Abstract
Subcortical vascular cognitive impairment (sVCI) is caused by lacunar infarcts or extensive and/or diffuse lesions in the white matter that may disrupt the white matter circuitry connecting cortical and subcortical regions and result in the degeneration of neurons in these regions. This study used structural magnetic resonance imaging (MRI) and high angular resolution diffusion imaging (HARDI) techniques to examine cortical thickness, subcortical shapes, and white matter integrity in mild vascular cognitive impairment no dementia (VCIND Mild) and moderate‐to‐severe VCI (MSVCI). Our study found that compared to controls (n = 25), VCIND Mild (n = 25), and MSVCI (n = 30) showed thinner cortex predominantly in the frontal cortex. The cortex in MSVCI was thinner in the parietal and lateral temporal cortices than that in VCIND Mild. Moreover, compared to controls, VCIND Mild and MSVCI showed smaller shapes (i.e., volume reduction) in the thalamus, putamen, and globus pallidus and ventricular enlargement. Finally, compared to controls, VCIND Mild, and MSVCI showed an increased mean diffusivity in the white matter, while decreased generalized fractional anisotropy was only found in the MSVCI subjects. The major axonal bundles involved in the white matter abnormalities were mainly toward the frontal regions, including the internal capsule/corona radiata, uncinate fasciculus, and anterior section of the inferior fronto‐occipital fasciculus, and were anatomically connected to the affected cortical and subcortical structures. Our findings suggest that abnormalities in cortical, subcortical, and white matter morphology in sVCI occur in anatomically connected structures, and that abnormalities progress along a similar trajectory from the mild to moderate and severe conditions. Hum Brain Mapp 35:2320–2332, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: vascular cognitive impairment, high angular resolution diffusion imaging, subcortical shapes, cortical thickness, white matter integrity
INTRODUCTION
Vascular cognitive impairment (VCI) is an umbrella term that describes a continuum of cognitive and behavioral deficits associated with cerebrovascular disease, ranging from mild deficits to severe dementia. Subcortical VCI (sVCI) is one of the most common subtypes of VCI [Jellinger, 2002] and is caused by lacunar infarcts or extensive and/or diffuse lesions in the white matter that appear as white matter hyperintensities (WMH) on MRI. It is believed that cognitive impairments in sVCI arise when infarcts in the white matter and WMH disrupt the white matter circuitry that connect the various cortical regions and subcortical structures, especially the frontal–subcortical circuitry and the long association fibers, and through which the cholinergic pathways pass [Bocti et al., 2005; Chui, 2007; Cummings, 1993]. These lesions also result in the secondary degeneration of neurons in connected cortical regions via processes known as “dying back” and Wallerian degeneration [Guimarães et al., 2009; Wang et al., 2012aa], manifesting as cortical atrophy which may also contribute to cognitive impairment.
Results from existing MRI studies show that both gray and white matter abnormalities are present in sVCI. A recent study found gray matter volume reductions in the frontal cortex and the superior and inferior temporal gyri of subjects with mild sVCI compared to controls [Yi et al., 2012]. Patients with mild sVCI or subcortical vascular dementia (sVaD) had widespread cortical thinning in the frontal cortex and sparse cortical thinning in the temporal and occipital cortices [Seo et al., 2010], which was also confirmed using another sample [Seo et al., 2012]. Using diffusion tensor imaging (DTI), subjects with sVCI showed reduced fractional anisotropy (FA) in the anterior corpus callosum (CC), frontal and parietal white matter regions [Zarei et al., 2009], and reduced mean FA values of the whole brain [Zhou et al., 2008, 2012] compared to controls.
Investigations of sVCI have thus far been limited to the examination of cortical thickness or white matter integrity assessed using FA of DTI, which is hence unable to demonstrate the connection between abnormalities in the two morphological findings. Moreover, very little has been done to examine the pattern of subcortical structural morphology in relation with severity of sVCI. Given interconnections among the cortex and subcortical structures through the white matter axons, it is critical to simultaneously examine the cortical, subcortical, and white matter morphology for the purpose of understanding coherence among morphological abnormalities in the brain gray and white matters as a function of sVCI severity.
Hence, the present study aimed to concurrently examine the cortical, subcortical and white matter structural morphology in patients with mild sVCI and moderate to severe sVCI using measures of cortical thickness, subcortical shape, and white matter structural integrity. Based on the previous findings [Yi et al., 2012, Zhou et al. 2008, 2011] we hypothesized concurrent and pronounced cortical thinning and white matter disruption in the frontal cortex in sVCI. Subcortical shape abnormalities in sVCI would be observed in the regions where the white matter axonal disruption anatomically connects to. In addition, the present study also aimed to compare the patterns of brain morphological abnormalities between the early and later stages of sVCI. While they are conceptualized as being on the same continuum, there remains a dearth of knowledge on the progression of brain abnormalities from the early stages of sVCI to the later stages of sVCI, especially in terms of cortical atrophy and subcortical shape deformations. We hypothesized that the pattern of brain structural abnormalities in the early and later stages of sVCI would be congruent and that the abnormalities in the later stages of sVCI would be more severe. Lastly, the present study aimed to identify patterns of gray and white matter abnormalities in sVCI that would be different from those seen in Alzheimer's disease (AD) and hence included AD subjects as a positive control group.
METHODS
Subjects
Subjects were selected from an ongoing longitudinal study of cognition approved by the Domain‐Specific Review Board of the National Healthcare Group. The source of controls was from community and clinics while patient groups with stroke or dementia were recruited from the stroke service and Memory clinics in Singapore. Informed consent was obtained from all participants before study procedures.
All subjects recruited were required to have sufficient language skills in English, Mandarin, or Malay for psychological tests, and had a knowledgeable informant for the subject's recent and past medical history. Exclusion criteria of the recruitment were as follows: hypoxic/anoxic, hypotensive, hypertensive, uremic, or hepatic encephalopathy; any disorder or infection affecting the central nervous system; intracranial hemorrhage; significant aphasia; dependence in function (modified Rankin Scale >4); cranial arteritis; inflammatory vasculitides; Moyamoya disease; medical illness requiring concomitant corticosteroid or immunosuppressant therapy; space occupying intracranial mass lesion; obstructive or normal pressure hydrocephalus; difficult to control epilepsy; current or past substance use disorder.
Normal controls (NC) were defined as subjects without any subjective cognitive complaints or functional loss who scored ≥23 on the Mini Mental State Examination (MMSE) if they had secondary/tertiary education, or scored ≥21 if they had primary/no education on initial screening and subsequently had no significant cognitive impairments on formal neuropsychological testing. The MMSE cutoff scores used in this study were relatively lower than those in western studies. Nevertheless, they were well validated in Singapore population [Chin, 2002]. Vascular cognitive impairment no dementia (VCIND) were patients who had an ischemic stroke within the past 6–24 months, neuroimaging evidence of cerebral infarction, and were impaired in at least one cognitive domain of a formal neuropsychological test battery, but did not meet Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM–IV) criteria for dementia. VCIND patients were further classified into VCIND Mild (impairment in ≤2 cognitive domains) and VCIND Moderate (impairment in ≥3 cognitive domains) [Narasimhalu et al., 2009]. Vascular Dementia (VaD) was diagnosed in accordance with the NINDS‐AIREN criteria [Wiederkehr et al., 2008]. Patients with VCIND Moderate and VaD were classified as moderate‐severe VCI [Dong et al., 2012]. AD was diagnosed in accordance with the NINCDS–ADRDA criteria [McKhann et al., 1984]. All NC and AD subjects were required to have no history of stroke, and no evidence of severe cerebrovascular disease on MRI, defined as the presence of an infarct (including lacunar infarcts) and/or the presence of significant white matter lesions, defined as a grade ≥2 in more than four regions (total score ≥8) on the age‐related white matter changes scale [Wahlund et al., 2001]. All clinical diagnoses were made at weekly consensus meetings attended by neurologists, clinicians, psychologists, and a neuroradiologist.
In the period of August 2010 to November 2012, a total of 172 subjects were recruited into the main study, of which 72 were excluded from the present analysis. The reasons for exclusion were: 16 subjects did not have MRI data, 6 subjects showed impairment in one or more cognitive domain but did not meet the requirements for VCIND, and 50 were NC or AD subjects that had a history of stroke or showed evidence of severe cerebrovascular disease on MRI. Hence, the present sample included 25 NC, 25 VCIND Mild, 18 VCIND Moderate, 12 VaD, and 20 AD subjects. The present study grouped 18 VCIND Moderate and 12 VaD subjects as moderate to severe VCI (MSVCI). Table 1 lists the demographic information in each group.
Table 1.
Demographics
| Demographics | Controls (25) | VCIND Mild (25) | MSVCI (30) | AD (20) |
|---|---|---|---|---|
| Age (SD) | 68.00 (6.33) | 66.20 (8.21) | 69.87 (9.61) | 74.25 (8.44) |
| Education (SD) | 9.10 (5.61) | 6.96 (4.62) | 6.03 (4.51) | 3.85 (3.98)a |
| MMSE score (SD) | 26.72 (2.32) | 25.64 (2.04) | 20.23 (4.62)a, b | 16.40 (4.74)a |
| Female (%) | 14 (56.0) | 6 (24.0) | 16 (53.3) | 14 (70.0) |
| TBV (SD) (cm3) | 1,047 (85.0) | 1,014 (96.0) | 971 (110.73) | 907 (90.1)a |
| WMH (SD) (mm3) | 3,357 (2,491) | 10,167 (10,987) | 20,002 (15,698) | 6,779 (5,960) |
| WMH to TBV ratio (×10−3) | 3.21 (2.31) | 10.43 (11.88)a | 20.03 (14.78)a, b | 7.14 (5.91) |
| Cardiovascular disease (%) | 0 (0.0) | 7 (28.0)a | 10 (33.3)a | 2 (10.0) |
| Diabetes (%) | 7 (28.0) | 8 (32.0) | 14 (46.7) | 11 (55.0) |
| Hypertension (%) | 15 (60.0) | 21 (84.0) | 23 (76.7) | 15 (75.0) |
| Hyperlipidemia (%) | 13 (52.0) | 22 (88.0)a | 23 (76.7) | 11 (55.0) |
| Smoking status (%) | 5 (20.0) | 8 (32.0) | 10 (33.3) | 5 (25.0) |
Group is significantly different from controls at P < 0.05.
Group is significantly different from the AD group at P < 0.05.
VCIND Mild, mild vascular cognitive impairment no dementia; MSVCI, moderate to severe vascular cognitive impairment; AD, Alzheimer's Disease; MMSE, Mini Mental State Examination; TBV, total brain volume; WMH, white matter hyperintensity; SD, standard deviation.
All subjects underwent the examination of cardiovascular diseases and risk factors through interview, laboratory, and physical examinations. In our study, cardiovascular disease was defined as a previous diagnosis of ischemic heart disease, congestive heart failure, and coronary interventional procedures (angioplasty, stenting, bypass graft). Hypertension was defined as previously diagnosed high blood pressure and on medications and/or systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg. Diabetes was defined as previously diagnosed diabetes and on treatment or glycated hemoglobin ≥6.5%. Hyperlipidemia was defined as previously diagnosed high lipid levels and on medications or total cholesterol levels ≥4.14 mmol/l following the National Cholesterol Education Programme Adult Treatment Panel III guidelines. Smoking is categorized into nonsmokers and smokers (past and current smokers).
MRI Acquisition and Analysis
Every subject underwent MRI scans that were performed on a 3T Siemens Magnetom Trio Tim scanner using a 32‐channel head coil at the Clinical Imaging Research Centre of the National University of Singapore. The image protocols were (i) high‐resolution T 1‐weighted magnetization prepared rapid gradient recalled echo (192 slices, 1 mm thickness, in‐plane resolution 1 mm, no interslice gap, sagittal acquisition, field of view = 256 × 256 mm, matrix = 256 × 256, repetition time = 2,300 ms, echo time = 1.9 ms, inversion time = 900 ms, flip angle = 9°); (ii) isotropic HARDI (single‐shot echo‐planar sequence; 48 slices of 3 mm thickness; with no interslice gaps; matrix: 96 × 96; field of view: 256 × 256 mm; repetition time: 6,800 ms; echo time: 85 ms; flip angle: 90°; 61 diffusion‐weighted images (DWIs) with b = 1,150 s/mm2, seven baseline images without diffusion weighting); (iii) T 2‐weighted imaging protocol (axial spin echo sequence, 48 slices with 3 mm slice thickness, no interslice gap, matrix = 232 × 256, field of view = 232 × 256 mm, repetition time = 2,600 ms, echo time = 99 ms, flip angle = 120°); (iv) fluid attenuated inversion recovery (FLAIR) imaging protocol (repetition time = 9,000 ms; echo time = 82 ms; inversion time = 2,500 ms; matrix size = 232 × 256, field of view = 232 × 256 mm, slice thickness = 3 mm, no slice gap, number of slices = 48).
In the structural MRI analysis, the gray matter, white matter, cerebral spinal fluid (CSF), lateral ventricles, and subcortical structures (amygdala, hippocampus, caudate, putamen, globus pallidus, thalamus) were automatically segmented from the intensity‐inhomogeneity corrected T 1‐weighted MR images [Sled et al., 1998]. For shape analysis, the subcortical and lateral ventricular shapes were generated using the prior shape information of an atlas that were created from 41 manually labeled individual structures via a large deformation diffeomorphic metric mapping (LDDMM) atlas generation procedure [Qiu et al., 2010a]. Shape variations of individual subjects relative to the atlas were characterized by the Jacobian determinant of the deformation in the logarithmic scale, where the deformation transformed the atlas shape to be similar to subjects. This measure, termed as the “deformation map,” represents the ratio of each subject's structural volume to the atlas volume in the logarithmic scale: i.e., positive values correspond to expansion, while negative values correspond to compression of the subject's structure relative to the atlas at each anatomical location. For cortical thickness analysis, an inner surface was constructed at the boundary between the white and gray matter and then propagated to the outer surface at the boundary between the gray matter and CSF. The cortical thickness was measured as the distance between the corresponding points on the inner and outer surfaces [Fischl and Dale, 2000]. A cortical surface mapping algorithm, LDDMM, was then applied to align individual cortical surfaces to an atlas cortical surface for group analysis of cortical thickness [Zhong and Qiu, 2010].
In HARDI analysis, the DWIs were corrected for motion and eddy current distortions using affine transformation to the image without diffusion weighting (b = 0). HARDI images were then aligned to an HARDI atlas using HARDI diffeomorphic metric mapping technique [Du et al., 2011, 2012], where the HARDI atlas was constructed based on 40 subjects selected from our sample using the HARDI atlas generation approach. Mean diffusivity (MD) and generalized fractional anisotropy (GFA, its value is between 0 and 1) were computed from HARDI images in the atlas space and were used for the following statistical analysis. Previous studies showed that GFA derived from HARDI is sensitive to multiple axonal orientations, which is more precise than FA derived from DTI [Tuch et al., 2002].
The T 1‐, T 2‐weighted, and FLAIR MRI data were used to segment WMH and infarction via the multistage segmentation algorithm proposed in [Wang et al., 2012b]. The segmentation accuracy was well evaluated and reported in [Wang et al., 2012b].
Statistical Analysis
Comparison of demographic variables between groups utilized one‐way ANOVAs with Bonferroni's correction for post hoc pairwise comparisons for continuous variables and chi‐square tests for categorical variables. The ratio of WMH volume to total brain volume (TBV) was log transformed for the comparison as it was negatively skewed.
Pairwise testing of cortical thickness and subcortical shape between any two diagnostic groups was done with linear regressions at each location of the brain. The diagnostic group was considered as a main factor. The cortical thickness and deformation maps were smoothed using a 30‐mm full‐width‐at‐half‐maximum Gaussian filter [Chung et al., 2005]. Results at each surface vertex were thresholded at the level of significance (P < 0.001) and then corrected for multiple comparisons at the cluster level of significance (corrected P < 0.05). Each cortical cluster size was greater than 242 mm2 and each subcortical cluster size was greater than 100 mm2, as determined by random field theory [Chung et al., 2010]. For analysis of white matter, voxel‐based analysis was performed to investigate pairwise group differences in MD and GFA using SPM8. Results at each voxel were thresholded at the level of significance (P < 0.001) and then corrected for multiple comparisons at the cluster level of significance (corrected P < 0.001). For each analysis, age and sex were added as covariates. Additionally, TBV was added as a covariate in the subcortical shape analysis. In statistical analysis on GFA and MD, we identified the infarction of the white matter using T 1‐, T 2‐weighted, and FLAIR MR images [Wang et al., 2012b] and then replaced the GFA or MD values of the infarction region by their corresponding group mean values. In this way, the slope for diagnosis in the regression model was not influenced due to the infarction; and hence, this method avoids comparing the white matter with CSF.
RESULTS
Sample Demographic
Sample demographics are listed in Table 1. Significant differences between the four diagnostic groups (NC, VCIND Mild, MSVCI, and AD) were found in age (F(3,96) = 3.789, P = 0.013), years of education (F(3,96) = 4.752, P = 0.004), sex ratio (χ 2 = 10.453, P = 0.015), TBV (F(3,96) = 7.705, P < 0.001), MMSE score (F(3,96) = 39.899, P < 0.001), WMH to TBV ratio (F(3,96) = 15.036, P < 0.001), history of cardiovascular disease (χ 2 = 12.237, P = 0.007), and history of hyperlipidemia (χ 2 = 10.254, P = 0.017). Post hoc tests revealed that controls had more years of education than the AD group, higher MMSE scores than the MSVCI and AD groups, larger TBV than the AD group, smaller WMH to TBV ratios than the VCIND Mild and MSVCI groups, lower rates of history of cardiovascular disease than the VCIND Mild and MSVCI groups and lower rates of history of hyperlipidemia than the VCIND Mild group. Additionally, the AD group had lower MMSE scores and lower WMH to TBV ratios than the MSVCI group. The patients in the AD group were older than those in the VCIND Mild group. The sex ratio was significantly different between the AD and VCIND Mild groups. There were no significant differences between groups in history of diabetes, history of hypertension, or smoking history.
All VCIND and MSVCI patients had subcortical infarcts, of which 18 extended to the cortex and 5 extended to the infratentorial region. Forty infarcts were limited to the subcortical white matter (including periventricular white matter), 4 were thalamic and 20 involved the basal ganglia, internal, and external capsular regions.
Morphological Comparisons of AD Patients with Controls
To demonstrate the robustness of the MRI analysis approaches used in this study, we first presented the morphological comparisons of AD patients with controls. The AD group showed thinner cortex than controls across the entire brain, especially in the superior and middle frontal gyri, medial temporal lobe, and precuneus (Fig. 1A), which is largely consistent with existing AD‐related studies [Dickerson et al., 2009; Seo et al., 2010; Thompson et al., 2003].
Figure 1.
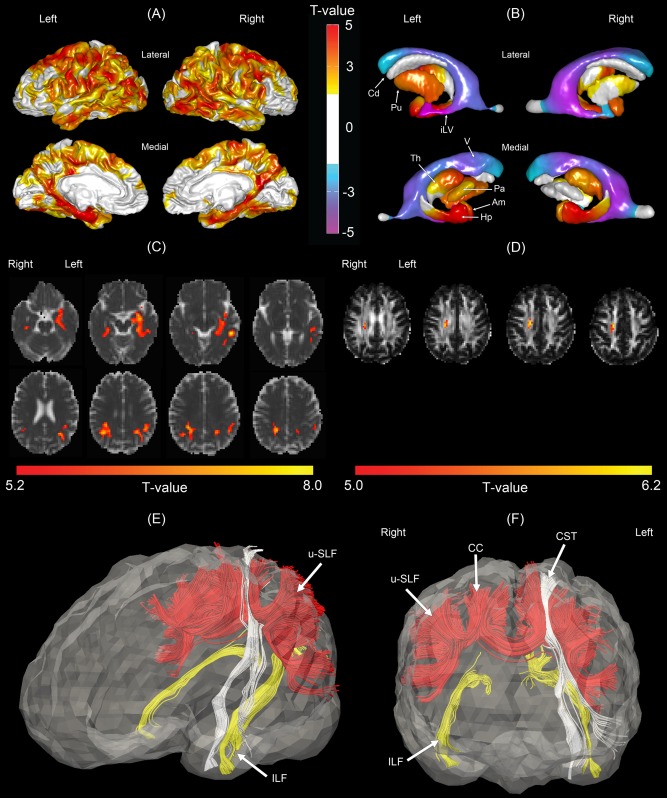
Morphological comparisons between the normal control (NC) and Alzheimer's disease (AD) groups. (A) Statistical map of cortical thickness. Warmer color indicates more severe cortical thinning in the AD group as compared to the NC group. (B) Statistical map of subcortical shapes. Warmer color indicates more severe subcortical shrinkage and cooler color indicates greater ventricular enlargement in the AD group as compared to the NC group. (C) Statistical map of mean diffusivity (MD). Brighter color indicates greater mean diffusivity in the AD group as compared to the NC group. (D) Statistical map of generalized fractional anisotropy (GFA). Brighter color indicates smaller GFA in the AD group. Panels (E, F) show affected fiber bundles passing through the regions highlighted in panel (C), which were determined in the atlas using HARDI tractography technique for the purpose of illustration. Am, amygdala; Cd, caudate; Hp, hippocampus; iLV, inferior lateral ventricle; Pa, globus pallidus; Th, thalamus; V, ventricle; CC, corpus callosum; CST, corticospinal tract; ILF, inferior longitudinal fasciculus; u‐SLF, U‐fibers of the superior longitudinal fasciculus.
Figure 1B illustrates the statistical map of subcortical shapes in AD. Compared to controls, the AD group showed smaller shape (i.e., volume reduction) in bilateral anterior hippocampi and amygdala and bilateral ventricular enlargement (i.e., volume expansion), which are similar to the patterns in subcortical shape abnormalities in the sample of Alzheimer's Disease Neuroimaging Initiative (ADNI) [Qiu et al., 2009]. Smaller shapes in AD were also seen in the left putamen, the dorsolateral aspect of the right putamen, the anterior aspect of the bilateral thalami, and the left globus pallidus. These findings were largely in agreement with the findings derived from subcortical volumetric analysis in AD [Fennema‐Notestine et al., 2009; Roh et al., 2011].
Figure 1C illustrates the MD statistical map in AD. Relative to controls, the AD group showed increased MD in the bilateral inferior longitudinal fasciculus (ILF), posterior corona radiata (PCR), and superior longitudinal fasciculus (SLF). Figure 1D showed decreased GFA in the right superior corona radiata in AD. HARDI deterministic tractography was then used to identify fiber tracts in the atlas that pass through the GFA and MD abnormal regions given in Figure 1C. Figure 1E,F shows the involvement of ILF, PCR, SLF, the left corticospinal tract (CST), and CC in the AD‐related white matter abnormalities. The major fiber bundles involved are posterior white matter bundles, which matches extant literature showing that white matter abnormalities are concentrated in the posterior regions in AD [Chua et al., 2008; Gold et al., 2012; Medina and Gaviria, 2008].
Morphological Comparisons Between Controls and Patients with VCIND Mild
Compared to controls, patients with VCIND Mild had thinner cortex in the bilateral superior and inferior frontal gyri, and the precuneus. Other areas affected were the pre and post‐central gyri, supramarginal gyrus, inferior parietal gyrus, middle temporal gyrus, and fusiform gyrus (Fig. 2A). Additionally, in the right hemisphere thinner cortex was observed in the superior parietal gyrus, posterior and isthmus cingulate, superior, middle, and inferior temporal gyri, temporal pole, entorhinal cortex, orbitofrontal cortex, and lateral occipital cortex.
Figure 2.
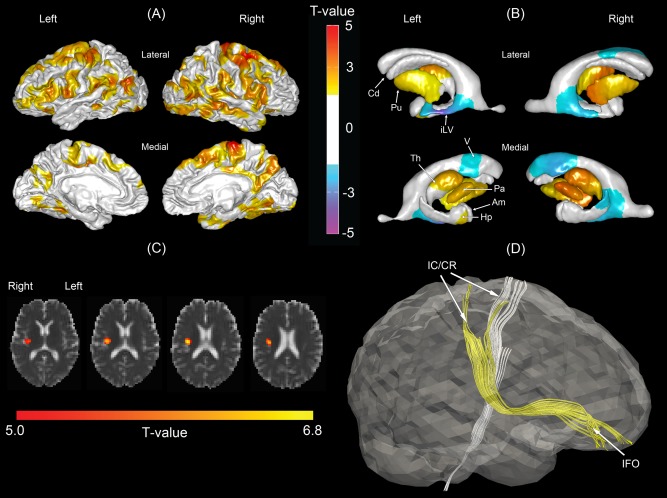
Morphological comparisons between the normal control and mild vascular cognitive impairment no demented (VCIND Mild) groups. (A) Statistical map of cortical thickness. Warmer color indicates more severe cortical thinning in the VCIND Mild group as compared to the NC group. (B) Statistical map of subcortical shapes. Warmer color indicates more severe subcortical shrinkage and cooler color indicates greater ventricular enlargement in the VCIND Mild group as compared to the NC group. (C) Statistical map of mean diffusivity (MD). Brighter color indicates greater mean diffusivity in the VCIND Mild group as compared to the NC group. (D) Affected fiber bundles passing through the region highlighted in panel (C), which were determined in the atlas using HARDI tractography technique. Am, amygdala; Cd, caudate; Hp, hippocampus; iLV, inferior lateral ventricle; Pa, globus pallidus; Th, thalamus; V, ventricle; IC/CR, internal capsule/corona radiata; IFO, inferior fronto‐occipital fasciculus. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Compared to controls, patients with VCIND Mild showed smaller shapes in bilateral globus pallidus and putamen, the right thalamus, the left anterior thalamus, and the left anterior hippocampus as well as the enlargement in the inferior temporal horn and anterior horn of the lateral ventricles (Fig. 2B).
Figure 2C illustrates the statistical map of MD in VCIND Mild group. Patients with VCIND Mild showed increased MD in the region where the right inferior fronto‐occipital fasciculus (IFO) and internal capsule/corona radiata (IC/CR) pass through as illustrated in (Fig. 2D). No significant differences in GFA were observed between the NC and VCIND Mild groups.
Morphological Comparisons of MSVCI Patients with Controls and VCIND Mild Patients
Figure 3A illustrates the statistical map of cortical thickness in the MSVCI group. Compared to the controls, MSVCI patients showed widespread thinner cortex in regions that were similar to those found in the VCIND Mild group such as the superior and inferior frontal cortices. Additionally, thinner cortex was observed in the superior frontal gyrus and motor cortices. Other areas showing thinner cortex were bilateral superior parietal gyrus, superior, middle, and inferior temporal gyri, temporal pole, entorhinal cortex, the isthmus and posterior cingulate, and the lateral occipital cortex.
Figure 3.
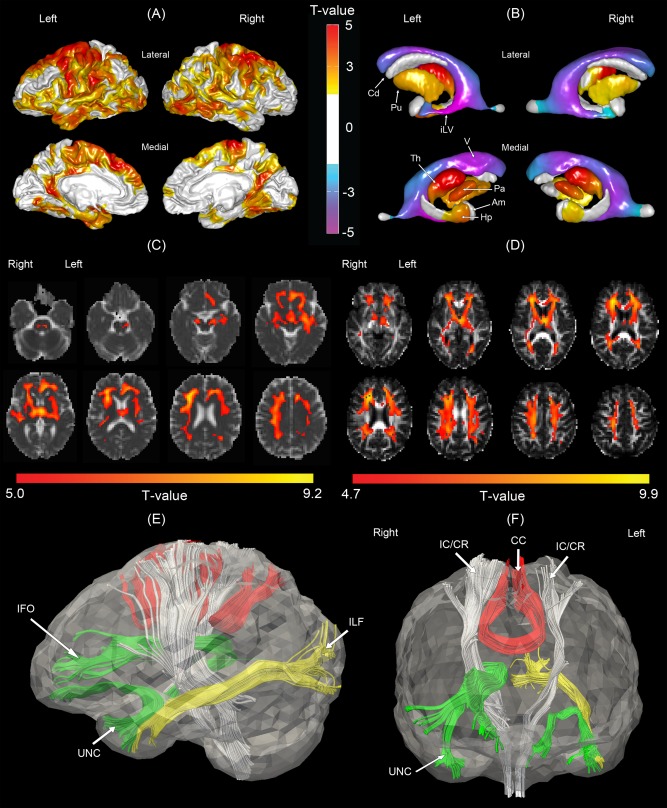
Morphological comparisons between the normal control and moderate to severe vascular cognitive impairment (MSVCI) groups. (A) Statistical map of cortical thickness. Warmer color indicates more severe cortical thinning in the MSVCI group as compared to the NC group. (B) Statistical map of subcortical shapes. Warmer color indicates more severe subcortical shrinkage and cooler colors indicates greater ventricular enlargement in the MSVCI group as compared to the NC group. (C) Statistical map of mean diffusivity (MD). Warmer color indicates more severe subcortical shrinkage and cooler color indicates greater ventricular enlargement in the MSVCI group as compared to the NC group. (D) Statistical map of generalized fractional anisotropy (GFA). Brighter color indicates smaller GFA in the MSVCI group. Panels (E, F) illustrate affected fiber bundles passing through the regions highlighted in panel (C), which were determined in the atlas using HARDI tractography technique. Am, amygdala; Cd, caudate; Hp, hippocampus; iLV, inferior lateral ventricle; Pa, globus pallidus; Th, thalamus; V, ventricle; CC, corpus callosum; IC/CR, internal capsule/corona radiata; IFO, inferior fronto‐occipital fasciculus; ILF, inferior longitudinal fasciculus; UNC, uncinate fasciculus. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 3B illustrates the statistical map of subcortical shapes in the MSVCI group. Relative to controls, smaller subcortical shape was observed in the MSVCI group bilaterally in the thalamus, globus pallidus, putamen, and anterior hippocampus and lateral ventricular enlargement across the entirety of the lateral ventricles. The findings were congruent with those in the VCIND Mild group (see Fig. 2B).
Compared to controls, the MSVCI group also showed increased MD and decreased GFA in the widespread white matter region of the brain (Fig. 3C,D). Specifically, increased MD and decreased GFA were observed bilaterally in regions corresponding to the SLF, ILF, IC/CR, corticopontine tract, CST, the body of CC, inferior fronto‐occipital fasciculus (IFO), thalamus, anterior and superior thalamic radiations, and the uncinate fasciculus (UNC). Increased MD was also observed in the right temporal lobe. Decreased GFA was observed in the sagittal striatum, forceps major, and posterior thalamic radiation. The major fiber bundles passing through the affected regions were the CC, IC/CR, and UNC bilaterally, the anterior section of the right IFO and the left ILF, which was determined in the atlas using HARDI deterministic tractography (Fig. 3E,F).
Compared to the VCIND Mild group, the MSVCI group showed thinner cortex in the left superior frontal gyrus, cingulate, temporal pole, and middle temporal gyrus (Fig. 4A). In contrast, there was not much difference between groups in cortical thickness in the right hemisphere. In the subcortical structures, we found smaller subcortical shapes in MSVCI subjects in the left anterior thalamus and left anterior hippocampus and greater shape expansion in bilateral lateral ventricles and the right posterior putamen (Fig. 4B). No significant differences in MD or GFA were found between the two groups.
Figure 4.

Morphological comparisons between the mild vascular cognitive impairment no dementia (VCIND) and moderate to severe vascular cognitive impairment (MSVCI) groups. (A) Statistical map of cortical thickness. Warmer color indicates more severe cortical thinning and cooler color indicates greater cortical thickness in the MSVCI group as compared to the VCIND Mild group. (B) Statistical map of subcortical shapes. Warmer color indicates more severe subcortical shrinkage and cooler color indicates greater subcortical expansion and enlargement in the MSVCI group as compared to the VCIND Mild group. Am, amygdala; Cd, caudate; Hp, hippocampus; iLV, inferior lateral ventricle; Pa, globus pallidus; Th, thalamus; V, ventricle. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Morphological Comparisons Between Patients with AD and MSVCI
A direct comparison between the AD and MSVCI groups showed that the AD group had thinner cortex bilaterally in the medial temporal lobe, parietal lobe and part of the middle and inferior frontal gyri, and the left occipital lobe (Fig. 5A). When comparing the AD and MSVCI groups (Fig. 5B), it was found that the AD group showed smaller subcortical shapes in the bilateral anterior hippocampus and amygdala, and subcortical shape expansion in the right globus pallidus compared to the MSVCI group. No significant differences in MD or GFA were found between the AD and MSVCI groups.
Figure 5.
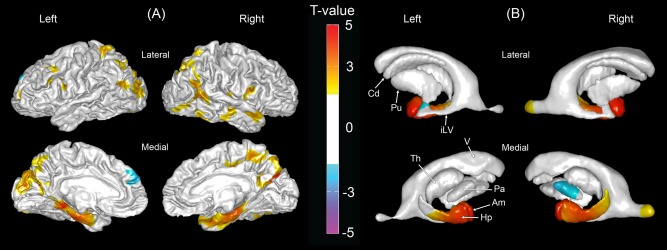
Morphological comparisons between the Alzheimer's disease (AD) and moderate to severe vascular cognitive impairment (MSVCI) groups. (A) Statistical map of cortical thickness. Warmer color indicates more severe cortical thinning and cooler color indicates greater cortical thickness in the MSVCI group as compared to the AD group. (B) Statistical map of subcortical shapes. Warmer color indicates more severe subcortical shrinkage and cooler color indicates greater subcortical expansion and enlargement in the MSVCI group as compared to the AD group. Am, amygdala; Cd, caudate; Hp, hippocampus; iLV, inferior lateral ventricle; Pa, globus pallidus; Th, thalamus; V, ventricle. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
The primary aims of this article were to describe the patterns of cortical, subcortical and white matter morphological abnormalities in patients with sVCI, and to determine the connection between the mild and moderate‐to‐severe conditions. Our hypothesis that concurrent and pronounced thinner cortex and white matter disruptions in the frontal cortex would be found in sVCI was supported. Additionally, we found shape abnormalities in subcortical structures anatomically connected to regions with disrupted white matter integrity. We showed that the patterns of cortical thickness and subcortical shape abnormalities were consistent in the VCIND Mild and MSVCI groups, suggesting that these two conditions lie on the continuum. Finally, we found key differences between the MSVCI and AD groups in cortical and subcortical structures, indicating that thinner cortex and subcortical shape abnormalities in MSVCI follow different trajectories from that in AD.
Our findings on cortical thickness in VCIND Mild and MSVCI are largely in agreement with those of the previous study in mild sVCI and sVaD [Seo et al., 2010] in which subjects with mild sVCI showed thinner cortex in the superior and inferior frontal gyri, superior temporal gyrus, and lingual gyrus and sVaD subjects showed additional thinner cortex in the prefrontal and temporal cortices. Kim et al. [2012] also found that SVaD subjects with 11C‐Pittsburgh compound B negative (PiB−) showed cortical thinning in the perisylvian area and posterior cingulate gyrus, which was also observed in our sample with MSVCI. In addition, our findings are also largely in agreement with those in recent DTI studies of sVCI. One study found that subjects with sVaD had reduced FA values in the anterior CC and frontal and parietal white matter regions [Zarei et al., 2009], while another showed that VCIND patients had reduced FA in both anterior and posterior white matter [Zhou et al., 2011]. In the MSVCI group, reduced GFA was observed in both anterior and posterior white matter, but concentrated in the anterior white matter. Hence, our findings are generally in line with existing literature.
Our study extends existing findings by concurrently assessing both gray and white matter as well as the subcortical structures. In the VCIND Mild subjects, thinner cortex was found in both hemispheres compared to controls. However, we only found increased MD in a small region of the right hemisphere white matter. The major axonal bundles passing through this region are the anterior section of the right IFO, which connects to the orbitofrontal cortex, and the right IC/CR, which connects to the subcortical structures as well as the sensorimotor cortices. This matches the observed thinner cortex in the prefrontal, motor, parietal, and orbitofrontal cortices of the right hemisphere. But it was not observed in the left hemisphere, which may partially due to the small sample size in our study. However, since the VCIND Mild group has a much higher WMH to TBV ratio compared to controls, we suspect that disruption in white matter may already occur. Additionally, we suspect that the white matter disruption may occur in the location connecting the subcortical structures and the cortex. In the VCIND Mild group, we found shape abnormalities in the anterior thalamus, lentiform nuclei, and anterior hippocampus of the left hemisphere. The thalamus is a key structure in implicated in executive function, motor function, language, attention, memory, and behavioral dysfunction, such as apathy, aboulia, and disinhibition [Schmahmann, 2003]. It has projections to most of the cortices [Herrero et al., 2002]. The putamen receives inputs from the association and sensorimotor areas of the cortex while the globus pallidus projects to the dorsomedial nuclei of the thalamus [Herrero et al., 2002]. The hippocampus, which is involved in memory processing, is connected to the temporal lobe structures and communicates with the association cortices as well as the thalamus and amygdala [Giap et al., 2000]. Abnormalities in the nuclei of these structures disrupt communication between multiple regions of the cortex, thereby contributing to cognitive and behavioral dysfunction, and deterioration of connected cortical neurons. Subcortical shape abnormalities may hence contribute to the some of the observed thinner cortex in the VCIND Mild group.
Compared to controls, patients with MSVCI showed abnormalities in large white matter regions involving multiple major axonal bundles, namely the IC/CR, UNC, ILF, IFO, and CC. These axonal bundles pass through or project to subcortical and cortical areas where thinner cortex was observed in the MSVCI group. For instance, the IC/CR contains fibers that project from the thalamus to the anterior and posterior cortices as well as fibers that descend from the frontoparietal cortex to the basal ganglia and to the spinal cord. UNC connects the anterior temporal lobe with the orbitofrontal cortex [Catani and Thiebaut de Schotten, 2008]. Concurrently, we observed widespread thinner cortex bilaterally in the prefrontal, motor, parietal, temporal and orbitofrontal cortices, and severe subcortical shape abnormalities in the thalamus and basal ganglia. In addition, we observed the involvement of the ILF that connects the occipital and temporal lobes. Consistent with this result we found that thinner cortex was more widespread in the temporal and occipital lobes. We also observed shape abnormalities in the anterior thalamus, lentiform nuclei, and anterior hippocampus of both hemispheres. These subcortical regions with abnormalities anatomically connected with cortical regions with cortical abnormalities through white matter fiber bundles. Thus, our results highlight the links between thinner cortex, subcortical shape abnormalities, and disrupted white matter integrity.
We also found a progressive pattern from VCIND Mild to MSVCI. Similar patterns of cortical thinning were seen in the VCIND Mild and MSVCI groups, concentrated in the frontal lobes. The severity of thinning in these structures was greater and more widespread in the MSVCI group. This is in agreement with a prior study showing that patterns of thinner cortex largely overlapped between sVaD and mild sVCI, and that their extent and severity were greater in SVaD than in sVCI [Seo et al., 2010]. Additionally, we showed that a similar pattern exists for shape abnormalities especially in the thalamus and lentiform nuclei in both VCIND Mild and MSVCI groups. In the white matter, we observed widespread increases in MD and decreases in GFA throughout most of the white matter structures in the MSVCI group while sparse findings were seen in the VCIND Mild group. However, no significant differences were found in GFA or MD between the VCIND Mild and MSVCI groups when we compared them directly, which may be due to small sample sizes in our study. Furthermore, similar fiber bundles were shown to be involved in both groups, namely, the right IC/CR and IFO. Taken together, these results suggest that brain morphological abnormalities in sVCI follow a similar trajectory as the disease progresses from mild to more advanced stages.
There were common morphological abnormalities in the AD and MSVCI groups, hence highlighting structures that are involved in both diseases. But, AD showed greater morphological abnormalities in the medial temporal lobe and posterior brain regions than MSVCI. Memory impairment has been identified as one of the key features of AD; in comparison, memory deficits generally tend to be less severe in VCI syndromes [Looi and Sachdev, 1999]. Medial temporal atrophy has been found to be able to distinguish AD from sVCI [Burton et al., 2009]. The amygdala and hippocampus are also known to show shape abnormalities and volume reductions even at the mild cognitive impairment stage of AD [Qiu et al., 2009; Roh et al., 2011]. Hence, it is not surprising that abnormalities in the medial temporal structures are more severe in AD than in sVCI. Furthermore, while no statistical differences in MD or GFA in the white matter were found, the fiber bundles involved in the AD and MSVCI groups were clearly different, with minimal overlap. In the AD group, the posterior fiber bundles such as the posterior U‐fibers of the SLF were involved (Fig.1E,F). This matches the known progression of the disease where pathology begins in the posterior regions of the brain before spreading to the anterior regions in the later stages of AD [Whitwell et al., 2007]. In contrast, the fiber bundles involved in the MSVCI group were toward the anterior regions of the brain, for example, the UNC and IC/CR (Fig. 3E,F). This supports the theory that the fronto‐subcortical white matter circuitry and long association fibers are implicated in the pathophysiology of sVCI [Chui, 2007; Cummings, 1993].
This study has several methodological strengths. First, our study used the state‐of‐art morphological shape analysis to examine subcortical morphological abnormalities in sVCI. Investigations of subcortical structures have thus far been limited to examination of volumetric analysis and are prone to missing localized abnormalities. Large deformation diffeomorphic metric mapping (LDDMM) [Qiu et al., 2010a; Qiu and Miller, 2008] is a powerful computational tool that provides detailed analysis of the morphological shape of subcortical brain regions, thereby allowing for precise examination of these structures, well beyond what has been examined in prior MRI studies of sVCI. Second, to our best knowledge, the present study was the first reporting the white matter integrity in AD and sVCI using HARDI instead of DTI. Compared to FA derived from DTI, GFA derived from HARDI is more sensitive to multiple axonal orientations and hence more precisely characterizes the coherence of white matter bundles [Tuch et al., 2002]. We also noticed that the present study has some limitation. While we have shown the close inter‐relatedness of abnormalities in the cortical, subcortical, and white matter structures, we are unable to determine the temporal order of cortical thinning, subcortical shape deformations and white matter degeneration and disconnectivity. Future longitudinal studies are needed to understand the progression of abnormalities in these brain structures, and to determine the direction of influence.
In conclusion, we investigated the patterns of cortical, subcortical, and white matter morphology in subjects with sVCI, and showed that the disease progresses along a similar trajectory from the mild to moderate and severe conditions. To our knowledge, this is the first study to integrate data on abnormalities in the cortical, subcortical, and white matter structures and demonstrate their congruency using multimodalities of MRI techniques. There are pronounced key differences in brain morphology between sVCI and AD, and much can be gained from utilizing information from cortical thickness, subcortical shape, and HARDI measures as a combined biomarker to distinguish one condition from the other.
REFERENCES
- Bocti C, Swartz RH, Gao F‐Q, Sahlas DJ, Behl P, Black SE (2005): A new visual rating scale to assess strategic white matter hyperintensities within cholinergic pathways in dementia. Stroke 36:2126–2131. [DOI] [PubMed] [Google Scholar]
- Burton EJ, Barber R, Mukaetova‐Ladinska EB, Robson J, Perry RH, Jaros E, Kalaria RN, O'Brien JT (2009): Medial temporal lobe atrophy on MRI differentiates Alzheimer's disease from dementia with Lewy bodies and vascular cognitive impairment: A prospective study with pathological verification of diagnosis. Brain 132:195–203. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M (2008): A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 44:1105–1132. [DOI] [PubMed] [Google Scholar]
- Chin J (2002): Screening for cognitive impairment in primary care: The role of objective tests. Singapore Fam Phys 28:62–68. [Google Scholar]
- Chua TC, Wen W, Slavin MJ, Sachdev PS (2008): Diffusion tensor imaging in mild cognitive impairment and Alzheimer's disease: A review. Curr Opin Neurol 21:83–92. doi: 10.1097/WCO.0b013e3282f4594b. [DOI] [PubMed] [Google Scholar]
- Chui HC (2007): Subcortical ischemic vascular dementia. Neurol Clin 25:717–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MK, Robbins SM, Dalton KM, Davidson RJ, Alexander AL, Evans AC (2005): Cortical thickness analysis in autism with heat kernel smoothing. Neuroimage 25:1256–1265. [DOI] [PubMed] [Google Scholar]
- Chung MK, Worsley KJ, Nacewicz BM, Dalton KM, Davidson RJ (2010): General multivariate linear modeling of surface shapes using SurfStat. Neuroimage 53:491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL (1993): FRontal‐subcortical circuits and human behavior. Arch Neurol 50:873–880. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD and others (2009): The cortical signature of Alzheimer's disease: Regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid‐positive individuals. Cereb Cortex 19:497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Venketasubramanian N, Chan BP, Sharma VK, Slavin MJ, Collinson SL, Sachdev P, Chan YH, Chen CL (2012): Brief screening tests during acute admission in patients with mild stroke are predictive of vascular cognitive impairment 3‐6 months after stroke. J Neurol Neurosurg Psychiatry 83:580–585. [DOI] [PubMed] [Google Scholar]
- Du J, Goh A, Qiu A (2011): Large deformation diffeomorphic metric mapping of orientation distribution functions. Information processing in medical imaging. Proc Conf 22:448–462. [DOI] [PubMed] [Google Scholar]
- Du J, Goh A, Qiu A (2012): Diffeomorphic metric mapping of high angular resolution diffusion imaging based on Riemannian structure of orientation distribution functions. IEEE Trans Med Imaging 31:1021–1033. [DOI] [PubMed] [Google Scholar]
- Fennema‐Notestine C, Hagler DJ Jr, McEvoy LK, Fleisher AS, Wu EH, Karow DS, Dale AM (2009): Structural MRI biomarkers for preclinical and mild Alzheimer's disease. Hum Brain Mapp 30(10):3238–3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM (2000): Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giap BT, Jong CN, Ricker JH, Cullen NK, Zafonte RD (2000): The hippocampus: Anatomy, pathophysiology, and regenerative capacity. J Head Trauma Rehabil 15:875–894. [DOI] [PubMed] [Google Scholar]
- Gold BT, Johnson NF, Powell DK, Smith CD (2012): White matter integrity and vulnerability to Alzheimer's disease: Preliminary findings and future directions. Biochim Biophys Acta 1822:416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães J, Freire M, Lima R, Souza‐Rodrigues R, Costa A, dos Santos C, Picanço‐Diniz C, Gomes‐Leal W (2009): Mechanisms of secondary degeneration in the central nervous system during acute neural disorders and white matter damage. Rev Neurol 48:304–310. [PubMed] [Google Scholar]
- Herrero M‐T, Barcia C, Navarro J (2002): Functional anatomy of thalamus and basal ganglia. Child's Nerv Syst 18:386–404. [DOI] [PubMed] [Google Scholar]
- Jellinger KA (2002): The pathology of ischemic‐vascular dementia: An update. J Neurol Sci 203–204:153–157. [DOI] [PubMed] [Google Scholar]
- Kim CH, Seo SW, Kim GH, Shin JS, Cho H, Noh Y, Kim S‐H, Kim MJ, Jeon S, Yoon U and others (2012): Cortical thinning in subcortical vascular dementia with negative (11)C‐PiB PET. J Alzheimer's Dis 31:315–323. [DOI] [PubMed] [Google Scholar]
- Looi JCL, Sachdev PS (1999): Differentiation of vascular dementia from AD on neuropsychological tests. Neurology 53:670. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984): Clinical diagnosis of Alzheimer's disease: Report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34:939–944. [DOI] [PubMed] [Google Scholar]
- Medina DA, Gaviria M (2008): Diffusion tensor imaging investigations in Alzheimer's disease: The resurgence of white matter compromise in the cortical dysfunction of the aging brain. Neuropsychiatr Dis Treat 4:737–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhalu K, Ang S, De Silva DA, Wong MC, Chang HM, Chia KS, Auchus AP, Chen C (2009): Severity of CIND and MCI predict incidence of dementia in an ischemic stroke cohort. Neurology 73:1866–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Brown T, Fischl B, Jun M, Miller MI (2010a): Atlas generation for subcortical and ventricular structures with its applications in shape analysis. Image Process IEEE Trans 19:1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Fennema‐Notestine C, Dale AM, Miller MI (2009): Regional shape abnormalities in mild cognitive impairment and Alzheimer's disease. Neuroimage 45:656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Miller MI (2008): Multi‐structure network shape analysis via normal surface momentum maps. Neuroimage 42:1430–1438. [DOI] [PubMed] [Google Scholar]
- Roh J, Qiu A, Seo S, Soon H, Kim J, Kim G, Kim M‐J, Lee J‐M, Na D (2011): Volume reduction in subcortical regions according to severity of Alzheimer's disease. J Neurol 258:1013–1020. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD (2003): Vascular syndromes of the thalamus. Stroke 34:2264–2278. [DOI] [PubMed] [Google Scholar]
- Seo SW, Ahn J, Yoon U, Im K, Lee J‐M, Tae Kim S, Ahn H‐J, Chin J, Jeong Y, Na DL (2010): Cortical thinning in vascular mild cognitive impairment and vascular dementia of subcortical type. J Neuroimaging 20:37–45. [DOI] [PubMed] [Google Scholar]
- Seo SW, Lee J‐M, Im K, Park J‐S, Kim S‐H, Kim ST, Ahn H‐J, Chin J, Cheong H‐K, Weiner MW and others (2012): Cortical thinning related to periventricular and deep white matter hyperintensities. Neurobiol Aging 33:1156–1167.e1. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC (1998): A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17:87–97. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, Semple J, Herman D, Hong MS, Dittmer SS, Doddrell DM and others (2003): Dynamics of gray matter loss in Alzheimer's disease. J Neurosci 23:994–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuch DS, Reese TG, Wiegell MR, Makris N, Belliveau JW, Wedeen VJ (2002): High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med 48:577–582. [DOI] [PubMed] [Google Scholar]
- Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjögren M, Wallin A, Ader H, Leys D, Pantoni L and others (2001): A new rating scale for age‐related white matter changes applicable to MRI and CT. Stroke 32:1318–1322. [DOI] [PubMed] [Google Scholar]
- Wang JT, Medress ZA, Barres BA (2012a): Axon degeneration: Molecular mechanisms of a self‐destruction pathway. J Cell Biol 196:7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Catindig JA, Hilal S, Soon HW, Ting E, Wong TY, Venketasubramanian N, Chen C, Qiu A (2012b): Multi‐stage segmentation of white matter hyperintensity, cortical and lacunar infarcts. Neuroimage 60:2379–2388. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Przybelski SA, Weigand SD, Knopman DS, Boeve BF, Petersen RC, Jack CR (2007): 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer's disease. Brain 130:1777–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederkehr S, Simard M, Fortin C, van Reekum R (2008): Validity of the clinical diagnostic criteria for vascular dementia: A critical review. Part II. J Neuropsychiatry Clin Neurosci 20:162–177. [DOI] [PubMed] [Google Scholar]
- Yi L, Wang J, Jia L, Zhao Z, Lu J, Li K, Jia J, He Y, Jiang C, Han Y (2012): Structural and functional changes in subcortical vascular mild cognitive impairment: A combined voxel‐based morphometry and resting‐state fMRI study. PLoS ONE 7:e44758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei M, Damoiseaux JS, Morgese C, Beckmann CF, Smith SM, Matthews PM, Scheltens P, Rombouts SA, Barkhof F (2009): Regional white matter integrity differentiates between vascular dementia and Alzheimer disease. Stroke 40:773–779. [DOI] [PubMed] [Google Scholar]
- Zhong J, Qiu A (2010): Multi‐manifold diffeomorphic metric mapping for aligning cortical hemispheric surfaces. NeuroImage 49:355–365. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Lin F‐c, Zhu J, Zhuang Z‐g, Li Y‐s, Tao J, Qian L‐j, Xu J‐r, Lei H (2008): Whole brain diffusion tensor imaging histogram analysis in vascular cognitive impairment. J Neurol Sci 268:60–64. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Qun X, Qin L‐d, Qian L‐j, Cao W‐w, Xu J‐r (2011): A primary study of diffusion tensor imaging‐based histogram analysis in vascular cognitive impairment with no dementia. Clin Neurol Neurosurg 113:92–97. [DOI] [PubMed] [Google Scholar]


