Abstract
Gyrification brain abnormalities are considered a marker of early deviations from normal developmental trajectories and a putative predictor of poor outcome in psychiatric disorders. The aim of this study was to explore cortical folding morphology in patients with anorexia nervosa (AN). A MRI brain study was conducted on 38 patients with AN, 20 fully recovered patients, and 38 healthy women. Local gyrification was measured with procedures implemented in FreeSurfer. Vertex‐wise comparisons were carried out to compare: (1) AN patients and healthy women; (2) patients with a full remission at a 3‐year longitudinal follow‐up assessment and patients who did not recover. AN patients exhibited significantly lower gyrification when compared with healthy controls. Patients with a poor 3‐year outcome had significantly lower baseline gyrification when compared to both healthy women and patients with full recovery at follow‐up, even after controlling for the effects of duration of illness and gray matter volume. No significant correlation has been found between gyrification, body mass index, amount of weight loss, onset age, and duration of illness. Brain gyrification significantly predicted outcome at follow‐up even after controlling for the effects of duration of illness and other clinical prognostic factors. Although the role of starvation in determining our findings cannot be excluded, our study showed that brain gyrification might be a predictor of outcome in AN. Further studies are needed to understand if brain gyrification abnormalities are indices of early neurodevelopmental alterations, the consequence of starvation, or the interaction between both factors. Hum Brain Mapp 36:5113–5122, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: anorexia nervosa, cortical folding, outcome, MRI, brain gyrification
INTRODUCTION
Anorexia nervosa (AN) is a severe psychiatric disorder characterized by a high female/male ratio, onset during adolescence [Favaro et al., 2009], and high levels of chronicity and mortality. Individuals suffering from AN reduce their food intake or increase their energy expenditure, have a distorted body image and often display low levels of insight about their condition. Although several studies have been conducted to explore the effects of having a diagnosis of AN on brain volumes, no study to date has been carried out to investigate the presence of cortical morphological abnormalities in this group of patients. With few exceptions [Brooks et al., 2011; Frank et al., 2013], AN has been associated with a reduction of both gray and white matter [Fonville et al., 2014; King et al., 2015; Seitz et al., 2014; Titova et al., 2014; Van den Eynde et al., 2012] which is usually considered a side effect of weight loss and malnutrition, given that an improvement of brain volumes has been observed in longitudinal studies [Seitz et al., 2014; Van den Eynde et al., 2012]. Whether any scarring effect might persist after full recovery is still a matter of debate, since some studies found a persistent reduction of gray matter volume in the anterior cingulate cortex of fully recovered AN patients [Muhlau et al., 2007], while others found no differences between recovered patients and healthy women [Wagner et al., 2006].
Since the effects of weight loss and malnutrition on brain volumes are known, although not completely characterized, and since malnutrition often begins a considerable amount of time before the onset of the disorder, only long‐term longitudinal studies of large cohorts of healthy adolescents would allow to infer the role of brain morphological aberrations in the risk of developing AN using volumetric studies. This question is of some importance since evidence about AN having a possible neurodevelopmental origin is increasing: the disorder has its onset during adolescence [Favaro et al., 2009], is associated with an excess of left‐handedness [Tenconi et al., 2010], specific neuropsychological impairments [Lopez et al., 2008; Roberts et al., 2007; Tenconi et al., 2010], and subtle neurological abnormalities [Gillberg et al., 1994]. In addition, prenatal and perinatal risk factors that have been implicated in other neurodevelopmental psychiatric disorders, such as autism and schizophrenia, seem to increase the risk of developing AN [Favaro et al., 2006, 2014; Tenconi et al., 2015]. Pregnancy and perinatal complications, for example, are twice more likely to occur in patients with AN than in healthy women [Favaro et al., 2006] and the presence of dysmaturity signs at birth (i.e., being small for gestational age or being hyporeactive/hypothermic at birth) are associated with an increased vulnerability to subsequent traumatic events [Favaro et al., 2010]. For this reasons, it is important to look for measures of brain morphology and function that are potentially not affected by malnutrition and other illness‐related factors.
In this study, we explored whether the investigation of brain gyrification can provide useful information about neurodevelopment outcome in AN. Cortical folding is known to reflect a person's prenatal development [Dubois et al., 2008]) and change only minimally after birth [White et al., 2010]: it is the result of a substantial expansion of cortical surface area despite a relatively minor gain in cortical thickness during evolutionary development [Sun and Hevner, 2014]. The processes leading to cortical gyrification are not fully understood, but recent theories consider cortical folding to be the result of underlying patterns of connectivity [Van Essen, 1997], thus emphasizing the possible functional significance of gyrification alterations [Sun and Hevner, 2014]. The gyrification index, which is the ratio between the inner folded contour and the length of the coronal outline, excluding the sulcal regions, has been found to increase dramatically during the third trimester of pregnancy, and then to remain relatively constant throughout development [Armstrong et al., 1995]: this means that the process of gyrification continues through the postnatal developmental period, maintaining this constant ratio throughout the process [White et al., 2010]. For this reason, the degree of folding is considered to be a crucial valid marker to our understanding of the timing and the nature of brain alterations during neurodevelopment [Dubois et al., 2008; White et al., 2010; Schaer et al., 2013]. Gyrification alterations have been found to be associated with prematurity [Giménez et al., 2006; Kesler et al., 2006] and exposure to obstetric complications [Haukvik et al., 2012], and to be frequent in neurodevelopmental psychiatric disorders such as autism and schizophrenia [Nanda et al., 2014; Nesvag et al., 2014; White and Hilgetag, 2011].
Furthermore, in first episode psychosis, cortical folding in specific brain areas seems to significantly predict response to antipsychotic treatment [Palaniyappan et al., 2013] and, in psychotic disorders, perinatal complications and neurological soft signs are considered factors of poor prognosis [Prikryl et al., 2007]. Despite the importance of having reliable predictors of poor response in AN, to our knowledge, no attempt to date has been made to explore the relationship between brain characteristics and outcome, although some earlier studies hypothesized a relationship between perinatal insults and outcome [Hamsher et al., 1981].
Finally, since the effects of malnutrition on the brain are not fully known, this study explored the relationship between cortical gyrification and nutritional parameters (body mass index, weight loss and body hydration) as well as age and duration of illness. Despite the relative stability of gyrification indices after birth, recent studies described cortical thinning and decreased gyrification index correlated to sulcal widening during adolescence [Aleman‐Gomez et al. 2013; Klein et al., 2014]. In this context, the impact of AN and malnutrition on brain morphology remains to be understood.
The aims of this study were twofold: (1) to investigate the pattern of brain gyrification in a sample of patients with AN and study the relationship between gyrification and relevant clinical variables, such as body mass index, the amount of weight loss, duration of illness and age of onset; (2) to explore the relationship between brain gyrification and the outcome at 3‐year follow‐up. We hypothesize that patients with AN exhibit aberrant gyrification patterns that are predictive of outcome at follow‐up.
METHODS
A total of 38 patients with acute AN and 38 healthy controls were included in this study. A further group of 20 patients in full remission from AN were included to test the state/trait nature of any MRI finding. Women who had recovered from AN were subjects who had had full AN in their lifetime, but were asymptomatic from at least 6 months at the time of scanning [mean remission time: 38.5 months (standard deviation = 33.2); range 6–96]. All recovered subjects were asked to participate during follow‐up visits carried out in our ED Unit after recovery. None of the subjects of this group relapsed in the year following the study. Table 1 describes the main characteristics of the sample (more details about recruitment are reported in Supporting Information).
Table 1.
Baseline characteristics of the three groups
| AN patients (n = 38) | Recovered AN patients (n = 20) | Healthy women (HW; n = 38) | AN vs. HW | recAN vs. HW | |
|---|---|---|---|---|---|
| mean (SD) | mean (SD) | mean (SD) | t (p) | t (p) | |
| Age | 26.1 (7.2) | 26.3 (7.1) | 25.3 (6.3) | 0.54 (0.59) | 0.59 (0.56) |
| Age at onset | 18.3 (5.1) | 17.7 (3.2) | – | – | – |
| Duration of illness (months) | 78.6 (81.3) | 45.7 (65.0) | – | – | – |
| Duration of recovery (months) | – | 45.4 (46.8) | – | – | – |
| Baseline BMI | 15.8 (1.8) | 19.6 (1.6) | 21.7 (2.9) | 10.51 (<0.001) | 2.91 (0.005) |
| Weight lossa | 7.1 (2.8) | 5.2 (3.1) | 3.4 (1.7) | 7.01 (<0.001) | 2.95 (0.005) |
| Lowest BMI | 14.0 (1.8) | 15.7 (1.4) | 19.8 (2.5) | 11.56 (<0.001) | 6.71 (<0.001) |
| Education | 14.2 (2.2) | 14.2 (2.7) | 15.5 (2.3) | 2.44 (0.02) | 1.97 (0.05) |
| Edinburgh laterality index | 57.2 (37.6) | 60.6 (35.2) | 55.1 (42.0) | 0.23 (0.82) | 0.50 (0.62) |
| Drive for thinness | 9.9 (6.1) | – | 2.3 (4.2) | 6.22 (<0.001) | – |
| Depression | 1.4 (0.8) | – | 0.7 (0.6) | 4.06 (<0.001) | – |
| Trait anxiety | 56.6 (9.7) | – | 39.3 (9.6) | 7.82 (<0.001) | – |
Weight loss is defined as the difference between highest and lowest lifetime body mass index.
Exclusion criteria for all subjects were male gender, history of head trauma or injury with loss of consciousness, history of any serious neurological or medical illness, active use of systemic steroids, pregnancy, active suicidality or major depression, history of substance/alcohol abuse or dependence, bipolar disorder or schizophrenia spectrum disorder, moderate mental impairment (IQ < 60) or learning disabilities, use of medications other than antidepressants, and known contraindications to conventional MRI. History of any psychiatric disorder and any first‐degree relatives with an eating disorder were additional exclusion criteria for healthy women. Amenorrhea, food restriction, bingeing, excessive exercise, fasting and purging in the last 6‐months were additional exclusion criteria for the recovered AN group.
Ethical permission was obtained from the ethics committee of the Padova Hospital. After completely describing the study to the subjects, informed written informed consent was obtained.
Clinical Assessment and Follow‐Up
In all subjects, diagnostic interviews were performed according to the eating disorders section of the Structured Clinical Interview for DSM‐IV [First et al., 1995] and a semi‐structured interview to gather socio‐demographic and clinical variables, as previously described [Favaro et al., 2012, 2013]. Subjects were also asked to complete the Hopkins Symptoms Checklist [Derogatis et al., 1974] to assess depressive and obsessive‐compulsive symptoms and the Eating Disorders Inventory [Garner et al., 1983] to assess eating psychopathology. Handedness was assessed by the Edinburgh Handedness Inventory [Oldfield, 1971].
All subjects were medically stable at the time of scanning and all were recruited at the Padova Hospital Eating Disorders Unit and all fulfilled all the diagnostic criteria for AN according to DSM‐IV at the time of scanning. The diagnostic subtype at the time of scanning was restrictive in 32 AN patients (84%) and binge eating/purging type in 6 patients, but 7 patients who were restrictive at the time of the present study reported previous recurrent binge eating and/or purging. Concerning the use of medications, 14 AN patients and 4 recovered women were under treatment with antidepressants at the time the study was conducted (acute AN: 1 case mirtazapine, 2 paroxetine, 2 escitalopram, 1 fluoxetine, 8 sertraline; recovered AN: 4 sertraline).
Follow‐up was performed 3 years later (on average 3.4 years, range 1.7–3.9) in acute AN patients. Both face‐to‐face interviews and reports from informants were used to gather diagnostic information. Patients were assessed using the eating disorders section of the Structured Clinical Interview for DSM‐IV [First et al., 1995]. Full recovery was defined as weight being in the normal range, regular menses, absence of binge eating, purging, food avoidance or restraint, excessive exercise, undue body dissatisfaction, or drive for thinness in the past 3 months.
MRI Data Acquisition
Data were collected on a Philips Achieva 1.5 Tesla scanner equipped for echo‐planar imaging. A high‐resolution 3D T1‐weighted anatomical image was also acquired, in a gradient‐echo sequence (repetition‐time = 20 s, echo time = 3.78 ms, flip angle = 20°, 160 sagittal slices, acquisition voxel size = 1 × 0.66 × 0.66 mm, field of view 21–22 cm).
Data Processing and Statistics
Surface extraction was completed using the FreeSurfer package (Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Boston) version 5.3.0. Preprocessing, cortical reconstruction and segmentation, and cortical thickness estimation were performed according to standard protocols [Desikan et al., 2006; Fischl and Dale, 2000; Fischl et al., 1999]. Details of image processing are reported in Supporting Information. Surface reconstruction and segmentation was manually inspected according to FreeSurfer user guidelines and edited with minor manual intervention when appropriate. The local Gyrification Index (lGI) takes into account the intrinsic three‐dimensional nature of the cortical surface and was developed to overcome limitations of previous two‐dimensional linear gyrification measures, which are susceptible to different types of bias. The lGI was measured at thousands of points of the reconstructed cortical surface using previously validated algorithms [Schaer et al., 2008]. LGI is a measurement of the degree of cortical folding that quantifies the amount of cortex buried with in the sulcal folds in the surrounding circular region. A general linear model controlling for the effects of age and intracranial volume was employed to estimate differences between AN patients and healthy women at each vertex of the right and left hemispheric surfaces. We also compared patients with full recovery to those not making a full recovery over the subsequent three years (and both of these groups were compared to healthy women) controlling for the effects of age, duration of illness, and intracranial volume. In order to reduce the possibility of biased findings due to the reduced cortical volume in AN patients, all vertex‐wise analyses were repeated including cortical thickness as a vertex‐wise covariate.
The Monte‐Carlo permutation approach (10,000 simulations) implemented in the FreeSurfer software was used for statistical correction of multiple comparisons with an additional Bonferroni correction to account for the two hemispheres. Clusterwise probability values of P < 0.01 were considered statistically significant in this study.
Statistics
General linear models were used to test differences in the overall gyrification index between groups using age and total intracranial volume (and duration of illness, when appropriate) as covariates. Nonparametric statistical tests were conducted in correlations and group comparisons for the other variables. Cohen's d effect sizes were calculated to investigate the strength of any group difference. These procedures were implemented with Statistical Product and Service Solutions software (SPSS, Chicago, Ill).
RESULTS
Diagnostic Group Comparisons
The overall lGI of both hemispheres was significantly lower in acute AN patients than in healthy women (left: 2.85 ± 0.09 vs. 2.90 ± 0.11; F(3,72) = 4.93; P = 0.03; Cohen's d = 0.50; right: 2.85 ± 0.10 vs. 2.90 ± 0.12; F(3,72) = 4.37; P = 0.04; Cohen's d = 0.45). AN patients who had recovered from the disorder showed no differences in comparison to healthy women in overall hemispheric lGI (left: 2.90 ± 0.09; F(3,54) = 0.11; P = 0.745; Cohen's d = 0; right: 2.90 ± 0.09; F(3,54) = 0.12; P = 0.730; Cohen's d = 0). Figure 1 shows the results of the vertex‐wise comparison between acute AN patients and healthy women: both in the left and in the right hemisphere an area in the parietal cortex showed significantly lower gyrification in acute AN patients. These areas mainly encompassed: in the left hemisphere: precentral, postcentral, superior parietal, supramarginal, and rostral‐middle‐frontal gyri; in the right hemisphere: precentral and superior‐frontal gyri. Performing the analysis including cortical thickness as a vertex‐wise covariate changed only marginally the brain areas of significant differences (Supporting Information Fig. S1). The comparison between recovered AN patients and healthy women revealed no cluster of significant difference after the multiple comparison correction.
Figure 1.
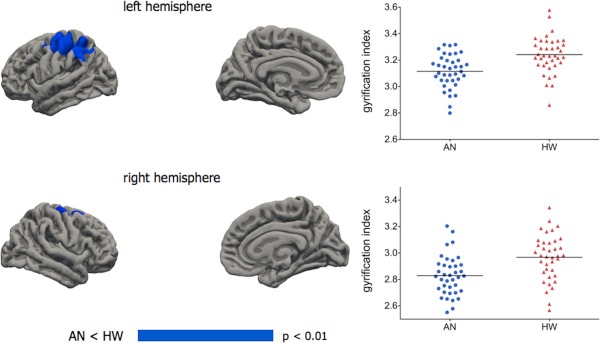
Vertex‐wise comparisons between AN patients (n = 38) and healthy women (n = 38). General linear models with age and total intracranial volume were used, applying the Monte Carlo method to control for multiple comparison and using a cluster‐wise threshold for inclusion of P < 0.01. Graphs show individual mean gyrification values in areas of significant difference. Left hemisphere: a cluster of 6.761 mm2 with a vertex of maximum difference (P = 0.0002) in the postcentral gyrus (−36, −36, 54). Right hemisphere: a cluster of 1.419 mm2 with a vertex of maximum difference (P = 0.002) in the superior frontal gyrus (21, 9, 53). No cluster showed significant results in the opposite direction. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
In the acute AN group, vertex‐wise correlation analyses did not reveal any significant correlation between gyrification and body mass index, amount of weight loss, age of onset, or duration of illness. Correlations with overall lGI showed a weak negative correlation between gyrification of the left hemisphere and duration of illness (Table 2). No significant differences emerged between patients who were taking antidepressants and those who were not in the acute AN group (left overall lGI: 2.84 ± 0.06 vs. 2.85 ± 0.11; F(3,34) = 0.28; P = 0.60; Cohen's d = 0.11; right overall lGI: 2.83 ± 0.06 vs. 2.86 ± 0.12; F(3,34) = 0.03; P = 0.87; Cohen's d = 0.32). In the 20 acute AN patients where bioimpedence analysis was performed, no significant correlation between gyrification and hydration index was found (left cluster: rho = 0.03; P = 0.91; right cluster: rho = 0.11; P = 0.65). Significant negative age correlations emerged in both the acute AN and healthy control groups, but no correlation × group interaction was found (Supporting Information Fig. S2). Acute AN patients also revealed several areas of significantly reduced cortical thickness (Supporting Information Table S1), that only partially overlapped with cortical areas displaying a reduced gyrification index (Supporting Information Fig. S3).
Table 2.
Correlations between hemispheric gyrification index and clinical variables, and differences in clinical characteristics in AN patients according to outcome at 3‐year follow‐up
| Correlation with left lGI | Correlation with right lGI | AN patients with recovery at follow‐up (n = 13) | AN patients without recovery (n = 24) | ||
|---|---|---|---|---|---|
| rho (p) | rho (p) | mean (SD) | mean (SD) | z (p) | |
| Age | −0.11 (0.51) | −0.16 (0.33) | 25.5 (6.8) | 26.7 (7.5) | 0.33 (0.74) |
| Age at onset | 0.20 (0.23) | 0.01 (0.94) | 20.8 (6.5) | 17.1 (3.8) | 2.26 (0.02)a |
| Duration of illness (months) | −0.36 (0.024)a | −0.30 (0.07) | 40.0 (46.2) | 101.2 (89.8) | 2.10 (0.04) |
| Baseline BMI | 0.03 (0.87) | 0.01 (0.80) | 14.9 (1.75) | 16.2 (1.6) | 2.23 (0.03) |
| Weight lossb | −0.10 (0.54) | 0.01 (0.94) | 7.0 (2.4) | 7.2 (3.1) | 0.19 (0.85) |
| Lowest BMI | 0.31 (0.06) | 0.17 (0.31) | 14.4 (2.0) | 13.7 (1.7) | 1.13 (0.26) |
| Drive for thinness | −0.02 (0.91) | −0.07 (0.70) | 12.2 (6.1) | 8.3 (5.6) | 1.82 (0.07) |
| Depression | 0.02 (0.92) | −0.02 (0.89) | 1.38 (0.68) | 1.39 (0.84) | 0.03 (0.98) |
| Trait anxiety | −0.26 (0.12) | −0.14 (0.40) | 55.6 (10.9) | 57.0 (9.3) | 0.40 (0.69) |
| Duration follow‐up (ys) | – | – | 3.2 (0.6) | 3.5 (0.5) | 1.48 (0.14) |
| Final BMI | 0.20 (0.24) | 0.20 (0.23) | 19.6 (2.1) | 17.7 (4.3) | 3.66 (<0.001)a |
AN: anorexia nervosa; lGI: local gyrification index; BMI: body mass index.
According to false discovery rate method, differences are significant at p<0.027 and correlations at P < 0.026.
Weight loss is defined as the difference between highest and lowest lifetime body mass index.
Outcome Analysis
Out of the acute AN patients who performed the 3 year follow‐up assessment (n = 37), 13 (35%) were completely recovered. Figure 2 and Table 2 shows the baseline differences between patients with full recovery and those not making a full recover over the subsequent 3 years. Performing the analysis including cortical thickness as a vertex‐wise covariate changed only marginally the brain areas of significant differences (Supporting Information Fig. S4). Areas of between‐group significant differences were located in bilateral superior parietal cortex and, in the left hemisphere, overlap with brain areas that showed significant difference in the comparison between the whole group of AN patients and healthy women. The group with poor outcome displayed significantly lower baseline overall gyrification both in comparison to healthy women (left: 2.82 ± 0.10 vs. 2.90 ± 0.11; F(3,58) = 6.98; P = 0.011; Cohen's d = 0.76; right: 2.82 ± 0.10 vs. 2.90 ± 0.12; F(3,58) = 6.60; P = 0.013; Cohen's d = 0.72) and in comparison to those AN patients who recovered (left: 2.82 ± 0.10 vs. 2.90 ± 0.06; F(3,33) = 4.75; P = 0.04; Cohen's d = 0.97; right 2.82 ± 0.10 vs. 2.90 ± 0.07; F(3,33) = 5.15; P = 0.03; Cohen's d = 0.93). No differences in gyrification emerged between good outcome AN patients (n = 13) and healthy women (n = 38). At vertex‐wise analysis, the AN group with poor outcome showed brain areas of significantly decreased gyrification in comparison to healthy women (Supporting Information Fig. S5) and to AN patients who did not recover at 3‐year follow‐up (Supporting Information Fig. S6).
Figure 2.
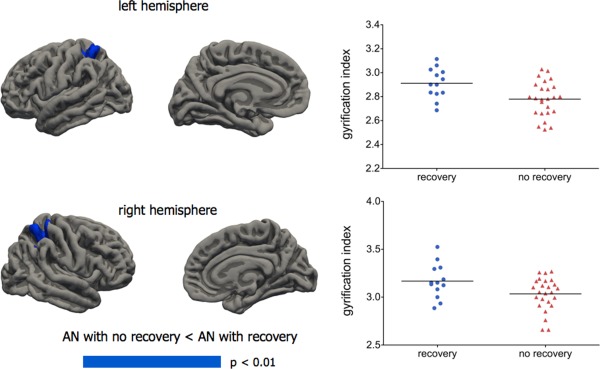
Vertex‐wise comparisons between acute AN patients who subsequently recovered at 3‐year follow‐up (n = 13) and those who still had an eating disorder at follow‐up (n = 24). General linear models with age, duration of illness, and total intracranial volume were used, applying the Monte Carlo method to control for multiple comparison and using a cluster‐wise threshold for inclusion of P < 0.01. Graphs show individual mean gyrification values in areas of significant difference. Left hemisphere: a cluster of 897 mm2 with a vertex of maximum difference (P = 0.02) in the superior parietal cortex (−17, −49, 62). Right hemisphere: a cluster of 1.993 mm2 with a vertex of maximum difference (P = 0.0002) in the postcentral gyrus (27, −38, 51). No cluster showed significant results in the opposite direction. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
No correlation emerged between overall hemispheric gyrification and weight gain at follow‐up (left lGI: rho = 0.18; P = 0.29; right lGI: rho = 0.21; P = 0.22). At vertex‐wise analysis, no significant correlation emerged between gyrification and weight gain.
Finally, we tested outcome predictive ability for both the overall hemispheric gyrification indices and the average gyrification index of clusters significantly differentiating patients with full recovery (n = 13) and those not making a full recover (n = 38). Both overall gyrification indices and those specific to significant clusters showed a significant predictive ability in identifying good outcome patients (Table 3). No relevant changes in the models were observed after including other possible prognostic variables, such as SSRI therapy, depressive/anxiety symptoms, diagnostic subtype, and severity of eating psychopathology.
Table 3.
Outcome predictive ability of gyrification indices (logistic regression analyses)
| OR (95% CI) | OR (95% CI) adjusted for age, age at onset, body mass index and duration of illness | |||
|---|---|---|---|---|
| Local gyrification indicesa | OR (95% CI) | % Individuals correctly classified | OR (95% CI) | % Individuals correctly classified |
| Overall lh LGI | 3.4 (1.1–10.3) P = 0.032 | 67.6% | 5.2 (1.1–23.9) P = 0.033 | 81.1% |
| Overall rh LGI | 2.9 (1.1–7.7) P = 0.033 | 73.0% | 3.8 (1.1–13.0) P = 0.031 | 83.8% |
| Left superior parietal cluster LGI | 2.5 (1.3–5.0) P = 0.008 | 73.0% | 4.3 (1.3–13.9) P = 0.015 | 83.8% |
| Right postcentral cluster LGI | 1.8 (1.0–3.0) P = 0.037 | 73.0% | 3.4 (1.2–9.4) P = 0.021 | 78.4% |
All LGI indices were multiplied by a factor of 10.
DISCUSSION
This study was the first to explore the presence of cortical folding aberrations in AN and the relationship between cortical morphology and outcome. In both hemispheres, AN patients showed decreased gyrification in comparison to healthy women in brain areas (superior parietal and frontal cortex) which are considered crucial for integration and elaboration of somatosensory perceptions, visuo‐spatial abilities, and executive functions. These cognitive functions may be related to body image disturbance, which is considered one of the “core” characteristics of this disorder [Favaro et al., 2012]. Functional neuroimaging studies relying on different measurement techniques have consistently implicated aberrations in the bilateral parietal cortex [Kaye et al., 2013; Van Kuyck et al., 2009] as possible markers of AN, given its role in body image elaboration. However, AN patients often also display problems in cognitive style, such as poor set‐shifting [Roberts et al., 2007; Tenconi et al., 2010] and decision making abilities [Bodell et al., 2014], these being considered to mainly rely on prefrontal cortex functioning. Future research should explore the pathways of possible relationships between alterations of cortical folding in patients with AN and those of underlying patterns of connectivity, as well as connections with psychopathology and cognitive abilities.
Although the mechanisms behind developmental aspects of cortical folds are still unclear, it is hypothesized that the adult pattern of gyrification somewhat reflects the integrity of the process of connectivity development during prenatal life [Dubois et al., 2008; White et al., 2010]. In this context, our findings would confirm a neurodevelopmental hypothesis for AN [Favaro, 2013], supported by evidence of similar patterns of hypogyria in other neurodevelopmental psychiatric disorders, such as schizophrenia [Palaniyappan and Liddle, 2012] and autism [Libero et al., 2014]. However, starvation in AN is associated with anatomical brain alterations that potentially could account for our findings. The lGI is not able to distinguish between a decrease in number or in development of sulci, from one hand, and reduced sulcal depth or increased sulcal width on the other [Palaniyappan et al., 2013]. In other words, a reduced gyrification index could imply either hypogiria or cortical flattening due to a decrease in cortical volume and thickness. To date, histological cortical changes associated with weight loss and malnutrition in AN have received little attention in literature [Neumarker et al., 1997]. The decrease in both gray and white matter volumes and their potential (at least partial) reversibility lead to the hypothesis that rather than neuronal death or other types of neurodegeneration, it is the reduced dendritic ramification and spine density, as well as the reduced number/volume of glia cells, that must be held accountable for these changes [Neumarker et al., 1997; Seitz et al., 2014]. Moreover, it is worth noting that the adolescent brain is characterized by developmental changes that physiologically imply reduced gray matter volume (due to cortical thinning) and increased white matter volume associated with the continuing process of myelination in the neuropil and deep intracortical layers [Aleman‐Gomez et al., 2013; Klein et al., 2014]. The impact of AN on brain developmental trajectories is not known and longitudinal studies would be of great importance for understanding the specific brain changes in this type of patients. In our cross‐sectional MRI study, we found both evidence in favor of as well as evidence against the hypothesis of an effect of weight loss in the determination of gyrification alterations. Our finding of an absence of gyrification alterations in the sample of recovered AN patients would support a weight loss effect hypothesis lacking long‐term consequences. This would be in line with previous studies about cortical thinning in AN [King et al., 2015] and many preceding voxel‐based morphometry studies [Van den Eynde et al., 2012; Seitz et al., 2014]. According to this hypothesis, decreased gyrification in our sample of acute AN patients could simply be the result of reduced cortical thickness. In contrast with this hypothesis, however, we found that maps of reduced cortical thickness and of reduced gyrification only show a marginal overlapping in our sample and including maps of cortical thickness as a vertex‐wise covariate did not affect our findings. In addition, we observed a decrease of brain gyrification along with age in large brain areas of both samples (as reported in previous studies on healthy individuals [Aleman‐Gomez et al., 2013; Klein et al., 2014]), with no evidence of differences between groups in the pattern of gyrification changes.
While analyzing our second objective, namely the impact of brain gyrification in predicting outcome at follow‐up, an alternative hypothesis emerged: in our analyses, we found that low levels of gyrification significantly predicted poor outcome at a 3‐year follow‐up, whereas the “good outcome” group showed no differences in gyrification index when compared to healthy women even though they were, on average, severely underweight.
The pattern of our findings could thus fit the hypothesis that abnormal brain gyrification is more evident in a subgroup of “poor outcome” AN patients, probably as the consequence of a more severe disruption of neurodevelopmental processes. Since the recovered AN group is “per definition” a group with a good prognosis, this hypothesis would explain why gyrification was not impaired in this group. Moreover, such result is in line with previous papers dealing with other psychiatric disorders which found that gyrification is not only a marker of integrity of normal cortical development [Palaniyappan and Liddle, 2012; Schaer et al., 2008], but also characterizes a subgroup of patients with low response to treatments [Palaniyappan et al., 2013]. Furthermore, in our sample, gyrification indices were able to predict outcome even after controlling for the effects of well known prognostic factors such as weight loss, body mass index, severity of psychopathology, and duration of illness. If confirmed by future studies, this finding would be of great importance for the identification of patients with poor response to standard treatments as well as for the provision, in these cases, of more intensive or alternative biological [Lipsman et al., 2013] or nonbiological [Tchanturia et al., 2014] treatments to obtain symptomatic relief. Since AN is an illness with relatively low levels of recovery and high levels of chronicization [Treasure et al., 2010], our findings might be taken as a warning against the tendency to generalize brain studies based on recovered patients. Indeed, in these studies although the effects of the presence of malnutrition on the brain are not present, it is very difficult to keep track of the effects of those favorable prognostic factors that made recovery possible.
This study has several strengths, as well as important limitations, which should be taken into consideration. It is the first to explore brain gyrification in terms of the possible relationships between brain characteristics and subsequent outcomes in a sample of AN patients. However, a particular word of caution must apply in interpreting brain findings in AN samples for which it is often difficult to disentangle the effects of early developmental factors on the brain and the consequences of starvation. In particular, although in our study no linear correlation has emerged between gyrification and duration of illness, we cannot exclude prolonged starvation as a possible cause of our findings as the poor outcome group had on average a duration of more than 8 years of illness. This is the reason why duration of illness was included as a covariate in the comparison between AN groups. Furthermore, according to a sort of ‘two‐hit’ model similar to that hypothesized for schizophrenia, our findings could be the result of an interaction between early developmental factors and later weight loss [Favaro, 2013; Schmitt et al., 2014]. Although in this study AN patients were assessed after their medical conditions had stabilized, hydration is another factor that could account for variations in the shape and volume of the brain cortex. However, we tested this possible correlation in a subsample of severely underweight patients and found no significant effects. Finally, this study was performed on patients and controls of female gender. Any inference about brain gyrification alterations in male patients with AN cannot be made and would be an interesting topic to explore in future studies.
According to data of the present study, cortical gyrification alterations are present in patients with AN, but they do not meet the criteria to be considered as endophenotype of the disorder or a sort of “biomarker” with a role in the identification of the disease. The specificity of gyrification alterations in terms of brain location and underlying connectivity dysfunctions in comparison with other types of psychiatric patients also needs to be tested when sufficient data becomes available in the literature to allow qualitative and quantitative reviews.
In conclusion, this study provides evidence of cortical gyrification alterations in the parietal and frontal cortex of patients with AN. These alterations did not show a linear relationship with body mass index, cortical thickness or dehydration and these changes were not present in patients with a good outcome, regardless of their body weight and recovery status. Longitudinal studies carried out on large cohorts of patients suffering from AN are needed to explore the effects of malnutrition and weight loss on cortical morphology and to understand how these interact with the duration of the illness, the response to treatment and aging. Moreover, to explore the possible involvement of these cortical aberrations in the psychopathology of AN and their clinical and scientific implications, it would be interesting to study at least two further aspects. One is the replicability of our findings in patients suffering from the illness for a short period of time or in high‐risk individuals who do not have AN. The other is the exploration of possible structural/functional connectivity alterations that, according to the Van Essen's hypothesis, underly aberrations of cortical folding [Van Essen, 1997]. Any possible hypothesis about a possible neurodevelopmental origin of these cortical aberrations requires a careful examination and exclusion of alternative hypotheses. However, if our findings will be replicated and supported by future studies, cortical folding alterations in AN patients, regardless of their origin, could represent a useful method for identifying patients who are resistant to conventional treatments at an early stage.
Supporting information
Supporting Information
Financial Disclosures: None of the authors reported any biomedical financial interests or potential conflicts of interest.
REFERENCES
- Aleman‐Gomez Y, Janssen J, Schnack H, Balaban E, Pina‐Camacho L, Alfaro‐Almagro F, Castro‐Fornieles J, Otero S, Baeza I, Moreno D, Bargallo N, Parellada M, Arango C, Desco M (2013): The human cerebral cortex flattens during adolescence. J Neurosci 33:15004–15010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K (1995): The ontogeny of human gyrification. Cereb Cortex 5:56–63. [DOI] [PubMed] [Google Scholar]
- Bodell LP, Keel PK, Brumm MC, Akubuiro A, Caballero J, Tranel D, Hodis B, McCormick LM (2014): Longitudinal examination of decision‐making performance in anorexia nervosa: Before and after weight restoration. J Psychiatr Res 56:150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SJ, Barker GJ, O'Daly OG, Brammer M, Williams SC, Benedict C, Schiöth HB, Treasure J, Campbell IC (2011): Restraint of appetite and reduced regional brain volumes in anorexia nervosa: A voxel‐based morphometric study. BMC Psychiatry 11:179. doi 10.1186/1471‐244X‐1 1‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L (1974): The Hopkins Symptom Checklist (HSCL): A self‐report symptom inventory. Behav Sci 19:1–15. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006): An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31:968–980. [DOI] [PubMed] [Google Scholar]
- Dubois J, Benders M, Borradori‐Tolsa C, Cachia A, Lazeyras F, Ha‐Vinh Leuchter R, Sizonenko SV, Warfield SK, Mangin JF, Hüppi PS (2008): Primary cortical folding in the human newborn: An early marker of later functional development. Brain 131:2028–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaro A (2013): Brain development and neurocircuit modeling are the interface between genetic/environmental risk factors and eating disorders. Int J Eat Disord 46:443–446. [DOI] [PubMed] [Google Scholar]
- Favaro A, Tenconi E, Santonastaso P (2006): Perinatal factors and the risk of developing anorexia nervosa and bulimia nervosa. Arch Gen Psychiatry 63:82–88. [DOI] [PubMed] [Google Scholar]
- Favaro A, Caregaro L, Tenconi E, Bosello R, Santonastaso P (2009): Time trends in age at onset of anorexia nervosa and bulimia nervosa. J Clin Psychiatry 70:1715–1721. [DOI] [PubMed] [Google Scholar]
- Favaro A, Tenconi E, Santonastaso P (2010): The interaction between perinatal factor and childhood abuse in the risk of developing anorexia nervosa. Psychol Med 40:657–665. [DOI] [PubMed] [Google Scholar]
- Favaro A, Santonastaso P, Manara R, Bosello R, Bommarito G, Tenconi E, Di Salle F (2012): Disruption of visuospatial and somatosensory functional connectivity in anorexia nervosa. Biol Psychiatry 72:864–870. [DOI] [PubMed] [Google Scholar]
- Favaro A, Clementi M, Manara R, Bosello R, Forzan M, Bruson A, Tenconi E, Degortes D, Titton F, Di Salle F, Santonastaso P (2013): Catechol‐O‐methyltransferase genotype modifies executive functioning and prefrontal functional connectivity in women with anorexia nervosa. J Psychiatry Neurosci 38:241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaro A, Tenconi E, Degortes D, Manara R, Santonastaso P (2014): Effects of obstetric complications on volume and functional connectivity of striatum in anorexia nervosa patients. Int J Eat Disord 47:686–695. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW (1995): Structured Clinical Interview for DSM‐IV Axis I Disorders. New York: Biometrics Research Department. [Google Scholar]
- Fischl B, Dale AM (2000): Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM (1999): Cortical surface‐based analysis. II: Inflation, flattening, and a surface‐based coordinate system. Neuroimage 9:195–207. [DOI] [PubMed] [Google Scholar]
- Fonville L, Giampietro V, Williams SC, Simmons A, Tchanturia K (2014): Alterations in brain structure in adults with anorexia nervosa and the impact of illness duration. Psychol Med 44:1965–1975. [DOI] [PubMed] [Google Scholar]
- Frank GK, Shott ME, Hagman JO, Mittal VA (2013): Alterations in brain structures related to taste reward circuitry in ill and recovered anorexia nervosa and in bulimia nervosa. Am J Psychiatry 170:1152–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner DM, Olmsted MP, Polivy J (1983): Development and validation of a multidimensional Eating Disorder Inventory for anorexia nervosa and bulimia. Int J Eat Disord 2:15–34. [Google Scholar]
- Gillberg C, Rastam M, Gillberg IC (1994): Anorexia nervosa: Physical health and neurodevelopment at 16 and 21 years. Dev Med Child Neurol 36:567–575. [DOI] [PubMed] [Google Scholar]
- Giménez M, Junqué C, Vendrell P, Narberhaus A, Bargalló N, Botet F, Mercader JM (2006): Abnormal orbitofrontal development due to prematurity. Neurology 67:1818–1822. [DOI] [PubMed] [Google Scholar]
- Hamsher KS, Halmi KA, Benton AL (1981): Prediction of outcome in anorexia nervosa from neuropsychological status. Psychiatry Res 4:79–88. [DOI] [PubMed] [Google Scholar]
- Haukvik UK, Schaer M, Nesvåg R, McNeil T, Hartberg CB, Jönsson EG, Eliez S, Agartz I (2012): Cortical folding in Broca's area relates to obstetric complications in schizophrenia patients and healthy controls. Psychol Med 42:1329–1337. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Wierenga CE, Bailer UF, Simmons AN, Bischoff‐Grethe A (2013): Nothing tastes as good as skinny feels: The neurobiology of anorexia nervosa. Trends Neurosci 36:110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Vohr B, Schneider KC, Katz KH, Makuch RW, Reiss AL, Ment LR (2006): Increased temporal lobe gyrification in preterm children. Neuropsychologia 44:445–453. [DOI] [PubMed] [Google Scholar]
- King JA, Geisler D, Ritschel F, Schober I, Seidel M, Roschinski B, Soltwedel L, Zwipp J, Pfuhl G, Marxen M, Roessner V, Ehrlich S (2015): Global cortical thinning in acute anorexia nervosa normalizes following long‐term weight restoration. Biol Psychiatry 77:624–632. [DOI] [PubMed] [Google Scholar]
- Klein D, Rotarska‐Jagiela A, Genc E, Sritharan S, Mohr H, Roux F, Han CE, Kaiser M, Singer W, Uhlhaas PJ (2014): Adolescent brain maturation and cortical folding: Evidence for reductions in gyrification. PLos One 9:e84914doi:10.1371/journal.pone.0084914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libero LE, DeRamus TP, Deshpande HD, Kana RK (2014): Surface‐based morphometry of the cortical architecture of autism spectrum disorders: Volume, thickness, area, and gyrification. Neuropsychologia 62:1–10. [DOI] [PubMed] [Google Scholar]
- Lipsman N, Woodside DB, Giacobbe P, Lozano AM (2013): Neurosurgical treatment of anorexia nervosa: Review of the literature from leucotomy to deep brain stimulation. Eur Eat Disord Rev 21:428–435. [DOI] [PubMed] [Google Scholar]
- Lopez C, Tchanturia K, Stahl D, Treasure J (2008): Central coherence in eating disorders: A systematic review. Psychol Med 38:1393–1404. [DOI] [PubMed] [Google Scholar]
- Muhlau M, Gaser C, Ilg R, Conrad B, Leibl C, Cebulla MH, Backmund H, Gerlinghoff M, Lommer P, Schnebel A, Wohlschläger AM, Zimmer C (2007): Gray matter decrease of the anterior cingulate cortex in anorexia nervosa. Am J Psychiatry 164:1850–1857. [DOI] [PubMed] [Google Scholar]
- Nanda P, Tandon N, Mathew IT, Giakoumatos CI, Abhishekh HA, Clementz BA, Pearlson GD, Sweeney J, Tamminga CA, Keshavan MS (2014): Local gyrification index in probands with psychotic disorders and their first‐degree relatives. Biol Psychiatry 76:447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesvag R, Schaer M, Haukvik UK, Westlye LT, Rimol LM, Lange EH, Hartberg CB, Ottet M‐C, Melle I, Andreassen OA, Jönsson EG, Agartz I, Eliez S (2014): Reduced brain cortical folding in schizophrenia revealed in two independent samples. Schizophr Res 152:333–338. [DOI] [PubMed] [Google Scholar]
- Neumarker KJ, Dudeck U, Meyer U, Neumarker U, Schulz E, Schonheit B (1997): Anorexia nervosa and sudden death in childhood: Clinical data and results obtained from quantitative neurohistological investigations of cortical neurons. Eur Arch Psychiatry Clin Neurosci 247:16–22. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Palaniyappan L, Liddle PF (2012): Aberrant cortical gyrification in schizophrenia: A surface‐based morphometry study. J Psychiat Neurosci 37:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyappan L, Marques TR, Taylor H, Handley R, Mondelli V, Bonaccorso S, Giordano A, DiForti M, Simmons A, David AS, Pariante CM, Murray RM, Dazzan P (2013): Cortical folding defects as markers of poor treatment response in first‐episode psychosis. JAMA Psychiatry 70:1031–1040. [DOI] [PubMed] [Google Scholar]
- Prikryl R, Ceskova E, Kasparek T, Kucerova H (2007): Neurological soft signs and their relationship to 1‐year outcome in first‐episode schizophrenia. Eur Psychiatry 8:499–504. [DOI] [PubMed] [Google Scholar]
- Roberts ME, Tchanturia K, Stahl D, Southgate L, Treasure J (2007): A systematic review and meta‐analysis of set‐shifting ability in eating disorders. Psychol Med 37:1075–1084. [DOI] [PubMed] [Google Scholar]
- Schaer M, Cuadra MB, Tamarit L, Lazeyras F, Eliez S, Thiran JP (2008): A surface‐based approach to quantify local cortical gyrification. IEEE Trans Med Imaging 27:161–170. [DOI] [PubMed] [Google Scholar]
- Schaer M, Ottet M‐C, Scariati E, Dukes D, Franchini M, Eliez S, Glaser B (2013): Decreased frontal gyrification correlates with altered connectivity in children with autism. Front Hum Neurosci 7: doi: 10.3389/fnhum.2013.00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A, Malchow B, Hasan A, Falkai P (2014): The impact of environmental factors in severe psychiatric disorders. Front Neurosci 8: doi: 10.3389/fnins.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz J, Buhren K, von Polier GG, Heussen N, Herpertz‐Dahlmann B, Konrad K (2014): Morphological changes in the brain of acutely ill and weight‐recovered patients with anorexia nervosa. A meta‐analysis and qualitative review. Z Kinder Jugendpsychiatr Psychother 42:7–18. [DOI] [PubMed] [Google Scholar]
- Sun T, Hevner RF (2014): Growth and folding of the mammalian cerebral cortex: From molecules to malformations. Nat Rev Neurosci 15:217–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchanturia K, Lounes N, Holttum S (2014): Cognitive remediation in anorexia nervosa and related conditions: A systematic review. Eur Eat Disord Rev 22:454–462. [DOI] [PubMed] [Google Scholar]
- Tenconi E, Santonastaso P, Degortes D, Bosello R, Titton F, Mapelli D, Favaro A (2010): Set‐shifting abilities, central coherence, and handedness in anorexia nervosa patients, their unaffected siblings and healthy controls: exploring putative endophenotypes. World J Biol Psychiatry 11:813–823. [DOI] [PubMed] [Google Scholar]
- Tenconi E, Santonastaso P, Monaco F, Favaro A (2015): Obstetric complications and eating disorders: A replication study. Int J Eat Disord 48:424–430. [DOI] [PubMed] [Google Scholar]
- Titova OE, Hjorth OC, Schiöth HB, Brooks SJ (2014): Anorexia nervosa is linked to reduced brain structure in reward and somatosensory regions: A meta‐analysis of VBM studies. BMC Psychiatry 13:110. doi: 10.1186/1471-244X-13-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treasure J, Claudino AM, Zucker N (2010): Eating disorders. Lancet 375:583–593. [DOI] [PubMed] [Google Scholar]
- Van den Eynde F, Suda M, Broadbent H, Guillaume S, Van den Eynde M, Steiger H, Israel M, Berlim M, Giampietro V, Simmons A, Treasure J, Campbell I, Schmidt U (2012): Structural magnetic resonance imaging in eating disorders: A systematic review of voxel‐based morphometry studies. Eur Eat Disord Rev 20:94–105. [DOI] [PubMed] [Google Scholar]
- Van Essen DC (1997): A tension‐based theory of morphogenesis and compact wiring in the central nervous system. Nature 385:313–318. [DOI] [PubMed] [Google Scholar]
- Van Kuyck K, Gerard N, Van Laere K, Casteels C, Pieters G, Gabriels L, Nuttin B (2009): Towards a neurocircuitry in anorexia nervosa: Evidence from functional neuroimaging studies. J Psychiatr Res 43:1133–1145. [DOI] [PubMed] [Google Scholar]
- Wagner A, Greer P, Bailer UF, Frank GK, Henry SE, Putnam K, Meltzer CC, Ziolko SK, Hoge J, McConaha C, Kaye WH (2006): Normal brain tissue volumes after long‐term recovery in anorexia and bulimia nervosa. Biol Psychiatry 59:291–293. [DOI] [PubMed] [Google Scholar]
- White T, Hilgetag CC (2011): Gyrification and neural connectivity in schizophrenia. Dev Psychopathol 23:339–352. [DOI] [PubMed] [Google Scholar]
- White T, Su S, Schmidt M, Kao C‐Y, Sapiro G (2010): The development of gyrification in childhood and adolescence. Brain Cogn 72:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


