Abstract
Neurofibrillary tangles are associated with cognitive dysfunction, and hippocampal atrophy with increased CSF tau markers. However, the plasma tau levels of Alzheimer's disease (AD) have not been well studied. We investigated plasma tau by using an immunomagnetic reduction assay in 20 patients with mild cognitive impairment (MCI) due to AD, 10 early AD dementia, and 30 healthy elders (HE). All received a 3D‐brain MRI scan and a set of cognitive function test. We explored their relationships with both brain structure and cognitive functions. Images were analyzed to determine the brain volumes and gray matter densities. Patients with MCI or early AD had significantly increased plasma tau levels compared with HE. Plasma tau levels were negatively associated with the performance of logical memory, visual reproduction, and verbal fluency; also negatively associated with volume of total gray matter, hippocampus, amygdala; and gray matter densities of various regions. Regression analyses indicated that logical memory explained 0.394 and hippocampus volume predicted .608 of the variance of plasma tau levels, both P < 0.001. Education years were negatively associated with the gray matter densities of the supramarginal (r = −0.407), middle temporal gyrus (r = −0.40) and precuneus (r = −0.377; all P < 0.05) in HE; and negatively associated with plasma tau levels in patients (r = −0.626). We propose that plasma tau may serve as a window to both structure and function of the brain. Higher education is a protective factor against AD and is associated with lower plasma tau levels in patients. Hum Brain Mapp 35:3132–3142, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: Alzheimer's disease, plasma total tau, MRI volumetry, voxel‐based morphometry, hippocampus, immunomagnetic reduction, biomarker
INTRODUCTION
Recent studies have provided new insights into the pathological processes associated with Alzheimer's disease that precede the onset of clinical dementia by many years (Perrin et al., 2009; Sperling et al., 2011). Although beta amyloid (Aβ) proteins are believed to play a central role in the pathogenesis of Alzheimer's disease [Ingelsson et al., 2004], their significance as a primary causative factor remains a subject of debate [Nelson et al., 2009]. The weak correlation between the accumulation of fibrillary Aβ in the brain and cognitive function that has been observed is a good example [Guannalopoulos et al., 2003]. However, the number of neurofibrillary tangles is significantly correlated with both cognitive function and clinical staging [Bennet et al., 2004; Guannalopoulos et al., 2003]. Published reports have also correlated hippocampal atrophy, a characteristic symptom of Alzheimer's disease, with tau markers in the cerebrospinal fluid (CSF) [Desikan et al., 2011; de Souza et al., 2012]. Recent studies have reached a general consensus that decreased levels of Aβ42 and increased total levels of tau in the CSF are potential biomarkers for AD [Vemuri et al., 2010]. Low levels of Aβ42 in the CSF reflect the deposition and entrapment of Aβ in amyloid plaques, whereas elevated levels of tau reflect abnormal tau accumulation in neurofibrillary tangles and neural threads. These findings are both indicative of a process of active axonal and neuronal destruction [Tapiola et al., 2009].
Biofunctionalized magnetic nanoparticles are useful tools for detecting biological molecules [Kötitz et al., 1999]. In particular, these nanoparticles may be used to label various types of target biological molecules; by measuring the magnetic signals from these nanoparticles, the concentrations of the target molecules can then be determined through the use of a magnetic field. This technique is referred to as a magnetically labeled immunoassay. Magnetically labeled immunoassays are able to quantitatively detect proteins, viruses, carcinogens, chemicals, and nucleic acids.
In our previous work, we developed an approach that is capable of quantifying the plasma levels of Aβ42 and Aβ40 [Chiu et al., 2012]. In these studies, we used an immunomagnetic reduction (IMR) method to investigate the association between Aβ and magnetic nanoparticles [Chiu et al., 2011, 2012; Yang et al., 2011].
The hypothesis that the total tau levels in the CSF may reflect axonal damage and neuronal degeneration is supported by previously published results. However, the plasma tau levels of patients with AD have not been thoroughly characterized. Thus, in this study, we sought to use the aforementioned IMR method to measure the plasma levels of tau in control subjects, subjects with mild cognitive impairment (MCI) and subjects with AD. We further explored the relationships between the plasma levels of tau and both the anatomical brain structures and the cognitive functions of the study participants.
METHODS
Participants
We recruited 20 subjects with MCI (mean age 71.2 ± 9.7; 11 women, 9 men; mean MMSE = 26.3 ± 2.7), 10 subjects with early AD (mean age 69.3 ± 9.4 years; 6 women, 4 men; mean MMSE = 22.7 ± 3.6), and 30 control subjects (mean age 64.4 ± 9.5; 17 women, 13 men; mean MMSE = 28.8 ± 1.6; Table 1). There were no significant group differences in the ages (F = 3.038, P = 0.056), gender (Pearson chi‐square = 0.068, P = 0.967) or APOE ε4 distributions (Pearson chi‐square = 2.125, P = 0.346). The subjects with AD were recruited from the memory clinic at the National Taiwan University Hospital. After undergoing routine tests at the memory clinic, each participant received a comprehensive clinical checkup that included a review of his or her medical history, physical, and neurological examinations, laboratory tests, and neuroimaging studies. All of the patients with dementia met diagnostic guidelines for probable AD dementia proposed by the National Institute on Aging‐Alzheimer's Association (NIA‐AA) workgroups in 2011 [McKhann et al., 2011]. The diagnosis of MCI due to AD also followed the recommendations from the NIA‐AA on diagnostic guidelines [Albert et al., 2011]. For the diagnosis of MCI due to AD we used formal cognitive test with the cut‐off value at or below the 4th percentile (lower than 1.5 SD) of scale score of the age and education matched control. To increase the certainty level of probable AD dementia we included evidence of the AD pathophysiological process with amyloid deposition by PIB‐PET, temporoparietal hypometabolism by FDG‐PET or decreased hippocampal volume (either by volumetry of lower than 95% of the healthy elderly controls or by Visual Rating System) [Scheltens et al., 1995]. Image biomarkers used in probable AD dementia were also incorporated in the research criteria for MCI due to AD group. The control subjects were selected from a group of healthy volunteers in an MCI project [Chen et al., 2009]. In this project, the volunteers were given a medical checklist to identify any major systemic diseases, operations, and/or hospitalizations. Volunteers who reported experiencing certain uncontrolled medical conditions including heart failure, recent myocardial infarction (within the past 6 months), malignancy (during the past 2 years), or poorly controlled diabetes (Hb A1C > 8.5) were excluded from the present study. The volunteers also received physical and neurological examinations, and they were scored on a short‐form Geriatric Depression Scale (GDS‐S). Volunteers, who had GDS‐S scores greater than 9, were also excluded from participation. Control subjects possessed normal cognitive function, as confirmed by a battery of neuropsychological tests. In addition to the MMSE, CDR and GDS that were used as screening tools to include subjects, all of the study participants including patients with probable AD dementia, MCI due to AD and healthy elders experienced the same neuropsychological test battery, which included the Wisconsin Card Sorting Test (WCST), two Trail Making Tests (A and B), two subtests of the WMS‐III [logical memory and visual reproduction; Hua et al., 2005], selected subtests of the WAIS‐III (digit span, matrix reasoning, block design, and digit symbol substitution), a test of verbal fluency; and a 3D T1‐weighted brain MRI scan which was obtained within one month of both the neuropsychological examination and the acquisition of a blood sample. At last, fluid‐attenuated inversion recovery (FLAIR) images were used to identify lacunar infarcts and diffusely confluent white matter hyperintensities (WMH). We excluded those subjects with the image findings of subcortical ischemic vascular dementia such as Fazekas WMH scale greater than 2 or multiple deep gray matter lacunes [Chen et al., 2009; Fazekas et al., 1987; Román et al., 2002].
Table 1.
Demographic data, cognitive functions, plasma tau levels of the subjects
| Group | Control (30) | MCI (20) | Early AD (10) |
|---|---|---|---|
| Age (years) | 64.4 ± 9.5 | 71.2 ± 9.7 | 69.3 ± 9.4 |
| Gender female/male | 17/13 | 11/9 | 6/4 |
| APOEε4 | 8 (1ε4/4) | 8 (1ε4/4) | 5 (3ε4/4) |
| Education (years) | 13.1 ± 3.0 | 12.0± 3.1 | 7.6 ± 3.2*† |
| CDR | 0 | .5 | .5/one with CDR1 |
| MMSE | 28.8 ± 1.6 | 26.3 ± 2.7* | 22.7 ± 3.0*† |
| Plasma tau level (pg/ml) | 15.6 ± 6.9 | 32.7 ± 5.8* | 53.9 ± 11.7*† |
Mean ± SD;
*: significant group difference between controls and MCI or early AD by using ANOVA at a significance level of P < 0.01;
†: significant group difference between MCI and Early AD at a significance level of P < 0.01; no significant between‐group difference in age, gender and APOEε4 distribution.
All of the study subjects or their primary caregivers provided informed consent prior to participating in this investigation. The study was approved by the ethics committee and institute review board of the university hospital.
Immunomagnetic Reduction Technique and Assessment of Plasma Tau
Immunomagnetic reduction technique
Immunomagnetic reduction (IMR) is a method to assay target molecules via measuring the reduction in the mixed frequency magnetic susceptibility of magnetic reagent due to binding of the target proteins to magnetic nanoparticles, in this study the tau protein (Fig. 1a,b). Under external multiple alternating current (ac) magnetic fields, magnetic nanoparticles oscillate with the multiple ac magnetic fields via magnetic interaction. Thus, the reagent under external multiple ac magnetic fields shows a magnetic property, called mixed‐frequency ac magnetocsusceptibility χac, (Fig. 1a). Through the anti‐tau antibody on the outer shell, magnetic nanoparticles bind with and magnetically label tau proteins. With the binding, magnetic nanoparticles become larger or clustered (Fig. 1b). The oscillation response of these larger magnetic nanoparticles to external multiple ac magnetic fields becomes much less than that of the originally unbound individual magnetic nanoparticles. Thus, the χac of the magnetic reagent is reduced due to the association between magnetic nanoparticles and tau proteins. The reduction percentage in χac of the magnetic reagent is referred to as IMR signals (Eq. 1). If the amount of tau proteins is increased, more magnetic nanoparticles become larger or clustered. The reduction in χac of the reagent is then increased.
| (1) |
Figure 1.
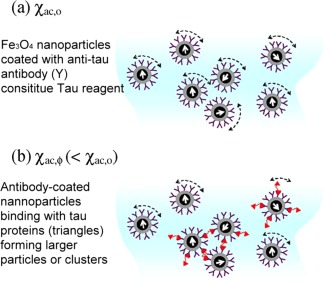
(a) The reagent under external multiple ac magnetic fields shows a magnetic property, called mixed‐frequency ac magnetosusceptibility χ ac,0. (b) With the binding, magnetic nanoparticles become larger or clustered. The oscillation response of these larger magnetic nanoparticles to external multiple ac magnetic fields becomes much less than that of the originally unbound individual magnetic nanoparticles. Thus, the magnetic reagent is reduced due to the association between magnetic nanoparticles and tau proteins χ ac,Φ. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Preparation of magnetic‐nanoparticle tau reagent
The magnetic nanoparticles used in this study were composed of Fe3O4 that was coated with dextran and dispersed in a PBS solution at a pH of 7.4 (MF‐DEX‐0060, MagQu). Antibodies against the tau protein (anti‐tau) (Tau‐441, Sigma) were covalently bound to the dextran coatings of the magnetic nanoparticles. A magnetic separation technique was used to separate the unbound antibodies from the magnetic solution. The magnetic solution that contained magnetic nanoparticles that had been biofunctionalized with anti‐tau is referred to as the tau reagent (MF‐TAU‐0060, MagQu).
Specimen collection and preparation
Participants were asked to provide a 10‐ml non‐fasting venous blood sample (K3 EDTA, lavender‐top tube). Each sample was assigned a registry number following the sampling sequence; hence, colleagues in the laboratory were blind to the clinical status and the demographic data of the subjects. In consideration of the possible circadian changes and food effects, all blood samples were collected between 10 AM and 2 PM and were postprandial. The blood samples were centrifuged (2500g for 15 min) within 1 h of collection, and plasma was aliquoted into cryotubes and stored at −80°C for less than three months until thawed for measurement. Cellular part was further used to extract DNA from WBC for gene analyses. Genotyping of the APOE epsilon alleles (ε2, ε3, and ε4) was conducted using sequence‐specific primer (SSR)‐PCR methodologies.
Measurement of plasma tau through IMR signal change
The percent reduction in the χac of the tau reagent was measured using an ac magnetosusceptometer (XacPro‐S, MagQu) that was equipped with a high‐Tc superconducting quantum interference device (SQUID) magnetometer, which functioned as a magnetic sensor. The degree of binding between plasma tau and biofunctionalized magnetic nanoparticles was assessed by mixing 60 μl of tau reagent with 60 μl of a solution containing a plasma sample and subsequently recording the time‐dependent χac signal of the mixture with the SQUID‐based ac magnetosusceptometer. The χac signal of the mixture decreases upon the binding of the nanoparticles to the tau proteins in the mixture; thus, the percent reduction in the χac signal for the sample can be determined from the starting and ending χac values. The percent reduction in the χac signal is referred to as the IMR signal. Once the tau concentration‐dependent IMR signal has been obtained, the level of the plasma tau can be determined using a logistic function (for technique details, please refer to our previously published work) [Chiu et al., 2011, 2012; Yang et al., 2011, Yang, 2013].
Test of the coefficient variance of the IMR signals
We used three concentration levels of tau protein to test the coefficient variance (CV) of our IMR method, i.e., 0.1 pg/ml, 10 pg/ml, and 1000 pg/ml. For each concentration, the IMR signals were measured in triplicate. Both the intra‐assay and inter‐assay CV are less than 5%.
The acquisition and analysis of magnetic resonance images
Image acquisition and preprocessing
High‐resolution structural brain MRI scans were acquired using a 1.5 T MRI scanner (EXCITE, General Electric, Milwaukee, USA). A whole‐brain T1‐weighted 3D spoiled gradient recovery (SPGR) sequence was used (TE = 9.3 ms, TR =3.9 ms, TI = 600 ms, flip angle = 12°, matrix size = 192 × 192, FOV = 25 cm), and a total of 170 contiguous sagittal slices that were 1.3 mm in thickness were acquired. The analyzed datasets include the T1‐weighted structural MRI scans from all available subjects (n = 60). The MRI images were corrected for intensity inhomogeneities and reoriented; then registered against MNI 152 space using FSL (FMRIB Software Library) tools [Smith et al., 2004].
Segmentation and volumetry
The converted T1‐weighted images were then processed; in particular, the FIRST and SIENAX FSL tools were used to extract the relevant regions of interest and calculate the total brain volume [Patenaude et al., 2011]. The FIRST tool is a model‐based segmentation/registration tool. The shape/appearance models used in FIRST are constructed from manually segmented images that are provided by the Center for Morphometric Analysis (CMA) at MGH, Boston. The shape and appearance models are based on multivariate Gaussian assumptions that allow the shape parameter can be expressed as a mean with certain modes of variation (principal components). FIRST then searches through several linear combinations of the modes of shape variation to determine the most probable shape given the observed intensities in the T1 images (Smith et al., 2004). We used this method to obtain the regional brain volumes of both the right and left hippocampi and the amygdala. Brain tissue volumes were normalized to the head sizes of the subjects and were estimated using the SIENAX [Smith et al., 2002], tool of the FSL. Tissue‐type segmentation with partial volume estimation was then performed to calculate the total volume of brain tissue for each study subject, including separate estimates for the gray matter, white matter, peripheral gray matter and ventricular CSF volumes [Jenkinson et al., 2001].
Voxel‐based morphometry
The structural data were further analyzed using the FSL‐VBM tool for a voxel‐based morphometry (VBM) analysis. In its simplest form, VBM involves a voxel‐wise comparison of the local gray matter concentrations of two groups of subjects [Good et al., 2001; Smith et al., 2004]. Tissue‐type segmentation was conducted using the FAST4 methodology [Zhang et al., 2001]. The resulting gray‐matter partial volume images were then aligned using the FLIRT affine registration tool [Jenkinson et al., 2001]; this alignment was followed by nonlinear registration using FNIRT [Andersson et al., 2007]. The modulated segmented images were then smoothed through the use of an isotropic Gaussian kernel with a sigma of 3 mm. The gray matter density images were then utilized for additional regional comparisons between the groups that were examined in this study (Fig. 2).
Figure 2.
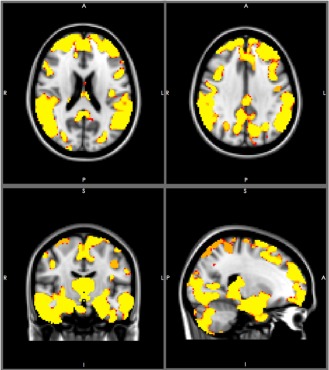
A comparison of voxel‐based morphometry (VBM) between 30 healthy elders and patients (20 MCI and 10 patients). The yellow color indicates a significance level of p < 0.001. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Statistical Analysis
Pearson chi‐square tests were used to examine the between group difference of the gender and the APOEε4 distributions. The significance levels of differences in ages, years of education, MMSE scores and plasma tau levels between groups was assessed through analyses of variance (ANOVAs). Pearson correlations were used to examine the pairwise associations between ages, years of education, plasma tau levels, global or regional brain volumes and neuropsychological test scores of the healthy elder group to identify possible confounding effect of age and years of education. A multivariate analysis of covariance (MANCOVA) with a Bonferroni correction was used to examine the between‐group differences in global and regional brain volume, gray matter density and cognitive test scores; in this analysis, age and years of education were considered to be covariates. Partial correlation using the same covariates was performed to identify factors to be included in the regression analysis. Finally, linear regression analyses (stepwise) were performed to evaluate the proportion of variance that was explained by each factor.
RESULTS
Demographic Data and Plasma Tau Levels
There were significant between‐group differences in MMSE scores (F = 27.58, P < 0.001), years of education (F = 11.975, P < 0.001) and plasma tau levels (F = 103.07, P < 0.001, Fig. 3). The AD group had the lowest average number of years of education (7.6 ± 3.2) and the lowest mean MMSE score (22.7 ± 3.1); the patients in the AD group also had the highest levels of plasma tau (53.9 ± 11.7 pg/ml; Table 1). Pearson correlations demonstrated significant negative associations between age and various aspects of brain structure in the healthy elder group, including the total gray matter volume (r = −0.430, P < 0.05), the total hippocampal volume (r = −0.591, P < 0.01) and the gray matter densities of various regions such as (r = −0.0707 for superior temporal gyrus; r = −0.704 for middle temporal gyrus, both P < 0.01). Similarly, for the healthy elder group, the number of years of education was also significantly correlated with the performance on various cognitive tests, such as the delayed recall of logical memory (r = 0.405, P < 0.05) and Trail Making Tests A and B (r= ‐0.586 for A, r = ‐0.529 for B, both P < 0.01) and so on. Because of these correlations, we controlled for the effects of both ages and years of education in all between‐group comparisons of both brain structures and cognitive test scores.
Figure 3.
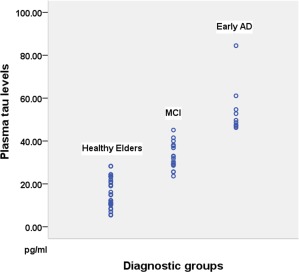
The plasma tau levels of different groups of subjects. The mean (±standard deviation) tau concentrations were 15.6 ± 6.9 pg/mL for the healthy elder group, 33.2 ± 5.4 pg/mL for the MCI group and 53.9 ± 11.7 pg/mL for the early AD group (F = 57.7, p < 0.01). Post hoc analyses identified significant differences between the control and early AD groups and between the MCI and early AD groups (both p < 0.01). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Between‐Group Comparison of Cognitive Functions and Brain Structures
Cognitive functions
MANCOVA tests that controlled for the effects of age and education indicated significant between‐group differences in all of the aspects of cognitive functioning that we assessed (all P < 0.001) with the exception of number of perseverative errors of WCST (P < 0.05) and digit span (p > .05). However, post‐hoc analyses revealed that only certain cognitive score were statistically different; in particular, these significant results included differences with respect to the delayed recall of both logical memory and visual reproduction; and semantic verbal fluency (Table 2). The statistically significant differences in cognitive test scores were typically between the healthy elder group and either AD or MCI patients; significant differences between AD and MCI patients were otherwise scanty.
Table 2.
Cognitive functions, brain volume, and gray matter density of the subjects
| Group | Control (30) | MCI (20) | Early AD (10) |
|---|---|---|---|
| WMS‐III | |||
| DLM | 78.7 ± 20.6 | 24.0 ± 22.1** | 2.6 ± 3.5** |
| DVR | 61.70 ± 22.6 | 30.6 ± 24.7** | 6.8 ± 5.5** |
| WCST | |||
| Categories completed | 5.1 ± 1.9 | 3.4 ± 2.2 | 1.6 ± 1.3**† |
| Perseverative errors | 3.7 ± 4.8 | 9.3 ± 10.5 | 10.0 ± 4.6 |
| Trail Making‐A | 46.1 ± 16.0 | 60.1 ± 32.1 | 86.0 ± 54.4 |
| Trail Making‐B | 95.5 ± 41.3 | 201.3 ± 195.2 | 241.7 ± 88.0 |
| WAIS‐III | |||
| Digit Span F:6 B:4 | 12.3 ± 2.4 | 12.3 ± 3.2 | 11.2 ± 2.8 |
| Matrix Reasoning | 11.4 ± 3.1 | 10.3 ± 2.4 | 7.8 ± 3.2 |
| Block Design | 11.1 ± 2.4 | 10.4 ± 2.2 | 7.8 ± 2.9 |
| DSS | 12.6 ± 2.7 | 10.6 ± 2.9 | 8.9 ± 3.1 |
| Semantic verbal fluency | 41.3 ± 7.5 | 27.2 ± 1.6** | 21.1 ± 6.7**† |
| Volumetry in cm3 | |||
| Gray matter | 753.56 ± 43.3 | 702.4 ± 47.1* | 699.49 ± 64.3* |
| Hippocampus | 7.66 ± .69 | 5.78 ± .65** | 6.05 ± .67** |
| Amygdala | 2.69 ± .29 | 2.13 ± .38** | 2.47 ± .23 |
| Voxel‐based morphometry | |||
| Superior frontal gyrus | .5398 ± .0158 | .5405 ± .0159* | .4968 ± .0258** |
| Middle frontal gyrus | .5390 ± .0167 | .5127 ± .0407* | .4894 ± .0291**† |
| Inferior frontal gyrus | .5595 ± .0166 | .5325 ± .0439* | .5258 ± .0222** |
| Sup temporal gyrus | .5313 ± .0199 | .4953 ± .0413** | .4914 ± .0339** |
| Middle temporal gyrus | .5888 ± .0260 | .5379 ± .0444** | .5352 ± .0422** |
| Hippocampus | .6531 ± .0341 | .6182 ± .0480 | .6242 ± .0395 |
| Precuneus | .5160 ± .0277 | .5013 ± .0297 | .4691 ± .0352**†† |
| Supramarginal gyrus | .5045 ± .0379 | .4644 ± .0441* | .4367 ± .0489** |
Mean ± SD; WMS‐III, Wechsler Memory Scale‐3rd edition; DLM, delayed recall of logistic memory scaled score; DVR, delayed recall of visual reproduction scaled score; WCST, Wisconsin Card Sorting Test; WAIS‐III, Wechsler Adult Intelligence Scale‐3rd edition; DSS: Digit Symbol‐Substitution;
*: significant group difference between controls and MCI or AD by using MANCOVA controlling age and years of education effects at a significance level of P < 0.05;
**: P < 0.01;
†: significant group difference between MCI and Early AD at a significance level of P < 0.05;
††: P < 0.01.
Brain Structures
The analyses of brain structures also revealed significant between‐group differences in the total volume of gray matter (P < 0.01) and the volumes of the hippocampus and the amygdala (P < 0.001). In terms of gray matter densities, almost all of the regions of interest had significant between‐group differences (all P < 0.01) except VBM of hippocampus (P > 0.05).
Genetic factor
Carriers of APOEε4 had higher plasma tau levels than non‐carriers of APOEε4, but the differences between the plasma tau levels of carriers and noncarriers did not reach statistical significance in the patient group (AD and MCI, 43.1 ± 16.6 vs. 37.3 ± 9.1 pg/mL; P > 0.1) or the healthy elder group (16.4 ± 5.9 vs. 15.3 ± 7.3 pg/mL; P > 0.1) but a marginal difference (32.9 ± 18.8 vs. 24.9 ± 13.6 pg/mL; P = 0.062) for all subjects.
Regression analysis
Partial correlation analysis controlling age and education between plasma tau levels and brain volumes revealed significant negative associations between plasma tau levels and total gray matter (r = −0.393, P < 0.01), total hippocampus (r = −0.570, P < 0.01) and total amygdala (r = −0.282, P < 0.05). Partial correlation analysis controlling age and education between plasma tau levels and gray matter densities (VBM) revealed significant negative associations between plasma tau levels and superior, middle and inferior frontal gyrus (r = −0.432, −0.411, −0.351 respectively, all P < 0.01), superior and middle temporal gyrus (r = −0.377, −0.445 respectively, both P < 0.01), supramarginal gyrus (r = −0.401, P < 0.01), thalamus (r = −0.397, P < 0.01) and precuneus (r = −0.314, P < 0.05). Partial correlation analysis controlling age and education between plasma tau levels and cognitive functions revealed significant negative associations between plasma tau levels and delayed recall of logical memory (r = −0.679, P < 0.01), delayed recall of visual reproduction (r = −0.576, P < 0.01) and verbal fluency (r = −0.588, P < 0.01).
The aforementioned independent variables obtained from the partial correlation analyses were then selected for linear regression analysis.
Stepwise linear regression analysis on brain structures demonstrated that the value of R 2 change of the total hippocampal volume was 0.394 (P < 0.001), followed by education 0.154 (P < 0.05) and superior frontal gyrus 0.045 (P < 0.05; Table 3). Stepwise linear regression analysis on cognitive functions demonstrated that the value of R 2 change of the delayed recall of logical memory 0.608 (P < 0.001), followed by education 0.031 (P < 0.05) and verbal fluency 0.026 (P < 0.05; Table 4).
Table 3.
Regression coefficients of brain structures association with plasma tau
| Model | B [95% CI] | β | P | R2 |
|---|---|---|---|---|
| Model I Hippocampal volume | −0.009 [−0.012∼−0.006] | −0.628 | .000 | .394 |
| Model II Hippocampal volume Education | −0.007 [−0.01∼−0.005] −1.806 [−2.626∼−0.985] | −0.519 −0.407 | .000 .000 | .548 |
| Model III Hippocampal volume Education Superior frontal VBM | −0.006 [−0.009∼−0.003] −1.727 [−2.516∼−0.939] −119.908 [−216.632∼−23.183] | −0.424 −0.389 −0.235 | .000 .000 .016 | .593 |
VBM: voxel‐based morphometry; CI: confidence interval; excluded variables in Model III (ExV‐Model III): age, gender, total gray matter volume, total amygdale volume, middle and inferior frontal gyrus, superior and middle temporal gyrus, supramarginal gyrus and precuneus. In Model I, all the ExV‐Model III and hippocampal volume were entered into stepwise regression analysis; in Model II, all the ExV‐Model III, hippocampal volume and education were entered; in Model III, all the EV‐Model III, hippocampal volume, education and superior frontal VBM were entered.
Table 4.
Regression coefficients of cognitive functions associations with plasma tau
| Model | B [95% CI] | β | P | R2 |
|---|---|---|---|---|
| Model I Logical memory | −0.336 [−0.407∼−0.265] | −0.78 | 0.000 | 0.608 |
| Model II Logical memory Education | −0.292 [−0.371∼−0.212] −0.900 [−1.719∼−0.080] | −0.677 −0.203 | 0.000 0.032 | 0.639 |
| Model III Logical memory Education Verbal fluency | −0.217 [−0.322∼0.111] −0.936 [−1.734∼−0.139] −0.342 [−0.670∼−0.013] | −0.503 −0.211 −0.235 | 0.000 0.022 0.042 | 0.665 |
CI, confidence interval; excluded variables in Model III (ExV‐Model III): age, gender, visual reproduction. In Model I, all the ExV‐Model III and logical memory were entered into stepwise regression analysis; in Model II, all the ExV‐Model III, logical memory and education were entered; in Model III, all EV‐Model III, logical memory, education and verbal fluency were entered.
Education predicts 0.154 of the variance of plasma tau levels in the association with brain structures. Partial correlations of education year association with brain structures or plasma tau levels controlling age in both control and patient groups were further examined. The results showed that education was negatively associated with VBM of the middle temporal gyrus (r = −0.440), precuneus (r = −0.377) and supramarginal gyrus (r = −0.407; all P < 0.05) in the healthy elder group while in the patient group education was negatively associated with the plasma tau levels (r = −0.626, P < 0.01) both controlling the effect of age.
DISCUSSION
The hypothesis that total tau levels in the CSF may reflect axonal damage and neuronal degeneration is supported by previous studies, which have shown marked increases in the total tau levels in the CSF of patients with certain neurological conditions, such as Creutzfeldt‐Jakob disease, acute stroke, and traumatic brain injury [Hesse et al., 2000; Kapaki et al., 2001; Zemlan et al., 1999]. Moreover, the results of approximately 50 studies that included a total of more than 5000 subjects have demonstrated that compared with controls, patients with AD have a threefold increase in CSF tau levels [Hampel et al., 2008]. As discussed above, the relationship between the tau level in the CSF and hippocampal atrophy has recently been studied [Desikan et al., 2011; de Souza et al., 2012; Hampel et al., 2005]. CSF tau levels may be regarded as an injury marker that may predict a rapid progression from MCI to AD‐associated dementia [van Rossum et al., 2012].
By contrast, the plasma tau levels in neurological diseases have not been well studied. One early study of plasma tau levels in small groups of patients with dementia produced discouraging results; in particular, the researchers reported a lack of any obvious increase in plasma tau levels in dementia patients [Ingelson et al., 1999]. Although the results of this previous study may have been limited by the relatively low sensitivity of the ELISA technology that it used, a more recent study that employed an ELISA technique with greater sensitivity to detect tau levels reported reduced plasma tau levels in patients with AD [Sparks et al., 2012]. Other studies that have used these more sensitive ELISA approaches have reported that plasma tau levels were increased in the late phase of comatose patients who had experienced cardiac arrest [Mörtberg et al., 2011] and in patients with Creutzfeldt‐Jakob disease, acute stroke, and traumatic brain injury [Bielewicz et al., 2011; Liliang et al., 2010; Noguchi‐Shinohara et al., 2011].
In this study, we found significantly increased plasma tau levels in subjects with MCI or early AD compared with the plasma tau levels of controls. A threefold increase in plasma tau levels in subjects with early AD is observed in this study and is similar to its CSF counterpart [Hampel et al., 2008]. This finding was supported by our experiment in this study that the CSF tau levels correlate well with the plasma tau levels (Spearman rho = 0.682, P < 0.01). A study of experimental brain injury in rats found that serum tau levels increased by more than fourfold in the hour following the insult [Liliang et al., 2010], indicating that the tau protein from the brain is readily excreted into the peripheral blood shortly after it is produced. Therefore, the elevated CSF tau levels that are observed in subjects with AD should result in elevated plasma tau levels that are similar to the levels that have been reported in the other aforementioned clinical studies [Bielewicz et al., 2011; Liliang et al., 2010; Mörtberg et al., 2011; Noguchi‐Shinohara et al., 2011]. Plasma tau can therefore be considered to be a window that reveals brain structure in terms of both total brain volume and the gray matter density of the hippocampus.
A regression analysis indicates that plasma tau levels were negatively associated with total hippocampus volume (which predicted 0.394 of the variance in the plasma tau level) and the gray matter density of the superior frontal (predicting 0.054 of the variance in the plasma tau level). In summary, the total hippocampus volume and the gray matter density of the superior frontal gyrus were the most valuable imaging factors for explaining the observed variance in the plasma tau levels of the study participants.
Furthermore, our regression analysis also identified significant negative associations between plasma tau levels and cognitive functions that scores of delayed recall of logical memory (predicting 0.608 of the variance in the plasma tau levels; P < 0.01) and verbal fluency contribute additional 0.026 (P < 0.05) of the variance in the plasma tau levels. This finding implies that plasma tau levels can also be regarded as a window that provides insights regarding the brain functions that are represented by verbal memory and executive function.
Education has long been known to be a protective factor against AD [Karp et al., 2004; Sattler et al., 2012]. The primary explanation of this phenomenon is the cognitive reserve hypothesis, which proposes that individuals with higher IQs, levels of education or levels of occupational attainment have lower risks of developing Alzheimer's disease [Meng et al., 2012]. The cognitive reserve hypothesis considers both protective and compensatory mechanisms. The compensatory mechanism claims that given the same clinical staging, patients with higher levels of education are likely to demonstrate greater pathological severity. In this study, we found that education years were negatively associated with gray matter densities of the precuneus, supramarginal gyrus and middle temporal gyrus (r = −0.377, −0.407, −0.400; all P < 0.05); while education years were negatively associated with the plasma tau levels of the patient group (r = −0.626, P < 0.01). The finding of negative associations between education years and brain structures supports the compensatory mechanism of cognitive reserve from education against the onset of dementia. However, education may have a further protection effect; even after the onset of cognitive impairment tau level remains lower in those people with higher education. This required further study to elucidate and validate this observation.
However, the study is not without limitations. The first limitation is that we measured the total tau of the plasma but not the phosphorylated tau (p‐tau) proteins. Indeed, total tau is not specific for degenerative disease as mentioned in our manuscript. However, we had excluded patients with those neurological conditions such as Creutzfeldt‐Jakob disease, acute stroke, and traumatic brain injury [Hesse et al., 2000; Kapaki et al., 2001; Zemlan et al., 1999]. Although p‐tau is more specific for degenerative dementia, it also has two problems. First, p‐tau has much lower concentration in both CSF and plasma (1/8∼1/5), it is much more difficult to measure the plasma levels of p‐tau (even lower than in CSF). Second, p‐tau proteins have different formats (e.g., p‐tau 181, 199, 231) and each has different clinical implication. For example, p‐tau 231 is useful for differentiating between AD and frontotemporal dementia, while p‐tau 181 is useful for differentiating AD from dementia of Lewy bodies [Hampel et al., 2010]. Still the task of measuring plasma p‐tau is our future work. The second limitation is that we do not have plasma‐CSF sample pairs from healthy controls for comparison. In our society, it is difficult to obtain CSF samples even from AD patients and we were not able to obtain CSF samples from healthy volunteers for measuring tau levels. The permeability of blood brain barrier may be changed by AD process (Matsumoto et al., 2007) and the relation of the plasma tau protein to its CSF counterparts in healthy controls remains unsolved in our study. Third, we did not have investigate the Aβ40 and 42 simultaneously and explore the Aβ42/tau ratios which can provide comparison with the findings from CSF studies. A final limitation is that we have quite a few different measures for cognitive functions and brain structures, although we have applied a strict statistical correction for multiple comparisons by using Bonferroni correction, in the generalization of our results it is better that readers refer only to those with higher significance (e.g., P < 0.01) to avoid possible false positives.
In conclusion, plasma tau levels are capable of differentiating between healthy elderly individuals and elderly patients with MCI or early AD. This study establishes associations that support its suggestion that plasma tau may serve as a window that reveals both the structure and the function of the brain.
REFERENCES
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH (2011): The diagnosis of mild cognitive impairment due to Alzheimer's disease: Recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S (2007): “Non‐linear optimization.” FMRIB technical report TR07JA1. Available at: http://www.fmrib.ox.ac.uk/analysis/techrep.
- Bennet DA, Schneider JA, Wilson RS, Bienas JL, Arnold SE (2004): Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol 61:378–384. [DOI] [PubMed] [Google Scholar]
- Bielewicz J, Kurzepa J, Czekajska‐chehab E, Stelmasiak Z, Bartosik‐Psujek H (2011): Does serum tau protein predict the outcome of patients with ischemic stroke? J Mol Neurosc 43:241–245. [DOI] [PubMed] [Google Scholar]
- Chen TF, Chen YF, Cheng TW, Hua MS, Liu HM, Chiu MJ (2009): Executive dysfunction and periventricular diffusion tensor changes in amnesic mild cognitive impairment and early Alzheimer's disease. Hum Brain Mapp 30:3826–3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TF, Lin CC, Chen YF, Liu HM, Hua MS, Huang YC, Chiu MJ (2009): Diffusion tensor changes in patients with amnesic mild cognitive impairment and various dementias. Psychiatry Res Neuroimaging 173:15–21. [DOI] [PubMed] [Google Scholar]
- Chiu MJ, Horng HE, Chieh JJ, Liao SH, Chen CH, Shih BY, Yang CC, Lee CL, Chen TF, Yang SY, Hong CY, Yang HC (2011): Multi‐channel SQID‐based ultra‐high‐sensitivity in‐vitro detections for biomarkers of Alzheimer's disease via immunomagnetic reduction. IEEE Trans Appl Supercond 21:477–480. [Google Scholar]
- Chiu MJ, Yang SY, Chen TF, Chieh JJ, Huang TZ, Yip PK, Yang HC, Cheng TW, Chen YF, Hua MS, Hong HE (2012): New assay for old markers—Plasma beta amyloid of mild cognitive impairement and Alzheimer's disease. Curr Alzheimer Res 9:1142–1148. [DOI] [PubMed] [Google Scholar]
- de Souza LC, Chupin M, Lamari F, Jardel C, Leclercq D, Colliot O, Lehéricy S, Dubois B, Sarazin M (2012): CSF tau markers are correlated with hippocampal volume in Alzheimer's disease. Neurobiol Aging 33:1253–1257. [DOI] [PubMed] [Google Scholar]
- Desikan RS, McEvoy LK, Thompson WT, Holland D, Roddey JC, Blennow K, Aisen PS, Brewer JB, Hyman BT, Dale AM; Alzheimer's Disease Neuroimaging Initiative (2011): Amyloid‐b associated volume loss occurs only in the presence of phospho‐Tau. Ann Neurol 70:657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas F, Chawluk JB, Alavi A, Hurtig HZ, Zimmerman RA (1987): MR signal abnormalities in Alzheimer's disease and normal aging. AJR Am J Roentgenol 49:351–356. [DOI] [PubMed] [Google Scholar]
- Good C, Johnsrude I, Ashburner J, Henson R, Friston K, Frackowiak R (2001): A voxel‐based morphometric study of ageing in 465 normal adult human brains. NeuroImage 14:21–36. [DOI] [PubMed] [Google Scholar]
- Guannalopoulos P, Herrmann FR, Bussiere T, Bouras C, Kövari E, Perl DP, Morrison JH, Gold G, Hof PR (2003): Tangles and neuron number but not amyloid load predict cognitive status in Alzheimer's disease. Neurology 60:1495–1500. [DOI] [PubMed] [Google Scholar]
- Hampel H, Bürger K, Pruessner JC, Zinkowski R, DeBernardis J, Kerkman D, Leinsinger G, Evans AC, Davies P, Möller HJ, Teipel SJ (2005): Correlation of cerebrospinal fluid levels of tau protein phosphorylated at threonine 231 with rates of hippocampal atrophy in Alzheimer disease. Arch Neurol 62:770–773. [DOI] [PubMed] [Google Scholar]
- Hampel H, Bürger K, Teipel SJ, Bokde AL, Zetterberg H, Blennow K (2008): Core candidate neurochemical and imaging biomarkers of Alzheimer's disease. Alzheimers Dement 4:38–48. [DOI] [PubMed] [Google Scholar]
- Hampel H, Blennow K, Shaw LM, Hoessler YC, Zetterberg H, Trojanowski JQ (2010): Total and phosphorylated tau protein as biological markers of Alzheimer's disease. Exp Gerontol 45:30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse C, Rosengren L, Vanmechelen E, Vanderstichele H, Jensen C, Davidsson P, Blennow K (2000): Cerebrospinal fluid markers for Alzheimer's disease evaluated after acute ischemic stroke. J Alzheimers Dis 2:199–206. [DOI] [PubMed] [Google Scholar]
- Hua MS, Chang BS, Lin KN, Yang JM, Lu SR, Chen SY. 2005. Wechsler memory scale, 3rd ed Chinese Behavioral Science Corporation. [Google Scholar]
- Ingelson M, Blomberg M, Benedikz E, Wahlund LO, Karlsson E, Vanmechelen E, Lannfelt L (1999): Tau immunoreactivity detected in human plasma, but no obvious increase in dementia. Dement Geriatr Cogn Disord 10:442–445. [DOI] [PubMed] [Google Scholar]
- Ingelsson M, Fukumoto H, Newell KL, Growdon JH, Hedley‐Whyte ET, Frosch MP, Albert MS, Hyman BT, Irizarry MC (2004): Early Aβ accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology 62:925–931. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith SM (2001): A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156. [DOI] [PubMed] [Google Scholar]
- Kapaki E, Kilidireas K, Paraskevas GP, Michalopoulou M, Patsouris E (2001): Highly increased CSF tau protein and decreased β‐amyloid (1–42) in sporadic CJD: A discrimination from Alzheimer's disease? J Neurol Neurosurg Psychiatry 71:401–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp A, Kareholt I, Qiu C, Bellander T, Winblad B, Fratiglioni L (2004): Relation of education and occupation‐based socioeconomic status to incident Alzheimer's disease. Am J Epidemiol 159:175–183. [DOI] [PubMed] [Google Scholar]
- Kötitz R, Weitschies W, Trahms L, Brewer W, Semmler WJ (1999): Determination of the binding reaction between avidin and biotin by relaxation measurements of magnetic nanoparticles. J Magn Magn Mater 194:62–68. [Google Scholar]
- Liliang PC, Liang CL, Lu K, Wang KW, Weng HC, Hsieh CH, Tsai YD, Chen HJ (2010): Relationship between injury severity and serum tau protein levels in traumatic brain injured rats. Resuscitation 10:1205–1208. [DOI] [PubMed] [Google Scholar]
- Liliang PC, Liang CL, Weng HC, Lu K, Wang KW, Chen HJ, Chuang JH (2010): Tau Proteins in Serum Predict Outcome After Severe Traumatic Brain Injury. J Surg Res 160:302–307. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Yanase D, Noguchi‐Shinohara M, Ono K, Yoshita M, Yamada M (2007): Blood‐brain barrier permeability correlates with medial temporal lobe atrophy but not with amyloid‐beta protein transport across the blood‐brain barrier in Alzheimer's disease. Dement Geriatr Cogn Disord 23:241–245. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011): The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, D'Arcy C (2012): Education and dementia in the context of the cognitive reserve hypothesis: A systemic review with meta‐analyses and qualitative analyses. PLoS One 7:e38268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mörtberg E, Zetterberg H, Nordmark J, Blennow K, Catry C, Decraemer H, Vanmechelen E, Rubertsson S (2011): Plasma tau protein in comatose patients after cardiac arrest with therapeutic hypothermia. Acta Anaesthesiol Scand 55:1132–1138. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Braak H, Markesbery WR (2009): Neuropathology and cognitive impairment in Alzheimer's disease: a complex but coherent relationship. J Neurol Exp Neurol 68:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi‐Shinohara M, Hamaguchi T, Nozaki I, Sakai K, Yamada M (2011): Serum tau protein as a marker for the diagnosis of Creutzfeldt‐Jakob disease. J Neurol 258:1464–1468. [DOI] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy D, Jenkinson M (2011): A Bayesian model of shape and appearance for subcortical brain. NeuroImage 6:907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin RJ, Fagan AM, Holtzman DM (2009): Multimodal techniques for diagnosis and prognosis of Alzheimer's disease. Nature 15:916–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC (2004): Mild cognitive impairment as a diagnostic entity. J Intern Med 256:183–194. [DOI] [PubMed] [Google Scholar]
- Roma'n GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC (2002): Subcortical ischaemic vascular dementia. Lancet Neurol 1:426–436. [DOI] [PubMed] [Google Scholar]
- Sattler C, Toro P, Schonknecht P, Schroder J (2012): Cognitive activity, education and socioeconomic status as preventive factors for mild cognitive impairment and Alzheimer's disease. Psychiatry Res 196:90–95. [DOI] [PubMed] [Google Scholar]
- Scheltens P, Launer LJ, Barkhof F, Weinstein HC, van Gool WA (1995): Visual assessment of medial temporal lobe atrophy on magnetic resonance imaging: interobserver reliability. J Neurol 242:557–560. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004): Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23:S208–S219. [DOI] [PubMed] [Google Scholar]
- Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, De Stefano N (2002): Accurate, robust and automated longitudinal and cross‐sectional brain change analysis. NeuroImage 17:479–489. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Kryscio RJ, Sabbagh MN, Ziolkowski C, Lin Y, Sparks LM, Liebsack C, Johnson‐Traver S (2012): Tau is reduced in AD plasma and validation of employed EISA methods. Am J Neurodegener Dis 1:99–106. [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison‐Bogorad M, Wagster MV, Phelps CH (2011): Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dementia 7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapiola T, Alafuzoff I, Herukka SK, Parkkinen L, Hartikainen P, Soininen H, Pirttilä T (2009): Cerebrospinal fluid β‐amyloid 42 and tau proteins as biomarkers of Alzheimer ‐type pathologic changes in the brain. Arch Neurol 2009;66:382–389. [DOI] [PubMed] [Google Scholar]
- van Rossum IA, Visser PJ, Knol DL, van der Flier WM, Teunissen CE, Barkhof F, Blankenstein MA, Scheltens P (2012): Injury markers but not amyloid markers are associated with rapid progression from mild cognitive impairment to dementia in Alzheimer's disease. J Alzheimers Dis 29:319–327. [DOI] [PubMed] [Google Scholar]
- Vemuri P, Wiste HJ, Weigand SD, Knopman DS, Trojanowski JQ, Shaw LM, Bernstein MA, Aisen PS, Weiner M, Petersen RC, Jack CR Jr; Alzheimer's Disease Neuroimaging Initiative (2010): Serial MRI and CSF biomarkers in normal aging, MCI, and AD. Neurology 75:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CC, Yang SY, Chieh JJ, Horng HE, Hong CY, Yang HC, Chen KH, Shih BY, Chen TF, Chiu MJ (2011): Bio‐functionalzied magnetic nanoparticles for specifically detecting biomarkers of Alzheimer's disease in vitro. ACS Chem Neurosci 2:500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemlan FP, Rosenberg WS, Luebbe PA, Campbell TA, Dean GE, Weiner NE, Cohen JA, Rudick RA, Woo D (1999): Quantification of axonal damage in traumatic brain injury: Affinity purification and characterization of cerebrospinal fluid tau proteins. J Neurochem 72:741–750. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S (2001): Segmentation of brain MR images through a hidden Markov random field model and the expectation maximization algorithm. IEEE Trans Med Imaging 20:45–57. [DOI] [PubMed] [Google Scholar]


