Abstract
Physiological and emotional stressors are associated with or provoke each migraine attack and cause structural and functional changes in the central nervous system. The hippocampus, a limbic structure important in anxiety‐related behavior, is vulnerable to long‐term stress. Given that catechol‐O‐methyltransferase (COMT) is widely distributed in the hippocampus and its genetic variation is thought to contribute to the interindividual variability in pain perception and anxiety regulation, whether or not migraine and COMT val158met genotype have an interactive effect in the key brain area related to maladaptive stress, the hippocampus, is still poorly understood. Using T1‐weighted and resting functional MRI, we evaluated the effect of COMT genetic variations on migraine and possible interactions between COMT and the disease in brain structure and function in 135 females with migraine without aura (MWoA) and 111 matched health controls (HC). Optimized voxel‐based morphometry (VBM) and functional connectivity (FC) analyses were applied. From the whole brain VBM analysis, we found a significant disease × genotype interaction in the hippocampus, which overlapped with disease‐related increase of gray matter (GM) in val homozygote migraineurs. In our results, increased GM in the hippocampus was only found in val homozygote MWoA compared to val homozygote HC. Moreover, FC between the hippocampus and the medial prefrontal cortex was significantly decreased in val homozygotes, and it was negatively correlated with self‐rating anxiety scale values.Our results indicated that brain structure and function of the hippocampus are differentially affected by migraine in val homozygotes compared with met carriers. Hum Brain Mapp 36:1782–1795, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: migraine, catechol‐O‐methyltransferase, structural change, functional connectivity, hippocampus
INTRODUCTION
Catechol‐O‐methyltransferase (COMT) is an enzyme that inactivates catecholamines, including noradrenaline, adrenaline and important neurotransmitters dopamine. The rs4680 variant in the COMT gene causes a substitution from a valine (val) to a methionine (met) at amine acid position 158 (val158met), leading to a significant decrease (three‐ to four‐times) in enzyme activity and increased dopamine availability [Bilder et al., 2004; Männistö and Kaakkola, 1999]. Recently, several neuroimaging studies have investigated the association between the effects of COMT val158met polymorphism and changes in synaptic dopamine availability, and they pointed out that COMT expression may be widely distributed in the hippocampus [Matsumoto et al., 2003a, 2003b]. Extensive evidence from animal models [Costa et al., 2012; Lisman and Grace, 2005] and human studies [Adcock et al., 2006; Pessiglione et al., 2006; Wittmann et al., 2005] suggested there was an interaction between dopamine transmission and hippocampal synaptic plasticity, which plays a critical role in cognitive and affective behavioral processes [Ghiglieri et al., 2011; Johnson et al., 2007; Shohamy and Adcock, 2010]. The proposed interrelationships among hippocampal functions, anxiety and emotional stressors, and COMT genetic variations are novel and little information is available regarding this interaction relationship in clinical states.
Migraine, as the most common neurological disease, is characterized by intermittent headache attacks that can be viewed as a number of repeated physiological and emotional stressors [Borsook et al., 2012]. While accompanied by light to heavy and infrequent to frequent headaches, migraine would cause selective alterations in gray matter (GM) and white matter (WM) involved in central pain processing system [Liu et al., 2013; Yu et al., 2013]. As the hippocampus plays a key role in providing inhibitory feedback to the hypothalamic‐pituitary‐adrenal axis that controls the reaction to stress [Gerritsen et al., 2011; Jacobson and Sapolsky, 1991], the functional changes in this region may be critical when facing uncertainty about upcoming pain [Ploghaus et al., 2000], exacerbation of pain by anticipatory anxiety [Ploghaus et al., 2001] and pain‐related negative emotions [Roy et al., 2009]. Morphometric results found a significantly larger bilateral hippocampal volume in low frequency migraine suffers as compared with healthy controls and high frequency migraineurs, and significantly higher deactivation responses in bilateral hippocampus were found for noxious stimulation in low frequency patients relative to high frequency patients [Maleki et al., 2013]. These results may reflect a contribution of hippocampal function during stress processing under the chronic pain condition [Maleki et al., 2013].
Zubieta et al. first pointed out that polymorphisms in COMT may influence the experience of pain and pain‐related brain responses in healthy volunteers [Zubieta et al., 2003]. Although COMT activity does not exhibit pain vulnerability to migraine [Tammimäki and Männistö, 2012], interindividual differences in COMT activity influenced the clinical response to drugs used for migraine treatment [Cargnin et al., 2013]. Additionally, the hippocampal formation is involved with anxiety‐related behavior and regulation of the stress response [Vachon‐Presseau et al., 2013], which is the common factor influenced by the COMT val158met polymorphism and the pathologic process of migraine. Hence, we hypothesized that migraine and COMT val158met may have an interactive effect in the hippocampus, which may be related to maladaptive stress in chronic pain.
In the current study, we evaluated the variation and possible interactions between COMT and the disease in the structural volumetric morphology in the patients with migraine without aura (MWoA) and gender‐, age‐, and education‐matched healthy controls (HC). Voxel‐based morphometry (VBM) and functional connectivity (FC) analyses were used.
MATERIALS AND METHODS
Much evidence showed that COMT genetic variations contributed to pain behaviors in mice and pain ratings in humans in a sex‐specific manner [Belfer et al., 2013]. Meanwhile, our group considered gender‐related differences for the resting state FC in migraine sufferers, and we found that migraine had a greater influence in females and led to more dysfunctional brain activity in their resting functional networks compared to males [Liu et al., 2011]. To avoid gender differences in the present study, only female subjects were enrolled in the current study.
All research procedures were approved by the West China Hospital Subcommittee on Human Studies and were conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from each participant.
Participants
Inclusion criteria for the migraine patient group were according to ICHD‐III (2013): (1) featuring at least two of the following characteristics: unilateral location, pulsating quality, and moderate‐to‐severe pain intensity, (2) headache attacks lasting 4–72 h (untreated or unsuccessfully treated), (3) presence of nausea and/or vomiting, photophobia, and phonophobia during the headache, (4) and the headache being disabling. The exclusion criteria were: (1) macroscopic brain T2‐visible lesions on MRI scans, (2) existence of a neurological disease, (3) pregnancy; (4) alcohol, nicotine or drug abuse, or (5) claustrophobia. All patients had no migraine 72 h prior to the scan and no symptoms of developing one attack during or 24 h after the scan.
One hundred and thirty five right‐handed MWoA who did not have any clinical affective disorder were recruited [female, 21.7 ± 2.1 years (mean age ± SD)]. One hundred and eleven age‐, education‐, and gender‐matched, healthy, right‐handed healthy controls (HC, age 21.3 ± 0.9 years) were recruited from the local community. The controls had experienced no headache during the previous year and had no family members who suffered regularly from a migraine or other headaches.
The drugs used for the prophylaxis of migraine were stopped 4 weeks before the experiment; however, the patients were allowed to take pirprofen when their headache was difficult to endure. Detailed information about the patients' drug intake was recorded.
Genotyping
Genomic deoxyribose nucleic acid (DNA) was isolated from 500 μL of whole blood using a TIANamp genomic DNA kit according to the manufacturer's instructions (TIANGEN BIOTECH, Beijing, China). Polymerase chain reaction (PCR) was carried out in a volume of 20 μL containing 10 ng of genomic DNA, 2.5 μL of 10×Taq buffer, 2 μL of dNTP Mixture (2.5 mM), 0.5 μL of Taq DNA Polymerase (2.5 U/μL, TIANGEN BIOTECH), 1 μL of each primer and ddH2O. PCRs were done using a BIO‐RAD cycle system. The primers were as follows: COMT, 5′‐CGAGATCAACCCCGACTGT‐3′ (forward), 5′‐CAGGCATGCACACCTTGTC‐3′ (reverse). The amplification conditions were initiated at 94°C for 5 min, followed by 30 cycles consisting of denaturation at 94°C for 30 s, annealing at 65°C for 30 s and extension at 72°C for 30 s with a final extension step of 5 min at 72°C. The PCR products were confirmed by 2% gel electrophoresis and purified by TIANgel Midi purification kit (TIANGEN BIOTECH). COMT gene sequence was implemented in Shanghai Bio‐tech (Shanghai, China). Genotypes were read by at least two researchers who were blinded to the experiment.
Headache Activity and Mood Rating
In the past 4 weeks before the MRI scan, all patients were required to keep a headache diary to record the pain intensity of the attacks (0–10 scale, with 10 being the most intense pain imaginable), migraine attack frequency (number of times), and migraine attack duration (hours). The Zung self‐rating anxiety scale (SAS) and Zung self‐rating depression scale (SDS) were used to quantify the anxiety/depression‐related symptoms of the patients. Both of the scales consisted of 20 items, and each item was scored from 1 to 4.
Imaging Acquisition
This experiment was carried out in a 3.0‐Tesla Signa GE scanner (GE Healthcare, Milwaukee, WI) with an 8‐channel phase array head coil at the Huaxi MR Research Center in Sichuan, China. For each subject, a high‐resolution structural image was acquired using a three‐dimensional MRI sequence with a voxel size of 1 × 1 × 1mm3 using an axial fast spoiled gradient recalled sequence with the following parameters: repetition time (TR) = 1,900 ms; echo time (TE) = 2.26 ms; data matrix = 256 × 256; field of view (FOV) = 256 × 256 mm2.
The functional images were obtained with an EPI (30 continuous slices with a slice thickness = 5 mm, TR = 2,000 ms, TE = 30 ms, FA = 90°, FOV =240 × 240 mm2, data matrix = 64 × 64). For each subject, a total of 205 volumes were acquired, resulting in a total scan time of 410 s. Subjects were instructed to rest with their eyes closed, not to think about anything in particular, and not to fall asleep. After the scan, the subjects were asked whether they remained awake during the whole procedure.
Voxel‐Based Morphometry Analysis
Two professional radiologists examined all subjects' structural images to exclude the possibility of clinically silent lesions. Structural data were analyzed with the FSL‐VBM protocol with the FMRIB Software Library (FSL) 4.1 software (http://fsl.fmrib.ox.ac.uk/fsl). First, the brain extracting tool (BET) was used to extract brain from all T1 images [Smith, 2002]. Second, tissue‐type segmentation was carried out using the FMRIB's automated segmentation tool (FAST) V4.1 [Zhang et al., 2001]. The resulting GM partial volume images were then aligned to MNI152 standard space using the FMRIB's linear image registration tool (FLIRT) [Jenkinson and Smith, 2001; Jenkinson et al., 2002], followed by nonlinear registration using FMRIB's nonlinear image registration tool as optional (FNIRT). Third, the resulting images were averaged to create a study‐specific template to which the native GM images were nonlinearly reregistered. The optimized protocol introduced a modulation for the contraction/enlargement caused by the nonlinear component of the transformation: each value of the voxel in the registered GM image was divided by the Jacobian of the warp field. Fourth, the modulated GM images were then smoothed with an isotropic Gaussian kernel with a sigma of 4 mm.
We determined that the GM in MWoA and HC implied that disease and genetic variation were related to morphometric differences, and the F‐test for two‐way ANOVA was performed using FEAT in FSL. The test evaluated the hypothesis that genetic variation, disease state and interaction effects were all the same against the alternative that they were not. More attention was focused on the significant interactions between COMT genetic variations and migraine, and we performed a test to determine which pairs of effects were significantly different. Correction for multiple comparisons was carried out using a cluster‐based thresholding method, with an initial cluster forming at a threshold of t = 2.0 [Smith and Nichols, 2009].
FC Analyses
Functional data was analyzed with both FSL and Automated Functional Neuro‐Imaging (http://afni.nimh.nih.gov/afni) software. Scripts containing the processing commands used in the current study have been released as part of the 1,000 Functional Connectomes Project (http://www.nitrc.org/projects/fcon_1000) [Biswal et al., 2010]. A standard data preprocessing strategy was performed. Specifically, (1) the first 5 echo‐planar imaging volumes were discarded to allow subjects to get used to the scanning environment and eliminate nonequilibrium effects of magnetization; (2) the remaining images were corrected by slice timing, 3‐dimensional head motion correction, time series despiking, spatial smoothing and 4‐dimensional mean based intensity normalization; (3) the time series from each voxel in the corrected images was temporally filtered with a band‐pass filter (0.01–0.08 Hz) and removed the linear and quadratic trends; (4) the remaining images were spatially normalized to the Montreal Neurological Institute 152 space and resampled to 2‐mm isotropic voxels; and (5) eight nuisance signals (WM, cerebrospinal fluid signals and six motion parameters) were regressed out.
FC analysis was performed using a method based on a seeding voxel correction approach [Lui et al., 2009]. The brain regions interacting with COMT genetic variations and migraine in VBM results were selected as the seed regions. In our study, the voxel cluster of hippocampus (left) whose corresponding P values passed the family‐wise error (FWE) correction (P < 0.03) were chosen (see results of interaction effect for disease × genotype). The individual seed connectivity map (Fisher's r‐to‐z transformation) was calculated by correlating the mean seed region time course with all other voxels in the brain. A two‐sample t‐test was used to evaluate the between‐group differences. False discovery rate (FDR) was used to correct the multiple comparisons.
Relation between Brain Alteration and SAS/SDS Values
We used Pearson's correlation coefficient to evaluate the relationship between the brain alteration (GM density and FC intensity) and SAS/SDS values for each patient group.
Head‐Motion Calculations
Subject head motion would lead to the changes in the time courses of resting state FC data, which may cause systematic but spurious correlation structures despite compensatory spatial registration and regression of motion estimates from the data [Power et al., 2012]. Here, to test whether our observations would be held when consider the effects of head motion, we eliminated volumes from each subject's rs‐fMRI time series that were associated with sudden head motion. Specifically, an index of framewise displacement (FD), calculated using the Euclidean norm for the six rigid‐body motion parameters, was applied to mark the volumes that tended to behave as burst noise. This would result temporal masks for our data and similar approaches have been used in several previous rs‐fMRI studies [Fair et al., 2012; Power et al., 2012]. For each subject, the flag volume was censored if its derivative values above 0.5 [Power et al., 2012].
RESULTS
Psychophysical and Biometric Results
As can be seen from Table 1, the genotype distribution in MWoA and HC did not deviate from those expected based on the Hardy–Weinberg equilibrium, and the allele frequencies did not differ significantly between migraine suffers and controls. Patients included in the analysis were matched for age, age of onset, gender, medication type, and disease duration. When merging the met homozygotes and met heterozygotes into a group of met carriers, there were no significant between‐group differences in migraine attack frequency, average pain intensity and attack duration (P > 0.05, Table 2).
Table 1.
The number of samples collected and distributions of COMT Val158Met polymorphism
| Subjects | N (%) | COMT genotypes (%) | Allele (%) | |||
|---|---|---|---|---|---|---|
| val/val | val/met | met/met | val | met | ||
| Healthy | 111 (45.12) | 54 (48.65) | 48 (43.24) | 9 (8.11) | 156 (70.27) | 66 (29.73) |
| Patients | 135 (54.88) | 77 (57.04) | 55 (40.74) | 3 (2.22) | 209 (77.41) | 61 (22.59) |
| Total | 246 (100.00) | 131 (53.25) | 103 (41.87) | 12 (4.88) | 365 (74.19) | 127 (25.81) |
Table 2.
Demographic characteristics of patients
| Information | Patients with migraine | P‐value | |
|---|---|---|---|
| val homozygotes (n = 77) | met carriers (n = 58) | ||
| Age (years) | 22.0 ± 1.9 | 21.4 ± 2.3 | 0.8 |
| Education (years) | 15.0 ± 1.1 | 14.7 ± 1.9 | 0.8 |
| Disease duration (months) | 62.6 ± 31.9 | 72.9 ± 37.6 | 0.2 |
| Migraine attacks during past four weeks | |||
| Attack frequency (times) | 6.6 ± 5.0 | 6.1 ± 5.1 | 0.7 |
| Average pain intensity (0–10) | 5.7 ± 1.4 | 5.7 ± 2.5 | 0.9 |
| Attack duration (hours) | 9.1 ± 11.8 | 7.0 ± 5.1 | 0.4 |
| SAS | 48.6 ± 8.2 | 41.7 ± 6.7 | <0.05 |
| SDS | 46.9 ± 12.1 | 45.7 ± 7.6 | 0.6 |
No significant differences in SAS and SDS were found between the HC groups (P > 0.05). The patient groups exhibited significantly higher SAS and SDS values as compared to each cohort HC group. Moreover, the val homozygote patient group showed higher SAS values than met carriers in MWoA (P < 0.05, Bonferroni corrected, Table 2), but no significant differences were found in SDS between patient groups (P >0.05, Table 2).
The Main Effect of Disease and Genotype
For the main effect of disease, significant GM increased were found in the hippocampus, parahippocampal gyrus, amygdala, cerebellum, and the occipital cortices in the MWoA as compared with the HC (P < 0.05, FWE corrected, Fig. 1A and Table 3); significant GM decreased were found in the middle frontal gyrus, superior frontal gyrus, inferior parietal lobule, supramarginal gyrus, temporal cortices, and the occipital cortices in the MWoA as compared with the HC (P < 0.05, FWE corrected, Fig. 1B and Table 3).
Figure 1.
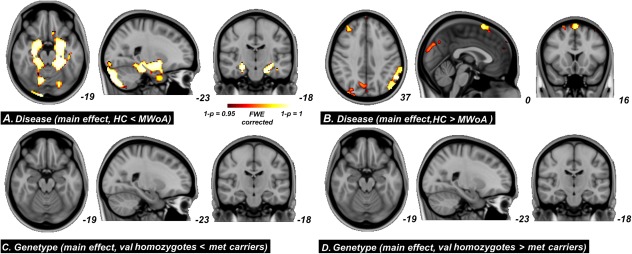
For the main effect of disease, significant GM increased were found in the hippocampus, parahippocampal gyrus, amygdala, cerebellum, and the occipital cortices in the MWoA as compared with the HC (P < 0.05, FWE corrected, (A); significant GM decreased were found in the middle frontal gyrus, superior frontal gyrus, inferior parietal lobule, supramarginal gyrus, temporal cortices, and the occipital cortices in the MWoA as compared with the HC (P < 0.05, FWE corrected, (B). For the main effect of genotype, no significant between‐group differences were found (C and D). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 3.
Main effect for ANOVA analysis (P < 0.05, FWE corrected)
| Brain Region | Side | Cluster Sizes | Voxels with maximum effect | |||
|---|---|---|---|---|---|---|
| Talairach | P‐value | |||||
| x | y | z | ||||
| (A) Disease (main effect, HC<MWoA) | ||||||
| Cerebellum | L | 3285 | −18 | −89 | −22 | 0.0002 |
| R | 2241 | 20 | −88 | −21 | 0.0004 | |
| Parahippocampal Gyrus | L | 764 | −26 | −30 | −15 | 0.0004 |
| R | 417 | 18 | 1 | −17 | 0.001 | |
| Hippocampus | L | 63 | −28 | −20 | −12 | 0.0010 |
| R | 67 | 28 | −20 | −12 | 0.0012 | |
| Amygdala | L | 131 | −26 | −7 | −18 | 0.0004 |
| R | 130 | 26 | −7 | −18 | 0.0016 | |
| Occipital cortices | L | 208 | −22 | −88 | −17 | 0.0010 |
| R | 207 | 22 | −88 | −17 | 0.0010 | |
| (B) Disease (main effect, HC>MWoA) | ||||||
| Middle Frontal Gyrus | L | 31 | −48 | 16 | 47 | 0.0400 |
| R | 369 | 34 | 35 | 37 | 0.0092 | |
| Superior Frontal Gyrus | L | 81 | −2 | 19 | 60 | 0.0100 |
| R | 519 | 4 | 17 | 60 | 0.0110 | |
| Inferior Parietal Lobule | L | 610 | −53 | −53 | 38 | 0.0028 |
| R | ||||||
| Precuneus | L | 29 | −42 | −72 | 35 | 0.0102 |
| R | 198 | 34 | −78 | 41 | 0.0162 | |
| Supramarginal Gyrus | L | 200 | −59 | −53 | 34 | 0.0028 |
| R | ||||||
| Inferior Temporal Gyrus | L | 42 | −63 | −11 | −18 | 0.0268 |
| R | ||||||
| Middle Temporal Gyrus | L | 117 | −53 | −63 | 22 | 0.0084 |
| R | ||||||
| Superior Temporal Gyrus | L | 131 | −51 | −57 | 23 | 0.0082 |
| R | ||||||
| Occipital cortices | L | 40 | −2 | −82 | 34 | 0.0270 |
| R | 55 | 2 | −82 | 34 | 0.0300 | |
| (C) Genotype (main effect, val homozygotes>met carriers) No between‐group differences | ||||||
| (D) Genotype (main effect, val homozygotes<met carriers) No between‐group differences | ||||||
For the main effect of genotype, no significant between‐group differences were found (Fig.1C,D).
Interaction Effect for Disease × Genotype
From the whole brain ANOVA analysis, we found a significant disease × genotype interaction in the hippocampus bilaterally (P = 0.03, FWE corrected, Fig. 2), which overlapped with disease‐related increase of GM in val homozygote migraineurs.
Figure 2.
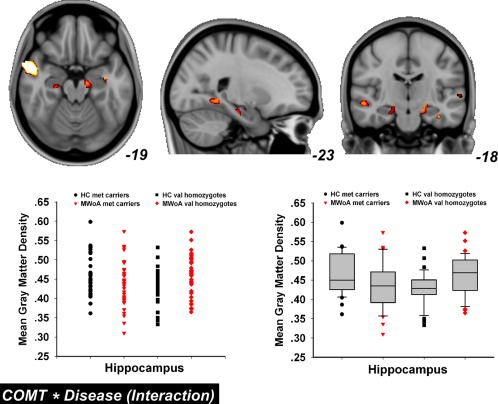
The disease × genotype interaction effect. From the whole brain ANOVA analysis, we found a significant interaction in the hippocampus bilaterally (P = 0.03, FWE corrected). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
No clear association between GM density and SAS/SDS value in val homozygote MWoA and/or other subject groups was found.
Post Hoc Analysis
Disease‐related differences were evaluated in each cohort separately. The HC versus MWoA comparisons in val homozygotes and met carrier subjects are shown in Figure 3. Significant disease‐related differences in val homozygote subjects were in the hippocampus, parahippocampal gyrus, amygdala, cerebellum, angular gyrus, and the occipital cortex (P < 0.05, FWE corrected, Fig. 3A,B, Table 4). The same comparison in the met carrier subjects exhibited more brain regions with GM alterations between HC versus MWoA including the medial prefrontal cortex (mPFC), mid cingulate cortex (MCC), orbitofrontal cortex (OFC), supplementary motor area, and the primary (SI) and secondary somatosensory cortices (P < 0.05, FWE corrected, Fig. 3C,D and Table 4). Within all these structural changes, increased GM was only found in val homozygote MWoA.
Figure 3.
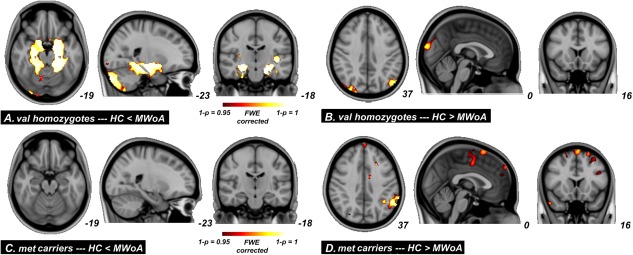
Significant between group differences from whole‐brain GM volume comparisons conducted on healthy versus migraine in val homozygotes and met carriers subjects. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 4.
Disease‐related difference in brain gray matter (P < 0.05, FWE corrected)
| Brain region | Side | Cluster sizes | Voxels with maximum effect | |||
|---|---|---|---|---|---|---|
| Talairach | P‐value | |||||
| x | y | z | ||||
| (A) val homozygotes (HC<MWoA) | ||||||
| Cerebellum | L | 3272 | −20 | −42 | −16 | 0.0002 |
| R | 2252 | 20 | −42 | −16 | 0.0008 | |
| Hippocampus | L | 74 | −24 | −11 | −21 | 0.0004 |
| R | 69 | 26 | −14 | −18 | 0.0006 | |
| Amygdala | L | 131 | −22 | −9 | −18 | 0.0002 |
| R | 124 | 28 | −4 | −12 | 0.0006 | |
| Parahippocampal Gyrus | L | 714 | −18 | −13 | −21 | 0.0002 |
| R | 514 | 24 | −18 | −19 | 0.0008 | |
| Occipital cortices | L | 128 | −14 | −43 | −3 | 0.0040 |
| R | 358 | 30 | −90 | −17 | 0.0050 | |
| (B) val homozygotes (HC>MWoA) | ||||||
| Occipital cortices | L | 19 | −2 | −92 | 18 | <0.0001 |
| R | 36 | 2 | −92 | 18 | <0.0001 | |
| Angular Gyrus | L | 63 | −51 | −66 | 31 | <0.0001 |
| (C) met carriers (HC<MWoA) No between‐group differences | ||||||
| (D) met carriers (HC>MWoA) | ||||||
| Medial frontal gyrus | L | 14 | −2 | 0 | 48 | 0.0024 |
| R | 32 | 4 | −3 | 48 | 0.0270 | |
| Middle frontal gyrus | L | 185 | −24 | 21 | 38 | 0.0043 |
| R | 64 | 36 | 37 | 39 | 0.0370 | |
| Superior frontal gyrus | L | 313 | −22 | 27 | 45 | 0.0038 |
| R | 24 | 4 | 19 | 62 | 0.0080 | |
| Precentral gyrus | L | 80 | −46 | −13 | 49 | 0.0012 |
| R | ||||||
| Mid cingulate | L | 196 | −6 | −4 | 43 | 0.0048 |
| R | 17 | 4 | −4 | 46 | 0.0276 | |
| Precuneus | L | 24 | −16 | −44 | 50 | 0.0400 |
| R | ||||||
| Inferior parietal lobule | L | 740 | −46 | −50 | 41 | 0.0030 |
| R | ||||||
| Postcentral gyrus | L | 52 | −40 | −19 | 51 | 0.0106 |
| R | ||||||
| Supramarginal gyrus | L | 317 | −57 | −55 | 36 | 0.0088 |
| R | ||||||
| Inferior temporal gyrus | L | 28 | −57 | −7 | −15 | 0.0079 |
| R | 24 | 59 | −5 | −17 | 0.0054 | |
| Middle temporal gyrus | L | 267 | −53 | −9 | −15 | 0.0031 |
| R | 293 | 53 | −3 | −12 | 0.0074 | |
| Superior temporal gyrus | L | 291 | −59 | −57 | 25 | 0.0020 |
| R | 24 | 48 | 1 | −14 | 0.0051 | |
The genotype‐related differences in MWoA and HC are presented in Figure 4. At the whole‐brain level, we found significant GM changes in the hippocampus in val homozygotes relative to met carriers in HC (P < 0.05, cluster size >10, FWE corrected, Fig. 4A,B). No significant GM changes were found in MWoA for val homozygotes versus met carriers comparisons (Fig. 4C,D).
Figure 4.
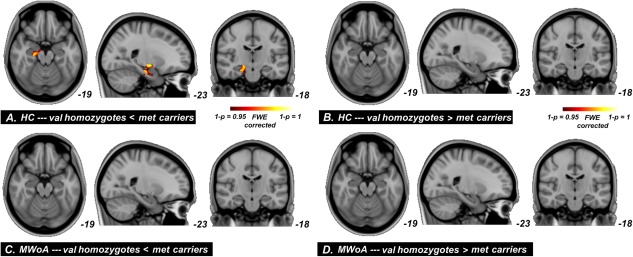
Significant between group differences from whole‐brain GM volume comparisons conducted on val homozygotes versus met carriers in healthy controls and migraineurs. As compare to each cohort separately, significant GM volume reduction was observed in the hippocampus in val homozygotes relative to met carriers in HC, and the other comparisons did not shown any significant difference. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
FC Analysis
In our results, the hippocampal volumes exhibited a significant interaction effect which was chosen as the seed for the resting FC analysis. Genotype‐related differences in brain FC were assessed, but no significant interaction effects were found. As shown in Figure 5 and Table 5, a significantly distinct hippocampal‐related connection pattern was found in different groups of MWoA. Specifically, as compared to each cohort separately, significantly decreased FC of the hippocampus were observed in the anterior cingulate cortex (ACC), MCC, insula, amygdala, thalamus, mPFC, dlPFC, OFC, precentral gyrus, and postcentral gyrus in val homozygote MWoA (P < 0.05, FDR corrected, Fig. 5A), and no significantly increased FC were found; significantly increased FC of the hippocampus were detected in the precentral gyrus, posterior cingulated, precuneus, and occipital cortices in met carriers MWoA (P < 0.05, FDR corrected, Fig. 5B), and no significantly decreased FC were found. The FC between the hippocampus and mPFC exhibited a negative correlation with SAS in val homozygote MWoA (P < 0.05, Bonferroni corrected), and no significant correlation was found in met carrier MWoA (Fig. 6).
Figure 5.
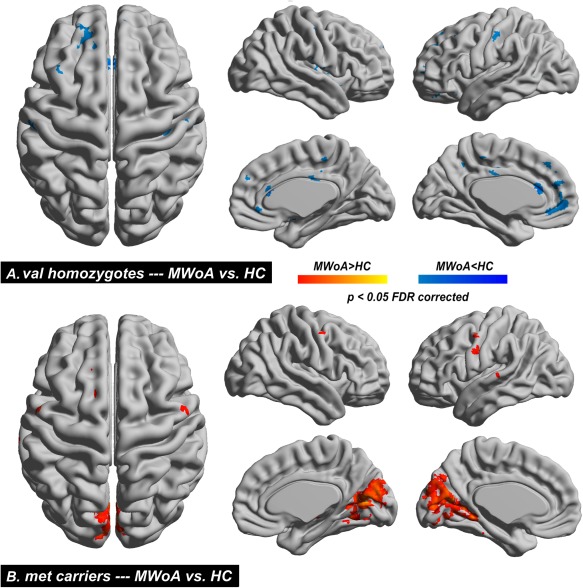
Functional connectivity (FC) changes. The hippocampus was chosen as the seed for the resting‐state FC. Significant between group differences from whole‐brain FC comparisons conducted on migraine versus healthy in val homozygotes (A) and met carriers subjects (B). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 5.
Between‐group differences of functional connectivity analysis in MWoA and HC (P < 0.05, FDR corrected)
| Brain Region | Side | Cluster Sizes | Voxels with maximum effect | |||
|---|---|---|---|---|---|---|
| Talairach | t‐value | |||||
| x | y | z | ||||
| A. MWoA > HC (val homozygotes) No between‐group differences | ||||||
| B. MWoA < HC (val homozygotes) | ||||||
| Medial Frontal Gyrus | L | 95 | −3 | 34 | −12 | −4.91 |
| R | 20 | 15 | 39 | 17 | −4.43 | |
| Middle Frontal Gyrus | L | 22 | −36 | 23 | 46 | −5.05 |
| Superior Frontal Gyrus | L | 54 | −6 | 32 | 56 | −5.18 |
| R | 10 | 12 | 48 | 25 | −4.4 | |
| Precentral Gyrus | L | 13 | −59 | −15 | 42 | −4.78 |
| R | 7 | 53 | −12 | 42 | −4.05 | |
| Anterior Cingulate | L | 32 | −6 | 46 | −5 | −4.64 |
| R | 20 | 3 | 47 | −2 | −4.58 | |
| Mid Cingulate | L | 32 | −3 | −19 | 29 | −4.74 |
| Insula | L | 31 | −42 | −9 | 0 | −4.64 |
| R | 67 | 42 | −11 | 17 | −5.42 | |
| Thalamus | L | 8 | −15 | −17 | 15 | −4.3 |
| R | 6 | 9 | −6 | 3 | −4.12 | |
| Amygdala | R | 6 | 24 | −1 | −18 | −4.28 |
| Postcentral Gyrus | L | 15 | −53 | −18 | 45 | −4.56 |
| R | 40 | 42 | −21 | 45 | −4.81 | |
| C. MWoA > HC (met carriers) | ||||||
| Precentral Gyrus | L | 25 | −53 | −1 | 44 | 4.96 |
| R | 8 | 56 | −4 | 44 | 4.31 | |
| Posterior Cingulate | L | 40 | −6 | −66 | 12 | 5.1 |
| R | 46 | 21 | −66 | 12 | 5.39 | |
| Precuneus | L | 21 | −3 | −72 | 23 | 4.74 |
| R | 11 | 3 | −72 | 23 | 4.52 | |
| Occipital cortices | L | 403 | −6 | −69 | 15 | 5.37 |
| R | 457 | 9 | −72 | 12 | 5.51 | |
| D. MWoA < HC (met carriers) No between‐group differences | ||||||
Figure 6.
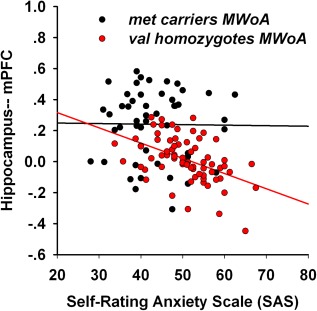
Association between functional connectivity (FC) of hippocampus‐mPFC and SAS. The Pearson correlation revealed significant negative correlation between FC of hippocampus‐mPFC and SAS in val homozygotes migraineurs. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Head‐Motion Analysis
In our study, value of 0.5 for FD was chosen to censor the rs‐fMRI volume. According to this criterion, forty‐five out of 246 subjects were contaminated with motion artifact. After motion censored, there were still more than 160 frames of data remaining within these 45 subjects. We re‐performed the FC analysis in each subject groups using censored time courses, and all our previous observations remain hold.
DISCUSSION
The current study investigated the potential role of COMT val158met genetic variation on brain structure and function in female val homozygotes and met carrier MWoA. There were three major findings: (1) val homozygote MWoA reported to have higher value of self‐rating anxiety; (2) disease × genotype interaction was found in the hippocampus, which was specific to val homozygote migraineurs with increased GM; and (3) as compared to each cohort separately, decreased hippocampal‐related FC were found within the mPFC and limbic brain structures in val homozygotes patients.
Prior experimental pain studies examined the close relationship between COMT influences of functional genetic polymorphism and the modulation of responses to sustained pain in healthy volunteers. For instance, Zubieta et al. found that individuals homozygous for the met allele had diminished regional mu‐opioid system responses to pain as compared to heterozygotes in HC [Zubieta et al., 2003]. Mobascher et al. reported that healthy subjects with the COMT met/met genotype showed a higher brain functional activity in the ACC in response to painful laser stimulation compared with val carriers [Mobascher et al., 2010]. As for the role of COMT polymorphism in the genetic susceptibility to stimulated pain, the current study found a significant disease × genotype interaction within migraine and COMT val158met genetic variation. Although no obvious association was found between COMT and migrainous headache [Tammimäki and Männistö, 2012], our results found an involvement of the val allele in the brain structural changes underlying migraine pathophysiology, which is a novel finding.
One of the main observations in the current study is that increased GM in the hippocampus was found in val homozygote MWoA compared to val homozygote HC. As previous studies on GM in healthy subjects have found decreased GM in the hippocampus and parahippocampal gyrus in val homozygotes relative to met carriers [Honea et al., 2009], the current results may indicate some specificity of altered function in these regions in val homozygote migraineurs. The hippocampus belongs to the limbic system and has high levels of glucocorticoid receptor, which makes it more vulnerable to stress than other brain regions [Joëls, 2008]. Research has consistently shown that lesions in the hippocampus or in the parahippocampal gyrus decreased neuroendocrine stress responses for unconditioned fear [Kjelstrup et al., 2002], blocked a stress‐induced behavioral response [Schulz‐Klaus, 2009] and suppressed the aversive information processing and emotional learning [Schulz et al., 2004]. For animal models in chronic pain studies, these independently exhibited hippocampal abnormalities including recognition memory deficiency [Kodama et al., 2011], alterations in cytokine expression [del Rey et al., 2011], and impaired enriched‐environment neurogenesis [Terada et al., 2008]. Moreover, Oler et al. characterized the neural circuitry associated with anxious temperament and the extent to which the function of this circuit was heritable in monkeys, and they found significant heritabilityof metabolic activity in anxious temperament associated hippocampal regions [Oler et al., 2010]. Together, these findings support a key role for the hippocampus during stressful circumstances than those from adaptive to maladaptive changes, which may be dependent on genetic, epigenetic and life experience to modify behaviors [Simons et al., 2013]. As we know, during irregular and debilitating episodes, migraine attacks are associated with or could be provoked with a number of physiological and emotional stressors [Borsook et al., 2012]. For repeated stressful challenges, frequent migraine may lead to abnormal glucocorticoid release and excitatory amino acid activity [McEwen, 1999], which in turn may result in deleterious effects on the hippocampus [Maleki et al., 2013]. While increased GM volume in the hippocampus has been reported in low frequency migraine [Mutso et al., 2012], our results provided additional evidence of the involvement of the hippocampus in the pathophysiology of the migraine. To our knowledge, few studies considered the COMT genetic variations for the neural functions of the hippocampus in migraine. Given that migraine changes stress‐related hormones and brain circuits differently across patients with different attack frequency and cognitive‐affective factors, the observation of altered volume in the hippocampus, while not new in chronic pain studies, is new in the context of migraine and its relationship with the potential role of COMT val158met genetic variation.
Altered structure in the hippocampus in val homozygote MWoA may have significant consequences on other systems, such as regulation of emotional behavior, as has been studied previously. As shown by our results, FC between the hippocampus‐mPFC and limbic systems was significantly decreased in val homozygote MWoA; moreover, FC intensity in the hippocampus‐mPFC was associated with SAS values, but was not found in met carriers. This suggested that the activity of some brain regions within the hippocampus‐related brain circuits may be affected by individual genetic variants. Clinical observations have shown that patients with chronic pain often generate anxiety that modulates functional activity within limbic structures involved in learning and memory, which indicates the critical roles of the affective component of pain and its maladaptive changes on cognition [Simons et al., 2013]. Numerous cognitive and emotional studies have demonstrated an important role for the mPFC in chronic pain and the modulation of anxiety, likely through its reciprocal connection within the limbic structures [Vertes, 2004]. Recently, Khan et al. found dysregulated hippocampus‐mPFC connectivity in burning mouth syndrome patients, and the connectivity was related to beck depression inventory scores [Khan et al., 2014]. Adhikari et al. pointed out that the range synchrony between the hippocampus and the mPFC was modulated by anxiety [Adhikari et al., 2010]. Based on the current finding, the decreased FC in val homozygote patients may suggest a diminished interaction within hippocampus‐related brain circuits involved in the affective‐motivational and cognitive‐evaluative dimensions of pain. Accordingly, we suspected that migraine had an additional effect on hippocampal function involved with the stress response related to episodic migraine attacks, which may cause anxiety‐like behaviors that became more prominent in val homozygote MWoA. Mismatching increase in GM volume and decrease in FC located in the hippocampus was difficult to explain. It is possible that impaired pruning or abnormal cell type distribution may cause abnormalities in hippocampal structure in val homozygote MWoA, which in turn disrupt hippocampus‐related FC. Possibilities also include that dysregulated FC in the hippocampus lead to maladaptive hippocampal structural changes in val homozygote MWoA.
Borsook et al. recently proposed a theoretical model for the effect of allostatic load on migraine, describing how maladaptive stress contributed to the triggering, amplification, progression and transformation from acute to chronic migraine [Borsook et al., 2012; Vachon‐Presseau et al., 2013]. The authors pointed out that stressful experiences related to activities of daily living may trigger migraine [Sauro and Becker, 2009], and specific stressors associated with migraine included emotional and physiological factors which may lead to failing habituation during headache [Borsook et al., 2012]. It seems that certain feelings and migraine‐related pain perception are closely linked and interact reciprocally [Price, 2000]. Conversely, specific studies emphasized the powerful effects of emotion on chronic pain. Rhudy et al. have demonstrated that viewing unpleasant pictures enhanced the nociceptive flexion reflex and pain [Rhudy et al., 2005]. Roy et al. found that pain‐related brain activity was modulated by emotions [Roy et al., 2009]. These studies showed a vicious cycle between anxiety and pain depicting dysfunctional brain responses as a result of continued stress accumulated over time. Sensory‐discriminative, cognitive‐evaluative, and affective‐motivational dimensions of pain may be altered in numerous ways resulting in further allostatic load [Borsook et al., 2012]. In our study, although we did not find any significant between‐group differences in migraine attack frequency, average pain intensity, and attack duration between patients, higher SAS values in val homozygote MWoA may constitute a factors of vulnerability for developing a maladaptive stress response when failure to shut down the stress response in a normal manner occurs. This indicated that this group of patients may have increased risk for the development of chronic pain [Vachon‐Presseau et al., 2013]. However, replication of these results in independent samples is necessary.
Several previous studies with Caucasian samples indicated that the COMT met allele was less proficient than val homozygotes in recognition of emotional expressions [Swart et al., 2011] and was associated with susceptibility for affective disorder [Smolka et al., 2005; Williams et al., 2010]. Finan et al. found that there was a met allele underlying interindividual differences in a reduced ability to regulate affective reactivity to pain in fibromyalgia [Finan et al., 2010]. Zubieta et al. also suggested that the met allele was less efficiently adapted to pain and other stressful stimuli [Zubieta et al., 2003]. Contrary to expectations, val homozygote MWoA reported more anxiety in the current study. As the associations between COMT val158met and its related cognitive performances have been found to vary by population [Wang et al., 2013], an explanation for our results may be that it was due to the ethnic differences in genetic effects [Kunugi et al., 1997]. In a Korean sample, Ham et al. found that subjects with the val homozygote genotype had significantly higher alexithymia scores on the Toronto Alexithymia Scale than those with met carrier genotypes [Ham et al., 2008], which may bolster our findings. Nevertheless, replication studies are required to investigate the existence of an ethnic difference in the role of COMT in anxiety.
CONCLUSIONS
In summary, a substantial difference in morphometric measures and region functional interaction was observed between val homozygotes and met carrier MWoA. The differences may suggest that there are interactions between COMT and migraine. We found that the val allele was associated with increased GM volume of the hippocampus with decreased hippocampus‐PFC connectivity in episodic migraine suffers, which may reflect additional changes in the brain structure and function in val homozygote MWoA in relation to maladaptive stress and the general state of allostatic load when faced with frequent headache.
Jixin Liu and Lei Lan contributed equally to this work.
REFERENCES
- Adcock RA, Thangavel A, Whitfield‐Gabrieli S, Knutson B, Gabrieli JD (2006): Reward‐motivated learning: Mesolimbic activation precedes memory formation. Neuron 50:507–517. [DOI] [PubMed] [Google Scholar]
- Adhikari A, Topiwala MA, Gordon JA (2010): Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron 65:257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfer I, Segall SK, Lariviere WR, Smith SB, Dai F, Slade GD, Rashid NU, Mogil JS, Campbell CM, Edwards RR (2013): Pain modality‐ and sex‐specific effects of COMT genetic functional variants. Pain 154:1368–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA (2004): The catechol‐O‐methyltransferase polymorphism: Relations to the tonic‐phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology 29:1943–1961. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo, X.‐N. , Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S (2010): Toward discovery science of human brain function. Proc Natl Acad Sci USA 107:4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Maleki N, Becerra L, McEwen B (2012): Understanding migraine through the lens of maladaptive stress responses: A model disease of allostatic load. Neuron 73:219–234. [DOI] [PubMed] [Google Scholar]
- Cargnin S, Magnani F, Viana M, Tassorelli C, Mittino D, Cantello R, Sances G, Nappi G, Canonico PL, Genazzani AA (2013): An opposite‐direction modulation of the COMT Val158Met polymorphism on the clinical response to intrathecal morphine and triptans. J Pain 14:1097–1106. [DOI] [PubMed] [Google Scholar]
- Costa C, Sgobio C, Siliquini S, Tozzi A, Tantucci M, Ghiglieri V, Di Filippo M, Pendolino V, de Iure A, Marti M (2012): Mechanisms underlying the impairment of hippocampal long‐term potentiation and memory in experimental Parkinson's disease. Brain 135:1884–1899. [DOI] [PubMed] [Google Scholar]
- del Rey A, Yau, H.‐J. , Randolf A, Centeno MV, Wildmann J, Martina M, Besedovsky HO, Apkarian AV (2011): Chronic neuropathic pain‐like behavior correlates with IL‐1β expression and disrupts cytokine interactions in the hippocampus. Pain 152:2827–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Nigg JT, Iyer S, Bathula D, Mills KL, Dosenbach NUF, Schlaggar BL, Mennes M, Gutman D, Bangaru S (2012): Distinct neural signatures detected for ADHD subtypes after controlling for micro‐movements in resting state functional connectivity MRI data. Front Syst Neurosci 6:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan PH, Zautra AJ, Davis MC, Lemery–Chalfant K, Covault J, Tennen H (2010): Genetic influences on the dynamics of pain and affect in fibromyalgia. Health Psychol 29:134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerritsen L, Comijs HC, van der Graaf Y, Knoops AJ, Penninx BW, Geerlings MI (2011): Depression, hypothalamic pituitary adrenal axis, and hippocampal and entorhinal cortex volumes—The SMART Medea study. Biol Psychiatry 70:373–380. [DOI] [PubMed] [Google Scholar]
- Ghiglieri V, Sgobio C, Costa C, Picconi B, Calabresi P (2011): Striatum–hippocampus balance: From physiological behavior to interneuronal pathology. Prog Neurobiol 94:102–114. [DOI] [PubMed] [Google Scholar]
- Ham B‐J, Lee M‐S, Lee Y‐M, Kim M‐K, Choi M‐J, Oh K‐S, Jung H, Lyoo I, Choi I‐G (2008): Association between the catechol O‐methyltransferase Val108/158Met polymorphism and alexithymia. Neuropsychobiology 52:151–154. [DOI] [PubMed] [Google Scholar]
- Honea R, Verchinski BA, Pezawas L, Kolachana BS, Callicott JH, Mattay VS, Weinberger DR, Meyer‐Lindenberg A (2009): Impact of interacting functional variants in COMT on regional gray matter volume in human brain. Neuroimage 45:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R (1991): The role of the hippocampus in feedback regulation of the hypothalamic‐pituitary‐adrenocortical Axis. Endocr Rev 12:118–134. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S (2001): A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith, S (2002); Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841. [DOI] [PubMed] [Google Scholar]
- Joëls M (2008); Functional actions of corticosteroids in the hippocampus. Eur J Pharmacol 583:312–321. [DOI] [PubMed] [Google Scholar]
- Johnson A, van der Meer MA, Redish AD (2007): Integrating hippocampus and striatum in decision‐making. Curr Opin Neurobiol 17:692–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SA, Keaser ML, Meiller TF, Seminowicz DA (2014): Altered structure and function in the hippocampus and medial prefrontal cortex in patients with burning mouth syndrome. Pain 155:1472–1480. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach, H.‐A. , Murison R, Moser EI, Moser M‐B(2002): Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci USA 99:10825–10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama D, Ono H, Tanabe M (2011): Increased hippocampal glycine uptake and cognitive dysfunction after peripheral nerve injury. Pain 152:809–817. [DOI] [PubMed] [Google Scholar]
- Kunugi H, Nanko S, Ueki A, Otsuka E, Hattori M, Hoda F, Vallada HP, Arranz MJ, Collier DA (1997): High and low activity alleles of catechol‐O‐methyltransferase gene: Ethnic difference and possible association with Parkinson's disease. Neurosci Lett 221:202–204. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Grace AA (2005): The hippocampal‐VTA loop: Controlling the entry of information into long‐term memory. Neuron 46:703–713. [DOI] [PubMed] [Google Scholar]
- Lui S, Deng W, Huang X, Jiang L, Ma X, Chen H, Zhang T, Li X, Li D, Zou L (2009): Association of cerebral deficits with clinical symptoms in antipsychotic‐naive first‐episode schizophrenia: An optimized voxel‐based morphometry and resting state functional connectivity study. Am J Psychiatry 166:196–205. [DOI] [PubMed] [Google Scholar]
- Liu J, Qin W, Nan J, Li J, Yuan K, Zhao L, Zeng F, Sun J, Yu D, Dong M (2011): Gender‐related differences in the dysfunctional resting networks of migraine suffers. PloS One 6:e27049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lan L, Li G, Yan X, Nan J, Xiong S, Yin Q, von Deneen KM, Gong Q, Liang F (2013): Migraine‐related gray matter and white matter changes at a 1‐year follow‐up evaluation. J Pain 14:1703–1708. [DOI] [PubMed] [Google Scholar]
- Männistö PT, Kaakkola S (1999): Catechol‐O‐methyltransferase (COMT): Biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev 51:593–628. [PubMed] [Google Scholar]
- Maleki N, Becerra L, Brawn J, McEwen B, Burstein R, Borsook D (2013): Common hippocampal structural and functional changes in migraine. Brain Struct Funct 218:903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Weickert CS, Akil M, Lipska B, Hyde T, Herman M, Kleinman J, Weinberger D (2003a): Catechol‐O‐methyltransferase mRNA expression in human and rat brain: Evidence for a role in cortical neuronal function. Neuroscience 116:127–137. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Weickert CS, Beltaifa S, Kolachana B, Chen J, Hyde TM, Herman MM, Weinberger DR, Kleinman JE (2003b): Catechol‐O‐methyltransferase (COMT) mRNA expression in the dorsolateral prefrontal cortex of patients with schizophrenia. Neuropsychopharmacology 28:1521–1530. [DOI] [PubMed] [Google Scholar]
- McEwen BS (1999): Stress and hippocampal plasticity. Annu Rev Neurosci 22:105–122. [DOI] [PubMed] [Google Scholar]
- Mobascher A, Brinkmeyer J, Thiele H, Toliat MR, Steffens M, Warbrick T, Musso F, Wittsack H‐J, Saleh A, Schnitzler A (2010): Research The val158met polymorphism of human catechol‐O‐methyltransferase (COMT) affects anterior cingulate cortex activation in response to painful laser stimulation. Mol Pain 6:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutso AA, Radzicki D, Baliki MN, Huang L, Banisadr G, Centeno MV, Radulovic J, Martina M, Miller RJ, Apkarian AV (2012): Abnormalities in hippocampal functioning with persistent pain. J Neurosci 32:5747–5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oler JA, Fox AS, Shelton SE, Rogers J, Dyer TD, Davidson RJ, Shelledy W, Oakes TR, Blangero J, Kalin NH (2010): Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature 466:864–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD (2006): Dopamine‐dependent prediction errors underpin reward‐seeking behaviour in humans. Nature 442:1042–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploghaus A, Tracey I, Clare S, Gati JS, Rawlins JNP, Matthews PM (2000): Learning about pain: The neural substrate of the prediction error for aversive events. Proc Natl Acad Sci USA 97:9281–9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, Matthews PM, Rawlins JNP, Tracey I (2001): Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci 21:9896–9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012): Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD (2000): Psychological and neural mechanisms of the affective dimension of pain. Science, 288:1769–1772. [DOI] [PubMed] [Google Scholar]
- Rhudy JL, Williams AE, McCabe KM, Rambo P (2005): Affective modulation of nociception at spinal and supraspinal levels. Psychophysiology 42:579–587. [DOI] [PubMed] [Google Scholar]
- Roy M, Piché M, Chen J‐I, Peretz I, Rainville P (2009): Cerebral and spinal modulation of pain by emotions. Proc Natl Acad Sci USA 106:20900–20905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauro KM, Becker WJ (2009): The stress and migraine interaction. Headache 49:1378–1386. [DOI] [PubMed] [Google Scholar]
- Schulz‐Klaus B (2009): Neurotoxic lesion of the rostral perirhinal cortex blocks stress‐induced exploratory behavioral changes in male rats. Stress 12:186–192. [DOI] [PubMed] [Google Scholar]
- Schulz B, Fendt M, Richardson R, Schnitzler HU (2004): Temporary inactivation of the perirhinal cortex by muscimol injections block acquisition and expression of fear‐potentiated startle. Eur J Neurosci 19:713–720. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Adcock RA (2010); Dopamine and adaptive memory. Trends Cogn Sci 14:464–472. [DOI] [PubMed] [Google Scholar]
- Simons LE, Elman I, Borsook D (2013): Psychological processing in chronic pain: A neural systems approach. Neurosci Biobehav Rev 39:61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM (2002): Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Nichols TE (2009): Threshold‐free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44:83–98. [DOI] [PubMed] [Google Scholar]
- Smolka MN, Schumann G, Wrase J, Grüsser SM, Flor H, Mann K, Braus DF, Goldman D, Büchel C, Heinz A (2005): Catechol‐O‐methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J Neurosci 25:836–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swart M, Bruggeman R, Larøi F, Alizadeh BZ, Kema I, Kortekaas R, Wiersma D, Aleman A (2011): COMT Val158Met polymorphism, verbalizing of emotion and activation of affective brain systems. Neuroimage 55:338–344. [DOI] [PubMed] [Google Scholar]
- Tammimäki A, Männistö, PT (2012): Catechol‐O‐methyltransferase gene polymorphism and chronic human pain: A systematic review and meta‐analysis. Pharmacogenet Genomics 22:673–691. [DOI] [PubMed] [Google Scholar]
- Terada M, Kuzumaki N, Hareyama N, Imai S, Niikura K, Narita M, Yamazaki M, Suzuki T, Narita M (2008): Suppression of enriched environment‐induced neurogenesis in a rodent model of neuropathic pain. Neurosci Lett 440:314–318. [DOI] [PubMed] [Google Scholar]
- Vachon‐Presseau E, Roy M, Martel, M.‐O. , Caron E, Marin, M.‐F. , Chen J, Albouy G, Plante I, Sullivan MJ, Lupien SJ (2013): The stress model of chronic pain: Evidence from basal cortisol and hippocampal structure and function in humans. Brain 136:815–827. [DOI] [PubMed] [Google Scholar]
- Vertes RP (2004): Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51:32–58. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li J, Chen C, Zhu B, Moysis R, Lei X, Li H, Liu Q, Xiu D, Liu B (2013): COMT rs4680 Met is not always the ‘smart allele’: Val allele is associated with better working memory and larger hippocampal volume in healthy Chinese. Genes Brain Behav 12:323–329. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Schott BH, Guderian S, Frey JU, Heinze H‐J, Düzel E (2005): Reward‐related FMRI activation of dopaminergic midbrain is associated with enhanced hippocampus‐dependent long‐term memory formation. Neuron 45:459–467. [DOI] [PubMed] [Google Scholar]
- Williams LM, Gatt JM, Grieve SM, Dobson‐Stone C, Paul RH, Gordon E, Schofield, PR (2010): COMT Val(108/158)Met polymorphism effects on emotional brain function and negativity bias. Neuroimage 53:918–925. [DOI] [PubMed] [Google Scholar]
- Yu D, Yuan K, Qin W, Zhao L, Dong M, Liu P, Yang X, Liu J, Sun J, Zhou G (2013): Axonal loss of white matter in migraine without aura: A tract‐based spatial statistics study. Cephalalgia 33:34–42. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S (2001): Segmentation of brain MR images through a hidden Markov random field model and the expectation‐maximization algorithm. IEEE Trans Med Imaging 20:45–57. [DOI] [PubMed] [Google Scholar]
- Zubieta, J.‐K. , Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D (2003): COMT val158met genotype affects µ‐opioid neurotransmitter responses to a pain stressor. Science 299:1240–1243. [DOI] [PubMed] [Google Scholar]


