Abstract
Both increases and decreases in resting state functional connectivity have been previously observed within the motor network during aging. Moreover, the relationship between altered functional connectivity and age‐related declines in bimanual coordination remains unclear. Here, we explored the developmental dynamics of the resting brain within a task‐specific motor network in a sample of 128 healthy participants, aged 18–80 years. We found that age‐related increases in functional connectivity between interhemispheric dorsal and ventral premotor areas were associated with poorer performance on a novel bimanual visuomotor task. Additionally, a control analysis performed on the default mode network confirmed that our age‐related increases in functional connectivity were specific to the motor system. Our findings suggest that increases in functional connectivity within the resting state motor network with aging reflect a loss of functional specialization that may not only occur in the active brain but also in the resting brain. Hum Brain Mapp 35:3945–3961, 2014. © 2014 Wiley Periodicals, Inc.
Keywords: aging, lifespan, resting state, motor network, bimanual coordination, functional connectivity
INTRODUCTION
Aging is characterized by an adaptive reorganization of brain networks that is commonly associated with impairments at the behavioral level. Of special interest are the prominent effects of aging on movement control in general, and bimanual coordination in particular (Lee et al., 2002; Seidler et al., 2010; Serbruyns et al., 2013; Serrien et al., 2000, 1996; Smith et al., 1999; Swinnen et al., 1998; Wishart et al., 2000). The impact that age‐related deficits in bimanual coordination have on daily life activities provided us with a strong impetus to investigate the neural correlates of this behavior in the resting brain of older individuals.
Generally, bimanual coordination is determined by different types of constraint, i.e., temporal, spatial and muscle grouping (Byblow et al., 1995; Carson et al., 2000; Kelso, 1984; Summers, 2002; Serrien, 2009; Temprado et al., 2003). Regarding the former, isofrequency patterns in which both hands move with the same relative velocity are more stable compared to multifrequency patterns (Li and Lindenberger, 1999). As for spatial constraints, isodirectionality in extrinsic space generally results in better performance than non‐isodirectionality (Swinnen et al., 1997). Muscle grouping refers to the body's natural preference to co‐activate homologous muscles, giving rise to the symmetrical or in‐phase mode being the most favorable state, and the asymmetrical or anti‐phase mode being less stable (Kelso, 1984; Swinnen et al., 1997; Swinnen, 2002). In the face of these constraints, converging evidence suggests an age‐related progressive decline in bimanual coordination and response switching performance (Coxon et al., 2010; Goble et al., 2010; Swinnen et al., 1998; Wishart et al., 2000).
Age‐related declines in bimanual coordination may be indicative of disruptions in the neural representations of the motor system. Task‐activated fMRI studies using various motor actions have shown that older adults exhibit both increased neural activity (Fang et al., 2005; Goble et al., 2010; Heuninckx et al., 2005, 2008; Mattay et al., 2002; Naccarato et al., 2006; Riecker et al., 2006; Van Impe et al., 2012a, 2012b, 2009, 2011; Ward and Frackowiak, 2003; Wu and Hallet, 2005) and interhemispheric functional connectivity (Heitger et al., 2013) compared to young adults in brain regions that typically constitute a motor network, such as the ventral and dorsal premotor cortices, supplementary motor area, primary motor cortex, and cingulate motor cortex. Increased connectivity across these regions have been found to be crucial for early movement preparation and temporal organization, selection and generation of motor sequences in complex bimanual tasks (Goerres et al., 1998; Jenkins et al., 1994; Sadato et al., 1997).
On the other hand, resting state functional connectivity MRI (rs‐fcMRI) has provided robust insights into the functional organization of the aging brain at rest, highlighting significant age‐related effects across different functional networks (see Van den Heuvel and Pol, 2010 for a review). In this regard, extensive literature has demonstrated an age‐related reduction in connectivity within the default mode network (DMN) that is related to poorer performance on cognitive tasks (Andrews‐Hanna et al., 2010, 2007; Damoiseaux et al., 2008; Gazzaley et al., 2005; Park et al., 2004). However, only a few studies have investigated age‐related differences in the motor network during the resting state, with results showing both increased and decreased functional connectivity strength. Age‐related increases in functional connectivity have been found between the somatosensory and motor cortices (Langan et al., 2010; Meier et al., 2013; Mowinckel et al., 2012; Tomasi and Volkow, 2012; Zuo et al., 2010). This finding supports the hypothesis that aging is associated with a loss of functional hemispheric asymmetry and reduced neural specialization (Bernard and Seidler 2012; Ferreira and Busatto, 2013; Mowinckel et al., 2012). In contrast, age‐related decreases in functional connectivity have been found between the premotor area and cingulate motor cortex, being associated with declines in motor performance (Wu et al., 2007a), and within an extended motor network including the thalamus, basal ganglia, and cerebellum (Wu et al., 2007b). One commonality among these resting state studies is that age‐related increases and decreases in functional connectivity are systematically observed across the same motor regions as the ones previously referred to in task‐related studies. Additionally, it is important to underscore that increases in functional connectivity within the motor network have been mainly observed in lifespan studies (Mowinckel et al., 2012; Tomasi and Volkow, 2012; Zuo et al., 2010), whereas decreases have been observed in studies comparing two smaller age groups (Wu et al., 2007a, b).
The inconclusive nature of these results suggests that further research is necessary. Therefore, we investigated the motor network in the resting brain across a large age span, ranging from 18 to 80 years. Specifically, we examined how aging influences functional connectivity within the resting state motor network and whether the observed alterations were associated with the commonly reported deficits in bimanual coordination. Consistent with previous research using large lifespan samples (Mowinckel et al., 2012; Tomasi and Volkow, 2012; Zuo et al., 2010), we predicted age‐related increases in functional connectivity between pairs of regions commonly involved in bimanual coordination, such as interhemispheric ventral and dorsal premotor areas, supplementary motor area, primary motor cortex, and cingulate motor cortex. It is worth noting that only one study to date has investigated the relationship between age‐related changes in the resting state motor network and motor performance (Wu et al., 2007a). Hence, the current work aims to extend this emerging line of research with a unique focus on bimanual coordination. We expected age‐related increases in functional connectivity of the motor network to be associated with declines in bimanual coordination. This hypothesis is based on previous research demonstrating a high correspondence between resting state and task‐related networks, supporting the stability of the functional organization of the brain across effortful and resting conditions (Smith et al., 2009).
We also examined the functional integrity of the DMN (Raichle et al., 2001) as a control measure in order to examine the specificity of age‐related effects on the resting state motor network and their association with bimanual coordination. Based on the previous work (Andrews‐Hanna et al., 2007), we hypothesized age‐related decreases in the functional connectivity of the DMN without any relation to bimanual coordination performance.
METHODS
Participants
One hundred and twenty eight participants (68 females) ranging in age from 18 to 80 years (mean age = 45.76, SD = 21.21 years) participated in the study. All were right‐handed, as verified by the Edinburgh Handedness Inventory (Oldfield, 1971) (mean laterality = 93.87, SD = 12.76, range 38–100), and had normal or corrected‐to‐normal vision. None of the participants reported a history of neurological, psychiatric, or vascular disease. Informed consent was obtained before testing. The experiment was approved by the local ethics committee for biomedical research, and was performed in accordance with the 1964 Declaration of Helsinki.
Procedures
Participants were tested in two different sessions. In the first session, they completed a behavioral assessment (duration = 55 minutes) that included the Montreal Cognitive Assessment scale (MoCA) and the bimanual visuomotor task. The second session was conducted on a different day and comprised the standard scanning protocol in the University Hospital of KU Leuven (duration = 60 minutes). It included a resting state protocol (lasting 10 min) during which subjects were instructed to keep their eyes open and fixated on a target point. No task‐related scans were acquired during the scanning session, leaving the resting state scan unaffected by potential changes due to administering the task.
Behavioral Measures
The MoCA
This scale (Nasreddine et al., 2005) was employed as a screening instrument for the detection of mild cognitive impairment (MCI). The MoCA detects MCI with 90% range sensitivity and 87% specificity, and Alzheimer's disease with 100% range sensitivity and 87% specificity (95% confidence interval), ensuring the applicability of this measure as a cognitive screening task.Only individuals above 60 years old (N = 43) were assessed with this scale. The suggested MoCA cut‐off score for normal controls is ≥26. In the current study, all participants obtained a score within normal limits (≥26, mean = 27.24, SD = 1.38, range = 26–30).
Bimanual visuomotor task
This task enables the evaluation of bimanual coordination accuracy, relying on the execution of complex bimanual patterns (Sisti et al., 2011). With some exceptions, bimanual coordination constraints such as temporal complexity, directionality, and muscle grouping, have often been investigated in separate experiments. However, here we used a bimanual coordination task that allowed us to address these key elements together to quantify the strength and interactions of these constraints.
Participants were seated in front of a computer monitor with both lower arms resting on two custom‐made adjustable ramps. At the end of the ramps, a dial was mounted on a horizontal support consisting of a flat disc (diameter 5 cm) and a vertical peg. The dials could be rotated by holding each peg between the thumb and the index finger (Fig. 1A). High precision shaft encoders were aligned with the axis of rotation of the dials to record angular displacement (Avago Technologies, sampling frequency = 100 Hz, accuracy = 0.089°). Direct vision of both hands and forearms was occluded by a horizontal table‐top bench, placed over the forearms of the subject.
Figure 1.
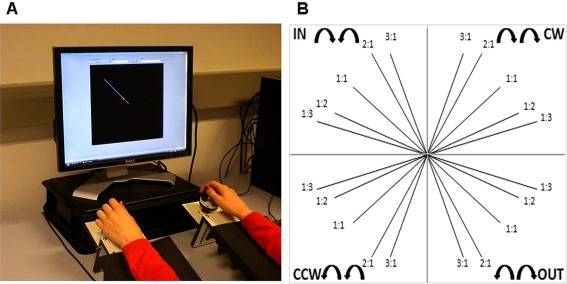
Experimental apparatus to assess bimanual coordination. (A) Custom‐made bimanual visuomotor task. Participants rotate two dials to track a target dot (hands are covered to prevent their vision). (B) We included five frequency ratios [1:1 (isofrequency, ISO), 1:2, 1:3, 2:1, and 3:1 (non‐isofrequency, N‐ISO)], and four coordination patterns: two with the left and right dials moving in the same direction (clockwise, CW; counterclockwise, CCW), and two with the left and right dial moving toward (inwards, IN) or away from each other (outwards, OUT) [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Each trial started with the presentation of a single blue target line with a distinct orientation. At the origin of this line, in the center of the display, a white target dot was presented, after which it began to move along the blue target line, toward the peripheral endpoint. The target dot moved at a constant rate and for a total duration of 10 s. After 10 s, the screen turned black, regardless of the subject's location on the screen, and the next target line would appear after a random interval of 4–6 s. Participants were instructed to rotate two dials in order to track the white target dot. The two dials controlled the movement of a red cursor serving as online visual feedback of tracking performance. The left and right dial controlled the movement of the subject's cursor along the vertical and horizontal axis, respectively. When the left‐hand dial was rotated to the right (clockwise, CW), the cursor moved up; when turned to the left (counterclockwise, CCW), the cursor moved down. When the right‐hand dial was rotated to the right (CW), the cursor moved to the right; when rotated to the left (CCW), the cursor moved to the left. The gain was set to 10 units (U) rotation−1, indicating that drawing a horizontal/vertical line on the screen of 150 U required 15 complete rotations of the right/left dial, respectively. The goal was to match the red cursor with the white target dot in both space and time, keeping the deviation as small as possible. In other words, participants had to rotate the dials as accurately as possible, generating the correct direction and velocity.
We manipulated two different variables: coordination pattern and frequency ratio. Four coordination patterns were introduced: two in which the left and right dial moved in the same direction, either CW or CCW; and two in which the left and right dial moved toward (inwards, IN) or away from each other (outwards, OUT). The coordination patterns had to be performed at different relative velocities of the hands (frequency ratios), represented by the precise slope of the target line. We included five frequency ratios (left hand first, right hand second): 1:1, 1:2, 1:3, 2:1, and 3:1. For example, a 3:1 frequency ratio indicated that the left hand moved three times faster than the right hand. The combination of coordination patterns (4) and frequency ratios (5) resulted in 20 experimental conditions (Fig. 1B).
Four blocks of 6 min with 3‐min rest in between were administered. A block consisted of 24 target lines (all 20 distinctive target lines plus the 1:1 frequency ratio repeated in all four coordination patterns), presented in a pseudorandom order. Prior to data recording, participants were allowed to practice 12 lines, for a period of 3 min, to become familiar with the task variants. The data were analyzed using both Labview 8.5 and MATLAB R2008a. The x‐ and y‐positions of the target dot and the subject cursor were sampled at 100 Hz per trial. Target deviation was calculated as a measure of accuracy, using the following multistep procedure:
- (a) Every 10 ms, the difference between the target position and the subject position, d, was calculated, using the Euclidean distance:
where and refer to the position of the subject's cursor on the x‐ and y‐axes, respectively, and and correspond to the position of the target dot on the x‐ and y‐axes, respectively. (b) At the end of each trial, the average of these distances was computed and defined as the trial's target deviation, expressed in units (U). A target deviation equal to 0 U would indicate that during the whole trial, the subject cursor was precisely on top of the target dot, representing perfect performance. Accordingly, larger target deviation scores reflect poorer performance.
To determine whether subjects generally met the task requirements, all data were transformed into z‐scores [(X‐MEAN/SD)]. Data were not normally distributed and required a square‐root transformation. After that, no outliers were removed from the data set (no z values were greater than │3│).
A preliminary series of repeated measures ANOVAs were conducted in order to explore the exact relationship between the different levels of the two variables manipulated across subjects. In this regard, a 4 × 5 (coordination pattern × frequency ratio) repeated measures ANOVA for target deviation was performed. Coordination pattern contained four levels, i.e., clockwise (CW), counterclockwise (CCW), inwards (IN), and outwards (OUT). Frequency ratio comprised five levels, i.e., 1:1, 1:2, 1:3, 2:1, and 3:1. Based on the results obtained from the repeated measures, we grouped those frequency ratios in which both hands were moving with different relative speeds (1:2, 1:3, 2:1, and 3:1) and performed a 4 × 2 (coordination pattern × frequency ratio) repeated measures ANOVA. At this stage, frequency ratio comprised two levels, i.e., isofrequency (ISO, 1:1), and non‐isofrequency (N‐ISO, 1:2, 1:3, 2:1, and 3:1 collapsed). Significant main and interaction effects were further explored by post‐hoc paired t‐tests using Bonferroni correction for each repeated measures ANOVA conducted. We also explored the effects of the different coordination patterns during isofrequency ratios. Inwards and outwards coordination patterns were grouped as in‐phase movements, and clockwise and counterclockwise coordination patterns were grouped as anti‐phase movements. in‐phase and anti‐phase movements were compared by means of paired t‐tests and corrected for multiple comparisons with Bonferroni.
Next, we explored the effect of aging on bimanual coordination. To this end, we calculated the correlation strength between age and the scores from the bimanual visuomotor task with Pearson correlation coefficients. The probabilities associated with all tests were FDR‐corrected for multiple comparisons. In the results section, we report FDR‐corrected (Q) statistics.
Image Acquisition
A Siemens 3 T Magnetom Trio MRI scanner (Siemens, Erlangen, Germany) with 12‐channel matrix head coil was used for image acquisition. Functional resting state data were acquired with a descending gradient echo‐planar imaging (EPI) pulse sequence for T 2 *‐weighted images (repetition time = 3000 ms; echo time = 30 ms; flip angle = 90°; 50 oblique axial slices each 2.8mm thick; inter‐slice gap = 0.028 mm; in‐plane resolution 2.5 × 2.5 mm; 80 × 80 matrix, 200 volumes). A 3D magnetization prepared rapid gradient echo (MPRAGE) high resolution T 1‐weighted structural image (repetition time = 2300 ms; echo time = 2.98 ms; 1 × 1 × 1.1 mm3 voxels; field of view = 240 × 256 mm2; 160 sagittal slices) was acquired for spatial normalization and group‐specific template generation.
Data Preprocessing
Standard preprocessing procedures were performed using SPM8 (Statistical Parametric Mapping software, SPM: Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm/) implemented in Matlab 7.7 (The Mathworks) following the signal processing pipeline developed by Mantini et al. (2011).
Functional images were slice‐time corrected to the middle slice (reference slice = 25), spatially realigned to the first image in the time series, normalized to the standard EPI template in Montreal Neurological Institute (MNI) space, and resampled into 3 mm isotropic voxels (Friston et al., 1995). Spatial smoothing was not applied in order to avoid introducing artificial local spatial correlations (Achard et al., 2006; Achard and Bullmore, 2007; Salvador et al., 2005). During fMRI data preprocessing, we quantified for each participant the root mean squared variance (rmsvariance) of head motion, on the basis of the head motion realignment parameters (Ebisch et al., 2011). Average subject rmsvariance of motion was 0.08 mm (SD = 0.05). No subject was characterized by movement larger than 0.3 mm during fMRI scanning.
Region Definition
Candidate ROIs were generated in an unbiased manner from a separate task‐related fMRI data set (Beets et al., 2013) of young (n = 26, range =17–27 years; mean = 21.15 years; SD = 2.33; 14 females) and older adults (n = 21, range = 60–80 years; mean = 69.02 years; SD = 1.98; 11 females). This study explored age‐related differences in motor activation while subjects performed the same bimanual visuomotor task used in our study. The main BOLD contrast of interest was bimanual visuomotor task performance vs baseline condition (no movement) in young and older adults. Young and old z‐score maps from the task‐based fMRI study were combined to find overlapping ROIs by means of a conjunction analysis [young (bimanual visuomotor task > baseline) ∩ old (bimanual visuomotor task > baseline)] (Nichols et al., 2005). The statistical threshold was set to P < 0.05 FWE corrected for multiple comparisons and a cluster size minimum of 20 voxels.
To define regions for our resting state connectivity analysis, we chose the peak voxel with the highest z‐score (z ≥ 4.69) in the positive group analysis. Our ROIs were composed of 6‐mm radius spheres centered on these peak voxels and were created using the MarsBAR toolbox (http://marsbar.sourceforge.net). The size of the spheres was selected to ensure that they contained voxels that were significantly activated in all cases. We defined the following a priori ROIs: supplementary motor area (SMA); middle cingulate (MCC); dorsal premotor area (PMd: R, L); ventral premotor area (PMv: R, L); primary motor cortex (M1: R, L); dorsal primary somatosensory area (S1d: R); ventral primary somatosensory area (S1v: R, L); and somatosensory association area (SAA: R). Regions, coordinates and peak z‐scores are listed in Table 1.
Table 1.
Regions defined for the resting state motor network
| Area | Hemisphere | x | y | z | z‐score |
|---|---|---|---|---|---|
| Movement>baseline | |||||
| Supplementary motor area (SMA) | bilateral | 2 | −8 | 52 | 5.64 |
| Middle cingulate(MCC) | bilateral | 2 | 8 | 46 | 5.15 |
| Dorsal premotor area (PMd) | R | 34 | −4 | 62 | 5.12 |
| L | −28 | −22 | 64 | 5.62 | |
| Ventral premotor area (PMv) | R | 32 | −8 | 52 | 5.79 |
| L | −26 | −8 | 54 | 5.39 | |
| Primary motor cortex (M1) | R | 34 | −32 | 62 | 5.74 |
| L | −42 | −22 | 54 | 6.09 | |
| Dorsal primary somatosensory area (S1d) | L | −40 | −42 | 60 | 4.99 |
| Ventral primary somatosensory area(S1v) | R | 36 | ‐26 | 46 | 6.53 |
| L | −30 | −30 | 48 | 6.19 | |
| Somatosensory association area (SAA) | R | 14 | −60 | 60 | 4.92 |
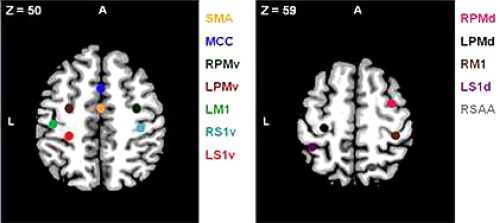
| |||||
Regions obtained after a conjunction analysis (young ∩ old) from a task‐based fMRI study, P FWE < 0.05, z ≥ 4.69. Spheres of 6‐mm radius centered on z‐peak voxels. R, right; L, left; A, anterior.
To the best of our knowledge, the most suitable approach to explore age‐related effects on resting state functional connectivity within a task‐specific motor network was to localize our ROIs from an independent task‐related fMRI data set in which young and older individuals performed the same bimanual visuomotor task used in our study. This rationale is supported by previous research that emphasizes the suitability of this approach to identify functionally specific regions in domains where the underlying function can be unequivocally localized from independent scans (Poldrack, 2007), as well as the importance of determining ROIs directly derived from the data and to avoid predefined ROIs in order to maximize accuracy (Smith et al., 2011).
To define the DMN, we conducted a seed‐based analysis after the conjunction analysis using the posterior cingulate cortex (PCC) as the seed region (created from the AAL atlas, Tzourio‐Mazoyer et al., 2002). PCC has previously been used in similar analyses (Andrews‐Hanna et al., 2007; Helmich et al., 2010) since it plays a central role in the DMN (Buckner et al., 2000, 2005; Fox et al., 2005). Correlation coefficients between the averaged time course of the PCC seed region and the time course for each voxel across the whole brain were calculated. The resulting correlation maps were converted to z values with Fischer's r‐to‐z transformation (Zar, 1998). Regions were defined as contiguous voxels showing significant correlations (at Q ≤ 0.01) with a cluster size minimum of 20 voxels. The following ROIs were created using the xjView toolbox (http://www.alivelearn.net/xjview8): dorsal medial prefrontal cortex (dMPFC), ventral medial prefrontal cortex (vMPFC), angular gyrus (r, l), middle temporal gyrus (MTG, r, l), posterior cingulate (PCC), and thalamus. Regions, coordinates and peak z‐scores are listed in Table 2.
Table 2.
Regions defined for the DMN
| Area | Hemisphere | x | y | z | z‐score |
|---|---|---|---|---|---|
| Dorsal medial prefrontal cortex (dMPFC) | bilateral | 0 | 58 | 12 | 8.08 |
| Ventral medial prefrontal cortex (vMPFC) | bilateral | 0 | 60 | −6 | 11.31 |
| Angular gyrus (AG) | R | 50 | −58 | 26 | 9.64 |
| L | −46 | −66 | 28 | 11.36 | |
| Middle temporal gyrus (MTG) | R | 64 | −6 | −14 | 8.00 |
| L | −64 | −18 | −16 | 8.73 | |
| Posterior cingulate(PCC) | bilateral | −6 | −46 | 34 | 13.60 |
| Thalamus | bilateral | −4 | −16 | 10 | 8.21 |
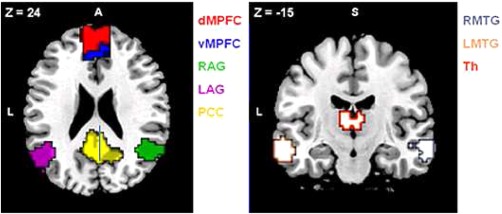
| |||||
Regions obtained from a seed‐based analysis using the PCC as the seed region. Regions defined as contiguous voxels with significant correlations, Q < 0.01, z ≥ 3.47, cluster size ≥ 20 voxels. R, right; L, left; A, anterior; S, superior.
Functional Connectivity Analysis
The same procedure was followed to study the motor and default mode networks. Before carrying out the connectivity analysis a number of additional preprocessing steps were taken to remove spurious sources of variance. We defined a small, bilateral region of interest in the ventricles, a region of interest in the deep white matter, and one covering the whole brain; we then calculated the average signals in these three regions, which are typically referred to as cerebrospinal fluid, white matter, and global signals (Fox et al., 2005, 2009). Next, we performed a regression analysis on the fMRI time courses, modeling the three aforementioned signals and the parameters obtained by rigid body head motion realignment (Fox et al., 2005, 2009), as well as their temporal derivatives, as regressors. The residuals of these regressions were then temporally band‐pass filtered (0.01–0.08 Hz) to reduce low‐frequency drift and high‐frequency noise. Previous studies indicate that only frequencies below 0.1 Hz contribute to regional BOLD response, whereas signals at higher frequencies are more likely to account for cardiac and respiratory factors (Biswal et al., 1995; Cordes et al., 2001; Fransson, 2005; Peltier and Noll, 2002).
For each subject, regional mean time series were extracted by averaging the functional MRI time series across all voxels in each ROI. Then, the correlation strength between every pair of ROIs was calculated using Pearson correlation coefficients creating a functional network captured by a 12 × 12 correlation matrix for the motor network and an 8 × 8 correlation matrix for the DMN. These Pearson correlation values were converted to Z‐scores by Fischer's r‐to‐z transformation (Zar, 1998), correcting the degrees of freedom for the autocorrelation in the time series (Shumway and Stoffer, 2006). Group‐level correlation matrices were created across subjects by using a random‐effects analysis (Ebisch et al., 2011; Pravata et al., 2011). All statistical analyses of correlation data were performed on z‐scores to ensure normality.
For subsequent analyses, all connections with a significant correlation (at P ≤ 0.05) were selected and correction for multiple comparisons was applied using the False Discovery Rate (FDR) method (Genovese et al., 2002). Next, we extracted single‐subject correlations from significant ROI pairs to examine whether functional connectivity was related to aging. We then calculated correlations between single‐subject connectivity metrics (expressed in Z‐scores) and age. Although we corrected for motion‐related artifacts before testing for the effects of age by regressing out estimated motion parameters, we additionally correlated our average measure of estimated motion across the times series of each subject (average subject rmsvariance of motion) with age. The absence of correlations between average estimated motion and age will ensure that the potential age‐effects are not a consequence of confounding motion artifacts.
Finally, we selected the ROI pairs showing a significant correlation with age and examined relationships with bimanual coordination. To this end, we calculated correlations between the surviving functional connectivity values and bimanual coordination scores (in Z‐scores). Additionally, we conducted a supplementary analysis for the resting state motor network. The sample of 128 participants was blindly split into two groups of 64 subjects. Both groups spanned the age range and matched gender and age (Group 1: age range = 18–80, mean age = 45.99, SD = 21.25 years; Group 2: age range = 18–80, mean age = 45.52, SD = 21.33 years). We calculated correlations between the abovementioned surviving functional connectivity values and bimanual coordination scores (Z‐scores) within each group in order to check the stability of the results across the entire lifespan sample and each cohort of 64 participants, respectively. This additional analysis was conducted in an effort to strengthen our results by showing consistency across different sample sizes after correcting for multiple comparisons.
In all cases, the probabilities associated with all tests were FDR‐corrected for multiple comparisons. In the following section, we report FDR‐corrected (Q) statistics.
RESULTS
Behavioral Results
The exploratory 4 × 5 (coordination pattern [CW, IN, CCW, OUT] × frequency ratio [1:1, 1:2, 1:3, 2:1, 3:1]) repeated measures ANOVA for target deviation revealed a significant main effect of coordination pattern, a main effect of frequency ratio, and a significant coordination pattern × frequency ratio interaction (see Table 3). For the latter, post‐hoc paired t‐tests corrected with Bonferroni (P < 0.005) revealed that the 1:1 ratio showed the statistically smallest target deviation compared to the other four frequency ratios across all coordination patterns. Consequently, we grouped 1:2, 2:1, 1:3, and 3:1 (N‐ISO) ratios for subsequent analysis, a procedure that has been previously reported (Gooijers et al., 2013; Sisti et al., 2011). Next, a 4 × 2 (coordination pattern [CW, IN, CCW, OUT] × frequency ratio [ISO, N‐ISO]) repeated measures ANOVA for target deviation was conducted. It revealed a main effect of coordination pattern, and frequency ratio. No significant interaction effect between coordination pattern and frequency ratio was found (Table 3). The smallest target deviation (indicative of better performance) was obtained for clockwise, followed by counterclockwise, inwards, and outwards patterns. Regarding frequency ratio, target deviation increased from isofrequency to non‐isofrequency ratios. Additionally, we explored the effect of coordination pattern on isofrequency ratios. We grouped inwards and outwards as in‐phase coordination, and clockwise and counterclockwise as anti‐phase coordination. Paired t‐tests showed the smallest target deviation for the anti‐phase as compared to the in‐phase coordination pattern when both hands moved at the same angular velocity (t 127 = 5.28, P < 0.001; in‐phase 3.81 ± 1.26; anti‐phase 3.41 ± 1.12).
Table 3.
Summary of ANOVA results on target deviation during bimanual performance
| Effect | df | F | P |
|---|---|---|---|
| 4 × 5 repeated measures ANOVA | |||
| Coordination pattern | 3 | 31.22 | <0.001 |
| Frequency ratio | 4 | 52.99 | <0.001 |
| Coordination pattern × frequency ratio | 12 | 4.56 | <0.001 |
| 4 × 2 repeated measures ANOVA | |||
| Coordination pattern | 3 | 29.71 | <0.001 |
| Frequency ratio | 1 | 205.89 | <0.001 |
| Coordination pattern × frequency ratio | 3 | 0.816 | >0.050 |
Finally, we calculated the correlations between age and the scores from the bimanual visuomotor task. Results showed a general decline of bimanual coordination with advancing age regardless of the coordination pattern or frequency ratio. The aging effect was also observed when we focused on isofrequency ratios. Positive correlations between age and target deviation are indicative of poorer performance (CW ISO: r = 0.39, Q < 0.001; IN ISO: r = 0.49, Q < 0.001; CCW ISO: r = 0.36, Q < 0.001; OUT ISO: r = 0.44, Q < 0.001; CW N‐ISO: r = 0.36, Q < 0.001; IN N‐ISO: r = 0.44, Q < 0.001; CCW N‐ISO: r = 0.44, Q < 0.001; OUT N‐ISO: r = 0.46, Q < 0.001; in‐phase: r = 0.49, Q < 0.001; anti‐phase: r = 0.40, Q < 0.001). Figure 2A, B summarizes these results. There was no significant correlation between age and gender (r = −0.07, Q = 0.45) suggesting that the observed results are not driven by participants' gender.
Figure 2.
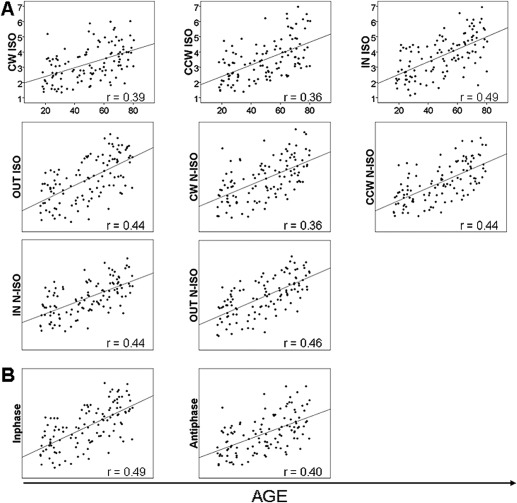
Significant correlations between the bimanual visuomotor task and age. Correlations between the scores from the bimanual visuomotor task (Y‐axis) and age (X‐axis). (A) Four coordination patterns (CW, CCW, IN, OUT) and two frequency ratios (ISO, N‐ISO) after grouping the initial five frequency ratios; (B) after grouping the initial four coordination patterns in two (in‐phase, anti‐phase) within the ISO ratios. Q < 0.001.
Functional Network Results
We studied low‐frequency functional correlations associated with 12 ROIs defined as the bilateral SMA, bilateral MCC, left and right PMd, left and right PMv, left and right M1, left S1d, left and right S1v, and right SAA. We calculated Pearson correlation coefficients between each pair of ROIs across subjects. Figure 3A shows the resulting 12 × 12 correlation matrix, which reflects the strength of functional connectivity between each pair of regions. Pairs of regions within the motor network and within the DMN showed strong positive correlations. Pairwise correlations between regions from the motor network and the DMN showed negative correlations, indicating system specificity. Figure 3B shows an example of time courses in a single subject for a pair of regions that are positively correlated, the SMA and left M1. Significant correlations were defined after correction for multiple comparisons using FDR (Q < 0.05). Only statistically significant correlations were considered for further analyses.
Figure 3.
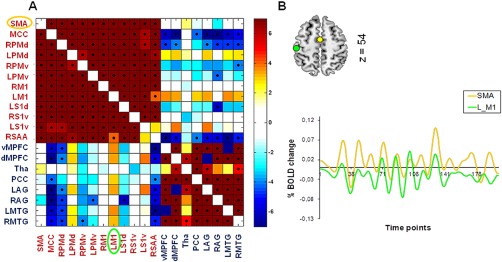
Increased functional connectivity between pairs of regions. (A) Correlation matrix showing the strength of functional connectivity between each pair of regions from the motor network and the DMN. Significant correlations (FDR‐corrected probability, Q < 0.05) are indicated with a black dot. Pairs of regions within the motor network and the DMN show positive correlations. Pairs of regions between the motor network and the DMN show negative correlations. Right bar indicates t values. (B) Time courses for a single subject are shown for the SMA (yellow) and the left M1 (green) which are positively correlated. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
We next examined if patterns of functional connectivity within our motor network were modulated as a function of aging. Results revealed 23 pairs of regions that showed increased functional connectivity with age, suggesting that age had a marked effect on functional connectivity patterns within our large‐scale motor network. This effect was observed in the following pairs: SMA/PM, SMA/S1, SMA/M1, MCC/PM, MCC/M1, interhemispheric PM, PM/M1, PM/S1, interhemispheric M1, and M1/S1. Table 4 shows the strength of correlation values between each pair of ROIs and age after multiple comparison correction with FDR.
Table 4.
Increased functional connectivity with age
| Pair of ROIs | Pearson correlation coefficient, r | P |
|---|---|---|
| SMA vs MCC | 0.34 | <0.0001 |
| SMA vs LPMv | 0.37 | <0.0001 |
| SMA vs LS1d | 0.23 | <0.05 |
| SMA vs LM1 | 0.24 | <0.001 |
| SMA vs LPMd | 0.24 | <0.05 |
| SMA vs RPMd | 0.29 | <0.001 |
| MCC vs LPMv | 0.32 | <0.0001 |
| MCC vs LM1 | 0.24 | <0.005 |
| MCC vs LPMd | 0.30 | <0.0001 |
| MCC vs RM1 | 0.22 | <0.05 |
| LMv vs LPMd | 0.33 | <0.0001 |
| RPMd vs RS1v | 0.23 | <0.01 |
| LM1 vs LPMd | 0.23 | <0.01 |
| LM1 vs RPMd | 0.25 | <0.005 |
| LM1 vs LPMv | 0.22 | <0.05 |
| LM1 vs RM1 | 0.31 | <0.0001 |
| LM1 vs RS1v | 0.25 | <0.005 |
| LPMd vs RPMd | 0.28 | <0.001 |
| LPMd vs RM1 | 0.28 | <0.01 |
| LPMd vs RPMv | 0.25 | <0.005 |
| LPMd vs RS1v | 0.26 | <0.01 |
| LS1d vs LM1 | 0.29 | <0.001 |
| LS1d vs LPMd | 0.29 | <0.001 |
Twenty‐three pairs of regions showing increased functional connectivity with age. R, right, L, left. Q < 0.05.
Correlations between the average measure of estimated motion (average subject rmsvariance) and age were not statistically significant (r = 0.11, P > 0.05). Hence, it is unlikely that the aging effects are a consequence of confounding motion artifacts.
Subsequent analysis, conducted to elucidate the behavioral significance of age‐related effects on functional connectivity, showed significant correlations between the left PMd/right PMv pair and bimanual coordination after FDR correction (Table 5). Specifically, age‐related increased functional connectivity in the left PMd/right PMv pair correlated with a greater target deviation (indicative of poorer performance) when participants performed the inwards or outwards patterns with both hands moving with the same or different relative velocity. Moreover, increased functional connectivity in the left PMd/right PMv pair was correlated with a greater target deviation for in‐phase movements (inwards and outwards collapsed) at isofrequency ratios. Figure 4 summarizes the significant correlations between age‐related increased functional connectivity and poorer bimanual coordination.
Table 5.
Correlations between functional connectivity measures and bimanual coordination performance
| IN ISO | OUT ISO | IN N‐ISO | OUT N‐ISO | In‐phase | |
|---|---|---|---|---|---|
| LPMd vs RPMv | r = 0.31 | r = 0.29 | r = 0.27 | r = 0.28 | r = 0.32 |
| P < 0.0001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.0001 | |
| Q < 0.001 | Q < 0.001 | Q < 0.001 | Q < 0.001 | Q < 0.05 | |
| MCC vs LPMd | r = 0.22 | r = 0.19 | r = 0.21 | ||
| P = 0.01 | P = 0.03 | P = 0.01 | |||
| Q = 0.07 | Q = 0.24 | Q = 0.12 | |||
| MCC vs LPMv | r = 0.24 | r = 0.18 | r = 0.20 | ||
| P = 0.00 | P = 0.04 | P = 0.03 | |||
| Q = 0.07 | Q = 0.47 | Q = 0.15 | |||
| MCC vs RM1 | r = 0.22 | r = 0.19 | r = 0.21 | ||
| P = 0.01 | P = 0.03 | P = 0.02 | |||
| Q = 0.07 | Q = 0.24 | Q = 0.12 | |||
| SMA vs MCC | r = 0.21 | r = 0.18 | |||
| P = 0.02 | P = 0.04 | ||||
| Q = 0.07 | Q = 0.15 | ||||
| SMA vs LPMv | r = 0.22 | r = 0.18 | |||
| P = 0.01 | P = 0.04 | ||||
| Q = 0.07 | Q = 0.15 | ||||
| MCC vs LM1 | r = 0.18 | ||||
| P = 0.05 | |||||
| Q = 0.16 |
Significant correlations between pairs of ROIs showing age‐related increases in functional connectivity and poorer bimanual coordination performance, before (P < 0.05) and after correcting for multiple comparisons with FDR (Q < 0.05). IN ISO, inwards isofrequency; OUT ISO, outwards isofrequency; IN N‐ISO, inwards, non‐isofrequency; OUT N‐ISO, outwards non‐isofrequency; r, Pearson correlation coefficient; R, right; L, left.
Figure 4.
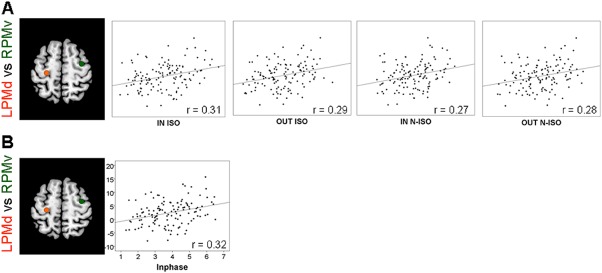
Correlations between functional connectivity and bimanual coordination. Z‐transformed correlations between the lPMd/rPMv pair with increased age‐related functional connectivity (Y‐axis) and the scores from the bimanual visuomotor task (X‐axis). r, Pearson coefficients. Q < 0.001. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
We also found correlations between pairs of ROIs and bimanual coordination performance that were below P < 0.05 but did not survive after FDR correction. In this regard, age‐related increased functional connectivity in the SMA/MCC, SMA/left PMv, MCC/left PMd, MCC/left PMv, MCC/right M1, and MCC/left M1 pairs correlated with a greater target deviation when participants performed the inwards pattern at isofrequency ratios, and also for in‐phase movements, except for the MCC/left M1 pair. Additionally, increased functional connectivity in the MCC/left PMd and MCC/right M1 pairs correlated with a greater error (target deviation) for the outwards pattern at isofrequency ratios. Finally, the MCC/left PMv pair correlated with the inwards pattern at non‐isofrequency ratios. Table 5 summarizes correlations between each pair of ROIs and bimanual coordination performance before and after correction with FDR.
Regarding the supplementary analysis after splitting the sample into two subgroups with matched gender and age, we observed significant correlations between the left PMd/right PMv pair and bimanual coordination within each cohort. These correlations were consistent with those previously reported for the entire sample. Specifically, age‐related increased functional connectivity in the left PMd/right PMv pair correlated with a greater target deviation for inwards and outwards patterns at iso‐ and non‐isofrequency ratios, and also for in‐phase movements, within each group (Group 1 IN ISO: r = 0.28, Q > 0.05, P < 0.05; OUT ISO: r = 0.38, Q < 0.01, P < 0.001; IN N‐ISO: r = 0.28, Q > 0.05, P < 0.05; OUT N‐ISO: r = 0.31, Q < 0.05, P < 0.01; in‐phase: r = 0.35, Q < 0.001, P < 0.001; Group 2 IN ISO: r = 0.36, Q < 0.01, P < 0.001; OUT ISO: r = 0.29, Q > 0.05, P < 0.05; IN N‐ISO: r = 0.34, Q < 0.01, P < 0.001; OUT N‐ISO: r = 0.26, Q > 0.05, P < 0.05; in‐phase: r = 0.31, Q < 0.05, P < 0.01). Hence, correlations between the left PMd/right PMv pair and bimanual coordination were found for each cohort, reflecting the stability of our results across different samples and, in the majority of the cases, after correction for multiple comparisons.
Default Network Control Analysis
Functional connectivity within the DMN was also modulated as a function of aging. Specifically, the dMPFC/vMPFC and dMPFC/lMTG pairs showed decreased functional connectivity with advancing age (dMPFC/vMPFC: r = −0.29, Q < 0.001; dMPFC/lMTG: r = −0.23, Q < 0.01). Subsequent analysis performed to investigate whether aging differences in the functional connectivity of the DMN predicted aging differences in performance showed that age‐related decreased functional connectivity in the abovementioned pairs of ROIs was not correlated with declines in bimanual coordination (CW ISO: r = −0.12, Q > 0.05; IN ISO: r = −0.18, Q > 0.05; CCW ISO: r = −0.15, Q > 0.05; OUT ISO: r = −0.12, Q > 0.05; CW N‐ISO: r = −0.13, Q > 0.05; IN N‐ISO: r = −0.12, Q > 0.05; CCW N‐ISO: r = −0.18, Q > 0.05; OUT N‐ISO: r = −0.12, Q > 0.05; in‐phase: r = −0.16, Q > 0.05; anti‐phase: r = −0.15, Q > 0.05).
As a final note, we would like to remark that in the present study age accounted for 10–20% of the variance in the investigated properties of resting state functional connectivity. Accordingly, Allen et al. (2011) also found that age accounted for 10–20% of the total variance in a multivariate analysis of different resting state networks across 603 participants. In this regard, previous research (Biswal et al., 2010) has demonstrated that although large sample sizes are recommended (>50 participants), results from these studies are likely to lead to Type II errors failing to detect true age‐effects.
DISCUSSION
We investigated the effects of aging on resting state functional connectivity within a task‐specific motor network supporting bimanual coordination with rs‐fcMRI recordings from 128 subjects, covering an age span of 18–80 years. Behavioral results revealed age‐related impairments in bimanual coordination for the different directional combinations and relative interhand velocities. Using rs‐fcMRI, we demonstrated that age has a robust effect on the resting state functional connectivity of the motor system. Specifically, we found age‐related increases in functional connectivity between 23 pairs of regions commonly involved in bimanual coordination, including the dorsal and ventral premotor cortices, primary motor cortex, supplementary motor area, cingulate motor cortex, and dorsal and ventral primary somatosensory areas. Interestingly, functional connectivity observed between the left dorsal premotor area and the right ventral premotor area showed the highest correlation with bimanual coordination performance. Additionally, both the resting state motor network and the DMN were negatively correlated with each other, and more importantly, age‐related decreases in DMN functional connectivity were not predictive of bimanual coordination performance. Altogether, our study shows that, within the task‐specific resting state motor network examined here, age‐related increases in dorsal and ventral premotor functional connectivity are paralleled by declines in bimanual performance. We discuss these findings in detail below.
Aging and Coordination Behavior
The bimanual visuomotor task employed here was sensitive to age‐related decline. This is consistent with previous work showing age‐related decreases during complex interlimb/bimanual coordination tasks requiring effortful processing (Bangert et al., 2010; Coxon et al., 2010; Fling et al., 2011; Serbruyns et al., 2013; Serrien et al., 2000; Summers et al., 2010; Wishart and Lee, 1997; Wishart et al., 2000). Additionally, we observed that participants showed a poorer performance for inwards and outwards compared to clockwise and counterclockwise directions, regardless of the frequency ratio. This observation requires further attention because it has been traditionally suggested that inwards and outwards directions are more stable and represent intrinsically more stable states than clockwise and counterclockwise directions, at least under isofrequency conditions (Carson et al., 1997; Swinnen et al., 1996, 1997; Summers et al., 1998). Our results do not support this finding, but are consistent with those obtained by Sisti et al. (2011) using the same bimanual visuomotor task as the present study. One plausible explanation for such divergent findings is the nature of the dial rotation task and its higher complexity compared to classical cyclical tasks employed to assess bimanual coordination. As stated before, the present bimanual coordination task integrates different constraints that have been classically addressed in separate experiments.
Aging Effects on the Resting State Functional Connectivity of the Motor System
We observed age‐related increases in functional connectivity across pairs of brain regions classically involved in bimanual coordination and representative of a core motor network (Coxon et al., 2010; Debaere et al., 2004a, 2004b; Goble et al., 2010; Goldberg et al., 2006; Halsband and Freund, 1993; Karabanov and Siebner, 2012; Lee and Quessy, 2003; Serrien et al., 2002; Swinnen, 2002; Swinnen and Wenderoth, 2004). Specifically, the dorsal and ventral premotor cortices, primary motor cortex, supplementary motor area, cingulate motor cortex, and dorsal and ventral primary somatosensory areas have been commonly involved in the preparation and/or execution of bimanual movements (Debaere et al., 2003, 2004a, b; Goerres et al., 1998; Jenkins et al., 1994; Sadato et al., 1997).
A central finding of the present study was the age‐related increase in functional connectivity of the resting state motor network. To investigate whether this increase generalized to other brain networks, we examined functional connectivity within the DMN across the lifespan and observed age‐related reduced functional connectivity that did not predict bimanual coordination performance. Furthermore, we found that the motor and default mode networks were negatively correlated, which is consistent with previous research showing negative correlations between two different resting state networks (Fransson, 2005; Fox et al., 2005; Greicius et al., 2004). In this regard, Fox et al. (2005) explained within‐network correlations and between‐network anticorrelations by means of two different neural mechanisms reflecting system specificity. The presence of age‐related increases in the resting state functional connectivity of the motor system together with decreases in the DMN suggests the idea of a non‐uniform mechanism of neural aging. Further research with a wider array of networks needs to be conducted in order to confirm the extent of system specificity.
Relationship Between Resting State Functional Connectivity and Bimanual Coordination with Age
In the present study, age‐related increased functional connectivity between the left dorsal and right ventral premotor areas in the resting brain was the best predictor of poorer bimanual coordination performance in the most difficult task variants. This prompts the question whether this particular connection is a unique predictor. On the one hand, we tend to qualify this statement because other pairwise connections of the motor network (including the supplementary motor area, primary motor cortex and middle cingulate) also showed a relation with bimanual performance but did not survive our stringent statistical correction procedure. On the other hand, we observed that this bilateral premotor functional connection stood out as a quite robust predictor. More specifically, we conducted an additional analysis and split the original sample into two cohorts with matched age and gender. Again, results showed that higher functional connectivity between the left dorsal and right ventral premotor areas at rest was associated with poorer bimanual coordination within each cohort, reinforcing the validity of our findings. This may point to a specific role of premotor interactions in bimanual coordination, as discussed next.
The premotor cortex is critical for rhythmical bimanual coordination (Christensen et al., 2013; Goerres et al., 1998; Hinder et al., 2012; Sadato et al., 1997; Szameitat et al., 2012), as part of a dorsal pathway that mediates sensory–motor interactions for externally guided movements (Goodale et al., 2003). In particular, age‐related increased functional connectivity between the left dorsal and right ventral premotor areas reflects their key role in the present bimanual coordination network with some lateralization specificity (see Jackson and Husain, 1996). Direct homotopic and non‐homotopic connections have been observed between the dorsal and ventral premotor areas in task‐related studies using unimanual motor tasks (Mochizuki et al., 2007), emphasizing the involvement of the dorsal premotor area in motor planning and execution of visually generated actions (Dum et al., 2002; Kurata, 1993; Szameitat et al., 2012; Wise and Mauritz, 1985), whereas the ventral premotor area is mainly involved in the sensory guidance of hand movements (Ehrsson et al., 2001; Graziano et al., 1994). These premotor areas are also prominently activated during visually‐guided bimanual coordination (Debaere et al., 2003, 2004a, b). Additionally, activation in the dorsal premotor area is modulated as a function of coordination complexity under increased movement frequencies (Debaere et al., 2004a; Meyer‐Lindenberg et al., 2002).
As previously stated, increased connectivity between the left dorsal and right ventral premotor areas also reflects a certain lateralization specificity, as shown in bimanual coordination tasks (van den Berg et al., 2010). In this regard, the right premotor cortex is involved in controlling complex bimanual movements (Aramaki et al., 2006; Sadato et al., 1997; van den Berg et al., 2010; Wenderoth et al., 2004), whereas the left premotor cortex is involved in unimanual movements of either side as well as bimanual movements, marking a generalized left lateralization of motor control in right‐handers (Serrien et al., 2006; Pollok et al., 2008). This functional lateralization of the premotor cortex can be interpreted within the framework of interhemispheric inhibition and facilitation (Koch et al., 2006). Premotor interhemispheric inhibition is required to prevent interference or motor overflow between simultaneous arm movements (Netz, 1999; Hoy et al., 2004). In this respect, the dominant premotor cortex (the left, in right‐handers) exerts both inhibitory and facilitatory influences on the non‐dominant premotor cortex (the right, in right‐handers), whereas the latter has primarily inhibitory influences on the former, preventing unwanted mirror movements (Koch et al., 2006; van den Berg et al., 2010). Age‐related increased connectivity of interhemispheric premotor regions at rest has been observed previously (Langan et al., 2010; Meier et al., 2013; Mowinckel et al., 2012; Zuo et al., 2010) and may reflect a shift from normal inhibitory/facilitatory balance toward increased facilitatory interhemispheric interactions with progressing age (Fling et al., 2011). This idea is supported by TMS literature showing age‐related declines in interhemispheric inhibition (Peinemann et al., 2001). Although beyond the scope of this study, this age‐related balance shifting hypothesis can only be truly tested by exploring the interhemispheric transfer of information via the corpus callosum. Structural changes of the corpus callosum with age impact upon cortical activity both at rest and during task performance, with important implications for motor behavior (Fling et al., 2011; Serbruyns et al., 2013). Hence, multimodal studies will be crucial to understand how age‐related functional and structural changes (O'Sullivan et al., 2001; Salat et al., 2005) determine motor behavior (Kalpouzos et al., 2012).
Finally, age‐related increases in functional connectivity between the left dorsal and right ventral premotor areas were associated with poorer performance in the most difficult bimanual task conditions. This is consistent with the hypothesis that age‐related declines in premotor interhemispheric inhibition at rest, resulting in increased connectivity, are associated with impairments in bimanual coordination. This, in turn, may be indicative of an age‐related generalized spreading of brain activity and loss of neural specialization that affects performance of highly demanding tasks (Li and Lindenberger, 1999), as the dedifferentiation hypothesis postulates.
Despite the prominent development of the classical dedifferentiation and compensation models in the context of task‐related brain activity and some experimental evidence for task‐related compensatory recruitment during interlimb coordination with age (Goble et al., 2010; Heuninckx et al., 2008), converging evidence suggests the prevalence of a dedifferentiation mechanism at rest (Bernard and Seidler, 2012; Mowinckel et al., 2012). In this regard, some authors have investigated the motor network modulations from the resting state to the motor task state in order to identify an underlying common mechanism. In this respect, Jiang et al. (2004) and Bressler and Kelso (2001) concluded that the functional motor network at rest maintains a dynamic equilibrium by keeping the brain ready to execute a future motor task. However, this important issue is beyond the scope of the present study and more research is required.
Conclusions
The current study focused on resting state functional connectivity within a task‐specific motor network supporting bimanual coordination. We assessed for the first time the relationships between the resting state motor network and bimanual coordination across the lifespan. We found that age‐related increases in resting state functional connectivity of interhemispheric dorsal and ventral premotor areas were associated with altered bimanual coordination functioning, as assessed by a novel visuomotor bimanual coordination task. Additionally, the control analysis conducted on the DMN showed that our results are specific to the motor system. This increased functional connectivity might reflect an altered balance between excitation and inhibition processes that is not uniformly beneficial for aged adults. Thus, a loss of neural specialization evident during rest might provide a window into age‐related deficits in motor functioning.
The authors have no actual or potential conflicts of interest to disclose.
This protocol was approved by the local ethics committee for biomedical research and subjects gave written informed consent prior to participation.
REFERENCES
- Achard S, Bullmore E (2007): Efficiency and cost of economical brain functional networks. PLoS Comput Biol 3(2):e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achard S, Salvador R, Whitcher B, Suckling J, Bullmore E (2006): A resilient, low‐frequency, small‐world human brain functional network with highly connected association cortical hubs. J Neurosci 26(1):63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, M Silva RF Havlicek, Rachakonda S, Fries J, Kalyanam R, Michael AM, Caprihan A, Turner JA, Eichel T, Adelsheim S, Bryan AD, Bustillo J, Clark VP, SW Feldstein Ewing, Filbey F, Ford CC, Hutchison K, Jung RE, Kiehl KA, Kodituwakku P, Komesu YM, Mayer AR, Pearlson GD, Phillips JP, Sadek JR, Stevens M, Teuscher U, Thoma RJ, Calhoun VD (2011): A baseline for the multivariate comparison of resting‐state networks. Front Syst Neurosci 5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL (2010): Functional‐anatomic fractionation of the brain's default network. Neuron 65(4):550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL (2007): Disruption of large‐scale brain systems in advanced aging. Neuron 56(5):924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramaki Y, Honda M, Okada T, Sadato N (2006): Neural correlates of the spontaneous phase transition during bimanual coordination. Cerebral Cortex 16(9):1338–1348. [DOI] [PubMed] [Google Scholar]
- Bangert AS, Reuter‐Lorenz PA, Walsh CM, Schachter AB, Seidler RD (2010): Bimanual coordination and aging: neurobehavioral implications. Neuropsychologia 48(4):1165–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beets IAM, Gooijers J, Boisgontier MP, Pauwels L, Coxon JP, Wittenberg G, Swinnen SP (2013): Reduced neural differentiation between feedback conditions after training bimanual coordination with and without augmented visual feedback. Cerebral Cortex (in press). [DOI] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD (2012): Evidence for motor cortex dedifferentiation in older adults. Neurobiol Aging 33(9):1890–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, S Whitfield‐Gabrieli, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP (1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Resonance Med 34(4):537–541. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, et al. (2010): Toward discovery science of human brain function. Proc Natl Acad Sci U S A 107(10):4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SL, Kelso JA (2001): Cortical coordination dynamics and cognition. Trends Cogn Sci 5(1):26–36. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Sanders AL, Raichle ME, Morris JC (2000): Functional brain imaging of young, nondemented, and demented older adults. J Cogn Neurosci 12 Suppl. 2:24–34. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA (2005): Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 25(34):7709–7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byblow WD, Chua R, Goodman D (1995): Asymmetries in coupling dynamics of perception and action. J Motor Behav 27(2):123–137. [DOI] [PubMed] [Google Scholar]
- Carson RG, Thomas J, Summers JJ, Walters MR, Semjen A (1997): The dynamics of bimanual circle drawing. Q J Exp Psychol A 50(3):664–83. [DOI] [PubMed] [Google Scholar]
- Carson RG, Riek S, Smethurst CJ, Parraga JFL, Byblow WD (2000): Neuromuscular‐skeletal constraints upon the dynamics of unimanual and bimanual coordination. Exp Brain Res 131(2):196–214. [DOI] [PubMed] [Google Scholar]
- Christensen MS, Ehrsson HH, Nielsen JB (2013): Seeing or moving in parallel: the premotor cortex does both during bimanual coordination, while the cerebellum monitors the behavioral instability of symmetric movements. Exp Brain Res 230(1):101–115. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME (2001): Frequencies contributing to functional connectivity in the cerebral cortex in “resting‐state” data. AJNR Am J Neuroradiol 22(7):1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Coxon JP, Goble DJ, Van Impe A, De Vos J, Wenderoth N, Swinnen SP (2010): Reduced basal ganglia function when elderly switch between coordinated movement patterns. Cerebral Cortex 20(10):2368–2379. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJS, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SARB (2008): Reduced resting‐state brain activity in the “default network” in normal aging. Cerebral Cortex 18(8):1856–1864. [DOI] [PubMed] [Google Scholar]
- Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP (2003): Internal vs external generation of movements: differential neural pathways involved in bimanual coordination performed in the presence or absence of augmented visual feedback. Neuroimage 19(3):764–776. [DOI] [PubMed] [Google Scholar]
- Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP (2004a): Cerebellar and premotor function in bimanual coordination: parametric neural responses to spatiotemporal complexity and cycling frequency. Neuroimage 21(4):1416–1427. [DOI] [PubMed] [Google Scholar]
- Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP (2004b): Changes in brain activation during the acquisition of a new bimanual coordination task. Neuropsychologia 42(7):855–867. [DOI] [PubMed] [Google Scholar]
- Dum RP, Li C, Strick PL (2002): Motor and nonmotor domains in the monkey dentate. Crebellum: Recent Developments in Cerebellar Research 978:289–301. [DOI] [PubMed] [Google Scholar]
- Ebisch SJH, Gallese V, Willems RM, Mantini D, Groen WB, Romani GL, Buitelaar JK, Bekkering H (2011): Altered intrinsic functional connectivity of anterior and posterior insula regions in high‐functioning participants with autism spectrum disorder. Human Brain Mapping 32(7):1013–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren E, Forssberg H (2001): Differential fronto‐parietal activation depending on force used in a precision grip task: an fMRI study. J Neurophysiol 85(6):2613–23. [DOI] [PubMed] [Google Scholar]
- Fang M, Li J, Lu G, Gong X, Yew DT (2005): A fMRI study of age‐related differential cortical patterns during cued motor movement. Brain Topogr 17(3):127–137. [DOI] [PubMed] [Google Scholar]
- Ferreira LK, Busatto GF (2013): Resting‐state functional connectivity in normal brain aging. Neurosci Biobehav Rev 37(3):384–400. [DOI] [PubMed] [Google Scholar]
- Fling BW, Walsh CM, Bangert AS, Reuter‐Lorenz PA, Welsh RC, Seidler RD (2011): Differential callosal contributions to bimanual control in young and older adults. J Cogn Neurosci 23(9):2171–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102(27):9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang DY, Snyder AZ, Raichle ME (2009): The global signal and observed anticorrelated resting state brain networks. J Neurophysiol 101(6):3270–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P (2005): Spontaneous low‐frequency BOLD signal fluctuations: an fMRI investigation of the resting‐state default mode of brain function hypothesis. Human Brain Mapp 26(1):15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R (1995): Analysis of fMRI time‐series revisited. Neuroimage 2(1):45–53. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D'Esposito M (2005): Top‐down suppression deficit underlies working memory impairment in normal aging (vol 8, pg 1298, 2005). Nat Neurosci 8(12):1791. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T (2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15(4):870–878. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Coxon JP, Van Impe A, De Vos J, Wenderoth N, Swinnen SP (2010): The neural control of bimanual movements in the elderly: brain regions exhibiting age‐related increases in activity, frequency‐induced neural modulation, and task‐specific compensatory recruitment. Human Brain Mapp 31(8):1281–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerres GW, Samuel M, Jenkins IH, Brooks DJ (1998): Cerebral control of unimanual and bimanual movements: an H2(15)O PET study. Neuroreport 9(16):3631–3638. [DOI] [PubMed] [Google Scholar]
- Goldberg RF, Perfetti CA, Schneider W (2006): Perceptual knowledge retrieval activates sensory brain regions. J Neurosci 26(18):4917–4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodale MA, Westwood DA, Milner AD (2003): Two distinct modes of control for object‐directed action. Roots Vis Awareness 144:131–144. [DOI] [PubMed] [Google Scholar]
- Gooijers J, Caeyenberghs K, Sisti HM, Geurts M, Heitger MH, Leemans A, Swinnen SP (2013): Diffusion tensor imaging metrics of the corpus callosum in relation to bimanual coordination: effect of task complexity and sensory feedback. Human Brain Mapp 34(1):241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano MSA, Yap GS, Gross CG (1994): Coding of visual space by premotor neurons. Science 266(5187):1054–1057. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V (2004): Default‐mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A 101(13):4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsband U, Freund HJ (1993): Motor learning. Curr Opin Neurobiol 3(6):940–949. [DOI] [PubMed] [Google Scholar]
- Heitger MH, Goble DJ, Dhollander T, Dupont P, Caeyenberghs K, Leemans A, Sunaert S, Swinnen SP (2013): Bimanual motor coordination in older adults is associated with increased functional brain connectivity—a graph‐theoretical analysis. PLoS One 8(4):e62133. doi: 10.1371/journal.pone.0062133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmich RC, Derikx LC, Bakker M, Scheeringa R, Bloem BR, Toni I (2010): Spatial remapping of cortico‐striatal connectivity in Parkinson's disease. Cerebral Cortex 20(5):1175–1186. [DOI] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Debaere F, Peeters R, Swinnen SP (2005): Neural basis of aging: the penetration of cognition into action control. J Neurosci 25(29):6787–6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Swinnen SP (2008): Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. J Neurosci 28(1):91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinder MR (2012): Interhemispheric connectivity between distinct motor regions as a window into bimanual coordination. J Neurophysiol 107(7):1791–1794. [DOI] [PubMed] [Google Scholar]
- Hoy KE, Fitzgerald PB, Bradshaw JL, Armatas CA, Georgiou‐Karistianis N (2004): Investigating the cortical origins of motor overflow. Brain Res Brain Res Rev 46(3):315–327. [DOI] [PubMed] [Google Scholar]
- Jackson SR, Husain M (1996): Visuomotor functions of the lateral pre‐motor cortex. Curr Opin Neurobiol 6(6):788–795. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Brooks DJ, Nixon PD, Frackowiak RS, Passingham RE (1994): Motor sequence learning: a study with positron emission tomography. J Neurosci 14(6):3775–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang TZ, He Y, Zang YF, Weng XC (2004): Modulation of functional connectivity during the resting state and the motor task. Human Brain Mapp 22(1):63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalpouzos G, Fischer H, Rieckmann A, Macdonald SW, Backman L (2012): Impact of negative emotion on the neural correlates of long‐term recognition in younger and older adults. Front Integr Neurosci 6:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabanov A, Siebner HR (2012): Unravelling homeostatic interactions in inhibitory and excitatory networks in human motor cortex. J Physiol Lond 590(22):5557–5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso JAS (1984): Phase‐transitions and critical‐behavior in human bimanual coordination. Am J Physiol 246(6):1000–1004. [DOI] [PubMed] [Google Scholar]
- Koch G, Franca M, Del Olmo MF, Cheeran B, Milton R, Alvarez Sauco M, Rothwell JC (2006): Time course of functional connectivity between dorsal premotor and contralateral motor cortex during movement selection. J Neurosci 26(28):7452–7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata K (1993): Premotor cortex of monkeys: set‐ and movement‐related activity reflecting amplitude and direction of wrist movements. J Neurophysiol 69(1):187–200. [DOI] [PubMed] [Google Scholar]
- Langan J, Peltier SJ, Bo J, Fling BW, Welsh RC, Seidler RD (2010): Functional implications of age differences in motor system connectivity. Front Syst Neurosci 4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Quessy S (2003): Activity in the supplementary motor area related to learning and performance during a sequential visuomotor task. J Neurophysiol 89(2):1039–1056. [DOI] [PubMed] [Google Scholar]
- Lee TD, Wishart LR, Murdoch JE (2002): Aging, attention, and bimanual coordination. Canadian J Aging Revue Canadienne Du Vieillissement 21(4):549–557. [Google Scholar]
- Li SC, Lindenberger U (1999): Cross‐level unification: a computational exploration of the link between deterioration of neurotransmitter systems and dedifferentiation of cognitive abilities in old age. Cognitive Neurosci Memory:103–146. [Google Scholar]
- Mantini D, Gerits A, Nelissen K, Durand JB, Joly O, Simone L, Sawamura H, Wardak C, Orban GA, Buckner RL, Vanduffel W (2011): Default mode of brain function in monkeys. J Neurosci 31(36):12954–12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, Weinberger DR (2002): Neurophysiological correlates of age‐related changes in human motor function. Neurology 58(4):630–635. [DOI] [PubMed] [Google Scholar]
- Meier MP, Ilmberger J, Fesl G, Ruge MI (2013): Validation of functional motor and language MRI with direct cortical stimulation. Acta Neurochir (Wien). [DOI] [PubMed] [Google Scholar]
- Meyer‐Lindenberg A, Ziemann U, Hajak G, Cohen L, Berman KF (2002): Transitions between dynamical states of differing stability in the human brain. Proc Natl Acad Sci U S A 99(17):10,948–10,953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki H, Furubayashi T, Hanajima R, Terao Y, Mizuno Y, Okabe S, Ugawa Y (2007): Hemoglobin concentration changes in the contralateral hemisphere during and after theta burst stimulation of the human sensorimotor cortices. Exp Brain Re 180(4):667–675. [DOI] [PubMed] [Google Scholar]
- Mowinckel AM, Espeseth T, Westlye LT (2012): Network‐specific effects of age and in‐scanner subject motion: a resting‐state fMRI study of 238 healthy adults. Neuroimage 63(3):1364–1373. [DOI] [PubMed] [Google Scholar]
- Naccarato M, Calautti C, Jones PS, Day DJ, Carpenter TA, Baron JC (2006): Does healthy aging affect the hemispheric activation balance during paced index‐to‐thumb opposition task? An fMRI study. Neuroimage 32(3):1250–1256. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005): The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53(4):695–699. [DOI] [PubMed] [Google Scholar]
- Netz J (1999): Asymmetry in transcallosal inhibition. Transcranial Magnetic Stimulation (51):137–144. [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB (2005): Valid conjunction inference with the minimum statistic. Neuroimage 25(3):653–660. [DOI] [PubMed] [Google Scholar]
- O'Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS (2001): Evidence for cortical “disconnection” as a mechanism of age‐related cognitive decline. Neurology 57(4):632–638. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9(1):97–113. [DOI] [PubMed] [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR (2004): Aging reduces neural specialization in ventral visual cortex. Proc Natl Acad Sci U S A 101(35):13091–13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinemann A, Lehner C, Conrad B, Siebner HR (2001): Age‐related decrease in paired‐pulse intracortical inhibition in the human primary motor cortex. Neuroscience Letters 313(1‐2):33–36. [DOI] [PubMed] [Google Scholar]
- Peltier SJ, Noll DC (2002): T(2)(*) dependence of low frequency functional connectivity. Neuroimage 16(4):985–992. [DOI] [PubMed] [Google Scholar]
- Poldrack RA (2007): Region of interest analysis for fMRI. Soc Cogn Affect Neurosci 2(1):67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollok B, Rothkegel H, Schnitzler A, Paulus W, Lang N (2008): The effect of rTMS over left and right dorsolateral premotor cortex on movement timing of either hand. Eur J Neurosci 27(3):757–764. [DOI] [PubMed] [Google Scholar]
- Pravata E, Sestieri C, Mantini D, Briganti C, Colicchio G, Marra C, Colosimo C, Tartaro A, Romani GL, Caulo M (2011): Functional connectivity MR imaging of the language network in patients with drug‐resistant epilepsy. AJNR Am J Neuroradiol 32(3):532–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci U S A 98(2):676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecker A, Groschel K, Ackermann H, Steinbrink C, Witte O, Kastrup A (2006): Functional significance of age‐related differences in motor activation patterns. Neuroimage 32(3):1345–1354. [DOI] [PubMed] [Google Scholar]
- Sadato N, Yonekura Y, Waki A, Yamada H, Ishii Y (1997): Role of the supplementary motor area and the right premotor cortex in the coordination of bimanual finger movements. J Neurosci 17(24):9667–9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, Dale AM (2005): Age‐related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging 26(8):1215–1227. [DOI] [PubMed] [Google Scholar]
- Salvador R, Suckling J, Coleman MR, Pickard JD, Menon D, Bullmore E (2005): Neurophysiological architecture of functional magnetic resonance images of human brain. Cerebral Cortex 15(9):1332–1342. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB (2010): Motor control and aging: links to age‐related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev 34(5):721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbruyns L GJ, Caeyenberghs K, Meesen RL, Cuypers K, Sisti HM, Leemans A, Swinnen SP (2013): Bimanual motor deficits in older adults predicted by diffusion tensor imaging metrics of corpus callosum subregions. Brain Struct Funct (in press). [DOI] [PubMed] [Google Scholar]
- Serrien DJ (2009): Interactions between new and pre‐existing dynamics in bimanual movement control. Exp Brain Res 197(3):269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrien DJ, Ivry RB, Swinnen SP (2006): Dynamics of hemispheric specialization and integration in the context of motor control. Nat Rev Neurosci 7(2):160–166. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Strens LHA, Oliviero A, Brown P (2002): Repetitive transcranial magnetic stimulation of the supplementary motor area (SMA) degrades bimanual movement control in humans. Neurosci Lett 328(2):89–92. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Swinnen SP, Stelmach GE (2000): Age‐related deterioration of coordinated interlimb behavior. J Gerontol B Psychol Sci Social Sci 55(5):P295–P303. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Teasdale N, Bard C, Fleury M (1996): Age‐related differences in the integration of sensory information during the execution of a bimanual coordination task. J Motor Behav 28(4):337–347. [DOI] [PubMed] [Google Scholar]
- Shumway RH, Stoffer DS (2006): Time Series Analysis and Its Applications. New York: Springer; 520 pp. [Google Scholar]
- Sisti HM, Geurts M, Clerckx R, Gooijers J, Coxon JP, Heitger MH, Caeyenberghs K, Beets IAM, Serbruyns L, Swinnen SP (2011): Testing multiple coordination constraints with a novel bimanual visuomotor task. Plos One 6(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Umberger GH, Manning EL, Slevin JT, Wekstein DR, Schmitt FA, Markesbery WR, Zhang Z, Gerhardt GA, Kryscio RJ, Gash DM (1999): Critical decline in fine motor hand movements in human aging. Neurology 53(7):1458–1461. [DOI] [PubMed] [Google Scholar]
- Smith SM, Miller KL, Salimi‐Khorshidi G, Webster M, Beckmann CF, Nichols TE, Ramsey JD, Woolrich MW (2011): Network modelling methods for FMRI. Neuroimage 54(2):875–891. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF (2009): Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A 106(31):13,040–13,045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers JJ, Byblow WD, Bysouth‐Young DF, Semjen A (1998): Bimanual circle drawing during secondary task loading. Motor Control 2(2):106–113. [DOI] [PubMed] [Google Scholar]
- Summers J (2002): Practice and training in bimanual coordination tasks: strategies and constraints. Brain Cognition 48(1):166–178. [DOI] [PubMed] [Google Scholar]
- Summers JJ, Lewis J, Fujiyama H (2010): Aging effects on event and emergent timing in bimanual coordination. Human Move Sci 29(5):820–830. [DOI] [PubMed] [Google Scholar]
- Swinnen SP (2002): Intermanual coordination: From behavioural principles to neural‐network interactions. Nat Rev Neurosci 3(5):350–361. [DOI] [PubMed] [Google Scholar]
- Swinnen SP, Jardin K, Meulenbroek R (1996): Between‐limb asynchronies during bimanual coordination: effects of manual dominance and attentional cueing. Neuropsychologia 34(12):1203–1213. [DOI] [PubMed] [Google Scholar]
- Swinnen SP, Van Langendonk L, Verschueren S, Peeters G, Dom R, De Weerdt W (1997): Interlimb coordination deficits in patients with Parkinson's disease during the production of two‐joint oscillations in the sagittal plane. Mov Disord 12(6):958–968. [DOI] [PubMed] [Google Scholar]
- Swinnen SP, Verschueren SMP, Bogaerts H, Dounskaia N, Lee TD, Stelmach GE, Serrien DJ (1998): Age‐related deficits in motor learning and differences in feedback processing during the production of a bimanual coordination pattern. Cognitive Neuropsychol 15(5):439–466. [DOI] [PubMed] [Google Scholar]
- Swinnen SP, Wenderoth N (2004): Two hands, one brain: cognitive neuroscience of bimanual skill. Trends Cogn Sci 8(1):18–25. [DOI] [PubMed] [Google Scholar]
- Szameitat AJ, McNamara A, Shen S, Sterr A (2012): Neural activation and functional connectivity during motor imagery of bimanual everyday actions. Plos One 7(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temprado JJ, Swinnen SP, Carson RG, Tourment A, Laurent M (2003): Interaction of directional, neuromuscular and egocentric constraints on the stability of preferred bimanual coordination patterns. Human Move Sci 22(3):339–363. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND (2012): Aging and functional brain networks. Mol Psychiatry 17(5):471, 549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15(1):273–289. [DOI] [PubMed] [Google Scholar]
- Van den Heuvel MP, Pol HEH (2010): Exploring the brain network: a review on resting‐state fMRI functional connectivity. Eur Neuropsychopharmacol 20(8):519–534. [DOI] [PubMed] [Google Scholar]
- van den Berg FE, Swinnen SP, Wenderoth N (2010): Hemispheric asymmetries of the premotor cortex are task specific as revealed by disruptive TMS during bimanual versus unimanual movements. Cerebral Cortex 20(12):2842–2851. [DOI] [PubMed] [Google Scholar]
- Van Impe A, Bruijn SM, Coxon JP, Wenderoth N, Sunaert S, Duysens J, Swinnen SP (2012a): Age‐related neural correlates of cognitive task performance under increased postural load. Age (Dordr). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Impe A, Coxon JP, Goble DJ, Doumas M, Swinnen SP (2012b): White matter fractional anisotropy predicts balance performance in older adults. Neurobiol Aging 33(9):1900–1912. [DOI] [PubMed] [Google Scholar]
- Van Impe A, Coxon JP, Goble DJ, Wenderoth N, Swinnen SP (2009): Ipsilateral coordination at preferred rate: effects of age, body side and task complexity. Neuroimage 47(4):1854–1862. [DOI] [PubMed] [Google Scholar]
- Van Impe A, Coxon JP, Goble DJ, Wenderoth N, Swinnen SP (2011): Age‐related changes in brain activation underlying single‐ and dual‐task performance: visuomanual drawing and mental arithmetic. Neuropsychologia 49(9):2400–2409. [DOI] [PubMed] [Google Scholar]
- Ward NS, Frackowiak RSJ (2003): Age‐related changes in the neural correlates of motor performance. Brain 126:873–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenderoth N, Debaere F, Sunaert S, van Hecke P, Swinnen SP (2004): Parieto‐premotor areas mediate directional interference during bimanual movements. Cerebral Cortex 14(10):1153–1163. [DOI] [PubMed] [Google Scholar]
- Wise SP, Mauritz KH (1985): Set‐related neuronal activity in the premotor cortex of rhesus monkeys: effects of changes in motor set. Proc R Soc Lond B Biol Sci 223(1232):331–354. [DOI] [PubMed] [Google Scholar]
- Wishart LR, Lee TD (1997): Effects of aging and reduced relative frequency of knowledge of results on learning a motor skill. Perceptual Motor Skills 84(3):1107–1122. [DOI] [PubMed] [Google Scholar]
- Wishart LR, Lee TD, Murdoch JE, Hodges NJ (2000): Effects of aging on automatic and effortful processes in bimanual coordination. J Gerontol B Psychol Sci Social Sci 55(2):P85–P94. [DOI] [PubMed] [Google Scholar]
- Wu T, Zang Y, Wang L, Long X, Li K, Chan P (2007a): Normal aging decreases regional homogeneity of the motor areas in the resting state. Neurosci Lett 423(3):189–193. [DOI] [PubMed] [Google Scholar]
- Wu T, Zang YF, Wang L, Long XY, Hallett M, Chen Y, Li KC, Chan P (2007b): Aging influence on functional connectivity of the motor network in the resting state. Neurosci Lett 422(3):164–168. [DOI] [PubMed] [Google Scholar]
- Wu T, Hallett M (2005): The influence of normal human ageing on automatic movements. J Physiol 562(Pt 2):605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar JH, 1998. Biostatistical Analysis. New York: Prentice‐Hall, Upper Saddle River; 450 pp. [Google Scholar]
- Zuo CT, Hua XY, Guan YH, Xu WD, Xu JG, Gu YD (2010): Long‐range plasticity between intact hemispheres after contralateral cervical nerve transfer in humans. J Neurosurg 113(1):133–140. [DOI] [PubMed] [Google Scholar]


