Abstract
Background
Bipolar disorder and Major depressive disorder are difficult to differentiate during depressive episodes, motivating research for differentiating neurobiological markers. Dysfunctional amygdala responsiveness during emotion processing has been implicated in both disorders, but the important rapid and automatic stages of emotion processing in the amygdala have so far never been investigated in bipolar patients.
Methods
fMRI data of 22 bipolar depressed patients (BD), 22 matched unipolar depressed patients (MDD), and 22 healthy controls (HC) were obtained during processing of subliminal sad, happy and neutral faces. Amygdala responsiveness was investigated using standard univariate analyses as well as pattern‐recognition techniques to differentiate the two clinical groups. Furthermore, medication effects on amygdala responsiveness were explored.
Results
All subjects were unaware of the emotional faces. Univariate analysis revealed a significant group × emotion interaction within the left amygdala. Amygdala responsiveness to sad>neutral faces was increased in MDD relative to BD. In contrast, responsiveness to happy>neutral faces showed the opposite pattern, with higher amygdala activity in BD than in MDD. Most of the activation patterns in both clinical groups differed significantly from activation patterns of HC—and therefore represent abnormalities. Furthermore, pattern classification on amygdala activation to sad>happy faces yielded almost 80% accuracy differentiating MDD and BD patients. Medication had no significant effect on these findings.
Conclusions
Distinct amygdala excitability during automatic stages of the processing of emotional faces may reflect differential pathophysiological processes in BD versus MDD depression, potentially representing diagnosis‐specific neural markers mostly unaffected by current psychotropic medication. Hum Brain Mapp 35:2995–3007, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: depression, bipolar disorder, fMRI, amygdala, subliminal emotion processing, pattern classification
INTRODUCTION
Bipolar disorder and Major depressive disorder rank among the most debilitating psychiatric diseases worldwide. Both disorders are predominantly characterized by depressive episodes [Judd et al., 2003, 2002; Kupka et al., 2007; Angst et al., 2010], making it extremely difficult to correctly differentiate depressed patients with unipolar depression (MDD) from those with bipolar depression (BD) [Almeida and Phillips, 2012]. Although some phenomenological differences have been described [Mitchell et al., 2008], up to 60% of bipolar patients are initially misdiagnosed with unipolar depression [Hirschfeld et al., 2003], leading to inappropriate treatment and often prolonged medical histories [Kupfer, 2005; Dudek et al., 2012]. There is growing consensus that current standard diagnostic tools do not satisfactorily differentiate between BD and MDDs [Phillips and Frank, 2006; Almeida and Phillips, 2012]. Objective biological markers reflecting different neural underpinnings of depressive episodes in unipolar versus bipolar disorders could significantly improve diagnostic accuracy [Strakowski et al., 2012]. Therefore, neuroimaging studies on differences in pathophysiological processes between BD patients and MDD patients are necessary, in particular probing the neural underpinnings of central emotion‐processing mechanisms already known to be abnormal in the disorders [Strakowski et al., 2012; Almeida and Phillips, 2012].
Neurobiological models for affective disorders suggest that amygdala dysfunction could be a core feature for differentiating BD from MDD [Almeida et al., 2009; Blond et al., 2012; Strakowski et al., 2012; Almeida and Phillips, 2012]. The amygdala is a key structure in (para)limbic‐cortical emotion networks, responsible in particular for emotional perception (i.e., salience detection) and emotion generation [Santos et al., 2011]. Projections from the thalamus enable the amygdala to process stimuli rapidly, even before they gain conscious awareness through slower cortical projections [Ledoux, 2000]. Unfortunately, to date, no study investigated early, automatic stages of emotion processing in the amygdala in BD patients, and thus, a comparison to MDD patients is also lacking, although these early processing stages seem to play a crucial role in depressive disorders [Dannlowski et al., 2006a,b] Studies employing subliminal stimulus presentation paradigms in acutely depressed unipolar patients showed a consistent pattern of amygdala hyper‐activation in response to negative stimuli and amygdala hypo‐activation in response to positive stimuli (Suslow et al., 2010a,b; Victor et al., 2010), associated with central psychopathological symptoms of depression. Previous work comparing neural emotion processing in acutely depressed bipolar and unipolar patients exclusively investigated conscious processing, reporting heterogeneous results concerning differences in amygdala responsiveness, as well as cortical abnormalities in anterior cingulate cortex (ACC), ventrolateral prefrontal cortex (VLPFC), amygdala‐orbitomedial prefrontal cortex (OMPFC), and in effective connectivity [Tavares et al., 2008; Almeida et al., 2010, 2009; Bertocci et al., 2012]—for review see [Almeida and Phillips, 2012]. Mourão‐Miranda et al. [2012] used Gaussian process classifiers on whole brain data to predict BD, MDD, and HC. Although the results of the between subjects approach were non‐significant after correction for multiple comparisons, differences in within‐subjects classifications indicated different neural patterns in these groups to the perception of happy faces. Only recently, Grotegerd et al. [2013] applied pattern classification to fMRI data of depressed BD and MDD patients. They reported high feature weights in the amygdala that were specific to MDD during conscious processing of negative facial expressions, whereas high feature weights during processing of happy facial expressions were related to BD. This result is suggestive of emotion‐specific amygdala responsiveness as a promising neurobiological marker for differentiating between MDD and BD. Such pattern‐classification approaches allow classifying subjects as belonging to a specific group, based on their underlying pathophysiology, which could be of high relevance for diagnostic purposes.
The goal of our present study was to investigate the unexplored automatic stages of emotion processing in depressed MDD and BD patients, using subliminal stimulus presentation. Standard fMRI analysis and pattern‐classification techniques were employed in parallel. The latter approach could provide additional evidence for discrete neuropsychological profiles in depressed patients with MDD or BD. The analysis focused on automatic amygdala dysfunction in the two patient groups. We contrasted sad>neutral, happy>neutral and sad>happy facial stimuli, because previous results showed abnormal emotion‐specific neural activity in the amygdala to these valences in MDD and BD patients [Lawrence et al., 2004; Chen et al., 2006; Almeida et al., 2010, 2009; Suslow et al., 2010a,b; Victor et al., 2010]. Given the data by Grotegerd et al. [2013], we hypothesized that compared to BD patients, MDD patients show automatic, increased amygdala responsiveness to negative>neutral stimuli, whereas BD patients show automatic, increased amygdala responsiveness to positive>neutral stimuli, relative to MDD patients. Furthermore, we used machine learning to examine the extent to which these patterns of amygdala activity to sad versus happy faces can discriminate between unipolar and bipolar depression.
METHODS AND MATERIALS
Participants
Datasets of 44 acutely depressed inpatients—22 with major depressive disorder (MDD) and 22 with bipolar I disorder (BD) according to DSM‐IV criteria [American Psychiatric Association, 2000] as diagnosed with the SCID‐I interview [Wittchen et al., 1997], and 22 matched healthy control subjects (HC) were tested. Patients were recruited from the inpatient service of the University of Muenster's Department of Psychiatry. There was no overlap of patients with our recent study reported by Grotegerd et al. The two clinical groups were recruited by carefully matching for age and gender. Both groups did not differ significantly in depression level (HAMD), current manic symptoms (YMRS), number of episodes of depression, age of onset, and illness duration (see Table 1 for details). All but two of the 44 patients were receiving medication (see Table 2 for details): while a higher proportion of BD patients were taking mood‐stabilizers, the groups did not differ significantly regarding the intake of antidepressant and antipsychotic medication. Therefore, medication was included as a covariate in between‐group analyses of the two patient samples. Exclusion criteria for all participants were any neurological abnormalities, substance‐related disorders or current benzodiazepine treatment (wash out of at least three half‐lives before study participation), and former electroconvulsive therapy. For control subjects, a further exclusion criterion was any current or former psychiatric disorder, also verified with the SCID‐Interview [Wittchen et al., 1997]. All participants had to fulfill the general MRI‐related requirements and head movement did not exceed > 2mm and/or 2° in any direction. The study protocol was approved by the Ethics Committee of the University of Muenster. Participants gave their full written informed consent and received a financial compensation.
Table 1.
Sociodemographic, questionnaire and clinical data of study participants; mean (SD)
| BPD (N = 22) | MDD (N = 22) | HC (N = 22) | P‐value according to χ 2‐tests or t‐tests between clinical groups | P‐value according to χ 2‐test or ANOVA between all groups | |
|---|---|---|---|---|---|
| Age | 42.0 (11.0) | 41.2 (11.8) | 41.1 (10.9) | 0.80 | 0.95 |
| Sex (male/female) | 11/11 | 11/11 | 11/11 | 1.0 | 1.0 |
| Total education time | 14.9 (1.9) | 15.3 (2.2) | 15.5 (2.1) | 0.47 | 0.62 |
| Verbal intelligence | 112.3 (16.2) | 116.9 (14.3) | 116.3 (13.0) | 0.33 | 0.53 |
| HAMD | 23.3 (4.6) | 25.1 (7.1) | 1.2 (1.8) | 0.30 | < 0.001a |
| YMRS | 2.1 (1.7) | 1.4 (2.1) | 0.4 (0.9) | 0.16 | 0.003a |
| Episodes of depression | 7.1 (6.0) | 5.7 (5.2) | n/a | 0.39 | – |
| Episodes of mania | 2.9 (3.4) | n/a | n/a | – | – |
| Age of onset | 28.4 (9.7) | 29.9 (12.8) | n/a | 0.67 | – |
| Duration of illness (in years) | 13.6 (10.0) | 11.3 (8.7) | n/a | 0.42 | – |
| Antidepressant medication (yes/no) | 13/9 | 18/4 | n/a | 0.10 | – |
| Antidepressive potencyb | 1.5 (1.1) | 1.4 (1.1) | n/a | 0.781 | – |
| Antipsychotic medication (yes/no) | 15/7 | 10/12 | n/a | 0.13 | – |
| Antipsychotic potencyc | 224.5 (324.2) | 53.9 (110.9) | n/a | 0.024a | – |
| Mood Stabilizer (yes/no) | 13/9 | 3/19 | n/a | 0.002a | – |
BPD, bipolar depressed patients; MDD, unipolar depressed patients; HC, healthy controls; SD, standard deviation; HAMD, Hamilton depression rating scale; YMRS, young mania rating scale.
Significant at statistical threshold P < 0.05.
Chlorpromazine equivalent. Patients not receiving antidepressants were coded as zero.
According to criteria proposed by Sackeim [2001]. Patients not receiving antipsychotics were coded as zero.
Table 2.
List of antidepressant, antipsychotic, and mood‐stabilizing medication in the two patient samples
| Number of unipolar patients | Number of bipolar patients | |
|---|---|---|
| Antidepressive medication | 18 | 13 |
| Agomelatine | 3 | 0 |
| Amytriptyline | 1 | 0 |
| Bupropion | 1 | 0 |
| Citalopram | 2 | 2 |
| Doxepin | 1 | 0 |
| Duloxetin | 1 | 1 |
| Escitalopram | 1 | 2 |
| Fluoxetine | 1 | 0 |
| Mirtazapin | 3 | 5 |
| Nortriptyline | 0 | 1 |
| Sertraline | 1 | 2 |
| Tranylcypromine | 0 | 1 |
| Venlafaxine | 7 | 3 |
| Antipsychotic medication | 10 | 15 |
| Amisulpride | 1 | 0 |
| Asenapine | 0 | 1 |
| Olanzapine | 1 | 1 |
| Pipamperone | 2 | 0 |
| Promethazine | 1 | 0 |
| Quetiapine | 6 | 14 |
| Mood stabilizer | 3 | 13 |
| Lamotrigine | 1 | 3 |
| Lithium | 2 | 9 |
| Valproic acid | 0 | 3 |
Values reflect number of patients treated with the respective medication. Due to combination treatments, the number of single substances within each medication class can exceed the number of patients treated with the respective medication class.
Task and Procedures
Subliminal affective priming paradigm
The paradigm is designed to investigate early, automatic stages of emotion processing and has been reliably used in healthy subjects and patient groups before [Suslow et al., 2010a,b; Reker et al., 2010; Dannlowski et al., 2012b, in press; Donges et al., 2012]. A detailed description can be found in Suslow et al. [2010a,b]. Briefly, facial stimuli consisted of grey‐scale normalized sad, happy, and neutral expressions [Ekman and Friesen, 1976]. Emotional and neutral faces were presented as primes for 33 ms, and subsequently masked by neutral faces. Eighty trials in two fixed pseudo‐random sequences were shown: 20 with sad, 20 with happy and 20 with neutral prime faces. In 20 trials no‐face primes were presented. In the no‐face primes, the central facial features (i.e., eyes, nose, and mouth) of neutral faces had been replaced by a smooth, skin‐colored surface. Each trial lasted 9 s. A fixation cross presented for 800 ms preceded a prime face shown for 33 ms, which was immediately followed by the same face with neutral expression, serving as mask and as target, and presented for 467 ms. A blank screen followed for 7.700 ms. During this time period, subjects had to evaluate whether the neutral (mask) face expressed negative or positive feelings, by pressing one of four buttons (−1.5, −0.5, +0.5, and +1.5). Evaluations and reaction times were registered.
Prime detection task
After the fMRI experiment, all subjects were questioned if they had noticed the subliminally presented faces in the affective priming task. After they were informed about the presence of the emotional prime faces, they took part in a forced‐choice prime‐detection task, intended to assess potential objective awareness. The prime‐detection task used facial stimuli from the fMRI experiment and consisted of 40 trials (33 ms prime presentation, followed by a neutral face mask for 467 ms). Participants were asked to indicate via button press which of the four prime conditions (sad, happy, neutral, no‐face) was presented before the neutral mask. As a nonparametric measure of sensitivity, including hit rates and false alarm rates, A′ was calculated for every prime condition. Chance level is indicated by A′ = 0.5 [Grier, 1971].
Behavioral Analysis
Sociodemographic, clinical, questionnaire, and behavioral data were analyzed, comparing all three groups (MDD, BD, HC), and also the two clinical groups only (see Tables 1 and 3).
Table 3.
Behavioural data: mean reaction times (in ms) and mean evaluative responses of study participants
| BPD (N = 22) | MDD (N = 21) | HC (N = 22) | P value according to t‐tests between clinical groups | Statistic and P‐value according to 3 (group) × 4 (emotion) ANOVA | |
|---|---|---|---|---|---|
| Mean evaluation | Main effect group: F(2,62) = 1.71; P = 0.19/Main effect prime: F(3,62) = 1.22; P = 3.03 | ||||
| Mean evaluation sad prime condition | 0.01 | −0.16 | −0.04 | 0.06 | |
| Mean evaluation happy prime condition | −0.04 | −0.21 | 0.01 | 0.10 | |
| Mean evaluation neutral prime condition | −0.03 | −0.16 | −0.02 | 0.26 | |
| Mean evaluation no‐face prime condition | 0.023 | −0.15 | 0.00 | 0.08 | |
| Mean reaction times | Main effect group: F(2,62) = 0.67; P = 0.51/Main effect prime: F(3,62) = 8.09; P < 0.001 | ||||
| RT sad prime condition | 1,589 | 1,623 | 1,479 | 0.85 | |
| RT happy prime condition | 1,555 | 1,529 | 1,384 | 0.89 | |
| RT neutral prime condition | 1,568 | 1,471 | 1,382 | 0.59 | |
| RT no face prime condition | 1,537 | 1,538 | 1,356 | 0.96 |
Statistical threshold P < 0.05. BPD, bipolar depressed patients; MDD, unipolar depressed patients; HC, healthy controls; RT, reaction times.
Image Acquisition
Functional‐imaging data were acquired at a 3 T scanner (Gyroscan Intera 3T, Philips Medical Systems, Best, NL), using a single shot echoplanar sequence with parameters selected to minimize distortion in the central region of interest, while retaining adequate signal to noise ratio (S/N) and sensitivity. Volumes consisting of 34 slices were acquired (matrix 64 × 64, resolution 3.6 × 3.6 × 3.6 mm; TR = 2.1 s, TE = 30 ms, FA = 90°). The slices were tilted 25° from the AC/PC line, in order to minimize drop‐out artifacts in the orbitofrontal and mediotemporal region.
Functional Neuroimaging Data Preprocessing
During preprocessing, data were motion‐corrected (using a set of six rigid body transformations determined for each image), spatially normalized to standard Montreal Neurological Institute (MNI) space and smoothed (Gaussian kernel, 8 mm FWHM) using Statistical Parametric Mapping (SPM8, http://www.fil.ion.ucl.ac.uk/spm). An event‐related analysis design was used modeling the onsets of each prime condition (33 ms prime presentation: sad, happy, neutral, no‐face), reducing the data to four averaged trials per subject. A vector of prime onset times of the emotional and neutral primes and the no‐face prime condition was convolved with a canonical hemodynamic response function, generating individual fixed‐effect contrast maps for the contrasts of interest (sad>neutral, happy>neutral, sad>happy). The six movement parameters from the realignment procedure were included as covariates of no interest into the first level model.
Statistical Analyses
Functional neuroimaging data were analyzed by applying two independent methodological approaches in parallel. On the one side, we used standard univariate fMRI analysis to perform a hypothesis‐driven region‐of‐interest analysis. On the other side, to investigate the discriminative abilities between the neural activation patterns of MDD and BD, a pattern classification approach was taken. This approach is based on multivariate statistics, hence relative differences of the activation patterns are taken into account in contrast to the standard univariate analysis. As the methods focus on different aspects of the data, results complementing one another would strengthen our conclusion.
Standard fMRI analysis
To test our hypothesis of distinct amygdala excitability in BD relative to MDD patients and HC, we first calculated a 3 (group: MDD vs. BD vs. HC) × 2 (emotion: sad>neutral vs. happy>neutral) ANOVA, using the flexible factorial model, with emotion as within‐subjects factor and group as between‐subjects factor. “Subjects” was included as a third factor in the model to account for the individual constants. This model was used to calculate the main effects of group and emotion, and the crucial group × emotion interaction. According to our hypothesis, a‐priori region‐of‐interest (ROI) analyses of the bilateral amygdala were performed. The amygdala was defined according to the AAL‐atlas [Tzourio‐Mazoyer et al., 2002] and the bilateral amygdala mask was created using the WFU‐pickatlas (http://fmri.wfubmc.edu/software/PickAtlas). To control for multiple statistical testing, we maintained a cluster‐level false positive detection rate at P < 0.05, using a voxel threshold of P < 0.05 with a cluster (k) extent empirically determined by Monte Carlo simulations implemented in the REST toolbox (http://restfmri.net/forum/index.php). This yielded a cluster‐extent threshold of k = 38 voxels for the bilateral amygdala. In case of significant emotion × group interaction effects, planned post hoc t‐tests with the bilateral amygdala mask were calculated to explore the nature of the interaction.
We also calculated the hypothesized group (MDD vs. BD) × emotion (sad>neutral vs. happy>neutral) interaction contrast for the two clinical groups alone, since a special focus of our analysis was the distinction between these two clinical groups. Furthermore, since the two patient groups differed in intake of mood‐stabilizing medication, intake (yes/no) of each medication class (antidepressants, antipsychotics and mood‐stabilizers) was entered as three covariates to assess potential confounding effects. In addition to mere presence/absence of medication classes, we further sought to explore medication dose effects. Antipsychotic potency was estimated by Chlorpromazine (CPZ) equivalents. Antidepressant potency was estimated by means of a classification proposed by Sackeim [2001], coding antidepressants in terms of dose and treatment duration in treatment levels from 1 to 4, as we [Dannlowski et al., 2006a,b; Dannlowski et al., 2007a,b; Dannlowski et al., 2008; Dannlowski et al., 2009; Stuhrmann et al., 2013] and others [Surguladze et al., 2005] have already used in previous fMRI and neuropsychological studies. Thus, we conducted a new model to estimate these medication dose effects by adding CPZ equivalents and antidepressant levels to the design instead of presence/absence of medication classes.
Pattern classification approach
A multivariate pattern classification approach was conducted, in parallel to the standard univariate analysis. All voxels of the bilateral amygdala were extracted from the corresponding SPM contrast images (sad>neutral, happy>neutral and sad>happy) and served as input pattern for machine learning computations. As pattern classification of neuroimaging data is still in development, discrimination between depressed MDD and BD patients was approached with two different algorithms in order to ensure the validity of these methods. The first concerned Support Vector Machines (SVM) [Vapnik and Chervonenkis, 1974] that were demonstrated to provide good results in similar studies [Fu et al., 2008a,b; Grotegerd et al., 2013]. In brief, an SVM calculates a hyperplane, which optimally separates between two classes of presented data. A linear SVM was used from the libsvm software package (http://www.csie.ntu.edu.tw/~cjlin/libsvm) [Chang and Lin, 2011]. Second, classifications were also repeated using Gaussian Process Classifiers, since this method has also been reported to be appropriate for depressive disorders [Hahn et al., 2010; Grotegerd et al., 2013; Mourão‐Miranda et al., 2012]. Computations were conducted using the GPML software package (http://www.gaussianprocess.org/gpml). Classifier performance was evaluated by using leave‐one‐out‐per‐group‐cross‐validation; average performance of all validation steps is reported as accuracy. In this context, sensitivity and specificity refer to the classification of bipolar depressive subjects. Statistical significance was empirically estimated by performing permutation tests (1,000 iterations).
RESULTS
Detection Task
After the fMRI procedure, none of the subjects reported having seen any of the briefly presented emotional prime faces that appeared before the neutral face mask, even after being informed about their presence (subjective awareness). Data from the detection task (objective awareness) confirmed subjects' unawareness of the emotional primes. According to t‐tests, the average prime‐detection sensitivity of healthy control subjects and patients did not differ significantly from chance level (all Ps > 0.12), neither for happy (control subjects: A′ = 0.6; MDD: A′ = 0.57; BD: A′ = 0.50), sad (control subjects: A′ = 0.52; MDD: A′ = 0.46; BD: A′ = 0.46), or neutral prime faces (control subjects: A′ = 0.49; MDD: A′ = 0.41; BP: A′ = 0.47). Behavioral data of two MDD patients were missing due to technical problems.
Behavioral Results
Table 3 lists mean reaction times and evaluative responses as a function of prime condition. Patients and controls did not differ in reaction times and behavioral responses, in any prime condition. Behavioral data of one MDD patient were missing owing to technical problems.
fMRI Results
The 3 (group) × 2 (emotion) ANOVA model revealed a significant main effect of emotion in the bilateral amygdala (right: x = 32, y = −2, z = −20; F(1,63) = 10.92; P = 0.002; k = 53 voxels, left: x = −24, y = 2, z = −16; F(1,63) = 9.22; p = 0.003; k = 43 voxels), resulting from overall higher activity for sad>neutral faces compared to happy>neutral faces. No significant main effect of group could be detected. However, the hypothesized emotion × group interaction yielded a significant cluster within the left amygdala (x = −22, y = −2, z = −12; F(2,63) = 7.14; P = 0.002; k = 42 voxels). A second cluster in the right amygdala (x = 34, y = 0, z = −20; F(2,63) = 5.01; P = 0.01; k = 14 voxels, n.s.) did not reach the cluster threshold corrected for multiple comparisons (Fig. 1). There were no significant associations of amygdala responsiveness to sad>neutral or happy>neutral faces with any behavioral measure (reaction times and evaluative responses).
Figure 1.
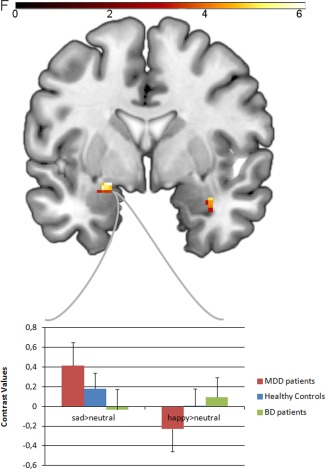
Top: coronal slice (MNI coordinate y = 0) depicting group (3) × emotion (2) interaction in the bilateral amygdala at uncorrected P < 0.05. Color bar, F values. Down: Bar graphs depicting the mean contrast values for sad>neutral faces and happy>neutral faces extracted from significant left amygdala group (3) × emotion (2) cluster (MNI coordinates x = −22, y = −2, z = −16; k = 42 voxels), dependent on emotion and study group. MNI = Montreal neurological Institute. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Post hoc bilateral amygdala analysis for sad>neutral faces showed significantly greater bilateral activity for sad faces in MDD, compared to BD patients and HC. Moreover, HC showed significantly greater left‐amygdala activity to sad faces than BD patients. Interestingly, the analysis for happy>neutral faces indicated the opposite pattern (Table 4): BD patients showed significantly greater activity than MDD patients for happy faces in right amygdala. Reactivity to happy faces in HC did not differ from BD patients, but again from MDD patients in bilateral amygdala.
Table 4.
Post hoc analysis of the group (3) × emotion (2) interaction (P < 0.05, uncorrected): differences between depressed unipolar patients (MDD), depressed bipolar patients (BD) and healthy controls (HC) in bilateral amygdala responses to subliminal sad and happy facial expressions, compared with neutral faces
| Side | Cluster size | T‐score | P‐value (uncorrected) | |
|---|---|---|---|---|
| Between group results (two t‐test) | ||||
| MDD > BD: sad>neutral faces | Left/Right | 1210 | 2.272.50 | P = 0.014/P = 0.008 |
| MDD > HC: sad>neutral faces | Left/Right | 2320 | 2.533.02 | P = 0.008/P = 0.002 |
| HC > BD: sad>neutral faces | Left | 4 | 2.54 | P = 0.007 |
| BD > MDD: happy>neutral faces | Right | 11 | 2.07 | P = 0.022 |
| HC > MDD: happy>neutral faces | Right | 11 | 2.09 | P = 0.019 |
| BD > HC: happy>neutral faces | – | – | – | – |
To determine the amygdala subregion where differential processing of emotion faces occurred, the SPM Anatomy toolbox Version 1.8 was administered [Eickhoff et al., 2005]. The highest emotion × group interaction effect was located in the superficial nuclei (SF) of the left amygdala, extending to the basolateral part (LB). In addition, the emotion × group interaction effect in the right amygdala (non‐significant after correction) was located predominantly in the basolateral part (LB).
This crucial group × emotion interaction in the left amygdala also remained highly significant analyzing the two clinical groups alone and including the three medication regressors (presence/absence of antidepressants, antipsychotics, and/or mood stabilizers): left: x = −22, y = −2, z = −11; t(63) = 3.64; P = 0.0003; k = 75 voxels; right: x = 22, y = −4, z = −12; t(63) = 2.64; P = 0.006; k = 16 voxels, n.s. It confirmed the pattern described above: MDD patients showed significantly greater amygdala reactivity to sad>neutral faces than BD patients, whereas BD patients showed significantly greater amygdala reactivity to happy>neutral faces than MDD patients. Dropping medication as additional covariates did not alter the pattern of results. Furthermore, using the dose estimations (CPZ equivalents and antidepressant levels) as additional nuisance regressors, this group × emotion interaction still remained practically unchanged.
Pattern Classification Results
Table 5 shows the results of the classifications between MDD and BD depressed patients. With up to 79.6% accuracy, the most significant results (P = 0.002) were achieved using the sad>happy contrast. While the sad>neutral faces contrast also yielded significant results, the contrast happy>neutral faces showed no significant effect. The combination of both contrasts resulted in intermediate accuracies, only significant when using a SVM. The differences between the two algorithms were marginal, indicating robust findings, independent of the classification method used.
Table 5.
Binary classifications between unipolar (MDD) and bipolar (BD) depressed patients (N = 44)
| Accuracy (%) | Sensitivity (%) | Specificity (%) | Positive predictive value | Negative predictive value | P‐value according to permutation tests | |
|---|---|---|---|---|---|---|
| Support vector machines | ||||||
| Sad>neutral faces | 68.2 | 63.6 | 72.7 | 0.700 | 0.667 | 0.028* |
| Happy>neutral faces | 61.4 | 59.1 | 63.6 | 0.619 | 0.636 | 0.12 |
| Sad>neutral faces and happy>neutral faces | 65.9 | 63.6 | 63.6 | 0.667 | 0.682 | 0.045* |
| Sad>happy faces | 75.0 | 63.6 | 86.4 | 0.820 | 0.704 | 0.008* |
| Gaussian process classifier | ||||||
| Sad>neutral faces | 65.9 | 68.2 | 63.6 | 0.652 | 0.667 | 0.043* |
| Happy>neutral faces | 56.8 | 59.1 | 54.6 | 0.565 | 0.571 | 0.263 |
| Sad>neutral faces and happy>neutral faces | 65.9 | 68.9 | 63.6 | 0.652 | 0.667 | 0.059 |
| Sad>happy faces | 79.6 | 81.8 | 77.3 | 0.782 | 0.810 | 0.002* |
Sensitivity and specificity refer to the classification of bipolar depressed subjects. *Significant at statistical threshold P < 0.05.
DISCUSSION
Our primary aim was to investigate automatic, emotion‐specific amygdala reactivity in the two patient groups, pointing to objective biological markers distinguishing unipolar from bipolar depression. To our knowledge, this is the first study probing neural activation patterns during automatic emotion processing, comparing depressed unipolar (MDD) and bipolar patients (BD). Actually, it is the first imaging study using subliminally presented stimuli in bipolar disorders. We employed two independent analysis approaches based on univariate as well as multivariate statistics in parallel, showing results complementing one another.
As hypothesized, the standard fMRI region‐of‐interest analysis showed greater amygdala activation to subliminal sad>neutral facial expressions in MDD patients than in BD patients. By contrast, greater amygdala responsivity to subliminal happy>neutral facial expressions was present in BD patients, but not in MDD patients. Except for the amygdala responsiveness to happy>neutral faces in BD patients, the differences observed between the two clinical groups represented abnormalities, relative to healthy controls. Furthermore, the differential activation patterns to sad>happy facial expressions provided high accuracy for distinguishing BD from MDD patients in multivariate pattern classification—closely in line with recent results by Grotegerd et al. [2013]. Taken together, the results suggest that automatic amygdala excitability in unipolar and bipolar depressed patients during unconscious processing of sad and happy facial stimuli may reflect differential pathophysiological processes in BD and MDD depression, possibly representing diagnosis‐specific neural markers.
In MDD patients, abnormally increased activation to negative (mood‐congruent) stimuli and reduced activation to positive (mood‐incongruent) stimuli seems to represent a core neural activation pattern, reported consistently in subliminal [Stuhrmann et al., 2013; Suslow et al., 2010a,b; Victor et al., 2010] as well as supraliminal emotion processing [Sheline et al., 2001; Siegle et al., 2002; Fu et al., 2004; Surguladze et al., 2005; Epstein et al., 2006; Abler et al., 2007; Siegle et al., 2007; Fu et al., 2008a,b; Peluso et al., 2009]—for a review see Stuhrmann et al. [2011]. As such “mood‐congruent” amygdala activation patterns were also found in unaffected persons at environmental [Tottenham et al., 2011; Dannlowski et al., 2012aa,b; van Harmelen et al., 2012; or genetic [Dannlowski et al., 2008; Domschke et al., 2008; Dannlowski et al., 2010; Domschke et al., 2010; Wolfensberger et al., 2008; Baune et al., 2010; Joormann et al., 2011] risk for depression, and further seem to persist in remitted patients [Victor et al., 2010], this pattern could reflect a trait marker of unipolar depression.
These data suggest that the mood‐congruent activation pattern in MDD patients during early, automatic emotion processing significantly differs from the pattern in BD patients. This is in line with observations by Grotegerd et al. [2013], who compared MDD and BD patients during conscious emotion processing. Furthermore, our findings are also consistent with amygdala activation to negative feedback [Tavares et al., 2008], which is prolonged in MDD relative to BD. Interestingly, Almeida et al. [2009] observed abnormally increased negative OMPFC‐amygdala connectivity in MDD patients, not seen in BD, during overt processing of happy faces. This points to suppression of amygdala responsiveness to positive emotions in MDD patients by the PFC, possibly leading to decreased amygdala responsiveness to happy facial stimuli in MDD patient compared to BD patients and HC, as observed in the presented data. Future studies applying functional connectivity analyses might prove this hypothesis. Taken together, exaggerated responsiveness to sad stimuli and diminished responsiveness to happy facial expressions in MDD relative to BD, even at unconscious processing stages, may represent the neural basis for negative, mood‐congruent attention biases. These are consistently observed in MDD patients, and probably associated with the development and maintenance of recurrent depressive episodes [Dannlowski et al., 2006a,b; Dannlowski et al., 2007a,b; Dannlowski et al., 2009].
Compared to MDD, neuronal activation patterns during emotion processing in BD patients appear more heterogeneous in the literature. Previous findings during conscious emotion processing concerning limbic emotional reactivity in BD patients demonstrated increased amygdala and striatal activity to positive [Malhi and Lagopoulos, 2004; Lawrence et al., 2004; Blumberg et al., 2005; Chen et al., 2006] as well as negative emotional stimuli [Lawrence et al., 2004; Hulvershorn et al., 2012]—for review see Delvecchio et al. [2012] and Almeida and Phillips [2012]. However, while all earlier studies used supraliminal stimuli, we opted for subliminal stimulus presentation, tapping early and automatic emotion processing stages. Increased amygdala responsiveness to positive stimuli in BD patients during conscious processing might be influenced by a diminished regulation of amygdala reactivity by the orbitomedial prefrontal cortex and perigenual anterior cingulate cortex—as connectivity results during processing of happy facial stimuli by Almeida et al. [2009] and Wang et al. [2009] suggest. This might apply less to the automatic processing of subliminal stimuli. Given previous findings of exaggerated amygdala reactivity to positive stimuli in manic patients [Bermpohl et al., 2009], remitted bipolar patients and their first‐degree relatives [Surguladze et al., 2010], it seems also plausible that automatic amygdala responsiveness to happy faces is stronger in BD than in MDD, even in the state of depression.
Furthermore, our results show decreased amygdala responsiveness to sad faces in BD patients, relative to both MDD patients and HC. The difference between BD and MDD is partly due to the highly exaggerated amygdala reactivity in MDD patients to sad facial expressions discussed above (Fig. 1). Nevertheless, abnormally reduced amygdala reactivity to subliminally presented sad facial expressions in BD relative to MDD is interesting, given the increased limbic responsiveness to negative stimuli during supraliminal emotion processing found for BD patients relative to MDD patients [Almeida et al., 2010; Hulvershorn et al., 2012] and HC [Hulvershorn et al., 2012]. These inconsistencies could be again a result of different paradigms, probing conscious versus unconscious neural processing. Conscious processes might depend on appraisal processes, involving prefrontal cortical regions interacting with limbic areas, whereas our paradigm rather relied on fast, automatic processing specific for the amygdala [Hariri et al., 2003]. In BD patients, exaggerated reactivity to negative stimuli might therefore depend on conscious appraisal processes relying on limbic‐prefrontal disruptions, rather than on early limbic reactivity. Furthermore, Almeida et al. [2010] observed increased amygdala reactivity specific for mildly sad and neutral faces in BD patients relative to MDD patients, possibly suggesting that exaggerated amygdala reactivity to negative facial expression in BD might be implicated in signaling ambiguity during conscious processing [Almeida et al., 2010] in comparison to rapidly signaling salience in MDD on early processing stages.
Our results indicate that in the left amygdala, the crucial emotion × group interaction was located in the superficial part (SF), extending to the basolateral nuclei (LB). Moreover, the (nonsignificant) emotion × group interaction in the right amygdala was predominantly located in the basolateral part. In sum, our results implicate that both, superficial as well as basolateral parts, might be relevant for differential emotion processing in MDD and BD depression. Previous studies suggest that the basolateral amygdala is critically involved in the processing of unconscious stimuli and in assigning emotional value to sensory stimuli [Davis and Whalen, 2001; Etkin et al., 2004]. The superficial part of the amygdala is a neighboring structure, but its function has been less investigated. However, due to the limited spatial resolution of our fMRI sequence, we are not able to reliably differentiate between amygdala subregions and standard atlas systems do not account for inter‐individual anatomical variability. Therefore these results should be taken with care.
In addition, one should draw attention to possible laterality effects. Our finding implicates differential emotion processing in MDD, BD and HC lateralized to the left during subliminal stimulus presentation. However, this result should be treated with caution, since we did not explicitly investigate laterality effects. In our case, the displayed laterality effect might rather depend on the corrected statistical threshold, since at a threshold of P < 0.05 (uncorrected) the analysis shows an emotion × group interaction effect also in the right amygdala (Fig. 1) with a similar activation pattern. Furthermore, left and right amygdala responsiveness to happy and sad faces were highly intercorrelated (r = 0.883 for sad faces, r = 0.914 for happy faces).
Taken together, our results suggest distinct neural correlates during automatic emotion processing underlying depressive episodes in BD and MDD—possibly representing important diagnostic biological markers. These findings from the standard univariate approach were corroborated by pattern‐classification techniques with high diagnostic accuracies of almost 80%, reached with the crucial sad>happy contrast—including a desirable balance between sensitivity and specificity. These results again fit well with Grotegerd et al. [2013], who reported high feature weights in the amygdala, specific to MDD for negative (sad and angry) facial expressions, whereas high feature weights for happy facial expressions were related to BD. However, Mourão‐Miranda et al. [2012] did not obtain robust discrimination between MDD and BD with whole‐brain pattern recognition based on happy and neutral faces. Indeed, this is not contradictory to our present results, since we focused on amygdala reactivity only, and applied very different presentation conditions (supraliminal vs. subliminal). Furthermore, our findings also indicate a weaker discriminability for happy>neutral facial expressions alone. Some MDD patients were wrongly classified as being bipolar, for instance GPC misclassified five MDD patients using the sad > happy contrast. However, in fact, it could be possible that these misclassified MDD patients might carry a risk for developing manic episodes in the future and therefore, it might well be the case that rather our current clinical diagnosis was wrong instead of our classification based on fMRI data. Overall, pattern recognition was only used twice before for discriminating between MDD and BD depressed patients, but again only data on conscious facial emotion processing were used in these studies [Grotegerd et al., 2013; Mourão‐Miranda et al., 2012]. Taken together, we suggest amygdala responsiveness measured during subliminal processing of sad faces (in contrast to happy or neutral faces) as a promising neurobiological pattern to predict MDD and BD depression, with conceivable applications supporting clinical diagnostics in future.
Some limitations must be acknowledged. First, our patient sample consisted of acutely depressed patients. It is thus difficult to decide whether the described neural differences represent state markers of current depressive episodes or would persist after remission. Second, most of the patients were medicated, a potential confound that we cannot rule out completely. Unfortunately, we did not assess a complete history of previous successful or unsuccessful treatment strategies and therefore, we cannot determine treatment resistance according to common definition. However, also due to the selection of MDD patients from a specialized ward in a university setting, the results might not generalize to outpatient samples. We did not exclude patients for recent changes in antidepressant and antipsychotic medication. However, all patients have been stable on their medication for at least 3 days. Although investigations in MDD patients reported some (overall “normalizing”) effects on amygdala responsiveness by antidepressant treatment [Sheline et al., 2001; Victor et al., 2010], findings in bipolar disorders implicated either no significant medication effects or rather ameliorative effects of medication on limbic activity—for reviews see Phillips et al. [2008] and Hafeman et al. [2012]. Although our key finding of an emotion × group interaction effect was not influenced by medication subgroup, these findings need replication in unmedicated patients, or in studies with a longitudinal design controlling for medication as proposed by Hafeman et al. [2012].
Concerning the standard univariate fMRI analysis one must note, that while the hypothesized emotion × group interaction effect was clearly significant, the post hoc t‐tests did not survive correction for multiple comparisons, albeit pointing to the hypothesized directions. Still this is an interesting finding since the emotion × group interaction clearly shows that BD is differently stimulated than unipolar depression.
Concerning pattern classification in general, our sample size is still quite small, albeit well in the range of previous fMRI pattern‐classification reports. Furthermore, the restriction of the pattern classification to an anatomical mask based on a‐priori hypotheses could be questioned with respect to whole‐brain analyses or data‐driven feature selection approaches. Nevertheless, whole‐brain approaches will carry numerous features with only little or no relevance, leading to weak discriminability. Finally, our paradigm was particularly designed for assessing automatic amygdala responsiveness and hence, relying on the amygdala as a core structure of automatic emotion processing appeared to be an effective procedure for the purpose of this investigation and has been conducted also in our previous report on pattern classification discriminating MDD and BD [Grotegerd et al., 2013]. However, it examines only one possible model of brain function.
Although pattern classification relying on the amygdala based on a priori knowledge is well‐founded regarding the present issue, future studies in unipolar and bipolar depressed patients using multi‐modal whole brain approaches should clarify the role of the amygdala (and other brain structures) for distinguishing these two disorders. Eventually, applicability and performance of the present method have to be validated with data of distinct, independent samples and accordingly of distinct studies. Regarding possible future clinical applications, the sensitivity and specificity of the classifiers would need adjustments to take account for the specific clinical query and for the unequal prevalence of both disorders within the population. Furthermore, such designs may be worthwhile to evaluate in first depressive episodes, as this might have future implications.
CONCLUSION
To conclude, our results demonstrate differential automatic amygdala responsiveness dependent on diagnosis and emotional valence. Hence, the current findings point to different pathophysiological processes underlying unipolar and bipolar depression. Together with the results of the additional pattern classification approach, the findings support the idea that valence‐specific neural activation pattern during subliminal emotion processing might represent characteristic neurobiological differences for discriminating bipolar from unipolar depression. Before possible diagnostic and therapeutic implications can be considered, further studies are needed to provide more evidence for this conceptualization.
ACKNOWLEDGMENTS
P. Zwanzger has received speaker fees from Pfizer, Servier, Lilly, AstraZeneca, and Bristol‐Myers Squibb, is on the advisory board of Pfizer, is a consultant for Ironwood Pharmaceuticals, and has received grants from Astra‐Zeneca. VA is a member of advisory boards of AstraZeneca, Lilly, Lundbeck, Servier, has received grants from Lundbeck and received payment for lectures and development of educational presentations from AstraZeneca, Lundbeck, Lilly and Servier. All other authors state that they have no conflicts of interest to declare, financial or otherwise for any aspect of the submitted work.
REFERENCES
- Abler B, Erk S, Herwig U, Walter H (2007): Anticipation of aversive stimuli activates extended amygdala in unipolar depression J Psychiatr Res 41:511–522. [DOI] [PubMed] [Google Scholar]
- Almeida JRC, Phillips ML (2012): Distinguishing between unipolar depression and bipolar depression: Current and future clinical and neuroimaging perspectives. Biol Psychiatry 73:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida JRC, Versace A, Hassel S, Kupfer DJ, Phillips ML (2010): Elevated amygdala activity to sad facial expressions: A state marker of bipolar but not unipolar depression. Biol Psychiatry 67:414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida JRC, Versace A, Mechelli A, Hassel S, Quevedo K, Kupfer DJ, Phillips ML (2009): Abnormal amygdala‐prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biol Psychiatry 66:451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (APA) (2000): Diagnostical and Statistical Manual of Mental Disorders: DSM‐IV‐TR, 4th ed Washington, DC, USA: Text Revision APA Press. [Google Scholar]
- Angst J, Cui L, Swendsen J (2010): Major depressive disorder with sub‐threshold bipolarity in the National Comorbidity Survey Replication. Am J Psychiatry 167:1194–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baune BT, Dannlowski U, Domschke K, Janssen DGA, Jordan MA, Ohrmann P, Bauer J, Biros E, Arolt V, Kugel H, Baxter AG, Suslow T (2010): The interleukin 1 beta (IL1B) gene is associated with failure to achieve remission and impaired emotion processing in major depression. Biol Psychiatry 67:543–549. [DOI] [PubMed] [Google Scholar]
- Bermpohl F, Dalanay U, Kahnt T, Sajonz B, Heimann H, Ricken R, Stoy M, Hägele C, Schlagenhauf F, Adli M, Wrase J, Ströhle A, Heinz A, Bauer M (2009): A preliminary study of increased amygdala activation to positive affective stimuli in mania. Bipolar Disord 11:70–75. [DOI] [PubMed] [Google Scholar]
- Bertocci M a, Bebko GM, Mullin BC, Langenecker S a, Ladouceur CD, Almeida JRC, Phillips ML (2012): Abnormal anterior cingulate cortical activity during emotional n‐back task performance distinguishes bipolar from unipolar depressed females. Psychol Med 42:1417–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blond BN, Fredericks C a, Blumberg HP (2012): Functional neuroanatomy of bipolar disorder: Structure, function, and connectivity in an amygdala‐anterior paralimbic neural system. Bipolar Disord 14:340–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg HP, Donegan NH, Sanislow CA, Collins SH, Lacadie CM, Skudlarski P, Gueorguieva R, Fulbright RK, McGlashan TH, Gore JC, Krystal JH (2005): Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology 183:308–313. [DOI] [PubMed] [Google Scholar]
- Chang C‐C, Lin C‐J(2011): LIBSVM: A Library for Support Vector Machines. ACM Trans Intell Syst Technol 2:27:1–27:27. [Google Scholar]
- Chen C‐H, Lennox BR, Jacob R, Calder AJ, Lupson V, Bisbrown‐Chippendale R, Suckling J, Bullmore ET (2006): Explicit and implicit facial affect recognition in manic and depressed States of bipolar disorder: A functional magnetic resonance imaging study. Biol Psychiatry 59:31–39. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Kersting A, Donges U‐S, Lalee‐Mentzel J, Arolt V, Suslow T (2006a): Masked facial affect priming is associated with therapy response in clinical depression. Eur Arch Psychiatry Clin Neurosci 256:215–221. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Kersting A, Lalee‐Mentzel J, Donges U‐S, Arolt V, Suslow T (2006b): Subliminal affective priming in clinical depression and comorbid anxiety: A longitudinal investigation. Psychiatry Res 143:63–75. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Konrad C, Kugel H, Zwitserlood P, Domschke K, Schöning S, Ohrmann P, Bauer J, Pyka M, Hohoff C, Zhang W, Baune BT, Heindel W, Arolt V, Suslow T (2010): Emotion specific modulation of automatic amygdala responses by 5‐HTTLPR genotype. Neuroimage 53:893–898. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Kugel H, Huber F, Stuhrmann A, Redlich R, Grotegerd D, Dohm K, Sehlmeyer C, Konrad C, Baune BT, Arolt V, Heindel W, Zwitserlood P, Suslow T (2012b): Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Human Brain Mapp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Deckert J, Hohoff C, Kugel H, Arolt V, Heindel W, Kersting A, Baune BT, Suslow T (2008): 5‐HTTLPR biases amygdala activity in response to masked facial expressions in major depression. Neuropsychopharmacology 33:418–424. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Kugel H, Arolt V, Heindel W, Kersting A, Baune BT, Suslow T (2007a): Amygdala reactivity to masked negative faces is associated with automatic judgmental bias in major depression: A 3 T fMRI study. J Psychiatry Neurosci 32:423–429. [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Kugel H, Arolt V, Heindel W, Suslow T (2007a): Amygdala reactivity predicts automatic negative evaluations for facial emotions. Psychiatry Res 154:13–20. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Konrad C, Domschke K, Bauer J, Kugel H, Hohoff C, Schöning S, Kersting A, Baune BT, Mortensen LS, Arolt V, Zwitserlood P, Deckert J, Heindel W, Suslow T (2009): Reduced amygdala‐prefrontal coupling in major depression: Association with MAOA genotype and illness severity. Int J Neuropsychopharmacology 12:11–22. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J, Lindner C, Postert C, Konrad C, Arolt V, Heindel W, Suslow T, Kugel H (2012a): Limbic scars: Long‐term consequences of childhood maltreatment revealed by functional and structural MRI. Biol Psychiatry 71:286–93. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen P (2001): The amygdala: Vigilance and emotion. Molecular psychiatry 6:13–34. [DOI] [PubMed] [Google Scholar]
- Delvecchio G, Fossati P, Boyer P, Brambilla P, Falkai P, Gruber O, Hietala J, Lawrie SM, Martinot JL, McIntosh AM, Meisenzahl E, Frangou S (2012): Common and distinct neural correlates of emotional processing in Bipolar Disorder and Major Depressive Disorder: A voxel‐based meta‐analysis of functional magnetic resonance imaging studies. Eur Neuropsychopharmacol 22:100–113. [DOI] [PubMed] [Google Scholar]
- Domschke K, Dannlowski U, Hohoff C, Ohrmann P, Bauer J, Kugel H, Zwanzger P, Heindel W, Deckert J, Arolt V, Suslow T, Baune BT (2010): Neuropeptide Y (NPY) gene: Impact on emotional processing and treatment response in anxious depression. Eur Neuropsychopharmacol 20:301–309. [DOI] [PubMed] [Google Scholar]
- Domschke K, Dannlowski U, Ohrmann P, Lawford B, Bauer J, Kugel H, Heindel W, Young R, Morris P, Arolt V, Deckert J, Suslow T, Baune BT (2008): Cannabinoid receptor 1 (CNR1) gene: Impact on antidepressant treatment response and emotion processing in major depression. Eur Neuropsychopharmacol 18:751–759. [DOI] [PubMed] [Google Scholar]
- Donges U‐S, Kugel H, Stuhrmann A, Grotegerd D, Redlich R, Lichev V, Rosenberg N, Ihme K, Suslow T, Dannlowski U (2012): Adult attachment anxiety is associated with enhanced automatic neural response to positive facial expression. Neuroscience 220:149–157. [DOI] [PubMed] [Google Scholar]
- Dudek D, Siwek M, Zielińska D, Jaeschke R, Rybakowski J (2012): Diagnostic conversions from major depressive disorder into bipolar disorder in an outpatient setting: Results of a retrospective chart review. J Affect Disorders 144:112–115. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K (2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25:1325–1335. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV (1976): Pictures of facial affect. Palo Alto: Consulting Psychologists Press. [Google Scholar]
- Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, Hochberg H, Murrough J, Strohmayer E, Stern E, Silbersweig DA (2006): Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry 163:1784–1790. [DOI] [PubMed] [Google Scholar]
- Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, Hirsch J (2004): Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron 44:1043–1055. [DOI] [PubMed] [Google Scholar]
- Fu CHY, Mourão‐Miranda J, Costafreda SG, Khanna A, Marquand AF, Williams SCR, Brammer MJ (2008a): Pattern Classification of Sad Facial Processing: Toward the Development of Neurobiological Markers in Depression. Biol Psychiatry 63:656–662. [DOI] [PubMed] [Google Scholar]
- Fu CHY, Williams SCR, Cleare AJ, Brammer M, Walsh ND, Kim J, Andrew CM, Pich EM, Williams PM, Reed LJ, Mitterschiffthaler MT, Suckling J, Bullmore ET (2004): Attenuation of the neural response to sad faces in major depression by antidepressant treatment: A prospective, event‐related functional magnetic resonance imaging study. Arch Gen Psychiatry 61:877–889. [DOI] [PubMed] [Google Scholar]
- Fu CHY, Williams SCR, Cleare AJ, Scott J, Mitterschiffthaler MT, Walsh ND, Donaldson C, Suckling J, Andrew C, Steiner H, Murray RM (2008a): Neural responses to sad facial expressions in major depression following cognitive behavioral therapy. Biol Psychiatry 64:505–512. [DOI] [PubMed] [Google Scholar]
- Grier JB (1971): Nonparametric indexes for sensitivity and bias: Computing formulas. Psychol Bull 75:424–429. [DOI] [PubMed] [Google Scholar]
- Grotegerd D, Suslow T, Bauer J, Ohrmann P, Arolt V, Stuhrmann A, Heindel W, Kugel H, Dannlowski U (2013): Discriminating unipolar and bipolar depression by means of fMRI and pattern classification: A pilot study. Eur Arch Psychiatry Clin Neurosci 263:119–131. [DOI] [PubMed] [Google Scholar]
- Hafeman DM, Chang KD, Garrett AS, Sanders EM, Phillips ML (2012): Effects of medication on neuroimaging findings in bipolar disorder: An updated review. Bipolar Disord 14:375–410. [DOI] [PubMed] [Google Scholar]
- Hahn T, Marquand A, Ehlis A‐C, Dresler T, Kittel‐Schneider S, Jarczok TA, Lesch KP, Jakob PM, Mourao‐Miranda J, Brammer MJ, Fallgatter AJ (2010): Integrating neurobiological markers of depression. Arch Gen Psychiatry 68:361–368. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR (2003): Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry 53:494–501. [DOI] [PubMed] [Google Scholar]
- Hirschfeld RMA, Lewis L, Vornik LA (2003): Perceptions and impact of bipolar disorder: How far have we really come? Results of the National Depressive and Manic‐Depressive Association 2000 Survey of Individuals With Bipolar Disorder. J Clin Psychiatry 64:161–174. [PubMed] [Google Scholar]
- Hulvershorn L a, Karne H, Gunn AD, Hartwick SL, Wang Y, Hummer TA, Anand A (2012): Neural activation during facial emotion processing in unmedicated bipolar depression, euthymia, and mania. Biol Psychiatry 71:603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Cooney RE, Henry ML, Gotlib IH (2011): Neural correlates of automatic mood regulation in girls at high risk for depression. J Abnorm Psychol 121:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Schettler PJ, Coryell W, Endicott J, Maser JD, Solomon DA, Leon AC, Keller MB (2003): A prospective investigation of the natural history of the long‐term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry 60:261–269. [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Schettler PJ, Endicott J, Maser JD, Solomon DA, Leon AC, Rice JA, Keller MB (2002): The long‐term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry 59:530–537. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ (2005): The increasing medical burden in bipolar disorder. JAMA 293:2528–2530. [DOI] [PubMed] [Google Scholar]
- Kupka RW, Altshuler LL, Nolen WA, Suppes T, Luckenbaugh DA, Leverich GS, Frye MA, Keck PE Jr, McElroy SL, Grunze H, Post RM (2007): Three times more days depressed than manic or hypomanic in both bipolar I and bipolar II disorder. Bipolar Disord 9:531–535. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze SA, Giampietro VP, Brammer M, Andrew CM, Frangou S, Ecker C, Phillips ML (2004): Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry 55:578–587. [DOI] [PubMed] [Google Scholar]
- Ledoux JE (2000): Emotion circuits in the brain. Ann Rev Neurosci 23:155–184. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Lagopoulos J (2004): Cognitive generation of affect in bipolar depression: An fMRI study. Eur J Neurosci 19:741–754. [DOI] [PubMed] [Google Scholar]
- Mitchell PB, Goodwin GM, Johnson GF, Hirschfeld RMA (2008): Diagnostic guidelines for bipolar depression: A probabilistic approach. Bipolar Disord 10:144–152. [DOI] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ (1999): A subcortical pathway to the right amygdala mediating “unseen” fear. Proc Natl Acad Sci USA 96:1680–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourão‐Miranda J, Almeida JR, Hassel S, de Oliveira L, Versace A, Marquand AF, Sato JR, Brammer M, Phillips ML (2012): Pattern recognition analyses of brain activation elicited by happy and neutral faces in unipolar and bipolar depression. Bipolar Disord 14:451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso MAM, Glahn DC, Matsuo K, Monkul ES, Najt P, Zamarripa F, Lancaster JL, Fox PT, Gao JH, Soares JC (2009): Amygdala hyperactivation in untreated depressed individuals. Psychiatry Res 173:158–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Frank E (2006): Redefining bipolar disorder: Toward DSM‐V. Am J Psychiatry 163:1135–1136. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Travis MJ, Kupfer DJ, Fagiolini A (2008): Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry 165:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reker M, Ohrmann P, Rauch AV, Kugel H, Bauer J, Dannlowski U, Arolt V, Heindel W, Suslow T (2010): Individual differences in alexithymia and brain response to masked emotion faces. Cortex 46:658–667. [DOI] [PubMed] [Google Scholar]
- Sackeim HA (2001): The definition and meaning of treatment‐resistant depression. J Clin Psychiatry 62:10–17. [PubMed] [Google Scholar]
- Santos A, Mier D, Kirsch P, Meyer‐Lindenberg A (2011): Evidence for a general face salience signal in human amygdala. Neuroimage 54:3111–3116. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA (2001): Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biol Psychiatry 50:651–658. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS (2002): Can't shake that feeling: Event‐related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry 51:693–707. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson WK, Carter CS, Steinhauer SR, Thase ME (2007): Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: Related and independent features. Biol Psychiatry 61:198–209. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Adler CM, Almeida J, Altshuler LL, Blumberg HP, Chang KD, DelBello MP, Frangou S, McIntosh A, Phillips ML, Sussman JE, Townsend JD (2012): The functional neuroanatomy of bipolar disorder: A consensus model. Bipolar Disord 14:313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhrmann A, Dohm K, Kugel H, Zwanzger P, Redlich R, Grotegerd D, Rauch AV, Arolt V, Heindel W, Suslow T, Zwitserlood P, Dannlowski U (2013): Mood‐congruent amygdala responses to subliminally presented facial expressions in Major Depression: Associations with anhedonia. J Psychiatry Neurosci 38:249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhrmann A, Suslow T, Dannlowski U (2011): Facial emotion processing in major depression: A systematic review of neuroimaging findings. Biol Mood Anxiety Disord 1:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguladze S, Brammer M, Keedwell P, Giampietro V, Young AW, Travis MJ, Williams SCR, Phillips ML (2005): A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry 57:201–209. [DOI] [PubMed] [Google Scholar]
- Surguladze SA, Marshall N, Schulze K, Hall M‐H, Walshe M, Bramon E, Phillips ML, Murray RM, McDonald C (2010): Exaggerated neural response to emotional faces in patients with bipolar disorder and their first‐degree relatives. Neuroimage 53:58–64. [DOI] [PubMed] [Google Scholar]
- Suslow T, Konrad C, Kugel H, Rumstadt D, Zwitserlood P, Schöning S, Ohrmann P, Bauer J, Pyka M, Kersting A, Arolt V, Heindel W, Dannlowski U (2010a): Automatic mood‐congruent amygdala responses to masked facial expressions in major depression. Biol Psychiatry 67:155–160. [DOI] [PubMed] [Google Scholar]
- Suslow T, Kugel H, Reber H, Bauer J, Dannlowski U, Kersting A, Arolt V, Heindel W, Ohrmann P, Egloff B (2010b): Automatic brain response to facial emotion as a function of implicitly and explicitly measured extraversion. Neuroscience 167:111–123. [DOI] [PubMed] [Google Scholar]
- Tavares JT, Clark LA, Furey ML (2008): Neural basis of abnormal response to negative feedback in unmedicated mood disorders. Neuroimage 42:1118–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin J, Casey BJ (2011): Elevated amygdala response to faces following early deprivation. Dev Sci 14:190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- van Harmelen A‐L, van Tol M‐J, Demenescu LR, van der Wee NJA, Veltman DJ, Aleman A, van Buchem MA, Spinhoven P, Penninx BW, Elzinga BM (2012): Enhanced amygdala reactivity to emotional faces in adults reporting childhood emotional maltreatment. Soc Cogn Affective Neurosci 8:362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vapnik V, Chervonenkis A (1974): Theory of Pattern Recognition. Moscow: Nauka; [in Russian]. [Google Scholar]
- Victor TA, Furey ML, Fromm S, Ohman A, Drevets WC (2010): Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Arch Gen Psychiatry 67:1128–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Kalmar JH, He Y, Jackowski M, Chepenik LG, Edmiston EE, Tie K, Gong G, Shah MP, Jones M, Uderman J, Constable RT, Blumberg HP (2009): Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biol Psychiatry 66:516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen H‐U, Wunderlich U, Gruschwitz S, Zaudig M (1997): SKID‐I. Strukturiertes Klinisches Interview für DSM‐IV. Göttingen: Hogrefe. [Google Scholar]
- Wolfensberger SPA, Veltman DJ, Hoogendijk WJG, Boomsma DI, Geus EJC (2008): Amygdala responses to emotional faces in twins discordant or concordant for the risk for anxiety and depression. Neuroimage 41:544–552. [DOI] [PubMed] [Google Scholar]


