Abstract
Neuroimaging studies have shown that task demands affect connectivity patterns in the human brain not only during task performance but also during subsequent rest periods. Our goal was to determine whether ongoing connectivity patterns during rest contain information about both the current rest state, as well as the recently terminated task. Our experimental design consisted of two types of active tasks that were followed by two types of low‐demand rest states. Using this design, we examined whether hippocampal functional connectivity during wakeful rest reflects both features of a recently terminated task and those of the current resting‐state condition. We identified four types of networks: (i) one whose connectivity with the hippocampus was determined only by features of a recently terminated task, (ii) one whose connectivity was determined only by features of the current resting‐state, (iii) one whose connectivity reflected aspects of both the recently terminated task and ongoing resting‐state features, and (iv) one whose connectivity with the hippocampus was strong, but not affected by any external factor. The left and right hippocampi played distinct roles in these networks. These findings suggest that ongoing hippocampal connectivity networks mediate information integration across multiple temporal scales, with hippocampal laterality moderating these connectivity patterns. Hum Brain Mapp 36:519–537, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: post‐task connectivity, resting‐state, hippocampus
INTRODUCTION
In recent years, neuroimaging studies have begun to reveal a general organizing principle of intrinsic brain activity where prior experiences directly influence subsequent resting‐state activity (post‐task rest; PTR). Effects of prior experiences on PTR have been linked to learning processes [e.g., Albert et al., 2009; Lewis et al., 2009], the content of recent experiences [e.g., Hasson et al., 2009], and to recovery from physical or cognitive effort [e.g., Barnes et al., 2009; Peltier et al., 2005]. It is well established that the hippocampal formation (HF) is vital to the consolidation of prior experiences [e.g., Eichenbaum et al., 2007; Milner and Penfield 1955; Scoville and Milner, 1957], and its role during PTR has been recently examined. Post‐task connectivity of the HF has been shown to be contingent on the prior task characteristics [e.g., Tambini et al., 2010] and HF activity in short post‐stimulus rest periods of ∼10 s predicted memory for the preceding stimulus [Ben‐Yakov and Dudai, 2011]. All these studies suggest that the HF is a pivotal structure in understanding PTR dynamics. However, in everyday experience, a cognitive task is often not immediately followed by a period of complete rest. Instead, it may be followed by different types of contexts that could potentially alter PTR processing mediated by the HF. The main goal of the current work was to identify brain regions whose functional connectivity with HF during rest varies as a function of prior task demands while additionally determining whether such connectivity is influenced by ongoing subtle attentional demands during rest.
Although the majority of studies investigating HF connectivity or activity during rest have used tasks involving explicit memory encoding paradigms [e.g., Tambini et al., 2010; Wegman and Janzen, 2011; Wig et al., 2008], two recent studies show that even passive experiences without explicit memory requirements can influence hippocampal PTR activity. After viewing brief movie scenes, PTR Blood oxygenation level dependent (BOLD) response in the right HF was higher for later remembered versus forgotten scenes [Ben‐Yakov and Dudai, 2011]. In another study [van Kesteren et al., 2010], PTR functional connectivity between a collapsed bilateral HF regressor and the ventromedial prefrontal cortex (vmPFC) was higher during subsequent rest for individuals who reconstructed the plot line from scrambled film‐clip sequences compared to those who saw the film‐clips in their correct sequence. This suggests that sorting or reordering a complex narrative schema continues to engage a HF–vmPFC circuit even after the stimuli are no longer present.
To develop a more comprehensive understanding of the role of HF connectivity networks during post‐task processing, we considered work by Barnes et al. [2009] showing gradual recovery of endogenous oscillation characteristics during PTR, and recent animal work showing that when switching to a new context, the HF is not only consolidating or replaying terminated experiences but also is concurrently processing features of the new, current context [Jadhav et al., 2012; Karlsson and Frank, 2009]. We intended to understand whether human HF functional connectivity networks also show sensitivity to features of both ongoing and recent contexts. To address this issue, we investigated HF connectivity after participants engaged in two tasks with different levels of attentional demand, while simultaneously manipulating the attentional demands of the current experience. Some aspects of HF‐connectivity (in the present) may be related to prior‐task characteristics, that is, connectivity of some regions could track features of the recently terminated experience rather than features of the present. Other aspects of HF‐connectivity may reflect features of the immediate, ongoing context, with a more limited temporal constant (i.e., a steeper decay). Further connectivity patterns might also reflect interactions between ongoing demands and prior task, thus indicating sensitivity to both temporal scales.
To study these questions, we constructed an experimental paradigm that manipulated both the demands of the prior tasks and the features of subsequent rest or rest‐like states (Fig. 1). Task demands were simple, requiring no specific learning or memory processes, but rather created environments of attentive and passive monitoring of stimuli, which were then followed by a period of more or less passive rest (PR). Specifically, in the task stage participants heard a series of tones for 5 min while either performing an attentive auditory monitoring task necessitating detection of changes in the transition‐probability structure that existed between auditory tones (attentive task; AT) or performed an easier visual monitoring task (passive task; PT). After performing either of these tasks, the audio was switched off and participants either rested in silence for 5 min while observing a fixation cross (PR) or rested while monitoring a fixation cross for very infrequent changes (attentive rest; AR). In this way, by orthogonally manipulating prior‐task demands and current‐rest demands, we intended to answer two related theoretical questions relating to organizing principles of hippocampal PTR patterns: (1) How does functional connectivity of the HF during rest change with different levels of attentional demands during rest and (2) how do these connectivity patterns interact with different levels of attentional demands from the prior task. We addressed these questions by examining both whole brain HF connectivity patterns, and HF connectivity patterns within functional regions of interest (fROIs) independently defined from a separate resting‐state scan collected for these participants.
Figure 1.
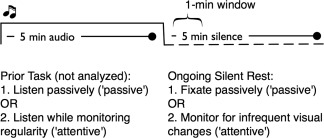
Study design and epochs of resting‐state data analysis.
It is important to note that prior work on HF resting‐state connectivity, both during task and rest, has typically assumed similar functions for the HF bilaterally and not accounted for the potential laterality differences in HF connectivity. This is seen, for example, in the collapsing of left and right HF seeds to examine HF connectivity [e.g., van Kesteren et al., 2010], or selecting one side as representative [e.g., Andrews‐Hanna et al., 2010; Buckner et al., 2008; Kahn et al., 2008; Tambini et al., 2010; Vincent et al., 2006]. This is surprising given prior literature demonstrating the lateralization of HF function and functional connectivity with regard to cognitive tasks. For example, Schott et al. [2013] recently found that the left and right HF play fundamentally different roles in networks mediating online encoding to memory. Lesion‐based studies have suggested a similar functional divergence between the left and right HF [Bonelli et al., 2010; Powell et al., 2005; Powell et al., 2007; Weber et al., 2007]. Also, whereas some brain regions (e.g., cingulate gyrus) are highly synchronized during rest (Pearson's r exceeding 0.85; Stark et al., 2008] the functional connectivity between the left and right HF is more moderate [Stark et al., 2008, mean correlation (Pearson's r) = 0.69; Salvador et al., 2005, mean correlation = 0.22]. Furthermore, the two HF have different features (clustering coefficient strength) when considered from a graph‐theoretical perspective [Supekar et al., 2008]. For these reasons, we treated the left and right hippocampi as distinct regions.
Recent work has questioned using only anatomically defined seed regions in HF resting state functional connectivity (rsFC) analyses, arguing that rsFC networks may be more accurately described using functionally defined seed regions [see Kahn et al., 2008]. To address this issue, we also functionally partitioned both the left and right hippocampi into anterior and posterior regions by identifying these regions' connectivity with functionally‐defined anterior and posterior memory networks (see Methods).
We conducted an independent resting‐state scan to define areas that are core nodes of the hippocampal connectivity network, which identified the typical HF resting‐state network. We were particularly interested in the superior frontal gyrus (SFG), posterior cingulate cortex (PCC), vmPFC, the anterior temporal cortex, and the angular gyrus (AG). It has been well demonstrated that functional connectivity within these regions, which constitute nodes of the default mode network (DMN), is sensitive to ongoing task demands and is stronger during rest than during cognitive tasks [e.g., Fransson, 2006; Hasson et al., 2009]. It has also been shown that functional connectivity of the AG and precuneus during rest varies as function of prior task [Hasson et al., 2009]. There is, therefore, reason to think that these nodes of the DMN may be involved in coding both features of past experience and concurrently ongoing experience. The role of the prefrontal regions was of particular interest since it has recently been shown [Hyman et al., 2012] that PFC neurons differentiate between environments, and do so more strongly than HF neurons. The HF‐vmPFC circuit is also involved in coding for contextual sequences [van Kesteren et al., 2010]. We, therefore, expected that after change from a task context to a rest context, the HF's connectivity with SFG or vmPFC might differ as a function of both the current context and the prior task.
We expected that a strong impact of prior task would be evident immediately upon switching from one context to another. There is strong evidence in recent work showing that dynamics of fMRI connectivity patterns within short time windows (∼30–60 s) can reveal connectivity patterns not observable when quantifying connectivity over time periods used in typical functional connectivity analyses (∼ 5 min or more, see e.g., Hutchison et al., 2013b]. Connectivity matrices constructed from time series as short as 30 s can accurately classify participants' engagement in different sorts of internal cognitions [Shirer et al., 2012], and even ∼10 s resting‐state HF BOLD data was predictive of memory for immediately preceding tasks [Ben‐Yakov and Dudai, 2011]. Taken together with the work of Barnes et al. [2009], which showed that immediate post‐task BOLD dynamics are most different from the modal resting‐state profile [see especially Barnes et al., 2009, their Fig. 2E], and that those dynamics recover over the course of minutes, we therefore hypothesized that immediately after a transition from a task context to a rest context, the HF's connectivity with SFG or vmPFC would differ as a function of the current context, the prior task, or both.
Figure 2.
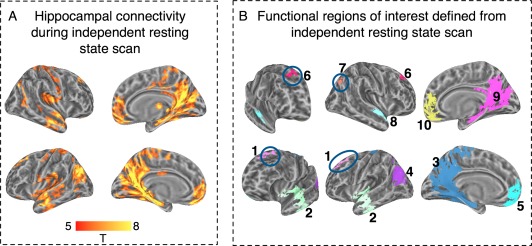
Hippocampal connectivity during independent resting‐state scan and definition of functional ROIs. Panel A: Whole brain connectivity. Panel B: Reference labels for functional ROIs: 1. Left SFG; 6. Right SFG; 2. Left STG; 8. Right STG; 3. Left PCC; 9. Right PCC; 5. Left vmPFC; 10. Right vmPFC; 4. Left Angular gyrus; and 7. Right Angular Gyrus. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
METHODS
Participants
Sixteen healthy right‐handed participants (8 F, Mean age 25.23, SD 3.49) with normal or corrected to normal vision were recruited from the surrounding community to participate in the experiment. Participants were interviewed by medical staff prior to beginning the study and were screened for history of psychiatric or neurological disorders, substance abuse, or use of psychoactive medications. The protocol was approved by the University of Trento's Ethics Committee and all participants provided written informed consent. All participants were compensated at rate of 15 Euro per hour for their time. Data from one participant were excluded from the analysis due to excessive head motion. Data from two other participants were of low quality for one of the four functional sessions as evident in low temporal signal‐to‐noise ratio, and due to the repeated measures design, they were excluded from all analyses as well.
Stimuli and Tasks
The experiment consisted of four runs in total each beginning with 5 min of auditory stimulation followed by 5 min of rest. The four runs consisted of two tasks followed by two types of rest periods. In the passive task (PT), participants listened to an auditory series of four tones while observing a central fixation cross, and pressed a button with the right hand when the central fixation cross was rotated ∼45° (the fixation cross rotated, on average, 2 times per min). In the attentive task (AT), participants were presented with the same auditory series, but were asked to observe a central fixation cross while listening to these series and to press a button with the right hand when they perceived a change in statistical regularity. Behavioral and neuroimaging data for these two auditory tasks have been reported elsewhere [Tobia et al., 2012], indicating that the attentive task was associated with higher arousal as seen in higher heart rate. Each of these two tasks could be followed by one of two types of silent episodes: (a) a “passive rest” (PR) epoch that consisted of observing a central fixation cross in silence, or (b) an “attentive rest” (AR) epoch that consisted of observing a central fixation cross in silence, and pressing a button with the right hand when the central fixation cross was rotated ∼45° (the fixation cross rotated, on average, 3 times per min). The nomenclature of “attentive” and “passive” pertains to the relative demands in the auditory and silent stages of the study; the passive task still demanded infrequent behavioral responses. The task‐rest conditions were rotated across participants so that there was no relation between experimental condition and its position in the experiment.
In addition to the main study, all participants were scanned in a separate 5 min resting‐state scan (independent resting state, IRS) at the beginning of the session, during which they were asked to focus on a fixation cross in the center of the screen. This IRS scan was used to define fROIs for the analysis of the main study.
Image Acquisition
A 4T Bruker/Siemens scanner was used to acquire a single 3D, T1‐weighted MPRAGE (TR/TE = 2,700/4 ms, 1 mm3 voxel, matrix 256 × 224 ×176), and 415 BOLD echo planar imaging (EPI) volumes for each experimental run (TR/TE = 1,500/34 ms, matrix 64 × 64, with 25 AC–PC parallel slices, voxel size 4 × 4 × 4 mm with 0.8 mm gap). The IRS scan consisted of 215 volumes and was acquired using the same protocol. Cardiac and respiratory data were recorded using a photoplethysmograph from the left index finger and a pneumatic belt strapped around the upper abdomen, both sampled at 50 Hz to obtain paired time series for each fMRI dataset.
Preprocessing of fMRI Data
Data preprocessing was conducted using AFNI [Cox, 1996]. Anatomical images were aligned to functional EPI data. The raw time series were corrected for physiological noise effects following the image‐based method for retrospective correction of physiological effects [RETROICOR: Glover et al., 2000]. The specific physiological noise correction implementation used AFNI's retrots.m routine that creates slice‐based physiological regressors. The first 15 volumes of each run were removed to allow for magnetization equilibration prior to subsequent analyses. These were followed by 200 functional volumes of auditory presentation, followed by 200 volumes of silence. These last 200 volumes of each run corresponded to the silence (rest) conditions and were the ones underlying all subsequent analyses. For the analysis of the IRS scan, we removed the first 15 volumes and analyzed the remaining 200 volumes.
After physiological noise removal, preprocessing of functional images included correction for slice acquisition time, volume registration to the first image in the scanning session, and despiking to suppress outliers. Prior to analysis of functional connectivity, in addition to physiological noise removal, several other nuisance sources of variance were removed from the times series, including linear and polynomial trends (fourth order), the six head motion parameters derived during functional volume registration, variance related to motor (button‐press) responses that was removed via a finite impulse response model, and white matter, cerebrospinal fluid, and out‐of‐brain voxel variance, removed via seed voxels in the respective regions. Data were then smoothed with a 6 mm (FWHM) Gaussian smoothing kernel to increase the temporal signal‐to‐noise ratio of the time series. After smoothing the residuals, functional connectivity maps were created using the residual time series as the predicted variable in a multiple regression, and using the variables of interest (left and right HF time series) as predicting variables (see Definition of Hippocampal ROIs section for definition of HF seed ROIs). Time series of the ROIs corresponding to HF resting‐state connectivity were created by averaging time series of all vertices in the region. Then, the Beta coefficients of the preprocessed time series were projected to the two‐dimensional cortical surface space using SUMA/FreeSurfer [Fischl et al., 1999]. This generated 8 values per surface‐domain vertex—2 (HF laterality) × 4 (experimental condition). To estimate the spatial smoothing factor needed for the simulation‐based familywise error (FWE) correction, the residuals from this last procedure were projected to the surface for estimating the FWHM of residuals in the surface domain, and that value was used for simulations.
The analysis of the 5 min independent resting‐state data was identical to the above‐reported workflow except that due to the absence of motor responses during that scan, there was no regressor modeling variance related to button presses. Data were not band‐pass filtered thus keeping low‐frequency components in the BOLD signal (however, low frequency signal drifts were removed from the data using 0–4th degree polynomials in the regression model described above, following Handwerker et al., [2012]). We chose to not implement a low‐pass filter to maintain relatively high frequencies (≥0.1 Hz) in this dataset because it has been shown that the hippocampus shows relatively fast dynamics [e.g., He, 2011], and its BOLD functional connectivity can be driven by frequencies as high as 0.14 Hz [Wu et al., 2008].
Definition of Hippocampal ROIs
Definition of left/right hippocampal ROIs
Left and right hippocampal masks were defined individually for each participant. These were derived using automated parcellation algorithms implemented in the FreeSurfer software package. The accuracy of this parcellation method has been shown to be comparable to human parcellation [Fischl et al., 2004]. We visually verified the resulting mask for each participant.
Definition of anterior/posterior hippocampal ROIs
Our main analysis, as detailed above, examined the impact of ongoing and recently completed demands on HF connectivity, and the potential modulation of HF laterality. We also examined whether functional connectivity networks of the anterior and posterior segments of HF (aHF, pHF) were differentially sensitive to ongoing and recent demands. Following Voets et al. [2014], we adopted a novel data‐driven approach for partitioning the hippocampus into anterior and posterior segments by categorizing the resting‐state functional connectivity profile of each hippocampal voxel [see also Zarei et al., 2012] obtained from the IRS scan. The details of this segmentation procedure are given in Supporting Information (Supplementary Methods). In brief, it is based on prior work showing that aHF voxels show stronger functional connectivity with regions defined as the anterior memory network—temporal pole, anterior parahippocampal gyrus, and orbitofrontal cortex—whereas pHF voxels show stronger connectivity with regions defined as the posterior memory network, including the cingulate gyrus, precuneus, middle frontal gyrus, fusiform, and thalamus. It is, therefore, possible to determine, for each HF voxel, whether it is preferentially connected to the anterior or posterior memory network, and in this way identify a boundary between the anterior and posterior HF. We applied this method successfully to our IRS data, finding preferential connectivity of aHF to the anterior memory network and of pHF to the posterior network, with a boundary approximately at the anterior 1/3 of HF length, which is highly consistent with prior applications of this method [Voets et al., 2014; Zarei et al., 2012].
Functional Connectivity During Wakeful Rest Epochs After Attentive and Passive Tasks
The 200 volumes of the silent rest epochs were separated for further analysis. Of these, the first 10 functional volumes (15 s) were discarded to allow for recovery of hemodynamic response from the listening period so that connectivity would not be affected by the transient hemodynamic drop carried over from the auditory stimulus or by any other potential sort of offset response that could occur when stimuli terminate [Gonzalez‐Castillo et al., 2012]. For the left‐ and right‐hippocampus, we constructed separate seed time series by averaging the time series of all voxels in each region. This resulted in distinct left‐hippocampus and right‐hippocampus seed time series for each of the four experimental conditions: PassiveRest‐postPassiveTask (PT→PR); PassiveRest‐postAttentiveTask (AT→PR); AttentiveRest‐postPassiveTask (PT→AR); AttentiveRest‐postAttentiveTask (AT→AR). This procedure returned eight “seed” time series in total per participant acquired during silence, described by the full crossover of the factors: 2 (HF laterality) × 2 (current rest) × 2 (prior task). Prior to establishing functional connectivity, outlier BOLD values introduced by head motion or scanner variance were identified by evaluating the motion plots (points of 1.5 mm motion and above declared extreme) and the outlier plots (using outputs from ANFI's outlier identifier, 3dToutcount). Functional volumes where outlier values were identified were removed from each time series before evaluating connectivity patterns, whether bilateral connectivity between hippocampi seeds themselves, or whole brain connectivity of the individual hippocampal seed regions.
In the whole‐brain connectivity analysis, for each participant, the beta values reflecting correlation strength were analyzed on a 2D cortical surface created by averaging all participants' cortical surfaces. Group‐level random effects analysis was conducted on the single‐voxel level via a 2 (prior task) × 2 (current rest) × 2 (HF laterality) ANOVA conducted for each surface vertex. These were thresholded using cluster extent thresholding to control for FWE, following the algorithms introduced by Forman et al. [1995] that were implemented in the 2D surface domain. Each simulation took into account the estimated spatial smoothing of the data (∼6 mm) and the maximal distance allowed between two nodes that passed the uncorrected threshold (2 mm). Five thousand simulations were constructed, where in each simulation, the size of the largest cluster satisfying these constraints was saved. This constructed a sampling distribution of cluster sizes likely to be generated by chance. The 95% cluster size of this sampling distribution was used as a lower‐bound value for cluster based thresholding. On the basis of these simulations, a reliable cluster was defined as a minimum 66 mm2 cluster where all the voxels passed an uncorrected alpha threshold of 0.005.
Given that changes in experimental contexts introduce strong changes to BOLD resting‐state connectivity [Barnes et al., 2009], and that effects of prior task on post‐task hippocampal activity may be relatively short lived, on the scale of 1 min [see Karlsson and Frank, 2009], we performed a connectivity analysis identical to the analysis described above using only 36 functional scans, corresponding to the period of 15–69 s after termination of the auditory sequence (see Fig. 1 for illustration of design and analysis epochs). This specific time window within the first minute was chosen for two reasons. We ignored the first 15 s, since BOLD in those time points could potentially directly reflect neural activity generated during the prior task. Second, we ended the time series 9 s after the termination of that minute, since activity up to ∼69 s could reflect activity during the first 1 min. Then, to verify that the findings identified for the first minute of rest were limited to that temporal period, a parallel analysis was conducted for the last minute of the rest period.
Prefacing the results, it was within this initial time window (15–69 s post‐task) that we found the main effects of prior task on ongoing connectivity, or interactions between prior and ongoing demands. For this reason, the analysis of differential connectivity of anterior and posterior HF was also conducted on data collected within this time window. The connectivity analysis followed the same procedures as the one described above. However, the left and right HF were analyzed separately, and for each HF, we conducted a group‐level random effects analysis on the single vertex level via a 2 (prior task) × 2 (current rest) × 2 (anterior/posterior segment) ANOVA. Control for FWE was implemented using the procedures described above.
Functional Connectivity in IRS Scan: Independent Identification of the Hippocampal Resting‐State Networks
To define hippocampal resting‐state functional connectivity, as defined in prior studies, single participant connectivity maps were constructed for the left and right HF seed regions, using data from an IRS scan obtained for each participant prior to the task‐rest experimental procedure that constituted the main study. The procedure for construction of these single‐participant connectivity maps was identical to that reported above in Functional Connectivity During Wakeful Rest Epochs After Attentive and Passive Tasks. So as not to bias the definition of fROIs, these were defined as clusters of contiguous vertices in which each vertex showed significant connectivity with both the left HF and right HF (each at an alpha level of P < 0.001, on the group level). FWE correction was implemented using cluster extent thresholding [Monte Carlo simulations following Forman et al., 1995], which was implemented in the surface domain (57 mm2 minimum cluster extent). We analyzed HF connectivity within this independent dataset to define group‐level fROIs capturing the main nodes of this HF resting‐state network (PCC, vmPFC, AG, and anterior lateral temporal cortex). This allowed us to specifically determine whether these regions (10 ROIs in total, see below, Results: Hippocampal Connectivity During IRS), which are intrinsically functionally connected to HF during rest, are modulated by the independent variables of interest in our study.
Statistical Thresholding Choices
All statistical analyses were controlled for FWE using cluster‐based correction at P < 0.05 on the cluster level. The single voxel (uncorrected) alpha threshold levels were set at P < 0.001, when there was very strong prior reason to think that effects would be widespread and large: (a) when defining group‐level HF resting‐state connectivity in the independent resting scan; (b) when defining connectivity (vs. baseline) during the first and last minute of the PTR state; or (c) when evaluating differences between the latter. Single‐voxel thresholds of 0.005 were used for the whole‐brain ANOVAs where the two‐ and three‐way interactions were of main theoretical interest and there was little prior literature to determine potential effect sizes.
RESULTS
Behavioral performance data for two participants were unavailable due to equipment malfunction. The remaining participants (N = 11) demonstrated high accuracy on the visual target change‐detection task during attentive rest, with a group mean of 98.79% (SD = 4.43), indicating that participants were alert. Average reaction time for the participant group was 797 ms (SD = 430).
Hippocampal Connectivity During IRS
The connectivity patterns during the IRS scan matched those previously reported in the literature, with strong connectivity in PCC, vmPFC, AG, lateral temporal cortex, and the SFG (Fig. 2A). In the analysis of the IRS, there was no impact of HF laterality (we evaluated several uncorrected single‐voxel alpha levels: 0.005, 0.001, 0.0001). Nonetheless, so as not to bias the definition of fROIs, these were defined as brain regions that showed significant functional connectivity to each HF seed region at an alpha level of P < 0.001 (on the group level). That is, as fROIs, we considered contiguous vertex‐clusters, exceeding 57 mm2, in which each vertex connectivity was significant at the P < 0.001 level against both HF seed regions. From this connectivity map, we defined 10 fROIs that included the anterior lateral temporal cortex, AG, SFG, PCC, and vmPFC, bilaterally, regions often linked to HF resting‐state connectivity [Vincent et al., 2006]. The locations of these fROIs are presented in Figure 2B. These fROIs served as a priori regions of interest in which we evaluated HF functional connectivity during passive and attentive rest as a function of prior task and hippocampal seed region.
Homotopic HF Connectivity
Prior to evaluating functional connectivity profiles, we evaluated the degree to which bilateral hippocampal activity was correlated during each type of silent rest period. A high correlation would obviate the need to analyze each HF separately. For each participant, the interhemispheric correlation between the two hippocampi was calculated for each of the four conditions. These correlation values were moderate (see Supporting Information Table 1), and very similar to those previously reported by Stark et al. [2008] who reported a mean correlation of 0.69 during complete rest and Buckner et al. [2008] who reported a correlation of 0.61. This validated the use of both seed regions in the following analyses. In addition, the degree of correlation between the two seeds was not affected by the independent variables as evident in a 2 (prior task) × 2 (current rest) ANOVA conducted on the (Fisher‐Z transformed) correlation values.
Table 1.
Summary of findings of whole‐brain analysis
| Effect | Region | Talairach coordinates (Cluster center of mass) | BA | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Ongoing Rest | 1. L. Parieto‐Occipital junction | −19 | −86 | 30 | 19 |
| HF‐laterality | 2. L. STG | −55 | −49 | 21 | 40 |
| 3. L. PHG | −19 | −11 | −21 | 28 | |
| HF‐laterality by Prior task | 4. L. Precuneus | −12 | −44 | 56 | 7 |
| 5. L. IPL/Postcentral G | −42 | −22 | 27 | 2 | |
| 6. L. Insula | −29 | 10 | 9 | 13 | |
| 7. R. PCC | 7 | −45 | 14 | 29 | |
| 8. R. Posterior insula | 33 | −29 | 16 | 13 | |
| Ongoing rest by HF‐laterality by Prior task | |||||
| 9. L. SFG | −13 | 50 | 34 | 9 | |
| 10. R. PHG/Uncus | 36 | −14 | −28 | 35 | |
An additional preliminary analysis examined whether functional connectivity of the HF during the 285 s task‐free epochs matched configurations previously reported in the literature. To this end, we constructed a connectivity map for the PR condition, when following the PT, an epoch arguably most similar to what are considered typical resting‐state conditions. As shown in Supporting Information Figure S1, connectivity patterns in this analysis were similar to those found in our IRS scan (compare vs. Fig. 2A) and those reported by Vincent et al. [2006]. (One exception is stronger connectivity with the insular cortex. which could be attributed to a number of factors including, but not limited to our use of a 4T scanner, the use of surface‐based alignment routines, or the direct use of physiological noise measurements as nuisance regressors rather than proxy measures.)
Context Sensitivity of Functional Connectivity in Core Regions of the HF Connectivity Network: A fROI Analysis
The main question addressed here was whether regions constituting core nodes of the HF resting‐state network show connectivity patterns that vary as a function of ongoing rest, prior task or interactions between the two factors (potentially further modulated by hippocampal laterality). We computed the functional connectivity estimate for each voxel indicating the degree of correlated activity with the (left or right) HF, during attentive or passive rest epochs that appeared after either an attentive or passive task. The procedure returned eight connectivity maps per participant, reflecting whole brain connectivity in the 285 s epochs with left/right HF as a function of prior task and current rest.
These eight connectivity maps were then analyzed using a fROI approach, with each fROI examined via a 2 (HF laterality) × 2 (current rest) × 2 (prior task) ANOVA collapsing across all surface vertices in the region, and with participants modeled as a random factor. These analyses revealed only one pattern: several fROIs showed stronger connectivity with the HF during PR than during AR. This pattern was seen for left PCC, F(1, 12) = 20.1, P < 0.001, left AG, F(1, 12) = 12.8, P < 0.004, left vmPFC, F(1, 12) = 7.7, P = 0.017, left SFG, F(1, 12) = 20.9, P < 0.001, right PCC, F(1, 12) = 15.3, P = 0.002, right vmPFC, F(1, 12) = 10.16, P = 0.008, right anterior lateral temporal cortex, F(1, 12) = 8.8, P = 0.011, right posterior middle temporal gyrus, F(1, 12) = 23.5, P < 0.001, and right SFG, F(1, 12) = 5.1, P = 0.04. The only other effect seen in this analysis was that the left SFG showed main effect of hemisphere laterality: F(1, 12) = 5.12, P = 0.04, because its connectivity was stronger with the left than right HF (M = 0.49 [SD = 0.27] versus M = 0.38 [SD = 0.24]). No other fROIs showed any other statistically significant main effects or interactions. These findings corroborate prior work indicating that connectivity of the DMN (of which the HF is a member; Buckner et al., [2008]) shows stronger connectivity during less demanding attentional contexts.
The previous analysis quantified functional connectivity collapsing over 5 min of post‐task silence epochs. Given this relatively long post‐task temporal period, it is perhaps not surprising that potential effects of prior task on subsequent functional connectivity would not be seen with connectivity aggregated over the entire 5 min post‐task epoch, since this epoch is associated with a dynamic process of return to the equilibrium resting state. Specifically, following task performance, resting state patterns in the DMN recover gradually, and reach pretask equilibrium levels within 6–7 min after performance of simple tasks [Barnes et al., 2009]. Other work suggests that effects of prior task on HF resting state activity may be relatively transient ([Karlsson and Frank, 2009], see their Fig. 5). In the following analysis we therefore concentrated on a shorter temporal epoch beginning 16 s after task termination, and continuing for 54 s afterward, and conducted the ROI analysis on functional connectivity estimates in this temporal window. Prior to this analysis, we examined the relative homotopicity between the two HF‐seed time series during this epoch. The results were highly similar to those seen when quantifying over the longer 285 s interval (see Supporting Information Table 1). We, therefore, again maintained HF laterality as a factor in the analysis.
Figure 5.
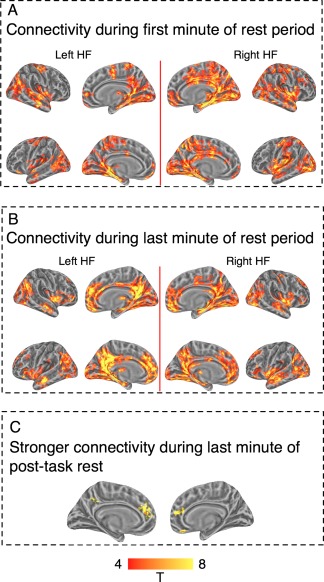
Whole brain connectivity of left hippocampus seed region (left HF) and right hippocampus seed region (right HF) during the first and last 1‐min epochs of a 5‐min rest period. Panel A: connectivity during first minute of rest. Panel B: connectivity during the last minute of rest. In Panels A and B, upper rows mark the right hemisphere cortical surface and lower rows mark the left hemisphere cortical surface. Panel C: direct comparison between connectivity in the first and last minute shows stronger connectivity with PCC and vmPFC during the last minute of rest. The figure shows that connectivity patterns settle into the well‐described hippocampal resting‐state configuration toward the end of the resting‐state epoch, but have a markedly different configuration at its beginning. Thresholded at single voxel alpha level of P < 0.001, familywise corrected for multiple comparisons using cluster‐extent thresholding. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
The fROI analysis of the 54 s epoch showed an impact of both prior task and current rest on functional connectivity patterns. The PCC connectivity patterns, bilaterally, depended on HF laterality and features of the prior task (Fig. 3). The left PCC showed a prior task × HF laterality interaction, F(1, 12) = 7.47, P = 0.02; the Left_HF ↔ Left_PCC connectivity was stronger after the active than the passive task, but the Right_HF ↔ Left_PCC connectivity was stronger after the passive than the active task. The right PCC showed the same interaction pattern, F(1, 12) = 7.12, P = 0.03, as well as an effect of current rest, as connectivity was stronger during passive rest, F(1, 12) = 4.87, P = 0.048.
Figure 3.
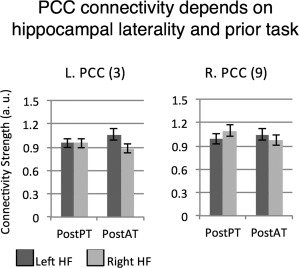
Post‐task connectivity of PCC. A functional ROI analysis shows that post‐task connectivity of PCC bilaterally is affected by features of prior task, but modulated by hippocampal laterality. PostPT: post passive task. PostAT: post attentive task. Numbers next to region names refer to region labels in Figure 2B.
Connectivity patterns of the vmPFC bilaterally and the left SFG showed sensitivity to both prior task and current rest features (Fig. 4). The left vmPFC showed a statistically significant prior task × current rest interaction, F(1, 12) = 5.4, P = 0.04. Connectivity during attentive rest was higher after the passive task, and connectivity during passive rest was higher after the attentive task. The right vmPFC showed the same interaction pattern, but only in its connectivity with the right HF, resulting in a statistically significant three‐way interaction, F(1, 12) = 7.83, P = 0.016. (The connectivity of right vmPFC with the left HF was not modulated by any experimental factor.) The left SFG showed the same pattern of results seen for right vmPFC, typified by a three‐way interaction, F(1, 12) = 9.8, P = 0.009: its connectivity with the right HF showed a prior task × current rest interaction F(1, 12)= 5.87, P = 0.03, but its connectivity with the left HF was not modulated by any factor. Finally, the angular gyri bilaterally showed three‐way interactions. For the left AG, the three‐way interaction was significant, F(1, 12) = 5.93, P = 0.03. To partition the AG interaction, we examined the connectivity of this region with left and right HF separately, but neither of the two was significant. A similar result was found for the right AG, F(1, 12) = 6.24, P = 0.028, but again, neither of the two‐way interactions was significant. To summarize, during the 54 s epoch, these fROIs showed sensitivity to prior task features or joint sensitivity to prior task and current rest, and posterior and anterior midline regions showed different response profiles. In addition, we note that the right SFG and the anterior temporal cortex (bilaterally) did not show any effects, suggesting they are relatively insensitive to contextual features.
Figure 4.
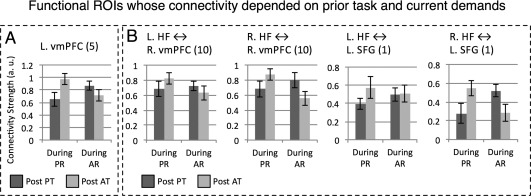
Functional ROIs where post‐task connectivity was jointly affected by features of current rest and prior task. These regions' connectivity was explained by a significant interaction between the two factors (Panel A) which could be further modulated by hippocampal laterality (Panel B). During AR: during attentive rest. During PR: during passive rest. PostPT: post passive task. PostAT: post attentive task. Numbers next to region names refer to region labels in Figure 2B.
As a control analysis, we applied the same fROI analysis to connectivity estimates for the last minute of rest. Here, we found that only one ROI showed sensitivity to prior task (PCC cluster F(1, 12) = 10.44, P = 0.009), and no ROI showed, during this last minute, the higher‐level interactions that were found during the first minute of rest. (Many ROIs showed an effect of current rest with stronger connectivity during passive rest, a point we do not expand on here.) Thus, the impact of prior task features on connectivity, and their interactions with ongoing demands, appears to largely terminate by 5 min.
Context Sensitivity of Functional Connectivity: Whole Brain Analysis
To identify other brain regions whose connectivity with the HF depended on the experimental factors, we conducted whole brain analyses using a group‐level random effects analysis. A 2 (prior task) × 2 (current rest) × 2 (HF laterality) ANOVA was applied to the data of each voxel, followed by cluster‐based thresholding (see Methods). As in the fROI analyses, one analysis was conducted on functional estimates for the entire 285 s epoch, and one for 54 s of data beginning 16 s from the onset of the silence period.
Whole brain analysis: Effects of prior task and current rest on hippocampal connectivity quantified over 285sec
The analysis of the entire 285 s post‐task epochs showed one result pattern: numerous regions showed stronger connectivity with the HF during passive than attentive rest. Supporting Information Figure S2 shows these areas, which by and large were limited to regions previously linked to hippocampal connectivity during rest [e.g., Vincent et al., 2006]. We did not find any brain region showing an impact of prior task, or an interaction between prior task and current rest. This finding is consistent with our fROI analysis of the 285 s epoch, and indeed many of the regions identified in the whole brain analysis are part of the DMN (see Supporting Information Fig. S2).
Whole brain analysis: Hippocampal connectivity for 16–69 s post‐task
Prior to examining whole‐brain modulations of functional connectivity within this 54 s epoch, we examined connectivity patterns in this period for the PR epoch that followed the PT, an epoch arguably most similar to what are considered typical resting‐state conditions. The HF connectivity pattern during this epoch is shown in Figure 5A. While there was clearly strong whole‐brain connectivity for both hippocampi, these did not resemble resting‐state networks previously documented in the literature, nor the resting‐state HF connectivity maps found for this group (Fig. 2A). In particular, connectivity with PCC, ACC, and vmPFC appeared reduced compared to previous reports suggesting that this initial transition period into rest was associated with unique connectivity patterns.
This observation was corroborated by two control analyses showing that this particular finding was not attributable to the shorter analysis window. First, when examining connectivity during passive rest after the passive task, for a comparable 54 s epoch at the end of the 5‐min resting period, we documented HF connectivity patterns that matched prior reports (Fig. 5B). Second, a direct comparison between connectivity maps during the last and first minute of the PTR period showed stronger connectivity with PCC, ACC, and vmPFC during the last minute of rest (Fig. 5C).
Whole brain analysis: Effects of prior task, current rest and hippocampal laterality on connectivity quantified for 16–69 s post‐task
The analysis of left and right HF connectivity during the first 1 min of silence revealed strong differentiation, with several regions identified in which connectivity during rest varied with (i) HF seed region (left vs. right), (ii) a combination of HF seed region and prior task, or (iii) a combination of HF seed region, prior task, and current rest demands. Table 1 summarizes the areas showing these effects, and the nature of the effects is detailed below. We briefly review these whole brain findings for the sake of completeness, as in some cases the whole brain analysis identified brain regions previously defined in the fROI analysis.
A cluster in the left parieto‐occipital junction showed a modulation of HF‐connectivity as function of current rest, with stronger connectivity during PR than during AR (M = 1.2, SE = 0.18 vs. M = 0.63, SE = 0.11; t(12) = 5.48, P < 0.0001; Fig. 6A).
Figure 6.
Impact of current‐rest features and hippocampal laterality. Panel A: stronger connectivity during passive than attentive rest. Panel B: regions showing stronger connectivity with left HF than with right HF. Here and in all subsequent figures colors mark different clusters. Cluster numbers match entries in Table 1. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Two clusters showed different connectivity strength with the left or right HF but without being affected by current rest or prior task features. These were the left posterior superior temporal gyrus (STG) extending into the supramarginal gyrus (SMG), and a cluster in the left parahippocampal gyrus (PHG; Fig. 6B). Both regions showed positive connectivity with bilateral HF, but with stronger connectivity for left HF (superior temporal sulcus and supramarginal gyrus: t(12) = 6.3, P < 0.001; PHG: t(12) = 4.4, P = 0.001)
In a number of areas, post‐task connectivity was determined by an interaction between features of the prior task and the HF seed region (but without any further modulation of the current rest properties; Fig. 7). Note, this was the same pattern identified in the fROI analysis for the PCC bilaterally. In the whole brain analysis, regions showing this interaction pattern included, on the left, the precuneus, anterior IPS/postcentral‐gyrus, and insula, and on the right, one cluster in the PCC and one in the superior temporal plane/posterior insula. In all the regions, the pattern of interaction was identical: stronger left HF connectivity than right HF after the AT, but stronger right HF connectivity than left HF after the PT. When collapsed across all surface vertices to obtain cluster means, the interaction was statistically significant for left precuneus (F(1, 12) = 22.5, P < 0.001), left IPS (F(1, 12) = 20.6, P = 0.001), left insula (F(1, 12) = 27.7, P < 0.001), right PCC (F(1, 12) = 26.4, P < 0.001), and right superior temporal plane, (F(1, 12) = 22.3, P < 0.001).
Figure 7.
Clusters where connectivity with left/right hippocampi varied as function of prior task. Cluster numbers match entries in Table 1. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
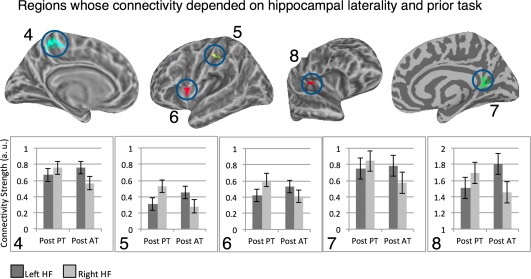
Two clusters, one in the left SFG and the other in the right PHG (on the border of the Uncus) showed the most differentiated connectivity pattern. For both, connectivity with HF varied according to HF laterality, prior task and current rest. The location of the left SFG cluster showing this pattern overlapped with the SFG fROI defined from the resting‐state scan, a fROI that showed the exact same three‐way interaction and will not be further discussed (Fig. 8A). For the right PHG/Uncus, when analyzed on the entire cluster level, a dominant pattern of interaction emerged, signaled by a significant three‐way interaction, F(1, 12) = 25.5, P < 0.001 (Fig. 8B). We found the region's connectivity with right HF was modulated by features of the current rest, with overall weaker connectivity during PR, F(1, 12) = 6.25, P = 0.03. Its connectivity with the left HF showed a statistically marginal interaction between current rest and prior task, with the same profile reported for the left SFG, F(1, 12) = 3.15, P = 0.1.
Figure 8.
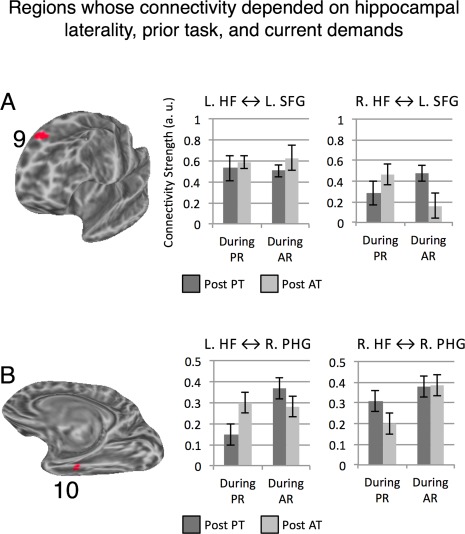
Clusters where hippocampal connectivity with given region was determined by an interaction between prior task demands, current rest features and hippocampal laterality. Cluster numbers match entries in Table 1. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
To summarize, within the first minute after task completion, a whole brain analysis documented strong indicators for regions whose connectivity with the HF varies as function of prior task (potentially modulated by HF laterality), as well as regions where connectivity depended on features of prior task and current rest. We replicated this analysis for connectivity patterns estimated for the last (fifth) minute of rest, and here the whole brain analysis did not identify any regions where connectivity varied as function of prior task, or showed an interaction with the prior task factor. This analysis identified only clusters showing an effect of current rest, which showed stronger connectivity during passive than attentive rest (see Supporting Information Supplementary Materials, Fig. S3).
Effects of prior task, current rest and anterior/posterior hippocampal division on connectivity quantified for 16–69 s post‐task
As a final analysis, we replicated the previous whole brain analysis, but examined whether anterior and posterior HF segments show different connectivity profiles as a function of prior and ongoing demands. This whole‐brain vertexwise analysis was performed separately for the left HF and right HF. For each vertex, we conducted a 2 (prior task) × 2 (current rest) × 2 (anterior/posterior segment) ANOVA.
For the left HF, we did not find any region whose connectivity showed an interaction between the experimental factors and the anterior/posterior division. (We also found numerous areas showing main effects of anterior vs. posterior HF divisions, as necessitated given the method used to define these regions, as well as areas showing effects of current rest. These results are nonindependent from what have been reported in prior sections or from the method defining the anterior/posterior division.)
For the right HF, we identified two regions—the left and right posterior IFG (on the border of pars opercularis and the ventral precentral gyrus)—whose connectivity was determined by a three‐way prior task × current rest × anterior/posterior interaction, which held for each vertex in those regions (Fig. 9). As we detail below, the IFG's connectivity (bilaterally) with the right aHF was impacted by both prior and ongoing demands, whereas IFG's connectivity (bilaterally) with the right pHF was not affected by either factor.
Figure 9.
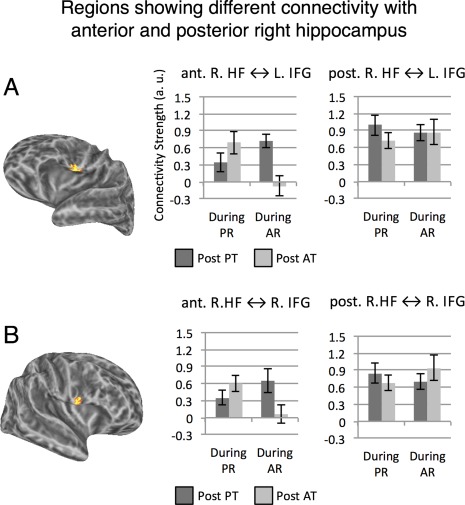
Regions showing different connectivity with anterior and posterior right HF. Panel A: Left Hemisphere. Panel B: Right Hemisphere. All vertices in warm colors showed a statistically significant three‐way interaction between prior task, current rest and anterior versus posterior HF segment, single‐vertex threshold for interaction tests P < 0.005 corrected for multiple comparisons using cluster extent threshold (FWE P < 0.05). For both left and right IFG, connectivity with posterior right HF was not modulated by the experimental manipulations (i.e., nonsignificant prior task × current rest interactions), but connectivity with anterior right HF was modulated by both factors (Ps < 0.05; see text). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
To obtain group‐level statistics for the right IFG cluster, for each participant data were averaged across all vertices in the cluster per each condition, and group‐level data were submitted to an ANOVA. Right IFG's connectivity with right aHF was modulated by ongoing and prior demands, but its connectivity with right pHF was not modulated by either factor, a pattern confirmed by a significant three‐way interaction at the cluster level, F(1, 12) = 19.92, P = 0.001. The pattern was examined by two follow‐up ANOVAs that partitioned the three‐way interaction into 2 two‐way interactions. These indicated that the right IFG's connectivity with right aHF showed a prior task × current rest interaction, F(1, 12) = 15.15, P = 0.002, but its connectivity with right pHF did not show significant modulation (P > 0.37). In addition, there was a main effect of anterior/posterior segment as right IFG's connectivity was stronger with pHF than aHF, F(1, 12) = 9.32, P = 0.01. Note that the stronger overall connectivity with the posterior compared to the anterior segment (see also Fig. 9) suggests the absence of significant experimental modulations of connectivity with the posterior segment was not due to a floor effect or lack of sensitivity.
The same analysis was repeated for the left IFG cluster, which showed very similar patterns, as evident in a significant three‐way interaction at the cluster level, F(1, 12) = 25. 89, P < 0.0001. The follow‐up analysis indicated that the left IFG's connectivity with the right aHF showed a prior task × current rest interaction, F(1, 12) = 7.14, P = 0.02, but its connectivity with right pHF did not show significant modulation (P > 0.25). In addition, connectivity was stronger overall with the right pHF compared to the aHF, F(1, 12) = 10.35, P = 0.007, again suggesting that the lack of connectivity‐modulation for pHF does not result from lack of power.
To summarize, we found an interesting dissociation of right HF connectivity, where connectivity of the aHF with IFG (bilaterally) showed connectivity highly sensitive to contextual features, whereas connectivity of pHF was not susceptible to any such effects.
DISCUSSION
The HF plays a central role in construing our experience of the world by consolidating recent experiences into long‐term memory systems. As we outlined in the Introduction, in the absence of exogenous input, activity and connectivity of the HF have been repeatedly linked to processing recent experiences, as demonstrated by brain:behavior correlations between its resting‐state activity profiles and behavioral outcomes for several tasks [Ben‐Yakov and Dudai, 2011; Schott et al., 2013; Tambini et al., 2010; van Kesteren et al., 2010; Wegman and Janzen, 2011; Wig et al., 2008]. Our goal was to determine whether these endogenous activity patterns found in the HF can be explained as reflecting an interaction between prior task and current attentional demands, and in doing so, determine whether the left and right HF areas subserve similar functions in this context, and whether functionally defined anterior/posterior divisions of the hippocampi show differential post‐task rsFC profiles. Our main finding is that HF connectivity does indeed vary with features of both current, ongoing demands as well as features of recently terminated experiences, and these effects are distributed in different networks. This finding points to a general principle of endogenous brain activity that has yet to be acknowledged in the literature on functional connectivity and HF functioning: that connectivity of some cortical networks (sampled in the present) is determined by both ongoing environmental features and those of a recently experienced environment that is no longer available.
The Dynamics of HF Connectivity Patterns After Task Termination
While the connectivity pattern for the HF during the first 1‐min rest epoch departed in some aspects from previously identified endogenous HF connectivity networks, the network connectivity pattern seen during the last 1‐min rest epoch strongly resembled those networks, in particular showing greater HF connectivity with the PCC, ACC, and vmPFC. Thus, endogenous activity in the HF soon after disengagement from an exogenous stimulus manifests a different organization of functional connectivity. This shows that endogenous HF connectivity does not immediately shift into a “rest‐like” topology when exogenous stimuli terminate, but eventually does settle into that configuration. This finding is consistent with work by Barnes et al. [2009] that examined the fractal complexity of time series during PTR states and showed that immediately post‐task, BOLD time series have lower temporal autocorrelation, and that it can take more than 5 min for the BOLD time series to return to their initial complexity levels. The flexible configuration of HF connectivity over relatively short periods is consistent with recent work showing that it is one of the brain regions demonstrating the highest variability in connectivity patterns [Park et al., 2012] and that its functional connectivity profile is mediated by relative high frequencies (≥0.1 Hz; Wu et al., 2008] accompanied by high variance and low time‐series autocorrelation [as emphasized by He, 2011, particulalry Figs. 3, 4 therein]. Our experimental findings showing an impact of recent experience on ongoing connectivity or interactions between the two factors were strongly observed during the 1‐min epoch sampled soon after termination of a task, but were largely absent when conducting a parallel analysis of the fifth minute of the rest period. This 1‐min duration is consistent with previous research showing that the duration of episodic hippocampal replay mechanisms is approximately 1 min when switching from task to rest [see Karlsson and Frank, 2009]. More generally, our findings are consistent with prior work showing that functional connectivity patterns in resting‐state networks are dynamic [for recent review, see Hutchison et al., 2013a] and that connectivity patterns observable on scales of 30–60 s may not be observable in longer temporal scales [Allen et al., 2014; Hutchison et al., 2013b].
With regard to the HF laterality effects in the first minute analyses, it is important to note that the correlation between the left and right HF time series was of similar magnitudes when quantified over both the entire 5‐min resting‐state period and over the shorter 1‐min epoch (Supporting Information Table 1). The absolute correlation values matched well those reported in prior examinations of endogenous HF bilateral connectivity [Stark et al., 2008]. Thus, the significant impact of laterality on the experimental effects in the analysis of the shorter epoch cannot be explained by stronger independence between the two HF regions in that epoch.
The dynamics of post‐task HF connectivity identified in the current study indicated that the strongest effects of prior‐task were found within the first minute post‐task. Only one region (identified in the fROI analysis; none in the whole brain) still showed sensitivity to prior task during the fifth minute after task completion. Our findings that point to a relatively short term impact of prior task on post‐task HF connectivity appear to diverge from prior results that documented longer lasting effects of task performance on post‐task connectivity [e.g. Lewis et al., 2009; Stevens et al., 2010; Stevens et al., 2012; Tambini et al., 2010]. There are several possible reasons for this divergence. First, many of the prior studies [including Lewis et al., 2009, Stevens et al., 2010, 2012] did not quantify hippocampal post‐task connectivity, so it is impossible to say whether or not the same patterns of post‐task HF connectivity hold in their data. This is not a matter of nuance: as mentioned, HF resting‐state patterns are particularly nonstationary and have higher variance in comparison to other brain regions [He, 2011; Wu et al., 2008]. For this reason, HF resting‐state connectivity may show faster dynamics than other regions. Second, prior work relied on long task epochs [e.g., 21 min in Tambini et al., 2010], consisting of highly specific and repetitive tasks [see also Jolles et al., 2013; Taubert et al., 2011]. For this reason, a long‐term impact of task on post‐task connectivity (quantified between task‐involved regions) could be a result of the prior task establishing more synchronized activation patterns between the regions in question during task execution, which could then sustain over relatively long post‐task periods or be more easily reinstated. For instance, Lewis et al. [2009] interpreted their findings as recapitulation of a “history of experience‐driven coactivation,” an interpretation not likely to hold in our case, since we document differential connectivity during post‐task epochs that were not found during task. Studies based on very extensive training such as that of Lewis et al. [2009] could also affect functional connectivity via a different route: even short amounts of training are sufficient for causing structural changes in brain activity [e.g., Taubert et al., 2011], and such structural changes could be manifested in a change in RS connectivity.
Prior and Ongoing Contexts Determine HF Connectivity Patterns
Our central finding is that connectivity of the HF with several cortical regions indicates an interaction between prior and current contextual demands, rather than either in isolation. While it has been shown that ongoing demands [e.g., Fransson, 2006], and prior demands [e.g., Hasson et al., 2009; Stevens et al., 2010] impact connectivity [see review by Northoff et al., 2010], a conjoint examination of both factors has not been carried out to date. These findings are consistent with other recent findings from animal experiments, which show that endogenous hippocampal activity reflects both the coding for features of the present and features of the recent past [Jadhav et al., 2012; Karlsson and Frank, 2009].
Our fROI analysis examined HF connectivity with regions defined from an independent resting‐state scan. Given that the HF is a node in the DMN [Buckner et al., 2008], the identified nodes were ones strongly associated with this network, including posterior and anterior midline regions, the SFG and anterior temporal cortex, bilaterally. Of these, the vmPFC and the SFG showed the most nuanced connectivity patterns, evident as an interaction between ongoing and prior contexts. This is consistent with animal work that has documented strong context sensitivity in vmPFC [Hyman et al., 2012], and with our own work [Hasson et al., 2009] showing that connectivity in right SFG during rest is sensitive to features of recently experienced contexts. In contrast, the PCC showed a simpler profile during this initial minute, with connectivity sensitive only to the recently terminated context (mediated by hippocampal laterality). Finally, the connectivity between HF and anterior temporal regions or right SFG was not modulated by any experimental factor.
These findings raise several points. First, they indicate that after task termination, core nodes of the DMN may be mediating different sorts of processes, with frontal regions (left SFG, vmPFC bilaterally) sensitive to ongoing and recent contexts, posterior regions to recent ones, and temporal regions and right SFG insensitive to either the previous or the current context. Thus, the DMN may be functionally heterogeneous with respect to its sensitivity to more or less recent temporal experiences [see e.g., Sestieri et al., 2011, for similar conclusions]. It has been shown that certain regions within the DMN form what might be considered an intrinsic network as their connectivity or temporal activation profiles are independent from the dynamics of externally presented stimuli [Golland et al., 2007]. Second, these findings raise the possibility that during wakeful rest, these aforementioned regions maintain similar functions to those documented here, with some processing experiences in the more‐ and less‐recent past, some focusing on the less‐recent past alone, and others engaged in as‐yet‐undetermined processes. Thus, these networks may mediate an internally driven default process by which they are sensitive to ongoing and prior task contexts (and potentially the relation between them) in absence of any external demand to do so. In this sense an intrinsic and default process does not have to be devoid of information processing related to the external environment, but can rather be endogenous in the sense that it mediates continuous background processes not triggered by any particular stimulus. It remains to be seen whether similar patterns indicating coding of both ongoing and recent past exist in other resting‐state networks, but it is unsurprising to find such effects for a network known to code, online, for both ongoing and recent information. Indeed, DMN connectivity has been shown to be affected by ongoing task features [Esposito et al., 2006; Fransson, 2006; Newton et al., 2011]; including relatively simple manipulations such as attending or ignoring scanner noise [Benjamin et al., 2010] or keeping eyes closed or open [Yan et al., 2009].
Our findings for SFG were found both in the fROI analysis (where SFG was defined from an independent rest scan) and in the whole brain analysis of connectivity during the first minute post task. The findings for vmPFC bilaterally were found only in the functionally defined fROIs. This could be due to the increased sensitivity of the fROI analysis: it relies on regions preselected due to their strong connectivity with the HF in a separate scan, which may indicate they are a priori involved in HF‐related processing. Furthermore, an fROI analysis aggregates over voxels in which the size of the effects of interest (main effects, interactions) may be relatively smaller than that necessitated by the more conservative single voxel thresholds used in whole‐brain analysis.
Impact of Left and Right Hippocampus Seed Regions, and Anterior/Posterior Differentiation
A dissociation between left and right HF was found when examining their sensitivity to the recent past context. Connectivity with PCC and several regions identified in the whole‐brain analysis (Fig. 7) showed a modulation that was only dependent on features of the recently terminated task, and was independent of the current demands (anterior insula, anterior IPS, the precuneus, and posterior cingulate). Each of these regions showed the same interaction pattern: following a passive task, connectivity with right HF was stronger than connectivity with left HF. Conversely, following an attentive task, connectivity with right HF was weaker than connectivity with left HF. This is a clear demonstration that communication between the HF and a network of distributed cortical regions continues to reflect aspects of recently terminated environments. However, understanding the differential role played by the left and right HF is more complicated. It may be that the two HF play different roles in processing ongoing versus recently terminated experiences. Specifically, the right HF may have a stronger role in coding for features of online experiences, a process that may have priority after relatively unchallenging recently terminated experiences. In contradistinction, the left HF could play a more dominant role in coding for features of recently terminated experiences, and thus have stronger connectivity with the identified brain regions after more demanding contexts. These differential connectivity profiles for left and right HF, as a function of prior task demands, may be related to different mechanisms implemented by these regions. The possibility that these mechanism relate to encoding is generally consistent with the recent work of Schott et al. [2013] who examined neural correlates of successful subsequent memory for words presented under shallow and deep encoding and documented qualitative differences between connectivity patterns of right and left HF during an active task context. For the deep encoding task, increased connectivity between the left HF and a broad frontoparietal network predicted subsequent memory. In contrast, the relationship between right‐HF connectivity and subsequent memory in this deep encoding task was minor; there were very few regions for which stronger connectivity with right‐HF predicted subsequent memory. When examining connectivity during words presented in the shallow task, an opposite picture emerged: there, transient increases in connectivity with right‐HF (within a broad network) predicted subsequent memory, whereas there were few regions where increases in connectivity with left‐HF predicted memory. These findings of Schott et al. [2013] suggest that in different cognitive contexts, fluctuations of right and left hippocampus play different roles, consistent with our current results.
The connectivity patterns of the left and right HF with left SFG (and right vmPFC) further support the postulation of different roles during endogenous activity. The connectivity of left SFG with left HF was consistently strongly positive, but was not modulated by any experimental factor in a statistically significant manner. In contrast, left SFG's connectivity with right HF was affected by both ongoing and prior task environments. This pattern indicates that these regions code for information over a longer temporal constant that considers both prior and ongoing contexts, and could indicate a relatively longer‐term temporal integration process mediated by hippocampal‐frontal connectivity. Specifically, the connectivity pattern may suggest sensitivity to attentional demands rather than sensitivity to exogenous stimulus per se. Relatively stronger connectivity was found for attentive rest following a passive task, and for passive rest following an attentive task. These were the two contextual sets that had similar visual attention requirements. The former required monitoring potential rotations of a fixation cross both during listening to audio and the subsequent silence epoch, whereas the latter did not necessitate sustained visual attention. Thus, the interaction patterns seen in Figure 4B suggests that the right HF might have a particularly important role in bridging between contexts, and the modulation of this circuit's activity as a function of both ongoing and prior context suggests it does not code solely features of the current experience but adaptively changes in strength depending on both current and prior demands.
Our analysis of contextually modulated connectivity of anterior versus posterior HF segments during the first 1 min of rest further suggests that the right HF may be involved in contextual integration. For the left HF, when analyzing connectivity of aHF versus pHF, we found no interaction of this factor with any other determinant of connectivity (prior task, current rest). Thus, in accord with the conclusions from the main analysis, left HF appeared to be relatively less sensitive to recently terminated contexts. However, for the right HF, we found dissociation between aHF and pHF connectivity, with strong contextual modulation of connectivity between IFG (bilaterally) and aHF, but not pHF. The right IFG has been implicated in explicit retrieval of previously heard melodies: Watanabe et al. [2008] showed that when asked to discriminate old vs. new music stimuli, the right HF (but not left) and left IFG (but not right) showed greater activity for retrieval success defined as hits versus correct rejections. These patterns of HF and IFG activity were interpreted in terms of successful retrieval of previous musical pieces, and we therefore suggest that in the current study, the connectivity of IFG and right aHF may indicate spontaneous retrieval of recently terminated auditory contexts. We also note that our analysis indicated that while the aHF and pHF segments show partly differential connectivity patterns during rest [Kahn et al., 2008], at the same time they maintained considerable correlation among themselves (see Supporting Information Supplementary Results.) Thus, future work on contextual‐modulated functional connectivity may want to analyze both the left and right HF as whole seed regions (particularly the left HF), and the separate left and right aHF and pHF contributions
Our findings also point to practical implications for the role of HF laterality in studying HF resting‐state networks. With respect to laterality, as detailed in Introduction, the vast majority of prior work examining resting‐state connectivity in the HF had not attended to potential connectivity differences between left and right HF, often collapsing data from both, or choosing one region as representative. On the one hand, our findings suggest that laterality of HF connectivity is not a significant factor when examining relatively long resting‐state periods as done in prior work. We did not find any effect of laterality in the analysis of the entire 5‐min post‐task epochs, or in the analysis of the independent 5‐min resting‐state scan. On the other hand, when examined from the perspective of shorter time scales such as the 1‐min transition between contexts or environments (i.e., in a nonstationary context), these laterality effects may be prevalent and important. Thus, the findings of the current work do not necessarily call for reinterpretation of prior work, but instead suggest that emerging work on dynamic functional connectivity [see Hutchison et al., 2013a, for a recent review], which typically focuses on shorter temporal windows, should consider HF laterality as a factor.
A Potential Uniqueness of HF Post‐Task Connectivity
It is an outstanding question whether and how modulations of post‐task HF connectivity may reflect invocation of processing carried out during the task itself. We have previously reported a detailed examination of brain activity during the two task periods per se [Tobia et al., 2012] where we identified multiple regions that differentiated the two tasks, but these did not include the HF; the HF connectivity in the two tasks was highly similar, and in both cases strongly resembled resting‐state networks. This analysis was undertaken given prior work [e.g., Harrison et al., 2006; Strange et al., 2005] linking HF to the coding of statistical features. Taken together with the current findings, this would suggest that the HF can exhibit post‐task connectivity impact of prior‐task without showing connectivity effects during the prior task itself. While this may seem counterintuitive, a similar pattern was identified by Tambini et al. [2010] in their study of post‐task HF connectivity. They documented that HF‐connectivity did not differ across their two types of associative encoding tasks, and yet its connectivity during the post‐task resting state did in fact vary depending on the prior task. This led Tambini et al. [2010] to conclude that “enhanced correlations during rest [do] not merely reflect, or mirror, previously induced correlations during behavior.” We suggest that HF networks very likely play a role in ongoing post‐experiential (post‐task) encoding in a multitude of cognitive contexts, including the sort of unchallenging and low‐demand experiences prevalent in everyday life that do not in and of themselves require mnemonic/HF computations. The interpretation we outline differs from the types of explanations given in some prior studies of post‐task RS [e.g., Stevens et al., 2010], where post‐task functional connectivity was examined in ROIs identified as playing a central role in task performance, interpreting the connectivity of the regions studied in the post‐task epochs vis‐a‐vis the computations performed during task.
CONCLUSION
The current work reflects an initial examination of how ongoing hippocampal connectivity is related to processing features of both ongoing and recently ended contexts. It shows that the medial temporal cortex, recently shown to continuously code for ongoing and recent information in animals [Jadhav et al., 2012; Karlsson and Frank, 2009] appears to be sensitive to multiple scales of experience in humans as well, as observed in specific connectivity patterns with a distributed set of regions. Whether other resting‐state networks show similar sensitivty patterns, and the temporal dynamics of those patterns are interesting questions for future work.
Supporting information
Supplementary Information
REFERENCES
- Albert NB, Robertson EM, Mehta P, Miall RC (2009): Resting state networks and memory consolidation. Commun Integr Biol 2:530–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen EA, Damaraju, E , Plis, SM , Erhardt, EB , Eichele, T , Calhoun, VD (2014): Tracking whole‐brain connectivity dynamics in the resting state. Cereb Cortex 24:663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL (2010): Functional‐anatomic fractionation of the brain's default network. Neuron 65:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes A, Bullmore ET, Suckling J (2009): Endogenous human brain dynamics recover slowly following cognitive effort. PLoS One 4:e6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben‐Yakov A, Dudai Y (2011): Constructing realistic engrams: Poststimulus activity of hippocampus and dorsal striatum predicts subsequent episodic memory. J Neurosci 31:9032–9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin C, Lieberman DA, Chang M, Ofen N, Whitfield‐Gabrieli S, Gabrieli JD, Gaab N (2010): The influence of rest period instructions on the default mode network. Front Hum Neurosci 4:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli SB, Powell RH, Yogarajah M, Samson RS, Symms MR, Thompson PJ, Koepp MJ, Duncan JS (2010): Imaging memory in temporal lobe epilepsy: Predicting the effects of temporal lobe resection. Brain 133:1186–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL (2008): The brain's default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Cox RW (1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C (2007): The medial temporal lobe and recognition memory. Annu Rev Neurosci 30:123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F, Bertolino A, Scarabino T, Latorre V, Blasi G, Popolizio T, Tedeschi G, Cirillo S, Goebel R, Di Salle F (2006): Independent component model of the default‐mode brain function: Assessing the impact of active thinking. Brain Res Bull 70:263–269. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM (1999): Cortical surface‐based analysis. II: Inflation, flattening, and a surface‐based coordinate system. Neuroimage 9:195–207. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM (2004): Automatically parcellating the human cerebral cortex. Cereb Cortex 14:11–22. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC (1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster‐size threshold Magn Reson Med 33:636–647. [DOI] [PubMed] [Google Scholar]
- Fransson P (2006): How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia 44:2836–2845. [DOI] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D (2000): Image‐based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med 44:162–167. [DOI] [PubMed] [Google Scholar]
- Golland Y, Bentin S, Gelbard H, Benjamini Y, Heller R, Nir Y, Hasson U, Malach R (2007): Extrinsic and intrinsic systems in the posterior cortex of the human brain revealed during natural sensory stimulation. Cereb Cortex 17:766–777. [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Castillo J, Saad ZS, Handwerker DA, Inati SJ, Brenowitz N, Bandettini PA (2012): Whole‐brain, time‐locked activation with simple tasks revealed using massive averaging and model‐free analysis. Proc Natl Acad Sci USA 109:5487–5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker DA, Roopchansingh V, Gonzalez‐Castillo J, Bandettini PA (2012): Periodic changes in fMRI connectivity. Neuroimage 63:1712–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison LM, Duggins A, Friston KJ (2006): Encoding uncertainty in the hippocampus. Neural Netw 19:535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Nusbaum HC, Small SL (2009): Task‐dependent organization of brain regions active during rest. Proc Natl Acad Sci USA 106:10841–10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ (2011): Scale‐free properties of the functional magnetic resonance imaging signal during rest and task. J Neurosci 31:13786–13795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, Della Penna S, Duyn JH, Glover GH, Gonzalez‐Castillo J, Handwerker DA, Keilholz S, Kiviniemi V, Leopold DA, de Pasquale F, Sporns O, Walter M, Chang C (2013a): Dynamic functional connectivity: Promise, issues, and interpretations. Neuroimage 80:360–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Gati JS, Everling S, Menon RS (2013b): Resting‐state networks show dynamic functional connectivity in awake humans and anesthetized macaques. Hum Brain Mapp 34:2154–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Ma L, Balaguer‐Ballester E, Durstewitz D, Seamans JK (2012): Contextual encoding by ensembles of medial prefrontal cortex neurons. Proc Natl Acad Sci USA 109:5086–5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav SP, Kemere C, German PW, Frank LM (2012): Awake hippocampal sharp‐wave ripples support spatial memory. Science 336:1454–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolles DD, van Buchem MA, Crone EA, Rombouts SA (2013): Functional brain connectivity at rest changes after working memory training. Hum Brain Mapp 34:396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn I, Andrews‐Hanna JR, Vincent JL, Snyder AZ, Buckner RL (2008): Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J Neurophysiol 100:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson MP, Frank LM (2009): Awake replay of remote experiences in the hippocampus. Nat Neurosci 12:913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CM, Baldassarre A, Committeri G, Romani GL, Corbetta M (2009): Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci USA 106:17558–17563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B, Penfield W (1955): The effect of hippocampal lesions on recent memory. Trans Am Neurol Assoc:42–48. [PubMed] [Google Scholar]
- Newton AT, Morgan VL, Rogers BP, Gore JC (2011): Modulation of steady state functional connectivity in the default mode and working memory networks by cognitive load. Hum Brain Mapp 32:1649–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Qin P, Nakao T (2010): Rest‐stimulus interaction in the brain: A review. Trends Neurosci 33:277–284. [DOI] [PubMed] [Google Scholar]
- Park B, Kim JI, Lee D, Jeong SO, Lee JD, Park HJ (2012): Are brain networks stable during a 24‐hour period? Neuroimage 59:456–466. [DOI] [PubMed] [Google Scholar]
- Peltier SJ, LaConte SM, Niyazov DM, Liu JZ, Sahgal V, Yue GH, Hu XP (2005): Reductions in interhemispheric motor cortex functional connectivity after muscle fatigue. Brain Res 1057:10–16. [DOI] [PubMed] [Google Scholar]
- Powell HW, Koepp MJ, Symms MR, Boulby PA, Salek‐Haddadi A, Thompson PJ, Duncan JS, Richardson MP (2005): Material‐specific lateralization of memory encoding in the medial temporal lobe: Blocked versus event‐related design. Neuroimage 27:231–239. [DOI] [PubMed] [Google Scholar]
- Powell HW, Richardson MP, Symms MR, Boulby PA, Thompson PJ, Duncan JS, Koepp MJ (2007): Reorganization of verbal and nonverbal memory in temporal lobe epilepsy due to unilateral hippocampal sclerosis. Epilepsia 48:1512–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador R, Suckling J, Coleman MR, Pickard JD, Menon D, Bullmore E (2005): Neurophysiological architecture of functional magnetic resonance images of human brain. Cereb Cortex 15:1332–1342. [DOI] [PubMed] [Google Scholar]
- Schott BH, Wustenberg T, Wimber M, Fenker DB, Zierhut KC, Seidenbecher CI, Heinze HJ, Walter H, Duzel E, Richardson‐Klavehn A (2013): The relationship between level of processing and hippocampal‐cortical functional connectivity during episodic memory formation in humans. Hum Brain Mapp 34:407–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B (1957): Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C, Corbetta M, Romani GL, Shulman GL (2011): Episodic memory retrieval, parietal cortex, and the default mode network: Functional and topographic analyses. J Neurosci 31:4407–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD (2012): Decoding subject‐driven cognitive states with whole‐brain connectivity patterns. Cereb Cortex 22:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark DE, Margulies DS, Shehzad ZE, Reiss P, Kelly AM, Uddin LQ, Gee DG, Roy AK, Banich MT, Castellanos FX, Milham MP (2008): Regional variation in interhemispheric coordination of intrinsic hemodynamic fluctuations. J Neurosci 28:13754–13764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens WD, Buckner RL, Schacter DL (2010): Correlated low‐frequency BOLD fluctuations in the resting human brain are modulated by recent experience in category‐preferential visual regions. Cereb Cortex 20:1997–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens WD, Kahn I, Wig GS, Schacter DL (2012): Hemispheric asymmetry of visual scene processing in the human brain: Evidence from repetition priming and intrinsic activity. Cereb Cortex 22:1935–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Duggins A, Penny W, Dolan RJ, Friston KJ (2005): Information theory, novelty and hippocampal responses: Unpredicted or unpredictable? Neural Netw 18:225–230. [DOI] [PubMed] [Google Scholar]
- Supekar K, Menon V, Rubin D, Musen M, Greicius MD (2008): Network analysis of intrinsic functional brain connectivity in Alzheimer's disease. PLoS Comput Biol 4:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambini A, Ketz N, Davachi L (2010): Enhanced brain correlations during rest are related to memory for recent experiences. Neuron 65:280–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubert M, Lohmann G, Margulies DS, Villringer A, Ragert P (2011): Long‐term effects of motor training on resting‐state networks and underlying brain structure. Neuroimage 57:1492–1498. [DOI] [PubMed] [Google Scholar]
- Tobia MJ, Iacovella V, Davis B, Hasson U (2012): Neural systems mediating recognition of changes in statistical regularities. Neuroimage 63:1730–1742. [DOI] [PubMed] [Google Scholar]
- van Kesteren MT, Fernandez G, Norris DG, Hermans EJ (2010): Persistent schema‐dependent hippocampal‐neocortical connectivity during memory encoding and postencoding rest in humans. Proc Natl Acad Sci USA 107:7550–7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL (2006): Coherent spontaneous activity identifies a hippocampal‐parietal memory network. J Neurophysiol 96:3517–3531. [DOI] [PubMed] [Google Scholar]
- Voets NL, Zamboni G, Stokes MG, Carpenter K, Stacey R, Adcock JE (2014): Aberrant functional connectivity in dissociable hippocampal networks is associated with deficits in memory. J Neurosci 34:4920–4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Yagishita S, Kikyo H (2008): Memory of music: Roles of right hippocampus and left inferior frontal gyrus. Neuroimage 39:483–491. [DOI] [PubMed] [Google Scholar]
- Weber B, Kugler F, Elger CE (2007): Comparison of implicit memory encoding paradigms for the activation of mediotemporal structures. Epilepsy Behav 10:442–448. [DOI] [PubMed] [Google Scholar]
- Wegman J, Janzen G (2011): Neural encoding of objects relevant for navigation and resting state correlations with navigational ability. J Cogn Neurosci 23:3841–3854. [DOI] [PubMed] [Google Scholar]
- Wig GS, Grafton ST, Demos KE, Wolford GL, Petersen SE, Kelley WM (2008): Medial temporal lobe BOLD activity at rest predicts individual differences in memory ability in healthy young adults. Proc Natl Acad Sci USA 105:18555–18560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CW, Gu H, Lu H, Stein EA, Chen JH, Yang Y (2008): Frequency specificity of functional connectivity in brain networks. Neuroimage 42:1047–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Liu D, He Y, Zou Q, Zhu C, Zuo X, Long X, Zang Y (2009): Spontaneous brain activity in the default mode network is sensitive to different resting‐state conditions with limited cognitive load. PLoS One 4:e5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei M, Beckmann CF, Binnewijzend MA, Schoonheim MM, Oghabian MA, Sanz‐Arigita EJ, Scheltens P, Matthews PM, Barkhof F (2012): Functional segmentation of the hippocampus in the healthy human brain and in Alzheimer's disease. Neuroimage 66C:28–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


