Abstract
The action observation network (AON) is supposed to play a crucial role when athletes anticipate the effect of others' actions in sports such as tennis. We used functional magnetic resonance imaging to explore whether motor expertise leads to a differential activation pattern within the AON during effect anticipation and whether spatial and motor anticipation tasks are associated with a differential activation pattern within the AON depending on participant expertise level. Expert (N = 16) and novice (N = 16) tennis players observed video clips depicting forehand strokes with the instruction to either indicate the predicted direction of ball flight (spatial anticipation) or to decide on an appropriate response to the observed action (motor anticipation). The experts performed better than novices on both tennis anticipation tasks, with the experts showing stronger neural activation in areas of the AON, namely, the superior parietal lobe, the intraparietal sulcus, the inferior frontal gyrus, and the cerebellum. When novices were contrasted with experts, motor anticipation resulted in stronger activation of the ventral premotor cortex, the supplementary motor area, and the superior parietal lobe than spatial anticipation task did. In experts, the comparison of motor and spatial anticipation revealed no increased activation. We suggest that the stronger activation of areas in the AON during the anticipation of action effects in experts reflects their use of the more fine‐tuned motor representations they have acquired and improved during years of training. Furthermore, results suggest that the neural processing of different anticipation tasks depends on the expertise level. Hum Brain Mapp 35:4016–4034, 2014. © 2014 Wiley Periodicals, Inc.
Keywords: action anticipation, motor expertise, cerebellum, instructions, action observation
INTRODUCTION
Anticipating an opponent's actions and deciding on an appropriate response are important skills in fast ball sports where athletes have to respond under time pressure. For example, tennis players have to select an appropriate response to their opponent's serve before the opponent strikes the ball otherwise there is not enough time to perform the return shot [Williams et al., 2011]. The substantial increases in serve velocity over the last two decades, with typical ball velocities now exceeding 240 kph, implies that anticipation is crucial to successful performance in professional tennis. Moreover, in team sports such as soccer and combat sports like karate athletes have to predict the specific outcomes of an opponent's movement ahead of the action itself. Similarly, in many professional occupations (e.g., aviation, surgery) and everyday activities (e.g., driving, crossing a road) the ability to anticipate future events is paramount to successful performance [e.g., Jackson et al., 2009; Koglbauer et al., 2011; Zheng et al., 2007].
Sport provides an excellent vehicle to examine how performers anticipate the actions of others, and particularly, the influence of the observer's experience on anticipation. When compared to novices, expert athletes possess a wealth of experience in executing sport‐specific movements along with an extraordinary ability to anticipate their opponents' movement behaviors [e.g., Abernethy et al., 2001; Cañal‐Bruland et al., 2011; Rowe and McKenna, 2001; Singer et al., 1996; Williams et al., 2002; for a review, see Williams et al., 2011]. For example, Abernethy and Russell [1987] showed that badminton experts perform better than novices when anticipating the direction of strokes from videos that were stopped before, at, or shortly after ball–racket contact. These results indicate that experts already know what action an opponent will perform well before ball‐flight information becomes available, potentially relying on information conveyed by the kinematics of their opponent's action rather than the ball per se [Abernethy and Russell, 1987; Aglioti et al., 2008; Huys et al., 2008; Urgesi et al., 2011; Williams et al., 2009].
Regarding the underlying neural mechanism, the so‐called action observation network (AON) is supposed to play a crucial role when observing the actions of others [Cross et al., 2009]. The AON embraces all brain areas activated by the mere observation of actions. According to a meta‐analysis of 104 studies, the inferior frontal gyrus (BA 44/45), dorsal and ventral premotor cortex (dPMC, vPMC), supplementary motor area (SMA), inferior parietal lobe (IPL), superior parietal lobe (SPL), and primary sensory cortex (SI) show enhanced activation during the observation of human actions [Caspers et al., 2010]. The involvement of these areas in action observation is supported by studies using repetitive transcranial magnetic stimulation to apply virtual lesions for a limited amount of time [Candidi et al., 2008; Pobric and Hamilton, 2006; Urgesi et al., 2007]. For example, a temporary lesion of the ventral premotor cortex impaired the performance of participants in a task that required the perception and processing of biological movements [Candidi et al., 2008].
Beyond the results of the meta‐analysis of Caspers et al. [2010], numerous researchers have reported activation in the cerebellum during the observation of actions [Buccino et al., 2004; Gallagher and Frith, 2004; Gazzola and Keysers, 2009; Gazzola et al., 2007; Molenberghs et al., 2012; Pilgramm et al., 2010]. Moreover, contrary to earlier publications where the cerebellum was regarded as a structure with functions exclusively in motor control [Glickstein, 1993; Holmes, 1939], many researchers have shown involvement of the cerebellum in non‐motor processes such as observation of biological movements as well as cognitive and emotional processing [Desmond and Fiez, 1998; Fink et al., 2000; Imaizumi et al., 1997; Rao et al., 1997; Schlösser et al., 1998; Stoodley and Schmahmann, 2009]. The cerebellum is part of the AON as well [Calvo‐Merino et al., 2006] and one the central functions of the AON may be the simulation of observed actions in order to predict the outcome of very specific movements [Abreu et al., 2012; Avenanti et al., 2013; Stadler et al., 2012; Zentgraf et al., 2011]. In particular, the premotor cortex is thought to be involved even in the anticipation of nonbiological events [Schubotz, 2007]. Abreu et al. [2012] reported activation in the AON when predicting the effects of basketball free throws. Basketball experts and novices showed similar activation in areas of the AON when the prediction of the throws was contrasted with a nonprediction task. Findings confirm that the AON is an important network for the perception of actions and the prediction of another person's actions. More precisely, the frontoparietal areas in the AON as well as the cerebellum [Abreu et al., 2012; Avenanti et al., 2013; Miall, 2003] are employed to simulate observed actions in order to predict their outcome.
Several researchers have focused on factors that may modulate neural activity in areas of the AON during action simulation. Calvo‐Merino et al. [2006] compared male and female ballet dancers who were watching gender‐specific dance videos in order to investigate the role of the observer's motor and visual expertise during action observation. They found stronger activation in the premotor cortex, the parietal cortex, and the cerebellum when dancers observed moves from their own motor repertoire compared to moves by the opposite gender with which they had only visual familiarity. These results indicate that activation in the AON and the cerebellum depends strongly on the motor expertise of the observer. Furthermore, it has been shown that different tasks or instructional modes modulate neural activation within specific areas of the AON [Zentgraf et al., 2005, 2011]. For example, there is evidence that experts demonstrate better performance on an action anticipation task when they have to perform a natural response depending on their motor repertoire compared to a verbal prediction [Farrow and Abernethy, 2003].
Thus far, no researchers have applied one design to test whether motor experts and novices prefer different anticipation strategies (motor vs. spatial anticipation) and, whether they engage different neural networks during such tasks. We examined the ability to anticipate an opponent's stroke in tennis using videos that were occluded at the moment of ball–racket contact. We applied a 2 × 2 design with two types of action anticipation task (within‐subject condition: motor vs. spatial anticipation) and two levels of tennis expertise (between‐subject condition: novices vs. experts). In one task, participants had to indicate the expected flight direction of the tennis ball (spatial task). In the other task, they had to decide on their own appropriate motor response to the observed stroke, namely, a forehand or a backhand stroke (motor task). Our main aim in the present study was to identify those areas of the AON involved in movement prediction. We expected, based on the results of Abreu et al. [2012] and Avenanti et al. [2013], neural activation in areas of the AON for all participants in the prediction task. The second aim was to explore whether motor expertise leads to a differential activation pattern within the AON (more precisely, within the premotor cortex, the posterior parietal cortex, and the cerebellum). According to Abreu et al. [2012], Calvo‐Merino et al. [2006], and Cross et al. [2009] there should be stronger activation within areas of the AON in a group with high motor expertise compared to novices. Consequently, we expected to find stronger AON activation in tennis experts during the prediction of stroke direction. A final aim was to assess whether different anticipation tasks (spatial vs. motor) are associated with a differential activation pattern within certain areas of the AON and to identify a possible interrelationship between motor expertise and task at behavioral and neural levels.
METHODS
Participants
Thirty two participants with normal or corrected to normal vision took part in the study. All participants were right‐handed according to the Edinburgh Handedness Inventory [Oldfield, 1971]. No participant reported any history of psychiatric or neurological disorders or current use of psychoactive medication. One‐half of the participants (eight females and eight males, mean age = 22.56, SD = 5.11) were tennis experts who played in one of the four highest level tennis leagues in Germany and trained an average of 4.56 times a week (SD = 2.16). The experts had a mean of 16 years (SD = 5.73) tennis‐playing experience and had played an average of 534 (SD = 300) tournament matches. The other 16 participants (8 females and 8 males, mean age = 25.38, SD = 3.88) were novices who had played only at a recreational level (mean experience in years = 0.19, SD = 0.54). All participants were paid or received course credits. The study was approved by the local ethics committee at the lead institution and participants gave their informed written consent in accordance with the Declaration of Helsinki.
Stimulus Material
Ninety‐six of the 144 video clips showed forehand ground strokes in tennis performed by a male and a female model (cf., Fig. 1A). More specifically, we recorded 48 strokes and presented each of these clips in two different response conditions, resulting in a total of 96 tennis stroke videos. Both models were right‐handed and played at a regional club level. All videos stopped at ball–racket contact and the clip duration ranged from 2,104 to 3,006 ms. The camera was positioned 2 m behind the net in the other half of the court, simulating the view of an opponent standing right behind the net waiting to play a volley. The players were instructed to aim for target zones in the left and the right corner of the court that had a width of one meter (from the sideline) and a length of three meters (from the baseline). Half of these tennis strokes showed hits of the target zone in the left‐hand corner, the other half hits of the target zone in the right‐hand corner. Another 24 clips displayed both models bouncing a ball with their racket in the right hand standing in the middle of the ground line (cf., Fig. 1B). The tennis stimuli were recorded using a Canon XM2 video camera with a sampling rate of 25 fps.
Figure 1.

Examples of all experimental conditions. Each of the 144 video clips lasted 2–3 s. (A) Male player performing a forehand stroke. The tennis stroke sequences were stopped at ball–racket contact and shown in both tennis anticipation conditions (motor anticipation, spatial anticipation). (B) Female player bouncing the ball with her racket (observation only condition). (C) Yellow ball moving down the screen on a vertical sinusoidal trajectory, disappearing at a random point in time (dashed line) (ball only condition). Please note that participants did not see the gray trajectory in the ball only condition.
To prevent such contradictory stimuli and to avoid neural effects related to face perception, the models' faces were blurred in all clips with Adobe Premiere (Adobe Systems Software Ireland Limited, Dublin, Ireland). A mismatch between gaze direction and kinematics of an actor can result in ambiguous movements that are used in sports to deceive the opponent about their own intention [e.g., the no‐look‐pass in basketball, see Kunde et al., 2011]. In the remaining 24 videos, participants watched a yellow ball moving down the screen on a vertical sinusoidal trajectory around a vertical line (cf. Fig. 1C). The trace was not visible (unlike in Fig. 1C in which it is depicted only to illustrate the task), and the ball disappeared at a random point in time. These stimuli were created using Microsoft PowerPoint (Microsoft Office Suite 2003, Redmond, USA). The duration of these ball bouncing and animated ball only stimuli was the same as the tennis strokes.
All stimuli were presented at a resolution of 720 × 576 pixels with a PC running Presentation software (Version 12.9, Neurobehavioral Systems, Albany, USA) and projected onto a screen behind the scanner so that the participants could watch them via a mirror attached to the head coil (visual field 188 mm in the horizontal and 168 mm in the vertical plane, rectangular aperture; visual angle ∼18° horizontal and 11° vertical).
Task
Participants had to respond to four different conditions. In the spatial anticipation condition, they watched tennis forehand strokes by the two models and were asked to anticipate the direction of the observed stroke and subsequently indicate the presumable flight direction of the ball. The response was given by pressing the left or right button on a two‐button response box. In this condition, the left button indicated a ball flying to the left‐hand corner and the right button a ball flying to the right‐hand corner. In the motor anticipation condition, participants watched the same 48 clips as in the spatial anticipation condition but were required to anticipate the direction of the observed stroke and subsequently choose an appropriate own motor response, namely, a forehand or a backhand stroke. To select a backhand stroke, participants pressed the left button, whereas for a forehand stroke the right button was pressed. Therefore, a correct forehand answer in the motor anticipation condition corresponded to a correct decision in favor of the right‐hand corner in the spatial anticipation condition. For both anticipation conditions, participants were asked to imagine themselves as the opposing player standing at the net (matching the camera view depicting a first‐person perspective). The latter instruction was used to prevent experts from thinking about playing an inside‐out stroke (i.e., playing a ball on the backhand side by running around the ball and performing a forehand stroke), because an inside‐out stroke is played only from the baseline and not when standing at the net to play a volley. We excluded inside‐out strokes because otherwise forehand and backhand would have been correct responses in trials showing a stroke to the backhand side.
To control for effects due to visual stimulation and the observation of biological movements, we added an observation only condition including the same two models in the same visual setting without any need for anticipation. The task in this observation only condition was merely to observe the models bouncing the ball and to press the left or right button immediately after the video. Which button to press was indicated in the instruction text before the video presentation and was identical for the six video clips of the observation only condition succeeding this instruction. In an additional nonbiological anticipation condition (ball only condition), participants were instructed to interpolate the trajectory of the unpredictably disappearing ball and to anticipate whether the ball would hit a horizontal line to the left or the right of mid‐line. As in the other conditions, they responded by pressing the left or right button. All responses in this study included motor reactions after the respective observation condition. The ratio of correct left and right reactions was balanced across all four conditions.
Procedure
Participants were given instructions for the experimental conditions illustrated with sample videos and figures. Before the start of the fMRI experiment, participants completed a short training session with two videos for each experimental condition to ensure their full understanding of the tasks. These videos were not used in the fMRI session. While lying in the scanner, participants had to complete 144 trials resulting in a total duration of about 35 min for the whole experiment. Every trial started with a black screen for 1 s and a fixation cross for another 5 s, followed by presentation of the video sequence lasting 2,104–3,006 ms. Directly after the video presentation, the screen turned blank and participants had 3 s to give their response by pressing the left or the right button on the response box with the index and middle finger of their right hand. When a button was pressed, the given response was displayed on the screen for the rest of the response time. During the experiment, participants did not receive any feedback on their performance.
The 144 trials were presented in miniblocks. Each miniblock consisted of an instruction lasting 8 s followed by six trials of the same condition. Miniblocks were organized into four bigger blocks. Each of these contained two motor anticipation, two spatial anticipation, one observation only, and one ball only miniblock. The sequence of miniblocks within the bigger blocks was balanced as well as the distribution of trials to the miniblocks (cf. Fig. 2).
Figure 2.

Configuration and procedure for the 24 miniblocks. Each miniblock started with an instruction followed by six trials of one of the four experimental conditions. Each trial began with a fixation cross, followed by one of the 144 video clips and a subsequent response. The order of the miniblocks as well as the distribution of the videos was randomized for each participant.
Behavioral Data Acquisition and Analysis
In each of the four experimental conditions, both correct answers and response times (defined as the time between the end of the video stimulation and the button press) were analyzed with SPSS (Version 18, SPSS, Chicago, IL). To investigate the influence of expertise and anticipation task (motor anticipation vs. spatial anticipation) on the number of correct responses, a 2 × 2 ANOVA with repeated measures was computed for anticipation task and expertise level. The same computation was employed for the response times. Additionally, a t test within each group assessed whether the number of correct responses in the motor and the spatial anticipation condition was significantly above chance level. For the ball only condition, a nonpaired t test between the two expertise groups was calculated to examine whether the number of correct responses differed significantly.
fMRI Data Acquisition and Preprocessing
The fMRI data were acquired using a 1.5 Tesla whole body scanner (Siemens symphony, Erlangen, Germany) with a standard head coil. The structural images consisted of 160 T1‐weighted sagittal images (slice thickness = 1 mm, TR = 1.99 s, TE = 4.18 ms, field of view = 250 × 250 mm2, base resolution = 256 × 256, orientation = sagittal). During the experiment, a total of 840 T2*‐weighted images were collected using a gradient echo‐planar‐imaging sequence (number of slices = 25, slice thickness = 5 mm, gap = 1 mm, TA = 100 ms per slice, TR = 2.5 s, TE = 55 ms, flip angle = 90°, field of view = 192 × 192 mm2, matrix size = 64 × 64). The axial slices recorded during the EPI sequence were oriented parallel to the AC–PC line. The onsets of the video clips were jittered within an interval between ± ½ TR to realize a better sampling of the HRF function.
Functional data were processed and analyzed using SPM8 (Wellcome Department of Cognitive Neurology, London, UK). The 840 volumes were realigned and unwarped, slice‐time corrected, and normalized into Montreal Neurological Institute (MNI) space. Finally, data were smoothed with 9‐mm Gaussian isotropic filter as recommended by Worsley [2007]. Furthermore, an additional movement correction was employed to reduce the impact of rapid head movements using software developed in‐house. The detection of outlier volumes was based on a comparison of each volume with its two neighbors in a motion‐corrected time series. This procedure was done by calculating the mean squared differences to the previous and the next volume. The smaller difference was used as the outlier score for each volume. Scores were thresholded using Hubert and van der Veeken's [2008] method of calculating a skewness‐corrected interquartile range. To threshold outlier scores, the range was multiplied by 1.5 and added to the 75th percentile. The correction of outlier volumes was done during the first‐level analysis by the usage of an additional regressor for each odd volume.
For the cerebellar data, a specific normalization method was applied to allow a more accurate localization of activation within the small structures of the cerebellum. Because of the low contrast in the 152 ICBM template (MNI space) of the cerebellum, a whole brain normalization that is used as a standard in SPM8 would have led to a large spatial variance between participants [Diedrichsen, 2006]. Therefore, we used the template of the SUIT toolbox for SPM8 (Version 2.5.2, Institute of Cognitive Neuroscience, London, UK), which is based on the average cerebellar anatomy of 20 participants. This procedure preserved the fine details of the cerebellum and improved the intersubject alignment compared to the standard normalization [Diedrichsen, 2006]. In a first step, the automatic isolation algorithm provided by the toolbox was used to segregate the cerebellum and the brainstem. If necessary, the isolation maps were corrected manually based on anatomical information and then these were normalized to the SUIT template via a nonlinear transformation. The resultant deformation maps were used to normalize the functional images of each participant. Contrary to the whole brain data, in which normalization and the ensuing smoothing were performed before the first‐level analysis, in the SUIT normalization, these steps were conducted after the functional data had been analyzed on the single subject level. On the second level, the whole brain and the cerebellar data were analyzed in exactly the same way.
Data Analysis
The first‐level analysis was computed for each participant separately on the basis of the general linear model (GLM). The signal was convoluted using the hemodynamic response function (HRF). The video observation of each trial in the four conditions was covered by this HRF matching the length of the video. Functional data were high‐pass filtered with a cutoff of 128 s to remove slow signal changes. The correct and incorrect trials of the four different experimental conditions (motor anticipation, spatial anticipation, ball only, and observation only) as well as the instruction, the response, and rest periods were entered into the model. Furthermore, six parameters resulting from the movement correction were added to the GLM as covariates. Autoregressive processing was applied to account for serial correlations.
In the second‐level analysis, one‐ and two‐sample t tests were conducted. First of all, we conducted a one‐sample t test for the contrast tennis anticipation (motor anticipation and spatial anticipation) > observation only for all 32 participants to identify brain areas activated during the anticipation of action effects in the whole group and to see whether we could replicate previous findings on activation in the AON during effect anticipation. To investigate the activation in areas of the AON for the contrast tennis anticipation > observation only with the influence of the anticipation performance eliminated, in a post‐hoc analysis, the covariate “correct responses” was fed into a one‐sample t test of the contrast tennis anticipation > observation only for all 32 participants. The covariate contained the percentages of correct responses in the tennis anticipation conditions. The statistical threshold for these comparisons was set at P = 0.05, corrected for multiple comparisons using the family‐wise error (FWE) criterion on the whole‐brain level. For these comparisons, the correction volume included all voxels of the brain mask provided by SPM8. The peak voxels of significant activations were labeled using the Anatomy Toolbox [Version 1.7, Eickhoff et al., 2005] for SPM8. This toolbox was used to label all activations on the basis of cytoarchitectonic probability maps. Significant results within the cerebellum were assigned to the cerebellar lobuli by means of the probabilistic atlas included in the SUIT toolbox [Diedrichsen et al., 2009]. The SUIT toolbox also provided the cerebellar mask that was used to define the correction volume for cerebellar activation. To investigate the influence of the tennis anticipation performance of the participants on the activation in areas of the AON, we conducted a second post‐hoc analysis considering the number of correct responses. A parameter “correct responses” containing the percentages of correct responses in the tennis anticipation conditions was used to examine the influence of the anticipation performance on the results of the one‐sample t test of the contrast tennis anticipation > observation only for all 32 participants. As we expected to find activation differences in the AON, we examined a small‐volume correction with a priori defined search volumes based on Caspers et al. [2010] (for details see section below).
To investigate the influence of expertise on the contrast tennis anticipation (motor anticipation and spatial anticipation) > observation only, an ANOVA was calculated. According to Penny and Henson [2007] a test for interaction requires the fitting of the first‐level models and the computation of the within‐subject contrasts, followed by the fitting of the second‐level models to test these contrasts between the two groups. Furthermore, an additional parameter “years of tennis experience,” containing the years of tennis experience of all 16 experts in whole numbers (7–29 years), was fed into a one‐sample t test of the contrast tennis anticipation > observation only in the expert group. The aim of this post hoc analysis was to investigate whether the brain activation of tennis experts depended on years of experience. To assess whether different anticipation tasks were associated with a differential activation pattern within certain areas of the AON, and to identify a possible interrelationship of motor expertise and task, the comparisons of the two different anticipation instructions, motor anticipation > spatial anticipation and spatial anticipation > motor anticipation, were calculated for all 32 participants, as well as in each expertise group separately, and in an ANOVA comparing the two tasks in a between‐subject comparison between both groups (Penny and Henson, 2007). To check whether the stronger activation in the experts was specific to the tennis anticipation task with which they had experience, we compared the anticipation performance in the ball only condition between the two groups.
We were particularly interested in brain activation within the areas of the AON and expected to find activation differences within these areas depending on expertise and different instructions. Therefore, we examined a small‐volume correction with a priori defined search volumes in the AON for the between‐subject comparison of the contrast tennis anticipation > observation only and for all contrasts comparing the motor and spatial anticipation tasks. The selection of these regions of interest (ROIs) was based on the results of Caspers et al.'s [2010] meta‐analysis and identical for all contrasts. We included the inferior parietal lobe (IPL), the intraparietal sulcus (IPS), the superior parietal lobe (SPL), the dorsal and ventral premotor cortex (dPMC and vPMC), the supplementary motor area (SMA), the somatosensory cortex (S1), and the inferior frontal gyrus (IFG). Because Caspers et al.'s [2010] meta‐analysis did not include the cerebellum, we chose ROIs in the cerebellum that had been reported to be activated during the execution [e.g., Dimitrova et al., 2006; Schmahmann et al., 2009] or the observation [e.g., Sokolov et al., 2010] of actions. These regions were Lobules I‐IV, VII, and VIII, as well as Crus I and Crus II. The cerebellar masks were based on the probabilistic atlas of the cerebellum provided by Diedrichsen et al. [2009], whereas the masks of the cerebral cortex were based on cytoarchitectonic data [Eickhoff et al., 2005]. All masks for this ROI analysis were created using FSL software [Smith et al., 2004] and included voxels with an at least 50% probability of being part of the specific regions. The statistical threshold for the ROI analysis was set at P = 0.05 (FWE‐corrected).
RESULTS
Behavioral Data
Considering motor and spatial anticipation together as an overall tennis stroke anticipation condition, participants in the novice group gave correct answers on an average of 57.29% (SD = 7.07) of strokes, whereas tennis experts recorded a mean accuracy score of 70.18% (SD = 10.08). When separated into both response conditions, tennis experts gave correct responses on 69.66% of trials (SD = 9.44) in the motor and 70.70% of trials (SD = 11.29) in the spatial condition, compared to 57.13% (SD = 7.64) in the motor and 57.42% (SD = 8.12) in the spatial condition for the novices. A 2 (expertise) × 2 (response condition) repeated measures ANOVA revealed a significant main effect of expertise, F(1,30) = 17.54, P < 0.001. No significant main effects were reported for the two different response conditions or the interaction. The number of correct responses in both groups was significantly above chance level, t experts(15) = 8.00, P < 0.001; t novices(15) = 4.13, P = 0.001.
Tennis experts had a mean response time (i.e., the time between the ball–racket contact and the button press) of 691 ms (SD = 380) in the motor and 674 ms (SD = 366) in the spatial condition, compared to 733 ms (SD = 259) in the motor and 675 ms (SD = 221) in the spatial condition for the novices. A 2 (expertise) × 2 (response condition) repeated measures ANOVA revealed a significant main effect of response condition, F(1,30) = 6.68, P < 0.05. Neither the main effect of expertise nor the interaction attained significance.
We found no significant differences for the percentage of correct responses between the two groups in the ball only condition (M correct experts = 53.39%; SD = 22.22; M correct novices = 51.82%; SD = 25.55), t(30) = .185, ns. In the ball‐bouncing condition (observation only), participants were asked to press either the left or right button depending on the instruction received before each miniblock. In this condition, all responses were correct, indicating that all participants had also maintained attention in the observation only condition during the whole experiment.
fMRI Data
In a first step, we were interested in the brain areas involved in anticipating observed actions in all participants irrespective of their tennis experience. We expected to replicate previous findings with activation in the AON being strongly evident during anticipation of action effects. To investigate whether motor expertise modulated this AON activation, we were particularly interested in differences within the AON between the two expertise levels when anticipating tennis strokes. In a further step, we assessed whether different instructions during effect anticipation were associated with differential activity within the areas of the AON.
Activation in the AON during anticipation of action effects
On the basis of the previous literature [Abreu et al., 2012; Avenanti et al., 2013], we expected to find neural activation in areas of the AON during the anticipation of action effects. To test this hypothesis, we ran a within‐subject comparison of the contrast tennis anticipation > observation only for all 32 participants. Because the ball‐bouncing control condition contained the observation of biological movements of the same players in the identical visual setting, the results of the contrast tennis anticipation > observation only reflect brain activation mainly due to anticipation and not to the mere observation of biological motion. The contrast revealed significant activation in the dorsal premotor cortex (dPMC), the superior parietal lobe (SPL), the inferior frontal gyrus (IFG), the primary sensory cortex (S1, area 2), the insula, the thalamus, the superior medial gyrus (SMG), the middle cingulate cortex (MCC), the hippocampus, the caudate nucleus, the visual cortices (V1, V2, V5), as well as Crus I, Crus II, Lobule VI, Lobule VIIIb, and Lobule IX of the cerebellum (P < 0.05, FWE‐corrected) (see Fig. 3). Table 1 presents a detailed summary of all activations. To eliminate the influence of the anticipation performance the covariate “correct responses” (varying from 49 to 88%, M = 63.74%, SD = 10.78) was fed into a one‐sample t test of the contrast tennis anticipation > observation only for all 32 participants in a post‐hoc analysis. The analysis revealed significant activation in the dPMC, the SPL, the IFG, the S1, the insula, the thalamus, the SMG, the MCC, the hippocampus, the caudate nucleus, the visual cortices (V1, V2, V5), as well as Crus I, Crus II, Lobule VI, Lobule VIIIb, Lobule IX and Lobule X of the cerebellum and therefore resulted in activation comparable to the same contrast without the covariate “correct responses” (P < 0.05, FWE‐corrected) (see Table 1). The division of the premotor cortex (BA 6) into the ventral and dorsal premotor cortex at Z = 50 was based on the recommendations of Rizzolatti et al. [2002].
Figure 3.
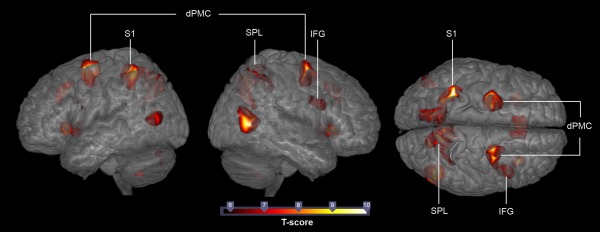
Significant brain activation in all 32 participants for the comparison tennis anticipation > observation only. T maps were thresholded at t = 5.81 (P < 0.05, FWE‐corrected). Activation is rendered on a high‐resolution T1 template (“colin brain”).
Table 1.
Brain areas identified by contrasting the anticipation of tennis strokes with the ball‐bouncing control condition in all 32 participants
| L/R | X | Y | Z | t value | SUIT | Covariatea | |
|---|---|---|---|---|---|---|---|
| Tennis anticipation > observation only (all 32 participants) | |||||||
| dPMC | L | −27 | −7 | 58 | 16.47 | ✓ | |
| dPMC | R | 27 | −4 | 58 | 11.12 | ✓ | |
| IFG (pars triangularis)b | R | 45 | 14 | 25 | 8.49 | ✓ | |
| IFG (pars opercularis) | L | −48 | 5 | 22 | 6.45 | ✓ | |
| IFG (pars opercularis)b | L | −51 | 2 | 34 | 6.13 | ✓ | |
| SPL (7A)b | R | 18 | −67 | 43 | 10.39 | ✓ | |
| SPL (5M) | R | 12 | −52 | 58 | 10.35 | ✓ | |
| Cerebellum, lobule IX | L | −12 | −54 | −49 | 8.40 | ✓ | ✓ |
| Cerebellum, lobule IX | R | 14 | −54 | −51 | 7.38 | ✓ | ✓ |
| Cerebellum, crus II | L | −8 | −80 | −37 | 7.71 | ✓ | ✓ |
| Cerebellum, crus II | R | 6 | −76 | −35 | 5.98 | ✓ | ✓ |
| Cerebellum, lobule VI | L | −10 | −76 | −25 | 7.43 | ✓ | ✓ |
| Cerebellum, lobule VI | L | −36 | −36 | −33 | 6.26 | ✓ | ✓ |
| Cerebellum, lobule VI | L | −24 | −60 | −29 | 5.10 | ✓ | |
| Cerebellum, lobule VIIIb | R | 28 | −42 | −45 | 5.75 | ✓ | ✓ |
| Cerebellum, crus I | R | 38 | −44 | −37 | 5.19 | ✓ | ✓ |
| Cerebellum, crus I | R | 38 | −64 | −29 | 5.01 | ✓ | |
| S1 (area 2) | L | −33 | −46 | 55 | 11.16 | ✓ | |
| V1 | L | −9 | −100 | 10 | 6.00 | ✓ | |
| V2 | L | −15 | −97 | 10 | 6.33 | ✓ | |
| V5/MT+ | R | 48 | −61 | 7 | 11.67 | ✓ | |
| V5/MT+ | L | −45 | −73 | 10 | 7.86 | ✓ | |
| Insulac | L | −30 | 20 | 1 | 10.58 | ✓ | |
| Insulac | R | 33 | 23 | −2 | 9.80 | ✓ | |
| SMGc | L | −9 | 20 | 40 | 9.08 | ✓ | |
| MCCc | L | −6 | 26 | 34 | 8.96 | ✓ | |
| MCCc | R | 9 | 17 | 43 | 7.69 | ✓ | |
| Caudate nucleusc | L | −9 | 14 | 1 | 7.76 | ✓ | |
| Caudate nucleusc | R | 12 | 14 | −2 | 6.27 | ✓ | |
| Caudate nucleusc | R | 9 | 5 | 1 | 6.11 | ✓ | |
| Thalamusc | L | −12 | −25 | 10 | 6.42 | ✓ | |
| Thalamusc | R | 6 | −13 | 10 | 6.09 | ✓ | |
| Hippocampusb | R | 9 | −31 | −5 | 6.28 | ✓ | |
| Hippocampusb | R | 15 | −31 | 4 | 6.21 | ✓ | |
Same activation found when a covariate “correct responses” was introduced.
Probabilistic labeling below threshold of 20%.
Anatomical labeling. MNI coordinates, P < 0.05, FWE‐corrected. For abbreviations, see text.
To examine whether the tennis anticipation performance modulated brain activation in the AON, we introduced the percentages of correct responses in the tennis anticipation conditions (varying from 49 to 88%, M = 63.74%, SD = 10.78) as an additional parameter. A post hoc ROI analysis of the influence of this parameter on the contrast tennis anticipation > observation only in all 32 participants revealed a performance‐related increase of activation in the SPL as well as in Crus II, Lobule I–IV, Lobule VIIb, Lobule VIIIa and Lobule VIIIb of the cerebellum (see Table 2).
Table 2.
Brain areas showing stronger activation as a function of correct responses in the tennis anticipation conditions when contrasting the anticipation of tennis strokes with the ball‐bouncing control condition in all 32 participants
| L/R | X | Y | Z | t value | SUIT | |
|---|---|---|---|---|---|---|
| Tennis anticipation > observation only (all 32 participants) | ||||||
| Cerebellum, crus II | L | −24 | −88 | −39 | 3.58 | ✓ |
| Cerebellum, lobule I–IV | R | 18 | −40 | −23 | 3.51 | ✓ |
| Cerebellum, lobule VIIIb | L | −22 | −42 | −51 | 3.43 | ✓ |
| Cerebellum, lobule VIIIa | L | −4 | −68 | −35 | 2.82 | ✓ |
| Cerebellum, lobule VIIb | L | −4 | −68 | −33 | 2.76 | ✓ |
| SPL (7PC) | R | 21 | −73 | 64 | 3.27 | |
| SPL (5Ci) | L | −15 | −34 | 46 | 2.76 | |
MNI coordinates, P < 0.05, FWE‐corrected, ROI analysis, ROI masks thresholded at 50%.
Differences in the activation of the AON between experts and novices during anticipation
To identify expertise‐related differences in activation of the AON during anticipation of action effects, we compared brain activation in experts versus novices during the anticipation of the tennis strokes (including motor and spatial anticipation). The between‐subject ROI analysis of the contrast tennis anticipation > observation only revealed higher activation in the tennis experts in the IFG, the IPS and the SPL as well as in the cerebellum in a cluster spreading into Crus I, Crus II, Lobules VIIb, and VIIIa and a second activation site in Lobule VIIIb (see Fig. 4). No area showed a higher activation in the novices. All results are summarized in Table 3. To assess whether the length of experience in playing tennis modulated the brain activation of experts, we introduced the years of tennis experience (varying from 7–29 years, M = 16.00, SD = 5.73) as an additional covariate. A post hoc ROI analysis of the influence of this covariate on the contrast tennis anticipation > observation only in the expertise group revealed an experience‐related increase of activation in Lobule VIIIb of the cerebellum (see Table 4). To test whether a stronger activation in the AON of the tennis experts was specific to the tennis anticipation task with which they had experience, we compared anticipation in the ball only condition between the two groups, ball onlyexperts > ball onlynovices and ball onlynovices > ball onlyexperts. This comparison revealed no significant activation differences between tennis experts and novices.
Figure 4.
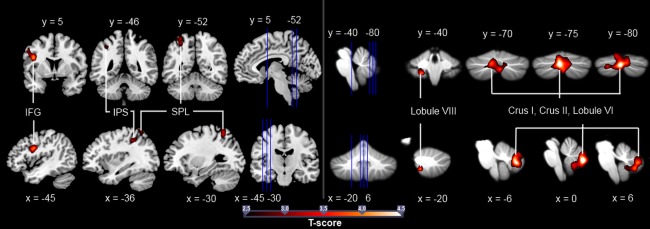
Significantly stronger activation in the tennis experts compared to the novices for the comparison tennis anticipation > observation only. The blue vertical lines indicate the slice positions. T maps were thresholded at t = 2.50 (P < 0.05, FWE‐corrected). Activation is rendered on a high‐resolution T1 template (“colin brain”) as well as on the cerebellar SUIT template [Diedrichsen, 2006].
Table 3.
Brain areas identified by the between‐subject comparison of tennis experts and novices when contrasting the anticipation of tennis strokes with the ball‐bouncing control condition
| L/R | X | Y | Z | t value | SUIT | |
|---|---|---|---|---|---|---|
| Tennis anticipation > observation only (experts > novices) | ||||||
| Cerebellum, crus I | L | −6 | −74 | −31 | 4.38 | ✓ |
| Cerebellum, crus II | L | −2 | −78 | −35 | 5.15 | ✓ |
| Cerebellum, crus II | R | 10 | −80 | −29 | 4,27 | ✓ |
| Cerebellum, lobule VIIb | L | −4 | −74 | −39 | 3.91 | ✓ |
| Cerebellum, lobule VIIIa | R | 4 | −72 | −43 | 3.91 | ✓ |
| Cerebellum, lobule VIIIb | L | −20 | −40 | −49 | 3.51 | ✓ |
| IFG (BA44) | L | −45 | 5 | 25 | 3.84 | |
| SPL (7P) | L | −30 | −52 | 64 | 3.19 | |
| IPS (hlP1) | L | −36 | −46 | 43 | 2.62 | |
| Tennis anticipation > observation only (novices > experts) | ||||||
| — | — | — | — | — | — | |
MNI coordinates, P < 0.05, FWE‐corrected, ROI analysis, ROI masks thresholded at 50%.
Table 4.
Brain areas showing stronger activation as a function of years of tennis experience when contrasting the anticipation of tennis strokes with the ball‐bouncing control condition within the expert group
| L/R | X | Y | Z | t value | SUIT | |
|---|---|---|---|---|---|---|
| Tennis anticipation > control (experts only) | ||||||
| Cerebellum, Lobule VIIIb | L | −20 | −44 | −51 | 3.98 | ✓ |
MNI coordinates, P < 0.05, FWE‐corrected, ROI analysis, ROI masks thresholded at 50%.
Differences between motor and spatial anticipation tasks
To investigate whether different instructions modulated activity in the AON during the anticipation of action effects, we contrasted the motor anticipation condition with the spatial anticipation condition and vice versa. The within‐subject ROI analysis of the contrast motor anticipation > spatial anticipation for all 32 participants revealed a stronger activation of the IFG, the S1, the IPL, and the medial SPL. The opposite comparison revealed stronger activation in the more lateral and anterior SPL. In the tennis experts, the contrast motor anticipation > spatial anticipation resulted in a stronger activation in the IPL, whereas the SPL was activated in the opposite comparison, spatial anticipation > motor anticipation (see Fig. 5B). In the novices, the motor anticipation task resulted in activation of the vPMC, the S1 (area 1), the pre‐SMA, and the SPL; the contrast spatial anticipation > motor anticipation did not reveal any activation differences (see Fig. 5C). To see whether this modulation was dependent on expertise, the comparison was performed for the between‐subject comparison of the two different expertise groups. In this between‐subject comparison, the novices showed stronger activation in the vPMC, the SMA proper, and the SPL for the contrast motor anticipation > spatial anticipation compared to the tennis experts (see Fig. 5A). All results are summarized in Table 5.
Figure 5.
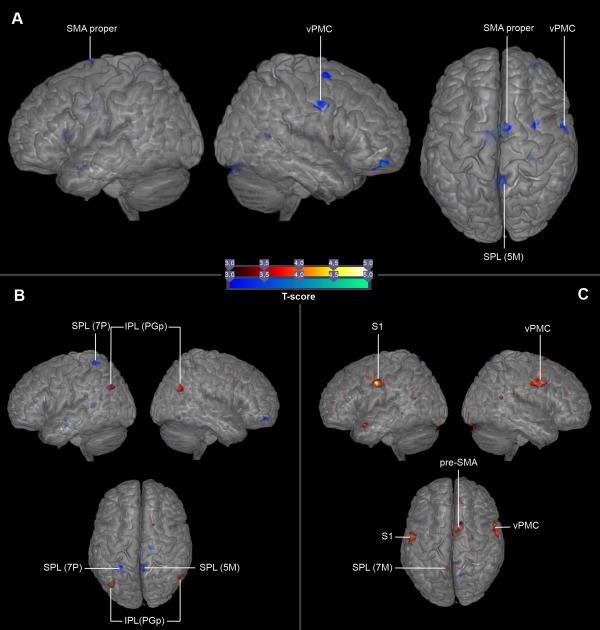
(A) Stronger activation in the novices in a between‐subject comparison of both groups for the contrast motor anticipation > spatial anticipation (blue marks). (B) Higher activation within the expert tennis players for the contrasts motor anticipation > spatial anticipation (red marks) and spatial anticipation > motor anticipation (blue marks). (C) Stronger activation within the novices for the contrast motor anticipation > spatial anticipation (red marks).T maps were thresholded at t = 3.00 (P < 0.05, FWE‐corrected). Activation is rendered on a high‐resolution T1 template (“colin brain”).
Table 5.
Brain areas identified when contrasting motor anticipation with spatial anticipation in a within‐subject comparison of all 32 participants and each group separately as well as in a between‐subject comparison of tennis experts and novices
| L/R | X | Y | Z | t value | SUIT | |
|---|---|---|---|---|---|---|
| Motor anticipation > spatial anticipation (all 32 participants) | ||||||
| IFG (BA44) | L | −57 | 11 | 31 | 4.03 | |
| S1 | L | −57 | −13 | 40 | 3.98 | |
| IPL (PGp) | L | −42 | −76 | 34 | 3.76 | |
| IPL (PGp) | R | 57 | −64 | 28 | 5.05 | |
| IPL (PGa) | R | 63 | −55 | 22 | 4.22 | |
| SPL (7M) | L | −3 | −70 | 37 | 2.98 | |
| SPL (7M) | R | 0 | −73 | 43 | 3.43 | |
| Spatial anticipation > motor anticipation (all 32 participants) | ||||||
| SPL (7A) | L | −21 | −61 | 70 | 3.83 | |
| SPL (7A) | R | 9 | −64 | 70 | 3.73 | |
| SPL (7P) | L | −33 | −52 | 67 | 3.02 | |
| Motor anticipation > spatial anticipation (experts > novices) | ||||||
| — | — | — | — | — | — | |
| Motor anticipation > spatial anticipation (novices > experts) | ||||||
| vPMC | R | 57 | 2 | 40 | 3.99 | |
| SMA proper | R | 6 | −1 | 73 | 3.80 | |
| SPL (5M) | R | 3 | −52 | 64 | 3.83 | |
| Motor anticipation > spatial anticipation (experts only) | ||||||
| IPL (PGp) | L | −39 | −73 | 37 | 4.34 | |
| IPL (PGp) | R | 57 | −64 | 28 | 4.30 | |
| Spatial anticipation > motor anticipation (experts only) | ||||||
| SPL (5M) | R | 3 | −49 | 64 | 3.79 | |
| SPL (7P) | L | −33 | −52 | 67 | 4.22 | |
| Motor anticipation > spatial anticipation (novices only) | ||||||
| vPMC | R | 57 | 5 | 40 | 5.04 | |
| S1 (area 1) | L | −57 | −7 | 40 | 5.62 | |
| pre‐SMA | R | 9 | 2 | 52 | 7.16 | |
| SPL (7M) | L | −3 | −70 | 37 | 2.73 | |
| Spatial anticipation > motor anticipation (novices only) | ||||||
| — | — | — | — | — | — | |
MNI coordinates, P < 0.05, FWE‐corrected, ROI analysis, ROI masks thresholded at 50%.
DISCUSSION
We used fMRI to identify the brain areas involved during anticipation of tennis strokes. Moreover, we used different prediction tasks (motor vs. spatial anticipation) to investigate differences in AON activation between tennis experts and novices during action anticipation. As expected, the tennis experts recorded higher accuracy scores on the task compared to novice players. On the neural level, the anticipation of tennis strokes resulted in enhanced activation in SPL, S1, IFG, and dPMC for all participants compared with a control condition which involved observation of the same models bouncing the ball. This activation was modulated by the anticipation performance of the participants, as better anticipation was correlated with stronger activation in the SPL and various areas of the cerebellum. During the anticipation task, experts showed stronger activation within the inferior frontal gyrus (BA 44), the intraparietal sulcus, the superior parietal lobe, and the cerebellum when compared with the tennis novices. Furthermore, experts' neural activation of the cerebellar lobule VIIIb was related linearly to years of tennis experience. Regarding the different anticipation instructions, the present data demonstrated that the motor anticipation task was related to increased activation with parietal activation sites in the IPL and the SPL as well as within the IFG (BA 44). Regarding the interrelation between expertise and task, the present data suggest that the neural processing of different anticipation tasks depends on expertise level, because novices showed an increased activation within the ventral section of the PMC, the adjoining SMA proper, and the SPL (Area 5M) for the contrast motor anticipation > spatial anticipation when compared to experts. The following sections will discuss the results and their implications in more detail.
Anticipation Performance in Expert and Novice Tennis Players
Our findings show that both groups of participants were able to successfully anticipate ball direction at levels above chance even in the absence of information from the ball's flight path. As ball‐flight information was not available, we suggest that participants had to base their decisions on the observed kinematic information. However, compared to their novice counterparts, experts showed a significantly higher number of correct responses. This result depends not on a speed–accuracy trade‐off, as response time did not differ significantly between the groups. Our findings support previous published reports involving anticipation of forehand strokes in tennis [Cañal‐Bruland et al., 2011; Williams et al., 2009] and provide additional support for the notion that the ability to pick up kinematic information from the opponent's initial movements is essential to successful performance in sport [Müller et al., 2006; Ward et al., 2002; Williams et al., 2002; for a review, see Williams et al., 2011].
Activation in the AON During Effect Anticipation
Based on results reported by Abreu et al. [2012], Avenanti et al. [2013], and Caspers et al. [2010], we expected increased neural activation in areas of the AON for all participants irrespective of their expertise level when the tennis anticipation task was contrasted with the ball‐bouncing condition (observation only). As expected, contrasting those two conditions in all participants regardless of their motor expertise revealed a stronger activation in the SPL, the S1, the dPMC, and the IFG during the anticipation of tennis strokes. The stronger activation of these areas is in line with the theory that a frontoparietal network is used to predict the effect of observed actions [Abreu et al., 2012; Avenanti et al., 2013; Blakemore and Decety, 2001; Blakemore and Frith, 2005; Calvo‐Merino et al., 2006; Caspers et al., 2010; Cross et al., 2009; Gazzola and Keysers, 2009; Iacoboni et al., 2005; Miall, 2003; Oztop et al., 2005; Prinz, 2006; Schütz‐Bosbach and Prinz, 2007; Shmuelof and Zohary, 2007; Stadler et al., 2011; Wolfensteller, 2009]. Because we found the well‐known activation pattern of motor and motor‐related areas belonging to the AON, we assume that action simulation, which is predictive in nature, is responsible for the prediction of the effects of others' actions.
The assumption that areas of the AON play a decisive role during the anticipation of action effects is further supported by a post‐hoc analysis including the parameter “correct responses” for the contrast tennis anticipation > observation only in all 32 participants. We found that the percentage of correct responses in the tennis anticipation conditions was associated with stronger activation in the SPL (5Ci, 7PC) as well as in Crus II, Lobule I–IV, Lobule VIIb, Lobule VIIIa and Lobule VIIIb of the cerebellum. To test whether this effect of anticipation performance was specific to areas within the AON, we ran additional analyses using ROIs outside of the AON, namely the middle frontal gyrus, the frontal operculum and the frontal pole. As these areas revealed no activation in dependence on the performance of the participants, the results indicate that areas of the AON are actually involved in the anticipation of action effects.
Differences in AON Activation Between Tennis Experts and Novices During Anticipation
Several researchers have demonstrated that biological motion perception is influenced by the observer's familiarity with the observed action [Bischoff et al., 2012; Calvo‐Merino et al., 2010, 2006; Cross et al., 2009]. In particular, previous reports involving experts underpin the notion that anticipation performance is related to motor representations. We investigated the underlying neural correlates of sports excellence during an anticipation task by measuring the hemodynamic response while tennis experts and novices observed tennis strokes. Our experts demonstrated an increased activation within the inferior frontal gyrus (BA44), the intraparietal sulcus (hIP1), the superior parietal lobe (7P), and broad sections of the cerebellum. Findings suggest that motor representations of athletes are activated more when they anticipate actions belonging to their domain of motor expertise. In contrast, novices did not demonstrate higher activations compared to experts. All reported structures are supposed to be a part of the AON [Abreu et al., 2012; Calvo‐Merino et al., 2006; Caspers et al., 2010] and are involved in the observation of actions and the prediction of the effects of observed actions [Abreu et al., 2012; Gazzola and Keysers, 2009; Stadler et al., 2011]. It is important to note that we used a control condition that included observation of the same players moving in the identical visual setting as well as a response with a button press at the end of the sequence (observation only condition) to investigate the neural activation resulting from the anticipation. Therefore, activation found when contrasting the anticipation conditions with the ball‐bouncing control condition could not be due to the observation of biological movements per se or the movement of the button press. To test whether a stronger activation in the AON of the tennis experts was specific to the tennis anticipation task, we compared the anticipation in the ball only condition between the experts and the novices. The lack of effects between the two groups is an indication that the tennis experts' use of areas of the AON is specific to the tennis anticipation task.
The most prominent activation cluster when comparing experts and novices during the anticipation of tennis strokes was identified in the cerebellum. This result is in line with data reported by Abreu et al. [2012], Calvo‐Merino et al. [2006], and Cross et al., [2009] that also demonstrated stronger activation of the cerebellum in a group with high motor expertise compared to novices. In accordance with the tennis‐stroke anticipation task, our study revealed stronger activation in the expert group in Crus I and Lobule VIII of the cerebellum that are both involved in the sensorimotor processing of, in particular, arm and hand movements [Dimitrova et al., 2006; Grodd et al., 2001; O'Reilly et al., 2010; Schmahmann et al., 2009; Stoodley and Schmahmann, 2009]. A broad body of literature, including neurophysiological and computational studies, has demonstrated the cerebellum as a principal brain structure for the storage of internal forward models that predict movement outcomes and therefore support predictive motor control [Bastian, 2006; Imamizu et al., 2000; Miall and King, 2008; Synofzik et al., 2008; Wolfensteller, 2009; Wolpert et al., 1998]. Within this framework, the cerebellum seems to be used to process sensory data and provide, for example, precise timing information for predictions [Gao et al., 1996; Ghajar and Ivry, 2008; O'Reilly et al., 2008]. As the provision of precise timing information for predictions plays a fundamental role in successful tennis‐stroke anticipation in our study, the activation of cerebellar areas supposed to be involved in motor prediction fits those assumptions perfectly. Furthermore, we observed that length of tennis expertise was associated with an increase of activation in the cerebellum within the expert group. This finding suggests that motor‐related cognitive functions of the cerebellum can be modified by training and, therefore, might exhibit neural plasticity [Calvo‐Merino et al., 2006; Iacoboni, 2001]. Similarly, Lotze, Scheler, Tan, Braun, and Birbaumer [2003] reported a correlation between years of practice and activation in the cerebellum when violin players actually performed a Mozart concerto, underpinning the notion of plasticity processes within this region that depend on experience and practice.
Because the cerebellum is interconnected with other brain areas belonging to the AON [Imamizu and Kawato, 2008; Miall, 2003; Ramnani, 2006], it is reasonable to assume that experts reveal increased activation within other cortical structures belonging to this network. In the literature, prominent candidates are posterior parietal as well as inferior frontal regions [e.g., Caspers et al., 2010]. The present data revealed increased activation within the intraparietal sulcus (IPS) and within the posterior parietal (SPL) as well as in the inferior frontal gyrus (BA 44) when experts performed the anticipation tasks. The center of posterior parietal activation was found within the posterior section of the SPL. According to execution studies, this area is related to predictable variations in motor control due to changing the accuracy demands of a motor task [Winstein et al., 1997]. With the growing accuracy demands of an executed aiming task, neural activity within these areas increases in line with the greater visuomotor processing demands. Within this framework, our data suggest that, especially in experts, the tennis anticipation task might trigger the activation of motor representations within areas whose activation relates clearly to the accuracy demands of a motor task. Motor expertise seems to enhance the use of specific internal sensorimotor representations during an anticipation process requiring accuracy. For example, expert tennis players know precisely which kind of response is required by observing their opponent's stroke.
To predict the effect of an opponent's action and to initiate an appropriate response, tennis experts also rely on contextual information such as the position from which the observed player is going to hit the ball and the type of strokes the opponent has played in former rallies. Several researchers have shown that experts improve their anticipation performance when they are provided with contextual, game‐related information [Crognier and Féry, 2005; McPherson and MacMahon, 2008; McRobert et al., 2011]. An alternative explanation for the SPL activation could be that experts use such contextual information during situations requiring the anticipation of their opponent's behavior. In support of this latter argument, Imamizu and Kawato [2008] found activation in the SPL when participants had to initiate movements based on prior expectations. These authors concluded that the SPL associates contextual information with an appropriate internal model located in the cerebellum to predict the consequences of an action.
Besides increased activation within cerebellar and superior parietal sites, the present data demonstrated that experts reveal increased activation compared to novices within the IFG (BA 44) during an anticipation task. Previously, researchers have linked action‐related activations in BA 44 to, for example, motor sequence learning, motor imagery, and action preparation [e.g., Binkofski et al., 1999; Johnson‐Frey et al., 2003; Krams et al., 1998; Mecklinger et al., 2002]. In particular, involvement of BA 44 in action preparation might explain the increased activation when experts engage in action anticipation. It might be argued that experts tend to prepare their motor response while observing the early movement phases of the opponent. This theory is further supported by the activation of the intraparietal sulcus, as the IPS is supposed to play a crucial role in the planning of actions as well as in the representation of the related action goals [for a review, see Tunik et al., 2007]. In summary, the present findings underpin the notion that action anticipation in experts is based on internally represented actions and their goal states, as well as on the predictions and internal computations made about the action in advance of its execution. These processes run on the basis of the expert's fine‐tuned motor representations that are built up over years of practice [e.g., Desmurget et al., 2009].
The Influence of Different Instructions on the Anticipation of Action Effects
A further aim in our study was to investigate whether the anticipation process as well as the underlying neural response could be manipulated by different instructions. Participants had to observe each tennis stroke with the instruction to either indicate the predicted direction of the stroke (spatial anticipation) or to decide on the own appropriate physical response that they would make to the observed action (motor anticipation). It has to be noted that in the present study we used identical stimulus material for both anticipation tasks. Therefore, stimulus differences can be ruled out as a confounding variable. As the participants had to press the left or the right button in both anticipation conditions we analyzed behavioral and neurophysiological differences to investigate whether participants used diverging strategies in both tasks. Previous reports suggest that different response types influence the performance of experts in an anticipation task [Farrow and Abernethy, 2003] as well as the brain areas activated during movement observation [Zentgraf et al., 2005].
The present data revealed longer response times as well as increased activation within the IFG (BA 44), the IPL (Areas PGp and PGa), and the SPL (Area 7M) when participants had to decide to play a forehand or backhand response rather than indicate the direction of the stroke—independent of expertise level. The opposite contrast revealed an increased activation within the anterior and adjoining posterior sections of the SPL. These findings indicate that the motor and the spatial instruction resulted in different anticipation strategies in the participants. When compared to experts, novices revealed increased activation within the ventral section of the PMC, the adjoining SMA proper, and the SPL (Area 5M) when the motor task was contrasted with the spatial anticipation task. The opposite comparison of the experts with the novices revealed no significant increases in activation.
On a behavioral level, Farrow and Abernethy [2003] conducted two experiments to examine the ability of tennis players to predict the direction of an opponent's serve. They used two different response conditions: Participants were required to make either a movement‐based or a verbal prediction of serve direction. Results provided clear evidence of superior prediction accuracy under the movement‐based response condition when early ball flight was available. Furthermore, the data revealed that the experts' superiority is more apparent for movement‐based predictions than for verbal predictions. They argued that different perceptual processes underlie anticipatory tasks that might depend on expertise level, the type of information presented, and the task requirements (motor vs. verbal).
Our findings provide support for the ideas proposed by Farrow and Abernethy [2003]. Neural activation increased within the IFG (BA 44), the IPL (Areas PGp and PGa), and the SPL (Area 7M) when the task required prediction of the required movement response rather than a prediction of ball direction. Moreover, greater activation of the SPL (Area 7P and Area 7A) was observed in the spatial compared with motor prediction task. Thus, the movement‐based prediction task captured broader sections of the AON, reflecting a sort of motor simulation running on motor representations lying in frontoparietal areas [Abreu et al., 2012; Avenanti et al., 2013; Blakemore and Decety, 2001; Blakemore and Frith, 2005; Calvo‐Merino et al., 2006; Caspers et al., 2010; Cross et al., 2009; Gazzola and Keysers, 2009; Iacoboni et al., 2005; Miall, 2003; Oztop et al., 2005; Prinz, 2006; Schütz‐Bosbach and Prinz, 2007; Shmuelof and Zohary, 2007; Stadler et al., 2011]. It could be argued that movement‐based prediction leads to a more action‐related performance with continuous functional linkages between perceptual information and the parameters being used for movement control [Bootsma, 1989; Farrow and Abernethy, 2003].
Regarding the influence of expertise, one focus of research during the last decade has been on investigating experts and novices using different performance strategies during action anticipation tasks. For example, it has been argued that especially motor experts solve anticipation tasks by using early information from the kinematics of the opponent's movement patterns that precede the availability of ball‐flight information [Williams et al., 1999, 2011]. On the neural level, the present data revealed increased activation within the ventral section of the premotor area, the SMA proper, and the SPL when performing the contrast motor anticipation > spatial anticipation in the novice group compared to the experts. This finding suggests that the novice group tended to respond to the instruction to solve the motor anticipation task by particularly applying a motor strategy. Experts, however, demonstrated no increased activation differences compared to novices with regard to the motor instruction. These data indicate that a motor instruction especially supports a motor‐processing strategy within the novice's brain.
When taking a closer look at the differences within the two expertise groups, it becomes apparent that the neural differences between the motor and spatial anticipation task were less pronounced in the expert group compared to the novices (see Table 5). Beilock et al. [2002] demonstrated that instructions directing a performer's attention to motor‐task‐relevant cues enhanced performance only in novices performing a soccer dribbling task, yet were detrimental in experts. It was reasoned that the motor‐related instructions disturbed the expert players' automatic processing of fine‐tuned motor knowledge and forced them to perform the task on a more explicit, perhaps more inefficient level. The present findings in the expert group point to a similar conclusion. Whereas the novices possibly used anticipation strategies depending on the given instruction, experts either did not or did to lesser degree. Thus, experts seem to exhibit similar neural processing under different instructions. Higher automaticity of perception–action coupling might align both processing strategies in experts.
Another explanation for the higher activation in motor related areas in novices during the motor anticipation condition could be that this increased activation is due to error processing, as incorrect trials were included in the analysis. For example, the predictive coding account of Kilner et al. [2007] claims that the anticipation of action effects is based on a process minimizing the prediction error, defined as the discrepancy between the predicted and the actual action effect, within the AON during the observation of actions. However, the anticipation performance of the novices in the motor and spatial anticipation condition did not differ. Therefore, the difficulty as well as the prediction error seems to be comparable between both conditions. Thus, one would expect activation related to error processing when the anticipation of tennis strokes (motor and spatial) in novices is contrasted with the anticipation of tennis strokes (motor and spatial) in experts, as novices made significantly more errors than the tennis experts. As can be seen in the results section as well as in Table 3, there was no stronger activation in novices compared to the experts in this contrast at all, indicating that higher activation in the motor anticipation condition in novices is not determined by error processing.
Potential Limitations
To control for effects due to visual stimulation and the observation of biological movements, we contrasted the tennis anticipation condition with an observation only condition without any need for anticipation. Although both conditions were comparable concerning the two depicted models, the tennis hall background, the perspective of the camera and the fact that both conditions involved the observation of biological movements that included a tennis racket and ball, there were some differences between the conditions. One limitation concerns the way the participants had to respond to the different conditions. In the tennis anticipation condition participants had to choose between the left and the right button depending on the tennis clip they had seen before. In the observation only condition the response button was instructed before the start of the video clip, so there was no response selection comparable to the tennis anticipation condition. A second limitation is related to the instructed response in the observation only condition. As the response was not associated with the upcoming video clip, one cannot be sure whether attention was comparable to the anticipation condition. A third potential criticism concerns the differences in task difficulty, as participants showed 100% correct responses in the observation only condition and a mean of 64% in the tennis anticipation condition. However, if a higher difficulty was responsible for stronger activation in the AON, one would expect a stronger AON activation in the novices compared to the experts; we found the opposite result. Additionally, when eliminating the influence of the anticipation performance on the activation of the AON for the contrast tennis anticipation > observation only for all 32 participants, the results were comparable to the same contrast without the covariate “correct responses.” These results indicate that the difficulty of the anticipation conditions had no influence on the activation of the AON.
Although these limitations should be acknowledged, we note that the same kind of control condition (observation only condition of ball bouncing) has been routinely used in published reports investigating brain activation during anticipation in sport (Abreu et al., 2012; Bishop et al., 2013; Wright and Jackson, 2007; Wright et al., 2010). Our findings are consistent with those previously reported. Furthermore, the comparison of anticipation conditions between experts and novices revealed activation in brain areas that were activated for the contrast of the tennis anticipation and the observation only condition in all 32 participants. As the influence of the observation only condition was minimized in the comparison of both expertise groups due to the fact that the observation only condition was used equally in both groups, activation differences we found can be assigned to anticipation processes that are modulated by expertise.
A further potential flaw concerning our interpretation could be related to neural activation differences between experts and novices as well as to different anticipation conditions resulting from different imagery strategy (i.e., kinesthetic or visual imagery). It might for example be argued that experts rely more on kinesthetic imagery as they have more direct experience in playing tennis whereas the novices might rely more on a visual mode of imagery. Thus, the increased activation of parietal and premotor areas during the anticipation condition in the expert group could be explained by different modes of imagery [Guillot et al., 2009]. However, in the present task, participants were asked to react immediately to the video clips and they were not explicitly instructed to imagine the opponents' action or their own movement response. Thus, first, the time might have been too short to generate a motor image. Second, as we not instructed motor or visual imagery, the used strategies might have been too heterogeneous to allow us to attribute the activation pattern to a certain imagery process. Furthermore, action observation and effect anticipation are viewed as stimulus driven processes whereas imagery is defined as a perception‐like process in the absence of external stimuli [Munzert and Zentgraf, 2009]. These arguments strongly argue against the possibility that the present neuronal differences mainly result from different motor imagery strategies.
CONCLUSION
Our data provide evidence that irrespective of expertise level, the anticipation of tennis strokes is associated with increased activation in areas that subserve the AON. Most importantly, tennis experts showed a stronger activation in the IFG, the SPL, the IPS and the cerebellum when anticipating the stroke direction irrespective of the given instruction. We consider that these activation differences between experts and novices reflect the use of the more fine‐tuned motor representations that experts have acquired and improved through years of training. Moreover, we reported that different anticipation tasks with different instructions (motor vs. spatial) invoke activity in different neural networks. We demonstrated increased activation within the frontoparietal areas independent of expertise especially when participants had to decide on an appropriate response to the observed action compared to indicating the direction of the ball flight. It appears that the neural processing of different anticipation tasks depends on the expertise level. When compared to experts, novices revealed an increased activation within the ventral section of the PMC, the adjoining SMA proper, and the SPL (Area 5M) when contrasting the motor with spatial anticipation task. This indicates that a motor instruction especially supports a motor processing strategy within the novice's brain.
ACKNOWLEDGMENTS
The authors thank Stefan Kindermann, Fabian Helm, and Kristin Zimmermann for their help in stimuli recording and data acquisition; Bertram Walter for statistical support; Jörn Diedrichsen for advice on using the SUIT toolbox; and all participants for taking part in our study.
REFERENCES
- Abernethy B, Russell DG (1987): The relationship between expertise and visual search strategy in a racquet sport. Hum Mov Sci 6:283–319. [Google Scholar]
- Abernethy B, Gill DP, Parks SL, Packer ST (2001): Expertise and the perception of kinematic and situational probability information. Perception 30:233–252. [DOI] [PubMed] [Google Scholar]
- Abreu AM, Macaluso E, Azevedo RT, Cesari P, Urgesi C, Aglioti SM (2012): Action anticipation beyond the action observation network: A functional magnetic resonance imaging study in expert basketball players. Eur J Neurosci 35:1646–1654. [DOI] [PubMed] [Google Scholar]
- Aglioti SM, Cesari P, Romani M, Urgesi C (2008): Action anticipation and motor resonance in elite basketball players. Nat Neurosci 11:1109–1116. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Annella L, Candidi M, Urgesi C, Aglioti SM (2013): Compensatory plasticity in the action observation network: Virtual lesions of STS enhance anticipatory simulation of seen actions. Cereb Cortex 23:570–580. [DOI] [PubMed] [Google Scholar]
- Bastian AJ (2006): Learning to predict the future: The cerebellum adapts feedforward movement control. Curr Opin Neurobiol 16:645–649. [DOI] [PubMed] [Google Scholar]
- Beilock SL, Carr TH, MacMahon C, Starkes JL (2002): When paying attention becomes counterproductive: Impact of divided versus skill‐focused attention on novice and experienced performance of sensorimotor skills. J Exp Psychol Appl 8:6–16. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buccino G, Stephan KM, Rizzolatti G, Seitz RJ, Freund HJ (1999): A parieto‐premotor network for object manipulation: Evidence from neuroimaging. Exp Brain Res 128:210–213. [DOI] [PubMed] [Google Scholar]
- Bischoff M, Zentgraf K, Lorey B, Pilgramm S, Balser N, Baumgartner E, Hohmann T, Stark R, Vaitl D, Munzert J (2012): Motor familiarity: Brain activation when watching kinematic displays of one's own movements. Neuropsychologia 50:2085–2092. [DOI] [PubMed] [Google Scholar]
- Bishop DT, Wright MJ, Jackson RC, Abernethy B (2013): Neural bases for anticipation skill in soccer: an FMRI study. J Sport Exerc Psychol 35:98–109. [DOI] [PubMed] [Google Scholar]
- Blakemore S, Decety J (2001): From the perception of action to the understanding of intention. Nat Rev Neurosci 2:561–567. [DOI] [PubMed] [Google Scholar]
- Blakemore S, Frith C (2005): The role of motor contagion in the prediction of action. Neuropsychologia 43:260–267. [DOI] [PubMed] [Google Scholar]
- Bootsma RJ (1989): Accuracy of perceptual processes subserving different perception‐action systems. Q J Exp Psychol 41 A:489–500. [DOI] [PubMed] [Google Scholar]
- Buccino G, Vogt S, Ritzl A, Fink GR, Zilles K, Freund H, Rizzolatti G (2004): Neural circuits underlying imitation learning of hand actions: An event‐related fMRI study. Neuron 42:323–334. [DOI] [PubMed] [Google Scholar]
- Calvo‐Merino B, Ehrenberg S, Leung D, Haggard P (2010): Experts see it all: Configural effects in action observation. Psychol Res 74:400–406. [DOI] [PubMed] [Google Scholar]
- Calvo‐Merino B, Grèzes J, Glaser DE, Passingham RE, Haggard P, Gre J (2006): Seeing or doing? Influence of visual and motor familiarity in action observation. Curr Biol 16:1905–1910. [DOI] [PubMed] [Google Scholar]
- Cañal‐Bruland R, van Ginneken WF, van der Meer BR, Williams AM (2011): The effect of local kinematic changes on anticipation judgments. Hum Mov Sci 30:495–503. [DOI] [PubMed] [Google Scholar]
- Candidi M, Urgesi C, Ionta S, Aglioti SM (2008): Virtual lesion of ventral premotor cortex impairs visual perception of biomechanically possible but not impossible actions. Soc Neurosci 3:388–400. [DOI] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB (2010): ALE meta‐analysis of action observation and imitation in the human brain. NeuroImage 50:1148–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crognier L, Féry Y (2005): Effect of tactical initiative on predicting passing shots in tennis. Appl Cogn Psychol 19:637–649. [Google Scholar]
- Cross ES, Hamilton AFC , de Kraemer DJM , Kelley WM, Grafton ST (2009): Sensitivity of the action observation network to physical and observational learning. Cereb Cortex 19:315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond JE, Fiez JA (1998): Neuroimaging studies of the cerebellum: Language, learning and memory. Trends Cogn Sci 2:355–362. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Reilly KT, Richard N, Szathmari A, Mottolese C, Sirigu A (2009): Movement intention after parietal cortex stimulation in humans. Science 324:811–813. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J (2006): A spatially unbiased atlas template of the human cerebellum. NeuroImage 33:127–138. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N (2009): A probabilistic MR atlas of the human cerebellum. NeuroImage 46:39–46. [DOI] [PubMed] [Google Scholar]
- Dimitrova A, Greiff A , de Schoch B , Gerwig M, Frings M, Gizewski ER, Timmann D (2006): Activation of cerebellar nuclei comparing finger, foot and tongue movements as revealed by fMRI. Brain Res Bull 71:233–241. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K (2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage 25:1325–1335. [DOI] [PubMed] [Google Scholar]
- Farrow D, Abernethy B (2003): Do expertise and the degree of perception—Action coupling affect natural anticipatory performance? Perception 32:1127–1139. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Shah NJ, Weiss PH, Halligan PW, Grosse‐Ruyken M, Ziemons K, Zilles K, Freund HJ (2000): Line bisection judgments implicate right parietal cortex and cerebellum as assessed by fMRI. Neurology 54:1324–1331. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD (2004): Dissociable neural pathways for the perception and recognition of expressive and instrumental gestures. Neuropsychologia 42:1725–1736. [DOI] [PubMed] [Google Scholar]
- Gao J, Parsons LM, Bower JM, Xiong J, Li J, Fox PT (1996): Cerebellum implicated in sensory acquisition and discrimination rather than motor control. Science 272:545–547. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Keysers C (2009): The observation and execution of actions share motor and somatosensory voxels in all tested subjects: Single‐subject analyses of unsmoothed fMRI data. Cereb Cortex 19:1239–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzola V, Rizzolatti G, Wicker B, Keysers C (2007): The anthropomorphic brain: The mirror neuron system responds to human and robotic actions. NeuroImage 35:1674–1684. [DOI] [PubMed] [Google Scholar]
- Ghajar J, Ivry RB (2008): The predictive brain state: timing deficiency in traumatic brain injury? Neurorehab Neural Re 22:217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickstein M (1993): Motor skills but not cognitive tasks. Trends Neurosci 16:450–451. [DOI] [PubMed] [Google Scholar]
- Grodd W, Hülsmann E, Lotze M, Wildgruber D, Erb M (2001): Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum Brain Mapp 13:55–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot A, Collet C, Nguyen VA, Malouin F, Richards C, Doyon J (2009): Brain activity during visual versus kinesthetic imagery: An fMRI study. Hum Brain Mapp 30:2157–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes G (1939): The cerebellum of man. Brain 62:1–30. [Google Scholar]
- Hubert M, van der Veeken S (2008): Outlier detection for skewed data. J Chemometr 22:235–246. [Google Scholar]
- Huys R, Smeeton NJ, Hodges NJ, Beek PJ, Williams AM (2008): On the dynamic information underlying visual anticipation skill. Percept Psychophys 70:1217–1234. [DOI] [PubMed] [Google Scholar]
- Iacoboni M (2001): Playing tennis with the cerebellum. Nat Neurosci 4:555–556. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Molnar‐Szakacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G (2005): Grasping the intentions of others with one's own mirror neuron system. PLoS Biol 3:e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi S, Mori K, Kiritani S, Kawashima R, Sugiura M, Fukuda H, Itoh K, Kato T, Nakamura A, Hatano K, Kojima S, Nakamura K (1997): Vocal identification of speaker and emotion activates different brain regions. Neuroreport 8:2809–2812. [DOI] [PubMed] [Google Scholar]
- Imamizu H, Kawato M (2008): Neural correlates of predictive and postdictive switching mechanisms for internal models. J Neurosci 28:10751–10765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Pütz B, Yoshioka T, Kawato M (2000): Human cerebellar activity reflecting an acquired internal model of a new tool. Nature 403:192–195. [DOI] [PubMed] [Google Scholar]
- Jackson L, Chapman P, Crundall D (2009): What happens next? Predicting other road users' behaviour as a function of driving experience and processing time. Ergonomics 52:154–164. [DOI] [PubMed] [Google Scholar]
- Johnson‐Frey SH, Maloof FR, Newman‐Norlund R, Farrer C, Inati S, Grafton ST (2003): Actions or hand‐object interactions? Human inferior frontal cortex and action observation. Neuron 39:1053–1058. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Friston KJ, Frith CD (2007): Predictive coding: An account of the mirror neuron system. Cogn Process 8:159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koglbauer I, Kallus KW, Braunstingl R, Boucsein W (2011): Recovery training in simulator improves performance and psychophysiological state of pilots during simulated and real visual flight rules. Int J Aviat Psychol 21:307–324. [Google Scholar]
- Krams M, Rushworth MF, Deiber MP, Frackowiak RS, Passingham RE (1998): The preparation, execution and suppression of copied movements in the human brain. Exp Brain Res 120:386–398. [DOI] [PubMed] [Google Scholar]
- Kunde W, Skirde S, Weigelt M (2011): Trust my face: Cognitive factors of head fakes in sports. J Exp Psychol Appl 17:110–127. [DOI] [PubMed] [Google Scholar]
- Lotze M, Scheler G, Tan HM, Braun C, Birbaumer N (2003): The musician's brain: Functional imaging of amateurs and professionals during performance and imagery. NeuroImage 20:1817–1829. [DOI] [PubMed] [Google Scholar]
- McPherson SL, MacMahon C (2008): How baseball players prepare to bat: Tactical knowledge as a mediator of expert performance in baseball. J Sport Exerc Psychol 30:755–778. [DOI] [PubMed] [Google Scholar]
- McRobert AP, Ward P, Eccles DW, Williams AM (2011): The effect of manipulating context‐specific information on perceptual–cognitive processes during a simulated anticipation task. Br J Psychol 102:519–534. [DOI] [PubMed] [Google Scholar]
- Mecklinger A, Gruenewald C, Besson M, Magnié M, von Cramon DY (2002): Separable neuronal circuitries for manipulable and non‐manipulable objects in working memory. Cereb Cortex 12:1115–1123. [DOI] [PubMed] [Google Scholar]
- Miall RC (2003): Connecting mirror neurons and forward models. Neuroreport 14:2135–2137. [DOI] [PubMed] [Google Scholar]
- Miall RC, King D (2008): State estimation in the cerebellum. Cerebellum 7:572–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs P, Cunnington R, Mattingley JB (2012): Brain regions with mirror properties: A meta‐analysis of 125 human fMRI studies. Neurosci Biobehav Rev 36:341–349. [DOI] [PubMed] [Google Scholar]
- Müller S, Abernethy B, Farrow D (2006): How do world‐class cricket batsmen anticipate a bowler's intention? Q J Exp Psychol 59:2162–2186. [DOI] [PubMed] [Google Scholar]
- Munzert J, Zentgraf K (2009): Motor imagery and its implications for understanding the motor system. Prog Brain Res 174:219–229. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- O'Reilly JX, Mesulam MM, Nobre AC (2008): The cerebellum predicts the timing of perceptual events. J Neurosci 28:2252–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen‐Berg H (2010): Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex 20:953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oztop E, Wolpert D, Kawato M (2005): Mental state inference using visual control parameters. Cognitive Brain Res 22:129–151. [DOI] [PubMed] [Google Scholar]
- Penny W, Henson R (2007): Analysis of variance In: Friston K, Ashburner J, Kiebel S, Nichols T, Penny W, editors. Statistical Parametric Mapping: The Analysis of Functional Brain Images. Amsterdam: Academic Press; pp 166–177; Chapter 13. [Google Scholar]
- Pilgramm S, Lorey B, Stark R, Munzert J, Vaitl D, Zentgraf K (2010): Differential activation of the lateral premotor cortex during action observation. BMC Neurosci 11:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobric G, Hamilton AFdC (2006): Action understanding requires the left inferior frontal cortex. Curr Biol 16:524–529. [DOI] [PubMed] [Google Scholar]
- Prinz W (2006): What re‐enactment earns to us. Cortex 42:515–517. [DOI] [PubMed] [Google Scholar]
- Ramnani N (2006): The primate cortico‐cerebellar system: anatomy and function. Nat Rev Neurosci 7:511–522. [DOI] [PubMed] [Google Scholar]
- Rao SM, Bobholz JA, Hammeke TA, Rosen AC, Woodley SJ, Cunningham JM, Cox RW, Stein EA, Binder JR (1997): Functional MRI evidence for subcortical participation in conceptual reasoning skills. Neuroreport 8:1987–1993. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V (2002): Motor and cognitive functions of the ventral premotor cortex. Curr Opin Neurobiol 12:149–154. [DOI] [PubMed] [Google Scholar]
- Rowe RM, McKenna FP (2001): Skilled anticipation in real‐world tasks: Measurement of attentional demands in the domain of tennis. J Exp Psychol Appl 7:60–67. [DOI] [PubMed] [Google Scholar]
- Schlösser R, Hutchinson M, Joseffer S, Rusinek H, Saarimaki A, Stevenson J, Dewey SL, Brodie JD (1998): Functional magnetic resonance imaging of human brain activity in a verbal fluency task. J Neurol Neurosurg Psychiatr 64:492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Macmore J, Vangel M (2009): Cerebellar stroke without motor deficit: Clinical evidence for motor and non‐motor domains within the human cerebellum. Neuroscience 162:852–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubotz RI (2007): Prediction of external events with our motor system: Towards a new framework. Trends Cogn Sci 11:211–218. [DOI] [PubMed] [Google Scholar]
- Schütz‐Bosbach S, Prinz W (2007): Perceptual resonance: Action‐induced modulation of perception. Trends Cogn Sci 11:349–355. [DOI] [PubMed] [Google Scholar]
- Shmuelof L, Zohary E (2007): Watching others' actions: Mirror representations in the parietal cortex. Neuroscientist 13:667–672. [DOI] [PubMed] [Google Scholar]
- Singer RN, Cauraugh JH, Chen D, Steinberg GM (1996): Visual search, anticipation, and reactive comparisons between highly‐skilled and beginning tennis players. J Appl Sport Psychol 8:9–26. [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen‐Berg H, Bannister PR, Luca M , de Drobnjak I , Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, Stefano N , de Brady JM , Matthews PM (2004): Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23 (Suppl 1):208–219. [DOI] [PubMed] [Google Scholar]
- Sokolov AA, Gharabaghi A, Tatagiba MS, Pavlova M (2010): Cerebellar engagement in an action observation network. Cereb Cortex 20:486–491. [DOI] [PubMed] [Google Scholar]
- Stadler W, Schubotz RI, Cramon DY , von Springer A , Graf M, Prinz W (2011): Predicting and memorizing observed action: Differential premotor cortex involvement. Hum Brain Mapp 32:677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD (2009): Functional topography in the human cerebellum: A meta‐analysis of neuroimaging studies. NeuroImage 44:489–501. [DOI] [PubMed] [Google Scholar]
- Synofzik M, Lindner A, Thier P (2008): The cerebellum updates predictions about the visual consequences of one's behavior. Curr Biol 18:814–818. [DOI] [PubMed] [Google Scholar]
- Tunik E, Rice NJ, Hamilton A, Grafton ST (2007): Beyond grasping: Representation of action in human anterior intraparietal sulcus. NeuroImage 36 (Suppl 2):T77–T86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urgesi C, Calvo‐Merino B, Haggard P, Aglioti SM (2007): Transcranial magnetic stimulation reveals two cortical pathways for visual body processing. J Neurosci 27:8023–8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urgesi C, Savonitto MM, Fabbro F, Aglioti SM (2011): Long‐ and short‐term plastic modeling of action prediction abilities in volleyball. Psychol Res 76:542–560. [DOI] [PubMed] [Google Scholar]
- Ward P, Williams AM, Bennett SJ (2002): Visual search and biological motion perception in tennis. Res Q Exerc Sport 73:107–112. [DOI] [PubMed] [Google Scholar]
- Williams AM, Davids K, Williams JG (1999): Visual perception and action in sport. London: E.& F.N. Spon. [Google Scholar]
- Williams AM, Ward P, Knowles JM, Smeeton NJ (2002): Anticipation skill in a real‐world task: Measurement, training, and transfer in tennis. J Exp Psychol Appl 8:259–270. [DOI] [PubMed] [Google Scholar]
- Williams AM, Huys R, Cañal‐Bruland R, Hagemann N (2009): The dynamical information underpinning anticipation skill. Hum Mov Sci 28:362–370. [DOI] [PubMed] [Google Scholar]
- Williams AM, Ford PR, Eccles DW, Ward P (2011): Perceptual‐cognitive expertise in sport and its acquisition: Implications for applied cognitive psychology. Appl Cogn Psychol 25:432–442. [Google Scholar]
- Winstein CJ, Grafton ST, Pohl PS, Angeles L (1997): Motor task difficulty and brain activity: Investigation of goal‐directed reciprocal aiming using positron emission tomography. J Neurophysiol 77:1581–1594. [DOI] [PubMed] [Google Scholar]
- Wolfensteller U (2009): Juggling with the brain—Thought and action in the human motor system. Prog Brain Res 174:289–301. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, Kawato M (1998): Internal models in the cerebellum. Trends Cogn Sci 2:338–347. [DOI] [PubMed] [Google Scholar]
- Worsley K (2007): Random field theory In: Friston K, Ashburner J, Kiebel S, Nichols T, Penny W, editors. Statistical Parametric Mapping: The Analysis of Functional Brain Images. Amsterdam: Academic Press; pp 232–236; Chapter 18. [Google Scholar]
- Wright MJ, Jackson RC (2007): Brain regions concerned with perceptual skills in tennis: An fMRI study. Int J Psychophysiol 63:214–220. [DOI] [PubMed] [Google Scholar]
- Wright MJ, Bishop DT, Jackson RC, Abernethy B (2010): Functional MRI reveals expert‐novice differences during sport‐related anticipation. Neuroreport 21:94–98. [DOI] [PubMed] [Google Scholar]
- Zentgraf K, Stark R, Reiser M, Künzell S, Schienle A, Kirsch P, Walter B, Vaitl D, Munzert J (2005): Differential activation of pre‐SMA and SMA proper during action observation: Effects of instructions. NeuroImage 26:662–672. [DOI] [PubMed] [Google Scholar]
- Zentgraf K, Munzert J, Bischoff M, Newman‐Norlund RD (2011): Simulation during observation of human actions—Theories, empirical studies, applications. Vis Res 51:827–835. [DOI] [PubMed] [Google Scholar]
- Zheng B, Swanström LL, MacKenzie CL (2007): A laboratory study on anticipatory movement in laparoscopic surgery: A behavioral indicator for team collaboration. Surg Endosc 21:935–940. [DOI] [PubMed] [Google Scholar]


