Abstract
Perceptual decision making involves a distributed cortical network including areas related to sensory feature extraction, decision formation, and finally signalling the decision through a motor response. Although these processing steps are supposed to occur in sequence, the seemingly instant mapping of a perceptual decision onto a motor response renders these processes almost indistinguishable. To dissociate cortical areas related to sensory decision making from areas that prepare the subsequent motor response, we performed functional magnetic resonance imaging during a tactile spatial pattern discrimination task with interleaved immediate and delayed response conditions. Decision difficulty was manipulated parametrically by adding spatial noise to the tactile patterns, resulting in a rise in decision time with increasing noise. We assumed that areas involved in making the decision should show a variation in their activation with decision time and irrespective of whether (immediate response condition) or not (delayed response condition) a motor response could be prepared in advance. To exhibit these putative decision areas, we used response time, as was obtained in the immediate response condition, as parametric predictor for the difficulty‐dependent variations of blood oxygenation level‐dependent (BOLD)‐activity in both response conditions. BOLD activations in right (contralateral) postcentral sulcus, right intraparietal sulcus (IPS) and bilateral anterior insula (aINS) reflected this parametric modulation in both response conditions, suggesting a role of these areas in tactile decisions independent of decision‐specific motor preparation. Furthermore, a multivariate pattern analysis performed on the BOLD responses in the delayed response condition for a single difficulty level independently validated IPS and aINS as decision‐related areas. Hum Brain Mapp 36:3339–3350, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: anterior insula, fMRI, intraparietal sulcus, multivariate decoding, tactile discrimination, somatosensory, BOLD, posterior parietal cortex, human
INTRODUCTION
In perceptual discrimination subjects decide on the category to which a perceived stimulus belongs. In order to come to a decision and finally to a corresponding motor response, various cortical areas collaborate while exhibiting different levels of engagement in sensory, cognitive and motor processes. Extraordinary insights into the networks of cortical areas involved in perceptual decision tasks come from electrophysiological studies in non‐human primates [e.g., Gold and Shadlen, 2007; Hernandez et al., 2010; Romo et al., 2006]. For instance, the recording of neural activities in multiple cortical areas in monkeys while they were performing a vibrotactile discrimination task, revealed detailed information about the processing latencies of these areas within the vibrotactile decision network [Hernandez et al., 2010; Lemus et al., 2007]. These studies suggest a task‐related hierarchy of processing that ranges from early sensory to frontal cognitive and motor execution areas.
However, the functional role of each cortical area in the processing hierarchy remains largely unclear. It is debated whether the flow of information from sensory to motor areas is strictly serial or whether these information forwarding steps involve the joint activation of multiple down‐ and upstream cortical areas. In particular, do the cortical areas that generate a perceptual decision also prepare a corresponding motor response [e.g., Bennur and Gold, 2011; Park et al., 2014]? This question faces several challenges. First, as extracellular recordings in multiple areas along the sensory‐motor pathway were usually not done simultaneously and could not cover the whole brain, it is difficult to infer a clear‐cut hierarchy from the processing latencies reported. Second, the neuronal signals recorded from an area do not necessarily reflect its real function as it could be the recipient of such signals due to recurrent connections within the decision circuit [for a review, see Wang, 2008]. Another complication refers to the fact that in various studies, it is not fully clear whether the tuning of decision‐related neurons is sensory, motor, or both, as often the sensory decisions and their motor signalling could be correlated [Gold and Shadlen, 2007].
To exhibit the processing hierarchy within a decision network, it is thus useful to capture activity within the whole network as such. In fact, various researchers have resorted to functional magnetic resonance imaging (fMRI) in order to have a high spatial resolution and an easy coverage of the entire brain to study perceptual decision making [for a review, see Heekeren et al., 2008; Noppeney et al., 2010; Thielscher and Pessoa, 2007]. However, it remains challenging, when resorting to the slow blood oxygenation level‐dependent (BOLD) signals (in the range of several seconds), to capture and dissociate the temporal dynamics of cortical activities during a perceptual decision (in the range of a few hundred milliseconds). Hence, several previous fMRI studies applied more elaborate experimental designs and analyses methods to distinguish the sensory and motor networks underlying perceptual decision making [Filimon et al., 2013; Ho et al., 2009; Kayser et al., 2010a,b; Liu and Pleskac, 2011; Ploran et al., 2007]. Several of these studies highlight inferior frontal/anterior insula (aINS) region as decision related. Because these perceptual decision making studies were mainly performed in the visual domain, it is not clear, however, whether the perceptual decision areas are more modality specific (i.e., reflecting the sensory percept) or whether they act in a domain‐general fashion (i.e., reflecting an abstract sensory decision). We were thus interested to investigate the perceptual decision networks in the tactile domain, focusing on dissociating the human cortical representations that generate a perceptual decision, from representations of motor preparation that translate the decision into a corresponding motor behavior.
In the current fMRI study we asked healthy human subjects to perform a tactile spatial discrimination task. To identify decision‐task related areas, we varied task difficulty while assuming that increasing task difficulty should lead to larger BOLD responses in areas related to perceptual decision making. This assumption is based on previous fMRI studies [Kayser et al., 2010a; Liu and Pleskac, 2011; Noppeney et al., 2010] using either a diffusion model‐based (for a recent review, see Ratcliff and McKoon, 2008] analysis of their fMRI data or a model‐free analysis [Filimon et al., 2013]. More importantly, to disentangle representations of perceptual decision from those of motor preparation, we pseudorandomly interleaved immediate and delayed response conditions: in the immediate condition subjects could prepare motor responses immediately while in the delayed condition they could prepare their response only after a delay since the response options were initially unknown to them. Thus in the delayed condition a perceptual decision could be dissociated from a specific motor preparation due to the delay.
Whereas in the immediate condition the parametric modulation of cortical responses could be indicative for multiple task‐related processes, such as perceptual decision formation, motor preparation, or the response itself; in the delayed condition such modulation would mainly indicate processes related to the perceptual decision. Right (contralateral) postcentral sulcus (PCS), right intraparietal sulcus (IPS) and bilateral aINS showed a parametric modulation of their responses as a function of task difficulty in the delayed condition. As attention and unspecific motor preparation could have likewise caused similar parametric modulation in these areas, we additionally confirmed our findings by means of a multivariate pattern analysis (MVPA) that verified that decisions could be decoded from the BOLD response patterns within IPS and aINS.
MATERIALS AND METHODS
Subjects
Fifteen healthy right‐handed subjects (7 females, age 25 ± 2.8 years) participated in the study and gave their written informed consent prior to the experiment. Handedness was assessed according to the Edinburgh Handedness Inventory [Oldfield, 1971]. All subjects had normal or corrected‐to‐normal vision. The protocol of this human study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee.
Tactile Discrimination Task
The vibrotactile stimuli that were used in our discrimination tasks were delivered by a square‐shaped MRI‐compatible stimulation panel containing a 4 × 4 pin matrix (pin size Ø 1.1 mm, panel size 1.1 × 1.1 cm2). Each pin was controlled by a piezoelectric device that was used to vibrate that pin with an amplitude of 1.5 mm at 24 Hz, that is, in the flutter frequency range [Mountcastle et al., 1990; Talbot et al., 1968]. Patterns for tactile discrimination always consisted of 8 (out of 16) simultaneously vibrating pins. The rest of the pins remained beneath the panel surface. Specifically, pattern stimuli were presented for three difficulty levels, including intact, partially scrambled and fully scrambled patterns (see Fig. 1A for some pattern examples). Intact patterns consisted of two neighbouring horizontal (2 × 4) or vertical lines (4 × 2) of vibrating pins. In the partially scrambled patterns, two pseudorandomly selected pins of one of these “object‐lines” were inactive but instead two of the remaining “non‐object” pins were vibrating and thus served as distracters. These two distracter pins never formed another horizontal or vertical line. In fully scrambled patterns two pseudorandomly selected pins of each of the two “object‐lines” were inactive and four of the remaining “non‐object” pins were vibrating. Care was taken that no horizontal or vertical 4‐pin line was formed in the fully scrambled pattern. Stimuli were always applied for 3 s to the tip of the left middle finger, on which the tactile stimulator was attached to with medical tape and with moderate force to ensure consistent tactile sensation. After the stimulation period subjects could no longer feel the pins because they were retrieved beneath the panel surface.
Figure 1.
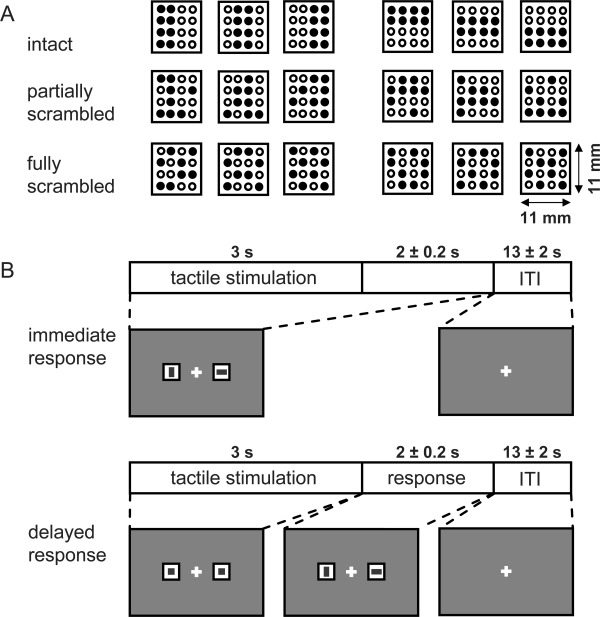
Tactile stimulus and trial structure. A. There were three difficulty levels in the tactile pattern discrimination task, using intact, partially scrambled and fully scrambled patterns representing either a vertical (left columns) or horizontal object (right columns). In intact patterns (top row), objects were defined by eight vibrating pins (filled circles; empty circles represent inactive pins that remain retrieved beneath the panel surface). In partially scrambled (middle row)/fully scrambled patterns (bottom row), 2/4 object‐pins were inactive while 2/4 nonobject pins were vibrating and served as distracters, respectively. B. There were two task conditions. In both conditions subjects initially received a tactile stimulation for 3 s in order to perform the discrimination task. In the immediate response condition, subjects were instructed to report the perceived orientation by making a quick saccade towards the corresponding icon (horizontal or vertical bar, randomly placed left or right) as soon as a decision was made. The response cues were present at the onset of the tactile stimulation and lasted 5 ± 0.2 s. In contrast, in the delayed response condition, they could only respond when the neutral icons (squares), presented during stimulation (3 s), turned into the response options (lasting 2 ± 0.2 s) at the offset of the tactile stimulus. Both types of trials, as well as the three difficulty levels, were presented pseudorandomly interleaved and were counterbalanced within and across sessions.
Visual task instructions (Fig. 1B) were back‐projected on a translucent screen at the head‐end of the MRI‐scanner. Subjects viewed that screen via a mirror that was mounted on the head coil of the scanner. Each trial started with the 3 s tactile stimulation epoch. Subjects were asked to discriminate whether the stimulus provided during this epoch consisted of either a vertical or a horizontal bar‐shaped pattern with respect to the finger axis. Subjects indicated their decision by either making a leftward or rightward saccade towards the corresponding icon (a horizontal or vertical bar at 4.2° eccentricity) on the response screen. We opted for using saccades instead of button presses as a response modality in order to better separate tactile and motor processes. Subjects were instructed to always maintain central visual fixation until the response screen was shown and until they have reached their final decision. After the saccade, subjects were instructed to return to the central fixation cross immediately and to maintain central fixation throughout the consecutive inter‐trial interval of 13 ± 2 s duration (randomly chosen in 200 ms steps).
There were two task conditions, which were identical with respect to the tactile stimulation (Fig. 1B). In the immediate response condition, the visual response cues were presented immediately at the onset of the tactile stimulation for 5 ± 0.2 s and the subjects were asked to respond as soon as a perceptual decision was made. In the delayed response condition, however, neutral cues (squares) were presented at tactile stimulation onset. Only at the offset of the tactile stimulation these cues then turned instantly into the response options and remained visible for 2 ± 0.2 s. Importantly, the positions of the horizontal and the vertical response cues (left or right) were randomized across trials so the direction of the saccade needed to indicate a perceptual decision was undefined until these response cues were presented. To minimize visual stimulus differences, every icon consisted of the same amount of grey and white pixels.
For each subject, we collected a total of 144 trials divided into four scanning sessions with short breaks in‐between. Each session started and ended with a long 18 ± 2 s fixation period. Within each session the immediate and delayed response conditions as well as the patterns within and across difficulty levels were interleaved and their order was pseudorandomized and counterbalanced. Prior to the fMRI measurement, every subject received about 50 training trials (depending on individual performance) until she/he was familiar with the tasks.
Behavioral Monitoring
For response recording, eye movements were monitored using an MRI‐compatible infrared camera‐system positioned over the right eye (Resonance Technology), which was used in combination with an eye‐tracking system from Arrington Research (Monocular PC‐60 integrator system, PCI framegrabber and ViewPoint eye‐tracking software). Eye calibration was done prior to each session. The eye movement data were sampled at 60 Hz and analyzed offline using custom written scripts in Matlab (MathWorks). Preprocessing included removal of eye closure and blinks. Next, saccades were detected using an absolute velocity threshold (20°/s).
The reaction times (RTs) defined the latency of subjects' behavioural decisions and were measured from the onset of the response options until the occurrence of the first horizontal saccade that was larger than 3.1° (thus excluding small fixational saccades). Across our 15 subjects a total of four trials, in which subjects did not respond or responded in anticipation (RT < 150 ms), were excluded from the RT‐ and further fMRI‐ analyses. To further examine subjects' quality of fixation we also assessed the number of trials in which horizontal saccades larger than 1° were preceding the actual response (detected at 3.1°). There was a total of 23 trials across all 15 subjects and we have not found any significant difference (2 × 3 repeated measures ANOVA) in their distribution, neither across response conditions (F [1,14] = 0.028, P = 0.87), nor across difficulty levels (F [2, 28] = 0.18, P = 0.84), nor for their interaction (F [2,28] = 0.18, P = 0.84). Due to such lack of a systematic bias we did not exclude those trials from analyses. In addition to the RTs we calculated hit rates, defined as the share of trials with a correct saccadic response relative to the overall number of trials. Note that for the fully scrambled patterns, the orientations of the original intact patterns from which they derive were used as their reference orientation. As no feedback about whether a response was correct or incorrect was provided, subjects could not learn how a fully scrambled pattern corresponded to a particular orientation. To ensure accuracy, saccadic data were also screened manually on a trial‐by‐trial basis. Statistical analysis of the behavioral data was carried out using SPSS (IBM).
fMRI Measurement
The experiment was conducted with a 3‐T MR scanner (Siemens Trio, Max Planck Institute of Biological Cybernetics, Tuebingen, Germany) equipped with a 12‐channel head coil. Each functional scan was acquired with an echo‐planar imaging sequence (TR = 2 s, TE = 35 ms, flip angle 90°), consisting of 30 axial slices (thickness: 3.2 mm) covering the whole cerebral cortex in a descending order, with a planar resolution of 3 × 3 mm2 and an inter‐slice gap of 0.8 mm. For every subject a high‐resolution (1 mm, isotropic) T1‐weighted structural image was acquired with an MPRAGE sequence directly after the functional imaging sessions.
fMRI Data Analysis
The fMRI data were analyzed using Brain Voyager QX 2.0 (Brain Innovation, Maastricht, the Netherlands). Specifically, we discarded the first two image volumes of a functional imaging session. Next, for each session we performed a slice scan time correction, a three‐dimensional rigid‐body motion correction, and temporal high‐pass filtering (0.0045 Hz, including linear trend removal). Inter‐session image alignment was done by aligning all functional data to the first volume of the session that was scanned closest to the anatomy session. Finally, functional images were coregistered to subjects' anatomical images. To allow a group analysis, segmented anatomical images were transformed into Talairach space, a polygon mesh was tessellated at the boundary of white and grey matter for each hemisphere and was smoothed to reconstruct the cortical surface for each subject. Then we performed a cortex‐based inter‐subject alignment to the average cortices of our subject pool. To achieve a better spatial correspondence of cortical areas among subjects, each cortical hemisphere was thereby morphed into a spherical representation [Fischl et al., 1999]. The resulting alignment parameters for a given subject were then used to “normalize” the coregistered functional images for subsequent fMRI group analysis.
A multisubject random‐effects general linear model (GLM) analysis was performed that built on individual subjects' GLMs, which included two main predictors (immediate and delayed response condition), each of which had one corresponding parametric modulation predictor, plus six regressors representing the parameter estimates of the 3D rigid‐body motion correction (x, y, z translation and rotation) and a constant term. The main predictors were modelled with a box car function (starting from the onset of the tactile stimulus till the offset of the response cue and independent from the RT), which was convolved with a two‐gamma hemodynamic response function (peak at 5 s, weight 1). In addition, a parametric predictor of the same duration (5 ± 0.2 s) was added for each response condition. Its weight was defined session‐wise by a correlate of the time when the decision was made, namely subject's average z‐scored RT, estimated in the immediate response condition and for each given task difficulty level.
These parametric predictors were supposed to capture any residual modulation of the BOLD response according to the RT differences, and thus would help to exhibit cortical areas that differ in their decision speeds for different task difficulties [Buchel et al., 1998]. Hence, we did not add three additional main predictors corresponding to the three difficulty levels to model the data, since the mean RTs for each difficulty level as the parametric predictors were supposed to per se capture the activation variations under different difficulty levels.
Specifically, the weights derived from the immediate response condition were applied as weights for the parametric predictors of both immediate and delayed response conditions. We adopted this approach since the immediate and delayed response trials were pseudorandomly interleaved within each session and since we reasoned that the perceptual decision speed should thus be similar for a given difficulty level in both response conditions within individual sessions. z‐Scoring the RT values ensured that the weights for the main and the parametric predictors are in the same scale. In addition, z‐scoring also helped to account for the inter‐subject variability in a way that it reflects the individual reliability of the modulation. Note that the weights of the parametric predictor thereby inversely reflected the decision speed: the shorter the RT, the faster the BOLD integration process, the smaller the BOLD response.
In addition, in order to test the robustness of using the averaged RTs to define the parametric weights in the immediate condition, we also used subjects' actual RTs for the parametric weighting in each individual trial. The GLM analysis revealed that results of the trial‐by‐trial RT weighting approach were very similar to the results of the average RT weighting approach in the immediate condition. Thus the average RT weighting approach was sensitive enough to capture the relative differences in BOLD responses elicited by different difficulty levels. Since in the delayed response condition the corresponding RT was not predictive for decision processes and in order to guarantee identical analyses across response conditions, we adopted the average RT of the immediate response condition as a predictor for the modelling of task difficulty in both, the immediate and the delayed response conditions.
At the second level, a conjunction analysis of the main and its corresponding parametric predictors was calculated across subjects to unveil task‐related cortical areas that displayed parametrically modulated BOLD‐responses in the immediate or the delayed response condition, respectively. We only report areas that survived our statistical threshold criterion of P = 0.05 (corrected). Multiple comparison correction was done by cluster thresholding (1000 permutations) via Monte Carlo simulations [Forman et al., 1995] as integrated in the BrainVoyager QX 2.0 software package.
MVPA Procedure
To re‐examine the areas revealed by the parametric modulation in the delayed response condition which were supposedly engaged in perceptual decision making, we adopted a MVPA. Using this approach we tried to classify “vertical” and “horizontal” decisions based on the patterns of BOLD activity across vertices within a region‐of‐interest (ROI) and for a single task difficulty. In case of an extended cluster of activation, the cluster was manually divided into several smaller ROIs according to both the local activation maxima and the anatomical borders of sulci and gyri. The MVPA approach promised several advantages over conventional model‐based analyses. First, it allowed to independently assess whether a given ROI has decision‐related information independent of any prior assumption. Second, since the seminal study by Haxby et al. [2001], MVPA has shown its great advantage over traditional univariate analyses in exhibiting distributed information hidden to the latter approach [Haynes and Rees, 2006; e.g., Kamitani and Tong, 2005; Kriegeskorte and Bandettini, 2007]. We performed MVPA with a combination of in‐house Matlab scripts and an open‐source Matlab toolbox for BrainVoyager (Neuroelf Version 0.9c by Jochen Weber) which implemented a Support Vector Machines (SVM) binary classifier (libSVM, http://www.csie.ntu.edu.tw/∼cjlin/libsvm/).
Our MVPA procedure (linear kernel function, constant cost parameter C = 1) is similar to what has been previously described in other fMRI studies [for a recent study, see Gallivan et al., 2011; for a detailed tutorial, see Pereira et al., 2009]. In short, we selected 100 vertices (the reconstructed cortical surface mesh for each hemisphere consists of 40,962 vertices) from each ROI by ranking vertices according to the t‐statistics of each vertex in the delayed response condition (conjunction of main and parametric predictors) and selecting the top‐ranked 100 vertices. There were three reasons why we followed this particular approach: First, we selected a fixed amount of vertices to ensure that decoding performance could not have been biased due to differences in ROI size. Second, across the four ROIs (aINS'_L: 105 vertices; PCS'_R: 351 vertices; IPS'_R: 232 vertices and aINS'_R: 135 vertices) we had to limit the maximum number of vertices considered according to the size of the smallest ROI (aINS'_L: 105 vertices). Third, Li and coworkers [2007] have used a similar approach to decode categorical decisions and they have shown that their classification accuracy reached a plateau at about 100 voxels, when decoding from the 1–200 top‐ranked (according to their t‐values) voxels. We are aware that there are superior methods for feature selection [such as nested‐cross‐validation, see Pereira et al., 2009]. Yet, these methods do typically require larger numbers of trials than were recorded in our experiment. Therefore, we did not systematically test different alternatives in feature selection and cross validation. Trial classification was restrained to the trials of the intact pattern quality (easy) as the trials of the other two pattern qualities had a strong imbalance between “vertical” and “horizontal” decision labels within each subject. This could have led to highly biased decoding results. Specifically, in easy trials, since the discrimination task was perhaps so easy, no consistent bias was found across subjects (P = 0.8, paired t‐test). There was a trend for such bias (P = 0.08) in the medium difficulty (partially scrambled) trials, and this bias became statistically significant (P = 0.01) in difficult (fully scrambled) trials. Nevertheless, this bias did not seem to affect the parametric modulation as it was present in individual subjects—irrespective of the direction of an individual's bias (see Fig. S2 in the Supporting Information).
The percent BOLD signal change at each time point of interest (0 to 6 s after tactile stimulus onset) in each vertex within a ROI was extracted and linearly interpolated (to 1 s temporal resolution) as input data for the classifiers. The baseline was defined as the average BOLD signal between −2 s and 0 s relative to tactile stimulus onset and calculated separately for each session. We chose these various time points of interest in order to exhibit decoding performance across time. After the extraction of BOLD percent signal change for each time point, we z‐scored the values for each vertex within a ROI across trials and sessions. Note that for the time period after the onset of the response cues in the delayed response condition, there is a potential limitation in the interpretation of decoding performance. The BOLD signal could then reflect a mixture of processes related to both decision making as well as response preparation. However, one also has to keep in mind that saccade response directions were not correlated with the actual perceptual decision, making it hard for a decoder to predict a decision solely based on uncorrelated motor information that might (or might not) have been present in any given ROI.
Classifiers were trained based on subjects' actual decisions, independent of their correctness. For most of the subjects, there were 12 trials for each decision label (three trials of immediate vertical or horizontal intact patterns per session times four sessions per subject). Yet, a few subjects had decided more often for one pattern orientation than for the other. In these subjects an equal number of trials were randomly drawn for each decision label. We used a “leave‐two‐trials‐out” cross‐validation to train and test the binary SVM classifiers, that is, two trials of each label (four trials in total) were left for testing the classifier and ten trials of each label (20 trials in total) were used for training the classifier (there were one or two trials variation for a few subjects). Considering the relatively few amount of trials subjected to the MVPA procedure and thus a potential bias in the accuracy of the decoding performance, we performed a full cross validation so that each individual trial was used equally often as a training trial, as well as a testing trial. This resulted in up to 4356 training‐and‐testing iterations per subject (12 trials for each label yielded 66 possible combinations when two trials were taken out for testing, leaving the rest ten trials for training. Thus a full combination to train and test both labels yielded 66 × 66 = 4356 combinations). This large number of iterations provided us a highly reliable and precise estimate of classification performance [Gallivan et al., 2011]. The decoding accuracy for each ROI in every subject was thereby calculated as the mean of all the iterations. The statistical significance of the decoding performance was evaluated across subjects with a two‐tailed t test, testing against 50% decoding performance, that is, chance level. A false discovery rate (FDR) correction of q ≤ 0.05 was applied for multiple comparison correction given all the t‐tests performed [Benjamini and Yekutieli, 2001].
RESULTS
Behavioural Data
In this study, subjects received a vibrating (24 Hz) pattern stimulus lasting 3 s on the left middle fingertip and had to decide whether the bar‐shaped pattern (Fig. 1A) was vertical or horizontal relative to their finger axis. Three task difficulty levels (provided by intact, partially scrambled and fully scrambled patterns) were used to manipulate the perceptual decision speed. In the immediate response condition, the visual response icons (Fig. 1B) for indicating a horizontal or vertical pattern were already available at the onset of the tactile stimulus and the subjects were asked to make a saccade toward the corresponding icon as soon as a perceptual decision was made. Their mean hit rate was 0.97 ± 0.04 (± standard deviation) for the intact patterns, 0.73 ± 0.10 for the partially scrambled and 0.49 ± 0.10 for the fully scrambled patterns (Fig. 2A). The corresponding mean RTs were 845 ± 200 ms, 1151 ± 292 ms, and 1347 ± 492 ms (Fig. 2B). RTs were measured from the onset of the response options until the onset of a horizontal saccade. In the delayed response condition, neutral visual cues (Fig. 1B) were provided during the tactile stimulation and the response options were only available at the offset of the tactile stimulus so that the subjects could not prepare a saccade in a specific direction in advance. Their mean hit rate was 0.96 ± 0.05 for the intact patterns, 0.73 ± 0.10 for the partially scrambled and 0.49 ± 0.11 for the fully scrambled patterns (Fig. 2A). The corresponding mean RTs were 437 ± 99 ms, 443 ± 127 ms, and 445 ± 125 ms (Fig. 2B).
Figure 2.
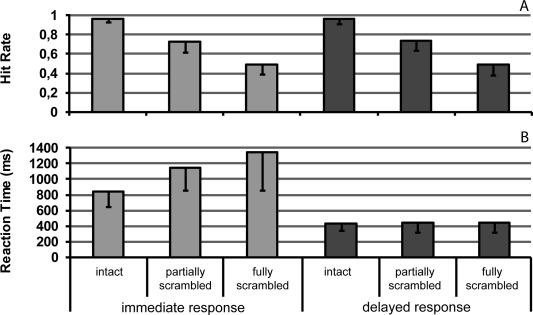
Behavioral results. A. Bars reflect subjects' mean hit rate as a function of task difficulty and response condition. Hit rates generally decreased with an increase in task difficulty. This is indicated by a 2 × 3 repeated‐measures ANOVA, which revealed a significant main effect of task difficulty (F [2, 28] = 217, P < 0.001) but no significant main effect of response condition (F [1, 14] = 0.007, P = 0.933) or significant interaction (F [2, 28] = 0.054, P = 0.940). B. Bars depict subjects' mean reaction time as a function of task difficulty and response condition. Both, the main effect of task difficulty (F [2, 28] = 29, P < 0.001) and of response condition (F [1, 14] = 101, P < 0.001), as well as their interaction (F [2, 28] = 32, P < 0.001) were significant. The effect of task difficulty thereby results from a decrease in reaction times in the immediate response condition, which is indicative for a putative increase in perceptual decision speed. The obvious lack of an influence of task difficulty on subjects' reaction times in the delayed response condition, however, is perhaps due to the fact that the saccadic response could only be prepared after stimulus offset and thus most likely after a perceptual decision had already been reached. Error bars denote standard deviations. The two gray tones of the bars serve to differentiate the two response conditions.
For the hit rate data a 2 × 3 (factor response condition: immediate vs. delayed response; factor task difficulty: intact, partially scrambled and fully scrambled pattern) repeated‐measures analysis of variance (ANOVA, Greenhouse‐Geisser correction for nonsphericity) revealed a significant main effect of task difficulty (F [2, 28] = 217, P < 0.001). Neither a significant effect of response condition (F [1, 14] = 0.007, P = 0.933) nor a significant interaction (F [2, 28] = 0.054, P = 0.940) was found. Therefore, the performances in the immediate and delayed response conditions are comparable. For the RT data, a 2 × 3 repeated‐measures ANOVA revealed significant effects for response condition (F [1, 14] = 101, P < 0.001), for task difficulty (F [2, 28] = 29, P < 0.001) and for their interaction (F [2, 28] = 32, P < 0.001). A post hoc one‐way repeated‐measures ANOVA revealed a significant difference in RTs across the difficulty levels in the immediate condition (F [2, 28] = 36, P < 0.001). This suggests that the perceptual decision speed was successfully manipulated across the tactile pattern quality in the immediate response condition. Moreover, a post hoc one‐way repeated‐measures ANOVA revealed no significant difference in RTs across difficulty levels in the delayed condition (F [2, 28] = 0.607, P = 0.520). This indicates that the RTs in the delayed response condition only reflected the time to initiate motor behavior but not the time to decide, that is, subjects already made their perceptual decision before the presentation of the response options.
Parametrically Modulated BOLD Activations
Conjunction analyses of main and parametric predictors were performed to exhibit task‐related areas that show decision‐related processes while minimizing false positives, that is, areas that manifest parametric modulation must at the same time also be activated during the task. In the immediate response condition, the conjunction analysis revealed a distributed cortical network that displayed parametrically‐modulated BOLD responses (Fig. 3A, P < 0.005 [P corrected = 0.05], cluster thresholding with 1000 Monte Carlo permutations [Forman et al., 1995] [left hemisphere: 181 mm2; right hemisphere: 221 mm2], see also Table S1 in the Supporting Information for the Talairach coordinates of each activated area). This network included regions within the right calcarine sulcus (primary visual cortex, V1), left (ipsilateral to the stimulated hand) dorsal premotor cortex (dPM), left medial intraparietal sulcus (mIPS); bilateral PCS, anterior intraparietal sulcus (aIPS), lateral intraparietal sulcus (ℓIPS), superior parietal lobule (SPL), ventral premotor cortex (vPM), aINS spilling over to the frontal operculum (FO), pre‐supplementary motor area (pre‐SMA), supplementary motor area (SMA), and anterior cingulate cortex (ACC).
Figure 3.
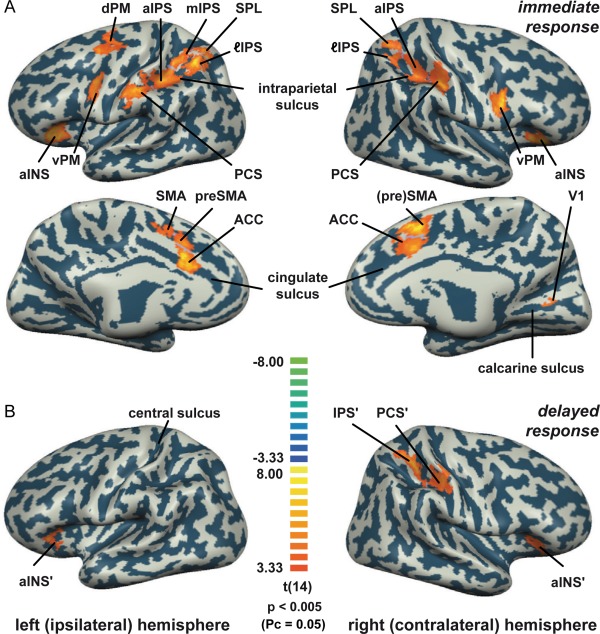
Task‐related areas exhibiting parametric modulation of BOLD response. A. Immediate response condition. Areas could represent perceptual decision, motor preparation, or the response itself. B. Delayed response condition. Depicted areas should be mainly involved in processes related to the decision. All BOLD activation maps were based on the results of the random‐effects conjunction analyses of the task‐related main and its parametric modulation predictor (Pcorrected = 0.05). The results were overlaid on the partially inflated cortices of a representative subject.
In the delayed response condition, a conjunction analysis of the main and the z‐scored parametric predictors derived from the average RT of the immediate response condition, revealed significant activation in regions within the right PCS, right IPS and bilateral aINS/FO (Fig. 3B, area labels marked with an apostrophe (') are introduced to allow differentiation from the corresponding areas mapped in the immediate condition, P < 0.005, [Pcorrected = 0.05], [left hemisphere: 119 mm2; right hemisphere: 185 mm2]). This indirectly suggests that areas related to the perceptual decision processes might behave similarly for both the immediate and the delayed response conditions. An overlay of the aforementioned areas for both immediate and delayed response conditions shows that the cortical network revealed in the delayed response condition was largely a subset of the network disclosed in the immediate response condition (see Fig. S1 B in the Supporting Information). In addition, we performed a conjunction analysis across both main predictors and their parametric modulators. The resulting map was actually identical to the activation map from the conjunction of both parametric predictors only (see Fig. S1 A in the Supporting Information). While the areas revealed for the immediate response condition could represent processes related to the perceptual decision, motor preparation, or the response itself, the areas revealed for the delayed response condition should be mainly involved in processes related to the decision.
MVPA Decoding Performance
In our primary analysis we assumed that activities within the perceptual decision areas would have similar parametric modulations according to decision difficulty levels for both response conditions. Using this approach, we were able to exhibit the right PCS, right IPS and bilateral aINS as perceptual decision‐related areas in our tactile discrimination task with the delayed behavioral response. To further examine whether these areas indeed carry decision‐related information independent of any prior assumption, and to also indirectly rule out that their parametric modulation was solely due to differences in subjects' attention to account for different task difficulty levels as well as unspecific motor preparation, we performed MVPA to decode subjects' perceptual decision in individual trials based on the spatial BOLD activation pattern within the putative perceptual decision areas mentioned above. Our decoding results showed that at as early as the 2nd second after tactile stimulus onset, tactile decisions could be predicted at above chance (Table 1, FDR q < 0.05 highlighted in gray) in two of the four ROIs, namely bilateral aINS (aINS'_R: accuracy: 56%, P uncorrected = 0.01, aINS'_L: accuracy: 56%, P uncorrected = 0.01). The remaining ROIs did not reach significant decoding performance at this early time point (PCS'_R: accuracy: 46%, P uncorrected = 0.05, IPS'_R: accuracy: 53%, P uncorrected = 0.29). Specifically, while decoding performance in bilateral aINS was above chance from the 2nd to the 6th second after stimulus onset, decoding performance in IPS'_R became only significant from the 4th second on, and in PCS'_R decoding performance was never higher than chance level at any of the time points tested. Hence, our decoding analysis provides an assumption‐free support for the notion that bilateral aINS and the right IPS do carry decision‐related information and that this information is already present at a time at which it cannot be corrupted by any decision‐specific motor preparation in the delayed response condition—at least in case of bilateral aINS.
Table 1.
Multivariate pattern decoding in parametrically modulated areas in the delayed response condition.
| Time | ROIs | PCS'_R | IPS'_R | alNS'_R | alNS'_L |
|---|---|---|---|---|---|
| 0 s | Accuracy | 53.01 | 50.38 | 49.82 | 49.08 |
| P uncorrected | 0.43 | 0.90 | 0.94 | 0.73 | |
| 1 s | Accuracy | 50.04 | 53.97 | 51.82 | 49.64 |
| P uncorrected | 0.99 | 0.25 | 0.45 | 0.83 | |
| 2 s | Accuracy | 46.48 | 52.77 | 56.37 | 56.00 |
| P uncorrected | 0.05 | 0.29 | 0.01 | 0.01 | |
| 3 s | Accuracy | 44.31 | 52.70 | 58.98 | 60.61 |
| P uncorrected | 0.01 | 0.19 | 0.01 | 0.01 | |
| 4 s | Accuracy | 46.42 | 57.39 | 57.32 | 57.64 |
| P uncorrected | 0.04 | 0.01 | 0.02 | 0.03 | |
| 5 s | Accuracy | 47.95 | 59.18 | 58.51 | 56.99 |
| P uncorrected | 0.30 | 0.01 | 0.01 | 0.02 | |
| 6 s | Accuracy | 50.70 | 63.13 | 57.46 | 56.73 |
| P uncorrected | 0.77 | 0.01 | 0.01 | 0.02 |
The percent BOLD signal change at each respective time point of interest (0 s to 6 s after tactile stimulus onset) in each vertex within a ROI was extracted as input data for the classification. The baseline was defined as the average BOLD signal between −2 s to 0 s relative to tactile stimulus onset and calculated separately for each session. The table displays the mean decoding accuracy (in %) for each ROI across subjects and the uncorrected P values (two‐tailed t test against 50% decoding performance, i.e., chance level, gray highlighted cells indicate significance with FDR correction of q ≤ 0.05 above chance) at each time point.
DISCUSSION
This fMRI study aimed at dissociating the cortical processes related to perceptual decision making and the subsequent motor preparation during human tactile pattern discrimination. Patterns of varying noise levels were used to manipulate the speed of perceptual decision making parametrically, as was confirmed by an analysis of subjects' response times. We found that the right PCS (i.e., contralateral to the stimulated hand), right IPS and bilateral aINS showed a correlated parametric modulation of their task‐related BOLD activation, irrespective of whether or not a saccade could be planned to signal the tactile decision. Furthermore, independent from any prior assumption, with MVPA we could further decode subjects' decisions from the BOLD signals of bilateral aINS already at the 2nd second after tactile stimulation onset, and at the 4th second from the BOLD signals of right IPS. This implies that these latter areas could function as tactile decision‐makers. In addition, right V1, right ACC, left dPM, left PCS extending to IPS and SPL; bilateral vPM and (pre‐) SMA exhibited parametrically‐modulated BOLD activation, but only in the immediate response condition. This suggests that these areas were more likely engaged in preparing the decision‐related eye‐movement response (and in processing its sensory consequences, i.e., in V1).
Parametric BOLD Modulation and Perceptual Decision Making
The rationale behind the identification of brain areas engaged in perceptual decisions has already been laid out in detail in Introduction. In short, we successfully identified decision‐related cortical representations by the parametric modulation of their BOLD responses as a function of task difficulty, based on an experimentally well‐established approach [Filimon et al., 2013; Ho et al., 2009; Kayser et al., 2010a; Liu and Pleskac, 2011; Noppeney et al., 2010]. Engaging a delayed response condition allowed us to additionally probe the impact of difficulty levels on decision speed without confounding motor involvement.
The observed parametric modulation of BOLD activity could, however, still be explained differently. For instance, the biases in perceptual decisions that systematically varied across difficulty levels, as was reported above in the methods section, could have accounted for the parametric modulation reported. Yet, the presence of perceptual biases was unlikely to explain the parametric modulation as it was present in individual subjects, irrespective of the respective direction of an individual's bias (see also Fig. S2 in the Supporting Information). The parametric modulation could also not be due to subjects' motor responses as there was no significant bias for the distribution of saccade directions (easy: P = 0.58; medium: P = 0.82; difficult: P = 0.29, paired t‐tests). One might also argue that more attention is required when discrimination is more difficult and this could likewise lead to stronger BOLD responses. Yet, importantly, using MVPA we could show that decisions for a single difficulty level could be significantly decoded in all of the putative decision areas except PCS. Since different levels of attention for different difficulty levels obviously cannot explain the results of the decoding obtained for a single difficulty level, we expect that attention—if at all—would only contribute to part of the activation we attributed to perceptual decision making in bilateral aINS and right IPS. Moreover, and in agreement with our decoding results, only the PCS has consistently been shown to be modulated by tactile attention [Macaluso et al., 2000; Meador et al., 2002; Roland, 1981; Schubert et al., 2008]. Thus, the parametric modulation of the right PCS activation might be mainly due to varying levels of attention that were modulated by task difficulty.
While PCS (Brodmann area 2, primary somatosensory cortex) is involved in somatosensory information processing and thus might not be a decision area per se (see also the MVPA results of the present study), IPS [Kayser et al., 2010a] and aINS contained tactile decision signals in our task and have also been implicated in perceptual decision processes of other sensory modalities [Ahlfors et al., 1999; Binder et al., 2004; Ho et al., 2009; Kayser et al., 2010a,b; Ploran et al., 2007; Thielscher and Pessoa, 2007]. Specifically, in monkey studies the lateral portion of the IPS has been shown to signal visual coherent motion decisions [Hanks et al., 2006; Shadlen and Newsome, 2001]. Furthermore, converging evidence has pointed to an essential role of aINS in human awareness, such as the “feeling of knowing” [Kikyo et al., 2002] and the “moment of recognition” [Ploran et al., 2007]—also in the context of decision tasks (for reviews, see Craig, 2009, 2011]. Interestingly, experimental approaches aiming to dissociate perceptual decisions from motor execution by linking a perceptual decision to different motor response modalities all revealed the involvement of aINS in decision making [Ho et al., 2009; Liu and Pleskac, 2011]. Taken together with our MVPA results, it appears that bilateral aINS could be where the perceptual decisions are formed as aINS showed the earliest successful decoding in our tactile task. Nevertheless, we cannot rule out that additional brain regions are involved in the decision process in our task that, however, were not picked up by our procedure. Moreover, moderate MVPA decoding performance might be further improved by larger number of trials. However, in another study on perceptual decision making [Li et al., 2007], similar ∼60% prediction accuracy was reported for frontal areas.
Decision‐Related Motor Preparation
Due to the instant mapping of a perceptual decision to the corresponding motor action, it remains a difficult challenge for researchers to separate the neural correlates underlying the formation of a perceptual decision from the preparation of a motor response signalling that decision. Our approach to this problem was to interleave trials, in which subjects could report their decision whenever ready, with another trial type, in which they could form a decision but could only report it after a short delay when the mapping between decision and motor responses was revealed to them. Apart from the abovementioned decision‐related areas that showed a decision‐time‐related parametric modulation in both response conditions, regions within the right V1, left dPM, left mIPS; bilateral PCS, aIPS, ℓIPS, SPL, vPM, aINS, pre‐SMA, SMA, and ACC exhibited a parametric BOLD modulation only in the immediate response condition, suggesting a role in motor action and, in case of V1, in processing the sensory consequences of the saccade. Several of these areas have been previously associated with perceptual decisions. Ploran and coworkers for instance suggested that besides the bilateral aINS/FO, also the SMA, the ACC, and some other areas (thalamus and right inferior parietal lobule) might be engaged in visual pattern discrimination and decision making [Ploran et al., 2007]. In comparison to our study, their experimental design (and that of several other of the aforementioned studies), however, did not permit a direct dissociation of processes related to decision making from decision‐related motor preparation. It is therefore likely that a subset of the areas they have reported, such as SMA and ACC, rather contributes to the preparation or the monitoring of the corresponding motor response.
Those “motor areas” could be holding the categorical perceptual decision (e.g., “horizontal” or “vertical”) online by unspecifically preparing all possible response options while waiting for the context cue that ultimately specifies which option to take [Cisek and Kalaska, 2005; Klaes et al., 2011]. Another area that could have served such function in our experiment is the putative homologue of monkey frontal eye field (here referred to as dPM), which also showed a parametric modulation of the BOLD response in the immediate but not in the delayed response condition.
CONCLUSION
Our present findings suggest that the aINS might reflect the ultimate stage within the processing hierarchy that translates sensory information into a categorical (abstract) decision. It might thereby reflect a key node that allows connecting a discrete perceptual decision to a corresponding motor behavior.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors thank Juergen Dax for technical support, and Fabienne Kernhof and Manuel Roth for their help with the data collection. They are grateful to the Max Planck Institute of Biological Cybernetics and especially to Frank Muehlbauer for their support of the fMRI measurement. They also thank the anonymous reviewers of a previous version of this manuscript for their helpful suggestions.
REFERENCES
- Ahlfors SP, Simpson GV, Dale AM, Belliveau JW, Liu AK, Korvenoja A, Virtanen J, Huotilainen M, Tootell RB, Aronen HJ, Ilmoniemi RJ (1999): Spatiotemporal activity of a cortical network for processing visual motion revealed by MEG and fMRI. J Neurophysiol 82:2545–2555. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Yekutieli D (2001): The control of the false discovery rate in multiple testing under dependency. Ann Stat 29:1165–1188. [Google Scholar]
- Bennur S, Gold JI (2011): Distinct representations of a perceptual decision and the associated oculomotor plan in the monkey lateral intraparietal area. J Neurosci 31:913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Liebenthal E, Possing ET, Medler DA, Ward BD (2004): Neural correlates of sensory and decision processes in auditory object identification. Nat Neurosci 7:295–301. [DOI] [PubMed] [Google Scholar]
- Buchel C, Holmes AP, Rees G, Friston KJ (1998): Characterizing stimulus‐response functions using nonlinear regressors in parametric fMRI experiments. Neuroimage 8:140–148. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF (2005): Neural correlates of reaching decisions in dorsal premotor cortex: Specification of multiple direction choices and final selection of action. Neuron 45:801–814. [DOI] [PubMed] [Google Scholar]
- Craig AD (2009): How do you feel now? the anterior insula and human awareness. Nat Rev Neurosci 10:59–70. [DOI] [PubMed] [Google Scholar]
- Craig ADB (2011): Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad Sci 1225:72–82. [DOI] [PubMed] [Google Scholar]
- Filimon F, Philiastides MG, Nelson JD, Kloosterman NA, Heekeren HR (2013): How embodied is perceptual decision making? Evidence for separate processing of perceptual and motor decisions. J Neurosci 33:2121–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM (1999): High‐resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp 8:272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC (1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster‐size threshold. Magn Reson Med 33:636–647. [DOI] [PubMed] [Google Scholar]
- Gallivan JP, McLean DA, Smith FW, Culham JC (2011): Decoding effector‐dependent and effector‐independent movement intentions from human parieto‐frontal brain activity. J Neurosci 31:17149–17168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN (2007): The neural basis of decision making. Annu Rev Neurosci 30:535–574. [DOI] [PubMed] [Google Scholar]
- Hanks TD, Ditterich J, Shadlen MN (2006): Microstimulation of macaque area LIP affects decision‐making in a motion discrimination task. Nat Neurosci 9:682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P (2001): Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science 293:2425–2430. [DOI] [PubMed] [Google Scholar]
- Haynes JD, Rees G (2006): Decoding mental states from brain activity in humans. Nat Rev Neurosci 7:523–534. [DOI] [PubMed] [Google Scholar]
- Heekeren HR, Marrett S, Ungerleider LG (2008): The neural systems that mediate human perceptual decision making. Nat Rev Neurosci 9:467–479. [DOI] [PubMed] [Google Scholar]
- Hernandez A, Nacher V, Luna R, Zainos A, Lemus L, Alvarez M, Vazquez Y, Camarillo L, Romo R (2010): Decoding a perceptual decision process across cortex. Neuron 66:300–314. [DOI] [PubMed] [Google Scholar]
- Ho TC, Brown S, Serences JT (2009): Domain general mechanisms of perceptual decision making in human cortex. J Neurosci 29:8675–8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamitani Y, Tong F (2005): Decoding the visual and subjective contents of the human brain. Nat Neurosci 8:679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser AS, Buchsbaum BR, Erickson DT, D'Esposito M (2010a): The functional anatomy of a perceptual decision in the human brain. J Neurophysiol 103:1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser AS, Erickson DT, Buchsbaum BR, D'Esposito M (2010b): Neural representations of relevant and irrelevant features in perceptual decision making. J Neurosci 30:15778–15789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikyo H, Ohki K, Miyashita Y (2002): Neural correlates for feeling‐of‐knowing: An fMRI parametric analysis. Neuron 36:177–186. [DOI] [PubMed] [Google Scholar]
- Klaes C, Westendorff S, Chakrabarti S, Gail A (2011): Choosing goals, not rules: Deciding among rule‐based action plans. Neuron 70:536–548. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Bandettini P (2007): Analyzing for information, not activation, to exploit high‐resolution fMRI. Neuroimage 38:649–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemus L, Hernandez A, Luna R, Zainos A, Nacher V, Romo R (2007): Neural correlates of a postponed decision report. Proc Natl Acad Sci U S A 104:17174–17179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Ostwald D, Giese M, Kourtzi Z (2007): Flexible coding for categorical decisions in the human brain. J Neurosci 27:12321–12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Pleskac TJ (2011): Neural correlates of evidence accumulation in a perceptual decision task. J Neurophysiol 106:2383–2398. [DOI] [PubMed] [Google Scholar]
- Macaluso E, Frith C, Driver J (2000): Selective spatial attention in vision and touch: Unimodal and multimodal mechanisms revealed by PET. J Neurophysiol 83:3062–3075. [DOI] [PubMed] [Google Scholar]
- Meador KJ, Allison JD, Loring DW, Lavin TB, Pillai JJ (2002): Topography of somatosensory processing: Cerebral lateralization and focused attention. J Int Neuropsychol Soc 8:349–359. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Steinmetz MA, Romo R (1990): Frequency discrimination in the sense of flutter: Psychophysical measurements correlated with postcentral events in behaving monkeys. J Neurosci 10:3032–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noppeney U, Ostwald D, Werner S (2010): Perceptual decisions formed by accumulation of audiovisual evidence in prefrontal cortex. J Neurosci 30:7434–7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychol 9:97–113. [DOI] [PubMed] [Google Scholar]
- Park IM, Meister MLR, Huk AC, Pillow JW (2014): Encoding and decoding in parietal cortex during sensorimotor decision‐making. Nat Neurosci 17:1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira F, Mitchell T, Botvinick M (2009): Machine learning classifiers and fMRI: A tutorial overview. Neuroimage 45:S199–S209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploran EJ, Nelson SM, Velanova K, Donaldson DI, Petersen SE, Wheeler ME (2007): Evidence accumulation and the moment of recognition: Dissociating perceptual recognition processes using fMRI. J Neurosci 27:11912–11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, McKoon G (2008): The diffusion decision model: Theory and data for two‐choice decision tasks. Neural Comput 20:873–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland PE (1981): Somatotopical tuning of postcentral gyrus during focal attention in man. A regional cerebral blood flow study. J Neurophysiol 46:744–754. [DOI] [PubMed] [Google Scholar]
- Romo R, Hernandez A, Zainos A, Lemus L, de Lafuente V, Luna R, Nacher V (2006): Decoding the temporal evolution of a simple perceptual act. Novartis Found Symp 270:170–186. [PubMed] [Google Scholar]
- Schubert R, Ritter P, Wustenberg T, Preuschhof C, Curio G, Sommer W, Villringer A (2008): Spatial attention related SEP amplitude modulations covary with BOLD signal in S1‐a simultaneous EEG‐fMRI study. Cerebral Cortex 18:2686–2700. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT (2001): Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J Neurophysiol 86:1916–1936. [DOI] [PubMed] [Google Scholar]
- Talbot WH, Darian‐Smith I, Kornhuber HH, Mountcastle VB (1968): The sense of flutter‐vibration: Comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol 31:301–334. [DOI] [PubMed] [Google Scholar]
- Thielscher A, Pessoa L (2007): Neural correlates of perceptual choice and decision making during fear‐disgust discrimination. J Neurosci 27:2908–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ (2008): Decision making in recurrent neuronal circuits. Neuron 60:215–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


