Abstract
Typical childhood development is characterized by the emergence of intrinsic connectivity networks (ICNs) by way of internetwork segregation and intranetwork integration. The impact of childhood epilepsy on the maturation of ICNs is, however, poorly understood. The developmental trajectory of ICNs in 26 children (8–17 years) with localization‐related epilepsy and 28 propensity‐score matched controls was evaluated using graph theoretical analysis of whole brain connectomes from resting‐state functional magnetic resonance imaging (fMRI) data. Children with epilepsy demonstrated impaired development of regional hubs in nodes of the salience and default mode networks (DMN). Seed‐based connectivity and hierarchical clustering analysis revealed significantly decreased intranetwork connections, and greater internetwork connectivity in children with epilepsy compared to controls. Significant interactions were identified between epilepsy duration and the expected developmental trajectory of ICNs, indicating that prolonged epilepsy may cause progressive alternations in large‐scale networks throughout childhood. DMN integration was also associated with better working memory, whereas internetwork segregation was associated with higher full‐scale intelligence quotient scores. Furthermore, subgroup analyses revealed the thalamus, hippocampus, and caudate were weaker hubs in children with secondarily generalized seizures, relative to other patient subgroups. Our findings underscore that epilepsy interferes with the developmental trajectory of brain networks underlying cognition, providing evidence supporting the early treatment of affected children. Hum Brain Mapp 35:5686–5700, 2014. © 2014 Wiley Periodicals, Inc.
Keywords: functional connectivity, intrinsic connectivity network, generalized tonic clonic (GTC), graph theoretical analysis, resting‐state fMRI
INTRODUCTION
Distributed brain networks demonstrate correlated low‐frequency fluctuations in blood oxygen level‐dependent (BOLD) signals at rest [Biswal et al., 1995; Raichle and Snyder, 2007; Raichle et al., 2001] that are correlated with underlying structural connectivity patterns [Greicius et al., 2009; van den Heuvel et al., 2009]. These fluctuations of BOLD signal may reflect large‐scale coherence of neurophysiological oscillations [Brookes et al., 2011a, b; de Pasquale et al., 2010; Nir et al., 2008] and/or the cross‐frequency coupling of oscillations among cortical regions [Foster and Parvizi, 2012]. Such intrinsic connectivity networks (ICNs, also known as resting‐state networks) comprises groups of brain regions exhibiting temporally correlated activity. The activity of certain ICNs may be relatively decreased during task performance, whereas other ICNs may express task‐dependent increases in connectivity. The former includes the default mode network [DMN; Raichle et al., 2001], which may support self‐referential thought, internally directed processing and mind‐wandering [Gusnard et al., 2001; Kim, 2012]. Task‐negative networks are also anticorrelated with regions that demonstrate increased activity during attention‐demanding tasks [Fox et al., 2005], such as the dorsal and ventral attention networks [Fox et al., 2006], as well as the cingulo‐insular or salience network [Seeley et al., 2007].
Typical development is characterized by increases in the strength of long‐range connections between brain regions associated with the same ICN, together with increasing segregation of cortical and subcortical structures associated with different ICNs [Dosenbach et al., 2010]. It has been shown previously that “connectivity gradients” between correlated and anticorrelated regions in major hubs of ICNs become stronger during adolescence and early adulthood with sharpening of the boundaries of the DMN and decreased correlation between the DMN and attention control networks [Anderson et al., 2011b]. The fractionation of brain regions into specific regional hubs also represents a transition from a local to distributed network organization, which is a hallmark of typical development [Fair et al., 2009]. This intranetwork integration and internetwork segregation contributes to the formation of mature distributed, functionally specialized networks seen in adults. Disruption of normative ICN organization is associated with a number of neurological and neuropsychiatric conditions, including neurodevelopmental disorders [Uddin et al., 2013; Washington et al., 2014].
Childhood epilepsy has long been known to interfere with typical childhood development [Keating, 1960]. Whereas adult‐onset epilepsy may result in a loss or interference with previously acquired function, epilepsy in children may manifest as a failure to progress along the expected developmental trajectory [Korman et al., 2013]. Furthermore, in contrast to adults, focal seizures in children may result in less specific and more global impairments [Hepworth and Smith, 2002; Smith et al., 2002, 2004], implicating dysfunction of the development of large‐scale networks as a putative underlying process. Knowledge is limited, however, whether or to what extent childhood epilepsy affects the development of ICNs. Children with epilepsy are known to have weaker ICNs than controls, although this has not been correlated with clinical features, including epilepsy duration and cognitive outcomes [Mankinen et al., 2012; Widjaja et al., 2013b]. Children with frontal lobe epilepsy also demonstrated an increase in the number of functionally isolated and dissociable brain modules within the frontal lobe compared to controls [Vaessen et al., 2012], suggesting that subnetworks are less interconnected in the patient population and different network combinations show both reduced and increased functional connectivity [Widjaja et al., 2013a]. Given that these networks are not static, it is remains important to evaluate the effects of focal epilepsy on the developmental trajectory of ICNs, to define optimal timing for surgical intervention and develop novel markers to longitudinally evaluate the efficacy of treatment. Such information is particularly valuable given the significant mean latent period of over 5 years and up to 17 years between diagnosis and surgical treatments for focal epilepsy [Lim et al., 2013].
Here, we hypothesize that the cumulative effects of prolonged, intractable, focal epilepsy interferes with the development of ICNs, and that disruption of ICN development would be associated with poor neuropsychological outcomes in children with epilepsy. We also investigated whether differing childhood epilepsy phenotypes (seizure semiology, epilepsy duration, and epileptic focus location) are associated with impairments in specific ICNs. To test these hypotheses, we performed a whole brain graph theoretical analysis of resting‐state functional connectivity magnetic resonance imaging data to identify brain regions demonstrating atypical development in children with epilepsy. The functional connectivity of specific ICNs identified as atypical was subsequently interrogated through seed‐based analyses. The organizational structure of ICNs in the children studied was further characterized through agglomerative hierarchical clustering. Network disturbances were then correlated with the children's neuropsychological function. These findings provide a first demonstration of the effects of epilepsy on the brain's developing ICNs, emphasize the importance of early surgical treatment to mitigate these effects, and following validation, may provide a benchmark to assess the efficacy of therapeutic interventions.
METHODS
Study Population
Forty‐seven children with localization related‐epilepsy were recruited from two separate institutions (Hospital for Children, Toronto, Canada and the Foothills Medical Centre, Calgary, Canada). Of these, 26 children met the stringent inclusion criteria (refer to Supporting Information Methods). The children ranged in age from 8 to 17 years with mean epilepsy duration (± standard deviation) of 4.34 ± 3.54 years. Eleven children (42%) had epileptic foci localized to the temporal lobe, whereas 15 children (58%) had extratemporal epilepsy and 14 children (54%) presented with secondarily generalized seizures (see Supporting Information for more details). Control fMRI data were collected from a large cohort of typically developing children who underwent similar acquisitions at The Hospital for Sick Children (see Supporting Information for demographics of control dataset). To identify appropriate controls, we performed propensity score matching [Rubin, 1997] using a multivariate logistic regression with group classification (epilepsy vs. controls) as the dependent variable. This approach balances selected covariates (age and sex) between the two study cohorts (Supporting Information Fig. S3). Propensity score matching was performed using R statistical software [Ho et al., 2007a, b].
Resting‐State fMRI Acquisition and Preprocessing
Structural and functional MRI data were collected using 3T scanners. Continuous fMRI data were collected, during which subjects were instructed to focus on a projected fixation cross. The scanners, protocols, and sequences used are presented in the Supporting Information Methods. We performed several validation studies to ensure that our findings were not attributable to differences in scanners, protocols, or discrepancies in the length of scans (presented in Supporting Information). The study complies with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and was approved by the Research Ethics Boards of the Hospital for Sick Children and the University of Calgary.
fMRI data were preprocessed using standard AFNI [Cox, 1996] and FMRIB Software Library (FSL) tools [Jenkinson et al., 2012]. Slice‐timing and motion correction were performed before aligning the data to the MNI152 T1 2 mm atlas via the subject's high‐resolution anatomical T1‐weighted images. Subjects with maximum head displacement greater than 2 mm from a reference volume for more than one‐third of their volumes or those with a total mean head displacement greater than 2 mm were excluded from the analysis. Of the 47 children with epilepsy initially recruited, eight were excluded because of excessive motion parameters (see Supporting Information for details). The mean head displacement for all subjects included in the analysis was less than 1 mm for both children with epilepsy and propensity‐matched controls. Next, data underwent spatial smoothing using a 6.6 mm FWHM Gaussian kernel and was bandpass filtered with a lower and upper cutoff frequency of 0.01 and 0.2 Hz, respectively. BOLD signal contributions from white matter and CSF and motion were regressed from the BOLD signal.
It has been postulated that task‐negative networks (such as the DMN) are anticorrelated with task‐positive networks [Fox et al., 2005; Fransson, 2005]. A body of literature, however, also suggests that this may be an artifact as a result of global signal regression during preprocessing, as it introduces greater anticorrelations in the data [Anderson et al., 2011a; Murphy et al., 2009; Saad et al., 2012]. Since regression of global variations in BOLD signal is advantageous in that it may reduce spurious correlations among brain regions [Weissenbacher et al., 2009], in the current study, we performed all analyses with and without regression of the global signal and found robust and reliable differences using both preprocessing pipelines. The results presented in this article show data preprocessed using whole brain signal regression.
Graph Theoretical Analysis of Whole Brain Connectomes
To identify and subsequently compare resting‐state hubs in children with localization‐related epilepsy and typically developing controls, we defined a whole brain connectome based on synchronized low‐frequency BOLD oscillations for each subject. Graph theoretical analysis was then performed to characterize and quantify each connectome's global organization. This approach is increasingly considered advantageous over other analytical methods to provide insight into functional topographic reconfiguration of the brain in response to various physiological and pathological conditions [refer to Wang et al., 2010 for review].
Whole brain connectomes were created for each individual subject by generating individual voxel‐based graphs, where each voxel represents a node. Each functional connection, indexed by the square of the Pearson correlation coefficient between the time series of any voxel pair was defined as an edge (Supporting Information Fig. S7). All voxels within a functional brain mask for each child were included in the analyses. The voxel‐based graph is a mathematical representation of the whole brain resting‐state connectome, from which network topologies may be inferred [Zuo et al., 2012]. A threshold was applied to the adjacency matrix for each subject to an Erdös‐Rényi entropy (S) of 2.0 [Watts and Strogatz, 1998], which ensures that the small‐world features of the network were consistent across all subjects [Gili et al., 2013]. Of note, for both children with epilepsy and propensity‐matched controls, minimal thresholding was required to reach an S‐value of 2.0. For both groups, over 90% of connections were included.
From the connectivity matrix, eigenvector centrality (EC) was calculated for each node using the Brain Connectivity Toolbox [Rubinov and Sporns, 2010]. EC is an index of the extent to which a given region represents a hub within a network. We chose this metric for several reasons. First, this topological measure directly evaluates a node's importance within a graph by assigning relative scores to nodes based on the principle that connections to high scoring nodes contribute more the score of a given node than equal connections to low scoring nodes. Functional connections to hub regions within a given network may, therefore, be prioritized while rejecting functional connections to nonhubs. Second, previous studies have demonstrated that EC is a robust measure to index changes in functional brain connectivity [Gili et al., 2013]. A final advantage of EC over other metrics that may identify brain hubs (such as betweenness or closeness centrality) is that it is computationally less expensive, allowing analysis of a large number of brain voxels. Once the EC maps were created, they were registered to the MNI152 template. Differences in hub‐like topographies between children with medically intractable epilepsy and normal controls as well as between different subgroups of children with epilepsy were evaluated using a nonparametric randomization algorithm [FSL randomize; Nichols and Holmes, 2002]. Significance thresholds for cluster‐based statistics were determined using 3dClustSim from the AFNI toolbox (429 contiguous voxels for a corrected P‐value of 0.04). Coding was performed using custom scripts written in‐house in MATLAB.
Seed‐Based Analysis
To evaluate brain regions to which identified hub regions were connected, we subsequently performed seed‐based connectivity analysis. Seed regions were chosen from selected ICNs that possessed differential hub properties in the previous whole brain connectome analysis. For example, if significant group differences in EC were identified in the posterior cingulate cortex (PCC), central nodes of the DMN were seeded. To account for intersubject variability in assigning seed points for connectivity analysis, a 15‐mm‐spherical region of interest (ROI) was defined and centered on published coordinates based on a review of literature [Brier et al., 2012]. An iterative algorithm adapted from prior work [Golestani and Goodyear, 2011] was then used whereby the ROI was eroded within each subject's functional space until an internal correlation of 0.7 was achieved. This method is advantageous as it may account for minor differences in the organization of intrinsic resting‐state networks [Golestani and Goodyear, 2011]. A detailed explanation of this method and intersubject variability in centroid locations of eroded ROIs is presented in Supporting Information Table S4.
First‐level analysis was performed by correlating the mean time series of the eroded ROIs with the time series of all voxels in the brain. This analysis was performed using FEAT (FMRI Expert Analysis Tool) Version 5.98, part of FSL (http://www.fmrib.ox.ac.uk/fsl). Timeseries statistical analysis was performed using FILM with local autocorrelation correction [Woolrich et al., 2001]. Mixed‐effects higher‐level analysis was subsequently performed to contrast the first‐level statistical parameter connectivity maps between children with epilepsy and propensity‐matched controls. Z (Gaussianised T/F) statistical images were thresholded using clusters determined by Z > 2.3 and a (corrected) cluster significance threshold of P < 0.05 [Worsley, 2001].
Hierarchical Clustering
To further characterize inter‐ and intranetwork patterns of connectivity, the mean BOLD time series of nodes of multiple ICNs were extracted. ICNs selection was based on a literature review performed by Brier et al. [2012]. Adjacency (correlation) matrices were constructed in a similar manner to the voxel‐based analysis previously described, but with each network region as the node and the Pearson correlation coefficient between any two nodes as the edge. Two network‐based graph theoretical measures were then calculated: the mean network clustering and characteristic network pathlength. The former is a measure of functional segregation by measuring the fraction of a node's neighbors that are also neighbors of each other [Rubinov and Sporns, 2010]. The latter is a measure of functional integration and describes the average pathlength between all pairs of nodes in the network [Watts and Strogatz, 1998].
Agglomerative hierarchical clustering was also performed on the adjacency matrices to visualize the segregation of nodes into individual networks based on the strength of the correlations between them. This algorithm creates a cluster tree (dendrogram) based on the shortest Euclidean distance between the correlation value of any two nodes. The clustering is based on the single linkage algorithm using Euclidean distances among the rows of the correlation matrix. Unweighted average distance (unweighted pair group method with arithmetic mean), also known as group average was used [Sokal and Michener, 1958]. The iterative algorithm continued until no further clusters could be created. Hierarchical clustering was performed in MATLAB (Natick, MA).
Neuropsychological Testing
To test whether the developmental impairments in the identified ICNs were associated with cognitive deficits, we correlated network measures with neuropsychological testing scores in children with epilepsy. Since the DMN connectivity has previously been associated with working memory [Hampson et al., 2006; Yakushev et al., 2013], forward and backward digit recall was measured using the Working Memory Test Battery for Children [Pickering and Gathercole, 2001]. The full‐scale intelligence quotient (IQ) of the Wechsler Intelligence Scale for Children was also correlated with neuroimaging data as a measure of global cognitive function [Wechsler, 2003]. Age‐adjusted z‐scores were derived from raw scores [Ruff and Parker, 1993; Wechsler, 2003]. Multivariate linear regression modeling was performed with network connectivity as the dependent variable and subject age, neuropsychological score and group classification as independent variables. Backward elimination, stepwise conditional regression was performed in MATLAB software using the sum squared error as the criterion to remove model terms [Hocking, 1976].
RESULTS
Children with Epilepsy Demonstrate Decreased Centrality in Important Hubs of Default Mode and Salience Networks
EC maps were calculated on connectivity matrices composed of approximately 30,000 voxels (Supporting Information Fig. S8). When the whole brain connectome of children with epilepsy was contrasted with propensity‐matched controls, significant group effects, and age interactions were identified in the centrality of important nodes of the DMN and salience networks. Children with epilepsy demonstrated significantly lower EC in the insulae bilaterally (Fig. 1; right insula: group effect: F (1,50) = 28.16; P < 0.01; age effect: F (1,50) = 0.5, P = NS; group × age interaction: F (1,50) = 0.30; P = NS; left insula: group effect: F (1,50) = 31.48; P < 0.01; age effect: F (1,50) = 0.14, P = NS; group × age interaction: F (1,50) = 0.73; P = NS). A significant age interaction was also observed in the PCC, where increases in centrality with age were significantly different between the two groups (group effect: F (1,50) = 11.47; P < 0.01; age effect: F (1,50) = 7.71, P = 0.021; group × age interaction: F (1,50) = 6.36; P = 0.015). Furthermore, whereas typically developing children demonstrated a decrease in centrality with age within a large cluster of the frontal lobe, those with epilepsy demonstrated persistence in frontal lobe centrality with age (group effect: F (1,50) = 4.69; P < 0.035; age effect: F (1,50) = 6.94, P = 0.011; group × age interaction: F (1,50) = 13.46; P < 0.01).
Impaired Functional Network Integration and Segregation in Children with Epilepsy
To characterize the patterns of connectivity of hub regions that showed significant differences between children with epilepsy and controls, ROI‐based functional connectivity analyses were performed. Brain regions that are known to be important nodes of the default mode (PCC and ventromedial prefrontal cortex [vmPFC]) and salience networks (right insula and anterior cingulate cortex [ACC]) were included as seeds for the ROI analysis. The mean connectivity maps for both children with intractable epilepsy and controls demonstrated expected anticorrelation of task‐positive and task‐negative ICNs (Fig. 2). Children with epilepsy, however, showed significantly greater connectivity between networks that are normally anticorrelated in comparison to controls. For instance, when evaluating two core nodes of DMN, the vmPFC and PCC, we found that the vmPFC showed greater connectivity to the bilateral insulae and somatosensory cortices in children with epilepsy. The PCC also showed greater connectivity to the bilateral insulae, as well as the thalami. In controls, the PCC demonstrated stronger connectivity to other regions within the DMN, namely the vmPFC and lateral parietal cortices. Similarly, when we evaluated core nodes of the salience networks, the right insulae and ACC, we found that controls demonstrate greater connectivity of the right insulae to the ACC, relative to children with epilepsy. Conversely, children with localization‐related epilepsy showed greater connectivity of the right insula and ACC to nodes of the DMN, the vmPFC, PCC, and lateral parietal cortices.
Figure 2.
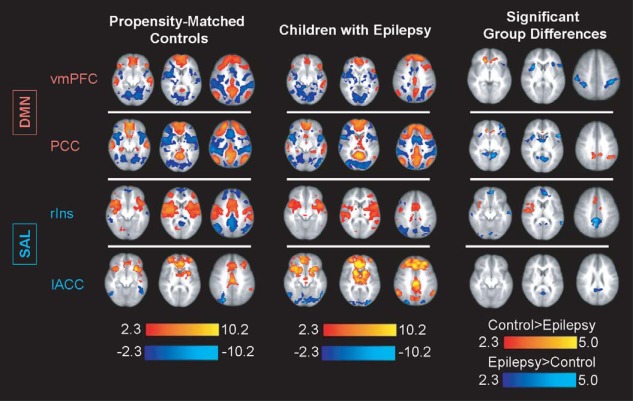
Seed‐based analysis reveals stronger internetwork connectivity and weaker intranetwork connectivity in children with epilepsy. Using core nodes of the DMN and salience networks as ROI, we determined that children with epilepsy demonstrate significantly stronger internetwork connectivity and weaker intranetwork connectivity compared to controls, suggesting a failure of network integration and segregation during development. Color bars represent z‐scores. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 1.
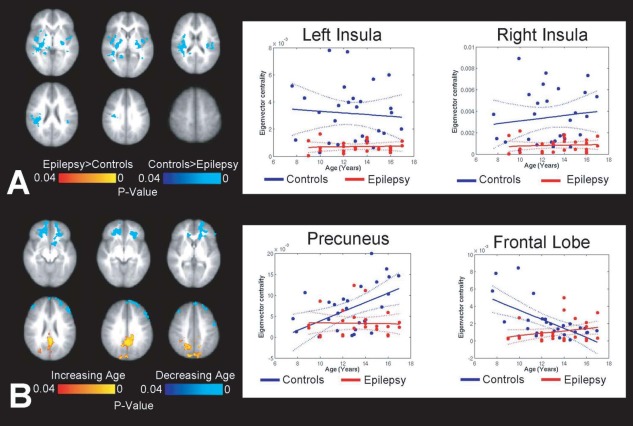
Differences in whole brain connectome hubs in children with epilepsy compared to controls. (A) Group effects show that insulae of children with epilepsy are weaker regional hubs compared to controls. (B) Age effects show that the PCC and frontal lobe demonstrate age‐related interactions in children with epilepsy. The PCC becomes more central as a function of age in controls compared with children with epilepsy, while the centrality of the frontal lobe fails to weaken with age in children with epilepsy. Axes scaled to show variance in data; steepest increases with age identified in the PCC (slopecontrols = 1.1 × 10−3; slopeepilepsy = 7.0 × 10−5). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
To quantify the extent of intranetwork connectivity, we extracted two graph theoretical properties: mean network clustering and average network pathlength (Fig. 3). With increasing age, the network topology of the DMN was characterized by greater clustering and lower characteristic pathlengths (network clustering: age: F (1,50) = 13.98; P < 0.01; group effect: F (1,50) = 0.33, P = NS; group × age interaction: F (1,50) = 0.08; P = NS; characteristic pathlength: age: F (1,50) = 11.26; P < 0.01; group effect: F (1,50) = 0.46, P = NS; group × age interaction: F (1,50) = 0.36; P = NS). Children with epilepsy also possessed lower clustering (F (1,50) = 6.78; P = 0.01) and higher pathlength (F (1,50) = 9.5; P < 0.01) of the salience network. Although internetwork correlations between important nodes of the DMN and salience networks decreased with age (PCC‐ventral ACC correlation; age effect: F (1,50) = 5.95; P = 0.018), children with epilepsy also demonstrated significantly higher internetwork correlations than controls (PCC‐right insula correlation; group effect: F (1,50) = 3.99; P = 0.05).
Figure 3.
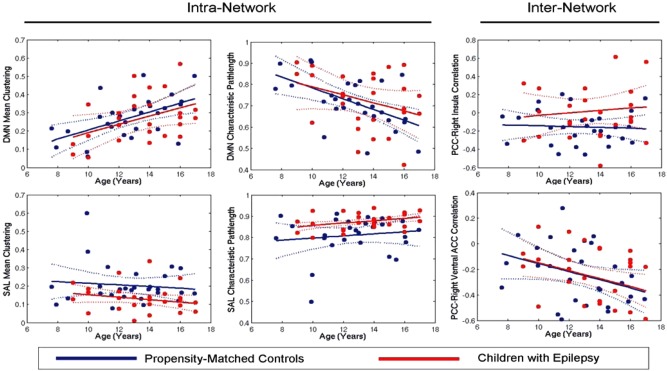
Differing trajectories for functional integration and segregation of DMN and salience networks with age among children with epilepsy and controls. Mean clustering and characteristic pathlength of the DMN increase and decrease with age, respectively. Although children with epilepsy demonstrated less steep slopes, the interaction was not significant. Mean clustering and characteristic pathlength of the salience network were significantly lower and greater in children with epilepsy, respectively. While internetwork correlations decreased with age, children with epilepsy demonstrated greater internetwork correlations than controls among various nodes. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Hierarchical Clustering Reveals Greater Network Disorganization in Children with Epilepsy
Given the evidence for weaker segregation and integration of resting‐state networks in children with epilepsy, we sought to further characterize our findings by extracting the times series of various nodes of the DMN and task/attention control networks. Connectivity matrices were constructed using correlations between mean ROI time series (Fig. 4). Using agglomerative hierarchical clustering, the ROIs within individual ICNs tended to cluster together in control subjects. In children with epilepsy, agglomerative clustering revealed greater disorganization of ICNs. When network edges that were significantly different between the two cohorts were identified and contrasted, it was determined that children with epilepsy possessed significantly more internetwork connections, whereas the controls possessed a greater number of intranetwork connections (P = 0.0005 uncorrected, P = 0.01 corrected; Fisher's Exact test).
Figure 4.
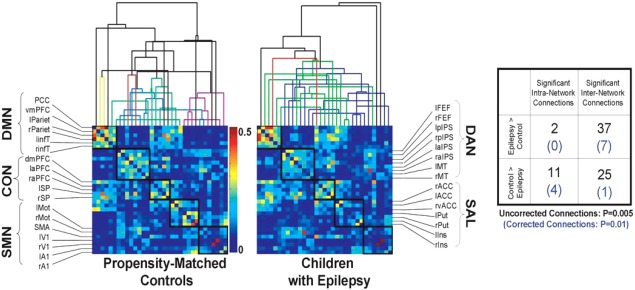
Agglomerative hierarchical clustering reveals more disorganized network architecture in children with epilepsy compared to controls. Less separation of individual networks was observed in children with epilepsy. Children with epilepsy also had a higher frequency of significant internetwork connection and fewer significant intranetwork connections relative to controls (P < 0.01, uncorrected; P = 0.01, corrected). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 5.
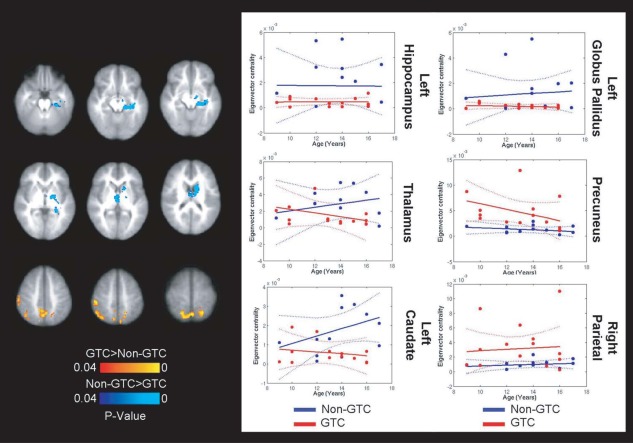
Differential intrinsic connectivity in children with secondarily generalized seizures. The connectomes of children with secondarily generalized demonstrated weaker hubs in the anterior thalamus, left hippocampus, and globus pallidus and greater hubs in nodes of the DMN. The DMN hubs also demonstrated pathological connectivity patterns in seed‐based analysis. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Epilepsy Duration, Location and Semiology Associated with Specific Network Disturbances
Whole brain connectome analyses were subsequently performed on subgroups of children with medically intractable epilepsy to determine the contributions of specific clinical features on connectome topology: epileptic focus (temporal vs. extratemporal), duration of epilepsy and semiology (secondarily generalized vs. nongeneralized). For all subgroup analyses, age was modeled as a covariate.
Children with secondarily generalized seizure semiologies demonstrated decreased centrality of left hippocampus (Fig. 5; F (1,22) = 11.38; P = 0.027), anterior thalamus (F (1,22) = 8.31; P = 0.0086), caudate head (F (1,22) = 10.19; P = 0.0042), and left globus pallidus (F (1,22) = 6.4; P = 0.019), relative to other children with epilepsy. Age was not a significant covariate in these analyses, although an interaction with age approached significance in the head of the caudate (F (1,22) = 3.67; P = 0.069). Children with secondarily generalized seizure semiology also demonstrated greater centrality of regions of the DMN, namely the precuneus (F (1,22) = 9.39; P = 0.0057) and right parietal cortex (F (1,22) = 7.03; P = 0.014). Seed‐based connectivity demonstrated that despite these regions being stronger hubs in children with secondarily generalized seizures, they are more strongly connected to brain regions outside of the DMN, such as the right insula and ventral ACC and less strongly connected to intranetwork nodes, such as the vmPFC (Supporting Information Fig. S9). Interestingly, the precuneus was a stronger hub in the whole‐brain connectome of children with greater seizure frequency (more than 1 seizure per day; Supporting Information Fig. S10).
To investigate the effects of epilepsy duration on ICNs, we included duration as a continuous covariate in the multivariate regression (Fig. 6). Increasing duration of epilepsy was associated with greater centrality of DMN regions, namely the precuneus (t (19) = 4.85; P < 0.001), vmPFC (t (19) = 6.08; P < 0.001) and right parietal cortex (t (19) = 5.20; P < 0.001). Again, although these regions were associated with greater centrality, seed‐based connectivity revealed greater internetwork connections involving these areas with increasing epilepsy duration (Supporting Information Fig. S11). A significant interaction was identified between duration of epilepsy and child age with respect to internetwork correlation. Children with a longer epilepsy duration exhibited greater correlation between core nodes of different ICNs (the PCC and ventral ACC) with increasing age, while children with a lesser duration of epilepsy showed age‐related anticorrelation between these two regions (age × duration interaction: P = 0.007). We also evaluated changes in ICNs as a function of the proportion of life with epilepsy, defined as the ratio of duration of epilepsy to child age, as well as age of seizure onset. These data yielded similar results to regression against epilepsy duration.
Figure 6.
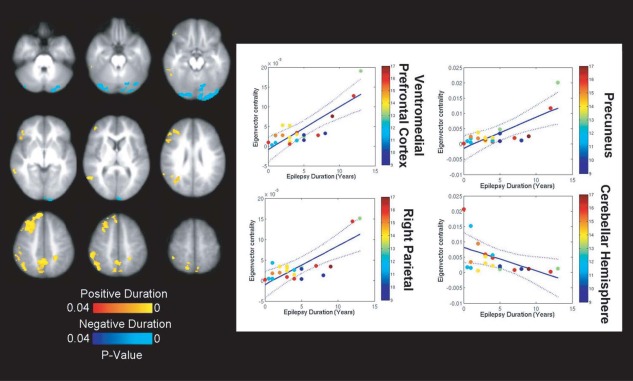
Duration of epilepsy is associated with weaker intranetwork integration and internetwork separation. With longer duration of epilepsy, nodes of the DMN express reduced EC and show greater disorganization. Similar patterns were observed when evaluating proportion of life with epilepsy and age of epilepsy onset as independent variables. Color bars represent distribution of subject ages. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Next, we evaluated whether the localization of epileptic foci was associated with unique functional connectome topologies. Children with epilepsy were dichotomized by the location of the seizure focus into those with temporal and those with extratemporal foci (Fig. 7). Compared to children temporal epileptic foci, those with extratemporal foci showed greater centrality of the temporal lobes and insula bilaterally (right: F (1,22) = 12.93; P = 0.0016; left: F (1,22) = 5.47; P = 0.029) as well as to the left posterior temporal cortex (F (1,22) = 9.82; P = 0.0048). The only region showing decreased centrality in children with temporal lobe seizure foci compared to extratemporal foci was the left parietal cortex (F (1,22) = 8.14; P = 0.0092), where an interaction with age was also significant (F (1,22) = 4.74; P = 0.04). No significant differences in DMN and salience network connectivity were identified using a seed‐based approach between children with temporal and extratemporal seizure foci.
Figure 7.
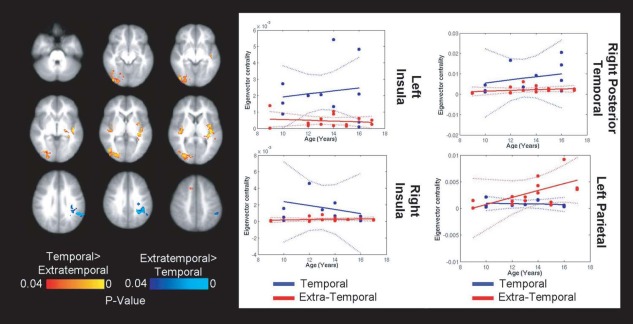
Extratemporal epilepsy location associated with weaker centrality in the salience network. Bilateral insular regions were weaker hubs in the connectomes of children with extratemporal epilepsy relative to children with temporal lobe epilepsy. The latter demonstrated greater centrality of the lateral parietal cortex (a component of the DMN). No significant differences were observed with seed‐based connectivity between the two groups. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DMN Network Integration Associated with Working Memory Capacity While Internetwork Correlations Associated with Global Deficits
On multivariate linear regression, after adjusting for child age, increased clustering of the DMN was associated with higher scores on neuropsychological testing for working memory (Supporting Information Fig. S12; forward digit span recall score: t (24)=2.10; P = 0.046; age effect: t (24) = 4.36; P < 0.01). There was no significant group effect (P = 0.70) or two‐way interactions, suggesting that maturation of DMN clustering is associated with improved working memory scores in both subject cohorts. Similarly, decreased pathlength of the DMN was associated with higher working memory scores (forward digit span score: t (24) = −2.28; P = 0.032; age effect: t (24) = −4.16; P < 0.01). Again no significant group effect (P = 0.64) or two‐way interactions were identified. The findings were specific to the DMN, as neither salience network clustering nor pathlength was associated with working memory function. Interestingly, although graph theoretical measures of DMN integration were associated with working memory, increased centrality of the PCC in itself was more strongly associated with forward digit span recall in controls than children with epilepsy, as indicated by the significant interaction term (Supporting Information Fig. S13; forward digit span: t (23)=2.19; P = 0.039; forward digit span × group interaction: t (23) = −2.08; P = 0.048; backward digit span: t (21) = 3.44; P < 0.01; age: t (21) = 2.98; P < 0.01; backward digit span × group: t (21) = −2.15; P = 0.043; age × group: t (21) = −2.46; P = 0.022).
Greater internetwork anticorrelation, indexed by the correlation coefficient between the PCC (DMN) and ventral anterior ACC (salience network), was also associated with higher full‐scale IQ scores in children with epilepsy (IQ score: t (16) = −3.20; P < 0.01; age effect: t (16) = −2.67, P = 0.016; IQ score × age interaction: t (16) = −1.04; P = 0.31). A trend toward an interaction was also identified between IQ score and group classification (IQ × group: t (41) = −1.82; P = 0.075), suggesting that the association between IQ and network segregation may be stronger in children with epilepsy compared to controls (Supporting Information Fig. S14). Weaker centrality of the frontal lobe was also associated with higher IQ scores in all children (IQ score: t (41) = −2.37; P = 0.022; age effect: t (46) = −4.60, P < 0.01; group effect: t (41) = −4.29; P < 0.01). There was no significant interaction between group and IQ with respect to centrality of the frontal lobe (P = 0.47), although, as previously noted, a significant interaction was observed between group and age (P < 0.001).
DISCUSSION
The intersection of childhood epilepsy, brain network development, and cognitive outcome is poorly understood. The current report provides evidence of impaired development of long‐range connectivity in children with medically intractable epilepsy, and demonstrates associations between abnormal ICN development and cognitive deficits exhibited by these patients, as previously suspected [Hepworth and Smith, 2002; Smith et al., 2002, 2004]. We also examine the effects of intractable epilepsy on the normative development of ICNs, and show that important regions of the default mode and salience networks demonstrate less hub‐like connectivity properties compared to propensity‐matched controls. We found that normative patterns of anticorrelation between task‐positive and task‐negative networks are disrupted with greater internetwork connectivity and weaker intranetwork integration in children with epilepsy and that these alterations in intrinsic brain connectivity are associated with poor cognitive outcome. Furthermore, we show that the duration of epilepsy is associated with altered developmental trajectory of these networks, supporting the model of earlier surgical treatment of children with localization‐related epileptic foci. By understanding impaired emergence of ICNs in children with epilepsy, we also hope to elucidate markers of impairment to benchmark therapeutic medical and surgical interventions.
Changes observed in DMN and attention control networks during neurodevelopment are consistent with other reports in the literature of increasing functional segregation of adjacent regions and integration of distributed hubs into common networks [Anderson et al., 2011b; Fair et al., 2008; Kelly et al., 2009]. Others have also shown that typical development is associated with increased cingulo‐insular connectivity and decreased fronto‐parietal connectivity [Dosenbach et al., 2010]. We demonstrate that localization‐related epilepsy interacts with normal developmental trajectories, resulting in deviations from expected patterns of intranetwork integration and internetwork segregation.
In our analysis of whole brain connectomes using graph theoretical metrics, we found a significant interaction between group and age in the PCC, which became a stronger hub as a function of age in controls compared to children with epilepsy. Intranetwork DMN connectivity involving the PCC has been shown to exhibit more rapid maturation over this age range than any other ICN [Kelly et al., 2009]; therefore, the interaction of typical development with epilepsy in this region may explain dysfunction of DMN activity that has been previously documented in young adults with epilepsy [Kay et al., 2013; McCormick et al., 2013; Zeng et al., 2013]. The finding that the PCC demonstrated significantly greater internetwork connectivity in children with epilepsy may explain why PCC centrality was not associated with digit span recall in this cohort, although increases in DMN clustering and decreases in pathlength were associated with working memory.
We have also shown that hub properties of a large portion of the frontal lobe fail to decrease with age in children with epilepsy, relative to the control children. Other studies have shown that the process of development over a similar age range involves a transition from “local” to “globally distributed” networks [Fair et al., 2009]. A hallmark of this transition is the loss of extensive cross‐correlations that exist within the frontal lobe and the fractionation of frontal lobe subregions that become integrated into various ICNs [Fair et al., 2009]. The interaction between age and group classification strongly suggests a disruption of typical fractionation of frontal regions into the task‐control and task‐negative regions, which was confirmed in the current study through seed‐based connectivity analyses. Interestingly, both decreasing frontal lobe centrality and greater internetwork segregation were associated with higher IQ, further supporting the claim that they may be inter‐related processes.
The association we identified between epilepsy duration (as well as proportion of life with the disease) and failure of network integration and segregation demonstrates that the burden of epilepsy adversely affects the development of brain networks. Previous studies have identified cognitive difficulties at the onset of epilepsy, suggesting that brain network dysfunction cannot be exclusively interpreted as the long‐term effects of the disease or seizures [Hermann et al., 2012; Jackson et al., 2013; Oostrom et al., 2003]. The majority of participants in the current study were children with chronic epilepsy. We show that the cumulative burden of epilepsy affects developmental trajectory, independent of the child's age. Importantly, our entire population also consisted of children with localization‐related medically intractable epilepsy, which is potentially amenable to surgical intervention. Given our findings, we suspect that early surgical treatment of affected children may mitigate the detrimental effects of chronic, repetitive seizure activity on the development of these networks. Future studies should also evaluate whether network disturbances return to the normative developmental trajectories following intervention, which would allow these findings to serve as markers of therapeutic effect. Indeed, a single case report recently suggested that following corpus callosotomy, ICNs showed greater internetwork separation [Pizoli et al., 2011].
Of particular importance was the finding that children with secondarily generalized seizure semiologies possessed less centrality in the anterior thalamus, left hippocampus, and left globus pallidus compared to those without secondarily generalized seizure activity. The laterality of these findings may be a reflection of the fact that the majority (73%) of children included with secondarily generalized seizures had epilepsy foci in the left hemisphere. Generalized seizures are recognized as the end result of a common pathway in seizure expression. While the corpus callosum is the primary pathway for interhemispheric propagation of seizure activity, other routes have been described involving basal ganglia, thalami, and brainstem reticular formation [Blumenfeld et al., 2009; Theodore et al., 1994]. Thalamocortical circuits are thought to play an important role in generalized seizure activity [Pulsipher et al., 2011]. Morphological studies in adults with idiopathic generalized epilepsy (IGE) have demonstrated gray matter deficits involving the thalamus, caudate, putamen, and cortical structures [Ciumas and Savic, 2006] and thalamic deactivation has been observed in response to generalized interictal discharges [Gotman et al., 2005; Moeller et al., 2008]. Other resting‐state studies have also proposed that functional alterations in subcortical structures are evident in adults with IGE [Wang et al., 2012; Zhang et al., 2011]. The current study revealed disengagement of the thalamus and other subcortical structures from whole brain connectomes of children with secondarily generalized seizures. These findings support the hypothesis that active inhibition of subcortical arousal systems may be involved in seizure semiologies characterized by decreased levels of consciousness. The translational utility of these findings is buttressed by the fact that stimulation of the anterior thalamus [Fisher et al., 2010] and hippocampi [McLachlan et al., 2010] are putative strategies for the treatment of medication‐resistant epilepsy. Our findings lead us to speculate that these interventions may exert a therapeutic effect by engaging these disconnected hubs within the network architecture of the brain.
Our study is unique in that we applied graph theoretical analysis and hierarchical clustering methods to examine the integration and segregation of ICNs during a critical developmental phase. Importantly, stringent quality‐assurance reduced the impact of head motion on connectivity results, as we excluded half of our original subject cohort due to excessive motion. Furthermore, whereas the majority of previous studies evaluated patients with IGE, all subjects herein were affected by localization‐related epilepsy, which is amenable to surgical treatment. Finally, our study is the first to examine the intersection of epilepsy and development of ICNs. Increased awareness of deviations from normative trajectories may yield markers to assess efficacy of future interventions.
Limitations and Future Directions
The manifestation of focal epilepsy in childhood is much more heterogeneous than adults. In contrast to adults, nearly half of children may present with extensive extratemporal or multilobar epileptic foci [Hemb et al., 2010; Kim et al., 2008]. Furthermore, surgically remediable syndromes are much more heterogeneous in children. We hypothesized that ICNs would vary depending on the expression of epilepsy syndromes in children and indeed identified differences based on epilepsy duration, seizure focus location, and clinical semiology. A larger database of functional imaging of children with intractable epilepsy is required to disentangle the effects of other variables.
Another consideration is the effect of antiepileptic drugs (presented in Supporting Information) on the findings observed. We have previously analyzed patterns of antiepileptic drug used in this patient cohort [Ibrahim et al., 2013] and determined that Levetiracetam was the most commonly used across in multiple drug regimens. It is unknown whether Levetiracetam affects resting‐state connectivity. The drug combinations administered were, however, distributed in a nonspecific pattern across patient subgroups, and therefore, unlikely to affect group differences.
Although we demonstrate that epilepsy affects the development of ICNs, the pathological processes that drive these deviations from typical developmental trajectories have yet to be elucidated. One putative mechanism involves interictal discharges, which have been shown to suspend DMN activity in previous studies evaluating adults with epilepsy [Gotman et al., 2005]. Ictal dynamics have also been previously shown to disrupt synchronization of neural oscillations in eloquent brain regions that may be distant from site of seizure origin [Ibrahim et al., 2012]. Such disruptions have been associated with specific clinical deficits that persist beyond the ictal period. Evaluation of ICNs in children with epilepsy using multiple modalities with varying temporo‐spatial resolutions as well as longer fMRI scans to capture BOLD variability is indicated to better characterize the mechanisms of network impairment.
CONCLUSIONS
The current study provides evidence for impaired development of ICNs in children with medically intractable localization‐related epilepsy. Regions of the brain that are central to the DMN and salience networks were weaker hubs in children with epilepsy compared to matched controls. Furthermore, affected children demonstrated weaker integration and segregation of ICNs. Atypical ICN development was associated with poor cognitive outcomes, suggesting that epilepsy may interfere with maturation of communication of functional brain networks, thereby contributing to cognitive deficits that are prevalent in this population. Patterns of network dysfunction also differed in various clinical epilepsy phenotypes. These findings support the model of early treatment of epilepsy and may provide a benchmark to assess efficacy of therapeutic interventions, once validated by longitudinal studies.
Supporting information
Supplementary Information
Conflicts of interest: The authors have no conflicts of interest to declare.
REFERENCES
- Anderson JS, Druzgal TJ, Lopez‐Larson M, Jeong EK, Desai K, Yurgelun‐Todd D (2011a): Network anticorrelations, global regression, and phase‐shifted soft tissue correction. Hum Brain Mapp 32:919–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JS, Ferguson MA, Lopez‐Larson M, Yurgelun‐Todd D (2011b): Connectivity gradients between the default mode and attention control networks. Brain Connect 1:147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34:537–541. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, Varghese GI, Purcaro MJ, Motelow JE, Enev M, McNally KA, Levin AR, Hirsch LJ, Tikofsky R, Zubal IG, Paige AL, Spencer SS (2009): Cortical and subcortical networks in human secondarily generalized tonic‐clonic seizures. Brain 132:999–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier MR, Thomas JB, Snyder AZ, Benzinger TL, Zhang D, Raichle ME, Holtzman DM, Morris JC, Ances BM (2012): Loss of intranetwork and internetwork resting state functional connections with Alzheimer's disease progression. J Neurosci 32: 8890–8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes MJ, Hale JR, Zumer JM, Stevenson CM, Francis ST, Barnes GR, Owen JP, Morris PG, Nagarajan SS (2011a): Measuring functional connectivity using MEG: Methodology and comparison with fcMRI. Neuroimage 56:1082–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes MJ, Woolrich M, Luckhoo H, Price D, Hale JR, Stephenson MC, Barnes GR, Smith SM, Morris PG (2011b): Investigating the electrophysiological basis of resting state networks using magnetoencephalography. Proc Natl Acad Sci USA 108:16783–16788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciumas C, Savic I (2006): Structural changes in patients with primary generalized tonic and clonic seizures. Neurology 67:683–686. [DOI] [PubMed] [Google Scholar]
- Cox RW (1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research 29:162–173. [DOI] [PubMed] [Google Scholar]
- de Pasquale F, Della Penna S, Snyder AZ, Lewis C, Mantini D, Marzetti L, Belardinelli P, Ciancetta L, Pizzella V, Romani GL, Corbetta M (2010): Temporal dynamics of spontaneous MEG activity in brain networks. Proc Natl Acad Sci USA 107:6040–6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, Wig GS, Vogel AC, Lessov‐Schlaggar CN, Barnes KA, Dubis JW, Feczko E, Coalson RS, Pruett JR Jr, Barch DM, Petersen SE, Schlaggar BL (2010): Prediction of individual brain maturity using fMRI. Science 329:1358–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL (2008): The maturing architecture of the brain's default network. Proc Natl Acad Sci USA 105:4028–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE (2009): Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol 5: e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, Oommen K, Osorio I, Nazzaro J, Labar D, Kaplitt M, Sperling M, Sandok E, Neal J, Handforth A, Stern J, DeSalles A, Chung S, Shetter A, Bergen D, Bakay R, Henderson J, French J, Baltuch G, Rosenfeld W, Youkilis A, Marks W, Garcia P, Barbaro N, Fountain N, Bazil C, Goodman R, McKhann G, Babu Krishnamurthy K, Papavassiliou S, Epstein C, Pollard J, Tonder L, Grebin J, Coffey R, Graves N, SANTE Study Group (2010): Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 51:899–908. [DOI] [PubMed] [Google Scholar]
- Foster BL, Parvizi J (2012): Resting oscillations and cross‐frequency coupling in the human posteromedial cortex. Neuroimage 60:384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102: 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME (2006): Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA 103:10046–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P (2005): Spontaneous low‐frequency BOLD signal fluctuations: An fMRI investigation of the resting‐state default mode of brain function hypothesis. Hum Brain Mapp 26:15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gili T, Saxena N, Diukova A, Murphy K, Hall JE, Wise RG (2013): The thalamus and brainstem act as key hubs in alterations of human brain network connectivity induced by mild propofol sedation. J Neurosci 33:4024–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golestani AM, Goodyear BG (2011): Regions of interest for resting‐state fMRI analysis determined by inter‐voxel cross‐correlation. Neuroimage 56:246–251. [DOI] [PubMed] [Google Scholar]
- Gotman J, Grova C, Bagshaw A, Kobayashi E, Aghakhani Y, Dubeau F (2005): Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc Natl Acad Sci USA 102:15236–15240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF (2009): Resting‐state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 19:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME (2001): Medial prefrontal cortex and self‐referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA 98:4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT (2006): Brain connectivity related to working memory performance. J Neurosci 26:13338–13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemb M, Velasco TR, Parnes MS, Wu JY, Lerner JT, Matsumoto JH, Yudovin S, Shields WD, Sankar R, Salamon N, Vinters HV, Mathern GW (2010): Improved outcomes in pediatric epilepsy surgery: The UCLA experience, 1986–2008. Neurology 74:1768–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth S, Smith M (2002): Learning and recall of story content and spatial location after unilateral temporal‐lobe excision in children and adolescents. Child Neuropsychol 8:16–26. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Jones JE, Jackson DC, Seidenberg M (2012): Starting at the beginning: The neuropsychological status of children with new‐onset epilepsies. Epileptic Disord 14:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho D, Imai K, King G, Stuart E (2007a): Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal 15:199–236. [Google Scholar]
- Ho D, Imai K, King G, Stuart E (2007b): Matchit: Nonparametric preprocessing for parametric causal inference. J Stat Softw 42:1–28. [Google Scholar]
- Hocking RR (1976): The analysis and selection of variables in linear regression. Biometrics 32:1–44. [Google Scholar]
- Ibrahim GM, Akiyama T, Ochi A, Otsubo H, Smith ML, Taylor MJ, Donner E, Rutka JT, Snead OC III, Doesburg SM (2012): Disruption of rolandic gamma‐band functional connectivity by seizures is associated with motor impairments in children with epilepsy. PLoS One 7:e39326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim GM, Rutka JT, Snead OC III (2013): Network analysis reveals patterns of antiepileptic drug use in children with medically intractable epilepsy. Epilepsy Behav 28:22–25. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Dabbs K, Walker NM, Jones JE, Hsu DA, Stafstrom CE, Seidenberg M, Hermann BP (2013): The neuropsychological and academic substrate of new/recent‐onset epilepsies. J Pediatr 162:1047–53.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012): FSL. NeuroImage 62:782–790. [DOI] [PubMed] [Google Scholar]
- Kay BP, DiFrancesco MW, Privitera MD, Gotman J, Holland SK, Szaflarski JP (2013): Reduced default mode network connectivity in treatment‐resistant idiopathic generalized epilepsy. Epilepsia 54:461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating LE (1960): A review of the literature on the relationship of epilepsy and intelligence in school children. J Ment Sci 106:1042–1059. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Margulies DS, Castellanos FX, Milham MP (2009): Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex 19:640–657. [DOI] [PubMed] [Google Scholar]
- Kim H (2012): A dual‐subsystem model of the brain's default network: Self‐referential processing, memory retrieval processes, and autobiographical memory retrieval. Neuroimage 61:966–977. [DOI] [PubMed] [Google Scholar]
- Kim SK, Wang KC, Hwang YS, Kim KJ, Chae JH, Kim IO, Cho BK (2008): Epilepsy surgery in children: Outcomes and complications. J Neurosurg Pediatr 1:277–283. [DOI] [PubMed] [Google Scholar]
- Korman B, Krsek P, Duchowny M, Maton B, Pacheco‐Jacome E, Rey G (2013): Early seizure onset and dysplastic lesion extent independently disrupt cognitive networks. Neurology 81:745–751. [DOI] [PubMed] [Google Scholar]
- Lim ME, Bowen JM, Snead OC III, Elliott I, Donner E, Weiss SK, Otsubo H, Ochi A, Drake J, Rutka JT, Worster A, Hopkins RB, Goeree R, Tarride JE (2013): Access to surgery for paediatric patients with medically refractory epilepsy: A systems analysis. Epilepsy Res 107:286–296. [DOI] [PubMed] [Google Scholar]
- Mankinen K, Jalovaara P, Paakki JJ, Harila M, Rytky S, Tervonen O, Nikkinen J, Starck T, Remes J, Rantala H, Kiviniemi V (2012): Connectivity disruptions in resting‐state functional brain networks in children with temporal lobe epilepsy. Epilepsy Res 100:168–178. [DOI] [PubMed] [Google Scholar]
- McCormick C, Quraan M, Cohn M, Valiante TA, McAndrews MP (2013): Default mode network connectivity indicates episodic memory capacity in mesial temporal lobe epilepsy. Epilepsia 54:809–818. [DOI] [PubMed] [Google Scholar]
- McLachlan RS, Pigott S, Tellez‐Zenteno JF, Wiebe S, Parrent A (2010): Bilateral hippocampal stimulation for intractable temporal lobe epilepsy: Impact on seizures and memory. Epilepsia 51:304–307. [DOI] [PubMed] [Google Scholar]
- Moeller F, Siebner HR, Wolff S, Muhle H, Boor R, Granert O, Jansen O, Stephani U, Siniatchkin M (2008): Changes in activity of striato‐thalamo‐cortical network precede generalized spike wave discharges. Neuroimage 39:1839–1849. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA (2009): The impact of global signal regression on resting state correlations: are anti‐correlated networks introduced? Neuroimage 44:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP (2002): Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp 15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir Y, Mukamel R, Dinstein I, Privman E, Harel M, Fisch L, Gelbard‐Sagiv H, Kipervasser S, Andelman F, Neufeld MY, Kramer U, Arieli A, Fried I, Malach R (2008): Interhemispheric correlations of slow spontaneous neuronal fluctuations revealed in human sensory cortex. Nat Neurosci 11:1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostrom KJ, Smeets‐Schouten A, Kruitwagen CL, Peters AC, Jennekens‐Schinkel A, Dutch Study Group of Epilepsy in Childhood (2003): Not only a matter of epilepsy: Early problems of cognition and behavior in children with “epilepsy only”—A prospective, longitudinal, controlled study starting at diagnosis. Pediatrics 112:1338–1344. [DOI] [PubMed] [Google Scholar]
- Pickering S, Gathercole S (2001): Working memory test batter for children (WMTB‐C) Manual. London: The Psychological Corporation. [Google Scholar]
- Pizoli CE, Shah MN, Snyder AZ, Shimony JS, Limbrick DD, Raichle ME, Schlaggar BL, Smyth MD (2011): Resting‐state activity in development and maintenance of normal brain function. Proc Natl Acad Sci USA 108:11638–11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulsipher DT, Dabbs K, Tuchsherer V, Sheth RD, Koehn MA, Hermann BP, Seidenberg M (2011): Thalamofrontal neurodevelopment in new‐onset pediatric idiopathic generalized epilepsy. Neurology 76:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ (2007): A default mode of brain function: a brief history of an evolving idea. Neuroimage 37:1083–1090; discussion 1097–1099. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci USA 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB (1997): Estimating causal effects from large data sets using propensity scores. Ann Intern Med 127:757–763. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O (2010): Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 52:1059–1069. [DOI] [PubMed] [Google Scholar]
- Ruff RM, Parker SB (1993): Gender‐ and age‐specific changes in motor speed and eye‐hand coordination in adults: Normative values for the finger tapping and grooved pegboard tests. Percept Mot Skills 76:1219–1230. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW (2012): Trouble at rest: How correlation patterns and group differences become distorted after global signal regression. Brain Connect 2:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Elliott IM, Lach L (2002): Cognitive skills in children with intractable epilepsy: Comparison of surgical and nonsurgical candidates. Epilepsia 43:631–637. [DOI] [PubMed] [Google Scholar]
- Smith ML, Elliott IM, Lach L (2004): Cognitive, psychosocial, and family function one year after pediatric epilepsy surgery. Epilepsia 45:650–660. [DOI] [PubMed] [Google Scholar]
- Sokal R, Michener C (1958): A statistical method for evaluating systematic relationships. University of Kansas Science Bulletin 38:1409–1438. [Google Scholar]
- Theodore WH, Porter RJ, Albert P, Kelley K, Bromfield E, Devinsky O, Sato S (1994): The secondarily generalized tonic‐clonic seizure: A videotape analysis. Neurology 44:1403–1407. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Lynch CJ, Khouzam A, Phillips J, Feinstein C, Ryali S, Menon V (2013): Salience network‐based classification and prediction of symptom severity in children with autism. JAMA Psychiatry 70:869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaessen MJ, Braakman HM, Heerink JS, Jansen JF, Debeij‐van Hall MH, Hofman PA, Aldenkamp AP, Backes WH (2012): Abnormal modular organization of functional networks in cognitively impaired children with frontal lobe epilepsy. Cereb Cortex 23:1997–2006. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RC, Kahn RS, Hulshoff Pol HE (2009): Functionally linked resting‐state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp 30:3127–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zuo X, He Y (2010): Graph‐based network analysis of resting‐state functional MRI. Front Syst Neurosci 4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zhang Z, Jiao Q, Liao W, Chen G, Sun K, Shen L, Wang M, Li K, Liu Y, Lu G (2012): Impairments of thalamic nuclei in idiopathic generalized epilepsy revealed by a study combining morphological and functional connectivity MRI. PLoS One 7:e39701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington SD, Gordon EM, Brar J, Warburton S, Sawyer AT, Wolfe A, Mease‐Ference ER, Girton L, Hailu A, Mbwana J, Gaillard WD, Kalbfleisch ML, Vanmeter JW (2014): Dysmaturation of the default mode network in autism. Hum Brain Mapp 35:1284–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH (1998): Collective dynamics of ‘small‐world’ networks. Nature 393:440–442. [DOI] [PubMed] [Google Scholar]
- Wechsler D (2003): WISC‐IV Administration Manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C (2009): Correlations and anticorrelations in resting‐state functional connectivity MRI: A quantitative comparison of preprocessing strategies. Neuroimage 47:1408–1416. [DOI] [PubMed] [Google Scholar]
- Widjaja E, Zamyadi M, Raybaud C, Snead OC, Smith ML (2013a): Abnormal functional network connectivity among resting‐state networks in children with frontal lobe epilepsy. AJNR Am J Neuroradiol 34:2386–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widjaja E, Zamyadi M, Raybaud C, Snead OC, Smith ML (2013b): Impaired default mode network on resting‐state fMRI in children with medically refractory epilepsy. AJNR Am J Neuroradiol 34:552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM (2001): Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage 14:1370–1386. [DOI] [PubMed] [Google Scholar]
- Worsley KJ (2001): Statistical analysis of activated images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An Introduction to Methods, Chapter 14. OUP, Oxford. [Google Scholar]
- Yakushev I, Chetelat G, Fischer FU, Landeau B, Bastin C, Scheurich A, Perrotin A, Bahri MA, Drzezga A, Eustache F, Schreckenberger M, Fellgiebel A, Salmon E (2013): Metabolic and structural connectivity within the default mode network relates to working memory performance in young healthy adults. Neuroimage 79:184–190. [DOI] [PubMed] [Google Scholar]
- Zeng H, Pizarro R, Nair VA, C, La Prabhakaran V (2013): Alterations in regional homogeneity of resting‐state brain activity in mesial temporal lobe epilepsy. Epilepsia 54:658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Liao W, Chen H, Mantini D, Ding JR, Xu Q, Wang Z, Yuan C, Chen G, Jiao Q, Lu G (2011): Altered functional‐structural coupling of large‐scale brain networks in idiopathic generalized epilepsy. Brain 134:2912–2928. [DOI] [PubMed] [Google Scholar]
- Zuo XN, Ehmke R, Mennes M, Imperati D, Castellanos FX, Sporns O, Milham MP (2012): Network centrality in the human functional connectome. Cereb Cortex 22:1862–1875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


