Abstract
Background and objectives: The thalamus exerts a pivotal role in pain processing and cortical excitability control, and migraine is characterized by repeated pain attacks and abnormal cortical habituation to excitatory stimuli. This work aimed at studying the microstructure of the thalamus in migraine patients using an innovative multiparametric approach at high‐field magnetic resonance imaging (MRI). Design: We examined 37 migraineurs (22 without aura, MWoA, and 15 with aura, MWA) as well as 20 healthy controls (HC) in a 3‐T MRI equipped with a 32‐channel coil. We acquired whole‐brain T1 relaxation maps and computed magnetization transfer ratio (MTR), generalized fractional anisotropy, and T2* maps to probe microstructural and connectivity integrity and to assess iron deposition. We also correlated the obtained parametric values with the average monthly frequency of migraine attacks and disease duration. Results: T1 relaxation time was significantly shorter in the thalamus of MWA patients compared with MWoA (P < 0.001) and HC (P ≤ 0.01); in addition, MTR was higher and T2* relaxation time was shorter in MWA than in MWoA patients (P < 0.05, respectively). These data reveal broad microstructural alterations in the thalamus of MWA patients compared with MWoA and HC, suggesting increased iron deposition and myelin content/cellularity. However, MWA and MWoA patients did not show any differences in the thalamic nucleus involved in pain processing in migraine. Conclusions: There are broad microstructural alterations in the thalamus of MWA patients that may underlie abnormal cortical excitability control leading to cortical spreading depression and visual aura. Hum Brain Mapp 35:1461–1468, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: multiparametric MRI, structural MRI, migraine
INTRODUCTION
The thalamus plays a central role in pain processing [Borsook, 2010] and in cortical excitability control [Poulet et al., 2012], and a number of studies have suggested that thalamic abnormalities may contribute to migraine pathophysiology. Migraine is in fact a pathological condition characterized by repeated pain attacks and abnormal cortical excitability [Akerman et al., 2010]. In a murine model, Burstein et al. 1999 showed that sensitization of thalamic neurons converging sensory impulses from the cranial meninges and extracephalic skin mediates transformation of headache into whole‐body allodynia/hyperalgesia, which is often described during a migraine attack. Moreover, Noseda et al. 2010 demonstrated that the posterior thalamus acts as an integration modulatory center of nociceptive sensory neurons from the dura and photosensitive retinal pathways, explaining why pain perception in migraine may be exacerbated by light. In addition, a recent experimental study [Eikermann‐Haerter et al., 2001] suggested that during migraine attacks, the thalamus may be affected by cortical spreading depression (CSD), a wave of depolarization thought to underlie the development of migraine with aura [Hadjikhani et al., 2010]. In this context, thalamic abnormalities due to repeated migraine attacks might contribute to additional increased cortical excitability.
In humans, electrophysiological studies indicate that migraine can be characterized interictally by a functional disconnection of the thalamus, leading to increased gamma‐band oscillations in the cortex and to reduced cortical habituation [Coppola et al., 2007]. In addition, functional magnetic resonance imaging (MRI) of migraineurs has shown the activation of thalamus contralateral to pain in acute migraine [Afridi et al., 2011].
Moreover, pharmacological studies have demonstrated that migraine preventive treatments such as propanolol [Shields and Goadsby, 2005], GABA inhibitors [Andreou et al., 2010], and triptans [Shields and Goadsby, 2006] modulate thalamic activity.
In light of these different findings, we aimed at investigating the presence of structural differences between the thalamus in migraineurs with aura (MWA), migraineurs without aura (MWoA), and healthy controls (HC) using multiparametric MRI at high magnetic resonance field (3 T). We used four different contrasts [quantitative T1, magnetization transfer ratio (MTR), quantitative T2*, and generalized fractional anisotropy (GFA)] to assess the presence of broad microstructural differences among groups (quantitative T1) and of specific biological alterations such as macromolecular differences (i.e., myelin and cell membrane proteins, MTR), iron deposition (quantitative T2*), and axonal integrity (GFA). Microstructural changes such as cellular proliferation (astrocytosis)14, iron deposition,16 and loss of axonal integrity [DaSilva et al., 1998] have been previously described in the migraineurs brain as a consequence of repeated CSD [Kraig et al., 1991], hypoperfusion [Petito et al., 1991], and repetitive stimulation of specific structures and pathways [DaSilva et al., 1998; Kruit et al., 2009; Welch et al., 2001].
Our hypothesis was that the thalamus of migraine patients would exhibit different microstructural features compared with that of control subjects. In particular, we expected that both MWA and MWoA patients would show similar abnormalities in the ventro‐postero‐medial thalamic nucleus, which is involved in pain pathophysiology of and that MWA subjects would exhibit greater microstructural abnormalities in other thalamic nuclei.
METHODS
Twenty‐two MWoA patients (19:3, F:M; age 39 ± 13, mean ± SD), 15 MWA patients (10:5, F:M; age 38 ± 11, mean ± SD), and 20 age‐matched HC (12:8, F:M; age 37 ± 12, mean ± SD) were enrolled in the study. Mean disease duration for MWoA patients was as follows: 24 ± 15 years, mean ± SD and for MWA: 21 ± 12 years, mean ± SD; attack frequency per month for MWoA patients was 6 ± 7, mean ± SD and for MWA patients was 4 ± 6, mean ± SD; number of migraine days for MWoA patients was 76 ± 107, mean ± SD and for MWA patients was 35 ± 31, mean ± SD. All MWA patients suffered from visual aura. One patient had in addition one episode of sensory aura and one episode of dysarthria. MWA, MWoA, and controls did not exhibit any difference in age (P > 0.6).
All participants underwent structural MRI at 3 T (Magnetom Trio, a Tim System, Siemens Healthcare, Erlangen, Germany) using a 32‐channel head coil.
Migraineurs were diagnosed based on the IHS criteria 2005. All patients were migraine free for at least 3 days before the MRI and had no prophylactic treatment for at least 6 months. Five patients with aura and seven patients without aura had at least one preventive treatment in the past for less than 1 year. Patients did not suffer from any other neurological or psychiatric disease, and had normal neurological examination. HCs had no history of migraine or other headache types, did not suffer of other neurological or psychiatric disease, and did not have any family history of migraine nor followed any pharmacological treatment in the 3 months before the study.
The scanning protocol included a MPRAGE scan [Jack et al., 2010] (TR/TE = 2,400/3 ms, TI: 900 ms, voxel size = 1 × 1 × 1.2 mm3, FoV = 256 × 240 × 212 mm, iPAT = 3, and ST = 3:47 min), a MP2RAGE acquisition [Marques et al., 2010] (TR/TE = 5,000/3 ms, TI1/TI2 = 700/2,500 ms, FA = 4°, voxel size = 1 × 1 × 1.2 mm3, FoV = 256 × 240 × 212 mm, iPAT = 3, and ST = 8:22 min), and magnetization transfer imaging (MTI) using an eight‐echo FLASH acquisition (TR = 48 ms, TE ranging from 2.3 to 23.1 ms, voxel size = 2 × 2 × 2 mm3, FoV = 256 × 256 × 192 mm, eight echoes, and ST = 2 × 3 min). All whole‐brain anatomical scans were performed in sagittal orientation. Diffusion spectrum imaging (DSI) was acquired as previously reported [Granziera et al., 2001] (TR/TE = 6,600/138 ms, FoV = 212 × 212 mm, 34 slices, 2.2 × 2.2 × 3 mm resolution, 258 diffusion directions, b = 8,000 s/mm2, and ST = 25 min).
T1 maps were obtained from the MP2RAGE acquisition [Marques et al., 2010]. MTR was computed from the MTI acquisition as follows: MTR = (M 0 − M T)/M 0*100, where M T and M 0 are, respectively, the image intensities acquired with and without magnetization transfer saturation pulse. T2* maps were calculated from the MTI acquisition by least‐squares fitting of an exponential decay to the multiple‐echo M 0 experimental data. GFA maps were calculated according to Tuch 2004. DSI scans from one patient with MWA, one patient with MWoA, and three controls could not be used because of motion artifacts.
Subsequently, T1, MTR, and T2* maps were aligned to the MPRAGE volume by a rigid‐body transform with six degrees of freedom and mutual information cost function using ELASTIX [Klein et al., 2010]. GFA maps were registered to MPRAGE images using an affine transformation with 12 degrees of freedom [Klein et al., 2010].
The thalamus was segmented on the right (R) and left (L) side according to Roche et al. 2011 (Fig. 1) and volumetric assessment was performed. T1, MTR, GFA, and T2* means were separately calculated for the thalami in the right and left hemispheres. Segmentation quality was manually confirmed by one neurologist and fine‐tuned when required.
Figure 1.
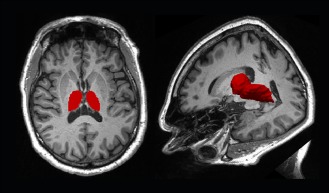
Segmentation of the thalamus as performed using the in‐house software [Roche et al., 2011]. A 2D cross‐section (left), a 3D surface rendering (right) of a thalamus segmentation in one healthy control is shown against an axial (right) and axial‐coronal‐sagittal (left) background of an anatomical MPRAGE data.
Statistical analysis was performed with R‐project software (http://www.r-project.org/) using permutation‐based univariate t‐tests and multivariate Hotelling tests [Prokhorov, 2001] with age and gender as covariates. Correction for family‐wise error rate was performed for multiple comparisons.
To map the specific location of the differences in the thalamus, we calculated whole‐brain pixel‐wise statistical parametric maps (T1, MTR, GFA, and T2*) performing a nonparametric two‐sample permutation test [10,000 permutations, using randomize; part of the FMRIB Software Library (FSL 4.X http://www.fmrib.ox.ac.uk/fsl/randomise/index.html)]. First, we registered each subject MPRAGE to the Montreal Neurological Institute (MNI) atlas [Mazziotta et al., 1995] with nonlinear registration (FNIRT) and then we applied the Monte Carlo approach to generate random permutations [Nichols and Holmes, 2002]. Although this approach is less powerful than parametric tests, it relies on minimal assumptions of the data and can be applied to parameters that do not follow a Gaussian distribution (like fractional anisotropy or in our case GFA) [Jones et al., 2005; Smith et al., 2006].
Control for multiple comparisons at voxel level was done using threshold‐free cluster enhancement (TFCE), with a corrected P < 0.05. TFCE is a newly proposed method to enhance cluster‐like structures in an image without having to define an initial cluster‐forming threshold or carry out a large amount of data [Smith and Nichols, 2009].
Thalamic nuclei were identified using the respective coordinates in the MNI atlas and visualized applying a tridimensional atlas of the human thalamus, generated from multiple histologic data [Krauth et al., 2010].
RESULTS
Univariate analysis on the thalamus revealed that: (i) mean T1 relaxation time in MWA was significantly shorter than in MWoA patients (left and right thalamus: P < 0.001) and HC (left thalamus: P = 0.07, right thalamus: P ≤ 0.01, Fig. 2); (ii) mean MTR was higher in MWA compared with MWoA and HC in both the L and R thalamus (P < 0.05, respectively, Fig. 2); (iii) mean T2* was significantly shorter in MWA patients compared with MWoA (P < 0.05, respectively, Fig. 2); (iv) and mean GFA did not show any significant differences (P > 0.1).
Figure 2.
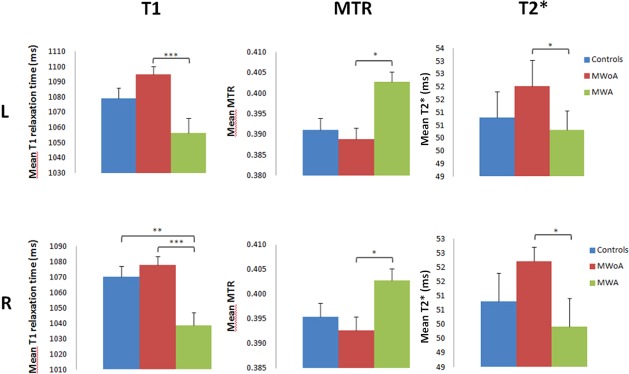
T1 relaxation time, MTR, and T2* relaxation time averages in left (L) and right (R) thalamus of MWA, MWoA, and control patients. ***P < 0.001, **P ≤ 0.01, and *P < 0.05.
Multivariate analysis based on T1, MTR, T2*, and GFA showed a significant difference between MWA and HC in the left thalamus (P < 0.05).
Whole‐brain pixel‐wise statistical maps revealed the localization of T1 changes: MWA patients had significantly shorter T1 values compared with HC and MWoA patients (Figs. 3 and 4) in a number of thalamic nuclei including the anterior nuclei (a), the laterodorsal (ld), and mediodorsal (md) nuclei as well as the ventrolateral (vl) nuclei, ventral‐postero‐lateral (vpl) nucleus, and the posterior group (pulvinar (p) and the lateroposterior (lp) nucleus].
Figure 3.
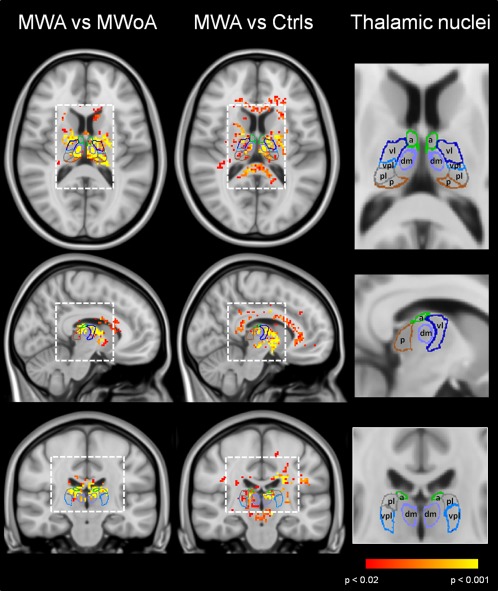
T1 statistical maps of MWA patients vs. HC. P < 0.05 was considered significant. Statistical maps were coregistered to the MNI atlas and to a tridimensional atlas of the human thalamus, generated from multiple histologic data [Krauth et al., 2010]. A, anterior nucleus; vl, ventrolateral nucleus; lp, lateroposterior nucleus; md, mediodorsal nucleus; p, pulvinar; vpl, ventro‐postero‐lateral nucleus.
Figure 4.
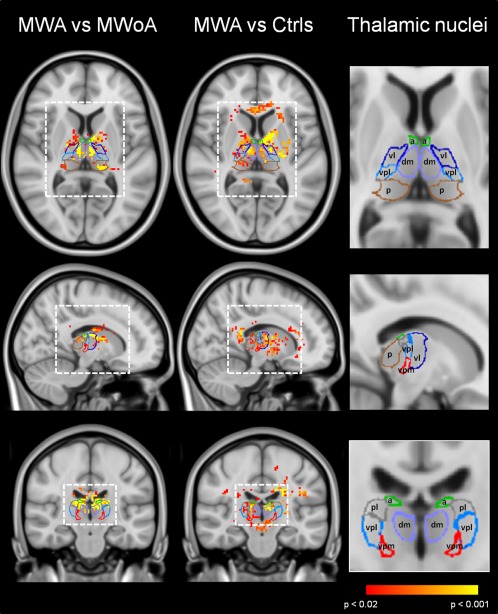
T1 statistical maps of MWA patients vs. HC. P < 0.05 was considered significant. Statistical maps were coregistered to the MNI atlas and to a tridimensional atlas of the human thalamus, generated from multiple histologic data [Krauth et al., 2010]. A, anterior nucleus; vl, ventrolateral nucleus; lp, lateroposterior nucleus; md, mediodorsal nucleus; p, pulvinar; vpl, ventro‐postero‐lateral nucleus; vpm, ventro‐postero‐medial nucleus.
MWA patients showed significantly shorter T1 in the ventral‐postero‐medial (vpm) and vpl nuclei if compared to HC but not with MWoA patients.
Significance was not reached in the whole‐brain MTR, GFA, and T2* statistical maps.
Significant correlations were neither found between T1, MTR, and GFA values and migraine frequency nor with migraine duration or days of migraine in MWA and MWoA patients.
As to the thalamus volume, there were no differences in the thalamus volume between migraine patients and HC (P > 0.1).
DISCUSSION
Our results show that the thalamus of MWA patients is structurally different than the thalamus in MWoA and HC. T1 relaxation time was significantly shorter in the thalamus of MWA patients compared with MWoA and HC. In addition, mean MTR showed higher values and T2* relaxation time was shorter in MWA compared with MWoA patients.
Multiparametric MRI may help revealing the biological substrate of brain alterations. In this study, we used four different parametric maps (T1, MTR, T2*, and GFA) to study the microstructural integrity of the thalamus in migraine patients and healthy subjects. We applied both a region‐of‐interest‐based analysis and a whole‐brain voxel‐based statistics to obtain complementary information. The first method has the advantage of being more sensitive and independent from spatial normalization issues and of atlas choice, whereas the second technique is more accurate to identify the spatial location of the observed differences [Astrakas and Argyropoulou, 2011]. Both methods revealed significant alterations of the thalamic microstructure of MWA patients compared with the two other groups.
We found significantly shorter T1 relaxation times in the thalamus of MWA patients compared with MWoA patients and HC. This finding may relate to two potential underlying phenomena: (i) increased cellular structure (neuronal and/or glial cells) and/or (ii) iron accumulation [Gelman et al., 2009; Rooney et al., 2007].
To investigate the biological substrate of the observed T1 differences, we analyzed T2* maps in our cohort of patients and HC and we found that MWA patients exhibited significantly shorter T2* relaxation times in the thalamus compared with MWoA patients, indicating a relatively higher iron accumulation [Helms et al., 1993]. Previous reports have suggested iron deposition in deep nuclei involved in pain processing in migraineurs. In 2001, Welch et al. found increased iron levels in the peri‐acqueductal gray matter (GM) and red nucleus of patients with migraine and chronic daily headache [Welch et al., 2001], and more recently, Kruit et al. showed higher iron deposition in the basal ganglia and red nucleus of young migraine patients with and without aura [Kruit et al., 2009]. Both studies measured T2‐based MR signals to quantify brain iron deposition, and suggested that free radical damage, associated to hyperemia during migraine attacks, was the underlying pathophysiological mechanisms [Kruit et al., 2009; Welch et al., 2001].
However, our data also showed an increased MTR in MWA compared with MWoA patients. These findings are in line with the observed shortening of T1 relaxation times and indicate an increase in structural material [Helms et al., 1993]. Alterations in the MTR in central nervous system have been often attributed to changes in myelin content [Dousset et al., 2011; Giacomini et al., 2009; Hiehle et al., 2004] and a previous study has shown histopathological evidence of a significant correlation between myelin and MTR [Schmierer et al., 2004]. However, other components could also influence the nonaqueous tissue content in GM structures like glial and or neuronal cells as well as changes in water content due to edema and inflammation [Henkelman et al., 1995; Serres et al., 2009]. Therefore, the observed increase in MTR in the thalamus of MWA patients, which is concomitant to T1 shortening, may reflect either an increase in myelin or an increased density of neuronal or glial cellular bodies.
Glia proliferation and neurogenesis have been reported in migraine with aura following repeated episodes of CSD [Cao et al., 2007; Hadjikhani et al., 2010; Kraig et al., 1991; Yanamoto et al., 2005] and/or hypoperfusion [Petito et al., 1991]; CSD was also recently shown to propagate to the thalamus in an animal model [Eikermann‐Haerter et al., 2001]. In this context, our results suggest that CSD (in cortical GM) or hypoperfusion (in GM, WM, deep GM nuclei, and thalamus) may lead to glial/neuronal proliferation resulting in an increased MTR and T1 shortening. Alternatively, the presence of increased myelin component and/or higher cellularity in the thalamus of MWA patients might be one of the factors contributing to the development of CSD in migraineurs suffering from aura; this latter hypothesis is supported by the absence of correlation between T1 data and migraine duration and frequency.
All together, these findings suggest that thalamic structural abnormalities might explain differences between MWA and MWoA patients as well as non‐migraineurs (i.e., the presence of aura and of CSD).
Another important result of our study is that a number of thalamic nuclei exhibited different microstructural properties in MWA patients compared with MWoA patients and HC (Figs. 3 and 4). Previous work from our group evidenced lower fractional anisotropy in a region possibly corresponding to the vpm thalamic nucleus of migraineurs with and without aura compared with people without migraine [DaSilva et al., 1998]. Our current multicontrast study suggests that broader structural alterations are present in the thalamus of patients with visual aura encompassing a broad number of thalamic nuclei.
Shorter T1 was observed in the anterior (a) and the md nuclei of the thalamus in MWA patients compared with MWoA patients and HC. These nuclei connect to limbic cortical areas and play a role in the modulation of alertness (a), learning and memory (an and md) and attention, planning, organization, abstract thinking, and multitasking (md). Previous clinical studies have reported deficits of attention, memory, and information speed that were worse in MWA patients than in MWoA and HC (for review, see [O'Bryant et al., 2006]). Future studies should aim at correlating structural alterations in these thalamic nuclei with neuropsychological tests assessing their functional performance.
MWA patients exhibited shorter T1 also in the vl nucleus and in the vpl nucleus compared with HC (Figs. 3 and 4). Interestingly, the vpl and the vpm, which are relies of the somatosensory and of trigeminal pathways, appeared to be structurally similar in MWA vs. MWoA patients but not in MWA patients vs. HC (Fig. 4), pointing at a common alteration in both migraineurs subgroups as previously suggested by DaSilva et al. 1998. This observation is in line with the presence of common physiopathological mechanisms leading to pain perception in migraineurs with and without aura.
In addition, shorter T1 was found in the lp nucleus and in the pulvinar (p) of MWA compared with both MWoA subjects and HC (Figs. 3 and 4). The lp and the p are highly interconnected with visual striate and extrastriate cortical areas, and area V3A, one of these cortical extrastriate visual areas, was shown to be the origin of CSD in one MWA patient [Hadjikhani et al., 2010].
The observed differences may underlie an altered modulation of cortical excitability in the visual areas leading to CSD and visual aura.
No differences were observed between groups in MTR, T2*, and GFA statistical maps most probably because of more important local variations and lower sensitivity in pixelwise comparisons.
CONCLUSION
Our study provides evidence of the involvement of the thalamus in the physiopathology of migraine with aura. Patients with aura exhibit broad changes in thalamic nuclei when compared with MWoA patients and HC. On the other hand, migraineurs with and without aura do not show any structural differences in the vpl and vpm, the thalamic nucleus involved in pain processing.
In the context of previous literature on migraine pathophysiology, the observed thalamic alterations in MWA may be associated with increased cortical excitability, CSD, and visual aura.
As to the nature of the observed differences, the multiparametric evaluation at 3‐T MRI suggests that they arise from a combination of iron deposition and increased structural material (myelin or cells).
Future longitudinal studies in younger cohorts of migraineurs may help establishing whether the observed changes are a cause or a consequence of repeated migraine with aura attacks.
REFERENCES
- Afridi SK, Giffin NJ, Kaube H, Friston KJ, Ward NS, Frackowiak RS, Goadsby PJ (2005): A positron emission tomographic study in spontaneous migraine. Arch Neurol 62:1270–1275. [DOI] [PubMed] [Google Scholar]
- Akerman S, Holland PR, Goadsby PJ (2011): Diencephalic and brainstem mechanisms in migraine. Nat Rev Neurosci 12:570–584. [DOI] [PubMed] [Google Scholar]
- Andreou AP, Shields KG, Goadsby PJ (2010): GABA and valproate modulate trigeminovascular nociceptive transmission in the thalamus. Neurobiol Dis 37:314–323. [DOI] [PubMed] [Google Scholar]
- Astrakas LG, Argyropoulou MI (2010): Shifting from region of interest (ROI) to voxel‐based analysis in human brain mapping. Pediatr Radiol 40:1857–1867. [DOI] [PubMed] [Google Scholar]
- Borsook D (2011): Neurological diseases and pain. Brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein R, Jakubowski M, Garcia‐Nicas E, Kainz V, Bajwa Z, Hargreaves R, Becerra L, Borsook D (2010): Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol 68:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Welch KM, Aurora S, Vikingstad EM (1999): Functional MRI‐BOLD of visually triggered headache in patients with migraine. Arch Neurol 56:548–554. [DOI] [PubMed] [Google Scholar]
- Coppola G, Ambrosini A, Di Clemente L, Magis D, Fumal A, Gerard P, Pierelli F, Schoenen J (2007): Interictal abnormalities of gamma band activity in visual evoked responses in migraine: an indication of thalamocortical dysrhythmia? Cephalalgia 27:1360–1367. [DOI] [PubMed] [Google Scholar]
- DaSilva AF, Granziera C, Tuch DS, Snyder J, Vincent M, Hadjikhani N (2007): Interictal alterations of the trigeminal somatosensory pathway and periaqueductal gray matter in migraine. Neuroreport 18:301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dousset V, Gayou A, Brochet B, Caille JM (1998): Early structural changes in acute MS lesions assessed by serial magnetization transfer studies. Neurology 51:1150–1155. [DOI] [PubMed] [Google Scholar]
- Eikermann‐Haerter K, Yuzawa I, Qin T, Wang Y, Baek K, Kim YR, Hoffmann U, Dilekoz E, Waeber C, Ferrari MD, van den Maagdenberg AM, Moskowitz MA, Ayata C (2011): Enhanced subcortical spreading depression in familial hemiplegic migraine type 1 mutant mice. J Neurosci 31:5755–5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman N, Ewing JR, Gorell JM, Spickler EM, Solomon EG (2001): Interregional variation of longitudinal relaxation rates in human brain at 3.0 T: relation to estimated iron and water contents. Magn Reson Med 45:71–79. [DOI] [PubMed] [Google Scholar]
- Giacomini PS, Levesque IR, Ribeiro L, Narayanan S, Francis SJ, Pike GB, Arnold DL (2009): Measuring demyelination and remyelination in acute multiple sclerosis lesion voxels. Arch Neurol 66:375–381. [DOI] [PubMed] [Google Scholar]
- Granziera C, Schmahmann JD, Hadjikhani N, Meyer H, Meuli R, Wedeen V, Krueger G (2009): Diffusion spectrum imaging shows the structural basis of functional cerebellar circuits in the human cerebellum in vivo. PLoS One 4:e5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, Sanchez Del Rio M, Wu O, Schwartz D, Bakker D, Fischl B, Kwong KK, Cutrer FM, Rosen BR, Tootell RB, Sorensen AG, Moskowitz MA (2001): Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A 98:4687–4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms G, Dathe H, Dechent P (2010): Modeling the influence of TR and excitation flip angle on the magnetization transfer ratio (MTR) in human brain obtained from 3D spoiled gradient echo MRI. Magn Reson Med 64:177–185. [DOI] [PubMed] [Google Scholar]
- Henkelman RM, Huang X, Xiang QS, Stanisz GJ, Swanson SD, Bronskill MJ (1993): Quantitative interpretation of magnetization transfer. Magn Reson Med 29:759–766. [DOI] [PubMed] [Google Scholar]
- Hiehle JF,Jr., Grossman RI, Ramer KN, Gonzalez‐Scarano F, Cohen JA (1995): Magnetization transfer effects in MR‐detected multiple sclerosis lesions: comparison with gadolinium‐enhanced spin‐echo images and nonenhanced T1‐weighted images. AJNR Am J Neuroradiol 16:69–77. [PMC free article] [PubMed] [Google Scholar]
- IHC (2004):The International Classification of Headache Disorders. 2nd ed, Blackwell Publishing, Oxford, UK. [Google Scholar]
- Jack CR,Jr., Bernstein MA, Borowski BJ, Gunter JL, Fox NC, Thompson PM, Schuff N, Krueger G, Killiany RJ, Decarli CS, Dale AM, Carmichael OW, Tosun D, Weiner MW (2010): Update on the magnetic resonance imaging core of the Alzheimer's disease neuroimaging initiative. Alzheimers Dement 6:212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Symms MR, Cercignani M, Howard RJ (2005): The effect of filter size on VBM analyses of DT‐MRI data. Neuroimage 26:546–554. [DOI] [PubMed] [Google Scholar]
- Klein S, Staring M, Murphy K, Viergever MA, Pluim JP (2010): elastix: a toolbox for intensity‐based medical image registration. IEEE Trans Med Imaging 29:196–205. [DOI] [PubMed] [Google Scholar]
- Kraig RP, Dong LM, Thisted R, Jaeger CB (1991): Spreading depression increases immunohistochemical staining of glial fibrillary acidic protein. J Neurosci 11:2187–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauth A, Blanc R, Poveda A, Jeanmonod D, Morel A, Szekely G (2010): A mean three‐dimensional atlas of the human thalamus: generation from multiple histological data. Neuroimage 49:2053–2062. [DOI] [PubMed] [Google Scholar]
- Kruit MC, Launer LJ, Overbosch J, van Buchem MA, Ferrari MD (2009): Iron accumulation in deep brain nuclei in migraine: a population‐based magnetic resonance imaging study. Cephalalgia 29:351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele PF, Gruetter R (2010): MP2RAGE, a self bias‐field corrected sequence for improved segmentation and T1‐mapping at high field. Neuroimage 49:1271–1281. [DOI] [PubMed] [Google Scholar]
- Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J (1995): A probabilistic atlas of the human brain: theory and rationale for its development. The International Consortium for Brain Mapping (ICBM). Neuroimage 2:89–101. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP (2002): Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseda R, Kainz V, Jakubowski M, Gooley JJ, Saper CB, Digre K, Burstein R (2010): A neural mechanism for exacerbation of headache by light. Nat Neurosci 13:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Bryant SE, Marcus DA, Rains JC, Penzien DB (2006): The neuropsychology of recurrent headache. Headache 46:1364–1376. [DOI] [PubMed] [Google Scholar]
- Petito CK, Juurlink BH, Hertz L (1991): In vitro models differentiating between direct and indirect effects of ischemia on astrocytes. Exp Neurol 113:364–372. [DOI] [PubMed] [Google Scholar]
- Poulet JF, Fernandez LM, Crochet S, Petersen CC (2012): Thalamic control of cortical states. Nat Neurosci. [DOI] [PubMed] [Google Scholar]
- Prokhorov AV (2001): Hotelling T2‐distribution In: Hazewinkel M, editor. Encyclopedia of Mathematics:Springer. [Google Scholar]
- Roche A, Ribes D, Bach‐Cuadra M, Kruger G (2011): On the convergence of EM‐like algorithms for image segmentation using Markov random fields. Med Image Anal. [DOI] [PubMed] [Google Scholar]
- Rooney WD, Johnson G, Li X, Cohen ER, Kim SG, Ugurbil K, Springer CS,Jr. (2007): Magnetic field and tissue dependencies of human brain longitudinal 1H2O relaxation in vivo. Magn Reson Med 57:308–318. [DOI] [PubMed] [Google Scholar]
- Schmierer K, Scaravilli F, Altmann DR, Barker GJ, Miller DH (2004): Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann Neurol 56:407–415. [DOI] [PubMed] [Google Scholar]
- Serres S, Anthony DC, Jiang Y, Campbell SJ, Broom KA, Khrapitchev A, Sibson NR (2009): Comparison of MRI signatures in pattern I and II multiple sclerosis models. NMR Biomed 22:1014–1024. [DOI] [PubMed] [Google Scholar]
- Shields KG, Goadsby PJ (2005): Propranolol modulates trigeminovascular responses in thalamic ventroposteromedial nucleus: a role in migraine? Brain 128:86–97. [DOI] [PubMed] [Google Scholar]
- Shields KG, Goadsby PJ (2006): Serotonin receptors modulate trigeminovascular responses in ventroposteromedial nucleus of thalamus: a migraine target? Neurobiol Dis 23:491–501. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen‐Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE (2006): Tract‐based spatial statistics: voxelwise analysis of multi‐subject diffusion data. Neuroimage 31:1487–1505. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE (2009): Threshold‐free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44:83–98. [DOI] [PubMed] [Google Scholar]
- Tuch DS (2004): Q‐ball imaging. Magn Reson Med 52:1358–1372. [DOI] [PubMed] [Google Scholar]
- Welch KM, Nagesh V, Aurora SK, Gelman N (2001): Periaqueductal gray matter dysfunction in migraine: cause or the burden of illness? Headache 41:629–637. [DOI] [PubMed] [Google Scholar]
- Yanamoto H, Miyamoto S, Tohnai N, Nagata I, Xue JH, Nakano Y, Nakajo Y, Kikuchi H (2005): Induced spreading depression activates persistent neurogenesis in the subventricular zone, generating cells with markers for divided and early committed neurons in the caudate putamen and cortex. Stroke 36:1544–1550. [DOI] [PubMed] [Google Scholar]


