Abstract
Objectives
Cortical areas involved in bimanual coordination have been regularly studied by functional neuroimaging and electroencephalography. However, the subcortical connectivity underlying this complex function has received less attention. Here, we used the technique of direct electrostimulation in awake patients who underwent surgery for brain glioma, with the goal to investigate the white matter pathways subserving bimanual coordination.
Experimental design
Eight patients were operated under local anesthesia for a frontal low‐grade glioma. Intraoperative subcortical electrostimulation mapping was used to search interference with bimanual coordination. The corresponding stimulation sites were reported on brain MRI.
Principal observations
All patients presented a complete arrest of the movement of both hands during unilateral subcortical stimulation of the white matter underneath the dorsal premotor cortex and the posterior part of the supplementary motor area, rostrally to the corticospinal tract, until the caudate nucleus and the anterior arm of the internal capsule. No movement deficits, especially no disturbances of bimanual coordination, were observed 3 months after surgery.
Conclusions
This is the first evidence of bilateral negative motor responses elicited by unilateral subcortical stimulation. Such findings support the existence of a bilateral cortico‐subcortical network connecting the premotor cortices, basal ganglia, and spinal cord, involved in the control of bimanual coordination. A better understanding of this modulatory motor circuit may have important implications in fundamental neurosciences as well as in brain surgery. Hum Brain Mapp 35:3439–3445, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: movement, bimanual coordination, direct electrical stimulation, awake surgery
INTRODUCTION
Although the cortical areas involved in the control of movement have been extensively studied, especially using neurophysiology, functional neuroimaging, and electroencephalography, the subcortical connectivity underlying this complex function has received less attention. Interestingly, the traditional view mainly based on pyramidal pathways coming from the primary motor cortex has already been modulated by the evidence of direct spinal projections running from the premotor cortex to the spinal cord [Dum and Strick, 1991; Maier et al., 2002]. However, even though many studies have reported the existence of a likely ipsilateral motor control [Gerloff and Andres, 2002; Gerloff et al., 1998; Chen et al., 2003], they did not identify the white matter tracts subserving this mechanism. Gerloff et al. suggested that there were several levels of control of movement, including an interhemispheric interaction through the transcallosal pathway as well as ipsilateral corticospinal projections [Gerloff et al., 1998], but no actual demonstration was clearly made. In addition, it is worth noting that the models trying to explain motor control did not account the possibility of a direct control on bimanual movement by unilateral network in a physiological state.
Remarkably, thanks to the use of direct cortical electrostimulation, beyond the detection of the classical primary motor area eliciting involuntary movement when stimulated (the so‐called Penfieldian homonculus), negative motor phenomenon has also been described [Lüders et al., 1987, 1995; Penfield, 1954]. Here, our purpose is to investigate the possible existence of a bilateral modulatory motor pathway (BMMP) playing a role in the bimanual coordination, and to study the fiber tracts underlying such network in humans. This hypothesis is made on the basis of a previous report demonstrating the existence of a cortico‐subcortical negative motor network subserved by fibers coming from the premotor cortex and running into the corona radiata and the anterior arm of the internal capsule, anterior to the primary motor fibers. Indeed, a recent electrostimulation study in awake patients who underwent surgery for brain glioma has shown that it was also possible to elicit negative motor phenomenon at the subcortical level, raising the question of the white matter connectivity involved in the control of movement [Schucht et al., in press]. Such findings are shedding the light on new aspects of the motor system, supporting the existence of a “modulatory motor network,” since the diverse interferences with motor function resulting in inhibition and acceleration imply a modulatory influence of the detected fiber network. However, only contralateral motor control has been identified and not ipsilateral/bilateral motor control. Therefore, although the functional and anatomical distinction between the different areas originating the corticospinal system is well known in animals, the pathways underlying bimanual coordination is still unclear in humans.
To this end, in a series of eight patients who underwent awake surgery for frontal low‐grade gliomas (LGG) near the classical pyramidal tract, we assessed the bimanual coordination during cortical and subcortical electrostimulation mapping. Indeed, we have already reported that some patients could experience permanent deficit in bimanual coordination and complex movement following resection of frontal glioma, especially when involving the supplementary motor area (SMA) [Krainik et al., 2001]. Consequently, our goal was to improve the onco‐functional balance of surgery, by maximizing the extent of resection while preserving the complex movement and thus the quality of life of patients, as previously demonstrated for language [Duffau et al., 2005]. Intraoperatively, all patients presented a bilateral inhibition of the bimanual movement during unilateral stimulation of a subcortical white matter bundle, allowing us to identify for the first time to our knowledge a new pathway involved in movement control. In the lights of these original findings, we suggest new insights into the pathophysiology of bimanual coordination.
MATERIALS AND METHODS
Patients
We report a prospective series of eight patients with a frontal World Health Organization grade II glioma (LGG) diagnosed because of seizures in all cases except one (incidental discovery). They were selected for surgical resection from May 2012 to August 2012. Due to the proximity of the tumor with motor structures, all patients underwent awake surgery with intraoperative cortical and subcortical electrostimulation mapping of bimanual movement.
A neurological examination as well as a language assessment using the Boston Diagnostic Aphasia Examination were both performed prior surgery for all patients. Informed consent was obtained before surgery from all patients.
Intraoperative Electrostimulation Mapping
All surgeries were performed under local anesthesia so that functional cortical and subcortical mapping could be carried out using direct brain stimulation, as already described [Duffau et al., 2005]. A bipolar electrode with 5‐mm spaced tips delivering a biphasic current (pulse frequency of 60 Hz, single pulse phase duration of 1 ms, amplitude from 1 to 4 mA‐Nimbus, Hemodia) was applied on the brain.
Firstly, ultrasonography was used to identify the tumor boundaries. Then, the cortical mapping was performed over the primary sensory‐motor area and ventral premotor cortex (during a counting and naming task) by progressively increasing the level of stimulation of 0.5 mA (from a baseline of 1 mA) until a positive response (involuntary movement, paresthesia, anarthria, or naming disturbances) was elicited, indicating the optimal threshold of stimulation. All positive stimulation sites were marked with a tag number.
During a second surgical stage, the removal of the tumor was performed according to the cortical and subcortical functional boundaries identified with electrical stimulation throughout the resection. The same electrical parameters were used at the subcortical level than at the cortical one. Language was analyzed by a speech therapist present during the awake phase. Moreover, our protocol of functional monitoring during tumor resection required patients to perform continuous movements of the controlateral upper extremity. This test consisted of repetitive and alternating flexion and extension of the arm, hand and fingers at a frequency at ~0.5 Hz. The same continuous movements of the contralateral lower extremity were also required during the resection of the postero‐mesial part of the frontal tumor. Indeed, we should insist on the fact that the patients are awake into the operating room during one to two hours throughout the mapping and tumor resection. This represents a strong constraint for the selection of the task. Repetitive movement at a frequency at approximately 0.5 Hz is feasible continuously by the patient, as already reported in previous surgical reports [Duffau, 2009; Schucht et al., in press].
A negative motor response (NMR) was defined as cessation of the movement without loss of consciousness [Lüders et al., 1987, 1995]. These NMRs were first searched for the contralateral hemibody to stimulation. Then, it was asked to the patient to perform bimanual movement, that is, a series of bilateral flexion and extension of the arms, hands, and fingers, in phase or in antiphase, at a frequency at ~0.5 Hz. This movement was analyzed by a neuropsychologist. The neuropsychologist checked (i) whether the movement was made continuously or whether it stopped, (ii) whether there was a modification of the frequency (e.g., acceleration or slowdown), and (iii) whether there was a modification of the bilateral coordination (e.g., in‐phase movement which became antiphase or vice‐versa). The stimulation was first done at the same subcortical sites which previously elicited controlateral NMR, and then around this area, that is, on the posterior part of the surgical cavity for frontal LGG. A NMR response was defined as bilateral and synchronous cessation of movement without loss of consciousness.
The corresponding subcortical sites were marked with a number in case of reproducible bimanual movement interference, namely when symptoms were induced by at least three stimulations. An intraoperative photography was taken to show the final cortico‐subcortical eloquent sites. Of note, the resection was extended up to functional boundaries assessed by stimulation mapping that respected both crucial cortical areas and essential subcortical pathways, to optimize the extent of resection while preserving brain functions [Duffau, 2009]. The exact localization of intraoperative subcortical stimulation sites was determined by its spatial relation to various anatomical (gyri, sulci, midline, deep gray nuclei, and lateral ventricle) and functional (motor, sensory, and language) landmarks as documented on intraoperative photography. The determined location of the stimulation site was plotted into the postoperative MRI. Indeed, beyond the assessment of the extent of resection, this imaging enabled the analysis of the anatomical location of the eloquent areas, that is, in essence at the periphery of the cavity, where the resection was stopped according to functional boundaries revealed by intrasurgical stimulation, a reliable methodology that we have previously reported [Duffau, 2009; Schucht et al., in press]. Therefore, it was also possible to plot these sites into the preoperative MRI (from the postoperative MRI). The neurological status was assessed immediately after surgery and again after 3 months analogical to the preoperative assessment.
RESULTS
Patients
The mean age was 41.7 years (range: 31–53 years). Of the 2 men and 6 women, 7 patients were right handed and 1 was left handed according to the Edinburgh inventory. All eight patients had a Karnofsky Performance Status score of 90–100%. Seizures were the presenting symptoms in all patients. None of them had motor deficits or language disorders on neurological examination and Boston Diagnostic Aphasia Examination, respectively. However, one patient had slight attentional disorders and one had a psychomotor slowness.
According to the preoperative MRI, the tumor involved the frontal lobe in the 8 cases (4 right and 4 left) within or close to at least one of the following structures: dorsal premotor cortex, SMA, anterior cingulate cortex (ACC), head of the caudate nucleus or anterior arm of the internal capsule (Table 1).
Table 1.
Patient characteristics, motor responses, and follow‐up
| Patient | Gender | Age | Handedness | Tumor location | Bilateral inhibition of both hands | Postoperative deficit | 3 months |
|---|---|---|---|---|---|---|---|
| 1 | M | 41 | R | Right preSMA Dorsal premotor cortex | Yes | No deficit | No deficit |
| 2 | F | 36 | R | Left SMA | Yes | Slight right upper limb paresis and slight speech disorders | Complete recovery |
| 3 | F | 42 | R | Left SMA | Yes | Mutism | Complete recovery |
| 4 | F | 41 | L | Right hemispheric | Yes | Apraxia | Complete recovery |
| 5 | F | 47 | R | Right frontocallosal | Yes | No deficit | No deficit |
| 6 | F | 43 | R | Left frontal | Yes | No deficit | No deficit |
| 7 | F | 31 | R | Right SMA | Yes | Slight left upper limb and face paresis | Complete recovery |
| 8 | M | 53 | R | Left SMA | Yes | No deficit | No deficit |
Abbreviations: M: male; F: female; R: right; L: left.
Characteristics of Stimulation Sites
Positive motor responses were found for each patient over the primary motor cortex, consisting of an involuntary muscle contraction of the contralateral upper limb or hemiface. The same positive responses were observed during subcortical stimulations of the corticospinal tract.
No NMR was elicited with cortical stimulation. Unilateral NMR (UNMR) were elicited at the subcortical level for each patient, immediately when the stimulation was applied on the brain. Sites of stimulations were located at the level of the white matter underneath the premotor cortex, immediately in front of the precentral sulcus. They were located veil‐like in a coronal plane.
In addition, bilateral NMR (BNMR) were elicited in all eight patient at the subcortical level, both for inphase and antiphase movements, again immediately when the stimulation was applied on the brain. Sites of stimulation were located at the level of the white matter underneath the dorsal premotor cortex and the posterior part of the SMA, rostrally to the corticospinal tract ‐ whatever the side. During the stimulation, we observed an immediate, synchronous and bilateral suspension of the arms, hands and fingers movement, without any loss of consciousness and without any loss of tonus (namely, the limbs did not fall at the moment of stimulation; see Supporting Information video 1). This bilateral movement arrest was observed by an “external visual inspection” without any cinematic or neurophysiologic quantification.
These BNMR were localized between the sites responsible of UNMR, in the same coronal plane. Subcortical fibers responsible for NMR were followed deeper throughout the resection. Sites of BNMR were found at the level of the anterior arm of the internal capsule and at the level of the head of the caudate nucleus (Figs. 1, 2, 3, 4).
Figure 1.
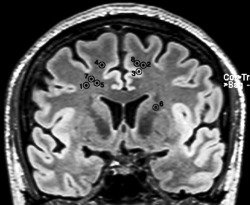
Projections of each site of BNMR on a coronal view in MRI. Numbers refer to patient in Table 1.
Figure 2.
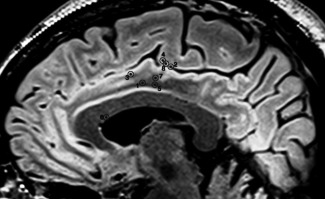
Projection of each site of stimulation on a sagittal view in MRI. Numbers refer to patient in Table 1.
Figure 3.
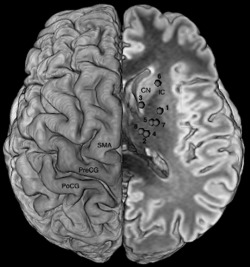
Superior view of the repartition of the subcortical sites eliciting BNMR reported on a unique side showing the course of the BMMP and its relation with cortical areas. PreCG: precentral gyrus; PoCG: postcentral gyrus; SMA: supplementary motor area; CN: head of the caudate nucleus; IC: anterior arm of the internal capsule. Numbers refer to patient in Table 1.
Figure 4.
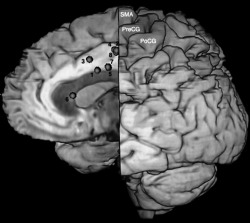
Medial view of the repartition of the subcortical sites eliciting BNMR reported on a unique side showing the course of the BMMP and its relation with cortical areas. PreCG: precentral gyrus; PoCG: postcentral gyrus; SMA: supplementary motor area.
Postoperative Course
All patients recovered well from surgery and were discharged home within 5 days following surgery. Two patients experienced a slight paresis of the upper limb and ataxia was noted in one case. One patient had a mustism and another patient had a slight dysarthria. All patients with neurological worsening underwent rehabilitation at home. On re‐examination at 3 months, all patients had regained their respective preoperative level, with no neurological deficit, especially no disorders of bimanual coordination (Table 1).
DISCUSSION
Identification of a BMMP
Recovery of controlateral hemiplegia and speech disorders following SMA resection has contributed to qualify the SMA syndrome as “reversible.” However, studies showed in animals and humans a persistence of bimanual coordination disorders after removal of the SMA [Brinkman, 1984; Krainik et al., 2001, 2004]. Schucht et al. assumed that sparing the fibers with “modulatory motor function” (namely fibers for which stimulations elicited UNMR) influenced the occurrence of the SMA syndrome and the long term recovery, that is, with no neurological deficits 3 months after surgery if this pathway was preserved [Schucht et al., in press]. Here, in the same vein, we performed awake mapping to identify structures eliciting UNMR when stimulated, and we avoided postoperative permanent worsening. Furthermore, we added a more complex intrasurgical protocol testing the bimanual coordination. This led us to identify a subcortical white matter pathway for which unilateral stimulation elicited an immediate, synchronous, and bilateral suspension of arms, hands, and fingers movement, without any loss of tonus, both during inphase and antiphase bilateral movement. Because of its proximity with sites eliciting UNMR, this pathway has probably been spared by Schucht et al. [in press], explaining that bimanual coordination was not worsened in their study. However, even if very close, BNMR sites were localized between the sites responsible of UNMR, in the same coronal plane. In addition, we acknowledge that we have induced only movement arrest in this study. Thus, this could be an aspecific inhibitory effect. However, in our previous report, we have also observed a possible acceleration of the movement during subcortical stimulation. This is the reason why we would like to leave a door opened by speaking about a “modulatory” network [as did in Schucht et al., in press] rather than to speak about a simple “inhibitory” network. Therefore, on the lights of our original results, we may hypothesize that these fibers belong to a BMMP playing a role in the bimanual coordination.
The BMMP Trajectory
We can only speculate on the cortical origin of BMMP as cortical stimulation did not elicit BNMR in our patients. Interestingly, cortical areas involved in the network subserving the bimanual coordination have already been discussed in the literature. Indeed, Debaere et al. specified the role of the SMA and the primary motor cortex in the interlimb coordination [Debaere et al., 2001]. The role of another medial area (namely the ACC), has also been demonstrated in the cognitive control of interlimb coordination [Wenderoth et al., 2005]. Moreover, Schucht et al. assumed that the subcortical fibers responsible of UNMR seem to take their origin in the premotor cortex, especially in the posterior part of the SMA‐proper [Schucht et al., in press]. Here, BNMR were elicited by stimulating white matter tracts underneath the dorsal premotor cortex, immediately in front of the precentral gyrus and at the level of the posterior part of the SMA proper. Therefore, we suggest that the origin of these fibers may be in the depth of the precentral sulcus, in the posterior part of the SMA proper, in the ACC and the dorsal premotor cortex. However, further investigations are needed to confirm this cortical origin, not clearly demonstrated here due to the limitations of our study.
Interestingly, we identified BNMR in several subcortical white matter sites confirming a downward course toward the anterior part of the striatum, since stimulation also elicited BNMR directly at the level of the anterior arm of the internal capsule and the head of the caudate nucleus (Fig. 5A,B). In addition, a large frontal lobectomy was performed in two patients who recovered following surgery, ruling out the possibility of a crucial anterior connectivity of this BMMP (Fig. 5). Therefore, we could presume that this fascicle is a part of the subcallosal fascicle (ScF) and/or the frontal aslant tract [Catani et al., 2012]. Indeed, both pathways are known to take their origin in the premotor region, especially in the SMA and cingulate area [Duffau et al., 2012; Leichnetz, 1981), and they are bilaterally represented in humans.
Figure 5.
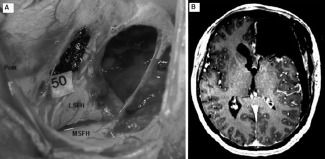
A: Preoperative view in patient 6 operated for a recurrence of a low grade glioma in the frontal lobe. The frontal horn of the left lateral ventricle is widely opened. Stimulation on number 50 elicited a BNMR and corresponds to the anterior arm of the internal capsule and the head of the caudate nucleus. Ant: anterior; Post: posterior; LSFH: lateral side of the frontal horn; MSFH: medial side of the frontal horn. B: Axial view of the postoperative MRI for patient 6. The wide opening of the ventricle and the absence of brain parenchyma anteriorly make easier the localization of the site eliciting BNMR on MRI and the link with the per‐operative view. Number 6 corresponds to the site of BNMR.
Because BNMR were elicited by stimulation of the head of the caudate nucleus, it could represent a distal termination of the BMMP. Indeed, it is noteworthy that the ScF has projections on the striatum [Duffau et al., 2012; Leichnetz, 1981). In addition, other cortico‐basal ganglia pathways originated from premotor areas and involved in the motor control have been described, namely the corticostriatal and cortisubthalamic pathways [Aron et al., 2007; Mathai and Smith, 2011] with a hyperdirect pathway [Jahfari et al., 2011]. Interestingly, these hyperdirect and indirect pathways play a role in stopping the action as well as the control of tonus, and they were demonstrated to have bilateral projections on both striatum in rodents and primates [Leichnetz, 1981; Mathai and Smith, 2011; Parent and Parent, 2006; Reiner et al., 2003], especially the ScF which has bilateral projections on the head of the caudate nuclei through the anterior arm of the internal capsule in monkeys [Leichnetz, 1981]. Such bilateral connectivity could explain why we elicited BNMR by performing unilateral stimulation, whatever the hemisphere, without any loss of tonus. In addition, because BNMR were also elicited by stimulation of the internal capsule, spinal cord projection cannot be excluded as a distal termination. In summary, we propose the existence of multiple targets of BMMP on the striatum and spinal cord, with bilateral projections, explaining our original findings during its stimulation.
Functional Role of BMMP
NMRs have previously been described [Lüders et al., 1987, 1995; Penfield, 1954] but their actual meaning remains unclear. Schucht et al. suggested that the neural network eliciting UNMR when stimulated might be involved in motor control [Schucht et al., in press]. Here, in the lights of our first observations of intraoperative BNMR induced by subcortical electrostimulation, and in agreement with Filevich et al. [2012], we suggest that the BMMP could modulate the excitatory output (“pyramidal” tract) through inhibitory signals, coming from each hemisphere at the same time, to synchronize the motor programs of both hands, and thus to allow bimanual coordination. Indeed, the absence of postoperative permanent deficit of bimanual coordination in our patients—despite a large resection within the frontal lobe, and even despite an extensive frontal lobectomy in several cases—is in favor of such a role of the BMMP, since this pathway was in essence preserved during surgery. Indeed, we have previously reported that, following removal of gliomas involving the frontal lobe, especially the SMA, without monitoring of NMR, patients experienced irreversible deficits of complex movement and bimanual coordination [Krainik et al., 2001].
Limitations of This Study
To optimize glioma resection while preserving quality of life, electrical stimulation was performed to identify functional boundaries and was therefore restricted to certain areas. As a consequence, we should acknowledge that further stimulation sites leading to various motor responses might have been missed, especially, other cortical areas and fibers involved in this complex network may have been ignored. In addition, the lack of kinematic data and electromyographic recordings are making our findings as just descriptive. Thus, the observation reported here is only the first step, on which other investigations should be performed to better understand the neuroanatomy of BMMP and its actual role.
CONCLUSION
This first evidence of BNMR elicited by unilateral subcortical stimulation gave new insights into the neural basis underlying control of bimanual coordination. Such findings plead in favor of a bilateral modulatory cortico‐subcortical network able to supervise the interlimb movement. A better understanding of this modulatory motor circuit could have important implications in fundamental neurosciences as well as in brain surgery. Therefore, further studies are needed to confirm our proposal and to better investigate the functional connectivity subserving complex bimanual movement.
Supporting information
Supplementary Information Video 1.
REFERENCES
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA (2007): Triangulating a cognitive control network using diffusion‐weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci 27:3743–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman C (1984): Supplementary motor area of the monkey's cerebral cortex: short‐and long‐term deficits after unilateral ablation and the effects of subsequent callosal section. J Neurosci 4:918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Dell'Acqua F, Vergani F, Malik F, Hodge H, Roy P, Valabreque R, Thiebaut de Schotten M (2012): Short frontal lobe connections of the human brain. Cortex 48:273–291. [DOI] [PubMed] [Google Scholar]
- Chen R, Yung D, Li JY (2003): Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol 89:1256–1264. [DOI] [PubMed] [Google Scholar]
- Debaere F, Swinnen SP, Béatse E, Sunaert S, Van Hecke P, Duysens J (2001): Brain areas involved in interlimb coordination: a distributed network. Neuroimage 14:947–958. [DOI] [PubMed] [Google Scholar]
- Duffau H (2009): Surgery of low‐grade gliomas: Towards a “functional neurooncology.” Curr Opin Oncol 21:543–549. [DOI] [PubMed] [Google Scholar]
- Duffau H, Capelle L, Sichez N, Denvil D, Lopes M, Sichez JP, Bitar A, Fohanno (2012): Intraoperative mapping of the subcortical language pathways using direct stimulations. An anatomo‐functional study. Brain 125:199–214. [DOI] [PubMed] [Google Scholar]
- Duffau H, Lopes M, Arthuis F, Bitar A, Sichez JP, van Effenterre R, Capelle L (2005): Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: A comparative study between two series without (1985‐96) and with (1996‐2003) functional mapping in the same institution. J Neurol Neurosurg Psychiatry 76:845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL (1991): The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci 11:667–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filevich E, Kühn S, Haggard P (2012): Negative motor phenomena in cortical stimulation: Implications for inhibitory control of human action. Cortex 48:1251–1261. [DOI] [PubMed] [Google Scholar]
- Gerloff C, Andres FG (2002): Bimanual coordination and interhemispheric interaction. Acta Psychol 110:161–186. [DOI] [PubMed] [Google Scholar]
- Gerloff C, Cohen LG, Floeter MK, Chen R, Corwell B, Hallett M (1998): Inhibitory influence of the ipsilateral motor cortex on responses to stimulation of the human cortex and pyramidal tract. J Physiol 510:249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahfari S, Waldorp L, van den Wildenberg WPM, Scholte HS, Ridderinkhof KR, Forstmann BU (2011): Effective connectivity reveals important roles for both the hyperdirect (fronto‐subthalamic) and the indirect (fronto‐striatal‐pallidal) fronto‐basal ganglia pathways during response inhibition. J Neurosci 31:6891–6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krainik A, Duffau H, Capelle L, Cornu P, Boch AL, Mangin JF, Le Bihan D, Marsault C, Chiras J, Lehéricy S (2004): Role of the healthy hemisphere in recovery after resection of the supplementary motor area. Neurology 62:1323–1332. [DOI] [PubMed] [Google Scholar]
- Krainik A, Lehericy S, Duffau H, Vlaicu M, Poupon F, Capelle L, Cornu P, Clemenceau S, Sahel M, Valery CA, Boch AL, Mangin JF, Bihan DL, Marsault C (2001): Role of the supplementary motor area in motor deficit following medial frontal lobe surgery. Neurology 57:871–878. [DOI] [PubMed] [Google Scholar]
- Leichnetz GR (1981): The median subcallosal fasciculus in the monkey: A unique prefrontal corticostriate and corticocortical pathway revealed by anterogradely transported horseradish peroxidase. Neurosci Lett 21:137–142. [DOI] [PubMed] [Google Scholar]
- Lüders H, Lesser RP, Morris HH (1987): Negative Motor Responses Elicited by Stimulation of the Human Cortex. Advances in Epileptology. New York: Raven Press; pp 229–231. [Google Scholar]
- Lüders HO, Dudley S, Dinner S (1995): The negative motor areas In: Fahn S, Hallett M, Lüders HO, Marsden CD, editors. Negative Motor Phenomenon. Philadelphia: Lippinscott‐Raven Publishers. [Google Scholar]
- Maier MA, Armand J, Kirkwood PA, Wang HW, Davis JN, Lemon RN (2002): Differences in the corticospinal projection from primary motor cortex and supplementary motor area to macaque upper limb motoneurons: An anatomical and electrophysiological study. Cereb Cortex 12:281–296. [DOI] [PubMed] [Google Scholar]
- Mathai A, Smith Y (2011): The corticostriatal and corticosubthalamic pathways: Two entries, one target. So what? Front Syst Neurosci 5:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent M, Parent A (2006): Single‐axon tracing study of corticostriatal projections arising from primary motor cortex in primates. J Comp Neurol 496:202–213. [DOI] [PubMed] [Google Scholar]
- Penfield W (1954): Mechanisms of voluntary movement. Brain 77:1–17. [DOI] [PubMed] [Google Scholar]
- Reiner A, Jiao Y, Del Mar N, Laverghetta AV, Lei W (2003): Differential morphology of pyramidal tract‐type and intratelencephalically projecting‐type corticostriatal neurons and their intrastriatal terminals in rats. J Comp Neurol 457:420–440. [DOI] [PubMed] [Google Scholar]
- Schucht P, Moritz‐Gasser S, Herbet G, Raabe A, Duffau H: Subcortical electrostimulation to identify network subserving motor control. Hum Brain Mapp (in press). DOI: 10.1002/hbm.22122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenderoth N, Debaere F, Sunaert S, Swinnen SP (2005): The role of anterior cingulate cortex and precuneus in the coordination of motor behaviour. Eur J Neurosci 22:235–246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information Video 1.


