Abstract
The biological model of extraversion and neuroticism identified by Eysenck has stimulated increasing interest in uncovering neurobiological substrate of the two fundamental dimensions. Here we aim to explore brain disturbances underlying extraversion and neuroticism in 87 healthy individuals using fractional amplitude of low‐frequency fluctuations (LFF) on resting‐state functional magnetic resonance imaging. Two different frequency bands, Slow‐5 (0.01–0.027 Hz) exhibiting higher power and involving larger brain regions, and Slow‐4 (0.027–0.073 Hz) exhibiting less power and emerging locally, were analyzed. Our results showed a positive correlation between LFF amplitude at Slow‐5 and extraversion in medial prefrontal cortex and precuneus, important portions of the default mode network, thus suggesting a link between default network activity and personality traits. LFF amplitude at Slow‐5 was correlated positively with neuroticism in right posterior portion of the frontal lobe, further validating neuroticism with frontal lateralization. In addition, LFF amplitude at Slow‐4 was negatively associated with extraversion and neuroticism in left hippocampus (HIP) and bilateral superior temporal cortex (STC) respectively, supporting the hypothesized (inverse) relationship between extraversion and resting arousal, also implying neural circuit underlying emotional process influencing on personality. Overall, these findings suggest the important relationships, between personality and LFF amplitude dynamic, depend on specific frequency bands. Hum Brain Mapp 35:331–339, 2014. © 2012 Wiley Periodicals, Inc.
Keywords: Eysenck, extraversion, neuroticism, LFF
INTRODUCTION
Extraversion and neuroticism are regarded as fundamental dimensions, with each deriving from a specific biological substrate [Eysenck, 1967, 1990]. Specifically, individual differences in the activity of the reticulo‐cortical loop are responsible for individual's position on extraversion. Introverts are characterized by higher levels of activity and are consequently cortically more aroused than extraverts [Eysenck and Eysenck, 1985]. Neuroticism, on the other hand, is explained in terms of activation thresholds in the limbic system. Individuals with higher neuroticism scores have greater activation levels and lower thresholds within subcortical structures [Eysenck, 1967, 1990].
The above biological explanation of extraversion and neuroticism has led increasing interest in uncovering neurobiological bases of the two basic traits. Studies using electroencephalography (EEG) have demonstrated that extraversion was positively related to the frontal alpha activity, while neuroticism was associated with greater right versus left frontal alpha activity [Gale, et al., 2001; Minnix and Kline, 2004; Schmidtke and Heller, 2004; Tran, et al., 2001]. With positron emission tomography (PET), photon emission computed tomography (SPECT), and magnetic resonance perfusion imaging technique, associations has been found in extraversion and neuroticism with regional cerebral blood flow (rCBF), glucose metabolism (rCMRglu) as well as perfusion in many regions, including cortical (e.g. prefrontal (PFC) and cingulate cortex) and subcortical structures (e.g. striatum, basal ganglia, and thalamus) [Deckersbach, et al. 2006; Ebmeier, et al. 1994; Fischer, et al. 1997; Johnson, et al. 1999; Kim, et al. 2008; O'Gorman, et al., 2006]. Those findings have highlighted the functional neural correlates of extraversion and neuroticism, and provided neurobiological evidence for the hypothesized biological model of Eysenck's personality.
Recently, resting‐state functional magnetic resonance imaging (RS‐fMRI), with better spatial resolution (compared to EEG) and no radiation exposure (compared to PET and SPECT), has been used as an effective noninvasive technique for exploring neural mechanisms underlying extraversion and neuroticism [Adelstein, et al., 2011; Hahn, et al., 2011; Kunisato, et al., 2011; Wei, et al., 2011]. Using resting‐state functional connectivity (RSFC) analyses, Adelstein et al. [2011] found that extraversion and neuroticism was encoded within RSFC between the precuneus (PCu) and lateral paralimbic regions and dorsomedial PFC, respectively. Besides, there is also evidence for local characteristics of resting brain function associated with personality traits [Hahn, et al. 2011; Kunisato, et al. 2011; Wei, et al. 2011]. Of particular interest to this work is the study by Kunisato et al. [2011], which investigated the relationship between the Big Five personality traits and the fractional amplitude of spontaneous low‐frequency fluctuations (fALFF). Kunisato et al. [2011] discovered that extraversion was correlated positively with fALFF in the striatum, PCu, and superior frontal gyrus (SFG), while neuroticism was correlated negatively with fALFF in the middle frontal gyrus (MFG) and PCu. Those results agree with the biological model for the Big Five traits [DeYoung and Gray, 2009, 2010]. Despite these advances, it remains largely unknown whether Eysenck's model of personality is related to specific frequency bands of fALFF. The primary reason we emphasized Eysenck's personality is that this personality theory compared to Big Five model has a strong biological component and explanation causal, more predictive power for investigation of extraversion and cortical arousal [Block, 2010; Kehoe, et al., 2011]. The aim of this study was to test the influential arousal hypothesis with fALFF serving as a measure of cortical arousal. Furthermore, we questioned whether specific frequency bands of fALFF associated with personality, on the basis of view that independent frequency bands are generated by distinct oscillators with specific properties and physiological functions [Buzsáki and Draguhn, 2004; Penttonen and Buzsáki, 2003]. By decomposing RS‐fMRI low‐frequency fluctuations (LFF) into four distinct frequency bands [Slow‐5 (0.01–0.027 Hz), Slow‐4 (0.027–0.073 Hz), Slow‐3 (0.073–0.198 Hz), and Slow‐2 (0.198–0.25 Hz)], Zuo et al. [2010] observed that gray matter‐related oscillatory amplitudes primarily occurred in the Slow‐5 and Slow‐4 ranges [Zuo, et al., 2010]. Moreover, several studies have suggested that the two frequency bands contribute differentially to the LFF amplitude [Baria, et al., 2011; Han, et al., 2010; Zuo, et al., 2010]. The Slow‐4 has greater test–retest reliability for LFF amplitude measure and more reliable BOLD fluctuation amplitude voxels than Slow‐5 [Zuo, et al., 2010]. In addition, the Slow‐5 showing higher power localizes more within default‐mode regions, while Slow‐4 exhibiting less power is more robust in basal ganglia [Baria, et al., 2011; Han, et al., 2010; Zuo, et al., 2010]. Those discoveries may reflect the fact that individual frequency bands could be associated with specific properties [Buzsáki and Draguhn, 2004]. Although the origins, relation, and specific physiological functions of different frequency bands have yet to be fully clarified, it has been found that the abnormalities of LFF amplitude detected in the clinical populations are associated with the choice of Slow‐5 and Slow‐4 bands, respectively [Di Martino, et al., 2008; Han, et al., 2012; Hoptman, et al., 2010], and the functional brain networks derived in the Slow‐4 are more reliable than those in Slow‐5 [Liang, et al., 2012]. These studies imply that the pattern of intrinsic brain activity is sensitive to specific frequency bands. Taken together, it would be necessary to differentiate the frequency bands to examine LFF amplitude in relation to personality therefore.
In this study, we sought to determine (i) whether extraversion and neuroticism are associated with LFF amplitude at specific frequency bands and (ii) whether these findings support for Eysenck's predication of extraversion and neuroticism. To address the aforementioned issues, extraversion and neuroticism was quantified by the Eysenck Personality Questionnare‐Revised, Short Scale for Chinese (EPQ‐RSC) [Eysenck, 1991; Qian, et al., 2000], and the fractional amplitude of low‐frequency fluctuation (fALFF) analysis, which may provide a more reasonable measure of low‐frequency oscillatory phenomena, was applied to calculate the value of LFF amplitude [Zou, et al., 2008, 2010], especially Slow‐5 and Slow‐4 frequency bands both broadly distributed through gray matter and thought to be mainly linked to neuronal fluctuations were analyzed [Biswal et al., 1995; Fox and Raichle, 2007; Han et al., 2010; Zuo et al., 2010]. Finally, multiple regression method was performed to assess the relationship between LFF amplitude and personality dimensions of extraversion and neuroticism.
MATERIALS AND METHODS
Participants
Resting‐state scans were acquired for 87 (48 males, age range: 17–36 years, mean age: 23.5 years) right‐handed healthy subjects in this study. All of these subjects participated in our previous study [Wei et al., 2011]. No subjects had history of psychiatric disorder or neurological illness. This study was approved by the local Medical Ethics Committee at Jinling Hospital, Nanjing University School of Medicine, and informed written consent was obtained from all subjects.
Personality Questionnaires
The participants completed the Eysenck Personality Questionnare‐Revised, Short Scale for Chinese (EPQ‐RSC) [Eysenck, 1991; Qian et al., 2000], a self‐report questionnaire that assesses four personality dimensions of Extraversion (E), Neuroticism (N), Psychoticism (P), and Lie (L) prior to MRI scanning. Subjects responded on each item in the questionnaire as “yes” or “no,” (coded as 1 and 0) depending on how applicable the statement was to them. Raw scores were converted to T scores using the formula reported in the Manual [Qian, et al., 2000]. We focused our analyses on E and N dimensions whose resultant T scores were used for measuring correlations with LFF amplitude. In the present sample, the mean score of E, N, and P was 56.33 (SD = 9.48), 44.22 (SD = 13.00), and 47.01 (SD = 7.99), respectively. The intercorrelations of E, N, and P dimensions, examined using Pearson's correlation, suggested nonsignificant relationship of E to N (r = ‐0.16; P = 0.135) and P (r = 0.013; P = 0.90) but moderate significant positive correlation between N and P (r = 0.21; P = 0.044).
Data Acquisition and Analysis
Imaging data were acquired using a 3‐T MRI scanner (Siemens‐Trio, Erlangen, German) located at the Jinling Hospital, Nanjing, China. Foam padding and headphones were used to limit head motion and reduce scanner noise. The subjects were instructed simply to rest with their eyes closed, not to think of anything in particular, and not to fall asleep. Functional images were collected transversely by using an echo‐planar imaging (EPI) sequence with the following settings: TR = 2,000 ms, TE = 30 ms, flip angle = 90°, FOV = 24 × 24 cm2, slices = 30, in‐plane matrix = 64 × 64, thickness = 4 mm, interslice gap = 0.2 mm, voxel size = 3.75 × 3.75 × 4.0 mm3. For each subject, a total of 255 volumes were acquired, resulting in a total scan time of 510 s. Three‐dimensional T1‐weighted anatomical images were collected axially using a 3‐D spoiled gradient recalled (SPGR) sequence (TR = 2,300 ms, TE = 2.98 ms, flip angle = 9°, slice thickness = 1 mm, FOV = 24 × 24 cm2, matrix size = 512 × 512 × 170, and voxel size = 0.5 × 0.5 × 1 mm3) on each subject.
Functional data preprocessing was carried out using the SPM8 package (http://www.fil.ion.ucl.ac.uk/spm). The first five volumes of the functional images were discarded due to instability of the initial MRI signal and adaptation of participants to the circumstance. The remaining 250 images were first corrected for acquisition delay between slices and head motion. One dataset was excluded from the further analysis according to the criteria that translational and rotational parameters exceeded ±1.5 mm or ±1.5°. Then, the resulting images were normalized to the standard SPM8 echo‐planar imaging (EPI) template, resampled to 3‐mm cubic voxels. The resultant normalized functional data underwent spatial smoothing [8‐mm full width at half maximum (FWHM) Gaussian kernel] and removal of linear trends. Finally, six head motion parameters were regressed, since an important recent study [Satterthwaite, et al., 2012] demonstrated that head motion had a confounding effect on LFF amplitude analysis.
Anatomical data were processed to separate the gray matter from the 3D T1‐wighted structural scans using the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.html). Anatomical images were bias‐corrected, tissue classified, and registered using linear (12‐parameter affine) and nonlinear transformations, within a unified model [Ashburner and Friston, 2005]. Then, the gray matter (GM) partitions were modulated to preserve actual GM values locally. The segmented gray matter volume was treated as an external regressor in the subsequent multiple regression analysis.
fALFF Calculation
fALFF analysis was performed by REST software (http://www.restfmri.net). The time series for each voxel was first transformed to the frequency domain and then the power spectrum was obtained. The square root of the power spectrum was computed, after that averaged across a predefined frequency interval. This averaged square root was taken as the amplitude of LFF (ALFF) [Zang et al., 2007]. Fractional ALFF (fALFF) is the fraction of ALFF in a given frequency band to the ALFF over the entire frequency range detectable in the given signal [Zou et al., 2008]. In this study, we divided the full frequency range (0–0.25 Hz) into five distinct bands: [Slow‐6 (0–0.01 Hz), Slow‐5 (0.01–0.027 Hz), Slow‐4 (0.027–0.073 Hz), Slow‐3(0.073–0.198 Hz), and Slow‐2 (0.198–0.25 Hz)] [Buzsáki and Draguhn, 2004; Han et al., 2010; Hoptman et al., 2010; Zuo et al., 2010]. The fALFF and then was computed at the Slow‐5 and Slow‐4 bands which mainly related to physiological meanings [Biswal et al., 1995; Han et al., 2010, 2012; Zuo et al., 2010]. Under the studied frequency ranges, fALFF of each voxel was computed for each subject, and it was further divided by the global mean value to reduce the global effects of variability across participants [Han et al., 2010; Zang et al., 2007].
Second‐level Analysis
Voxel‐based multiple regression analysis was carried out by SPM8 with voxel‐wise fALFF value as dependent variable, each E or N score as a covariate of interest, and with gender, age, averaged gray‐matter volumes and mean relative displacement [Satterthwaite, et al., 2012] as external regressors to control for their effects, for previous evidences indicated that gender, age, and gray‐matter volume were influencing factors of the association between personality traits and brain function [Blankstein, et al., 2009; DeYoung, et al., 2010; Gardini, et al., 2009; Sutin, et al., 2009; Wright, et al., 2007; Yamasue, et al., 2007], as well as head motion was a confounding factor in LFF amplitude analysis [Power, et al., 2011; Satterthwaite, et al., 2012; Van Dijk, et al., 2011]. We set at P < 0.05 (combined height threshold of P < 0.01 and a minimum cluster size of 74 voxels), using the AlphaSim program in the REST Software.
RESULTS
LFF Amplitude at Slow‐5 Frequency Band Correlated With E and N
Positive correlation was observed in the MPFC and PCu between LFF amplitude at Slow‐5 and E score (P < 0.05, AlphaSim corrected; Fig. 1, Table 1). Regarding LFF amplitude at Slow‐5 related to N score, positive correlation emerged in the right precentral gyrus (PreCG) (P < 0.05, AlphaSim corrected; Fig. 1, Table 1).
Figure 1.
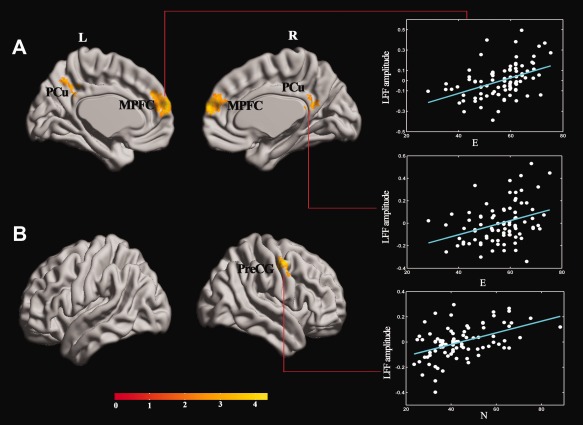
(A) Correlation of E score and LFF amplitude (P < 0.05, corrected by Alphasim, a combined threshold of P < 0.01, and a minimum cluster size of 74 voxels) in the MPFC (0, 60, 18) (r = 0.44, P < 0.001), and the PCu (−9, −48, 36) (r = 0.34, P < 0.001). (B) Correlation of N score and LFF amplitude (P < 0.05, corrected by Alphasim, a combined threshold of P < 0.01, and a minimum cluster size of 74 voxels) in the R PreCG (54, −3, 48) (r = 0.46, P < 0.001). The LFF amplitude values in the figure are extracted from the significant clusters after a linear regression of age, gender, and grey matter volumes of each subject. More details of these regions are described in Table 1. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 1.
Brain regions in which LFF amplitude at slow‐5 was associated with E and N
| Brain regions in which LFF amplitude at Slow‐5 was associated with E and N | |||||
|---|---|---|---|---|---|
| Anatomical region | MNI (x, y, z) | BA | Voxels | T | |
| E | MPFC | 0, 60, 18 | 9/10 | 283 | 4.39 |
| PCu | −9, −48, 36 | 7/31 | 81 | 3.09 | |
| N | R PreCG | 54, −3, 48 | 4/6 | 104 | 4.43 |
MNI, Montreal Neurologic Institute; BA, Brodmann's area; E, extraversion; N, neuroticism; MPFC, medial prefrontal cortex; PCu, precuneus; R, right; PreCG, precentral gyrus. All the coordinates are donated by Montreal Neurological Institute (MNI) space coordinates. T score represents the statistical value of peak voxel showing LFF amplitude correlated with E and N. Positive and negative T values indicate positive and negative correlations, respectively, between LFF amplitude value and E/N score.
LFF Amplitude at Slow‐4 Frequency Band Correlated With E and N
LFF amplitude at Slow‐4 band was correlated negatively with E score in the left hippocampus (HIP), while negatively with N score in the bilateral superior temporal cortex (STC) (P < 0.05, AlphaSim corrected; Fig. 2, Table 2).
Figure 2.
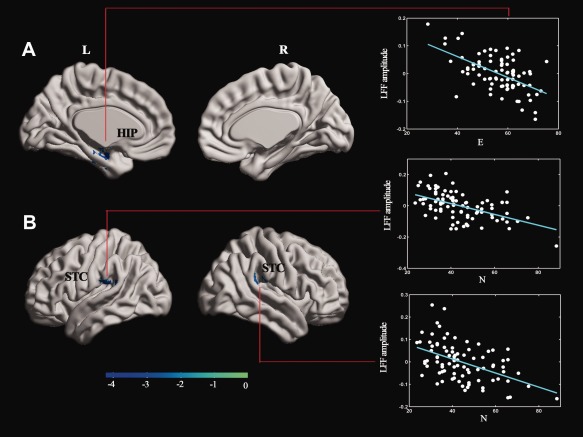
(A) Correlation of E score and LFF amplitude (P < 0.05, corrected by Alphasim, a combined threshold of P < 0.01, and a minimum cluster size of 74 voxels) in the L HIP (−22, −9, −24) (r = −0.56, P < 0.001). (B) Correlation of N score and LFF amplitude (P < 0.05, corrected by Alphasim, a combined threshold of P < 0.01, and a minimum cluster size of 74 voxels) in L STC (−48, −33, 18) (r = −0.53, P < 0.001), R STC (63, −33, 21) (r = −0.49, P < 0.001). The LFF amplitude values in the figure are extracted from the significant clusters after a linear regression of age, gender, and grey matter volumes of each subject. More details of these regions are described in Table 2. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 2.
Brain regions in which LFF amplitude at Slow‐4 was associated with E and N
| Brain regions in which LFF amplitude at Slow‐4 was associated with E/N score | |||||
|---|---|---|---|---|---|
| Anatomical region | MNI (x, y, z) | BA | Voxels | T | |
| E | L HIP | −22, −9, −24 | 20/35 | 154 | −3.84 |
| N | L STC | −48, −33, 18 | 13 | 139 | −4.70 |
| R STC | 63, −33, 21 | 40 | 80 | −3.97 | |
MNI, Montreal Neurologic Institute; BA, Brodmann's area; E, extraversion; N, neuroticism; L, left; R, right; HIP, hippocampus; STC, superior temporal cortex. All the coordinates are donated by Montreal Neurological Institute (MNI) space coordinates. T score represents the statistical value of peak voxel showing LFF amplitude correlated with E and N. Negative T values indicate negative correlation between LFF amplitude value and E/N score.
DISCUSSION
In this study, two of Eysenck's personality dimensions, extraversion and neuroticism, are of particular interest because their biological bases have been fully clarified and achieved near universal acceptance [Matthews and Gilliland, 1999]. The neuropsychology of the third dimension of psychoticism, in contrast, has not been worked out in detail. Eysenck has suggested that psychoticism is inversely related to serotonergic function [Eysenck, 1992], but, subsequently, psychoticism is linked to dopamine [Eysenck, 1997]. The above may imply that considerable debate persists regarding the importance, nature, and definition of the psychoticism. Here we aimed to test Eysenck's predication for extraversion and neuroticism by examining the association between the two basic traits and LFF amplitude at two different frequency bands (Slow‐4 and Slow‐5 bands). We found that extraversion and neuroticism were correlated with LFF amplitude at Slow‐4 and Slow‐5 bands in specific regions, respectively. These findings shed light on the important relationships between LFF amplitude and Eysenck's personality, and further suggest the relationships are relied on specific frequency bands.
LFF Amplitude at Slow‐5 Frequency Band Correlated With E and N
The Slow‐5, relative to Slow‐4, has the higher power and is more dominant within prefrontal, parietal, and occipital cortex, especially within several default‐mode regions (the posterior cingulate cortex (PCC)/ PCu, MPFC and inferior parietal lobule (IPL)) [Baria et al., 2011; Han et al., 2010; Zuo et al., 2010]. In this study, we found LFF amplitude at Slow‐5 was positively correlated with extraversion in the MPFC and PCu. A large body of neuroimaging evidence has confirmed that the MPFC involved in affective, social and self‐referential processes is associated with extraversion [Blankstein et al., 2009; Johnson et al., 1999; Kumari et al., 2004; Sheng et al., 2010; Sutin et al., 2009; Wei et al., 2011; Wright et al., 2006, 2007]. For example, Johnson et al. [1999] indicated that extraverts, relative to introverts, showed decreased rCBF in the MPFC Kumari et al. [2004] discovered that measures of extraversion were negatively related to resting fMRI signals in prefrontal cortex. Besides, EEG studies have validated extraversion was positivity correlated with frontal alpha activity [Hagemann et al., 2009; Tran et al., 2001, 2006]. Together, those findings support Eysenck's predication for extraverts with lower arousal level in the frontal lobe [Eysenck, 1967, 1990; Tran et al., 2001, 2006]. The current finding shows that individuals with high extraversion scores have increased LFF amplitude in the MPFC, which disagree with the aforementioned argument. This conflicting result could partly be due to experimental situations and the frequency bands chosen for correlation analysis. According to EEG studies, the inverse relation between extraversion and cortical arousal is observed when subjects are instructed to open and close their eyes [Gale, 1973, 1981; Tran et al., 2001]. Nevertheless, participants in this study are instructed simply to rest with their eyes closed. The difference in experimental conditions might contribute to the conflicting result, since LFF amplitude has been demonstrated to be significantly different in specific regions between eyes open and eyes closed [Yan et al., 2009; Yang et al., 2007]. Therefore, experimental condition with repeated opening and closing of the eyes may be more reasonable for investigating relationship between LFF amplitude and extraversion. The second possible source of confounding could be the frequency bands applied for correlation analysis. The oscillations of the brain cover a wide range of frequencies and each of these oscillatory bands is generated by different mechanisms and has different physiological functions [Buzsáki and Draguhn, 2004; Penttonen and Buzsáki, 2003]. Thus, extraversion might be negatively related to LFF amplitude in frontal lobe at other frequency bands. To further test Eysenck's assumption of extraversion in future works, subjects would be instructed to rest with their eyes open and closed and multiple frequency bands would be chosen for correlation analysis. In addition, LFF amplitude at Slow‐5 was correlated positively with extraversion in the PCu, consistent with early findings [Kunisato et al., 2011; Ryman et al., 2011]. The PCu has effect on one's personality characteristics probably due to its functional role in self‐related mental representations during rest [Cavanna and Trimble, 2006]. In combination, the relationships between LFF amplitude at Slow‐5 and extraversion were found within brain regions (MPFC and PCu) overlapping the so‐called default node network, which suggest that individual differences in default network activity might be able to explain differences in personality.
Our results also showed that LFF amplitude at Slow‐5 was correlated positively with neuroticism in the right PreCG. The emerging neuroanatomy study has demonstrated brain morphometry varies with neuroticism scores in the PreCG [DeYoung et al., 2010]. It is noteworthy that the PreCG is located in posterior portion of the frontal lobe [Kido et al., 1980], and the frontal asymmetry involved in emotion dimensions of valence (pleasant‐unpleasant) has been elucidated in previous studies [Heller, 1990, 1993]. Greater left frontal lateralization is associated with an increase in tendency towards pleasant affect, while greater right frontal lateralization with an increase in tendency towards unpleasant affect [Canli et al., 1998; Heller, 1990, 1993; Mathersul et al., 2008; Tomarken, et al., 1992]. These arguments provide a framework for exploring relationship between neuroticism and patterns of brain activity in the frontal cortex, based on the literature linking neuroticism with unpleasant affect [Larsen and Diener, 1992; McCrae and Costa, 1999]. To date, several studies have demonstrated that neuroticism is correlated with greater right frontal activity, whereas emotional stability with greater left frontal activity [Kim et al., 2008; Minnix and Kline, 2004; Schmidtke and Heller, 2004; Tran et al., 2006; Wei et al., 2011]. In this study, the association of LFF amplitude and neuroticism detected in frontal regions provides further evidence for neuroticism with lateralized frontal activation.
LFF amplitude at Slow‐4 Frequency Band Correlated With E and N
Compared to Slow‐5, Slow‐4 exhibiting less power is localized more within temporal cortex and subcortical structures [Baria et al., 2011; Han et al., 2010; Zuo et al., 2010]. The negative relationship between extraversion and LFF amplitude at Slow‐4 in the HIP found here confirms earlier findings with PET [Johnson et al., 1999] and supports the influential arousal hypothesis [Eysenck, 1983, 1967; Gray, 1970, 1972]. The HIP, along with the septum, forms the neural substrate of the “behavioral inhibition system,” which is initiated during anxiety‐provoking or conflict situations [Gray, 1982]. Previous investigation has provided considerable evidence for linking the HIP with anxiety‐related personality traits [DeYoung et al., 2010; Kalisch et al., 2005; Naghavi et al., 2009; Whittle et al., 2008; Yamasue et al., 2007]. Harm avoidance (HA), as a frequently measured anxiety‐related trait, was related to reduced regional gray matter volume in the HIP [Yamasue, et al., 2007]. Individuals with high neuroticism score, enhancing susceptibility to anxiety states, also represented smaller hippocampal volume [DeYoung et al., 2010]. Besides, the role of HIP in depression and stress has been elaborated in former studies [Bremner et al., 2000; Hasler et al., 2004; Pitman et al., 2001].The above has reaffirmed the involvement of HIP in negative affect processing. In this study, increased LFF amplitude in the HIP may be related to an enhanced processing of negative cues, and thereby with an increased negative affect that introverts are known for.
Significant negative relation between LFF amplitude at Slow‐4 and neuroticism was detected in the bilateral STC. There has been PET research demonstrating negative correlation between neuroticism and rCMRglu in the left STC [Deckersbach et al., 2006], and a fMRI study showing strong inverse relation between neuroticism and activity in bilateral superior/middle temporal cortex in response to threat of electric shocks [Kumari et al., 2007]. The temporal cortex, according to former research, plays an important part in emotional process [Canli et al., 2001; Fredrikson et al., 1995; Hagemann et al., 1999; Lane et al., 1997; Wik et al., 1993]. The STC implicated in affective process may thereby have the relationship with neuroticism. The function of the STC is very complex and may vary depending on the nature of network co‐activations with different regions in the frontal cortex and medial‐temporal lobe [Hein and Knight, 2008]. Further studies, thus, should aim to clarify the underlying mechanism of STC associated with neuroticism in depth.
CONCLUSION
In summary, we investigated the associations between Eysenck's personality and LFF amplitude at two different frequency bands (Slow‐4 and Slow‐5 bands). Positive correlation for LFF amplitude at Slow‐5 and extraversion was found in the MPFC and PCu, implying a possible link between default network activity and personality traits. Regarding LFF amplitude at Slow‐5 associated with neuroticism, positive correlation detected in the right frontal region, providing further evidence for the hypothesis of neuroticism and frontal asymmetry. In addition, LFF amplitude at Slow‐4 was correlated negatively with extraversion and neuroticism in the HIP and STC respectively, supporting Eysenck's assumption that extraversion with lower cortical arousal, also suggesting brain structures involved in affective process accounting for modulation and shaping of personality. Together, our findings strongly suggest that personality dimensions of extraversion and neuroticism are associated with LFF amplitude dynamic at specific frequency bands, and contribute to a growing literature which suggests that human personality traits are based on individual difference in brain function.
REFERENCES
- Adelstein JS, Shehzad Z, Mennes M, DeYoung CG, Zuo XN, Kelly C, Margulies DS, Bloomfield A, Gray JR, Castellanos FX (2011): Personality is reflected in the brain's intrinsic functional architecture. PLoS one 6:e27633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston K (2005): Unified segmentation. NeuroImage,26:839–851. [DOI] [PubMed] [Google Scholar]
- Baria AT, Baliki MN, Parrish T, Apkarian AV (2011): Anatomical and functional assemblies of brain BOLD oscillations. J Neurosci 31:7910–7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin F, Haughton V, Hyde J (1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34:537–541. [DOI] [PubMed] [Google Scholar]
- Blankstein U, Chen JYW, Mincic AM, McGrath PA, Davis KD (2009): The complex minds of teenagers: Neuroanatomy of personality differs between sexes. Neuropsychologia 47:599–603. [DOI] [PubMed] [Google Scholar]
- Block J (2010): The five‐factor framing of personality and beyond: Some ruminations. Psychol Inquiry 21:2–25. [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS (2000): Hippocampal volume reduction in major depression. Am J Psychiatry 157:115–117. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Draguhn A (2004): Neuronal oscillations in cortical networks. Science 304:1926. [DOI] [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Glover G, Gabrieli JDE (1998): Hemispheric asymmetry for emotional stimuli detected with fMRI. Neuroreport 9:3233. [DOI] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Desmond JE, Kang E, Gross J, Gabrieli JDE (2001): An fMRI study of personality influences on brain reactivity to emotional stimuli. Behav Neurosci 115:33. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR (2006): The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129:564. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Miller KK, Klibanski A, Fischman A, Dougherty DD, Blais MA, Herzog DB, Rauch SL (2006): Regional cerebral brain metabolism correlates of neuroticism and extraversion. Depression and anxiety,23:133–138. [DOI] [PubMed] [Google Scholar]
- DeYoung CG, Gray JR (2009): 20 Personality neuroscience: Explaining individual differences in affect, behaviour and cognition. The Cambridge Handbook of Personality Psychology 323.
- DeYoung CG, Hirsh JB, Shane MS, Papademetris X, Rajeevan N, Gray JR (2010): Testing predictions from personality neuroscience. Psychol Sci 21:820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Ghaffari M, Curchack J, Reiss P, Hyde C, Vannucci M, Petkova E, Klein DF, Castellanos FX (2008): Decomposing intra‐subject variability in children with attention‐deficit/hyperactivity disorder. Biol Psychiatry 64:607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck H (1983): Psychophysiology and personality: Extraversion, neuroticism and psychoticism. Physiol Correlates Hum Behav 3:13–30. [Google Scholar]
- Eysenck HJ (1967): The biological basis of personality. Transaction Pub 199:1031–1034. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ (1990): Biological dimensions of personality. pp 244–276.
- Eysenck H (1991): Manual of the Eysenck Personality Scales (EPS Adult).London:Hodder & Stoughton. [Google Scholar]
- Eysenck HJ (1992): The definition and measurement of psychoticism. Personality and Individual Differences 13:757–785. [Google Scholar]
- Eysenck HJ (1997): Personality and experimental psychology: The unification of psychology and the possibility of a paradigm. J Personality Social Psychol 73:1224. [Google Scholar]
- Eysenck HJ, Eysenck MW (1985): Personality and Individual Differences: A Natural Science Approach.Plenum Press; New York. [Google Scholar]
- Fox MD, Raichle ME (2007): Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Rev Neurosci 8:700–711. [DOI] [PubMed] [Google Scholar]
- Fredrikson M, Wik G, Annas P, Fricson K, Stoneelander S (1995): Functional neuroanatomy of visually elicited simple phobic fear: Additional data and theoretical analysis. Psychophysiology 32:43–48. [DOI] [PubMed] [Google Scholar]
- Gale A (1973): New Approaches in Psychological Measurement.New York:Wiley; pp211–256. [Google Scholar]
- Gale A (1981): EEG studies of extraversion‐introversion: What's the next step. Dimensions of Personality: Papers in Honour of HJ Eysenck. pp 181–207. [Google Scholar]
- Gale A, Edwards J, Morris P, Moore R, Forrester D (2001): Extraversion‐introversion, neuroticism‐stability, and EEG indicators of positive and negative empathic mood. Personality and Individual Differences 30:449–461. [Google Scholar]
- Gardini S, Cloninger C, Venneri A (2009): Individual differences in personality traits reflect structural variance in specific brain regions. Brain Res Bull 79:265–270. [DOI] [PubMed] [Google Scholar]
- Gray JA (1970): The psychophysiological basis of introversion‐extraversion. Behav Res Therapy 8:249–266. [DOI] [PubMed] [Google Scholar]
- Gray JA (1972): The psychophysiological basis of introversion‐extraversion: A modification of Eysenck's theory. Biol Bases Individual Behav 8:182–205. [Google Scholar]
- Gray JA (1982): The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo‐hippocampal System.Clarendon Press:Oxford. [Google Scholar]
- Hagemann D, Naumann E, Lürken A, Becker G, Maier S, Bartussek D (1999): EEG asymmetry, dispositional mood and personality. Personality Individual Diff 27:541–568. [Google Scholar]
- Hagemann D, Hewig J, Walter C, Schankin A, Danner D, Naumann E (2009): Positive evidence for Eysenck's arousal hypothesis: A combined EEG and MRI study with multiple measurement occasions. Personality Individual Diff 47:717–721. [Google Scholar]
- Hahn T, Dresler T, Ehlis AC, Pyka M, Dieler AC, Saathoff C, Jakob PM, Lesch KP, Fallgatter AJ (2011): Randomness of resting‐state brain oscillations encodes Gray's personality trait. NeuroImage 59:1842–1845 [DOI] [PubMed] [Google Scholar]
- Han Y, Wang J, Zhao Z, Min B, Lu J, Li K, He Y, Jia J (2010): Frequency‐dependent changes in the amplitude of low‐frequency fluctuations in amnestic mild cognitive impairment: A resting‐state fMRI study. NeuroImage 55:287–295 [DOI] [PubMed] [Google Scholar]
- Han Y, Lui S, Kuang W, Lang Q, Zou L, Jia J (2012): Anatomical and functional deficits in patients with amnestic mild cognitive impairment. PLoS one 7:e28664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS (2004): Discoveringendophenotypes for major depression. Neuropsychopharmacology 29:1765–1781. [DOI] [PubMed] [Google Scholar]
- Hein G, Knight RT (2008): Superior temporal sulcus—It's my area: Or is it? J Cogn Neurosci 20:2125–2136. [DOI] [PubMed] [Google Scholar]
- Heller W (1990): The neuropsychology of emotion: Developmental patterns and implications for psychopathology. Psychol Biol Approach Emotion 1:67–211. [Google Scholar]
- Heller W (1993): Neuropsychological mechanisms of individual differences in emotion, personality, and arousal. Neuropsychology 7:476. [Google Scholar]
- Hoptman MJ, Zuo XN, Butler PD, Javitt DC, D'Angelo D, Mauro CJ, Milham MP (2010): Amplitude of low‐frequency oscillations in schizophrenia: A resting state fMRI study. Schizophrenia Res 117:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DL, Wiebe JS, Gold SM, Andreasen NC, Hichwa RD, Watkins GL, Ponto LLB (1999): Cerebral blood flow and personality: A positron emission tomography study. Am J Psychiatry 156:252–257. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Schubert M, Jacob W, Keβler MS, Hemauer R, Wigger A, Landgraf R, Auer DP (2005): Anxiety and hippocampus volume in the rat. Neuropsychopharmacology 31:925–932. [DOI] [PubMed] [Google Scholar]
- Kehoe EG, Toomey JM, Balsters JH, Bokde ALW (2011): Personality modulates the effects of emotional arousal and valence on brain activation. Social Cogn Affect Neurosci 11–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido D, LeMay M, Levinson A, Benson W (1980): Computed tomographic localization of the precentral gyrus. Radiology 135:373–377. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hwang JH, Park HS, Kim SE (2008): Resting brain metabolic correlates of neuroticism and extraversion in young men. Neuroreport 19:883. [DOI] [PubMed] [Google Scholar]
- Kumari V, Williams SCR, Gray JA (2004): Personality predicts brain responses to cognitive demands. J Neurosci 24:10636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Das M, Wilson GD, Goswami S, Sharma T (2007): Neuroticism and brain responses to anticipatory fear. Behav Neurosci 121:643. [DOI] [PubMed] [Google Scholar]
- Kunisato Y, Okamoto Y, Okada G, Aoyama S, Nishiyama Y, Onoda K, Yamawaki S (2011): Personality traits and the amplitude of spontaneous low‐frequency oscillations during resting state. Neurosci Lett 492:109–113 [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, Davidson RJ, Schwartz GE (1997): Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia 35:1437–1444. [DOI] [PubMed] [Google Scholar]
- Larsen RJ, Diener E (1992): Promises and problems with the circumplex model of emotion In: Clark MS, editor. Review of Personality and Social Psychology Emotion. 13:25–59. [Google Scholar]
- Liang X, Wang J, Yan C, Shu N, Xu K, Gong G, He Y (2012): Effects of different correlation metrics and preprocessing factors on small‐world brain functional networks: A resting‐state functional MRI study. PLoS one 7:e32766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathersul D, Williams LM, Hopkinson PJ, Kemp AH (2008): Investigating models of affect: Relationships among EEG alpha asymmetry, depression, and anxiety. Emotion 8:560. [DOI] [PubMed] [Google Scholar]
- Matthews G, Gilliland K (1999): The personality theories of HJ Eysenck and JA Gray: A comparative review. Personality Individual Diff 26:583–626. [Google Scholar]
- McCrae RR, Costa PT Jr (1999): A five‐factor theory of personality. Handbook Personality: Theory Res 2:139–153. [Google Scholar]
- Minnix JA, Kline JP (2004): Neuroticism predicts resting frontal EEG asymmetry variability. Personality Individual Diff 36:823–832. [Google Scholar]
- Naghavi HR, Lind J, Nilsson LG, Adolfsson R, Nyberg L (2009): Personality traits predict response to novel and familiar stimuli in the hippocampal region. Psychiatry Res: Neuroimag 173:94–99. [DOI] [PubMed] [Google Scholar]
- O'Gorman R, Kumari V, Williams S, Zelaya F, Connor S, Alsop D, Gray J (2006): Personality factors correlate with regional cerebral perfusion. NeuroImage 31:489–495. [DOI] [PubMed] [Google Scholar]
- Penttonen M, Buzsáki G (2003): Natural logarithmic relationship between brain oscillators. Thalamus Relat Syst 2:145–152. [Google Scholar]
- Pitman RK, Shin LM, Rauch SL (2001): Investigating the pathogenesis of posttraumatic stress disorder with neuroimaging. J Clin Psychiatry 62:47–54. [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2011): Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian M, Wu G, Zhu R, Zhang S (2000): Development of the revised Eysenck personality questionnaire short scale for Chinese (EPQ‐RSC). Acta Psychol Sin 32:317–323. [Google Scholar]
- Ryman SG, Gasparovic C, Bedrick EJ, Flores RA, Marshall AN, Jung RE (2011): Brain biochemistry and personality: A magnetic resonance spectroscopy study. PLoS one 6:e26758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, Gur RC, Gur RE (2012): Impact of in‐scanner head motion on multiple measures of functional connectivity: Relevance for studies of neurodevelopment in youth. NeuroImage 60:623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtke JI, Heller W (2004): Personality, affect and EEG: Predicting patterns of regional brain activity related to extraversion and neuroticism. Personality Individual Diff 36:717–732. [Google Scholar]
- Sheng T, Gheytanchi A, Aziz‐Zadeh L (2010): Default network deactivations are correlated with psychopathic personality traits. PLoS one 5:e12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin A, Beason‐Held L, Resnick S, Costa P (2009): Sex differences in resting‐state neural correlates of openness to experience among older adults. Cerebral Cortex 19:2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Wheeler RE, Doss RC (1992): Individual differences in anterior brain asymmetry and fundamental dimensions of emotion. J Personality Social Psychol 62:676. [DOI] [PubMed] [Google Scholar]
- Tran Y, Craig A, McIsaac P (2001): Extraversion‐introversion and 8‐13 Hz waves in frontal cortical regions. Personality Individual Diff 30:205–215. [Google Scholar]
- Tran Y, Craig A, Boord P, Connell K, Cooper N, Gordon E (2006): Personality traits and its association with resting regional brain activity. Int J Psychophysiol 60:215–224. [DOI] [PubMed] [Google Scholar]
- Van Dijk KRA, Sabuncu MR, Buckner RL (2011): The influence of head motion on intrinsic functional connectivity MRI. NeuroImage 59:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Duan X, Yang Y, Liao W, Gao Q, Ding J, Zhang Z, Zeng W, Li Y, Lu G (2011): The synchronization of spontaneous BOLD activity predicts extraversion and neuroticism. Brain Res 1419:68–75. [DOI] [PubMed] [Google Scholar]
- Whittle S, Yücel M, Fornito A, Barrett A, Wood SJ, Lubman DI, Simmons J, Pantelis C, Allen NB (2008): Neuroanatomical correlates of temperament in early adolescents. J Am Acad Child Adolesc Psychiatry 47:682–693. [DOI] [PubMed] [Google Scholar]
- Wik G, Fredrikson M, Ericson K, Eriksson L, Stone‐Elander S, Greitz T (1993): A functional cerebral response to frightening visual stimulation. Psychiatry Res: Neuroimaging 50:15–24. [DOI] [PubMed] [Google Scholar]
- Wright CI, Williams D, Feczko E, Barrett LF, Dickerson BC, Schwartz CE, Wedig MM (2006): Neuroanatomical correlates of extraversion and neuroticism. Cerebral Cortex 16:1809. [DOI] [PubMed] [Google Scholar]
- Wright C, Feczko E, Dickerson B, Williams D (2007): Neuroanatomical correlates of personality in the elderly. NeuroImage 35:263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasue H, Abe O, Suga M, Yamada H, Inoue H, Tochigi M, Rogers M, Aoki S, Kato N, Kasai K (2007): Gender‐common and‐specific neuroanatomical basis of human anxiety‐related personality traits. Cerebral Cortex 18:46–52. [DOI] [PubMed] [Google Scholar]
- Yan C, Liu D, He Y, Zou Q, Zhu C, Zuo X, Long X, Zang Y (2009): Spontaneous brain activity in the default mode network is sensitive to different resting‐state conditions with limited cognitive load. PLoS one 4:e5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Long XY, Yang Y, Yan H, Zhu CZ, Zhou XP, Zang YF, Gong QY (2007): Amplitude of low frequency fluctuation within visual areas revealed by resting‐state functional MRI. NeuroImage 36:144–152. [DOI] [PubMed] [Google Scholar]
- Zang Y, Yong H, Chao‐Zhe Z, Qing‐Jiu C, Man‐Qiu S, Meng L, Li‐Xia T, Tian‐Zi J, Yu‐Feng W (2007): Altered baseline brain activity in children with ADHD revealed by resting‐state functional MRI. Brain Dev 29:83–91. [DOI] [PubMed] [Google Scholar]
- Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, Wang YF, Zang YF (2008): An improved approach to detection of amplitude of low‐frequency fluctuation (ALFF): For resting‐state fMRI: fractional ALFF. J Neurosci Meth 172:137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG, Klein DF, Castellanos FX, Biswal BB, Milham MP (2010): The oscillating brain: Complex and reliable. NeuroImage 49:1432–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]


