Abstract
Obese adolescents suffer negative social experiences, but no studies have examined whether obesity is associated with dysfunction of the social brain or whether social brain abnormalities relate to disadvantageous traits and social decisions. We aimed at mapping functional activation differences in the brain circuitry of social decision making in adolescents with excess versus normal weight, and at examining whether these separate patterns correlate with reward/punishment sensitivity, disordered eating features, and behavioral decisions. In this fMRI study, 80 adolescents aged 12 to 18 years old were classified in two groups based on age adjusted body mass index (BMI) percentiles: normal weight (n = 44, BMI percentiles 5th–84th) and excess weight (n = 36, BMI percentile ≥ 85th). Participants were scanned while performing a social decision‐making task (ultimatum game) in which they chose to “accept” or “reject” offers to split monetary stakes made by another peer. Offers varied in fairness (Fair vs. Unfair) but in all cases “accepting” meant both players win the money, whereas “rejecting” meant both lose it. We showed that adolescents with excess weight compared to controls display significantly decreased activation of anterior insula, anterior cingulate, and midbrain during decisions about Unfair versus Fair offers. Moreover, excess weight subjects show lower sensitivity to reward and more maturity fears, which correlate with insula activation. Indeed, blunted insula activation accounted for the relationship between maturity fears and acceptance of unfair offers. Excess weight adolescents have diminished activation of brain regions essential for affective tracking of social decision making, which accounts for the association between maturity fears and social decisions. Hum Brain Mapp, 36:–237, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: obesity, social decision making, anterior insula, fMRI
INTRODUCTION
Adolescent obesity is a major public health problem that has rapidly attained epidemic levels [Gee et al., 2013; Ji, 2008; Rudolf et al., 2004; Strauss and Pollack, 2001]. Neuroscience models posit that major societal changes have transferred the obesity problem to the decision‐making field: in plentiful environments, decision making is essential to prioritize what to eat (i.e., health‐wise versus rewarding unhealthy food) [Zheng and Berthoud, 2007]. In fitting with this notion, we have shown that adolescents with excess weight have decreased activation of risk‐sensitive brain regions and increased activation of reward‐signaling brain regions during decisions about small safe rewards versus high risky gains [Delgado‐Rico et al., 2013]. However, the relevance of decision making to adolescent obesity goes beyond factoring personal rewards, and extends to the social‐evaluative domain. Adolescents with excess weight suffer significantly more peer bullying, marginalization, and social isolation [Ludwig, 2007; Strauss and Pollack, 2003]. These negative social experiences are the main predictor of poor psychosocial adjustment in children and adolescents with obesity [Gunnarsdottir et al., 2012]. Moreover, social stress is known to decrease prosocial choices in adolescents [Youssef et al., 2012], and preclinical studies indicate that this detrimental impact is mediated by neuroadaptations in prefrontal and limbic regions [Baarendse et al., 2013; McEwen, 2007]. Therefore, excess weight adolescents are likely to experience social stress and social decision‐making deficits, which should manifest in prefrontal‐limbic neuroadaptations.
The ultimatum game (UG) is a social decision‐making task in which two parties (the proposer and the respondent) negotiate how to share a specified amount of money. The proposer makes the offer (sharing around 15, 25, or 50% of the stake) and the respondent chooses to either accept, in which case the money is split the way is offered, or reject, in which case none of the parties get any money. Set this way, the task raises a conflict between the cognitive choice (accepting the offer, getting the money) and the emotional response to unfairness (unfair offers elicit negative affect and increase rejection) [van't Wout et al., 2006]. The typical neural network activated during unfair versus fair offers involve the anterior insula, the dorsolateral prefrontal cortex and the anterior cingulate cortex, purportedly involved in perception of unfairness, cognitive evaluation, and conflict between emotion and cognition, respectively, [King‐Casas et al., 2008; Knoch et al., 2006; Sanfey et al., 2003]. Moreover, brain regions involved in reward prediction and emotional learning further contribute to subjective feelings about the offers and behavioral decisions to accept/reject [Gospic et al., 2011; Hollmann et al., 2011]. Therefore, the UG poses an interpersonal decision‐making conflict in which brain regions typically involved in emotion and reward processing come into play in the social domain [Xiang et al., 2013]. Further, the degree of engagement of this circuitry in response to unfair offers might be sensitive to psychological characteristics of the excess weight population that are disadvantageous in the social domain. Specifically, obesity has been associated with high maturity fears, which reflects the anxiety of facing the social‐evaluative demands of adult life [Garner, 1994]. These fears are the most potent determinant of social maturation during adolescence [Westenberg et al., 2004]. Further, obese populations typically display low sensitivity to reward and high sensitivity to punishment [Davis, 2009], which are known to impact social function specifically during adolescence [Harms et al., 2014].
In this study, we aimed at mapping the activation of the social decision‐making brain circuitry as measured by the UG in adolescents with excess versus normal weight and examining the association between separate patterns of activation (in excess vs. normal weight groups) and psychological traits including reward sensitivity and disordered eating features that are central to obesity and social decision‐making behavior. On the basis of previous evidence, we expect that excess weight adolescents display blunted activation of regions involved in social decisions (i.e., anterior cingulate, insula) and social rewards (i.e., striatum, amygdala), and that these separate patterns correlate with reward sensitivity, obesity‐related traits, and behavioral decisions of accept/reject.
MATERIALS AND METHODS
Participants
Eighty adolescents aged between 12 and 18 years participated in the study. They were classified in two groups (normal weight [n = 44] and excess weight [n = 36]) based on their age adjusted body mass index (BMI) percentile [Cole and Lobstein, 2012]. The classification of the two groups was conducted in alignment with the guidelines of the International Obesity Task Force and the Centers for Disease Control and Prevention: Normal weight participants had age adjusted BMI percentiles in the range between the 5th and the 84th percentile, and excess weight participants had age adjusted BMI percentiles ≥ 85 (see Table 1). Participants' sociodemographic characteristics, BMIs, percentage fat, and blood count‐based biochemical parameters are as well displayed in Table 1. Participants were recruited from the paediatrics and endocrinology services of the Hospital “Virgen de las Nieves” in Granada (Spain), and from schools located in the same geographical area. The inclusion criteria for participants were defined as follows: (i) age range between 12 and 18 years; (ii) BMI percentiles falling within the intervals categorized as overweight or obesity (≥85: Excess weight group), or normal weight (5–84: Normal weight group); (iii) absence of history or current evidence of neurological or psychiatric disorders, assessed by participants and parents interviews and the Eating Disorder Inventory [Garner, 1994]; (iv) absence of significant abnormalities on magnetic resonance imaging (MRI) or any contraindications to MRI scanning (including claustrophobia and implanted ferromagnetic objects). All participants had normal or corrected‐to‐normal vision.
Table 1.
Demographic and body characteristic, scores from SPSRQ and EDI‐2 and behavioral performance during the UG inside the scanner
| Normal weight | Excess weight | ||
|---|---|---|---|
| (n =44) | (n = 36) | P‐value | |
| mean (SD) | mean (SD) | ||
| Demographic variables | |||
| Age | 15.32 (1.69) | 15.06 (1.88) | 0.514 |
| Gender (male/female) | 19/25 | 12/24 | 0.375 |
| BMI | 20.96 (2.31) | 29.11 (3.90) | <0.001 |
| Range of BMI percentiles | 9–84 | 85–97 | |
| Fat (%) | 18.49 (33.89) | 33.89 (8.33) | <0.001 |
| Biochemical parameters | |||
| Insulin | 33.61 (37.78) | 40.26(50.91) | 0.548 |
| Basal glucose | 90.61 (10.42) | 90.85 (7.85) | 0.910 |
| Triglycerides | 66.70 (28.63) | 83.11 (35.02) | 0.025 |
| HDL | 59.04 (14.86) | 55.38 (12.90) | 0.253 |
| Total cholesterol | 150.23 (24.52) | 163.86 (29.77) | 0.029 |
| Sensitivity to punishment and reward | |||
| Sensitivity to punishment | 10.52 (5.15) | 10.39 (5.03) | 0.907 |
| Sensitivity to reward | 11.84 (4.15) | 9.78 (3.50) | 0.020 |
| Eating disorders scales | |||
| Drive for thinness | 3.00 (4.27) | 7.63 (5.81) | <0.001 |
| Bulimia | 1.33 (2.18) | 1.16 (1.76) | 0.716 |
| Body dissatisfaction | 5.50 (5.97) | 12.25 (7.32) | <0.001 |
| Ineffectiveness | 3.22 (3.53) | 3.34 (3.82) | 0.892 |
| Perfectionism | 5.25 (4.10) | 5.44 (3.50) | 0.841 |
| Interpersonal distrust | 3.56 (3.00) | 2.50 (2.75) | 0.137 |
| Interoceptive awareness | 4.17 (3.92) | 4.09 (3.60) | 0.937 |
| Maturity fears | 5.50 (2.81) | 8.31 (4.84) | 0.006 |
| Asceticism | 3.64 (2.65) | 3.91 (2.52) | 0.672 |
| Impulse regulation | 3.39 (3.79) | 4.00 (4.17) | 0.529 |
| Social insecurity | 4.14 (3.45) | 3.75 (3.62) | 0.652 |
| UG behavioral performance | |||
| Accepted offers (%) | |||
| All offers | 58.69 (19.74) | 55.90 (21.18) | 0.543 |
| Fair offers | 82.36 (19.49) | 83.84 (21.11) | 0.870 |
| Unfair offers | 46.70 (28.41) | 42.05 (29.53) | 0.476 |
| Response time (s) | |||
| All offers | 1.010 (0.297) | 1.029 (0.275) | 0.777 |
| Fair offers | 0.934 (0.293) | 0.971 (0.284) | 0.568 |
| Unfair offers | 1.046 (0.316) | 1.053 (0.279) | 0.912 |
SD, standard deviation; BMI, body mass index; s, seconds; HDL, high‐density lipoprotein.
The study was approved by the Ethics Committee for Human Research of the Universidad de Granada. Both participants and parents signed an informed consent form.
Experimental Task
We utilized an fMRI suitable previously validated UG task [Crockett et al., 2008] involving one proposer and one responder. Participants always played the responder's role. To enhance the credibility and the interpersonal appeal of the game, participants were told that the proposer was another participant of the research project, who had left a picture of himself/herself and a list of proposals after his/her own scanning session. We told them that this proposer had been randomly selected from the pool of previous participants, and that they could see his/her picture during the game. In addition, they were told they would have the opportunity to play the role of the proposer with other volunteers who would participate in the future, if they would allow their photograph to be taken and used in future sessions, and submit their own proposals for several stake sizes. In reality, the picture of the proposer was taken from a web pool of images and utilized with all participants to minimize potential confounders associated with social identification.
In each trial, participants were initially prompted with a picture of the proposer (2 s), followed by a graphical depiction of the money available to split in that particular trial (indicated by the number expressed in Euro and the length of a horizontal light‐colored bar) and the amount of money that the proposer offered to share (indicated by the number expressed in Euro and the proportion of the above bar filled in red; 1 s). Once the offer was presented, participants had 3 s to accept or reject the offer using designated buttons in a button‐box response pad. They were told that if they accepted the proposer's offer, both players were supposed to be paid in the specified way. Conversely, by rejecting the offer, none of them would get the money. After the response, each trial was followed by 3 s of baseline during which a fixation cross was presented in the screen until the next trial started, for a total trial duration of 9 s (see Fig. 1). Event onsets were jittered with respect to scan onsets across trials [Henson and Mouchlianitis, 2007].
Figure 1.
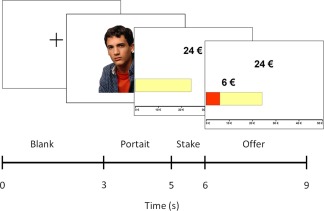
Schematic representation of the UG task through depiction of one experimental trial. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
The task included two types of offers varying on degree of fairness: Fair offers, in which the proposer offered to share around 46% of the money, and Unfair offers, in which the proposer offered to share between 15 and 25% of the money. Participants were informed that payments were hypothetical. Our main interest was to contrast group differences in brain activations involved in (1) making decisions about Unfair versus Fair offers (indexing the conflict between perception of unfairness and cognitive evaluation); and (2) deciding to Reject versus Accept the offers (indexing emotion‐based decisions involving missing reward vs. strategic decisions).
Inside scanner behavioral measures
Acceptance rates (% of offers accepted) and response times were calculated for each participant as a function of offer type.
Outside scanner behavioral measures
The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) [Torrubia et al., 2001]
It is a 48 yes–no response item questionnaire that measures trait sensitivity to reward (24 items) and punishment (24 items). The SPSRQ has demonstrated sound psychometric properties, construct validity, and significant associations with biologically plausible brain systems [Costumero et al., 2013].
The Eating Disorder Inventory—Second Edition (EDI‐2) [Garner, 1994]
It is a 64‐item self‐report measure assessing disadvantageous psychological traits commonly associated with eating disorders. Responses are made on a 6‐point Likert‐type scale ranging from never to always. The EDI‐2 has demonstrated sound psychometric properties and construct validity [Elder and Grilo, 2007; Reas et al., 2006].
Imaging Data Acquisition
A 3.0 T clinical MRI scanner, equipped with an eight‐channel phased‐array head coil was used (Intera Achieva, Philips Medical Systems, Eindhoven, The Netherlands). During task performance, a T2*‐weighted echo‐planar imaging (EPI) was collected, (repetition time (TR) = 2000 ms, echo time (TE) = 35 ms, field of view (FOV) = 230 × 230 mm, 96 × 96 matrix, flip angle = 90°, 21 4 mm axial slices, 1‐mm gap, 442 scans). A sagittal three‐dimensional T1‐weighted turbo‐gradient‐echo sequence (3D‐TFE; 160 slices, TR = 8.3 ms, TE = 3.8 ms, flip angle = 8°, FOV = 240 × 240, 1 mm3 voxels) was obtained in the same experimental session for anatomical reference. Stimuli were presented through magnetic resonance‐compatible liquid crystal display goggles (Resonance Technology, Northridge, CA) and responses were recorded through Evoke Response Pad System (Resonance Technology).
Imaging Data Processing and Analysis
The functional images were analyzed using Statistical Parametric Mapping (SPM8) software (Wellcome Department of Cognitive Neurology, Institute of Neurology, Queen Square, London, UK), running under MATLAB R2009 (MathWorks, Natick, MA). Preprocessing included reslicing to the first image of the time series, slice timing correction, normalization, using affine and smoothly nonlinear transformations, to an EPI template in the Montreal Neurological Institute (MNI) space, and spatial smoothing by convolution with a 3D Gaussian kernel (full width at half maximum = 8 mm).
Data Analysis
Behavioral analyses
We used the Statistical Package for the Social Sciences version 19 (SPSS 19; Chicago, IL) for these analyses. We conducted independent‐sample t‐tests (two‐tailed) to compare the two groups on relevant sociodemographic variables and inside and outside scanner behavioral measures.
fMRI, main task effects
The conditions of interest were modeled from the time at which the offer was presented to the time at which participants responded. Two contrasts of interest were defined at the first‐level (single‐subject) and between‐group analyses: (1) “Unfair > Fair offers,” (2) “Reject > Accept unfair offers.” The BOLD response at each voxel was convolved with the SPM8 canonical hemodynamic response function and a high‐pass filter was used to remove low‐frequency noise (1/128 Hz). The resulting first‐level contrast images were then carried forward to subsequent second‐level random‐effect (group) analyses. Main task effects were assessed with one‐sample t‐test while two‐sample t‐tests were used to assess between‐group differences. The results were corrected for multiple comparisons with a combination of voxel intensity and cluster extent thresholds. The spatial extent threshold was determined by 1000 Monte Carlo simulations using AlphaSim as implemented in the SPM REST toolbox [Song et al., 2011; Ward, 2013 2013]. For one‐sample t‐tests, input parameters included a brain mask of 161,455 voxels, an individual voxel threshold probability of 0.005, and a cluster connection radius of 5 mm, considering the actual smoothness of data after model estimation. A minimum cluster extent (KE) of 436 voxels was estimated to satisfy a P FWE < 0.05. Significance in two‐sample t‐tests was assessed using the same input parameters, masking results on the basis of activation and deactivation maps derived from the one‐sample t‐tests. Therefore, for contrasts 1 and 2, respectively, a minimum cluster extent (KE) of 54 and 87 voxels (within brain masks of 17,968 and 18,266 voxels), was estimated to satisfy a P FWE < 0.05. All analyses were conducted both including and not including age as a nuisance variable. As in both cases we obtained the same results, we only report uncorrected effects.
Correlation analyses
Correlation analyses were performed in SPSS. Specifically, the beta eigenvalues from the peak coordinates of each cluster of significant brain results were extracted for each participant, and then correlated with behavioral measures within each group. Correlation analyses were complemented with structural equation modeling (SEM) analyses aimed at testing and estimating causal relationships between the different variables involved in our imaging and behavioral assessments. Specifically, we examined whether obesity‐related psychological traits were associated with the decision of accepting or rejecting social offers through the activation/deactivation of specific brain regions. Thus, we estimated the direct effect of trait measures on the behavioral response, the effect of trait measures on brain activity (the brain‐trait pathway), the effect of brain activity on the behavioral response after controlling for trait measures (the brain‐state pathway), and the indirect relationship (through activation/deactivation of specific brain regions) between trait measures and the behavioral response. The effect and statistical significance of the different paths were estimated using the mediation toolbox (http://wagerlab.colorado.edu/files/tools/mediation.html).
RESULTS
Behavioral Results
Independent‐sample t‐tests showed no significant between‐group differences on acceptance rates or response times to any type of offer (P > 0.1 in all cases). Participants with excess weight showed significantly lower scores in sensitivity to reward, and significantly higher scores in drive for thinness, body dissatisfaction, and maturity fears compared to normal weight peers.
Imaging Results
Unfair > Fair offers.
Intra‐group activations
One‐sample t‐tests showed that Normal weight participants significantly activated medial wall regions (including the anterior cingulate cortex, the medial frontal gyrus, and the supplementary motor area), the superior and middle fontal gyrus, the thalamus (extending to midbrain and amygdala), and the right precentral gyrus (somatosensory cortex encompassing posterior insula). Normal weight participants also showed significant deactivations in left parietal and occipital cortices. Excess weight participants did not show significantly increased activations. However, they showed significant deactivations in a large cluster including left anterior insula, frontal operculum, and superior temporal gyrus. Similar to normal weight adolescents, they also showed deactivations in bilateral parietal and occipital cortices (extending to the fusiform gyri; Fig. 2 and Supporting Information Table SI).
Figure 2.

Brain activations, deactivations, and group differences during “Unfair > Fair” contrast. Note: (A) Top panel displays the brain regions showing activations and deactivations during “Unfair > Fair” offers in both groups. Warm colors reflect normal weight group and cold colors reflect excess weight group. (B) Bottom panel displays the differences between groups. Bottom‐right panel displays the correlations between “Unfair offers accepted” and peaks activation at dorsal ACC and anterior insula/frontal operculum in the “Unfair > Fair” comparison. X, Y and Z denote coordinate in standard MNI space. Right hemisphere is displayed on the right. Color bar indicates T value. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Group differences
Excess weight adolescents showed significantly reduced activations in dorsal anterior cingulate cortex, left anterior insula/frontal operculum, superior temporal gyrus, and thalamus and midbrain (extending to the amygdala) compared to normal weight peers (Fig. 2 and Supporting Information Table SI).
Correlations between brain activation patterns and behavioral measures
Regarding acceptance rates, the proportion of unfair offers accepted positively correlated with dorsal anterior cingulate cortex activation in normal and excess weight subjects, although such correlation was statistical significant only in the former (r = 0.321, P = 0.034, and r = 0.283, P = 0.105, respectively). By contrast, the proportion of unfair offers accepted correlated negatively with anterior insula/frontal operculum activation in excess weight participants (r = −0.353, P = 0.038), whereas this correlation was positive and nonsignificant in the normal weight group (r = 0.241, P = 0.114). The direct comparison between these correlations revealed a significant group difference, with z = 2.63, P = 0.008 (Fig. 2).
As for psychological traits, both maturity fears and sensitivity to reward scores negatively correlated with anterior insula/frontal operculum activation in excess weight participants (r = −0.443, P = 0.011 and r = −0.335, P = 0.046, respectively), whereas these correlations were positive and nonsignificant in the normal weight group (r = 0.245, P = 0.149 and r = 0.121, P = 0.433). The direct comparison between these correlations revealed a significant group difference, with z = 2.85, P = 0.004 (for maturity fears) and z = 2.01, P = 0.044 (for sensitivity to reward; Fig. 3). We found no significant correlations between drive for thinness or body dissatisfaction and brain activation patterns.
Figure 3.

Scatterplots displaying the correlation between behavioral measures (“Sensitivity to Reward” and “Maturity Fears”) and brain activation patterns during the “Unfair > Fair” contrast (peak activation at the anterior insula/frontal operculum region). The data of excess weight and normal weight participants are represented with different symbols (crosses and circles, respectively) to illustrate the different direction of the correlation as a function of group. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
As in excess weight participants, anterior insula/frontal operculum activation was significantly related to both the behavioral response (i.e., the proportion of unfair offers accepted) and specific trait measures (maturity fears and sensitivity to reward); in a post hoc analysis, we studied the relationships between these variables using a SEM approach. Specifically, we observed that maturity fears were indirectly and positively associated with the acceptance of unfair offers through the decreased anterior insula/frontal operculum activation observed in excess weight participants (z = 2.17, P = 0.03; Fig. 4). Of note, the direct correlation between maturity fears (X) and acceptance of unfair offers (Y) was negative, although nonsignificant (zero‐order or c: z = −0.44, P = 0.660). The lack of significance of direct effects indicates that the association between maturity fears and acceptance of unfair offers is exclusively accounted for the pattern of insula activation. Moreover, the opposite signs observed between direct (c') and indirect (ab) effects further supports that the association between these behavioral variables is specifically conveyed by the pattern of insula activation. None of these effects were observed in control participants. Likewise, we did not observe any significant relationship between sensitivity to reward and behavioral responses in the task.
Figure 4.
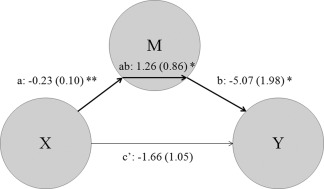
Path diagram showing the relationships between maturity fears (X), percentage of unfair offers accepted (Y), and insula activation (M) during the UG task in excess weight participants. X was inversely related to M (a, or the brain‐trait pathway), while M was also inversely related to Y (b, or the brain‐state pathway). X was not directly related to Y (c'), but these two measurements were indirectly related through M (a*b). *P < 0.05, **P < 0.01.
Reject > Accept Unfair offers
Intra‐group activations
One‐sample t‐test showed that during rejected offers, both groups showed significant activations in the dorsal anterior cingulate cortex, the somatosensory cortices, the insula, and the adjacent temporal cortices. However, in normal weight participants, the activation in the postcentral gyri extended to the precentral gyri and additional activations involved the supplementary motor area and the thalamus extending to putamen, midbrain, and the left amygdala (Fig. 5 and Supporting Information Table SII).
Figure 5.

Brain regions activated during “Reject > Accept” unfair offers in both groups. Note: Warm colors reflect normal weight group and cold colors reflect excess weight group. X, Y, and Z denote coordinate in standard MNI space. Right hemisphere is displayed on the right. Color bar indicates T value. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Group differences
We did not observe significant differences between the groups at the selected threshold.
Correlations between brain activation patterns and behavioral decisions
Regarding acceptance rates, the proportion of unfair offers accepted positively correlated with amygdala activation in excess weight participants (r = 0.448, P = 0.032), whereas this correlation was negative, albeit nonsignificant, in the normal weight group (r = −0.227, P = 0.205). The direct comparison between these correlations revealed a significant group difference, with z = 2.47, P = 0.013 (Fig. 6).
Figure 6.
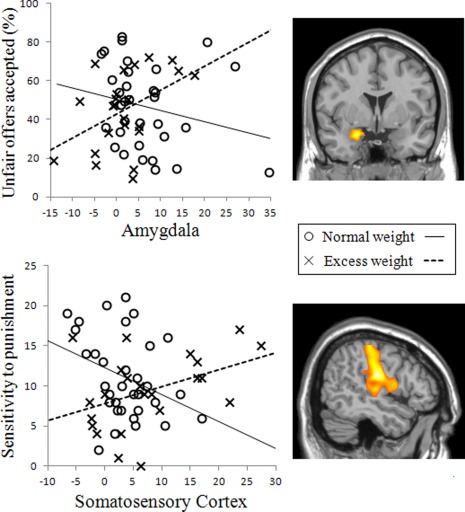
Scatterplots displaying the correlation between behavioral measures (“Unfair offers accepted” and “Sensitivity to Punishment” and brain activation patterns during the “Reject > Accept” contrast (peak activations at the amygdala and the postcentral cortex regions, respectively). The data of excess weight and normal weight participants are represented with different symbols (crosses and circles, respectively) to illustrate the different direction of the correlation as a function of group. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
As for psychological traits, sensitivity to punishment positively correlated with right somatosensory cortex activation in excess weight participants (r = 0.415, P = 0.049), whereas this correlation was negative and nearly significant in the normal weight group (r = −0.339, P = 0.053). The direct comparison between these correlations revealed a significant group difference, with z = 2.75, P = 0.006 (Fig. 6). We found no significant correlations between drive for thinness or body dissatisfaction and brain activation patterns.
DISCUSSION
We showed that adolescents with excess weight have reduced activation of brain regions involved in emotion and reward processing including the anterior cingulate cortex, the insula, and the thalamus during social decision making. Furthermore, we showed that deactivation of the anterior insula correlates with higher sensitivity to reward and higher maturity fears uniquely in the excess weight group and that such deactivation accounts for an indirect relationship between maturity fears and a higher probability of accepting unfair offers. Moreover, somatosensory cortex activation during rejection of unfair offers positively correlates with sensitivity to punishment, and amygdala activation positively correlates with acceptance of unfair offers uniquely in the excess weight group. Collectively, our findings indicate that adolescents with excess weight display blunted activation of the social decision‐making circuitry, which correlates with disadvantageous traits and interpersonal decisions.
Our social decision‐making task (UG) induced a reliable pattern of brain activations including the dorsal anterior cingulate cortex, the insula and thalamic/limbic regions, which is in fitting with previous evidence [Gospic et al., 2011; Sanfey et al., 2003]. Moreover, the activation of the anterior cingulate cortex correlated with acceptance rates in both groups, indicating good fit between brain activation measures and behavioral decisions [Glascher et al., 2012]. In this context, adolescents with excess weight exhibited significantly decreased activation of dorsal anterior cingulate cortex and thalamic regions and concomitant deactivation of the anterior insula/frontal opercular region. The dorsal anterior cingulate and the thalamus are functionally associated with the generation of emotional responses to social stressors [Güroglu et al., 2011]. The anterior insula is generally associated with perception of bodily signals and emotional awareness [Wager et al., 2009]. Moreover, in the context of the UG, insula activation is specifically associated with subjective feelings of unfairness [Sanfey et al., 2003]. Therefore, the decreased activation of this set of regions suggests decreased affective tracking of social unfairness in the excess weight group.
The central question is whether this brain activation pattern is relevant and potentially disadvantageous for the social behavior of adolescents with excess weight. Correlation analyses strongly suggest this is the case. First, insula deactivation correlated with higher acceptance rates, suggesting that reduced affective tracking of social unfairness is linked to more acceptances of unfair offers. As the contribution of the affective neural circuitry is essential for adequate social functioning [Bar‐On et al., 2003], this pattern is likely to impact the real‐life interpersonal decisions of obese adolescents. Moreover, the opposite pattern (positive correlations, hence more insula greater acceptance rates) has been previously demonstrated in healthy adolescents [Güroglu et al., 2011], similar to what we showed in our control group. Second, we found that insula deactivation correlated with increased maturity fears. Fears of social‐evaluative situations are indicative of poor social‐cognitive development [Westenberg et al., 2004]. Therefore, this finding is the first to demonstrate an association between poor affective tracking of social unfairness (indexed by insula deactivation) and this hindering trait of adolescent obesity. Finally, and linking the two above described relationships, we also showed that maturity fears are indirectly associated to social decision making through blunted insula activation. Such findings demonstrate for the first time a significant association between a trait marker of eating disorders and the social decision‐making behavior of excess weight adolescents conveyed by a particular pattern of brain activity. This notion agrees with developmental models that highlight the insula as a key region for the maturation of social decision‐making systems during adolescence [Smith et al., 2014]. At difference with maturity fears, we found no significant associations between brain activation patterns and body dissatisfaction and drive for thinness. This discrepancy is reasonable as the latter traits basically reflect the difficulties associated with physical weight gain, whereas maturity fears capture personality and interpersonal aspects of obesity [Garner, 1994]. Therefore, this negative finding conceivably speaks of the specificity of the UG task as a biomarker of social disadvantage in adolescent obesity.
Moreover, insula deactivation also correlated with higher sensitivity to reward. Sensitivity to reward has been associated with higher risk‐taking in social scenarios specifically during adolescence [Chein et al., 2011]. Furthermore, lower insula activations predict greater risk taking [Mohr et al., 2010] and this notion has been linked to insula‐mediated sensitization of the reward system [Smith et al., 2014]. More broadly, sensitivity to reward has been associated with several aspects of unhealthy eating, including binge eating patterns [Ziauddeen et al., 2012]. Uncoupling of reward (i.e., wanting) from emotion (i.e., liking) processing is as well reminiscent of addictive features, in which sensitivity to rewarding stimuli increases while the hedonic quality of these stimuli decreases [Robinson and Berridge, 2003]. Also in this case, the opposite pattern is typically found in healthy adolescents [Jarcho et al., 2012], consistent with what we showed in our control group. Collectively, our findings suggest that the anterior insula, which is strongly involved in the processing of highly appetizing food [Wang et al., 2004], may consequently lose control over more complex reward‐related choices including social decisions.
We did not find significant group differences in brain activation as a function of type of choice (reject vs. accept). However, the relationship between behavioral responses and brain activity was again distinctive in the excess weight group. Specifically, reject‐related amygdala activation correlated with greater probability of accepting unfair offers. Such finding suggests that excess weight adolescents display a paradoxically increased negative emotional reactivity in situations where they adopt a more assertive social role. Therefore, this finding suggests an abnormal role of the amygdala in directing social behavior, as greater amygdala activation is typically associated with rapid rejection of unfair offers [Gospic et al., 2011]. Moreover, the pattern of somatosensory activation contingent to rejecting offers (entailing losing reward) was positively correlated with sensitivity to punishment in the excess weight group, and negatively correlated with this trait in controls. Somatosensory regions are relevant to anticipate reinforcement outcome [Bieszczad and Weinberger, 2012], but in excess weight subjects the activation of these regions seem to reflect a trait susceptibility to reward omission or punishment. Hence, similar to those results and to the reward sensitivity‐insula correlations reported above, this finding indicates that the association between reinforcement‐based temperamental traits and somatosensory regions is abnormal in excess weight adolescent populations.
Our findings show that excess weight adolescents show dysfunctional engagement of brain regions involved in emotion perception and reward during social decisions. These early deficits may not only predict poor clinical prognosis [Ludwig, 2007] but also lie at the root of well‐described socioeconomic disadvantage in the adult obesity population, including wage penalty and hiring discrimination [Agerström and Rooth, 2011; Baum and Ford, 2004; Caliendo and Lee, 2013; Latner et al., 2012]. This study has several strengths, including a large sample size, detailed medical and psychological characterization of participants, and novel use of a social decision‐making paradigm in this population. Nonetheless, findings should also be assessed in the context of several limitations. First, our sample spans a 6‐year adolescent period characterized by intense maturational processes, which may have impacted results. However, the psychological features addressed in the UG seem to be already optimized by the age of 9 [Güroglu et al., 2009], and analyses covaried by age showed equivalent results. Second, we used a simple version of the UG because our main interest was to raise the conflict between emotion and cognition in an interpersonal scenario. We are aware of the existence of a more specific UG literature; however, the purpose of this study was not to experimentally characterize the task but to make it instrumental to understand a clinical population. Similarly, we did not detect behavioral differences between the groups in the UG task. This is likely due to the fact that this UG task was specifically designed for fMRI experiments, seeking maximization of engagement of relevant brain circuitry but not of potential behavioral differences. Future studies that utilize UG tasks more sensitive to behavioral profiles are warranted to reveal if our brain activation findings are mirrored by conceptually compatible behavioral group differences. Finally, future studies and longitudinal designs are warranted to address whether these deficits precede excess weight problems or arise as a consequence of weight gain or related psychosocial burden. Similarly, future studies are warranted to investigate whether these patterns can predict clinical prognosis and socioeconomic disadvantage during adulthood.
CONCLUSION
We show that excess weight adolescents display impaired activation of affective brain regions during social decision making, and that blunted activation of this circuitry accounts for the association between maturity fears and social decisions. The study yields a high translational value as the UG neural circuitry may serve as a dimensional biomarker of the risk of social disadvantage in obesity and of the effectiveness of novel treatments that focus on the social burden of obesity.
Acknowledgements
We acknowledge Elena Delgado‐Rico for assistance in recruitment and data collection.
Supporting information
Supplementary Information Table 1.
Supplementary Information Table 2.
Conflict of interest: The authors declare not having competing financial interests in relation to the work described.
REFERENCES
- Agerström J, Rooth DO (2011): The role of automatic obesity stereotypes in real hiring discrimination. J Appl Psychol 96:790–805. [DOI] [PubMed] [Google Scholar]
- Baarendse PJ, Counotte DS, O'Donnell P, Vanderschuren LJ (2013): Early social experience is critical for the development of cognitive control and dopamine modulation of prefrontal cortex function. Neuropsychopharmacology 38:1485–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar‐On R, Tranel D, Denburg NL, Bechara A (2003): Exploring the neurological substrate of emotional and social intelligence. Brain 126:1790–1800. [DOI] [PubMed] [Google Scholar]
- Baum CL, Ford WF (2004): The wage effects of obesity: A longitudinal study. Health Econ 13:885–899. [DOI] [PubMed] [Google Scholar]
- Bieszczad KM, Weinberger NM (2012): Extinction reveals that primary sensory cortex predicts reinforcement outcome. Eur J Neurosci 35:598–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliendo M, Lee WS (2013): Fat chance! Obesity and the transition from unemployment to employment. Econ Hum Biol 11:121–133. [DOI] [PubMed] [Google Scholar]
- Chein J, Albert D, O'Brien L, Uckert K, Steinberg L (2011): Peers increase adolescent risk taking by enhancing activity in the brain's reward circuitry. Dev Sci 14:F1–F10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TJ, Lobstein T (2012): Extended international (IOTF) body mass index cut‐offs for thinness, overweight and obesity. Pediatr Obes 7:284–294. [DOI] [PubMed] [Google Scholar]
- Costumero V, Barros‐Loscertales A, Bustamante JC, Ventura‐Campos N, Fuentes P, Ávila C (2013): Reward sensitivity modulates connectivity among reward brain areas during processing of anticipatory reward cues. Eur J Neurosci 38:2399–2407. [DOI] [PubMed] [Google Scholar]
- Crockett MJ, Clark L, Tabibnia G, Lieberman MD, Robbins TW (2008): Serotonin modulates behavioral reactions to unfairness. Science 320:1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C (2009): Psychobiological traits in the risk profile for overeating and weight gain. Int J Obes 33(Suppl 2):S49–S53. [DOI] [PubMed] [Google Scholar]
- Delgado‐Rico E, Soriano‐Mas C, Verdejo‐Roman J, Schmidt Rio‐Valle J, Verdejo‐Garcia A (2013): Decreased insular and increased midbrain activations during decision‐making under risk in adolescents with excess weight. Obesity 21:1662–1668. [DOI] [PubMed] [Google Scholar]
- Elder KA, Grilo CM (2007): The Spanish language version of the Eating Disorder Examination Questionnaire: Comparison with the Spanish language version of the eating disorder examination and test‐retest reliability. Behav Res Ther 45:1369–1377. [DOI] [PubMed] [Google Scholar]
- Garner DM. 1994: Eating Disorders Inventory‐2. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Gee S, Chin D, Ackerson L, Woo D, Howell A (2013): Prevalence of childhood and adolescent overweight and obesity from 2003 to 2010 in an integrated health care delivery system. J Obes 2013:417907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glascher J, Adolphs R, Damasio H, Bechara A, Rudrauf D, Calamia M, Paul LK, Tranel D (2012): Lesion mapping of cognitive control and value‐based decision making in the prefrontal cortex. Proc Natl Acad Sci USA 109:14681–14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospic K, Mohlin E, Fransson P, Petrovic P, Johannesson M, Ingvar M (2011): Limbic justice–amygdala involvement in immediate rejection in the Ultimatum Game. PLoS Biol 9:e1001054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnarsdottir T, Njardvik U, Olafsdottir AS, Craighead LW, Bjarnason R (2012): Teasing and social rejection among obese children enrolling in family‐based behavioural treatment: Effects on psychological adjustment and academic competencies. Int J Obes 36:35–44. [DOI] [PubMed] [Google Scholar]
- Güroglu B, van den Bos W, Crone EA (2009): Fairness considerations: Increasing understanding of intentionality during adolescence. J Exp Child Psychol 104:398–409. [DOI] [PubMed] [Google Scholar]
- Güroglu B, van den Bos W, van Dijk E, Rombouts SA, Crone EA (2011): Dissociable brain networks involved in development of fairness considerations: Understanding intentionality behind unfairness. NeuroImage 57:634–641. [DOI] [PubMed] [Google Scholar]
- Harms MB, Zayas V, Meltzoff AN, Carlson SM (2014): Stability of executive function and predictions to adaptive behavior from middle childhood to pre‐adolescence. Front Psychol 5:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Mouchlianitis E (2007): Effect of spatial attention on stimulus‐specific haemodynamic repetition effects. NeuroImage 35:1317–1329. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Rieger JW, Baecke S, Lützkendorf R, Müller C, Adolf D, Bernarding J (2011): Predicting decisions in human social interactions using real‐time fMRI and pattern classification. PloS One 6:e25304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho JM, Benson BE, Plate RC, Guyer AE, Detloff AM, Pine DS, Leibenluft E, Ernst M (2012): Developmental effects of decision‐making on sensitivity to reward: An fMRI study. Dev Cogn Neurosci 2:437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji CY (2008): The prevalence of childhood overweight/obesity and the epidemic changes in 1985‐2000 for Chinese school‐age children and adolescents. Obes Rev 9:78–81. [DOI] [PubMed] [Google Scholar]
- King‐Casas B, Sharp C, Lomax‐Bream L, Lohrenz T, Fonagy P, Montague PR (2008): The rupture and repair of cooperation in borderline personality disorder. Science 321:806–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoch D, Pascual‐Leone A, Meyer K, Treyer V, Fehr E (2006): Diminishing reciprocal fairness by disrupting the right prefrontal cortex. Science 314:829–832. [DOI] [PubMed] [Google Scholar]
- Latner JD, Ebneter DS, O'Brien KS (2012): Residual obesity stigma: An experimental investigation of bias against obese and lean targets differing in weight‐loss history. Obesity 20:2035–2038. [DOI] [PubMed] [Google Scholar]
- Ludwig DS (2007): Childhood obesity—The shape of things to come. N Engl J Med 357:2325–2327. [DOI] [PubMed] [Google Scholar]
- McEwen BS (2007): Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev 87:873–904. [DOI] [PubMed] [Google Scholar]
- Mohr PN, Biele G, Heekeren HR (2010): Neural processing of risk. J Neurosci 30:6613–6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reas DL, Grilo CM, Masheb RM (2006): Reliability of the Eating Disorder Examination‐Questionnaire in patients with binge eating disorder. Behav Res Ther 44:43–51. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC (2003): Addiction. Annu Rev Psychol 54:25–53. [DOI] [PubMed] [Google Scholar]
- Rudolf MC, Greenwood DC, Cole TJ, Levine R, Sahota P, Walker J, Holland P, Cade J, Truscott J (2004): Rising obesity and expanding waistlines in schoolchildren: A cohort study. Arch Dis Child 89:235–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD (2003): The neural basis of economic decision‐making in the Ultimatum Game. Science 300:1755–1758. [DOI] [PubMed] [Google Scholar]
- Smith AR, Steinberg L, Chein J (2014): The role of the anterior insula in adolescent decision making. Dev Neurosci 36:196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF (2011): REST: A toolkit for resting‐state functional magnetic resonance imaging data processing. PLoS One 6:e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss RS, Pollack HA (2001): Epidemic increase in childhood overweight, 1986–1998. JAMA 286:2845–2848. [DOI] [PubMed] [Google Scholar]
- Strauss RS, Pollack HA (2003): Social marginalization of overweight children. Arch Pediatr Adolesc Med 157:746–752. [DOI] [PubMed] [Google Scholar]
- Torrubia R, Avila C, Molto J, Caseras X (2001): The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) as a measure of Gray's anxiety and impulsivity dimensions. Pers Individ Dif 31:837–862. [Google Scholar]
- van't Wout M, Kahn RS, Sanfey AG, Aleman A (2006): Affective state and decision‐making in the Ultimatum Game. Exp Brain Res 169:564–568. [DOI] [PubMed] [Google Scholar]
- Wager TD, van Ast VA, Hughes BL, Davidson ML, Lindquist MA, Ochsner KN (2009): Brain mediators of cardiovascular responses to social threat, part II: Prefrontal subcortical pathways and relationship with anxiety. Neuroimage 47:836–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Telang F, Jayne M, Ma J, Rao M, Zhu W, Wong CT, Pappas NR, Geliebter A, Fowler JS (2004): Exposure to appetitive food stimuli markedly activates the human brain. NeuroImage 21:1790–1797. [DOI] [PubMed] [Google Scholar]
- Ward BD (2013) AFNI and NIFTI server at NIMH in Bethesda, MD USA. Simultaneous inference for FMRI data. Available at:http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf, accessed on September 2013.
- Westenberg PM, Drewes MJ, Goedhart AW, Siebelink BM, Treffers PD (2004): A developmental analysis of self‐reported fears in late childhood through mid‐adolescence: Social‐evaluative fears on the rise? J Child Psychol Psychiatry 45:481–495. [DOI] [PubMed] [Google Scholar]
- Xiang T, Lohrenz T, Montague PR (2013): Computational substrates of norms and their violations during social exchange. J Neurosci 33:1099–1108a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef FF, Dookeeram K, Basdeo V, Francis E, Doman M, Mamed D, Maloo S, Deganner J, Dobo L, Ditshotlo P, Legall G (2012): Stress alters personal moral decision making. Psychoneuroendocrinology 37:491–498. [DOI] [PubMed] [Google Scholar]
- Zheng H, Berthoud HR (2007): Eating for pleasure or calories. Curr Opin Pharmacol 7:607–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziauddeen H, Farooqi IS, Fletcher PC (2012): Obesity and the brain: How convincing is the addiction model? Nat Rev Neurosci 13:279–286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information Table 1.
Supplementary Information Table 2.


