Abstract
Prospective memory (PM) refers to the ability to remember to do something in the future, either in response to an event (event‐based) or after a certain amount of time has elapsed (time‐based). While the distinction between event‐ and time‐based PM is widely acknowledged in the literature, little is known about the processes they share and those they do not. This is particularly true concerning their brain substrates, as almost all neuroimaging studies so far have focused on event‐based PM. We proposed a functional magnetic resonance imaging paradigm assessing both event‐based and time‐based PM to 20 healthy young individuals. Analyses revealed that event‐ and time‐based PM both induced activation in the posterior frontal and parietal cortices, and deactivation in the medial rostral prefrontal cortex. In addition, activation more specific to each condition, which may underlie differences in strategic monitoring, was highlighted. Thus, occipital areas were more activated during event‐based PM, probably reflecting target‐checking, while a network comprising the dorsolateral prefrontal cortex, the cuneus/precuneus and, to a lesser extent, the inferior parietal lobule, superior temporal gyrus, and the cerebellum, was more activated in time‐based PM, which may reflect the involvement of time‐estimation processes. These results confirm the allocation of attentional resources to the maintenance of intention for event‐based and time‐based PM, as well as the engagement of distinct mechanisms reflecting the monitoring strategies specific to each condition. Hum Brain Mapp 35:3066–3082, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: attention, frontal lobe, functional brain imaging, memory, young adult
INTRODUCTION
Long neglected, the investigation of the mechanisms subserving the realization of delayed intentions, also known as prospective memory (PM), has been the subject of a growing number of studies over the past two decades. Two components are fundamental to correctly execute delayed intentions: a prospective component and a retrospective one [Einstein and McDaniel, 1990]. The prospective component is specific to PM and involves remembering that something has to be done at the appropriate time. The retrospective component refers to the content of the intention, in other words, remembering what has to be done. PM thus involves encoding an intention, maintaining it over time and, at the appropriate moment, reinstating that intention and executing the action associated with it [Kliegel et al., 2002]. Finally, individuals have to verify and then suppress the intention, to avoid its detrimental repetition [Einstein et al., 1998]. A key aspect of PM, compared with “classic” episodic memory, lies in the retrieval of intention. No explicit instruction for recall is given and individuals have to initiate the recall by themselves at a given point in the future. Intention is triggered either by the occurrence of an external event, namely the prospective cue (e.g., “take the cake out of the oven when the timer rings”), or after a defined amount of time (e.g., “take the cake out of the oven after 30 minutes”); respectively called event‐based PM (EBPM) and time‐based PM (TBPM) [Einstein and McDaniel, 1990]. TBPM is usually judged to be harder than EBPM, because the former relies on self‐initiated processes for the retrieval of intention, whereas the latter is cued by the occurrence of an event [Einstein et al., 1995; Khan et al., 2008; Kliegel et al., 2001; Park et al., 1997]. Nevertheless, this is not always the case and, under some conditions, EBPM may be as vulnerable as TBPM, if not more [Cheng et al., 2008; Cuttler and Graf, 2009; d'Ydewalle et al., 2001; Gonneaud et al., 2011].
Several authors tried to explain PM functioning, and most of them referred to the multiprocess theory [Einstein et al., 2005; McDaniel and Einstein, 2000, 2007]. This theory, developed to account for EBPM performance, highlights the involvement of either automatic or controlled processes, depending on the task. More specifically, it suggests that PM processes lie on a continuum from automatic to controlled, depending on the characteristics of the ongoing task, the PM cue and the individual. This suggestion has been confirmed by studies reporting better PM performance when relatively few attentional resources are devoted to the ongoing activity [Einstein et al., 1997; Kidder et al., 1997; Marsh and Hicks, 1998; Marsh et al., 2002], when detecting the PM cue relies on the same processes as the ongoing task (i.e., focal cue) [Brewer et al., 2010; Marsh et al., 2000; Scullin et al., 2010] or when the PM cue is particularly distinctive [Brandimonte and Passolunghi, 1994; McDaniel and Einstein, 1993; West et al., 2003]. In the same manner, the individual's motivation, consciousness or cognitive abilities may affect the kind of processes engaged in PM [Brandimonte et al., 2010; Kliegel et al., 2004; Smith et al., 2011]. Although the multiprocess theory was developed to account for EBPM processes, it could easily be extended to time‐based situations. Insofar as they rely far more on controlled processes, TBPM intentions are generally more vulnerable than EBPM ones. Moreover, like EBPM, TBPM is negatively affected by particularly demanding ongoing tasks [Khan et al., 2008; Occhionero et al., 2010] and positively affected by the degree of motivation allocated to executing the task [Altgassen et al., 2010; Kliegel et al., 2001].
Regarding the cognitive substrates of PM, links have been highlighted between EBPM performance and retrospective episodic memory, executive functioning, working memory, and binding processes [Hainselin et al., 2011; Logie et al., 2004; Martin et al., 2003; Salthouse et al., 2004; Wang et al., 2010]. TBPM performance largely depends on time‐estimation processes and, more specifically, on accurate time‐monitoring (i.e., number of times that individuals check the clock during the task) behavior [Harris and Wilkins, 1982; Khan et al., 2008; Kvavilashvili and Fisher, 2007; Mäntylä et al., 2009]. Direct comparisons of EBPM and TBPM indicate that they depend on partially different sets of cognitive functions. In a previous study [Gonneaud et al., 2011], we found that although age‐related impairment of both EBPM and TBPM was related to processing speed and inhibition, age‐related impairment of EBPM alone was mediated by binding and retrospective episodic memory processes, and that of TBPM by inhibition processes. Another study, however, reported different results suggesting that, in healthy aging, EBPM could be predicted by inhibition, while TBPM could be predicted by shifting abilities [Kliegel et al., 2003]. These results point out the existence of distinct cognitive substrates for EBPM and TBPM that rely, at least in part, on distinct brain regions. Lesion‐based studies support this idea, reporting impairment specific to TBPM after thalamic lesions [Cheng et al., 2010] and specific to EBPM in the case of focal prefrontal lesions [Cheng et al., 2008].
Most neuroimaging studies have focused on EBPM. Positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) studies fairly consistently indicate the involvement of the rostral prefrontal cortex (RPFC; Brodmann area [BA] 10) in PM. More specifically, they report increased activity in the lateral RPFC during PM, compared with the ongoing task alone, associated with decreased activity in the medial part of the RPFC [see Burgess et al., 2011 for a review]. This involvement of the RPFC reflects active maintenance of intention [Burgess et al., 2001] and appears to be common to every EBPM task, whatever the characteristics of the cues or the ongoing task [Burgess et al., 2003, see also 2011 for a review]. This result has been interpreted within the framework of the gateway hypothesis [Burgess et al., 2007a,b] according to which the lateral part of the RPFC subserves stimulus‐independent attending and the medial part stimulus‐oriented attending. In other words, activation of the lateral part of the RPFC allows for stimulus‐independent cognition, such as the maintenance of an intention while performing another task. By contrast, the medial part of the RPFC subserves processes directed toward the environment. Deactivation of this area reflects disengagement from the ongoing task in order to maintain intention. According to Gilbert et al. [Gilbert, 2011; Gilbert et al., 2012], activation of the lateral RPFC is specific to the storage stage of PM but does not directly support the content of delayed intentions. Rather, this region may act through its interaction with the other regions involved in PM, notably the posterior regions that decode the content of intention. However, in a study where the storage stage was not occupied by an ongoing activity, thus allowing participants to focus their attention exclusively on the intention, Haynes et al. [2007] concluded that the medial anterior and lateral prefrontal cortex encodes intentional content. As a result, Gilbert [2011] suggested that the role played by the RPFC in PM may vary according to the experimental design. Consistent with this, conditions requiring different amounts of attentional resources, depending on the complexity of the intention [Simons et al., 2006], the specificity of the PM instructions [Gilbert et al., 2009], or the characteristics of the PM cue [Rea et al., 2011], lead to varying degrees of involvement of the lateral RPFC.
Kalpouzos et al. [2010] conducted a virtual reality‐based task conjointly with fMRI to investigate the neural substrates of each PM stage. Participants were immersed in a virtual town and asked to execute a list of intentions in this environment. This task allowed the authors to highlight the brain and cognitive substrates of each PM stage, and propose a dynamic model for PM. A frontoparietal network was found to be involved throughout the entire task for the maintenance of intentions, in addition to the activation of the occipital cortex during target‐searching and the activation of a fronto‐hippocampal network during the retrieval of intentional content.
To our knowledge, only one fMRI study [Momennejad and Haynes, 2012] and one PET study [Okuda et al., 2007] have used a TBPM paradigm. The former assessed the prospective (i.e., “when”) and retrospective (i.e., “what”) components of TBPM. Participants were asked to categorize numbers and to change the categorization criteria after a specific interval. As no clock was displayed, participants had to self‐estimate the passage of time. TBPM maintenance and retrieval were associated with activation of the medial and lateral PFC. To our knowledge, the latter study [Okuda et al., 2007] is the only one to have sought to identify the neural substrates of EBPM and TBPM using PET. Interestingly, TBPM was assessed with two tasks: one that involved self‐estimation of time and another in which this process of time estimation was minimized by adding a clock on the screen. Results revealed that EBPM induced activation of the lateral RPFC, accompanied by medial deactivation, while TBPM induced medial RPFC activation. Moreover, the authors found that TBPM recruited far more prefrontal regions than EBPM did. The involvement of these areas differed according to whether participants had to estimate time or could check a clock, with the former condition requiring more superior areas of the prefrontal cortex than the latter. Nevertheless, the differing degrees of difficulty of the EBPM and TBPM tasks probably resulted in differences in the allocation of controlled processes between conditions, and thus in the involvement of the RPFC.
Altogether, these studies suggest that TBPM and EBPM rely on partially distinct processes and neural substrates. Nevertheless, although PM studies point out the greater involvement of several subregions of the RPFC in TBPM than in EBPM [Momennejad and Haynes, 2012; Okuda et al., 2007], this question has yet to be unraveled. The present study was therefore designed to better characterize the similarities and differences between EBPM and TBPM within the same group of healthy young individuals, using fMRI and EBPM and TBPM tasks that were as equivalent as possible.
METHOD
Participants
Twenty healthy individuals aged 18–35 years (mean = 25.15 years, standard deviation = 5.14; 9 women and 11 men) participated in this study. They were all native French speakers and all right‐handed on the Edinburgh inventory [Oldfield, 1971]. We ensured that participants did not have history of neurological or psychiatric disorders, and had at least seven years of schooling (mean = 14.45 years; standard deviation = 2.56). The study was approved by the regional ethics committee (CPP Nord Ouest III) and all the participants gave their written informed consent prior to participation.
fMRI Tasks
The PM task was inspired by the design devised by Reynolds et al. [2009], featuring a succession of short blocks with different instructions. Participants had to complete blocks of an ongoing task and, for some of these blocks, a PM instruction was added. There were three experimental conditions: ongoing task only (OG‐only), EBPM, and TBPM. A fourth condition was included in the design, to assess the retrospective component of EBPM, but will not be described here. Prior to scanning, and as already done in other studies [see notably Reynolds et al., 2009; Simons et al., 2006], participants were trained to perform the task under each condition, the learning procedure being repeated as many times as it took for each participant to succeed. This allowed us to ensure participants fully understand the procedure and perform the task correctly latter. fMRI data were acquired in three runs, each of them encompassing all the conditions (i.e., OG‐only, EBPM, and TBPM, as well as retrospective component of EBPM assessment). The order of presentation of each condition was randomized between runs. After the fMRI scanning session, participants were debriefed, to ensure that they had not encountered any difficulty during the scanning.
Ongoing task
Participants performed a semantic categorization task. They were asked to classify 480 color pictures as “natural” or “manmade” items. Each object was displayed within a 280 × 280‐pixel white square with a 20‐pixel colored border. Nine different border colors were used, and colors were randomly changed between pictures. A digital countdown was displayed in the upper right‐hand corner of the screen. The border colors and the countdown were used for PM conditions (see below). To avoid any difficulty arising from a failure to remember the response keys, a reminder was displayed at the bottom of the screen, representing the forefinger–category association. Subjects had to evaluate 12 sets of 40 pictures. Among those 12 sets, only nine were used; the three others corresponded to the assessment of the retrospective component of EBPM. Each set took place as follows (Fig. 1): first of all, the instructions were displayed on the screen for 8 s. Half of the participants had to answer with the left forefinger for natural items and the right forefinger for manmade items. The other half had to answer the other way round. In order to artificially create a delay between the instructions and the beginning of the task, participants were asked to predict their ability to succeed on the upcoming set of picture on a 5‐point scale ranging from very badly to very well. This question remained on the screen for 8 s and was followed by a fixation cross for 1 s, and then the 40 pictures were displayed one by one. Each picture remained on the screen for 2 s, after which it was replaced by a mask (i.e., random black‐and‐white checkerboard with a multicolored border) for 1 or 2 s, used as temporal jitter. Although the participants were asked to answer as quickly and accurately as possible, answers given after the mask appeared were also taken into account. The pictures' order of presentation was entirely randomized between participants. At the end of each set of pictures, participants were asked retrospectively to assess their performance on that set on the same 5‐point scale as before (from very badly to very well). After 8 s, a fixation cross appeared and a new set began.
Figure 1.
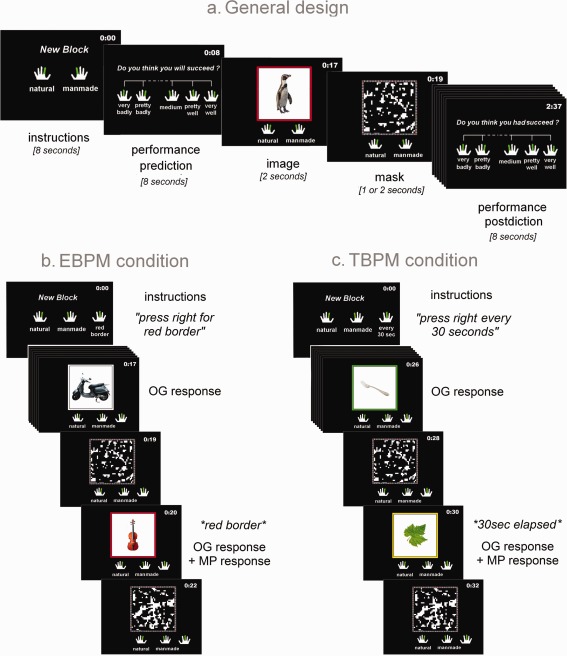
Design of the fMRI task. Each set includes instructions, performance prediction, 40 trials (picture + mask), and performance postdiction. Here are illustrated the general procedure and OG‐only condition (a), the EBPM (b), and TBPM (c) conditions [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
PM task
Participants were told that we were also interested in their ability to remember to do something in the future. Consequently, for some sets of pictures, they were asked to give an additional answer, using their middle finger (the right one for half the participants, and the left one for the other half). The sequencing of answers was not imposed to avoid any additional cost to the task. In fact, pretests of the task showed that such instructions increased dramatically the difficulty for the subjects. In addition, this choice allowed us to take into account correct answers that were just a little delayed from the appearance of the cue/time, but remained correct. In the TBPM condition, participants were asked to press a key with their middle finger every 30 s. To do so, they could look at the countdown, always present in the upper right‐hand corner of the screen. In the EBPM condition, participants had to press a key with their middle finger in response to a specific border color (for instance for each red border). A total of five PM answers were expected for each set of pictures. Participants were specifically told that for PM items, they had to provide both the PM answer (i.e., pressing with their middle finger) and the ongoing answer (i.e., pressing with their forefinger for the natural/manmade decision task). The EBPM and TBPM conditions were made as similar as possible, to ensure that differences in activation would be due solely to differences in strategic monitoring and detection processes. First, the appearance of the PM cue in the EBPM condition (i.e., color border) was always synchronized with the beginning of a trial (i.e., picture display) resulting in potential conflict between OG and PM answers. As a result, to reproduce the conjoint appearance of the picture and the PM cue in the TBPM condition, the end of the 30‐s periods always coincided with the beginning of a trial. Thus, the detection of the appropriate time to act always took place while a picture to classify appeared, and not during the two seconds after its appearance or during the mask. In addition, to mimic the TBPM condition, EBPM cue appeared once in each 30‐s interval, though not every 30 s, to avoid either conscious or unconscious expectation strategies [see Okuda et al., 2011]. EBPM cues were also designed not to be focal, because in essence TBPM is nonfocal. In order to keep a tight control over visual stimulation, the countdown was displayed on the screen in all three conditions and the color border systematically changed between pictures. Finally, to reduce the retrospective component cost, the middle finger used for the PM answers was the same for both EBPM and TBPM (right middle finger for half the participants and left middle finger for the other half), and was depicted at the bottom of the screen throughout each PM condition (see Fig. 1).
fMRI Data Acquisition
Two scanning sessions were performed on a Philips (Eindhoven, The Netherlands) Achieva 3.0 T scanner. In the first acquisition session, a high‐resolution T1‐weighted anatomical image was first acquired using a 3D fast field echo sequence (3D‐T1‐FFE sagittal, repetition time (TR) = 20 ms; echo time (TE) = 4.6 ms; flip angle = 20°; 170 slices; slice thickness = 1 mm; field of view (FOV) = 256 × 256 mm2; matrix = 256 × 256; acquisition voxel size = 1 × 1 × 1 mm3), followed by a high‐resolution T2‐weighted anatomical image (2D‐T2‐SE sagittal, SENSE factor = 2; TR = 5,500 ms; TE = 80 ms; flip angle = 90°; 81 slices; slice thickness = 2 mm; FOV = 256 × 256 mm2; matrix = 256 × 256; acquisition voxel size = 2 × 1 × 1 mm3) and a non‐EPI T2 Star image (2D‐T2 Star‐FFE axial, SENSE factor = 2; TR = 3,505 ms; TE = 30 ms; flip angle = 90°; 70 slices; slice thickness = 2 mm; FOV = 256 × 256 mm2; matrix = 128 × 128; acquisition voxel size = 2 × 2 × 2 mm3). In the second acquisition session, a non‐EPI T2 Star image, similar to that of the anatomical session was acquired. Functional data were then acquired using an interleaved 2D T2 Star EPI sequence designed to reduce geometrical distortions and magnetic susceptibility artifacts (2D‐T2 Star‐FFE‐EPI axial, SENSE factor = 2; TR = 2,600 ms; TE = 30 ms; flip angle = 80°; 46 slices; slice thickness = 3 mm; matrix = 80 × 80; FOV = 224 × 224 mm2; acquisition voxel size = 2.8 × 2.8 × 3.0 mm3; 266 volumes per run). Three functional runs were acquired during the session, each including the four conditions (i.e., OG‐only, EBPM, TBPM, and retrospective component of EBPM, this latter being not analyzed in the present article). The six initial volumes of each run were discarded to control for magnetic saturation effects.
Behavioral Data Analysis
ANOVAs were conducted to assess accuracy and reaction times for correct answers in each condition (OG‐only vs. EBPM vs. TBPM). First, we looked at whether participants' PM answers were equally accurate in both the EBPM and TBPM conditions. To do so, we ran a one‐way ANOVA comparing the percentages of correct middle‐finger presses (i.e., PM answers) in the EBPM and TBPM conditions. For TBPM, answers were deemed to be correct if they were provided at the target times (i.e., during the picture display or the mask). It should be noted that all the participants either answered during this interval or not at all. Second, to assess the interference effect of the PM task on the ongoing task, an ANOVA was conducted to compare the percentages of correct answers for ongoing items (excluding PM items) in the OG‐only, EBPM and TBPM conditions. The same procedure was then used with the reaction times for correct answers on ongoing items (excluding PM items). Lastly, Tukey's HSD tests were used for post hoc comparisons.
fMRI Data Processing and Analysis
fMRI data were analyzed using SPM5 (Statistical Parametric Mapping software; http://www.fil.ion.ucl.ac.uk/spm). Preprocessing was conducted as follows. Briefly, EPI data were checked to ensure the absence of artifacts and realigned with the first volume of the first run. Geometric EPI distortions were then corrected as follows: the mean EPI image was coregistered onto the non‐EPI T2 Star volume of the functional session, the non‐EPI T2 Star volume of the functional session was coregistered onto the anatomical one, the non‐EPI T2 Star volume of the anatomical session was then coregistered onto the T2 image, and finally the T2 volume was coregistered onto the T1 image according to the procedure described by Villain et al. [2010]. T1 was segmented and normalized on the Montreal Neurological Institute (MNI) template and the resulting parameters were applied to the T1, non‐EPI T2 star volumes and EPI images. Finally, the EPI images were smoothed using an 8‐mm full width at half maximum (FWHM) Gaussian kernel.
The general linear model was used to assess the effects of the different conditions (OG‐only vs. EBPM vs. TBPM). In a first‐level analysis, each event was specified individually. Each correct trial (picture + mask) was specified as an event. In addition, instructions, questions, and errors were modeled as regressors of no interest. For each participant, main effect of the OG‐only, EBPM, and TBPM conditions were estimated, specifying the onset and full duration of every trial in each condition (ongoing items and PM items) resulting in a correct answer. Correct responses on OG items and on PM items were modeled separately at the first level. For PM items, both correct ongoing answers and correct PM answers were required. Finally, to account for potential differences in reaction times between conditions, reaction time for each item was added in the model as a parametric modulator.
Second‐level analyses consisted in comparisons between the OG‐only condition and each of the two PM conditions to highlight the brain substrates of EBPM and TBPM. These analyses aimed at revealing the neural substrates of each PM condition, independently from each other. To do so, subtractions between each PM condition and the OG‐only one were performed. Then, in order to identify areas specifically involved in each PM condition compared to the OG‐only one, main effects of each PM condition were exclusively masked by the main effect of OG‐only (exclusive mask at P < 0.05). Then, to pinpoint the similarities and differences between EBPM and TBPM, we performed conjunction and subtraction analyses. Conjunction analyses were performed using main effects of the two PM conditions (conjunction of EBPM + TBPM) and were restricted to the regions which were not involved in OG‐only, using the main effect of this latter condition as exclusive mask (at P < 0.05 uncorrected). This method was used both for activations and deactivations. Subtractions between EBPM and TBPM main effects were then assessed. As both conditions included the same ongoing task, we assume that such subtractions highlight substrates independent from the ongoing ones. Finally, to identify more precisely the substrates of EBPM and TBPM detection and execution, an additional analysis was conducted, contrasting correct responses on PM items only for EBPM and TBPM.
Statistical maps were thresholded at P < 0.05 using FWE correction. For finer effect, a less stringent threshold was also adopted at P < 0.001 uncorrected at the voxel level and a minimal cluster size was calculated using 3dClustSim (http://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html) to obtain a significance level corrected for multiple comparisons. Thus, the probability of false positive for the entire functional volume was P < 0.05, as estimated by Monte Carlo simulation. This value depends on the final smoothness of the analyses, which differed between analyses of the whole condition and the PM items only. Nevertheless, in order to make results coherent, we applied the same threshold adopting the stricter of them (i.e., 87 voxels).
RESULTS
Behavioral Results
Accuracy rates (i.e., percentages of correct responses) and reaction times (in milliseconds) for ongoing items and PM items are reported in Table 1. A one‐way ANOVA was performed to compare the percentages of correct responses to PM items in the EBPM and TBPM conditions. Participants had a high level of performance and there was no difference in accuracy between EBPM and TBPM on PM items [F(1,19) = 0.28, P = 0.60]. To assess the interference effect of PM, ANOVAs were also performed on accuracy rates and reaction times for ongoing items in each condition (OG‐only vs. EBPM vs. TBPM). No effect of condition was found on accuracy [F(2,38) = 0.6, P = 0.53]. However, a significant effect of condition was found on reaction times [F(2,38) = 48.22, P < 0.0001]. Tukey's HSD post hoc comparison revealed that reaction times differed significantly between all three conditions (all P‐values < 0.03). More specifically, reaction times for correct answers on ongoing items were significantly faster in the OG‐only condition than in the TBPM condition, in which responses to ongoing items were faster than those in the EBPM condition.
Table 1.
Behavioral performance
| OG‐only | EBPM | TBPM | ||
|---|---|---|---|---|
| PM items | Accuracy (%) | — | 95.17 | 96.17 |
| (SD) | — | (6.96) | (6.78) | |
| Ongoing items | Accuracy (%) | 98.67 | 98.26 | 98.29 |
| (SD) | (1.46) | (1.29) | (1.32) | |
| Ongoing items | Reaction time (ms) | 791.68a, b | 910.97c | 825.76 |
| (SD) | (138.77) | (143.29) | (132.05) |
Mean percentages of correct answers to PM items, and correct answers to ongoing items (excluding PM items), and mean reaction times for correct answers to ongoing items (excluding PM items). SD: standard deviation
Significant difference from EBPM at P < 0.001.
Significant difference from TBPM condition at P < 0.05.
Significant difference from TBPM condition at P < 0.001.
Imaging Results
Activation and deactivation related to EBPM
First, we assessed the neural substrates of EBPM by subtracting the OG‐only condition from the EBPM condition (see Fig. 2a and Table 2). This contrast highlighted regions that were more highly activated in the EBPM condition than in the OG‐only one, in other words regions involved in the maintenance and execution of EBPM intentions. There was significant activation across a broad network encompassing the occipital regions, with significant peaks in the cuneus, the lingual, fusiform, and middle occipital gyri. This activation was mainly bilateral and extended to the cerebellum. Activation was also significantly higher for EBPM in the bilateral parietal lobule, the left postcentral and inferior frontal gyri, and the middle frontal gyrus bilaterally, although these frontal regions were much more dorsal and posterior than the RPFC regions that are classically activated in PM. These parietal and frontal activations seem to be specific of PM processes as they remained significant when the regions of OG‐only were excluded (main effect of EBPM exclusively masked by OG‐only main effect; data not shown). The reverse contrast (OG‐only minus EBPM) highlighted the involvement of various regions. However, extracting signal values in these clusters, we found that, instead of reflecting greater activation, these differences actually reflected that these regions were more deactivated in EBPM condition than in OG‐only one. Those regions were located in the left medial RPFC, middle frontal gyrus, angular gyrus, precuneus and cuneus, and bilateral posterior cingulate gyri. Angular and frontal deactivations were specific to EBPM as they remained significant when regions deactivated in OG‐only were excluded (negative main effect of EBPM exclusively masked by OG‐only negative main effect; data not shown).
Figure 2.
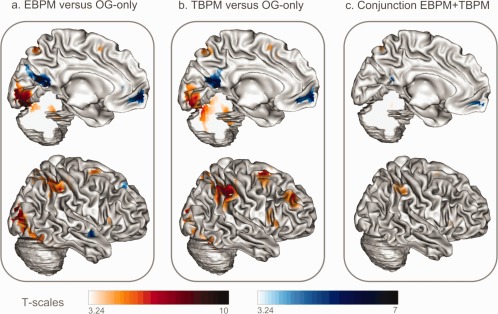
Brain activations (red scale) and deactivations (blue scale) in the EBPM (a) and TBPM (b) conditions relative to the OG‐only condition, and (c) in both EBPM and TBPM conditions (conjunction masked by OG‐only at P < 0.05 uncorrected). Contrasts are displayed at P < 0.001 uncorrected, with a minimum cluster size of 87 voxels, and superimposed onto an inflated reconstruction of the MNI template brain using the Anatomist software (http://www.brainvisa.info).
Table 2.
Regions showing significant BOLD changes in the contrasts between the EBPM and OG‐only conditions
| Contrast | Region | Side | BA | MNI coordinates | Z value | k | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| EBPM>OG‐only | Cuneus | L | 18 | −6 | −98 | 4 | 7.36 | 5267 |
| Lingual gyrus | L | 18 | −6 | −86 | −10 | 7.25 | ||
| Middle occipital gyrus | R | 18 | 20 | −92 | 10 | 7.24 | ||
| Inferior parietal lobule | L | 40 | −50 | −32 | 48 | 6.24 | 1388 | |
| Superior parietal lobule | L | 7 | −26 | −66 | 46 | 6.14 | ||
| Inferior parietal lobule | R | 40 | 42 | −46 | 48 | 5.80 | 393 | |
| Postcentral gyrus | L | 3 | −60 | −18 | 22 | 5.51 | 38 | |
| Inferior frontal gyrus | L | 9 | −52 | 6 | 36 | 5.22 | 61 | |
| Middle frontal gyrus | L | 6 | −26 | −2 | 48 | 5.17 | 27 | |
| Middle frontal gyrus | R | 6 | 32 | 0 | 62 | 4.85 | 14 | |
| Fusiform gyrus | R | 19 | 44 | −74 | −18 | 4.74 | 5 | |
| Superior parietal lobule | R | 7 | 28 | −68 | 56 | 4.64 | 6 | |
| OG‐only>EBPM | Precuneus | L | 31 | −12 | −66 | 18 | 5.43 | 194 |
| Posterior cingulate | L | 30 | −8 | −56 | 16 | 5.14 | ||
| Angular gyrus | L | 39 | −42 | −68 | 32 | 5.14 | 56 | |
| Medial frontal gyrus | L | 10 | −8 | 56 | −6 | 5.03 | 42 | |
| Middle frontal gyrus | L | 8 | −22 | 26 | 46 | 5.00 | 34 | |
| Cuneus | L | 18 | −10 | −80 | 28 | 4.83 | 12 | |
| Posterior cingulate | R | 23 | 8 | −58 | 16 | 4.73 | 5 | |
Note: Reported results show BOLD changes at P < 0.05 (FWE correction at the voxel level)
L = left, R = right, BA = Brodmann area, k = number of voxels. Where several peaks were observed within the same cluster, the coordinates refer to the strongest activation.
Activation and deactivation related to TBPM
Contrasting the TBPM and OG‐only conditions highlighted what seems to be a more diffuse activation network than for EBPM (Fig. 2b and Table 3). Significant activation was observed in the occipital regions (including the cuneus and lingual gyrus, extending to the cerebellum), the left postcentral gyrus, the superior and right inferior parietal lobule, the right precuneus, and the insula, as well as the middle and superior frontal gyri. As with EBPM, subtracting the activation map of the TBPM condition from that of the OG‐only condition did not reveal greater activation in the OG‐only condition, but instead greater deactivation of the posterior cingulate gyrus, the left middle temporal gyrus, and the medial RPFC during TBPM. The same localizations, highly reduced in size (notably for the occipital clusters), were found to be specific to TBPM when activity of OG‐only was excluded (TBPM main effects exclusively masked by OG‐only main effects; data not shown), both for activations and deactivations.
Table 3.
Regions showing significant BOLD changes in the contrasts between the TBPM and OG‐only conditions
| Contrast | Region | Side | BA | MNI coordinates | Z value | k | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| TBPM>OG‐only | Cuneus | R | 18 | 16 | −94 | 18 | 7.36 | 2601 |
| 17 | 20 | −92 | 6 | 6.97 | ||||
| Lingual gyrus | R | 17 | 12 | −92 | 2 | 6.69 | ||
| Cerebellum | L | −38 | −58 | −26 | 6.48 | 749 | ||
| Inferior parietal lobule | R | 40 | 56 | −40 | 46 | 6.48 | 939 | |
| Middle frontal gyrus | R | 6 | 32 | 4 | 62 | 6.01 | 151 | |
| Insula | R | 13 | 36 | 20 | 8 | 5.90 | 120 | |
| Cerebellum | R | 40 | −62 | −24 | 5.88 | 428 | ||
| Superior frontal gyrus | R | 9 | 38 | 40 | 36 | 5.64 | 134 | |
| Middle frontal gyrus | R | 10 | 36 | 36 | 24 | 4.71 | ||
| Postcentral gyrus | L | 40 | −50 | −34 | 52 | 5.41 | 156 | |
| Superior parietal lobule | R | 7 | 16 | −72 | 60 | 5.39 | 120 | |
| Precuneus | R | 7 | 6 | −64 | 54 | 4.85 | ||
| Superior parietal lobule | L | 7 | −10 | −70 | 56 | 5.08 | 31 | |
| Middle frontal gyrus | L | 46 | −38 | 34 | 30 | 5.03 | 42 | |
| Insula | L | 13 | −42 | 14 | 6 | 4.92 | 12 | |
| Inferior occipital gyrus | L | 19 | −36 | −80 | −14 | 4.79 | 11 | |
| Superior frontal gyrus | R | 6 | 18 | 16 | 64 | 4.74 | 5 | |
| OG‐only>TBPM | Posterior cingulate | L | 30 | −8 | −56 | 16 | 5.68 | 243 |
| R | 23 | 10 | −56 | 16 | 5.24 | |||
| Medial frontal gyrus | R | 11 | 2 | 60 | −12 | 5.36 | 17 | |
| Medial frontal gyrus | L | 10 | −2 | 58 | −12 | 5.32 | 32 | |
| Middle temporal gyrus | L | 21 | −58 | −6 | −12 | 5.29 | 73 | |
| Middle temporal gyrus | L | 39 | −44 | −66 | 28 | 5.13 | 37 | |
Note: Reported results show BOLD changes at P < 0.05 (FWE correction at the voxel level).
L = left, R = right, BA = Brodmann area, k = number of voxels. Where several peaks were observed within the same cluster, the coordinates refer to the strongest activation.
Commonalities Between Neural Substrates in EBPM and TBPM Conditions
To explore the substrates of PM shared by EBPM and TBPM, we performed conjunction analyses (Fig. 2c and Table 4). Positive and negative main effects were tested to reveal those regions that were engaged and those that were disengaged during PM storage and execution. To ensure that this activation was specific to PM processes, the activation and deactivation maps were exclusively masked by those of the OG‐only condition at P < 0.05 (uncorrected). We first applied a strict threshold of P < 0.05 corrected for family wise error. Only a few clusters within the cerebral networks elicited by EBPM and TBPM (see Tables 2 and III) remained significantly activated or deactivated in both conditions. The conjunction analysis of the positive main effects revealed activation common to the two PM conditions in the inferior parietal lobules, the left superior parietal lobule, and postcentral gyrus. The conjunction analysis of the negative main effects revealed significant deactivation in the left hemisphere in the medial RPFC and the middle temporal gyri. Nevertheless, according to our objective to better delineate common substrates of EBPM and TBPM and the strong hypothesis of the involvement of frontal regions in PM, we looked at results at a less stringent threshold, which was nevertheless corrected at the cluster level using 3dClustSim (P < 0.001 uncorrected, k > 87). With this more permissive threshold, activations were also found in the inferior and middle frontal gyri, the insula, and the cerebellum. For the deactivations, they were also observed in the right medial RPFC as well as in the left middle temporal gyrus.
Table 4.
Regions showing significant BOLD increases or decreases for both EBPM and TBPM conditions
| Contrast | Region | Side | BA | MNI coordinates | Z value | k | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Activated regions | Postcentral gyrus | L | 40 | −48 | −34 | 54 | 5.18a | 1543 |
| Inferior parietal lobule | L | 40 | −44 | −36 | 46 | 5.00a | ||
| Superior parietal lobule | L | 7 | −12 | −70 | 58 | 4.89a | ||
| Inferior parietal lobule | R | 40 | 48 | −40 | 42 | 4.79a | 906 | |
| Insula | R | 13 | 34 | 20 | 8 | 4.52 | 182 | |
| Inferior frontal gyrus | R | 47 | 52 | 20 | −2 | 3.59 | ||
| Middle frontal gyrus | L | 6 | −34 | −6 | 64 | 4.16 | 134 | |
| Cerebellum | R | — | 28 | −60 | −28 | 4.15 | 314 | |
| L | — | −10 | −52 | −18 | 4.13 | |||
| Deactivated regions | Middle temporal gyrus | L | 39 | −44 | −68 | 30 | 4.99a | 205 |
| Medial frontal gyrus | L | 10 | −6 | 54 | −8 | 4.73a | 274 | |
| R | 11 | 2 | 58 | −12 | 4.36 | |||
| Middle temporal gyrus | L | 21 | −60 | −6 | −10 | 4.14 | 127 | |
| Superior Temporal gyrus | L | 22 | −54 | −10 | −6 | 3.54 | ||
Note: Reported results show BOLD changes at P < 0.001 (unc), with a minimum cluster size of 87 voxels. Conjunction results were obtained with exclusive masking by the ongoing main effect (mask threshold P = 0.05).
Indicates Z value significant at P < 0.05 for an FWE correction (voxel level); L = left, R = right, BA = Brodmann area, k = number of voxels. Where several peaks were observed within the same cluster, the coordinates refer to the strongest activation.
Distinct Substrates of EBPM and TBPM
To better evaluate the processes involved in each kind of PM, direct comparisons of EBPM and TBPM were conducted for PM + ongoing items (whole condition) and for PM items only. Regarding the fact that the ongoing task is present and similar in both conditions, we assume that this subtraction method allows highlighting the substrates of EBPM and TBPM regardless of the regions involved in the ongoing task. Results are reported in Table 5.
Table 5.
Regions showing significant BOLD changes between the EBPM and TBPM conditions as a whole (PM + ongoing items) and specifically for the prospective items in each condition
| Contrast | Region | Side | BA | MNI coordinates | Z value | k | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Analysis for the whole conditions | ||||||||
| EB>TB | Lingual gyrus | L | 18 | −24 | −80 | −10 | 3.97 | 125 |
| Fusiform gyrus | L | 19 | −28 | −70 | −14 | 3.58 | ||
| Middle occipital gyrus | L | 18 | −22 | −92 | 12 | 3.78 | 100 | |
| 19 | −32 | −86 | 10 | 3.24 | ||||
| TB>EB | Middle frontal gyrus | R | 9 | 30 | 38 | 40 | 4.88a | 736 |
| Superior frontal gyrus | R | 10 | 24 | 42 | 26 | 4.36 | ||
| Cuneus | R | 18 | 6 | −82 | 22 | 4.65a | 362 | |
| Precuneus | R | 19 | 18 | −86 | 38 | 4.19 | ||
| Cuneus | R | 17 | 14 | −82 | 8 | 3.15 | ||
| Analysis for the prospective items only | ||||||||
| EB>TB | Lingual gyrus | L | 18 | −20 | −76 | −12 | 4.26 | 579 |
| Fusiform gyrus | L | 19 | −26 | −66 | −14 | 3.94 | ||
| Middle occipital gyrus | R | 19 | 38 | −84 | 12 | 4.15 | 209 | |
| Posterior cingulate | R | 31 | 32 | −72 | 18 | 3.39 | ||
| Superior parietal lobule | L | 7 | −24 | −68 | 50 | 3.73 | 122 | |
| Cuneus | L | 18 | −6 | −96 | 4 | 3.57 | 156 | |
| 17 | −10 | −80 | 6 | 3.54 | ||||
| TB>EB | Cuneus | R | 19 | 8 | −84 | 34 | 3.83 | 90 |
Note: Reported results show BOLD changes at P < 0.001 (unc), with a minimum cluster size of 87 voxels.
Indicates Z value significant at P < 0.05 for an FWE correction (voxel level); L = left, R = right, BA = Brodmann area, k = number of voxels. Where several peaks were observed within the same region, the coordinates refer to the strongest activation.
Concerning the EBPM condition, higher activation appeared only in the posterior part of the left occipital lobe (left fusiform gyrus extending to the lingual gyrus and left middle occipital gyrus). In contrast, the maintenance and realization of TBPM intentions elicited higher activation in different parts of the right hemisphere, notably the middle and superior frontal gyri, the cuneus, and precuneus (Fig. 3a). According to the strong hypothesis of the involvement of time estimation processes in TBPM, we used a more permissive threshold (P < 0.005 uncorrected at the voxel level and a cluster size of at least 185 to obtain a significance level corrected for multiple comparisons), to disclose a wider network, potentially reflecting such processes. With this permissive threshold an increased activity was additionally found for EBPM compared to TBPM in the left inferior frontal gyrus ([−48, 32, 18], Z = 3.78), in addition to a wider occipital activity. For TBPM, a network comprising—in addition to the right middle and superior frontal gyri, cuneus, and the precuneus—the left cerebellum ([−34, −54, −32], Z = 3.84), a cluster more rostral in the right precuneus ([6, −46, 48], Z = 3.41), and the right superior temporal gyrus ([54, −44, 14], Z = 3.21) extending to the inferior parietal lobule ([52, −40, 26], Z = 3.07) was highlighted.
Figure 3.
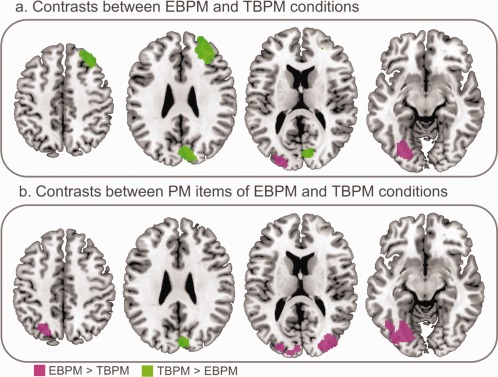
Networks specific to EBPM (pink clusters) and TBPM (green clusters) for (a) the whole condition and (b) PM items only. Contrasts are displayed at P < 0.001 uncorrected, with a minimum cluster size of 87 voxels, and superimposed onto sections of the MNI template (z = 46, 28, 14, and −10; from left to right) using MRIcroN (http://www.mccauslandcenter.sc.edu/mricro/mricron/).
To explore further the distinction between EBPM and TBPM and highlight the activation specific to the detection–execution stage of PM, the same contrasts were performed on PM items only (Fig. 3b). While contrasting the whole conditions indicated that far more regions were activated in TBPM than in EBPM, contrasting just the PM items revealed a much more extensive network in EBPM than in TBPM. This suggests that detecting the target time in TBPM involved fewer processes than target detection in EBPM, probably because its appearance had been predicted upstream. More specifically, intention retrieval and execution in the EBPM condition induced higher activation of a broad occipital network, extending to the cerebellum (see Table 5 for details). The reverse contrast (PM items in the TBPM condition minus those in the EBPM condition) showed only one cluster in the right cuneus.
DISCUSSION
Although a distinction is classically made in the PM literature between EBPM and TBPM, few studies have tried directly to identify those processes they share and those they do not. We therefore devised an fMRI study to assess these two kinds of PM in a semantic categorization task, in the same group of 20 healthy young individuals. Behavioral data revealed that participants were highly accurate in remembering and executing EBPM and TBPM intentions, while maintaining a high level of efficiency in the ongoing task, without any difference between the two conditions. This is to note that some fMRI studies on PM previously found such pattern of results [see for example Burgess et al., 2003; Okuda et al., 2007]. Nevertheless, a PM interference effect was found, with an increase in reaction times for PM conditions compared with the condition without intention (i.e., OG‐only). This interference effect is classic and reflects the cognitive cost of maintaining an intention while performing another task [Hicks et al., 2005; Loft et al., 2008]. These behavioral results suggest that additional processes were engaged in PM relative to the ongoing task alone, as reported in previous studies [see notably Smith, 2003]. This was confirmed by the neuroimaging data, which showed that both EBPM and TBPM elicited enhanced activation during the maintenance of intention of a broad cerebral network that included parts of the occipital lobe, parietal lobe, and frontal regions, the latter being more posterior than the ones reported in previous PM studies. Interestingly, by excluding the regions involved in the ongoing task, the conjunction analyses revealed a network that was specific to PM, independently of the nature of the retrieval (event‐based or time‐based). In addition, our data highlighted regions whose involvement depended on the nature of the PM (event‐based vs. time‐based).
Shared Substrates of EBPM and TBPM—Frontal Activity During PM
The literature on PM suggests that intention maintenance leads to increased activation of the lateral RPFC, compared with the situation of an uncontaminated ongoing task, while the medial part of this area is deactivated [see Burgess et al., 2011 for a review]. Our results confirmed the disengagement of the medial RPFC during intention maintenance, and consistently so for both EBPM and TBPM. This deactivation, coupled with slower reaction times for ongoing items in the two PM conditions, is consistent with the gateway hypothesis [Burgess et al., 2007a,b], suggesting that it reflects attending to stimuli while performing the ongoing task. This finding allows us to draw two conclusions. First, although Okuda et al. [2007] suggested that medial RPFC deactivation is specific to EBPM, our results instead indicate that the deactivation of the medial RPCF occurs in TBPM, as well as in EBPM. Second, we demonstrate that deactivation of the medial RPFC is a genuine PM‐related phenomenon and not an artifact arising from the experimental design. In previous studies, the ongoing task was always administered on its own, prior to the condition featuring the PM instruction. As a result, Burgess et al. [2011] recently suggested that medial RPFC deactivation might be due to the order of condition administration. Our data show that this is not the case, because the medial RPFC deactivation was present and robust in both PM conditions, even though they did not always follow the uncontaminated ongoing condition (random presentation of the conditions in the three runs).
Concerning the lateral RPFC activation in PM maintenance, several studies have failed to detect activation in this area. For example, Kalpouzos et al. [2010], using a virtual reality paradigm in fMRI, did not observe any activation of the lateral RPFC during the maintenance stage between the formation of the intention and its execution, only recording it in the phase immediately following execution. The authors suggested that this reflected the search for a new intention in mind. Okuda et al. [2011], who also failed to find evidence of lateral RPFC involvement in their fMRI study, suggested that the lateral RPFC is related to conscious, strategic controlled processes, which were not predominant in their experiment. In our study, we expected PM to require such controlled processes in the TBPM and EBPM conditions, as the cues were nonfocal and nonsalient [see McDaniel and Einstein, 2000, 2007]. However, we cannot completely rule out the hypothesis that our ongoing task (i.e., semantic categorization test) was not sufficiently complex to elicit the strong involvement of controlled processes requiring attention to be shared between the PM task and the ongoing one, and thus inducing lateral RPFC activation. Moreover, in our study, participants were instructed to provide both prospective and ongoing responses when they encountered a cue or when the time interval had elapsed, whereas in previous studies, participants were asked to inhibit the ongoing answer and only respond to the PM task [Benoit et al., 2012; Burgess et al., 2001; den Ouden et al., 2005; Gilbert, 2011; Gilbert et al., 2012]. As a result, in our design, participants engaged dual‐task processes in PM rather than inhibition processes. This methodological difference may have influenced the processes involved in the cognitive control of the task, as already suggested by an ERP study [Bisiacchi et al., 2009].
By contrast, more posterior parts of the frontal cortex were activated during PM in our study, notably in EBPM where they are strongly caudal (at least with a very strict threshold). This is consistent with theories of a rostrocaudal gradient in the PFC [Badre, 2008; Koechlin and Summerfield, 2007]. They postulate that the most anterior parts of the prefrontal cortex subserve “branching” functions, namely the management of multiple cognitive tasks at the same time, while the most posterior regions are engaged in situations where action is more sensory‐driven. The design adopted in our study may have relied on the branching function to a lesser extent than that of previous studies. Further experiments are needed to explore the implication of the lateral RPCF in PM according to the characteristics of the design.
Shared Substrates of EBPM and TBPM—Importance of the Parietal Cortex
In addition to the frontal regions, maintenance of intention for both EBPM and TBPM relies on the parietal regions, right insula, and cerebellum. This network is usually described in the PM literature. Even though authors usually focus on RPFC activity, the precuneus and parietal lobe, as well as the anterior cingulate cortex, have frequently been identified in PM [see Burgess et al., 2011, for a review], but their respective roles have not been discussed.
In our study, parietal areas appeared to play a role in both the EBPM and TBPM conditions. The involvement of the parietal lobe in attention and memory retrieval is well documented [see Hutchinson et al., 2009; Wagner et al., 2005, for review]. Two distinct frontoparietal networks have been identified in attention [Cabeza et al., 2008; Corbetta and Shulman, 2002]. The dorsal attention network, which includes the superior parietal lobule, subserves top‐down processes, namely the voluntary allocation of attention to prepare and apply goals. The ventral attention network, involving the inferior parietal lobule, subtends bottom‐up processes and underlies the detection of behaviorally relevant stimuli in a reflexive way, particularly when cues are salient. This dorsal–ventral distinction has been applied to memory retrieval, giving rise to the attention to memory (AtoM) model [Ciaramelli et al., 2008]. The extensive activation of the parietal lobule in our study, as in previous studies of PM, may reflect the involvement of attentional processes directed toward the maintenance of intention as already suggested by previous studies [Kalpouzos et al., 2010; Ramaekers et al., 2009; Rusted et al., 2011].
Specificity of EBPM and TBPM
Our behavioral results demonstrated that the interference effect of PM instructions on the execution of the ongoing task was stronger in the EBPM condition than in the TBPM one. At first sight, this result may be surprising, as most existing research suggests that TBPM requires more self‐initiated processes than EBPM [Craik, 1986; Einstein et al., 1995; Khan et al., 2008; Kliegel et al., 2001; Park et al., 1997]. Dividing cognitive resources between PM and the ongoing task should thus be more detrimental to the ongoing task in the TBPM condition. This view is, however, easily refutable considering that PM interference is due to the allocation of resources to the strategic monitoring of the environment. Whereas the time of execution of the EBPM intention is unpredictable, requiring constant checking of the environment for PM cues, TBPM execution is eminently predictable and requires only periodic checking of the time. Our data support this distinction between constant and periodic monitoring [see also Cona et al., 2012; Hicks et al., 2005]. A post‐experiment debriefing (data not shown) confirmed that subjects used a constant target checking in the EBPM condition, whereas in the TBPM condition, only periodic monitoring was made, with rare clock checking at the beginning of each 30 s period, becoming more frequent while the target time approached.
Looking at the activation and deactivation patterns elicited by EBPM and those elicited by TBPM, quite similar regions seem to be implicated when a PM instruction is added to the ongoing task. Nevertheless, and consistent with our predictions, the EBPM and TBPM conditions generated partially distinct patterns of activation.
First, concerning EBPM, our results indicate that this condition involved stronger activation in occipital areas compared with TBPM. This occipital activation may subtend the constant target‐checking in the EBPM condition. Monitoring in our EBPM task required visual attention to be directed to the stimuli, especially given that participants had to pay attention to colors [see Kalpouzos et al., 2010 for a similar result]. As no other region was specifically engaged in EBPM, except one cluster in the left frontal inferior gyrus, we propose that this mode of target‐checking constitutes the signature of the maintenance stage of EBPM intentions. This result is in line with a recent ERP study contrasting EBPM and TBPM [Cona et al., 2012]. These authors hypothesized that EBPM and TBPM have similar retrieval modes (i.e., cognitive sets to process stimuli as cues for memory retrieval), but differ in terms of target‐checking [Guynn, 2003]. Consistent with this, for both EBPM and TBPM, they demonstrated increased positivity, broadly distributed across the scalp, but especially strong in the frontal and prefrontal regions. Additionally, EBPM was characterized by increased positivity across parietal and occipital regions, reflecting the continuous monitoring of the environment until PM cues were encountered. The left frontal cluster found with a permissive threshold is coherent, with the only cluster found to be more activated in EBPM compared to TBPM in Okuda et al.'s study [Okuda et al., 2007].
Second, our investigation of the substrates of TBPM revealed a right‐sided network including the middle and superior frontal gyri, the cuneus, and precuneus. The dorsolateral frontal activation, previously reported in time estimation paradigms [Coull, 2004; Macar et al., 2002; Pouthas et al., 2005; Rao et al., 2001], may reflect the involvement of such processes in TBPM. Quite consistently, the only previous fMRI study of TBPM highlighted a strong involvement of those regions in TBPM [Momennejad and Haynes, 2012]. This is also consistent with Okuda's PET study [Okuda et al., 2007] which reported higher involvement of prefrontal regions in TBPM than in EBPM. They notably interpreted this as reflecting of time estimation processes. At a quite permissive threshold, the appearance of a wider network, including the inferior parietal lobule and cerebellum, classically involved in time estimation [see Rubia, 2006 for a review] supports this interpretation. Moreover, the right lateralization of our results is classic in studies of time perception and discrimination, notably when the experimental design features longer durations (i.e., several seconds) [see Macar et al., 2002; Pouthas et al., 2005]. This network may allow for the allocation of sustained attention to time.
As a whole, these results nicely fits with previous studies contrasting time and color judgments, which underlie occipital networks in color judgment tasks and the association of frontal, parietal, temporal, and cerebellar areas in time estimation [see Coull et al., 2004; Morillon et al., 2009]. Results are also consistent with those reported by Okuda et al. [2007]. In fact, they highlighted much more frontal activity in TBPM than EBPM, with only one left‐sided cluster in the superior frontal gyrus (RPFC) for the latter condition. They suggested that the differences of localizations between frontal regions involved in EBPM and TBPM, and the strength of their activation, could depend on the requirement in episodic memory, multitasking (i.e., dual‐task), and mentalizing processes of the tasks, as well as the predictability of PM occurrence. This may be the case in our study too. Nevertheless, considering the reduced threshold and the possible confounding variables of the design (e.g., absence of measure of time‐monitoring, constant presence of the clock), these results need stronger confirmation.
Interestingly, while the TBPM‐specific network as a whole involved far more brain areas than the EBPM network, the reverse picture emerged when these conditions were contrasted on PM items alone (i.e., detection and execution stages of PM only). This result is consistent with previous arguments concerning strategic monitoring in EBPM and TBPM. As EBPM occurrence is unpredictable, correct cue detection probably involves many processes (e.g., for detecting and ensuring that the color of the border matches the expected one), thus eliciting massive occipital activation. By contrast, as TBPM target times can be predicted, detecting the appropriate time for answering may engage far fewer processes.
As this is the first fMRI study to investigate the commonalities and differences in the neural substrates of EBPM and TBPM, further studies are warranted to characterize more finely those two forms of PM.
Toward an Attentional Account of PM?
Neuroimaging studies have fairly consistently identified the implication of the frontal (more specifically the RPFC) and parietal cortices in PM. The involvement of these regions may lie on a continuum and their activation may depend on the amount of controlled versus automatic processes required by the task [McDaniel and Einstein, 2000, 2007; Okuda et al., 2011]. It has been previously suggested that this frontoparietal network could reflect an implication of attentional processes during PM condition [Cabeza et al., 2008; Corbetta and Shulman, 2002]. The deactivation of other regions (comprising notably the medial RPFC), in line with previous studies of PM [see notably Hashimoto et al., 2011; Simons et al., 2006], can also be interpreted in an attentional view. In fact, studies of other cognitive functions have shown that complex cognitive task cause higher deactivation in these regions, corresponding in part to the default mode network [see Mevel et al., 2011] than tasks with lower cognitive load [see notably McKiernan et al., 2003]. Taking together, this pattern of results suggests that adding PM instruction may only affect the amount of attentional processes involved in the task.
Nevertheless, given that PM has an episodic (i.e., episodic memory) dimension, we would expect the medial temporal lobe and the hippocampus to play an important role in this cognitive function. While the prospective component of PM may be mediated by executive/frontal processes, the retrospective component may be subserved by regions associated with “classic” (i.e., retrospective) episodic memory, such as medial temporal areas [Umeda et al., 2006]. Consistent with this, Martin et al. [2007], using magnetoencephalography, reported the implication of both frontoparietal areas for noticing PM cues, and the hippocampal region for retrieving intention in memory. Other studies have found a link between hippocampal regions and PM, using either brain imaging or lesion‐based studies [Adda et al., 2008; Kalpouzos et al., 2010; Umeda et al., 2006; Volle et al., 2011]. Our study failed to find such involvement. This is not surprising, as we had deliberately weakened the retrospective component of intention in order to focus on the similarities and differences between TBPM and EBPM, which chiefly concern the prospective component. The “what has to be done” component remained the same throughout the entire procedure, whatever the PM condition (i.e., press with middle finger) and a reminder at the bottom of the screen indicated which middle finger was required. As a result, once participants had correctly detected that there was something to do, the retrieval of the action that had to be performed was extremely easy and did not rely on memory processes supported by the hippocampus.
In addition, according to the characteristics of our design, which promote active monitoring of PM cues or time, the involvement of attentional processes in PM, probably close to dual‐task processes, would have been expected. A design with less frequent PM items and fewer cues (e.g., response key, constant countdown) would trigger processes less dependent of attentional ones. Accordingly, we can assume that the cerebral network highlighted in the present study reflected the substrates of the prospective component of PM and active monitoring, rather than PM as a whole (i.e., both prospective and retrospective components for more delayed and less frequent intentions).
CONCLUSION
Our results suggest that a broadly similar cerebral network is involved in both EBPM and TBPM, composed mainly of frontal and parietal regions. The engagement of these regions most likely accounts for the cost of maintaining an intention in the mind, which is the same for both EBPM and TBPM. Our data, taken together with the findings of previous neuroimaging studies of PM, support an attentional account of PM, probably specific to the prospective component of PM. We also observed activation specific to either EBPM or TBPM, corresponding to the strategic monitoring of the environment. More specifically, while the EBPM condition engaged occipital areas, presumably reflecting checking of the environment for visual cues during PM maintenance, the TBPM condition resulted in activations in regions that may reflect the involvement of time estimation processes. Further neuroimaging investigations of PM are needed to clarify the involvement of the lateral RPFC, in relation to the characteristics of the PM tasks and the ongoing activity, as well as the substrates of the retrospective component of PM.
ACKNOWLEDGMENTS
The authors would like to thank F. Lamberton for his help in the fMRI sequences, J. Chavant, J. Dayan, C. Lebouleux, M.H. Noel, M.C. Onfroy, A. Quillard, and C. Schupp for data acquisition and volunteers recruitment, as well as E. Wiles‐Portier for reviewing the English style. Finally, the authors are grateful to A. Bejanin, S. Egret, M. Fouquet, M. Gaubert, M. Hainselin, C. Harand, R. La Joie, H. Platel, and S. Segobin for their help in various stages of this study. The authors declare that they have no conflict of interest.
REFERENCES
- Adda CC, Castro LHM, Além‐Mar e Silva LC, De Manreza MLG, Kashiara R (2008): Prospective memory and mesial temporal epilepsy associated with hippocampal sclerosis. Neuropsychologia 46:1954–1964. [DOI] [PubMed] [Google Scholar]
- Altgassen M, Kliegel M, Brandimonte M, Filippello P (2010): Are older adults more social than younger adults? Social importance increases older adults' prospective memory performance. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 17:312–328. [DOI] [PubMed] [Google Scholar]
- Badre D (2008): Cognitive control, hierarchy, and the rostro‐caudal organization of the frontal lobes. Trends Cogn. Sci (Regul Ed) 12:193–200. [DOI] [PubMed] [Google Scholar]
- Benoit RG, Gilbert SJ, Frith CD, Burgess PW (2012): Rostral prefrontal cortex and the focus of attention in prospective memory. Cereb. Cortex 22:1876–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisiacchi PS, Schiff S, Ciccola A, Kliegel M (2009): The role of dual‐task and task‐switch in prospective memory: Behavioural data and neural correlates. Neuropsychologia 47:1362–1373. [DOI] [PubMed] [Google Scholar]
- Brandimonte MA, Passolunghi MC (1994): The effect of cue‐familiarity, cue‐distinctiveness, and retention interval on prospective remembering. Q J Exp Psychol A 47:565–587. [DOI] [PubMed] [Google Scholar]
- Brandimonte MA, Ferrante D, Bianco C, Villani MG (2010): Memory for pro‐social intentions: When competing motives collide. Cognition 114:436–441. [DOI] [PubMed] [Google Scholar]
- Brewer GA, Knight JB, Marsh RL, Unsworth N (2010): Individual differences in event‐based prospective memory: Evidence for multiple processes supporting cue detection. Mem Cogn 38:304–311. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Quayle A, Frith CD (2001): Brain regions involved in prospective memory as determined by positron emission tomography. Neuropsychologia 39:545–555. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Scott SK, Frith CD (2003): The role of the rostral frontal cortex (area 10) in prospective memory: A lateral versus medial dissociation. Neuropsychologia 41:906–918. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Dumontheil I, Gilbert SJ (2007a): The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends Cogn Sci (Regul Ed) 11:290–298. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Gilbert SJ, Dumontheil I (2007b): Function and localization within rostral prefrontal cortex (area 10). Philos Trans R Soc Lond B Biol Sci 362:887–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PW, Gonen‐Yaacovi G, Volle E (2011): Functional neuroimaging studies of prospective memory: What have we learnt so far? Neuropsychologia 49:2246–2257. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M (2008): The parietal cortex and episodic memory: An attentional account. Nat Rev Neurosci 9:613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H‐D, Wang K, Xi C‐H, Niu C‐S, Fu X‐M (2008): Prefrontal cortex involvement in the event‐based prospective memory: Evidence from patients with lesions in the prefrontal cortex. Brain Inj 22:697–704. [DOI] [PubMed] [Google Scholar]
- Cheng H, Tian Y, Hu P, Wang J, Wang K (2010): Time‐based prospective memory impairment in patients with thalamic stroke. Behav Neurosci 124:152–158. [DOI] [PubMed] [Google Scholar]
- Ciaramelli E, Grady CL, Moscovitch M (2008): Top‐down and bottom‐up attention to memory: A hypothesis (AtoM) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia 46:1828–1851. [DOI] [PubMed] [Google Scholar]
- Cona G, Arcara G, Tarantino V, Bisiacchi PS (2012): Electrophysiological correlates of strategic monitoring in event‐based and time‐based prospective memory. PLoS One 7:e31659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL (2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3:201–215. [DOI] [PubMed] [Google Scholar]
- Coull JT (2004): fMRI studies of temporal attention: Allocating attention within, or towards, time. Brain Res Cogn Brain Res 21:216–226. [DOI] [PubMed] [Google Scholar]
- Craik FIM (1986): A functional account of age differences in memory In Lix F, Hagendorf H, éditor. Human Memory and Cognitive Capabilities: Mechanisms and Performances. Amsterdam: Elsevier Science; pp 409–422. [Google Scholar]
- Cuttler C, Graf P (2009): Sub‐clinical compulsive checkers show impaired performance on habitual, event‐ and time‐cued episodic prospective memory tasks. J Anxiety Disord 23:813–823. [DOI] [PubMed] [Google Scholar]
- den Ouden HEM, Frith U, Frith C, Blakemore S‐J (2005): Thinking about intentions. Neuroimage 28:787–796. [DOI] [PubMed] [Google Scholar]
- d'Ydewalle G, Bouckaert D, Brunfaut E (2001): Age‐related differences and complexity of ongoing activities in time‐ and event‐based prospective memory. Am J Psychol 114:411–423. [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA (1990): Normal aging and prospective memory. J Exp Psychol Learn Mem Cogn 16:717–726. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA, Richardson SL, Guynn MJ, Cunfer AR (1995): Aging and prospective memory: Examining the influences of self‐initiated retrieval processes. J Exp Psychol Learn Mem Cogn 21:996–1007. [DOI] [PubMed] [Google Scholar]
- Einstein GO, Smith RE, McDaniel MA, Shaw P (1997): Aging and prospective memory: The influence of increased task demands at encoding and retrieval. Psychol Aging 12:479–488. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA, Smith RE, Shaw P (1998): Habitual prospective memory and aging: Remembering intentions and forgetting actions. Psychol Sci 9:284–288. [Google Scholar]
- Einstein GO, McDaniel MA, Thomas R, Mayfield S, Shank H, Morrisette N, Breneiser J (2005): Multiple processes in prospective memory retrieval: factors determining monitoring versus spontaneous retrieval. J Exp Psychol Gen 134:327–342. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ (2011): Decoding the content of delayed intentions. J Neurosci 31:2888–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SJ, Gollwitzer PM, Cohen A‐L, Burgess PW, Oettingen G (2009): Separable brain systems supporting cued versus self‐initiated realization of delayed intentions. J Exp Psychol Learn Mem Cogn 35:905–915. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Armbruster DJN, Panagiotidi M (2012): Similarity between brain activity at encoding and retrieval predicts successful realization of delayed intentions. J Cogn Neurosci 24:93–105. [DOI] [PubMed] [Google Scholar]
- Gonneaud J, Kalpouzos G, Bon L, Viader F, Eustache F, Desgranges B (2011): Distinct and shared cognitive functions mediate event‐ and time‐based prospective memory impairment in normal ageing. Memory 19:360–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guynn MJ (2003): A two‐process model of strategic monitoring in event‐based prospective memory: Activation/retrieval mode and checking. Int J Psychol 38:245–256. [Google Scholar]
- Hainselin M, Quinette P, Desgranges B, Martinaud O, Hannequin D, De La Sayette V, Viader F, Eustache F (2011): Can we remember future actions yet forget the last two minutes? Study in transient global amnesia. J Cogn Neurosci 23:4138–4149. [DOI] [PubMed] [Google Scholar]
- Harris JE, Wilkins AJ (1982): Remembering to do things: A theoretical framework and an illustrative experiment. Hum Learn 1:123–136. [Google Scholar]
- Hashimoto T, Umeda S, Kojima S (2011): Neural substrates of implicit cueing effect on prospective memory. Neuroimage 54:645–652. [DOI] [PubMed] [Google Scholar]
- Haynes J‐D, Sakai K, Rees G, Gilbert S, Frith C, Passingham RE (2007): Reading hidden intentions in the human brain. Curr Biol 17:323–328. [DOI] [PubMed] [Google Scholar]
- Hicks JL, Marsh RL, Cook GI (2005): Task interference in time‐based, event‐based, and dual intention prospective memory conditions. J Mem Lang 53:430–444. [Google Scholar]
- Hutchinson JB, Uncapher MR, Wagner AD (2009): Posterior parietal cortex and episodic retrieval: Convergent and divergent effects of attention and memory. Learn Mem 16:343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalpouzos G, Eriksson J, Sjölie D, Molin J, Nyberg L (2010): Neurocognitive systems related to real‐world prospective memory. PLoS One 5:e13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A, Sharma NK, Dixit S (2008): Cognitive load and task condition in event‐ and time‐based prospective memory: An experimental investigation. J Psychol 142:517–531. [DOI] [PubMed] [Google Scholar]
- Kidder DP, Park DC, Hertzog C, Morrell RW (1997): Prospective memory and aging: The effects of working memory and prospective memory task load. Aging, Neuropsychol Cogn 4:93–112. [Google Scholar]
- Kliegel M, Martin M, McDaniel MA, Einstein GO (2001): Varying the importance of a prospective memory task: Differential effects across time‐ and event‐based prospective memory. Memory 9:1–11. [DOI] [PubMed] [Google Scholar]
- Kliegel M, Martin M, McDaniel MA, Einstein GO (2002): Complex prospective memory and executive control of working memory: A process model. Psychologische Beitrage 44:303–318. [Google Scholar]
- Kliegel M, Ramuschkat G, Martin M (2003): [Executive functions and prospective memory performance in old age: An analysis of event‐based and time‐based prospective memory]. Z Gerontol Geriatr 36:35–41. [DOI] [PubMed] [Google Scholar]
- Kliegel M, Martin M, McDaniel MA, Einstein GO (2004): Importance effects on performance in event‐based prospective memory tasks. Memory 12:553–561. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Summerfield C (2007): An information theoretical approach to prefrontal executive function. Trends Cogn Sci (Regul Ed) 11:229–235. [DOI] [PubMed] [Google Scholar]
- Kvavilashvili L, Fisher L (2007): Is time‐based prospective remembering mediated by self‐initiated rehearsals? Role of incidental cues, ongoing activity, age, and motivation. J Exp Psychol Gen 136:112–132. [DOI] [PubMed] [Google Scholar]
- Loft S, Kearney R, Remington R (2008): Is task interference in event‐based prospective memory dependent on cue presentation? Mem Cogn 36:139–148. [DOI] [PubMed] [Google Scholar]
- Logie R, Maylor E, Della Sala S, Smith G (2004): Working memory in event‐ and time‐based prospective memory tasks: Effects of secondary demand and age. Eur J Cogn Psychol 16:441–456. [Google Scholar]
- Macar F, Lejeune H, Bonnet M, Ferrara A, Pouthas V, Vidal F, Maquet P (2002): Activation of the supplementary motor area and of attentional networks during temporal processing. Exp Brain Res 142:475–485. [DOI] [PubMed] [Google Scholar]
- Mäntylä T, Missier FD, Nilsson L‐G (2009): Age differences in multiple outcome measures of time‐based prospective memory. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 16:708–720. [DOI] [PubMed] [Google Scholar]
- Marsh RL, Hicks JL (1998): Event‐based prospective memory and executive control of working memory. J Exp Psychol Learn Mem Cogn 24:336–349. [DOI] [PubMed] [Google Scholar]
- Marsh RL, Hicks JL, Hancock TW (2000): On the interaction of ongoing cognitive activity and the nature of an event‐based intention. Appl Cognitive Psychology 14: S29–S41. [Google Scholar]
- Marsh RL, Hancock TW, Hicks JL (2002): The demands of an ongoing activity influence the success of event‐based prospective memory. Psychon Bull Rev 9:604–610. [DOI] [PubMed] [Google Scholar]
- Martin M, Kliegel M, McDaniel MA (2003): The involvement of executive functions in prospective memory performance of adults. Int J Psychol 38:195–206. [Google Scholar]
- Martin T, McDaniel MA, Guynn MJ, Houck JM, Woodruff CC, Bish JP, Moses SN, Kicić D, Tesche CD (2007): Brain regions and their dynamics in prospective memory retrieval: A MEG study. Int J Psychophysiol 64:247–258. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Einstein GO (1993): The importance of cue familiarity and cue distinctiveness in prospective memory. Memory 1:23–41. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Einstein GO (2000): Strategic and automatic processes in prospective memory retrieval: A multiprocess framework. Appl Cogn Psychol 14:S127–S144. [Google Scholar]
- McDaniel MA, Einstein GO (2007): Prospective Memory: An Overview and Synthesis of an Emerging Field Thousand Oaks (CA): Sage Publications. [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera‐Thompson J, Binder JR (2003): A parametric manipulation of factors affecting task‐induced deactivation in functional neuroimaging. J Cogn Neurosci 15:394–408. [DOI] [PubMed] [Google Scholar]
- Mevel K, Chételat G, Eustache F, Desgranges B (2011): The default mode network in healthy aging and Alzheimer's disease. Int J Alzheimers Dis 535816‐. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momennejad I, Haynes J‐D (2012): Human anterior prefrontal cortex encodes the “what” and “when” of future intentions. Neuroimage 61:139–148. [DOI] [PubMed] [Google Scholar]
- Morillon B, Kell CA, Giraud A‐L, (2009): Three Stages and Four Neural Systems in Time Estimation. Journal of Neuroscience 29:14803–14811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Occhionero M, Esposito MJ, Cicogna PC, Nigro G (2010): The effects of ongoing activity on time estimation in prospective remembering. Appl Cogn Psychol 24:774–791. [Google Scholar]
- Okuda J, Fujii T, Ohtake H, Tsukiura T, Yamadori A, Frith CD, Burgess PW (2007): Differential involvement of regions of rostral prefrontal cortex (Brodmann area 10) in time‐ and event‐based prospective memory. Int J Psychophysiol 64:233–246. [DOI] [PubMed] [Google Scholar]
- Okuda J, Gilbert SJ, Burgess PW, Frith CD, Simons JS (2011): Looking to the future: Automatic regulation of attention between current performance and future plans. Neuropsychologia 49:2258–2271. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Park DC, Hertzog C, Kidder DP, Morrell RW, Mayhorn CB (1997): Effect of age on event‐based and time‐based prospective memory. Psychol Aging 12:314–327. [DOI] [PubMed] [Google Scholar]
- Pouthas V, George N, Poline J‐B, Pfeuty M, Vandemoorteele P‐F, Hugueville L, Ferrandez A‐M, Lehéricy S, Lebihan D, Renault B (2005): Neural network involved in time perception: An fMRI study comparing long and short interval estimation. Hum Brain Mapp 25:433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers JG, Kuypers KPC, Wingen M, Heinecke A, Formisano E (2009): Involvement of inferior parietal lobules in prospective memory impairment during acute MDMA (ecstasy) intoxication: An event‐related fMRI study. Neuropsychopharmacology 34:1641–1648. [DOI] [PubMed] [Google Scholar]
- Rao SM, Mayer AR, Harrington DL (2001): The evolution of brain activation during temporal processing. Nat Neurosci 4:317–323. [DOI] [PubMed] [Google Scholar]
- Rea M, Kullmann S, Veit R, Casile A, Braun C, Belardinelli MO, Birbaumer N, Caria A (2011): Effects of aversive stimuli on prospective memory. An event‐related fMRI study. PLoS One 6:e26290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JR, West R, Braver T (2009): Distinct neural circuits support transient and sustained processes in prospective memory and working memory. Cereb Cortex 19:1208–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K (2006): The Neural Correlates of Timing Functions. Timing the Future: The Case for a Time‐Based Prospective Memory. London: World Scientific; pp 213–238. [Google Scholar]
- Rusted J, Ruest T, Gray MA (2011): Acute effects of nicotine administration during prospective memory, an event related fMRI study. Neuropsychologia 49:2362–2368. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Berish DE, Siedlecki KL (2004): Construct validity and age sensitivity of prospective memory. Mem Cogn 32:1133–1148. [DOI] [PubMed] [Google Scholar]
- Scullin MK, McDaniel MA, Shelton JT, Lee JH (2010): Focal/nonfocal cue effects in prospective memory: Monitoring difficulty or different retrieval processes? J Exp Psychol Learn Mem Cogn 36:736–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Schölvinck ML, Gilbert SJ, Frith CD, Burgess PW (2006): Differential components of prospective memory? Evidence from fMRI. Neuropsychologia 44:1388–1397. [DOI] [PubMed] [Google Scholar]
- Smith RE (2003): The cost of remembering to remember in event‐based prospective memory: Investigating the capacity demands of delayed intention performance. J Exp Psychol Learn Mem Cogn 29:347–361. [DOI] [PubMed] [Google Scholar]
- Smith RE, Persyn D, Butler P (2011): Prospective memory, personality, and working memory: A formal modeling approach. Zeitschrift fur Psychologie 219:108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda S, Nagumo Y, Kato M (2006): Dissociative contributions of medial temporal and frontal regions to prospective remembering. Rev Neurosci 17:267–278. [DOI] [PubMed] [Google Scholar]
- Villain N, Landeau B, Groussard M, Mevel K, Fouquet M, Dayan J, Eustache F, Desgranges B, Chételat G (2010): A simple way to improve anatomical mapping of functional brain imaging. J Neuroimaging 20:324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volle E, Gonen‐Yaacovi G, Costello A L, de Gilbert SJ, Burgess PW (2011): The role of rostral prefrontal cortex in prospective memory: A voxel‐based lesion study. Neuropsychologia 49:2185–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL (2005): Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci (Regul Ed) 9:445–453. [DOI] [PubMed] [Google Scholar]
- Wang W‐C, Dew ITZ, Giovanello KS (2010): Effects of aging and prospective memory on recognition of item and associative information. Psychol Aging 25:486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R, Wymbs N, Jakubek K, Herndon RW (2003): Effects of intention load and background context on prospective remembering: An event‐related brain potential study. Psychophysiology 40:260–276. [DOI] [PubMed] [Google Scholar]


