Abstract
Appetitive conditioning is an important mechanism for the development, maintenance, and treatment of psychiatric disorders like substance abuse. Therefore, it is important to identify genetic variations, which impact appetitive conditioning. It has been suggested that the Val158Met‐polymorphism in the Catechol‐O‐Methyl‐Transferase (COMT) is associated with the alteration of neural processes of appetitive conditioning due to the central role of the dopaminergic system in reward processing. However, no study has so far investigated the relationship between variations in the COMT Val158Met‐polymorphism and appetitive conditioning. In this fMRI study, an appetitive conditioning paradigm was applied, in which one neutral stimulus (CS+) predicted appetitive stimuli (UCS) while a second neutral stimulus (CS−) was never paired with the UCS. As a main result, we observed a significant association between the COMT Val158Met‐genotype and appetitive conditioning: skin conductance responses (SCRs) revealed a significant difference between CS+ and CS− in Val/Val‐allele carriers but not in the other genotype groups. Val/Val‐allele carriers showed increased hemodynamic responses in the amygdala compared with the Met/Met‐allele group in the contrast CS+ > CS−. In addition, psychophysiological‐interaction analysis revealed increased effective amygdala/ventromedial prefrontal cortex connectivity in Met/Met‐allele carriers. The increased amygdala activity points to facilitated appetitive conditioning in Val/Val‐allele carriers while the amygdala/prefrontal connectivity results could be regarded as a marker for altered emotion regulation during conditioning, which potentially impacts appetitive learning sensitivity. The SCRs finding indicates a stronger conditioned response in the Val/Val‐allele group and dovetails with the neural differences between the groups. These findings contribute to the current research on COMT in emotional processing. Hum Brain Mapp 36:1093–1101, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: classical conditioning, Catechol‐O‐Methyl‐Transferase, imaging genetics, fMRI, positive emotion, reward
INTRODUCTION
Appetitive conditioning is assumed to play an important role in the etiology of many psychiatric disorders, like substance abuse, eating disorders, or pathological gambling [Martin‐Soelch et al., 2007]. Thus, the identification of specific individual differences, which influence neural mechanisms underlying appetitive conditioning, could shed light on (mal)adaptive human behavior. In appetitive conditioning paradigms, a neutral stimulus (CS+) is paired with pleasant stimuli (UCS) while a second neutral stimulus (CS−) is never paired with the UCS. After few repetitions, the CS+ typically elicits conditioned reactions (CRs) like increased skin conductance responses (SCRs), changes in valence ratings, as well as changes in brain activity [Kirsch et al., 2003; Klucken et al., 2013; Martin‐Soelch et al., 2007]. The underlying network that plays the main role in the neural processing of appetitive conditioning includes the amygdala, the anterior cingulate cortex (ACC), the nucleus accumbens (NAcc), the midbrain, the insula, the hippocampus, the (orbito)frontal cortex, and the occipital cortex [Martin‐Soelch et al., 2007]. In detail, the amygdala is considered to be crucial for the automatic CS/UCS association while the (dorsal) ACC seems to contribute to CS+/CS− discrimination learning [Martin‐Soelch et al., 2007; Mechias et al., 2010]. Activity of the NAcc and the midbrain have been observed to shift from the delivery of the UCS to the onset of the CS as the reward becomes more predictable [O'Doherty et al., 2004; Schultz et al., 1997] and might also be crucially important for the development of CS/UCS contingency awareness [Klucken et al., 2009a, b]. Finally, the orbitofrontal cortex has been linked to the conscious evaluation of the CS valence [O'Doherty, 2007].
Genetic variations influencing dopaminergic transmission have been proposed to be an important factor for individual differences in appetitive conditioning, because dopamine plays a central role in reward processing, anticipation, and in the valence transfer from the UCS to the CS [Blum et al., 2011; Day and Carelli, 2007; Schultz et al., 1997; Tunbridge et al., 2012]. Recently, the Val158Met‐polymorphism in the Catechol‐O‐Methyl‐Transferase (COMT) has gained increased interest in the context of appetitive conditioning and reward anticipation, but research is only at the beginning [Tunbridge et al., 2012]. COMT catabolizes catecholamines like dopamine and the insertion of methionine (Met) instead of valine (Val) decreases its activity [Lachman et al., 1996]. Carriers of the homozygote valine‐allele have a 30–40 % higher enzyme activity compared with homozygote methionine allele carriers while heterozygote carriers (Val/Met‐allele) are assumed to exhibit intermediate levels of enzyme activity [Lachman et al., 1996].
The Val158Met‐polymorphism has been linked to differences in emotional as well as cognitive processes [Mier et al., 2010]. Numerous studies confirmed that COMT regulates tonic and phasic dopamine levels in the prefrontal cortex (PFC) and probably also in other brain regions like amygdala, ventral striatum, hippocampus, and midbrain partly due to regulatory influences of the PFC [Bertolino et al., 2006; Meyer‐Lindenberg et al., 2005; Rasch et al., 2010; Schmack et al., 2008]. It is well established that Met/Met‐allele carriers show better attentional control and cognitive stability [Bilder et al., 2004], most likely because the PFC works more efficiently with higher tonic DA concentrations [Caldú et al., 2007]. Regarding the association between affective learning and the COMT genotype, a more puzzling picture emerges: it has been reported that Val/Val‐allele carriers adjust to changing contingencies between cues and rewarding stimuli more flexibly [Frank et al., 2009; Krugel et al., 2009; Tunbridge et al., 2012]. Moreover, greater neural differentiations in Val/Val‐allele carriers were found during the anticipation of winning as compared to loosing [Camara et al., 2010; Marco‐Pallarés et al., 2009; Tunbridge et al., 2012]. Other studies, however, found contrary results [Tunbridge et al., 2012] or no group differences between the groups during reward or fear anticipation [Forbes et al., 2009; Lonsdorf et al., 2009].
In addition to the analysis of the mere strength of hemodynamic responding to explain altered vulnerability for psychiatric disorders, measures of connectivity between brain areas have recently gained increased attention [Delgado et al., 2008; Domschke and Dannlowski, 2010; Meyer‐Lindenberg, 2009]. In the context of conditioning, the connectivity between amygdala and the ventromedial prefrontal cortex (vmPFC) is of special interest. The vmPFC has been hypothesized to be involved in the (down‐) regulation of amygdala activity, for example, during emotion regulation and during the extinction of conditioned fear [Meyer‐Lindenberg, 2009]. This connectivity has also been observed to be influenced by the COMT Val158Met‐polymorphism: two studies observed increased amygdala‐prefrontal connectivity in Met/Met‐allele carriers as compared to Val/Val‐allele carriers, which has been interpreted to reflect inflexible and rigid responding to affective stimuli [Drabant et al., 2006; Rasch et al., 2010].
Based on the aforementioned findings, this study aimed to investigate the following: first, we investigated potential differences in the strength of neural responses during appetitive conditioning dependent on the Val158Met‐polymorphism. We assumed greater neural differentiation in Val/Val‐allele compared with Met/Met‐allele carriers. Second, we were interested in differences in amygdala/vmPFC coupling associated with the Val158Met‐polymorphism. We expected increased amygdala/vmPFC coupling in Met/Met‐allele carriers as compared to Val/Val‐allele carriers.
MATERIAL AND METHODS
Participants
One hundred subjects (Caucasian; 51 females; M Age = 25.3; SDAge = 4.7) were recruited. All were native German speakers with European background, right handed, and had normal or corrected‐to‐normal vision. Subjects reporting current or past mental, sexual, or chronic problems or a consumption of psychotropic drugs were excluded. Subjects gave informed consent and received 30 € for their participation. The study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of the German Psychological Society.
Genotyping
DNA was obtained from buccal cells with an extraction kit (High Pure PCR Template Preparation Kit; Roche, Mannheim, Germany) in a MagNA Pure1 LC System (Roche). Subjects were genotyped for COMT by Polymerase Chain Reaction and gel electrophoresis [for a detailed protocol: see Ott et al., 2005] and for other genotypes (DRD2 and 5‐HTTLPR; partly reported in [Klucken et al., 2013]). Technical problems during the experiment compromised the data of nine subjects. Regarding fMRI analysis, 11 subjects (out of 91) had to be excluded due to irregular head movements and normalization problems. Thus, the final sample of 80 subjects comprised 20 Val/Val‐Carriers, 41 Val/Met‐Carriers, and 19 Met/Met‐Carriers with no significant deviation from Hardy‐Weinberg‐Equilibrium (χ 2(1) = 0.05; P > 0.9). There were no differences between the groups with respect to age, sex, educational status, or other genotypes (all P > 0.26).
Conditioning Procedure
The appetitive conditioning procedure consisted of 42 trials (21 per CS). Each trial started with the presentation of one of two geometrical squares (blue vs. yellow). The CS+ was presented for 8 s and was always followed by one of the 21 UCS pictures (duration: 2.5 s) while the other stimulus (CS−) was never paired with the UCS. Due to the strong association between dopaminergic transmission and the processing of sexual stimuli, motivation, and anticipation, erotic stimuli were used as UCS [Brom et al., 2014; Georgiadis and Kringelbach, 2012; Georgiadis et al., 2012]. The erotic pictures contained scenes with couples (one male, one female) having vaginal intercourse in different positions. In a pilot study, all pictures had been rated as highly pleasant by females and males alike. The scenes were presented in color with 800 × 600 pixel resolution. A fixation cross was displayed during the inter‐trial‐interval, which ranged from 12 to 14.5 s. All trials were presented in a pseudorandomized order: the same CS was not presented more than twice in succession. The two CS were presented equally often in the first and the second half of the acquisition. The first two trials (one Cs+ trial and one CS− trial) were discarded from the analysis because learning could not have taken place yet [Klucken et al., 2012; Phelps et al., 2004]. All stimuli were projected onto a screen at the end of the scanner and were viewed through a mirror (visual field = 18°) mounted on the head coil. A compatible video camera was used to check if subjects watched the stimuli. The CS+ and the CS− were rated before and after the experiment; the erotic pictures were only rated after the experiment to avoid UCS pre‐exposure effects.
Subjective Ratings
Participants rated valence and arousal of CS+ and CS− on a 9‐point Likert scale and UCS‐expectancy on a 10‐point Likert scale before and after the experiment. The UCS were rated after the experiment. The CS ratings were analyzed by ANOVA in a 2 (CS‐type: CS+ vs. CS−) × 3 (COMT genotype) design followed by post hoc tests using SPSS 22 (SPSS, Chicago, Illinois).
Skin Conductance Measuring
SCRs were sampled using Ag/AgCl electrodes filled with isotonic (0.05 M NaCl) electrolyte medium placed at the nondominant left hand. The 1–8 s after onset of CS+ or CS−, respectively, were defined as analysis window for the CRs. The 1–3 s after onset of the UCS or the comparable interval following the CS− were defined as analysis window for the unconditioned reactions. The maximum response within the analysis windows was extracted using a customized version of Ledalab 3.4.4 [Benedek and Kaernbach, 2010]. The maximum response is defined as the highest difference between a maximum and the minimum that directly precedes it. The preceding minimum has to fall within the predefined response window. These maximum responses were log (µS + 1) transformed to correct for violation of normal distribution of the data. Mean SCRs to CS+ and CS− were analyzed via analysis of variance (ANOVA) in a 2 (CS‐type: CS+ vs. CS−) × 3 (COMT genotype: Val/Val, Val/Met, Met/Met) design followed by post hoc tests using SPSS 22 (SPSS).
Magnetic Resonance Imaging
The subjects were scanned using a 1.5 Tesla whole‐body tomograph (Siemens Symphony with a quantum gradient system) with a standard head coil. 160 T1‐weighted images (MPRage, 1 mm slice thickness) were acquired in sagittal orientation. Functional imaging consisted of 420 images in a T2*‐weighted echo‐planar imaging sequence. The whole brain was covered with 25 slices (slice thickness = 5 mm; 1 mm gap; descending slice procedure; TR = 2.5 s; TE = 55 ms; flip angle = 90°; field of view 192 × 192 mm; matrix size 64 × 64). The orientation of the axial slices was parallel to the OFC tissue‐bone transition. Preprocessing and data analysis was performed using SPM8 (Wellcome Department of Cognitive Neurology, London, UK; 2008) implemented in MATLAB 7.14 (Mathworks, Sherbourn, MA). Preprocessing comprised realignment and unwarping (b‐spline interpolation), slice time correction, coregistration of the anatomical data to the functional data, normalization to standard stereotactic space (Montreal Neurological Institute brain), and smoothing with a Gaussian filter, set at 9 mm full width at half maximum. The data were then checked for extensive head motion (>2 mm or 2°) and failure to normalize to the MNI stereotactic space and excluded, if necessary.
The functional data were analyzed using the General Linear Model (GLM). The experimental conditions were CS+, CS−, UCS+, and UCS− (time corresponding to the UCS after the CS−; [Klucken et al. 2009a, c]) and were split into a first half (CS+1/CS−1; UCS+1/UCS−1) and a second half (CS+2/CS−2; UCS+2/UCS−2) to investigate potential group differences in the early phase of acquisition, which cannot be seen in models without considering this division [e.g., Straube et al., 2007; Tabbert et al., 2005]. Each of the conditions was modeled by a stick function convolved with a hemodynamic response function in the GLM. The six movement parameters estimated in the realignment were entered into the model as covariates. The voxel‐based time series was filtered with a high pass filter (time constant = 128 s). On the group level, a factorial analysis was computed in SPM8. Because we did not find any additional region in the first half of the acquisition, we analyzed the whole acquisition phase with the within‐subject factor CS‐type (CS+1 + CS+2; CS−1 + CS−2) and the between‐subjects factor COMT (Val/Val, Val/Met, Met/Met). The same procedure was conducted for analyzing the UCS results (UCS+1 + UCS+2; UCS−1 + UCS−2). The threshold for whole brain as well as Region of Interest (ROI) analyses was set to P < 0.05 FWE‐corrected. Based on previous studies investigating appetitive conditioning and the effects of COMT on brain responses, the following ROIs were chosen: amygdala, NAcc, insula, midbrain, ACC, hippocampus, occipital cortex (fusiform gyrus), and vmPFC. Anatomical masks were taken from “Harvard Oxford cortical and subcortical structural atlases” (probability threshold 25%) provided by Harvard Center for Morphometric Analysis while the mask for the vmPFC was kindly provided by other authors [Hermann et al., 2009].
Effective connectivity analyses
A psychophysiological interaction (PPI) analysis was conducted [Gitelman et al., 2003] investigating the effective connectivity between a seed region and other brain areas in interaction with an experimental task (CS+ > CS−). We used the amygdala and the midbrain as seed regions and extracted the first eigenvariate as implemented in SPM8. Then, the interaction term was created by multiplying the extracted signal with the contrast of interest (CS+ > CS−) for each subject. First level analysis was conducted for each subject, including the three regressors (psychological variable, physiological variable, interaction term) in the design matrix [Gitelman et al., 2003]. At the second level, we analyzed group differences in effective connectivity between the amygdala and the vmPFC.
RESULTS
Subjective Ratings
The 2 × 3 ANOVA of post‐conditioning ratings revealed main effects of CS‐type in expectancy (F (1, 77) = 286.11; P < 0.001), and arousal (F (1, 77) = 33.62; P < 0.001), and valence ratings (F (1, 77) = 10.65; P = 0.002). The CS+ was rated as more positive, more arousal‐inducing, and with a higher UCS‐expectancy (see Table 1). No effects of the COMT genotype were observed (all F < 1).
Table 1.
Mean subjective ratings (SD) for the CS+ and the CS− for each genotype group separately
| Val/Val (N = 20) | Val/Met (N = 41) | Met/Met (N = 19) | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | ||
| UCS expectancy | CS+ | 4.75 (2.36) | 9.10 (2.86) | 4.95 (2.51) | 9.93 (1.88) | 5.32 (1.83) | 10.00 (2.16) |
| CS− | 5.25 (2.38) | 2.15 (1.73) | 5.05 (2.30) | 1.88 (1.82) | 5.79 (2.07) | 2.16 (2.34) | |
| Valence | CS+ | 5.35 (1.84) | 5.65 (1.79) | 5.10 (1.46) | 5.76 (1.86) | 5.21 (1.65) | 6.05 (1.84) |
| CS− | 5.35 (1.87) | 4.70 (1.84) | 5.29 (1.50) | 4.73 (1.95) | 5.21 (1.44) | 4.21 (1.75) | |
| Arousal | CS+ | 3.85 (2.32) | 5.05 (2.33) | 3.63 (2.05) | 5.12 (2.36) | 3.58 (1.95) | 5.53 (2.12) |
| CS− | 4.05 (2.24) | 3.15 (2.20) | 3.68 (1.89) | 2.51 (1.82) | 4.57 (1.95) | 3.47 (1.90) | |
Skin Conductance Responses
The 2 × 3 ANOVA of SCRs showed a main effect of CS‐type (F (1, 77) = 5.40; P = .023), with greater responses to the CS+ as compared to the CS−. While there was no main effect of COMT genotype on SCRs (F < 1), there was, however, a significant CS‐type × COMT genotype interaction (F (2, 77) = 3.34; P = 0.04). We conducted paired t‐tests to follow up on this interaction and compared the SCRs to CS+ and CS− separately for the three COMT genotypes. Interestingly, only Val/Val‐allele carriers showed a significant difference between CS+ and CS− (t(19) = 2.32; P = 0.032). Neither Val/Met‐allele carriers (t(40) = 0.09; P = 0.93) nor Met/Met‐allele carriers (t(18) = 0.66; P = 0.52) showed conditioned SCRs. This indicates that the main effect of CS‐type was mainly driven by the Val/Val‐allele (see Fig. 1). Analysis of the unconditioned reaction revealed a main effect of UCS (F (1, 77) = 4.7; P = 0.033) but no effect of COMT genotype (all F < 1).
Figure 1.
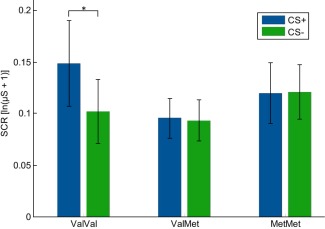
Mean SCRs to the CS+ and CS− by COMT genotype. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Hemodynamic Responses
Main effect of stimulus (CS+ > CS−)
Whole‐brain analysis showed increased hemodynamic responses to the CS+ compared with the CS− in the frontal lobe, the insula, the parietal lobe, and the occipital lobe (see Supporting Information for exact coordinates and statistics). Analyzing the predefined ROIs added several findings: a main effect of stimulus was found in the bilateral NAcc, ACC, vmPFC, insula, hippocampus, the left amygdala, and a trend in the right amygdala. The observed hemodynamic responses point toward a successful conditioning of BOLD responses (see Table 2). In addition, we also investigated the UCS to further look for potential differences. We observed strong activated hemodynamic responses in all ROI (all P < 0.05 FWE‐corrected).
Table 2.
Localization and statistics of the peak voxels for the contrast CS+ > CS− and for group differences (Val/Val‐allele group vs. Met/Met‐allele group)
| Group analysis | Contrast | Structure | Side | k | x | y | z | Z max | P corr |
|---|---|---|---|---|---|---|---|---|---|
| Main effect of stimulus | CS+ > CS− | Amygdala | L | 18 | −15 | −4 | −14 | 3.14 | 0.033 |
| Amygdala | R | 12 | 18 | −4 | −11 | 2.85 | 0.074 | ||
| dACC | L | 158 | −9 | 26 | 25 | 3.89 | 0.010 | ||
| dACC | R | 191 | 9 | 17 | 40 | 5.33 | <0.001 | ||
| Occipital cortex | L | 271 | −21 | −88 | −8 | 13.71 | <0.001 | ||
| Occipital cortex | R | 259 | 24 | −88 | −5 | 14.81 | <0.001 | ||
| Insula | L | 133 | −36 | 23 | 1 | 5.35 | <0.001 | ||
| Insula | R | 161 | 33 | 26 | 4 | 5.97 | <0.001 | ||
| Midbrain | R | 120 | 9 | −28 | −5 | 4.43 | 0.001 | ||
| NAcc | L | 24 | −6 | 5 | −8 | 3.25 | 0.012 | ||
| NAcc | R | 24 | 6 | 14 | −2 | 3.35 | 0.008 | ||
| vmPFC | L | 169 | −6 | 56 | −5 | 3.49 | 0.037 | ||
| vmPFC | R | 22 | 18 | 17 | −17 | 3.66 | 0.023 | ||
| Val/Val‐allele > Met/Met allele group | CS+ > CS− | Amygdala | L | 52 | −30 | −1 | −20 | 3.23 | 0.025 |
| Amygdala | R | 77 | 30 | −4 | −20 | 2.92 | 0.062 | ||
| dACC | R | 81 | 9 | 26 | 40 | 3.44 | 0.042 | ||
| Occipital cortex | R | 128 | 33 | −64 | −17 | 3.47 | 0.029 | ||
| Insula | L | 88 | −42 | 11 | −11 | 3.98 | 0.009 | ||
| Midbrain | L | 83 | −12 | −28 | −11 | 4.63 | <0.001 | ||
| Hippocampus | L | 85 | −15 | −34 | −5 | 3.91 | 0.005 |
The threshold was P < 0.05 (FWE‐corrected; small volume correction according to SPM8). All coordinates are given in MNI space. L: left hemisphere, R: right hemisphere.
Main effect of the COMT genotype
No group differences were found in whole‐brain analysis. ROI‐analyses revealed a main effect of COMT genotype in the contrast CS+ > CS−. The Val/Val‐allele group showed increased activation in the amygdala (see Fig. 2), the midbrain, the hippocampus, the dACC, and the insula, as well as in the occipital cortex (see Table 2) in contrast to the Met/Met‐allele group. No significant effect was detected in the opposite direction (Met/Met‐allele group > Val/Val‐allele group). In addition, only few differences were found comparing the homozygote groups with the heterozygote group: increased activity in the contrast CS+ > CS− was observed in the right insula (x = 39; y = −10; z = 10; Z max = 3.38; P < 0.05; FWE‐corrected) in Val/Val‐allele as compared to the Val/Met‐allele carriers while again no group differences occurred in the opposite direction. The Val/Met group as compared to the Met/Met group showed increased activity in the occipital cortex (x = 24; y = −88; z = −5; Z max = 3.39; P = 0.032; FWE‐corrected) and the midbrain (x = −12; y = −28; z = −14; Z max = 3.31; P = 0.027; FWE‐corrected) while in the opposite direction a stronger activation in left (x = −9; y = −65; z = −5; Z max = 4.17; P = 0.003; FWE‐corrected) and right (x = 9; y = 65; z = −2; Z max = 3.78; P = 0.013; FWE‐corrected) vmPFC occurred. Regarding the UCS, we did not find group differences.
Figure 2.
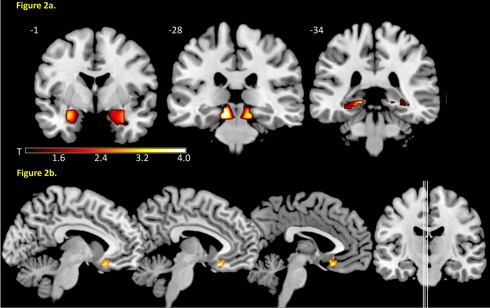
(a) Increased amygdala, midbrain, and hippocampal reactivity in Val/Val‐allele compared with Met/Met‐allele carriers in the contrast CS+ > CS− and (b) increased neural coupling in the vmPFC in Met/Met‐allele compared with Val/Val‐allele carriers [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Effective connectivity
In addition to the analyses of the strength of hemodynamic responses, we explored effective connectivity with the amygdala and the midbrain as seed regions using PPI analysis. PPI analysis detects brain structures correlated with a seed region of interest in a task‐dependent manner. Group analysis showed increased amygdala/vmPFC coupling (x = −6; y = 23; z= −17, Z max= 3.52, P = 0.025) in the Met/Met‐allele group as compared to the Val/Val‐allele carriers (see Fig. 2b). No significant differences occurred between the heterozygote and the two homozygote groups (Met/Met‐allele group and Val/Val‐allele group). No group differences were observed in the PPI analysis with the midbrain as seed region.
DISCUSSION
This study aimed to investigate two main topics: first, due to the association of COMT with reward processing, reward sensitivity, and learning [Tunbridge et al., 2012], we were interested in the influence of the COMT Val158Met‐polymorphism on appetitive conditioning. Second, differences in effective amygdala/vmPFC connectivity were explored because of its hypothesized involvement in emotion regulation processes and regulating of CRs like extinction processes [Delgado et al., 2008]. Regarding the first aim, we found stronger CS+/CS− differentiation in Val/Val‐allele carriers in SCRs and greater neural differentiation compared with the Met/Met‐allele group. Regarding effective connectivity, stronger amygdala/vmPFC connectivity was observed in the Met/Met‐allele as compared to the Val/Val‐allele group.
The increased hemodynamic responses in the contrast CS+ > CS− in Val/Val‐allele carriers are in line with previous studies reporting greater neural activity and a more flexible adjustment to changing stimulus‐reward contingencies [Tunbridge et al., 2012]. Increased amygdala responses to the CS+ during appetitive conditioning have been associated with the development of the CS/UCS association and the production of CRs. For instance, it has been shown that the amygdala is crucially involved in the transmission process which renders neutral into appetitive stimuli [Martin‐Soelch et al., 2007]. The observed group differences in the contrast CS+ > CS− in Val/Val carriers might thus reflect a facilitated acquisition process, which may finally lead to “wanting” processes [Mahler and Berridge, 2009]. This increased “wanting” is assumed as a vulnerability factor for addiction disorders, because it provokes increased approach behavior. In addition, we also found stronger hemodynamic responses in the ACC in Val/Val‐allele carriers as compared to the Met/Met‐allele group. Influential models assume that ACC activations are important for the processing of discrimination learning and the anticipation as well as preparation of action outcomes and guided behavior [Botvinick, 2007; Martin‐Soelch et al., 2007; Mechias et al., 2010]. Exaggerated activity in the fusiform gyrus of the occipital lobe in response to the CS+ might indicate the increased salience of the (CS+) stimulus, which is probably driven by amygdala and ACC activity [Bradley et al., 2003] and is often called motivated attention [Bradley et al., 2003]. The increased midbrain responses combined with the increased hippocampal activity might reflect the interaction of these regions in forming reward‐dependent long‐term‐memory of the CS+ [Adcock et al., 2006]. For example, reward‐predicting cues that were later remembered elicited a greater activation in the midbrain and hippocampus [Wittmann et al., 2005]. Both regions have previously been shown to be influenced by COMT genotype [Bertolino et al., 2006; Meyer‐Lindenberg et al., 2005]. The stronger activation in the Val/Val‐allele group might, therefore, reflect a stronger memory formation for the reinforced CS. Taken together, group differences were not exclusively detected in core regions of the reward network, but also in other brain structures associated with appetitive conditioning. This finding indicates that alterations in dopaminergic transmission may have more systemic rather than isolated effects on the brain. It further confirms the strong interconnection between different brain regions and underlines that brain structures and functions should not be regarded isolated.
Regarding the second aim of this study, we found enhanced amygdala/vmPFC coupling in Met/Met‐allele carriers compared with the Val/Val‐allele group. Several explanations are possible: considering the currently hypothesized inverse relationship of the amygdala and the vmPFC, it could be argued that the vmPFC inhibits amygdala activity during appetitive conditioning and thus leads to impeded learning in the Met/Met‐allele group. In line with this assumption, several studies have shown that the vmPFC is involved in the downregulation of the amygdala and emotion regulation in general. In our study, emotion regulation might have led to reduced CRs reflected in altered amygdala/vmPFC connectivity. For instance, in the study by Delgado et al., emotion regulation diminished CRs in a similar way as extinction processes and both processes elicited increased amygdala/PFC connectivity [Delgado et al., 2008]. However, it should be noted that participants in our study were not explicitly instructed to use emotion regulation strategies. Therefore, this argumentation should be treated with caution until an independent replication is available.
With respect to clinical implications, our results provide a biological mechanism that might explain the increased vulnerability of Val/Val‐allele carriers for addiction disorders [e.g., in nicotine addiction; Colilla et al., 2005; Munafò et al., 2008; Tammimäki and Männistö, 2010]. Neutral stimuli might be associated with reward more easily in Val/Val carriers and thus may trigger more craving, which ultimately may lead to more consumption. In accordance, elevated amygdala reactivity in appetitive conditioning processes has been proposed as a maintaining factor in several drug‐related and non‐drug related psychiatric disorders [Jasinska et al., 2014].
Limitations
In the present study, 21 pictures were used as UCS to avoid rapid habituation effects because the repeated presentation of the same picture would let to, reduced attention, etc. We used pictures with comparable valence and arousal ratings chosen in a large, multistep rating procedure (see Wehrum et al., 2013). In addition, UCS ratings did not differ between the groups. Nevertheless, it cannot be ruled out that different learning curves exist between subjects. In contrast to these results, other studies reported greater neural activity in response to salient stimuli in Met/Met‐allele carriers [Mier et al., 2010; Tunbridge et al., 2012]. In face of these inconsistencies, it is important to point out that design differences and/or sample sizes may have affected the divergent outcomes: it has been assumed that Met/Met‐allele carriers are more sensitive for aversive stimuli and for tasks, in which contingencies are clearly instructed and learning processes are irrelevant [Tunbridge et al., 2012]. Therefore, one explanation for the contrary findings could be that the subjects in the present study had to learn the contingencies during the experiment. In addition, in contrast to other studies, which investigated the neural correlates of reward and loss anticipation, we were interested in classical appetitive conditioning and did, therefore, not include aversive conditions. Regarding sample sizes, this study compared both homozygote groups with each other. Previous studies often assumed a dominant effect from one allele, which resulted in investigating one homozygote group plus the heterozygote group (e.g., Met/Met‐allele group plus Val/Met‐allele carriers) against the other homozygote group Val/Val‐allele carriers.
Regarding the UCS, in a meta‐analysis, Mier et al. [Mier et al., 2010] showed increased neural differentiation toward affective stimuli in Met/Met‐allele carriers. Interestingly, analyzing the UCS (appetitive pictures) we did not find group differences. This may indicate that the COMT genotype is more associated to reward learning and conditioning rather than the mere processing of reward stimuli. This is in line with previous studies, which found group differences during the processing of aversive stimuli only [Smolka et al., 2005; Smolka et al., 2007]. Finally, the understanding of COMT on appetitive conditioning or even more complex genes × brain interaction effects is to date far from comprehensive and results should be interpreted with caution until replication is available.
Conclusion
In sum, the results clearly support that the COMT genotype influences appetitive conditioning. We observed increased BOLD‐responses in subcortical and cortical structures in Val/Val‐allele carriers as compared to the Met/Met‐allele group. Moreover, we found increased amygdala/vmPFC connectivity in Met/Met‐allele carriers, which may have decreased CRs in this group. Thus, our findings contribute to the current debate on the influence of genetic variations on appetitive conditioning and reward processing, and provide a potential mechanism through which genetic variations may be linked to a higher risk for addiction disorders.
Supporting information
Supplementary Information
REFERENCES
- Adcock RA, Thangavel A, Whitfield‐Gabrieli S, Knutson B, Gabrieli John DE (2006): Reward‐motivated learning: Mesolimbic activation precedes memory formation. Neuron 50:507–517. [DOI] [PubMed] [Google Scholar]
- Benedek M, Kaernbach C (2010): A continuous measure of phasic electrodermal activity. J Neurosci Methods 190:80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Blasi G, Latorre V, Rubino V, Rampino A, Sinibaldi L, Caforio G, Petruzzella V, Pizzuti A, Scarabino T, Nardini M, Weinberger DR, Dallapiccola B (2006): Additive effects of genetic variation in dopamine regulating genes on working memory cortical activity in human brain. J Neurosci 26:3918–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA (2004): The catechol‐O‐methyltransferase polymorphism: Relations to the tonic‐phasic dopamine hypothesis and neuropsychiatric phenotypes. Psychol Sci 29:1943–1961. [DOI] [PubMed] [Google Scholar]
- Blum K, Liu Y, Shriner R, Gold MS (2011): Reward circuitry dopaminergic activation regulates food and drug craving behavior. Curr Pharm Des 17:1158–1167. [DOI] [PubMed] [Google Scholar]
- Botvinick MM (2007): Conflict monitoring and decision making: Reconciling two perspectives on anterior cingulate function. Cogn Affect Behav Neurosci 7:356–366. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Sabatinelli D, Lang PJ, Fitzsimmons JR, King W, Desai P (2003): Activation of the visual cortex in motivated attention. Behav Neurosci 117:369–380. [DOI] [PubMed] [Google Scholar]
- Brom M, Both S, Laan E, Everaerd W, Spinhoven P (2014): The role of conditioning, learning and dopamine in sexual behavior: A narrative review of animal and human studies. Neurosci Biobehav Rev 38:38–59. [DOI] [PubMed] [Google Scholar]
- Caldú X, Vendrell P, Bartrés‐Faz D, Clemente I, Bargalló N, Jurado MA, Serra‐Grabulosa JM, Junqué C (2007): Impact of the COMT Val108/158 Met and DAT genotypes on prefrontal function in healthy subjects. NeuroImage 37:1437–1444. [DOI] [PubMed] [Google Scholar]
- Camara E, Krämer UM, Cunillera T, Marco‐Pallarés J, Cucurell D, Nager W, Mestres‐Missé A, Bauer P, Schüle R, Schöls L, Tempelmann C, Rodriguez‐Fornells A, Münte TF (2010): The effects of COMT (Val108/158Met) and DRD4 (SNP‐521) dopamine genotypes on brain activations related to valence and magnitude of rewards. Cereb Cortex 20:1985–1996. [DOI] [PubMed] [Google Scholar]
- Colilla S, Lerman C, Shields PG, Jepson C, Rukstalis M, Berlin J, DeMichele A, Bunin G, Strom BL, Rebbeck TR (2005): Association of catechol‐O‐methyltransferase with smoking cessation in two independent studies of women. Pharmacogenet Genomics 15:393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Carelli RM (2007): The nucleus accumbens and Pavlovian reward learning. Neuroscientist 13:148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Gillis MM, Phelps EA (2008): Regulating the expectation of reward via cognitive strategies. Nat Neurosci 11:880–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domschke K, Dannlowski U (2010): Imaging genetics of anxiety disorders. NeuroImage 53:822–831. [DOI] [PubMed] [Google Scholar]
- Drabant EM, Hariri AR, Meyer‐Lindenberg A, Munoz KE, Mattay VS, Kolachana BS, Egan MF, Weinberger DR (2006): Catechol O‐methyltransferase val158met genotype and neural mechanisms related to affective arousal and regulation. Arch Gen Psychiat 63:1396–1406. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR (2009): Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol Psychiat 14:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Doll BB, Oas‐Terpstra J, Moreno F (2009): Prefrontal and striatal dopaminergic genes predict individual differences in exploration and exploitation. Nat Neurosci 12:1062–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiadis JR, Kringelbach ML (2012): The human sexual response cycle: Brain imaging evidence linking sex to other pleasures. Prog Neurobiol 98:49–81. [DOI] [PubMed] [Google Scholar]
- Georgiadis JR, Kringelbach ML, Pfaus JG (2012): Sex for fun: A synthesis of human and animal neurobiology. Nat Rev Urol 9:486–498. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ (2003): Modeling regional and psychophysiologic interactions in fMRI: The importance of hemodynamic deconvolution. NeuroImage 19:200–207. [DOI] [PubMed] [Google Scholar]
- Hermann A, Schäfer A, Walter B, Stark R, Vaitl D, Schienle A (2009): Emotion regulation in spider phobia: Role of the medial prefrontal cortex. Soc Cogn Affect Neurosci 4:257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y (2014): Factors modulating neural reactivity to drug cues in addiction: A survey of human neuroimaging studies. Neurosci Biobehav Rev 38:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P, Schienle A, Stark R, Sammer G, Blecker C, Walter B, Ott U, Burkart J, Vaitl D (2003): Anticipation of reward in a nonaversive differential conditioning paradigm and the brain reward system: An event‐related fMRI study. NeuroImage 20:1086–1095. [DOI] [PubMed] [Google Scholar]
- Klucken T, Schweckendiek J, Merz CJ, Tabbert K, Walter B, Kagerer S, Vaitl D, Stark R (2009a): Neural activations of the acquisition of conditioned sexual arousal: Effects of contingency awareness and sex. J Sex Med 6:3071–3085. [DOI] [PubMed] [Google Scholar]
- Klucken T, Tabbert K, Schweckendiek J, Merz CJ, Kagerer S, Vaitl D, Stark R (2009b): Contingency learning in human fear conditioning involves the ventral striatum. Hum Brain Mapp 30:3636–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucken T, Schweckendiek J, Merz CJ, Tabbert K, Walter B, Kagerer S, Vaitl D, Stark R (2009c): Neural activations of the acquisition of conditioned sexual arousal: Effects of contingency awareness and sex. J Sex Med 6:3071–3085. [DOI] [PubMed] [Google Scholar]
- Klucken T, Schweckendiek J, Koppe G, Merz CJ, Kagerer S, Walter B, Sammer G, Vaitl D, Stark R (2012): Neural correlates of disgust‐ and fear‐conditioned responses. Neuroscience 201:209–218. [DOI] [PubMed] [Google Scholar]
- Klucken T, Wehrum S, Schweckendiek J, Merz CJ, Hennig J, Vaitl D, Stark R (2013): The 5‐HTTLPR polymorphism is associated with altered hemodynamic responses during appetitive conditioning. Hum Brain Mapp 34:2549–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugel LK, Biele G, Mohr PNC, Li S‐C, Heekeren HR (2009): Genetic variation in dopaminergic neuromodulation influences the ability to rapidly and flexibly adapt decisions. Proc Natl Acad Sci USA 106:17951–17956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM (1996): Human catechol‐O‐methyltransferase pharmacogenetics: Description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics 6:243–250. [DOI] [PubMed] [Google Scholar]
- Lonsdorf TB, Weike AI, Nikamo P, Schalling M, Hamm AO, Ohman A (2009): Genetic gating of human fear learning and extinction: Possible implications for gene‐environment interaction in anxiety disorder. Psychol Sci 20:198–206. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Berridge KC (2009): Which cue to "want?" Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. J Neurosci 29:6500–6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco‐Pallarés J, Cucurell D, Cunillera T, Krämer UM, Càmara E, Nager W, Bauer P, Schüle R, Schöls L, Münte TF, Rodriguez‐Fornells A (2009): Genetic variability in the dopamine system (dopamine receptor D4, catechol‐O‐methyltransferase) modulates neurophysiological responses to gains and losses. Biol Psychiatry 66:154–161. [DOI] [PubMed] [Google Scholar]
- Martin‐Soelch C, Linthicum J, Ernst M (2007): Appetitive conditioning: Neural bases and implications for psychopathology. Neurosci Biobehav Rev 31:426–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechias M, Etkin A, Kalisch R (2010): A meta‐analysis of instructed fear studies: Implications for conscious appraisal of threat. NeuroImage 49:1760–1768. [DOI] [PubMed] [Google Scholar]
- Meyer‐Lindenberg A (2009): Neural connectivity as an intermediate phenotype: Brain networks under genetic control. Hum Brain Mapp 30:1938–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer‐Lindenberg A, Kohn PD, Kolachana B, Kippenhan S, McInerney‐Leo A, Nussbaum R, Weinberger DR, Berman KF (2005): Midbrain dopamine and prefrontal function in humans: Interaction and modulation by COMT genotype. Nat Neurosci 8:594–596. [DOI] [PubMed] [Google Scholar]
- Mier D, Kirsch P, Meyer‐Lindenberg A (2010): Neural substrates of pleiotropic action of genetic variation in COMT: A meta‐analysis. Mol Psychiat 15:918–927. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Johnstone EC, Guo B, Murphy MF, Aveyard P (2008): Association of COMT Val108/158Met genotype with smoking cessation. Pharmacogenet Genomics 18:121–128. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ (2004): Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science 304:452–454. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP (2007): Lights, camembert, action! The role of human orbitofrontal cortex in encoding stimuli, rewards, and choices. Ann N Y Acad Sci 1121:254–272. [DOI] [PubMed] [Google Scholar]
- Ott U, Reuter M, Hennig J, Vaitl D (2005): Evidence for a common biological basis of the Absorption trait, hallucinogen effects, and positive symptoms: Epistasis between 5‐HT2a and COMT polymorphisms. Am J Med Genet 137B:29–32. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE (2004): Extinction Learning in Humans: Role of the Amygdala and vmPFC. Neuron 43:897–905. [DOI] [PubMed] [Google Scholar]
- Rasch B, Spalek K, Buholzer S, Luechinger R, Boesiger P, Quervain DJ , de Papassotiropoulos A (2010): Aversive stimuli lead to differential amygdala activation and connectivity patterns depending on catechol‐O‐methyltransferase Val158Met genotype. NeuroImage 52:1712–1719. [DOI] [PubMed] [Google Scholar]
- Schmack K, Schlagenhauf F, Sterzer P, Wrase J, Beck A, Dembler T, Kalus P, Puls I, Sander T, Heinz A, Gallinat J (2008): Catechol‐O‐methyltransferase val158met genotype influences neural processing of reward anticipation. NeuroImage 42:1631–1638. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR (1997): A neural substrate of prediction and reward. Science 275:1593–1599. [DOI] [PubMed] [Google Scholar]
- Smolka MN, Schumann G, Wrase J, Grüsser SM, Flor H, Mann K, Braus DF, Goldman D, Büchel C, Heinz A (2005): Catechol‐O‐methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J Neurosci 25:836–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolka MN, Bühler M, Schumann G, Klein S, Hu X, Moayer M, Zimmer A, Wrase J, Flor H, Mann K, Braus DF, Goldman D, Heinz A (2007): Gene‐gene effects on central processing of aversive stimuli. Mol Psychiat 12:307–317. [DOI] [PubMed] [Google Scholar]
- Straube T, Weiss T, Mentzel H, Miltner WHR (2007): Time course of amygdala activation during aversive conditioning depends on attention. NeuroImage 34:462–469. [DOI] [PubMed] [Google Scholar]
- Tabbert K, Stark R, Kirsch P, Vaitl D (2005): Hemodynamic responses of the amygdala, the orbitofrontal cortex and the visual cortex during a fear conditioning paradigm. Int J Psychophysiol 57:15–23. [DOI] [PubMed] [Google Scholar]
- Tammimäki AE, Männistö PT (2010): Are genetic variants of COMT associated with addiction? Pharmacogenet. Genomics 20:717–741. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Huber A, Farrell SM, Stumpenhorst K, Harrison PJ, Walton ME (2012): The role of catechol‐O‐methyltransferase in reward processing and addiction. CNS Neurol Disord Drug Targets 11:306–323. [DOI] [PubMed] [Google Scholar]
- Wehrum S, Klucken T, Kagerer S, Walter B, Hermann A, Vaitl D, Stark R (2013): Gender commonalities and differences in the neural processing of visual sexual stimuli. J Sex Med 10:1328–1342. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Schott BH, Guderian S, Frey JU, Heinze H, Düzel E (2005): Reward‐related FMRI activation of dopaminergic midbrain is associated with enhanced hippocampus‐dependent long‐term memory formation. Neuron 45:459–467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


