta‐analysis included 697 individuals with MCI and 628 healthy controls while the AD‐related meta‐analysis included 421 AD patients and 512 healthy controls. The characteristics of the included studies are summarized in Table 1. There were 17 and 8 studies included in the MCI‐related and AD‐related resting‐state fMRI meta‐analyses, respectively (details please see the Supporting Information). The characteristics of the included studies are summarized in Supporting Information Table I.
Figure 1.
Flow chart of the study selection process for MCI‐related and AD‐related task‐based fMRI meta‐analyses. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 1.
Characteristics of task‐fMRI studies included in the meta‐analysis of MCI and AD
| Study | N | Age (SD) | Education (SD) | MMSE (SD) | fMRI task type | Group contrasts | Number of foci |
|---|---|---|---|---|---|---|---|
| MCI versus healthy controls | |||||||
| Alichniewicz et al. (2012) | 39 MCI | 62.3 (8.6) | 13.1 (3.0) | 28.6 (1.2) | Working memory | MCI < Controls | 5 |
| 24 controls | 60.7 (7.2) | 13.7 (2.0) | 29.2 (0.9) | ||||
| Baglio et al. (2012) | 16 MCI | 71.0 (5.8) | 9.9 (4.8) | 27.0 (1.8) | Theory of mind | MCI < Controls | 1 |
| 15 controls | 66.9 (6.4) | 10.8 (3.5) | 29.0 (1.3) | MCI > Controls | 7 | ||
| Bokde et al. (2008) | 16 MCI | 69.9 (7.8) | 13.2 (3.3) | 27.2 (1.5) | Visuospatial | MCI > Controls | 16 |
| 17 controls | 66.7 (4.2) | 12.8 (2.9) | 29.2 (1.0) | ||||
| Bokde et al. (2010a) | 8 MCI | 70.8 (5.3) | 26.6 (1.3) | Working memory | MCI < Controls | 8 | |
| 8 controls | 66.6 (3.9) | 30.0 (0.0) | MCI > Controls | 18 | |||
| Bosch et al. (2010) | 15 MCI | 74.6 (6.9) | 25.5 (2.0) | Language processing | MCI > Controls | 3 | |
| 15 controls | 72.2 (5.8) | 27.7 (1.5) | |||||
| Celone et al. (2006) | 27 MCI | 77.3 (6.1) | 16.3 (3.1) | 29.0 (1.0) | Memory encoding | MCI > Controls | 6 |
| 15 controls | 75.5 (6.0) | 16.5 (2.1) | 29.5 (0.5) | ||||
| Clément and Belleville (2010) | 26 MCI | 67.9 (8.5) | 14.4 (3.9) | 27.7 (1.6) | Memory encoding | MCI > Controls | 6 |
| 14 controls | 67.2 (6.8) | 14.6 (3.8) | 29.3 (1.1) | MCI < Controls | 2 | ||
| Clément and Belleville (2012) | 26 MCI | 67.9 (8.5) | 14.5 (3.9) | 27.7 (1.6) | Memory retrieval | MCI < Controls | 1 |
| 14 controls | 67.2 (6.8) | 14.6 (3.8) | 29.3 (1.1) | MCI > Controls | 10 | ||
| Clément et al. (2013) | 24 MCI | 68.4 (9.1) | 14.5 (4.1) | 28.0 (1.8) | Executive function | MCI < Controls | 3 |
| 14 controls | 67.2 (6.8) | 14.6 (3.8) | 29.3 (1.1) | MCI > Controls | 7 | ||
| Dannhauser et al. (2005) | 10 MCI | 72.0 (7.7) | 10.3 (1.8) | 24.5 (1.5) | Divided attention | MCI < Controls | 1 |
| 10 controls | 68.0 (13.5) | 10.1 (1.4) | 28.3 (1.6) | ||||
| Dannhauser et al. (2008) | 10 MCI | 72.0 (7.7) | 10.3 (1.8) | 24.5 (1.5) | Memory encoding | MCI < Controls | 1 |
| 10 controls | 68.0 (13.5) | 10.1 (1.4) | 28.3 (1.6) | ||||
| Faraco et al. (2013) | 16 MCI | 75.1 (6.5) | 14.2 (3.5) | Visuoattention | MCI < Controls | 7 | |
| 24 controls | 74.2 (5.5) | 17.0 (2.3) | Working memory | MCI > Controls | 94 | ||
| Giovanello et al. (2012) | 12 MCI | 75.2 (4.3) | 16.3 (2.9) | 27.8 (1.7) | Memory retrieval | MCI < Controls | 7 |
| 12 controls | 72.6 (5.9) | 15.6 (3.1) | 29.5 (0.9) | MCI > Controls | 4 | ||
| Hämäläinen et al. (2007) | 14 MCI | 72.4 (7.3) | 8.1 (2.6) | 25.6 (3.1) | Memory encoding | MCI < Controls | 1 |
| 21 controls | 71.2 (4.9) | 7.9 (2.9) | 27.7 (2.0) | MCI > Controls | 13 | ||
| Hanseeuw et al. (2011) | 16 MCI | 72.6 (7.9) | 13.5 (2.7) | 27.3 (1.6) | Memory encoding | MCI < Controls | 5 |
| 15 controls | 69.4 (4.8) | 14.9 (2.4) | 28.7 (1.5) | ||||
| Heun et al. (2007) | 20 MCI | 69.7 (7.1) | 26.6 (1.5) | Memory retrieval | MCI > Controls | 3 | |
| 28 controls | 67.5 (5.4) | 28.9 (1.1) | |||||
| Jacobs et al. (2012) | 18 MCI | 65.1 (4.5) | 27.6 (2.3) | Visuospatial | MCI > Controls | 10 | |
| 18 controls | 64.6 (3.4) | 28.9 (1.0) | |||||
| Jin et al. (2012) | 8 MCI | 60.9 (3.2) | 16.9 (1.9) | 28.1 (1.1) | Memory encoding | MCI < Controls | 10 |
| 8 controls | 60.6 (8.3) | 16.9 (2.1) | 29.6 (0.5) | Memory retrieval | MCI > Controls | 5 | |
| Johnson et al. (2006) | 14 MCI | 73.7 (6.9) | 16.2 (2.7) | 28.6 (1.5) | Memory retrieval | MCI < Controls | 6 |
| 14 controls | 72.5 (5.7) | 17.3 (2.9) | 29.4 (0.8) | ||||
| Kaufmann et al. (2008) | 6 MCI | 69.8 (5.3) | 24.8 (1.2) | Executive function | MCI > Controls | 24 | |
| 9 controls | 68.3 (7.5) | 29.0 (1.2) | |||||
| Kircher et al. (2007) | 29 MCI | 69.7 (7.0) | 26.6 (1.4) | Memory encoding | MCI > Controls | 4 | |
| 21 controls | 67.8 (5.4) | 28.8 (1.2) | |||||
| Kochan et al. (2011) | 35 MCI | 78.0 (3.9) | 12.6 (3.9) | 27.9 (1.6) | Working memory | MCI < Controls | 5 |
| 22 controls | 77.2 (3.3) | 11.4 (3.7) | 29.3 (1.0) | MCI > Controls | 2 | ||
| Lenzi et al. (2011) | 15 MCI | 72.5 | 10.3 | 25.1 | Language processing/memory retrieval/attention | MCI > Controls | 3 |
| 14 controls | 64.3 | 13.6 | 28.6 | ||||
| LeyHe et al. (2009) | 11 MCI | 75.0 (6.7) | 13.4 (3.3) | 27.6 (1.4) | Executive function | MCI < Controls | 1 |
| 15 controls | 70.6 (11.8) | 13.7 (3.0) | 29.7 (0.5) | MCI > Controls | 7 | ||
| Li et al. (2013) | 34 MCI | 64.4 (7.5) | 11.1 (2.4) | 26.0 (2.0) | Memory encoding | MCI < Controls | 9 |
| 25 controls | 62.5 (5.4) | 11.4 (3.3) | 28.6 (1.4) | ||||
| Machulda et al. (2009) | 31 MCI | 76.6 (6.8) | 14.9 (3.4) | memory encoding | MCI < Controls | 33 | |
| 29 controls | 73.0 (7.0) | 14.1 (2.4) | Memory retrieval | ||||
| Mandzia et al. (2009) | 14 MCI | 68.6 (7.4) | 13.4 (2.8) | 27.7 (1.1) | Memory encoding | MCI < Controls | 36 |
| 14 controls | 72.2 (6.4) | 15.4 (2.8) | 28.6 (1.1) | Memory retrieval | MCI > Controls | 4 | |
| Papma et al. (2012) | 42 MCI | 73.4 (4.4) | 27.2 (2.0) | Visuospatial | MCI < Controls | 13 | |
| 25 controls | 71.6 (5.2) | 28.8 (1.2) | Working memory | MCI > Controls | 6 | ||
| Petrella et al. (2006) | 20 MCI | 75.0 (7.6) | 15.0 (2.2) | 26.7 (1.5) | Memory encoding | MCI < Controls | 11 |
| 20 controls | 71.2 (4.5) | 15.9 (2.9) | 28.4 (1.4) | Memory retrieval | MCI > Controls | 2 | |
| Poettrich et al. (2009) | 13 MCI | 60.5 (6.6) | 28.3 (0.9) | Memory retrieval | MCI > Controls | 3 | |
| 13 controls | 59.8 (5.3) | 29.1 (0.9) | |||||
| Risacher et al. (2013) | 18 MCI | 72.3 (6.3) | 16.3 (2.9) | 26.6 (2.8) | Memory encoding | MCI < Controls | 6 |
| 20 controls | 71.4 (4.7) | 17.1 (2.4) | 29.1 (0.9) | MCI > Controls | 6 | ||
| Staffen et al. (2012) | 12 MCI | 71.8 (5.2) | 27.0 (1.8) | Executive function | MCI < Controls | 33 | |
| 13 controls | 68.4 (7.9) | 28.0 (1.1) | |||||
| Trivedi et al. (2008) | 16 MCI | 73.1 (5.5) | 14.9 (3.3) | 26.3 (2.3) | Memory encoding | MCI < Controls | 8 |
| 23 controls | 77.0 (8.4) | 16.2 (3.0) | 28.8 (1.2) | MCI > Controls | 1 | ||
| Van Dam et al. (2013) | 8 MCI | 77.6 (7.0) | 14.6 (3.2) | 27.1 (1.8) | Executive function | MCI < Controls | 20 |
| 8 controls | 74.6 (9.2) | 16.9 (2.4) | 28.8 (1.4) | MCI > Controls | 41 | ||
| Vandenbulcke et al. (2007) | 13 MCI | 65.8 (6.8) | 12.7 (2.7) | Language processing | MCI< Controls | 2 | |
| 13 controls | 65.9 (6.3) | 12.9 (2.6) | |||||
| van der Meulen et al. (2012) | 13 MCI | 69.2 (8.2) | 13.0 (2.3) | 26.7 (2.3) | Memory encoding | MCI < Controls | 28 |
| 15 controls | 68.1 (7.2) | 14.3 (2.6) | 29.5 (0.8) | Memory retrieval | |||
| Vannini et al. (2007) | 13 MCI | 58.6 (5.3) | 14.7 (3.5) | Visuospatial | MCI < Controls | 2 | |
| 13 controls | 58.5 (6.4) | 15.9 (3.1) | MCI > Controls | 5 | |||
| Xu et al. (2007) | 10 MCI | 77.0 (4.5) | 13.7 (2.7) | 27.8 (1.5) | Memory encoding | MCI < Controls | 1 |
| 12 controls | 70.0 (3.9) | 15.6 (2.1) | 29.6 (0.8) | ||||
| Yetkin et al. (2006) | 9 MCI | 72.0 (8.0) | 13.0 (1.0) | 28.4 (1.9) | Working memory | MCI < Controls | 11 |
| 8 controls | 65.0 (7.0) | 16.0 (3.0) | 30.0 (0.0) | MCI > Controls | 12 | ||
| AD versus healthy controls | |||||||
| Bokde et al. (2010b) | 12 AD | 71.2 (6.9) | 25.3 (2.3) | Visuospatial | AD > Controls | 18 | |
| 14 controls | 67.1 (4.0) | 29.2 (1.2) | |||||
| Bosch et al. (2010) | 15 AD | 75.3 (5.7) | 21.4 (3.1) | Language processing | AD > Controls | 2 | |
| 15 controls | 72.2 (5.8) | 27.7 (1.5) | |||||
| Celone et al. (2006) | 10 AD | 77.6 (8.0) | 21.1 (3.2) | Memory encoding | AD > Controls | 2 | |
| 15 controls | 75.5 (6.0) | 29.5 (0.5) | (ICA) | ||||
| Cole et al. (2006) | 14 AD | 79.0 (5.0) | 19.4 (5.7) | Emotion processing | AD > Controls | 17 | |
| 15 controls | 79.0 (4.0) | 29.3 (0.1) | |||||
| Donix et al. (2013) | 12 AD | 69.6 (6.1) | 14.5 (3.2) | 24.5 (2.5) | Episodic encoding | AD < Controls | 5 |
| 12 controls | 62.1 (5.4) | 15.0 (2.2) | 29.6 (0.5) | ||||
| Golby et al. (2005) | 7 AD | 69.0 (8.0) | 20.8 (2.0) | Memory encoding | AD < Controls | 7 | |
| 7 controls | 66.0 (11.0) | 29.4 (0.5) | |||||
| Gould et al. (2005) | 12 AD | 77.3 (4.9) | 26.3 (2.1) | Memory encoding | AD < Controls | 7 | |
| 12 controls | 77.3 (4.8) | 29.1 (0.9) | Memory retrieval | AD > Controls | 11 | ||
| Grön et al. (2002) | 12 AD | 61.7 (5.0) | 25.9 (3.5) | Memory encoding | AD < Controls | 17 | |
| 12 controls | 59.8 (2.6) | 30.0 (0.0) | Memory retrieval | AD > Controls | 6 | ||
| Grossman et al. (2003a) | 11 AD | 73.0 (4.9) | 15.3 (2.9) | 20.2 (6.1) | Emotion processing | AD < Controls | 7 |
| 16 controls | 73.9 (3.6) | 13.8 (1.8) | 29.7 (0.8) | AD > Controls | 2 | ||
| Grossman et al. (2003b) | 11 AD | 73.0 (4.9) | 15.3 (2.9) | 20.2 (6.1) | Memory encoding | AD < Controls | 2 |
| 16 controls | 73.9 (3.6) | 13.8 (1.8) | 29.7 (0.8) | AD > Controls | 4 | ||
| Hämäläinen et al. (2007) | 15 AD | 73.1 (6.7) | 8.2 (2.7) | 21.7 (3.7) | Memory encoding | AD < Controls | 5 |
| 21 controls | 71.2 (4.9) | 7.9 (2.9) | 27.1 (2.0) | AD > Controls | 2 | ||
| Kato et al. (2001) | 7 AD | 73.6 (2.9) | Memory encoding | AD < Controls | 4 | ||
| 8 controls | 65.1 (1.8) | ||||||
| Kircher et al. (2005) | 10 AD | 71.8 (12.0) | 22.3 (3.9) | Memory encoding | AD < Controls | 1 | |
| 10 controls | 67.2 (5.1) | 29.3 (0.6) | |||||
| Lee et al. (2013) | 12 AD | 76.7 (5.2) | 1.9 (3.4) | 18.3 (3.4) | Emotion processing | AD < Controls | 3 |
| 12 controls | 72.3 (6.2) | 4.7 (4.4) | 26.8 (2.9) | ||||
| LeyHe et al. (2009) | 15 AD | 71.5 (7.9) | 13.5 (3.2) | 22.9 (2.8) | Executive function | AD < Controls | 17 |
| 15 controls | 70.6 (11.8) | 13.7 (3.0) | 29.7 (0.5) | AD > Controls | 2 | ||
| Lim et al. (2008) | 12 AD | 69.5 (5.6) | 10.8 (4.3) | 20.3 (1.4) | Working memory | AD < Controls | 4 |
| 12 controls | 68.6 (6.2) | 11.3 (3.1) | 29.1 (1.2) | AD > Controls | 2 | ||
| McGeown et al. (2008) | 11 AD | 79.0 (7.4) | 11.6 (3.3) | 21–26 | Language processing | AD < Controls | 3 |
| 9 controls | 75.1 (1.6) | 11.7 (2.3) | 27–30 | Working memory | AD > Controls | 6 | |
| Meulenbroek et al. (2010) | 21 AD | 72.4 (7.1) | 16.1 (3.9) | 24.8 (3.4) | Memory retrieval | AD > Controls | 4 |
| 22 controls | 69.6 (8.6) | 16.5 (3.2) | |||||
| Olichney et al. (2010) | 15 AD | 72.9 (8.6) | 14.7 (2.3) | 24.4 | Language processing | AD < Controls | 7 |
| 15 controls | 68.7 (12.1) | 15.5 (2.4) | AD > Controls | 20 | |||
| Pariente et al. (2005) | 12 AD | 70.9 (6.4) | 12.9 (2.3) | 25.1 (1.8) | Memory encoding | AD < Controls | 7 |
| 17 controls | 70.6 (5.6) | 13.20 (3.8) | 29.0 (1.0) | Memory retrieval | AD > Controls | 13 | |
| Parra et al. (2013) | 10 AD | 78.0 (7.56) | 23.6 (3.37) | Emotional retrieval | AD < Controls | 2 | |
| 10 controls | 74.0 (8.89) | 29.1 (1.60) | |||||
| Peelle et al. (2014) | 12 AD | 68.8 (10.18) | 16.7 (2.99) | 22.5 (6.1) | Semantic processing | AD < Controls | 3 |
| 21 controls | 65.0 (9.22) | 15.2 (2.3) | 28.0 (1.3) | ||||
| Petrella et al. (2007) | 13 AD | 71.4 (6.8) | 12.7 (2.3) | 24.6 (2.4) | Memory encoding | AD < Controls | 8 |
| 28 controls | 72.0 (4.9) | 16.3 (2.8) | 28.3 (1.4) | AD > Controls | 10 | ||
| Pihlajamaki et al. (2008) | 15 AD | 78.3 (6.9) | 13.3 (3.2) | 23.3 (4.2) | Memory encoding | AD < Controls | 4 |
| 29 controls | 74.2 (5.6) | 15.6 (2.6) | 29.7 (0.5) | AD > Controls | 6 | ||
| Pihlajamaki et al. (2010) | 15 AD | 78.3 (7.1) | 13.3 (3.2) | Memory encoding | AD > Controls | 14 | |
| 30 controls | 74.0 (5.5) | 15.6 (2.7) | |||||
| Rémy et al. (2004) | 7 AD | 70.4 (10.3) | 13.1 (2.8) | 20.7 (7.4) | Executive function | AD < Controls | 8 |
| 11 controls | 65.9 (5.7) | 13.3 (2.6) | 29.4 (0.5) | AD > Controls | 1 | ||
| Rémy et al. (2005) | 8 AD | 72.2 (10.8) | 13.1 (2.8) | 21.2 (6.4) | Memory encoding | AD < Controls | 24 |
| 11 controls | 65.9 (5.7) | 13.3 (2.6) | 29.4 (0.5) | Memory retrieval | AD > Controls | 8 | |
| Saykin et al. (1999) | 9 AD | 79.0 (5.0) | 17.0 (2.0) | Language processing | AD < Controls | 11 | |
| 6 controls | 71.0 (4.0) | 16.0 (2.0) | AD > Controls | 10 | |||
| Shanks et al. (2007) | 9 AD | 74.9 (10.1) | 12.2 (3.7) | 27–30 | Selective attention | AD < Controls | 3 |
| 9 controls | 75.1 (1.6) | 11.7 (2.3) | 27–30 | AD > Controls | 3 | ||
| Sperling et al. (2003) | 7 AD | 80.6 (6.9) | 22.6 (2.2) | Memory encoding | AD < Controls | 15 | |
| 10 controls | 74.1 (7.3) | AD > Controls | 16 | ||||
| Thiyagesh et al. (2009) | 12 AD | 76.4 (7.0) | 9.9 (1.4) | 22.6 (4.0) | Visuospatial | AD < Controls | 12 |
| 13 controls | 71.2 (4.9) | 11.5 (2.0) | 28.5 (1.0) | AD > Controls | 3 | ||
| Thiyagesh et al. (2010) | 10 AD | 76.0 (6.5) | 9.8 (1.3) | 24.1 (3.5) | Visuospatial | AD > Controls | 3 |
| 11 controls | 70.2 (4.4) | 11.3 (1.9) | 28.8 (0.8) | ||||
| Vannini et al. (2008) | 13 AD | 68.9 (6.9) | 12.5 (3.6) | 25.5 (2.3) | Visuospatial | AD < Controls | 22 |
| 13 controls | 68.7 (7.8) | 13.2 (3.9) | AD > Controls | 1 | |||
| Vidoni et al. (2012) | 9 AD | 69.0 (7.2) | 14.8 (7.9) | 21.7 (3.4) | Visuomotor | AD < Controls | 2 |
| 10 controls | 73.6 (6.3) | 16.1 (2.8) | 29.8 (0.4) | AD > Controls | 9 | ||
| Yetkin et al. (2006) | 9 AD | 68.0 (10.0) | 14.0 (3.0) | 23.1 (3.1) | Working memory | AD < Controls | 7 |
| 8 controls | 65.0 (7.0) | 13.0 (1.0) | 30.0 (0.0) | AD > Controls | 19 | ||
| Zamboni et al. (2013) | 17 AD | 76.7 (5.4) | 14.3 (4.0) | 22.2 (3.0) | Self‐awareness | AD < Controls | 12 |
| 17 controls | 75.5 (4.8) | 14.9 (2.8) | 29.85 (0.7) | ||||
Abbreviations: MMSE, Mini‐Mental State Examination; fMRI, functional magnetic resonance imaging; ICA, independent components analysis
MCI‐Related Meta‐Analysis
In the overall meta‐analysis of task‐based fMRI studies, MCI‐related hypoactivation relative to healthy elderly was observed in the right putamen, right insula, right hippocampus, left inferior and middle frontal gyrus, and left middle temporal gyrus; MCI‐related hyperactivation relative to healthy elderly was mainly found in the left superior temporal gyrus, bilateral insula, left claustrum, right inferior frontal gyrus, right middle frontal gyrus, left parahippocampus, right inferior parietal lobule, and right supramarginal gyrus (Table 2, Fig. 2).
Table 2.
ALE results for MCI‐related and AD‐related task‐based fMRI meta‐analyses
| Volume (mm3) | Weighted center | Extrema value | Maximum ALE value | BA | Anatomical label | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | |||||
| Healthy controls > MCI (276 foci, 30 experiments) | ||||||||||
| 1 | 1552 | 33.21 | 16.11 | −12.87 | 0.014661 | 30 | 10 | −14 | Putamen | |
| 0.014532 | 34 | 22 | −10 | 47 | Insula (frontoparietal) | |||||
| 2 | 1144 | 28.25 | −33.46 | −9.08 | 0.015837 | 30 | −34 | −10 | Hippocampus | |
| 3 | 720 | −45.42 | 16.37 | −16.16 | 0.014161 | −48 | 16 | −14 | 47 | Inferior frontal gyrus (limbic) |
| 0.013944 | −42 | 16 | −20 | 47 | Inferior frontal gyrus (limbic) | |||||
| 4 | 624 | −60.12 | −46.9 | 0.64 | 0.014217 | −60 | −44 | 0 | 21 | Middle temporal gyrus (default) |
| 0.010393 | −56 | −60 | 2 | 37 | Middle temporal gyrus (dorsal attention) | |||||
| 5 | 512 | −49.44 | 12.44 | 35.3 | 0.012688 | −50 | 12 | 36 | 9 | Middle frontal gyrus (frontoparietal) |
| 6 | 448 | −41.34 | 23.04 | 14.46 | 0.011563 | −44 | 22 | 16 | 46 | Middle frontal gyrus (frontoparietal) |
| MCI > Healthy controls (322 foci, 28 experiments) | ||||||||||
| 1 | 1104 | −50.46 | −17 | 4.52 | 0.014216 | −50 | −16 | 4 | 22 | Superior temporal gyrus (somatomotor) |
| 0.011706 | −40 | −20 | 2 | 13 | Insula (somatomotor) | |||||
| 0.011561 | −56 | −10 | 4 | 22 | Superior temporal gyrus (somatomotor) | |||||
| 0.010957 | −60 | −26 | 8 | 41 | Superior temporal gyrus (somatomotor) | |||||
| 0.009894 | −38 | −12 | 4 | Claustrum (ventral attention) | ||||||
| 2 | 992 | 49.42 | 19.95 | 16.7 | 0.013785 | 56 | 22 | 20 | 9 | Inferior frontal gyrus (frontoparietal) |
| 0.012846 | 46 | 16 | 10 | 13 | Insula | |||||
| 0.011527 | 46 | 20 | 16 | 46 | Middle frontal gyrus | |||||
| 3 | 824 | −15.63 | −14.29 | −13.01 | 0.013940 | −14 | −14 | −14 | 28 | Parahippocampal gyrus |
| 0.013257 | −18 | −20 | −14 | 35 | Parahippocampal gyrus | |||||
| 4 | 584 | 62.22 | −29.16 | 24.9 | 0.014395 | 62 | −30 | 24 | 40 | Inferior parietal lobule (ventral attention) |
| 5 | 528 | 45.71 | −36.85 | 40.11 | 0.011823 | 44 | −40 | 42 | 40 | Supramarginal gyrus (dorsal attention) |
| Healthy controls > AD (244 foci, 29 experiments) | ||||||||||
| 1 | 2808 | −31.9 | −23.39 | −15.79 | 0.013243 | −26 | −20 | −18 | Hippocampus | |
| 0.012076 | −34 | −30 | −20 | 36 | Parahippocampal gyrus | |||||
| 0.009811 | −22 | −30 | −4 | 27 | Parahippocampal gyrus | |||||
| 2 | 1256 | −44.38 | −75.59 | 0.56 | 0.015756 | −46 | −74 | 0 | 37 | Inferior temporal gyrus (visual) |
| 3 | 1128 | −11.14 | 22.68 | 49.36 | 0.012152 | −6 | 26 | 48 | 8 | Medial frontal gyrus (frontoparietal) |
| 0.009945 | −20 | 16 | 48 | 32 | Medial frontal gyrus (default) | |||||
| 0.009657 | −22 | 22 | 48 | 6 | Superior frontal gyrus (default) | |||||
| 4 | 1000 | −28.72 | −79.51 | −6.37 | 0.016688 | −28 | −80 | −6 | 19 | Lingual gyrus |
| 5 | 744 | 29.95 | −20.11 | −18.4 | 0.011889 | 28 | −18 | −20 | Parahippocampal gyrus | |
| 0.011831 | 32 | −20 | −16 | Parahippocampal gyrus | ||||||
| 6 | 720 | −44.68 | −21.46 | −4.33 | 0.012743 | −44 | −20 | −6 | 13 | Insula |
| 7 | 592 | −49.36 | −3.87 | 28.36 | 0.012419 | −50 | −4 | 28 | 6 | Precentral gyrus (somatomotor) |
| 8 | 584 | 25.49 | −23.85 | 1.51 | 0.012015 | 30 | −26 | 0 | Thalamus | |
| 0.010222 | 22 | −22 | 2 | Ventral posterior lateral nucleus | ||||||
| AD > Healthy controls (201 foci, 28 experiments) | ||||||||||
| 1 | 2240 | −12.3 | −60.9 | 40.5 | 0.013268 | −10 | −58 | 46 | 7 | Precuneus (default) |
| 0.011640 | −10 | −64 | 32 | 31 | Precuneus (default) | |||||
| 0.010246 | −20 | −64 | 42 | 7 | Precuneus (dorsal attention) | |||||
| 2 | 1768 | 13.7 | −61.29 | 37.95 | 0.017395 | 12 | −64 | 38 | 7 | Cuneus (default) |
| 3 | 856 | 44.17 | 14.04 | 33.89 | 0.014982 | 44 | 14 | 36 | 9 | Precentral gyrus (frontoparietal) |
| 4 | 600 | 9.96 | −16.73 | 37.94 | 0.013933 | 10 | −16 | 38 | 24 | Cingulate gyrus (ventral attention) |
| 5 | 584 | −52.46 | 5.01 | 16.3 | 0.012293 | −52 | 6 | 18 | 44 | Inferior frontal gyrus (ventral attention) |
| 6 | 544 | −21.14 | −11.39 | −0.79 | 0.012364 | −20 | −12 | 0 | Lentiform nucleus | |
| 0.008278 | −28 | −6 | 0 | Putamen | ||||||
Figure 2.
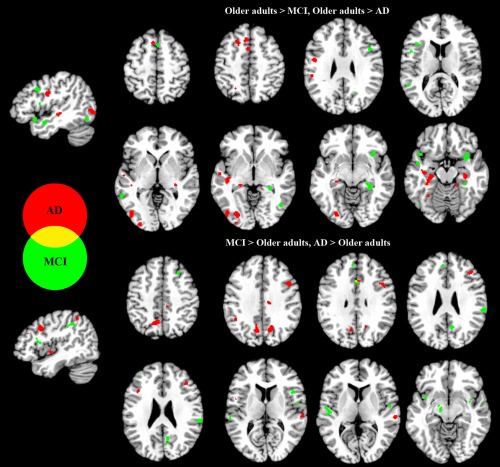
Regions exhibiting significantly greater activation when comparing MCI patients and healthy older adults, and AD patients and healthy older adults. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
We also compared the functional activation between MCI patients and healthy elderly during several cognitive tasks. In tasks related to memory encoding, MCI‐related hypoactivation relative to healthy elderly was observed in left inferior frontal gyrus, left insula, right fusiform gyrus, left superior temporal gyrus, and left inferior parietal lobule while MCI‐related hyperactivation relative to healthy elderly was found in the left lateral globus pallidus, left parahippocampus, right inferior frontal gyrus, and right middle frontal gyrus (Supporting Information Table II). The meta‐analysis of memory‐retrieval tasks revealed MCI‐related hypoactivation of bilateral hippocampus, right parahippocampus, bilateral middle frontal gyrus, right insula, left inferior parietal lobule, and the left precuneus while no hyperactivation cluster was found in MCI (Supporting Information Table III). In executive function and working memory tasks, no MCI‐related hypoactivation brain regions were found, whereas MCI‐related hyperactivation was found in the right precentral gyrus, left insula, left postcentral gyrus, left claustrum, left superior temporal gyrus, right precuneus, left fusiform gyrus, right cingulate gyrus, and left paracentral lobule (Supporting Information Table IV). Due to the limited number of relevant studies, no significant hyperactivation or hypoactivation was found in tasks of attention and visuospatial processing, emotional processing, or language processing. The results of resting‐state fMRI studies in MCI patients can be found in Supporting Information Results (Supporting Information Table V).
AD‐Related Meta‐Analysis
The meta‐analysis on AD revealed that in the overall meta‐analysis of task‐based fMRI studies, AD‐related hypoactivation relative to healthy elderly was observed mainly in subcortical regions, including the left hippocampus, bilateral parahippocampal gyri, right thalamus, left insula, right ventral posterior lateral nucleus, left inferior temporal gyrus, left medial and superior frontal gyrus, left lingual gyrus, and left precentral gyrus. In contrast, AD‐related hyperactivation relative to healthy elderly was found mainly in left precuneus, left cuneus, right precentral gyrus, right cingulate gyrus, left inferior frontal gyrus, and left lentiform gyrus/putamen (Table 2, Fig. 2).
In memory‐encoding tasks, AD‐related hypoactivation relative to healthy elderly was observed in bilateral hippocampi, left parahippocampus, left putamen, and left inferior parietal lobule; AD‐related hyperactivation relative to healthy elderly was observed in right cuneus, bilateral precuneus, left amygdala, and right middle frontal gyrus (Supporting Information Table II). In memory‐retrieval tasks, we detected AD‐related hypoactivation of the left parahippocampal gyrus, no significant AD‐related hyperactivation was found (Supporting Information Table III). For tasks of executive function and working memory, AD‐related hypoactivation was observed in the left insula; AD‐related hyperactivation was found in left cingulate gyrus (Supporting Information Table IV). Attention and visuospatial processing yielded AD‐related hypoactivation mainly in the left inferior temporal gyrus (−46, −72, 2) and no AD‐related hyperactivation clusters were found. AD patients exhibited decreased activation of lingual gyrus (−28, −80, −6) in language processing tasks. The results of resting‐state fMRI studies in AD patients can be found in Supporting Information Table V.
Relationship with Neuronal Networks
Based on the seven neuronal network parcellations of the human brain, we identified the percentage of significant voxels located in each network. For task‐based fMRI meta‐analysis, MCI‐related hypoactivation was found mainly in the default mode (29.9%) and frontoparietal (24.5%) networks while hyperactivation was found mainly in the ventral attention (29.8%), somatomotor (27.7%), frontoparietal (19.1%), and default mode (16.9%) networks (Fig. 3). The distribution of MCI‐related hypoactivation and hyperactivation between the networks differed significantly (χ 2 > 100, P < 0.0001).
Figure 3.
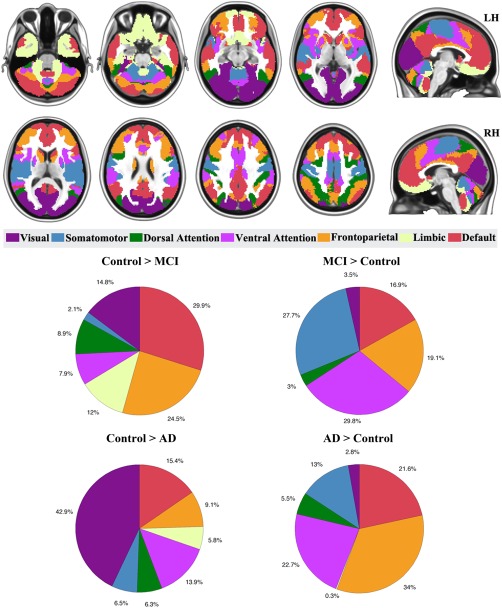
Proportions of MCI‐related, and AD‐related hypoactivation or hyperactivation in the overall meta‐analyses of task‐based fMRI studies, merged from cortical, cerebellar, and striatal networks. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
For task‐based fMRI meta‐analysis, AD‐related hypoactivation voxels were mainly in the visual (42.9%), default mode network (15.4%), and ventral attention (13.9%) networks. AD‐related hyperactivation voxels were found mainly in frontoparietal (34%), ventral attention (22.7%), and default mode (21.6%) networks (Fig. 3). The distribution of hypoactivation and hyperactivation between the networks differed significantly (χ 2 > 100, P < 0.0001).
The neuronal network results of the specific tasks and resting state in MCI and AD patients can be found in Supporting Information Results.
DISCUSSION
To the best of our knowledge, this work is the first meta‐analysis exploring the large‐scale neuronal network dysfunctions in MCI and AD. The results revealed that MCI and AD patients presented different pathological while shared similar compensatory mechanisms in large‐scale networks during fulfilling the cognitive tasks.
MCI‐ and AD‐Related Task‐Based fMRI Meta‐Analysis
MCI and AD patients presented different decreased patterns, implying that the neuronal network dysfunctions might be a consequence of the disease progression. MCI individuals showed hypoactivation in regions within the frontoparietal and default mode network relative to controls while AD patients showed hypoactivation in regions within the visual network relative to controls. Frontoparietal network was considered to be an important flexible hub for initiating and modulating cognitive control [Cole et al., 2013; Dosenbach et al., 2008]. The decreased activation in frontoparietal network suggested the reduced cognitive control ability in MCI patients. Moreover, further task‐specific analysis revealed that the decreased frontoparietal network in MCI was mainly contributed by the memory‐retrieval tasks. The findings were partly consistent with previous memory retrieval meta‐analysis [Schwindt and Black, 2009], perhaps reflecting worse self‐monitoring in MCI individuals [Christoff and Gabrieli, 2000]. With the progression to AD, patients presented decreased activation in visual network. Visual cortex has been reported dysfunctional in AD; the underlying pathology might be due to the neurofibrillary tangles and amyloid plaques [Lewis et al., 1987; Morrison et al., 1991]. Other studies also suggested that the reduction choline acetyltransferase activity in primary visual cortex resulted in the cognitive deficits in AD [Ikonomovic et al., 2005]. These findings indicated that the visual network deficits might be due to the consequence of the disease, meanwhile, inefficiently visual network activation might interfere the following higher cognitive processing in AD patients. Many fMRI studies have reported decreased activity of the default network in MCI [Cha et al., 2013; Jin et al., 2012; Zhu et al., 2013] and AD [Brier et al., 2012; Greicius et al., 2004; Wang et al., 2006; Zhou et al., 2010]. In this MCI‐ and AD‐related meta‐analyses, we found most of the decreased regions located in the posterior aspect of the default network, these parts were considered to be related to memory retrieval [Buckner et al., 2008] and played neurodegenerative roles during cognitive decline [Jacobs et al., 2013].
The increased patterns of neuronal networks were similar between MCI and AD patients, implying the similar compensatory mechanisms underlying the functional brain activity. The frontoparietal compensatory hypothesis has been consistently reported in previous meta‐analyses in MCI and AD [Browndyke et al., 2013; Schwindt and Black, 2009]. Many previous task‐based fMRI studies found that MCI patients recruited more activity than healthy controls in frontal and parietal regions across memory encoding and retrieval, working memory, executive function, and perception tasks [Bokde et al., 2010b; Bokde et al., 2008; Hämäläinen et al., 2007; Kaufmann et al., 2008; LeyHe et al., 2009; Poettrich et al., 2009; Yetkin et al., 2006]. Moreover, increased activity in frontal and parietal areas was consistently found in AD patients across a variety of tasks, including memory encoding and retrieval, working memory, perception, and language processing [Bokde et al., 2010a; Bosch et al., 2010; Hämäläinen et al., 2007; Yetkin et al., 2006]. The present findings implicated that ventral attention, somotomotor, and default networks were also involved in the compensatory processing besides frontoparietal network. Ventral attention network are considered to be responsible for the endogenous attention orienting process [Corbetta and Shulman, 2002]. Somatomotor network is involved in the episodic memory, action recognition, and spatial navigation [Russ et al., 2003]. The increased default network regions were mainly in the anterior regions; the anterior aspect of the default network was more associated with self‐referential thoughts and cognitive control [Buckner et al., 2008] and played compensatory roles in the degenerative process [Jacobs et al., 2013].
Meta‐Analysis for Specific Task
The large‐scale functional network disruptions presented differentially patterns across cognitive tasks in MCI and AD patients. Episodic memory deficit was the core characteristics of cognitive decline in MCI and AD. For memory encoding meta‐analyses, MCI patients showed hypoactivation in visual, dorsal attention, and ventral attention networks, more percentages of hypoactivation voxels were found in these networks in AD patients, these results suggested that the reduced visual processing and the attentional orienting might influence subsequent encoding of the stimuli [Corbetta et al., 2008; Corbetta and Shulman, 2002]. Moreover, the hyperactivation voxels in MCI were focused exclusively in frontoparietal network while frontoparietal and default networks occupied 98% of the hyperactivation voxels in AD‐related memory encoding meta‐analysis, these results implied that frontoparietal network played an important role in dealing with the memory decline in MCI and AD. Moreover, default network was also involved in this compensatory process when the disease progressed to AD. Several other cognitive tasks also revealed interesting and differential results, for AD patients, they presented 94.1% of the hypoactivation voxels in default network in executive function and working memory tasks while demonstrated 92% of the hypoactivation voxels in visual networks in attention and visuospatial tasks; these results reflected that the dysfunctional large‐scale networks in MCI and AD are influenced by specific type of cognitive task. However, due to the limited number of studies, the results should be treated cautiously.
MCI‐ and AD‐Related Resting‐State fMRI Meta‐Analyses
During the resting‐state fMRI meta‐analysis, MCI patients showed similar lower activation in default, frontoparietal, and limbic networks than healthy controls, as well as task‐based fMRI meta‐analysis. The results further suggested that reduced self‐monitoring and executive control ability resulted in the cognitive decline in MCI patients. MCI patients presented exclusively higher activity in default network. Recent studies revealed increased functional connectivity in default network might be compensated to disruptions of other networks [De Vogelaere et al., 2012; Esposito et al., 2013; Jin et al., 2012; Li et al., 2012]. Functional differentiation of the default network may result in the bidirectional significant clusters that were observed in MCI patients. People with MCI have been reported to demonstrate both increased and decreased activity in the default network, which suggests that both deficits and functional compensation may coexist in the default network [Qi et al., 2010]. Regarding the fact that only eight resting‐state fMRI studies contributed to the AD‐related meta‐analysis, the results should be treated carefully.
General Discussion
The large‐scale brain network approach has become increasingly important in understanding the neural mechanisms of cognitive decline in pathological aging [Bressler and Menon, 2010; Menon, 2011], and it was deemed to be a promising biomarker for disease diagnosis and monitoring of MCI and AD. Actually, varying levels of biomarkers may be related to disease progression from MCI and AD [Jack et al., 2010]. Previous studies have consistently found amyloid‐β and tau pathology in the default mode network during the progression of AD [Buckner et al., 2005; Kapogiannis and Mattson, 2011; Small et al., 2006]. Although amyloid‐β and tau pathology were considered to be most sensitive biomarkers for AD, however, any single biomarker cannot predict the conversion to AD. Multiple biomarkers must be combined to detect and predict disease progression. The present findings suggested that the functional neuronal networks might be a useful imaging biomarker that may have important implications in elucidating the underlying pathologic mechanisms in pathological aging.
Although cognitive decline is consistently described in pathologically aging populations, meta‐analyses of cognitive intervention revealed cognitive plasticity in MCI [Li et al., 2011] and AD patients [Sitzer et al., 2006]. Recent cognitive intervention studies have reported that training‐related brain plasticity is observed in individuals with MCI [Belleville et al., 2011; Li et al., 2014]. The present results imply that the neuronal networks may be useful to evaluate the effects of cognitive intervention and investigate the underlying mechanisms of brain plasticity.
Limitations
Several limitations must be considered in this meta‐analysis. First, participants across selected studies were heterogeneous, which may potentially influence the meta‐analysis results. In the MCI‐related and AD‐related meta‐analyses, diagnostic and inclusion criteria of MCI and AD, age, gender, handedness, behavioral performance, duration of illness, pathology severity, medication dosage, and other clinical symptoms experienced by MCI and AD patients are not the same, and these differences may influence brain activation. Second, this ALE model could not evaluate the relative weights among studies that used differing criteria for statistical significance. Third, we did not concern the scattered deactivation coordinates or studies only focused on deactivation. Because only few studies reported deactivation coordinates, it would not be beneficial to explore deactivation patterns at present. Finally, although these seven neuronal networks cover the cerebral neuronal network and the functional projections to the cortex from the cerebellum and striatum, some important subcortical memory related regions, such as the hippocampus and parahippocampus, were not included in the neuronal networks. These regions played important roles in pathological aging; therefore, the proportions of MCI‐related and AD‐related hypoactivation and hyperactivation may be influenced due to the lack of consideration memory network regions.
CONCLUSIONS
These meta‐analyses demonstrated the extent and nature of the functional abnormal activation and large‐scale neuronal network dysfunction in MCI and AD. The decreased activation was mainly detected in frontoparietal and default networks in MCI whereas AD patients showed more hypoactivation voxels in visual network. Similar frontoparietal, ventral attention, somatomotor, and default networks were involved in the compensatory process in these two populations. This large‐scale network approach reveals neuronal network changes in cognitive decline and may provide potential insights in evaluating brain pathological aging at a system level.
Supporting information
Supplementary Information
Supplementary Information Figure S1.
Supplementary Information Figure S2.
Supplementary Information Figure S3.
Supplementary Information Tabel R1.
The authors have no conflict of interest to report.
REFERENCES
- Alichniewicz KK, Brunner F, Klunemann HH, Greenlee MW (2012): Structural and functional neural correlates of visuospatial information processing in normal aging and amnestic mild cognitive impairment. Neurobiol Aging 33:2782–2797. [DOI] [PubMed] [Google Scholar]
- Andrews‐Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL (2007): Disruption of large‐scale brain systems in advanced aging. Neuron 56:924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaiz E, Almkvist O (2003): Neuropsychological features of mild cognitive impairment and preclinical Alzheimer's disease. Acta Neurol Scand Suppl 179:34–41. [PubMed] [Google Scholar]
- Baglio F, Castelli I, Alberoni M, Blasi V, Griffanti L, Falini A, Nemni R, Marchetti A (2012): Theory of mind in amnestic mild cognitive impairment: An FMRI study. J Alzheimers Dis 29:25–37. [DOI] [PubMed] [Google Scholar]
- Belleville S, Clément F, Mellah S, Gilbert B, Fontaine F, Gauthier S (2011): Training‐related brain plasticity in subjects at risk of developing Alzheimer's disease. Brain 134:1623–1634. [DOI] [PubMed] [Google Scholar]
- Bokde AL, Lopez‐Bayo P, Born C, Dong W, Meindl T, Leinsinger G, Teipel SJ, Faltraco F, Reiser M, Möller H‐J, Hampel H (2008): Functional abnormalities of the visual processing system in subjects with mild cognitive impairment: An fMRI study. Psychiatry Res 163:248–259. [DOI] [PubMed] [Google Scholar]
- Bokde AL, Lopez‐Bayo P, Born C, Ewers M, Meindl T, Teipel SJ, Faltraco F, Reiser MF, Moller HJ, Hampel H (2010a): Alzheimer disease: functional abnormalities in the dorsal visual pathway. Radiology 254:219–226. [DOI] [PubMed] [Google Scholar]
- Bokde AL, Karmann M, Born C, Teipel SJ, Omerovic M, Ewers M, Frodl T, Meisenzahl E, Reiser M, Möller H‐J, Hampel H (2010b): Altered brain activation during a verbal working memory task in subjects with amnestic mild cognitive impairment. J Alzheimers Dis 21:103–118. [DOI] [PubMed] [Google Scholar]
- Bosch B, Bartres‐Faz D, Rami L, Arenaza‐Urquijo EM, Fernandez‐Espejo D, Junque C, Sole‐Padulles C, Sanchez‐Valle R, Bargallo N, Falcon C, Molinuevo JL (2010): Cognitive reserve modulates task‐induced activations and deactivations in healthy elders, amnestic mild cognitive impairment and mild Alzheimer's disease. Cortex 46:451–461. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Menon V (2010): Large‐scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci 14:277–290. [DOI] [PubMed] [Google Scholar]
- Brier MR, Thomas JB, Snyder AZ, Benzinger TL, Zhang DY, Raichle ME, Holtzman DM, Morris JC, Ances BM (2012): Loss of Intranetwork and Internetwork Resting State Functional Connections with Alzheimer's Disease Progression. J Neurosci 32:8890–8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browndyke JN, Giovanello K, Petrella J, Hayden K, Chiba‐Falek O, Tucker KA, Burke JR, Welsh‐Bohmer KA (2013): Phenotypic regional functional imaging patterns during memory encoding in mild cognitive impairment and Alzheimer's disease. Alzheimers Dement 9:284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC (2005): Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 25:7709–7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL (2008): The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT (2011): The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 106:2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, DePeau K, Rentz DM, Selkoe DJ, Blacker D, Albert MS, Sperling RA (2006): Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J Neurosci 26:10222–10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J, Jo HJ, Kim HJ, Seo SW, Kim HS, Yoon U, Park H, Na DL, Lee JM (2013): Functional alteration patterns of default mode networks: comparisons of normal aging, amnestic mild cognitive impairment and Alzheimer's disease. Eur J Neurosci 37:1916–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Sperling RA (2012): Functional MRI of mnemonic networks across the spectrum of normal aging, mild cognitive impairment, and Alzheimer's disease. J Alzheimers Dis 31(Suppl 3):S155–S167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EY, Yeo BT, Buckner RL (2012): The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol 108:2242–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Gabrieli JDE (2000): The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology 28:168–186. [Google Scholar]
- Clément F, Belleville S (2010): Compensation and disease severity on the memory‐related activations in mild cognitive impairment. Biol Psychiat 68:894–902. [DOI] [PubMed] [Google Scholar]
- Clément F, Belleville S (2012): Effect of disease severity on neural compensation of item and associative recognition in mild cognitive impairment. J Alzheimers Dis 29:109–123. [DOI] [PubMed] [Google Scholar]
- Clément F, Gauthier S, Belleville S (2013): Executive functions in mild cognitive impairment: emergence and breakdown of neural plasticity. Cortex 49:1268–1279. [DOI] [PubMed] [Google Scholar]
- Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS (2013): Multi‐task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci 16:1348–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole LJ, Farrell MJ, Duff EP, Barber JB, Egan GF, Gibson SJ (2006): Pain sensitivity and fMRI pain‐related brain activity in Alzheimer's disease. Brain 129:2957–2965. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL (2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3:215–229. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL (2008): The reorienting system of the human brain: from environment to theory of mind. Neuron 58:306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, Castellanos FX (2012): Toward Systems Neuroscience of ADHD: A Meta‐Analysis of 55 fMRI Studies. Am J Psychiat 169:1038–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannhauser TM, Shergill SS, Stevens T, Lee L, Seal M, Walker RW, Walker Z (2008): An fMRI study of verbal episodic memory encoding in amnestic mild cognitive impairment. Cortex 44:869–880. [DOI] [PubMed] [Google Scholar]
- Dannhauser TM, Walker Z, Stevens T, Lee L, Seal M, Shergill SS (2005): The functional anatomy of divided attention in amnestic mild cognitive impairment. Brain 128:1418–1427. [DOI] [PubMed] [Google Scholar]
- De Vogelaere F, Santens P, Achten E, Boon P, Vingerhoets G (2012): Altered default‐mode network activation in mild cognitive impairment compared with healthy aging. Neuroradiology 54:1195–1206. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Sperling RA (2009): Large‐scale functional brain network abnormalities in Alzheimer's disease: insights from functional neuroimaging. Behav Neurol 21:63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donix M, Jurjanz L, Meyer S, Amanatidis EC, Baeumler D, Huebner T, Poettrich K, Smolka MN, Holthoff VA (2013): Functional imaging during recognition of personally familiar faces and places in Alzheimer's disease. Arch Clin Neuropsychol 28:72–80. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE (2008): A dual‐networks architecture of top‐down control. Trends Cogn Sci 12:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito R, Mosca A, Pieramico V, Cieri F, Cera N, Sensi SL (2013): Characterization of resting state activity in MCI individuals. PeerJ 1:e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraco CC, Puente AN, Brown C, Terry DP, Miller LS (2013): Lateral temporal hyper‐activation as a novel biomarker of mild cognitive impairment. Neuropsychologia 51:2281–2293. [DOI] [PubMed] [Google Scholar]
- Giovanello KS, De Brigard F, Ford JH, Kaufer DI, Burke JR, Browndyke JN, Welsh‐Bohmer KA (2012): Event‐related functional magnetic resonance imaging changes during relational retrieval in normal aging and amnestic mild cognitive impairment. J Int Neuropsychol Soc 18:886–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golby A, Silverberg G, Race E, Gabrieli S, O'Shea J, Knierim K, Stebbins G, Gabrieli J (2005): Memory encoding in Alzheimer's disease: An fMRI study of explicit and implicit memory. Brain 128:773–787. [DOI] [PubMed] [Google Scholar]
- Gould RL, Brown RG, Owen AM, Bullmore ET, Williams SCR, Howard RJ (2005): Functional neuroanatomy of successful paired associate learning in Alzheimer's disease. Am J Psychiatry 162:2049–2060. [DOI] [PubMed] [Google Scholar]
- Gould RL, Brown RG, Owen AM, Bullmore ET, Howard RJ (2006): Task‐induced deactivations during successful paired associates learning: An effect of age but not Alzheimer's disease. Neuroimage 31:818–831. [DOI] [PubMed] [Google Scholar]
- Grön G, Bittner D, Schmitz B, Wunderlich AP, Riepe MW (2002): Subjective memory complaints: Objective neural markers in patients with Alzheimer's disease and major depressive disorder. Ann Neurol 51:491–498. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V (2004): Default‐mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA 101:4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Koenig P, DeVita C, Glosser G, Moore P, Gee J, Detre J, Alsop D (2003a): Neural basis for verb processing in Alzheimer's disease: An fMRI study. Neuropsychology 17:658–674. [DOI] [PubMed] [Google Scholar]
- Grossman M, Koenig P, Glosser G, DeVita C, Moore P, Rhee J, Detre J, Alsop D, Gee J (2003b): Neural basis for semantic memory difficulty in Alzheimer's disease: An fMRI study. Brain 126:292–311. [DOI] [PubMed] [Google Scholar]
- Hämäläinen A, Pihlajamaki M, Tanila H, Hanninen T, Niskanen E, Tervo S, Karjalainen PA, Vanninen RL, Soininen H (2007): Increased fMRI responses during encoding in mild cognitive impairment. Neurobiol Aging 28:1889–1903. [DOI] [PubMed] [Google Scholar]
- Hanseeuw B, Dricot L, Kavec M, Grandin C, Seron X, Ivanoiu A (2011): Associative encoding deficits in amnestic mild cognitive impairment: A volumetric and functional MRI study. Neuroimage 56:1743–1748. [DOI] [PubMed] [Google Scholar]
- He Y, Chen Z, Gong G, Evans A (2009): Neuronal networks in Alzheimer's disease. Neuroscientist 15:333–350. [DOI] [PubMed] [Google Scholar]
- Heun R, Freymann K, Erb M, Leube DT, Jessen F, Kircher TT, Grodd W (2007): Mild cognitive impairment (MCI) and actual retrieval performance affect cerebral activation in the elderly. Neurobiol Aging 28:404–413. [DOI] [PubMed] [Google Scholar]
- Husain MM, Garrett RK (2005): Clinical diagnosis and management of Alzheimer's disease. Neuroimaging Clin N Am 15:767–777. [DOI] [PubMed] [Google Scholar]
- Ikonomovic MD, Mufson EJ, Wuu J, Bennett DA, DeKosky ST (2005): Reduction of choline acetyltransferase activity in primary visual cortex in mild to moderate Alzheimer's disease. Arch Neurol 62:425–430. [DOI] [PubMed] [Google Scholar]
- Jack CR Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ (2010): Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol 9:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs HI, Gronenschild EH, Evers EA, Ramakers IH, Hofman PA, Backes WH, Jolles J, Verhey FR, Van Boxtel MP (2012): Visuospatial processing in early Alzheimer's disease: A multimodal neuroimaging study (in press) [DOI] [PubMed] [Google Scholar]
- Jacobs HI, Radua J, Luckmann HC, Sack AT (2013): Meta‐analysis of functional network alterations in Alzheimer's disease: Toward a network biomarker. Neurosci Biobehav Rev 37:753–765. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Moritz CH, Meyerand ME, Rowley HA, Alexander AL, Hansen KW, Gleason CE, Carlsson CM, Ries ML, Asthana S, Chen K, Reiman EM, Alexander GE (2006): Activation of brain regions vulnerable to Alzheimer's disease: The effect of mild cognitive impairment. Neurobiol Aging 27:1604–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin MW, Pelak VS, Cordes D (2012): Aberrant default mode network in subjects with amnestic mild cognitive impairment using resting‐state functional MRI. Magn Reson Imaging 30:48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapogiannis D, Mattson MP (2011): Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer's disease. Lancet Neurol 10:187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Knopman D, Liu HY (2001): Dissociation of regional activation in mild AD during visual encoding—A functional MRI study. Neurology 57:812–816. [DOI] [PubMed] [Google Scholar]
- Kaufmann L, Ischebeck A, Weiss E, Koppelstaetter F, Siedentopf C, Vogel SE, Gotwald T, Marksteiner J, Wood G (2008): An fMRI study of the numerical Stroop task in individuals with and without minimal cognitive impairment. Cortex 44:1248–1255. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Weis S, Freymann K, Erb M, Jessen F, Grodd W, Heun R, Leube DT (2007): Hippocampal activation in patients with mild cognitive impairment is necessary for successful memory encoding. J Neurol Neurosurg Psychiatry 78:812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher TTJ, Erb M, Grodd W, Leube DT (2005): Cortical activation during cholinesterase‐inhibitor treatment in Alzheimer disease—Preliminary findings from a pharmaco‐fMRI study. Am J Geriat Psychiatry 13:1006–1013. [DOI] [PubMed] [Google Scholar]
- Koch W, Teipel S, Mueller S, Benninghoff J, Wagner M, Bokde ALW, Hampel H, Coates U, Reiser M, Meindl T (2012): Diagnostic power of default mode network resting state fMRI in the detection of Alzheimer's disease. Neurobiol Aging 33:466–478. [DOI] [PubMed] [Google Scholar]
- Kochan NA, Breakspear M, Slavin MJ, Valenzuela M, McCraw S, Brodaty H, Sachdev PS (2011): Functional alterations in brain activation and deactivation in mild cognitive impairment in response to a graded working memory challenge. Dement Geriatr Cogn Disord 30:553–568. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT (2005): ALE meta‐analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp 25:155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas‐Gutiérrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT (2007): Bias between MNI and Talairach coordinates analyzed using the ICBM‐152 brain template. Hum Brain Mapp 28:1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TM, Sun D, Leung MK, Chu LW, Keysers C (2013): Neural activities during affective processing in people with Alzheimer's disease. Neurobiol Aging 34:706–715. [DOI] [PubMed] [Google Scholar]
- Lenzi D, Serra L, Perri R, Pantano P, Lenzi GL, Paulesu E, Caltagirone C, Bozzali M, Macaluso E (2011): Single domain amnestic MCI: A multiple cognitive domains fMRI investigation. Neurobiol Aging 32:1542–1557. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Campbell MJ, Terry RD, Morrison JH (1987): Laminar and regional distributions of neurofibrillary tangles and neuritic plaques in Alzheimer's disease: a quantitative study of visual and auditory cortices. J Neurosci 7:1799–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyhe T, Erb M, Milian M, Eschweiler GW, Ethofer T, Grodd W, Saur R (2009): Changes in cortical activation during retrieval of clock time representations in patients with mild cognitive impairment and early Alzheimer's disease. Dement Geriatr Cogn Disord 27:117–132. [DOI] [PubMed] [Google Scholar]
- Li H, Li J, Li N, Li B, Wang P, Zhou T (2011): Cognitive intervention for persons with mild cognitive impairment: A meta‐analysis. Ageing Res Rev 10:285–296. [DOI] [PubMed] [Google Scholar]
- Li R, Wu X, Fleisher AS, Reiman EM, Chen K, Yao L (2012): Attention‐related networks in Alzheimer's disease: A resting functional MRI study. Hum Brain Mapp 33:1076–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Zhu X, Yin S, Niu Y, Zheng Z, Huang X, Wang B, Li J (2014): Multimodal intervention in older adults improves resting‐state functional connectivity between the medial prefrontal cortex and medial temporal lobe. Front Aging Neurosci 10:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zheng L, Zhang JY, Zhou XQ, Ma C, Chen YJ, Shu N, Zhang ZJ (2013): Differences in functional brain activation and hippocampal volume among amnestic mild cognitive impairment subtypes. Curr Alzheimer Res 10:1080–1089. [DOI] [PubMed] [Google Scholar]
- Lim HK, Juh R, Pae CU, Lee BT, Yoo SS, Ryu SH, Kwak KR, Lee C, Lee CU (2008): Altered verbal working memory process in patients with Alzheimer's disease: An fMRI investigation. Neuropsychobiology 57:181–187. [DOI] [PubMed] [Google Scholar]
- Machulda MM, Senjem ML, Weigand SD, Smith GE, Ivnik RJ, Boeve BF, Knopman DS, Petersen RC, Jack CR (2009): Functional magnetic resonance imaging changes in amnestic and nonamnestic mild cognitive impairment during encoding and recognition tasks. J Int Neuropsychol Soc 15:372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandzia JL, McAndrews MP, Grady CL, Graham SJ, Black SE (2009): Neural correlates of incidental memory in mild cognitive impairment: An fMRI study. Neurobiol Aging 30:717–730. [DOI] [PubMed] [Google Scholar]
- McGeown WJ, Shanks MF, Venneri A (2008): Prolonged cholinergic enrichment influences regional cortical activation in early Alzheimer's disease. Neuropsychiatr Dis Treat 4:465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V (2011): Large‐scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 15:483–506. [DOI] [PubMed] [Google Scholar]
- Meulenbroek O, Rijpkema M, Kessels RP, Rikkert MG, Fernandez G (2010): Autobiographical memory retrieval in patients with Alzheimer's disease. Neuroimage 53:331–340. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR, Bouras C (1991): An anatomic substrate for visual disconnection in Alzheimer's disease. Ann N Y Acad Sci 640:36–43. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Taylor JR, Chan SH, Yang JC, Stringfellow A, Hillert DG, Simmons AL, Salmon DP, Iragui‐Madoz V, Kutas M (2010): fMRI responses to words repeated in a congruous semantic context are abnormal in mild Alzheimer's disease. Neuropsychologia 48:2476–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papma JM, den Heijer T, de Koning I, Mattace‐Raso FU, van der Lugt A, van der Lijn F, van Swieten JC, Koudstaal PJ, Smits M, Prins ND (2012): The influence of cerebral small vessel disease on default mode network deactivation in mild cognitive impairment. Neuroimage Clin 2:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariente J, Cole S, Henson R, Clare L, Kennedy A, Rossor M, Cipoloti L, Puel M, Demonet JF, Chollet F (2005): Alzheimer's patients engage an alternative network during a memory task. Ann Neurol 58:870–879. [DOI] [PubMed] [Google Scholar]
- Parra MA, Pattan V, Wong D, Beaglehole A, Lonie J, Wan HI, Honey G, Hall J, Whalley HC, Lawrie SM (2013): Medial temporal lobe function during emotional memory in early Alzheimer's disease, mild cognitive impairment and healthy ageing: An fMRI study. BMC Psychiatry 13:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Powers J, Cook PA, Smith EE, Grossman M (2014): Frontotemporal neural systems supporting semantic processing in Alzheimer's disease. Cogn Affect Behav Neurosci 14:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrella JR, Krishnan S, Slavin MJ, Tran TTT, Murty L, Doraiswamy PM (2006): Mild cognitive impairment: Evaluation with 4‐T functional MR imaging. Radiology 240:177–186. [DOI] [PubMed] [Google Scholar]
- Petrella JR, Wang LH, Krishnan S, Slavin MJ, Prince SE, Tran TTT, Doraiswamy PM (2007): Cortical deactivation in mild cognitive impairment: High‐field strength functional MR Imaging. Radiology 245:224–235. [DOI] [PubMed] [Google Scholar]
- Pihlajamaki M, DePeau KM, Blacker D, Sperling RA (2008): Impaired medial temporal repetition suppression is related to failure of parietal deactivation in Alzheimer disease. Am J Geriat Psychiat 16:283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajamaki M, O'Keefe K, Bertram L, Tanzi RE, Dickerson BC, Blacker D, Albert MS, Sperling RA (2010): Evidence of Altered Posteromedial Cortical fMRI Activity in Subjects at Risk for Alzheimer Disease. Alz Dis Assoc Dis 24:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC (2004): Mild cognitive impairment as a diagnostic entity. J Intern Med 256:183–194. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E (1999): Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 56:303–308. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B (2001): Current concepts in mild cognitive impairment. Arch Neurol 58:1985–1992. [DOI] [PubMed] [Google Scholar]
- Poettrich K, Weiss PH, Werner A, Lux S, Donix M, Gerber J, von Kummer R, Fink GR, Holthoff VA (2009): Altered neural network supporting declarative long‐term memory in mild cognitive impairment. Neurobiol Aging 30:284–298. [DOI] [PubMed] [Google Scholar]
- Qi Z, Wu X, Wang Z, Zhang N, Dong H, Yao L, Li K (2010): Impairment and compensation coexist in amnestic MCI default mode network. Neuroimage 50:48–55. [DOI] [PubMed] [Google Scholar]
- Rémy F, Mirrashed F, Campbell B, Richter W (2004): Mental calculation impairment in Alzheimer's disease: A functional magnetic resonance imaging study. Neurosci Lett 358:25–28. [DOI] [PubMed] [Google Scholar]
- Rémy F, Mirrashed F, Campbell B, Richter W (2005): Verbal episodic memory impairment in Alzheimer's disease: A combined structural and functional MRI study. Neuroimage 25:253–266. [DOI] [PubMed] [Google Scholar]
- Risacher SL, Wang Y, Wishart HA, Rabin LA, Flashman LA, McDonald BC, West JD, Santulli RB, Saykin AJ (2013): Cholinergic enhancement of brain activation in mild cognitive impairment during episodic memory encoding. Front Psychiatry 4:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts SA, Barkhof F, Goekoop R, Stam CJ, Scheltens P (2005): Altered resting state networks in mild cognitive impairment and mild Alzheimer's disease: an fMRI study. Hum Brain Mapp 26:231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ MO, Mack W, Grama CR, Lanfermann H, Knopf M (2003): Enactment effect in memory: evidence concerning the function of the supramarginal gyrus. Exp Brain Res 149:497–504. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Flashman LA, Frutiger SA, Johnson SC, Mamourian AC, Moritz CH, O'Jile JR, Riordan HJ, Santulli RB, Smith CA, Weaver JB (1999): Neuroanatomic substrates of semantic memory impairment in Alzheimer's disease: Patterns of functional MRI activation. J Int Neuropsychol Soc 5:377–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt GC, Black SE (2009): Functional imaging studies of episodic memory in Alzheimer's disease: a quantitative meta‐analysis. Neuroimage 45:181–190. [DOI] [PubMed] [Google Scholar]
- Shanks MF, McGeown WJ, Forbes‐McKay KE, Waiter GD, Ries M, Venneri A (2007): Regional brain activity after prolonged cholinergic enhancement in early Alzheimer's disease. Magn Reson Imaging 25:848–859. [DOI] [PubMed] [Google Scholar]
- Sitzer DI, Twamley EW, Jeste DV (2006): Cognitive training in Alzheimer's disease: a meta‐analysis of the literature. Acta Psychiatr Scand 114:75–90. [DOI] [PubMed] [Google Scholar]
- Small GW, Kepe V, Ercoli LM, Siddarth P, Bookheimer SY, Miller KJ, Lavretsky H, Burggren AC, Cole GM, Vinters HV (2006): PET of brain amyloid and tau in mild cognitive impairment. New Engl J Med 355:2652–2663. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Chua EF, Cocchiarella AJ, Rentz DM, Rosen BR, Schacter DL, Albert MS (2003): fMRI studies of associative encoding in young and elderly controls and mild Alzheimer's disease. J Neurol Neurosurg Psychiatry 74:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffen W, Ladurner G, Holler Y, Bergmann J, Aichhorn M, Golaszewski S, Kronbichler M (2012): Brain activation disturbance for target detection in patients with mild cognitive impairment: An fMRI study. Neurobiol Aging 33:1002 e1001–e1016. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R (1991): Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol 30:572–580. [DOI] [PubMed] [Google Scholar]
- Thiyagesh SN, Farrow TFD, Parks RW, Accosta‐Mesa H, Young C, Wilkinson ID, Hunter MD, Woodruff PWR (2009): The neural basis of visuospatial perception in Alzheimer's disease and healthy elderly comparison subjects: An fMRI study. Psychiat Res‐Neuroim 172:109–116. [DOI] [PubMed] [Google Scholar]
- Thiyagesh SN, Farrow TFD, Parks RW, Accosta‐Mesa H, Hunter MD, Young C, Wilkinson lD, Woodruff PWR (2010): Treatment effects of therapeutic cholinesterase inhibitors on visuospatial processing in Alzheimer's disease: A longitudinal functional MRI study. Dement Geriatr Cogn 29:176–188. [DOI] [PubMed] [Google Scholar]
- Touchon J, Ritchie K (1999): Prodromal cognitive disorder in Alzheimer's disease. Int J Geriatr Psychiatry 14:556–563. [DOI] [PubMed] [Google Scholar]
- Trivedi MA, Murphy CM, Goetz C, Shah RC, Gabrieli JDE, Whitfield‐Gabrieli S, Turner DA, Stebbins GT (2008): fMRI activation changes during successful episodic memory encoding and recognition in amnestic mild cognitive impairment relative to cognitively healthy older adults. Dement Geriatr Cogn Disord 26:123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA (2002): Meta‐analysis of the functional neuroanatomy of single‐word reading: method and validation. Neuroimage 16:765–780. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P (2012): Minimizing within‐experiment and within‐group effects in activation likelihood estimation meta‐analyses. Hum Brain Mapp 33:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dam NT, Sano M, Mitsis EM, Grossman HT, Gu XS, Park Y, Hof PR, Fan J (2013): Functional neural correlates of attentional deficits in amnestic mild cognitive impairment. Plos One 8:e54035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meulen M, Lederrey C, Rieger SW, van Assche M, Schwartz S, Vuilleumier P, Assal F (2012): Associative and semantic memory deficits in amnestic mild cognitive impairment as revealed by functional magnetic resonance imaging. Cogn Behav Neurol 25:195–215. [DOI] [PubMed] [Google Scholar]
- Vandenbulcke M, Peeters R, Dupont P, Van Hecke P, Vandenberghe R (2007): Word reading and posterior temporal dysfunction in amnestic mild cognitive impairment. Cereb Cortex 17:542–551. [DOI] [PubMed] [Google Scholar]
- Vannini P, Almkvist O, Dierks T, Lehmann C, Wahlund L‐O(2007): Reduced neuronal efficacy in progressive mild cognitive impairment: A prospective fMRI study on visuospatial processing. Psychiatry Res 156:43–57. [DOI] [PubMed] [Google Scholar]
- Vannini P, Lehmann C, Dierks T, Jann K, Viitanen M, Wahlund LO, Almkvist O (2008): Failure to modulate neural response to increased task demand in mild Alzheimer's disease: fMRI study of visuospatial processing. Neurobiol Dis 31:287–297. [DOI] [PubMed] [Google Scholar]
- Vidoni ED, Thomas GP, Honea RA, Loskutova N, Burns JM (2012): Evidence of altered corticomotor system connectivity in early‐stage Alzheimer's disease. J Neurol Phys Ther 36:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Antuono PG, Jones J, Xu Y, Wu G, Ward D, Li SJ (2007): Perfusion fMRI detects deficits in regional CBF during memory‐encoding tasks in MCI subjects. Neurology 69:1650–1656. [DOI] [PubMed] [Google Scholar]
- Wang L, Zang Y, He Y, Liang M, Zhang X, Tian L, Wu T, Jiang T, Li K (2006): Changes in hippocampal connectivity in the early stages of Alzheimer's disease: evidence from resting state fMRI. Neuroimage 31:496–504. [DOI] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC (2004): Mild cognitive impairment ‐ beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 256:240–246. [DOI] [PubMed] [Google Scholar]
- Woodard JL, Sugarman MA (2012): Functional magnetic resonance imaging in aging and dementia: detection of age‐related cognitive changes and prediction of cognitive decline. Curr Top Behav Neurosci 10:113–136. [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR (2011): The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetkin FZ, Rosenberg RN, Weiner MF, Purdy PD, Cullum CM (2006): FMRI of working memory in patients with mild cognitive impairment and probable Alzheimer's disease. Eur Radiol 16:193–206. [DOI] [PubMed] [Google Scholar]
- Zamboni G, Drazich E, McCulloch E, Filippini N, Mackay CE, Jenkinson M, Tracey I, Wilcock GK (2013): Neuroanatomy of impaired self‐awareness in Alzheimer's disease and mild cognitive impairment. Cortex 49:668–678. [DOI] [PubMed] [Google Scholar]
- Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, Kramer JH, Weiner M, Miller BL, Seeley WW (2010): Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain 133:1352–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu DC, Majumdar S, Korolev IO, Berger KL, Bozoki AC (2013): Alzheimer's disease and amnestic mild cognitive impairment weaken connections within the default‐mode network: a multi‐modal imaging study. J Alzheimers Dis 34:969–984. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Information Figure S1.
Supplementary Information Figure S2.
Supplementary Information Figure S3.
Supplementary Information Tabel R1.


