Abstract
The existence of a behavioral advantage of bilinguals over monolinguals during executive tasks is controversial. A new approach to this issue is to investigate the effect of bilingualism on neural control when performing these tasks as a window to understand when behavioral differences are produced. Here, we tested if early bilinguals use more language‐related networks than monolinguals while performing a go/no‐go task that includes infrequent no‐go and go trials. The RTs and accuracy in both groups did not differ. An independent component analyses (ICA) revealed, however, that bilinguals used the left fronto‐parietal network and the salience network more than monolinguals while processing go infrequent cues and no‐go cues, respectively. It was noteworthy that the modulation of these networks had opposite correlates with performance in bilinguals and monolinguals, which suggests that between‐group differences were more qualitative than quantitative. Our results suggest that bilinguals may differently develop the involvement of the executive control networks that comprise the left inferior frontal gyrus during cognitive control tasks than monolinguals. Hum Brain Mapp 36:5101–5112, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: bilingualism, executive control, language
Abbreviations
- AC‐PC
anterior‐posterior commissure
- DMN
default mode network
- DAN
dorsal attention network
- ICA
independent component analyses
- PCA
principal component analysis
INTRODUCTION
Since the first positive results were obtained [Bialystok et al., 2004; Costa et al., 2008], different studies have questioned if bilinguals exhibit enhanced cognitive control and better inhibitory control when compared to monolinguals [Hilchey and Klein, 2011; Duñabeitia et al., 2014]. A different approach to this issue stems from cognitive neuroscience and relates bilingualism to different development of brain networks [Grady et al., 2015; Stocco et al., 2014], which may bias toward use of language brain areas during control tasks [Bialystok et al., 2005 ; Bialystok, 2011; Buchweitz and Prat, 2013; Garbin et al., 2010]. A recent neurocognitive model has proposed the mechanism that underlies the differences between bilinguals and monolinguals [Stocco et al., 2014]. In brief, they propose that bilinguals experience stronger activity in the basal ganglia as a result of their need to control which language they use. This extensive practice would modify the activity and connectivity between the striatum and the language prefrontal areas, and would override the bottom‐up cortico‐cortical processing, and facilitate the implementation of top–down influences. As a result, the authors propose that bilinguals would display better performance during the executive tasks that require top‐down processing, but would probably display worse performance during the tasks that require bottom‐up processing.
Processes such as inhibitory control or set shifting are mainly right‐lateralized in the inferior frontal cortex [Robbins, 2007]. However, participation of other areas, including the anterior cingulate cortex or the left inferior frontal cortex, may be found in situations that require actively maintaining semantic information throughout the task [Bari and Robbins, 2013]. The participation of the left or the right inferior frontal cortex implies two complementary forms of responding: the former relates to the top‐down control of the task, whereas the latter relates more to inhibitory control. Bilingual language control throughout life (i.e. selecting, inhibiting, and switching between languages) may impact organization of the cognitive control network in a qualitative (and not only in a quantitative) way, leading to the involvement of language control brain areas in nonlinguistic executive tasks [Buchweitz and Prat, 2013; Stocco et al., 2014]. Some previous studies have shown that bilinguals use the left lateral prefrontal cortex and the left striatum more than monolinguals during executive tasks [Garbin et al., 2010; Rodríguez‐Pujadas et al., 2013], whereas monolinguals display greater use of other areas, e.g. the right inferior frontal cortex [Garbin et al., 2010] or the anterior cingulate [Abutalebi et al., 2012; Rodríguez‐Pujadas et al., 2014]. These studies have suggested that both language groups employ different brain networks to perform executive tasks: monolinguals use the “classical” areas involved in executive processing more, whereas bilinguals also involve the left lateral prefrontal cortex and the striatum. Thus the determination of these brain networks would facilitate the comprehension of behavioral differences when they appear between both groups.
The current study aimed to investigate differences for the functional networks involved in cognitive control in bilingualism by applying an independent component analysis (ICA) to a go/no‐go task. ICA is a statistical method used to discover hidden factors from a set of measurements or observed data so that sources are maximally independent. The ICA method most commonly used to the analysis of fMRI data is spatial ICA, which identifies the temporally coherent networks that are spatially and maximally independent [see Calhoun et al. 2003 and 2009 for review). One advantage of ICA is that it does not require a priori models of brain activity or connectivity to generate functional networks because it is a data‐driven approach. Furthermore, the application of general linear model (GLM) to the temporal signals estimated with ICA can provide new insights into the brain's functional organization, which are not observed in conventional GLM analyses [Xu et al. 2013]. It also offers other advantages as it reduces the number of comparisons and is more sensitive to detect between‐group differences [Congdon et al., 2010]. Among those involved in cognitive control, there are three relevant networks called the left and right fronto‐parietal networks (FPN), and one bilateral cingulo‐opercular network (i.e. salience) that comprises the inferior frontal gyrus, the insula, and the dorsal anterior cingulate [Allen et al., 2011; Dosenbach et al., 2007, 2008], and in some cases, the striatum [García‐García et al., 2013]. Given the previous results using a GLM approach, all three may be relevant to help understand neural differences as a function of bilingualism [Grady et al., 2015]. FPN networks have been proposed to actively maintain task‐relevant information about one or a small number of trials, and to implement control parameter adjustments more rapidly [Dosenbach et al., 2008]. It is noteworthy that the left FPN involves the brain areas related to language control and is associated more with verbal working memory, whereas the right FPN involves the brain areas related to stimulus‐driving orienting of attention [Allen et al., 2011]. The salience network is related to processing relevant unexpected stimuli [Seeley et al., 2007].
The objective of the present study was to investigate how bilingualism modulates the activity of cognitive control networks when participants perform a nonlinguistic task that requires cognitive control. To this end, we carried out an ICA to explore any possible differences in the relevant networks between early high‐proficient bilinguals and monolinguals when performing a go/no‐go task. According to the Stocco et al. [2014] model and the above‐cited results, we hypothesized that the modulation of networks, which include the brain areas involved in language control (mainly the left IFG and left caudate), would be more prominent in bilinguals than in monolinguals when responding to situations that require more cognitive control (i.e. infrequent go and no‐go cues), whereas the right inferior frontal gyrus and the ACC would be more relevant for monolinguals.
MATERIALS AND METHODS
Participants
Twenty early high proficient Catalan‐Spanish bilinguals (12 females; mean age = 21.1; SD = 1.4) and 19 Spanish monolinguals (10 females; mean age = 20.5; SD = 2.9) took part in this study. There were no statistically significant between‐group differences in age and gender. All the participants were undergraduates; this means they all had to pass university entrance Spanish exams, and bilinguals had to pass the same exams also in Catalan. Participants were physically and psychologically healthy, with no history of mental disorders, head trauma, or current psychotropic medication use. Participants provided written informed consent prior to participating in this study and were paid for their participation. The study was approved by the Ethical Committee of the Universitat Jaume I.
All the participants were subjected to a preliminary interview on their use of languages, and their personal and familial language history, which allowed them to be assigned to a bilingual or a monolingual group. The interview was conducted by a fully Spanish‐Catalan bilingual, who also checked the ability to speak in both languages. Monolinguals lived in a monolingual community and reported Spanish to be their only communicating language. Bilinguals lived in a Spanish‐Catalan bilingual community and reported that they had used both Spanish and Catalan regularly since early infancy. We also ensured group assignment through self‐reported questionnaires. The linguistic competence questionnaire completed by bilinguals showed that they had acquired Spanish and Catalan simultaneously in early years of infancy (Spanish AoA, in years: mean = 0.35, SD = 0.29; Catalan AoA: mean = 0.76, SD = 0.61). Their mean competence level in Spanish and Catalan was 4 on a 4‐point scale, which measured listening, reading, speaking, and writing. All the monolinguals were exposed to Spanish since early infancy, and self‐reported a competence level of 4 in Spanish. Despite the fact that all the participants had learned English as a second language at school, they reported low‐frequency English use, and low proficiency in English or any other language.
Experimental Design and Stimuli
Participants performed a go/no‐go task adapted from Chikazoe et al. [2009], while undergoing fMRI scanning. Visual stimuli consisted of colored circles, where color indicated trial type: frequent‐go (gray); infrequent‐go (blue); no‐go (yellow). In the frequent‐go and infrequent‐go trials, participants were required to respond to visual stimuli as quickly as possible with a button press, whereas they were instructed not to respond to visual stimuli in the no‐go trials. Each colored circle was presented for 400 ms, followed by a 400‐ms intertrial interval. The task consisted of eight runs of identical duration (2:24 minutes), which threw 1280 trials, of which 992 (77.5%) were frequent‐go trials. Infrequent‐go and no‐go trials were equally frequent with 144 trials (11.25%) each. This modification of the traditional go/no‐go task, introduced by Chikazoe et al. [2009], allows the effects of response inhibition and stimulus frequency to be dissociated. Reaction times (RT) and correct responses (hits) were recorded during the fMRI session. A short (1‐run) practice session was administered prior to scanning to minimize later learning effects.
The task was programmed and presented with the Presentation software (Neurobehavioral Systems, Albany, CA, USA). Visual stimuli were displayed in the scanner with Visuastim goggles (Resonance Technology, Northridge, CA, USA). Stimulus presentation was synchronized by scanner acquisition with SyncBox (Nordic NeuroLab, Bergen, Norway), and behavioral task performance was recorded with ResponseGrip (Nordic NeuroLab).
Behavioral Analyses
RT and correct responses were recorded during scanner sessions as behavioral data. In order to investigate between group differences in RT, a 2 × 2 mixed ANOVA was performed, which included the RTs for frequent‐go and infrequent‐go as the levels of within‐subjects factor and group as the between‐subjects factor. The between‐group differences in accuracy were also investigated by a 2 × 2 mixed ANOVA, where group was the between‐subjects factor, and the within‐subjects factor was the percentage of correct responses for the frequent‐go and infrequent‐go conditions. The between‐group differences in the percentage of correct inhibitions under the no‐go condition were also investigated by a two‐sample t‐test.
Image Acquisition
Image acquisition was performed in a 1.5‐T Siemens Avanto MRI scanner (Siemens, Erlangen, Germany). Participants were placed inside the scanner in the supine position and their heads were immobilized with cushions. Functional images were acquired with a T2*‐weighted echo‐planar imaging sequence (TE = 55 ms, TR = 2670 ms, FOV = 224 × 224, matrix = 64 × 64, voxel size = 3.5 × 3.5, 4 mm slice thickness, Flip angle = 90°). In each run 52 volumes were acquired. Each volume consisted of 29 interleaved axial slices, acquired parallel to the anterior‐posterior commissure (AC‐PC) plane covering the entire brain. Prior to the functional MR sequence, an anatomical 3D volume was acquired by an MPRAGE sequence (TE = 3.79 ms, TR = 2200 ms, FOV = 256 mm, matrix = 256 × 256 × 160, voxel size 1 × 1 × 1, 1 mm slice thickness).
Image Preprocessing
Image preprocessing was carried out using SPM8 (Wellcome Trust Center for Neuroimaging, London, UK). It included artifact correction (automatic detection and repairing bad slices) with the ArtRepair toolbox for SPM (http://cibsr.stanford.edu/tools/humanbrain-project/artrepair-software.html), slice time correction, realignment to correct for motion‐related artifacts, spatial normalization after extracting normalization parameters from the segmentation of each participant's high‐resolution anatomical acquisition (see Image Acquisition) and smoothing with an 8‐mm (FWHM) Gaussian kernel.
Independent Component Analysis
The group spatial ICA [Calhoun et al., 2001] was performed to obtain the functional brain networks that underlie fMRI data. This analysis assumes that fMRI data are linear mixtures of independent source signals, and it attempts to maximally extract independent signals and their mixing coefficients. The driving principle behind ICA is that these independent source signals represent coherent groupings of MRI activations, often referred to as component maps, which implies the representation of a functionally connected network [see Calhoun et al., 2003 and 2009 for reviews]. The group spatial ICA is performed on all the subjects at once and provides an independent component spatial map and a single associated ICA time course for every component, subject and session. Significant between‐group differences are determined by a second‐level analysis of the ICA results. The objective of our study was to investigate the between‐groups differences in the functional networks implicated in go/no‐go tasks. Thus we performed a GLM analysis in the components' time courses, estimated by ICA, to determine how the different brain networks are modulated by the experimental conditions. Then between‐group comparisons using the beta‐weights obtained from the GLM were made by second‐level analyses. The ICA procedure performed in our study is described in detail below.
First, the optimal number of components was estimated. There were 21 independent components based on minimum description length criteria [Li et al. 2007]. Then, Group ICA [Calhoun et al., 2001] was done with the Gift toolbox (v3.0a, http://icatb.sourceforge.net) using the Infomax algorithm [Bell and Sejnowski, 1995]. Twenty iterations of the ICA analysis were performed with the ICASSO software [Himberg et al., 2004] to ensure the stability of the estimated components. Prior to the ICA, the intensity of images was normalized and data dimensionality was reduced through a principal component analysis (PCA) following a two‐step data reduction approach. In the first step, the functional data for each subject and session were reduced to 32 dimensions. In the second step, the compressed datasets were concatenated in a single dataset and reduced again to 21 dimensions. After ICA decomposition, individual independent components maps and time courses were computed by the GICA‐3 back‐reconstruction approach. In our study, there were 39 subjects, 8 runs, and 21 components. Thus 6552 (39*8*21) independent component spatial maps (each associated with a single ICA time course) were estimated.
Statistical analysis of spatial maps
The individual spatial maps generated by ICA were used to identify the brain regions associated with each component in the whole sample. On the individual spatial maps, the voxel values represented its contribution to the associated time course. Thus the brain regions that significantly related with each component time course were determined in the whole group through second‐level analyses. Following previous studies [Kim et al., 2009a], the spatial maps were first averaged across runs within their respective components and subjects, and then one‐sample t‐tests at the second‐level analyses were performed with SPM8 (at P < 1 × 10−13 FDR‐corrected; k = 30).
Statistical analysis of component time courses
In order to study how functional networks were modulated during the go/no‐go task, a GLM was applied to the individual components' time courses using the design matrix that represented the task. This analysis is analogous to the GLM fit, which is performed on each voxel of the preprocessed data in conventional task fMRI analyses. However in this case, it was performed on the components' time course. Thus it yielded a set of beta‐weights, which represented the modulation of the components' time courses by the GLM regressors in relation to the baseline. The GLM design matrix included separate regressors to model the correct responses for the three trial types (frequent‐go, infrequent‐go and no‐go). The model also included incorrect responses for the go trials, incorrect responses for the no‐go trials and the parameters that modeled residual motion as regressors of no interest. Regressors were convolved with the canonical hemodynamic response function and included time derivatives. Once the analyses were performed, the beta‐weights associated with the onsets of frequent‐go, infrequent‐go and no‐go conditions were averaged across runs within their respective component in each participant, and were then used to perform the second‐level analyses.
Component selection
As ICA constitutes a data‐driven approach and some components may represent motion‐related or physiological signals, group analyses were performed in a small set of components. Given the hypothesis of the study, the networks of interests were the left FPN, the right FPN and the salience network. We performed visual inspection among the estimated group spatial maps of the components to identify those components that spatially fitted the brain areas associated with our networks of interest in previous studies (Allen et al., 2011; Dosenbach et al., 2007, 2008]. From the remaining components, for which we had no prior hypothesis, we also looked for other possible components of interest following a two‐step selection criterion based on previous studies. The first step involved correlating the estimated group spatial map of the components with the prior probabilistic maps of gray matter, white matter and cerebral spinal fluid (CSF), provided by the MNI templates of SPM8 [Kim et al., 2009a, 2009b; Ye et al., 2014]. The components with a spatial correlation greater than r 2=0.02, when compared with white matter, or r 2=0.05 when compared with CSF, or with a lower correlation with gray matter than CSF or white matter, were not considered to be primarily located within gray matter and were removed. The second step of the selection criteria was based on the involvement of the functional networks in the task [Kim et al., 2009a, 2009b]. In order to determine which functional networks were modulated during the go/no‐go task, the components' average beta‐weights for frequent‐go, infrequent‐go, and no‐go were compared separately for each group with a one‐way repeated measures ANOVA. Those components that did not show significant differences at a statistical threshold of P<0.05 FDR‐corrected in any group were considered unrelated to the task and were removed from subsequent analyses.
Second‐level between‐groups analyses
After component selection, second‐level analyses were performed to investigate how functional networks are affected by bilingualism. First a 3 × 2 mixed ANOVA was run for each network, which included as the within‐subjects factor the beta‐weights that represented the modulation of functional networks by experimental conditions, and group as the between‐subjects factor. Correlation analyses were done to study the association between the modulation of functional networks and behavioral performance. Thus the beta‐weights for the modulation of functional networks under the experimental conditions correlated with RTs and percentage of hits. These analyses were performed for the whole sample and for both groups separately.
RESULTS
Behavioral Results
The median of the RTs and percentage of hits for each condition and group are presented in Table 1. The 2 × 2 mixed ANOVA, performed to investigate differences in RTs, showed a main effect for Condition (F (1,37)=18.81; P<0.001), with the RTs for infrequent‐go trials being slower than the RTs for frequent‐go trials. No main effect of the Language Group or the Language Group × Condition interaction reached significance, thus the RTs for bilinguals and monolinguals were similar. The same 2 × 2 ANOVA was run to investigate differences in the percentage of hits for the go conditions. This analysis also showed a main effect for Condition (F (1,37)=11.24; P = 0.002), with a higher percentage of hits for the frequent‐go condition than for the infrequent‐go one. Once again, no significant effect for Language Group and Group × Condition was significant. Finally, no between‐group differences were shown for the percentage of correct inhibition (P > 0.1).
Table 1.
Behavioral results
| Bilinguals (n = 21) | Monolinguals (n = 20) | Differencea | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | (P < 0.05) | |
| Reaction time (msec.) | |||
| Frequent Go | 220.64 (30.57) | 205.80 (53.79) | n.s. |
| Infrequent Go | 228.26 (34.13) | 211.54 (54.52) | n.s. |
| Hits (%) | |||
| Frequent Go | 91.08 (9.14) | 88.99 (11.85) | n.s. |
| Infrequent Go | 87.81 (10.94) | 87.11 (9.45) | n.s. |
| No go | 55.07 (14.00) | 50.62 (17.06) | n.s. |
| Efficiency | 0.06 (9.21) | −0.07 (12.86) | n.s. |
Differences between groups (two sample t test).
n = number of participants; n.s., no significance; SD, standard deviation; msec, miliseconds.
ICA Results
By inspecting the components' spatial maps, we identified the three brain networks of interest for our study in C12 (right FPN), C18 (left FPN) and C19 (salience network) (Fig. 1 and Table 2). The FPN components were strongly lateralized on the right and the left hemisphere, respectively, which comprised similar regions of the inferior parietal cortex, middle frontal cortex and inferior frontal cortex. C19 was the only component that comprised the core regions of the salience network: the bilateral frontal operculum/insula and the dorsal anterior cingulate cortex [Seeley et al., 2007]. Two different components passed the selection criteria: C11 and C21 (See Table 2). C11, which involved occipital areas, the fusiform gyrus and superior parietal areas, was identified as a dorsal attention network (DAN), as previously described by Corbetta and Shulman [2002]. C21 was identified as the default mode network (DMN) as it involved the precuneus, inferior parietal areas and the medial frontal cortex [Greicius et al., 2003; Raichle et al. 2001]. As previously noted, the selection criteria were based not only on the correlations of the component spatial maps with the prior probabilistic maps provided by SPM, but also on the differences found in the modulation of the component time courses by the task conditions. At this point, it is important to note that all the components that represented the networks of interest for our study (C12, C18 and C19) passed the first step of the selection criteria. C12 and C19 also passed the second step of the selection criteria, while C18 showed a significant differential modulation among the conditions in one of the groups (bilinguals) with a lower statistical threshold of P < 0.05 uncorrected.
Figure 1.
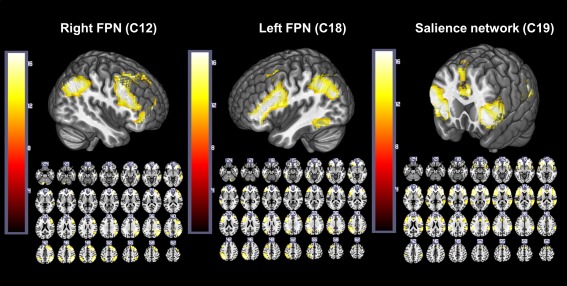
Spatial maps for the left FPN, right FPN, and salience network. Images are presented in neurological convention (left is left). The statistical threshold is P < 1x10−13 FDR‐corrected, with a minimum extent threshold of 30 contiguous voxels. The color bar represents the t values applicable to the image, while the numbers in the images correspond to the z MNI coordinates. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 2.
Brain regions belonging to functional networks involved in go/no go task
| Network | Regionsa | MNI coordinates | t Value | K | Brodmann areasb |
|---|---|---|---|---|---|
| DAN (C11) | Occipital middle left | −36 −88 7 | 23.08 | 1350 | 19, 18, 37 |
| Fusiform left | −36 −52 −17 | 20.72 | |||
| Occipital inferior left | −45 −70 −8 | 19.45 | |||
| Temporal inferior right | 48 −64 −8 | 21.82 | 1484 | 19, 18, 37 | |
| Temporal middle right | 48 −67 −2 | 21.80 | |||
| Fusiform right | 27 −67 −14 | 21.64 | |||
| Parietal inferior left | −54 −22 46 | 15.46 | 327 | 40, 2, 3 | |
| Parietal superior left | −24 −61 55 | 15.01 | |||
| Postcentral left | −39 −31 49 | 14.57 | |||
| Angular right | 27 −64 46 | 13.80 | 50 | 7 | |
| Parietal superior right | 27 −58 58 | 12.27 | |||
| Right FPN (C12) | Frontal middle right | 36 53 7 | 23.80 | 246 | 10 |
| Frontal inferior orbitalis right | 39 32 −8 | 12.39 | |||
| Frontal middle right | 48 14 40 | 23.27 | 1297 | 8, 9, 6, 46 | |
| Frontal inferior triangularis right | 45 26 28 | 22.62 | |||
| Frontal inferior opercularis right | 48 17 34 | 22.47 | |||
| Parietal inferior right | 42 −58 43 | 23.00 | 896 | 40, 7, 39 | |
| Cerebellum left | −15 −82 −26 | 19.58 | 112 | ‐ | |
| Temporal inferior right | 60 −25 −17 | 17.33 | 160 | 21 | |
| Temporal middle right | 63 −31 −8 | 17.07 | |||
| Parietal inferior left | −48 −52 46 | 16.80 | 193 | 40 | |
| Angular left | −36 −61 46 | 15.11 | |||
| Cingulum middle right | 6 −34 37 | 16.61 | 35 | 31 | |
| Cerebellum left | −39 −70 −35 | 14.26 | 55 | ‐ | |
| Left FPN (C18) | Frontal inferior triangularis left | −45 32 16 | 24.62 | 1201 | 9, 6, 46, 8 |
| Frontal middle left | −30 11 58 | 19.48 | |||
| Orbitofrontal inferior left | −27 29 −8 | 15.45 | |||
| Parietal inferior left | −33 −73 40 | 23.73 | 985 | 40, 7, 39, 19 | |
| Angular left | −42 −70 31 | 18.13 | |||
| Parietal superior left | −12 −76 52 | 12.26 | |||
| Temporal middle left | −57 −46 −8 | 23.22 | 228 | 37 | |
| Temporal inferior left | −51 −55 −14 | 22.74 | |||
| Cerebellum right | 12 −82 −26 | 15.45 | 49 | ‐ | |
| Frontal inferior triangularis right | 45 32 16 | 15.16 | 91 | 46 | |
| Frontal inferior opercularis right | 54 20 31 | 12.60 | |||
| Occipital middle right | 33 −70 37 | 13.27 | 31 | 19 | |
| Angular right | 33 −61 40 | 11.59 | |||
| Salience network (C19) | Insula left | −39 11 −5 | 30.97 | 1290 | 13, 45, 47, 44, 46 |
| Frontal inferior triangularis left | −42 29 4 | 23.15 | |||
| Frontal inferior opercularis left | −54 14 13 | 19.42 | |||
| Frontal inferior triangularis right | 51 35 4 | 25.60 | 1321 | 13, 47, 45, 46, 44 | |
| Insula right | 42 20 −8 | 23.07 | |||
| Frontal inferior opercularis right | 54 14 13 | 21.13 | |||
| Supramarginal right | 60 −40 25 | 19.13 | 714 | 40, 22 | |
| Temporal middle right | 63 −49 16 | 18.30 | |||
| Temporal superior right | 60 −43 19 | 17.84 | |||
| Supramarginal left | −60 −40 31 | 18.79 | 445 | 40 | |
| Inferior parietal left | −63 −37 37 | 18.70 | |||
| Temporal middle left | −57 −55 4 | 13.93 | |||
| Cingulum middle left | −6 20 31 | 17.38 | 261 | 32, 24 | |
| Cingulum anterior left | −3 26 22 | 17.37 | |||
| Cingulum anterior right | 6 26 25 | 16.52 | |||
| Supplementary motor area right | 9 17 61 | 16.38 | 108 | 8 | |
| Supplementary motor area left | −3 8 61 | 12.22 | |||
| DMN (C21) | Precuneus right | 3 −61 25 | 28.31 | 1251 | 31, 30, 7, 29 |
| Precuneus left | −3 −49 13 | 27.23 | |||
| Cuneus left | −12 −61 19 | 23.60 | |||
| Temporal middle left | −42 −61 22 | 23.18 | 587 | 39, 19 | |
| Angular left | −45 −73 28 | 20.93 | |||
| Occipital middle left | −33 −79 37 | 20.12 | |||
| Angular right | 45 −70 31 | 20.30 | 1023 | 39, 19, 22, 37 | |
| Occipital middle right | 36 −79 28 | 20.24 | |||
| Parahippocampal right | 30 −34 −11 | 20.10 | |||
| Fusiform left | −27 −40 −11 | 18.92 | 185 | 37 | |
| Frontal medial orbital right | 3 50 −8 | 18.19 | 101 | 10 | |
| Temporal middle right | 57 −7 −17 | 16.14 | 77 | 21 | |
| Temporal inferior left | −51 −58 −11 | 13.48 | 53 | 8 |
For clusters involving different regions the areas of the three most significant peak coordinates are reported.
For clusters involving different regions the Brodmann areas with more than 30 voxels within the cluster are reported.
Statistical threshold at P < 1 × 10−13 FDR‐corrected, K = 30.
Abbreviations: C, component; FPN, fronto‐parietal network; DAN, dorsal attention network; DMN, default mode network; K, cluster.
ANOVA Results
After identifying the relevant components for our study, second‐level analyses, which consisted in 2 (Language Group) × 3 (Condition) mixed ANOVA and correlations with behavioral data, were performed for each network of interest.
According to our hypothesis, the ANOVA analysis revealed a trend for a significant main effect of Group (F (1,37)=3.31; P = 0.07) in the left FPN, which indicates that bilinguals showed a higher modulation of this network across all the conditions than monolinguals. This main effect was driven by the significant Group x Condition interaction (F (2,74) = 3.11; P = 0.05), which showed increased left FPN modulation under the infrequent‐go condition than the frequent‐go condition in bilinguals compared with monolinguals (see Fig. 2A).
Figure 2.
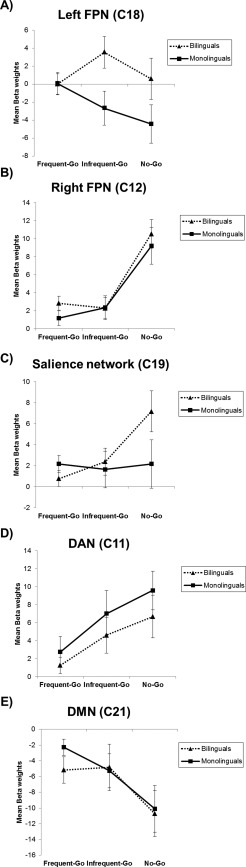
Mean and standard error bars for the beta‐weights of each functional network. C, component; FPN, fronto‐parietal network; DAN, dorsal attention network; DMN, default mode network.
The 2 × 3 mixed ANOVA, performed to investigate bilingualism effects on right FPN modulation, showed no significant main effects for either Group or the Group x Condition interaction. This finding indicates that the modulations of the right FPN by the task conditions were similar in both bilinguals and monolinguals (Fig. 2B). Nevertheless, this analysis revealed a significant main effect of Condition (F (2,74)=22.12; P < 0.001), which suggests that the right FPN was more positively modulated by the no‐go condition than the frequent‐go (P < 0.001) and infrequent‐go (P < 0.001) conditions.
The ANOVA analysis for the salience network yielded a significant main effect for Condition (F (2,74)= 3.39; P = 0.039), and a higher modulation by the no‐go than the go conditions (P < 0.05). As expected, the Condition x Group interaction reached significance (F (2,74)=3.07; P = 0.05), which implies greater salience network modulation under the no‐go condition than the frequent‐go condition in bilinguals than in monolinguals (P < 0.05; see Fig. 2C).
The ANOVA performed to investigate the modulation of the DAN showed a significant main effect for Condition (F (2,74)=10.08; P < 0.001), which indicates greater positive modulation for the no‐go (p=0.001) and infrequent‐go (P = 0.018) conditions than the frequent‐go condition. Finally, the ANOVA results for the DMN network also obtained a Condition main effect (F (2,74)=11.06; P < 0.001), with a greater negative modulation for the no‐go condition than the frequent‐go (P = 0.001) and infrequent‐go (P = 0.002) conditions. These results indicate that these networks are involved in the task (Fig. 2D,E).
Correlations Between Brain Activation Patterns and Behavioral Measures
The correlation analyses between performance measures and modulation in the relevant networks under the frequent‐go, infrequent‐go and no‐go conditions were performed for each language group. Regarding accuracy, the percentage of correct inhibitions correlated negatively with salience network modulation during the infrequent trials in bilinguals (r = −0.48, P = 0.033), whereas this correlation was nonsignificant in monolinguals (r = 0.12, p > 0.1). The direct comparison made between these correlations, using the Fisher's Z test, revealed a significant group difference with z = 1.90, P < 0.05 (Fig. 3A). In bilinguals, the RTs in the frequent and infrequent go responses correlated significantly and negatively with salience network modulation in the infrequent trials (r = −0.50, P = 0.024 and r = −0.45, P = 0.049, respectively), and with left FPN network modulation in the no‐go trials (r = −0.52, P = 0.019 and r = −0.43, P = 0.059, respectively). The same correlations for the monolingual group were all non significant (Fig. 3B). The direct comparisons of these correlations were significant in all four cases (z > 1.67, P < 0.05).
Figure 3.
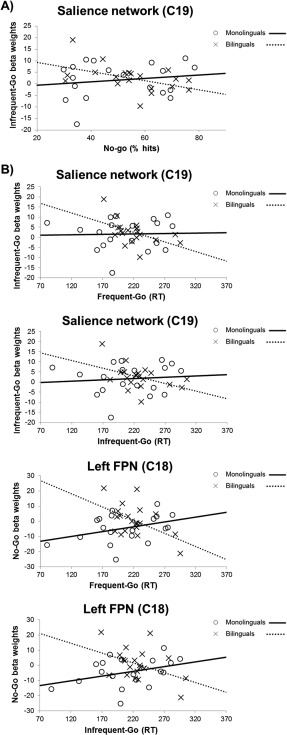
Scatterplots that display the correlation between behavioral measures and network modulation. The data of the monolingual and bilingual participants are represented with different symbols (circles and crosses, respectively) to illustrate the different direction of the correlation according to Group. A: Correlations with percentage of hits. B: Correlations with RTs. C, component; FPN, fronto‐parietal; RT, reaction times.
DISCUSSION
Using ICA, the present study investigated the different involvement of the executive control networks in bilinguals and monolinguals during a nonlinguistic go/no‐go task. We obtained three main results. First bilinguals used the left FPN during infrequent‐go trials more than monolinguals. Second the salience network in bilinguals was more activated during no‐go trials than in monolinguals. Third the correlation analyses revealed a different use of both the left FPN and salience network for bilinguals and monolinguals. Overall, our results demonstrated that early bilingualism, even when performance differences were lacking, was associated with a qualitatively different use of the brain networks involved in language processing during the nonverbal tasks that require cognitive control. As proposed in previous studies, these differences may arise from stronger functional connectivity in the specific networks related to the lateral prefrontal cortex, which allows greater modulation of executive control tasks [Grady et al., 2015]. This different use of lateral prefrontal areas may be shaped by bilinguals' extensive practice in language control [Stocco et al., 2014].
Previous studies that used inhibitory control tasks, like the go/no‐go task or stop, have consistently related the inhibitory process with the right lateral prefrontal cortex and the right FPN [Aron and Poldrack, 2006; Chikazoe et al., 2009; Hirose et al., 2012; Swick et al., 2008]. The role proposed for this brain area was to reorient attention toward inhibitory cues [Aron et al., 2014; Zhang and Li, 2012] or, in general, to monitor for cues that signal a change in action [Swick and Chatham, 2014]. The present results confirmed a prominent similar role of this area in inhibitory control for both bilinguals and monolinguals, but found no significant between‐groups differences [Grady et al., 2015]. Therefore, our results differed from those obtained in a previous study by our lab, which showed a more marked use of the right lateral prefrontal cortex in monolinguals during switching tasks that require maintaining or switching the response set [Garbin et al., 2010].
As expected, bilinguals used the left FPN in a qualitatively different way during the go/no‐go task than monolinguals. First our results showed a relevant role of the left FPN in bilinguals, but not in monolinguals. This indicates that only the former group used language control brain areas when performing difficult cognitive control tasks [Garbin et al., 2010; Rodríguez‐Pujadas et al., 2013]. Training in language selection and inhibition early in infancy may determine a different brain strategy to perform these cognitive control tasks. This could explain why bilinguals were more efficient throughout the task performed in some circumstances than monolinguals [Hilchey and Klein, 2011].
The second result obtained herein demonstrated a specific stronger left FPN modulation for the infrequent‐go stimulus in bilinguals than in monolinguals. This result is consistent with our previous observations, in which early bilinguals used language control brain areas to a greater extent when performing switching tasks than monolinguals [Garbin et al., 2010]. It is quite relevant that such brain differences have also been found even in the absence of statistically significant behavioral differences between groups [Rodríguez‐Pujadas et al., 2013]. This suggests that, in agreement with our hypothesis, the greater involvement of language control brain areas in bilinguals when performing nonlinguistic control tasks does not necessarily imply advantages in bilinguals, but merely differences in the cognitive control network. Hence this result would extend previous evidence which has demonstrated that early bilinguals use language control brain areas more than monolinguals when processing nonverbal stimuli that require executive control [Green and Abutalebi, 2013].
Lastly the correlation analyses evidenced differences between bilinguals and monolinguals in the link between left FPN modulation and behavioral measures. Specifically, the bilinguals who displayed greater left FPN modulation in the no‐go trials obtained faster RTs, whereas the left FPN modulation in the same trials done by monolinguals did not correlate with RTs. Thus for RTs, only bilinguals would benefit from greater left FPN involvement in inhibitory trials, which evidenced a different pattern in monolinguals. These differences are consistent with previous research, which has revealed greater functional connectivity between the inferior frontal cortex and the posterior areas of the brain in bilinguals [Luk et al., 2011], and has related this stronger functional connectivity to their participation in executive control tasks [Grady et al., 2015].
The role of the left lateral prefrontal cortex in executive tasks is controversial. Lesion studies have demonstrated that this area is related directly to inhibitory control, especially when the frequency of the no‐go signals is low [Swick et al., 2008]. Using the same task as that employed in our study, Hirose et al. [2012] reported that the left lateral prefrontal cortex is related to efficiency in processing an infrequent‐go stimulus [see Chao et al., 2009 and Li et al., 2006; for similar results using different tasks]. Similarly, Zhang and Li [2012] proposed that the joint action of the left FPN and motor networks is responsible for implementing an appropriate response in inhibitory tasks. Hence the left FPN implements the response according to the cue's semantic meaning [see Bari and Robbins, 2013, for a similar proposal]. Our data are consistent with this interpretation for the bilingual group as a whole, but not for the monolingual group.
The last relevant result obtained herein reveals stronger salience network modulation in bilinguals than in monolinguals during no‐go trials. The salience network involves the bilateral frontal operculum, the inferior frontal cortex and the dorsal anterior cingulate cortex. As this network is related to establishing which stimuli are relevant, its activity during go/no‐go tasks was mainly connected with the processing of no‐go stimuli. The salience network is typically associated with error processing. Both the insula and operculum have been previously shown to be consistently activated while performing monitoring, and to be modulated by error awareness. They have also been reported to play a key role in adjusting human behavior vKlein et al., 2007; Menon and Uddin, 2010]. As in the left FPC network, the behavioral correlates of salience network modulation differed for both groups. The modulation of this network during infrequent go trials in bilinguals correlated more with fast go responses, but with less accuracy in no‐go trials. These correlations were not significant in monolinguals. Thus bilinguals' different use of the salience network allows them to more rapidly process cues and to process fewer errors.
The anatomical link between the two relevant executive control networks is the left anterior insula/frontal operculum node [see Grady et al., 2015]. This area is common to both the salience and left fronto‐parietal networks. Previous research has demonstrated that this area plays a pivotal role in identifying new salient stimuli, and that it decouples its activity from the left FPN network and increases intrinsic activity in the salience network. Thus bilinguals better use both networks to process infrequent‐go and no‐go stimuli, which suggests a different form to cope with cognitive control tasks. The pattern of the results also showed different behavioral correlates for both networks in the two groups, which is consistent with the idea of qualitative (more than merely quantitative) differences existing in the development of executive control functions. Bilinguals and monolinguals may confer a distinct function to executive control networks in the cognitive control context, but not even this fact necessarily led to different behavioral results. This view is consistent with the model described by Stocco et al. [2014] since the left lateral prefrontal cortex was proposed as the main frontal candidate of the output of bilingual experience. Unfortunately, our networks neither involved the basal ganglia as an isolate network [Mcfadden et al., 2014], nor included basal ganglia in the salience network [García‐García et al., 2013], and we were unable to test for a possible role of these nuclei in our task. However, our results were consistent with the idea included in this model, which indicated that the left lateral prefrontal cortex, as a result of bilingualism, may perform different executive functions in bilinguals (probably more influenced by basal ganglia) and monolinguals (probably more linked to other cortical areas).
Our study has not found any brain networks that display more modulation in monolinguals than in bilinguals. This result is consistent with the recent findings obtained in Bialystok's lab. These authors proposed that (older) bilinguals had greater anterior‐posterior functional connectivity than monolinguals [Luk et al., 2011], which allows the more efficient modulation of these networks during executive control tasks [Grady et al., 2015]. Thus the results presented herein agree with this model and demonstrate a higher modulation of bilinguals' left FPN while processing salient stimuli, which will allow more efficient processing during these tasks [Hilchey and Klein 2011].
In general terms, the results of this study are consistent with our previous research [Garbin et al., 2010; Rodríguez‐Pujadas et al., 2014, 2013] and with the idea that early bilinguals and monolinguals may display different development for the brain networks associated with cognitive executive control functions. The fact that the greater modulation of the networks involved in the left opercular area in bilinguals while performing the cognitive control functions involved in this go/no‐go task suggests some cross‐talk between the brain areas that house language control and those involved in general‐purpose cognitive control systems [Moritz‐Gasser and Duffau, 2009]. This is likely to happen as a result of bilinguals' early extensive experience in managing and controlling two languages. The fact that these brain functional differences are observed, even when behavioral differences between both two groups are lacking, supports the idea that the functional brain dynamics associated with bilingualism do not necessarily lead to more efficient behavioral performance [Rodríguez‐Pujadas et al., 2014].
ACKNOWLEDGMENT
The authors declare that they have no conflict of interest.
REFERENCES
- Abutalebi J, Della Rosa PA, Green DW, Hernandez M, Scifo P, Keim R, Cappa SF, Costa A (2012): Bilingualism tunes the anterior cingulate cortex for conflict monitoring. Cereb Cortex 22:2076–2086. [DOI] [PubMed] [Google Scholar]
- Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, Silva RF, Havlicek M, Rachakonda S, Fries J, Kalyanam R, Michael AM, Caprihan A, Turner JA, Eichele T, Adelsheim S, Bryan AD, Bustillo J, Clark VP, Feldstein Ewing SW, Filbey F, Ford CC, Hutchison K, Jung RE, Kiehl KA, Kodituwakku P, Komesu YM, Mayer AR, Pearlson GD, Phillips JP, Sadek JR, Stevens M, Teuscher U, Thoma RJ, Calhoun VD (2011): A baseline for the multivariate comparison of resting‐state networks. Front Syst Neurosci 5:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA (2006): Cortical and subcortical contributions to stop signal response inhibition: Role of the subthalamic nucleus. J Neurosci 26:2424–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. (2014): Inhibition and the right inferior frontal cortex: One decade on . Trends Cogn Sci 18:177–185. [DOI] [PubMed] [Google Scholar]
- Bari A, Robbins TW (2013): Inhibition and impulsivity: Behavioral and neural basis of response control. Prog Neurobiol 108:44–79. [DOI] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ (1995): An information‐maximization approach to blind separation and blind deconvolution. Neural Comput 7:1129–1159. [DOI] [PubMed] [Google Scholar]
- Bialystok E (2011): Reshaping the mind: The benefits of bilingualism. Can J Exp Psychol 65:229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialystok E, Craik FIM, Grady C, Chau W, Ishii R, Gunji A, Pantev C (2005): Effect of bilingualism on cognitive control in the Simon task: Evidence from MEG. Neuroimage 24:40–49. [DOI] [PubMed] [Google Scholar]
- Bialystok E, Craik FIM, Klein R, Viswanathan M (2004): Bilingualism, aging, and cognitive control: Evidence from the Simon task. Psychol Aging 19:290–303. [DOI] [PubMed] [Google Scholar]
- Buchweitz A, Prat C (2013): The bilingual brain: Flexibility and control in the human cortex. Phys Life Rev 10:428–443. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ (2001): A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp 14:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adalı T, Hansen LK, Larsen J, Pekar JJ (2003): ICA of functional MRI data: An overview. In: 4th International Conference on Independent Component Analysis and Blind Source Separation. Nara, Japan, pp 281–288.
- Calhoun VD, Liu J, Adali T (2009): A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage 45:S163–S172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao HHa, Luo X, Chang JLK, Li C‐SR (2009): Activation of the pre‐supplementary motor area but not inferior prefrontal cortex in association with short stop signal reaction time—An intra‐subject analysis. BMC Neurosci 10:75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Asari T, Yamashita K, Morimoto H, Hirose S, Miyashita Y, Konishi S (2009): Functional dissociation in right inferior frontal cortex during performance of go/no‐go task. Cereb Cortex 19:146–152. [DOI] [PubMed] [Google Scholar]
- Congdon E, Mumford JA, Cohen JR, Galvan A, Aron AR, Xue G, Miller E, Poldrack RA (2010): Engagement of large‐scale networks is related to individual differences in inhibitory control. Neuroimage 53:653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL (2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3:201–215. [DOI] [PubMed] [Google Scholar]
- Costa A, Hernández M, Sebastián‐Gallés N (2008): Bilingualism aids conflict resolution: Evidence from the ANT task. Cognition 106:59–86. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair Da, Cohen AL, Schlaggar BL, Petersen SE (2008): A dual‐networks architecture of top‐down control. Trends Cogn Sci 12:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach Rat, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE (2007): Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A 104:11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duñabeitia JA, Hernández JA, Antón E, Macizo P, Estévez A, Fuentes LJ, Carreiras M (2014): The inhibitory advantage in bilingual children revisited: Myth or reality? Exp Psychol 61:234–251. [DOI] [PubMed] [Google Scholar]
- Garbin G, Sanjuan A, Forn C, Bustamante JC, Rodriguez‐Pujadas a, Belloch V, Hernandez M, Costa A, Ávila C (2010): Bridging language and attention: Brain basis of the impact of bilingualism on cognitive control. Neuroimage 53:1272–1278. [DOI] [PubMed] [Google Scholar]
- García‐García I, Jurado MÁ, Garolera M, Segura B, Sala‐Llonch R, Marqués‐Iturria I, Pueyo R, Sender‐Palacios MJ, Vernet‐Vernet M, Narberhaus A, Ariza M, Junqué C (2013): Alterations of the salience network in obesity: A resting‐state fMRI study. Hum Brain Mapp 34:2786–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Luk G, Craik FIM, Bialystok E (2015): Brain network activity in monolingual and bilingual older adults. Neuropsychologia 66:170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DW, Abutalebi J (2013): Language control in bilinguals: The adaptive control hypothesis. J Cogn Psychol 25:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V (2003): Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 100:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilchey MD, Klein RM (2011): Are there bilingual advantages on nonlinguistic interference tasks? Implications for the plasticity of executive control processes. Psychon Bull Rev 18:625–658. [DOI] [PubMed] [Google Scholar]
- Himberg J, Hyvärinen A, Esposito F (2004): Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage 22:1214–1222. [DOI] [PubMed] [Google Scholar]
- Hirose S, Chikazoe J, Watanabe T, Jimura K, Kunimatsu A, Abe O, Ohtomo K, Miyashita Y, Konishi S (2012): Efficiency of go/no‐go task performance implemented in the left hemisphere. J Neurosci 32:9059–9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Il Manoach DS, Mathalon DH, Turner JA, Mannell M, Brown GG, Ford JM, Gollub RL, White T, Wible C, Belger A, Bockholt HJ, Clark VP, Lauriello J, O'Leary D, Mueller BA, Lim KO, Andreasen N, Potkin SG Calhoun VD (2009a): Dysregulation of working memory and default‐mode networks in schizophrenia using independent component analysis, an fBIRN and MCIC study. Hum Brain Mapp 30:3795–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Il Mathalon DH, Ford JM, Mannell M, Turner J, a Brown GG, Belger A, Gollub R, Lauriello J, Wible C, O'Leary D, Lim K, Toga A, Potkin SG, Birn F Calhoun VD (2009b): Auditory oddball deficits in schizophrenia: An independent component analysis of the fMRI multisite function BIRN study. Schizophr Bull 35:67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TA, Endrass T, Kathmann N, Neumann J, von Cramon DY, Ullsperger M (2007): Neural correlates of error awareness. Neuroimage 34:1774–1781. [DOI] [PubMed] [Google Scholar]
- Li CR, Huang C, Constable RT, Sinha R (2006): Imaging response inhibition in a stop‐signal task: Neural correlates independent of signal monitoring and post‐response processing. J Neurosci 26:186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y‐O, Adali T, Calhoun VD (2007): Estimating the number of independent components for functional magnetic resonance imaging data. Hum Brain Mapp 28:1251–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk G, Bialystok E, Craik FIM, Grady CL (2011): Lifelong bilingualism maintains white matter integrity in older adults. J Neurosci 31:16808–16813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden KL, Tregellas JR, Shott ME, Frank GKW (2014): Reduced salience and default mode network activity in women with anorexia nervosa. J Psychiatry Neurosci 39:178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ (2010): Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct: 214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz‐Gasser S, Duffau H (2009): Cognitive processes and neural basis of language switching: Proposal of a new model. Neuroreport 20:1577–1580. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod am, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci U S A 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW (2007): Shifting and stopping: Fronto‐striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci 362:917–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez‐Pujadas A, Sanjuán A, Fuentes P, Ventura‐Campos N, Barrós‐Loscertales A, Ávila C (2014): Differential neural control in early bilinguals and monolinguals during response inhibition. Brain Lang 132:43–51. [DOI] [PubMed] [Google Scholar]
- Rodríguez‐Pujadas A, Sanjuán A, Ventura‐Campos N, Román P, Martin C, Barceló F, Costa A, Avila C (2013): Bilinguals use language‐control brain areas more than monolinguals to perform non‐linguistic switching tasks. PLoS One 8:e73028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco A, Yamasaki B, Natalenko R, Prat CS (2014): Bilingual brain training: A neurobiological framework of how bilingual experience improves executive function. Int J Biling 18:67–92. [Google Scholar]
- Swick D, Chatham CH (2014): Ten years of inhibition revisited. Front Hum Neurosci 8: 329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken AU (2008): Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci 9:102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Potenza MN, Calhoun VD (2013): Spatial ICA reveals functional activity hidden from traditional fMRI GLM‐based analyses. Front Neurosci 7:154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Doñamayor N, Münte TF (2014): Brain network of semantic integration in sentence reading: insights from independent component analysis and graph theoretical analysis. Hum Brain Mapp 35:367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li CR (2012): Functional networks for cognitive control in a stop signal task: Independent component analysis. Hum Brain Mapp 33:89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]


