Abstract
Although there is emerging data on the effects of blast‐related concussion (or mTBI) on cognition, the effects of blast exposure itself on the brain have only recently been explored. Toward this end, we examine functional connectivity to the posterior cingulate cortex, a primary region within the default mode network (DMN), in a cohort of 134 Iraq and Afghanistan Veterans characterized for a range of common military‐associated comorbidities. Exposure to a blast at close range (<10 meters) was associated with decreased connectivity of bilateral primary somatosensory and motor cortices, and these changes were not different from those seen in participants with blast‐related mTBI. These results remained significant when clinical factors such as sleep quality, chronic pain, or post traumatic stress disorder were included in the statistical model. In contrast, differences in functional connectivity based on concussion history and blast exposures at greater distances were not apparent. Despite the limitations of a study of this nature (e.g., assessments long removed from injury, self‐reported blast history), these data demonstrate that blast exposure per se, which is prevalent among those who served in Iraq and Afghanistan, may be an important consideration in Veterans' health. It further offers a clinical guideline for determining which blasts (namely, those within 10 meters) are likely to lead to long‐term health concerns and may be more accurate than using concussion symptoms alone. Hum Brain Mapp 36:911–922, 2015. Published 2014. This article is a U.S. Government work and is in the public domain in the USA.
Keywords: fMRI, Veterans, blast injuries, traumatic brain injury, mild traumatic brain injury
INTRODUCTION
Combat‐related traumatic brain injuries (TBIs) are often caused by exposure to detonation of explosive munitions [Cernak and Noble‐Haeusslein, 2010]. Although there is emerging data on the possible detrimental neurobiological effects of even mild TBI (or concussion) due to blasts [Goldstein et al., 2012; Han et al., 2014; Mac Donald et al., 2011; McKee and Robinson, 2014; Omalu et al., 2011], the effect of blast exposure itself on brain structure and function is still virtually unknown. According to a survey of Veterans [Hoge et al., 2008], not only were 75% of the TBIs during their service in Iraq due to explosive blasts, such as those from improvised explosive devices (IEDs), but over half of the soldiers who did not report TBIs were still exposed to two or more nearby IEDs. Animal models demonstrate numerous deleterious effects of blast at both the cellular [Goldstein et al., 2012; Saljo et al., 2000, 2002; Svetlov et al., 2009] and behavioral [Cernak et al., 2001; Tompkins et al., 2013; VandeVord et al., 2012] level in the absence of blunt trauma; however, only recently have the effects of blast exposure in humans been considered.
Blunt TBIs due to contact or acceleration traumas produce injuries through tissue shearing and rapid changes in pressure within the cranial cavity as solids and fluids in the brain react at different speeds. Each of these can be caused by blast pressure waves [Cernak and Noble‐Haeusslein, 2010], and the TBIs due to blunt and blast traumas have been shown to produce similar secondary injury cascades at the cellular level [Cernak and Noble‐Haeusslein, 2010]. Studies in humans have found that, although concussions due to blast or blunt forces produce similar symptoms [Lippa et al., 2010], they are associated with different patterns of brain activation as measured by functional imaging [Fischer et al., 2014]. The over‐ and under‐pressurization from the blast shock wave can cause changes in the solubility of blood gasses [DePalma et al., 2005], providing a distinct mechanism from blunt TBI that may have important consequences on neural health. In animal models, controlled blast exposure has been shown to be sufficient to cause behavioral [Cernak et al., 2001; Tompkins et al., 2013; VandeVord et al., 2012] and histological [Goldstein et al., 2012; Saljo et al., 2000, 2002; Svetlov et al., 2009] changes. Further, recent studies have found differences in diffusion imaging measures [Bazarian et al., 2012; Taber et al., in press] in Veterans with blast exposure.
Resting state functional connectivity magnetic resonance imaging (fcMRI) provides an index of inter‐regional communication in the brain [Biswal et al., 1995]. The strength of functional connectivity among regions within the “default mode” network (DMN) has been examined in several studies of clinical phenomena, as this network is reliably detected and well‐characterized. Further, the regions comprising this network are highly connected to additional extra‐network regions, potentially making these connections sensitive to changes with disease [Lee et al., 2013; Shehzad et al., 2009]. Previous studies in individuals with post‐traumatic stress disorder (PTSD) [Sripada et al., 2012], and individuals with TBI in the acute phase of the injury [Sharp et al., 2011] as well as in a period of chronic symptomatology [Mayer et al., 2011], have demonstrated that functional connectivity within the DMN can be a useful tool for understanding the types of phenomena that are likely to affect Veterans.
Unlike many other conditions affecting Veteran health, there are no established methods for classifying the severity of a blast in the absence of physical injury or acute symptoms of concussion. While severity of brain injury is most likely proportional to the intensity of the pressure wave, this measurement is rarely, if ever, available. Judging blast severity by concussion symptomatology, as is common practice for both research and clinical applications, may be misleading. In fact, some researchers have proposed separate symptom‐based severity guidelines [Ling et al., 2009]. Blast neurotrauma without concurrent blunt concussion may result in an altogether different constellation of symptoms, and those that might occur are likely to be obscured by comorbid psychological trauma, as they are for military mild TBI [Lippa et al., 2010]. Therefore, as a practical measure, we have defined blast injury severity by spatial proximity, using the classifications from the Boston Assessment of Traumatic Brain Injury‐Lifetime (BAT‐L) [Fortier et al., 2013].
Research into the nature of any single military hazard, such as blast exposure, is complicated by other coexisting conditions. Military blast exposure not only increases the risk of blunt‐force traumatic brain injury, it may also increase the risk of psychological consequences such as PTSD; both of which may affect brain structure and function independently of the direct physical forces of the blast itself. Furthermore, explosive devices may result in the individual being exposed to toxic fumes, thrown by the explosion, or struck by debris [DePalma et al., 2005]. Military exposure including this type of physical trauma subsequently contributes to a range of psychiatric phenomena including PTSD, sleep disorder, chronic pain, depression, and alcohol or drug abuse [Otis et al., 2011; Seal et al., 2007].
We examined how self‐reported blast exposure and evidence of TBI was related to functional connectivity in a cohort of Operation Enduring Freedom/Operation Iraqi Freedom/Operation New Dawn (OEF/OIF/OND) Veterans from the Translational Research Center for TBI and Stress Disorders (TRACTS) cohort. Many individuals in this study experienced one or more exposures to blast or TBIs during their deployment(s). Each participant was characterized through extensive neuropsychological testing and clinical interviews as described previously [Fortier et al., 2013; Lindemer et al., 2013]. Using this characterization, we examined the influence of close‐range blast exposure on neural integrity considering a range of other potential influences. The data demonstrate that close‐range blast exposure is associated with alterations in functional connectivity that are not apparent in mTBI (as defined by symptoms) or blast exposures at greater distances, and are not explained by other demographic or clinical factors. In all, this suggests that Veterans with close‐range blast exposure (but no associated symptoms) may represent an overlooked population with neural integrity equally influenced by their service as those with concussions due to blasts.
MATERIALS AND METHODS
Participants
The first 203 consecutively recruited participants in the TRACTS VA RR&D Center of Excellence were included. A full‐time recruitment specialist who attended Yellow Ribbon Events, Task Force Meetings, and other Veteran‐associated activities in the Boston metropolitan area recruited participants, who were primarily OEF/OIF/OND service members and Veterans, but approximately 5% of the sample had not yet been deployed.
Twelve individuals were excluded because of history of seizures; prior serious medical illness; current Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) diagnosis of bipolar disorder, schizophrenia, or other psychotic disorder (except psychosis not otherwise specified due to trauma‐related hallucinations); or cognitive disorder not caused by TBI. Of those remaining, 159 were MRI eligible; however, seven of these did not complete the imaging. A further 13 participants were excluded due to excessive motion. Five of the 139 remaining participants had lifetime history of TBI that was either moderate or severe. These participants were included in analyses unless otherwise noted. Five participants (including one with a history of moderate or severe TBI) had incomplete neuropsychological or clinical interview data, and had to be excluded from those analyses that relied on these data. Complete records for this study after these exclusions were available from 134 participants (114 men; ages 19–62, mean 33.0 ± 8.6 y).
The institutional review board at the VA Boston Healthcare System approved all research procedures. All participants gave informed consent prior to participating in the study.
Demographic, Psychological, and Behavioral Assessment
Demographic and deployment information was collected using self‐report questionnaires. Combat exposure was assessed using the Deployment Risk and Resilience Inventory (DRRI) Combat Experiences Scale [King et al., 2006]. Intelligence Quotient (IQ) was estimated using the Wechsler Test of Adult Reading (WTAR) [Holdnack, 2001].
Pain and sleep quality were assessed using self‐report questionnaires. Pain was assessed using the Short Form McGill Pain Questionnaire [Melzack, 1987]. Current pain was defined as current severity level of “mild” or greater. The Pittsburgh Sleep Quality Index (PSQI) was administered to measure sleep quality, with the recommended score of 5 or greater used to define sleep disturbance [Buysse et al., 1989].
History of blast and TBI were acquired using the Boston Assessment of TBI‐ Lifetime (BAT‐L) [Fortier et al., 2013]. This is a semistructured interview based on the Department of Defense diagnostic criteria that addresses the lifetime history of brain injury. It includes self‐reported history of head trauma and blast exposure, including classification of each blast exposure event into a distance range (0–10 m, 11–25 m, 26–100 m). The diagnostic tool was developed at the TRACTS Center of Excellence, and is intended to focus on injuries that are unique to Veterans from this specific cohort, including a comprehensive assessment of blast exposure and blast‐related TBI.
Current psychological conditions were assessed using a structured interview administered by doctoral‐level psychologists. Posttraumatic Stress Disorder (PTSD) symptoms were quantified using the Clinician Administered PTSD Scale (CAPS) [Blake et al., 2006]. The structured clinical interview for DSM‐IV Axis I disorders non‐patient edition (SCID) [First et al., 1996] was administered to assess for other Axis I diagnoses and to screen for psychotic disorders. Diagnoses of TBI, PTSD, and other psychological disorders were determined through a consensus meeting consisting of at least three doctoral‐level psychologists.
Magnetic Resonance Imaging
Neuroimaging data were acquired on a 3‐Tesla Siemens (Erlangen, Germany) TIM Trio scanner, using a 12‐channel brain array. Two MPRAGE (Magnetization Prepared Rapid Gradient Echo) T1‐weighted anatomical scans (TR/TE: 2530/3.32 ms, flip angle: 7°, 1 mm isotropic) were acquired for surface reconstruction, functional connectivity seed placement, and inter‐participant registration. Resting state functional data were acquired in two runs (gradient echo echo‐planar imaging, TR/TE: 3000/30 ms, flip angle: 90°, 3 × 3 × 3.75 mm, 38 slices, 6 min per run). Prior to each run, participants were instructed to keep their eyes open and stay awake.
Image Processing
Neuroimaging data were processed using a combination of FreeSurfer [Fischl et al., 1999a], AFNI [Cox, 1996], and FSL [Jenkinson et al., 2012], based primarily on the FSFAST processing stream (http://freesurfer.net/fswiki/FsFast). Surface models were reconstructed from anatomical images using FreeSurfer as described previously [Lindemer et al., 2013]. Resting state fMRI scans for each subject were processed using a standard stream (motion correction, time shifting, concatenation of scans, motion regressed from time series, regression of the global mean and the average time courses from the white matter and the ventricles, band pass filtering between 0.01 and 0.1 Hz). Time points, runs, and sessions with excessive motion were excluded (0.5 mm/TR; 20 TRs/run; 30 TRs/session). Data were sampled to and smoothed on the surface, and each brain was warped to a surface‐based template (fsaverage) [Fischl et al., 1999b]. Seed regions were derived from surface‐based parcellations of the cortex [Fischl et al., 2002]. The bilateral superior third of the isthmus of the cingulate, as defined within each participant's native space, was used as a seed region. The vertex‐wise partial correlation to the seed was used for further group‐level analyses.
Statistical Analysis
Group differences and associations (see Table 1) were computed over the cortex using multiple linear regression with FreeSurfer's mri_glmfit, and MATLAB (Mathworks; Natick, MA). Current CAPS score, age, gender, and WTAR estimated IQ were included in all models as nuisance regressors, unless otherwise noted. These factors were chosen because they showed relationships with connectivity when analyzed individually in preliminary analyses (data not shown). Where possible, all participants that fit the analysis criteria were included. When this produced greatly unbalanced groups, comparisons instead used age‐ and CAPS‐matched participants. For these analyses, age was matched within 2 years and CAPS was matched within a score of 5 where available. For the matched close‐range blast exposure analysis, participants were matched for history of moderate or severe TBI, current PTSD diagnosis, current diagnosis of substance dependence, number of lifetime TBIs (within 1), total number of blast exposures within 100 m (log of the total within 0.3, i.e., not more than double), WTAR Estimated IQ (within 10 points), age (within 5 years), and gender. Family‐wise corrections for multiple comparisons were simulated using mri_glmfit‐sim, with vertex‐ and cluster‐wise thresholds of P = 0.05.
Table 1.
Summary of Imaging Analyses and Results
| Analysis | n (group n) | Selection Method | Additional Controlling Variablesa | Significant Regions |
|---|---|---|---|---|
| Close‐range Blast Exposure versus No Close‐range Blast | 134 (39/95) | All available | None; Log10(LOC); months deployed; months since deployment; pain; sleep | Bilateral primary somatosensory and motor cortices, bilateral supplementary motor area |
| Blast TBI versus no Blast TBI | 40 (20/20) | Age/PTSD matched | None | n.s. |
| Blast‐TBI versus Close blast only | 40 (20/20) | All available | None; PTSD not included | n.s. |
| Lifetime TBI versus No TBI | 134 (44/90) | All available | None | n.s. |
| Military TBI Comorbid with PTSD versus PTSD only | 73 (36/37) | All available | None | n.s. |
| Lifetime TBI Comorbid with PTSD versus PTSD only | 35 (17/18) | Age/PTSD matched | None | n.s. |
| 3 or more TBI versus 2 or less | 38 (19/19) | Age/PTSD matched | None; CB | n.s. |
| Log10(Combined time Loss‐of‐consciousness) | 134 | All available | None; CB | L. sup. temp, R. somatosensory cortex |
| Log10(Combined time Post‐traumatic Amnesia) | 134 | All available | None; CB | n.s. |
| Log10(Combined time Altered Mental State) | 134 | All available | None; CB | L. sup. temp, R. somatosensory cortex |
| Log10(Combined time Loss‐of‐consciousness) | 130 | Moderate and severe TBIs removed | None; CB | n.s. |
Unless otherwise noted, current PTSD severity score (CAPS), age, gender, and WTAR Estimated IQ were included as nuisance variables. CB = close blast status; LOC =loss‐of‐consciousness; n.s. = not significant.
RESULTS
In this report, we consider blast exposure independently of concussive symptoms associated with TBI, under the hypothesis that some brain injuries due to blast may not produce symptoms traditionally associated with concussion due to blunt trauma. Most participants (72%) had blast exposures at distances up to 100 meters, so focus was given to individuals who experienced at least one blast exposure within close proximity, defined as self‐report of blast <10 meters away. We then examined the significance of this proximity threshold, and examined other demographic and clinical factors that differed between participants with and without close‐range blast exposure and might provide an alternative explanation for the effects found. We considered close‐range blast exposure in the context of TBI, and, finally, examined measures of TBI itself for comparison (see Table 1).
Demographic Factors
For participants who were included in the imaging analysis, those with close‐range blast exposure differed from those without in number of TBIs, deployment history, and PTSD (Table 2). The entire cohort (including those that were not eligible for MRI scanning) additionally differed in age, gender, pain, and sleep (Supporting Information Table S1). Age, gender, and PTSD severity (as well as WTAR Estimated IQ, which did not differ between groups; see Methods) were included in all regression analyses below, unless noted otherwise.
Table 2.
Demographic and Current Psychiatric and Behavioral Characteristics of Participants with and without Close‐range Blast Exposure
| Total (n = 139) | Close‐range Blast (n = 41) | No Close‐range Blast (n = 98) | P‐value | |
|---|---|---|---|---|
| Age | 33.0 ± 8.7 | 31.2 ± 8.5 | 33.7 ± 8.8 | 0.127 |
| Men | 86.3% | 90.2% | 84.7% | 0.385 |
| Education | 13.9 ± 1.9 | 13.6 ± 1.9 | 14.0 ± 1.9 | 0.175 |
| WTAR estimated IQ | 100.8 ± 10.8 | 99.9 ± 10.9 | 101.2 ± 10.9 | 0.551 |
| OEF/OIF Deployments | ||||
| Number of deployments | 1.2 ± 0.6 | 1.5 ± 0.7 | 1.1 ± 0.5 | 0.002 |
| Duration (months) | 13.2 ± 8.3 | 16.8 ± 9.6 | 11.8 ± 7.3 | 0.004 |
| Months since deployment | 31.9 ± 26.2 | 33.9 ± 26.8 | 31.1 ± 26.0 | 0.567 |
| Combat exposure | 14.3 ± 11.5 | 22.7 ± 11.6 | 10.7 ± 9.4 | <0.001 |
| Traumatic Brain Injurya | ||||
| Number of blasts | 2, 9, 61 | 11, 30, 65 | 1, 4, 51 | 0.125 |
| Number of lifetime TBIs | 1, 2, 4 | 2, 3, 5 | 1, 1, 3 | <0.001 |
| Number of military TBIs | 0, 1, 2 | 1, 2, 3 | 0, 0, 1 | <0.001 |
| Number of blast TBIs | 0, 0, 1 | 0, 1, 2 | 0, 0, 0 | <0.001 |
| Loss‐of‐consciousness (min) | 0, 1, 20 | 0, 1, 30 | 0, 1, 15 | 0.341 |
| Post‐traumatic amnesia (min) | 0, 5, 121 | 0.2, 5, 67 | 0, 5, 121 | 0.306 |
| Altered mental state (min) | 3, 43, 1560 | 10.3, 92, 4350 | 1, 10.3, 365 | 0.191 |
| Psychiatric Conditions | ||||
| PTSD | 55.4% | 75.6% | 46.9% | 0.002 |
| Mood disorders | 23.7% | 22.0% | 24.5% | 0.748 |
| Panic disorder | 5.0% | 7.3% | 4.1% | 0.426 |
| Social phobia | 7.9% | 7.3% | 8.2% | 0.867 |
| Generalized Anxiety Disorder (GAD) | 6.5% | 7.3% | 6.1% | 0.794 |
| Other anxiety disorder | 5.0% | 9.8% | 3.1% | 0.195 |
| Alcohol abuse/dependence | 18.7% | 24.4% | 16.3% | 0.266 |
| Other abuse/dependence | 4.3% | 4.9% | 4.1% | 0.833 |
| Current pain | 68.5% | 74.3% | 66.3% | 0.387 |
| Average pain in 30 days | 25.3 ± 22.3 | 29.3 ± 25.2 | 23.7 ± 21.0 | 0.210 |
| Sleep impairment | 68.4% | 77.5% | 64.5% | 0.140 |
| Sleep quality | 8.9 ± 4.7 | 9.6 ± 4.7 | 8.6 ± 4.7 | 0.259 |
All subjects in the cohort are included, even those that were unable to participate in MRI scans. Note: The percentages displayed in this table are column percentages. Comparisons that are significant at P < 0.05 are indicated in bold.
Due to skewed distribution, the median, 75th percentile, and 95th percentile values are included rather than mean and standard deviation.
There were no differences in the number of volumes censored (P > 0.7), nor in the mean (t‐test, P > 0.4) or distribution (Kolmogorov–Smirnoff test, P > 0.4) of TR‐to‐TR motion during the scan between participants with and without close‐range blast exposure.
Blast Exposure
Individuals exposed to blasts at close range had significantly different connectivity between the seed and the primary somatosensory cortex and the pre‐supplementary motor area than those who had not experienced a blast at this proximity (regardless of TBI status) (Table 1, Figs. 1 and 2). This difference was not influenced by the removal of participants with moderate or severe TBI. Time since and duration of deployment in OEF/OIF, average pain, sleep quality, and summed time of TBI‐induced loss‐of‐consciousness were each added (separately) as potential confounders in the group comparison. These factors did not influence the group results.
Figure 1.

Effect of close‐range blast and TBI on connectivity. Group average correlation maps are shown for participants without (a) and with (b) history of close‐range blast, and without (d) and with (e) history of TBI. Group difference significance map for close‐range blast exposure (c) and TBI history (f). Yellow outlines indicate regions of significant anti‐correlation in the group average of all subjects, vertex‐wise significance is uncorrected. Reduced functional connectivity in the close‐range blast/lifetime concussion groups are indicated in blue. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 2.
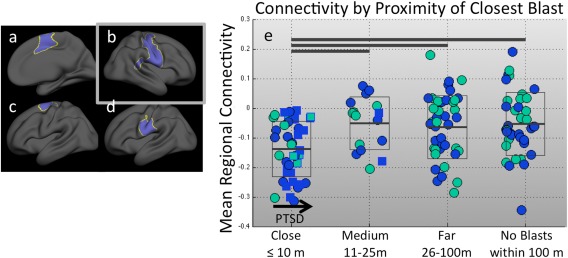
Effect of blast proximity on connectivity. (a–d) Significant clusters from the close‐range blast group analysis are shown in blue. (e) Standard box plot of connectivity in the region shown in (b) for all participants, grouped by proximity of nearest blast. Horizontal bars at the top of the chart indicate significant group differences at P < 0.05. Within groups, the x‐axis indicates current PTSD symptom severity score, where the leftmost box border is a CAPS score of 0, the rightmost is a CAPS score of 117 (the maximum of the group), and the midpoint is a CAPS score of 59. Blue fill color indicates a history of blunt TBI, while green indicates no history of blunt TBI. Square markers with blue outlines indicate participants with blast TBI history. Only the close‐range blast group demonstrates the lowered connectivity. Participants with blasts at greater distances have very similar means to those participants with no blast exposure. Thus, no gradual change with increasing proximity is apparent. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Closely matched participants (n = 16) were chosen with consideration for number of lifetime TBIs, total number of blast exposures (at any distance), current PTSD diagnosis, current diagnosis of substance dependence, WTAR Estimated IQ, and age (see Methods). All of these participants were male, and none had a history of moderate or severe TBI. Participants were not matched on CAPS score, but there was no mean difference between the groups in CAPS score. When these groups were compared, they also show significant differences in bilateral somatosensory cortex. These participants were then compared on the factors listed in Table 2, and did not significantly differ for any demographics except number of blast TBIs and summed time of LOC and PTA.
Figure 1 shows that the boundaries of significant group difference for close‐range blast corresponded to the boundaries of regions showing “anti‐correlation” to the seed region (see Discussion). Thus, the effect of close‐range blast was to increase the extent of the regions exhibiting significant anticorrelation. That is to say, the default mode connectivity (after global signal regression) in this area was more negative in the close‐range blast group (Fig. 1).
Blast Proximity
To examine 10 meters as a threshold of clinical significance, participants were grouped by the proximity range (based on categorizations used in the BAT‐L) of the closest blast they reported. Ranges for close, medium, and far blast are 10 m or less, 11–25 m, and 26–100 m, respectively [Fortier et al., 2013]. A final group reported no blast exposures within 100 m. The suppressed connectivity was only apparent in the group with close‐range blast (included in Fig. 2 for reference). There was no difference between the other three groups, suggesting that this was not a gradual change, but rather that the regions identified by the group analysis were, in fact, specific to the close blast group. A one‐way ANOVA demonstrated that the medium‐range, far‐range, and no blast exposure groups were not statistically different for any region identified in the close‐range blast group analysis (P > 0.8).
Blast TBI
It should be noted that nearly all (18/20) participants with blast TBI (that is, those who reported concussion symptoms after a blast exposure) had been exposed to at least one blast within 10 m, and 16 of the 20 blast‐related TBIs were due to blasts at distances less than 10 m. The median self‐reported (and self‐estimated) distance of blasts associated with blast TBIs was 3 m.
Participants with history of blast TBI (n = 20) did not differ in functional connectivity from age‐ and PTSD symptom‐matched participants without history of blast TBI. These control participants may have had either blast exposure or other TBIs, as participants without these could not be matched on age and PTSD symptoms. Participants with blast TBI were compared to participants with close‐range blast exposure but no blast TBIs (n = 20), and did not significantly differ from them. These groups were not well matched for PTSD, which was significantly higher in the blast TBI group; however, regardless of inclusion of PTSD severity in the regression model, significant group differences were not apparent.
TBI History
Figure 3 shows connectivity in the regions identified by the close‐range blast group analysis (in the right sensorimotor areas) in participants grouped into mutually exclusive groups based on combined blast and TBI history (Note: participants were grouped as blast TBI even if they had never experienced a close‐range blast, or their blast TBI was due to a blast of greater distance). These analyses confirmed earlier analyses that connectivity in this region was similar between participants with and without TBI due to blast, and suppressed compared to any other TBI/blast history.
Figure 3.

Functional connectivity by blast and TBI history. (a) Comparison of connectivity to DMN seed in region shown in (b). Participants are categorized by TBI and blast history, as illustrated in (c). Within groups, the x‐axis represents current PTSD symptom severity score, as described in Figure 2. Participants who had reported any history of moderate or severe TBI are indicated with square markers with red outlines. Blue fill color indicates history of blunt TBI and green fill color indicates no history of blunt TBI. The mean correlation map is shown for each group (threshold at ρ = 0.10). The blast TBI (1) and close‐range blast (2) groups were significantly different from all remaining groups. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table I shows analyses of cortex‐wide group differences based on TBI history, which is given more clinical consideration than blast exposure. Group divisions were made in multiple ways. Where possible, close‐range blast exposure was included in the model, but in some cases this was confounded with group assignments and could not be included. First, any participant who had experienced at least one TBI was compared to participants who had never experienced a TBI (as shown in Fig 1). Given the potentially confounding influence of PTSD in this population and its association with TBI, the analysis was repeated, but restricted to only those participants with a current diagnosis of PTSD, and those TBIs occurring during military service. Potential effects of repetitive trauma were assessed by comparing participants with 3 or more TBIs to those with less than three, matched by age and PTSD severity. For each participant, the total time over all TBI events of loss‐of‐consciousness, post‐traumatic amnesia, and altered mental state were summed (separately) and transformed to a logarithmic scale to mitigate influential points. The correlation of connectivity to these summary TBI measures was calculated. Most of these analyses did not exhibit statistical group differences. The exception is the correlation of connectivity to length of loss‐of‐consciousness and also, to a lesser extent, altered mental state summed over all TBIs in the individual's lifetime. However, when participants with moderate or severe TBI were removed from the analysis, the effect was no longer apparent. Therefore, there were no significant associations with mTBI and connectivity in this cohort.
DISCUSSION
These data demonstrate that exposure to a munitions blast less than 10 meters away is associated with alterations in resting state functional connectivity to the isthmus of the cingulate, a primary region within the default mode network (DMN), as measured in a cohort of OEF/OIF/OND Veterans. In contrast, our analyses did not find evidence that history of a diagnosed mild TBI had an influence on DMN connectivity, nor did blasts at a greater distance. Connectivity differences due to close‐range blast were not influenced by whether or not the blast had resulted in a concussion at the time; when subjects with blast TBI and close‐range blast exposure were compared, there were no significant differences. The differences were also not attributable to sleep quality, pain, length of or time since deployment, or PTSD symptoms, although these clinical factors were associated with close‐range blast exposure in the larger cohort.
Regions of group difference due to close‐range blast exposure fell between areas of “anti‐correlation” to the seed (Fig. 1), which appeared to spread into regions associated with the sensorimotor network. The data processing procedures (namely, the inclusion of the global mean time course in the regression model), which were used to reduce inter‐subject variability, result in correlation measures that are relative to other brain regions. These “anti‐correlated” brain regions, generally associated with salience, central executive, and dorsal attention networks, are those regions of the brain that were least correlated (but not necessarily negatively correlated [Murphy et al., 2009]) to the seed time course. The physiological implications of this relationship to the DMN, even in healthy populations, are not well understood. It is telling that areas of apparent anti‐correlation are consistent across subjects [Van Dijk et al., 2010], arise from other noise correction processes [Keller et al., 2013], and are in relative agreement with more direct measures of neural activity [Chai et al., 2012]. However, because this is a particularly indirect measure of neural function and can be sensitive to many neural and non‐neural features, it is currently unclear what a change in the relative connectivity near the anti‐correlation would mean neurophysiologically. The spread presented here could be explained by hyperactivity (and decreased variability) in salience and attention networks or a change in vascular coupling that modulates the functional MRI signal itself. In a preliminary analysis (data not shown) using a seed in primary visual areas, significant group differences were observed at a more limited scale than those seen using the DMN, but they did not correspond to the anti‐correlation to the visual network. However, the sensorimotor network, which exhibited changes in connectivity to the isthmus of the cingulate seed region, has been shown previously to be affected in mTBI, and thus could equally be the source of the group difference. It has been shown to have altered within‐network fMRI connectivity [Kasahara et al., 2010], and transcranial magnetic stimulation (TMS) excitability [DeBeaumont et al., 2007] in mild TBI. Further, similar regions were found to be abnormal in a recent functional connectivity study using graph theoretical methods to compare blast‐exposed and blast TBI Veterans [Han et al., 2014].
The absence of significant effects of concussion in this cohort is notable. It is unlikely that the lack of significant differences is due to insufficient power in the sample because there was sufficient power to detect differences with blast exposure. With the full number of participants included in the analyses, we should be able to detect a group difference in correlation to the seed of approximately 0.04, which is half the size of the difference seen in the close‐range blast analysis. The expected size of the smallest detectable difference will be larger for the analyses that utilize only a subgroup, however, these are still not larger than what was detected in the close‐range blast difference. Thus, any of the analyses presented should have been able to detect an effect of similar size to what was observed in association with close‐range blast exposure. While other studies of TBI using DMN connectivity have demonstrated that it is sensitive to the injury, those studies generally select for TBI patients that are symptomatic, either chronically or acutely [Mayer et al., 2011; Sharp et al., 2011]. In this study, we drew from a large cohort of at‐risk individuals, who were, on average, 36 months post‐deployment, and 5 years removed from their most severe TBI at the time of participation. Although it is possible that some participants were experiencing prolonged symptoms from TBI, it is generally found that in Veterans, prolonged neuropsychological symptoms are better attributed to pain, depression, or PTSD, rather than mild TBI [Lippa et al., 2010]. Thus, it is likely that most, if not all, of these participants have recovered from previous concussions, and the TBIs that were observed in this study are not comparable to those described elsewhere as having resulted in changes in functional connectivity. If this is the case, it implies that the effects of close‐range blast exposure are more persistent than those of blunt concussion. It is also notable that studies of connectivity changes in TBI often report altered connectivity within the network [Kasahara et al., 2010; Mayer et al., 2011; Sharp et al., 2011], whereas our findings were altered connectivity between the DMN and regions outside the network. This may mean that, in addition to resolving on a different time scale, the primary blast injury is affecting the brain in a fundamentally different way than has been previously observed in blunt TBI. There is some evidence that the vasculature may be particularly vulnerable to blast rather than blunt trauma [Cernak and Noble‐Haeusslein, 2010; Ling et al., 2009; McKee and Robinson, 2014], and vascular damage is a plausible explanation for the results presented, however, the current data do not address this directly.
In this work, distance from a blast was treated as though it were a reliable proxy to the magnitude of a pressure wave; however, this is known to be inaccurate. In addition to the variability of the blasts themselves, pressure waves due to any given blast can deviate greatly from an open‐field prediction based on distance due to obstacles in the blast field that can magnify or attenuate the pressure wave [Nakagawa et al., 2011]. We do not know the explosive equivalent for any given blast event or the surrounding environment's effect on the reflecting wave, but it may be reasonable to assume that the probability of an intervening shielding obstacle increases with increasing distance. Thus, 10 meters is probably not a distance of particular mechanical significance—it is unlikely that this could be reliably translated to an injury threshold of pressure—but rather serves as a useful clinical guideline for assessing the significance of a blast or the blast history of a patient. It is worth noting that, while blasts within 10 meters are predictive of differences in functional connectivity, the variability within each group was such that functional connectivity measurements themselves are not able to provide a reliable diagnostic tool or biomarker at this time.
This study used a large, well‐characterized cohort design, allowing us to examine the observed changes in the context of other relevant co‐existing conditions. This is especially important in the Veteran population due to the numerous comorbidities that are common. Participants in the study were not recruited specifically for any particular trauma, and constitute a representative sample of service members from the Boston metropolitan area [Department of Defense, 2012]. However, there is still a possible selection bias for participants who are willing and able to come to the VA hospital for research studies. Participants with close‐range blast exposure were compared to the remainder of the participants on a number of clinical and demographic factors that may bias the results. The MRI‐eligible participants with close‐range blast exposure were more likely to have a diagnosis of PTSD; had more numerous deployments, which totaled a greater number of months; and had a greater number of TBIs. The larger cohort (including those who were not MRI‐eligible) additionally differed in sleep quality and pain. While the goal of this study was not to establish clinical effects of close‐range blast exposure, it is noteworthy that these group differences exist, and they will be explored further in future work. None of these factors were able to account for the group difference in connectivity. However, there may be numerous other factors that could simultaneously affect brain connectivity and alter the probability of a participant experiencing or reporting a close‐range blast exposure.
Changes in brain activity and connectivity, such as those reported here, are not necessarily accompanied by acute clinical symptoms. In the blunt TBI literature, particularly in sports‐related TBI, there is mounting evidence that subclinical (i.e., asymptomatic) head trauma may produce changes that can still be measured through neuropsychological testing [Moser et al., 2005] or fMRI [Breedlove et al., 2012]; can increase severity of subsequent injuries [Longhi et al., 2005]; and may lead to long‐term health concerns [McKee et al., 2013]. Similarly, it is possible that these alterations in connectivity mark an injury where Veterans are predisposed to further injury or health problems [McKee and Robinson, 2014]. While this is of great concern, the current study cannot address whether or not this is the case. Analyses of non‐concussion symptoms and health effects associated with close‐range blast exposure, as well as longitudinal observation, would be valuable, and are left for future study with this cohort.
Blast exposure is not often studied as an injury in itself unless it results in a TBI. For this reason, the long‐term effects of blast exposure are not clear. In the TRACTS cohort, roughly one third of the participants had a history of either close‐range blast exposure or blast TBI, and 72% reported at least one blast exposure within 100 meters. This suggests that any health effects of these brain changes could result in tremendous costs to the Veterans healthcare system, potentially similar or greater than the estimated $100 million annually due to TBI [RAND, 2008]. Although TBI in military populations receives considerable attention from the media and the research community, this may not accurately describe the complete picture of the healthcare concerns of Veterans when they return home.
CONCLUSION
In summary, blast exposure at close range (<10 m) is associated with altered DMN functional connectivity, whereas mild TBI and blasts at greater distance are not. This is true for close‐range blast exposures even when they did not result in concussion symptoms at the time. This is especially important to consider given the high incidence rate of head injuries and blast exposures in this cohort, and the high estimated cost of care. At present, the clinical effects of these changes are unknown; nevertheless, they are concerning and merit further consideration by the Veteran healthcare community.
Supporting information
Supplementary Information Table 1.
ACKNOWLEDGMENTS
The authors would like to acknowledge Michael Esterman, PhD, Sara Lippa, PhD and Benjamin Trotter, for helpful feedback; Vincent Corbo, PhD and Deborah LaFlamme for assistance with imaging data collection; Pedram Parva, MD, Melissa Amick, PhD, Catherine Fortier, PhD, and Alexandra Kenna, PhD for clinical evaluations; Walter Musto CMSgt (Ret), RING for tireless efforts in recruiting participants on our behalf; and the TRACTS team for all their hard work.
REFERENCES
- Bazarian J, Donnelly K, Peterson D, Warner G, Zhu T, Zhong J (2012): The relation between posttraumatic stress disorder and mild traumatic brain injury acquired during Operations Enduring Freedom and Iraqi Freedom. J Head Trauma Rehabil 28:1–12. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin F, Haughton V, Hyde J (1995): Functional Connectivity in the motor cortex ofthe resting human brain using echo‐planar MRI. Magn Reson Med 34:537–541. [DOI] [PubMed] [Google Scholar]
- Blake D, Weathers F, Nagy L, Kaloupek D, Gusman F, Charney D, Keane T (2006): The development of a clinician‐administered PTSD scale. J Trauma Stress 8:75–90. [DOI] [PubMed] [Google Scholar]
- Breedlove E, Robinson M, Talavage T, Morigaki K, Yoruk U, O'Keefe K, King J, Leverenz L, Gilger J, Nauman E (2012): Biomechanical correlates of symptomatic and asymptomatic neurophysiological impairment in high school football. J Biomech 45:1265–1272. [DOI] [PubMed] [Google Scholar]
- Buysse D, Reynolds C, Month T, Berman S, Kupfer D (1989): The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res 28:193–213. [DOI] [PubMed] [Google Scholar]
- Cernak I, Noble‐Haeusslein L (2010): Traumatic brain injury: An overview of pathobiology with emphasis on military populations. J Cereb Blood Flow Metab 30:255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernak I, Wang Z, Jiang J, Bian X, Savic J (2001): Cognitive deficits following blast injury‐induced neurotrauma: Possible involvement of nitric oxide. Brain Inj 15:593–612. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Castanon AN, Ongur D, Whitfield‐Gabrieli S (2012): Anticorrelations in resting state networks without global signal regression. Neuroimage 59:1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R (1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173. [DOI] [PubMed] [Google Scholar]
- DeBeaumont L, Lassonde M, Leclerc S, Theoret H (2007): Long‐term and cumulative effects of sports concussion on motor cortex inhibition. Neurosurgery 61:329–337. [DOI] [PubMed] [Google Scholar]
- DePalma R, Burris D, Champion H, Hodgson M (2005): Blast Injuries. N Engl J Med 352:1335–1342. [DOI] [PubMed] [Google Scholar]
- Department of Defense (2012): Demographics Profile of the Military Community. Department of Defense: Washington DC. [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. 1996. Structured Clinical Interview for the DSM‐IV Axis I Disorders. Biometrics Research Department, New York.
- Fischer B, Parsons M, Durgerian S, Reece C, Mourany L, Lowe M, Beall E, Koenig K, Jones S, Newsome M, Scheibel R, Wilde E, Troyanskaya M, Merkley R, Walker M, Levin H, Rao S (2014): Neural Activation during response inhibition differentiates blast from mechanical causes of mild to moderate traumatic brain injury. J Neurotrauma 31:169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM (1999a): Cortical surface‐based analysis. II: Inflation, flattening, and a surface‐based coordinate system. Neuroimage 9:195–207. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM (1999b): High‐resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp 8:272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002): Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355. [DOI] [PubMed] [Google Scholar]
- Fortier CB, Amick MM, Grande L, McGlynn S, Kenna A, Morra L, Clark A, Milberg WP, McGlinchey RE (2013): The boston assessment of traumatic brain injury‐lifetime (BAT‐L) semistructured interview: Evidence of research utility and validity. J Head Trauma Rehabil 29:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LE, Fisher AM, Tagge CA, Zhang X‐L, Velisek L, Sullivan JA, Upreti C, Kracht JM, Ericsson M, Wojnarowicz MW, Goletiani CJ, Maglakelidze GM, Case N, Moncaster JA, Minaeva O, Moir RD, Nowinski CJ, Stern RA, Cantu RC, Geiling J, JK Blusztajn, BL Wolozin, T Ikezu, TD Stein, AE Budson, NW Kowall, D Chargin, A Sharon, S Saman, GF Hall, WC Moss, RO Cleveland, RE Tanzi, PK Stanton, A McKee (2012): Chronic traumatic encephalopathy in blast‐exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med 4:ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K, Mac Donald CL, Johnson AM, Barnes Y, Wierzechowski L, Zonies D, Oh J, Flaherty S, Fang R, Raichle ME, Brody DL (2014): Disrupted modular organization of resting‐state cortical functional connectivity in US military personnel following concussive 'mild' blast‐related traumatic brain injury. NeuroImage 84:76–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA (2008): Mild traumatic brain injury in US soldiers returning from Iraq. N Engl J Med 358:453–463. [DOI] [PubMed] [Google Scholar]
- Holdnack H (2001): Wechsler Test of Adult Reading: WTAR. San Antonio. Psychological Corporation.
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012): Fsl. Neuroimage 62:782–790. [DOI] [PubMed] [Google Scholar]
- Kasahara M, Menon D, Salmond C, Outtrim J, Tavares JT, Carpenter T, Pickard J, Sahakian B, Stamatakis E (2010): Altered functional connectivity in the motor network after traumatic brain injury. Neurology 75:168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CJ, Bickel S, Honey CJ, Groppe DM, Entz L, Craddock RC, Lado FA, Kelly C, Milham M, Mehta AD (2013): Neurophysiological investigation of spontaneous correlated and anticorrelated fluctuations of the BOLD signal. J Neuroscience 33:6333–6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LA, King DW, Vogt DS, Knight J, Samper RE (2006): Deployment Risk and Resilience Inventory: A collection of measures for studying deployment‐related experiences of military personnel and veterans. Mil Psychol 18:89. [Google Scholar]
- Lee M, Smyser C, Shimony J (2013): Resting‐state fMRI: A review of methods and clinical applications. Am J Neuroradiol 34:1866–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemer ER, Salat DH, Leritz EC, McGlinchey RE, Milberg WP (2013): Reduced cortical thickness with increased lifetime burden of PTSD in OEF/OIF veterans and the impact of comorbid TBI. NeuroImage: Clin 2:601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling G, Bandak F, Armonda R, Grant G, Ecklund J (2009): Explosive blast neurotrauma. J Neurotrauma 26:815–825. [DOI] [PubMed] [Google Scholar]
- Lippa SM, Pastorek NJ, Benge JF, Thornton G (2010): Postconcussive symptoms after blast and nonblast‐related mild traumatic brain injuries in Afghanistan and Iraq war veterans. J Int Neuropsychol Soc 16:856–866. [DOI] [PubMed] [Google Scholar]
- Longhi L, Saatman KE, Fujimoto S, Raghupathi R, Meaney DF, Davis J, McMillan A, Conte V, Laurer HL, Stein S, Stocchetti N, McIntosh TK (2005): Temporal window of vulnerability to repetitive experimental concussive brain injury. Neurosurgery 56:364–374. [DOI] [PubMed] [Google Scholar]
- Mac Donald CL, Johnson AM, Cooper D, Nelson EC, Werner NJ, Shimony JS, Snyder AZ, Raichle ME, Witherow JR, Fang R, Flaherty SF, Brody DL (2011): Detection of blast‐related traumatic brain injury in US military personnel. N Engl J Med 364:2091–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Mannell MV, Ling J, Gasparovic C, Yeo RA (2011): Functional connectivity in mild traumatic brain injury. Hum Brain Mapp 32:1825–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Robinson ME (2014): Military‐related traumatic brain injury and neurodegeneration. Alzheimer's Dementia 10(3, Suppl.):S242–S253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Stern RA, Nowinski CJ, Stein TD, Daneshvar DH, Alvarez VE, Lee H‐S, Hall G, Wojtowicz SM, Baugh CM, Riley DO, Kubilus CA, Cormier KA, Jacobs MA, Martin BR, Abraham CR, Ikezu T, Reichard RR, Wolozin BL, Budson AE, LE Goldstein, NW Kowall, RC Cantu (2013): The spectrum of disease in chronic traumatic encephalopathy. Brain 136:43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R (1987): The short‐form McGill Pain Questionnaire. Pain 30:191–197. [DOI] [PubMed] [Google Scholar]
- Moser RS, Schatz P, Jordan BD (2005): Prolonged effects of concussion in high school athletes. Neurosurgery 57:300–306. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA (2009): The impact of global signal regression on resting state correlations: Are anti‐correlated networks introduced? Neuroimage 44:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa A, Manley GT, Gean AD, Ohtani K, Armonda R, Tsukamoto A, Yamamoto H, Takayama K, Tominaga T (2011): Mechanisms of primary blast‐induced traumatic brain injury: Insights from shock‐wave research. J Neurotrauma 28:1101–1119. [DOI] [PubMed] [Google Scholar]
- Omalu B, Hammers JL, Bailes J, Hamilton RL, Kamboh MI, Webster G, Fitzsimmons RP (2011): Chronic traumatic encephalopathy in an Iraqi war veteran with posttraumatic stress disorder who committed suicide. Neurosurg Focus 31:E3. [DOI] [PubMed] [Google Scholar]
- Otis JD, McGlinchey R, Vasterling JJ, Kerns RD (2011): Complicating factors associated with mild traumatic brain injury: Impact on pain and posttraumatic stress disorder treatment. J Clin Psychol Med Settings 18:145–154. [DOI] [PubMed] [Google Scholar]
- RAND (2008): Invisible wounds of war: Psychological and cognitive injuries, their consequences, and services to assist recovery. Rand Corporation. [Google Scholar]
- Saljo A, Bao F, Haglid KG, Hansson H‐A (2000): Blast exposure causes redistribution of phosphorylated neurofilament subunits in neurons of the adult rat brain. J Neurotrauma 17:719–726. [DOI] [PubMed] [Google Scholar]
- Saljo A, Bao F, Jingshan S, Hamberger A, Hansson H‐A, Haglid KG (2002): Exposure to short‐lasting impulse noise causes neuronal c‐Jun expression and induction of apoptosis in the adult rat brain. J Neurotrauma 19:985–991. [DOI] [PubMed] [Google Scholar]
- Seal KH, Bertenthal D, Miner CR, Sen S, Marmar C (2007): Bringing the war back home: Mental health disorders among 103 788 US veterans returning from Iraq and Afghanistan seen at Department of Veterans Affairs Facilities. Arch Int Med 167:476–482. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Beckmann CF, Greenwood R, Kinnunen KM, Bonnelle V, De Boissezon X, Powell JH, Counsell SJ, Patel MC, Leech R (2011): Default mode network functional and structural connectivity after traumatic brain injury. Brain 134:2233–2247. [DOI] [PubMed] [Google Scholar]
- Shehzad Z, Kelly AC, Reiss PT, Gee DG, Gotimer K, Uddin LQ, Lee SH, Margulies DS, Roy AK, Biswal BB, Petkova E, Castellanos X, Milham MP (2009): The resting brain: Unconstrained yet reliable. Cereb cortex 19:2209–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, King AP, Welsh RC, Garfinkel SN, Wang X, Sripada CS, Liberzon I (2012): Neural dysregulation in posttraumatic stress disorder: Evidence for disrupted equilibrium between salience and default mode brain networks. Psychosomatic Med 74:904–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svetlov SI, Larner SF, Kirk DR, Atkinson J, Hayes RL, Wang KK (2009): Biomarkers of blast‐induced neurotrauma: Profiling molecular and cellular mechanisms of blast brain injury. J Neurotrauma 26:913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber K, Hurley R, Haswell C, Rowland J, Hurt S, Lamar C, Morey R: White matter compromise in veterans exposed to primary blast forces. J Head Trauma Rehabil (in press). 10.1097/HTR.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins P, Tesiram Y, Lerner M, Gonzalez LP, Lightfoot S, Rabb CH, Brackett DJ (2013): Brain injury: Neuro‐inflammation, cognitive deficit, and magnetic resonance imaging in a model of blast induced traumatic brain injury. J Neurotrauma 30:1888–1897. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL (2010): Intrinsic functional connectivity as a tool for human connectomics: Theory, properties, and optimization. J Neurophysiol 103:297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VandeVord PJ, Bolander R, Sajja VSSS, Hay K, Bir CA (2012): Mild neurotrauma indicates a range‐specific pressure response to low level shock wave exposure. Ann Biomed Eng 40:227–236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information Table 1.


