Abstract
This study combines functional and structural magnetic resonance imaging to test the “asymmetric sampling in time” (AST) hypothesis, which makes assertions about the symmetrical and asymmetrical representation of speech in the primary and nonprimary auditory cortex. Twenty‐three volunteers participated in this parametric clustered‐sparse fMRI study. The availability of slowly changing acoustic cues in spoken sentences was systematically reduced over continuous segments with varying lengths (100, 150, 200, 250 ms) by utilizing local time‐reversion. As predicted by the hypothesis, functional lateralization in Heschl's gyrus could not be observed. Lateralization in the planum temporale and posterior superior temporal gyrus shifted towards the right hemisphere with decreasing suprasegmental temporal integrity. Cortical thickness of the planum temporale was automatically measured. Participants with an L > R cortical thickness performed better on the in‐scanner auditory pattern‐matching task. Taken together, these findings support the AST hypothesis and provide substantial novel insight into the division of labor between left and right nonprimary auditory cortex functions during comprehension of spoken utterances. In addition, the present data yield support for a structural‐behavioral relationship in the nonprimary auditory cortex. Hum Brain Mapp 35:1779–1789, 2014. © 2013 Wiley‐Periodicals, Inc.
Keywords: anatomical asymmetry, asymmetric sampling in time, auditory cortex, functional lateralization, speech perception
INTRODUCTION
Long before modern neuroimaging methods have been established, behavioral evidence collected from healthy and brain‐injured individuals illustrated that temporal characteristics of speech influence the cortical lateralization during speech perception [Efron, 1963; Schwartz and Tallal, 1980; Studdert‐Kennedy and Shankweiler, 1970; Tallal et al., 1996; Zurif and Mendelsohn, 1972]. Based on reports of neurological symptoms, namely, pure word deafness, and the psychophysiological properties of speech, Poeppel [2001, 2003] introduced the “asymmetric sampling in time” hypothesis (AST). This framework makes assertions about asymmetrical representation of speech in the auditory‐related cortex on the posterior supratemporal plane, which is dependent on the temporal characteristics of the speech signal. It is well established that acoustic information in speech is conveyed over multiple time scales [Rosen, 1992]. For instance, the difference between the utterances “date” and “gate” is encoded in rapidly changing formant patterns at the level of acoustic fine structure (subsegmental cues at a scale of several milliseconds up to tens of milliseconds; see also Best et al., 1981]. Conversely, prosodic elements at both the word and sentence levels (e.g., intonation contour, tempo, rhythm, syllabic structure, and stress) are transmitted via slower fluctuations in the speech signal at a scale of hundreds of milliseconds (suprasegmental cues). In functional terms, these parameters convey pivotal information. For instance, the intonation contour (melody) of an utterance can mark the vocalization as either a question or a statement (”That is all?”/“That is all!”). According to the AST hypothesis, the continuously inflowing auditory signal is chunked via temporal integration windows of different sizes. After a suggested initial symmetrical representation of auditory input in core auditory cortex, the acoustic signal is then processed in an asymmetrical manner in the non‐primary auditory cortex, presumably the planum temporale (PT). Left non‐primary cortex is assumed to predominantly accommodate neuronal ensembles that preferentially integrate over relatively short time windows (up to 50 ms), while right non‐primary areas are surmised to harbor neurons that preferentially integrate over relatively long time windows (around 150 ‐ 250 ms)1. In terms of speech as an incoming acoustic signal, transient phonetic cues at the subsyllabic level (e.g. formant transitions, voice onset time) preferentially drive neurons residing in the left non‐primary auditory cortex, while information that unfolds more slowly (at the syllabic level and beyond, e.g. intonation contour) preferentially drive the right non‐primary auditory cortex. The framework is not restricted to speech but also applies to all auditory non‐speech signals, for instance music.
The AST hypothesis has found support from various empirical studies. In a combined EEG‐fMRI study, Giraud et al. 2007 have corroborated the notion of endogenous leftward lateralization of fast oscillators (gamma frequencies) and rightward lateralization of slow oscillators (theta frequencies) in the auditory cortex. In general, those oscillations can be seen as foundation of speech processing [Giraud and Poeppel, 2012]. Utilizing an array of behavioral and neuroimaging methods, it has been convincingly demonstrated that the processing of rapidly changing acoustic cues results in a right ear advantage and left‐lateralized recruitment of auditory‐related cortex, respectively [Belin et al., 1998; Jamison et al., 2006; Jäncke et al., 2002; Liégeois‐Chauvel et al., 1999; Meyer et al., 2005; Obleser et al., 2008; Schönwiesner et al., 2005; Schwartz and Tallal, 1980; Slevc et al., 2011; Studdert‐Kennedy and Shankweiler, 1970; Warrier et al., 2009; Zaehle et al., 2007a, 2004a, 2009; Zatorre and Belin, 2001; Zatorre et al., 2002]. In contrast, evidence for the preference of the right hemisphere for slowly changing acoustic cues is less numerous and less straightforward. Boemio et al. 2005 reported a rightward STS lateralization for slowly fluctuating non‐speech stimuli. Meyer et al. found the right PT to be especially sensitive to isolated intonation contour, as well as to speech lacking dynamic pitch variations [Meyer et al., 2002, 2004]. These findings correspond with a study by Gandour et al. 2003 that also examined intonation contour. Another suprasegmental domain of speech perception, in which lateralization in posterior temporal regions can be observed, is sentence‐level rhyme and rhythm processing. Hurschler et al. 2012 have shown that an explicit sentence‐level rhyming task implicates the right auditory‐related cortex more strongly than the left auditory‐related cortex. Geiser et al. 2008 reported right auditory‐related cortex activation during a sentence rhythm detection task. Furthermore, Zhang et al. 2010 observed right‐lateralized temporal lobe brain activation for processing rhythm and intonation in pseudospeech. Taken together, these results indicate a left hemispheric advantage of auditory‐related cortex for the processing of rapidly changing acoustic cues in complex sounds and speech, as well as a right hemispheric preference for slowly changing cues. Nevertheless, due to the present lack of systematic investigations, further studies that examine the processing of slowly changing acoustic cues and the interplay between left and right auditory‐related cortex are warranted.
Since the AST hypothesis suggests continuous temporal scales, rather than a temporal dichotomy, the strongest evidence for, or against, the previously postulated predictions might come from a parametric study design. Several studies have utilized complex nonspeech sounds in combination with temporal manipulation [e.g., Boemio et al., 2005, Schönwiesner et al., 2005]; nevertheless, parametrically designed neuroimaging studies that employ natural speech as stimuli are scarce and are usually limited to very brief stimuli [Britton et al., 2009]. However, aforementioned prosodic elements are more pronounced, or only exist in speech exceeding the subsegmental level, for example sentences, as recently emphasized by McGettigan and Scott 2012. Hence, it is of vital interest to test whether the predictions of the AST model are also accurate for longer, more natural sounding, parametrically manipulated speech stimuli.
In order to contribute to a deeper understanding of this issue, the present study examines the effect of temporal integrity of speech input on the functional lateralization in the auditory‐related cortex. The current investigation employed an fMRI design, using spoken sentences as stimuli and a stimulus manipulation procedure initially introduced by Saberi and Perrott 1999 and further developed by Walker et al. 2008. Saberi and Perrott 1999 parametrically manipulated the temporal integrity of speech by segmenting an utterance into consecutive time windows and then by locally time reversing those windows. The longer the length of the locally reversed time segment, the worse the participants performance became; thereby, indicating that the decreasing temporal integrity led to a decrease in comprehensibility. In this study, four different segment lengths (100–250 ms) were utilized, in order to alter the integrity of slow modulations in the speech signal. Additionally, normal sentences (0 ms) were presented as a control condition. First, in order to test the two main predictions of Poeppel's AST model, which posits functional symmetry at the primary auditory cortex, followed by temporally dependent asymmetry at non‐primary regions, we investigated the functional lateralization in Heschl's gyrus (HG), PT and the posterior superior temporal gyrus (pSTG) as a function of the stimuli's temporal integrity. We hypothesized that while the lateralization of BOLD response collected from HG remains uninfluenced by the stimuli's temporal integrity, activity in PT and pSTG should shift to the right hemisphere when the availability of intact slowly changing acoustic cues decreases. It has previously been shown that decreasing the amount of acoustic information available in speech stimuli yields increases in activation in auditory‐related temporal and frontal regions [Davis and Johnsrude, 2003; Meyer et al., 2004]. Therefore, it is reasonable to assume a relatively enhanced signal increase in the right auditory‐related cortex when the suprasegmental temporal integrity of stimuli is decreased, resulting in a rightward shift of lateralization in PT and pSTG. Second, the correlation between cortical anatomy, functional and behavioral measurements increasingly gathered attention over the last years. For instance, manual measurements of auditory cortex anatomy were used by Wong et al. 2008 to demonstrate a link between anatomy and success in a pitch learning task, or by Warrier et al. 2009 to show a connection between hemodynamic response and cortical volume. Therefore, we also added an exploratory surface‐based morphometry analysis. Here, we inspected the relationship of anatomical PT asymmetry, functional PT lateralization and task performance, in order to explore whether there might be a connection between specific patterns of neuroarchitecture, function and individual differences in task performance during auditory cognition. While the aforementioned studies focused on the relationship between basic acoustic processing and HG anatomy, we chose to focus on the anatomy of the PT, since our task involved more complex speech processing.
MATERIALS AND METHODS
Participants
Twenty‐three healthy native (Swiss‐) German speakers took part in this study (12 female, M age = 25 (SD = 3)). All of the participants were right‐handed [Annett, 1970] and had no musical training. Subjects were screened for (developmental) speech and/or hearing impairments, neurological and/or psychiatric history, as well as MR‐compatibility. Volunteers gave written informed consent prior to the experiment and were paid for their participation. The Canton of Zurich Ethics Committee approved this study (application E‐40/2009).
Stimuli
One hundred and eighty German sentences were binaurally presented in the course of this experiment. They were spoken by a trained female speaker and recorded in a soundproof chamber at the University of Zurich Phonetics Lab. Stimuli were normalized with regards to mean intensity. Each stimulus was presented only once. The temporal structure of the stimuli was manipulated following the approach introduced by Saberi and Perrott 1999. Sentences were split into segments and then locally time reversed. Segment length was varied (100, 150, 200, and 250 ms) to create conditions of differential suprasegmental temporal integrity. Since time reversing segments leads to temporal discontinuities resulting in audible artifacts at the border of two segments, overlapping cosine windows were utilized. This was done via a procedure introduced by Walker et al. 2008 and resulted in artifact free stimuli (cf. Fig. 1). In addition, unaltered sentences (0 ms) were presented. The sentences' duration was between 2.2 and 3.5 s (M = 2.9 s). The conditions did not differ with regards to sentence duration, stimulus intensity, and intonation contour. Furthermore, probe stimuli were used to implement a pattern‐matching task. Each probe stimulus was created from the original, not manipulated version of the stimulus. This was done by cutting an interval with a duration of 10% of the total sentence duration and a random onset. Subsequently, it was assured that the probe stimuli did not contain extended silent periods.
Figure 1.
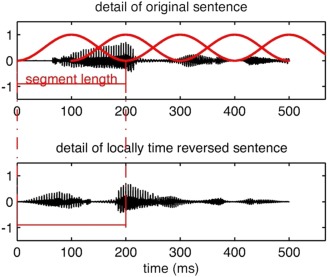
Stimulus manipulation. The top portion illustrates a detail of the waveform of an original sentence, which is multiplied by the cosine windows, and then locally time reversed. In this example, segment length is 200 ms. This procedure results in a bottom waveform. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Design and Procedure
Participants were familiarized with the stimuli outside the scanner. In addition to the sound attenuating (30 dB) MR headphones (NordicNeuroLab, Bergen, Norway), participants received protective earplugs. The headphones are equipped with high‐precision electrostatics transducers and show a flat frequency response between 8 Hz and 35 kHz. Participants were placed inside the scanner and were instructed to keep their eyes open and to look at a fixation cross. Participants saw the cross on a screen via a coil‐mounted mirror. Each trial consisted of one sentence, one probe stimulus, and the response phase. The auditory stimuli were presented in a scanner‐silent period. The response was given during the three seconds of image acquisition (see Fig. 2 for the exact timing).
Figure 2.
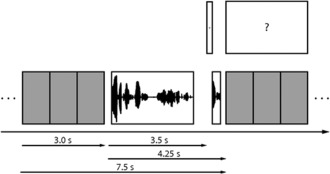
Sequence of a single trial. Gray squares: volume acquisitions; Long waveform: sentence stimulus; Short waveform: probe stimulus; +: fixation cross; ?: response screen.
Participants performed a pattern‐matching task. They were asked to press a specific button on a button‐box to indicate whether or not the probe stimulus was a sample from the original version of the sentence (right index finger for a match response, right middle finger for a no‐match response). Sentences were pseudorandomly presented over two runs, balanced across participants. Each of the five conditions (0, 100, 150, 200, 250 ms) was presented 36 times over the course of the experiment. Within each condition, 50% of the probe stimuli were matching fragments. Additionally, 36 empty baseline trials, lacking any sentence and probe stimuli, were presented. During these trials, participants were asked to randomly press a button to control for motor‐related brain activity.
MRI Data Acquisition
Data was acquired at the University Hospital Zurich on a Philips 3T Achieva whole‐body MR unit (Philips HealthCare, Best, The Netherlands) equipped with an eight‐channel head coil. Single‐shot echo‐planar images were recorded using a clustered‐sparse acquisition scheme [Liem et al., 2012aa; Schmidt et al., 2008; Zaehle et al., 2007bb]. Three volumes were acquired per cluster. In total, 648 functional brain volumes (3 × 216 trials) were collected for each participant (echo planar imaging, 16 transversal slices, in‐plane resolution = 2.75 x 2.75 mm, slice thickness = 4 mm, inter‐slice gap = 2 mm, matrix size = 80 x 80, field of view [FOV] = 220 x 220 mm, cluster‐onset asynchrony [COA] = 7.5 s, acquisition time [TA] = 1 s, echo time [TE] = 35 ms, flip angle = 68°, SENSE factor = 2). When previously using this protocol, we were able to demonstrate that a COA of 7.5 s is more advantageous than standard repetition times/COAs in sparse/clustered‐sparse acquisition schemes, which range from 10 s to 15 s [Liem et al., 2012aa]. The functional volumes covered the entire perisylvian and extrasylvian cortex. Additionally, a 3D T1‐weighted volume was acquired with a gradient echo sequence (turbo field echo, 160 saggital slices, in‐plane resolution = 0.94 x 0.94 mm, slice thickness = 1 mm, matrix size = 256 x 256, FOV = 240 x 240 mm, repetition time [TR] = 8.17 ms, TE = 3.7 ms, flip angle = 8°).
MRI Data Analysis
The T1‐weighted images were analyzed via the Freesurfer software (5.0.0; http://surfer.nmr.mgh.harvard.edu/), a) to create individual regions of interest (ROIs) for each subject, which were then employed in the functional ROI analysis and b) to obtain measurements of cortical thickness (CT) and cortical surface area [CSA; Dale et al., 1999; Dale and Sereno, 1993; Fischl and Dale, 2000; Fischl et al., 2001, 2002, 2004a, 1999a, 1999b, 2004b; Jovicich et al., 2006; Ségonne et al., 2004]. In short, Freesurfer reconstructs models of the white matter surface (border between white and gray matter) and the cortical surface (border between gray matter and cerebrospinal fluid). Subsequently, a parcellation of the cortex is performed, based on gyral and sulcal structure [Desikan et al., 2006; Fischl et al., 2004bb]2. Freesurfer provides objective, reliable [Han et al., 2006], and valid [Kuperberg et al., 2003; Rosas et al., 2002; Salat et al., 2004] surface‐based measures of cortical anatomy. Each participant's reconstruction was manually checked for accuracy. Measurements of CT and CSA were collected from the a2009s parcellation [Destrieux et al., 2010]. Volumetric ROI masks of HG, PT, and pSTG were exported into native space individually for each participant, in order to be used in the context of functional (BOLD related) ROI analysis.
Functional brain volumes were analyzed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) in a Matlab (R2009a) environment (The MathWorks, MA). We performed a whole brain analysis to check for the general integrity of our data. For the whole brain analysis, the functional volumes were corrected for head movement and coregistered onto the T1 image. The T1 image was normalized using the unified segmentation approach [Ashburner and Friston, 2005]. The resulting normalization matrix was applied to the functional volumes; thereby, transforming them into MNI space (new voxel size = 3 x 3 x 3 mm). Functional images were smoothed with an isotropic 6 mm FWHM Gaussian kernel. A general linear model was applied in which each stimulus was modeled as an event. The hemodynamic response was modeled by means of a boxcar function (Finite Impulse Response, 1st order, 3 s window length). To account for the overall T1 signal decay along the functional volumes of a single cluster, two regressors of no interest were included in the model as established by Zaehle et al. [2007bb]. Contrast images of the general linear model were entered into a random effects group analysis.
Since we had clear hypotheses with regards to the regions of interest and lateralization, the main results are presented in the ROI analysis. The ROI analysis was performed in an identical manner as the whole brain analysis, with the exception of normalization. To avoid unnecessary interpolation, the ROI analysis was performed in native space. After motion correction, coregistration, smoothing, and modeling the hemodynamic response as mentioned above, mean effect sizes (beta values) within the ROI masks were collected from the model's contrast images for each participant. ROI masks were anatomically defined and created for each subject individually via Freesurfer. We then calculated lateralization indices (LI) for the HG, PT and pSTG ROI (LI = (L ‐ R) / (L + R); possible range of LI: ‐1 to +1; positive values indicating leftward lateralization, negative values indicating lateralization to the right hemisphere; all entered raw values were positive). The LI values were entered into ANOVAs. Huynh‐Feldt correction was used when the assumption of sphericity was violated. When the ANOVAs revealed significant effects, trend analyses (1st, 2nd and 3rd order) were calculated to utilize the parametric study design.
RESULTS
Behavioral Results
The in‐scanner performance of the pattern‐matching task was calculated as the percentage of correct answers per condition. Task performance significantly decreases with increasing segment length (see Fig. 3; ANOVA for repeated measures, IV: condition, DV: percent correct; F(3, 64) = 116.53, P < 0.001, η p 2 = 0.841).
Figure 3.
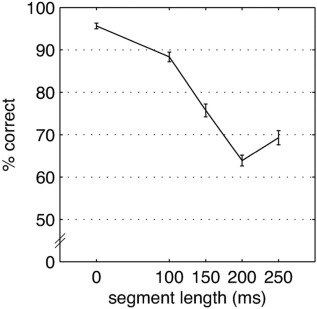
Percent of correct answers from the in‐scanner pattern‐matching task (±1 SE).
MRI Results
Whole brain analysis
In order to check the general integrity of our data, a whole brain group analysis was performed. For each condition, contrasts against the control condition made up of silent trials were calculated. These contrasts yielded bihemispheric suprathreshold activation in the posterior superior temporal lobe (FWE corrected, P < 0.05), most notably in the posterior superior temporal lobe. As these contrasts are not of primary interest, they are depicted as Supporting Information (Supporting Information Fig. 1).
ROI analysis
Functional lateralization of PT and pSTG, but not HG, depends on suprasegmental temporal integrity of stimuli
Functional lateralization indices were calculated for HG, PT and pSTG for each condition (for the raw values cf. Table 1). While the lateralization in HG is unaffected by the temporal integrity of the stimuli, lateralization in PT and pSTG is significantly influenced. In those two regions, lateralization shifts to the right with increasing segment length. This is demonstrated by a more negative LI (see Fig. 4; ANOVAs for repeated measures, IV: condition, DV: LI; HG: F(3, 74) = 2.15, ns., η p 2 = 0.089; PT: F(3, 62) = 6.79, P < 0.001, η p 2 = 0.236, linear trend: F(1, 22) = 26.44, P < 0.001, η p 2 = 0.546, higher order trends: ns.; pSTG: F(2, 52) = 12.6, P < 001, η p 2 = 0.365, linear trend: F(1, 22) = 17.23, P < 0.001, η p 2 = 0.439, higher order trends: ns.). This pattern indicates that PT and pSTG are indeed sensitive to the temporal integrity of auditory stimuli.
Table 1.
Raw values (mean beta‐values) for all conditions (segment lengths) in the ROIs
| Segment length (ms) | 0 | 100 | 150 | 200 | 250 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Regions | M | SD | M | SD | M | SD | M | SD | M | SD |
| LH | ||||||||||
| HG | 5.05 | 1.35 | 5.53 | 1.46 | 5.56 | 1.50 | 5.56 | 1.45 | 5.54 | 1.42 |
| PT | 2.54 | 1.08 | 2.73 | 1.15 | 2.54 | 1.09 | 2.61 | 1.15 | 2.59 | 1.14 |
| pSTG | 3.67 | 1.14 | 3.85 | 1.18 | 3.61 | 1.25 | 3.73 | 1.12 | 3.69 | 1.14 |
| RH | ||||||||||
| HG | 5.01 | 1.38 | 5.54 | 1.50 | 5.50 | 1.54 | 5.46 | 1.41 | 5.44 | 1.64 |
| PT | 2.82 | 1.31 | 3.26 | 1.43 | 3.20 | 1.40 | 3.29 | 1.44 | 3.36 | 1.39 |
| pSTG | 4.24 | 1.00 | 4.56 | 1.12 | 4.45 | 1.07 | 4.57 | 0.96 | 4.62 | 1.00 |
HG, Heschl's gyrus; PT, planum temporale; pSTG, posterior superior temporal gyrus. N = 23.
Figure 4.
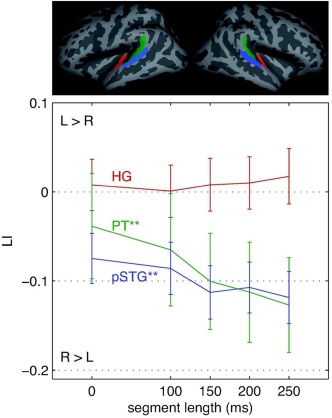
Here, the functional lateralization index (LI; ± 1 SE) in anatomically defined ROIs is displayed; positive values indicate L > R lateralization; HG, Heschl's gyrus (red); PT, planum temporale (green); pSTG, posterior superior temporal gyrus (blue). **P < 0.001.
PT anatomy predicts task performance
To explore to what extent the anatomical asymmetry of the PT may influence the performance in the pattern‐matching task, LIs for CTPT and CSAPT were calculated (for the raw values cf. Table 2). LIs for CTPT and CSAPT did not exhibit sex differences and did not correlate with age or brain volume (CTPT: sex: t(21) = 0.16, ns., age: r = 0.07, ns., intracranial brain volume (ICV): r = 0.06, ns.; CSAPT: sex: t(21) = −0.72, ns., age: r = −0.05, ns., ICV: r = −0.06, ns.). LIs for CT and CSA were not correlated to each other (r = −0.355, ns.). Following Warrier et al. 2009, participants were split into two groups: L > R and R > L, for PT thickness and surface area, respectively (for details see Table 3). Both groups did not differ with regards to basic characteristics (CT: sex: X2(1) = .354, ns., age: t (21) = −0.457, ns., ICV: t(21) = 0.051, ns.; CSA: sex: X2(1) = 0.683, ns., age: t (21) = 0.667 ns., ICV: t(21) = 1.173, ns.). The thickness‐split groups differed significantly with respect to task performance. Participants with L > R thickness perform better than participants with the reverse lateralization pattern (see Fig. 5; ANOVA, between‐subjects IV: LICT, PT group, within‐subjects IV: condition, DV: LICT, PT; F group(1, 21) = 5.65, P < 0.05, ηp 2 = .212, F condition(3, 67) = 111.17, P < 0.001, η p 2 = 0.841, F group X condition(3, 67) = 1.82, ns., η p 2 = 0.080). A closer inspection of the data revealed that the relationship between anatomical asymmetry and behavioral scores is not linear (Pearson correlation between LICT, PT and mean correct answers: r = 0.26, ns.). Instead, as Supporting Information Figure 2 shows, there seems to exist a categorical difference between R > L and L > R subjects with a lower mean and a larger variability of behavioral performance in the R > L group.
Table 2.
Raw values from surface‐based morphometry analysis for the planum temporale
| CT (mm) | CSA (mm2) | ||||||
|---|---|---|---|---|---|---|---|
| LH | RH | LH | RH | ||||
| M | SD | M | SD | M | SD | M | SD |
| 2.66 | 0.19 | 2.69 | 0.18 | 675.30 | 116.58 | 596.65 | 139.90 |
CT, cortical thickness; CSA, cortical surface area. N = 23.
Table 3.
Anatomical asymmetry of PT in groups split according to lateralization
| CT | CSA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L>R group | R > L group | L>R group | R > L group | ||||||||
| N | LI | SD | N | LI | SD | N | LI | SD | N | LI | SD |
| 9 | 0.03 | 0.02 | 14 | ‐0.03 | 0.03 | 17 | 0.12 | 0.08 | 6 | ‐0.08 | 0.06 |
CT, cortical thickness; CSA, cortical surface area; LI, lateralization index.
Figure 5.
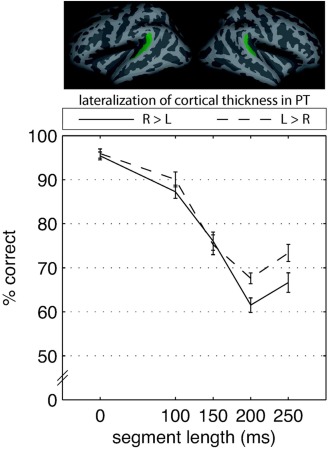
Here, the percent of correct answers from the in‐scanner pattern‐matching task (±1 SE) is depicted separately for the two groups of cortical thickness lateralization in planum temporale (PT). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
No significant group‐related effects could be found for CSA (F group(1, 21) = 0.07, ns., η p 2 = 0.003, F condition(3, 62) = 88.25, P < 0.001, η p 2 = 0.808, F group X condition(3, 62) = 1.08, ns., η p 2 = 0.049).
Relationship between PT function and task performance
To assess the relationship between functional lateralization in PT and task performance, we correlated the mean task performance with the functional lateralization in PT for all five conditions separately. This revealed only one significant correlation and two trends towards a significant correlation (r 0ms = −0.36, P < 0.1; r 100ms = −0.46, P < 0.05; r 150ms = −0.25, ns.; r 200ms = −0.37, P < 0.1; r 250ms = −0.29, ns.). Here, functional lateralization to the right is associated with better task performance.
Relationship between PT anatomy, function and task performance
Finally, we calculated correlations and partial correlations between PT anatomical asymmetry (for cortical thickness and surface area separately), PT functional lateralization (mean of all five conditions) and behavioral performance (mean percent correct). None of the correlations or partial correlations reached statistical significance. However, it has to be noted that CT seems to be more closely related to behavior and function than CSA. Furthermore, the (nonsignificant) correlation coefficient between cortical thickness asymmetry and functional lateralization was negative; hinting at an inverse relationship between lateralization of structure and function (cf. Supporting Information Fig. 3).
DISCUSSION
This study investigated the AST framework's central hypothesis pertaining to functional lateralization in HG, PT, and pSTG [Poeppel, 2001, 2003]. In a parametric event‐related clustered‐sparse fMRI design, auditory sentences were presented with varying degrees of suprasegmental temporal integrity. Whereas functional lateralization in HG was not present and was actually independent of temporal integrity, a functional shift to the right hemisphere was observed in PT and pSTG when diminishing temporal information with increasing time windows. These findings corroborate the AST framework's notion of a symmetrical representation of the auditory stream in primary auditory cortex and asymmetrical representation in nonprimary auditory fields. While left nonprimary auditory fields preferentially process rapidly changing acoustic cues, right nonprimary fields are supposed to show a preference for slowly changing cues [Poeppel, 2001, 2003]. To our knowledge, this is the first study testing the AST's prediction in the context of an fMRI design that employs parametrically manipulated spoken sentences as stimuli. Previous studies using carefully composed sound stimuli [e.g., Boemio et al., 2005; Overath et al., 2008] could not show a clear lateralization in PT or the superior temporal plane. The PT has been termed “computational hub” for complex sound processing that compares the auditory input signal with already stored templates [Griffiths and Warren, 2002]. Previous studies have found that the PT is critically involved both in basic auditory and speech processing [Brechmann and Scheich, 2005; Meyer et al., 2002, 2004, 2005; Obleser et al., 2007bb, 2008; Xu et al., 2006; Zaehle et al., 2004].
One might be concerned that our results may be driven by a manipulation of “intelligibility,” not the manipulation of acoustic cues per se. However, this reasoning contradicts recent evidence that attributed “intelligibility” to the anterior temporal lobe, either in the left or both hemispheres [Friederici et al., 2010; Obleser et al., 2007ba; Scott et al., 2000]. Therefore, there is no indication that effects in the regions we investigated (HG, PT, pSTG) were driven by variation of “intelligibility”.
We also explored individual differences in cortical anatomy and the relationship to auditory task performance. Recent studies have provided insightful evidence for structural‐behavioral relationship in the auditory‐related cortex [e.g. Golestani et al., 2007; Wong et al., 2008]. In the present experiment, lateralization of cortical thickness and surface area in PT was assessed separately as it has been shown that these parameters should be considered independently [Lyttelton et al., 2009]. Anatomical PT measurements collected from our sample are well in line with previous reports. A quantitative review by Shapleske et al. 1999 showed that an overwhelming majority of studies report a leftward asymmetry of planum temporale surface area. This review shows that, across studies, the mean portion of subjects demonstrating leftward PT asymmetry is around 78 percent. This fits well with our study where 74 percent of subjects demonstrated leftward surface area asymmetry. Regarding the asymmetry of cortical thickness measures in PT, studies are surprisingly scarce. A study examining post‐mortem brains found a rightward lateralization of cortical thickness in the PT [Harasty et al., 2003]. Around 71 percent of the post‐mortem brains demonstrated rightward asymmetry of PT thickness. This is in agreement with our sample where 61 percent of subjects demonstrated a rightward asymmetry. Our results show that while the lateralization of surface area did not predict task performance, the lateralization of cortical thickness did; subjects with L > R lateralization performed better at the auditory pattern‐matching task than subjects with R > L lateralization, especially if temporal integrity was severely diminished. It has to be noted that the relationship between the asymmetry of cortical thickness and task performance is not a linear one. It rather seems that there exist a threshold phenomenon, in that rightward CT asymmetry, per se, results in worse task performance.
As cortical thickness in auditory‐related cortex tends to be right‐lateralized [Harasty et al., 2003] and the left auditory‐related cortex exhibits stronger myelination than the right [Sigalovsky et al., 2006], one might argue that subjects with a left‐lateralized cortical thickness, thus thinner cortex on the right, might show larger right‐hemispheric myelination than subjects with a rightward thickness asymmetry. One might wonder whether this constellation might better support the auditory analysis of suprasegmental cues. Reports of a relationship between cortical thickness in the superior temporal lobes and proficiency in auditory tasks or expertise are relatively scarce [e.g., Bermudez et al., 2009; Foster and Zatorre, 2010]. Therefore, this topic deserves more attention in future studies.
The correlational analysis of hemodynamic response and task performance yielded a mixed picture. Better task performance tended to be associated with a rightward functional lateralization in the planum temporale. This suggest that a more right‐lateralized hemodynamic response better supports suprasegmental analyses required by the task.
Finally, correlation analysis of anatomical asymmetry in PT, functional PT lateralization and task performance did not yield significant results. Nevertheless, this analysis suggests, first, that lateralization of cortical thickness might have a closer relationship to behavior and the lateralization of hemodynamic response than cortical surface area. Second, if anything, lateralization of cortical thickness and hemodynamic response seem to be inversely related. That is, left‐lateralized thickness tends to be associated with right‐lateralized function. This is in line with a study by Lu et al. 2009 which found an inverse relationship of cortical thickness and hemodynamic response during an orthographic task. Furthermore, a recent study showed that thinner cortex in auditory‐related regions is connected to stronger electrophysiological response during auditory perception [Liem et al., 2012bb]. The inverse direction of this relationship stands in contrast to the report by Warrier et al. 2009. However, it has to be considered that these authors demonstrated a correlation between cortical volume and extent of the functional response. As we reported cortical thickness and effect sizes of functional response, these results are difficult to compare directly.
CONCLUSIONS
In conclusion, we have presented further evidence for the validity of the AST hypothesis in particular with regards to the symmetrical representation of suprasegmental speech in the primary auditory cortex and the asymmetric representation of speech in posterior non‐primary auditory cortex. Moreover, we have shown a relationship between asymmetry of planum temporale cortical thickness and behavioral performance during an auditory pattern‐matching task.
Supporting information
Supporting Information
Supporting Information Video 1.
Supporting Information Video 2.
Supporting Information Video 3.
Supporting Information Video 4.
Supporting Information Video 5.
ACKNOWLEDGMENTS
The authors are indebted to Sarah McCourt Meyer for her helpful comments on this manuscript, Bianca Riesner, Katharina Rufener and Anita Wildi for their help with data acquisition, and Kerry M. M. Walker for sharing the stimulus manipulation procedure.
References
- Annett M (1970): A classification of hand preference by association analysis. Br J Psychol 61:303–321. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005): Unified segmentation. NeuroImage 26:839–851. [DOI] [PubMed] [Google Scholar]
- Belin P, Zilbovicius M, Crozier S, Thivard L, Fontaine A, Masure MC, Samson Y (1998): Lateralization of speech and auditory temporal processing. J Cogn Neurosci 10:536–540. [DOI] [PubMed] [Google Scholar]
- Bermudez P, Lerch JP, Evans AC, Zatorre RJ (2009): Neuroanatomical correlates of musicianship as revealed by cortical thickness and voxel‐based morphometry. Cereb Cortex 19:1583–1596. [DOI] [PubMed] [Google Scholar]
- Best CT, Morrongiello B, Robson R (1981): Perceptual equivalence of acoustic cues in speech and nonspeech perception. Percept Psychophys 29:191–211. [DOI] [PubMed] [Google Scholar]
- Boemio A, Fromm S, Braun A, Poeppel D (2005): Hierarchical and asymmetric temporal sensitivity in human auditory cortices. Nat Neurosci 8:389–395. [DOI] [PubMed] [Google Scholar]
- Brechmann A, Scheich H (2005): Hemispheric shifts of sound representation in auditory cortex with conceptual listening. Cereb Cortex 15:578–587. [DOI] [PubMed] [Google Scholar]
- Britton B, Blumstein SE, Myers EB, Grindrod C (2009): The role of spectral and durational properties on hemispheric asymmetries in vowel perception. Neuropsychologia 47:1096–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI (1999): Cortical surface‐based analysis. I. Segmentation and surface reconstruction. NeuroImage 9:179–194. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI (1993): Improved localizadon of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: A linear approach. J Cogn Neurosci 5:162–176. [DOI] [PubMed] [Google Scholar]
- Davis MH, Johnsrude IS (2003): Hierarchical processing in spoken language comprehension. J Neurosci 23:3423–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006): An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31:968–980. [DOI] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E (2010): Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage 53:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron R (1963): Temporal Perception, Aphasia and Déjà Vu. Brain 86:403–424. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM (2000): Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM (2001): Automated manifold surgery: Constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging 20:70–80. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002): Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJW, Makris N, Ségonne F, Quinn BT, Dale AM (2004a): Sequence‐independent segmentation of magnetic resonance images. NeuroImage 23 (Suppl 1):S69–S84. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM (1999a): Cortical surface‐based analysis. II. Inflation, flattening, and a surface‐based coordinate system. NeuroImage 9:195–207. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM (1999b): High‐resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp 8:272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM (2004b): Automatically parcellating the human cerebral cortex. Cereb Cortex 14:11–22. [DOI] [PubMed] [Google Scholar]
- Foster NEV, Zatorre RJ (2010): Cortical structure predicts success in performing musical transformation judgments. NeuroImage 53:26–36. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Kotz SA, Scott SK, Obleser J (2010): Disentangling syntax and intelligibility in auditory language comprehension. Hum Brain Mapp 31:448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandour J, Dzemidzic M, Wong D, Lowe M, Tong Y, Hsieh L, Satthamnuwong N, Lurito J (2003): Temporal integration of speech prosody is shaped by language experience: An fMRI study. Brain Lang 84:318–336. [DOI] [PubMed] [Google Scholar]
- Geiser E, Zaehle T, Jancke L, Meyer M (2008): The neural correlate of speech rhythm as evidenced by metrical speech processing. J Cogn Neurosci 20:541–552. [DOI] [PubMed] [Google Scholar]
- Giraud A‐L, Kleinschmidt A, Poeppel D, Lund TE, Frackowiak RSJ, Laufs H (2007): Endogenous cortical rhythms determine cerebral specialization for speech perception and production. Neuron 56:1127–1134. [DOI] [PubMed] [Google Scholar]
- Giraud A‐L, Poeppel D (2012): Cortical oscillations and speech processing: Emerging computational principles and operations. Nat Neurosci 15:511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golestani N, Molko N, Dehaene S, Lebihan D, Pallier C (2007): Brain structure predicts the learning of foreign speech sounds. Cereb Cortex 17:575–582. [DOI] [PubMed] [Google Scholar]
- Griffiths TD, Warren JD (2002): The planum temporale as a computational hub. Trends Neurosci 25:348–353. [DOI] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Busa E, Pacheco J, Albert M, Killiany R, Maguire P, Rosas D, Makris N, Dale A, Dickerson B, Fischl B (2006): Reliability of MRI‐derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. NeuroImage 32:180–194. [DOI] [PubMed] [Google Scholar]
- Harasty J, Seldon HL, Chan P, Halliday G, Harding A (2003): The left human speech‐processing cortex is thinner but longer than the right. Laterality Asymmetries Body Brain Cogn 8:247–260. [DOI] [PubMed] [Google Scholar]
- Hurschler MA, Liem F, Jancke L, Meyer M (2012): Right and left perisylvian cortex and left inferior frontal cortex mediate sentence‐level rhyme detection in spoken language as revealed by sparse fMRI. Hum Brain Mapp, ahead of print. doi: 10.1002/hbm.22134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison HL, Watkins KE, Bishop DVM, Matthews PM (2006): Hemispheric specialization for processing auditory nonspeech stimuli. Cereb Cortex 16:1266–1275. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Wüstenberg T, Scheich H, Heinze H‐J (2002): Phonetic perception and the temporal cortex. NeuroImage 15:733–746. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, Kennedy D, Schmitt F, Brown G, Macfall J, Fischl B, Dale A (2006): Reliability in multi‐site structural MRI studies: Effects of gradient non‐linearity correction on phantom and human data. NeuroImage 30:436–443. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SCR, van der Kouwe AJW, Salat DH, Dale AM, Fischl B (2003): Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry 60:878–888. [DOI] [PubMed] [Google Scholar]
- Liem F, Lutz K, Luechinger R, Jäncke L, Meyer M (2012a): Reducing the interval between volume acquisitions improves “sparse” scanning protocols in event‐related auditory fMRI. Brain Topogr 25:182–193. [DOI] [PubMed] [Google Scholar]
- Liem F, Zaehle T, Burkhard A, Jancke L, Meyer M (2012b): Cortical thickness of supratemporal plane predicts auditory N1 amplitude. NeuroReport 23:1026–1030. [DOI] [PubMed] [Google Scholar]
- Liégeois‐Chauvel C, de Graaf JB, Laguitton V, Chauvel P (1999): Specialization of left auditory cortex for speech perception in man depends on temporal coding. Cereb Cortex 9:484–496. [DOI] [PubMed] [Google Scholar]
- Lu LH, Dapretto M, O'Hare ED, Kan E, McCourt ST, Thompson PM, Toga AW, Bookheimer SY, Sowell ER (2009): Relationships between brain activation and brain structure in normally developing children. Cereb Cortex 19:2595–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyttelton OC, Karama S, Ad‐Dab'bagh Y, Zatorre RJ, Carbonell F, Worsley K, Evans AC (2009): Positional and surface area asymmetry of the human cerebral cortex. NeuroImage 46:895–903. [DOI] [PubMed] [Google Scholar]
- McGettigan C, Scott SK (2012): Cortical asymmetries in speech perception: What's wrong,what's right and what's left? Trends Cogn Sci 16:269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Alter K, Friederici AD, Lohmann G, Cramon von DY (2002): FMRI reveals brain regions mediating slow prosodic modulations in spoken sentences. Hum Brain Mapp 17:73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Steinhauer K, Alter K, Friederici AD, Cramon von DY (2004): Brain activity varies with modulation of dynamic pitch variance in sentence melody. Brain Lang 89:277–289. [DOI] [PubMed] [Google Scholar]
- Meyer M, Zaehle T, Gountouna V‐E, Barron A, Jancke L, Turk A (2005): Spectro‐temporal processing during speech perception involves left posterior auditory cortex. NeuroReport 16:1985–1989. [DOI] [PubMed] [Google Scholar]
- Obleser J, Eisner F, Kotz SA (2008): Bilateral speech comprehension reflects differential sensitivity to spectral and temporal features. J Neurosci 28:8116–8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obleser J, Wise RJS, Alex Dresner M, Scott SK (2007a): Functional integration across brain regions improves speech perception under adverse listening conditions. J Neurosci 27:2283–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obleser J, Zimmermann J, Van Meter J, Rauschecker JP (2007b): Multiple stages of auditory speech perception reflected in event‐related FMRI. Cereb Cortex 17:2251–2257. [DOI] [PubMed] [Google Scholar]
- Overath T, Kumar S, Kriegstein von K, Griffiths TD (2008): Encoding of spectral correlation over time in auditory cortex. J Neurosci 28:13268–13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeppel D (2001): Pure word deafness and the bilateral processing of the speech code. Cogn Sci 25:679–693. [Google Scholar]
- Poeppel D (2003): The analysis of speech in different temporal integration windows: cerebral lateralization as “asymmetric sampling in time. ” Speech Commun 41:245–255. [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B (2002): Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology 58:695–701. [DOI] [PubMed] [Google Scholar]
- Rosen S (1992): Temporal information in speech: Acoustic, auditory and linguistic aspects. Philos Trans R Soc Lond, B, Biol Sci 336:367–373. [DOI] [PubMed] [Google Scholar]
- Saberi K, Perrott DR (1999): Cognitive restoration of reversed speech. Nature 398:760. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E, Morris JC, Dale AM, Fischl B (2004): Thinning of the cerebral cortex in aging. Cereb Cortex 14:721–730. [DOI] [PubMed] [Google Scholar]
- Schmidt CF, Zaehle T, Meyer M, Geiser E, Boesiger P, Jancke L (2008): Silent and continuous fMRI scanning differentially modulate activation in an auditory language comprehension task. Hum Brain Mapp 29:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönwiesner M, Rübsamen R, Cramon von DY (2005): Hemispheric asymmetry for spectral and temporal processing in the human antero‐lateral auditory belt cortex. Eur J Neurosci 22:1521–1528. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Tallal P (1980): Rate of acoustic change may underlie hemispheric specialization for speech perception. Science 207:1380–1381. [DOI] [PubMed] [Google Scholar]
- Scott SK, Blank CC, Rosen S, Wise RJ (2000): Identification of a pathway for intelligible speech in the left temporal lobe. Brain 123 ( Part 12):2400–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ségonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B (2004): A hybrid approach to the skull stripping problem in MRI. NeuroImage 22:1060–1075. [DOI] [PubMed] [Google Scholar]
- Shapleske J, Rossell SL, Woodruff PW, David AS (1999): The planum temporale: a systematic, quantitative review of its structural, functional and clinical significance. Brain Res Brain Res Rev 29:26–49. [DOI] [PubMed] [Google Scholar]
- Sigalovsky IS, Fischl B, Melcher JR (2006): Mapping an intrinsic MR property of gray matter in auditory cortex of living humans: A possible marker for primary cortex and hemispheric differences. NeuroImage 32:1524–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slevc LR, Martin RC, Hamilton AC, Joanisse MF (2011): Speech perception, rapid temporal processing, and the left hemisphere: A case study of unilateral pure word deafness. Neuropsychologia 49:216–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studdert‐Kennedy M, Shankweiler D (1970): Hemispheric specialization for speech perception. J Acoust Soc Am 48:579–594. [DOI] [PubMed] [Google Scholar]
- Tallal P, Miller SL, Bedi G, Byma G, Wang X, Nagarajan SS, Schreiner C, Jenkins WM, Merzenich MM (1996): Language comprehension in language‐learning impaired children improved with acoustically modified speech. Science 271:81–84. [DOI] [PubMed] [Google Scholar]
- Walker KMM, Ahmed B, Schnupp JWH (2008): Linking cortical spike pattern codes to auditory perception. J Cogn Neurosci 20:135–152. [DOI] [PubMed] [Google Scholar]
- Warrier C, Wong P, Penhune V, Zatorre R, Parrish T, Abrams D, Kraus N (2009): Relating structure to function: Heschl's gyrus and acoustic processing. J Neurosci 29:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PCM, Warrier CM, Penhune VB, Roy AK, Sadehh A, Parrish TB, Zatorre RJ (2008): Volume of left Heschl's Gyrus and linguistic pitch learning. Cereb Cortex 18:828–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Gandour J, Talavage T, Wong D, Dzemidzic M, Tong Y, Li X, Lowe M (2006): Activation of the left planum temporale in pitch processing is shaped by language experience. Hum Brain Mapp 27:173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaehle T, Jancke L, Herrmann CS, Meyer M (2009): Pre‐attentive spectro‐temporal feature processing in the human auditory system. Brain Topogr 22:97–108. [DOI] [PubMed] [Google Scholar]
- Zaehle T, Jancke L, Meyer M (2007a): Electrical brain imaging evidences left auditory cortex involvement in speech and non‐speech discrimination based on temporal features. Behav Brain Funct BBF 3:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaehle T, Schmidt CF, Meyer M, Baumann S, Baltes C, Boesiger P, Jancke L (2007b): Comparison of “silent” clustered and sparse temporal fMRI acquisitions in tonal and speech perception tasks. NeuroImage 37:1195–1204. [DOI] [PubMed] [Google Scholar]
- Zaehle T, Wüstenberg T, Meyer M, Jäncke L (2004): Evidence for rapid auditory perception as the foundation of speech processing: A sparse temporal sampling fMRI study. Eur J Neurosci 20:2447–2456. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Belin P (2001): Spectral and temporal processing in human auditory cortex. Cereb Cortex 11:946–953. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Belin P, Penhune VB (2002): Structure and function of auditory cortex: Music and speech. Trends Cogn Sci 6:37–46. [DOI] [PubMed] [Google Scholar]
- Zhang L, Shu H, Zhou F, Wang X, Li P (2010): Common and distinct neural substrates for the perception of speech rhythm and intonation. Hum Brain Mapp 31:1106–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurif E, Mendelsohn M (1972): Hemispheric specialization for perception of speech sounds: Influence of intonation and structure. Percept Psychophys 11:329–332. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information Video 1.
Supporting Information Video 2.
Supporting Information Video 3.
Supporting Information Video 4.
Supporting Information Video 5.


