Abstract
The in vivo detection of subpial cortical gray matter lesions in multiple sclerosis is challenging. We quantified the spatial extent of subpial decreases in the magnetization transfer ratio (MTR) of cortical gray matter in subjects with multiple sclerosis, as such reductions may indicate regions of cortical demyelination. We exploited the unique geometry of cortical lesions by using two‐dimensional parametric surface models of the cortex instead of traditional three‐dimensional voxel‐wise analyses. MTR images were mapped onto intermediate surfaces between the pial and white matter surfaces and were used to compute differences between secondary‐progressive MS (n = 12), relapsing‐remitting MS (n = 12), and normal control (n = 12) groups as well as between each individual patient and the normal controls. We identified large regions of significantly reduced cortical MTR in secondary‐progressive patients when compared with normal controls. We also identified large regions of reduced cortical MTR in 11 individual patients (8 secondary‐progressive, 3 relapsing‐remitting). The secondary‐progressive patients showed larger areas of abnormally low MTR compared with relapsing‐remitting patients both at the group level and on an individual basis. The spatial distributions of abnormal MTR preferentially involved cingulate cortex, insula, and the depths of sulci, in agreement with pathological descriptions of subpial gray matter lesion distribution. These findings suggest that our method is a plausible in vivo imaging technique for quantifying subpial cortical demyelinating lesions in patients with multiple sclerosis and, furthermore, can be applied at the typical clinical field strength of 1.5 T. Hum Brain Mapp 35:3402–3413, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: multiple sclerosis, MTR, subpial, demyelination, cortical lesion
INTRODUCTION
Although multiple sclerosis (MS) has traditionally been viewed as a disease of the white matter (WM), the involvement of gray matter (GM) was noted over a hundred years ago in some early pathological studies of chronic MS cases [Dinkler, 1904; Taylor, 1894]. In these case studies, researchers noted that demyelination affected the cortex at least as much as the WM and, in some cases, even more. Almost a century later, with advances in immunohistochemical staining techniques sensitive to myelin [Kutzelnigg et al., 2005; Peterson et al., 2001; Stadelmann et al., 2008], the MS research community is only beginning to appreciate how extensive GM damage can be [Bo et al., 2003a; Kutzelnigg et al., 2007; Peterson et al., 2001].
Several schemas have emerged for classifying cortical GM lesions in MS. For simplicity, we use the taxonomy defined by Trapp and Nave [2008] and enumerate three types of cortical lesions: Type I lesions are leukocortical areas of demyelination that involve both subcortical WM and cortex. Type II lesions are small, perivascular, and purely intracortical and do not significantly contribute to cortical lesion load. Type III lesions are characterized by subpial demyelination that follows the shape of the cortical mantle, often extending over multiple gyri and often stopping at cortical layer III or IV [Trapp and Nave, 2008]. Although the exact distribution of each type of lesion has varied by study, subpial demyelinating lesions are the most prevalent, accounting for half or more of the cortical lesions seen on histology [Bo et al., 2003a; Geurts et al., 2005a] and up to 67% of the total demyelinated cortical area [Bo et al., 2003a]. In addition, these lesions have been found to be more extensive in progressive cases of MS than in cases of relapsing‐remitting (RR) MS [Kutzelnigg et al., 2005].
Despite the prevalence of lesions in cortical GM (cGM) in MS, conventional magnetic resonance imaging (MRI) is not able to detect most cGM pathology. Newer imaging techniques such as double inversion recovery (DIR), in which the signal from both the WM and the cerebrospinal fluid (CSF) is suppressed, have increased the detection of cGM pathology compared with more conventional sequences [Geurts et al., 2005b], but this increase has been limited to juxtacortical and purely intracortical lesions (Types I and II) [Calabrese et al., 2007; Geurts et al., 2005b; Pouwels et al., 2006]. The difficulty with using conventional imaging to detect subpial cortical demyelination (Type III) is that these lesions do not appear to be associated with an appreciable influx of inflammatory cells or edema and consequently show little alteration of T2 or T1 relaxation times [Bo et al., 2003b; Geurts et al., 2005a; Peterson et al., 2001]. Increasing the signal‐to‐noise ratio has been purported to increase the sensitivity of conventional MRI to detect cGM pathology; however, as with the DIR sequences, this improvement was limited to juxtacortical and intracortical lesions and has not significantly helped in the detection of Type III subpial demyelinations [Bagnato et al., 2006; Geurts et al., 2008]. Scanning at ultrahigh field (e.g., ≥7 T) has been able to identify Type III lesions ex vivo [Kangarlu et al., 2007; Pitt et al., 2010; Schmierer et al., 2010]; however, even at such high field strengths, these lesions may [Mainero et al., 2009] or may not [de Graaf et al., 2012; Kollia et al., 2009] be visualized in vivo. In addition, the extremely limited access to such scanners, combined with technical challenges (i.e., B0 and B1 field inhomogeneities, higher energy deposition, etc.), makes ultrahigh‐field MRI for the detection of subpial demyelination clinically impractical at the present time.
Microscopic changes seen in Type III cortical lesions include microglial activation, axonal transection, and apoptotic neurons [Peterson et al., 2001]; importantly, however, compared with WM lesions, these subpial cortical lesions in patients with long‐standing MS show a significantly less pronounced inflammatory response (lack of T‐cell and B‐cell infiltration, microglial activation, and astrogliosis) [Bo et al., 2003b; Peterson et al., 2001]. That is not to say that all subpial demyelinating lesions are devoid of inflammation as a recent study of patients with early MS found T‐cell infiltrates and myelin‐laden macrophages [Lucchinetti et al., 2011]; still, the predominant feature of the Type III cortical lesion is demyelination. This characteristic suggests the potential usefulness of studying such lesions using magnetization transfer imaging, a methodology that, in the present context, has two very important strengths: first, as we will describe below, it is sensitive to myelin content; second, it can be easily acquired on standard clinical MRI scanners.
Magnetization transfer imaging, specifically, the magnetization transfer ratio (MTR), has been shown to be sensitive to changes in myelin content in WM [Chen et al., 2007, 2008; Dousset et al., 1992; Giacomini et al., 2009; Pike et al., 2000; Schmierer et al., 2004], a marker of intrinsic GM damage [Fisniku et al., 2009; Jure et al., 2010], and significant decreases and increases in MTR have been shown to be associated with de‐ and remyelination, respectively, of WM lesions on postmortem histology [Chen et al., 2007, 2008]. Though MTR may not be a purely specific marker for myelin as it may be affected by axonal loss and, to a lesser degree, inflammation, it does appear to be weighted toward myelination [Dousset et al., 1992; Schmierer et al., 2004, 2007], and in a recent postmortem 9.4 T imaging study of cortical lesions [Schmierer et al., 2010], MTR was strongly correlated to the intensity of myelin basic protein staining (r = 0.52, P = 0.02). Based on these findings, we sought to determine whether cortical MTR has the potential to provide a measure of subpial demyelination.
Macroscopically, subpial demyelinating lesions are characterized as extending over multiple gyri, following the shape of the cortical mantle [Bo et al., 2003a], and preferentially affecting the outermost layers of the cortex [Kutzelnigg et al., 2005; Trapp and Nave, 2008]. Therefore, to increase sensitivity, we exploited this unique geometry and performed our analyses on two‐dimensional (2D) parametric surface models of the cortex instead of using the traditional three‐dimensional (3D) voxel‐wise analyses. These surface models were reconstructed at a subvoxel resolution that enabled the quantification of MRI signal(s) (in this case MTR) at given depth(s) of the cortex [Dale et al., 1999; Fischl et al., 1999b]. Blurring along these surface models also avoids the problems associated with 3D voxel‐wise blurring (e.g., the signal from the voxels of two separate gyri can be erroneously blurred together as they are considered adjoining in 3D space because of the particular folding pattern but, in reality, are not neighbors along the cortical surface). Indeed, the superior power and precision of surface‐based techniques have been most notably demonstrated in cortical thickness analyses [Lerch and Evans, 2005]. When combined with the difficulties of 3D voxel‐based GM segmentation methods [Derakhshan et al., 2010a], a surface‐based approach seems intrinsically better suited to the analysis of the cerebral cortex or, more specifically in this case, subpial demyelination that follows the geometry of the cortical mantle.
As our appreciation for the importance of cGM pathology grows, reliable imaging methods are essential to accurately measure and analyze cGM pathology in MS. In this report, we present a novel surface‐based method developed by our group [Derakhshan et al., 2009, 2010b] to quantify the extent of subpial decreases in MTR that may correspond to regions of cortical demyelination. Our method was applied to group data from patients with MS and normal controls (NCs), as well as to data from individuals to identify areas that differed from the control group. Importantly, these differences were detected using conventional‐resolution images such as those typically obtained in clinical practice.
METHODS
Subjects
Magnetic resonance scans of healthy controls and patients with RR and secondary‐progressive (SP) MS recruited from the MS Clinic at the Montreal Neurological Institute and Hospital for a previous study were analyzed in this retrospective analysis. Of the possible 26 SP MS patients in the original study, 14 were included based on availability of MTR data and adequate scan quality. The SP group was further limited to 12 subjects as we were unable to obtain accurate cortical reconstructions for two of the subjects. Further selection was then performed to match all groups for population size, sex, and mean age, given that age‐related changes in the MTR of both the GM [Benedetti et al., 2006; Ge et al., 2002; Lee et al., 2004] and WM [Ge et al., 2002; Lee et al., 2004] of NCs have been demonstrated. Demographics for the 12 patients with SP MS, 12 patients with RR MS, and 12 NC are presented in Table 1. Patients were not being treated with disease‐modifying drugs at the time of the scan. Local ethics board approval and informed consent from each participant were obtained.
Table 1.
Subject demographics
| M/F | Age (years) | DD (years) | EDSS | NBV (cc) | T2LV (cc) | cThx (mm) | |
|---|---|---|---|---|---|---|---|
| NC | 4/8 | 44 (28–60) | — | — | 1510.6 (1397.6–1685.6) | — | 2.43 (2.27–2.56) |
| RR | 4/8 | 45 (30–59) | 13 (1–33) | 2.5 (1.0–4.0) | 1491.2 (1299.6–1661.2) | 7.6 (0.8–27.3) | 2.42 (2.29–2.53) |
| SP | 4/8 | 47 (30–62) | 14 (3–26) | 5.7 (3.5–8.0) | 1461.7 (1335.0–1581.7) | 33.1 (4.0–80.1) | 2.25 (1.89–2.44) |
All values are mean (range). DD: disease duration; EDSS: Kurtzke's‐expanded disability status scale; T2LV: manually identified white matter lesion volume on the T2‐weighted scan; NBV: normalized brain volume output using SIENAx; cThx: global cortical thickness; NC: normal controls; SP: secondary‐progressive; RR: relapsing‐remitting.
MRI Acquisition
Subjects were scanned on a 1.5 T Philips ACS II scanner (Philips Medical Systems, Best, the Netherlands). Oblique axial T1‐weighted (T1w) images were acquired parallel to the antero‐posterior commissural line using a 3D spoiled gradient‐recalled echo sequence (TR = 35 ms, TE = 10 ms, 256 × 256 matrix, 250 mm field‐of‐view, 60 partitions, 3‐mm partition thickness, 1 signal average, voxel size = 0.98 × 0.98 × 3.0 mm3). Images were acquired without and with a 1.2 ms on‐resonance, bipolar (1‐2′‐1) magnetization transfer pulse (20 μT RF field strength) placed just before each slice‐selective excitation. To calculate the MTR for each patient, the magnetization transfer image volume acquired with the saturation pulse (Sat) was first linearly registered (mritoself, McConnell Brain Imaging Centre [Collins et al., 1994]) to the volume without the saturation pulse (NoSat), and the MTR image volume was then calculated as 100 × (NoSat − Sat)/NoSat. To remove outliers caused by noise and possible data discretization errors, the MTR image was clamped so that all values were within the theoretical limits of 0 and 100.
MRI Processing
Cortical reconstruction was performed using the T1w image volume without the magnetization saturation pulse (NoSat) as input to the FreeSurfer image analysis suite (v4.0.5, http://surfer.nmr.mgh.harvard.edu) [Dale et al., 1999; Fischl et al., 1999a]. We visually checked the cortical reconstruction of all patients and controls, and manual corrections (inclusion of WM control points, corrections for WM lesions including Type I juxtacortical lesions, and pial surface edits) were performed as necessary to ensure accurate surfaces. As mentioned above, two patients were excluded due to inaccurate surfaces that could not be corrected. From the generated pial and WM surfaces, intermediate surfaces were created by traveling 25%, 50%, or 75% of the distance along the vector linking a vertex on the WM surface to its corresponding vertex on the pial surface. See Figure 1 for examples of the surface extractions.
Figure 1.
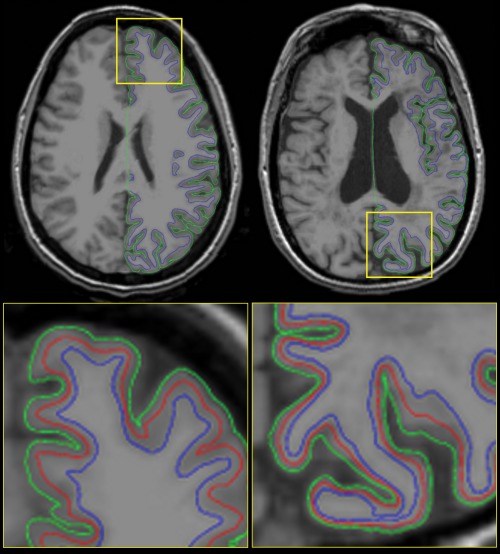
Examples of the cortical surface extractions for a NC (left) and an atrophic MS patient (right). Top: axial view showing the extractions of the right hemisphere; bottom: zoomed‐in axial view of the yellow squares showing the pial (green), white matter (blue), and the intermediate surfaces at 50% depth (red). Subjects were selected at random as an accurate representation of the data, and not just the best possible slice of the best possible subject. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
The information from the MTR image volume was interpolated onto every vertex on each surface. As Lerch et al. demonstrated that 2D geodesic blurring yields less error and bias than conventional 3D blurring for surface analyses [Lerch and Evans, 2005], the MTR data was blurred along the surface with a 10 mm full‐width‐at‐half‐maximum geodesic kernel. The kernel size was chosen to preferentially detect the large subpial Type III cortical lesions over the small, punctate Type II lesions [Geurts et al., 2005a; Kidd et al., 1999; Trapp and Nave, 2008].
Statistical Analysis
To facilitate group comparisons, the cortical models were nonlinearly registered to a spherical atlas that uses individual cortical folding patterns to match cortical geometry across subjects [Fischl et al., 1999a], after which a general linear model (GLM) was performed to test for MTR differences between groups. The GLM was run at each vertex of each hemisphere, producing t‐statistics that were then thresholded for significance and corrected for multiple comparisons using a false discovery rate (FDR) of 0.05. A mask of the cortex (lh.cortex.label and rh.cortex.label provided by FreeSurfer) was used to ensure that the FDR was not erroneously influenced by the noncortical, central regions of the brain (e.g., corpus callosum, third ventricle, and diencephalon) that are by default included on the medial surface of each hemisphere (see the noncolored region in the bottom three views of Fig. 5A). Statistical analyses were run using the mni.cortical.statistics library (courtesy of Jason Lerch) for R [Ihaka and Gentleman, 1996].
Figure 5.
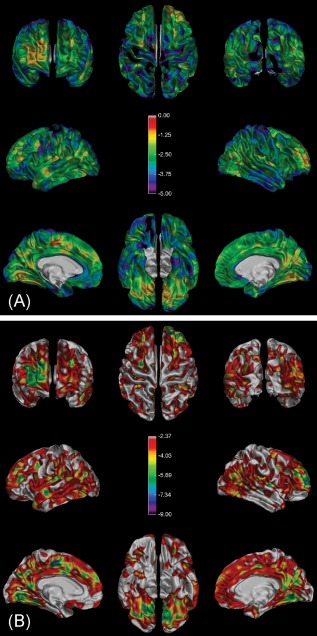
A: The spatial distribution of the calculated theoretical decrease in MTR percentage units (p.u.) needed at each point on the surface to detect a significant difference (uncorrected) from the NC group. The color bar ranges from 0 MTR p.u. (in red) to ‐5 MTR p.u. (in purple). B: The spatial distribution of significant differences (false‐discovery‐rate‐corrected t‐ratios) detected using a simulated subject dataset for which each point on the surface was 4 MTR p.u. lower than the average NC value at that vertex. Note that the highlighted areas represent a 74.2% detection of the surface area. Simulations with global decreases of 3 MTR p.u. or less resulted in no significant detections (not shown), whereas increasing the simulated decrease to 6 MTR p.u. gave almost complete (98.1%) detection.) [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
The above procedure was followed for each group comparison (SP vs. NC, RR vs. NC) and for each individual patient compared with the group of NC (e.g., Subject 1 vs. NC, Subject 2 vs. NC, etc.).
Sensitivity Analysis
To assess the sensitivity of our proposed method to cortical MTR changes, we calculated the amount of MTR decrease that would be necessary to be detected using two methods: (1) direct calculation of a minimum detectable difference map and (2) simulated decreases in MTR.
Sensitivity Analysis: Direct Calculation
As indicated in the statistical analysis section above, the magnitude of the MTR difference at each vertex is tested for significance using a standard two‐tailed t‐test. However, one could directly calculate the minimum MTR difference required at each vertex to be deemed significant by rearranging the formula for a t‐test, given that we know the cortical MTR values for each control subject and can look up the significant t‐ratio threshold in a t‐table (using our known degrees of freedom). Although this method provides us with a minimum detectable difference map, we are unable to correct for multiple comparisons via FDR using this model because the t‐ratio is the same for each vertex; FDR correction requires a variance in the to‐be‐corrected t‐ratios. To overcome this limitation and obtain a more accurate map of the minimum detectable differences, we used simulated data (described below) that took FDR correction into account.
Sensitivity Analysis: Simulations
Six datasets were created with a simulated decrease in MTR as follows: First, the average MTR value at each vertex, along each intermediate surface, was calculated for the group of 12 NC. These MTR values were then uniformly decreased by 1–6 MTR percentage units (p.u.) below the average value to create six new simulated datasets. For example, the intermediate surface (50% depth) of simulated dataset number 1 would be the same as the average of the 12 NC's intermediate surface MTR values, decreased by 1 MTR p.u. at every vertex, dataset 2 would have each value decreased by 2 MTR p.u., and so forth. Each of the six simulated datasets was then treated as an individual subject and run though the identical surface‐based analysis method described above (surface‐based registration, blurring, and statistical analysis), where each vertex of the decreased MTR surfaces was compared to the group of 12 NC and FDR corrected.
RESULTS
Group Differences
Figure 2 shows the average cortical MTR maps for each group, with clearly evident decreases from the NC to the RR and SP groups. The groupwise mean MTR value in percentage units and the groupwise mean of the standard deviations over the entire intermediate surface were 25.49 (1.41), 24.24 (2.60), and 23.80 (2.29) for the NC, RR, and SP groups, respectively. The mean MTR value for each group differed significantly from those for other groups (P < 0.0001), as evidenced by a Tukey–Kramer HSD test.
Figure 2.
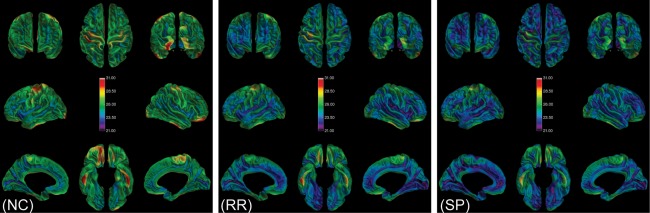
Average (n = 12) maps of cortical MTR for each group. From left to right, there is a visually discernible decline in MTR going from healthy NCs to the RR and SP groups. The color bars in each panel show the identical windowing used for display purposes going from 21 to 31 MTR percentage units (p.u.).The eight views presented in each panel starting from the upper left and going clockwise are: front, top, back, right hemisphere temporal, right hemisphere medial, bottom, left hemisphere medial, and left hemisphere temporal. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Also observed in Figure 2 are regions where the MTR appeared higher than that of its surroundings. This is best illustrated in the NC group where the occipital pole, motor cortex, and inferior surface of the brain appear brighter, but it can also be seen to a lesser extent in the RR and SP groups. The observation of relatively high MTR in occipital and motor cortex is consistent with the known increased density of myelin in these regions [Glasser and van Essen, 2011].
The highlighted areas in Figure 3 map the spatial distribution of significantly low cortical MTR (after FDR correction) in the SP group compared with the NC group. The SP group shows large contiguous areas of low MTR covering ∼34% of the cortex. The RR group as a whole showed no such areas, likely because of more sparsely distributed areas of low focal cortical MTR [Kutzelnigg et al., 2005] and spatial variability in their location across individuals, rather than as an indication that all RR patients have normal cortical MTR.
Figure 3.
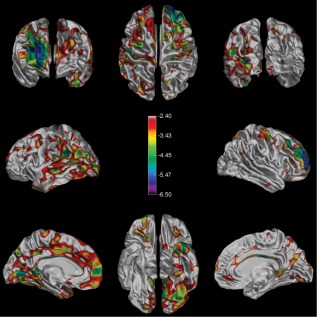
Significant differences in cortical MTR between the SP and NC groups. The color bar shows the false‐discovery‐rate‐corrected t‐statistics, such that any highlighted area on the surface represents a statistically significant decrease in MTR between groups. The RR cohort showed no such significant differences. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Individual Differences
Each of the 24 subjects with MS (12 RR and 12 SP) was compared with the NC group to reveal differences at the individual level. Figure 4 shows the 8 SP and 3 RR subjects for whom a localized significant decrease in MTR was detected, as well as a more detailed view of the median subject in each cohort in terms of area of decreased MTR detected.
Figure 4.
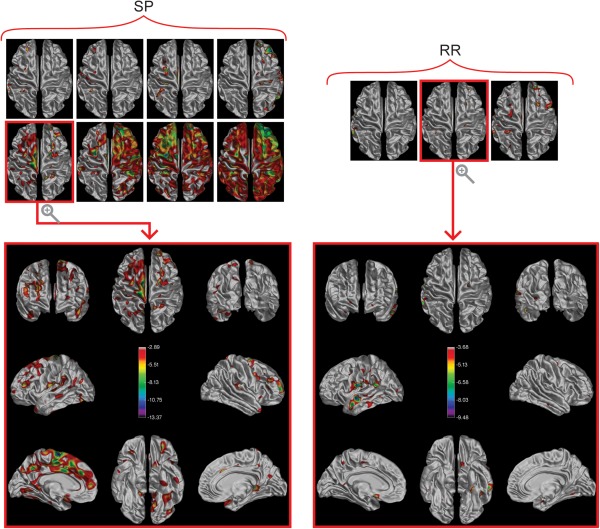
Significant differences in cortical MTR between individuals and the NC group. On the left is a montage of the top views of the cortical surfaces of the 8/12 individual SP subjects for whom a significant difference from the NC group was detected. The 3/12 individual RR subjects are shown on the right. The median subject (in terms of affected surface area) is presented with all surface views visible below each respective montage. The color bar shows the false‐discovery‐rate‐corrected t‐statistics, such that any highlighted area on the surface represents a statistically significant decrease in MTR between the individual and the NC group. Notice the range of affected surface area detected progressing from small clusters to almost complete coverage in the SP cohort. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Sensitivity Analysis: Direct Calculation
The map showing the minimum MTR difference required at each vertex to detect a significant change on an individual basis is shown in Figure 5A. The mean (standard deviation) decrease in cortical MTR p.u. was 2.63 (0.84).
Sensitivity Analysis: Simulations
The first three simulated subjects with a global decrease of 3 or fewer MTR p.u. did not show any vertices being detected as having significantly low MTR after FDR correction. A global decrease of 4 p.u. resulted in 74.2% of the vertices being detected as having low MTR and is shown in Figure 5B. Further decreases of 5 and 6 p.u. resulted in 94.0% and 98.1% of vertices being detected, respectively.
DISCUSSION
The complex geometry and thinness of the cortex induce variable amounts of partial volume at the cortical boundaries, and the pathology we are trying to detect (subpial demyelination) is often thinner than the largest voxel dimension of our MTR images. These factors severely limit the sensitivity of traditional 3D voxel‐based methods to localized changes in MTR. By using a 2D surface that follows the cortical mantle, we have developed a new analytical method that is better suited to the task of detecting regional MTR abnormalities in the cortex that are of the shape expected for subpial demyelinating lesions. In addition to matching the profile of these lesions, our surface‐based method has the advantage of not diluting our signal of interest. In a traditional 3D voxel‐wise approach, the focal MTR decrease elicited by Type III cortical lesions would be diluted by information perpendicular to the plane of interest at the blurring stage of the analysis. Conversely, in our surface‐based blurring scheme, we use only the information from adjacent vertices at the same depth of cortex. Although we present this technique in the context of detecting subpial demyelination using MTR images, the method can be applied to any imaging modality (e.g., T2‐relaxometry data and double‐inversion recovery) to look for aberrances in the cortex of an individual (or population) and possible correlations to cognitive or behavioral data.
Understanding cGM pathology in MS is of great interest to the field, and a number of studies have been looking to fully describe this pathology using ultrahigh‐field imaging, often combined with highly specific immunohistochemical staining. The primary aim of this study was not to provide the same degree of insight into the disease pathology, but rather to assess the sensitivity and feasibility of a new technique to detect differences in the cGM of subjects with MS, and to do so using images easily acquired on standard clinical MRI scanners, which—once validated with postmortem data—would make this approach feasible for use as a measure of subpial demyelination in clinical trials of patients with MS—trials that will most likely still be acquiring data on 1.5–3.0 T scanners in the foreseeable future.
Group Differences
To compare the MS patients with the NC group, we assessed the spatial distribution of the areas of decreased cGM MTR by thresholding for significant differences using a false discovery rate of 0.05. Compared with the NC group, only the SP group showed large, contiguous areas of significantly decreased MTR. The highlighted areas in Figure 3 indicate the regions where the decreases in cGM MTR over the entire group were large enough to be considered significant when compared with the NC group. That the RR group did not show large group differences in regional MTR does not mean that there were no regional decreases in individuals, but rather that the extent and spatial distribution of any such decreases were not consistent enough between RR subjects to be detected across the entire group.
As can be seen in Table 1, the RR cohort in this study is somewhat atypical. By design, the RR subjects were matched for age to the SP and NC groups to control for the age‐related changes that have been seen in the MTR of the NC group [Benedetti et al., 2006; Ge et al., 2002; Lee et al., 2004]. Without age matching, the detected differences in MTR may simply have been the result of normal aging. Consequently, in this study, we presented an RR group with an uncharacteristically elevated age, as well as a mean disease duration that does not differ from the SP group as would be expected. While this limits the generalization of the disease‐related findings, it does not take away from the primary aim of this study.
Individual Differences
When individual subjects were compared with the NC group, 8/12 SP patients, and 3/12 RR patients showed large, contiguous areas of decreased cortical MTR, with spatial distributions consistent with the Type III subpial GM lesions described pathologically [Bo et al., 2003a]. An examination of the median subject (in terms of affected area) in each group indicated a much larger affected area in the median SP subject than in the median RR subject. This higher frequency of patients with a detectable difference in the SP group than in the RR group, and generally greater extent of the differences seen, is consistent with pathological observations that the later stages of MS are characterized by an increase in cortical subpial demyelinating lesions [Lassmann and Lucchinetti, 2008].
Cortical Layers
In addition to carrying out our analysis on the intermediate (50%) surface, we also looked at parallel surfaces at differing depths (25 and 75%) between the pial and WM surfaces. We observed that, qualitatively, in both group comparisons and in those individuals who displayed large areas of decreased cGM MTR, the amount of affected surface area increased closer to the pial surface and decreased toward the WM surface. These observations are consistent with what has been seen on postmortem histology, namely, that subpial demyelination preferentially affects the outermost layers of the cortex, often stopping at cytoarchitectonic layer III or IV [Kutzelnigg et al., 2005; Trapp and Nave, 2008].
Sensitivity Analysis
To assess the sensitivity of our technique, we generated the theoretical map of minimum detectable differences shown in Figure 5A. The map varies spatially and reflects the variability of MTR in the NC group (i.e., changes in MTR were most easily detected in areas of the cortex where there was little variation in the MTR of our control group). This method provides the MTR difference needed at each vertex to achieve significance; however, it does not correct for multiple comparisons, as is actually done in this sort of analysis. The simulations shown in Figure 5B do correct for multiple comparisons and also show that, with this technique, acquisition protocol, and number of controls, the minimum detectable decrease of MTR is somewhere between 3 and 4 p.u. We expect that higher resolution acquisitions would reduce variability due to partial volume effects and increase sensitivity (i.e., reducing the minimum MTR change that can be detected).
Voxel Geometry
Because our study aimed to analyze data that is typically used in clinical research, we did not employ high field strengths or long scanning times to obtain high‐resolution images. The T1w images input into FreeSurfer were 0.98 × 0.98 × 3.0 mm3, which is indeed suboptimal in comparison to the 1 mm isotropic recommended resolution; however, we feel that we were able to obtain precise surface extractions (see Fig. 1 for examples) for the following reasons: (i) FreeSurfer relies heavily on WM and GM contrast to identify brain surfaces; (ii) our T1w sequences were suited to providing this contrast; and (iii) each surface was carefully visually examined and corrected as needed. The effects of voxel size and anisotropy on the reliability of FreeSurfer have been previously investigated [Wonderlick et al., 2009], and the reliability of the morphometric measures has been reported to be generally high and largely unaffected by differences in voxel geometry (reliability was also largely unaffected by parallel acceleration factors or the use of high‐bandwidth multiecho techniques). Larger, anisotropic voxel sizes did, however, result in a significant measurement bias—the cortical thickness was higher than that from a sequence with smaller isotropic voxels—to which our study may be subject. However, all the subjects in our study were acquired with the same sequence parameters and therefore all experienced the same amount of said bias; thus, tests looking for differences between groups or individuals would not be affected.
Blurring Kernels
We investigated the effect of 2D versus 3D blurring by examining how the type and size of blurring kernel affected the variance in the cortical MTR of our cohorts (Fig. 6). The 3D analysis was done by obtaining the median MTR values of each subject within a mask of the cerebral cortex (provided by the volumetric stream of FreeSurfer), while the 2D analysis was performed as previously described—by obtaining the mean MTR value along the intermediate surface and using surface‐based blurring. For each method, and for each of the 0‐, 2‐, 4‐, 6‐, 8‐, and 10‐mm kernel sizes tried, an ANOVA was performed to reveal how much of the variance in cGM MTR values was explained by the three cohorts (i.e., NC, RR, and SP) (higher r 2 means the groups are better separated). We found that the r 2 value remained high and the P value remained low regardless of the blurring kernel used for the 2D analysis, but that for the 3D analysis, there was much variation depending on the blurring kernel size. Intuitively, this makes sense because the median cGM MTR value in the 3D case was altered by signal outside the cortex, whereas in the 2D scenario, the mean values remained consistent, as one would expect, because the cGM signal was blurred by signal within the cortex. Also worth noting is that, for all the 2D analysis blurring kernels (0–10 mm), a Tukey–Kramer HSD test revealed that the NC cGM MTR values were significantly different from those for the RR and SP groups, but for the 3D analysis method, this was only the case for smaller kernels (2–8 mm). There was actually no separation of the groups where there was no blurring (0 mm has P > 0.05). In the 3D analysis, the r 2 value did plateau to a value slightly higher than that in the 2D analysis for the larger blurring kernels, but as the kernel size is increased to 10 mm and higher, only the SP group became significantly different from the NC group. The dependence of the 3D analysis on technicalities such as the blurring kernel size—which varies immensely study by study—combined with the consistent result of the 2D analysis, suggests that the 2D analysis is better suited to investigating abnormalities within the cortex.
Figure 6.
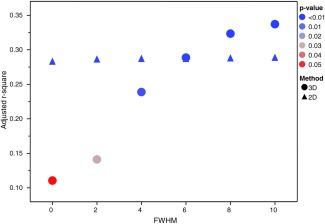
Adjusted r 2 values at each of the full‐width‐at‐half‐maximum (FWHM) blurring kernel sizes for a 2D and a 3D analysis looking for group differences in the cGM MTR values. The r 2 of the ANOVA reveals how much of the variance in cGM MTR values is explained by the three groups (NC, RR, and SP) (higher r 2 means the groups are better separated), while the color of each datapoint represents the associated P value. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Partial Volume Effects
As with any finite sampling MRI acquisition, we are burdened by partial volume effects whereby the signal represented in a single voxel may consist of contributions from more than one type of tissue. In particular, the cGM voxels in the MTR image will be a mixture of pure GM tissue and the CSF and WM tissues that abut the cortex. This is of concern because subjects with more brain atrophy have thinner cortices and larger sulci; thus, the cGM voxels in these subjects are more likely to have some CSF contamination that could lower MTR values. Unfortunately, the quantitative effects of partial volume on cortical surface MTR is not well described; however, by looking at the cortical thickness and MTR in the cortex of a NC subject, we can see if more partial volume is associated with a lower MTR value, as would be evidenced by an expected positive correlation. We found no such correlation (r 2 < 0.01), and thus we are confident that the subpial decreases in MTR we detect with this method are biologically driven and not artifactual.
CONCLUSIONS
In this study, we have presented a novel surface‐based method for the detection of local subpial decreases in MTR. This new approach represents an important advancement for the detection of putative Type III cortical lesions in vivo, as conventional methods to date have had only limited success. Our method exploits what is known about both the spatial organization and the microscopic composition of these lesions and produces results that are very consistent with postmortem pathological studies. Our method identifies focal, cortical pathology that increases in the later stages of disease, affects the outermost layers of the cortex more than the deepest layers, and preferentially involves the same regions that have been identified to be preferentially involved in histopathological studies (e.g., cingulate cortex, insula, and the depths of sulci) [Bo et al., 2003a; Gilmore et al., 2009; Kutzelnigg and Lassmann, 2006]. Importantly, our method seems to allow us to do this using data that can be acquired with conventional clinical scanners, which makes this approach feasible for use in clinical trials interested in measuring cortical pathology in patients with MS. For all of these reasons, we believe that surface‐based MTR analysis is an extremely promising approach for the detection of Type III cortical demyelinating lesions in vivo that merits further development and validation.
REFERENCES
- Bagnato F, Butman JA, Gupta S, Calabrese M, Pezawas L, Ohayon JM, Tovar‐Moll F, Riva M, Cao MM, Talagala SL, McFarland HF (2006): In vivo detection of cortical plaques by MR imaging in patients with multiple sclerosis. AJNR Am J Neuroradiol 27:2161–2167. [PMC free article] [PubMed] [Google Scholar]
- Benedetti B, Charil A, Rovaris M, Judica E, Valsasina P, Sormani MP, Filippi M (2006): Influence of aging on brain gray and white matter changes assessed by conventional, MT, and DT MRI. Neurology 66:535–539. [DOI] [PubMed] [Google Scholar]
- Bo L, Vedeler CA, Nyland H, Trapp BD, Mork SJ (2003a): Intracortical multiple sclerosis lesions are not associated with increased lymphocyte infiltration. Mult Scler 9:323–331. [DOI] [PubMed] [Google Scholar]
- Bo L, Vedeler CA, Nyland HI, Trapp BD, Mork SJ (2003b): Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J Neuropathol Exp Neurol 62:723–732. [DOI] [PubMed] [Google Scholar]
- Calabrese M, De Stefano N, Atzori M, Bernardi V, Mattisi I, Barachino L, Morra A, Rinaldi L, Romualdi C, Perini P, Battistin L, Gallo P (2007): Detection of cortical inflammatory lesions by double inversion recovery magnetic resonance imaging in patients with multiple sclerosis. Arch Neurol 64:1416–1422. [DOI] [PubMed] [Google Scholar]
- Chen JT, Kuhlmann T, Jansen GH, Collins DL, Atkins HL, Freedman MS, O'Connor PW, Arnold DL (2007): Voxel‐based analysis of the evolution of magnetization transfer ratio to quantify remyelination and demyelination with histopathological validation in a multiple sclerosis lesion. Neuroimage 36:1152–1158. [DOI] [PubMed] [Google Scholar]
- Chen JT, Collins DL, Atkins HL, Freedman MS, Arnold DL (2008): Magnetization transfer ratio evolution with demyelination and remyelination in multiple sclerosis lesions. Ann Neurol 63:254–262. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC (1994): Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18:192–205. [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI (1999): Cortical surface‐based analysis. I. Segmentation and surface reconstruction. Neuroimage 9:179–194. [DOI] [PubMed] [Google Scholar]
- Derakhshan M, Caramanos Z, Narayanan S, Collins DL, Arnold DL (2009): Regions of Reduced Cortical Magnetization Transfer ratio Detected in MS Patients using Surface‐Based Techniques. Honolulu, USA: p 338. [Google Scholar]
- Derakhshan M, Caramanos Z, Giacomini PS, Narayanan S, Maranzano J, Francis SJ, Arnold DL, Collins DL (2010a): Evaluation of automated techniques for the quantification of grey matter atrophy in patients with multiple sclerosis. Neuroimage 52:1261–1267. [DOI] [PubMed] [Google Scholar]
- Derakhshan M, Caramanos Z, Narayanan S, Arnold DL, Collins DL (2010b): Surface‐Based Techniques Reveal Regions of Reduced Cortical Magnetization Transfer Ratio in Patients with MS. Stockholm, Sweeden: p 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkler M (1904): Zur Kasuistik der multiplen Herdsklerose des Gehirns und Rückenmarks. J Neurol 26:233–247. [Google Scholar]
- Dousset V, Grossman RI, Ramer KN, Schnall MD, Young LH, Gonzalez‐Scarano F, Lavi E, Cohen JA (1992): Experimental allergic encephalomyelitis and multiple sclerosis: Lesion characterization with magnetization transfer imaging. Radiology 182:483–491. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM (1999a): Cortical surface‐based analysis. II: Inflation, flattening, and a surface‐based coordinate system. Neuroimage 9:195–207. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM (1999b): High‐resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp 8:272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisniku LK, Altmann DR, Cercignani M, Tozer DJ, Chard DT, Jackson JS, Miszkiel KA, Schmierer K, Thompson AJ, Miller DH (2009): Magnetization transfer ratio abnormalities reflect clinically relevant grey matter damage in multiple sclerosis. Mult Scler 15:668–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL (2002): Age‐related total gray matter and white matter changes in normal adult brain. Part II: Quantitative magnetization transfer ratio histogram analysis. AJNR Am J Neuroradiol 23:1334–1341. [PMC free article] [PubMed] [Google Scholar]
- Geurts JJ, Bo L, Pouwels PJ, Castelijns JA, Polman CH, Barkhof F (2005a): Cortical lesions in multiple sclerosis: Combined postmortem MR imaging and histopathology. AJNR Am J Neuroradiol 26:572–577. [PMC free article] [PubMed] [Google Scholar]
- Geurts JJ, Pouwels PJ, Uitdehaag BM, Polman CH, Barkhof F, Castelijns JA (2005b): Intracortical lesions in multiple sclerosis: Improved detection with 3D double inversion‐recovery MR imaging. Radiology 236:254–260. [DOI] [PubMed] [Google Scholar]
- Geurts JJ, Blezer EL, Vrenken H, van der Toorn A, Castelijns JA, Polman CH, Pouwels PJ, Bo L, Barkhof F (2008): Does high‐field MR imaging improve cortical lesion detection in multiple sclerosis? J Neurol 255:183–191. [DOI] [PubMed] [Google Scholar]
- Giacomini PS, Levesque IR, Ribeiro L, Narayanan S, Francis SJ, Pike GB, Arnold DL (2009): Measuring demyelination and remyelination in acute multiple sclerosis lesion voxels. Arch Neurol 66:375–381. [DOI] [PubMed] [Google Scholar]
- Gilmore CP, Donaldson I, Bo L, Owens T, Lowe J, Evangelou N (2009): Regional variations in the extent and pattern of grey matter demyelination in multiple sclerosis: A comparison between the cerebral cortex, cerebellar cortex, deep grey matter nuclei and the spinal cord. J Neurol Neurosurg Psychiatry 80:182–187. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Van Essen DC (2011): Mapping human cortical areas in vivo based on myelin content as revealed by T1‐ and T2‐weighted MRI. J Neurosci 31:11597–11616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf WL, Zwanenburg JJ, Visser F, Wattjes MP, Pouwels PJ, Geurts JJ, Polman CH, Barkhof F, Luijten PR, Castelijns JA (2012): Lesion detection at seven Tesla in multiple sclerosis using magnetisation prepared 3D‐FLAIR and 3D‐DIR. Eur Radiol 22:221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihaka R, Gentleman R (1996): R: A language for data analysis and graphics. J Comput Graph Stat 5:299–314. [Google Scholar]
- Jure L, Zaaraoui W, Rousseau C, Reuter F, Rico A, Malikova I, Confort‐Gouny S, Cozzone PJ, Pelletier J, Ranjeva JP, Audoin B (2010): Individual voxel‐based analysis of brain magnetization transfer maps shows great variability of gray matter injury in the first stage of multiple sclerosis. J Magn Reson Imaging 32:424–428. [DOI] [PubMed] [Google Scholar]
- Kangarlu A, Bourekas EC, Ray‐Chaudhury A, Rammohan KW (2007): Cerebral cortical lesions in multiple sclerosis detected by MR imaging at 8 Tesla. AJNR Am J Neuroradiol 28:262–266. [PMC free article] [PubMed] [Google Scholar]
- Kidd D, Barkhof F, McConnell R, Algra PR, Allen IV, Revesz T (1999): Cortical lesions in multiple sclerosis. Brain 122 (Pt 1):17–26. [DOI] [PubMed] [Google Scholar]
- Kollia K, Maderwald S, Putzki N, Schlamann M, Theysohn JM, Kraff O, Ladd ME, Forsting M, Wanke I (2009): First clinical study on ultra‐high‐field MR imaging in patients with multiple sclerosis: Comparison of 1.5T and 7T. AJNR Am J Neuroradiol 30:699–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutzelnigg A, Lucchinetti CF, Stadelmann C, Bruck W, Rauschka H, Bergmann M, Schmidbauer M, Parisi JE, Lassmann H (2005): Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 128:2705–2712. [DOI] [PubMed] [Google Scholar]
- Kutzelnigg A, Lassmann H (2006): Cortical demyelination in multiple sclerosis: A substrate for cognitive deficits? J Neurol Sci 245:123–126. [DOI] [PubMed] [Google Scholar]
- Kutzelnigg A, Faber‐Rod JC, Bauer J, Lucchinetti CF, Sorensen PS, Laursen H, Stadelmann C, Bruck W, Rauschka H, Schmidbauer M, Lassmann H (2007): Widespread demyelination in the cerebellar cortex in multiple sclerosis. Brain Pathol 17:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H, Lucchinetti CF (2008): Cortical demyelination in CNS inflammatory demyelinating diseases. Neurology 70:332–333. [DOI] [PubMed] [Google Scholar]
- Lee KY, Kim TK, Park M, Ko S, Song IC, Cho IH (2004): Age‐related changes in conventional and magnetization transfer MR imaging in elderly people: Comparison with neurocognitive performance. Korean J Radiol 5:96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch JP, Evans AC (2005): Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage 24:163–173. [DOI] [PubMed] [Google Scholar]
- Lucchinetti CF, Popescu BF, Bunyan RF, Moll NM, Roemer SF, Lassmann H, Bruck W, Parisi JE, Scheithauer BW, Giannini C, Weigand SD, Mandrekar J, Ransohoff RM (2011): Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med 365:2188–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainero C, Benner T, Radding A, van der Kouwe A, Jensen R, Rosen BR, Kinkel RP (2009): In vivo imaging of cortical pathology in multiple sclerosis using ultra‐high field MRI. Neurology 73:941‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JW, Bo L, Mork S, Chang A, Trapp BD (2001): Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol 50:389–400. [DOI] [PubMed] [Google Scholar]
- Pike GB, De Stefano N, Narayanan S, Worsley KJ, Pelletier D, Francis GS, Antel JP, Arnold DL (2000): Multiple sclerosis: Magnetization transfer MR imaging of white matter before lesion appearance on T2‐weighted images. Radiology 215:824–830. [DOI] [PubMed] [Google Scholar]
- Pitt D, Boster A, Pei W, Wohleb E, Jasne A, Zachariah CR, Rammohan K, Knopp MV, Schmalbrock P (2010): Imaging cortical lesions in multiple sclerosis with ultra‐high‐field magnetic resonance imaging. Arch Neurol 67:812–818. [DOI] [PubMed] [Google Scholar]
- Pouwels PJ, Kuijer JP, Mugler JP III, Guttmann CR, Barkhof F (2006): Human gray matter: Feasibility of single‐slab 3D double inversion‐recovery high‐spatial‐resolution MR imaging. Radiology 241:873–879. [DOI] [PubMed] [Google Scholar]
- Schmierer K, Scaravilli F, Altmann DR, Barker GJ, Miller DH (2004): Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann Neurol 56:407–415. [DOI] [PubMed] [Google Scholar]
- Schmierer K, Tozer DJ, Scaravilli F, Altmann DR, Barker GJ, Tofts PS, Miller DH (2007): Quantitative magnetization transfer imaging in postmortem multiple sclerosis brain. J Magn Reson Imaging 26:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmierer K, Parkes HG, So PW, An SF, Brandner S, Ordidge RJ, Yousry TA, Miller DH (2010): High field (9.4 Tesla) magnetic resonance imaging of cortical grey matter lesions in multiple sclerosis. Brain 133:858–867. [DOI] [PubMed] [Google Scholar]
- Stadelmann C, Albert M, Wegner C, Bruck W (2008): Cortical pathology in multiple sclerosis. Curr Opin Neurol 21:229–234. [DOI] [PubMed] [Google Scholar]
- Taylor EW (1894): Zur pathologischen Anatomie der multiplen Sklerose. J Neurol 5:1–26. [Google Scholar]
- Trapp BD, Nave KA (2008): Multiple sclerosis: An immune or neurodegenerative disorder? Annu Rev Neurosci 31:247–269. [DOI] [PubMed] [Google Scholar]
- Wonderlick JS, Ziegler DA, Hosseini‐Varnamkhasti P, Locascio JJ, Bakkour A, van der Kouwe A, Triantafyllou C, Corkin S, Dickerson BC (2009): Reliability of MRI‐derived cortical and subcortical morphometric measures: Effects of pulse sequence, voxel geometry, and parallel imaging. Neuroimage 44:1324–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]


