Abstract
Microbial biofilms are ubiquitous in nature, and they pose a serious threat to public health. Staphylococcus epidermidis is the most common clinical isolate from healthcare-and medical device-related biofilm infections. No antibiotic currently on the market can eradicate pathogenic biofilms, which contain complex defense mechanisms composed of slimelike extracellular polymeric substances. Understanding the need to develop alternative approaches, we examine 600 Da branched polyethylenimine (BPEI) against methicillin-resistant Staphylococcus epidermidis (MRSE) bio-films. Here, a microtiter biofilm model is used to test the synergistic effects between the two components of our combination treatment: BPEI and β-lactam antibiotics. Electron microscopy was used to confirm the growth of MRSE biofilms from the model. Minimum biofilm eradication concentration assays, crystal violet assays, and biofilm kill curves suggest that BPEI exhibits antibiofilm activity and can potentiate β-lactams to eradicate MRSE biofilms.
INTRODUCTION
Staphylococcus epidermidis belongs to the Gram-positive Staphylococcus genus. It has emerged as one of the most common causes of healthcare-associated infections due to the increasing use of medical implant devices.1,2 Unlike the coagulase-positive Staphylococcus aureus, S. epidermidis does not produce coagulase and therefore is classified under coagulase-negative staphylococci (CoNS). Accounting for about 70% of all CoNS on human skin, S. epidermidis is a leading cause of severe bloodstream infections. Approximately 80,000 cases of central venous catheter infections per year in the U.S. are caused by S. epidermidis.3 S. epidermidis is an opportunistic pathogen, like most of the CoNS, because they lack aggressive virulence factors (like those in S. aureus) and instead owe their pathogenic success to the ability to form biofilms.
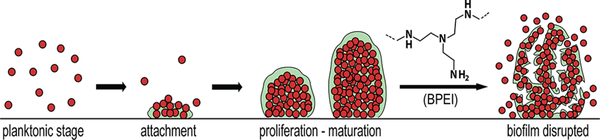
Biofilms are multicellular agglomerations of microorganisms enclosed in a matrix of extracellular polymeric substances (EPS). Containing polysaccharides, proteins, and extracellular DNA, the EPS matrix acts as a shield that protects the organisms from host defenses and antibiotics. Biofilms can adhere to either biotic or abiotic surfaces, such as cardiac pacemakers and catheters, and have a highly regulated defense mechanism that grants intrinsic resistance against antimicrobial agents.4 Biofilm development starts with an initial attachment of planktonic cells to a surface, which then grow into clusters of multicellular colonies (Figure 1). Subsequent cell–cell adhesions, divisions, and secretion of EPS create a threedimensional architecture designed to channel water and supply nutrients to the inner layers, thereby allowing for biofilm maturation. While the outer-layer cells remain metabolically active, the inner-layer cells are persister bacteria that often stay in a dormant state and thus are the most difficult to eradicate with antimicrobial treatments that only target growing organisms.5 During biofilm maturation, part of the biofilm can detach and disperse planktonic cells, which spread to colonize new surfaces. Mechanisms of biofilm maturation and detachment are poorly understood, but studies suggest that dispersed cells are more virulent and heighten the risk of acute infections.5,6
Figure 1.
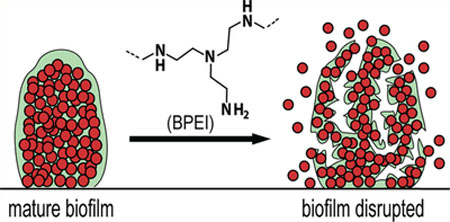
Biofilm formation stages and biofilm-disrupting ability of BPEI. BPEI disrupts established biofilm by collapsing the structure of EPS, releasing the cells into planktonic stage so that β-lactam antibiotics can actively target and eradicate them.
Biofilm defense mechanisms reduce antibiotic efficacy. The antibiotic concentrations required to eradicate biofilms are ten-to a thousand-fold higher than the concentrations required to kill planktonic bacteria, creating a burden on both public health and the economy from increased medical costs. Removal of biofilm-infected indwelling medical devices complicates treatments and interferes with the healing process.4 Additionally, persistent biofilms are also a leading cause of chronic wound infections. Around 60% of chronic wound specimens, compared to only 6% for acute wounds, were found to contain biofilms in which the prevalent species was Staphylococci.7 Thus, few publications offer information on S. epidermidis biofilm properties and antibiofilm testing, and the virulence and resistance factors of S. epidermidis biofilms are poorly understood.5 Nevertheless, it is necessary to investigate and to find a treatment for these dangerous biofilm infections.
Bacterial biofilms that are impenetrable to antibiotics pose an even greater threat when they are created by drug-resistant bacteria, such as methicillin-resistant S. epidermidis (MRSE). MRSA, MRSE, and their biofilms lead to chronic wound infections (i.e., wounds that have not proceeded through a reparative process in three months) that affect millions of Americans each year.8–10 With a dwindling arsenal of new antibiotics, existing drugs and regimens must be coupled with potentiators and re-evaluated as combination treatments for biofilms and antibiotic-resistant diseases. Our lab previously examined the efficacy of branched polyethylenimine (BPEI), a cationic polymer with a low molecular weight of 600 Da, against planktonic MRSE bacteria. Cationic BPEI with many primary and secondary amine groups indirectly targets the resistant factor PBP2a by electrostatically binding to anionic wall teichoic acid required for PBP2a activity. In this manner, BPEI disabled resistance in MRSE strains, restoring their susceptibility to traditional β-lactam antibiotics.11 In this report, we further investigate the application of our technology to MRSE biofilms where the EPS contains numerous anionic biomacromolecules. The EPS creates a hydrophobic barrier that increases the tolerance of bacteria toward antimicrobials by reducing or preventing the antibiotics from reaching their target(s). When BPEI binds to EPS, two changes will occur. First, the anionic character of the biofilm will be neutralized leading to a disruption of the EPS matrix. Second, because BPEI is a hydrophilic polymer, it will disrupt hydrophobic barriers that hinder diffusion of aqueous antibiotics. Thus, BPEI functions as a potentiator by inhibiting and disrupting the EPS matrix allowing antibiotics to enter and kill the bacteria. Using the minimum biofilm eradication concentration (MBEC) assay, which is represented in Figure 2 as a schematic flow, our study demonstrates that BPEI possesses antibiofilm activity itself at high concentrations. However, we also observe synergy between lower concentrations of BPEI and β-lactams against MRSE biofilms. Because it can both disable resistance mechanisms and eradicate biofilms, BPEI is a dual-function potentiator, making it a promising means of preventing and treating healthcare-associated S.epidermidis biofilms.
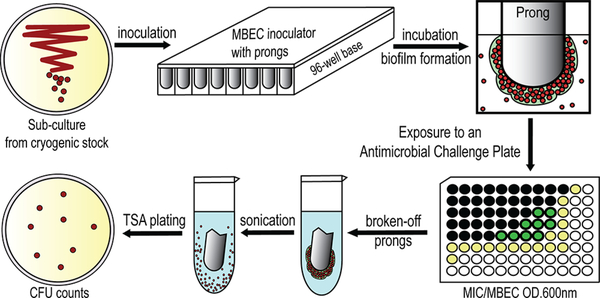
Figure 2.
A schematic experimental procedure of our microtiter biofilm model for synergistic effect screening against methicillin-resistant S. epidermidis (MRSE) biofilms. MBEC assays were carried out using MBEC inoculator, which is a microtiter plate lid with protruding prongs attached. Each prong fits into each well and allows bacterial biofilm to form and grow. Details are given in the Experimental Procedures. The method of CFU counting is detailed in Biofilm Kill Curve.
EXPERIMENTAL PROCEDURES
Materials.
In this experiment, the Staphylococcus epidermidis bacteria were purchased from the American Type Culture Collection (ATCC 29887, methicillin-resistant/biofilm-producer; ATCC 35984, methicillin-resistant/biofilm-producer; and ATCC 12228, methicillin-susceptible/nonbiofilm producer). Chemicals were purchased from Sigma-Aldrich (DMSO, growth media, and electron microscopy fixatives). Antibiotics were purchased from Gold Biotechnology. BPEI (600 Da) was purchased from Polysciences, Inc. MBEC Biofilm Inoculator with 96-well base plates were purchased from Innovotech, Inc.
MBEC Assay.
The MBEC assay is adapted from previous literature reports. 12–14
Inoculation and Biofilm Formation.
A subculture of MRSE was grown from the cryogenic stock on an agar plate overnight at 35 °C. The MBEC plate was inoculated with 150 μL of TSB/well plus 1 μL of a stock culture made from 1 colony/mL of MRSE in TSB. The MBEC inoculator plate was sealed with parafilm and incubated for 24 h at 35 °C with 100 rpm shaking to facilitate biofilm formation on the prongs. Following biofilm formation, the lid of the MBEC inoculator was removed and placed in a rinse plate containing 200 μL of sterile PBS for 10 s. Biofilm growth check (BGC) was performed by breaking a few prongs off using sterile pliers, submerging them in 1 mL PBS, and sonicating them on high for 30 min to dislodge the biofilm. After sonication, the biofilm solution was serial-diluted and spot-plated on agar plates for CFU counting to determine the biofilm density on the prongs.
Antimicrobial Challenge.
A challenge plate was made in a new presterilized 96-well plate in a checkerboard-assay pattern (following the methods of Lam et al.11) to test the synergistic activity of BPEI + antibiotic combinations. Antimicrobial solutions were serial-diluted and added to the 96-well plate, which contained 200 μL of cation-adjusted Mueller-Hinton broth (MHB) per well. Following the rinsing step and biofilm growth check, the MBEC inoculator lid was immediately transferred into the prepared antimicrobial challenge plate and incubated at 35 °C for 20–24 h.
Recovery and Quantitative MBEC.
After the challenge period, the MBEC inoculator lid was transferred into a recovery plate containing 200 μL of MHB per well, sonicated on high (Branson B-220, frequency of 40 kHz) for 30 min to dislodge the biofilm and then incubated at 35 °C for 20–24 h to allow the surviving bacterial cells to grow. After incubation, the OD600 (optical density at 600 nm) of the recovery plate was measured using a Tecan Infinite M20 plate reader to determine the MBEC of the antimicrobial compounds tested. A change in OD600 greater than 0.05 indicated positive growth. Likewise, the OD600 for the base of the challenge plate was measured immediately after inoculation to determine the MICs of the antimicrobial compounds. The fractional inhibitory concentration index (FICI) calculated based on the established equation15 was used to determine synergy (FICI < 0.5), additivity (0.5 < FICI < 1), and no synergy (FICI = 1).
Scanning Electron Microscopy.
MRSE 35984 cells were inoculated from 0.5% of an overnight culture and grown at 35 °C with shaking in the MBEC biofilm inoculator for 24 h to facilitate biofilm formation on the prongs. Prongs were broken off the plate using a sterile plier, submerged, treated with primary fixative (5% glutaraldehyde in 0.1 M cacodylate buffer) in a capped vial, and incubated at 4 ± 2 °C for 2 days. The prongs were removed from the fixing solution and air-dried for 72 h in a fume hood. They were mounted on aluminum stubs with carbon tape and sputter-coated with AuPd. A Zeiss NEON SEM was used to image the samples at 5 kV accelerating voltage.
In a different experiment, MRSE 35984 cells were inoculated from 0.5% of an overnight culture and grown at 35 °C with shaking in the MBEC biofilm inoculator for 3 days to ensure maturation of biofilms on the prongs. Nutrient media was replaced every 24 h. After 3 days, biofilms on the prongs were submerged into a new 96-well base with BPEI (512 μg/mL) for 24 h of treatment. Then, the prongs were broken off the plate using a sterile plier, submerged, fixed with primary fixative (5% glutaraldehyde in 0.1 M cacodylate buffer) in a capped vial, and incubated at 4 ± 2 °C for 2 days. The prongs were removed from the fixing solution and air-dried for 72 h in a fume hood. They were mounted on aluminum stubs with carbon tape and sputter-coated with AuPd. A Zeiss NEON SEM was used to image the samples at 5 kV accelerating voltage.
Biofilm Disrupting Assay.
Two similar sets of the experiment were conducted: one used 600 Da BPEI and the other used 10 000 Da BPEI. A subculture of MRSE 35984 was grown from the cryogenic stock on an agar plate overnight at 35 °C. A presterilized 96-well tissue-culture treated plate was inoculated with 100 μL of TSB/well plus 1 μL of a stock culture made from 1 colony/mL of MRSE in TSB. The plate was incubated at 35 °C for 24 h to form established biofilm.16,17 Planktonic bacteria were removed by washing five times with water. Crystal violet solution (0.1%) was used to stain the biofilm by adding 100 μL of the solution to each well for 15 min. The plate was then washed five times with water to remove all excess cells and dye. The plate was turned upside down and air-dried overnight.
Six separate treatments were performed on the preformed biofilm plate (total volume of 100 μL/well): untreated (negative control), BPEI-treated (32, 64, 128, and 256 μg/mL), and 30% acetic acid-treated (positive control). The treated samples were incubated at room temperature overnight to test the biofilm-disrupting ability of BPEI. Carefully, without touching the bottom of the plate, the solubilized solution in each well was transferred to a new flat-bottom plate for an absorbance measurement of OD550.The OD550 represents the amount of MRSE biofilm that was disrupted by BPEI, allowing for quantitative comparison of the controls and treated samples. Statistical data analysis among treated samples was performed using t test, n = 10.
Minimum Biofilm Inhibitory Concentration (MBIC).
MBIC assays were conducted in a similar fashion as checkerboard assays. The full procedure for checkerboard assays are outlined in Lam et al.11 In brief, a checkerboard assay was made in a 96-well plate to examine the synergy between BPEI and an antibiotic. After an overnight incubation, the cell suspension in the plate was discarded and washed with 100% methanol to retain only the biofilm attached on the well surface. Next, crystal violet solution (0.1%) was used to stain the bacterial biofilm for 15 min then washed three times with water. Stained biofilm was then dissolved with 95% ethanol for an OD550 (optical density at 550 nm) measurement. Data were subtracted from positive control values and reported.
Biofilm Kill Curve.
Biofilm was grown in an MBEC inoculator plate for 24 h with shaking to facilitate biofilm formation. At time zero, the prongs were sonicated in PBS for 30 min and then plated on agar for CFU counting. Four separate treatments were performed in a new 96-well base: Group 1 was the untreated control, Group 2 had 64 μg/mL of BPEI, Group 3 had 16 μg/mL of oxacillin, and Group 4 had a combination of 64 μg/mL of BPEI + 16 μg/mL of oxacillin. The prongs on the MBEC inoculator were washed in PBS for 10 s and then transferred into the new treated base plate and incubated. Agar CFU plating was performed at 2, 4, 8, and 24 h for each treatment group. All the agar plating was incubated at 35 °C and counted for colony forming units the next day. Each trial was done in duplicate.
RESULTS AND DISCUSSION
During the staphylococcal biofilm attachment stage, bacteria adhere to a surface through noncovalent interactions (e.g., electrostatic bonds) via microbial surface components recognizing adhesive matrix molecules. The next stages are biofilm proliferation and maturation, during which EPS (containing proteins, polysaccharide intercellular adhesin PIA/PNAG, teichoic acids, and eDNA) and channel architecture are produced. During the last stage, biofilm detachment and dispersal, phenol soluble modulin peptides disrupt the noncovalent interactions established in the attachment stage. To survive in the human body, pathogens need to cope with the host defense mechanisms: the innate immune system, which includes neutrophils and antimicrobial peptides (AMPs) and the acquired immune system, which includes antigen-dependent T and B cells. The latter is ineffective against MRSE infections for reasons that are not well understood.18 Because they have been colonizing human skin for millennia, perhaps S. epidermidis strains have evolved ways to evade the host defenses. These recalcitrant biofilms particularly threaten immunocompromised patients and those who need prosthetic limbs or artificial implant devices because biofilms can survive on abiotic surfaces for weeks to months.6 Motivated to join in the fight against MRSE biofilm infections, we are testing our combination treatment of BPEI and β-lactams in an MBEC microtiter biofilm model.
Confirmation of MRSE Biofilms.
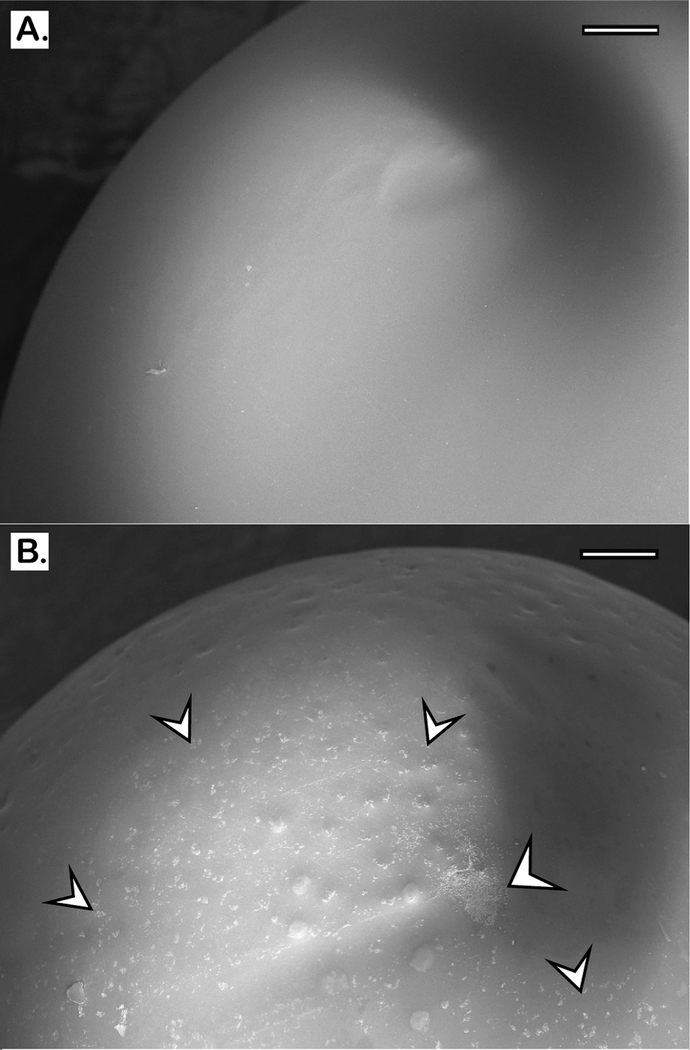
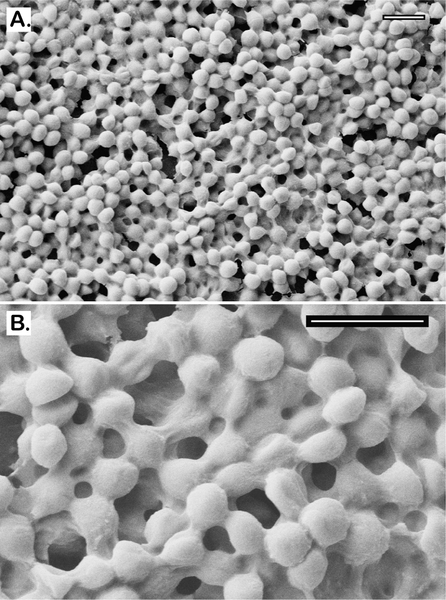
The MBEC plates with protruding-prong lids (shown in Figure 2) were used in our experiments to determine the antibiofilm activity of BPEI and conventional antibiotics. The prong lids with established biofilms can fit into regular 96-well microtiter plates for further antimicrobial assays. Many biofilm studies fail to confirm biofilm presence before applying treatments. In this study, scanning electron microscopy (SEM) was performed to confirm that MRSE biofilms formed on the prongs after 24 h inoculation. Compared to the smooth surface of the control prong (Figure 3A), numerous microcolonies of MRSE were found on the inoculated prong (Figure 3B), indicating that these prongs provide surfaces for biofilm attachment and development. To better characterize the MRSE biofilm morphology, higher magnifications were obtained. Images depict spherical cocci of MRSE bacteria enfolded in a “blanketlike” coat of EPS matrix (Figure 4A). The layers of bacteria are intertwined throughout the matrix, confirming the three-dimensional architecture and the existence of EPS in biofilms (Figure 4B). Among many substances in the EPS matrix, the poly-N-acetylglucosamine (PNAG, also known as PIA) polymer in particular was suggested to have a critical impact on S. epidermidis biofilms both in vitro19,20 and in vivo.21–23 Generated from the ica locus, this homopolymer is believed to interact with surface proteins and protect against host defense mechanisms during biofilm formation. Another important protective exopolymer is the pseudopeptide polymer poly-γ-DL-glutamic acid (PGA), which is encoded by the cap gene. Although PGA is produced in very small amounts, it plays a pivotal role in S. epidermidis resistance against host AMPs and leukocyte phagocytosis.24 These biopolymers, along with teichoic acids and eDNA, comprise the slimelike EPS coat. The SEM images confirm that established MRSE biofilms have formed before treatment with BPEI and β-lactam combinations.
Figure 3.
Scanning electron micrographs of the tip of MBEC prongs. A control prong with no bacteria is shown (A). MRSE 35984 biofilm colonies were formed after 24 h of inoculation (B); the arrows highlight some of the biofilm microcolonies. Scale bars = 200 μm.
Figure 4.
Scanning electron micrographs of MRSE 35984 biofilm. The intercellular matrices of EPS are captured as they wrap around every bacterium (A). At higher magnification, the EPS matrix is clearly shown to be sheltering the whole bacterial colony in an amorphous coat (B). Scale bars = 2 μm.
Efficacy of BPEI and β-Lactams against MRSE Biofilms.
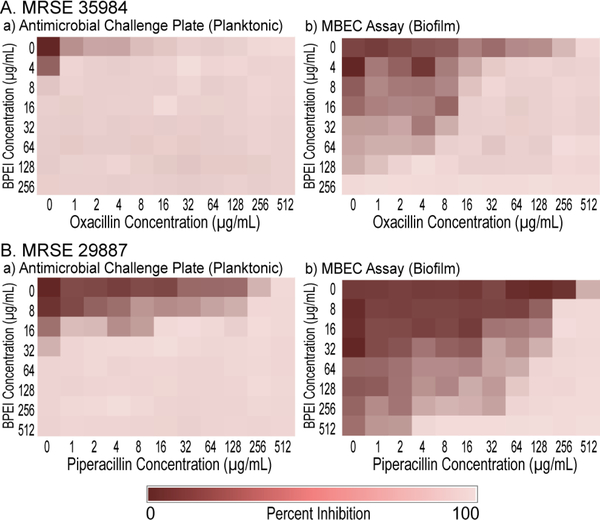
In our previous study, disabling PBP2a with 600 Da BPEI resensitizes MRSE to β-lactams.11 Here, we investigated a combination of BPEI and β-lactam antibiotics (oxacillin and piperacillin) against biofilms formed by two MRSE strains, MRSE ATCC 35984, and MRSE ATCC 29887. The MICs of BPEI and the antibiotics were found using the antimicrobial challenge plates, which measured the change in OD600 of planktonic bacteria. The results are consistent with our previously published study.11 Sonication of the prongs into a recovery plate allows us to measure the MBEC values, which were found to be much higher than the corresponding MIC values. This illustrates the intrinsic resistance of biofilms. For MRSE 35984, the oxacillin MIC is 16 μg/mL, whereas the oxacillin MBEC is 512 μg/mL (Figure 5A,a). Likewise, for BPEI the MIC is 8 μg/mL whereas the MBEC is 256 μg/mL (Figure 5A,b). Synergy occurs when an FICI is less than 0.5.15 With the addition of 8 μg/mL of BPEI, a synergistic effect lowered the MBEC of oxacillin from 512 to 32 μg/mL (FICI = 0.19). Higher amounts of BPEI lowered oxacillin MBEC values further, for instance, 32 jWg/mL of BPEI leads to an 8 jWg/mL MBEC value for oxacillin (FICI = 0.28). For MRSE 29887, the piperacillin MIC is 512 μg/mL, and the BPEI MIC is 64 μg/ mL (Figure 5B,a). Although the MBEC values for this strain were found to exceed 512 μg/mL (Figure 5B,b), synergy between 64 μg/mL piperacillin and 128 μg/mL BPEI (FICI = 0.19) eradicated the biofilms.
Figure 5.
Synergistic effects of BPEI and antibiotics against MRSE 35984 (A) and MRSE 29887 (B) on a 96-well checkerboard pattern. The synergy was seen both on the planktonic challenge plates (A,a and B,a) and the biofilm MBEC assays (A,b and B,b).
BPEI Possesses Biofilm-Disrupting Potential.
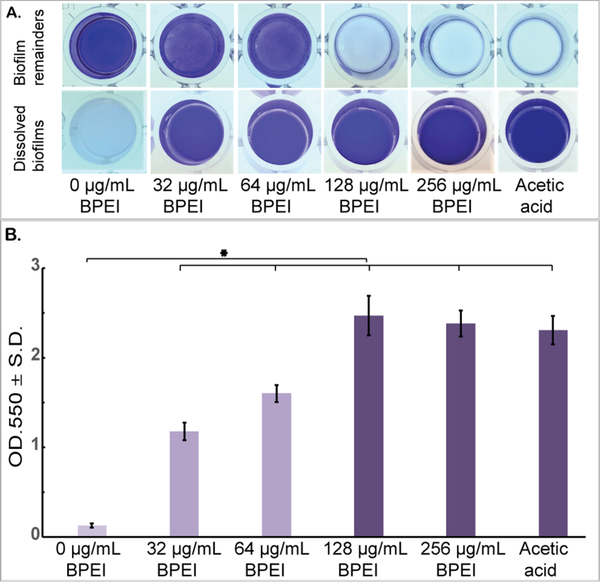
Antibio-film activity of BPEI was supported by the biofilm disrupting assay (Figure 6). Established biofilms of MRSE 35984 were stained with crystal violet and then treated with 32, 64, 128, and 256 μg/mL of 600 Da BPEI. A negative control (0 μg/mL BPEI) and a positive control (acetic acid) were also performed. After 20 h of treatment, BPEI-treated data was compared with the negative control using Student’s t test, and the results indicated that the MRSE biofilms were significantly dissolved by 600 Da BPEI (n = 10, p-value < 0.01). The dissolved biofilm solutions were carefully transferred to a new plate (without touching the bottom of the wells) for OD550 measurement. As shown in Figure 6A, MRSE biofilm remained intact in the bottom of the negative control well, while the biofilm in the 32 and 64 μg/mL BPEI-treated wells were partially dissolved into solution. Biofilms treated with 128 and 256 μg/mL BPEI were completely dissolved, as was the biofilm treated with the positive control of acetic acid. Figure 6B shows the OD550 values of the crystal violet absorbance, which represent the amount of biofilm dissolved in each treatment.
Figure 6.
Established MRSE 35984 biofilms stained with crystal violet were treated with 600-Da BPEI for 20 h, as well as the negative and positive controls. The dissolved biofilm solutions were transferred to a new plate, and the biofilm remainders are shown as top-down view (A). The mean OD550 of the dissolved biofilms was measured (B). Error bars denote standard deviation (n = 10). The MRSE biofilms were significantly dissolved by 600 Da BPEI (t test, p-value < 0.01, significant difference between the negative control and each treatment is indicated with an asterisk).
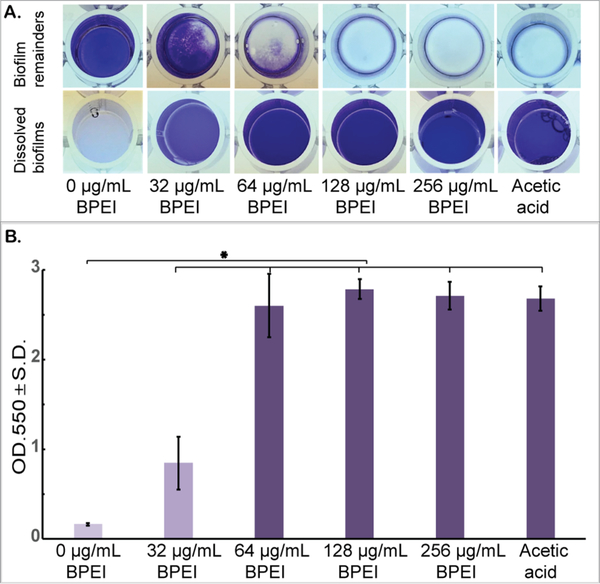
A similar experiment was conducted using 10 000 Da BPEI (Figure 7). As with 600Da BPEI, the t test indicated that 10 000 Da BPEI dissolved MRSE biofilms (n = 10, p-value < 0.01). Greater biofilm disruption effects were seen at 64 μg/ mL of 10 000-Da BPEI-treated samples (OD550 = 2.60, Figure 7B) than at 64 μg/mL of 600-Da BPEI-treated samples (OD550 = 1.59, Figure 6B). According to Wiegand et al., even though high molecular weight BPEIs (over 25 000 Da) are toxic, 600 Da BPEI has high biocompatibility and a low likelihood for mutagenesis.25 We have been able to confirm these observations using in vitro nephrotoxicity assay.26 Further-more, 600 Da BPEI is not toxic toward colon, kidney, and HeLa cells unless the concentration is orders-of-magnitude higher than the amount required for potentiation.26,27 These factors suggest that a combination treatment for biofilms of bacteria with or without antibiotic resistance could be accomplished with antibiotics given topically, orally, or intravenously while BPEI potentiators are administered topically. Using BPEI in this manner would disable biofilms and resistance mechanisms while lowering concerns of BPEI toxicity. Additional work is required to measure the trans-dermal flux and determine if toxic levels of 600 Da BPEI can reach the dermal and subcutaneous skin layers and thereby conceivably reach the bloodstream.
Figure 7.
Established MRSE 35984 biofilms stained with crystal violet were treated with 10 000 Da BPEI for 20 h, as well as the negative and positive controls. The dissolved biofilm solutions were transferred to a new plate, and the biofilm remainders are shown as top-down view (A). The mean OD550 of the dissolved biofilms was measured (B). Error bars denote standard deviation (n = 10). The MRSE biofilms were significantly dissolved by 10 000 Da BPEI (t test, p-value < 0.01, significant difference between the negative control and each treatment is indicated with an asterisk).
Biofilm Inhibition and Eradication Using a Combination of BPEI + β-Lactams.
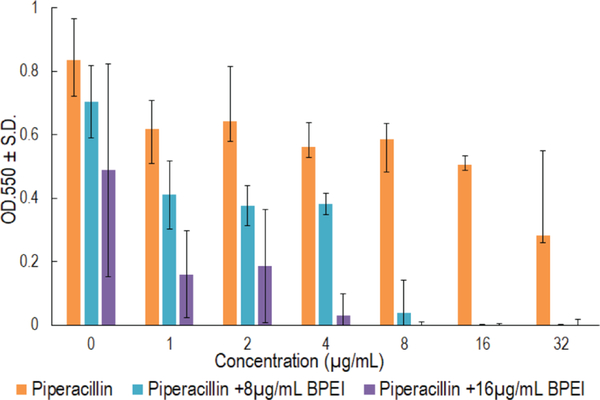
Crystal violet assays were used to demonstrate that BPEI synergizes with piperacillin to inhibit MRSE biofilm formation. This assay is similar to the checkerboard assay used in our previous study.11 However, we tested the antibiofilm activity instead of the MICs, so a modified procedure was used. Twenty-four hours after inoculation in a 96-well checkerboard plate containing combinations of 600 Da BPEI and piperacillin, the cell suspension supernatant was discarded, leaving the attached biofilms, which were then stained with crystal violet for measurement at OD550 to quantify the remaining biomass. The MBIC of BPEI was found to be 64 μg/mL, and the MBIC of piperacillin was 64 μg/mL. As shown in Figure 8, less biofilm formed in BPEI + piperacillin combination wells than in the piperacillin wells. Additionally, higher concentrations of BPEI corresponded to greater inhibition of biofilm formation. For example, 8 μg/mL of BPEI and 16 μg/mL of piperacillin prevented biofilm growth, however 16 μg/mL of BPEI also prevented biofilm growth when combined with 8 μg/mL of piperacillin. These results confirm that 600 Da BPEI possesses inhibitory activity against MRSE biofilms.
Figure 8.
Crystal violet absorbance represents MRSE 35984 biofilm biomass. Strong antibiofilm formation synergy between BPEI and piperacillin was observed, compared to individual piperacillin or BPEI-treated samples. Error bars denote standard deviation (n = 3). PIP, piperacillin.
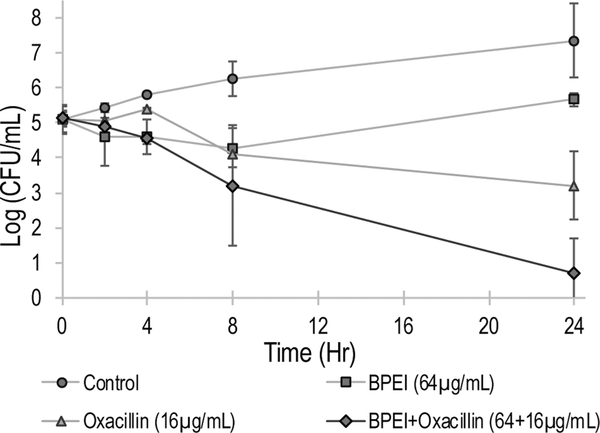
No antibiotic currently on the market can eradicate pathogenic biofilms but the combination treatment of BPEI + oxacillin showed promise. Established biofilms of MRSE 35984 were treated in four different groups: untreated control, BPEI-treated, oxacillin-treated, and combination (BPEI + oxacillin)-treated. A kill curve was generated to compare the antibiofilm activities of the treatments (Figure 9). Before treatment, all four groups had the same cell density of approximately 105 CFU/mL of bacteria. After treatments, the cell densities of each treated group were monitored by serial-diluting and agar-plating the sonicated prongs. Neither BPEI-treated nor oxacillin-treated groups could eradicate the biofilms, though they did inhibit the rate of the bacterial growth compared to the untreated control. At time 24 h, cell densities were ~107 CFU/mL in the control group, ~105 CFU/mL in the BPEI-treated group, and ~103 CFU/mL in the oxacillin-treated groups. Because implantable medical devices have ample surface area for bacterial colonization, even a low bacterial inoculum (~102 CFU/mL S. aureus27) can provoke an infection. Oxacillin did eradicate some biofilm, as indicated by its declining kill curve in Figure 9, but the remaining persister bacteria within the biofilm on the treated prongs (>103 CFU/mL at 24 h) are sufficient to grow and spread to new niches. Compared to the control group at time 24 h (~107 CFU/mL), the combination treatment of BPEI + oxacillin reduced the cell density of the biofilms by 100 000-fold (<101 CFU/mL), illustrating the combination’s potential to eradicate biofilms.
Figure 9.
Biofilm kill curve of MRSE 35984. Only the combination treatment of BPEI + oxacillin (64 μg/mL +16 μg/mL), the diamond-curve, could eradicate MRSE 35984 biofilms. Error bars denote standard deviation (n = 2). CFU, colony forming units.
Efficacy of BPEI on 3 Day Old Biofilms.
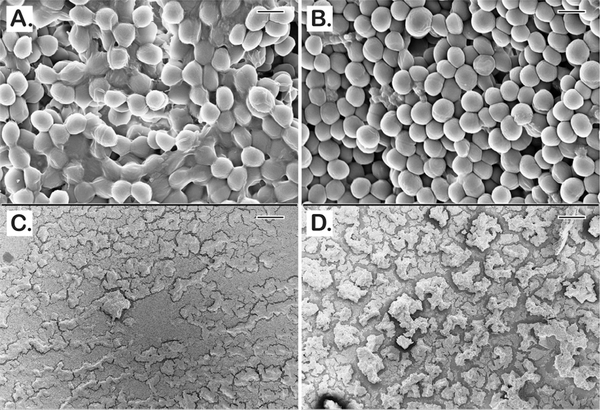
To test our technology against a more realistic chronic wound model, we qualitatively investigated BPEI’s effects on a 3 day old MRSE biofilm. MRSE 35984 was grown on the MBEC device for 3 days prior to treatment. Then, the untreated control and the BPEI-treated (512 μg/mL) samples were fixed and imaged for microscopic analysis. As shown in Figure 10, the untreated MRSE biofilms were thick and encased in EPS (Figure 10A) and they densely occupied the entire prong surface (Figure 10C). In contrast, after BPEI treatment, the EPS coat was visibly disrupted which reveals the bacterial cells with a thin or nonexistent EPS coating (Figure 10B) and a greater proportion of the prong surface was exposed (Figure 10D). These results indicate that BPEI not only can effectively potentiate antibiotics against planktonic cells but also against the established biofilms through an EPS-disruption mechanism. The exposure of the individual cells without the EPS protection would make them more vulnerable to antimicrobial agents, increasing the likelihood of clinical treatment success against persistent pathogenic biofilms.
Figure 10.
Scanning electron micrographs of established MRSE 35984 biofilms (3 day old). The untreated control sample shows thick EPS enfolding every bacterial cell (A). BPEI-treated sample shows disrupted EPS and significant number of exposed cells without the EPS (B). At lower magnification, the untreated control (C) biofilms appear with full and tightly occupied biofilms, whereas the BPEI-treated sample (D) shows disjointed biofilms by many revealed surfaces. Scale bars (A,B) = 1 μm. Scale bars (C,D) = 100 μm.
Many studies have tested cationic agents against pathogenic biofilms. In one study, Bottcher et al. used norspermidine, a natural trigger for Bacillus subtilis biofilm disassembly, to synthesize mimetic cationic compounds. Their compounds were able to disrupt established biofilms of B. subtilis and S. aureus. A library of synthetic guanidine and biguanidine polyamines were shown to inhibit biofilm formation although few were able to disrupt established biofilms.28 Our approach to biofilm treatment is unique because BPEI, a small macromolecule, can be a biofilm inhibitor and disruptor against not only the susceptible strains but also the resistant staphylococcal strains with the added benefit of disabling antimicrobial resistance mechanisms of planktonic cells. As a dual-function potentiator, 600 Da BPEI is a more powerful and versatile therapeutic agent than other cationic polymers.
CONCLUSION
Bacteria within a biofilm differ from their planktonic counterparts in a number of ways, including using the matrix of EPS, extracellular DNA, and extracellular WTA as barriers against antimicrobial agents.29–39 By targeting WTA-mediated resistance in MRSA/MRSE, 600 Da BPEI acts as a dual-function potentiator that can improve wound care outcomes by restoring potency to existing antibiotics.
Overall, the strong synergy between BPEI and β-lactam antibiotics make this combination a promising treatment for S. epidermidis biofilms because 600 Da BPEI’s in vitro cytotoxicity is low, and lower antibiotic concentrations reduce adverse side effects. Future experiments will be conducted to elucidate the antibiofilm mechanism of our combination therapy and to test our technology against more virulent superbugs. Potentially, the combined approach described in this study will make a positive change in the ongoing battle against MRSE biofilms
ACKNOWLEDGMENTS
This work was possible due to the kindness and contributions of Prof. Robert Cichewicz, Scott Russell, Ph.D., and Preston Larson, Ph.D. We would also like to acknowledge the valuable insight and suggestions provided by Prof. Robert Brennan, University of Central Oklahoma.
Funding
Funding was provided by the National Institutes of Health (CVR, R03AI142420–01) and Oklahoma Center of Advancement of Science and Technology (CVR, HR16–084-3) and The University of Oklahoma
ABBREVIATIONS
- MRSE
methicillin-resistant Staphylococcus epidermidis
- CoNS
coagulase-negative staphylococci
- BPEI
branched polyethylenimine
- SEM
scanning electron microscopy
- PBP
penicillin-binding protein
- PIA/PNAG
polysaccharide intercellular adhesin/poly-N-acetyl glucosamine
- DMSO
dimethyl sulfoxide
- PBS
phosphate-buffered saline
- MIC
minimum inhibitory concentration
- MBEC
minimum biofilm eradication concentration
- MBIC
minimum biofilm inhibitory concentration
- FICI
fractional inhibitory concentration index
- AuPd
gold palladium
- PIP
piperacillin
- HMDS
hexamethyldisilazane
- OD600
optical density at 600 nm
- OD550
optical density at 550 nm
- TSB
tryptic soy broth
- TSA
tryptic soy agar
- Da
dalton
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Percival SL; Suleman L; Vuotto C; Donelli G. Healthcare-associated infections, medical devices and biofilms: risk, tolerance and control. J. Med. Microbiol 2015, 64, 323–34. [DOI] [PubMed] [Google Scholar]
- (2).Nery PB; Fernandes R; Nair GM; Sumner GL; Ribas CS; Menon SM; Wang X; Krahn AD; Morillo CA; Connolly SJ; Healey JS Device-related infection among patients with pacemakers and implantable defibrillators: incidence, risk factors, and consequences. J. Cardiovasc Electrophysiol 2010, 21, 786–90. [DOI] [PubMed] [Google Scholar]
- (3).Maki DG; Kluger DM; Crnich CJ The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin. Proc 2006, 81, 1159–71. [DOI] [PubMed] [Google Scholar]
- (4).Romling U; Balsalobre C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med 2012, 272, 541–61. [DOI] [PubMed] [Google Scholar]
- (5).Wolfmeier H; Pletzer D; Mansour SC; Hancock REW New Perspectives in Biofilm Eradication. ACS Infect. Dis 2018, 4, 93–106. [DOI] [PubMed] [Google Scholar]
- (6).Otto M. Staphylococcus epidermidis-the ‘accidental’ pathogen. Nat. Rev. Microbiol 2009, 7, 555–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).James GA; Swogger E; Wolcott R; Pulcini E; Secor P; Sestrich J; Costerton JW; Stewart PS Biofilms in chronic wounds. Wound Repair Regen 2008, 16, 37–44. [DOI] [PubMed] [Google Scholar]
- (8).Wolcott R Disrupting the biofilm matrix improves wound healing outcomes. J. Wound Care 2015, 24, 366–71. [DOI] [PubMed] [Google Scholar]
- (9).Jones RE ; Foster DS; Longaker MT. Management of Chronic Wounds-2018. JAMA 2018, 320, 1481–1482. [DOI] [PubMed] [Google Scholar]
- (10).Sen CK; Gordillo GM; Roy S; Kirsner R; Lambert L; Hunt TK; Gottrup F; Gurtner GC; Longaker MT Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009, 17, 763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Lam AK; Hill MA; Moen EL; Pusavat J; Wouters CL; Rice CV Cationic Branched Polyethylenimine (BPEI) Disables Antibiotic Resistance in Methicillin-Resistant Staphylococcus epidermidis (MRSE). ChemMedChem 2018, 13, 2240–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Ceri H; Olson M; Morck D; Storey D; Read R; Buret A; Olson B, The MBEC assay system: Multiple equivalent biofilms for antibiotic and biocide susceptibility testing In Methods in Enzymology; Elsevier, 2001; Vol. 337, pp 377–385. [DOI] [PubMed] [Google Scholar]
- (13).Ceri H; Olson ME; Stremick C; Read RR ; Morck D; Buret A. The Calgary Biofilm Device: New Technology for Rapid Determination of Antibiotic Susceptibilities of Bacterial Biofilms. J. Clin. Microbiol 1999, 37 (6), 1771–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Olson ME; Ceri H; Morck DW; Buret AG; Read R R Biofilm bacteria: formation and comparative susceptibility to antibiotics. Can. J. Vet Res 2002, 66, 86–92. [PMC free article] [PubMed] [Google Scholar]
- (15).Odds FC Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [DOI] [PubMed] [Google Scholar]
- (16).Schuch R; Khan BK; Raz A; Rotolo JA; Wittekind M. Bacteriophage Lysin CF-301, a Potent Antistaphylococcal Biofilm Agent. Antimicrob. Agents Chemother. 2017, 61, No. e02666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Pabst B; Pitts B; Lauchnor E; Stewart PS Gel-Entrapped Staphylococcus aureus Bacteria as Models of Biofilm Infection Exhibit Growth in Dense Aggregates, Oxygen Limitation, Antibiotic Tolerance, and Heterogeneous Gene Expression. Antimicrob. Agents Chemother. 2016, 60, 6294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Pourmand MR; Clarke SR; Schuman RF ; Mond JJ; Foster SJ. Identification of antigenic components of Staphylococcus epidermidis expressed during human infection. Infect. Immun 2006, 74, 4644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Heilmann C; Gerke C; Perdreau-Remington F; Tz FG Characterization of Tn917 Insertion Mutants of Staphylococcus epidermidis Affected in Biofilm Formation. INFECT. IMMUN 1996, 64, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Heilmann C; Schweitzer O; Gerke C; Vanittanakom N; Mack D; Gotz F. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol 1996, 20, 1083–91. [DOI] [PubMed] [Google Scholar]
- (21).Rupp ME; Fey PD; Heilmann C; Gotz F. Characterization of the importance of Staphylococcus epidermidis autolysin and polysaccharide intercellular adhesin in the pathogenesis of intra-vascular catheter-associated infection in a rat model. J. Infect. Dis 2001, 183, 1038–42. [DOI] [PubMed] [Google Scholar]
- (22).Rupp ME; Ulphani JS; Fey PD; Mack D. Characterization of Staphylococcus epidermidis polysaccharide intercellular adhesin/hemagglutinin in the pathogenesis of intra-vascular catheter-associated infection in a rat model. Infect. Immun 1999, 67, 2656–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Chokr A; Leterme D; Watier D; Jabbouri S. Neither the presence of ica locus, nor in vitro-biofilm formation ability is a crucial parameter for some Staphylococcus epidermidis strains to maintain an infection in a guinea pig tissue cage model. Microb. Pathog 2007, 42, 94–7. [DOI] [PubMed] [Google Scholar]
- (24).Kocianova S; Vuong C; Yao Y; Voyich JM; Fischer ER; DeLeo FR; Otto M. Key role of poly-gamma-DL-glutamic acid in immune evasion and virulence of Staphylococcus epidermidis. J. Clin. Invest 2005, 115, 688–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Wiegand C; Bauer M; Hipler UC; Fischer D. Poly(ethyleneimines) in dermal applications: biocompatibility and antimicrobial effects. Int. J. Pharm 2013, 456, 165–74. [DOI] [PubMed] [Google Scholar]
- (26).Foxley MA; Wright SN; Lam AK; Friedline AW; Strange SJ; Xiao MT; Moen EL; Rice CV Targeting Wall Teichoic Acid in Situ with Branched Polyethylenimine Potentiates beta-Lactam Efficacy against MRSA. ACS Med. Chem. Lett 2017, 8, 1083–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Foxley MA; Friedline AW; Jensen JM; Nimmo SL; Scull EM; King JB; Strange S; Xiao MT; Smith BE; Thomas Iii KJ; Glatzhofer DT; Cichewicz RH; Rice CV Efficacy of ampicillin against methicillin-resistant Staphylococcus aureus restored through synergy with branched poly(ethylenimine). J. Antibiot 2016, 69, 871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Bottcher T; Kolodkin-Gal I; Kolter R; Losick R; Clardy J. Synthesis and activity of biomimetic biofilm disruptors. J. Am. Chem. Soc 2013, 135, 2927–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Jennings LK; Storek KM; Ledvina HE; Coulon C; Marmont LS; Sadovskaya I; Secor PR; Tseng BS; Scian M; Filloux A; Wozniak DJ; Howell PL; Parsek MR Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 11353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Sadovskaya I; Vinogradov E; Li J; Jabbouri S. Structural elucidation of the extracellular and cell-wall teichoic acids of Staphylococcus epidermidis RP62A, a reference biofilm-positive strain. Carbohydr. Res 2004, 339, 1467–73. [DOI] [PubMed] [Google Scholar]
- (31).Vinogradov E; Sadovskaya I; Li J; Jabbouri S. Structural elucidation of the extracellular and cell-wall teichoic acids of Staphylococcus aureus MN8m, a biofilm forming strain. Carbohydr. Res 2006, 341, 738–43. [DOI] [PubMed] [Google Scholar]
- (32).Wagstaff JL; Sadovskaya I; Vinogradov E; Jabbouri S; Howard MJ Poly-N-acetylglucosamine and poly(glycerol phosphate) teichoic acid identification from staphylococcal biofilm extracts using excitation sculptured TOCSY NMR. Mol BioSyst 2008, 4, 170–4. [DOI] [PubMed] [Google Scholar]
- (33).Holland LM; Conlon B; O’Gara JP Mutation of tagO reveals an essential role for wall teichoic acids in Staphylococcus epidermidis biofilm development. Microbiology 2011, 157, 408–18. [DOI] [PubMed] [Google Scholar]
- (34).Joo HS; Otto M. Mechanisms of resistance to antimicrobial peptides in staphylococci. Biochim. Biophys. Acta, Biomembr 2015, 1848, 3055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Krismer B; Weidenmaier C; Zipperer A; Peschel A. The commensal lifestyle of Staphylococcus aureus and its interactions with the nasal microbiota. Nat. Rev. Microbiol 2017, 15, 675–687. [DOI] [PubMed] [Google Scholar]
- (36).Laverty G; Gorman SP; Gilmore BF Biomolecular mechanisms of staphylococcal biofilm formation. Future Microbiol 2013, 8, 509–24. [DOI] [PubMed] [Google Scholar]
- (37).Arciola CR; Campoccia D; Speziale P; Montanaro L; Costerton JW Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 2012, 33, 5967–82. [DOI] [PubMed] [Google Scholar]
- (38).Bucher T; Oppenheimer-Shaanan Y; Savidor A; Bloom-Ackermann Z; Kolodkin-Gal I. Disturbance of the bacterial cell wall specifically interferes with biofilm formation. Environ. Microbiol. Rep 2015, 7, 990–1004. [DOI] [PubMed] [Google Scholar]
- (39).O’Neill E; Pozzi C; Houston P; Smyth D; Humphreys H; Robinson DA; O’Gara JP Association between methicillin susceptibility and biofilm regulation in Staphylococcus aureus isolates from device-related infections. J. Clin Microbiol 2007, 45, 1379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]