Abstract
A functional polymorphism (5‐hydroxytryptamine transporter linked polymorphic region [5‐HTTLPR]) in the promoter region of human serotonin transporter gene has been found to be associated with several dimensions of neuroticism and psychopathology, especially anxiety. However, the neural basis underlying the association between 5‐HTTLPR and anxiety is less clear. Here, we explored how 5‐HTTLPR influenced anxiety by modulating the spontaneous brain activities in Han Chinese. First, we found an association between 5‐HTTLPR and anxiety only in the male and not in the female population, where male S/S homozygotes had a significantly higher level of anxiety than male L allele carriers. Then, we examined how 5‐HTTLPR influenced anxiety at both regional and network levels in the brain at rest. At the regional level, we found a significantly higher fractional amplitude of low‐frequency fluctuations in the amygdala in male S/S homozygotes relative to male L allele carriers. At the network level, male S/S homozygotes showed a weaker resting‐state functional connectivity (RSFC) between the amygdala and various regions, including the insula, Heschl's gyrus, lateral occipital cortex, superior temporal gyrus, and hippocampus, and a stronger RSFC between the amygdala and various regions, including the supramariginal gyrus and middle frontal gyrus. However, at both levels, only was the amygdala–insula RSFC correlated with anxiety. Mediation analyses further revealed that the amygdala–insula RSFC mediated the association between 5‐HTTLPR and anxiety. In short, our study provided the first empirical evidence that the amygdala–insula RSFC served as the neural basis underlying the association between 5‐HTTLPR and anxiety, suggesting a potential neurogenetic susceptibility mechanism for anxiety. Hum Brain Mapp 36:2732–2742, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: 5‐HTTLPR, anxiety, amygdala, resting‐state functional connectivity
INTRODUCTION
Research concerning the genetic background of traits and cognitive abilities has been rapidly expanding [Jonassen and Landrø, 2014; Montag and Reuter, 2014; Papageorgiou and Ronald, 2013]. In humans, two common alleles, short (S) and long (L), in a variable repeat sequence of the promoter region of the serotonin transporter gene (5‐hydroxytryptamine transporter linked polymorphic region, 5‐HTTLPR) have been associated with anxiety‐related behaviors in healthy subjects. Specifically, the S allele reduces transcriptional efficiency compared with the L allele; therefore, S allele carriers demonstrate a higher level of anxiety and neuroticism than L/L homozygotes [Katsuragi et al., 1999; Lesch et al., 1996; Osher et al., 2000; Schinka et al., 2004; Sen et al., 2004; Xenia Gonda et al., 2009].
Because the amygdala is identified as a critical region for emotion processing and neuroticism [Aghajani et al., 2013; Costafreda et al., 2008; Koelsch et al., 2013], previous studies have investigated the effect of the 5‐HTTLPR polymorphism on the functionality of the amygdala. Stronger amygdala activation in response to negative stimuli [Canli et al., 2005, Costafreda et al., 2013; Hariri et al., 2002; Heinz et al., 2004; Kobiella et al., 2011; Munafò et al., 2008] and higher resting‐state cerebral blood flow in the amygdala [Canli et al., 2006; Rao et al., 2007] in S allele carriers have been consistently observed in healthy individuals. Significant differences have also been found in the functional connectivity between the amygdala and other regions during affective tasks in different 5‐HTTLPR genotype groups. Specifically, the functional connectivity between the amygdala and cingulate decreases in the S allele carriers when processing emotional faces compared with L/L homozygotes [Costafreda et al., 2013; Pezawas et al., 2005], whereas greater functional connectivity between the amygdala and ventromedial prefrontal cortex during the processing of emotional stimuli is observed in S allele carriers compared with L/L homozygotes [Heinz et al., 2004]. Furthermore, the magnitude of the functional connectivity between the amygdala and cingulate during the processing of emotional faces predicts individual differences in anxiety [Pezawas et al., 2005]. Here, we investigated whether the effect of 5‐HTTLPR polymorphism on the amygdala is maintained at rest (i.e., when no affective stimuli are processed) and whether such an effect can be read out to account for individuals' anxiety.
To this end, we recruited a large sample of Han Chinese participants and measured their levels of anxiety, genotypes of 5‐HTTLPR polymorphism, and spontaneous brain activities at rest with resting‐state functional magnetic resonance imaging (rs‐fMRI). First, we attempted to replicate findings that genetic variations of 5‐HTTLPR are associated with individual differences in anxiety [Katsuragi et al., 1999; Lesch et al., 1996; Osher et al., 2000; Schinka et al., 2004; Sen et al., 2004; Xenia Gonda et al., 2009]. Second, we explored the effect of 5‐HTTLPR polymorphism on the functionality of the amygdala at both regional and network levels at rest. Specifically, at the regional level, we used an increasingly popular measure of low‐frequency BOLD oscillations: fractional amplitude of low‐frequency fluctuations [fALFF, Zou et al., 2008], which is a normalized index of the amplitude of low‐frequency fluctuations. Previous studies have shown that fALFF is associated with individual differences in a variety of cognitive abilities and personality traits in the healthy population [Cox et al., 2012; Mennes et al., 2011; Wei et al., 2014]. At the network level, we used resting‐state functional connectivity (RSFC) to evaluate how the amygdala is functionally connected to the rest of the brain. After we identified the effect of 5‐HTTLPR polymorphism on the amygdala in these two measures, we examined whether such an effect was associated with behaviorally observed anxiety.
MATERIALS AND METHODS
Participants
Two hundred and sixty‐two Han Chinese students (104 males, 158 females, mean age = 22.14 years, SD = 0.83 years) from Beijing Normal University (BNU) participated in this study. Participants reported no past or current psychiatric illness or history of neurological disorders (e.g., epilepsy, traumatic brain injury, neurodegenerative disorders and cerebro‐vascular disease), mental retardation or significant systemic medical illness. All participants provided blood samples, and finished the behavioral tests and fMRI scan (N = 262). The study was approved by the institutional review board of BNU. Prior to testing, written informed consent was obtained from the participants.
Behavioral Measurements and Data Analyses
Assessment of anxiety
The participants' trait anxiety was assessed with the anxiety facet in Neuroticism dimension of the NEO‐PI‐R (the Revised NEO Personality Inventory, Costa and McCrae, 1995] questionnaire. The anxiety facet consists of four items: “I am not a worrier,” “I rarely feel fearful or anxious,” “I often feel tense and jittery,” and “I often worry about things that might go wrong.” The items were translated into Chinese for the ease of comprehension. Participants were instructed to select one of the five statements ranging from 0 (strongly disagree) to 4 (strongly agree) in a Likert scale. Coefficient alpha for the present sample was 0.75, showing reasonably high reliability to assess the participants' anxiety. The participants' anxiety was indexed based on the total score of four items, with higher scores indicating a higher level of anxiety.
Assessment of general intelligence
The participants' general intelligence was measured with the Chinese version of the Raven's advanced progressive matrix [Raven et al., 1998] to control for the effect of general intelligence on the association between 5‐HTTLPR and anxiety. The scale contains 36 nonverbal items. For each item, the participants were required to select the missing piece of a 3 × 3 matrix from eight options [Takeuchi et al., 2010]. The participants' general intelligence was indexed based on the number of correct answers in 30 min. The Cronbach's Alpha of intelligence assessment was 0.80 for the present sample.
rs‐fMRI Data Acquisition and Analyses
Image acquisition
Images were acquired using a 3T scanner (Siemens Magnetom Trio, A Tim System) with a 12‐channel, phase‐arrayed coil at BNU Imaging Center for Brain Research, Beijing, China. During the rs‐fMRI scan, participants were instructed to relax without engaging in any specific task while remaining still (and awake) with the eyes closed. The resting state scanning consisted of 240 contiguous echo planar imaging (EPI) volumes (repetition time (TR) = 2,000 ms; echo time (TE) = 30 ms; flip angle = 90°; number of slices = 33; matrix = 64 × 64; Field of View (FOV) = 200 × 200 mm2; acquisition voxel size = 3.1 × 3.1 × 3.6 mm3). Moreover, high‐resolution T1‐weighted images were also acquired with a magnetization prepared rapid acquisition gradient echo sequence (MPRAGE: TR/TE/inversion time (TI) = 2,530/3.39/1,100 ms; flip angle = 7°; matrix = 256 × 256) for spatial registration. One hundred and twenty‐eight contiguous sagittal slices were obtained with a 1 × 1 mm2 in‐plane resolution and 1.33‐mm slice thickness.
Data preprocessing
The images were preprocessed using the functional MRI of the brain (FMRIB) Software Library (FSL, http://www.fmrib.ox.ac.uk/fsl/). For the rs‐fMRI data, the first four volumes were discarded for signal equilibrium. The preprocessing steps of rs‐fMRI data consisted of spatial Gaussian smoothing (full‐width at half maximum (FWHM) = 6 mm), realignment, motion correction (by aligning each volume to the middle volume of the image with motion correction FLIRT (MCFLIRT)), intensity normalization, and removing linear trends. The registration of each participant's T1‐weighted anatomic image to a common stereotaxic space (the Montreal Neurological Institute 152‐brain template, Montreal Neurological Institute (MNI)152, 2 × 2 × 2 mm3 resolution) was accomplished using a two‐step process [Andersson et al., 2007]. First, a 12‐degrees‐of‐freedom linear affine was carried out with FMRIB's Linear Image Registration Tool (FLIRT) [Jenkinson and Smith, 2001; Jenkinson et al., 2002]. Second, the registration was further refined with FLIRT nonlinear registration [Andersson et al., 2007]. Each participant's rs‐fMRI image to the T1‐weighted anatomic image was registered with FLIRT to produce a six‐degrees‐of‐freedom affine transformation matrix.
Calculation of fALFF
For some participants, part of the frontal lobe, parietal lobe, and cerebellum was missed in the rs‐fMRI data because their heads were too large. Thus, a mask was defined by intersecting the images from all participants and excluding the cerebellar regions. Moreover, because low‐frequency fluctuations are sensitive to the signal in the gray matter, voxels in the mask with a mean gray matter volume lower than 0.2 across the same group of participants were excluded based on the voxel‐based morphometry analysis. In total, 131,983 voxels were in the mask.
For a time series in each voxel, the sum of amplitudes within a low‐frequency range (0.01–0.1 Hz) was extracted. The fALFF was then computed as the fractional sum of amplitudes within the low‐frequency range that was divided by the sum of amplitude across the entire frequency range [0–0.25 Hz; Zuo et al., 2010]. As a normalized index of ALFF, fALFF is less susceptible to artifacts in regions located within the vicinity of vessels and/or significant pulsatile motion [Zou et al., 2008; Zuo et al., 2010]. The fALFF maps were then registered to the MNI152 space by applying the previously calculated transformation information.
Calculation of RSFC
To eliminate physiological noise, such as fluctuations related to motion and cardiac and respiratory cycles, nuisance signals were regressed out using the methods described in previous studies [Biswal et al., 2010; Fox et al., 2005]. Nuisance regressors included the averaged cerebrospinal fluid signal, averaged white matter signal, global signal averaged across the whole brain, six head realignment parameters obtained by rigid‐body head motion correction, and the derivatives of each of these signals. The 4‐D residual time series obtained after removing the nuisance covariates were registered to the MNI standard space by applying the previously computed linear transformation matrix.
To obtain the RSFC, we calculated the mean time series of the seed region of interest (ROI) for each participant. For the same gray matter mask applied in the above regional analysis, we calculated the correlation coefficients (r) between the time series of the seed and other voxels to obtain an r map for each participant. Fisher z score transformations were conducted for correlation coefficients (r) to generate a z‐functional connectivity map for each participant.
Genetic Data Acquisition and Analyses
Genotyping
Each participant provided a 4‐ml venous blood sample, and genomic DNA was isolated from blood samples using standard techniques. Forward (5′‐GGC GTT GCC GCT CTG AAT TGC‐3′) and reverse (5′‐GAG GGA CTG AGC TGG ACA ACC AC‐3′) primers were used to generate 484 and 528 bp fragments of the 5‐HTTLPR polymorphism. Polymerase chain reaction (PCR) was performed according to a standard protocol [Collier et al., 1996] on a PE‐9700 or a PE‐2400 thermal cycle (Perkin Elmer). After an initial denaturation at 95 °C for 4 min, 35 cycles were carried out at 96 °C for 45 s, 61 °C for 90 s, and 72 °C for 90 s, followed by a final step of elongation at 72 °C for 10 min. The PCR products were then separated on a 2% agarose gel supplemented with ethidium bromide for 3 h. Finally, the genotype of each sample was detected and recorded with the Gel Doc 2000 imaging system. The L allele represented the fragment of 528 bp, and the S allele represented the fragment of 488 bp. The genotypes were determined by at least two researchers, and ambiguous or unidentifiable results were reamplified and rescored. Samples that continued to be amplified poorly were eliminated from the study.
5‐HTTLPR—behavior analyses
To examine whether the variance of 5‐HTTLPR polymorphism was associated with individual differences in anxiety, we compared the anxiety scores between different genotype groups. Most previous studies using Caucasian samples compared S allele carriers with the L/L homozygotes [e.g., Lesch et al., 1996; Osher et al., 2000; Xenia Gonda et al., 2009]. However, the distribution of the 5‐HTTLPR genotype differs between the Caucasian and Chinese populations. The frequency of S/S is approximately 12–24% in Caucasians but ranges from 45 to 74% in Asians [Goldman et al., 2010]. Therefore, we compared S/S homozygotes with L allele carriers in this study. Furthermore, the effect of gender was taken into account because previous studies have reported a gender difference. For example, a significant genetic contribution of 5‐HTTLPR to emotion disorders in the Han Chinese population was only found in males [Wang et al., 2014]. Thus, a two‐way ANOVA with 5‐HTTLPR (L allele carriers vs. S/S homozygotes) by gender (male vs. female) was performed.
Regional level analyses on the relation between 5‐HTTLPR and fALFF
To compare the fALFF of the amygdala between different 5‐HTTLPR genotypes, we first identified the amygdala using a probabilistic map from the Harvard‐Oxford Subcortical Atlas in FSL with a threshold of 50%, that is, voxels that have a 50% or greater probability of being labeled as the amygdala were included as the amygdala and used in further analyses (left amygdala: 1,816 mm3, right amygdala: 2,224 mm3). The fALFF of the right (or left) amygdala was calculated by averaging the fALFF of all voxels in the right (or left) amygdala. The mean fALFF was obtained by averaging the fALFF of all voxels in the bilateral amygdala. To examine the effect of 5‐HTTLPR polymorphism on the fALFF of the amygdala, an independent two‐sample t‐test was conducted to compare the fALFF of the amygdala between L allele carriers and S/S homozygotes.
To explore whether regions other than the amygdala were also influenced by 5‐HTTLPR polymorphism, a whole‐brain analysis was performed on the fALFF of each voxel in the brain with a general linear model. The 5‐HTTLPR genotype (L allele carriers vs. S/S homozygotes) was treated as the independent variable, and the mean fALFF of the whole brain was treated as a confounding covariate. Fisher z transformations were conducted from correlation coefficients (β) to generate a z map. Based on Monte Carlo simulations (3dClustSim, AFNI, http://afni.nimh.nih.gov), the threshold was a combination of a voxelwise P value of <0.005 and a cluster size greater than 67 voxels, above which the probability of a type I error was below 0.05.
Network level analyses on the relation between 5‐HTTLPR and RSFC
To explore the effect of 5‐HTTLPR polymorphism on the RSFC between the amygdala and other cortical regions, two 4‐mm‐radius spheres in the amygdala were defined as seeds, centering at the voxels showing the strongest association between fALFF and 5‐HTTLPR in the left and right amygdala. To get a more stable signal, the time series of the voxels in the seeds were then averaged. To obtain the RSFC map for each participant, the correlation coefficient (r) between the mean time series of the seeds and that of each voxel in the brain was calculated. Fisher z score transformations were conducted for the correlation coefficients (r) to generate a z‐RSFC map for each participant.
To identify the RSFC that reflected the variance of the 5‐HTTLPR genotypes, regression analyses were conducted in each voxel across participants to examine whether the z‐RSFC value of the voxel significantly differed between L allele carriers and S/S homozygotes. The 5‐HTTLPR (L allele carriers vs. the S/S homozygotes) and z‐RSFC value of each voxel were treated as the independent variable and dependent variable, respectively. Fisher z score transformations were conducted from the regression coefficient (β) to generate a z map. The z map was thresholded using a voxel‐based threshold of P < 0.005. Significant effects were reported when the volume of a cluster was greater than the Monte Carlo simulation determined minimum cluster size (67 voxels), above which the probability of a type I error was below 0.05.
The behavioral relevance of 5‐HTTLPR‐related neural substrates
Next, we explored whether the 5‐HTTLPR‐related neural substrates at both the regional and network levels accounted for individual differences in anxiety. At the regional level, correlational analyses were performed to examine the association between the fALFF in the amygdala and anxiety. The fALFF of the right (or left) amygdala was calculated by averaging the fALFF of all voxels in the right (or left) amygdala, which was obtained in the 5‐HTTLPR—fALFF whole brain analyses (P < 0.005). Similar analyses were performed at the network level. The mean time course of each region whose RSFC with the amygdala was associated with the variance of 5‐HTTLPR was calculated by averaging the time courses of all voxels in the region. The RSFC between the amygdala and the region was then recalculated by correlating the mean time course of the seeds in the amygdala and that of the region. Fisher z score transformations were conducted to normalize the RSFC. Finally, correlational analyses were conducted between the RSFC and anxiety.
Mediation analysis
Mediation analysis was used to test the mediation effect of neural substrates in the association between 5‐HTTLPR polymorphism and anxiety. In other words, we aimed to test whether the genetic variation of 5‐HTTLPR influenced individuals' anxiety by modulating the neural substrates found above. The mediation analysis was conducted with the 5‐HTTLPR genotype as the predictor, the neural substrates as the mediator and self‐reported anxiety as the outcome. In this study, the unbiased estimate of the indirect effects and 95% confidence interval (CI) was obtained with a bootstrapping procedure (n = 5,000). A 95% CI not containing zero was considered a significant indirect effect (i.e., P < 0.05).
RESULTS
The Association Between 5‐HTTLPR Polymorphism and Anxiety
The distribution of genotypes in our sample satisfied the Hardy‐Weinberg equilibrium (χ 2 = 0.10, df = 2, P = 0.95). The frequency of the L allele in our participants was 26.9%, similar to previous reports on Asian populations [Kunugi et al., 1997]. Because only a minority of participants possessed the L/L genotype (6.87%), we compared the L allele carriers and S/S homozygotes in the following analyses, which is consistent with previous studies on Asian participants [Ohira et al., 2009]. Table 1 shows the study group characteristics.
Table 1.
Study group characteristics (mean ± SD) according to gender
| Female (N = 158) | Male (N = 104) | Test statistic (two‐tailed) | |
|---|---|---|---|
| Age | 20.10 ± 0.85 | 20.21 ± 0.81 | t = 1.19, P = 0.24 |
| General intelligence | 25.54 ± 4.05 | 25.76 ± 5.17 | t = 0.39, P = 0.70 |
| Anxiety | 8.04 ± 3.27 | 8.34 ± 3.28 | t = 0.72, P = 0.47 |
| 5‐HTTLPR polymorphism | χ 2 = 1.95, P = 0.38 | ||
| L/L | 13 | 5 | |
| L/S | 59 | 46 | |
| S/S | 86 | 53 |
To replicate previous findings that the 5‐HTTLPR genotype is associated with individual differences in anxiety, we compared the anxiety scores between L‐allele carriers and S/S homozygotes. The effect of gender was also taken into account because previous studies have reported a gender difference in the association between 5‐HTTLPR and anxiety [Cerasa et al., 2014; Du et al., 2000; Mizuno et al., 2006] and association between 5‐HTTLPR and emotion disorders [Wang et al., 2014]. A two‐way ANOVA with the 5‐HTTLPR genotype (L allele carriers vs. S/S homozygotes) by gender (male vs. female) showed a significant interaction (F(1, 258) = 7.45, P = 0.007, η 2 = 2.78%, Fig. 1), indicating that the 5‐HTTLPR polymorphism exerted different effects in males and females. A post‐hoc two sample t‐test further revealed that male S/S homozygotes demonstrated a higher level of anxiety than male L allele carries (t(102) = 2.79, P = 0.006, cohen'd = 0.55), whereas no significant difference was found between the two genotypes in females (t(156) = −0.94, P = 0.35, cohen'd = −0.15). Neither the main effect of the 5‐HTTLPR genotype (F(1,258) = 2.33, P = 0.13, η 2 = 0.88%) nor that of gender (F(1,258) = 0.405, P = 0.53, η 2 = 0.15%) reached significance. This finding suggests that 5‐HTTLPR is only associated with anxiety in the male Han Chinese population. Therefore, further analyses on the neural basis through which 5‐HTTLPR influenced anxiety were performed in male participants only (N = 104).
Figure 1.

The association between the 5‐HTTLPR polymorphism and anxiety. The bars indicate the mean of anxiety scores in each group, and the error bars indicate the standard error of mean (S.E.M.) of anxiety scores across participants. The dots show the distribution of anxiety scores, with the size of the dots representing the number of participants. * P < 0.01.
Neural Substrates at the Regional Level
First, we focused on the amygdala, which is a central region for emotional processing and neuroticism [Aghajani et al., 2013; Costafreda et al., 2008; Koelsch et al., 2013]. To this end, we compared the fALFF of the amygdala between L allele carriers and S/S homozygotes in males. The fALFF of the bilateral amygdala in S/S homozygotes was significantly higher than that in L allele carriers (t(102) = 2.94, P = 0.004, cohen'd = 0.58, Fig. 2A), indicating that the spontaneous brain activities of the amygdala in S/S homozygotes was stronger than that in L allele carriers. A similar pattern was observed in both the right (t(102) = 2.63, P = 0.01, cohen'd = 0.52) and left (t(102) = 3.03, P = 0.003, cohen'd = 0.59) amygdala. The whole brain analysis further revealed that S/S homozygotes showed a significantly higher fALFF in the left amygdala and left parietal operculum and a significantly lower fALFF in the anterior middle temporal gyrus than L allele carriers (P < 0.005, corrected; Table 2). Among all of these regions, the left amygdala is the region showing the largest difference in fALFF between the S/S homozygotes and L allele carriers, suggesting that the amygdala is the primary region affected by the 5‐HTTLPR polymorphism. A cluster in the right amygdala also showed a higher fALFF in S/S homozygotes than in L‐allele carriers, although the significance did not pass the correction for multiple comparisons. Figure 2b shows the association between 5‐HTTLPR and fALFF in the bilateral amygdala from the whole brain analysis.
Figure 2.

The fALFF of the amygdala was modulated by the 5‐HTTLPR polymorphism. (A) The fALFF value in the amygdala was higher in male S/S homozygotes than in male L allele carriers. The error bars indicate the SEM of fALFF value in the amygdala across participants. * P < 0.01. (B) The whole‐brain analysis on the association between 5‐HTTLPR and fALFF revealed two clusters in the bilateral amygdala, where S/S homozygotes showed significantly greater fALFF value than L allele carriers. Color bar indicates Z scores. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 2.
Regions where the regional fALFF was modulated by the 5‐HTTLPR genotype
| Region | Hemisphere | Volume (mm3) | Z‐max | MNI coordinate | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Amygdala | L | 1,992 | −4.74 | −26 | −2 | −10 |
| Parietal operculum | L | 880 | −4.32 | −50 | −10 | 18 |
| Anterior middle temporal gyrus | R | 1,128 | 4.62 | 60 | −2 | −28 |
Abbreviations: MNI, Montreal Neurological Institute; L, left; R, right.
Neural Substrates at the Network Level
At the network level, we explored the effect of the 5‐HTTLPR polymorphism on the RSFC between the amygdala and the remaining brain. In the S/S homozygotes, we found that the RSFC was significantly weaker between the amygdala and several regions, including the insula, Heschl's gyrus, lateral occipital cortex, superior temporal gyrus, and hippocampus (P < 0.005, corrected). In addition, S/S homozygotes showed a significantly stronger RSFC between the amygdala and several regions, including the supramarginal gyrus and middle frontal gyrus (P < 0.005, corrected). The details of these regions are reported in Table 3.
Table 3.
Regions whose RSFC with the amygdala was modulated by the 5‐HTTLPR polymorphism
| Region | Hemisphere | Volume (mm3) | Z‐max | MNI coordinate | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Heschl's gyrus | R | 3,080 | −3.78 | 50 | −14 | 8 |
| Lateral occipital cortex | R | 3,056 | −4.38 | 22 | −70 | 34 |
| Superior temporal gyrus | L | 1,152 | −4.03 | −50 | −16 | −4 |
| Hippocampus | R | 624 | −3.63 | 30 | −30 | −12 |
| Insula | L | 544 | −3.96 | −40 | −12 | 4 |
| Supramarginal gyrus | L | 28,80 | 4.35 | −56 | −42 | 34 |
| Middle frontal gyrus | R | 1,040 | 3.81 | 38 | 32 | 28 |
Abbreviations: MNI, Montreal Neurological Institute; L, left; R, right.
The Behavioral Correlates of the Neural Signature
After revealing the effect of 5‐HTTLPR on the amygdala in the resting state at both the regional and network levels, we investigated whether this effect accounted for individual differences in anxiety. At the regional level, individual differences in the fALFF of the amygdala were not correlated with anxiety (r = 0.06, P = 0.55). Therefore, although 5‐HTTLPR polymorphism modulated the fALFF of the amygdala, it likely did not contribute to anxiety because the fALFF was unrelated to anxiety. In contrast, at the network level, the RSFC between the amygdala and insula was negatively correlated with participants' anxiety, indicating that a weaker amygdala–insula RSFC corresponded to a higher level of anxiety (r = −0.26, P = 0.008; Bonferroni corrected, P < 0.05; Fig. 3). To further illustrate that the amygdala–insula RSFC is the neural basis underlying the association between 5‐HTTLPR polymorphism and anxiety, a mediation analysis was performed with the 5‐HTTLPR genotype, the amygdala–insula RSFC, and anxiety, as the predictor, mediator, and outcome, respectively. The effect of 5‐HTTLPR polymorphism on anxiety dropped from β = −0.27 (P = 0.006) to β = −0.21 (P = 0.03) after the amygdala–insula RSFC was added as a mediator. The indirect effect of 5‐HTTLPR polymorphism on anxiety as mediated by amygdala–insula RSFC was significant (bootstrapping test, P = 0.048; Fig. 4). Therefore, the 5‐HTTLPR polymorphism may influence individuals' anxiety by modulating the strength of RSFC between the amygdala and insula. Moreover, the results were similar after controlling the variance of intelligence and age (bootstrapping test, P = 0.053), suggesting that the significant indirect effect was not due to the effect of intelligence and age. Besides, recent studies have shown that ignoring head motion may drastically underestimate or overestimate short range and long range connections [e.g., Murphy et al., 2013; Power et al., 2012, 2014; Satterthwaite et al., 2012; Van Dijk et al., 2012]. To examine whether head motion affected our finding, we excluded participants whose head motion was greater than 1.0° or 1.0 mm throughout the rs‐fMRI scan, which resulted in the exclusion of 14 male participants. We found a similar result. That is, the amygdala–insula RSFC was significantly weaker in S/S homozygotes than that in the L allele carriers (t(100) = 2.48, P = 0.015) in the remaining sample (N = 90). The amygdala–insula RSFC was also negatively correlated with participants' anxiety (r = −0.29, P = 0.006). The indirect effect of 5‐HTTLPR polymorphism on anxiety as mediated by amygdala–insula RSFC was marginally significant (indirect effect = −0.54, bootstrapping test, P = 0.06). Therefore, head motion apparently did not drastically affect the neural phenotype of anxiety identified.
Figure 3.

The amygdala–insula RSFC that was modulated by 5‐HTTLPR polymorphism predicted the level of anxiety. (A) The cluster in the insula whose RSFC with the amygdala was modulated by 5‐HTTLPR polymorphism. The color bar indicates Z scores. (B) The scatter plot shows the correlation between the amygdala–insula RSFC and anxiety. X axis denotes the normalized strength of the amygdala–insula RSFC, and Y axis indicates the level of self‐reported trait anxiety. Each dot represents data from one participant. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 4.
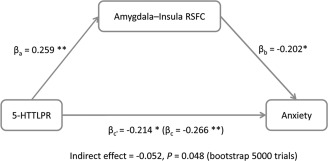
The mediation model of 5‐HTTLPR genotype, the amygdala–insula RSFC and anxiety. The amygdala–insula RSFC serves as a mediator in the association between 5‐HTTLPR polymorphism and anxiety in the mediation analysis. Path coefficients are shown next to the arrows that indicate links in the analysis. All values represent standardized betas. *P < 0.05; **P < 0.01.
Although the female participants did not have genotype differences in the anxiety examined, it is possible that variability on anxiety within the females might relate to neural phenotypes (i.e., the amygdala–insula RSFC). To test this possibility, we calculated females' amygdala–insula RSFC by selecting the amygdala and insula seed regions identified in the analysis on the male participants. We found that the amygdala–insula RSFC was not significantly correlated with the anxiety score of the female participants (r = 0.002, p = 0.98), suggesting that the neural basis underlying the association between 5‐HTTLPR and anxiety was specific to males.
DISCUSSION
In this study, we investigated the neural basis by which 5‐HTTLPR polymorphism influenced individuals' anxiety in Han Chinese participants. First, we only found an association of 5‐HTTLPR and anxiety in males and not females; male S/S homozygotes had a significantly higher level of anxiety than L allele carriers. Neurally, 5‐HTTLPR polymorphism modulated the spontaneous brain activities at both the regional and network levels. At the regional level, the fALFF of the amygdala was significantly higher in male S/S homozygotes than male L allele carriers. At the network level, male S/S homozygotes showed a weaker RSFC between the amygdala and various regions, including the insula, Heschl's gyrus, lateral occipital cortex, superior temporal gyrus, and hippocampus, and showed a stronger RSFC between the amygdala and several regions, including the supramariginal gyrus and middle frontal gyrus. At both the regional and network levels, only did the amygdala–insula RSFC account for individual differences in anxiety. Our study provides the first empirical evidence that the amygdala–insula RSFC serves as the neural basis underlying the association between 5‐HTTLPR polymorphism and anxiety.
Our finding fits nice with previous studies based on functional [Canli et al., 2006; Costafreda et al., 2013; Hariri et al., 2002; Heinz et al., 2004; Munafò et al., 2008; Rao et al., 2007] and structural magnetic resonance imaging (MRI) [Pezawas et al., 2005]. Our finding supplements these findings by showing that the spontaneous activities of the amygdala were also modulated by 5‐HTTLPR polymorphism [Canli and Lesch, 2007], suggesting that 5‐HTTLPR polymorphism affects a broad range of neural properties in the amygdala. However, we found that the fALFF was not associated with anxiety, which suggested that the effect of 5‐HTTLPR polymorphism on the amygdala affects psychological traits other than anxiety. This idea is supported by both functional and structural studies, which showed that neither 5‐HTTLPR‐related functional activation [Hariri et al., 2002] nor 5‐HTTLPR‐related gray matter volume [Pezawas et al., 2005] is correlated with anxiety. Future studies are needed to elucidate the effect of 5‐HTTLPR polymorphism on the amygdala.
Our finding that 5‐HTTLPR polymorphism affected the amygdala‐centered network extends previous studies [Heinz et al., 2004; Pezawas et al., 2005]. Importantly, we found that the amygdala–insula RSFC mediated the association between 5‐HTTLPR polymorphism and anxiety, suggesting that the network formed by the amygdala and insula is the neural basis underlying the association between 5‐HTTLPR polymorphism and anxiety. In fact, both the amygdala and insula are key nodes in network processing saliency and emotion; the reciprocal anatomical connections of the amygdala and insula are extensive [Mesulam and Mufson, 1982; Mufson et al., 1981], and these structures are frequently coactivated [Etkin and Wager, 2010; Kober et al., 2008; Stein et al., 2007]. Amygdala–insula RSFC is negatively associated with neuroticism in healthy individuals [Aghajani et al., 2013] and significantly decreases in individuals suffering from anxiety or depression disorders [Etkin et al., 2009; Perlman et al., 2012; Ramasubbu et al., 2014; Veer et al., 2011]. Importantly, the functional connectivity between the amygdala and insula positively correlated with habituation during repeated exposure to aversive stimuli [Denny et al., 2014]. Moreover, S allele carriers show weaker amygdala habituation to angry faces than L/L homozygotes [Lonsdorf et al., 2011], and habituation to fearful faces in the amygdala is negatively correlated with self‐rating anxiety [Hare et al., 2008]. Therefore, the decreased amygdala–insula RSFC in S/S homozygotes observed in our study may impair behavioral habituation and enhance the sensitivity to negative emotional cues, which leads to a higher level of anxiety. Alternatively, because both the amygdala and insula play a crucial role in socio‐emotional behavior, such as understanding others' emotions [Decety, 2010; Singer, 2006; Singer et al., 2009], weaker functional connectivity between the amygdala and insula may hinder the process of understanding and interpreting social cues, a process crucial to social emotional functioning [Hughes and Dunn, 1998; Singer, 2006], which increases the level of anxiety.
In our study, the association between 5‐HTTLPR polymorphism and anxiety was only found in the male population. Similar male‐specific associations between the S allele and anxiety [Du et al., 2000] or emotion disorders [Wang et al., 2014] have been reported in previous studies. The gender difference may account for the inconsistent results [Flory et al., 1999; Lang et al., 2004; Ohira et al., 2009; Shi et al., 2008;] for the association between 5‐HTTLPR polymorphism and neuroticism‐related traits and disorders reported by others. Note that some studies of female Caucasians found an association between 5‐HTTLPR polymorphism and neuroticism‐related traits [Melke et al., 2001; Xenia Gonda et al., 2009]. The inconsistency may reflect that the effect of 5‐HTTLPR polymorphism on anxiety may differ by ethnic groups or that different measures of anxiety were used in different studies. Future studies are needed to examine the gender‐specific effect of 5‐HTTLPR polymorphism on anxiety.
In short, our study demonstrates that the amygdala–insula RSFC serves as a neural basis by which 5‐HTTLPR polymorphism modulates individual differences in anxiety. Thus, this study provides an example of how genetic variations modulate human personality traits via the human brain. However, several limitations need to be addressed in future studies. First, our study was based on a healthy population; therefore, the relevance of our finding to people with affective disorders remains unclear. According to previous studies, the amygdala–insula RSFC significantly decreases in individuals suffering from anxiety or depression disorders [Etkin et al., 2009; Perlman et al., 2012; Ramasubbu et al., 2014; Veer et al., 2011], and these affective disorders are associated with the S allele of the 5‐HTTLPR [Bellivier et al., 1998, 2002; Caspi et al., 2003]. Future studies are needed to investigate whether 5‐HTTLPR polymorphism is associated with affective disorders by modulating the amygdala–insula RSFC. Second, based on previous studies [Denny et al., 2014; Hare et al., 2008; Lonsdorf et al., 2011], the 5‐HTTLPR was proposed to influence individuals' habituation to aversive stimuli via the functional connectivity between the amygdala and insula. Future studies are needed to examine this hypothesis by directly examining the relationship between 5‐HTTLPR polymorphism, individual differences in habituation to aversive stimuli, amygdala–insula RSFC, and anxiety. Finally, we only explored the effect of 5‐HTTLPR on the brain and behavioral anxiety in this study. Previous studies also found a significant interaction between 5‐HTTLPR and stressful life events in the development of affective disorders [Caspi et al., 2003; Karg et al., 2011]. Future studies are needed to explore the neural basis underlying the interaction between 5‐HTTLPR polymorphism and stress life events.
REFERENCES
- Aghajani M, Veer I, van Tol MJ, Aleman A, van Buchem MA, Veltman DJ, Rombouts SA, van der Wee NJ (2013): Neuroticism and extraversion are associated with amygdala resting‐state functional connectivity. Cogn Affect Behav Neurosci 14:836–848. [DOI] [PubMed] [Google Scholar]
- Andersson J, Jenkinson M, Smith S (2007): TR07JA2: Non‐linear registration, aka spatial normalisation. FMRIB Analysis Group Technical Reports.
- Bellivier F, Henry C, Szöke A, Schürhoff F, Nosten‐Bertrand M, Feingold J, Launay JM, Leboyer M, Laplanche JL (1998): Serotonin transporter gene polymorphisms in patients with unipolar or bipolar depression. Neurosci lett 255:143–146. [DOI] [PubMed] [Google Scholar]
- Bellivier F, Leroux M, Henry C, Rayah F, Rouillon F, Laplanche JL, Leboyer M (2002): Serotonin transporter gene polymorphism influences age at onset in patients with bipolar affective disorder. Neurosci lett 334:17–20. [DOI] [PubMed] [Google Scholar]
- Biswal BB, M Mennes, XN Zuo, S Gohel, C Kelly, SM Smith, CF Beckmann, JS Adelstein, RL Buckner, S Colcombe, AM Dogonowski, M Ernst, D Fair, M Hampson, MJ Hoptman, JS Hyde, VJ Kiviniemi, R Kötter, SJ Li, CP Lin, MJ Lowe, C Mackay, DJ Madden, KH Madsen, DS Margulies, HS Mayberg, K McMahon, CS Monk, SH Mostofsky, BJ Nagel, JJ Pekar, SJ Peltier, SE Petersen, V Riedl, SA Rombouts, B Rypma, BL Schlaggar, S Schmidt, RD Seidler, GJ Siegle, C Sorg, GJ Teng, J Veijola, A Villringer, M Walter, L Wang, XC Weng, S Whitfield‐Gabrieli, P Williamson, C Windischberger, YF Zang, HY Zhang, FX Castellanos, MP Milham (2010): Toward discovery science of human brain function. Proc Natl Acad Sci USA 107:4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Lesch KP (2007): Long story short: The serotonin transporter in emotion regulation and social cognition. Nat neurosci 10:1103–1109. [DOI] [PubMed] [Google Scholar]
- Canli T, Omura K, Haas BW, Fallgatter A, Constable RT, Lesch KP (2005): Beyond affect: A role for genetic variation of the serotonin transporter in neural activation during a cognitive attention task. Proc Natl Acad Sci USA 102:12224–12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Qiu M, Omura K, Congdon E, Haas BW, Amin Z, Herrmann MJ, Constable RT, Lesch KP (2006): Neural correlates of epigenesis. Proc Natl Acad Sci USA 103:16033–16038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R (2003): Influence of life stress on depression: Moderation by a polymorphism in the 5‐HTT gene. Science 301:386–389. [DOI] [PubMed] [Google Scholar]
- Cerasa A, Quattrone A, Piras F, Mangone G, Magariello A, Fagioli S, Girardi P, Muglia M, Caltagirone C, Spalletta G (2014): 5‐HTTLPR, anxiety, and gender interaction moderates right amygdala volume in healthy subjects. Soc Cogn Affect Neurosci 9:1537–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier D, Stober G, Li T, Heils A, Catalano M, Di Bella D, Arranz MJ, Murray RM, Vallada HP, Bengel D, Müller CR, Roberts GW, Smeraldi E, Kirov G, Sham P, Lesch KP (1996): A novel functional polymorphism within the promoter of the serotonin transporter gene: Possible role in susceptibility to affective disorders. Mol Psychiatry 1:453–460 [PubMed] [Google Scholar]
- Costa PT Jr, McCrae RR (1995): Domains and facets: Hierarchical personality assessment using the Revised NEO Personality Inventory. J Pers Assess 64:21–50. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CH (2008): Predictors of amygdala activation during the processing of emotional stimuli: A meta‐analysis of 385 PET and fMRI studies. Brain Res Rev 58:57–70. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, McCann P, Saker P, Cole JH, Cohen‐Woods S, Farmer AE, Aitchison KJ, McGuffin P, Fu CH (2013): Modulation of amygdala response and connectivity in depression by serotonin transporter polymorphism and diagnosis. J Affect Disord 150:96–103. [DOI] [PubMed] [Google Scholar]
- Cox CL, Uddin LQ, Di Martino A, Castellanos FX, Milham MP, Kelly C (2012): The balance between feeling and knowing: Affective and cognitive empathy are reflected in the brain's intrinsic functional dynamics. Soc Cogn Affect Neurosci 7:727–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J (2010): The neurodevelopment of empathy in humans. Dev neurosci 32:257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny BT, Fan J, Liu X, Guerreri S, Mayson SJ, Rimsky L, New AS, Siever LJ, Koenigsberg HW (2014): Insula–amygdala functional connectivity is correlated with habituation to repeated negative images. Soc Cogn Affect Neurosci 9:1660–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Bakish D, Hrdina PD (2000): Gender differences in association between serotonin transporter gene polymorphism and personality traits. Psychiatr Genet 10:159–164. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD (2010): Brain systems underlying anxiety disorders: A view from neuroimaging In: Simpson HB, Schneier F, Neria Y, Lewis‐Fernandez R, editors. Anxiety Disorders: Theory, Research and Clinical Perspectives. UK: Cambridge University Press, pp 192–203. [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD (2009): Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry 66:1361–1372. [DOI] [PubMed] [Google Scholar]
- Flory JD, Manuck SB, Ferrell RE, Dent KM, Peters DG, Muldoon MF (1999): Neuroticism is not associated with the serotonin transporter (5‐HTTLPR) polymorphism. Mol Psychiatry 4:93–96. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman N, Glei DA, Lin YH, Weinstein M (2010): The serotonin transporter polymorphism (5‐HTTLPR): Allelic variation and links with depressive symptoms. Depress Anxiety 27:260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey B (2008): Biological substrates of emotional reactivity and regulation in adolescence during an emotional go‐nogo task. Biol Psychiatry 63:927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR (2002): Serotonin transporter genetic variation and the response of the human amygdala. Science 297:400–403. [DOI] [PubMed] [Google Scholar]
- Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, Klein S, Grüsser SM, Flor H, Schumann G, Mann K, Büchel C (2004): Amygdala‐prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci 8:20–21. [DOI] [PubMed] [Google Scholar]
- Hughes C, Dunn J (1998): Understanding mind and emotion: Longitudinal associations with mental‐state talk between young friends. Dev Psychol 34:1026–1037. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S (2001): A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S (2002): Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841. [DOI] [PubMed] [Google Scholar]
- Jonassen R, Landrø N (2014): Serotonin transporter polymorphisms (5‐HTTLPR) in emotion processing: Implications from current neurobiology. Prog Neurobiol 117:41–53. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S (2011): The serotonin transporter promoter variant (5‐HTTLPR), stress, and depression meta‐analysis revisited: Evidence of genetic moderation. Arch Gen Psychiatry 68:444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuragi S, Kunugi H, Sano A, Tsutsumi T, Isogawa K, Nanko S, Akiyoshi J (1999): Association between serotonin transporter gene polymorphism and anxiety‐related traits. Biol Psychiatry 45:368–370. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss‐Moreau E, Lindquist K, Wager TD (2008): Functional grouping and cortical–subcortical interactions in emotion: A meta‐analysis of neuroimaging studies. Neuroimage 42:998–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobiella A, Reimold M, Ulshöfer D, Ikonomidou V, Vollmert C, Vollstädt‐Klein S, Rietschel M, Reischl G, Heinz A, Smolka MN (2011): How the serotonin transporter 5‐HTTLPR polymorphism influences amygdala function: The roles of in vivo serotonin transporter expression and amygdala structure. Transl Psychiatry 1:e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelsch S, Skouras S, Jentschke S (2013): Neural correlates of emotional personality: A structural and functional magnetic resonance imaging study. PLoS One 8:e77196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunugi H, Hattori M, Kato Ta, Tatsumi M, Sakai T, Sasaki T, Hirose T, Nanko S (1997): Serotonin transporter gene polymorphisms: Ethnic difference and possible association with bipolar affective disorder. Mol Psychiatry 2:457–462. [DOI] [PubMed] [Google Scholar]
- Lang UE, Bajbouj M, Wernicke C, Rommelspacher H, Danker‐Hopfe H, Gallinat J (2004): No association of a functional polymorphism in the serotonin transporter gene promoter and anxiety‐related personality traits. Neuropsychobiology 49:182–184. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Müller CR, Hamer DH, Murphy DL (1996): Association of anxiety‐related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274:1527–1531. [DOI] [PubMed] [Google Scholar]
- Lonsdorf TB, Golkar A, Lindstöm KM, Fransson P, Schalling M, Öhman A, Ingvar M (2011): 5‐HTTLPR and COMTval158met genotype gate amygdala reactivity and habituation. Biol Psychol 87:106–112. [DOI] [PubMed] [Google Scholar]
- Melke J, Landén M, Baghei F, Rosmond R, Holm G, Björntorp P, Westberg L, Hellstrand M, Eriksson E (2001): Serotonin transporter gene polymorphisms are associated with anxiety‐related personality traits in women. Am J Med Genet 105:458–463. [DOI] [PubMed] [Google Scholar]
- Mennes M, Zuo XN, Kelly C, Di Martino A, Zang YF, Biswal B, Castellanos FX, Milham MP (2011): Linking inter‐individual differences in neural activation and behavior to intrinsic brain dynamics. Neuroimage 54:2950–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Mufson EJ (1982): Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol 212:38–52. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Aoki M, Shimada Y, Inoue M, Nakaya K, Takahashi T, Itoyama Y, Kanazawa M, Utsumi A, Endo Y, Nomura T, Hiratsuka M, Mizugaki M, Goto J, Hongo M, Fukudo S (2006): Gender difference in association between polymorphism of serotonin transporter gene regulatory region and anxiety. J Psychosom Res 60:91–97. [DOI] [PubMed] [Google Scholar]
- Montag C, Reuter M (2014): Disentangling the molecular genetic basis of personality: From monoamines to neuropeptides. Neurosci Biobehav Rev 43:228–239. [DOI] [PubMed] [Google Scholar]
- Mufson E, Mesulam MM, Pandya D (1981): Insular interconnections with the amygdala in the rhesus monkey. Neuroscience 6:1231–1248. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Brown SM, Hariri AR (2008): Serotonin transporter (5‐HTTLPR) genotype and amygdala activation: A meta‐analysis. Biol Psychiatry 63:852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Bandettini PA (2013): Resting‐state fMRI confounds and cleanup. Neuroimage 80:349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohira H, Matsunaga M, Isowa T, Nomura M, Ichikawa N, Kimura K, Kanayama N, Murakami H, Osumi T, Konagaya T, Nogimori T, Fukuyama S, Shinoda J, Yamada J (2009): Polymorphism of the serotonin transporter gene modulates brain and physiological responses to acute stress in Japanese men. Stress 12:533–543. [DOI] [PubMed] [Google Scholar]
- Osher Y, Hamer D, Benjamin J (2000): Association and linkage of anxiety‐related traits with a functional polymorphism of the serotonin transporter gene regulatory region in Israeli sibling pairs. Mol Psychiatry 5:216–219. [DOI] [PubMed] [Google Scholar]
- Papageorgiou KA, Ronald A (2013): “He who sees things grow from the beginning will have the finest view of them” A systematic review of genetic studies on psychological traits in infancy. Neurosci Biobehav Rev 37:1500–1517. [DOI] [PubMed] [Google Scholar]
- Perlman G, Simmons AN, Wu J, Hahn KS, Tapert SF, Max JE, Paulus MP, Brown GG, Frank GK, Campbell‐Sills L, Yang TT (2012): Amygdala response and functional connectivity during emotion regulation: A study of 14 depressed adolescents. J Affect Disord 139:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer‐Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR (2005): 5‐HTTLPR polymorphism impacts human cingulate‐amygdala interactions: A genetic susceptibility mechanism for depression. Nat Neurosci 8:828–834. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012): Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Petersen SE (2014): Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage 105:536–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasubbu R, Konduru N, Cortese F, Bray S, Gaxiola‐Valdez I, Goodyear B (2014): Reduced intrinsic connectivity of amygdala in adults with major depressive disorder. Front Psychiatry 5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao H, Gillihan SJ, Wang J, Korczykowski M, Sankoorikal GMV, Kaercher KA, Brodkin ES, Detre JA, Farah MJ (2007): Genetic variation in serotonin transporter alters resting brain function in healthy individuals. Biol Psychiatry 62:600–606. [DOI] [PubMed] [Google Scholar]
- Raven J, Raven JC, Court JH (1998): Manual for Raven's Progressive Matrices and Vocabulary Scales. Section 4: The Advanced Progressive Matrices. San Antonio, TX: Harcourt Assessment.
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, Gur RC, Gur RE (2012): Impact of in‐scanner head motion on multiple measures of functional connectivity: Relevance for studies of neurodevelopment in youth. Neuroimage 60:623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinka J, Busch R, Robichaux‐Keene N (2004): A meta‐analysis of the association between the serotonin transporter gene polymorphism (5‐HTTLPR) and trait anxiety. Mol Psychiatry 9:197–202. [DOI] [PubMed] [Google Scholar]
- Sen S, Burmeister M, Ghosh D (2004): Meta‐analysis of the association between a serotonin transporter promoter polymorphism (5‐HTTLPR) and anxiety‐related personality traits. Am J Med Genet B: Neuropsychiatr Genet 127:85–89. [DOI] [PubMed] [Google Scholar]
- Shi M, Hu J, Dong X, Gao Y, An G, Liu W, Chen L, Sun X (2008): Association of unipolar depression with gene polymorphisms in the serotonergic pathways in Han Chinese. Acta Neuropsychiatr 20:139–144. [DOI] [PubMed] [Google Scholar]
- Singer T (2006): The neuronal basis and ontogeny of empathy and mind reading: Review of literature and implications for future research. Neurosci Biobehav Rev 30:855–863. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K (2009): A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci 13:334–340. [DOI] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer‐Lindenberg A (2007): A validated network of effective amygdala connectivity. Neuroimage 36:736–745. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Sekiguchi A, Fukushima A, Kawashima R (2010): Regional gray matter volume of dopaminergic system associate with creativity: Evidence from voxel‐based morphometry. NeuroImage 51:578–85. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL (2012): The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 59:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veer IM, Oei NY, Spinhoven P, Van Buchem MA, Elzinga BM, Rombouts SA (2011): Beyond acute social stress: Increased functional connectivity between amygdala and cortical midline structures Neuroimage 57:1534–1541. [DOI] [PubMed] [Google Scholar]
- Wang TY, Lee SY, Chen SL, Chang YH, Chen SH, Huang SY, Tzeng NS, Wang CL, Yeh PH, Chen KC, Lee IH, Yeh TL, Yang YK, Lu RB (2014): Gender‐specific association of the SLC6A4 and DRD2 gene variants in bipolar disorder. Int J Neuropsychopharmacol 17:211–222. [DOI] [PubMed] [Google Scholar]
- Wei L, Duan X, Zheng C, Wang S, Gao Q, Zhang Z, Lu G, Chen H (2014): Specific frequency bands of amplitude low‐frequency oscillation encodes personality. Hum Brain Mapp 35:331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xenia Gonda M, Fountoulakis KN, Zoltan Rihmer M, Lazary J, Akiskal HS, Gyorgy Bagdy M (2009): Association of the s allele of the 5‐HTTLPR with neuroticism‐related traits and temperaments in a psychiatrically healthy population. Eur Arch Psychiatry and Clin Neurosci 259:106–113. [DOI] [PubMed] [Google Scholar]
- Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, Wang YF, Zang YF (2008): An improved approach to detection of amplitude of low‐frequency fluctuation (ALFF) for resting‐state fMRI: Fractional ALFF. J Neurosci Meth 172:137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG, Klein DF, Castellanos FX, Biswal BB, Milham MP (2010): The oscillating brain: Complex and reliable. Neuroimage 49:1432–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]


