Abstract
Chinese processing has been suggested involving distinct brain areas from English. However, current functional localization studies on Chinese speech processing use mostly “indirect” techniques such as functional magnetic resonance imaging and electroencephalography, lacking direct evidence by means of electrocortical recording. In this study, awake craniotomies in 66 Chinese‐speaking glioma patients provide a unique opportunity to directly map eloquent language areas. Intraoperative electrocortical stimulation was conducted and the positive sites for speech arrest, anomia, and alexia were identified separately. With help of stereotaxic neuronavigation system and computational modeling, all positive sites elicited by stimulation were integrated and a series of two‐ and three‐dimension Chinese language probability maps were built. We performed statistical comparisons between the Chinese maps and previously derived English maps. While most Chinese speech arrest areas located at typical language production sites (i.e., 50% positive sites in ventral precentral gyrus, 28% in pars opercularis and pars triangularis), which also serve English production, an additional brain area, the left middle frontal gyrus (Brodmann's areas 6/9), was found to be unique in Chinese production (P < 0.05). Moreover, Chinese speakers’ inferior ventral precentral gyrus (Brodmann's area 6) was used more than that in English speakers. Our finding suggests that Chinese involves more perisylvian region (extending to left middle frontal gyrus) than English. This is the first time that direct evidence supports cross‐cultural neurolinguistics differences in human beings. The Chinese language atlas will also helpful in brain surgery planning for Chinese‐speakers. Hum Brain Mapp 36:4972–4985, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: Chinese, glioma, language mapping, intraoperative stimulation mapping, neurosurgery
Abbreviations
- ALE
Activation likelihood estimation
- FWHM
Full width half maximum
- ISM
Intraoperative stimulation mapping
- MAC
Monitored anesthesia care
- MNI
Montreal Neurological Institute
- ROI
region‐of‐interest
INTRODUCTION
The preservation of language function is critical to the neurosurgical procedures adjacent to the language areas [De Witt Hamer et al., 2012; Sanai et al., 2008]. It concerns the patients’ postoperative quality of life and influences their survival time [McGirt et al., 2009]. Intraoperative stimulation mapping (ISM) is the gold standard for localizing language areas [De Witt Hamer et al., 2012; Maldonado et al., 2011; Ojemann et al., 1989] by using direct electrocortical stimulation to inhibit neural activity during awake surgery while the patient is performing various types of language tasks, e.g., counting, picture naming, reading, listening [Coello et al., 2013]. As a widely accepted method for eloquent language area mapping, ISM overcomes the disadvantages of functional MRI (fMRI)‐based mapping because it is a direct measurement, while the blood oxygenation level‐dependent signal obtained from fMRI is an indirect and complex reflection of the underlying neuronal activity. Thus, the ISM technique gives researchers a unique opportunity to map language topographical configurations and provides an input gate into brain functional networks [Mandonnet et al., 2010].
Several studies have used ISM to investigate the language area distribution and have generated language maps for English or other alphabetic languages [Duffau et al., 2003; Lubrano et al., 2010; Sanai et al., 2008; Tate et al., 2014]. Sanai et al. [2008] systemically mapped the ISM positive sites in English speakers performing three tasks (number counting, picture naming and word reading). They generated the cerebral distribution maps for English language processing by using the largest population of brain tumor patients to date. This was the first time that researchers manipulated the brain to obtain systematic, group‐level language probabilistic maps and illustrated how cortical language sites for speech arrest, anomia and alexia are distributed within the dominant hemisphere of the cortex. They found that the critical sites related to speech function were variably located and went well beyond the classic language areas. In addition, in a recent study, Tate et al. [2014] generated the first bilateral probabilistic maps for the essential cortical epicenters of speech production and naming based on data from France. They concluded that Broca's area was not the speech output region because the speech arrest regions localized to the ventral premotor cortex, not to the classical Broca's area. They also reported that anomia regions were located not only in the classical Wernicke's areas but also in the middle and inferior frontal gyri. However, it remains unclear whether those language maps generated by ISM based on the Indo‐European language family (e.g., English) can be completely applied to the neurosurgical protection of language function in native Chinese speakers.
Chinese and English are the most widely spoken languages in the world, but they differ in several aspects, including their scripts, orthography, and tonality. Substantial fMRI evidence has shown that Chinese and English language processing involve different neural networks for the spoken and written languages [Bolger et al., 2005; Booth et al., 2006; Cao et al., 2009, 2010, 2013a, 2013b; Chen et al., 2009; Wu et al., 2012; Zhu et al., 2014]. In a series of studies using fMRI, Tan and his colleagues [Siok et al., 2004, 2008; Tan et al., 2000, 2001, 2005] compared brain activation in Chinese (logographic) and English (alphabetic) reading. They first reported that the left middle frontal gyrus (LMFG, BA9) might play a crucial role in orthography‐to‐phonography and orthography‐to‐semantics conversion during Chinese reading. Their theory was further validated by many other centers [Chen et al., 2008; Wu et al., 2012]. In addition to reading, a recent study revealed that there were different dynamic brain systems between Chinese and English speakers during speech comprehension [Ge et al., 2015], which indicated that Chinese (tonal) might differ from English (atonal) in semantic processing. Furthermore, because the pronunciation of Chinese words involves consonants and vowels together with tones [Liu et al., 2006], word production in Chinese may involve coordination of different parts of the speech articulators. In a bilingual study by Liu et al. [2010], Chinese was found to have a neural dissociation with English in word production. Therefore, because of these differences between Chinese and English, neurosurgeons should pay more attention to neural specialization, especially for cross‐linguistic neurosurgical concerns, when operating within different brain regions.
This study aimed to generate Chinese probability maps in a quantitative way by using ISM during awake surgery based on a large group of patients with brain tumors and to investigate the commonality and particularity between Chinese and English. As in the previous study [Sanai et al., 2008], the three most common tasks for language mapping (i.e., number counting, picture naming, word reading) were applied in Chinese speakers. Both qualitative and quantitative comparisons with English language maps [Sanai et al., 2008] were conducted. We hypothesized that the speech processing of these two languages involves both common and distinct brain areas.
MATERIALS AND METHODS
Subject Information
Between December 2010 and June 2013, a total of 75 patients with glioma underwent awake surgery and intraoperative language mapping in the Neurosurgical Department of Huashan Hospital, Shanghai, China. The eligibility and exclusion criteria for awake language mapping are listed in our previous study [Lu et al., 2013a]. To generate the left‐sided language maps, six patients with right dominant hemispheres were further excluded. All of the remaining 69 patients were adults of Han ethnicity and native Chinese speakers (Chinese official language, Mandarin). All the lesions were located in the peri‐insular area of the left‐dominant hemisphere [Li, 1983]. Three patients with oversized tumors and/or edema leading to obvious brain deformation in the frontal lobe were excluded because the purpose of this study was to generate a language production map; oversized intracranial lesions may lead to serious displacement or significant functional reorganization of the nearby language‐related areas. Such an assessment was based on anatomical imaging (T1 or T2‐flair image). Consequently, 66 patients (41.8 ± 12.7 years old, 43 males) from eight provinces of China entered the following data analyses.
All patients were cognitively normal as evaluated by the Mini‐Mental State Examination, and all but nine patients had normal overall preoperative language function. Information on demographics, clinical manifestations, tumor location, tumor grade, size and histopathology, as well as gross total resection rate and postoperative functional outcome, are listed in Table 1. For more details of language/cognitive state evaluation and tumor resection, please see Supporting Information. The study protocol was approved by the Huashan Institutional Review Board, and all patients or their families provided written inform consent before this study.
Table 1.
Demographic, clinical, and pathological information
| Variables | Values | Variables | Values |
|---|---|---|---|
| Age (yrs) | WHO tumor grading, Histologic type—no. (%) | ||
| Mean ± std | 41.8 ± 12.7 | I, dysembryoplastic neuroepithelial tumor | 1 (1.5) |
| Range | 19–76 | II, astrocytoma | 30 (45.4) |
| Males—no. (%) | 43 (65.2) | II, oligodendroglioma | 9 (13.6) |
| Right‐handed (%) | 66 (100) | II, oligoastrocytoma | 4 (6.1) |
| Educational level (yr) | 11.9 ± 3.6 | III, anaplastic astrocytoma | 4(6.1) |
| MMSE (tot. = 30) | 26.6 ± 2.6 | III, anaplastic oligodendroglioma | 2 (3.0) |
| Presurgical language function score | IV, glioblastoma multiforme | 16 (24.2) | |
| Spontaneous speech (tot. = 20) | 19.3 ± 1.6 | Tumor volume (cm3) | |
| Repetition (tot. = 100) | 94.1 ± 11.1 | Mean ± std | 57.1 ± 33.2 |
| Naming (tot. = 100) | 93.2 ± 9.2 | Range | 4.3–146.1 |
| Comprehension (tot. = 230) | 216.1 ± 19.4 | Gross total resection—no./tot. no. (%) | |
| Aphasia quotient (AQ)a | 94.84b | All grades | 44/66 (66.7) |
| Symptoms at presentation—no. (%) | I/II | 26/44 (59.1) | |
| Seizure | 38 (57.6) | III/IV | 18/22 (81.8) |
| Headache | 15 (22.7) | Functional outcome—no. (%)c | |
| Language deficit | 9 (13.6) | 1 month | 12 (18.2) |
| Others | 4 (6.1) | 6 month | 3 (4.5) |
WHO, World Health Organization.
aAQ was calculated according to the method in Western Aphasia Battery (Kertesz and Poole, 1974), AQ = (S+R/10+N/10+C/23)*2.
bA score of 93.8 is usually treated as the cut‐off between aphasia and non‐aphasia.
cNew or increased postoperative language deficits, e.g., aphasia, anomia.
Awake Anesthesia and ISM
The monitored anesthesia care (MAC) approach was adopted for awake anesthesia in all patients. The detailed awake anesthesia procedures have been documented in our previous intraoperative awake studies [Lu et al., 2013a; Lu et al., 2013b].
ISM for language mapping was performed with a 5‐mm‐wide bipolar electrode stimulator (Epoch XP, Axon Systems) that produces biphasic square‐wave pulse at 60 Hz. The recording needle electrodes were implanted in the patient's scalp, tendon and muscle belly of the contralateral target muscles to record compound muscle action potential. The current intensity was initiated at a minimum of 1 mA and then increased to a maximum of 6 mA. A stimulus duration of 3–4 s was required for language mapping. While ISM was in progress, a 4‐ or 6‐contact strip subdural electrode was used to record after‐discharge activity. The presence of after‐discharge potentials indicated that the stimulation current was too high. The intensity was then reduced by 0.5–1 mA until no after‐discharge was detected. This threshold was adopted as the upper limit for the sequential stimulation current. We stimulated the whole exposed cortical area in square‐centimeter patches. Functional sites were marked with 0.5 × 0.5 cm2 sterile tags. The limits of cortical area exposure and ISM positive site for language production were then snapshotted by neuronavigation. Finally, all 1 × 1 cm2 patches of the exposed cortex were examined. Sites suspected of altering language ability were stimulated a minimum of three times to obtain consistent results.
During the ISM, the patient completed a battery of language tasks that consist of (1) number counting from 1 to 50, (2) picture naming, and (3) word reading. Before the operation, the patient was trained to conduct these tasks by a neuropsychologist (Y Zhou). In the picture naming and word reading tasks, the pictures or words were presented on slides, which were projected onto a screen before the patient's face by an in‐house‐developed interactive system (Chinese patent No. ZL201220303939.1). The stimulation started just before the presentation of the item, and the patient was asked to produce a short introductive phrase, ‘this picture (or word) is…’ in Chinese before naming or reading. The neuropsychologist (Y Zhou) and neurophysiologist (G Xu) evaluated and categorized the language disturbance together. The transitory language deficits in counting, naming, and reading tasks caused by electrical stimulation were classified as speech arrest, anomia, and alexia, respectively. The three definitions are fundamentally different from each other. We applied the same definitions with the study by Sanai et al. [2008].
Chinese Language Map Generation
We generated three types of the Chinese language maps: first, the two‐dimensional patch‐like probability maps (2D) for the three tasks to be consistent with the English maps of Sanai et al. [2008] for comparison; second, three‐dimensional surface‐based scatter maps (3D) for more accurate illustration. These two maps were based on a fixed‐effect model (i.e., producing probability maps for the specific group we studied). The third type was based on a random‐effect model using activation likelihood estimation (ALE)‐based “meta‐analysis” producing a statistical parameter map. The schematic illustration of the generation of these maps is shown in Figure 1. They are described in detail below.
Figure 1.
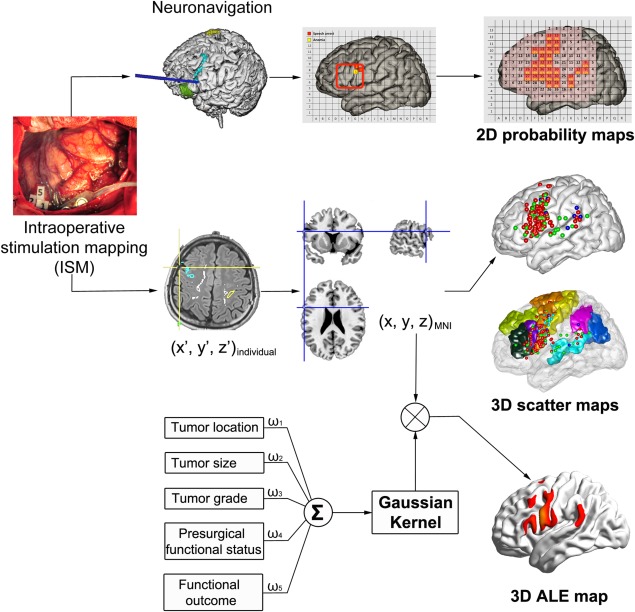
Flowchart for the generation of 2D patch‐like probability maps, 3D scatter maps and activation likelihood estimation (ALE) maps. With the reference of intraoperative photography and neuronavigation, the location of ISM positive and negative sites were labeled on the surface of the Collin's template based on the correspondence of anatomical landmarks to generate 2D patch‐like probability maps. Based on neuronavigation results, individual coordinates (x′, y′, z′) were obtained. Then, those individual coordinates were transformed to standard Montreal Neurological Institute (MNI) coordinates. Then, the group‐wise ISM positive points were integrated to generate 3D scatter maps and 3D ALE maps (based on a meta‐analysis‐like analysis considering tumor characteristics, functional evaluations before and after surgery) for number counting, picture naming and word reading.
2D Patch‐like probability map
We followed the procedure described by Sanai et al. [2008]. First, we rotated the Collin's template (“ch2 template”) to match Sanai et al. [2008]'s underlying brain template. Then, we generated a lattice to segment this brain template into 234 patches with 13 horizontal and 18 vertical lines to match Sanai et al.'s lattice in the frontal, temporal and parietal lobes. With the reference of intraoperative photography and neuronavigation, the location of ISM positive and negative sites for all the patients were labeled on the lattice based on the correspondence of anatomical landmarks (by JF Lu [five‐year neurosurgical experience] and confirmed by JS Wu [senior neurosurgeon]). Then, we calculated the positive rate by dividing the total number of positive sites by the total number of stimuli. In addition, the positive rate in all patches was up‐sampled and plotted in pseudo‐color for better illustration and for providing a reference map for future usage using MATLAB (R2012b, the MathWorks, Inc) with the cubic interpolation method. Totally three 2D patch‐like probability maps were generated for the three intraoperative tasks, respectively.
3D Surface‐based scatter map
To generate the 3D language map for following region‐of‐interest (ROI) analyses, we further transformed individual coordinates for positive sites to standard (MNI) coordinates based on the snapshot of neuronavigation and anatomical landmark. Then, the ch2 template was used to localize and output the MNI coordinates with help of a visualization tool “BrainNet Viewer” [Xia et al., 2013]. To increase the accuracy and reduce subjective error, the MNI coordinates were confirmed by a senior neurosurgeon (JS Wu). Similarly, three 3D surface‐based scatter maps were generated.
ALE statistical map
To reduce bias and potential mislocation when building the spatial correspondence from individual to standard space in the above two methods and to reduce potential tumor‐induced deformation of the eloquent areas, we introduced an ALE‐based meta‐analysis approach [Eickhoff et al., 2009, 2012; Turkeltaub et al., 2012] to ISM‐based language mapping during number counting (for speech arrest), generating a statistical parameter map that can be inferred to the whole population (i.e., random‐effect analysis). To do this, the coordinates in stereotaxic space for each ISM positive point were treated as “peak coordinates” from “the report from a single study”; then Gaussian smoothing was applied to this focus with a corresponding full width half maximum (FWHM) representing the confidence level parameterizing the extent to which we trusted this ISM positive point. After that, all blurred foci across all the patients were integrated, and statistical testing was performed, resulting in the ALE maps (with P values). The confidence level was quantified based on comprehensive assessment of tumor location, tumor size, tumor grade, patient's presurgical functional status and postoperative functional outcomes based on the consensus of our group, which consisted of neurosurgeons, neuroscientists and radiologists. For detailed methods, please refer to Supporting Information. For other two tasks, because of the inadequate ISM positive points, we did not perform ALE‐based analysis.
ROI Analyses
For each intraoperative task, we calculated the percentage of the ISM positive sites lying within each of the language‐related ROIs for comprehensive evaluation. The ROIs included Broca's area (BA 44 and 45), Wernicke's area (BA 40 and 22), LMFG (BA 6 and 9) and other Brodmann areas (BA 2/4/42/43), which were chosen, based on BA template and prior knowledge. With these ROIs, we further quantified the similarity and distinction of the involved brain areas among speech arrest, anomia, and alexia.
Statistical Analysis
To compare the language maps in Chinese speakers with those in English speakers, we performed the chi‐square test for positive rates (or Fisher's exact probabilities test if the chi‐square test was not applicable) between our 2D maps and the data from Sanai et al. [2008] (with permission from Dr. Berger at UCSF) patch‐by‐patch for the speech arrest and anomia tasks (we did not conduct an analysis for the alexia task because we did not have a sufficient number of positive sites). The statistical analyses were performed using SPSS version 21 (IBM, Inc.). A P value ≤ 0.05 was considered statistically significant. The P values were not corrected for multiple comparisons because the unit for which we performed statistical comparison was the patch and because the number of patches was limited; moreover, the comparisons were conducted between two independent studies, thus alleviating the concern of the multiple comparison problems. The comparison areas were restricted to the regions where ISM was conducted in both the current study and in the study by Sanai et al. [2008]. Therefore, for speech arrest, only areas in the frontal lobe were compared; for anomia, frontal, temporal and parietal lobes were included. The same comparison method was also applied to compare the patterns of speech arrest and anomia tasks based on Chinese data (we did not extend this analysis to include the alexia task because we did not have a sufficient number of positive sites).
RESULTS
ISM Information
All patients in the series completed the counting task. The picture‐naming task was completed by 53 patients and the word‐reading task by 48 patients. In the speech arrest task, a total of 1402 stimuli resulted in 132 positive sites (2 ± 1.8 per subject). For the anomia task, there were 1030 stimuli and 43 positive sites (0.8 ± 1.1 per subject). For the alexia task, these numbers were 925, 11 and 0.2 ± 0.6.
2D Patch‐Like Maps and 3D Surface‐Based Scatter Maps
Figure 2A shows the total number of intraoperative stimulations exerted to each patch (pink areas with Arabic numbers) across subjects. It also shows the probability of an ISM positive case (percentages in yellow) integrated from 66 subjects during speech arrest. Speech arrest areas were located mainly at the frontal cortex and perisylvian fissure. The positive rates were highest in the VPCG and pars opercularis (up to 49% of sites). The stimulation sites and probability (in blue) of anomia across 53 subjects are shown in Figure 2B. Figure 2C shows the results for alexia, with a scattered spatial pattern and reduced probabilities compared with the previous two conditions. The exact number (rather than probability) of ISM positive sites for the three tasks is also shown in Figure 3A–C: speech arrest occurred more often and was distributed more focally than the other two conditions; the alexia areas were distributed more evenly in the frontal and parietal lobes, while the speech arrest and anomia areas were located more in the frontal lobe than other lobes. Figure 3D–F presents the 3D scatter maps of the brain surface, with more accurate spatial positions for the three tasks in different colors (red for speech arrest, green for anomia and dark blue for alexia). The combined version is shown in Figure 3G, where we also show the brain sites with dysarthria (in light blue). This is mainly for differentiating from speech arrest because dysarthria was only caused by motor responses in the mouth or pharyngeal muscle.
Figure 2.
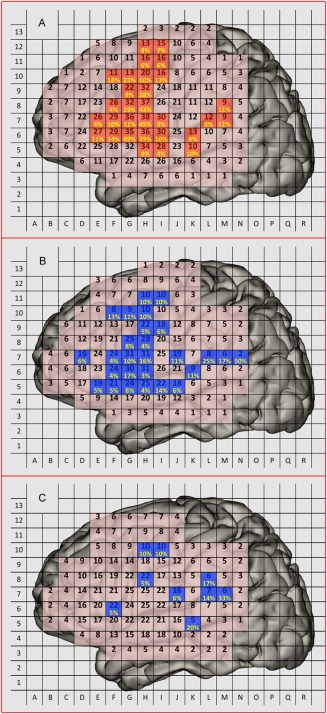
2D patch‐like probability maps for Chinese. (A) The position (in pink) and the number (black Arabic number) of the electrocortical stimulation across subjects and the sites of ISM positive results and the correspondent probability (i.e., ISM positive rate, calculated by dividing the ISM positive time by total electrocortical stimulation time, in yellow) for speech arrest; (B) ISM sites and positive sites (in blue) and positive probability (in yellow) for anomia; (C) ISM sites (in blue) and positive sites (in blue) for alexia.
Figure 3.
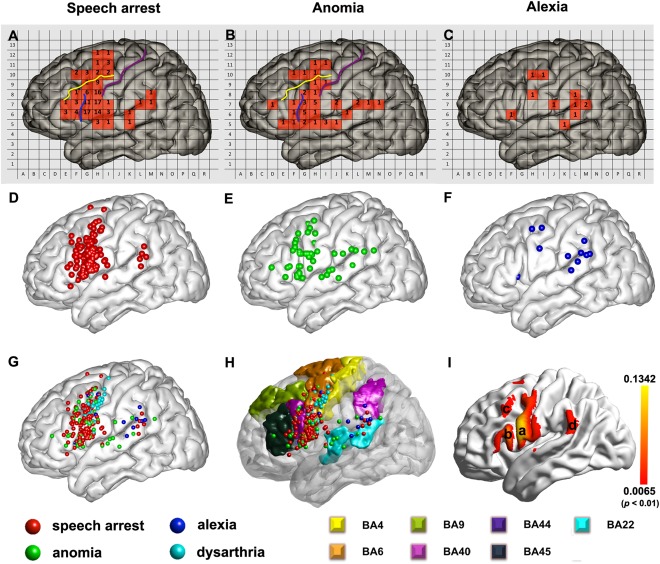
Three types of language maps for Chinese. (A, B, and C) The total number of positive sites for speech arrest, anomia and alexia in relevant landmarks in a 2D patch‐like view. (D, E, and F) 3D scatter maps of the ISM positive sites for speech arrest, anomia, and alexia. (G) The combination of all ISM positive sites, including speech arrest, anomia, alexia and dysarthria (this condition was shown to differentiate from the speech arrest condition because dysarthria was only caused by motor responses in the mouth or pharyngeal muscle). (H) The relationship between the ISM positive sites and the typical language‐related Brodmann areas (BA). (I) ALE map for speech arrest. (For detailed ALE statistics map generation method, please see Supporting Information). Region (a): ventral precentral gyrus, (b): pars opercularis, (c): middle frontal gyrus, (d): temporo‐parietal junction. The threshold was P < 0.01, uncorrected (the corresponding value of ALE statistics threshold was 0.0065).
ROI Analysis for Language Maps
We segmented the frontal lobe into four regions in the 2D patch‐like maps according to the relative position to the central sulci (purple curve in Fig. 3A,B), inferior frontal sulci (yellow curve in Fig. 3A,B) and the ascending branch of the Sylvian fissure (blue curve in Fig. 3A,B). Those regions (as shown in Fig. 3H) were (1) the superior frontal gyrus; (2) the middle frontal gyrus; (3) the anterior two‐thirds of the inferior frontal gyrus (pars triangularis, BA45, and pars orbitalis, BA47); and (4) the posterior third of the inferior frontal gyrus (pars opercularis, BA44) and the VPCG (BA6). Ninety‐two (69.7%) speech arrest sites were located in the VPCG (ventral part of BA6, 66 of the 92) and pars opercularis (BA44, part of Broca's area, 26 of the 92), followed by the LMFG (13 sites, 9.8%), the pars triangularis (12 sites, 9.1%), and the superior frontal gyrus (two sites, 1.5%). For the anomia condition, 21 (48.8%) of 43 ISM positive sites were located at the pars opercularis and VPCG. The LMFG, superior temporal gyrus and supramarginal gyrus had five (11.6%), 4 (9.3%), 6 (14.0%), and three (7.0%) anomia‐positive sites, respectively. For the alexia condition, four sites were located in the frontal lobe, four in the supramarginal gyrus and angular gyrus, and two in the temporal lobe. The ROI analysis based on 3D scatter maps indicated that the speech arrest sites were located mainly at BA6/44 (over 85%), while the anomia sites were distributed more evenly (over 70% within BA6/44/22). The alexia sites were distributed at BA6/22/40 and other regions. Compared with the former two conditions, the alexia region had a more distributed and less prominent and consistent (across subjects) pattern. Due to the small number of ISM positive sites in the alexia condition, further interpretation should be more careful. For better illustration of the ROI analysis result for each condition, please see Supporting Information Figure 1 for bar plots.
ALE Map
Our “meta‐analysis” using the ALE approach revealed that the “activation” of language production was mainly located in the VPCG (region a in Fig. 3I), IFG (region b in Fig. 3I), LMFG (c in Fig. 3I), and supramarginal gyrus (d in Fig. 3I). The threshold was set to P < 0.01, uncorrected. The peak intensity in the frontal lobe was at (−66, 8 14) in MNI space, belonging to BA 6. The peak in the parietal lobe was at (−66 −40 26), located in the supramarginal gyrus, posterior part of BA 22.
Comparisons of Patterns Among Speech Arrest, Anomia, and Alexia
By statistical comparison in the corresponding patches between speech arrest (number counting) and anomia (picture naming), two patches in the VPCG (Fig. 4) have higher rates in speech arrest than in anomia (P < 0.05). Although no patches showed a significantly higher rate for anomia than for speech arrest, the pattern of cortical representation in anomia suggests an obvious bias in three regions: the posterior superior temporal gyrus/inferior parietal lobule, the middle part of the superior temporal gyrus and the posterior middle frontal gyrus/adjacent precentral gyrus. As for alexia, we did not conduct statistical analyses for the other two conditions due to the low frequency of positive sites (Fig. 3C). However, from a general view, there are two regions in common among these three different patterns. One such area was located at the posterior part of LMFG (BA9, BA6). The other was the supramarginal gyrus/angular gyrus (BA40, BA39).
Figure 4.
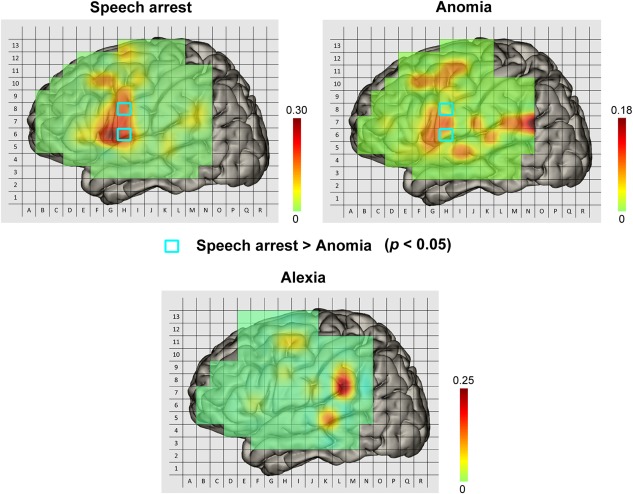
Comparison of patterns among speech arrest, anomia and alexia for Chinese data. The light blue squares indicate that the positive rates of speech arrest in these two sites were significantly higher than that of anomia. At no sites was the positive rate of anomia found to be significantly higher than that of speech arrest. Due to the lower frequency of positive sites in alexia, we did not perform a statistical comparison between alexia and the other two sites. In addition, these three patterns had two brain regions in common: the posterior middle frontal gyrus (BA9, BA6) and the supramarginal gyrus/angular gyrus (BA40, BA39).
Difference in Language Maps Between Chinese and English Based on ISM
Our data are comparable to those of Sanai et al. [2008] in demographic information (i.e., gender and age), gross total resection (including those for all grades, I/II and III/IV tumors) and functional outcome (P > 0.05), but not in tumor volume (study by Sanai et al. 71 cm3; this study: 57.1 cm3) and WHO grade distribution (more glioblastomas in Sanai's and more astrocytoma in this study). The smaller tumor volume and fewer glioblastomas in our study indicate a lesser impact of the tumor on the language mapping. For the speech arrest condition (Fig. 5, upper two panels), statistical comparisons in the corresponding patches between Chinese and English languages showed that the positive rate for Chinese was higher (P < 0.05) than that for English in three patches in the VPCG and opercularis (BA6/44) and one patch in the LMFG (BA6/9). In addition, another Chinese > English patch in the LMFG came close to statistical significance (P = 0.051). In contrast, the English > Chinese areas were one patch in the dorsal part of the inferior frontal gyrus (P = 0.029). During anomia (Fig. 5, lower two panels), one patch in the VPCG (P = 0.023) and a neighboring one in the pars opercularis (P = 0.022) had a higher positive rate for Chinese than for English. Another patch in the pars triangularis showed a higher positive rate for English than for Chinese (P = 0.034). In general, the differences between the two languages in the speech arrest condition and the anomia condition were consistent. In addition, although no significant differences in LMFG were found between Chinese and English for both anomia and alexia, the LMFG had more positive sites in Chinese than English.
Figure 5.
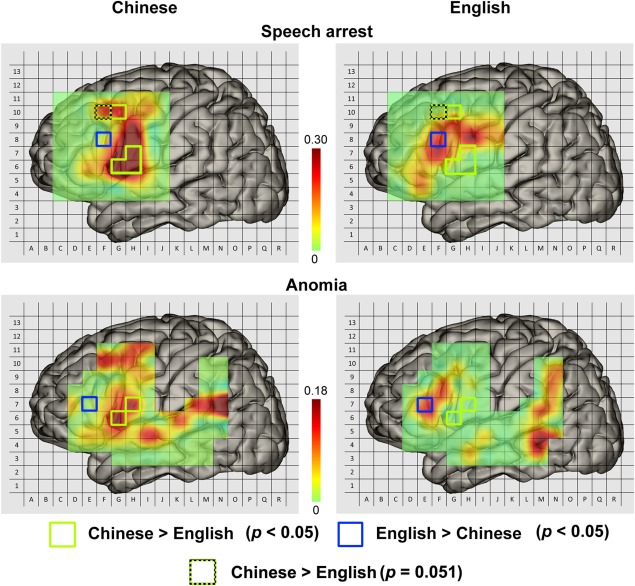
Pseudo‐colored 2D probability maps for ISM positive regions in speech arrest and anomia conditions for Chinese and English data. The green and blue (dotted blue) squares outlined the significant difference in language production areas between Chinese‐speakers and English‐speakers. Please note that the figures in the right panels were modified according to the study by Sanai et al. [2008]. (With the permission from Dr. Berger).
Functional Outcome
One month after surgery, 12 patients (18.2%) demonstrated a worsening of their language function or new language deficits. By 6 months post‐surgery, three patients (4.5%) still had not returned to their preoperative level (Fig. 1). In other words, the permanent language deficit observed for using the individualized identification of eloquent areas for Chinese is 4.5%.
DISCUSSION
In this study, we established Chinese probabilistic production maps by applying ISM and assessed the similarity and distinctions between Chinese and English maps (Fig. 5). We generated different language maps for different purposes: a 2D patch‐like probability map for comparison with English language mapping results, a 3D surface‐based scatter map for more accurate location and ROI analyses, and an ALE statistical map for group inference and further reference by future study (Figs. 2, 3). Moreover, we systematically assessed three commonly used but essentially different tasks for eloquent language area mapping and found overlapping patterns but with descending frequency for ISM positivity in number counting (corresponding to speech arrest condition), picture naming (corresponding to anomia) and word reading (corresponding to alexia).
To the best of our knowledge, this is the first study creating Chinese maps or atlases using a direct neurophysiological technique based on a relatively large and homogeneous tumor patient cohort. These maps are group‐level probability maps that integrate all of the individual‐level evidence, so it could be expected that this eloquent area mapping would be more thorough and systematic. The ISM we used is a direct reflection of neuronal activity and has been considered the gold standard for functional mapping [De Witt Hamer et al., 2012; Duffau, 2010; Sanai et al., 2008]. From numerous clinical reports [Coello et al., 2013; Giussani et al., 2010] and our experience, the results should be more accurate than those from other indirect technologies (e.g., preoperative fMRI). Probability maps such as ours will be useful as a reference for brain surgical planning, especially as they will provide a priori knowledge or guidance of the language areas for invasive surgery planning during craniotomy to reduce the time of awake surgery and the stress on patients. Moreover, we produced an ALE map for speech arrest and reported the peak coordinates for each cluster, both of which might be helpful for basic cognitive neuroscience studies on Chinese language. We will discuss the comparison of speech arrest, anomia and alexia for Chinese data and our findings on the similarities and potential differences between Chinese and English language, emphasizing the newly found node in the LMFG areas in Chinese speech production.
Comparison of Patterns Among Speech Arrest, Anomia, and Alexia
Speech arrest, anomia and alexia showed different neural basis in language processing. Speech arrest elicited by ISM during number counting was defined by “a total impossibility to speak while being still able to perform rapid alternating movements of the tongue” [Duffau et al., 2014; Matsumoto et al., 2004]. It was a complete disruption of the phonetic encoding stage or speech planning [Duffau et al., 2014; Tate et al., 2014]. Anomia during picture naming was defined as an inability to name the picture while still being able to speak. It stemmed from damage to the semantic system. In other words, the semantic selection was disrupted, and/or access to the phonological code was completely blocked [Duffau et al., 2014]. Alexia was referred to as making errors when reading words. It might be associated with the disruption of the conversion between orthography and phonology. Therefore, the pattern of speech arrest represented the cortical regions that are necessary for speech planning, whereas that of anomia reflected the critical regions for semantic computation and that of alexia for orthography‐phonology transition.
Our results suggested that speech arrest was more easily elicited than anomia in the VPCG at BA6, which was reported to be responsible for speech programming and articulation [Fox et al., 2000; Shuster and Lemieux, 2005]. With respect to the pattern of anomia, no region was found to be more activated than speech arrest. One reason for this was the less frequent anomia elicited by cortical stimulation during naming [Corina et al., 2010]. However, the anomia activations observed in the posterior superior temporal gyrus/supramarginal gyrus (BA22, BA40) and the middle part of superior temporal gyrus (BA22) were strikingly consistent with the anomia pattern found in previous studies [Sanai et al., 2008; Tate et al., 2014]. This result indicates that both BA22 and BA40 were involved in semantic processing during picture naming, which was universally accepted in fMRI studies [Vigneau et al., 2006]. In addition, although the posterior middle frontal gyrus/adjacent precentral gyrus (BA6, BA9) was not reported by Sanai et al. [2008], both Tate et al. [2014] and this study found that stimulating this region could elicit anomia. One possible interpretation is that this region is responsible for orthography‐to‐semantics conversion [Siok et al., 2004]. However, its precise function during picture naming remains to be explored in future work. Finally, the alexia pattern also demonstrated several main brain regions, despite the low occurrence of positive sites. The supramarginal gyrus/angular gyrus (BA40, BA39), which had the highest positive rate and is involved in the conversion between orthography and phonology, lends support to Booth et al. [2002]'s fMRI‐based results. In addition, the activation of alexia in the posterior part of LMFG, which has only been found in Chinese data, might be supposed to participate in the orthography‐to‐phonology mapping that is unique to the Chinese language [Tan et al., 2003, 2005].
Similarities Between Chinese and English Production Maps
Our results share several similarities with the cortical representation of English production (number counting and picture naming). The general probability patterns of the ISM positive sites in our study included traditional speech production areas [Friederici, 2011; Hickok, 2012], which mainly encompassed Broca's area (BA 44/45) and the premotor cortex (BA 6). Our results also indicate that other areas, including a small portion of Wernicke's area (BA 22) and the Sylvian fissure at the parieto‐temporal boundary (temporal‐parietal junction/TPJ, BA 40) [Benzagmout et al., 2007; De Witt Hamer et al., 2011; McGirt et al., 2009], take part in language production (Figs. 2 and 3). This is consistent with the recent findings that indicate that the temporal‐parietal junction is another speech production area with functions of auditory‐motor integration and sending projections to the Broca's and premotor areas [Hickok, 2012; Hickok and Poeppel, 2007]. Moreover, both we and Sanai et al. [2008] detected tremendous inter‐individual variability in language areas (the maximum positive rate in our study was only 49% and in Sanai's was 30%). This indicates that individual‐based language mapping is extremely important for surgical planning, especially for protecting higher‐order language functions, as the anomia and alexia distributions were even more scattered. Finally, the ISM positive areas we found consisted of the dorsal stream of language processing in the previously established “dual‐stream model” [Hickok and Poeppel, 2004], which maps acoustic signals to frontal articulatory networks. In this pathway, the temporal‐parietal junction serves as a sensorimotor interface [Hickok, 2012]; the posterior half of the superior temporal gyrus mediates phonological code retrieval, and the left posterior IFG, syllabification. Future electrophysiological studies should determine the precise timing of each node to map the direction of information flow.
Differences Between Chinese and English Production Maps
In addition to the universal areas, we found that the LMFG, anterior to the superior portion of the ventral premotor cortex, was specific to Chinese compared with English maps for not only speech arrest but also anomia and alexia. In an fMRI meta‐analysis study, Tan et al. [2005] found that the LMFG plays a unique role in the phonological processing of Chinese reading. It was proposed to be responsible for phonology and the coordination of orthographic, phonological, and semantic information for Chinese reading [Booth et al., 2006; Cao et al., 2009; Kuo et al., 2001, 2003; Perfetti et al., 2005; Siok et al., 2004; Tan et al., 2003, 2005]. Because the tasks of number counting, picture naming and reading in our study were overt speech, language production involved at least two stages of processing: word level and phonological level [Hickok, 2012; Indefrey and Levelt, 2004; Levelt et al., 1999]. The inhibition of any stage by electrical stimulation might elicit positive results. We speculated that because reading and speech production might share a common pathway in the phonology component, the blocking of phonological processing in Chinese speakers would result in the difference between Chinese and English production maps in the LMFG. Another possible interpretation for this finding, according to Tang et al. [2006], is that there are differences in the brain representation for number processing between Chinese and English speakers. They reported that a region in‐between BA6, BA8, and BA9 in native Chinese speakers had much larger brain activation than did native English speakers during number processing. They believed that cultural variations may influence the representation of numerical concepts and that these factors may cause differential brain processes.
Moreover, we found that the VPCG (ventral part of BA6) is involved in Chinese more ventrally than in Sanai et al. [2008]'s English language study. They found activation in the superior part of the VPCG that did not extend to the Sylvian fissure. Sanai et al. [2008] did not find the inferior part of VPCG activations possibly because of registration errors from individuals to a common lattice in both our and study by Sanai et al. or errors in building correspondence between our and study by Sanai et al. lattice. Despite this slight difference, the studies share an important similarity in that the site with the largest percentage of total stimulations (peak coordinate) was located in the VPCG, which further confirmed the significance of VPCG to neurosurgical preservation of language function [Bizzi et al., 2012]. Recent PET and fMRI evidence also suggests that the ventral part of BA6 (the anterior part of VPCG) is involved in speech programming or planning [Fox et al., 2000; Shuster and Lemieux, 2005]. The involvement of VPCG in speech programming causes speech arrest (at positive sites) while stimulating the cortex of VPCG and making it a suitable target for the arcuate fascicle/superior longitudinal fasciculus [Bernal and Ardila, 2009]. This finding is consistent with a recent study generating a probabilistic map of critical functional regions in French speakers [Tate et al., 2014], which proposed that the VPCG, not the classical Broca's area, is the speech output region. In our previous study [Wu et al., 2015], we questioned their conclusion by analyzing the distribution of the speech output region and reconstructing the structure basis of function [Anwander et al., 2007], the frontal terminal territories of the arcuate fascicle/superior longitudinal fasciculus in 18 healthy subjects and in 34 patients. Our results supported the finding that the VPCG plays a key role in speech output. However, in some subjects (approximately 20%), the role of Broca's area (especially for pars opercularis) in speech output should also be considered [Friederici, 2011], not just the high‐order functions of language processing (e.g., lexical retrieval, phonological assembly, working memory) [Friederici, 2011; Vigneau et al., 2006]. In other words, both Broca's area and the VPCG are involved in speech output [Wu et al., 2015], which coincides with the patterns of both Chinese and English probabilistic maps (Fig. 5).
Results Validation
First, we constructed the pseudo‐colored maps of the total electrocortical stimulation sites for this study and for the study by Sanai et al. [2008]. Supporting Information Fig. 2 shows similar distributions and comparable bone windows, which indicated that the ISM positive rates were comparable between the two studies. In addition, differences between the two studies due to the methods per se were unlikely because we adopted exactly the same 2D patch‐like map generation method as Sanai et al. [2008].
Moreover, we confirmed the electrocortical technique‐based language maps with the data from fMRI‐based language mapping. We generated probability maps of the task activation for two language production tasks (verb generation (Supporting Information Fig. 3A) and covert recitation (Supporting Information Fig. 3B) across 15 patients who also underwent fMRI scan out of the 66 patients we involved (See Supporting Information). These probability maps resembled the ISM‐generated maps (Fig. 2A).
Finally, to address the concern whether tumor tissue induced bias in our language mapping, we conducted a picture naming fMRI task on 15 patients and on another group of 10 healthy volunteers (see Supporting Information). Then, we also created the probability maps of the picture naming task activation (Supporting Information Fig. 3D) for the healthy volunteers, which were quite similar to the patients’ result (Supporting Information Fig. 3C). This evidence suggests that the tumors did not obviously affect the language mapping. Moreover, the meta‐analysis‐like method we applied (producing the ALE map) added further evidence for our language mapping by considering the potential effect of the tumor location, size, grade, and the pre‐/post‐operative clinical evaluations.
Limitations of This Study
There were two limitations to this study. First, the number of patients involved in this study was still not large enough for building language maps because language areas have large inter‐individual variability. However, we had applied stringent inclusion and exclusion criteria to increase subject homogeneity and to reduce such a concern. Second, the observed difference in VPCG between Chinese and English production might be (at least partially) due to a mismatch to the underlay brain template or systemic error. Thus, the distinct in the VPCG should be interpreted carefully because both English and Chinese language could use this region.
To conclude, we for the first time mapped Chinese language production by means of intraoperative electrophysiology in a relatively large and homogeneous tumor patient's cohort. Our electrophysiological evidence confirms that the VPCG (ventral part of BA 6) and the pars opercularis (BA 44) play critical roles in speech output. In addition, the LMFG was shown to be more important in language production for Chinese speakers. The series of Chinese language production maps we produced may be important not only for basic linguistic cognition research but also for brain surgery planning for Chinese speakers.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors thank the neuropsychologists (Yan Zhou) for the language assessments; the nurses (Qiuyue Wu, Chunmei Li, Ye Wang) for their cooperation; MRI technician (Zhong Yang) for the collection of MRI data and Jianbing Shi, Geng Xu for technical support of neuronavigation and electrophysiological monitoring. They also do appreciate Dr. Berger for allowing us to use their precious data.
REFERENCES
- Anwander A, Tittgemeyer M, von Cramon DY, Friederici AD, Knosche TR (2007): Connectivity‐based parcellation of Broca's area. Cereb Cortex 17:816–825. [DOI] [PubMed] [Google Scholar]
- Benzagmout M, Gatignol P, Duffau H (2007): Resection of World Health Organization Grade II gliomas involving Broca's area: Methodological and functional considerations. Neurosurgery 61:741–752. discussion 752‐3. [DOI] [PubMed] [Google Scholar]
- Bernal B, Ardila A. (2009): The role of the arcuate fasciculus in conduction aphasia. Brain 132(Pt 9):2309–2316. [DOI] [PubMed] [Google Scholar]
- Bizzi A, Nava S, Ferre F, Castelli G, Aquino D, Ciaraffa F, Broggi G, DiMeco F, Piacentini S (2012): Aphasia induced by gliomas growing in the ventrolateral frontal region: Assessment with diffusion MR tractography, functional MR imaging and neuropsychology. Cortex 48:255–272. [DOI] [PubMed] [Google Scholar]
- Bolger DJ, Perfetti CA, Schneider W (2005): Cross‐cultural effect on the brain revisited: Universal structures plus writing system variation. Hum Brain Mapp 25:92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM (2002): Functional anatomy of intra‐ and cross‐modal lexical tasks. Neuroimage 16:7–22. [DOI] [PubMed] [Google Scholar]
- Booth JR, Lu D, Burman DD, Chou TL, Jin Z, Peng DL, Zhang L, Ding GS, Deng Y, Liu L (2006): Specialization of phonological and semantic processing in Chinese word reading. Brain Res 1071:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F, Peng D, Liu L, Jin Z, Fan N, Deng Y, Booth JR (2009): Developmental differences of neurocognitive networks for phonological and semantic processing in Chinese word reading. Hum Brain Mapp 30:797–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F, Lee R, Shu H, Yang Y, Xu G, Li K, Booth JR (2010): Cultural constraints on brain development: Evidence from a developmental study of visual word processing in mandarin Chinese. Cereb Cortex 20:1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F, Tao R, Liu L, Perfetti CA, Booth JR (2013a): High proficiency in a second language is characterized by greater involvement of the first language network: Evidence from Chinese learners of English. J Cogn Neurosci 25:1649–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F, Vu M, Chan DH, Lawrence JM, Harris LN, Guan Q, Xu Y, Perfetti CA (2013b): Writing affects the brain network of reading in Chinese: A functional magnetic resonance imaging study. Hum Brain Mapp 34:1670–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Vaid J, Bortfeld H, Boas DA (2008): Optical imaging of phonological processing in two distinct orthographies. Exp Brain Res 184:427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Xue G, Mei L, Chen C, Dong Q (2009): Cultural neurolinguistics. Prog Brain Res 178:159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coello AF, Moritz‐Gasser S, Martino J, Martinoni M, Matsuda R, Duffau H (2013): Selection of intraoperative tasks for awake mapping based on relationships between tumor location and functional networks. J Neurosurg 119:1380–1394. [DOI] [PubMed] [Google Scholar]
- Corina DP, Loudermilk BC, Detwiler L, Martin RF, Brinkley JF, Ojemann G (2010): Analysis of naming errors during cortical stimulation mapping: Implications for models of language representation. Brain Lang 115:101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Witt Hamer PC, Moritz‐Gasser S, Gatignol P, Duffau H (2011): Is the human left middle longitudinal fascicle essential for language? A brain electrostimulation study. Hum Brain Mapp 32:962–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Witt Hamer PC, Robles SG, Zwinderman AH, Duffau H, Berger MS (2012): Impact of intraoperative stimulation brain mapping on glioma surgery outcome: A meta‐analysis. J Clin Oncol 30:2559–2565. [DOI] [PubMed] [Google Scholar]
- Duffau H, Capelle L, Denvil D, Gatignol P, Sichez N, Lopes M, Sichez JP, Van Effenterre R (2003): The role of dominant premotor cortex in language: A study using intraoperative functional mapping in awake patients. Neuroimage 20:1903–1914. [DOI] [PubMed] [Google Scholar]
- Duffau H, Moritz‐Gasser S, Mandonnet E (2014): A re‐examination of neural basis of language processing: Proposal of a dynamic hodotopical model from data provided by brain stimulation mapping during picture naming. Brain Lang 131:1–10. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT (2009): Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: A random‐effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 30:2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT (2012): Activation likelihood estimation meta‐analysis revisited. Neuroimage 59:2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Ingham RJ, Ingham JC, Zamarripa F, Xiong JH, Lancaster JL. (2000): Brain correlates of stuttering and syllable production. A PET performance‐correlation analysis . Brain 123 (Pt 10):1985–2004. [DOI] [PubMed] [Google Scholar]
- Friederici AD (2011): The brain basis of language processing: from structure to function. Physiol Rev 91:1357–1392. [DOI] [PubMed] [Google Scholar]
- Ge J, Peng G, Lyu B, Wang Y, Zhuo Y, Niu Z, Tan LH, Leff AP, Gao JH (2015): Cross‐language differences in the brain network subserving intelligible speech. Proc Natl Acad Sci USA 112:2972–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- C Giussani, FE Roux, J Ojemann, EP Sganzerla, D Pirillo, C Papagno. (2010): Is preoperative functional magnetic resonance imaging reliable for language areas mapping in brain tumor surgery? Review of language functional magnetic resonance imaging and direct cortical stimulation correlation studies. Neurosurgery 66:113–120. [DOI] [PubMed] [Google Scholar]
- Hickok G (2012): Computational neuroanatomy of speech production. Nat Rev Neurosci 13:135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D (2004): Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition 92:67–99. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D (2007): The cortical organization of speech processing. Nat Rev Neurosci 8:393–402. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJ. (2004): The spatial and temporal signatures of word production components. Cognition 92:101–144. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Poole E (1974): The aphasia quotient: the taxonomic approach to measurement of aphasic disability. Can J Neurol Sci 1:7–16. [PubMed] [Google Scholar]
- Kuo WJ, Yeh TC, Duann JR, Wu YT, Ho LT, Hung D, Tzeng OJ, Hsieh JC (2001): A left‐lateralized network for reading Chinese words: A 3 T fMRI study. Neuroreport 12:3997–4001. [DOI] [PubMed] [Google Scholar]
- Kuo WJ, Yeh TC, Lee CY, Wu Y, Chou CC, Ho LT, Hung DL, Tzeng OJ, Hsieh JC (2003): Frequency effects of Chinese character processing in the brain: An event‐related fMRI study. Neuroimage 18:720–730. [DOI] [PubMed] [Google Scholar]
- Levelt WJ, Roelofs A, Meyer AS. (1999): A theory of lexical access in speech production. Behav Brain Sci 22:1–38; discussion 38–75. [DOI] [PubMed] [Google Scholar]
- Li XT (1983): The distribution of left and right handedness in Chinese people. Acta Psychol Sinica 15:268–276. [Google Scholar]
- Liu L, Peng D, Ding G, Jin Z, Zhang L, Li K, Chen C (2006): Dissociation in the neural basis underlying Chinese tone and vowel production. Neuroimage 29:515–523. [DOI] [PubMed] [Google Scholar]
- Liu H, Hu Z, Guo T, Peng D (2010): Speaking words in two languages with one brain: Neural overlap and dissociation. Brain Res 1316:75–82. [DOI] [PubMed] [Google Scholar]
- Lu JF, Wu JS, Yao CJ, Zhuang DX, Qiu TM, Hu XB, Zhang J, Gong X, Liang WM Mao Y, others. (2013a): Awake language mapping and 3‐Tesla intraoperative MRI‐guided volumetric resection for gliomas in language areas. J Clin Neurosci 20:1280–1287. [DOI] [PubMed] [Google Scholar]
- Lu JF, Zhang H, Wu JS, Yao CJ, Zhuang DX, Qiu TM, Jia WB, Mao Y, Zhou LF (2013b): “Awake” intraoperative functional MRI (ai‐fMRI) for mapping the eloquent cortex: Is it possible in awake craniotomy? NeuroImage: Clinical 2:132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubrano V, Draper L, Roux FE (2010): What makes surgical tumor resection feasible in Broca's area? Insights into intraoperative brain mapping. Neurosurgery 66:868–875; discussion 875. [DOI] [PubMed] [Google Scholar]
- Maldonado IL, Moritz‐Gasser S, Duffau H (2011): Does the left superior longitudinal fascicle subserve language semantics? A brain electrostimulation study. Brain Struct Funct 216:263–274. [DOI] [PubMed] [Google Scholar]
- Mandonnet E, Winkler PA, Duffau H (2010): Direct electrical stimulation as an input gate into brain functional networks: Principles, advantages and limitations. Acta Neurochir (Wien) 152:185–193. [DOI] [PubMed] [Google Scholar]
- Matsumoto R, Nair DR, LaPresto E, Najm I, Bingaman W, Shibasaki H, Luders HO. (2004): Functional connectivity in the human language system: A cortico‐cortical evoked potential study. Brain 127(Pt 10):2316–2330. [DOI] [PubMed] [Google Scholar]
- McGirt MJ, Mukherjee D, Chaichana KL, Than KD, Weingart JD, Quinones‐Hinojosa A. (2009): Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery 65:463–469; discussion 469–470. [DOI] [PubMed] [Google Scholar]
- Ojemann G, Ojemann J, Lettich E, Berger M (1989): Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg 71:316–326. [DOI] [PubMed] [Google Scholar]
- Perfetti CA, Liu Y, Tan LH (2005): The lexical constituency model: Some implications of research on Chinese for general theories of reading. Psychol Rev 112:43–59. [DOI] [PubMed] [Google Scholar]
- Sanai N, Mirzadeh Z, Berger MS (2008): Functional outcome after language mapping for glioma resection. N Engl J Med 358:18–27. [DOI] [PubMed] [Google Scholar]
- Shuster LI, Lemieux SK (2005): An fMRI investigation of covertly and overtly produced mono‐ and multisyllabic words. Brain Lang 93:20–31. [DOI] [PubMed] [Google Scholar]
- Siok WT, Perfetti CA, Jin Z, Tan LH (2004): Biological abnormality of impaired reading is constrained by culture. Nature 431:71–76. [DOI] [PubMed] [Google Scholar]
- Siok WT, Niu Z, Jin Z, Perfetti CA, Tan LH (2008): A structural‐functional basis for dyslexia in the cortex of Chinese readers. Proc Natl Acad Sci USA 105:5561–5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LH, Spinks JA, Gao JH, Liu HL, Perfetti CA, Xiong J, Stofer KA, Pu Y, Liu Y, Fox PT (2000): Brain activation in the processing of Chinese characters and words: A functional MRI study. Hum Brain Mapp 10:16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LH, Liu HL, Perfetti CA, Spinks JA, Fox PT, Gao JH (2001): The neural system underlying Chinese logograph reading. Neuroimage 13:836–846. [DOI] [PubMed] [Google Scholar]
- Tan LH, Spinks JA, Feng CM, Siok WT, Perfetti CA, Xiong J, Fox PT, Gao JH (2003): Neural systems of second language reading are shaped by native language. Hum Brain Mapp 18:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LH, Laird AR, Li K, Fox PT (2005): Neuroanatomical correlates of phonological processing of Chinese characters and alphabetic words: A meta‐analysis. Hum Brain Mapp 25:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Zhang W, Chen K, Feng S, Ji Y, Shen J, Reiman EM, Liu Y (2006): Arithmetic processing in the brain shaped by cultures. Proc Natl Acad Sci USA 103:10775–10780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate MC, Herbet G, Moritz‐Gasser S, Tate JE, Duffau H. (2014): Probabilistic map of critical functional regions of the human cerebral cortex: Broca's area revisited. Brain 137(Pt 10):2773–2782. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P (2012): Minimizing within‐experiment and within‐group effects in activation likelihood estimation meta‐analyses. Hum Brain Mapp 33:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, Mazoyer B, Tzourio‐Mazoyer N (2006): Meta‐analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. Neuroimage 30:1414–1432. [DOI] [PubMed] [Google Scholar]
- Wu CY, Ho MH, Chen SH (2012): A meta‐analysis of fMRI studies on Chinese orthographic, phonological, and semantic processing. Neuroimage 63:381–391. [DOI] [PubMed] [Google Scholar]
- Wu J, Lu J, Zhang H, Zhang J, Mao Y, Zhou L. (2015): Probabilistic map of language regions: Challenge and implication. Brain 138(Pt 3):e337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M, Wang J, He Y (2013): BrainNet viewer: A network visualization tool for human brain connectomics. PLoS One 8:e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Nie Y, Chang C, Gao J‐H, Niu Z (2014): Different patterns and development characteristics of processing written logographic characters and alphabetic words: An ALE meta‐analysis. Hum Brain Mapp 35:2607–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


