Abstract
The association between functional activation and gray matter (GM) structure has been revealed in clinical studies and studies of aging involving a small number of subjects. The purpose of this study was to investigate the association between functional activation maps and GM structures in young adults who do not show apparent GM atrophy and to investigate in detail the nature of this association using a large number of subjects. We used voxel‐by‐voxel regression analyses to investigate voxel‐by‐voxel associations between GM concentration (GMC) and contrast estimate images of brain activity during n‐back working memory tasks. Associations were assessed for each voxel after regressing out the effects of age, sex, and mean signal intensity during functional magnetic resonance imaging scanning at each voxel using data from 248 normal, right‐handed, young adult subjects. In our study, the concept of “the greater the GMC, the greater the task‐related activation increase/task‐related activation decrease (or the greater the task‐related activation change from baseline)” was true for a wide range of activated and deactivated areas. However, in some minor regions, the other pattern of “the greater the GMC, the smaller the task‐related activation increase” was observed. The first pattern is often observed at the borders of GM structures. These findings may have to be taken into consideration when group/individual differences in functional activation are investigated. Hum Brain Mapp 35:185–198, 2014. © 2012 Wiley Periodicals, Inc.
Keywords: working memory, gray matter, brain activation, deactivation
INTRODUCTION
Group differences in functional activation are sometimes observed in regions with group differences in gray matter (GM) structure [e.g., Hayasaka et al., 2006]. Furthermore, a positive correlation between functional activation and certain cognitive functions is sometimes observed in regions where a positive correlation between GM structure and certain cognitive functions is reported. In case of working memory (WM) performance, regions in the lateral prefrontal cortex (PFC) and parietal cortex, especially the inferior parietal lobule and intraparietal lobule, are involved with the WM system [Takeuchi et al., 2010a] and are activated during WM tasks. The activities of these regions are correlated with WM performance [Callicott et al., 1999; Klingberg, 2006; Olesen et al., 2003]. Previous anatomical studies revealed that amount of GM in PFC and the parietal regions is correlated with WM performance in clinical and nonclinical populations [Amici et al., 2007; Antonova et al., 2005; Takeuchi et al., 2011d, 2011f].
These overlaps of functional and structural differences can be due to underlying common physiological mechanisms. In particular, blood‐oxygen‐level‐dependent (BOLD) responses in functional magnetic resonance imaging (fMRI) correlate well with local field potentials (LFPs) [Logothetis et al., 2001]. LFP is mostly a weighted average of synchronized dendrosomatic components of the input signals of a neural population [Juergens et al., 1999; Mitzdorf, 1987], and potential correlates of size of GM include the number and size of neurons and glias, the level of synaptic bulk, and the number of neurites [Draganski et al., 2004; May and Gaser, 2006]. Thus, increase in neuronal structures in a certain region may lead to an increase in GM structures and functional activation. On the other hand, these overlaps may be caused by the extent of GM structures alone. In other words, differences in functional activations may be caused by the differences in the extent of GM areas where functional activities occur, instead of the differences in the magnitude of functional activities in GM areas of the same extent. In the normalized images, the extent of GM corresponds to GM concentration (GMC; relative GM existence probability compared with that of the white matter and cerebrospinal fluid). fMRI typically has higher spatial resolution compared with other functional imaging methods such as positron emission tomography, but because the images are smoothed when analyzed, the problem remains the same.
In studies of functional activation using fMRI that investigate group or individual differences, the effects of GM structure are not usually considered, and thus, the extent and nature of the effect of GM structure on functional activation is a matter of interest.
An association between functional activation and GM structure has been revealed in clinical studies using a small number of subjects [Peiffer et al., 2009] as well as in studies of aging [Hayasaka et al., 2006]. These studies showed some overlaps as well as differences between regions of age‐related functional activation decrease and those of age‐related GM decrease. However, (a) whether these associations between functional activation maps and GM structures are also observed in normal young adults who do not show apparent GM atrophy, (b) whether the association between functional activation maps and GM structures is universal, and (c) what the nature of this association between functional activation and GM structure is (such as where it is stronger in normal subjects) remain unknown. It is important to investigate this issue in normal young adults because they are the ones who often become the subjects of typical fMRI studies. As for (a), GM structures among patients of advanced age are characterized by advanced cortical thinning [Taki et al., 2004]) and those of young adults may well be different. In addition, in young adults, while superior intellectual abilities are often associated with decreased functional activation in the relevant region, they are also often associated with more GM structures in the relevant regions [Neubauer and Fink, 2009]. Thus, patterns other than “the greater the GMC, the greater the task‐related activation increase” (Fig. 1a) may be observed in young adults.
Figure 1.
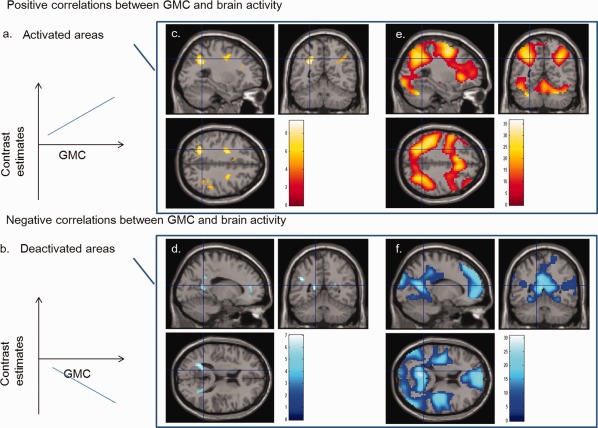
Patterns confirming to the statement “the greater the GMC, the greater the task‐related activation increase/task‐related activation decrease” (or the greater the task‐related activation change from baseline). (a) A schematic of positive correlation between GMC and brain activity in activated areas (the greater the GMC, the greater the task‐related activation increase). (b) A schematic of negative correlation between GMC and brain activity in deactivated areas (the greater the GMC, the greater the task‐related activation decrease). (c) Regions with a significant positive correlation between brain activity during the 2‐back task and GMC (P < 0.05, corrected for voxel‐level FWE). The regions with correlation were overlaid on a single subject T 1 image of SPM5. GMC was significantly and positively correlated with brain activity during the 2‐back task in the anatomical cluster around the left inferior parietal lobule together with other regions. (d) Regions with significant negative correlations between brain activity during the 2‐back task and GMC (P < 0.05, corrected for voxel‐level FWE). The regions with correlations were overlaid on a T 1 SPM5 image of a single subject. GMC was significantly and negatively correlated with brain activity during the 2‐back task in the anatomical cluster that spread around the left precuneus together with other regions. (e) Regions that were activated during the 2‐back task are displayed at a height threshold of 0.05, corrected for voxel‐level FWE. Note that the voxels of positive correlation observed in (c) are included in the regions that were activated during the 2‐back task. (f) Regions that were deactivated during the 2‐back task are displayed at a height threshold of 0.05, corrected for voxel‐level FWE. Note that the regions with a negative correlation observed in (d) are largely located at the borders of the regions that were deactivated during the 2‐back task.
The purpose of this study was to investigate these issues. We used voxel‐by‐voxel regression analyses to investigate voxel‐by‐voxel associations between GMC and contrast estimate images of brain activity during tasks after regressing out the effects of age, sex, and mean signal intensity during fMRI scanning at each voxel using data from 248 normal young adult subjects. fMRI was used to map task‐induced activation changes. The n‐back task, a typical task in fMRI studies that has 0‐back (simple cognitive processes) and 2‐back (WM) conditions, was used. The n‐back task consistently recruits brain regions involved in a wide range of externally directed attention‐demanding tasks and deactivates brain regions that are deactivated by a wide range of externally directed attention‐demanding tasks [Buckner et al., 2008; Owen, 2000].
We hypothesized that the concept of “the greater the GMC, the greater the task‐related activation increase/task‐related activation decrease (or the greater the task‐related activation change from baseline)” (Fig. 1a,b) holds true for a wide range of activated and deactivated areas.
METHODS
Subjects
Two hundred forty‐eight healthy, right‐handed individuals (126 men and 122 women) participated in this study as part of our ongoing project investigating associations among brain imaging, cognitive functions, and aging [Takeuchi et al., 2010b, 2010c, 2011b, in press; Taki et al., 2010], and data from some of the subjects in this study was used in another study [Takeuchi et al., 2011b]. Data derived from subjects in this project will be used in future studies. Furthermore, some of the subjects who participated in this project also became subjects of our intervention studies (psychological and imaging data recorded before intervention were used in this study) [Takeuchi et al., 2011a, in press]. Psychological tests and MRI scanning not described in this study were performed as part of our ongoing project. The mean age of the subjects was 21.1 years [standard deviation, 1.8]. All subjects were university students or postgraduates. All subjects had normal vision and none had a history of neurological or psychiatric illnesses. Handedness was evaluated using the Edinburgh Handedness Inventory [Oldfield, 1971], and we used scores of > 0 as the cut‐off value.
fMRI Tasks
fMRI was used to map task‐related activations changes during typical cognitive tasks. The n‐back task was performed during fMRI scanning, as described in our previous study [Takeuchi et al., 2011a, 2011b]. Participants received instructions and practiced the tasks before entering the scanner. During scanning, they viewed stimuli on a screen via a mirror mounted on a head coil. Visual stimuli were presented using Presentation software (Neurobehavioral Systems, Albany, CA). A fiber‐optic, light‐sensitive key press interface with a button box was used to record participants' behavior.
We used a simple block design and the n‐back WM task [Callicott et al., 1999] to tap brain activities during the WM task. There were two conditions (0‐ and 2‐back). Each condition had six blocks, and all n‐back tasks were performed in one session. The subjects were instructed to recall stimuli [visually presented four types of Japanese letters) seen “n” times previously (e.g., two letters shown previously for the 2‐back task and the currently presented letter for the 0‐back task). Two buttons were used during the 0‐back task, and the subjects were asked to push the first button when the target stimuli were presented and the second button when the other stimuli were presented. During the 2‐back task, the subjects were asked to push the first button when the currently presented stimuli and the stimuli presented two letters previously were the same and to push the second button when the currently presented stimuli and the stimuli presented two letters previously were different. Our version of the n‐back task was designed to require individuals to push buttons continuously during the task period. The task level of the memory load was found to be above the stimuli for 2 s before the task started (cue phase) and remained unchanged during the task period. Each letter was presented for 0.5 s, and a fixation cross was presented for 1.5 s between each item. Each block consisted of 10 stimuli. Thus, each block lasted for 20 s. A baseline fixation cross was presented for 13 s between the task and the presentation of the next condition's task level (2 s). Thus, the rest period lasted for 15 s. There were six blocks for each 2‐ and 0‐back condition.
Image Acquisition and Analysis
All MRI data acquisition was performed with a 3‐T Philips Achieva scanner. Using an magnetization‐prepared rapid gradient‐echo (MPRAGE) sequence, high‐resolution T 1‐weighted structural images (240 × 240 matrix, repetition time (TR) = 6.5 ms, echo time (TE) = 3 ms, field of view (FOV) = 24 cm, 162 slices, 1.0 mm slice thickness) were collected. Forty‐two transaxial gradient‐echo images (echo time = 30 ms, flip angle = 90°, slice thickness = 3 mm, FOV = 192 mm, matrix = 64 × 64) covering the entire brain were acquired at a repetition time of 2.5 s using an echo planar sequence. For the n‐back session, 174 functional volumes were obtained. Three images without diffusion weighting (b value = 0 s/mm2; b = 0 images) were obtained from 176 subjects and one b = 0 image was obtained from 72 subjects; the b = 0 images were acquired using a spin‐echo echo‐planar imaging (EPI) sequence (TR = 10,293 ms, TE = 55 ms, FOV = 22.4 cm, 2 × 2 × 2‐mm3 voxels, 60 slices). These b = 0 images were acquired from the scans used to obtain diffusion weighted images [see Takeuchi et al., 2010c for details]. A mean of the three b = 0 images (for the 176 subjects) or the single b = 0 image (for the 72 subjects) was used for preprocessing the imaging data.
Preprocessing and Individual Level Functional Imaging Data Analysis
Preprocessing and data analysis were performed using statistical Parametric Mapping software (SPM5; Wellcome Department of Cognitive Neurology, London, UK) and implemented in Matlab (Mathworks, Natick, MA), as described in our previous study [Takeuchi et al., 2011b]. Before the analysis, the BOLD images were realigned and resliced to fit the mean image of the series and corrected for slice timing. The skull and skin parts of the mean image of all BOLD images of each subject were then stripped by masking the raw BOLD images of each subject with a threshold of given signal intensity in the spatially smoothed [8‐mm full width at half maximum (FWHM)] mean image of all BOLD images of each subject. By doing this, we were able to delete the skin and skull parts of the images but not the brain parenchyma parts [Takeuchi et al., 2011b]. The first smoothing for skull stripping was performed to make mask images for skull stripping the unsmoothed BOLD images. Thus, the processed BOLD images that were used in the next processing step were unsmoothed images.
The masked BOLD images were coregistered to a skull‐stripped b = 0 image, which was created by a method similar to the one used for making skull‐stripped BOLD images, and they were spatially normalized onto a skull‐stripped b = 0 image template, which was created from data obtained using our scanner [Takeuchi et al., 2010c]. We did not use T 1‐weighted structural images for coregistering because coregisteration of the BOLD images taken in our studies to the T 1‐weighted structural images used in our laboratory often fails when visually inspected. This failure might possibly be caused by differences in the two images due to distortion of the BOLD images. We used b = 0 images for the normalization procedures as they had relatively higher anatomical clarity and were compatible with the BOLD images taken in this study during coregistration [Takeuchi et al., 2011b]. The normalization step resulted in BOLD images with 3 × 3 × 3 mm voxels. The flow chart illustrating these preprocessing procedures is shown in Figure 2. A mean image of the normalized and smoothed (8‐mm FWHM) images obtained during fMRI scanning was used in second‐level multiple regression analyses described below.
Figure 2.
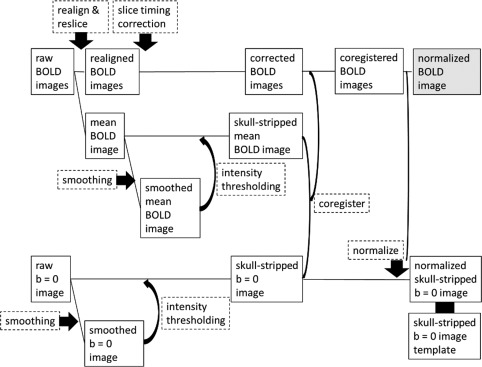
Flow chart for the preprocessing of BOLD images.
Individual‐level statistical analyses were performed using a general linear model. A design matrix was fitted to each participant with one regressor in each task condition (0‐ or 2‐back in the n‐back task) using a standard hemodynamic response function. The cue phases of the n‐back task were modeled in the same manner but were not analyzed further. Several sources of spurious correlations were regressed out by adding the following regressors: (i) six parameters obtained by rigid body correction of head motion across scans, (ii) time courses of brain signal changes in unsmoothed normalized images averaged over the brain masks of white matter and cerebrospinal fluid (CSF) areas. The brain masks of white matter and CSF consisted of areas of tissue probability > 0.95 in the SPM5 tissue probability maps for each tissue. The design matrix weighted each raw image according to its overall variability to reduce the impact of movement artifacts [Diedrichsen and Shadmehr, 2005]. We removed low‐frequency fluctuations with a high‐pass filter using a cut‐off value of 128 s. After estimation, the beta images were smoothed (8‐mm FWHM) and were used for the multiple regression analyses described below. For the fMRI analysis, contrast images from the 0‐back and 2‐back tasks compared with the resting period as well as for the 2‐back–0‐back task were estimated for each subject after preprocessing. Data from four subjects whose contrast images could not be assessed due to errors were removed from the analysis. When we used the method of Diedrichsen and Shadmehr 2005, an autoregressive AR(1) model, which accounts for serial correlations in fMRI series due to aliased biorhythms and unmodeled neuronal activity, was not used as this is the default option for this method. In low‐frequency models such as ours, neither an autoregressive model nor a high‐pass filter are necessary to prevent false positives [Della‐Maggiore et al., 2002]. In addition, the use of these substantially lowers the statistical power, and thus, they are not recommended for models such as ours [Della‐Maggiore et al., 2002].
One‐sample t‐tests using these first‐level contrast images without covariates were used to create maps of voxels that showed activation/deactivation (P < 0.05, corrected for multiple comparisons at voxel‐level family wise error (FWE) at the whole‐brain level using random field theory as implemented in SPM5). These maps were used in the Results section tables and figures to determine whether the regions that showed significant correlations with brain activity, and GMC were the same regions that were activated/deactivated during the task.
Preprocessing of T 1‐Weighted Structural Data
Preprocessing of the morphological data was performed with VBM2 software [Gaser, 2007], an extension of SPM2. Default parameter settings were used [Gaser, 2007].
To decrease the scanner‐specific bias, we used a customized GM anatomical template and prior probability maps of gray and white matter images created from the T 1‐weighted structural images taken in the same scanner used in our previous study [Takeuchi et al., 2010b]. The T 1‐weighted structural image of each subject was segmented into gray and white matter partitions using the aforementioned customized gray and white matter prior probability maps. The resulting images included extracted gray and white matter partitions in the native space. The GM partition was then normalized to the aforementioned customized GM probability map. The normalization parameters determined from this initial step were then applied to the native T 1‐weighted structural images. These normalized T 1‐weighted structural images were then segmented into gray and white matter partitions. The segmented images were then resliced into 3 × 3 × 3 mm3 voxels, which is the same voxel size as that of the normalized fMRI images. Subsequently, all images were smoothed by convolving them with an isotropic Gaussian kernel of 8‐mm FWHM. The resulting maps representing the GMC were then forwarded to the multiple regression analyses described below.
VBM2 was used for the preprocessing of T 1‐weighted structural imaging data instead of VBM5 or VBM8 because the T 1‐weighted images obtained using the aforementioned MPRAGE sequence were incompatible with preprocessing using VBM5/SPM5 and VBM8/SPM8. When VBM5 or SPM5 was used, many apparent segmentation errors occurred, unlike when the optimized protocol of VBM2 was used. Furthermore, when VBM8 was used, the test–retest reliability of the total GM volume in 50 subjects who underwent a 1‐week longitudinal intervention study in which a T 1‐weighted structural image was taken on the first day of the experiment and 1 week thereafter [Takeuchi et al., 2011a] was 0.746 (it was 0.980 when VBM2 was used). These procedures (preprocessing with VBM2 and statistical analyses with different versions of SPM/VBM) were also used in previous studies [Ilg et al., 2008; Takeuchi et al., 2010b, 2011c, 2011e]. Although the aforementioned data do not indicate preprocessing with VBM5/VBM8 is worse, they do indicate something about the compatibility between the T 1‐weighted structural images of certain sequences and VBM5/VBM8.
Although the normalization parameters differed between BOLD and T 1‐weighted structural images, this cannot be avoided for accurate normalization due to the distortion of EPI images in 3 T MRI regardless of the method used. For further details on this problem, see the Discussion section.
Statistical Analysis
To perform multimodal voxel‐wise multiple regression analyses to investigate correlations between changes of BOLD signals (task‐induced activation and task‐induced deactivation (TID)) and GMC, we used the biological parametric mapping toolbox for SPM [Casanova et al., 2007], which examines correlations between different types of images. We performed the following three analyses: an analysis of the association between 0‐back task‐related activity and GMC; an analysis of the association between 2‐back task‐related activity and GMC; and an analysis of the association between 2‐back–0‐back task‐related activity and GMC. In these multiple regression analyses, the dependent variable at each voxel was the GMC signal intensity at the voxel and the independent variables at the voxel were the beta estimate value of the contrast image of corresponding brain activity (0‐back, 2‐back, and 2‐back–0‐back tasks) at the voxel, the signal intensity of the mean smoothed and normalized image of all fMRI scans at the voxel, age, and sex. The analyses were performed in all the voxels that all subjects had signals in both of GMC maps and individual level functional activation maps. As a result, the voxels of low GMC signals were also included in the analyses if they had signals in the functional activation maps. This is because these voxels would be included if the functional activation images were analyzed in the normal manner and because whether the magnitude of functional activities in these voxels correlates with GMC is a matter of interest in this study if these voxels are included in the normal functional activation analyses. A multiple comparison correction was performed using the voxel‐level FWE approach at the whole‐brain level using random field theory as implemented in SPM5.
In the Results tables, we provided information on the proportion of the significantly correlated voxels in each cluster that belong to the area of certain GMC probability (the average GMC signal intensity of all subjects). This GMC probability map was constructed by averaging the smoothed (8‐mm FWHM) GMC maps of all subjects. We provided information on the proportion of significant clusters belonging to areas of (1) GMC probability 0–0.1, (2) GMC probability 0.1–0.2, (3) GMC probability 0.2–0.3, and (4) GMC probability ≥ 0.3 (note that because of smoothing, thin GM areas can present a not very high GMC, such as 0.3–0.4 probability, even when the voxel is located in the deep GM areas in the MNI coordinates).
RESULTS
Positive Correlation Between GMC and Brain Activity During the 0‐Back Task
After controlling for sex, age, and mean BOLD signal during scanning at each voxel, multiple regression analysis revealed that brain activity during the 0‐back task at each voxel was significantly and positively correlated with GMC at the voxels in areas in and/or close to the left precentral gyrus, left superior parietal lobule, and left middle cingulate gyrus/left supplementary motor area. These regions were activated during the 0‐back task. These findings indicate that in these areas, the greater GMC is associated with a greater 0‐back task‐related activation increase (Fig. 1a; Table 1). All voxels that showed correlations of this pattern were included in areas that were activated during the task. As can be seen in Table 1, much of the significant voxels belonged to areas of low GMC (GMC value: 0.1–0.2) or medium GMC (GMC value: 0.2–0.3), suggesting that these significant clusters are located in areas in the periphery of the GM areas.
Table 1.
Brain regions with significant positive correlations between GMC and brain activity during the 0‐back task
| Area | x | y | z | T score | Corrected P value (FWE) | Cluster size (mm3) | Activated regions (%)a | Deactivated regions (%)a | GMC 0–0.1 (%) | GMC 0.1–0.2 (%) | GMC 0.2–0.3 (%) | GMC ≥ 0.3 (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgyral (close to the precentral gyrus) | L | −24 | −6 | 39 | 6.30 | < 0.001 | 486 | 100 | 0 | 55.6 | 33.3 | 11.1 | 0 |
| Superior parietal lobule | L | −24 | −57 | 42 | 6.15 | < 0.001 | 378 | 100 | 0 | 0 | 35.7 | 42.8 | 0 |
| Precentral gyrus | L | −30 | −21 | 51 | 5.60 | 0.001 | 270 | 100 | 0 | 10.0 | 30.0 | 50.0 | 0 |
| Middle cingulate gyrus | L | −12 | 12 | 42 | 4.95 | 0.015 | 135 | 100 | 0 | 0 | 0 | 100 | 0 |
Percentage of voxels that showed activation/deactivation during the 0‐back task in a one‐sample t‐test (P < 0.05, corrected for multiple comparisons at voxel‐level FWE at the whole‐brain level).
Negative Correlation Between GMC and Brain Activity During the 0‐Back Task
The analysis also revealed that brain activity during the 0‐back task at each voxel was significantly and negatively correlated with GMC at the voxels in areas in and/or close to the bilateral precuneus, left cuneus, left precentral gyrus, and left superior frontal gyrus. The voxels in the left precuneus/cuneus mainly consisted of regions that were deactivated during the 0‐back task, while the voxel in the left superior frontal gyrus was close to the areas that were deactivated during the 0‐back task. These findings indicate that in this area, the greater GMC was associated with a greater 0‐back task‐related activation decrease (Fig. 1b). These voxels tended to be located at the periphery of the deactivated areas, as in analysis of the 2‐back task. For all the results, see Table 2.
Table 2.
Brain regions with significant negative correlations between GMC and brain activity during the 0‐back task
| Area | x | y | z | T score | Corrected P value (FWE) | Cluster size (mm3) | Activated regions (%)a | Deactivated regions (%)a | GMC 0–0.1 (%) | GMC 0.1–0.2 (%) | GMC 0.2–0.3 (%) | GMC ≥ 0.3 (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cuneus/precuneus | L | −18 | −63 | 27 | 5.04 | 0.010 | 54 | 0 | 100 | 0 | 0 | 50.0 | 50.0 |
| Superior frontal gyrus | L | −15 | 36 | 39 | 4.74 | 0.032 | 27 | 0 | 0 | 0 | 100 | 0 | 0 |
| Precuneus | R | 24 | −66 | 33 | 4.73 | 0.034 | 27 | 0 | 0 | 0 | 0 | 0 | 100 |
| Precentral gyrus | L | −57 | −9 | 45 | 4.66 | 0.045 | 27 | 100 | 0 | 0 | 0 | 0 | 100 |
Percentage of voxels that showed activation/deactivation during the 0‐back task in a one‐sample t‐test (P < 0.05, corrected for multiple comparisons at voxel‐level FWE at the whole‐brain level).
Positive Correlation Between GMC and Brain Activity During the 2‐Back Task
After controlling for sex, age, and mean signal intensity during fMRI scanning at each voxel, a multiple regression analysis revealed that brain activity during the 2‐back task at each voxel was significantly and positively correlated with GMC at the voxel in a wide range of areas.
Among these areas, those that mainly consisted of regions activated during the 2‐back task (i.e., those that had the pattern of “the greater the GMC, the greater the task‐related activation increase”; Fig. 1a) were the bilateral anatomical clusters around the bilateral inferior parietal lobules and contingent parietal regions, bilateral dorsolateral PFCs, left inferior frontal gyrus, left precentral gyrus, supplementary motor area, and thalamus (Fig. 1c,e). All voxels that showed correlations of this pattern were included in areas that were activated during the task (Table 3). As can be seen in Table 3, much of these significant voxels belonged to areas of very low GMC (GMC value: 0–0.1) or low GMC (GMC value: 0.1–0.2), suggesting that the significant clusters are located in areas in the periphery of the GM areas.
Table 3.
Brain regions with significant positive correlations between GMC and brain activity during the 2‐back task
| Area | x | y | z | T score | Corrected P value (FWE) | Cluster size (mm3) | Activated regions (%)a | Deactivated regions (%)a | GMC 0–0.1 (%) | GMC 0.1–0.2 (%) | GMC 0.2–0.3 (%) | GMC≥ 0.3 (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Middle occipital lobe/inferior parietal lobule/angular gyrus | L | −27 | −57 | 33 | 9.42 | < 0.001 | 3,159 | 100 | 0 | 28.2 | 25.6 | 22.2 | 23.0 |
| Precentral gyrus/middle frontal gyrus | L | −24 | −3 | 39 | 8.42 | < 0.001 | 1,863 | 100 | 0 | 39.1 | 27.5 | 21.7 | 11.7 |
| Middle frontal gyrus | R | 24 | 3 | 42 | 7.49 | < 0.001 | 1,080 | 100 | 0 | 45.0 | 30.0 | 17.5 | 7.5 |
| Inferior parietal lobule | R | 33 | −48 | 33 | 7.08 | < 0.001 | 2,214 | 100 | 0 | 26.8 | 30.5 | 26.8 | 15.9 |
| Precentral gyrus | L | −36 | −3 | 27 | 7.02 | < 0.001 | 729 | 100 | 0 | 25.9 | 37.0 | 22.2 | 14.9 |
| Supplementary motor area | L | −9 | 12 | 48 | 6.71 | < 0.001 | 513 | 100 | 0 | 0 | 0 | 68.4 | 31.6 |
| Subgyral (close to the Inferior frontal gyrus) | L | −30 | 24 | 18 | 6.56 | < 0.001 | 405 | 100 | 0 | 40.0 | 53.3 | 6.6 | 0 |
| Ventricle area (close to the caudate) | L | −9 | 12 | 18 | 5.49 | 0.002 | 189 | 0 | 0 | 0 | 57.1 | 42.9 | 0 |
| Superior frontal gyrus | L | −24 | 42 | 12 | 5.44 | 0.002 | 54 | 100 | 0 | 0 | 50.0 | 50.0 | 0 |
| Ventricle area (close to the caudate) | L | −12 | −21 | 24 | 5.21 | 0.006 | 270 | 0 | 0 | 0 | 30.0 | 60.0 | 0 |
| Ventricle area (close to the thalamus) | 3 | −9 | 18 | 5.11 | 0.009 | 54 | 100 | 0 | 0 | 0 | 0 | 100 | |
| Subgyral (close to the inferior frontal gyrus) | L | −36 | 15 | 24 | 4.89 | 0.022 | 54 | 100 | 0 | 0 | 0 | 0 | 100 |
| Supramarginal gyrus | L | −45 | −42 | 36 | 4.87 | 0.023 | 54 | 100 | 0 | 0 | 0 | 0 | 100 |
| Ventricle area (close to the caudate) | L | −12 | −27 | 21 | 4.84 | 0.027 | 54 | 0 | 0 | 0 | 50.0 | 50.0 | 0 |
| Caudate | L | −12 | 18 | 12 | 4.73 | 0.041 | 27 | 0 | 0 | 0 | 0 | 0 | 100 |
| Ventricle area (close to the caudate) | L | −18 | −36 | 15 | 4.70 | 0.046 | 27 | 0 | 0 | 0 | 100 | 0 | 0 |
Percentage of voxels that showed activation/deactivation during the 2‐back task in one‐sample t‐test (P < 0.05, corrected for multiple comparisons at voxel‐level FWE at the whole‐brain level).
In addition, among the areas that had significant positive correlations between brain activity during the 2‐back task and GMC, those that mainly consisted of regions that were not activated or deactivated during the 2‐back task (i.e., those that had patterns indicative of “the greater the GMC, the greater the task‐related activation increase” and “the lower the GMC, the greater the task‐related activation decrease”; Fig. 3a) included anatomical clusters in or close to the thalamus and left caudate (Fig. 3c–e).
Figure 3.
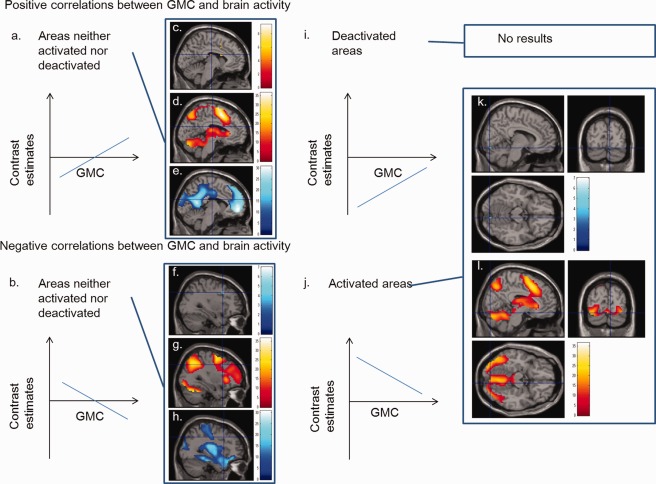
Minor patterns of correlations between GMC and brain activity. (a, b, i, j) schematics of correlations between GMC and brain activity. (a, i) Positive correlations in (a) areas that were neither activated nor deactivated and (i) deactivated areas (“the greater the GMC, the smaller the task‐related activation decrease). (b, j) Negative correlations in (b) areas that were neither activated nor deactivated and (j) activated areas (“the greater the GMC, the smaller the task‐related activation increase). (c) A region with a significant positive correlation between brain activity during the 2‐back task and GMC in an area close to the thalamus (P < 0.05, corrected for voxel‐level FWE), which was not activated nor deactivated during the 2‐back task. The regions with correlation were overlaid on a single subject T 1 image of SPM5. (f, k) Regions with significant negative correlations between brain activity during the 2‐back task and GMC (P < 0.05, corrected for voxel‐level FWE) (f) in an area of the right middle frontal gyrus, which was neither activated nor deactivated during the task and (k) in an area of the lingual gyrus, which was activated during the 2‐back task. (d, g, l) Regions that were activated during the 2‐back task are displayed at a height threshold of 0.05, corrected for voxel‐level FWE. (e, h) Regions that were deactivated during the 2‐back task are displayed at a height threshold of 0.05, corrected for voxel‐level FWE.
For all the results, see Table 3.
Negative Correlation Between GMC and Brain Activity During the 2‐Back Task
After adjusting for sex, age, and mean signal intensity during fMRI scanning at each voxel, a multiple regression analysis revealed that brain activity during the 2‐back task at each voxel was significantly and negatively correlated with GMC at the voxel in a wide range of areas.
Among these areas, those that mainly consisted of regions that were deactivated during the 2‐back task (i.e., those that had the pattern of “the greater the GMC, the greater the task‐related activation decrease”; Fig. 1b) included anatomical clusters in and/or around the bilateral angular gyrus, medial PFC and anterior cingulate cortex, left middle temporal gyrus, precuneus, and middle/posterior cingulate gyrus. The majority of these voxels displayed functional deactivation during the task. These regions also often bordered areas that were deactivated during the 2‐back task (Fig. 1d,f). Many of the large clusters in this analysis consisted of voxels that were not deactivated during the tasks, meaning the clusters were located in the periphery of the deactivated areas (Fig. 3d,f; Table 4). As can be seen in Table 4, much of these significant voxels belonged to areas of low GMC (GMC value: 0.1–0.2) or medium GMC (GMC value: 0.2–0.3), suggesting that these significant clusters tended to be located in areas in the periphery of the GM areas.
Table 4.
Brain regions with significant negative correlations between GMC and brain activity during the 2‐back task
| Area | x | y | z | T score | Corrected P value (FWE) | Cluster size (mm3) | Activated regions (%)a | Deactivated regions (%)a | GMC 0–0.1 (%) | GMC 0.1–0.2 (%) | GMC 0.2–0.3 (%) | GMC ≥ 0.3 (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Precuneus/angular gyrus | L | −24 | −60 | 18 | 7.01 | < 0.001 | 2,997 | 3.6 | 59.4 | 4.5 | 24.3 | 29.7 | 41.5 |
| Middle temporal gyrus | L | −45 | −9 | −21 | 6.96 | < 0.001 | 270 | 0 | 100 | 0 | 20.0 | 40.0 | 40.0 |
| Posterior cingulate gyrus | R | 18 | −45 | 33 | 6.13 | < 0.001 | 378 | 0 | 28.6 | 42.9 | 50.0 | 7.1 | 0 |
| Precuneus | R | 27 | −57 | 18 | 6.03 | < 0.001 | 432 | 0 | 68.8 | 6.3 | 50.0 | 37.5 | 6.3 |
| Anterior cingulate gyrus | L | −15 | 42 | 12 | 5.54 | 0.001 | 162 | 0 | 100 | 0 | 0 | 83.3 | 16.7 |
| Angular gyrus | R | 39 | −57 | 24 | 6.10 | 0.006 | 189 | 0 | 28.6 | 0 | 0 | 14.3 | 85.7 |
| Middle frontal gyrus | R | 33 | 15 | 39 | 5.17 | 0.007 | 108 | 0 | 0 | 0 | 25.0 | 75.0 | 0 |
| Precuneus | L | −15 | −48 | 30 | 5.16 | 0.007 | 81 | 0 | 100 | 0 | 66.7 | 33.3 | 0 |
| Middle cingulate gyrus | R | 12 | −9 | 39 | 5.11 | 0.009 | 54 | 0 | 100 | 0 | 0 | 100 | 0 |
| Anterior cingulate gyrus | L | −15 | 42 | −6 | 5.00 | 0.014 | 54 | 0 | 100 | 0 | 0 | 100 | 0 |
| Middle cingulate gyrus | L | −15 | −33 | 33 | 4.97 | 0.016 | 108 | 0 | 100 | 0 | 100 | 0 | 0 |
| Inferior frontal gyrus | L | −57 | 21 | 24 | 4.83 | 0.027 | 27 | 0 | 0 | 0 | 100 | 0 | 0 |
| Lingual gyrus | R | 9 | −84 | −15 | 4.79 | 0.032 | 54 | 100 | 0 | 0 | 0 | 0 | 100 |
| Precuneus | L | −18 | −69 | 30 | 4.68 | 0.049 | 27 | 0 | 0 | 0 | 0 | 0 | 100 |
Percentage of voxels that showed activation/deactivation during the 2‐back task in one‐sample t‐test (P < 0.05, corrected for multiple comparisons at voxel‐level FWE at the whole‐brain level).
Among the areas with significant positive correlations between brain activity during the 2‐back task and GMC, those that mainly consisted of regions that were activated during the 2‐back task (i.e., those that had the pattern of “the greater the GMC, the smaller the task‐related activation increase”; Fig. 3j) included anatomical clusters in the right lingual gyrus (Fig. 3k,l). Unlike the negative correlations in the deactivated areas, the voxels with negative correlations in the activated areas were located in areas of high GMC (GMC value: ≥ 0.3).
Among the areas with significant negative correlations between brain activity during the 2‐back task and GMC, those that mainly consisted of regions that were not activated or deactivated during the 2‐back task (i.e., those that had a pattern of “greater the GMC, the greater the task‐related activation decrease” and “the lower the GMC, the greater the task‐related activation increase”; Fig. 3b) included clusters in the right middle frontal gyrus, left inferior frontal gyrus, and left precuneus (Fig. 3f–h).
For all the results, see Table 4.
Positive/Negative Correlations Between GMC and Brain Activity Related to the 2‐Back–0‐Back Task
To confirm the differences between 2‐back and 0‐back task‐related activity in association with GMC, we investigated the positive/negative correlations between GMC and the contrast image of the 2‐back–0‐back task.
Significant positive and negative correlations are shown in Tables 5 and 6, respectively. The regions of significant correlation were generally similar to those identified by analyses of the correlation between GMC and brain activity related to the 2‐back task, although the statistical values and the extent of association was generally weaker.
Table 5.
Brain regions with significant positive correlations between GMC and brain activity related to the 2‐back–0‐back task
| Area | x | y | z | T score | Corrected P value (FWE) | Cluster size (mm3) | Activated regions (%)a | Deactivated regions (%)a | GMC 0–0.1 (%) | GMC 0.1–0.2 (%) | GMC 0.2–0.3 (%) | GMC ≥ 0.3 (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angular gyrus | L | −30 | −54 | 30 | 7.29 | < 0.001 | 1,053 | 100 | 0 | 41.0 | 35.9 | 23.1 | 0 |
| Middle frontal gyrus | R | 24 | 3 | 42 | 6.72 | < 0.001 | 945 | 100 | 0 | 40.0 | 48.6 | 11.4 | 0 |
| Subgyral (close to the inferior frontal gyrus) | L | −30 | 27 | 21 | 6.55 | < 0.001 | 513 | 100 | 0 | 31.6 | 52.6 | 15.8 | 0 |
| Inferior parietal lobule | R | 33 | −48 | 33 | 5.74 | < 0.001 | 648 | 100 | 0 | 41.7 | 33.3 | 16.7 | 8.3 |
| Middle frontal gyrus | L | −24 | 3 | 45 | 5.24 | 0.004 | 297 | 100 | 0 | 27.3 | 0 | 27.3 | 45.4 |
| Subgyral (close to the angular gyrus) | R | 30 | −63 | 27 | 5.02 | 0.011 | 162 | 100 | 0 | 0 | 33.3 | 33.3 | 33.3 |
| Supplementary motor area | −6 | 9 | 48 | 4.95 | 0.014 | 54 | 100 | 0 | 0 | 0 | 100 | 0 | |
| Inferior parietal lobule | R | 45 | −57 | 42 | 4.90 | 0.018 | 54 | 100 | 0 | 0 | 0 | 0 | 100 |
| Superior frontal gyrus | L | −24 | 42 | 12 | 4.86 | 0.021 | 27 | 100 | 0 | 0 | 100 | 0 | 0 |
| Medial frontal gyrus | R | 18 | 57 | −3 | 4.82 | 0.025 | 27 | 0 | 0 | 0 | 0 | 0 | 100 |
| Precentral gyrus | L | −36 | 0 | 27 | 4.79 | 0.028 | 54 | 100 | 0 | 0 | 50.0 | 50.0 | 0 |
| Inferior parietal lobule | L | −42 | −60 | 42 | 4.75 | 0.031 | 54 | 100 | 0 | 0 | 0 | 0 | 100 |
Percentage of voxels that showed activation/deactivation related to the 2‐back–0‐back task in a one‐sample t‐test (P < 0.05, corrected for multiple comparisons at voxel‐level FWE at the whole‐brain level).
Table 6.
Brain regions with significant positive correlations between GMC and brain activity related to the 0‐back–2‐back task
| Area | x | y | z | T score | Corrected P value (FWE) | Cluster size (mm3) | Activated regions (%)a | Deactivated regions (%)a | GMC 0–0.1 (%) | GMC 0.1–0.2 (%) | GMC 0.2–0.3 (%) | GMC ≥ 0.3 (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angular gyrus | L | −42 | −54 | 27 | 7.42 | < 0.001 | 945 | 14.3 | 8.6 | 2.9 | 14.3 | 31.4 | 51.4 |
| Angular gyrus | R | 39 | −60 | 24 | 5.53 | 0.001 | 162 | 16.7 | 0 | 0 | 16.7 | 16.7 | 66.7 |
| Subgyral (close to the middle cingulate gyrus) | L | −24 | −33 | 42 | 5.16 | 0.006 | 81 | 0 | 0 | 100 | 0 | 0 | 0 |
| Subgyral (close to the middle temporal gyrus) | L | −30 | −63 | 18 | 5.07 | 0.009 | 54 | 50 | 0 | 100 | 0 | 0 | 0 |
| Precuneus | L | −15 | −51 | 9 | 4.97 | 0.014 | 54 | 0 | 100 | 0 | 0 | 0 | 100 |
| Middle temporal gyrus | L | −48 | −9 | −18 | 4.95 | 0.015 | 135 | 0 | 100 | 0 | 20.0 | 20.0 | 60.0 |
| Postcentral gyrus | L | −42 | −42 | 63 | 4.71 | 0.037 | 27 | 100 | 0 | 0 | 0 | 100 | 0 |
Percentage of voxels that showed activation/deactivation related to the 0‐back–2‐back task in a one‐sample t‐test (P < 0.05, corrected for multiple comparisons at voxel‐level FWE at the whole‐brain level).
DISCUSSION
This is the first study to investigate the association between functional activation maps and GM structures in young adults who do not show apparent GM atrophy as well as the first to investigate the nature of this association using a large number of subjects. Consistent with our hypothesis, the concept of “the greater the GMC, the greater the task‐related activation increase/task‐related activation decrease (or the greater task‐related activation change from baseline)” (Fig. 1a,b) held true for a wide range of activated and deactivated areas. However, two other patterns were also observed. First, in fewer regions, one of the opposite patterns, namely “the greater the GMC, the smaller the task‐related activation increase” (Fig. 3j) was observed. Second, the pattern of “the greater the GMC, the greater the task‐related activation decrease” (Fig. 1b) seemed to be particularly concentrated at the borders of GM structures and regions that were deactivated during the task (Tables 2 and 4, Fig. 1d,f); the pattern of “the greater the GMC, the greater the task‐related activation increase” (Fig. 1a) was also observed at the borders of GM structures (Tables 1 and 3, Fig. 1c). Third, the function–structure correlations were observed more often in the 2‐back condition than in the 0‐back condition, and results of analyses of the 2‐back–0‐back task were similar to but weaker than those for the 2‐back task. This may have been because of there being more variances in the 2‐back condition as a result of the greater incidence of activation and deactivation.
The “greater the GMC, the greater the task‐related activation increase/task‐related activation decrease (or the greater the task‐related activation change from baseline)” pattern (Fig. 1a,b) was observed in a wide range of activated/deactivated areas. With regard to the “greater the GMC, the greater the task‐related activation increase” (Fig. 1a) pattern, this was observed in all major areas activated during the 2‐back task. “The greater the GMC, the greater the task‐related activation decrease” pattern (Fig. 1b) was also observed in almost all of the major areas that were deactivated during the 2‐back task. Thus, the fact that these structure–function associations exist regardless of the location of the region and regardless of whether the regions were activated or deactivated should be taken into consideration when interpreting the functional activation results. As described in the Introduction section, if we do not assume any special mechanism, the same extent of GM in the same space should produce same amount of functional activity in the space. Thus, a greater extent of GM should be associated with a greater task‐related activation increase in regions activated during the task and with a greater task‐related activation decrease in regions deactivated during the task. This may be the major reason why “greater the GMC, the greater the task‐related activation increase/task‐related activation decrease (or the greater the task‐related activation change from baseline)” pattern was widely observed.
The pattern of “the greater the GMC, the greater the task‐related activation decrease” (Fig. 1b) seemed to be particularly concentrated at the borders of GM structures and regions that were deactivated during the task, as is clearly observed in Fig. 3d,f and Table 4 (2‐back) and Table 2 (0‐back). On the other hand, “the greater the GMC, the greater the task‐related activation increase” pattern (Fig. 1a) seemed to be more common in regions that were activated during the task (Tables 1 and 3). In case of the 0‐back and 2‐back tasks, all voxels of the clusters that displayed “the greater the GMC, the greater the task‐related activation increase” pattern (Fig. 1a) were included in areas that were activated during the task (Tables 1 and 3). However, “the greater the GMC, the greater the task‐related activation increase” pattern also seemed to be concentrated at the borders of the GM structures (Fig. 1c, Tables 1 and 3). These associations may have been often observed at the borders of GM structures because individual differences and variances may be larger in these areas. Furthermore, it may be that the magnitude of the task‐related activation increase/decrease is critically dependent on whether there are GM structures in low GMC areas. In extreme cases, these may be the regions that show positive/negative correlations in areas that are neither activated nor deactivated. This finding is consistent with another of our studies [Takeuchi et al., Associations among imaging measures (3): Imaging measure correlates of functional connectivity during rest, submitted for publication], which investigated the relationship between functional connectivity during the resting state and GMC; this study showed a clear correlation between the magnitude of functional connectivity and GMC at the borders of GM structures. Thus, at the borders of GM structures, regardless of whether we measure cerebral blood flow, task‐related activation changes (activation and deactivation), or resting‐FC, partial volume effects should be considered. In clear contrast, voxels of the clusters that formed the pattern “the greater the GMC, the greater the task‐related activation increase” were consistently included in areas that showed activations (Fig. 1c,e, Tables 1 and 3), but this did not hold true for the voxels of the clusters that formed the pattern “the greater the GMC, the greater the task‐related activation decrease” (Fig. 1d,f, Tables 2 and 4). We can only speculate the reasons of this clear contrast. This phenomenon seems to be related to the fact that regions that showed a task‐related activation increase during the tasks performed in this study were well spread out in the white matter regions, but the regions that showed a task‐related activation decrease did not spread far beyond the GM areas (Fig. 1e,f) and both correlation patterns were spread around the periphery of the GM areas. This may be because cerebral blood flow cannot decrease any further in the white matter regions as cerebral blood flow in these regions is relatively low [Wong et al., 1998]. On the other hand, Raichle et al. 2001 performed quantitative measurements of the oxygen‐extraction fraction and argued that the areas that showed a task‐related activation decrease in this study were not persistently activated at rest and the other regions are not deactivated at rest either. Therefore, cerebral blood flow at rest in the regions that showed a task‐related activation increase may be comparable with that in the regions that showed a task‐related activation decrease [see Raichle et al., 2001]. Thus, while cerebral blood flow at rest may have room for an increase in white matter areas with a task‐related activation increase, there may not be any room for a decrease in areas with a task‐related activation decrease. However, this is highly speculative and future studies are needed to investigate this issue.
These concentrations of correlations at the borders of GM structures cannot be explained by decreased accuracy of normalization of images in these areas from insufficient matches related to the different normalizing procedures used for BOLD and structural images, as the possible lowered accuracy resulting from matching different images in these areas must lead to lowered sensitivity of analysis (thus, weaker or insignificant results). However, if the degree of distortion of EPI images in 3 T MRI is associated with the amount of GM in the space around the area, this might complicate the association between functional activation and GM in analyses, and it might contribute to the association between GM and functional activation around areas with strong distortion. Regardless, when the individual or group differences in functional activations are observed at these borders of GM structures, the possibility that these functional differences directly reflect differences in the GM structures should be considered.
Some positive and negative correlations between GMC and brain activity were seen in areas that were not activated or deactivated during the tasks. The former pattern is indicative of “the greater the GMC, the greater the task‐related activation increase” and “the lower the GMC, the greater the task‐related activation decrease (Fig. 3a),” whereas the latter is indicative of the opposite pattern (Fig. 3b). Another possibility is that because these voxels often do not belong to an area of high GMC (Tables 3 and 4), most of the areas were neither activated nor deactivated during the task, but the small number of subjects who tended to have more GM structures in these areas may show activation/deactivation in this region; this may result in correlations between GMC and task‐related activation increases/decreases (but not significant activation/deactivation among all subjects). However, another possibility is the case shown in Figure 3b with the pattern in the bilateral dorsolateral prefrontal cortex (DLPFCs) (Fig. 3f); because these voxels are located at the periphery of the areas that were activated during the task but not close to the areas that were deactivated during the task, these areas were usually not recruited during the 2‐back task. However, subjects with less GMC in this area may have to recruit additional regions (as described below), and this may result in negative correlations between GMC and task‐related activation increases (but not significant activation among all subjects). Partly congruent with this concept is the fact that patients with schizophrenia, who are characterized by deficits of WM, have been shown to have higher activity than control subjects during WM tasks in peripheral areas that are activated during the task in the PFC [Callicott et al., 2003].
Negative correlations between brain activity during the 0‐ and 2‐back tasks and GMC were observed in some clusters that mainly consisted of areas that were activated during these tasks. This means that in these areas, the greater GMC was associated with less brain activity (Fig. 3j) during the 0‐ and 2‐back tasks. As described above, the bilateral DLPFCs may fall into this category. In the case of the results related to the 2‐back task, these regions have multiple roles in WM, such as a central executive function (bilateral DLPFCs) and passive information storage systems (the lingual gyrus) [Baddeley, 2003; Wager and Smith, 2003]. On the other hand, in the case of the results related to the 0‐back task, the left precentral gyrus seemed to be involved in the button press during the 0‐back task, which is a simple motor execution task. These regions may show characteristic patterns because they play a key role in processing task execution through the above mechanisms. Perhaps the more developed GM structures (increased GMC), which are possibly underlain by more neuronal structures, in these areas lead to more efficient information processing and thus lessen the cognitive load (processing load), requiring less brain activity in these areas. These associations may underlie the often‐observed negative correlation between intellectual ability and functional activity and the positive correlation between intellectual abilities and GMC [for review, see Neubauer and Fink, 2009].
In summary, this is the first study to investigate the associations between functional activation maps and GM structures in normal young adults who do not show apparent GM atrophy, as well as the first to investigate the nature of this association using a large number of subjects. An association between functional activation and GM structure has been revealed in clinical studies as well as aging studies using small numbers of subjects. We are the first to reveal associations in young adults without apparent GM atrophy. We also found that the concept of “the greater the GMC, the greater the task‐related activation increase/task‐related activation decrease (or the greater the task‐related activation change from baseline)” (Fig. 1a,b) held true for a wide range of areas that were activated/deactivated during the task. In addition, the pattern of “the greater the GMC, the smaller the task‐related activation decrease” (Fig. 2j) was observed in fewer minor regions. Furthermore, “the greater the GMC, the greater the task‐related activation increase/task‐related activation decrease (or the greater the task‐related activation change from baseline)” pattern was often observed at the borders of GM structures. These findings may have to be taken into consideration when group/individual differences in functional activation are investigated.
ACKNOWLEDGMENTS
The authors thank Yuki Yamada for operating the MRI scanner, the participants, and all their other colleagues at IDAC, Tohoku University for their support. They also thank Enago (http://www.enago.jp) for the English language review.
REFERENCES
- Amici S, Brambati SM, Wilkins DP, Ogar J, Dronkers NL, Miller BL, Gorno‐Tempini ML (2007): Anatomical correlates of sentence comprehension and verbal working memory in neurodegenerative disease. J Neurosci 27:6282–6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonova E, Kumari V, Morris R, Halari R, Anilkumar A, Mehrotra R, Sharma T (2005): The relationship of structural alterations to cognitive deficits in schizophrenia: A voxel‐based morphometry study. Biol Psychiatr 58:457–467. [DOI] [PubMed] [Google Scholar]
- Baddeley A (2003): Working memory: Looking back and looking forward. Nat Rev Neurosci 4:829–839. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL (2008): The brain's default network. Ann NY Acad Sci 1124(The Year in Cognitive Neuroscience 2008):1–38. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, Goldberg TE, Weinberger DR (1999): Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cerebr Cortex 9:20–26. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR (2003): Complexity of prefrontal cortical dysfunction in schizophrenia: More than up or down. Am J Psychiatr 160:2209–2215. [DOI] [PubMed] [Google Scholar]
- Casanova R, Srikanth R, Baer A, Laurienti PJ, Burdette JH, Hayasaka S, Flowers L, Wood F, Maldjian JA (2007): Biological parametric mapping: A statistical toolbox for multimodality brain image analysis. Neuroimage 34:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della‐Maggiore V, Chau W, Peres‐Neto PR, McIntosh AR (2002): An empirical comparison of SPM preprocessing parameters to the analysis of fMRI data. Neuroimage 17:19–28. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Shadmehr R (2005): Detecting and adjusting for artifacts in fMRI time series data. Neuroimage 27:624–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A (2004): Neuroplasticity: Changes in grey matter induced by training. Nature 427:311–312. [DOI] [PubMed] [Google Scholar]
- Gaser C. (2007): VBM Toolbox for SPM2, VBM Toolbox for SPM5. Available at: http://dbm.neuro.uni-jena.de/vbm/. Accessed on 2009/January/19th.
- Hayasaka S, Du AT, Duarte A, Kornak J, Jahng GH, Weiner MW, Schuff N (2006): A non‐parametric approach for co‐analysis of multi‐modal brain imaging data: Application to Alzheimer's disease. Neuroimage 30:768–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilg R, Wohlschlager AM, Gaser C, Liebau Y, Dauner R, Woller A, Zimmer C, Zihl J, Muhlau M (2008): Gray matter increase induced by practice correlates with task‐specific activation: A combined functional and morphometric magnetic resonance imaging study. J Neurosci 28:4210–4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juergens E, Guettler A, Eckhorn R (1999): Visual stimulation elicits locked and induced gamma oscillations in monkey intracortical‐and EEG‐potentials, but not in human EEG. Exp Brain Res 129:247–259. [DOI] [PubMed] [Google Scholar]
- Klingberg T (2006): Development of a superior frontal‐intraparietal network for visuo‐spatial working memory. Neuropsychologia 44:2171–2177. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A (2001): Neurophysiological investigation of the basis of the fMRI signal. Nature 412:150–157. [DOI] [PubMed] [Google Scholar]
- May A, Gaser C (2006): Magnetic resonance‐based morphometry: A window into structural plasticity of the brain. Curr Opin Neurol 19:407–411. [DOI] [PubMed] [Google Scholar]
- Mitzdorf U (1987): Properties of the evoked potential generators: Current source‐density analysis of visually evoked potentials in the cat cortex. Int J Neurosci 33:33–59. [DOI] [PubMed] [Google Scholar]
- Neubauer AC, Fink A (2009): Intelligence and neural efficiency. Neurosci Biobehav Rev 33:1004–1023. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Nagy Z, Westerberg H, Klingberg T (2003): Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto‐parietal network. Cognit Brain Res 18:48–57. [DOI] [PubMed] [Google Scholar]
- Owen AM. (2000): The role of the lateral frontal cortex in mnemonic processing: The contribution of functional neuroimaging. Exp Brain Res 133:33–43. [DOI] [PubMed] [Google Scholar]
- Peiffer AM, Hugenschmidt CE, Maldjian JA, Casanova R, Srikanth R, Hayasaka S, Burdette JH, Kraft RA, Laurienti PJ. (2009): Aging and the interaction of sensory cortical function and structure. Hum Brain Mapp 30:228–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. (2001): A default mode of brain function. Proc Natl Acad Sci 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Kawashima R (2010a)Effects of working memory training on cognitive functions and neural systems. Rev Neurosci 21:427–450. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R (2010b): Regional gray matter volume of dopaminergic system associate with creativity: Evidence from voxel‐based morphometry Neuroimage 51:578–585. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R (2010c)White matter structures associated with creativity: Evidence from diffusion tensor imaging. Neuroimage 51:11–18. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Hashizume H, Sassa Y, Nagase T, Nouchi R, Kawashima R (2011a)Effects of training of processing speed on neural systems. J Neurosci 31:12139–12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Hashizume H, Sassa Y, Nagase T, Nouchi R, Kawashima R (2011b)Failing to deactivate: The association between brain activity during a working memory task and creativity. Neuroimage 55:681–687. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R (2011c)Regional gray matter density associated with emotional intelligence: Evidence from voxel‐based morphometry. Hum Brain Mapp 32:1497–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R (2011d)Verbal working memory performance correlates with regional white matter structures in the fronto‐parietal regions. Neuropsychologia 49:3466–3473. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R (2011e): Working memory training using mental calculation impacts regional gray matter of the frontal and parietal regions. PLoS ONE 6:e23175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Nagase T, Nouchi R, Fukushima A, Kawashima R (2011f): White matter structures associated with emotional intelligence: Evidence from diffusion tensor imaging. Human brain mapping: Epub ahead of publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Kawashima R (2012a): Effects of processing speed training on cognitive functions and neural systems. Reviews in the Neurosciences 23:289–301. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Nagase T, Nouchi R, Fukushima A, Kawashima R (2012b): Regional gray and white matter volume associated with Stroop interference: Evidence from voxel‐based morphometry. Neuroimage 59:2899–2907. [DOI] [PubMed] [Google Scholar]
- Taki Y, Goto R, Evans A, Zijdenbos A, Neelin P, Lerch J, Sato K, Ono S, Kinomura S, Nakagawa M (2004): Voxel‐based morphometry of human brain with age and cerebrovascular risk factors. Neurobiol Aging 25:455–463. [DOI] [PubMed] [Google Scholar]
- Taki Y, Hashizume H, Sassa Y, Takeuchi H, Asano M, Asano K, Kawashima R (2010): Breakfast staple types affect brain gray matter volume and cognitive function in healthy children. PLoS ONE 5:e15213:1–e15213:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Smith EE (2003): Neuroimaging studies of working memory: A meta‐analysis. Cognit Affect Behav Neurosci 3:255–274. [DOI] [PubMed] [Google Scholar]
- Wong EC, Buxton RB, Frank LR (1998): Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II). Magn Reson Med 39:702–708. [DOI] [PubMed] [Google Scholar]


