Abstract
There are limited resting‐state functional magnetic resonance imaging (fMRI) studies in major depressive disorder (MDD). Of these studies, functional connectivity analyses are mostly used. However, a new method based on the magnitude of low frequency fluctuation (LFF) during resting‐state fMRI may provide important insight into MDD. In this study, we examined the amplitude of LFF (ALFF) within the whole brain during resting‐state fMRI in 30 treatment‐naïve MDD subjects and 30 healthy control (HC) subjects. When compared with HC, MDD subjects showed increased ALFF in the frontal cortex (including the bilateral ventral/dorsal anterior cingulate cortex, orbitofrontal cortex, premotor cortex, ventral prefrontal cortex, left dorsal lateral frontal cortex, left superior frontal cortex), basal ganglia (including the right putamen and left caudate nucleus), left insular cortex, right anterior entorhinal cortex and left inferior parietal cortex, together with decreased ALFF in the bilateral occipital cortex, cerebellum hemisphere, and right superior temporal cortex. These findings may relate to characteristics of MDD, such as excessive self‐referential processing and deficits in cognitive control of emotional processing, which may contribute to the persistent and recurrent nature of the disorder. Hum Brain Mapp 35:4979–4988, 2014. © 2014 The Authors Human Brain Mapping Published by Wiley Periodicals, Inc.
Keywords: depression, resting‐state fMRI, amplitude of low‐frequency fluctuation, prefrontal cortex, cerebellum
INTRODUCTION
Major depressive disorder (MDD) is a debilitating illness characterized by depressed mood, anhedonia, anergia, feelings of guilt and helplessness, as well as sleep, appetite, and cognitive disturbances. Its debilitating impact on the lives of affected individuals stems from its persistent and recurrent nature. Several factors may contribute to the perpetuation of MDD, such as repeated exposure to negative life events and genetic vulnerability. Of particular interest among these factors is the cognitive framework in which individuals with MDD perceive and interact with the world. Cognitive and emotional processing appears to be biased in depressive states with selective processing of negative external stimuli, leading to internal milieu colored by negative self‐referential beliefs [Hasler et al., 2004]. In individuals with MDD, this internal milieu appears to be maintained even in the absence of explicit environmental stimuli. How these phenomena are perpetuated by the brain is not well understood; however, functional neuroimaging offers potential insight into the neural mechanisms involved.
Functional neuroimaging allows us to examine the brain under two conditions: during a particular task or stimuli (task or stimuli‐dependent state) and during the absence of overt stimuli (resting state). Of these two conditions, resting state may offer greater insight into the neural mechanisms involved in perpetuating the negative internal milieu observed in MDD as spontaneous brain activity at rest may be involved in the internal processes underlying the representation of self separate from the environment. Recently, Northoff et al. [2011] proposed the “resting‐state hypothesis” of MDD based on an extensive resting‐state literature in MDD and pointed to the “neural predisposition” at rest as a fundamental mechanism in the pathophysiology of MDD. Furthermore, they suggested that an altered resting state led to disturbed rest‐stimulus interaction and subsequently altered task‐evoked responses [Northoff et al., 2011].
Multiple brain regions implicated in self‐referential processing and cognitive/emotional processing are of great interest in the study of MDD. Regions in the medial prefrontal cortex (mPFC) are believed to be the neural correlate of self‐referential processing. As the anterior node of the default mode network (DMN), the mPFC region is highly active at rest but has suppressed activity during cognitive and emotional processing. Converging evidence suggests the involvement of altered mPFC functional connectivity in the development of MDD [Greicius et al., 2007; Lui et al., 2011]. Furthermore, an altered resting state in MDD could adversely affect activity during cognitive/emotional regulation, particularly in the dorsal PFC (dPFC). Thus, dPFC regions are also implicated in the perpetuation of negative self‐referential states.
Current understanding of resting‐state brain activity in MDD relies primarily on positron emission tomography (PET) and single photon emission computed tomography studies. These studies have shown metabolic abnormalities in multiple brain regions of the frontal‐limbic system in MDD, although there are inconsistent findings regarding the direction of these abnormalities. These inconsistencies are thought to be in part due to variations in patient selection criteria, depressive states, and treatment history [Seminowicz et al., 2004].
In recent years, functional magnetic resonance imaging (fMRI) has opened new avenues of understanding the resting‐state neuropathophysiology in MDD. fMRI allows examination of fluctuations in blood oxygenation level dependent (BOLD) signals, and its advantages for studying resting states include minimally invasive procedures and refined temporal and spatial resolution [Greicius et al., 2007]. Recent resting‐state fMRI studies are largely comprised of functional connectivity analyses, which examine the inter‐regional temporal correlation between predefined seed regions and related functional regions. An alternative analysis approach is to examine low frequency fluctuations (LFF) in this disorder. LFF in resting‐state fMRI encode physiologically meaningful indicators of intrinsic brain function in the absence of explicit input [Zhou et al., 2010]. In particular, the amplitude of LFF (ALFF) is thought to reflect the intensity of spontaneous neural activity at rest. ALFF has been used as a reliable and sensitive measure in the study of both healthy and clinical populations, including studies of individuals with attention‐deficit/hyperactivity disorder, schizophrenia, and post‐traumatic stress disorder [Huang et al., 2010; Yang et al., 2007; Yu‐Feng et al., 2007]. However, we are not aware of any prior studies using this method to examine resting‐state abnormalities in treatment‐naïve MDD patients.
In this study, we examined differences in resting‐state activity as measured by ALFF in treatment‐naïve MDD subjects compared to healthy control (HC) subjects. We hypothesized that MDD subjects would have altered resting‐state activity in brain regions subserving self‐referential processing and cognitive/emotional regulation.
MATERIALS AND METHODS
Subjects
Thirty treatment‐naïve MDD subjects (13 males, 17 females), with mean age 29.8 ± SD 8.9 years), were recruited from the outpatient clinics at the Department of Psychiatry, First Affiliated Hospital of China Medical University and the Mental Health Center of Shenyang. MDD subjects were independently evaluated by two trained psychiatrists using the Structured Clinical Interview for DSM‐IV Disorders (SCID) and met the following inclusion criteria: (1) fulfilled DSM‐IV criteria for MDD, (2) did not have comorbid Axis I diagnosis, (3) had a score of at least 24 on the 17‐item Hamilton Depression Rating Scale (HDRS‐17), and (4) did not have a history of psychopharmacotherapy, electroconvulsive therapy, or psychotherapy. The mean years of education were 13.1 ± SD 3.2, the mean duration of illness was 13.3 ± SD 15.4 months, and the mean HDRS‐17 score was 28.5 ± SD 5.1.
Thirty HC subjects (15 males, 15 females), with mean age 30.1 ± SD 8.4 years and mean years of education 14.3 ± SD 2.7, were recruited from the community of Shenyang, China via advertisements. The absence of DSM‐IV Axis I disorders in HC subjects was independently confirmed by two psychiatrists using the SCID. Individuals with a history of DSM‐IV Axis I disorders in their first‐degree relatives were excluded.
For all subjects, individuals were excluded for any contraindications for MRI, such as history of head injury, neurological disorders, or concomitant medical disorders. All subjects were right‐handed, scanned within 24 h of initial contact, and rated on the HDRS‐17 at the time of scanning. The participants provided written informed consent after detailed description of the study as approved by the Institutional Review Board of the China Medical University.
MRI Data Acquisition
fMRI data was acquired using a 3‐T GE MR scanner (General Electric, Milwaukee, USA) at the First Affiliated Hospital of China Medical University with a spin echo planar imaging (EPI) sequence, parallel to the anterior–posterior commissure plane, using the following scan parameters: repetition time = 2,000 ms; echo time = 40 ms; image matrix = 64 × 64; field of view = 24 × 24 cm2; 35 contiguous slices of 3 mm and without gap; scan time 6 min and 40 s. Head motion was minimized with restraining foam pads. A standard head coil was used for radiofrequency transmission and reception of the nuclear magnetic resonance signal. Subjects were asked to keep their eyes closed, remain awake, and keep their mind blank during scanning.
fMRI Image Processing
Resting‐state fMRI data preprocessing was carried out by using Data Processing Assistant for Resting‐State fMRI (DPARSF, V2.0_101025, http://www.restfmri.net), which was based on SPM 8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8) and the Resting State fMRI Data Analysis Toolkit (REST, V1.5_101101, http://www.restfmri.net). Ten volumes were discarded to allow for steady‐state magnetization. Further data preprocessing included slice timing correction, head motion correction, spatial normalization, and smoothing. Spatial normalization was performed by using the standard EPI template from the Montreal Neurological Institute (MNI). Spatial smoothing was done with a 4‐mm full‐width half‐maximum Gaussian filter. Then, linear detrending and temporal bandpass (0.01–0.08 Hz) filtering were performed to remove low‐frequency drifts and physiological high‐frequency noise [Cordes et al., 2001].
ALFF Calculation
ALFF was calculated using REST software. Briefly, for a given voxel, the time series was first converted to the frequency domain using a Fast Fourier Transform. The square root of the power spectrum was computed and then averaged across a predefined frequency interval. This averaged square root was termed the ALFF at the given voxel [Zang et al., 2007]. ALFF measures the absolute strength or intensity of spontaneous low frequency oscillations (typically 0.01–0.1 Hz). Under the studied frequency ranges, ALFF at each voxel was computed for each subject, and it was further divided by the global mean value to reduce the global effects of variability across participants [Zang et al., 2007].
Statistical Analyses
Independent‐sample t tests and χ 2 tests were used to compare demographic data between the MDD and HC groups with SPSS 13.0 software (SPSS, Chicago, IL). Voxel‐based two‐sample (MDD vs. HC) t‐tests were performed in SPM5 to assess group differences in ALFF. A gray matter mask was created in SPM5 template with a probability higher than 0.2 to confine the analyses to gray matter. Findings were considered statistically significant at a significance level of P < 0.05 (corrected for multiple comparisons) by combining individual voxel P value < 0.005 with cluster size > 324 mm3 (12 voxels) based on Monte Carlo simulations [Ledberg et al., 1998]. Additionally, potential correlations in MDD participants between the ALFF measures and HDRS scores were examined in regions with significant differences between the HC and MDD groups.
RESULTS
Voxel‐based analysis showed clusters with significantly increased ALFF in the bilateral ventral/dorsal anterior cingulate cortex (dACC), orbitofrontal cortex, premotor cortex, ventral prefrontal cortex, left dorsal lateral frontal cortex, left superior frontal cortex, left insular cortex, left inferior parietal cortex, left caudate nucleus, right anterior entorhinal cortex, and right putamen in the MDD group when compared with HC group (see Table 1, Fig. 1). Significantly decreased ALFF was found in the bilateral occipital cortex, cerebellum, and right superior temporal cortex in the MDD subjects (see Table 2, Fig. 2). After controlling for the effect of head motion and white matter and cerebrospinal fluid signals, the overall patterns of between‐group differences were preserved, except for differences in the left hippocampus becoming significant and those in the left insular losing significance in the MDD > HC comparison (see Supporting Information Figs. S1 and S2). Additional exploratory analysis did not yield significant correlations of HDRS scores and ALFF measures in the MDD participants (P > 0.05).
Table 1.
Areas of increased amplitude of low frequency fluctuation in subjects with major depressive disorder compared to healthy controls
| MNI coordinates | |||||
|---|---|---|---|---|---|
| Brain regions (Brodmann areas) | Cluster size | x | y | z | T values |
| Left dorsal lateral frontal cortex (BA9) | 68 | −36 | 48 | 33 | 5.33 |
| −27 | 48 | 24 | 3.12 | ||
| Right premotor cortex (BA6) | 80 | 12 | 3 | 57 | 4.66 |
| 15 | 9 | 63 | 4.19 | ||
| 12 | 15 | 57 | 3.47 | ||
| Right dorsal anterior cingulate cortex (BA32) | 57 | 9 | 45 | 27 | 4.39 |
| 21 | 51 | 9 | 4.16 | ||
| 12 | 57 | 18 | 3.47 | ||
| Bilateral ventral anterior cingulate cortex (BA25) | 18 | −9 | 0 | −18 | 4.27 |
| −12 | 12 | −18 | 3.06 | ||
| Left dorsal anterior cingulate cortex (BA32) | 32 | −12 | 48 | 30 | 4.13 |
| −15 | 45 | 18 | 3.94 | ||
| −15 | 33 | 21 | 3.52 | ||
| Right premotor cortex (BA6) | 13 | 24 | 21 | 60 | 4.12 |
| Right putamen | 30 | 21 | 6 | 9 | 4.04 |
| 27 | 12 | 6 | 3.29 | ||
| 30 | 3 | 0 | 2.97 | ||
| Left caudate | 30 | −15 | 21 | 12 | 3.95 |
| Left premotor cortex (BA6) | 14 | −24 | 18 | 66 | 3.84 |
| Left insular | 24 | −27 | 27 | 9 | 3.77 |
| −39 | 24 | 0 | 2.95 | ||
| Right orbitofrontal cortex (BA11) | 18 | 21 | 21 | −6 | 3.67 |
| Left orbitofrontal cortex (BA47, 10) | 20 | −33 | 30 | −18 | 3.60 |
| −36 | 42 | −12 | 3.06 | ||
| Left superior frontal cortex (BA6) | 21 | −51 | 0 | 6 | 3.48 |
| −45 | 6 | 6 | 2.99 | ||
| −51 | 9 | 0 | 2.89 | ||
| Right anterior entorhinal cortex (BA34) | 27 | 27 | 9 | −15 | 3.43 |
| 18 | 12 | −18 | 3.21 | ||
| Left inferior parietal cortex (BA40) | 16 | −51 | −36 | 24 | 3.35 |
| −63 | −39 | 21 | 2.91 | ||
| Right ventral prefrontal cortex (BA47) | 13 | 51 | 42 | −6 | 3.31 |
| 51 | 36 | −12 | 2.69 | ||
| Left ventral prefrontal cortex (BA10) | 12 | −24 | 66 | −6 | 3.24 |
| Left insular | 37 | −36 | 12 | 12 | 3.17 |
| Left caudate | −12 | 21 | 0 | 3.11 | |
| −30 | 9 | 6 | 3.06 | ||
Figure 1.
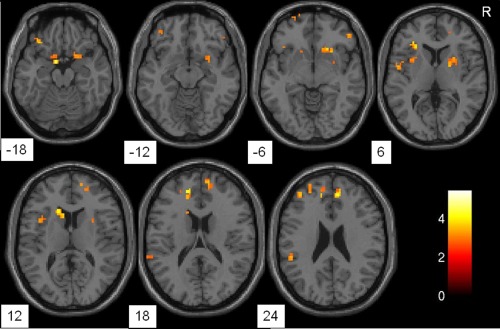
Axial brain slices showing regions of significantly increased amplitude of low frequency fluctuation in the major depressive disorder group when compared with the healthy control group, including the orbitofrontal cortex, ventral/dorsal anterior cingulate cortex, dorsal lateral frontal cortex, premotor cortex, anterior entorhinal cortex, insular cortex, and caudate nucleus. Clusters are shown after controlling for multiple comparisons (P < 0.05). The color bar represents the range of T values. R = right.
Table 2.
Areas of reduced amplitude of low frequency fluctuation in subjects with major depressive disorder compared to healthy controls
| MNI coordinates | |||||
|---|---|---|---|---|---|
| Brain regions (Brodmann areas) | Cluster size | x | y | z | T values |
| Right cerebellum | 919 | 27 | −60 | −27 | 5.16 |
| 12 | −75 | −15 | 4.73 | ||
| 36 | −57 | −33 | 4.64 | ||
| Left cerebellum | 33 | −18 | −42 | −30 | 4.15 |
| −27 | −42 | −30 | 3.04 | ||
| Right occipital cortex (BA19) | 34 | 42 | −66 | 3 | 3.94 |
| 42 | −75 | 0 | 3.07 | ||
| 48 | −57 | 0 | 2.90 | ||
| Right superior temporal cortex (BA22) | 24 | 57 | −24 | 12 | 3.87 |
| Left cerebellum | 54 | −21 | −75 | −27 | 3.84 |
| −24 | −87 | −24 | 3.54 | ||
| Left occipital cortex (BA19) | 21 | −48 | −78 | 6 | 3.74 |
| −39 | −78 | 6 | 3.07 | ||
| Right occipital cortex (BA18) | 29 | 24 | −93 | 6 | 3.61 |
| 18 | −96 | 15 | 3.39 | ||
| Right occipital cortex (BA19) | 27 | 6 | −81 | 33 | 3.54 |
| 12 | −69 | 27 | 3.27 | ||
| Left cerebellum | 18 | −30 | −63 | −33 | 3.51 |
Figure 2.
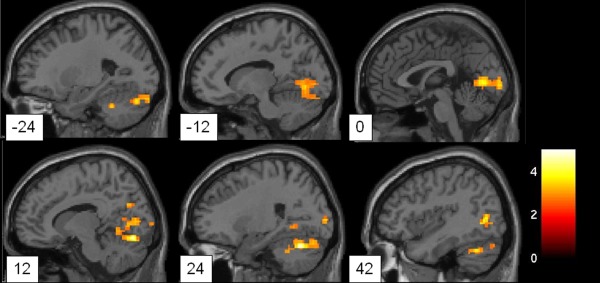
Sagittal brain slices showing regions of significantly decreased amplitude of low frequency fluctuation in the major depressive disorder group when compared with the healthy control group, including the occipital cortex, superior temporal cortex, and cerebellum. Clusters are shown after controlling for multiple comparisons (P < 0.05). The color bar represents the range of T values.
DISCUSSION
In this study, we adopted a novel strategy to map resting‐state functional topology based on the magnitude of spontaneous neural activity in MDD. Using measures of ALFF, we found altered brain activity in MDD subjects in several clusters within the brain that included the medial PFC, dPFC, basal ganglia, occipital lobe, and cerebellum. Interestingly, there was a distinctive regional pattern in altered resting activity in MDD: increased ALFF primarily in the forebrain while decreased ALFF in posterior brain regions.
Increased ALFF
We found significantly increased ALFF in the mPFC, dACC, dorsolateral prefrontal cortex (DLPFC), and basal ganglia. Extensive evidence implicates the role of the mPFC (BA6, 9, 10) in self‐referential processing. BA9 and 10 have long been thought to be involved in the generation of self‐referential processing [Gusnard et al., 2001; McKiernan et al., 2003]. With decreased cognitive demand, BA 6, 9, and 10 have increased activity concurrent with a rise in stimulus‐independent thought. Activations in these regions have been positively correlated with the frequency of mind‐wandering [Mason et al., 2007]. We speculate that the overactive resting‐state mPFC can be related to exaggerated self‐referential bias in MDD that is hallmarked by chronic self‐focus and negative rumination [Sheline et al., 2001].
Our findings of increased ALFF in the dACC and DLPFC are of particular interest. Both the ACC and DLPFC are two central components in cognitive control systems involved in detecting incongruence between expectations and outcomes during decision‐making processes [Vincent et al., 2008]. Studies have found altered activity in both ventral and dorsal subregions of ACC in MDD [Davidson et al., 2002; Mayberg, 2003; Mayberg et al., 1997]. Altered ALFF was primarily found in the dACC in this study. The dACC, as opposed to the ventral ACC, is interconnected with the supplementary motor cortex and DLPFC to form a dorsal attention system [Devinsky et al., 1995]. It subserves on‐line performance supervision and sends signals to the DLPFC to initiate attentional adjustments based on conflict paradigms [Kerns et al., 2004]. Therefore, we speculate that increased resting activity in the dACC may underlie the phenomenon of amplified error detection coupled with failure to evoke cognition control seen in MDD. Increased resting activity in the rostral/ventral ACC has been reported in MDD [Davidson et al., 2002; Mayberg, 2003; Mayberg et al., 1997; Pizzagalli et al., 2001]. Basal metabolism and activity in rostral ACC demonstrated by PET and electromagnetic tomography have been suggested to predict treatment outcomes in MDD [Mayberg et al., 1997; Pizzagalli et al., 2001]. The treatment‐naïve feature of our study sample may have contributed to the lack of findings of resting‐state abnormalities in the rostral ACC in this study.
As previously mentioned, the DLPFC plays an important role in cognitive control of perception and decision‐making. Typically, the interplay between resting‐state and task‐related activity determines how well the brain attends to and processes ongoing salient environmental stimuli. With altered tonic activity, the DLPFC is more likely to be insufficiently activated for top–down regulation of stimulus‐bound processing. Previous studies have demonstrated attenuated activity in both the DLPFC and dACC during performing various cognitive tasks in MDD [Elliott et al., 1997; Halari et al., 2009; Okada et al., 2003]. Failure to monitor performance and make rapid adjustments based on external feedback can result in suboptimal decision‐making, repeated behavioral failures, persistent feelings of frustration, and thus perpetuation and persistence of depressed affect [Pizzagalli, 2011]. We speculate deficits in cognitive resource allocation to external stimuli can perpetuate depressive internal thoughts and suppress attempts for appropriate top–down regulation. Even in the context of overall decrease in DLPFC activation during goal‐directed behavior, stimuli for self‐referential processing were able to preferentially activate the DLPFC to process with a negative bias, reinforcing hard‐wired depressive thinking, and cognitive appraisal [Kerestes et al., 2012; Lemogne et al., 2009].
External tasks have been shown to effectively suppress ruminative thoughts in individuals with MDD and improve their performance in subsequent cognitive tasks by redistributing cognitive resources to diminish intrinsic maladaptive thinking [Watkins and Brown, 2002]. However, abnormalities within the mPFC, dACC, and DLPFC and compromised interactions among these neural nodes are speculated to hinder the processing of external “distraction” tasks and therefore to constitute a vicious cycle in the maintenance of clinical manifestations of MDD. Recorded event‐related potentials have shown that ACC activation failed to predict subsequent DLPFC activation in the Stroop task in individuals with MDD, indicating exaggerated error negativity and lack of recruitment of cognitive control from the DLPFC in MDD [Holmes and Pizzagalli, 2008].
The caudate nucleus is functionally connected with the DMN via dopaminergic projections [Robinson et al., 2012]. Dopaminergic modulation of the interaction between the caudate and DMN, including the mPFC, has been demonstrated, suggesting that striatal dopamine circuits may be involved in modulating the DMN to establish balance between processing of external stimuli and internal, self‐directed processing [Kelly et al., 2009; Robinson et al., 2012].
Decreased ALFF
We found significantly decreased ALFF in the occipital cortex (BA18 and 19) and cerebellum in MDD subjects. There has been evidence in the occipital cortex of elevated glutamate concentrations together with reduced gamma‐aminobutyric acid concentrations in MDD [Sanacora et al., 2004]. Further insight regarding occipital involvement in MDD is still quite limited. In contrast, there is growing awareness of the role of the cerebellum in emotional and cognitive function. The traditional role of the cerebellum as a motor control center has been revised to encompass its involvement in more complex higher order cognitive and emotional functions. Lesions in the cerebellum have shown disruptions in multiple domains of cognitive and affective control in the cerebellar cognitive affective syndrome [Schmahmann and Pandya, 1997]. Cerebellar abnormality, at both the anatomical and functional level, is a common manifestation across various psychiatric disorders [Villanueva, 2012; Womer et al., 2009]. The cerebellum receives projections from the ventral tegmental area, ACC and cerebral input directly or via the thalamus relay [Schmahmann, 2001]. Functionally parallel pathways between the cerebellum and anterior, dorsolateral, and medial prefrontal seeds have been previously shown [Krienen and Buckner, 2009]. In studies of MDD, seed regions in the cerebellum showed reduced connectivity with the ventromedial prefrontal cortex, which was crucial in the modulation of emotional processing [Alalade et al., 2011]. A meta‐analysis identified decreased cerebellar activity to emotionally laden stimuli as a characteristic of MDD [Fitzgerald et al., 2008]. Information circulated between the cortex and cerebellum, which relies on feed‐forward and feedback connections within the cerebello‐thalamo‐cortical circuit, mediates the refinement of behavioral adjustments [Koziol et al., 2010]. In this sense, decreased cerebellar ALFF in MDD may indicate suppressed capability to perform proper adjustments of the internal milieu in response to environmental demands as the consequence of disruptions in the cerebro‐cerebellar interaction in MDD. Additionally, decreased ALFF in the occipital cortex and cerebellum in MDD may reflect the effects of PFC abnormalities, mediated by the compromised cross‐talk between anterior and posterior brain regions.
Exploratory Correlation Analysis
We did not find significant correlations between ALFF in regions with between‐group differences and HDRS‐17 ratings. Recent evidence has demonstrated robust associations of resting‐state brain activity and measures of ruminative tendency, primarily the Ruminative Responses Scale, but not the HDRS‐17 [Berman et al., 2011; Hamilton et al., 2011]. Nonspecificity of the HDRS‐17 in evaluation of rumination could in part account for the insignificant correlations found herein. Furthermore, the MDD subjects in the present study scored higher than 24 on the HDRS‐17 due to our stringent inclusion criteria to ensure depressed mood state in the MDD subjects at the time of scanning. The limited range and variability in HDRS scores may have limited the statistical power to detect significant correlations. Alternatively, the lack of correlation between ALFF and HDRS scores may imply that ALFF abnormalities are stable traits in MDD that are independent of symptom severity.
The Relation Between ALFF and Conventional Connectivity‐Based Approach
Conventional connectivity methods are devoted mainly to the spatial coherence of resting‐state brain activity in a set of closely related regions. At the heart of connectivity methods is a particular emphasis on the global integration of neural network by means of indicators reflecting inter‐regional co‐fluctuations. Hierarchical organization of neural activity is theorized to function in accordance to two key principles: segregation and integration [Zeki and Shipp, 1988]. However, the development of available analytical tools for resting‐state fMRI data primarily examines integration‐related connectivity measures. The other aspect of brain's functional integrity, the functional segregation or regional specialization, is largely overlooked. This methodological void is a consequence of challenges to reliably quantify intrinsic brain activity in the absence of exogenous stimuli [Zhou et al., 2010]. The introduction of ALFF provides a novel method to bridge gaps in our understanding of the brain's resting state by measuring the amplitude of intra‐regional brain activity at rest. Hence, ALFF analysis extends and enriches the current understanding of MDD, which primarily stems from connectivity analyses that examine global functional integration within the brain.
To enhance interpretation of the ALFF results in the context of mainstream connectivity approach, we have conducted an exploratory connectivity analysis, focusing on the regions in which MDD patients showed altered ALFF compared with the HC subjects (as reported in the Supporting Information). Interestingly, at P uncorrected < 0.05, regions with increased activation amplitude (i.e., ALFF) in the resting state tended to be more functionally connected with each other in the MDD group, whereas regions with decreased amplitude were less functionally connected (Supporting Information Fig. S3). Because of the explorative nature, the correspondence between ALFF and functional connectivity was most pronounced at a subthreshold level in our analyses. This combined approach, however, highlights the importance of applying a multilevel marker in characterizing brain dysfunctions [Dai et al., 2012]. The reason that ALFF appears to be more sensitive as compared with connectivity index is not well understood. One possible account is that regional alterations could progressively deteriorate into dysfunctions at network level with the development of MDD. Future studies with longitudinal imaging will provide important clues to the question of whether connectivity abnormalities in MDD are secondary to ALFF changes.
The ALFF findings herein support the important role of the dACC and mPFC in MDD. The exploratory functional connectivity analyses suggest that these regions with disproportionately high activation may co‐fluctuate at rest and form a highly connected community (i.e., a module in the brain's functional architecture). The mPFC‐centered module has long been suspected to be the neural basis of self‐referential mental activity in MDD. Additionally, our analyses also indicate the involvement of posterior brain regions in MDD with findings of decreased ALFF in the occipital cortex and cerebellum and possibly weakened connectivity between these regions. We postulate that disrupted activation in each individual region can hinder their mutual communication, conceivably leading to a gradual disintegration in the posterior module spanning the cerebellum and occipital cortex. Furthermore, the interaction between region‐specific changes in activation amplitude and functional connectivity of the frontal and posterior areas could contribute to trait‐based features of MDD, such as internally generated negative schemata. However, further studies are needed to clarify the implications of combined ALFF and functional connectivity alterations in MDD. The significance of the convergence between these two approaches, if confirmed by future studies, goes beyond simple replication; it would allow closer examination of the characteristic brain activation in MDD from a systematic perspective by combining intensity and spatial scale.
Limitations and Future Prospects
There are also some limitations in this study. First, generalizations from our findings should be made with caution because our findings are based on a population of treatment‐naive MDD individuals with relatively high HDRS‐17 ratings. Future studies with more participants, especially including asymptomatic MDD subjects, who are euthymic or remitted, are needed to clarify whether the altered resting‐state ALFF is indeed one biological trait of MDD and to further develop the neurobiological model of MDD we describe herein. Second, analysis of resting‐state fMRI data can be sensitive to motion and physiological artifacts; our main findings, however, are maintained after adjustment for these possible confounding factors. Still, the presence of hippocampus in the additional analysis, especially given other evidence for its neurobiological role in MDD, warrants further study. Third, the loci where ALFF differed between MDD and HC participants were located in gray matter, with certain clusters straddling the boundary of gray‐white matter. It is possibly due to the mask threshold (P > 0.2) and BOLD signals in white matter. A body of recent evidence has suggested that white matter BOLD activation, once considered as artifacts and noise, turns out to be a stable result and has its own neuropsychological implications [Fabri et al., 2011; Gawryluk et al., 2011a,b; Makedonov et al., 2013; Mazerolle et al., 2010, 2013; McWhinney et al., 2012]. Future studies are required to address the common biological bases of gray/white matter dysfunctions and distinctive roles in producing depressive mood. Fourth, it is difficult to determine from this study whether the resting‐state abnormalities represent pre‐existing susceptibility versus consequences of the disorder. Altered resting‐state function may manifest even at early stages of MDD; study in youths at risk and at first onset may provide important insights into the development of the ALFF abnormalities observed.
CONCLUSIONS
To summarize, we observed altered patterns of brain activity in MDD at rest, with increased ALFF mainly in the PFC and basal ganglia and decreased ALFF in the occipital lobe and cerebellum. In particular, the recurrent tendency to ruminate and the deficiency of effective cognitive control in MDD are potentially related to a concerted mechanism through distinct regional alterations of resting‐state activity in the mPFC, DLPFC, and cerebellum. Our findings of abnormal resting activity in a unique frontal‐posterior pattern, together with previous studies on task‐elicited activity, may enhance the understanding of the neurobiological underpinnings in MDD and identify potential key targets for treatment of this disorder.
Supporting information
Supplementary Information
ACKNOWLEDGMENTS
The authors thank Dr. Yu‐Feng Zang for his constructive comments during revision of this manuscript.
REFERENCES
- Alalade E, Denny K, Potter G, Steffens D, Wang L (2011): Altered cerebellar‐cerebral functional connectivity in geriatric depression. PLoS ONE 6:e20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J (2011): Depression, rumination and the default network. Soc Cogn Affect Neurosci 6:548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME (2001): Frequencies contributing to functional connectivity in the cerebral cortex in “resting‐state” data. AJNR Am J Neuroradiol 22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Dai Z, Yan C, Wang Z, Wang J, Xia M, Li K, He Y (2012): Discriminative analysis of early Alzheimer's disease using multi‐modal imaging and multi‐level characterization with multi‐classifier (M3). Neuroimage 59:2187–2195. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K (2002): Depression: Perspectives from affective neuroscience. Annu Rev Psychol 53:545–574. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA (1995): Contributions of anterior cingulate cortex to behaviour. Brain 118 (Part 1):279–306. [DOI] [PubMed] [Google Scholar]
- Elliott R, Baker SC, Rogers RD, O'Leary DA, Paykel ES, Frith CD, Dolan RJ, Sahakian BJ (1997): Prefrontal dysfunction in depressed patients performing a complex planning task: A study using positron emission tomography. Psychol Med 27:931–942. [DOI] [PubMed] [Google Scholar]
- Fabri M, Polonara G, Mascioli G, Salvolini U, Manzoni T (2011): Topographical organization of human corpus callosum: An fMRI mapping study. Brain Res 1370:99–111. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ (2008): A meta‐analytic study of changes in brain activation in depression. Hum Brain Mapp 29:683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawryluk JR, D'Arcy RC, Mazerolle EL, Brewer KD, Beyea SD (2011a): Functional mapping in the corpus callosum: A 4T fMRI study of white matter. Neuroimage 54:10–15. [DOI] [PubMed] [Google Scholar]
- Gawryluk JR, Mazerolle EL, Brewer KD, Beyea SD, D'Arcy RC (2011b): Investigation of fMRI activation in the internal capsule. BMC Neurosci 12:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF (2007): Resting‐state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 62:429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME (2001): Medial prefrontal cortex and self‐referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci USA 98:4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halari R, Simic M, Pariante CM, Papadopoulos A, Cleare A, Brammer M, Fombonne E, Rubia K (2009): Reduced activation in lateral prefrontal cortex and anterior cingulate during attention and cognitive control functions in medication‐naive adolescents with depression compared to controls. J Child Psychol Psychiatry 50:307–316. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH (2011): Default‐mode and task‐positive network activity in major depressive disorder: Implications for adaptive and maladaptive rumination. Biol Psychiatry 70:327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS (2004): Discovering endophenotypes for major depression. Neuropsychopharmacology 29:1765–1781. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, Pizzagalli DA (2008): Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Arch Gen Psychiatry 65:179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X‐Q, Lui S, Deng W, Chan RCK, Wu Q‐Z, Jiang L‐J, Zhang J‐R, Jia Z‐Y, Li X‐L, Li F, L Chen, T Li, QY Gong (2010): Localization of cerebral functional deficits in treatment‐naive, first‐episode schizophrenia using resting‐state fMRI. Neuroimage 49:2901–2906. [DOI] [PubMed] [Google Scholar]
- Kelly C, de Zubicaray G, Di Martino A, Copland DA, Reiss PT, Klein DF, Castellanos FX, Milham MP, McMahon K (2009): l‐Dopa modulates functional connectivity in striatal cognitive and motor networks: A double‐blind placebo‐controlled study. J Neurosci 29:7364–7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerestes R, Ladouceur CD, Meda S, Nathan PJ, Blumberg HP, Maloney K, Ruf B, Saricicek A, Pearlson GD, Bhagwagar Z, Phillips ML (2012): Abnormal prefrontal activity subserving attentional control of emotion in remitted depressed patients during a working memory task with emotional distracters. Psychol Med 42:29–40. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW III, Cho RY, Stenger VA, Carter CS (2004): Anterior cingulate conflict monitoring and adjustments in control. Science 303:1023–1026. [DOI] [PubMed] [Google Scholar]
- Koziol LF, Budding DE, Chidekel D (2010): Adaptation, expertise, and giftedness: Towards an understanding of cortical, subcortical, and cerebellar network contributions. Cerebellum 9:499–529. [DOI] [PubMed] [Google Scholar]
- Krienen FM, Buckner RL (2009): Segregated fronto‐cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex 19:2485–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledberg A, Akerman S, Roland PE (1998): Estimation of the probabilities of 3D clusters in functional brain images. Neuroimage 8:113–128. [DOI] [PubMed] [Google Scholar]
- Lemogne C, le Bastard G, Mayberg H, Volle E, Bergouignan L, Lehericy S, Allilaire JF, Fossati P (2009): In search of the depressive self: Extended medial prefrontal network during self‐referential processing in major depression. Soc Cogn Affect Neurosci 4:305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui S, Wu Q, Qiu L, Yang X, Kuang W, Chan RC, Huang X, Kemp GJ, Mechelli A, Gong Q (2011): Resting‐state functional connectivity in treatment‐resistant depression. Am J Psychiatry 168:642–648. [DOI] [PubMed] [Google Scholar]
- Makedonov I, Black SE, Macintosh BJ (2013): BOLD fMRI in the white matter as a marker of aging and small vessel disease. PLoS One 8:e67652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN (2007): Wandering minds: The default network and stimulus‐independent thought. Science 315:393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS (2003): Modulating dysfunctional limbic‐cortical circuits in depression: Towards development of brain‐based algorithms for diagnosis and optimised treatment. Br Med Bull 65:193–207. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva JA, McGinnis S, Glass TG, Martin CC, Fox PT (1997): Cingulate function in depression: A potential predictor of treatment response. Neuroreport 8:1057–1061. [DOI] [PubMed] [Google Scholar]
- Mazerolle EL, Beyea SD, Gawryluk JR, Brewer KD, Bowen CV, D'Arcy RC (2010): Confirming white matter fMRI activation in the corpus callosum: Co‐localization with DTI tractography. Neuroimage 50:616–621. [DOI] [PubMed] [Google Scholar]
- Mazerolle EL, Gawryluk JR, Dillen KN, Patterson SA, Feindel KW, Beyea SD, Stevens MT, Newman AJ, Schmidt MH, D'Arcy RC (2013): Sensitivity to white matter FMRI activation increases with field strength. PLoS One 8:e58130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera‐Thompson J, Binder JR (2003): A parametric manipulation of factors affecting task‐induced deactivation in functional neuroimaging. J Cogn Neurosci 15:394–408. [DOI] [PubMed] [Google Scholar]
- McWhinney SR, Mazerolle EL, Gawryluk JR, Beyea SD, D'Arcy RC (2012): Comparing gray and white matter fMRI activation using asymmetric spin echo spiral. J Neurosci Methods 209:351–356. [DOI] [PubMed] [Google Scholar]
- Northoff G, Wiebking C, Feinberg T, Panksepp J (2011): The ‘resting‐state hypothesis' of major depressive disorder‐a translational subcortical‐cortical framework for a system disorder. Neurosci Biobehav Rev 35:1929–1945. [DOI] [PubMed] [Google Scholar]
- Okada G, Okamoto Y, Morinobu S, Yamawaki S, Yokota N (2003): Attenuated left prefrontal activation during a verbal fluency task in patients with depression. Neuropsychobiology 47:21–26. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D, Pascual‐Marqui RD, Nitschke JB, Oakes TR, Larson CL, Abercrombie HC, Schaefer SM, Koger JV, Benca RM, Davidson RJ (2001): Anterior cingulate activity as a predictor of degree of treatment response in major depression: Evidence from brain electrical tomography analysis. Am J Psychiatry 158:405–415. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA (2011): Frontocingulate dysfunction in depression: Toward biomarkers of treatment response. Neuropsychopharmacology 36:183–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Cools R, Carlisi CO, Sahakian BJ, Drevets WC (2012): Ventral striatum response during reward and punishment reversal learning in unmedicated major depressive disorder. Am J Psychiatry 169:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, Krystal JH, Mason GF (2004): Subtype‐specific alterations of gamma‐aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry 61:705–713. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD (2001): The cerebrocerebellar system: Anatomic substrates of the cerebellar contribution to cognition and emotion. Int Rev Psychiatry 13:247–260. [Google Scholar]
- Schmahmann JD, Pandya DN (1997): The cerebrocerebellar system. Int Rev Neurobiol 41:31–60. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, Rafi‐Tari S (2004): Limbic‐frontal circuitry in major depression: A path modeling metanalysis. Neuroimage 22:409–418. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA (2001): Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biol Psychiatry 50:651–658. [DOI] [PubMed] [Google Scholar]
- Villanueva R (2012): The cerebellum and neuropsychiatric disorders. Psychiatry Res 198:527–532. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL (2008): Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol 100:3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins E, Brown RG (2002): Rumination and executive function in depression: An experimental study. J Neurol Neurosurg Psychiatry 72:400–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womer FY, Wang F, Chepenik LG, Kalmar JH, Spencer L, Edmiston E, Pittman BP, Constable RT, Papademetris X, Blumberg HP (2009): Sexually dimorphic features of vermis morphology in bipolar disorder. Bipolar Disord 11:753–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Long X‐Y, Yang Y, Yan H, Zhu C‐Z, Zhou X‐P, Zang Y‐sF, Gong Q‐Y (2007): Amplitude of low frequency fluctuation within visual areas revealed by resting‐state functional MRI. Neuroimage 36:144–152. [DOI] [PubMed] [Google Scholar]
- Yu‐Feng Z, Yong H, Chao‐Zhe Z, Qing‐Jiu C, Man‐Qiu S, Meng L, Li‐Xia T, Tian‐Zi J, Yu‐Feng W (2007): Altered baseline brain activity in children with ADHD revealed by resting‐state functional MRI. Brain Dev 29:83–91. [DOI] [PubMed] [Google Scholar]
- Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF (2007): Altered baseline brain activity in children with ADHD revealed by resting‐state functional MRI. Brain Dev 29:83–91. [DOI] [PubMed] [Google Scholar]
- Zeki S, Shipp S (1988): The functional logic of cortical connections. Nature 335:311–317. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Wang K, Liu Y, Song M, Song SW, Jiang T. (2010): Spontaneous brain activity observed with functional magnetic resonance imaging as a potential biomarker in neuropsychiatric disorders. Cogn Neurodyn 4:275–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


