Abstract
Imagining future events while performing an intertemporal choice task can attenuate the devaluation of future rewards. Here, we investigated whether this effect and its neural basis depend on the degree of personal prior experience associated with the simulated future scenarios. Functional magnetic resonance imaging was combined with a modified intertemporal choice task in which the delayed options were either purely monetary, or linked with a social event. Subject‐specific events differed regarding familiarity, that is, meeting a close, familiar person or a celebrity in a café. In line with recent hypotheses on episodic construction, the simulation of future familiar and unfamiliar events equally attenuated delay discounting behavior in comparison with the control condition and both were imagined with similar richness. Imaging data, however, indicate that these results rely on differential neural activation patterns. The hippocampus was particularly involved in the simulation of unfamiliar future scenarios, probably reflecting enhanced construction processes when personal experience with similar past events is lacking. Consequently, functional coupling of the hippocampus with neural valuation signals in the anterior cingulate cortex predicted the subjective value only of rewards offered in the unfamiliar context. In contrast, valuation of rewards in a familiar context was predicted by activation in key nodes of emotional and autobiographical memory retrieval and dynamically modulated by frontal‐striatal connectivity. The present data emphasize that the mechanisms underlying neural valuation of prospective rewards largely depend on the pre‐experience with the context in which they are offered. Hum Brain Mapp 36:4210–4221, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: decision‐making, intertemporal choice, prospection, autobiographical memory, hippocampus
INTRODUCTION
Farsighted decisions are strongly biased by the tendency to devaluate rewards as a function of time to delivery [Peters and Büchel, 2011]. The resistance to the temptation of an immediate gratification for the benefit of a larger but delayed payoff can be increased when connecting it to an anticipated future event (“tag‐effect”) [Daniel et al., 2013a, 2013b; Kwan et al., 2015; Peters and Büchel, 2010], when the reward consumption itself is simulated [Benoit et al., 2011; Palombo et al., 2015] and when a future‐oriented mindset is induced via prospective imagery [Cheng et al., 2012]. Highlighting the impact of episodic prospection on this effect, neural activation in response to episodic tags strongly overlaps with a core network implicated in remembering and imagining events [Race et al., 2011; Schacter et al., 2012], including the ventromedial prefrontal cortex, precuneus, and posterior cingulate cortex [Benoit et al., 2011; Peters and Büchel, 2010]. This fits with suggestions that constructing coherent future scenarios involves the flexible recombination of episodic memories [Schacter and Addis, 2007b, 2009].
The extent to which prospective thinking requires such flexible recombination is certainly affected by the degree to which a particular event relies on self‐experience. Along those lines, lacking self‐experience during the imagination of unfamiliar future events may be compensated by stronger reliance on episodic information from other sources, such as media and third person experiences [Anderson, 2012]. Findings are mixed whether such additional recombination demands affect the quality of imagination with some studies reporting richer episodic thought when based on prior experience [D'Argembeau and Van der Linden, 2012; Szpunar and McDermott, 2008] and others reporting the quality of imagination to be unaffected by familiarity [Anderson, 2012]. In the same vein, it is unclear whether the neural circuits modulating the tag‐effect are different for familiar and unfamiliar future events. It seems likely that the hippocampus is generally involved in the episodic construction of future events [Hassabis et al., 2007; Maguire and Mullally, 2013]. Moreover, there is evidence suggesting that the hippocampus is even stronger engaged when self‐experience with simulated events is lacking. Specifically, increased hippocampal activation has been observed during the imagination of novel compared to remembering episodic events [Addis et al., 2011; Okuda et al., 2003; Schacter and Addis, 2007a; Weiler et al., 2010b ]. In addition, a recent study that explicitly controlled encoding related processing in the simulation of unfamiliar events demonstrated that activity within the posterior hippocampus was significantly modulated by construction effort [Gaesser et al., 2013]. When construction demands are reduced in the context of familiarity, however, episodic simulation seems to be less dependent on hippocampal processing [Weiler et al., 2010a] and to rely more on posterior parietal regions [Szpunar et al., 2009], which together with occipital and temporal regions are part of the core network implicated in autobiographical memory retrieval [Cabeza and St Jacques, 2007; Martinelli et al., 2013; Svoboda et al., 2006].
In the context of the tag‐effect, episodic prospection has so far either been restricted to familiar scenarios [Palombo et al., 2015] or familiarity was not controlled at all [Daniel et al., 2013a, 2013b; Peters and Büchel, 2010]. However, the quality [Lebreton et al., 2013; Palombo et al., 2015; Peters and Büchel, 2010] and emotional valence [Benoit et al., 2011; Liu et al., 2013] of imagination, which might rely on the personal experience with future events, has found to be crucial for the magnitude of the tag‐effect. Recent mixed findings about presence [Kwan et al., 2015] or absence [Palombo et al., 2015] of the tag‐effect in amnestic patients with hippocampal lesions further indicate a differential impact of future event features on the neurobehavioral manifestation of the tag‐effect. To fill this gap, this study combined functional magnetic resonance imaging (fMRI) with a modified intertemporal choice task in which future rewards were linked to subject‐specific social events differing systematically in familiarity. An established hyperbolic model of delay discounting [Mazur, 1987] and extensive interview data were used to explore potential behavioral differences or similarities between episodic conditions. On the neural level, we used factorial and functional coupling analyses to focus on condition dependent interactions between neural decision‐making and episodic future‐thinking networks. Specifically, the hippocampus might critically mediate the construction of unfamiliar events and consequently impact on neural valuation signals more strongly in the unfamiliar condition. In contrast, neural networks related to autobiographical and emotional memory retrieval might have a larger impact on neural valuation in the context of familiar event simulation.
MATERIALS AND METHODS
Participants
Twenty‐three healthy young adults (21–30 years; M = 24.96; SD = 2.79; 12 men) participated in this study. Participants were recruited via an online‐announcement and from an existing database and gave written informed consent before participating. Exclusion criteria were neurological, psychiatric, and other serious physical conditions. Participants were financially compensated with 10 Euros per hour. In addition, one chosen reward from the delay discounting task was randomly selected and paid out with the respective delay. The study was approved by the local ethics committee.
Study Design
Following established procedures [Peters and Büchel, 2009], all participants were invited in on a separate day, prior to testing, to complete a computer‐based delay‐discounting procedure. This pretest was used to construct subject‐specific trials for the main discounting experiment to ensure that participants would choose the delayed option in 50% of the trials. Individual choices from this pretest were fitted via a hyperbolic discounting function of the form
to estimate the individual discount rate for a reward of 20€ (SV = subjective value; A = amount of the delayed reward; D = delay in days; k = discount rate) [Mazur, 1987]. The discount rate was then used to calculate indifference amounts for six delays, randomly drawn from one of two sets [1, 2], [6, 7], [13, 15], [28, 32], [85, 95], [170, 190]. Next, the six delays were paired with six amounts lying equally above and below the respective indifference point. Monetary amounts ranged from 20.5€ to 79.50€. In this fashion, six blocks of 36 trials each were constructed for the fMRI experiment. While minimum and maximum amount for each delay were close to the extreme values for each participants, values varied individually between these extremes. In order to get an impression of such interindividual variability, Table 1 shows the average interquartile ranges (IQR) computed as the difference between the 75% minus the 25% percentile of the rewards across the six sessions and their standard deviation for each of the six delays (for complete information on the individual IQRs, see Table S1 in the Supporting Information).
Table 1.
Interindividual variability in the reward ranges at each delay
| Delay | M (IQR) | SD |
|---|---|---|
| [1, 2] | 21.30 | 3.11 |
| [6, 7] | 21.26 | 3.58 |
| [13, 15] | 20.39 | 3.66 |
| [28, 32] | 20.05 | 5.76 |
| [85, 95] | 21.14 | 9.11 |
| [170, 190] | 25.13 | 10.40 |
Means (M) and standard deviations (SD) of the individual IQR of the delayed rewards from the fMRI experiment are reported separately for each of the six delays. IQRs are computed for each participant by subtracting the 25% percentile of the rewards across all sessions from the 75% percentile.
Two of these six blocks served as the control condition, which involved standard delay discounting without episodic prospection, while the remaining four blocks were assigned to the episodic tag conditions. In each of these four blocks, participants were instructed to imagine meeting a person in a café for the day of delayed reward delivery. To ensure not only temporal but also spatial specificity of the imaginations, participants were familiarized with five images depicting scenes of a typical café before the experiment. In two of these blocks, this event was related to prior experiences and involved meeting with a familiar social partner (e.g., Mother). In the two other blocks, the event was unfamiliar and involved meeting with a famous person from the media who had not been met in person before (e.g., Angela Merkel). A standardized interview (adopted from [Carstensen and Fredrickson, 1998]) was used to identify these two familiar and two novel social partners: For the identification of the familiar social partners, participants had to imagine moving to a foreign country on their own and to appoint familiar persons with whom they would like to spend the last hours before their departure. To identify the famous, novel partners, participants imagined conducting an interview for the local newspaper with persons of public interest whom they had never met in person before.
The applied fMRI paradigm was a modified version of the task used by Peters and Büchel [2010] (Fig. 1). The three conditions were presented in six blocks (2 blocks per condition) of 36 trials each. To avoid various types of confounding sequence effects, the presentation of the three conditions was randomized but the two blocks of each condition were always presented successively. Between blocks, participants were given a five minutes break to relax. In each trial, participants were required to choose between a fixed immediate reward option of 20 € (which was not shown on the screen) and a larger but delayed amount. During episodic conditions, this delayed reward option was presented together with the name of the social partner with whom they had to imagine a meeting in a café for the date of the delayed reward delivery. In the control condition, delayed options were presented together with placeholder strings (“XXXX” or “YYYY”) and participants were explicitly instructed not to imagine anything. Participants were trained on the task before scanning. After the task and without being scanned, participants remained lying in the scanner for approximately ten minutes for an interview about the richness of their imagination for the four events. During this interview, participants were asked to describe their imaginations for the four scenarios as detailed as possible. Answers were recorded and later transliterated.
Figure 1.
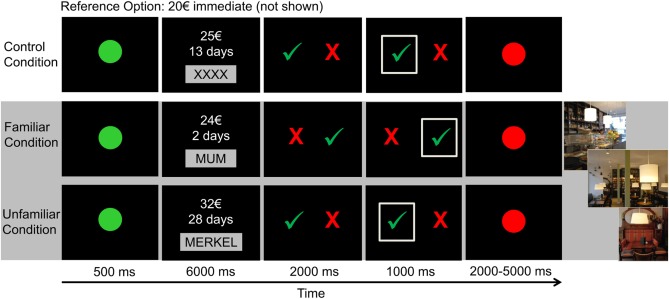
Outline of the paradigm. Each trial started with a green dot, signaling the start of the trial. Next, the delayed reward option was presented for 6 s. Participants had to indicate their choice by selecting the red cross for the immediate reward (20 € that were not shown) or the green check mark for the delayed reward option. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Outside the scanner, participants were given questionnaires to rate the emotionality and curiosity they associated with the four partners as well as their motivation to meet the partners for each event on scales ranging from 1 to 7.
Data Acquisition
We used Presentation software (Neurobehavioral Systems) for stimulus presentation and recording. FMRI data were acquired on a 3 tesla system (Magnetom Trio, Siemens) equipped with a 32‐channel head coil. Each volume comprised 41 transversal slices (2mm thickness, 1mm gap, TR = 2460 ms, TE = 25 ms, FOV = 216 × 216 mm2, in‐plane resolution 2 × 2 mm2, GRAPPA factor 2). After functional imaging, high‐resolution anatomical MR images were acquired using a T1‐weighted MPRAGE sequence (1 × 1 × 1 mm).
Behavioral Data Analysis
Behavioral data analysis was performed with Matlab (Mathworks). Individual‐subject choice data were fitted using maximum likelihood estimation by combining a hyperbolic discounting function with softmax action selection [Peters et al., 2012] separately for each experimental condition. This yielded two free parameters per condition, the hyperbolic discounting constant k, where higher values reflect greater impatience, and the inverse temperature parameter β of the softmax choice function, where greater values reflect more decision noise (see Table 2 for medians and IQRs of the absolute single‐subject maximum likelihood parameter estimates, model fit criterion and reaction time data). A square‐root transformation was applied to the resulting k parameters prior to analyses [Ballard and Knutson, 2009; Peters et al., 2012], accounting for their skewed distributions.
Table 2.
Model parameters
| k | β | AIC | RT | |||||
|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | |
| Control | 0.0086 | 0.0099 | 1.30 | 2.31 | 40.27 | 36.17 | 745.58 | 96.88 |
| Familiar | 0.0060 | 0.0093 | 1.12 | 1.92 | 38.11 | 26.40 | 752.97 | 191.79 |
| Unfamiliar | 0.0066 | 0.0105 | 1.60 | 2.44 | 37.42 | 29.51 | 748 | 240.35 |
For each of the three conditions, medians and IQRs are reported for the model estimates of the discounting parameter (k) and temperature parameter (ß), the Akaike information criterion (AIC) as a measure of goodness‐of‐fit, as well as for the reaction times (RT).
Descriptions of the imaginations for the four events were analyzed with respect to the level of episodic richness using a similar procedure to the Autobiographical Interview Analysis [Levine et al., 2002]. Details were categorized as internal (episodic information relating to the given future event) or external (nonepisodic information). Internal details were categorized further into one of five categories adapted from Levine et al.: time, place, perceptual, emotions/thoughts, and event details (examples of narratives coded into internal and external details are presented in Figure S1 in the Supporting Information). External details comprised semantic details, repetitions and other metacognitive statements. A second independent rater coded details into the same categories, yielding a reliability between the raters of cronbach's alpha = 0.97 for internal details and cronbach's alpha = 0.89 for external details. Where appropriate, degrees of freedom were adjusted using the Greenhouse‐Geisser procedure to correct for potential violations of the sphericity assumption.
FMRI Data Analysis
FMRI data processing and statistical analyses were performed using statistical parametric mapping (SPM8; Wellcome Department of Imaging Neuroscience, London, UK).
Functional data were corrected for slice timing, rigid body motion and susceptibility artefacts (“realign and unwarp”). Next, the individual structural T1 image was coregistered to the mean functional image generated during realignment. Coregistered T1 images were then segmented using the ‘New Segment’ routine in SPM8. Resulting tissue‐class images for gray and white matter were subsequently used for spatial normalization of the functional images using the DARTEL toolbox. Data were smoothed with a 6‐mm full‐width at half maximum (FWHM) isotropic Gaussian kernel.
Data analysis was performed using the general linear model (GLM) approach as implemented in SPM. Sustained activation during the presentation of the delayed option (i.e., from option onset until button press) was modeled by boxcar regressors that were convolved with the canonical hemodynamic response function. Condition‐specific k‐parameters from the scanning session were used for the calculation of the subjective value of each delayed option and included as a parametric regressor in the GLM. Error trials (trials in which participants responded too early/late) were modelled separately. All first‐level analyses also contained a set of regressors that modelled images contaminated by movement (using an adaptive velocity cutoff with a criterion of 0.4 mm/TR).
For each subject, contrast images for each condition (control/familiar/unfamiliar) and for the respective subjective value regressor were constructed. For differential analyses, these contrast images were entered into a second‐level random effects ANOVA model.
Finally, we used psychophysiological interaction analyses (PPI) [Friston et al., 1997] to test for potentially different connectivity patterns of neural valuation regions for the familiar and unfamiliar condition. To this end, we designed first‐level models for each participant consisting of the following three regressors: (1) the time course of the seed region; (2) the psychological variable (i.e. the subjective value of the delayed option folded with the haemodynamic response function); and (3) the product of the former two.
To correct for multiple comparisons in the hippocampus, we used anatomical masks obtained from the Harvard‐Oxford atlas (probability threshold 50%). In addition, due to the ventral striatums' role in coding delayed reward signals [Miedl et al., 2014] and emotional aspects of autobiographical memories [Speer et al., 2014], 8mm‐sphreres were centred around established coordinates: x,y,z: ±14, 8, −8 mm [O'Doherty et al., 2004; Yacubian et al., 2006]. The threshold of small volume corrections was set to P < 0.05 corrected for multiple comparisons using the family wise error rate (FWE < 0.05). Other regions were reported when passing a whole‐brain corrected cluster‐threshold of FWE < 0.05 (cluster forming threshold P < 0.005 uncorrected).
RESULTS
Behavioral Results
The condition specific discounting parameters (transformed k‐values) were subjected to a repeated measures ANOVA, yielding a significant main effect of condition (control/familiar/unfamiliar), F(2,44) = 5.83, P < 0.01. Post‐hoc comparisons revealed that participants' discounting behaviour was significantly lower in both episodic conditions compared with the control condition (familiar > control: t(21) = 2.71, P < 0.01; unfamiliar > control: t(21) = 2.93, P < 0.01). No significant difference was observed between the conditions (P = 0.92; Fig. 2).
Figure 2.
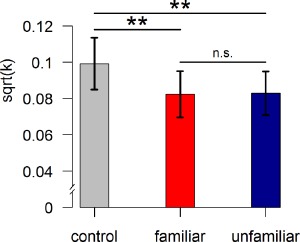
Behavioral data. Square‐root transformed delay discounting rate k, plotted separately for the three conditions. Error bars represent standard errors of the mean. ** P < 0.01, n.s.: not significant. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Post‐Hoc Ratings
Confirming presumed categorization, post‐hoc interviews revealed an equal motivation to meet the familiar (M = 5.96, SD = 1.30) and unfamiliar social partners (M = 5.96, SD = 1.03), P > 0.99. Moreover, familiar partners (M = 6.65, SD = 0.51) were rated as significantly higher on emotional closeness than the unfamiliar partners (M = 2.52, SD = 1.01), t(22) = 15.91, P < 0.001. Analysis of the autobiographical Interview indicated that participants imagined familiar and unfamiliar events with similar amounts of internal, t(22) = 0.06, P > .94 and external details, t(22) = 1.31, P > .20 (Table 3). Neither for the familiar nor for the unfamiliar condition was the tag‐effect directly correlated with the amount of internal details.
Table 3.
Level of detail and episodic richness of simulations across future event scenarios
| Familiar | Unfamiliar | |||
|---|---|---|---|---|
| M | SD | M | SD | |
| Internal Details | 10.70 | 4.80 | 10.74 | 5.85 |
| Event Details | 4.35 | 2.21 | 3.70 | 2.96 |
| Perceptual Details | 2.09 | 2.13 | 2.26 | 1.91 |
| Place Details | 1.57 | 0.79 | 1.52 | 0.79 |
| Emotion/Thought Details | 2.48 | 1.93 | 3.30 | 3.15 |
| External Details | 1.09 | 1.48 | 0.87 | 1.33 |
| Semantic Details | 0.39 | 0.84 | 0.39 | 0.66 |
| Repetition | 0.48 | 0.79 | 0.39 | 0.78 |
| Other | 0.22 | 0.85 | 0.22 | 0.67 |
Means (M) and standard deviations (SD) are reported for the amount of details imagined for familiar and unfamiliar events with further divisions into subcategories of internal and external detail categories. Due to the link of the imagined scenarios to specific time delays, time details were only rarely reported and are therefore not listed in the table.
FMRI Data
We first analyzed differences in the condition regressors without parametric modulation. The comparison of both episodic conditions versus the control condition was associated with greater BOLD signals in a network consisting of the left medial rostral and ventromedial prefrontal cortex, left middle temporal gyrus, left retrosplenial cortex/posterior cingulate cortex and left lateral parietal cortex (all P < 0.05 FWE; Table 4, Fig. 3).
Table 4.
Regions in which the BOLD signal was significantly increased in the episodic (familiar + unfamiliar) compared with the control condition and significantly differed between the episodic conditions (familiar > <unfamiliar)
| Brain Region | MNI (peak) | |||||
|---|---|---|---|---|---|---|
| Side | x | y | z | Cluster size | Z‐Score | |
| Episodic > Control | ||||||
| Medial rostral PFC | l | −6 | 62 | 22 | 798 | 3.87 |
| Ventromedial PFC | l | −6 | 58 | −6 | Same Cluster | 4.05 |
| Middle Temporal Gyrus | l | −64 | −6 | −12 | 346 | 4.22 |
| Retrosplenial Cortex | l | −10 | −44 | 4 | 1865 | 4.65 |
| Posterior Cingulate Cortex | l | −4 | −52 | 36 | Same Cluster | 4.47 |
| Lateral Parietal Cortex | l | −50 | −72 | 28 | 943 | 4.03 |
| Familiar > Unfamiliar | ||||||
| Amygdala | r | 16 | −6 | −12 | 661 | 4.04 |
| Thalamus | l | −8 | −12 | 8 | Same Cluster | 3.90 |
| Ventral Tegmental Area | l | −10 | −22 | −8 | Same Cluster | 3.79 |
| Middle Temporal Gyrus | l | −52 | −52 | −2 | 329 | 3.70 |
| Unfamiliar > Familiar | ||||||
| Hippocampus | l | −16 | −38 | 2 | 26 | 3.51a |
Montreal Neurological Institute and Hospital (MNI) coordinates and z values are reported for peak voxels and local maxima within each cluster. All P < 0.05 FWE. l: left; r: right.
Figure 3.
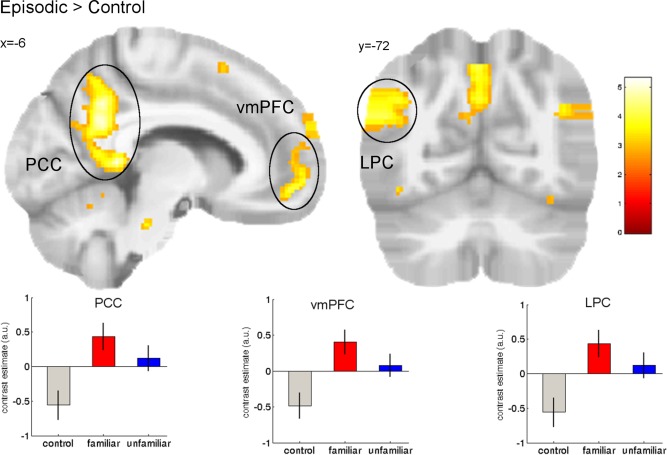
Greater activation for both episodic (familiar and unfamiliar) conditions compared with the control condition was observed in the left posterior cingulate cortex (PCC), the left ventromedial prefrontal cortex (vmPFC) and the left lateral parietal cortex (LPC) (all P < 0.05 FWE). Activations are overlaid on the mean structural image of all participants (display threshold P < 0.005 uncorrected). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
When comparing the familiar with the unfamiliar condition (familiar > unfamiliar), whole‐brain analyses revealed a significant increase in the BOLD response in the right extended amygdala, the left thalamus, the left ventral tegmental area, as well as the left middle temporal gyrus (all P < 0.05 FWE see Fig. 4A and Table 4). In the reverse contrast (unfamiliar > familiar), a higher BOLD signal was observed in the left posterior hippocampus (P < 0.05 FWE, see Fig. 4B). No further regions exhibited significant activation in this contrast on the whole brain level.
Figure 4.
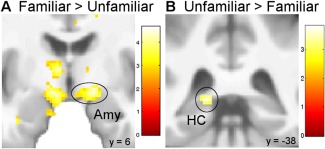
A: Activation in the right extended amygdala was significantly increased in the familiar compared with the unfamiliar condition (P < 0.05 FWE). B: The reverse contrast yielded a greater signal in the left posterior hippocampus (P < 0.05 FWE). Activations are overlaid on the mean structural image of all participants (display threshold P < 0.005 uncorrected). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
In order to investigate whether differences in the neural tag effect could be predicted by interindividual differences in the discounting behavior, we next performed a simple regression analysis including the single‐subject contrast images and transformed k values. Results revealed that across both episodic conditions there was a significant correlation between individual discounting parameters and the ACC (0,42,0, z = 4.64, cluster size = 429, P < 0.05 FWE). As demonstrated in Figure 5, this correlation did not differ between episodic conditions.
Figure 5.
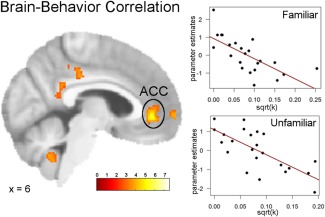
Simple regression of the discounting parameter across the two episodic conditions and neural activation during the two episodic conditions yielded a significant correlation in the bilateral ACC (P < 0.05 FWE). Separate regression plots of parameter estimates in the peak voxel of the ACC and the individual discounting parameters of the familiar and unfamiliar condition confirm the presence of a correlation in both conditions. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Next, we were interested in whether the neural substrates of subjective valuation underlying both episodic conditions differ from each other. To this aim, individual contrast images coding for parametric modulation by subjective values were entered into the analysis. The main effect across both conditions revealed significant clusters in the ACC, the right orbitofrontal cortex, the bilateral ventral striatum, left posterior and middle frontal regions, the bilateral middle temporal gyrus, the right mid cingulum and the left hippocampus to be significantly modulated by subjective value (all P < 0.05 FWE). Subsequent differential analyses for the contrast familiar > unfamiliar revealed a stronger positive subjective value correlation in a cortical network including inferior and middle frontal gyrus, inferior and middle temporal gyrus, precuneus and the cerebellum (all P < 0.05 FWE; Table 5, Fig. 6). No significant differences were observed in the reverse contrast (unfamiliar > familiar).
Table 5.
Regions in which the BOLD signal was significantly modulated by the subjective value of the delayed reward option in the episodic conditions
| MNI (peak) | ||||||
|---|---|---|---|---|---|---|
| Brain Region | Side | x | y | z | Cluster size | Z‐Score |
| SV (familiar + unfamiliar) | ||||||
| Middle Frontal Gyrus | l | −36 | 40 | 32 | 311 | 4.24 |
| Anterior Cingulate Cortex | l | −12 | 48 | 6 | 1972 | 3.69 |
| Orbital Frontal Cortex | l | −6 | 44 | −10 | Same Cluster | 3.82 |
| Ventral Striatum | r | 8 | 12 | −8 | 78 | 4.05a |
| l | −6 | 8 | −8 | 70 | 3.53a | |
| Posterior−Medial Frontal | l | −18 | −6 | 62 | 1472 | 4.42 |
| Mid Cingulum | r | 10 | −16 | 38 | 498 | 3.81 |
| Hippocampus | l | −20 | −20 | −18 | 63 | 3.57a |
| Middle Temporal Gyrus | r | 46 | −56 | 2 | 1645 | 4.41 |
| l | −48 | −62 | −14 | 1083 | 4.44 | |
| SV (familiar > unfamiliar) | ||||||
| Inferior Frontal Gyrus | l | −34 | 24 | −18 | 351 | 3.77 |
| Middle Frontal Gyrus | r | 10 | 6 | 70 | 1323 | 3.84 |
| Middle Temporal Gyrus | r | 50 | −24 | −6 | 406 | 3.97 |
| Inferior Temporal Gyrus | r | 52 | −62 | −6 | 2935 | 4.29 |
| Precuneus | r | 8 | −72 | 20 | Same Cluster | 4.42 |
| Cerebellum | l | −24 | −76 | −18 | 618 | 4.88 |
Montreal Neurological Institute and Hospital (MNI) coordinates and z values are reported for peak voxels and local maxima within each cluster. All P < 0.05 FWE. l: left; r: right.
Figure 6.
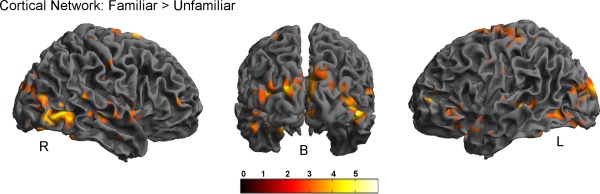
Cortical activation in middle and inferior frontal gyrus, middle and inferior temporal gyrus, precuneus and the cerebellum was significantly increased during subjective value processing in the familiar compared with the unfamiliar condition (P < 0.05 FWE). L, left; R, right; B back. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Given our aforementioned findings demonstrating the ACC to be involved in individual discounting behavior and parametric valuation as well as previous evidence for a key role of dynamic ACC‐limbic interactions on choice behavior [Peters and Büchel, 2010; Roiser et al., 2009], we next analyzed whether the valuation signals in the ACC are differentially connected with other brain regions in familiar versus unfamiliar trials. In other words, we were interested in whether the contribution of brain regions to the valuation in the ACC changes with the episodic context.
To this end, PPI were conducted using a 4mm sphere around the ACC peak from the SV main effect analysis (−12, 48, 6) as the seed region. Results revealed a stronger coupling between ACC and left hippocampus (−22, −26, −14, z = 3.56, P < 0.05 FWE) in the unfamiliar compared with the familiar condition. For the reverse contrast (familiar > unfamiliar), stronger coupling was detected between the ACC and the left ventral striatum (−10, 10, −14, z = 3.50, P < 0.05 FWE; Fig. 7). No further regions showed significant differences in coupling with the ACC between the two conditions.
Figure 7.
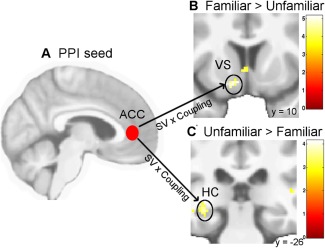
A: The seed for the PPI analysis was placed in the left anterior cingulate cortex (ACC; −12,48,6, with 4 mm sphere). B: In comparison to the familiar condition, functional coupling between the ACC and the left hippocampus during subjective value (SV) processing was increased in the unfamiliar condition (P < 0.05 FWE). C: The reverse contrast revealed increased functional coupling between ACC and left ventral striatum for the familiar compared with the unfamiliar condition (P < 0.05 FWE). Activations are overlaid on the mean structural image of all participants (display threshold P < 0.005 uncorrected). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
We investigated the role of familiarity in the episodic modulation of delay discounting using fMRI combined with a modified intertemporal choice task. Behaviorally, reward delay discounting was attenuated by episodic future event imagination regardless of the degree of personal prior experience associated with the simulated future scenarios. In the same vein, interview data showed that both familiar and unfamiliar scenarios were imagined with similar richness. The neuroimaging data highlight the hippocampus to be particularly crucial for the construction of unfamiliar events and for triggering frontal valuation signals when rewards are presented in an unfamiliar context. In contrast, the valuation of delayed rewards in a familiar context more strongly relied on key nodes of emotional and autobiographical memory circuits.
Our observation of a behavioral tag‐effect further confirms suggestions of an adaptive function of episodic prospection in future decision‐making. Both the imagination of familiar and unfamiliar episodes increased activity in a network of brain regions involved in the recollection of previous events and in the construction of potential future scenarios, including the ventromedial PFC (vmPFC), the posterior cingulate cortex and the lateral parietal cortex [Hassabis and Maguire, 2007; Peters and Büchel, 2011; Schacter et al., 2012].
The simulation of unfamiliar future events was specifically mirrored by additional hippocampal engagement. According to the constructive episodic simulation hypothesis, episodic memories of individual events can be flexibly recombined to construct future scenarios [Schacter and Addis, 2007a, 2007b] and such recombination has been related to hippocampal activity [Addis and Schacter, 2008, 2011; Schacter and Addis, 2007a]. It is, however, unclear how much the result of such construction processes depends on the availability of self‐experienced, similar episodes. While some data demonstrated richer and more detailed simulations when strong personal prior experience of similar previous events was available [Arnold et al., 2011; Szpunar and McDermott, 2008], other findings suggest no impact of personal familiarity on the quality of future event simulations [Anderson, 2012]. The latter findings have been attributed to the cognitive flexibility to recruit alternative sources for future event construction, such as media and third party reports, when personal experience is lacking.
In our paradigm, unfamiliar events, that is, events that have previously not been experienced, consisted of meeting a famous person about whom participants certainly possessed media‐sourced information. It seems likely that the cover story used to identify novel contacts specifically encouraged participants to choose persons about whom they possessed such detailed information. Examples from our post‐scan imagination interviews support this assumption: “During our meeting, he [an author] always looked like on this photograph on the back of his new book”; “He [the politician] smoked cigarettes throughout our meeting as he is always doing on TV” (see also Supporting Information Fig. S1 for sample narratives).
Contacts were identified in this way to ensure a similar attractiveness of familiar and unfamiliar events, which was confirmed by our ratings indicating equal motivation towards both events. It is possible, however, that if participants had been asked to imagine personally insignificant and rather unknown persons, results might have been different. In this case, the lack of information might have led to declines of episodic richness and hippocampal engagement [Rabin and Rosenbaum, 2012; Rabin et al., 2012; Westmacott et al., 2004]. In the present study, participants could not rely on self‐experience while simulating the event with the famous person, thus they needed to construct the scenario by combining episodic details from different sources [Anderson, 2012]. They seemed to master this construction successfully since the level of episodic details generated by participants and the impact of future thinking on delay discounting was unaffected by personal experience and plausibility in our study. In sum, we speculate that increased hippocampal engagement during such successful construction of unfamiliar events reflects an increased need or effort to combine information from disparate sources for which no pre‐association exist (e.g., categorical memory of “having a coffee” combined with media‐sourced information about the person) [Weiler et al., 2010a].
The simulation of an event including a familiar person, with whom participants described significantly higher emotional closeness than with an unfamiliar person, induced specific activation in affective brain regions, i.e., the extended amygdala and the ventral tegmental area. Hence, these results extend findings of an overlap of brain networks engaged in remembering events and future memory construction to brain regions relevant for emotional processing [Cabeza and St Jacques, 2007; Scheele et al., 2013]. Such impact of emotional, autobiographical memory on simulating and evaluating future familiar events is further supported by the finding of a direct correlation between a cortical fronto‐tempo‐parietal network and valuation of future rewards in a familiar context. This cortical network has previously been implicated in representing distributed semantic and visuospatial features of consolidated autobiographical memories [Cabeza and St Jacques, 2007; Martinelli et al., 2013; Svoboda et al., 2006].
Across both episodic conditions, the ACC signal correlated with subjective values as expected from its role for cost‐benefit computations [Hillman and Bilkey, 2012; Hosokawa et al., 2013] and adaptive decision‐making [Rushworth and Behrens, 2008]. This function is further highlighted by our finding of a direct correlation between ACC activity and individual discounting rates. The ACC exhibits bidirectional anatomical connections with regions of the reward and memory circuits, including the hippocampal formation and the striatum [Haber and Knutson, 2009]. Since general ACC activation predicted both discounting rates in familiar and unfamiliar conditions, we further investigated whether its modulation by other brain regions differs between the two conditions. Results of psychophysiological interaction analyses revealed a significantly stronger connectivity of the ACC valuation signal with the hippocampus in the unfamiliar condition. The hippocampus has previously been related to subjective value computation [Lebreton et al., 2009, 2013]. Previous data showed an increased ACC‐hippocampal coupling to predict decreased discounting rates in the context of future episodic thinking even though these studies did not explicitly control for familiarity of future events [Benoit et al., 2011; Peters and Büchel, 2010]. It has been argued that hippocampal neurons may modulate medial prefrontal information processing through their role in mentally simulating potential future outcomes [Schacter et al., 2012], that is, by providing episodic predictions of decision outcomes [Johnson and Redish, 2007; Lee et al., 2012]. Our results suggest that such modulation of frontal valuation regions might crucially depend on the required construction effort, which was amplified in the unfamiliar condition of the present paradigm. Intriguingly, in a very recent study by Gaesser et al. [2013] a subregion of the left hippocampus, very similar to the region in our study, was engaged during construction of unfamiliar event stimulation, indicating that the location might play a critical role in this context.
When imagined events were familiar, the ACC valuation signal was specifically mediated by activity in the striatum. Given recent data indicating that positive emotional quality of autobiographical information may be modulated by the striatum [Scheele et al., 2013; Speer et al., 2014], such coupling possibly signals the emotional value of reactivated autobiographical memories.
More generally, our data may contribute to the ongoing debate about the role of the hippocampus in episodic prospection [Addis and Schacter, 2011; Schacter et al., 2012]. Controversial findings stem from hippocampal lesion patients who often [Andelman et al., 2010; Hassabis et al., 2007; Race et al., 2011], but not always [Cooper et al., 2011; Maguire et al., 2010; Squire et al., 2010], show impairments in memory construction. Our findings suggest a critical role of familiarity in this context by showing that future events that strongly draw on autobiographical, familiar experiences seem to depend less on hippocampal construction activity but increasingly engage neocortical areas containing already consolidated autobiographical memories. Moreover, due to pre‐scan familiarization with the spatial context (café) and participants' strong personal experience with the familiar contact in our study, simulation might have included more generic/semantic memory representations (e.g., Mum always drinks cappuccino), which might be less hippocampus dependent (e.g., as discussed by [Moscovitch et al., 2006]) than scene construction involving the processing of dispersed memories [Maguire and Mullally, 2013]. Since both imagination richness and external details were similar for both conditions, this idea, however, is rather speculative at present [Verfaellie et al., 2014].
It has been shown that reduced delay discounting can rely on shifts of attentional resources to the future [Radu et al., 2011]. These shifts also depend on how distant time is described (e.g., specific date versus unspecific delay) [Read et al., 2005]. Hence, it seems likely that future event construction, as required in our study, additionally binds attentional resources to the delayed option [Marchetti, 2014]. To disentangle incremental effects of attention and episodic prospection on temporal discounting, future studies should therefore compare effects of episodic tags with those of other attentional manipulations. Existing findings showing the tag‐effect to be parametrically modulated by episodic network activity and imagination quality [Palombo et al., 2015; Peters and Büchel, 2010], however, strongly argue for a specific impact of episodic simulation in the present context. Whereas our neural findings further support this assumption, we did not observe direct correlations between episodic richness and the behavioral tag‐effect. The lacking correlation might be caused by individual ceiling effects in imagination quality due to the block‐wise presentation of prioritized contacts but also by the limited sensitivity of a post‐scan interview on imagination. Future studies might be able to provide more sensitive insights into relations with imagination quality by assessing trial‐wise imagination scores during the task without influencing behavior in the primary intertemporal choice task.
Taken together, our findings strengthen and extend previous assumptions about the impact of episodic prospection on reduced delay discounting. By applying different analytical approaches, we were able to specify a general engagement of the imagination/prospection network in response to episodic tags whose activation strength in some core regions (i.e., ACC) predicted discounting behavior and the subjective value of a reward on a trial‐by‐trial basis. Depending on episodic construction demands, this neural valuation signal was directly modulated by dynamic interactions with different limbic brain structures. Most importantly, our findings indicate a critical role of the hippocampus when future simulations include scenarios that have not yet been encountered. While healthy young adults, as in our study, were capable to master the construction demands by hippocampal engagement, it could be speculated that patients with hippocampal lesions or older people with hippocampal atrophy might show a reduced tag‐effect when unfamiliar future events are included. So far, findings regarding the presence of the tag‐effect in amnestic patients are mixed [Kwan et al., 2015; Palombo et al., 2015], thus supporting the notion that additional factors such as prior experience with a prospective event may mediate the impact of hippocampal involvement on intertemporal choice. Our findings may be especially important for future studies in which potentially beneficial consequences of future thinking on decision‐making may be used in the context of interventions, e.g. in clinical populations characterized by steep discounting, such as substance abuse or pathological gambling [Bickel et al., 2014].
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors confirm that they have no financial interests in the subject matter or materials discussed in the manuscript. The manuscript has not been published previously nor is it concurrently submitted for publication elsewhere.
REFERENCES
- Addis DR, Schacter DL (2008): Constructive episodic simulation: Temporal distance and detail of past and future events modulate hippocampal engagement. Hippocampus 18:227–237. [DOI] [PubMed] [Google Scholar]
- Addis DR, Schacter DL (2011): The hippocampus and imagining the future: Where do we stand? Front Hum Neurosci 5:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Cheng T, Roberts RP, Schacter DL (2011): Hippocampal contributions to the episodic simulation of specific and general future events. Hippocampus 21:1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andelman F, Hoofien D, Goldberg I, Aizenstein O, Neufeld MY (2010): Bilateral hippocampal lesion and a selective impairment of the ability for mental time travel. Neurocase 16:426–435. [DOI] [PubMed] [Google Scholar]
- Anderson RJ (2012): Imagining novel futures: The roles of event plausibility and familiarity. Memory 20:443–451. [DOI] [PubMed] [Google Scholar]
- Arnold KM, McDermott KB, Szpunar KK (2011): Imagining the near and far future: The role of location familiarity. Mem Cognit 39:954–967. [DOI] [PubMed] [Google Scholar]
- Ballard K, Knutson B (2009): Dissociable neural representations of future reward magnitude and delay during temporal discounting. Neuroimage 45:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RG, Gilbert SJ, Burgess PW (2011): A neural mechanism mediating the impact of episodic prospection on farsighted decisions. J Neurosci 31:6771–6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Koffarnus MN, Moody L, Wilson AG (2014): The behavioral‐ and neuro‐economic process of temporal discounting: A candidate behavioral marker of addiction. Neuropharmacology 76:518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, St Jacques P (2007): Functional neuroimaging of autobiographical memory. Trends Cogn Sci 11:219–227. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Fredrickson BL (1998): Influence of hiv status and age on cognitive representations of others. Health Psychol 17:494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y‐Y, Shein PP, Chiou W‐B (2012): Escaping the impulse to immediate gratification: The prospect concept promotes a future‐oriented mindset, prompting an inclination towards delayed gratification. Br J Psychol 103:129–141. [DOI] [PubMed] [Google Scholar]
- Cooper JM, Vargha‐Khadem F, Gadian DG, Maguire EA (2011): The effect of hippocampal damage in children on recalling the past and imagining new experiences. Neuropsychologia 49:1843–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel TO, Stanton CM, Epstein LH (2013a): The future is now: Comparing the effect of episodic future thinking on impulsivity in lean and obese individuals. Appetite 71:120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel TO, Stanton CM, Epstein LH (2013b): The future is now: Reducing impulsivity and energy intake using episodic future thinking. Psychol Sci 24:2339–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Argembeau A, Van der Linden M (2012): Predicting the phenomenology of episodic future thoughts. Conscious Cogn 21:1198–1206. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ (1997): Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6:218–229. [DOI] [PubMed] [Google Scholar]
- Gaesser B, Spreng RN, VC McLelland, DR Addis, DL Schacter (2013): Imagining the future: evidence for a hippocampal contribution to constructive processing. Hippocampus 23:1150–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B (2009): The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 35:4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA (2007): Deconstructing episodic memory with construction. Trends Cogn Sci 11:299–306. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Vann SD, Maguire EA (2007): Patients with hippocampal amnesia cannot imagine new experiences. Proc Natl Acad Sci 104:1726–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman KL, Bilkey DK (2012): Neural encoding of competitive effort in the anterior cingulate cortex. Nat Neurosci 15:1290–1297. [DOI] [PubMed] [Google Scholar]
- Hosokawa T, Kennerley SW, Sloan J, Wallis JD (2013): Single‐neuron mechanisms underlying cost‐benefit analysis in frontal cortex. J Neurosci 33:17385–17397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, Redish AD (2007): Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. J Neurosci 27:12176–12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan D, Craver CF, Green L, Myerson J, Gao F, Black SE, Rosenbaum RS (2015): Cueing the personal future to reduce discounting in intertemporal choice: Is episodic prospection necessary? Hippocampus 25:432–443. [DOI] [PubMed] [Google Scholar]
- Lebreton M, Jorge S, Michel V, Thirion B, Pessiglione M (2009): An automatic valuation system in the human brain: Evidence from functional neuroimaging. Neuron 64:431–439. [DOI] [PubMed] [Google Scholar]
- Lebreton M, Bertoux M, Boutet C, Lehericy S, Dubois B, Fossati P, Pessiglione M (2013): A critical role for the hippocampus in the valuation of imagined outcomes. PLoS Biol 11:e1001684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Ghim J‐W, Kim H, Lee D, Jung M (2012): Hippocampal neural correlates for values of experienced events. J Neurosci 32:15053–15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M (2002): Aging and autobiographical memory: Dissociating episodic from semantic retrieval. Psychol Aging 17:677–689. [PubMed] [Google Scholar]
- Liu L, Feng T, Chen J, Li H (2013): The value of emotion: How does episodic prospection modulate delay discounting? PLoS One 8:e81717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Mullally SL (2013): The hippocampus: A manifesto for change. J Exp Psychol Gen 142:1180–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Vargha‐Khadem F, Hassabis D (2010): Imagining fictitious and future experiences: Evidence from developmental amnesia. Neuropsychologia 48:3187–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti G (2014): Attention and working memory: Two basic mechanisms for constructing temporal experiences. Front Psychol 5:880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli P, Sperduti M, Piolino P (2013): Neural substrates of the self‐memory system: New insights from a meta‐analysis. Hum Brain Mapp 34:1515–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE (1987): An adjusting procedure for studying delayed reinforcement Quantitative Analyses of Behavior: The Effect of Delay and of Intervening Events on Reinforcement Value, Vol. 5 Hillsdale, New Jersey: Erlbaum; pp 55–73. [Google Scholar]
- Miedl SF, Büchel C, Peters J (2014): Cue‐induced craving increases impulsivity via changes in striatal value signals in problem gamblers. J Neurosci 34:4750–4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M, Nadel L, Winocur G, Gilboa A, Rosenbaum RS (2006): The cognitive neuroscience of remote episodic, semantic and spatial memory. Curr Opin Neurobiol 16:179–190. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ (2004): Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science 304:452–454. [DOI] [PubMed] [Google Scholar]
- Okuda J, Fujii T, Ohtake H, Tsukiura T, Tanji K, Suzuki K, Kawashima R, Fukuda H, Itoh M, Yamadori A (2003): Thinking of the future and past: The roles of the frontal pole and the medial temporal lobes. Neuroimage 19:1369–1380. [DOI] [PubMed] [Google Scholar]
- Palombo DJ, Keane MM, Verfaellie M (2015): The medial temporal lobes are critical for reward‐based decision making under conditions that promote episodic future thinking. Hippocampus 25:345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Büchel C (2009): Overlapping and distinct neural systems code for subjective value during intertemporal and risky decision making. J Neurosci 29:15727–15734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Büchel C (2010): Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal‐mediotemporal interactions. Neuron 66:138–148. [DOI] [PubMed] [Google Scholar]
- Peters J, Büchel C (2011): The neural mechanisms of inter‐temporal decision‐making: Understanding variability. Trends Cogn Sci 15:227–239. [DOI] [PubMed] [Google Scholar]
- Peters J, Miedl SF, Büchel C (2012): Formal comparison of dual‐parameter temporal discounting models in controls and pathological gamblers. PLoS One 7:e47225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin JS, Rosenbaum RS (2012): Familiarity modulates the functional relationship between theory of mind and autobiographical memory. Neuroimage 62:520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin JS, Carson N, Gilboa A, Stuss DT, Rosenbaum RS (2012): Imagining other people's experiences in a person with impaired episodic memory: The role of personal familiarity. Front Psychol 3:588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race E, Keane MM, Verfaellie M (2011): Medial temporal lobe damage causes deficits in episodic memory and episodic future thinking not attributable to deficits in narrative construction. J Neurosci 31:10262–10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radu PT, Yi R, Bickel WK, Gross JJ, McClure SM (2011): A mechanism for reducing delay discounting by altering temporal attention. J Exp Anal Behav 96:363–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read D, Frederick S, Orsel B, Rahman J (2005): Four score and seven years from now: The date/delay effect in temporal discounting. Manag Sci 51:1326–1335. [Google Scholar]
- Roiser JP, Martino B, de Tan GCY, Kumaran D, Seymour B, Wood NW Dolan RJ (2009): A genetically mediated bias in decision making driven by failure of amygdala control. J Neurosci 29:5985–5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MFS, Behrens TEJ (2008): Choice, uncertainty and value in prefrontal and cingulate cortex. Nat Neurosci 11:389–397. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR (2007a): Constructive memory: The ghosts of past and future. Nature 445:27–27. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR (2007b): The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Philos Trans R Soc Lond B Biol Sci 362:773–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR (2009): On the nature of medial temporal lobe contributions to the constructive simulation of future events. Philos Trans R Soc Lond B Biol Sci 364:1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK (2012): The future of memory: Remembering, imagining, and the brain. Neuron 76:677–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D, Wille A, Kendrick KM, Stoffel‐Wagner B, Becker B, Güntürkün O, Maier W, Hurlemann R (2013): Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proc Natl Acad Sci USA 110:20308–20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer ME, Bhanji JP, Delgado MR (2014): Savoring the past: Positive memories evoke value representations in the striatum. Neuron 84:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Horst AS, van der McDuff SGR, Frascino JC, Hopkins RO Mauldin KN (2010): Role of the hippocampus in remembering the past and imagining the future. Proc Natl Acad Sci 107:19044–19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B (2006): The functional neuroanatomy of autobiographical memory: A meta‐analysis. Neuropsychologia 44:2189–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK, McDermott KB (2008): Episodic future thought and its relation to remembering: Evidence from ratings of subjective experience. Conscious Cogn 17:330–334. [DOI] [PubMed] [Google Scholar]
- Szpunar KK, Chan JCK, McDermott KB (2009): Contextual processing in episodic future thought. Cereb Cortex 19:1539–1548. [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Bousquet K, Keane MM (2014): Medial temporal and neocortical contributions to remote memory for semantic narratives: Evidence from amnesia. Neuropsychologia 61:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler JA, Suchan B, Daum I (2010a): Foreseeing the future: occurrence probability of imagined future events modulates hippocampal activation. Hippocampus 20:685–690. [DOI] [PubMed] [Google Scholar]
- Weiler JA, Suchan B, Daum I (2010b): When the future becomes the past: Differences in brain activation patterns for episodic memory and episodic future thinking. Behav Brain Res 212:196–203. [DOI] [PubMed] [Google Scholar]
- Westmacott R, Black SE, Freedman M, Moscovitch M (2004): The contribution of autobiographical significance to semantic memory: evidence from Alzheimer's disease, semantic dementia, and amnesia. Neuropsychologia 42:25–48. [DOI] [PubMed] [Google Scholar]
- Yacubian J, Gläscher J, Schroeder K, Sommer T, Braus DF, Büchel C (2006): Dissociable systems for gain‐ and loss‐related value predictions and errors of prediction in the human brain. J Neurosci 26:9530–9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


