Abstract
This study assesses the patterns of gray matter (GM) and white matter (WM) damage in patients with Parkinson's disease and mild cognitive impairment (PD‐MCI) compared with healthy controls and cognitively unimpaired PD patients (PD‐Cu). Three‐dimensional T1‐weighted and diffusion tensor (DT) magnetic resonance imaging (MRI) scans were obtained from 43 PD patients and 33 healthy controls. Cognition was assessed using a neuropsychological battery. Tract‐based spatial statistics was applied to compare DT MRI indices between groups on a voxel‐by‐voxel basis. Voxel‐based morphometry was performed to assess GM atrophy. Thirty PD patients were classified as MCI. Compared with healthy controls, PD‐Cu and PD‐MCI patients did not have GM atrophy. No region of WM damage was found in PD‐Cu patients when compared with healthy controls. Relative to healthy controls and PD‐Cu patients, PD‐MCI patients showed a distributed pattern of WM abnormalities in the anterior and superior corona radiata, genu, and body of the corpus callosum, and anterior inferior fronto‐occipital, uncinate, and superior longitudinal fasciculi, bilaterally. Subtle cognitive decline in PD is associated with abnormalities of frontal and interhemispheric WM connections, and not with GM atrophy. DT MRI might contribute to the identification of structural changes in PD‐MCI patients prior to the development of dementia. Hum Brain Mapp 35:1921–1929, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: MRI, Parkinson's disease, mild cognitive impairment, white matter damage, diffusion tensor MRI
INTRODUCTION
Mild cognitive impairment (MCI) is a well‐defined nonmotor manifestation of Parkinson's disease (PD), which is characterized by executive and visuospatial dysfunctions, as well as deficits of attention and short‐term recall [Emre, 2003]. In contrast to Alzheimer's disease, language and praxis remain relatively preserved [Emre, 2003]. Patients who have been newly diagnosed with PD are twice as likely to develop MCI than healthy elderly individuals, with 20–57% of PD patients being affected by Parkinson's disease and mild cognitive impairment (PD‐MCI) within the first 3–5 years from diagnosis [Caviness et al., 2007; Williams‐Gray et al., 2007]. Compared with cognitively unimpaired PD cases (PD‐Cu), PD‐MCI patients have a higher risk to develop dementia, which can occur in up to 80% of PD patients over the long term [Hely et al., 2008].
Pathological and psychopharmacological studies have suggested that cognitive decline in PD can be secondary to cortical Lewy bodies (LBs) accumulation, Alzheimer's pathology, dysfunction of dopaminergic and nondopaminergic neurotransmitter systems, and vascular lesions [Kehagia et al., 2010]. Reduced cerebral glucose uptake in several cortical regions, particularly the frontal and parietal association areas, occurs in PD‐MCI and PD with dementia (PDD) compared with PD‐Cu patients [Huang et al., 2008; Peppard et al., 1992]. In agreement with pathological findings [Kehagia et al., 2010], magnetic resonance imaging (MRI) studies have shown gray matter (GM) atrophy in PDD [Apostolova et al., 2010; Beyer et al., 2007; Burton et al., 2004; Lee et al., 2010a; Melzer et al., 2012; Nagano‐Saito et al., 2005; Ramirez‐Ruiz et al., 2005; Song et al., 2011; Summerfield et al., 2005; Tam et al., 2005], whereas the few studies which assessed GM atrophy in PD‐MCI patients showed no [Dalaker et al., 2010; Hattori et al., 2012] or only slight frontal, temporal, and parietal atrophy [Beyer et al., 2007; Lee et al., 2010c; Melzer et al., 2012; Song et al., 2011]. Although the small sample sizes and the lack of consistent criteria for defining MCI may be the reasons for such inconclusive findings, it is possible that structural abnormalities other than those occurring in the GM might contribute to the development of cognitive deficits in PD patients.
White matter (WM) damage is emerging as an important pathological substrate of cognitive deficits in PD patients [Agosta et al., 2012; Bertrand et al., 2010; Deng et al., 2013; Hattori et al., 2012; Lee et al., 2010aa,b; Matsui et al., 2007; Rae et al., 2012]. Diffusion tensor (DT) MRI in vivo allows to investigate the WM microstructure by measuring directional changes of water diffusivity [Basser et al., 1994]. DT MRI studies of cognitively normal, early, idiopathic PD patients showed subtle WM alterations [Karagulle Kendi et al., 2008], which were found to be associated with executive [Matsui et al., 2007; Rae et al., 2012] and color discrimination deficits [Bertrand et al., 2010]. A large study of idiopathic nondemented PD cases at different disease stages showed that WM damage is more marked with increasing PD severity and is associated with the degree of global cognitive impairment [Agosta et al., 2012]. So far, only a few studies used DT MRI to explore WM tract abnormalities in PD‐MCI patients, showing an involvement of the corpus callosum, cingulum, and major association WM tracts [Deng et al., 2013; Hattori et al., 2012]. However, a detailed neuropsychological status of the patients was not obtained [Hattori et al., 2012], PD‐MCI was diagnosed only on the basis of the Clinical Dementia Rating score [Hattori et al., 2012], or a regions of interest‐based analysis was performed [Deng et al., 2013].
The aim of this study was to assess WM tract damage in patients with PD‐MCI, defined according to the recent Movement Disorder Society (MDS) Task force guidelines for the diagnosis of MCI in PD [Litvan et al., 2012], compared to healthy controls and PD‐Cu patients. The patterns of GM atrophy were also investigated.
Materials and Methods
Subjects
Thirty patients with PD‐MCI in accordance with the new MDS Task Force criteria, Level I, [Litvan et al., 2012] were recruited from the outpatient population attending the Centre for Neurodegenerative Diseases, University of Belgrade, Serbia (Table 1). Thirteen PD‐Cu patients were enrolled consecutively to match PD‐MCI patients in terms of demographic and motor clinical features (Table 1). All patients received a comprehensive assessment in ON state including neurological examination, neuropsychological testing, and MRI scan. Clinical assessments were performed by an experienced neurologist blinded to the MRI results. Disease stage was defined using the Hoehn and Yahr stage score, and disease severity was quantified using the Unified Parkinson's Disease Rating Scale (UPDRS) III. Patients were excluded if they had parkin, leucine‐rich repeat kinase 2, and glucocerebrosidase gene mutations; depression according to the Structured Clinical Interview for DSM‐IV Axis I Disorders [Association, 1994]; dementia according to the MDS diagnostic criteria for PDD [Dubois et al., 2007]; cerebrovascular disorders, a history of traumatic brain injury, or intracranial masses; any other major neurological and medical conditions. To make a diagnosis of PDD in keeping with the MDS diagnostic criteria [Dubois et al., 2007], the impairment in more than one cognitive domain has to be associated with a decreased global cognitive efficiency (i.e., Mini Mental State Examination [MMSE] score, <26), and cognitive deficits severe enough to impair daily life. Thirty‐three healthy controls with no history of neurologic and psychiatric disease were recruited from spouses of patients and by word of mouth (Table 1). HC underwent a multidimensional assessment, including neurological and global cognitive evaluation, and were included in the study only when all items were normal.
Table 1.
Demographic, clinical, and conventional MRI data from healthy controls and PD patients stratified according to their cognitive status
| HC | PD | P‐valuea | PD‐Cu | PD‐MCI | P‐valueb | |
|---|---|---|---|---|---|---|
| Number | 33 | 43 | — | 13 | 30 | — |
| Women/men | 16/17 | 14/29 | 0.24 | 7/6 | 7/23 | 0.07 |
| Age at MRI (years) | 64 ± 7.3 (48–81) | 65.8 ± 7.9 (49–82) | 0.24 | 63.9 ± 7.1 (49–79) | 66.6 ± 8.2 (49–82) | 0.24 |
| Education (years) | 12.7 ± 3.1 (4–16) | 11.7 ± 3.1 (4–17) | 0.18 | 11.7 ± 4.1 (4–16) | 11.7 ± 2.7 (5–17) | 0.37 |
| Age at onset (years) | — | 57.0 ± 9.8 (36–71) | — | 53.7 ± 9.6 (36–69) | 58.3 ± 9.7 (43–71) | 0.18 |
| Disease duration (years) | — | 9.1 ± 6.2 (2–25) | — | 10.0 ± 7.1 (2–25) | 8.7 ± 5.9 (2–21) | 0.64 |
| Side initially affected (right/left) | — | 24/19 | — | 7/6 | 17/13 | 0.74 |
| H&Y | — | 2.4 ± 0.8 (1–4) | — | 2.4 ± 0.7 (1.5–4) | 2.3 ± 0.8 (1–4) | 0.53 |
| UPDRS III | — | 32.6 ± 10.0 (12–54) | — | 28.3 ± 11.4 (12–50) | 34.3 ± 9.1 (18–54) | 0.12 |
| MMSE | 28.9 ± 1.0 (26–30) | 27.6 ± 1.8 (23–30) | 0.003 | 28.9 ± 1.0 (28–30) | 27.1 ± 1.8 (23–30)*# | <0.001 |
| HDRS | 6.0 ± 4.2 (0–17) | 8.3 ± 5.5 (0–18) | 0.06 | 7.6 ± 5.5 (0–18) | 8.6 ± 5.6 (0–18) | 0.16 |
| Levodopa equivalent dose (mg) | — | 607.1 ± 228.8 (0–1,101) | — | 566.8 ± 178.9 (300–801) | 623.2 ± 246.8 (0–1,101) | 0.39 |
| WMH load (mL) | 0.7 ± 1.2 (0–5) | 1.1 ± 1.6 (0–6) | 0.19 | 0.8 ± 1.1 (0–4) | 1.3 ± 1.9 (0–6) | 0.36 |
Values are means ± SDs (range) or number of subjects. Clinical and cognitive evaluations were performed in ON state. P‐values refer to Fisher's exact test, Kruskal–Wallis test, or Mann–Whitney U‐test (for further details, see the text).
The comparison between HC and all PD patients.
The comparisons between HC, PD‐Cu and PD‐MCI patients, or between PD‐Cu and PD‐MCI patients, as appropriate. Post hoc comparisons: P < 0.05 compared with HC* and PD‐Cu# patients.
Abbreviations: HC, healthy controls; HDRS, Hamilton Depression Rating Scale; HY, Hoehn and Yahr stage score; MMSE, Mini Mental State Examination; MRI, magnetic resonance imaging; PD‐Cu, cognitively unimpaired Parkinson's disease patients; PD‐MCI, Parkinson's disease patients with mild cognitive impairment; UPDRS III, Unified Parkinson's Disease Rating Scale III; WMH, white matter hyperintensity.
Local Ethical Committee approval and written informed consent from all subjects were obtained prior to study initiation.
Neuropsychological Assessment
Within 48 h from MRI, an experienced neuropsychologist, who was unaware of the clinical and MRI data, administered an abbreviated neuropsychological and behavioral evaluation. MMSE score and Hamilton Depression Rating Scale (HDRS) score [Hamilton, 1960] were obtained from all participants. PD patients also underwent the Addenbrooke's Cognitive Assessment‐revised (ACE‐R) [Mioshi et al., 2006], and the Frontal Assessment Battery (FAB) [Dubois et al., 2000]. In case of memory impairment documented by the ACE‐R battery, the Rey Auditory Verbal Learning Test (RAVLT) [Rey, 1964] was also administered. Memory was evaluated using both the subtests of the ACE‐R and the RAVLT; attention, visuo‐spatial abilities, and language with the subtests of ACE‐R; and executive functions with the verbal fluency subtest of the ACE‐R and the subscales of FAB. Patients were defined as having MCI in accordance with the Level I of the new operational criteria [Litvan et al., 2012], that is, if they performed at least 1.0 standard deviation (SD) below the normative mean score in at least two cognitive tests within relevant cognitive domains (including executive functions, attention, visuo‐spatial abilities, memory, and language). The normative mean scores were obtained from 56 healthy controls (35 men), aged 63.9 years (SD 7.3), years of education 13.3 (SD 3.0), MMSE 29.1 (SD 0.8), and HDRS 4.9 (SD 4.1).
MRI Acquisition
MRI scans were obtained on a 1.5 Tesla Avanto system (Siemens, Erlangen, Germany). The following sequences were acquired: (i) dual‐echo (DE) turbo spin‐echo (SE) (repetition time [TR] = 2,650 ms, echo time [TE] = 28/113 ms, echo train length = 5, 50 axial slices, thickness = 2.5 mm, matrix size = 256 × 256, field of view [FOV] = 250 mm2); (ii) three‐dimensional (3D) T1‐weighted magnetization‐prepared rapid acquisition gradient echo (frequency direction = inferior–superior; TR = 2,000 ms, TE = 4.72 ms, flip angle = 12°, matrix size = 256 × 224 × 208 [inferior–superior, anterior–posterior, left–right], FOV = 236 × 236 × 236 mm); and (iii) pulsed gradient SE single‐shot echo‐planar (TR = 8,100, TE = 95, flip angle = 90°, matrix size = 128 × 128, FOV = 240 mm2; 50 contiguous, 2.5‐mm thick, axial slices), with diffusion‐encoding gradients applied in 12 noncollinear directions, coded as default in the scanner (b–factor = 1,000 s/mm2; eight averages). The maximum amplitude of the diffusion gradients was 33 mT/m and a multiple channel head coil was used for signal reception.
MRI Analysis
All MRI postprocessing was performed by a single experienced observer, blinded to clinical and cognitive findings. WM hyperintensities (WMHs), if any, were identified on DE scans. WMH load was measured using the Jim software package (Version 5.0, Xinapse Systems, Northants, United Kingdom, http://www.xinapse.com).
GM atrophy: VBM
VBM was performed using SPM8 and the Diffeomorphic Anatomical Registration using Exponentiated Lie algebra (DARTEL) registration method [Ashburner, 2007]. Briefly, (i) T1‐weighted images were segmented using VBM5.1 toolbox (http://dbm.neuro.uni-jena.de) [Ashburner and Friston, 2005] to produce tissue probability maps in the Montreal Neurological Institute (MNI) space, (ii) the images were imported in DARTEL, rigidly aligned, and segmented into GM, WM and cerebrospinal fluid (CSF) (using the segmentation parameters from step [i]) and resampled to 1.5 mm isotropic voxels; (iii) GM segments were coregistered simultaneously using the fast diffeomorphic image registration algorithm [Ashburner, 2007]; (iv) the flow fields were then applied to the rigidly aligned segments to warp them to the common DARTEL space and then modulated using the Jacobian determinants; and (v) the modulated images from DARTEL were normalized to the MNI template using an affine transformation estimated from the DARTEL GM template and the a priori GM probability map without resampling (http://brainmap.wisc.edu/normalizeDARTELtoMontreal Neurological Institute) [McLaren et al., 2009]. Prior to the statistical computations, the images were smoothed with an 8‐mm full‐width half‐maximum Gaussian filter.
WM damage: TBSS
DT MRI analysis was carried out using a in‐house software. DT MR images were first corrected for distortion induced by eddy currents [Studholme et al., 1997], then the DT was estimated by linear regression [Basser et al., 1994], and mean diffusivity (MD) and fractional anisotropy (FA) maps were computed [Pierpaoli et al., 1996]. TBSS version 1.2 (http://www.fmrib.ox.ac.uk/fsl/tbss/index.html) was used to perform the multisubject DT MRI analysis [Smith et al., 2006]. FA volumes were aligned to a target image using the following procedure: (i) a target image was selected automatically as the most representative FA image by the FMRIB's Non‐linear Image Registration Tool (FNIRT), (ii) the nonlinear transformation that mapped each subject's FA to the target image was compute using FNIRT, (iii) the target image was transformed affinely to the MNI 152 standard space, and (iv) the same transformation was used to align each subject's FA to the standard space. A mean FA image was then created by averaging the aligned individual FA images, and thinned to create a FA skeleton representing WM tracts common to all subjects [Smith et al., 2006]. The FA skeleton was thresholded at 0.2 to exclude voxels with low FA values, which are likely to include GM or CSF. Individual MD and FA data were projected onto this common skeleton. Two WM atlases within FSL (http://fsl.fmrib.ox.ac.uk/fsl/data/atlas-descriptions.html), the Johns Hopkins University WM, tractography atlas, and the ICBM‐DTI WM labels atlas guided the identification of WM tracts.
Statistical Analysis
Demographic and clinical variables were compared between groups using the Fisher's exact test for categorical variables and the Mann–Whitney U‐test or the Kruskal–Wallis test for continuous variables. These analyses were run using SPSS 13.0. A P‐value of <0.05 was considered as significant.
Analyses of covariance were performed to assess GM volume differences between groups adjusting for age, gender, and total intracranial volume (TICV), at P < 0.05, family‐wise error (FWE)‐corrected for multiple comparisons. UPDRS III was used as additional covariate in the comparison between patient groups. The following contrasts were tested: all PD patients versus healthy controls, PD‐Cu versus healthy controls, PD‐MCI patients versus healthy controls, and PD‐MCI versus PD‐Cu.
DT MRI voxelwise statistics were performed using a permutation‐based inference tool for nonparametric statistical thresholding (“randomize,” part of FSL) [Nichols and Holmes, 2002]. The number of permutations was set at 5,000 [Nichols and Holmes, 2002]. MD and FA values within the skeleton were compared between groups using permutation‐based two‐sample t‐tests, adjusting for subject's age, gender, and UPDRS III (in case of patient group comparison). The contrasts tested were the same run for VBM. The resulting statistical maps were thresholded at P < 0.05 FWE‐corrected for multiple comparisons, at a cluster level using the threshold‐free cluster enhancement option [Smith and Nichols, 2009].
To assess whether cognitive scores (i.e., MMSE, ACE‐R total, and FAB scores) were associated with GM atrophy and WM damage in PD patients, regression models in SPM8 (adjusted for TICV and age) and FSL (adjusted for age) were run. Correlations were tested at P < 0.05 FWE‐corrected.
RESULTS
Demographic, Clinical, and Cognitive Findings
PD patients and healthy controls were similar in terms of age, gender, years of education, and WMHs load (Table 1). PD‐MCI and PD‐Cu patients were similar in age, gender, years of education, age at onset, disease duration, disease stage and severity, side initially affected, HDRS score, levodopa equivalent dose, and WMH load (Table 1). Compared to PD‐Cu patients, PD‐MCI patients showed a lower MMSE score and worse performances at all cognitive tests, except for the language subtest of the ACE‐R (Tables 1 and 2). In PD‐MCI patients, the cognitive domains most frequently affected were executive functions (87%) and memory (57%), followed by orientation and attention (43%), visuospatial abilities (27%), and language (27%). Twenty‐seven PD‐MCI patients presented with a multidomain impairment, one patient had a memory deficit, and two patients presented with an isolated deficit in the executive functions.
Table 2.
Neuropsychological data of PD‐Cu and PD‐MCI
| Cutoff | PD‐Cu | PD‐MCI | P‐value | |
|---|---|---|---|---|
| Number | — | 13 | 30 | — |
| ACE‐R | ||||
| Total | 79.1 | 93.0 ± 2.9 (89–97) | 81.6 ± 8.9 (63–91) | <0.001 |
| Memory | 20.03 | 24.9 ± 1.1 (23–26) | 20.5 ± 3.7 (13–25) | <0.001 |
| Orientation and attention | 16.87 | 17.6 ± 0.5 (17–18) | 16.4 ± 1.5 (13–18) | 0.02 |
| Verbal fluency | 8.53 | 10.5 ± 1.8 (7–14) | 8.3 ± 2.1 (5–14) | 0.002 |
| Language | 21.36 | 24.3 ± 1.5 (20–26) | 23.0 ± 3.0 (16–26) | 0.29 |
| Visuospatial ability | 12.57 | 15.3 ± 0.5 (15–16) | 13.4 ± 2.4 (8–16) | 0.01 |
| RAVLT | ||||
| Total | 34.66 | — | 33.3 ± 5.2 (25–42) | — |
| Delay recall | 4.91 | — | 5.8 ± 1.7 (3–8) | — |
| FAB | ||||
| Total | 14.71 | 16.6 ± 1.2 (15–18) | 13.2 ± 2.6 (8–16) | <0.001 |
| Motor series (programming) | 2.22 | 2.8 ± 0.4 (2–3) | 2.1 ± 0.7 (1–3) | 0.01 |
| Conflicting instructions (sensitivity to interference) | 2.17 | 2.9 ± 0.4 (2–3) | 2.0 ± 0.9 (0–3) | 0.002 |
| Go–No go (inhibitory control) | 1.96 | 2.8 ± 0.4 (2–3) | 2.1 ± 0.8 (0–3) | 0.01 |
Values are means ± SDs (ranges) or number of patients. P‐values refer to the Mann–Whitney U‐test between PD‐MCI and PD‐Cu patients. Cutoff values are mean values of normative population ± 1 SD (for further details, see the text).
Abbreviations: ACE‐R, The Addenbrooke's Cognitive Examination Revised; FAB, Frontal Assessment Battery; PD‐Cu, cognitively unimpaired PD patients; PD‐MCI, PD patients with mild cognitive impairment; RAVLT, Rey Auditory Verbal Learning Test.
GM Atrophy
No GM volume difference was found in all the investigated contrasts.
WM damage: group comparisons
All PD patients versus healthy controls
No DT MRI differences were found between all PD patients and healthy controls.
PD‐Cu patients versus healthy controls
No DT MRI differences were found between PD‐Cu patients and healthy controls.
PD‐MCI patients versus healthy controls
Compared with healthy controls, PD‐MCI patients showed decreased FA values in the anterior and superior corona radiata bilaterally, body of the corpus callosum, and anterior superior longitudinal fasciculus (SLF) (Fig. 1, P < 0.05 FWE‐corrected). No regions of increased FA or MD difference were found in PD‐MCI patients compared with healthy controls.
Figure 1.
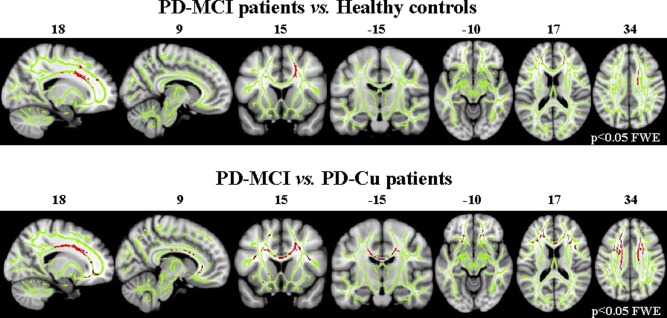
Tract‐based spatial statistics results in PD‐MCI patients compared with healthy controls and PD‐Cu patients. Voxelwise group differences are shown in red (decreased FA). Results are overlaid on the WM skeleton (light green) and displayed on the sagittal, coronal, and axial sections of the MNI standard brain in neurological convention (right is right) at P < 0.05 FWE‐corrected. Abbreviations: FA, fractional anisotropy; FWE, family‐wise error; PD‐Cu, cognitively unimpaired PD patients; PD‐MCI, PD patients with mild cognitive impairment.
PD‐MCI versus PD‐Cu patients
Compared with PD‐Cu, PD‐MCI patients showed decreased FA values in the anterior and superior corona radiata, genu, and body of the corpus callosum, anterior inferior fronto‐occipital fasciculus (IFOF)/uncinate, and anterior SLF, bilaterally (Fig. 1, P < 0.05 FWE‐corrected). No regions of increased FA or MD difference were found in PD‐MCI compared with PD‐Cu patients.
Correlations
In PD patients, cognitive scores did not correlate with GM volumes and DT MRI variables.
DISCUSSION
In this study, we investigated brain GM volumetry and WM diffusivity in PD‐MCI patients diagnosed according to the Level I of new MDS Task Force criteria [Litvan et al., 2012]. We found that PD‐MCI is associated with a distributed pattern of structural WM damage, mainly located in the frontal brain regions. On the contrary, PD‐MCI was not associated with GM atrophy. These findings suggest that DT MRI might contribute to the identification of structural brain abnormalities in PD‐MCI patients prior to the development of overt dementia.
PD‐Cu patients did not show WM abnormalities compared with healthy controls, whereas PD‐MCI patients experienced decreased FA values in the anterior and superior corona radiata, genu, and body of the CC, major corticocortical pathways linking the frontal lobe with temporal, parietal, and occipital regions (i.e., SLF, uncinate, and IFOF). The pattern of decreased FA observed in our patients is similar to that reported by the only previous whole‐brain DT MRI study assessing WM damage in PD‐MCI cases [Hattori et al., 2012], where PD‐MCI was defined according to Petersen's criteria [Petersen et al., 1999]. The involvement of the genu of the corpus callosum and SLF was also reported in another group of nondemented PD patients [Karagulle Kendi et al., 2008]; however, these authors did not asses the cognitive status of the patients. Our findings are also in line with the DT MRI studies that showed a widespread involvement of frontal WM areas in patients with PDD [Hattori et al., 2012; Lee et al., 2010b]. A decreased FA of the CC and frontal, parietal, and occipital WM was also observed in patients with dementia with LBs [Bozzali et al., 2005; Hattori et al., 2012; Lee et al., 2010b].
The pattern of a prevalent frontoparietal WM tract damage in PD‐MCI patients fits with their cognitive features as they showed primarily, as expected [Caviness et al., 2007], an executive dysfunction syndrome, associated with attention deficits in about half of the cases. The frequency of memory disturbance in our PD patients is similar to that reported by the previous studies [Caviness et al., 2007; Sollinger et al., 2010]. Fifty‐seven percent of PD‐MCI patients had memory deficits, but in most cases they were in association with one or more additional cognitive domain dysfunction (mainly executive and attention deficits). Functional MRI (fMRI) studies of healthy subjects demonstrated that the performance at tests assessing executive functions relies on the communication among a number of broadly distributed, functionally specialized regions of the frontal and parietal lobes [Zakzanis et al., 2005]. The prefrontal cortex is central in performance monitoring, and is involved in the allocation and coordination of attentional resources [Sohn et al., 2000]. The performance of various executive tasks in healthy humans is also associated with the recruitment of parietal areas, which are involved in the attentional shifting necessary to maintain simultaneously activated auditory and visual information [Collette et al., 2005]. fMRI studies of PD patients suggested that a decreased recruitment of the frontoparietal network is likely to be the neural correlate of attentional control impairment [Williams‐Gray et al., 2008]. A previous DT MRI study showed that executive performance of PD‐Cu patients was associated with decreased FA and increased MD of the prefrontal WM and anterior corpus callosum [Rae et al., 2012]. Together with the previous findings, our results suggest that subtle cognitive decline in PD is associated with damage to frontal and frontoparietal WM pathways connecting cortical/(sub)cortical structures.
PD‐MCI patients had no GM atrophy compared with healthy controls and PD‐Cu patients. Previous MRI studies of nondemented PD patients, where those with MCI were not always analyzed separately, have generally found only a subtle GM atrophy, if any [Whitwell and Josephs, 2007]. Moreover, studies which investigated GM atrophy in PD‐MCI patients were negative [Dalaker et al., 2010; Hattori et al., 2012], or reported only a limited GM atrophy in the temporal, parietal, and frontal cortices [Beyer et al., 2007; Lee et al., 2010c; Melzer et al., 2012; Song et al., 2011]. Furthermore, only one study showed GM findings surviving correction for multiple comparisons in PD‐MCI patients [Melzer et al., 2012]. A 12‐month, longitudinal study of PD patients found no association between the rate of GM atrophy development and the cognitive decline [Burton et al., 2005]. Together with these results, our own findings suggest that GM atrophy is not a major contributor to cognitive impairment in nondemented PD patients.
Pathological studies have shown that cortical LB disease and concomitant Alzheimer pathology are likely to be among the main structural substrates of cognitive impairment in PD [Kehagia et al., 2010]. According to such a notion, the DT MRI abnormalities we observed in PD‐MCI patients may be interpreted as secondary to axonal degeneration after injury of cortical neurons. However, no GM atrophy was found in PD‐MCI patients. As a consequence, it is plausible that microstructural WM abnormalities may become detectable earlier than GM atrophy with available MR technology. Nevertheless, it is noteworthy that VBM may not be the most appropriate procedure to assess cortical pathology as its normalization/smoothing steps reduce the ability to anatomically characterize sulci and gyri. Indeed, a recent study has reported that cortical thickness is reduced in PD patients in the absence of VBM abnormalities [Pereira et al., 2011]. Although cellular characteristics cannot be quantified in vivo using MRI, cortical thickness is likely to reflect cytoarchitectural abnormalities more closely than cortical volume. In fact, VBM provides a mixed measure of GM, including cortical surface area and/or cortical folding as well as cortical thickness; in contrast, cortical thickness is a topographical measurement that is an indicator of the integrity of cytoarchitecture of the cortex. Studies based on the cortical thickness measurements are, therefore, warranted to explore GM damage in PD‐MCI patients. It is also tempting to speculate that a primary damage to the WM may occur in PD. Ubiquitin and α‐synuclein‐positive inclusions are found in portions of the axons of LB disease cases [Braak et al., 1999]. There is also evidence for an accumulation of axonally transported substances in cortical neurons of these patients [Katsuse et al., 2003], suggesting that an impaired axonal transport may be associated with PD. Finally, WMHs are likely to contribute not only to the motor symptoms but also to the cognitive status of PD patients, possibly via disrupting subcortical–cortical tracts involved in gait, balance, and cognition [Bohnen and Albin, 2011]. A pathological study found that comorbid WMHs increase the risk of dementia more than twofold in patients with PD [Choi et al., 2010]. Higher burdens of WMH, mainly in the periventricular and deep WM, were found in PDD compared with PD patients [Beyer et al., 2006; Lee et al., 2010d]. Although we excluded subjects with evident vascular changes, a microvascular pathology cannot be completely ruled out.
This study is not without limitations. First, it was conducted in a university setting specifically investigating cognition in PD. This may have inflated the proportion of patients with cognitive deficits. The smaller sample of PD‐Cu patients compared with the other two groups may also explain the fact that PD‐MCI showed more extensive FA reduction compared with PD‐Cu patients than with healthy controls. Second, the neuropsychological battery used was designed to detect a multidomain more than a single‐domain cognitive impairment. PD MCI was diagnosed according to the Level I criteria of the MDS Task Force [Litvan et al., 2012], because comprehensive testing was not available. As Level I criteria provide less diagnostic certainty than Level II [Litvan et al., 2012], we cannot exclude the presence of false negatives in the PD‐Cu group. In addition, the validation of the cognitive tests in the Serbian population has been performed at the Centre for Neurodegenerative Diseases, University of Belgrade, Serbia, but still not published. Finally, this was a cross‐sectional study.
CONCLUSIONS
In conclusion, we found that MCI in PD is associated with damage to the frontoparietal, frontotemporal, and interhemispheric WM tracts, and not with GM atrophy. Our study contributed to identify a priori hypotheses to be tested in a prospective, longitudinal study of the present cohort, which hopefully will identify early DT MRI markers able to contribute to the prediction of dementia in PD patients.
ACKNOWLEDGMENT
The authors thank Dr. Melissa Petrolini and Dr. Stefania Sala (Neuroimaging Research Unit, INSPE, Division of Neuroscience, Scientific Institute San Raffaele, Milan, Italy) for their support in the MRI analysis.
REFERENCES
- Agosta F, Canu E, Stojkovic T, Pievani M, Tomic A, Sarro L, Dragasevic N, Copetti M, Comi G, Kostic VS, Filippi M (2012): The topography of brain damage at different stages of Parkinson's disease. Hum Brain Mapp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova LG, Beyer M, Green AE, Hwang KS, Morra JH, Chou YY, Avedissian C, Aarsland D, Janvin CC, Larsen JP, Cummings JL, Thompson PM (2010): Hippocampal, caudate, and ventricular changes in Parkinson's disease with and without dementia. Mov Disord 25:687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J (2007): A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005): Unified segmentation. Neuroimage 26:839–851. [DOI] [PubMed] [Google Scholar]
- Association AP (1994): Diagnostic and Statistical Manual of Mental Disorders. Association AP. [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D (1994): Estimation of the effective self‐diffusion tensor from the NMR spin echo. J Magn Reson B 103:247–254. [DOI] [PubMed] [Google Scholar]
- Bertrand JA, Bedetti C, Postuma RB, Monchi O, Genier Marchand D, Jubault T, Gagnon JF (2010): Color discrimination deficits in Parkinson's disease are related to cognitive impairment and white‐matter alterations. Mov Disord 27:1781–1788. [DOI] [PubMed] [Google Scholar]
- Beyer MK, Aarsland D, Greve OJ, Larsen JP (2006): Visual rating of white matter hyperintensities in Parkinson's disease. Mov Disord 21:223–229. [DOI] [PubMed] [Google Scholar]
- Beyer MK, Janvin CC, Larsen JP, Aarsland D (2007): A magnetic resonance imaging study of patients with Parkinson's disease with mild cognitive impairment and dementia using voxel‐based morphometry. J Neurol Neurosurg Psychiatry 78:254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Albin RL (2011): White matter lesions in Parkinson disease. Nat Rev Neurol 7:229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzali M, Falini A, Cercignani M, Baglio F, Farina E, Alberoni M, Vezzulli P, Olivotto F, Mantovani F, Shallice T, Scotti G, Canal N, Nemni R (2005): Brain tissue damage in dementia with Lewy bodies: An in vivo diffusion tensor MRI study. Brain 128:1595–1604. [DOI] [PubMed] [Google Scholar]
- Braak H, Sandmann‐Keil D, Gai W, Braak E (1999): Extensive axonal Lewy neurites in Parkinson's disease: A novel pathological feature revealed by alpha‐synuclein immunocytochemistry. Neurosci Lett 265:67–69. [DOI] [PubMed] [Google Scholar]
- Burton EJ, McKeith IG, Burn DJ, Williams ED, O'Brien JT (2004): Cerebral atrophy in Parkinson's disease with and without dementia: A comparison with Alzheimer's disease, dementia with Lewy bodies and controls. Brain 127:791–800. [DOI] [PubMed] [Google Scholar]
- Burton EJ, McKeith IG, Burn DJ, O'Brien JT (2005): Brain atrophy rates in Parkinson's disease with and without dementia using serial magnetic resonance imaging. Mov Disord 20:1571–1576. [DOI] [PubMed] [Google Scholar]
- Caviness JN, Driver‐Dunckley E, Connor DJ, Sabbagh MN, Hentz JG, Noble B, Evidente VG, Shill HA, Adler CH (2007): Defining mild cognitive impairment in Parkinson's disease. Mov Disord 22:1272–1277. [DOI] [PubMed] [Google Scholar]
- Choi SA, Evidente VG, Caviness JN, Shill HA, Sabbagh MN, Connor DJ, Hentz JG, Adler CH, Beach TG (2010): Are there differences in cerebral white matter lesion burdens between Parkinson's disease patients with or without dementia? Acta Neuropathol 119:147–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collette F, Olivier L, Van der Linden M, Laureys S, Delfiore G, Luxen A, Salmon E (2005): Involvement of both prefrontal and inferior parietal cortex in dual‐task performance. Brain Res Cogn Brain Res 24:237–251. [DOI] [PubMed] [Google Scholar]
- Dalaker TO, Zivadinov R, Larsen JP, Beyer MK, Cox JL, Alves G, Bronnick K, Tysnes OB, Antulov R, Dwyer MG, Aarsland D (2010): Gray matter correlations of cognition in incident Parkinson's disease. Mov Disord 25:629–633. [DOI] [PubMed] [Google Scholar]
- Deng B, Zhang Y, Wang L, Peng K, Han L, Nie K, Yang H, Zhang L, Wang J (2013): Diffusion tensor imaging reveals white matter changes associated with cognitive status in patients with Parkinson's disease. Am J Alzheimers Dis Other Demen 28:154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Slachevsky A, Litvan I, Pillon B (2000): The FAB: A Frontal Assessment Battery at bedside. Neurology 55:1621–1626. [DOI] [PubMed] [Google Scholar]
- Dubois B, Burn D, Goetz C, Aarsland D, Brown RG, Broe GA, Dickson D, Duyckaerts C, Cummings J, Gauthier S, Korczyn A, Lees A, Levy R, Litvan I, Mizuno Y, McKeith IG, Olanow CW, Poewe W, Sampaio C, Tolosa E, Emre M (2007): Diagnostic procedures for Parkinson's disease dementia: Recommendations from the movement disorder society task force. Mov Disord 22:2314–2324. [DOI] [PubMed] [Google Scholar]
- Emre M (2003): Dementia associated with Parkinson's disease. Lancet Neurol 2:229–237. [DOI] [PubMed] [Google Scholar]
- Hamilton M (1960): A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T, Orimo S, Aoki S, Ito K, Abe O, Amano A, Sato R, Sakai K, Mizusawa H (2012): Cognitive status correlates with white matter alteration in Parkinson's disease. Hum Brain Mapp 33:727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG (2008): The Sydney multicenter study of Parkinson's disease: The inevitability of dementia at 20 years. Mov Disord 23:837–844. [DOI] [PubMed] [Google Scholar]
- Huang C, Mattis P, Perrine K, Brown N, Dhawan V, Eidelberg D (2008): Metabolic abnormalities associated with mild cognitive impairment in Parkinson disease. Neurology 70:1470–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagulle Kendi AT, Lehericy S, Luciana M, Ugurbil K, Tuite P (2008): Altered diffusion in the frontal lobe in Parkinson disease. AJNR Am J Neuroradiol 29:501–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuse O, Iseki E, Marui W, Kosaka K (2003): Developmental stages of cortical Lewy bodies and their relation to axonal transport blockage in brains of patients with dementia with Lewy bodies. J Neurol Sci 211:29–35. [DOI] [PubMed] [Google Scholar]
- Kehagia AA, Barker RA, Robbins TW (2010): Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet Neurol 9:1200–1213. [DOI] [PubMed] [Google Scholar]
- Lee JE, Park B, Song SK, Sohn YH, Park HJ, Lee PH (2010a): A comparison of gray and white matter density in patients with Parkinson's disease dementia and dementia with Lewy bodies using voxel‐based morphometry. Mov Disord 25:28–34. [DOI] [PubMed] [Google Scholar]
- Lee JE, Park HJ, Park B, Song SK, Sohn YH, Lee JD, Lee PH (2010b): A comparative analysis of cognitive profiles and white‐matter alterations using voxel‐based diffusion tensor imaging between patients with Parkinson's disease dementia and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 81:320–326. [DOI] [PubMed] [Google Scholar]
- Lee JE, Park HJ, Song SK, Sohn YH, Lee JD, Lee PH (2010c): Neuroanatomic basis of amnestic MCI differs in patients with and without Parkinson disease. Neurology 75:2009–2016. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Kim JS, Yoo JY, Song IU, Kim BS, Jung SL, Yang DW, Kim YI, Jeong DS, Lee KS (2010d): Influence of white matter hyperintensities on the cognition of patients with Parkinson disease. Alzheimer Dis Assoc Disord 24:227–233. [DOI] [PubMed] [Google Scholar]
- Litvan I, Goldman JG, Troster AI, Schmand BA, Weintraub D, Petersen RC, Mollenhauer B, Adler CH, Marder K, Williams‐Gray CH, Aarsland D, Kulisevsky J, Rodriguez‐Oroz MC, Burn DJ, Barker RA, Emre M (2012): Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord 27:349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H, Nishinaka K, Oda M, Niikawa H, Komatsu K, Kubori T, Udaka F (2007): Wisconsin Card Sorting Test in Parkinson's disease: Diffusion tensor imaging. Acta Neurol Scand 116:108–112. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Kosmatka KJ, Kastman EK, Bendlin BB, Johnson SC (2009): Rhesus macaque brain morphometry: A methodological comparison of voxel‐wise approaches. Methods 50:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer TR, Watts R, MacAskill MR, Pitcher TL, Livingston L, Keenan RJ, Dalrymple‐Alford JC, Anderson TJ (2012): Grey matter atrophy in cognitively impaired Parkinson's disease. J Neurol Neurosurg Psychiatry 83:188–194. [DOI] [PubMed] [Google Scholar]
- Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR (2006): The Addenbrooke's Cognitive Examination Revised (ACE‐R): A brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry 21:1078–1085. [DOI] [PubMed] [Google Scholar]
- Nagano‐Saito A, Washimi Y, Arahata Y, Kachi T, Lerch JP, Evans AC, Dagher A, Ito K (2005): Cerebral atrophy and its relation to cognitive impairment in Parkinson disease. Neurology 64:224–229. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP (2002): Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp 15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppard RF, Martin WR, Carr GD, Grochowski E, Schulzer M, Guttman M, McGeer PL, Phillips AG, Tsui JK, Calne DB (1992): Cerebral glucose metabolism in Parkinson's disease with and without dementia. Arch Neurol 49:1262–1268. [DOI] [PubMed] [Google Scholar]
- Pereira JB, Ibarretxe‐Bilbao N, Marti MJ, Compta Y, Junque C, Bargallo N, Tolosa E (2011): Assessment of cortical degeneration in patients with Parkinson's disease by voxel‐based morphometry, cortical folding, and cortical thickness. Hum Brain Mapp 33:2521–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E (1999): Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol 56:303–308. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G (1996): Diffusion tensor MR imaging of the human brain. Radiology 201:637–648. [DOI] [PubMed] [Google Scholar]
- Rae CL, Correia MM, Altena E, Hughes LE, Barker RA, Rowe JB (2012): White matter pathology in Parkinson's disease: The effect of imaging protocol differences and relevance to executive function. Neuroimage 62:1675–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez‐Ruiz B, Marti MJ, Tolosa E, Bartres‐Faz D, Summerfield C, Salgado‐Pineda P, Gomez‐Anson B, Junque C (2005): Longitudinal evaluation of cerebral morphological changes in Parkinson's disease with and without dementia. J Neurol 252:1345–1352. [DOI] [PubMed] [Google Scholar]
- Rey A (1964): L'examen Clinique en Psychologie. Paris: Presses Universitaires. [Google Scholar]
- Smith SM, Nichols TE (2009): Threshold‐free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44:83–98. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen‐Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE (2006): Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. Neuroimage 31:1487–1505. [DOI] [PubMed] [Google Scholar]
- Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS (2000): The role of prefrontal cortex and posterior parietal cortex in task switching. Proc Natl Acad Sci USA 97:13448–13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollinger AB, Goldstein FC, Lah JJ, Levey AI, Factor SA (2010): Mild cognitive impairment in Parkinson's disease: Subtypes and motor characteristics. Parkinsonism Relat Disord 16:177–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Lee JE, Park HJ, Sohn YH, Lee JD, Lee PH (2011): The pattern of cortical atrophy in patients with Parkinson's disease according to cognitive status. Mov Disord 26:289–296. [DOI] [PubMed] [Google Scholar]
- Studholme C, Hill DL, Hawkes DJ (1997): Automated three‐dimensional registration of magnetic resonance and positron emission tomography brain images by multiresolution optimization of voxel similarity measures. Med Phys 24:25–35. [DOI] [PubMed] [Google Scholar]
- Summerfield C, Junque C, Tolosa E, Salgado‐Pineda P, Gomez‐Anson B, Marti MJ, Pastor P, Ramirez‐Ruiz B, Mercader J (2005): Structural brain changes in Parkinson disease with dementia: A voxel‐based morphometry study. Arch Neurol 62:281–285. [DOI] [PubMed] [Google Scholar]
- Tam CW, Burton EJ, McKeith IG, Burn DJ, O'Brien JT (2005): Temporal lobe atrophy on MRI in Parkinson disease with dementia: A comparison with Alzheimer disease and dementia with Lewy bodies. Neurology 64:861–865. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Josephs KA (2007): Voxel‐based morphometry and its application to movement disorders. Parkinsonism Relat Disord 13:S406–S416. [DOI] [PubMed] [Google Scholar]
- Williams‐Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA (2007): Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain 130:1787–1798. [DOI] [PubMed] [Google Scholar]
- Williams‐Gray CH, Hampshire A, Barker RA, Owen AM (2008): Attentional control in Parkinson's disease is dependent on COMT val 158 met genotype. Brain 131:397–408. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK, Mraz R, Graham SJ (2005): An fMRI study of the Trail Making Test. Neuropsychologia 43:1878–1886. [DOI] [PubMed] [Google Scholar]


