Abstract
Objective
To assess the role of the superior longitudinal fascicle, the inferior fronto‐occipital fascicle, and the posterior parietal lobe in visuospatial attention in humans during awake brain surgery.
Experimental design
Seven patients with hemispheric gliomas (six in the right hemisphere) entered the study. During surgery in asleep/awake anesthesia, guided by Diffusion Tensor Imaging Fiber Tractography, visuospatial neglect was assessed during direct electrical stimulation by computerized line bisection.
Principal observations
A rightward deviation, indicating left visuospatial neglect, was induced in six of seven patients by stimulation of the parietofrontal connections, in a location consistent with the trajectory of the second branch of the superior longitudinal fascicle. Stimulation of the medial and dorsal white matter of the superior parietal lobule (corresponding to the first branch of the superior longitudinal fascicle), of the ventral and lateral white matter of the supramarginal gyrus (corresponding to the third branch of the superior longitudinal fascicle), and of the inferior occipitofrontal fasciculus, was largely ineffective. Stimulation of the superior parietal lobule (Brodmann's area 7) caused a marked rightward deviation in all of the six assessed patients, while stimulation of Brodmann's areas 5 and 19 was ineffective.
Conclusions
The parietofrontal connections of the dorso‐lateral fibers of the superior longitudinal fascicle (i.e., the second branch of the fascicle), and the posterior superior parietal lobe (Brodmann's area 7) are involved in the orientation of spatial attention. Spatial neglect should be assessed systematically during awake brain surgery, particularly when the right parietal lobe may be involved by the neurosurgical procedure. Hum Brain Mapp 35:1334–1350, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: cerebral cortex, diffusion tensor imaging, hemispatial neglect, electrical stimulation of the brain, glioma, neuropsychology, neurosurgery, right cerebral hemisphere
INTRODUCTION
The neural correlates of the syndrome of left unilateral spatial neglect [Heilman and Valenstein, 2011; Husain, 2008; Karnath and Rorden, 2012; Molenberghs et al., 2012; Vallar, 2001] have been investigated in the last decades by mapping and overlapping the lesions of series of right‐brain‐damaged patients showing the deficit, as contrasted with the lesions of patients without the disorder. The relevant network, as suggested by lesion‐clinical correlation studies in right‐brain‐damaged patients, comprises: (a) the inferior parietal lobule (IPL) (supramarginal gyrus (SMG) at the temporo‐parietal junction (TPJ) [Heilman and Valenstein, 1972; Vallar and Perani, 1986; Golay et al., 2008; Verdon et al., 2010], angular gyrus (AG) [Hillis et al., 2005; Mort et al., 2003]), (b) the ventral premotor cortex in the frontal lobe [Committeri et al., 2007; Husain and Kennard, 1996], and (c) the superior temporal gyrus (STG) [Karnath et al., 2001, 2004, with a greatest lesion overlap in the middle part of the STG; Committeri et al., 2007]. Impairments in visuoexploratory tasks, such as cancellation, appear to be more closely associated with frontal damage, while posterior damage brings about a disproportionate rightward bias in line bisection [Daini et al., 2002; Golay et al., 2008; Rorden et al., 2006; Verdon et al., 2010].
It has long been known that vascular lesions involving and confined to right‐sided white matter fiber tracts and the right internal capsule [Cappa and Vallar, 1992, see Table 2 for review; Hier et al., 1983; Stein and Volpe, 1983, case #3; Vallar and Perani, 1986] may cause left spatial neglect, disconnecting relevant cerebral areas [Cappa and Vallar, 1992; Catani and Mesulam, 2008a; Hier et al., 1983, for a recent discussion). Spatial neglect caused by damage confined to the white matter was found to be an infrequent deficit, however, with its more frequent neuropathological association being extensive cortico‐subcortical lesions [Cappa and Vallar, 1992; Hier et al., 1983]. Later neuroimaging‐clinical correlation studies have highlighted the contribution of disconnection mechanisms, and of damage to the white matter. CT‐Scan assessed lesions involving the anterior, central and posterior white matter [superior longitudinal fascicle (SLF), and inferior longitudinal fascicle (ILF)], as well as the parietal, sensorimotor, and anterior cingulate cortices, are more extensive in patients with left visuospatial neglect; damage to the right anterior cingulate and the parietal regions, particularly the supramarginal gyrus, is a significant predictor of left visuospatial neglect [Leibovitch et al., 1998].
The SLF is the largest of the fiber bundles that connect the perisylvian frontal, parietal, and temporal cortices, as described by Déjérine 1892 in his classical neuroanatomical work. Déjérine considered this large fascicle to include the arcuate fasciculus (AF), using the term “faisceau longitudinal supérieur” or “arqué” in his descriptions [Déjérine, 1914]. A Diffusion Tensor Magnetic Resonance Imaging (DTI‐MR) study in the human brain [Makris et al., 2005] demonstrated that the SLF includes three branches (I–III, arranged dorso‐ventrally) plus the AF, and provides the major parietofrontal connections. In the recent imaging literature there is a wide variability in the definition of the different branches of the SLF and the AF [Catani et al., 2005; Kaplan et al., 2010; Makris et al., 2005], and a large overlapping between these connections. Indeed, the horizontal portion of the AF (hAF, or frontoparietal or anterior segment) runs parallel to the SLF II and the SLF III, and, in many DTI‐MR studies, it is generally considered to be indistinguishable from fibers of the SLF III [Kaplan et al., 2010]. The ILF connects the parahippocampal gyrus with the posterior part of the IPL [Rushworth et al., 2006; Seltzer and Pandya, 1984].
The association of damage to these fiber tracts with left visuospatial neglect has been elucidated in nonhemianopic right‐brain‐damaged patients with lesions broadly extending in the vascular territory of the middle cerebral artery [Doricchi and Tomaiuolo, 2003; He et al., 2007, for related evidence; see also Ptak and Schnider, 2010], and in hemianopic right‐brain‐damaged patients with lesions in the territory of the posterior cerebral artery [Bird et al., 2006, ILF]. In a recent study [Verdon et al., 2010], the damage associated with visuospatial neglect involves, in the white matter, the SLF and the superior occipitofrontal fascicle [Catani et al., 2002]. A reanalysis of the data of Doricchi and Tomaiuolo 2003 has suggested that the white matter damage associated with left neglect involves the SLF II and III, rather than the AF [Thiebaut de Schotten et al., 2008]. A study using Diffusion Tensor Imaging (DTI)‐MR tractography [Urbanski et al., 2011] shows that six right‐brain‐damaged patients with left spatial neglect had damage to the inferior fronto‐occipital fasciculus (IFOF), and the anterior segment of the AF, as compared with six right brain damaged patients with no spatial neglect; the anterior segment of the AF links the IPL with the posterior Broca territory/premotor cortex, and, as also discussed above, may correspond to the SLF III [see also Catani et al., 2005]. A recent MRI study in brain‐damaged patients, with mixed, mainly vascular, etiology, suggests a role of damage to the SLF and the IFOF in patients with left neglect [Chechlacz et al., in press]. A recent case report [Ciaraffa et al., in press] associates severe visual neglect in the acute poststroke stage with focal damage to the AF, in its anterior segment, and in its long, direct segment, a well‐established pathway which connects the Wernicke territory with the Broca territory [e.g., Rodrigo et al., 2007].
At variance from these findings, a recent longitudinal study in a larger sample of stroke right‐brain‐patients (using MRI‐ and CT‐based voxel‐wise lesion mapping, and no tractography) points to a role of damage to a set of regions including the superior and middle temporal gyri, the basal ganglia, the IFOF, and the right uncinate fascicle (UF) in chronic patients with spatial neglect [Karnath et al., 2011]; the UF connects the anterior part of the temporal lobe with the orbital and polar frontal cortices [Catani et al., 2002; Schmahmann et al., 2007].
While most patients with left spatial neglect have extensive corticosubcortical lesions, the relative role of cortical vs. white matter damage is a matter of controversy, with some studies assigning a main role to white matter damage. Particularly, the suggestion has been made that a fronto‐parietal disconnection plays a main causal role in spatial neglect [Bartolomeo et al., 2007; Corbetta and Shulman, 2011; Corbetta et al., 2005; He et al., 2007], highlighting the role of a fronto‐parietal system in spatial attention and representation [Vallar, 1998; Mesulam, 1981, 1999, 2002; Rizzolatti and Matelli, 2003]. In line with this view, two right brain‐damaged patients with left spatial neglect showed damage to the cortex and the SLF, but not the ILF [Rode et al., 2010]. Damage to frontal‐occipital fiber bundles (i.e., the IFOF) may be also relevant [Urbanski et al., 2008, 2011]. By contrast, one study [Karnath et al., 2009] keeps assigning a main role to the cortical damage; within white matter fiber tracts, damage to the IFOF, but not to the SLF, predicts spatial neglect.
A different approach involves direct electrical stimulation (DES) during brain surgery [Foerster and Penfield, 1930; Ojemann et al., 1989; see Borchers et al., 2011, for a recent discussion]: restricted cerebral regions are temporarily inactivated in awaken patients, while they perform tasks of interest. The patients' behavior during DES allows an online correlation between the deficit and the inactivated area. Electrocortical stimulation mapping of the posterior temporal region and the IPL by subdural electrodes may produce spatial neglect [Kleinman et al., 2007]. In one patient with a right temporo‐parietal tumor, intraoperative electrical stimulation of the middle portion of the STS disrupted visual search, with, however, no lateral asymmetries, indicating left spatial neglect, while stimulation of the more caudal portion of the STS was ineffective [Gharabaghi et al., 2006]. In a seminal study [Thiebaut de Schotten et al., 2005, see also correction in Science, 2007, 317, n. 5838, p. 597], during brain surgery for tumor resection in two right brain damaged left‐handed patients (CAL and SB), the stimulation of the right SMG, and of the caudal portion of the STS, caused a rightward error in line bisection, indicating left spatial neglect. In patient SB the greatest deviation was produced by the stimulation of the SLF II. These conclusions were reached in two patients, who were both left‐handed, leaving open the possibility of a noncanonical organization of cognitive functions and of their neural underpinnings [Witelson, 1985; Witelson and Kigar, 1988]. The assessment was made with a paper‐and‐pencil clinical method, and the two patients used the left (dominant) hand, contralateral to the damaged hemisphere (contralesional). Right brain damaged patients with left spatial neglect, when performing line bisection tasks, typically use the right hand, ipsilateral to the damaged hemisphere (ipsilesional), and unaffected by sensorimotor deficits [e.g., Vallar et al., 2000].
In this study, using a computerized line bisection task in seven patients who underwent brain surgery for a glioma, we investigated the effects of DES of white matter fiber tracts, the SLF [Makris et al., 2005] and the IFOF [Catani et al., 2002; Makris et al., 2007] in producing visuospatial neglect. The effects of DES on some posterior parietal cortical regions were also assessed.
MATERIALS AND METHODS
Patients
Seven male patients entered this study. The protocol was carried out in accordance with the ethical standards of the Declaration of Helsinki (BMJ 1991; 302: 1194), in compliance of a protocol approved by the local Ethical Committee. Clinical and demographical data are reported in Table 1. Six of the seven patients came for clinical observation because of epileptic seizures, one patient (AS) presented with a transient weakness of the left limbs. The preoperative neurological examination of all patients was normal. In particular, no motor, somatosensory, and visual half‐field deficits were observed. Each patient was submitted to an extensive standard neuropsychological battery in the week before the surgical procedure. The evaluation included tests assessing: nonverbal intelligence; verbal and visuospatial short‐ and long‐term memory; selective and divided attention; orofacial, ideomotor and constructional apraxia, language and spatial cognition [see Bello et al., 2006 for details]. The results of the neuropsychological evaluation are summarized in Supporting Information. Handedness was assessed by means of The Edinburgh Inventory Questionnaire, which provided a 12‐point score, with 10 questions assessing the hand, one the eye and one the foot; a 10 plus 2 score denoted a complete right‐handedness, a 0 score a complete left‐handedness [Oldfield, 1971]. Functional MR imaging (fMRI) was performed to assess language dominance by using a verb generation and a picture‐naming task. Tumor volume was calculated on T2‐weighted MRI scans for low‐grade gliomas, and on postcontrast T1‐weighted MRI scans for high‐grade gliomas via a computerized system [Bello et al., 2007]. Histology was classified according to the WHO brain tumor classification [Rousseau et al., 2008].
Table 1.
Clinical and Demographical Data of Seven Patients
| Patient | Handedness (score) | Age/Sex | Education (years of schooling) | Lesion side/site | Histology/grade |
|---|---|---|---|---|---|
| P1‐AS | L (0) | 32/M | 8 | R/F‐P mesial, lateral | Oligodendroglioma/II |
| P2‐DS | R (10+1) | 37/M | 17 | L/P mesial | Astrocytoma/III |
| P3‐LT | R (10+2) | 29/M | 8 | R/F‐P mesial | Oligodendroglioma/II |
| P4‐FV | L (0+1) | 35/M | 8 | R/F‐T‐P lateral | Oligodendroglioma/II |
| P5‐FF | R (10+1) | 37/M | 8 | R/T‐P lateral | Astrocytoma/III |
| P6‐AGC | R (8+1) | 47/M | 13 | R/P mesial | Oligodendroglioma/II |
| P7‐ME | R (9+2) | 31/M | 17 | R/P mesial | Oligodendroglioma/II |
M: male. L/R: left‐right. F/T/P: frontal, temporal, parietal.
Baseline Assessment for Visuospatial Neglect
In the visuomotor tasks patients used the ipsilesional hand, unaffected by sensorimotor deficits: the six right brain‐damaged patients used their right hand, left‐brain‐damaged patient P2‐DS his left hand. The following test were used: (1) line bisection [Ronchi et al., 2009]; (2) line [Albert, 1973], letter [Diller and Weinberg, 1977; Vallar et al., 1994], and star [Wilson et al., 1987] cancellation; (3) sentence reading [Pizzamiglio et al., 1992]; (4) drawing: copying [Gainotti et al., 1972; Fortis et al., 2010], and drawing from memory the hours of a clock. Details about the baseline tests for visuospatial neglect are provided in Supporting Information. The neuropsychological battery was administered to six patients, while one patient (P6‐AGC) was not available for testing. None of the patients showed preoperative clinical evidence of visuospatial neglect.
Touch Screen Line Bisection
The participant's task was to touch the subjective midpoint of horizontal black lines 19.6 cm in length, and 1 mm in width. Stimulus generation and presentation were controlled by a PC, connected to a touch screen, and running E‐Prime (Psychology Software Tools, Inc.). Patients performed the task reclined on their contralesional shoulder, using the upper limb and the index finger of the ipsilesional hand. The six right brain damaged patients were reclined on their left shoulder and used their right hand. Left brain damaged patient P2‐DS was reclined on his right shoulder, and used his left hand. The experimenter initiated each trial. After a random interval a beep alerted the participant, and 200 ms afterwards a line appeared. In order to prevent patients from performing the task just pointing straight ahead, the position of the line was randomized so that its midpoint fell within an imaginary square of 1 cm2 centered on the rectangular screen. In different trials, each line position was jiggled, so that its midpoint was positioned randomly within an imaginary square of 1 cm2, in the center of the screen. The mean overall deviation of the midpoint of the line from the objective center of the screen was −0.08 mm (SD ±3.02, range +0.5 to −0.5 mm) in the horizontal (x) axis, and +0.18 mm (SD ±2.91, range +0.5 to −0.5 mm) in the vertical (y) axis. It is unlikely that these minor (±5 mm maximum from the center of the screen) and random displacements of the objective midpoint of the line affected the patients' performances. Significant effects of the position of the to‐be‐bisected line have been reported when the stimulus was placed entirely to the left or to the right of the midsagittal plane of the body, with a more severe neglect for left‐sided lines [Heilman and Valenstein, 1979; Schenkenberg et al., 1980; Vallar et al., 2000]. Furthermore, these random displacements occurred in both the stimulation and the non‐stimulation trials.
During surgery, by an auditory warning when delivering the DES, the neurosurgeon prompted the neuropsychologist to start each bisection trial on the PC. As soon as participants gave their response the line disappeared, with no time response limits. No visual feedback was provided as to the localization of the participant's response on the touch screen. The patient's response was recorded in terms of touch‐screen coordinates in pixels, subsequently converted in millimeters. The score was the deviation of the participant's unseen touch from the objective midpoint. Positive score denoted a rightward displacement, negative scores a leftward displacement.
Control data were collected from 10 neurologically unimpaired male participants (mean age 28.5, SD ±6.35, range 21–40), who performed the bisection task (30 trials) reclined on their left shoulder, using their right hand, and from five neurologically unimpaired male participants (mean age 34.8 years, SD ±4.91, range 27–39), who performed the bisection task (30 trials) reclined on their right shoulder, using their left hand.
Statistical Analysis
The average bisection scores of each patient during each stimulation session were compared with the patient's own bisection scores under condition of no cerebral stimulation by t tests [Crawford and Garthwaite, 2002]. The average scores after stimulation of the different brain regions in the individual patients were compared by a mixed model analysis of variance [Raudenbush and Bryk, 2002].
Diffusion Tensor Imaging
Preoperative MR imaging was performed on a Philips Intera 3.0‐T (Best, The Netherlands) system with a maximum field gradient strength of 80 mT/m. A multi‐channel head coil was used for the reception of the MR signal. DTI data were acquired using a single‐shot echo planar imaging (EPI) sequence (TR/TE 8,986/80 ms) with parallel imaging (SENSE factor, R = 2.5). Thirty‐two diffusion gradient directions (b = 1,000 s/mm2), and one image set without diffusion‐weighting were obtained. A field of view measuring 240 × 240 mm2 and a data matrix of 96 × 96 were used, leading to isotropic voxel dimensions (2.5 × 2.5 × 2.5 mm3). The data were interpolated in‐plane to a matrix of 256 × 256 leading to voxel size of 0.94 × 0.94 × 2.5 mm3. Acquisition coverage extended from the medulla oblongata to the brain vertex (56 slices, no gap). The sequence was repeated twice consecutively, and data were averaged off‐line to increase signal‐to‐noise ratio. DTI Studio software —see below— automatically performed an average of the two sequences acquired separately. DTI datasets were aligned offline to the echo‐planar volume without diffusion weighing on a PC workstation using the AIR (Automatic Image Registration) software to correct artifacts due to rigid body movement during scan acquisition. Three‐dimensional Fast Field Echo (FFE) T1‐weighted imaging (TR 8 ms; TE 4 ms; image resolution equal to DTI) was performed for anatomic guidance. Images were analyzed using DTI Studio version 2.4.01 software (H. Jiang and S. Mori, Johns Hopkins University, Kennedy Krieger Institute, Baltimore, MD) [Basser et al., 1994; Jiang et al., 2006], obtaining main eigenvector and fractional anisotropy (FA) maps. From their combination, color maps were generated with conventional color‐coding [Pajevic and Pierpaoli, 1999]. Color was assigned for each voxel using the main eigenvector (corresponding to the largest eigenvalue) of the diffusion tensor: red voxel in the maps represented right–left (or left–right) direction, green indicated anterior–posterior (or posterior–anterior) direction, and blue represented superior–inferior (or inferior–superior) direction.
Subcortical connections were reconstructed using the “fiber assignment by continuous tracking” (FACT) method [Mori and van Zijl, 2002; Mori et al., 1999]. The eigenvector associated with the largest eigenvalue was assumed to represent the main fiber direction in the voxel. An FA threshold of 0.1 and a turning angle >55° were used as criteria to start and stop tracking [Bello et al., 2008], allowing reconstruction of fibers also through regions of tumor infiltration, in order to guide intraoperative stimulation.
To reconstruct the SLF, a first region of interest (ROI) was placed on a coronal section at the level of a high‐anisotropy region on color maps laterally to the central part of the lateral ventricle; a second ROI was placed in a peritrigonal site at the level of the descending branch of the tract. Then, dissection of the three subcomponents of the SLF, and of the AF, was obtained using AND operation. ROIs were selected on color maps according to anatomic knowledge and to previous literature [Makris et al., 2005], in sections perpendicular to the main course of the stem portions of the SLF I, SLF II, SLF III, and the AF. Specifically, progressing through coronal and axial sections on color maps, the voxels pertaining to the stem portions of fiber pathways were determined by their relative location and the orientation of diffusion properties of the tissue, as described by Makris et al. 2005:
The SLF I is located in the white matter of the superior parietal and superior frontal lobes, and extends to the dorsal premotor and dorsolateral prefrontal regions; voxels anterior–posteriorly oriented on color maps contained within the dorsomedial part of the hemisphere in the frontal and parietal lobes were selected, to isolate only fibers of the SLF I with AND operation.
The SLF II extends from the AG to the caudal‐lateral prefrontal regions; voxels with tensor information that was oriented anterior–posteriorly on coronal sections of color maps in the region above the insula, the extreme capsule, the claustrum, the external capsule, the lenticular nucleus, and the internal capsule were selected with AND operation to dissect fibers of the second subdivision of the SLF.
The SLF III is situated in the white matter of the parietal and frontal opercula, and extends from the SMG to the ventral premotor and prefrontal regions; voxels anterior–posteriorly oriented on color maps within the frontoparietal opercular region were selected, to isolate only fibers of the SLF III with AND operation.
The AF extends from the caudal part of the STG to the lateral prefrontal cortex, in contiguity to the ventral surface of the SLF II fibers. For this tract, voxels anterior–posteriorly oriented on color maps located laterally to the trigonal region of the lateral ventricle were selected on axial sections on color maps at the level of the vertical part of the AF with AND operation.
All the dissections were accurately inspected in order to ensure the reliability of the subcortical trajectory of each component.
To reconstruct the IFOF, a two‐ROIs approach was used, with a first ROI placed on a coronal section at the level of the anterior part of the external capsule, on a coronal plane passing through the junction of the frontal and temporal lobes; at this level the IFOF runs in contiguity with the UF; a second posterior ROI was placed on a coronal section at the level of the occipital lobe, to isolate only fibers of the IFOF with AND operation [Bello et al., 2008].
Finally, reconstructed white matter tracts were superimposed on volumetric postcontrast T1‐weighted images, previously coregistered to the mean of all diffusion‐weighted images using the SPM5 software (Statistical Parametric Mapping software, Wellcome Trust Centre for Neuroimaging, University College London, UK; available at: http://www.fil.ion.ucl.ac.uk/spm). This allowed evaluating the anatomical relationship between the tracts and the tumor lesions. Tractography reconstructions of the three branches of the SLF, plus the AF, and of the IFOF were represented in different colors (see Fig. 1): SLF I fibers were depicted in green, SLF II in blue, SLF III in yellow, and AF in red; IFOF fibers were shown in azure.
Figure 1.
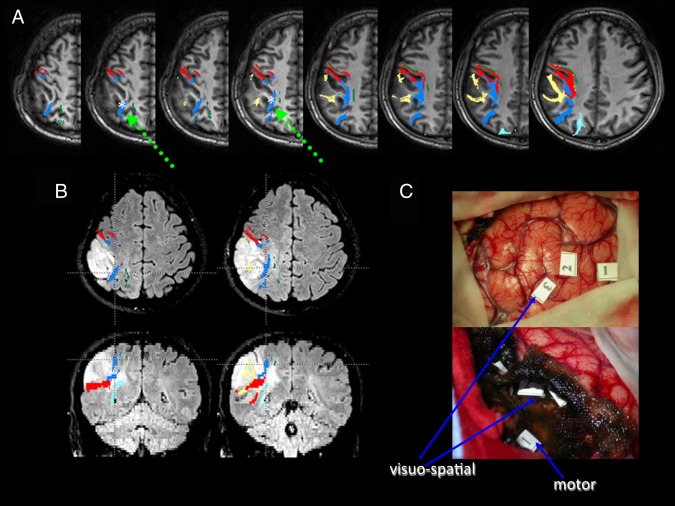
Patient P4. A) Preoperative deterministic tractography reconstructions superimposed on axial T1‐weighted images; SLF I fibers are depicted in green, SLF II in blue, SLF III (that includes fibers of the horizontal part of the AF) in yellow, AF in red, and IFOF in azure. The white asterisk and the green arrow show the DES site of the SLF II, which caused a rightward bisection error during intraoperative stimulation. B) Morphologic FLAIR axial (upper) and coronal (lower) images of the tumor, showing both the displacement and the infiltration of SLF fibers along the tumor margins: specifically, the second branch was much closer to the tumor border than the third branch and the AF, which was located more laterally and ventrally, and the first branch, which was displaced superiorly. This information was available during surgery, because tractography reconstructions were loaded into the neuronavigation system. At surgery, tumor resection was immediately performed at a subcortical level close to these tracts, in order to reduce the risk of brain shift. The patient was asked to perform the bisection test, with the sites corresponding to bisection deviations being registered into the neuronavigation system. Axial and coronal sections correspond to the positive sites at stimulation. These sites were found in the subcortical white matter of the parietal lobe, in a location consistent with the trajectory of the SLF II at a dorsal level: note that fibers of this branch lie dorsally to the fibers of the AF. C) Intraoperative pictures of the surgical cavity at the beginning (upper) and at the end of tumor resection (lower); numbers correspond to the sites where DES induced intraoperatively a rightward bisection error at cortical (“3” indicates BA7b), and subcortical (SLF II) levels; “1” and “2” indicate BA 39; at a subcortical level also a motor response was induced.
DTI‐fiber tractography (FT) data were saved as a compatible format (DICOM) to be transferred to the Neuronavigational System (BrainLab, Munich, Germany) using Medx Software (Medical Numerics, Inc.). The Neuronavigational System performed an automatic coregistration between tractography datasets and the preoperative MR images acquired with references applied on the skull of the patient by a voxel‐by‐voxel intensity matching non‐linear algorithm. The result was the fusion in the same space of anatomical and functional datasets. As an estimate for the clinical navigation accuracy, the target registration error localizing a separate fiducial, which was not used for registration, was documented. Furthermore, repeated landmark checks were performed during surgery to ensure overall ongoing clinical navigation accuracy.
To substantiate preoperative findings, postoperative deterministic and probabilistic tractographies were also performed. Postoperative deterministic tractography was performed with an FA threshold of 0.2 and fiber angles of less than 40° and 55° between two connected pixels [Mori et al., 2005; Oishi et al., 2008]. These FA and angular thresholds were more selective than those used preoperatively [Bello et al., 2008]. Seed ROI were placed on axial sections in correspondence to the stimulation sites previously recorded on the neuronavigational system, at the level of the subcortical white matter of the angular gyrus; then, a coronal ROI was delineated around the white matter of the frontal lobe on a section passing through the anterior commissure, to isolate with AND operation all the parietofrontal fibers from stimulation areas.
Postoperative probabilistic tractography analyses on preoperative scans were performed in all patients, by using stimulation sites recorded during interventions as seeding masks. All scans were transferred to a Linux‐based Sun workstation; the DICOM files of each DTI acquisition were converted into a single multivolume ANALYZE 7.5 file, and were then corrected for eddy currents using the ‘eddycorrect’ algorithm implemented in FSL v4.1 (available at: http://www.fmrib.ox.ac.uk/fsl/). After this coregistration step, the two b = 0 volumes of each patient were extracted and averaged. A multitensor model was fitted to the diffusion data using the FDT (FMRIB's Diffusion Toolbox) v2.0 software tool (available at: http://www.fmrib.ox.ac.uk/fsl/fdt/), and allowed modeling multiple fiber orientations per voxel [Behrens et al., 2007]. Probabilistic tractography analysis was carried out using the method described by Behrens et al. 2003, extended to multiple fibers, as implemented in the FDT software tool [Behrens et al., 2007]. The program FSL view (available at: http://www.fmrib.ox.ac.uk/fsl/fslview/) was used to visualize images and create masks. Seed masks for tractography were placed in correspondence to the stimulation sites previously marked on the volumetric postcontrast T1‐weighted scan on the neuronavigational system (see above), and then co‐registered to the mean of all diffusion‐weighted images using SPM5. For all these sites, seed masks were identified and positioned in the subcortical white matter from axial views. A coronal waypoint mask at the level of the white matter of the frontal lobe was also used, in order to include from the calculation of the connectivity distribution only tracts that passed through both these masks. An exclusion mask at the midline level on a sagittal plane was used, to restrict the pathways to the hemisphere ipsilateral to the seed mask, so that all streamlines from the seed region that intersected with the exclusion mask were discarded.
Surgical Procedure
Surgery was performed in asleep/awake anesthesia to monitor both motor and language function, at the cortical and subcortical level. Six patients had a right frontoparietal craniotomy exposing the parietal lobe, the superior temporal sulcus, and the precentral and postcentral gyri. One patient (P2) had a left fronto‐parietal craniotomy. Neuronavigation was available and loaded with volumetric T2, FLAIR and postgadolinium T1 imaging, and DTI data including the corticospinal tract, the three branches (I, II, III) of the SLF, the AF [Schmahmann and Pandya, 2006; Schmahmann et al., 2007], and the IFOF [Catani and Thiebaut de Schotten, 2008]. Repeated checks were performed with external and internal fiducials to assess the maintenance of the neuronavigation accuracy. Surgery was performed according to functional boundaries by the aid of motor, language, and visuospatial cortical and subcortical mapping. We planned to resect tumor tissue according to functional boundaries. The finding of subcortical motor, language, and visuospatial tracts constituted the limit of resection. EEG, electrocorticography (ECoG), and motor evoked potentials (MEPs) were also available during the entire duration of the surgery, to detect the occurrence of afterdischarges and electric seizures, as well as the integrity of motor pathways. The brain mapping procedure was video‐ and audio‐recorded, and reviewed postoperatively by two surgeons and two neuropsychologists, in order to verify the stimulation sites and the corresponding responses. Resection was started at the lateral and superior‐anterior border, with the aim of detecting subcortical responses, and localizing functional tracts, without removing the main tumor mass, in order to maintain the accuracy of the neuronavigation system. At the time of surgery, the location of each subcortical site where anomia, phonemic or semantic paraphasias, and lateral deviations in line bisection were induced, was recorded during the various phases of the resection, using the neuronavigation system on the preoperative MR scan, in which the tractography data were loaded. In addition, each subcortical site identified during subcortical mapping was marked with a sterile numbered tag, and a digital picture of the surgical cavity was taken at the end of the resection. On the immediate postoperative MR scan, it was evaluated the anatomical location of the subcortical pathways, i.e., the periphery of the surgical cavity, where the resection was stopped according to the functional responses elicited by intraoperative stimulation. Technically, this was accomplished by transferring immediate postoperative MR images of each patient on a Brain Lab workstation, and fusing them with preoperative MR images, on which tractography data for each fascicle were merged.
RESULTS
Baseline and Postsurgical Assessments
Both in the baseline and in the postintervention assessment all six patients had an errorless performance in the line, letter, and star cancellation tasks, as well as in the copy drawing and clock drawing tasks, and in the sentence reading test. In the bisection task the average deviation scores of the six patients were +0.17 mm (SD ±1.40, range, −1.11 to +2.16) before the operation, and −0.60 mm (SD ±0.75, range −1.5 to +0.16) after the operation (Wilcoxon Signed‐Rank Test, Z = 1.78, P = 0.074). In the bisection task all individual average scores were within the normal range (mean −0.70 mm, SD ±0.98, range −7.5 to +1.80), with the exception of the preoperative bisection score of left brain‐damaged patient P2 (+2.16), which was outside the controls' range, but did not differ from their average score (P = 0.10). This rightward error in a left brain damaged patient does not indicate contralesional spatial neglect, but might be considered an ipsilesional variety of the deficit [“ipsilesional neglect,” see Kwon and Heilman, 1991], that, in any case, was not found postsurgically.
Tractography
Figure 1 illustrates the preoperative deterministic tractography reconstruction and FLAIR images of the tumor, and the intraoperative photograph of patient P4.
Postoperative deterministic and probabilistic tractographies confirmed the preoperative data in all patients but left brain damaged patient P2, in whom the SLF II and the AF proved to be difficult to distinguish. P2, however, showed no visuospatial neglect during DES. Figure 2 shows the postoperative deterministic and probabilistic tractography reconstructions of patient P4. Pre‐ and postoperative deterministic tractography and postoperative probabilistic tractography reconstructions of patients P1–P3, P5, and P7 are shown in Supporting Information.
Figure 2.
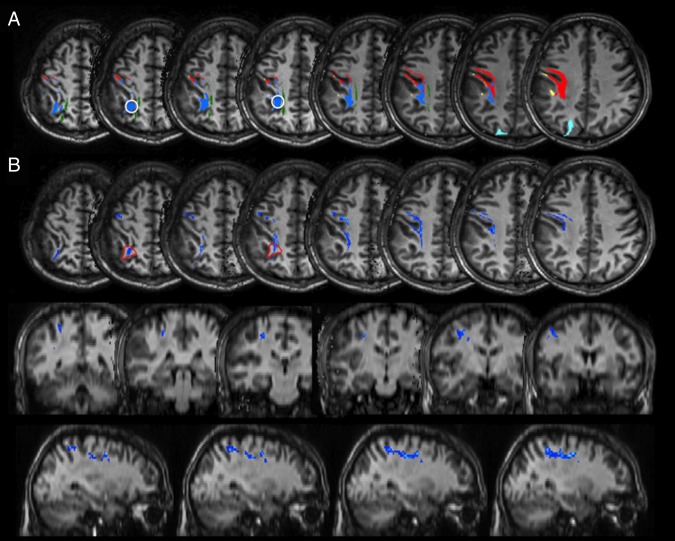
Patient P4. A) Postoperative MR tractography analysis on preoperative DTI acquisitions, starting from positive stimulation points, FA threshold >0.2. White circles: ROIs positioned in the sites of positive stimulation regions: depiction of the blue fibers, corresponding to the second branch of the SLF, at a higher and less “permissive” FA threshold. B) Postoperative probabilistic tractography superimposed on axial, coronal and sagittal T1‐weighted images; tracking of parietofrontal connections from stimulation sites (the red line depicts the seed mask in A). Voxels are color‐coded from 20 (blue) to 200 (light blue) samples passing through the voxel. Multi‐fiber tractography identifies dorsal and lateral parietofrontal connections linking the inferior parietal lobule to the posterior regions of the superior and middle frontal gyri, belonging to the second branch of the SLF.
The DES sites based on the preoperative reconstruction are indicated in Figures 3 and 4 for each patient.
Figure 3.
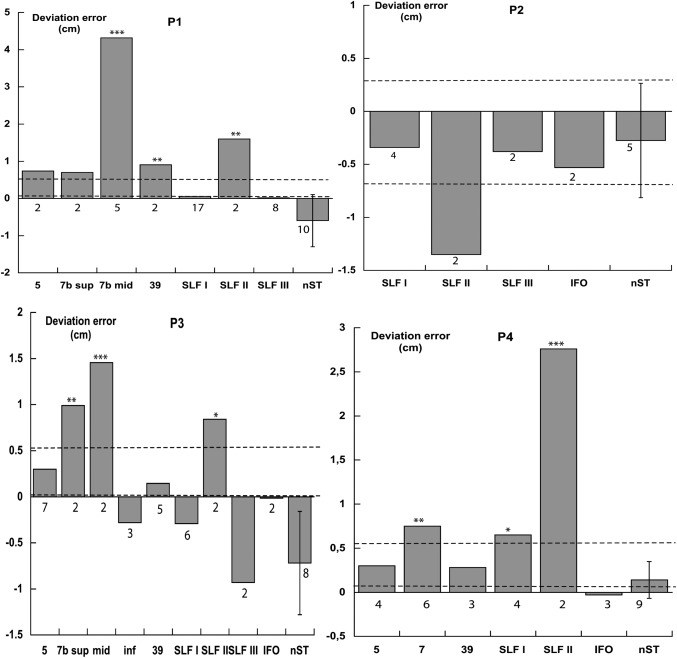
Patients P1, P2, P3, and P4. Deviations in cm in line bisection. Each column refers to a stimulated brain region (BAs 5, 7, 39; SLF I‐III, IFO fasciculus). The right‐sided column shows the patient's average bisection error (SD) under conditions of no stimulation (nST). Numbers under each column indicate the number of bisection trials. The two dashed horizontal lines indicate the range of the bisection error of ten neurologically unimpaired participants using their right hand (control for P1, P3, P4), and five neurologically unimpaired participants using their left hand (control for P2). T tests *(P < 0.05), **(P < 0.025), ***(P < 0.01).
Figure 4.
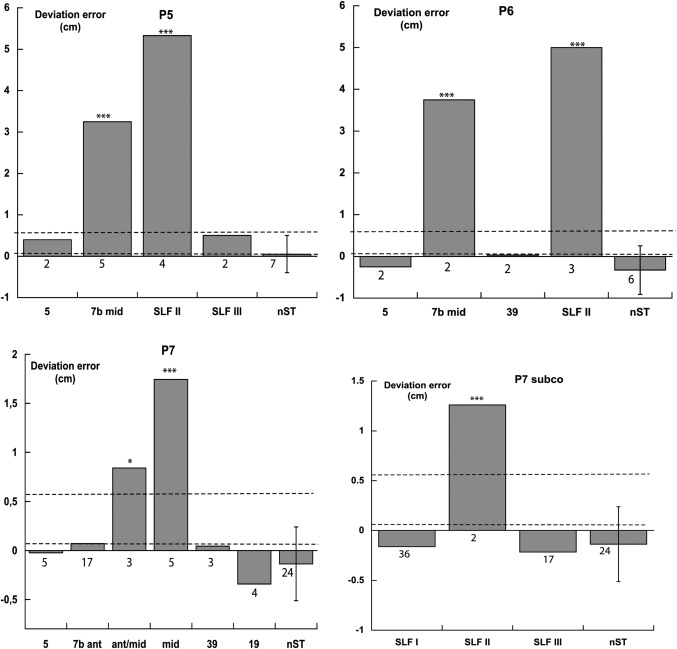
Patients P5, P6, and P7. Deviations in cm in line bisection. Each column refers to a stimulated brain region (BAs 5, 7, 39, 19; SLF I–III). nST trials, dashed horizontal lines (range of the bisection error of ten neurologically unimpaired participants using their right hand), and t tests as in Figure 3.
Line Bisection During DES
The bisection errors of the seven patients, performed during DES, are shown in Figures 3 and 4. SLF II stimulation brought about a rightward bisection error in six out of the seven assessed patients (P1, P3, P4, P5, P6, and P7; no effects in left brain‐damaged patient P2). SLF I stimulation caused a mild rightward error in patient P4, but not in patients P1, P2, P3, and P7. SLF III and IFOF stimulations were ineffective. Stimulation of BA 7 (particularly the middle part) produced a rightward error in all six assessed patients (P1, P3, P4, P5, P6, and P7). Stimulation of BA 39 caused a rightward error in one (P1) out of the five assessed patients (P1, P3, P4, P6, and P7). Stimulations of BAs 5 (six patients: P1, P3, P4, P5, P6, and P7) and 19 (patient P7) were ineffective. The visual monitoring of the patients' performances did not show any misreaching error, in addition to the lateral bias, with patients being fully accurate in reaching the to‐be‐bisected line, with no evidence, therefore, of optic ataxia [Rossetti et al., 2003].
In the mixed model analysis of variance the fixed factor included the brain regions where bisection scores during DES had been recorded from more than one patient (BA 5; BA 7, collapsing the superior, anterior, middle, and inferior subregions; BA 39, SLF I; SLF II; SLF III; IFO), and the no stimulation trials. The random factor included the seven patients. The fixed effect was significant (F= 25.08; df = 7, 267.34; P < 0.001), indicating differences among the various stimulated regions. Pairwise Bonferroni comparisons showed that the error scores after DES of BA 7 (mean 1.24 cm, SD ±1.69, n = 52), and of SLF II (mean 2.69 cm, SD ±2.40, n = 17) differed from the no‐stimulation trials (mean −0.24 cm, SD ±0.53, n = 69). The scores of all other stimulated regions did not differ from the no‐stimulation trials: BA 5 (mean 0.18 cm, SD ±0.41, n = 22), BA 39 (mean 0.16 cm, SD ±0.54, n = 15), SFL I (mean −0.12 cm, SD ±0.53, n = 67), SLF III (mean −0.16 cm, SD ±0.54, n = 31), and IFO (mean −0.02 cm, SD ±0.46, n = 7). The SLF II bisection scores differed from those of all other stimulated regions, the BA 7 from those of all other regions, but the IFO.
DISCUSSION
The first main result of this study is that, within the white matter, it is the interference with the function of dorsolateral parietofrontal connections, in a location consistent with the trajectory of the second branch of the SLF, which causes a consistent left visuospatial neglect, as assessed by line bisection. The deficit is present in six out of the seven patients (over 85%). Conversely, stimulation of more dorsomedial or ventrolateral fibers of the SLF, namely the two other components of the SLF (I and III), as well as of the IFOF, is ineffective, with the exception of a mild rightward error during DES of SLF I in patient P4, who, however, shows the largest bias during DES of SLF II.
The SLF II connects the posterior inferior parietal region (BA 39, AG) with the prefrontal and premotor regions BAs 46, 8, and 6 [Makris et al., 2005], and, in the monkey, the caudal part of the IPL with the dorsal premotor and prefrontal cortices [Schmahmann et al., 2007]. Consistent with these findings, stimulation of BA 39 brings about left neglect in one (P1) out of four patients. The lack of effects of stimulation of BA 39 in patients P3, P4, P6, and P7 could be related to plastic cortical changes, due to the slowly growing neoplastic lesion [Thiel et al., 2001]. Also the possibility that the role of this portion of the IPL [Mort et al., 2003] in line bisection performance is comparatively minor should be considered [see Rorden et al., 2006, for damage to the junction between the middle occipital gyrus and the middle temporal gyrus; Verdon et al., 2010, for evidence for the IPL].
Damage to both the inferior parietal [Mort et al., 2003; Verdon et al., 2010], and the premotor frontal [Committeri et al., 2007; Verdon et al., 2010] regions may cause left extra‐personal neglect in humans, possibly with a major role of damage to the IPL [Verdon et al., 2010] to bring about visuospatial neglect evidenced by tasks such as line bisection, and to the frontal cortex [Verdon et al., 2010; see also Rengachary et al., 2011, for related evidence] for spatial neglect revealed by motor exploratory tasks [see also Binder et al., 1992, for early evidence for this dissociation]. The SLF I connects the superior and medial parietal cortices [superior parietal lobule (SPL), BAs 5 and 7, and precuneus medial BA 7] with the supplementary motor region [Makris et al., 2005]. Damage to these regions is not an anatomical correlate of spatial neglect [Committeri et al., 2007; Vallar and Perani, 1986; Verdon et al., 2010], although lesions to the supplementary motor region have been associated with “motor neglect” of the limbs contralateral to the side of the lesion [Meador et al., 1986]. The SLF III (including part of the fibers of the anterior segment of the AF) connects the SMG (BA 40), with BA 44 and ventral BA 6 in the frontal lobe [Makris et al., 2005], and, in the monkey, the rostral IPL to the ventral part of the prefrontal and premotor cortices [Schmahmann et al., 2007]. In humans, the role of lesions of the IPL in bringing about extra‐personal visuospatial neglect is definite [Vallar, 2001]: within this region, however, the specific contributions of damage to the SMG [Golay et al., 2008; Leibovitch et al., 1998; Vallar and Perani, 1986; Verdon et al., 2010], and to the AG [Hillis et al., 2005; Mort et al., 2003] are less clear [see also, Committeri et al., 2007]. In both patients studied by Thiebaut de Schotten et al. (CAL and SB, [2005]) stimulation of the SMG brought about a left visuospatial neglect in line bisection. These findings are consistent with the results of a TMS study in neurologically unimpaired participants, that assessed the role of the SMG and the AG in setting the midline of a segment [Oliveri and Vallar, 2009]. In the present study, due to neurosurgical constraints, the contribution of the SMG, and of other putatively relevant regions, such as BAs 6 and 44, could not be assessed.
Stimulation of the dorsal and lateral parietofrontal connections, at the level of the subcortical white matter of the right AG (in a location consistent with the trajectory of the second subdivision of the SLF, and not of the more ventral SLF III fibers), disrupts line bisection performance. These findings are by and large in line with the general conclusion that the SLF II is important for spatial perception and awareness, providing DES evidence for a role of this branch in a ventral parietofrontal attentional network [Corbetta et al., 2008]. The SLF III in the left hemisphere may be relevant for articulatory processes: in humans, the articulatory component of language [Makris et al., 2005; Schmahmann et al., 2007]. In the right hemisphere, the SLF III contributes to spatial cognition [see the re‐analysis by Thiebaut de Schotten et al., 2008 of the data of Doricchi and Tomaiuolo, 2003; Urbanski et al., 2011, for evidence suggesting a role of the anterior segment of the AF/SLF III].
A recent study in a series of 50 right‐brain‐damaged patients with various types of tumor [low and high grade glioma, metastases, cavernoma, Roux et al., 2011] shows that DES of both cortical (inferior frontal and postcentral gyri, inferior parietal lobule, posterior part of the superior and middle temporal gyri) and subcortical (AF and fronto‐occipital fasciculus, SLF II) brings about shifts in line bisection. These findings highlight the role of both cortical and subcortical regions in spatial attention and representation, as in the present study. However, deviations caused by subcortical DES were very infrequent (about 5% of stimulations), compared with cortical DES; evidence of left visuospatial neglect was found only after DES of the AF [see also Urbanski et al., 2011, for related evidence about the role of the anterior segment of the right AF/SLF III], but not of the SLF II. Secondly, and importantly, both leftward and rightward bisection deviations are reported, with rightward errors being slightly greater. Overall, these findings cannot be readily interpreted in terms of spatial neglect for the contralesional side of space, suggesting instead that also ipsilesional neglect [Kwon and Heilman, 1991] may be possibly elicited by right hemispheric DES, sometimes in the same patient.
Evidence converging with the present results comes from a study in the monkey [Gaffan and Hornak, 1997] showing that parietal leucotomy (after removal of the cortex in the lateral posterior bank of the intraparietal sulcus), which may disrupt the SLF II [Schmahmann et al., 2007], brings about a contralesional visuospatial neglect. The present findings are also in line with the re‐analysis by Bartolomeo et al. [2007, p. 12] of the original statements by Thiebaut de Schotten et al. 2005, concluding that the fascicle whose stimulation caused the maximal rightward bias in left‐handed patient SB was likely to be the SLF II. In line with these findings, a role of the SLF II in visuospatial attention is suggested by a correlation in neurologically unimpaired individuals between the amount of “pseudoneglect” [namely the leftward error in line bisection, see Jewell and McCourt, 2000], and its size; such a correlation does not hold for the SLF branches I and III; furthermore, volumetric measurements show a right lateralization of the SLF III, with some trend for the SLF II [Thiebaut de Schotten et al., 2011].
Finally, stimulation of the IFOF (in two patients the right, and in one the left) was ineffective. Recent evidence indicates a role of this fascicle [Urbanski et al., 2008]: two right brain damaged patients, with a lesion to the IFOF, and with sparing of the SLF and of the ILF, showed left visuospatial neglect, and one such patient (#3 in the series) exhibited a disproportionate rightward bias in line bisection [see also Urbanski et al., 2011]. On the other hand, two patients undergoing surgery for brain tumors within or near the posterior portions of the right lateral ventricle, who showed left neglect, had damage to the SLF, but neither to the ILF nor to the IFOF; this indicates a specific role of the SLF, as compared with the ILF and the IFOF [Shinoura et al., 2009]. The IFOF connects areas, namely the ventral occipital lobe and the orbito‐frontal cortex [Catani and Thiebaut de Schotten, 2008], whose damage is not usually associated with spatial neglect [Vallar, 2001]. In sum, while damage to the IFOF may be associated with visuospatial neglect [Doricchi et al., 2008, for review; Urbanski et al., 2008], the present evidence from DES in awake surgery suggests that its role may be minor, as compared with the contribution of the SLF II.
The second main result of this study is that stimulation of BA 7b, particularly its middle portion, surrounding the superior parietal sulcus, causes a rightward shift in line bisection. This finding differs from the neuropsychological evidence from right brain damaged patients, showing that visuospatial neglect, as assessed also by line bisection tasks, is associated with more ventral damage, including the right IPL [Mort et al., 2003; Verdon et al., 2010], in line with early evidence [Heilman et al., 1994; Hécaen et al., 1956; Leibovitch et al., 1998; Vallar and Perani, 1987]. A number of functional imaging studies in neurologically unimpaired participants show, however, that perceptual bisection tasks (“Landmark” test) activate bilaterally, although overall predominantly in the right hemisphere, not only the IPL, but also the SPL (BA 7) [Fink et al., 2000, 2001], and the right intraparietal sulcus (IPS), for both perceptual and manual line bisection [Ciçek et al., 2009]. In a spatial attention and memory task, requiring the report of target detections, activations include not only the IPS, but also the SPL, in the right hemisphere [Szczepanski et al., 2010]. The SPL is activated in tasks involving a spatial shift of attention, such as a displacement in the location of the target [Molenberghs et al., 2007]. The discrepancy between the neuropsychological evidence from brain‐damaged patients, indicating a role of ventral regions such as the IPL and the TPJ, and the neuroimaging activation studies in neurologically unimpaired participants pointing to a contribution also from the SPL in spatial attention has been repeatedly noted [Szczepanski et al., 2010; Vallar et al., 2003]. The suggestion has been made that the lesions typically associated with spatial neglect, “ventral”, perisylvian, involving the IPL, the TPJ, the STG, and the ventral premotor cortex [Committeri et al., 2007; Mort et al., 2003; Vallar, 2001; Verdon et al., 2010] cause a “dorsal” functional imbalance in the IPS‐SPL, with a relative left hemisphere SPL hyperactivity being related to neglect severity [Corbetta et al., 2005, 2008, for discussion]. Somewhat similarly, it has been proposed that, in right‐brain damaged patients with damage to the IPL‐TPJ, the deficit of spatial attention may result from a disconnection between these ventral regions and the SPL [Szczepanski et al., 2010]. Accounts of this sort predict that interference by DES with the function of the right SPL may bring about spatial neglect, causing a temporary left–right imbalance, with a right hemispheric dysfunction, and are consistent with the present findings, although the weak association between structural damage to the SPL and spatial neglect remains to be explained [see also Corbetta, 2002; Shulman et al., 2010].
There is neuropsychological evidence that the SPL is involved in spatial attentional functions. Two patients [one left brain damaged, HH, one right brain damaged NV, Gillebert et al., 2011], with lesions involving the IPS and the SPL (in NV), and sparing the IPL, were impaired in orienting to and discriminating contralesional targets under conditions of bilateral stimulation, and of invalid cueing to contralesional stimuli [review in Vandenberghe et al., 2012]. These results complement the finding that damage to the right IPL (particularly, the AG), the ascending posterior branch of the superior temporal sulcus, and the IPS (specifically, the lower bank of the horizontal segment) is related to defective contralesional orienting under conditions of bilateral symmetrical stimulation [Molenberghs et al., 2008]. At the clinical assessment, patient HH had a right neglect on line bisection, while patient NV showed no impairment. One of the five right brain damaged patients with IPL damage showed left neglect in both cancellation and bisection tasks. Overall, these findings [Gillebert et al., 2011], based on a few patients with parietal lesions, suggest that damage to the SPL may bring about deficits of spatial attention, and, sometimes, signs of spatial neglect.
The selectivity of the effects of DES of BA 7 in the SPL is supported by the finding that DES of BA 5, performed in six patients, did not cause any bisection bias. This region, which in humans occupies the anterior part of the SPL [Zilles, 2004], is not a neuropathological correlate of spatial neglect [Vallar, 2001; Vallar et al., 2003], and was not found to be activated in the neuroimaging studies mentioned above [Ciçek et al., 2009; Fink et al., 2000, 2001].
The present pattern of results cannot be explained in terms of non‐typical neurofunctional organization, due to left handedness, as in the two right brain damaged patients (CAL and SB) reported by Thiebaut de Schotten et al. 2005. Four out of six right brain damaged patients were right‐handed, as well as the one left brain damaged patient (P2), and no differences related to this variable are apparent. Furthermore, all patients showed visuospatial neglect, neither before and after the surgery, nor during surgery in the no‐stimulation trials. These findings indicate that the neural network [Mesulam, 1990, 2002; Vallar, 1998] supporting spatial attention was largely preserved (or, at least, adequately reorganized, as commented in the following sentence), so that any observed effect may be specifically traced back to DES. A second possible confound to be taken into consideration is the cerebral neurofunctional plastic rearrangement, due to the slow growing features of the neoplastic lesion [Papagno et al., 2011b; Thiel et al., 2001]. Relevant as this factor is, independent evidence from human and animal data corroborates the involvement in spatial attention of white matter fascicles (the SLF II), and of cortical regions (BAs 7b, and, to a lesser extent in the present study, BA 39), as discussed previously. The consistent and major effects of stimulation of the SLF II suggest that, if possible, lateral fibers of this fascicle should be spared by the neurosurgical intervention.
A note of caution concerns the variability in the number of bisection trials performed by the patients during DES of different sites, and in the no‐stimulation condition. This was due to neurosurgical constraints. Nevertheless, both the analysis of the bisection performances of the individual patients and the group analysis converge in suggesting the role of the SLF II and the SPL.
A further cautionary note concerns the inherent limitations of the deterministic preoperative tractography algorithm used in this study, yet largely used in clinical practice: the FACT method is unable to resolve white matter architecture where more than one fiber population occupies the same voxel, and this can affect the reliability of tracking mainly in regions that contain multiple crossing white matter pathways, or where a competing pathway is significantly stronger. As an example, in our patients we were able to demonstrate only a part of the fibers of such a small tract like the first branch of the SLF, and mainly its dorsomedial anteroposterior connections in the white matter of the medial portion of the SPL before the intersection with descending corticospinal tract fibers. The shortcomings of DTI tractography have driven the development of probabilistic algorithms [Behrens et al., 2007], that allow modeling the distribution of multiple fiber populations in a voxel, and of more accurate acquisition techniques, based on advanced high angular resolution diffusion imaging and Spherical Deconvolution tractography [Dell'acqua et al., 2010; Thiebaut de Schotten et al., 2011], in order to provide a more accurate tractography of parietofrontal networks. Diffusion‐tensor streamline tractography still remains the most widely used method in clinical settings and preoperative assessment [Bello et al., 2008]. In addition, the distortion of the normal white matter architecture in the presence of infiltrating tumors, that disrupt the directional organization of fiber tracts causing reduced FA, and altered color patterns on directional maps, possibly affects their fully accurate localization by the DTI FACT method. Nevertheless, the low anisotropy threshold used for reconstructions, previously established on the basis of combination of DTI tractography with functional intraoperative data [Bello et al., 2008], allows to obtain reliable reconstruction of white matter tracts also through regions of tumor infiltration (see Fig. 1A,B). Moreover, in this patients' series displaced white matter tracts are prevalent with respect to infiltrated ones (see also Table 1). With reference to the prevalent site of subcortical involvement, when the mesial portion of the parietal lobe was prevalently involved, the subcortical tracts were mainly displaced laterally; when the lateral portion of the parietal lobe was prevalently involved, the subcortical tracts were displaced medially. Finally, it should be also pointed out that the selection of seed regions of interest for tractography dissections used in this work relied on a priori knowledge of the anatomical and topographical details of the bundles, that are necessary for the accurate delineation and interpretation of each tracts' subcortical trajectory.
Does the involvement of the SLF II, found in this study, mean that spatial neglect is to be conceived as a disconnection syndrome? With reference to the traditional box‐and‐arrow neurofunctional models of cognition [Lichtheim, 1885; Wernicke, 1874], spatial neglect should not be regarded as a classical disconnection syndrome, since the white matter lesion does not interrupt neural connections between brain areas subserving specific and discrete functional cognitive representations, bringing about a functionally defined disconnection syndrome. This is the traditional account of deficits such as “conduction aphasia” [see a recent review in Catani and Mesulam, 2008b], and “pure alexia” [Cohen et al., 2003; Déjérine, 1892].
CONCLUSIONS
The available evidence from extra‐personal spatial neglect supports the involvement of a premotor frontal‐posterior parietal network in spatial representation and attention [Rengachary et al., 2011; Verdon et al., 2010], although observations for a role of the superior temporal gyrus are on record [Committeri et al., 2007; Karnath et al., 2001, 2004]. The specific contribution of damage to the frontal vs. parietal components of the network has been elucidated so far in terms of “motor exploratory” versus “perceptual” differences (also related to the tasks used, such as line bisection and target cancellation) in the manifestations of the neglect syndrome [Binder et al., 1992; Vallar and Mancini, 2010, for review; Verdon et al., 2010]. In the present study, white matter (i.e., mainly the SLF II) and cortical (i.e., mainly BA 7) DES brings about a defective performance (namely, a disproportionate ipsilesional shift in line bisection). These findings are compatible with network [Bartolomeo et al., 2007; Cappa and Vallar, 1992; Doricchi et al., 2008; Mesulam, 1981, 1990, 1998; Vallar, 1998], and hodological (pathway‐based) [Catani and Mesulam, 2008a] approaches to the neural basis of sensorimotor and cognitive functions, where both the grey and the white matter matter.
Finally, this study, as also suggested by Papagno et al. [2011a, 2011b], highlights the need of testing cognitive functions during neurosurgery also for subcortical pathways, in addition to cortical regions, in patients with cerebral tumors.
Supporting information
Supporting Information Figure 1. Patient P1. A) Pre‐operative deterministic tractography reconstructions superimposed on axial T1‐weighted images; SLF I fibers are depicted in green, SLF II in blue, SLF III (that includes fibers of the horizontal part of the AF) in yellow and AF in red. White asterisks show the DES site of the SLF II, which caused a rightward bisection error during intraoperative stimulation. SLF II fibers are displaced laterally by the tumor. B) Postoperative MR tractography analysis on preoperative DTI acquisitions, with FA threshold > 0.2. White circles: starting ROIs for tractography reconstructions positioned in the sites of positive stimulation regions: depiction of the blue fibers, corresponding to the SLF II, at a higher and less “permissive” FA threshold. C) Post‐operative probabilistic tractography superimposed on axial, coronal and sagittal T1‐weighted images; tracking of parieto‐frontal connections from stimulation sites (the red line depicts the seed mask on axial sections). Voxels are color‐coded from 20 (blue) to 200 (light blue) samples passing through the voxel. Multi‐fiber tractography identifies dorsal and lateral parieto‐frontal connections linking the inferior parietal lobule to the posterior regions of the superior and middle frontal gyri, belonging to the SLF II.
Supporting Information Figure 2. Patient P2. A) Pre‐operative deterministic tractography reconstructions superimposed on axial T1‐weighted images (color code idem as in figure 1) in left‐brain‐damaged patient P2. SLF II fibers are difficult to be distinguished from AF fibers. White asterisks show the DES site of the SLF II. P2 showed no visuo‐spatial neglect during DES. B) Postoperative MR tractography analysis on preoperative DTI acquisitions, with FA threshold > 0.2. Starting ROIs for tractography reconstructions were positioned in the sites of DES stimulation regions. SLF II fibers run very closely to AF fibers. C) Post‐operative probabilistic tractography superimposed on axial, coronal and sagittal T1‐weighted images; tracking of connections from stimulation sites (the red line depicts the seed mask on axial sections). Voxels are color‐coded from 20 (blue) to 150 (light blue) samples passing through the voxel. Multi‐fiber tractography identifies lateral fronto‐temporal connections probably belonging to the AF.
Supporting Information Figure 3. Patient P3. A) Pre‐operative deterministic tractography reconstructions superimposed on axial T1‐weighted images (color code idem as in figure 1). White asterisks show the DES site of the SLF II, which caused a rightward bisection error during intraoperative stimulation. SLF II fibers pass through the hypo‐intense tumoral tissue area, and are infiltrated by the tumor. B) Postoperative MR tractography analysis on preoperative DTI acquisitions, with FA threshold > 0.2. White circles: starting ROIs for tractography reconstructions positioned in the sites of positive stimulation regions. C) Post‐operative probabilistic tractography superimposed on axial, coronal and sagittal T1‐weighted images; tracking of parieto‐frontal connections from stimulation sites (the red line depicts the seed mask on axial sections). Voxels are color‐coded from 20 (blue) to 150 (light blue) samples passing through the voxel. Multi‐fiber tractography identifies dorsal and lateral parieto‐frontal connections belonging to the SLF II.
Supporting Information Figure 4. Patient P5. A) Pre‐operative deterministic tractography reconstructions superimposed on axial T1‐weighted images (color code idem as in figure 1). White asterisks show the DES site of the SLF II, which caused a rightward bisection error during intraoperative stimulation. SLF II fibers are displaced medially and superiorly by the tumor. B) Postoperative MR tractography analysis on preoperative DTI acquisitions, FA threshold > 0.2. White circles: starting ROIs for tractography reconstructions positioned in the sites of positive stimulation regions; depiction of the blue fibers, corresponding to the SLF II. C) Post‐operative probabilistic tractography superimposed on axial, coronal and sagittal T1‐weighted images; tracking of parieto‐frontal connections from stimulation sites (the red line depicts the seed mask on axial sections). Voxels are color‐coded from 20 (blue) to 200 (light blue) samples passing through the voxel. Multi‐fiber tractography identifies dorsal and lateral parieto‐frontal connections linking the inferior parietal lobule to the posterior regions of the superior and middle frontal gyri, belonging to the SLF II.
Supporting Information Figure 5. Patient P7. A) Pre‐operative deterministic tractography reconstructions superimposed on axial T1‐weighted images (color code idem as in figure 1). White asterisks show the DES site of the SLF II, which caused a rightward bisection error during intraoperative stimulation. SLF II fibers lie laterally to the tumor. B) Postoperative MR tractography analysis on preoperative DTI acquisitions, FA threshold > 0.2. White circles: starting ROIs for tractography reconstructions positioned in the sites of positive stimulation regions: depiction of the blue fibers, corresponding to the SLF II. C) Post‐operative probabilistic tractography superimposed on axial, coronal and sagittal T1‐weighted images; tracking of parieto‐frontal connections from stimulation sites (the red line depicts the seed mask on axial sections). Voxels are color‐coded from 20 (blue) to 200 (light blue) samples passing through the voxel. Multi‐fiber tractography identifies dorsal and lateral parieto‐frontal connections linking the inferior parietal lobule to the posterior regions of the superior and middle frontal gyri, belonging to the SLF II.
Supporting Information
Supporting Information Table 1.
ACKNOWLEDGMENTS
The authors are grateful to Prof. Marcello Gallucci for statistical advice.
REFERENCES
- Albert ML (1973): A simple test of visual neglect. Neurology 23:658–664. [DOI] [PubMed] [Google Scholar]
- Bartolomeo P, Thiebaut de Schotten M, Doricchi F (2007): Left unilateral neglect as a disconnection syndrome. Cereb Cortex 17:2479–2490. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D (1994): MR diffusion tensor spectroscopy and imaging. Biophys J 66:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW (2007): Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage 34:144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen‐Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM (2003): Characterization and propagation of uncertainty in diffusion‐weighted MR imaging. Magn Reson Med 50:1077–1088. [DOI] [PubMed] [Google Scholar]
- Bello L, Acerbi F, Giussani C, Baratta P, Taccone P, Songa V, Fava M, Stocchetti N, Papagno C, Gaini SM (2006): Intraoperative language localization in multilingual patients with gliomas. Neurosurgery 59:115–125. [DOI] [PubMed] [Google Scholar]
- Bello L, Gallucci M, Fava M, Carrabba G, Giussani C, Acerbi F, Baratta P, Songa V, Conte V, Branca V, Stocchetti N, Papagno C, Gaini SM (2007): Intraoperative subcortical language tract mapping guides surgical removal of gliomas involving speech areas. Neurosurgery 60:67–82. [DOI] [PubMed] [Google Scholar]
- Bello L, Gambini A, Castellano A, Carrabba G, Acerbi F, Fava E, Giussani C, Cadioli M, Blasi V, Casarotti A, Papagno C, Gupta AK, Gaini SM, Scotti G, Falini A (2008): Motor and language DTI Fiber Tracking combined with intraoperative subcortical mapping for surgical removal of gliomas. Neuroimage 39:369–382. [DOI] [PubMed] [Google Scholar]
- Binder J, Marshall R, Lazar R, Benjamin J, Mohr JP (1992): Distinct syndromes of hemineglect. Arch Neurol 49:1187–1194. [DOI] [PubMed] [Google Scholar]
- Bird CM, Malhotra P, Parton A, Coulthard E, Rushworth MF, Husain M (2006): Visual neglect after right posterior cerebral artery infarction. J Neurol Neurosurg Psychiatry 77:1008–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchers S, Himmelbach M, Logothetis N, Karnath H‐O (2011): Direct electrical stimulation of human cortex—The gold standard for mapping brain functions? Nat Rev Neurosci 13:63–70. [DOI] [PubMed] [Google Scholar]
- Cappa SF, Vallar G (1992): Neuropsychological disorders after subcortical lesions: Implications for neural models of language and spatial attention In: Vallar G, Cappa SF, Wallesch C‐W, editors. Neuropsychological Disorders Associated with Subcortical Lesions. Oxford:Oxford University Press; pp7–41. [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK (2002): Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage 17:77–94. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Ffytche DH (2005): Perisylvian language networks of the human brain. Ann Neurol 57:8–16. [DOI] [PubMed] [Google Scholar]
- Catani M, Mesulam M (2008a): What is a disconnection syndrome? Cortex 44:911–913. [DOI] [PubMed] [Google Scholar]
- Catani M, Mesulam M (2008b): The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex 44:953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M (2008): A diffusion tensor imaging tractography atlas for virtual in vivo dissection. Cortex 44:1105–1132. [DOI] [PubMed] [Google Scholar]
- Chechlacz M, Rotshtein P, Hansen PC, Deb S, Riddoch MJ, Humphreys GW:The central role of the temporo‐parietal junction and the superior longitudinal fasciculus in supporting multi‐item competition: Evidence from lesion‐symptom mapping of extinction. Cortex; (in press). [DOI] [PubMed] [Google Scholar]
- Ciaraffa F, Castelli G, Parati EA, Bartolomeo P, Bizzi A: Visual neglect as a disconnection syndrome? A confirmatory case report. Neurocase (in press). [DOI] [PubMed] [Google Scholar]
- Ciçek M, Deouell LY, Knight RT (2009): Brain activity during landmark and line bisection tasks. Front Hum Neurosci 3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Martinaud O, Lemer C, Lehéricy S, Samson Y, Obadia M, Slachevsky A, Dehaene S (2003): Visual word recognition in the left and right hemispheres: anatomical and functional correlates of peripheral alexias. Cereb Cortex 13:1313–1333. [DOI] [PubMed] [Google Scholar]
- Committeri G, Pitzalis S, Galati G, Patria F, Pelle G, Sabatini U, Castriota‐Scanderbeg A, Piccardi L, Guariglia C, Pizzamiglio L (2007): Neural bases of personal and extrapersonal neglect in humans. Brain 130:431–441. [DOI] [PubMed] [Google Scholar]
- Corbetta M (2002): Two neural systems for visual orienting and the pathophysiology of unilateral spatial neglect In: Karnath H‐O, Milner AD, Vallar G, editors. The Cognitive and Neural Bases of Spatial Neglect. Oxford:Oxford University Press; pp259–273. [Google Scholar]
- Corbetta M, Kincade MJ, Lewis C, Snyder AZ, Sapir A (2005): Neural basis and recovery of spatial attention deficits in spatial neglect. Nat Neurosci 8:1603–1610. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL (2008): The reorienting system of the human brain: from environment to theory of mind. Neuron 58:306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL (2011): Spatial neglect and attention networks. Annu Rev Neurosci 34:569–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford JR, Garthwaite PH (2002): Investigation of the single case in neuropsychology: Confidence limits on the abnormality of test scores and test score differences. Neuropsychologia 40:1196–1208. [DOI] [PubMed] [Google Scholar]
- Daini R, Angelelli P, Antonucci G, Cappa SF, Vallar G (2002): Exploring the syndrome of spatial unilateral neglect through an illusion of length. Exp Brain Res 144:224–237. [DOI] [PubMed] [Google Scholar]
- Déjérine J (1892): Contribution à l'étude anatomo‐pathologique et clinique des différentes variétés de cécité verbale. Mém Soc Biol 4:61–90. [Google Scholar]
- Déjérine J (1914):Sémiologie des affections du système nerveux. Paris:Masson. [Google Scholar]
- Dell'acqua F, Scifo P, Rizzo G, Catani M, Simmons A, Scotti G, Fazio F (2010): A modified damped Richardson‐Lucy algorithm to reduce isotropic background effects in spherical deconvolution. Neuroimage 49:1446–1458. [DOI] [PubMed] [Google Scholar]
- Diller L, Weinberg J (1977): Hemi‐Inattention in rehabilitation. The evolution of a rational remediation program In: Weinstein EA, Friedland RP, editors. Hemi‐inattention and Hemisphere Specialization. New York:Raven Press; pp62–82. [Google Scholar]
- Doricchi F, Thiebaut de Schotten M, Tomaiuolo F, Bartolomeo P (2008): White matter (dis)connections and gray matter (dys)functions in visual neglect: Gaining insights into the brain networks of spatial awareness. Cortex 44:983–895. [DOI] [PubMed] [Google Scholar]
- Doricchi F, Tomaiuolo F (2003): The anatomy of neglect without hemianopia: a key role for parietal‐frontal disconnection? NeuroReport 14:2239–2243. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Shah NJ, Weiss PH, Halligan PW, Grosse‐Ruyken M, Ziemons K, Zilles K, Freund HJ (2000): Line bisection judgments implicate right parietal cortex and cerebellum as assessed by fMRI. Neurology 54:1324–1331. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Weiss PH, Zilles K (2001): The neural basis of vertical and horizontal line bisection judgments: an fMRI study of normal volunteers. Neuroimage 14:S59–67. [DOI] [PubMed] [Google Scholar]
- Foerster O, Penfield W (1930): The structural basis of traumatic epilepsy and results of radical operation. Brain 53:99–119. [Google Scholar]
- Fortis P, Maravita A, Gallucci M, Ronchi R, Grassi E, Senna I, Olgiati E, Perucca L, Banco E, Posteraro L, Tesio L, Vallar G (2010): Rehabilitating patients with left spatial neglect by prism exposure during a visuomotor activity. Neuropsychology 24:681–697. [DOI] [PubMed] [Google Scholar]
- Gaffan D, Hornak J (1997): Visual neglect in the monkey. Representation and disconnection. Brain 120:1647–1657. [DOI] [PubMed] [Google Scholar]
- Gainotti G, Messerli P, Tissot R (1972): Qualitative analysis of unilateral spatial neglect in relation to laterality of cerebral lesions. J Neurol Neurosurg Psychiatry 35:545–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharabaghi A, Fruhmann Berger M, Tatagiba M, Karnath H‐O (2006): The role of the right superior temporal gyrus in visual search‐insights from intraoperative electrical stimulation. Neuropsychologia 44:2578–2581. [DOI] [PubMed] [Google Scholar]
- Gillebert CR, Mantini D, Thijs V, Sunaert S, Dupont P, Vandenberghe R (2011): Lesion evidence for the critical role of the intraparietal sulcus in spatial attention. Brain 134:1694–1709. [DOI] [PubMed] [Google Scholar]
- Golay L, Schnider A, Ptak R (2008): Cortical and subcortical anatomy of chronic spatial neglect following vascular damage. Behav Brain Funct 4:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M (2007): Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron 53:905–918. [DOI] [PubMed] [Google Scholar]
- Hécaen H, Penfield W, Bertrand C, Malmo R (1956): The syndrome of apractognosia due to lesions of the minor cerebral hemisphere. Arch Neurol Psychiatry 75:400–434. [PubMed] [Google Scholar]
- Heilman KM, Valenstein E (1972): Auditory neglect in man. Arch Neurol 26:32–35. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Valenstein E (1979): Mechanisms underlying hemispatial neglect. Ann Neurol 5:166–170. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Valenstein E (2011):Clinical Neuropsychology, 5th ed Oxford:Oxford University Press. [Google Scholar]
- Heilman KM, Watson RT, Valenstein E (1994): Localization of lesions in neglect and related disorders In: Kertesz A, editor. Localization and Neuroimaging in Neuropsychology. San Diego:Academic Press; pp495–524. [Google Scholar]
- Hier DB, Mondlock J, Caplan LR (1983): Behavioral abnormalities after right hemisphere stroke. Neurology 33:337–344. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Newhart M, Heidler J, Barker PB, Herskovits EH, Degaonkar M (2005): Anatomy of spatial attention: insights from perfusion imaging and hemispatial neglect in acute stroke. J Neurosci 25:3161–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain M (2008): Hemispatial neglect In: Goldenberg G, Miller BL, editors. Handbook of Clinical Neurology. Amsterdam:Elsevier, B. V. pp359–372. [DOI] [PubMed] [Google Scholar]
- Husain M, Kennard C (1996): Visual neglect associated with frontal lobe infarction. J Neurol 243:652–657. [DOI] [PubMed] [Google Scholar]
- Jewell G, McCourt ME (2000): Pseudoneglect: A review and meta‐analysis of performance factors in line bisection tasks. Neuropsychologia 38:93–110. [DOI] [PubMed] [Google Scholar]
- Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S (2006): DtiStudio: Resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed 81:106–116. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Naeser MA, Martin PI, Ho M, Wang Y, Baker E, Pascual‐Leone A (2010): Horizontal portion of arcuate fasciculus fibers track to pars opercularis, not pars triangularis, in right and left hemispheres: a DTI study. Neuroimage 52:436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnath H‐O, Ferber S, Himmelbach M (2001): Spatial awareness is a function of the temporal not the posterior parietal lobe. Nature 411:950–953. [DOI] [PubMed] [Google Scholar]
- Karnath H‐O, Fruhmann Berger M, Küker W, Rorden C (2004): The anatomy of spatial neglect based on voxelwise statistical analysis: a study of 140 patients. Cereb Cortex 14:1164–1172. [DOI] [PubMed] [Google Scholar]
- Karnath H‐O, Rennig S, Johannsen L, Rorden C (2011): The anatomy underlying acute versus chronic spatial neglect: a longitudinal study. Brain 134:903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnath H‐O, Rorden C (2012): The anatomy of spatial neglect. Neuropsychologia 50:1010–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnath H‐O, Rorden C, Ticini LF (2009): Damage to white matter fiber tracts in acute spatial neglect. Cereb Cortex 19:2331–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman JT, Sepkuty JP, Hillis AE, Lenz FA, Heidler‐Gary J, Gingis L, Crone NE (2007): Spatial neglect during electrocortical stimulation mapping in the right hemisphere. Epilepsia 48:2365–2368. [DOI] [PubMed] [Google Scholar]
- Kwon SE, Heilman KM (1991): Ipsilateral neglect in a patient following a unilateral frontal lesion. Neurology 41:2001–2004. [DOI] [PubMed] [Google Scholar]
- Leibovitch FS, Black SE, Caldwell CB, Ebert PL, Ehrlich LE, Szalai JP (1998): Brain‐behavior correlations in hemispatial neglect using CT and SPECT: The Sunnybrook Stroke Study. Neurology 50:901–908. [DOI] [PubMed] [Google Scholar]
- Lichtheim L (1885): On aphasia. Brain 7:433–484. [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Pandya DN (2005): Segmentation of subcomponents within the superior longitudinal fascicle in humans: A quantitative, in vivo, DT‐MRI study. Cereb Cortex 15:854–869. [DOI] [PubMed] [Google Scholar]
- Makris N, Papadimitriou GM, Sorg S, Kennedy DN, Caviness VS, Pandya DN (2007): The occipitofrontal fascicle in humans: A quantitative, in vivo, DT‐MRI study. Neuroimage 37:1100–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador KJ, Watson RT, Bowers D, Heilman KM (1986): Hypometria with hemispatial and limb motor neglect. Brain 109:293–305. [DOI] [PubMed] [Google Scholar]
- Mesulam M‐M (1981): A cortical network for directed attention and unilateral neglect. Ann Neurol 10:309–325. [DOI] [PubMed] [Google Scholar]
- Mesulam M‐M (1990): Large‐scale neurocognitive networks and distributed processing for attention, language and memory. Ann Neurol 28:597–613. [DOI] [PubMed] [Google Scholar]
- Mesulam M‐M (1999): Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci 354:1325–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M‐M (2002): Functional anatomy of attention and neglect: from neurons to networks In: Karnath H‐O, Milner AD, Vallar G, editorsThe Cognitive and Neural Bases of Spatial Neglect. Oxford:Oxford University Press; pp33–45. [Google Scholar]
- Mesulam MM (1998): From sensation to cognition. Brain 121:1013–1052. [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Gillebert CR, Peeters R, Vandenberghe R (2008): Convergence between lesion‐symptom mapping and functional magnetic resonance imaging of spatially selective attention in the intact brain. J Neurosci 28:3359–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs P, Mesulam MM, Peeters R, Vandenberghe RR (2007): Remapping attentional priorities: differential contribution of superior parietal lobule and intraparietal sulcus. Cereb Cortex 17:2703–2712. [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Sale MV, Mattingley JB (2012): Is there a critical lesion site for unilateral spatial neglect? A meta‐analysis using activation likelihood estimation. Front Hum Neurosci 6:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC (1999): Three dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 45:265–269. [DOI] [PubMed] [Google Scholar]
- Mori S, van Zijl PC (2002): Fiber tracking: principles and strategies—A technical review. NMR Biomed 15:468–480. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Nagae‐Poetscher LM, van Zijl PC (2005):MRI Atlas of Human White Matter. Amsterdam, The Netherlands:Elsevier. [Google Scholar]
- Mort DJ, Malhotra P, Mannan SK, Rorden C, Pambakian A, Kennard C, Husain M (2003): The anatomy of visual neglect. Brain 126:1986–1997. [DOI] [PubMed] [Google Scholar]
- Oishi K, Zilles K, Amunts K, Faria A, Jiang H, Akhter K, Hua K, Woods R, Toga AW, Pike GB, Rosa‐Neto P, Evans A, Zhang J, Huang H, Miller MI, van Zijl PC, Mazziotta J, Mori S (2008): Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. Neuroimage 43:447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann G, Ojemann J, Lettich E, Berger M (1989): Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg 71:316–326. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Vallar G (2009): Parietal versus temporal lobe components in spatial cognition: Setting the mid‐point of a horizontal line. J Neuropsychol 3:201–211. [DOI] [PubMed] [Google Scholar]
- Pajevic S, Pierpaoli C (1999): Color schemes to represent the orientation of anisotropic tissues from diffusion tensor data: Application to white matter fiber tract mapping in the human brain. Magn Reson Med 42:526–540. [PubMed] [Google Scholar]
- Papagno C, Gallucci M, Casarotti A, Castellano A, Falini A, Fava E, Giussani C, Carrabba G, Bello L, Caramazza A (2011a): Connectivity constraints on cortical reorganization of neural circuits involved in object naming. Neuroimage 55:1306–1313. [DOI] [PubMed] [Google Scholar]
- Papagno C, Miracapillo C, Casarotti A, Romero Lauro LJ, Castellano A, Falini A, Casaceli G, Fava E, Bello L (2011b): What is the role of the uncinate fasciculus? Surgical removal and proper name retrieval. Brain 134:405–414. [DOI] [PubMed] [Google Scholar]
- Pizzamiglio L, Antonucci G, Judica A, Montenero P, Razzano C, Zoccolotti P (1992): Cognitive rehabilitation of the hemineglect disorder in chronic patients with unilateral brain damage. J Clin Exp Neuropsychol 14:901–923. [DOI] [PubMed] [Google Scholar]
- Ptak R, Schnider A (2010): The dorsal attention network mediates orienting toward behaviorally relevant stimuli in spatial neglect. J Neurosci 30:12557–12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS (2002):Hierarchical Linear Models, 2nd ed Thousand Oaks:Sage Publications. [Google Scholar]
- Rengachary J, He BJ, Shulman GL, Corbetta M (2011): A behavioral analysis of spatial neglect and its recovery after stroke. Front Hum Neurosci 5:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Matelli M (2003): Two different streams form the dorsal visual system: anatomy and functions. Exp Brain Res 153:146–157. [DOI] [PubMed] [Google Scholar]
- Rode G, Cotton F, Revol P, Jacquin‐Courtois S, Rossetti Y, Bartolomeo P (2010): Representation and disconnection in imaginal neglect. Neuropsychologia 48:2903–2911. [DOI] [PubMed] [Google Scholar]
- Rodrigo S, Naggara O, Oppenheim C, Golestani N, Poupon C, Cointepas Y, Mangin JF, Le Bihan D, Meder JF (2007): Human subinsular asymmetry studied by diffusion tensor imaging and fiber tracking. Am J Neuroradiol 28:1526–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronchi R, Posteraro L, Fortis P, Bricolo E, Vallar G (2009): Perseveration in left spatial neglect; drawing and cancellation tasks. Cortex 45:300–312. [DOI] [PubMed] [Google Scholar]
- Rorden C, Fruhmann Berger M, Karnath H‐O (2006): Disturbed line bisection is associated with posterior brain lesions. Brain Res 1080:17–25. [DOI] [PubMed] [Google Scholar]
- Rossetti Y, Pisella L, Vighetto A (2003): Optic ataxia revisited: visually guided action versus immediate visuomotor control. Exp Brain Res 153:171–179. [DOI] [PubMed] [Google Scholar]
- Rousseau A, Mokhtari K., Duyckaerts C (2008): The 2007 WHO classification of tumors of the central nervous system—What has changed? Curr Opin Neurol 21:720–727. [DOI] [PubMed] [Google Scholar]
- Roux FE, Dufor O, Lauwers‐Cances V, Boukhatem L, Brauge D, Draper L, Lotterie JA, Démonet JF (2011): Electrostimulation mapping of spatial neglect. Neurosurgery 69:1218–1231. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE, Johansen‐Berg H (2006): Connection patterns distinguish 3 regions of human parietal cortex. Cereb Cortex 16:1418–1430. [DOI] [PubMed] [Google Scholar]
- Schenkenberg T, Bradford DC, Ajax ET (1980): Line bisection and unilateral visual neglect in patients with neurologic impairment. Neurology 30:509–517. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN (2006):Fiber pathways of the brain. Oxford:Oxford University Press. [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D'Arceuil HE, de Crespigny AJ, Wedeen VJ (2007): Association fibre pathways of the brain: Parallel observations from diffusion spectrum imaging and autoradiography. Brain 130:630–653. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN (1984): Further observations on parieto‐temporal connections in the rhesus monkey. Exp Brain Res 55:301–312. [DOI] [PubMed] [Google Scholar]
- Shinoura N, Suzuki Y, Yamada R, Tabei Y, Saito K, Yagi K (2009): Damage to the right superior longitudinal fasciculus in the inferior parietal lobe plays a role in spatial neglect. Neuropsychologia 47:2600–2603. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Pope DL, Astafiev SV, McAvoy MP, Snyder AZ, Corbetta M (2010): Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. J Neurosci 30:3640–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein S, Volpe BT (1983): Classical “parietal” neglect syndrome after subcortical right frontal lobe infarction. Neurology 33:797–799. [DOI] [PubMed] [Google Scholar]
- Szczepanski SM, Konen CS, Kastner S (2010): Mechanisms of spatial attention control in frontal and parietal cortex. J Neurosci 30:148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell'Acqua F, Forkel SJ, Simmons A, Vergani F., Murphy DG, Catani M (2011): A lateralized brain network for visuospatial attention. Nat Neurosci 14:1245–1246. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Kinkingnéhun S, Delmaire C, Lehéricy S, Duffau H, Thivard L, Volle E, Levy R, Dubois B, Bartolomeo P (2008): Visualization of disconnection syndromes in humans. Cortex 44:1097–1103. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Urbanski M, Duffau H, Volle E, Levy R, Dubois B, Bartolomeo P (2005): Direct evidence for a parietal‐frontal pathway subserving spatial awareness in humans. Science 309:2226–2228. [DOI] [PubMed] [Google Scholar]
- Thiel A, Herholz K, Koyuncu A, Ghaemi M, Kracht LW, Habedank B, Heiss WD (2001): Plasticity of language networks in patients with brain tumors: A positron emission tomography activation study. Ann Neurol 50:620–629. [DOI] [PubMed] [Google Scholar]
- Urbanski M, Thiebaut de Schotten M, Rodrigo S, Catani M, Oppenheim C, Touzé E, Chokron S, Méder JF, Lévy R, Dubois B, Bartolomeo P (2008): Brain networks of spatial awareness: Evidence from diffusion tensor imaging tractography. J Neurol Neurosurg Psychiatry 79:598–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski M, Thiebaut de Schotten M, Rodrigo S, Oppenheim C, Touzé E, Méder JF, Moreau K, Loeper‐Jeny C, Dubois B, Bartolomeo P (2011): DTI‐MR tractography of white matter damage in stroke patients with neglect. Exp Brain Res 208:491–505. [DOI] [PubMed] [Google Scholar]
- Vallar G (1998): Spatial hemineglect in humans. Trends Cogn Sci 2:87–97. [DOI] [PubMed] [Google Scholar]
- Vallar G (2001): Extrapersonal visual unilateral spatial neglect and its neuroanatomy. Neuroimage 14:S52–S58. [DOI] [PubMed] [Google Scholar]
- Vallar G, Bottini G, Paulesu E (2003): Neglect syndromes: The role of the parietal cortex In: Siegel AM, Andersen RA, Freund H‐J, Spencer DD, editors. Advances in Neurology Vol. 93 The Parietal Lobes. Philadelphia:Lippincott Williams & Wilkins; pp293–319. [PubMed] [Google Scholar]
- Vallar G, Daini R, Antonucci G (2000): Processing of illusion of length in spatial hemineglect. A study of line bisection. Neuropsychologia 38:1087–1097. [DOI] [PubMed] [Google Scholar]
- Vallar G, Mancini F (2010): Mapping the neglect syndrome onto neurofunctional streams In:Perception, Action, and Consciousness. Sensorimotor Dynamics and Two Visual Systems. Oxford:Oxford University Press; pp183–215. [Google Scholar]
- Vallar G, Perani D (1986): The anatomy of unilateral neglect after right hemisphere stroke lesions. A clinical CT/Scan correlation study in man. Neuropsychologia 24:609–622. [DOI] [PubMed] [Google Scholar]
- Vallar G, Perani D (1987): The anatomy of spatial neglect in humans In:Neurophysiological and Neuropsychological Aspects of Spatial Neglect. Amsterdam:Elsevier Science Publishers B. V., North‐Holland; pp235–258. [Google Scholar]
- Vallar G, Rusconi ML, Fontana S, Musicco M (1994): Tre test di esplorazione visuo‐spaziale: taratura su 212 soggetti normali. Arch Psicol Neurol Psichiatr 55:827–841. [Google Scholar]
- Vandenberghe R, Molenberghs P, Gillebert CR (2012): Spatial attention deficits in humans: the critical role of superior compared to inferior parietal lesions. Neuropsychologia 50:1092–1103. [DOI] [PubMed] [Google Scholar]
- Verdon V, Schwartz S, Lovblad KO, Hauert CA, Vuilleumier P (2010): Neuroanatomy of hemispatial neglect and its functional components: A study using voxel‐based lesion‐symptom mapping. Brain 133:880–894. [DOI] [PubMed] [Google Scholar]
- Wernicke C (1874): The symptom complex of aphasia. Boston Stud Philos Sci Proc Boston Colloquium Philos Sci 4:34–97. [Google Scholar]
- Wilson B, Cockburn J, Halligan PW (1987):Behavioural inattention test. Titchfield, Hants:Thames Valley Test Company. [Google Scholar]
- Witelson SF (1985): The brain connection: the corpus callosum is larger in left‐handers. Science 229:665–668. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Kigar DL (1988): Asymmetry in brain function follows asymmetry in anatomical form: Gross, microscopic, postmortem and imaging studies In: Boller F, Grafman J, editors. Handbook of Neuropsychology. Amsterdam:Elsevier; pp111–142. [Google Scholar]
- Zilles K (2004):Architecture of the human cerebral cortex. Regional and laminar organization In: Paxinos G, Mai JK, editors. The Human Nervous System, 2nd ed Amsterdam:Elsevier; pp 997–1055. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure 1. Patient P1. A) Pre‐operative deterministic tractography reconstructions superimposed on axial T1‐weighted images; SLF I fibers are depicted in green, SLF II in blue, SLF III (that includes fibers of the horizontal part of the AF) in yellow and AF in red. White asterisks show the DES site of the SLF II, which caused a rightward bisection error during intraoperative stimulation. SLF II fibers are displaced laterally by the tumor. B) Postoperative MR tractography analysis on preoperative DTI acquisitions, with FA threshold > 0.2. White circles: starting ROIs for tractography reconstructions positioned in the sites of positive stimulation regions: depiction of the blue fibers, corresponding to the SLF II, at a higher and less “permissive” FA threshold. C) Post‐operative probabilistic tractography superimposed on axial, coronal and sagittal T1‐weighted images; tracking of parieto‐frontal connections from stimulation sites (the red line depicts the seed mask on axial sections). Voxels are color‐coded from 20 (blue) to 200 (light blue) samples passing through the voxel. Multi‐fiber tractography identifies dorsal and lateral parieto‐frontal connections linking the inferior parietal lobule to the posterior regions of the superior and middle frontal gyri, belonging to the SLF II.
Supporting Information Figure 2. Patient P2. A) Pre‐operative deterministic tractography reconstructions superimposed on axial T1‐weighted images (color code idem as in figure 1) in left‐brain‐damaged patient P2. SLF II fibers are difficult to be distinguished from AF fibers. White asterisks show the DES site of the SLF II. P2 showed no visuo‐spatial neglect during DES. B) Postoperative MR tractography analysis on preoperative DTI acquisitions, with FA threshold > 0.2. Starting ROIs for tractography reconstructions were positioned in the sites of DES stimulation regions. SLF II fibers run very closely to AF fibers. C) Post‐operative probabilistic tractography superimposed on axial, coronal and sagittal T1‐weighted images; tracking of connections from stimulation sites (the red line depicts the seed mask on axial sections). Voxels are color‐coded from 20 (blue) to 150 (light blue) samples passing through the voxel. Multi‐fiber tractography identifies lateral fronto‐temporal connections probably belonging to the AF.
Supporting Information Figure 3. Patient P3. A) Pre‐operative deterministic tractography reconstructions superimposed on axial T1‐weighted images (color code idem as in figure 1). White asterisks show the DES site of the SLF II, which caused a rightward bisection error during intraoperative stimulation. SLF II fibers pass through the hypo‐intense tumoral tissue area, and are infiltrated by the tumor. B) Postoperative MR tractography analysis on preoperative DTI acquisitions, with FA threshold > 0.2. White circles: starting ROIs for tractography reconstructions positioned in the sites of positive stimulation regions. C) Post‐operative probabilistic tractography superimposed on axial, coronal and sagittal T1‐weighted images; tracking of parieto‐frontal connections from stimulation sites (the red line depicts the seed mask on axial sections). Voxels are color‐coded from 20 (blue) to 150 (light blue) samples passing through the voxel. Multi‐fiber tractography identifies dorsal and lateral parieto‐frontal connections belonging to the SLF II.
Supporting Information Figure 4. Patient P5. A) Pre‐operative deterministic tractography reconstructions superimposed on axial T1‐weighted images (color code idem as in figure 1). White asterisks show the DES site of the SLF II, which caused a rightward bisection error during intraoperative stimulation. SLF II fibers are displaced medially and superiorly by the tumor. B) Postoperative MR tractography analysis on preoperative DTI acquisitions, FA threshold > 0.2. White circles: starting ROIs for tractography reconstructions positioned in the sites of positive stimulation regions; depiction of the blue fibers, corresponding to the SLF II. C) Post‐operative probabilistic tractography superimposed on axial, coronal and sagittal T1‐weighted images; tracking of parieto‐frontal connections from stimulation sites (the red line depicts the seed mask on axial sections). Voxels are color‐coded from 20 (blue) to 200 (light blue) samples passing through the voxel. Multi‐fiber tractography identifies dorsal and lateral parieto‐frontal connections linking the inferior parietal lobule to the posterior regions of the superior and middle frontal gyri, belonging to the SLF II.
Supporting Information Figure 5. Patient P7. A) Pre‐operative deterministic tractography reconstructions superimposed on axial T1‐weighted images (color code idem as in figure 1). White asterisks show the DES site of the SLF II, which caused a rightward bisection error during intraoperative stimulation. SLF II fibers lie laterally to the tumor. B) Postoperative MR tractography analysis on preoperative DTI acquisitions, FA threshold > 0.2. White circles: starting ROIs for tractography reconstructions positioned in the sites of positive stimulation regions: depiction of the blue fibers, corresponding to the SLF II. C) Post‐operative probabilistic tractography superimposed on axial, coronal and sagittal T1‐weighted images; tracking of parieto‐frontal connections from stimulation sites (the red line depicts the seed mask on axial sections). Voxels are color‐coded from 20 (blue) to 200 (light blue) samples passing through the voxel. Multi‐fiber tractography identifies dorsal and lateral parieto‐frontal connections linking the inferior parietal lobule to the posterior regions of the superior and middle frontal gyri, belonging to the SLF II.
Supporting Information
Supporting Information Table 1.


