Abstract
Recognition memory, that is, the ability to judge whether an item has been previously encountered in a particular context, depends on two factors: discriminability and criterion setting. Discriminability draws on memory processes while criterion setting (i.e., the application of a threshold resulting in a yes/no response) is regarded as a process of cognitive control. Discriminability and criterion setting are assumed to draw on distinct anatomical structures, but definite evidence for this assumption is lacking. We applied voxel‐based and region of interest‐based lesion‐symptom mapping to 83 patients in the acute phase of ischemic stroke to determine the anatomical correlates of discriminability and criterion setting in verbal recognition memory. Recognition memory was measured with the Rey Auditory Verbal Learning Test. Signal‐detection theory was used to calculate measures for discriminability and criterion setting. Lesion‐symptom mapping revealed that discriminability draws on left medial temporal and temporo‐occipital structures, both thalami and the right hippocampus, while criterion setting draws on the right inferior frontal gyrus. Lesions in the right inferior frontal gyrus were associated with liberal response bias. These findings indicate that discriminability and criterion setting indeed depend on distinct anatomical structures and provide new insights in the anatomical correlates of these cognitive processes that underlie verbal recognition memory. Hum Brain Mapp 36:1292–1303, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: recognition memory, discriminability, criterion setting, response bias, lesion‐symptom mapping
INTRODUCTION
Recognition memory can be defined as the ability to judge whether an item has been previously encountered in a particular context [Berry et al., 2008]. According to signal‐detection theory, recognition depends on two factors: (1) discriminability and (2) a decision criterion, according to which the item is judged to be old or new [Berry et al., 2008; Wixted, 2007]. Discriminability reflects the process of gathering the “strength of evidence” of having previously encountered a specific item and depends on memory processes. Criterion setting (the actual decision whether an item is old or new based on the evidence, i.e., the application of a threshold) is regarded as a process of cognitive control [Jacoby et al., 2005]. Thus, an individual is assumed to decide whether an item is old or new by assessing the “strength of evidence” relative to a subjective decision criterion located at some point along this continuum. If the strength of evidence of having previously encountered the item exceeds the criterion then it will be judged to be old, otherwise it will be judged new [Berry et al., 2008].
Functional and lesion studies have provided insight in the anatomical correlates of several aspects of explicit memory, for example, working memory (dorsolateral parts of prefrontal cortices and lateral parietal cortices) and encoding for long term memory (medial temporal lobes, fornices, mammillary bodies, amygdala, thalamus) [Markowitsch, 2008; Rosen and Viskontas, 2008; van Strien et al., 2009; Vann et al., 2009]. Regarding recognition memory, there is substantial evidence for distinct anatomical correlates of the processes that underlie discriminability: recollection depends on a system centering on the hippocampus, whereas familiarity depends on a system centering on parahippocampal structures [Brown et al., 2010; Vann et al., 2009; Yonelinas et al., 2010]. Discriminability and criterion setting are also assumed to have distinct anatomical correlates, although there is no conclusive evidence from lesion studies that have attempted to compare the correlates of these two processes. There is some evidence for a role of the medial temporal lobes and thalamus in discriminability [Wixted and Squire, 2010; Yonelinas, 2002]. Functional studies have suggested that the parietal cortices might be involved in discriminability as well, although lesion studies have never been able to confirm these fMRI findings [Schoo et al., 2011; Vilberg and Rugg, 2008]. Lesion studies that have attempted to identify the anatomical correlates of criterion setting (also referred to as “response bias”) mainly focused on the frontal lobes. Two case studies have reported on patients with focal frontal lesions (one in the left, the other in the right prefrontal cortex), who showed remarkable high false alarm rates (i.e., false recognition); in both studies it was suggested that this may be due to extremely liberal response bias [Parkin et al., 1996; Schacter et al., 1996]. In a series of 14 patients with acquired brain damage, nonamnesic patients with isolated frontal lesions had a higher false alarm rate in a verbal word recognition test than patients with medial temporal lobe lesions, but the false alarm rate was not increased compared with controls [Melo et al., 1999]. In a more recent series of 46 patients, no difference in discriminability was found between nonamnesic patients with frontal lesions and controls in a visual word recognition test, whereas several of these patients with frontal lesions did demonstrate pathologically elevated false alarm rates [Verfaellie et al., 2004]. A recent lesion‐symptom mapping study, which applied voxel‐wise analyses in 11 stroke patients, demonstrated an association between right fronto‐temporal lesions and impaired discriminability for pictures and sounds and between right frontal lesions and liberal response bias (i.e., tendency toward high false alarm rate) for sounds and visually presented words [Haramati et al., 2008]. In summary, lesion studies on criterion setting in recognition memory have thus far been limited by low spatial resolution (grouping patients as either “frontal” or “medial temporal”), small sample size, and have yielded conflicting results. Moreover, in all but one of these studies the analyses were driven by the hypothesis that criterion setting takes place in either the frontal or temporal lobe, meaning potential involvement of other brain areas was not assessed. Thus, definite evidence regarding the anatomical correlates of criterion setting is lacking.
In this study, we therefore aimed to further clarify the anatomical correlates of recognition memory by applying lesion‐symptom mapping in a cohort of 88 patients with first‐ever ischemic stroke. We used the Rey Auditory Verbal Learning Test (RAVLT) to assess verbal memory [Rey, 1958]. Signal‐detection theory was used to calculate measures for discriminability and response bias [Donaldson, 1992; Snodgrass et al., 1985]. We subsequently performed assumption‐free voxel‐based lesion‐symptom mapping (VLSM) and region of interest‐based analyses to determine the anatomical correlates of recognition memory and, more specifically, discriminability and criterion setting.
MATERIAL AND METHODS
Subjects
A flowchart of the inclusion of patients for this study is provided in Supporting Information Fig. 1. Neuropsychological examination was performed in ischemic stroke patients who are admitted to our service in the setting of standard clinical care, if their condition permitted testing and testing facilities were available. All 243 ischemic stroke patients who were admitted from November 2005 through December 2012 and underwent neuropsychological assessment during admission were eligible for the present study (see Supporting Information Fig. 1). We subsequently applied a stepwise exclusion procedure to select patients without interfering pre‐existent neurological conditions or brain lesions, in whom the ischemic lesion could be segmented on CT or MRI, and with available data on recognition memory. In the first step, we excluded 79 patients with pre‐existent neurological conditions or imaging abnormalities: 26 patients with (probable) pre‐existent cognitive impairment (history of cognitive impairment (n = 7), traumatic brain injury (n = 5), brain tumor (n = 4), epilepsy (n = 1), multiple sclerosis (n = 1), moyamoya disease (n = 1), or severe white matter hyperintensities, reflected in large confluent lesions on CT/MRI (i.e., Fazekas grade 3; see Fazekas et al., 1987; n = 7), 21 patients with prior stroke, 30 patients with old (silent) infarcts on brain imaging, and 2 patients with recurrent stroke between brain imaging and neuropsychological examination. In the second step, we excluded 43 patients for whom no brain imaging was available (no follow‐up imaging in 24 patients, no ischemic lesion detected on follow‐up imaging in 19 patients). In the final step, we excluded 20 patients who had no data on the RAVLT, 12 patients who were not right‐handed, and one patient who performed far below chance on the recognition test (recognition score −11 [4/30 correct]). The application of these exclusion criteria resulted in the inclusion of 88 patients. This study was approved by the institutional review board of the University Medical Center Utrecht. Neuropsychological examinations and brain imaging were performed in the setting of standard clinical care.
Neuropsychological Assessment
Neuropsychological assessment was performed within 1 month after ischemic stroke (mean 7.6 days, range 1–30 days). We have previously demonstrated that the applied cognitive assessment battery is feasible and reliable in the acute stage (first days to weeks) of ischemic stroke [Nys et al., 2005]. Verbal memory was assessed with the Dutch version of the RAVLT, the Groningen Vijftienwoorden Test (Groningen Fifteen Words Test) [Brand and Jolles, 1985; Rey, 1958; Van der Elst et al., 2005]. Participants were presented with 15 common, monosyllabic words in auditory format. Directly after the presentation, they were asked to recall as many of the presented words as possible. The trial was repeated four more times, in which the same words were presented in identical order. Following the five consecutive trials, a battery of unrelated tests was conducted for approximately 20 min. After the delay, participants had again to recall the words of the initial test. This was immediately followed by a delayed recognition test: the 15 words of the initial test (target words) were mixed with 15 new words (distracter words) and the participants were asked to indicate for each word whether it was a target or not (yes/no). Educational level was divided into seven categories (scored according to [Verhage, 1964]) with scores ranging from unfinished primary school education (Category 1) to an academic degree (Category 7) according to the Dutch educational system).
Generation of Lesion Maps
Infarcts were manually delineated on transversal slices of either follow‐up CT (n = 59), or MRI scans (n = 29) by two trained raters. Slice thickness (i.e., voxel‐size along the z‐axis) ranged from 4–6 mm; the in‐plane voxel‐size (i.e., along x‐ and y‐axis) of the original CT and MRI scans was less than 1 mm in all cases. The infarct maps were registered to the T1 MNI‐152 (Montreal Neurological Institute) template utilizing a lesion‐masking approach [Brett et al., 2001; Fonov et al., 2009]. Registration of MRI images was performed using elastix; CT images were registered using an in‐house developed algorithm which is described elsewhere [Klein et al., 2010; Kuijf et al., 2013]. A detailed description of the generation, registration, and quality checks of the lesion maps is provided in the online supplementary methods.
Statistics
Performance on the recognition test of the RAVLT was used to calculate the following measure for recognition: number of correct hits minus the number of false positives, with a score of 0 indicating chance performance. This recognition measure was norm‐corrected for age, sex, and level of education, and transformed into z‐scores using norms that were obtained in a large cohort of healthy Dutch individuals [Van der Elst et al., 2005]. Performance below the fifth percentile was considered abnormal. As the focus of this study was on the mechanisms involved in recognition memory, we did not consider the preceding recall scores of the RAVLT.
To study recognition memory in more detail, we used a previously described nonparametric model that estimates discriminability and response bias based on the hit rate (H = number of correct hits/number of old items) and the false alarm rate (FA = number of false alarms/number of new items) [Donaldson, 1992; Snodgrass et al., 1985]. Discriminability (A′; varies from 0 to 1 with 0.5 indicating chance performance, and 1 indicating flawless performance) was calculated using the following formula: A′ = ½ + [(H – FA) (1 + H – FA)]/[4H (1 – FA)]. Response bias ( ; values >0 indicate conservative bias, values <0 indicate liberal bias) was calculated using the following formula: = [(1 – H) (1 – FA) – HFA]/[(1 – H) (1 – FA) + HFA]. For a detailed description of the theory behind these formulas, see Donaldson, 1992. This nonparametric model can reliably estimate discriminability and response bias when an individual has a hit rate of 1 or a false alarm rate of 0, whereas the parametric alternative to calculating discriminability (d′) and bias (C) cannot be used when a subject has a hit rate of 1 or a false alarm rate of 0. Several methods have been proposed to correct the parametric model for such floor and ceiling effects, although these do not resolve the issue entirely [Stanislaw and Todorov, 1999]. Because a significant proportion of patients had a hit rate of 1 or a false alarm rate of 0, we chose to use the nonparametric model in the current study. Discriminability and response bias were calculated for 83 out of 88 patients, because data on the hits and false alarms had not been registered for the remaining five patients. Discriminability and response bias were corrected for age, sex, and level of education using linear regression because no norms are available for these measures. Mean corrected z‐scores for discriminability and response bias were related to the location of ischemic lesions using a Student's t‐test.
Voxel‐based lesion‐symptom mapping (VLSM) was used to determine the relationship between verbal memory measures and the location of brain injury [Kimberg et al., 2007; Rorden and Karnath, 2004; Rorden et al., 2007]. One major advantage of this method over traditional approaches to lesion‐symptom mapping is that instead of grouping of patients with or without lesions in one or more pre‐defined areas of interest, it allows for assumption‐free calculation of association at each voxel. VLSM analyses were done on z‐scores for performance on the RAVLT using Non‐Parametric Mapping (most recent version, December 2012) [Rorden et al., 2007]. The Non‐Parametric Mapping software provides two tests for VLSM: the parametric t‐test and the nonparametric Brunner–Munzel (BM) statistic. Because the t‐test has higher power than the BM statistic in small sample sizes, and because the t‐test is particularly robust as it becomes conservative rather than liberal (i.e., reporting false alarms) when the underlying assumptions are violated, we chose to use the t‐test in our main analyses [Rorden et al., 2007]. We additionally performed a supplementary analysis using the BM statistic. Voxels affected by ischemic lesions in less than 3 patients were not considered for analysis (the threshold for the minimum number of patients with a lesion per voxel is arbitrary, but generally in the range of 3–5) [Biesbroek et al., 2013; Haramati et al., 2008; Knutson et al., 2014; Molenberghs and Sale, 2011; Schwartz et al., 2009; Thothathiri et al., 2012]. Correction for multiple testing was achieved using a false discovery rate (FDR) threshold with q < 0.05.
In the next step, we complemented the VLSM analysis with a region of interest‐based approach because these techniques have complementary strengths and weaknesses. Region of interest‐based analyses have limited power for detecting patterns that are only present in a subset of voxels in the region; VLSM is more sensitive for detecting such patterns due to its high spatial resolution. Conversely, VLSM requires a far greater number of statistical tests and should, therefore, be followed by correction for multiple testing, which reduces statistical power [Kimberg et al., 2007; Thothathiri et al., 2012]. Ideally, the discordance in anatomical correlates of discriminability and criterion setting should, therefore, be confirmed in the region of interest‐based analysis to rule out method‐dependent type 2 errors.
For this purpose, regions of interest for 90 cerebral cortical regions were extracted from the automatic anatomical labeling (AAL) atlas [Tzourio‐Mazoyer et al., 2002]. These 90 regions were projected on the VLSM results and the amount of voxels with a statistically significant correlation within each region was quantitatively assessed. Regions that appeared to be involved in discriminability or criterion setting (operationally defined as at least 5% of tested voxels having a statistically significant association between the presence of a lesion and performance, with a total of no less than 100 significant voxels) were selected and used to calculate regional infarct volume within these regions for every patient. These regional infarct volumes were entered as independent variables in a linear regression model with either discriminability or response bias as dependent variables, before and after adding total infarct volume to the model. The rationale behind adding infarct volume as a covariate was that brain regions that are crucial when performing a certain task should predict performance, independent of total infarct volume [Karnath et al., 2004].
RESULTS
Clinical characteristics of the study cohort are provided in Table 1. Twenty‐two out of 88 patients had impaired recognition. Impaired recognition was more common in patients with isolated left hemispheric lesions (11/30; 37%) than with isolated right hemispheric lesions (7/38; 18%; Table 2). Out of the 83 patients with complete data on the number of hits and false alarms, 24 patients had a hit rate of 1 (15/15 old items correctly identified), and 44 patients had a false alarm rate of 0 (no false alarms). Seventeen patients had perfect recognition performance (hit rate of 1 and a false alarm rate of 0). Mean corrected discriminability scores (A′) were lower in patients with isolated left hemispheric lesions (mean z‐score −0.29 (SD1.25)) than in patients with isolated right hemispheric lesions (mean z‐score 0.22 (SD 0.62); P = 0.05). In contrast, mean corrected response bias scores ( , low score indicates liberal bias) were lower in patients with right hemispheric lesions (mean z‐score −0.19 (SD 1.04) than in patients with left hemispheric lesions (mean z‐score 0.31 (SD 0.75); P = 0.04).
Table 1.
Characteristics of the study cohort
| Characteristics | Study cohort (n = 88) |
|---|---|
| Demographic characteristics | |
| Age, mean (SD) | 61.1 (13.5) |
| Male, n (%) | 55 (63) |
| Education, mean (SD)a | 5.0 (1.4) |
| Time interval between stroke and NPE in days, mean (SD; range) | 7.6 (5.2; 1–30) |
| RAVLT results (raw scores) | |
| Recognition, mean (SD; range) | 11.2 (3.8; −2 to 15) |
| Discriminability (A′), mean (SD; range)b | 0.92 (0.11; 0.38–1.00) |
| Bias ( ), median (range)b | 0.19 (−1.00 to 1.00) |
NPE: neuropsychological examination. RAVLT: Rey Auditory Verbal Learning Test.
Education scored according to Verhage scoring system (1: Did not finish primary school, 2: finished primary school, 3: did not finish secondary school, 4: finished secondary school, low level, 5: finished secondary school, medium level, 6: finished secondary school, highest level, and/or college degree, 7: university degree).
Based on 83 patients with data on the number of false and true positive and negative responses in the recognition test.
Table 2.
Location of ischemic lesion in relation to the presence of impaired verbal recognition memory
| Lesion location | ||||
|---|---|---|---|---|
| Left (n = 30) | Right (n = 38) | Infratentorial (n = 12) | Multiple (n = 8) | |
| Impaired recognition | ||||
| Yes (n = 22) | 11/30 (37%) | 7/38 (18%) | 1/12 (8%) | 3/8 (38%) |
| No (n = 66) | 19/30 (63%) | 31/38 (82%) | 11/12 (92%) | 5/8 (63%) |
Left: left cerebral hemisphere. Right: right cerebral hemisphere. Infratentorial: cerebellum and/or brainstem. Multiple: lesion located at multiple sites.
Voxel‐Based Lesion‐Symptom Mapping
The spatial distribution of infarcts is illustrated by the lesion prevalence map of the 88 included patients (Fig. 1). The lesion prevalence was highest for voxels in the right cerebral hemisphere in the vascular territory of the middle cerebral artery. Lesion frequency was relatively low for left hemispheric voxels. As a consequence, some left temporal, occipital, insular, inferior frontal, and thalamic regions could be included in the analyses while the remaining left hemispheric regions were lesioned in less than three patients and could not be included in the VLSM analyses (Fig. 1). VLSM identified clusters of voxels with a statistically significant association between the presence of a lesion and poor recognition and discriminability (entered as continuous variables; corrected for age, sex, and level of education). Poor recognition and discriminability were associated with lesions in the left medial temporal lobe and left temporo‐occipital structures (hippocampus, parahippocampal, inferior temporal, fusiform, lingual, inferior and medial occipital gyrus, and calcarine gyri), the left and right thalamus, and the right hippocampus. Liberal response bias (i.e., low ) was associated with lesions in the opercular part of the right inferior frontal gyrus. The VLSM results for recognition, discriminability, and response bias are provided in Figure 2. The number of significant voxels for each region is provided in Table 3.
Figure 1.
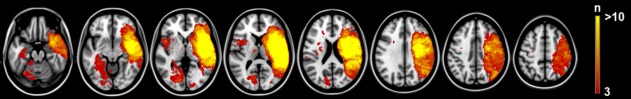
Distribution of ischemic lesions. Voxels that are damaged in at least three patients are projected on the 1 mm MNI‐152 template (Z coordinates: −20, −10, 0, 10, 20, 30, 40, 50). Bar indicates the number of patients with a lesion for each voxel. The right hemisphere is depicted on the right. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 2.
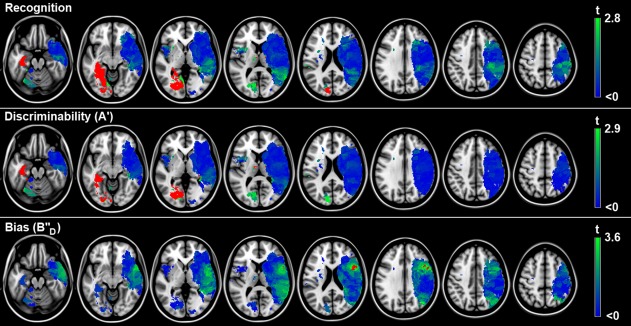
Voxel‐based lesion‐symptom mapping results. Map of the voxelwise association (t‐statistic) between the presence of a lesion and cognitive performance. Voxels exceeding the FDR threshold (q = 0.05) are rendered in red. Nonsignificant voxels are rendered on a scale from blue (t < 0) to bright green (t‐value just below threshold). Recognition was norm‐corrected for age, sex, and level of education; discriminability and bias were corrected for age, sex, and level of education using linear regression. Negative t‐values (meaning the presence of a lesion was associated with better cognitive performance or with a conservative bias) were not statistically significant. The results are projected on the MNI 1‐mm template (Z coordinates: −20, −10, 0, 10, 20, 30, 40, 50). The right hemisphere is depicted on the right. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 3.
Voxel‐based lesion‐symptom mapping results for discriminability and criterion setting: tested and significant voxels for each AAL region
| Anatomical regions (AAL atlas) | Patients with lesion (n)a | Region size in voxels (n) | Tested voxels (n) | Significant voxels criterion setting (n [%]) | Significant voxels discriminability (n [%]) |
|---|---|---|---|---|---|
| Inferior frontal opercular R | 25 | 11174 | 9385 | 868 (9.25) | 0 |
| Precentral R | 24 | 27058 | 14536 | 185 (1.27) | 0 |
| Rolandic operculum R | 26 | 10733 | 10025 | 30 (0.30) | 0 |
| Inferior frontal triangular R | 20 | 17132 | 8863 | 17 (0.19) | 0 |
| Superior temporal pole R | 20 | 10654 | 6266 | 6 (0.10) | 0 |
| Middle temporal R | 18 | 35484 | 28161 | 16 (0.06) | 0 |
| Superior temporal R | 25 | 25258 | 22465 | 7 (0.03) | 0 |
| Insula R | 31 | 14128 | 14091 | 2 (0.01) | 0 |
| Hippocampus L | 7 | 7469 | 850 | 0 | 850 (100) |
| Parahippocampal L | 4 | 7891 | 843 | 0 | 843 (100) |
| Fusiform L | 9 | 18333 | 3875 | 0 | 3875 (100) |
| Inferior temporal L | 5 | 25647 | 514 | 0 | 514 (100) |
| Inferior occipital L | 5 | 7536 | 1207 | 0 | 1174 (97.27) |
| Lingual L | 12 | 16932 | 4542 | 0 | 4185 (92.14) |
| Medial occipital L | 8 | 25989 | 455 | 0 | 392 (86.15) |
| Thalamus L | 9 | 8700 | 324 | 0 | 100 (30.86) |
| Calcarine L | 10 | 18157 | 3029 | 0 | 655 (21.62) |
| Hippocampus R | 17 | 7606 | 1934 | 0 | 106 (5.48) |
| Thalamus R | 13 | 8399 | 1587 | 0 | 40 (2.52) |
| Parahippocampal R | 7 | 9028 | 289 | 0 | 4 (1.38) |
R: right. L: left. AAL regions in which no significant voxels were observed for either discriminability or criterion setting are not shown.
Indicates how many of the 83 patients had a lesion (≥1 voxel) within the specified region of interest.
Region of Interest‐Based Analyses
Next, we analyzed the impact of lesion volumes in specific cortical regions of interest (Table 4). These regions were selected based on the VLSM results to reproduce their involvement and quantify the impact of regional lesion volumes on discriminability and criterion setting. Age, sex, and level of education explained 9% of variance in discriminability and only 3% of variance in response bias. Infarct volume within the left hippocampus, parahippocampal, inferior temporal, fusiform, lingual, inferior and medial occipital, and calcarine gyri, and the left thalamus inversely correlated with discriminability. The increase in explained variance in discriminability was highest for lesion volume within the left inferior occipital gyrus (additional explained variance of 37%; Table 4). Infarct volume within the opercular part of the right inferior frontal gyrus inversely correlated with response bias and explained an additional 11% of variance. The results of the linear regression analyses remained essentially the same after additional adjustment for total infarct volume (Supporting Information Table 1).
Table 4.
Results of linear regression models with z‐scores of cognitive performance as outcome
| Discriminability (A′) | Response bias ( ) | ||||||
|---|---|---|---|---|---|---|---|
| Model | Independent variables | R 2 | P ΔR 2 | B (95% CI) | R 2 | P ΔR 2 | B (95% CI) |
| 1 | Age, sex, level of education | 0.09 | 0.053 | — | 0.03 | 0.516 | — |
| 2 | Model 1 + total IV | 0.11 | 0.169 | −0.00 (−0.01 to 0.00) | 0.06 | 0.110 | −0.00 (−0.01 to 0.00) |
| 3a | Model 1 + IV R inferior frontal opercular part | 0.09 | 0.826 | 0.01 (−0.09 to 0.11) | 0.14 | 0.002 | −0.15 (−0.24 to −0.06) |
| 3b | Model 1 + IV L hippocampus | 0.45 | <0.001 | −0.85 (−1.09 to −0.61) | 0.03 | 0.848 | −0.03 (−0.35 to 0.29) |
| 3c | Model 1 + IV L parahippocampal gyrus | 0.45 | <0.001 | −0.86 (−1.11 to −0.62) | 0.03 | 0.884 | −0.02 (−0.35 to 0.30) |
| 3d | Model 1 + IV L fusiform gyrus | 0.43 | <0.001 | −0.29 (−0.38 to −0.21) | 0.03 | 0.900 | −0.01 (−0.12 to 0.11) |
| 3e | Model 1 + IV L lingual gyrus | 0.42 | <0.001 | −0.33 (−0.42 to −0.23) | 0.03 | 0.854 | 0.01 (−0.12 to 0.14) |
| 3f | Model 1 + IV L inferior temporal gyrus | 0.42 | <0.001 | −0.30 (−0.39 to −0.21) | 0.03 | 0.989 | 0.00 (−0.12 to 0.12) |
| 3g | Model 1 + IV L inferior occipital gyrus | 0.46 | <0.001 | −1.19 (−1.52 to −0.86) | 0.03 | 0.897 | −0.03 (−0.47 to 0.41) |
| 3h | Model 1 + IV L medial occipital gyrus | 0.19 | 0.003 | −0.69 (−1.13 to −0.24) | 0.03 | 0.779 | −0.07 (−0.56 to 0.42) |
| 3i | Model 1 + IV L Calcarine gyrus | 0.22 | 0.001 | −0.35 (−0.55 to −0.16) | 0.03 | 0.650 | −0.05 (−0.27 to 0.17) |
| 3j | Model 1 + IV L thalamus | 0.17 | 0.009 | −0.62 (−1.08 to −0.16) | 0.05 | 0.229 | −0.30 (−0.79 to 0.19) |
| 3k | Model 1 + IV R hippocampus | 0.12 | 0.100 | −0.40 (−0.88 to 0.08) | 0.06 | 0.128 | −0.39 (−0.88 to 0.11) |
The explained variance (R 2) in discriminability and response bias is given for each model with the corresponding P‐value for the difference in explained variance (P ΔR 2) between the model and the previous model. Unstandardized coefficients (B) with corresponding 95% CIs are provided. The unstandardized coefficient applies to the change in z‐score for every 1 ml increase in infarct volume. IV: infarct volume. R: right. L: left. Low response bias corresponds with liberal bias.
Nonparametric Analyses
It should be noted that the distribution of z‐scores for recognition, discriminability, and response bias were skewed, as is often the case when using cognitive performance of stroke patients as the dependent variable (e.g., skewness for the residuals of the multivariate model in which we corrected raw scores for age, sex and level of education was −2.6 (SE 0.26) for discriminability and −0.41 (SE 0.26) for response bias). To assess the robustness of our findings, we therefore also performed two nonparametric analyses: a lesion‐subtraction analysis and a voxel‐wise BM statistic. The qualitative lesion‐subtraction analysis using dichotomized measures of discriminability (z‐score below or above group mean) and response bias (liberal versus neutral or conservative) reproduced our main finding of distinct anatomical correlates for discriminability (left temporo‐occipital regions and left thalamus) and criterion setting (right frontal regions; Fig. 3). The results of the nonparametric BM statistic (Supporting Information Fig. 2) also essentially showed the same pattern as the t‐test results (Fig. 2), although in this less sensitive BM analysis the association remained statistically significant in only a few voxels after correction for multiple testing.
Figure 3.
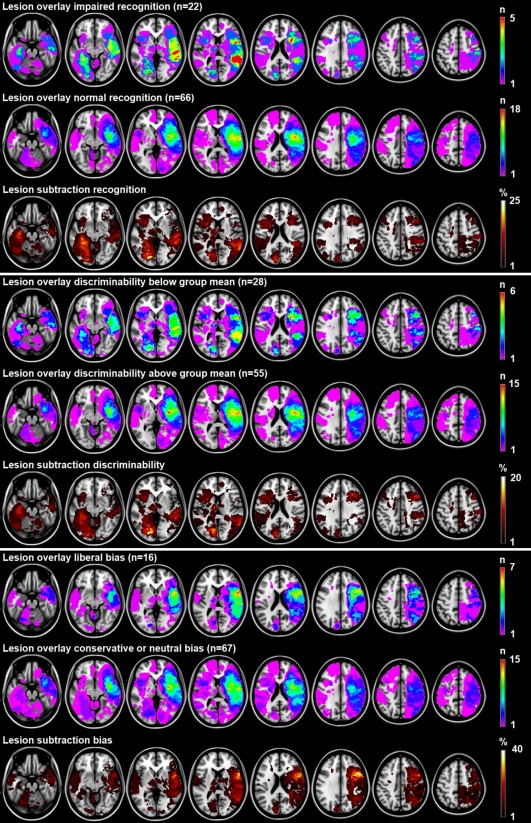
Lesion subtraction analyses. Lesion overlay and subtraction plots of dichotomized measures of recognition (impaired yes/no based on previously described norms), discriminability (impaired defined as performance below group mean because norms are not available), and response bias (liberal versus neutral or conservative). The overlay plots show the number of patients with a lesion for a given voxel separately for patients with impaired and normal performance. The lesion subtraction plots show which voxels are more frequently affected in patients with impaired performance compared to patients with normal performance. For example, the recognition overlay plots show that 4 out of 22 (18%) patients with impaired recognition have a lesion in the left hippocampus, whereas none of the 66 (0%) patients with normal recognition have a lesion in the left hippocampus. The lesion subtraction plot shows the resulting 18% difference in lesion prevalence. This finding suggests a crucial role of the left hippocampus in recognition. Because dichotomization of performance results in a decrease in statistical power and does not account for severity of the deficit, we chose to use the continuous outcome (analyzed with t‐test) in our main analyses. These qualitative lesion subtraction analyses (lesion subtraction does not provide measures of statistical significance) are presented here to assess the robustness of our results. The results are essentially the same: left medial temporal and temporo‐occipital structures were most consistently damaged in patients with impaired recognition and discriminability and spared in patients with normal recognition and discriminability (maximum difference in lesion density of 23% and 21%, respectively). The right inferior frontal gyrus was most consistently damaged in patients with liberal bias (maximum difference in lesion density of 36%). The right hemisphere is depicted on the right. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
In this study, we determined the anatomical correlates of cognitive processes underlying verbal recognition memory by performing lesion‐symptom mapping in patients with first‐ever ischemic stroke. Our findings indicate that the left medial temporal lobe, left temporo‐occipital structures, both thalami, and the right hippocampus are crucial structures underlying discriminability, while the right inferior frontal gyrus plays a crucial role in criterion setting. More specifically, lesions in the right inferior frontal gyrus are associated with liberal response bias but not with impaired discriminability.
The main strengths of this study are the substantial sample size, the assumption‐free nature of the analyses (as opposed to hypothesis‐driven analyses, in which the analyses are focused on predefined regions of interest), and the application of quantitative voxel‐wise analyses that provide high spatial resolution.
Our findings regarding involvement of the left medial temporal lobe, right hippocampus, and both thalami in verbal recognition memory corroborate and extend previous findings [Wixted and Squire, 2010; Yonelinas, 2002]. Functional studies have suggested that the parietal cortices might be involved in recognition memory, although lesion studies have never been able to confirm these fMRI findings [Schoo et al., 2011; Vilberg and Rugg, 2008]. We found no associations between right parietal lesions and poor recognition, which is in line with these previous findings. Regarding the previously assumed role of the frontal lobe in recognition, we have demonstrated that the inferior frontal gyrus is involved in criterion setting while frontal structures do not appear to be involved in discriminability.
Based on several case reports and lesion studies with relatively small sample size, it has previously been suggested that the frontal lobes play an important role in criterion setting [Haramati et al., 2008; Melo et al., 1999; Parkin et al., 1996; Schacter et al., 1996; Verfaellie et al., 2004]. Our results provide statistical evidence for the previously assumed crucial role of the right frontal lobe in criterion setting, and further localize this function in the inferior frontal gyrus. To our knowledge, this study is the first to demonstrate a crucial role of the right inferior frontal gyrus in criterion setting in verbal recognition memory. Criterion setting depends on context (instructional motivation, proportion of old test items) and is to some extent subject to trait‐like variability, as introvert persons tend to use more conservative recognition criteria than extroverts as they exercise greater “response cautiousness” [Kantner and Lindsay, 2012; McNally et al., 2009]. Pathological processes that are known to influence response bias typically lead to extremely liberal bias (resulting in false recognition); to our knowledge there have been no reports of patients with extremely conservative bias following brain damage. It has been hypothesized that false recognition may be induced by several conditions: (1) partial memory for the study lists, (2) the inability to extract the semantic gist of the list, and (3) defective strategic monitoring of cognitive processes, which is assumed to depend on frontal structures in particular and may result in a false sensation of familiarity [Melo et al., 1999]. Our findings indicate that discriminability and criterion setting have separate anatomical correlates. Thus, our results suggest that patients with medial temporal, temporo‐occipital, or thalamic lesions may show false recognition due to impaired discriminability (reflecting condition 1 and 2), whereas patients with isolated frontal lesions might show false recognition due to liberal bias (reflecting condition 3).
Despite the fact that we have used well accepted lesion‐symptom mapping techniques that are commonly used, some challenges and limitations that are inherent to these techniques should be taken into account. These include how deal with low lesion frequencies in certain anatomical regions, skewed data, and the problem of identical origins. We will discuss these limitations in the following section. First, a potential limitation of this study is the relatively low lesion frequency in the left cerebral hemisphere (despite the substantial number of patients with left hemispheric lesions). This is explained by the fact that neuropsychological examination is not always feasible in patients with severe global aphasia, especially when applying tests that require processing of verbal information. Most voxels in the left cerebral hemisphere were therefore not included in the VLSM analysis. Due to this limitation, we cannot draw any conclusions regarding the involvement of the left frontal lobe in criterion setting in verbal recognition memory. Similarly, we cannot draw any strong conclusions regarding the role of left parietal and frontal structures in discriminability. Second, there was some skewing of measures for discriminability and response bias (see results section). Our main findings (Fig. 2) were supported by the results of the qualitative lesion‐subtraction analysis (Fig. 3). Moreover, the results of the supplementary analysis using the nonparametric BM statistic showed essentially the same pattern as the t‐test results (Supporting Information Fig. 2). However, in this analysis most of the voxels lost statistical significance after correction for multiple testing which is explained by the fact that the BM statistic has lower power than the t‐test when applied to voxels with less than 10 patients with a lesion [Rorden et al., 2007]. Finally, the region of interest‐based analyses indicated that infarct volume within the regions that were identified by the t‐test inversely correlated with discriminability and response bias, independent of total infarct size. The converging findings of these different analytical approaches indicate that our findings are robust, although the point estimates and P‐values of the regression coefficients that are provided by the linear regression model should be interpreted with some caution. Third, we cannot rule out that the observed correlates of discriminability in left occipital structures might in fact be driven by hippocampal damage, because both regions are vascularized by the posterior cerebral artery and were, therefore, frequently lesioned together (i.e., were intercorrelated), as is shown in Supporting Information Figure 3. This limitation, which is referred to as the problem of identical origins, is inherent to VLSM studies, especially when lesion frequency is low. However, it should be noted that the differences in anatomical correlates of discriminability and criterion setting as observed in the current study cannot be attributed to this problem of identical origins. New methods for lesion‐symptom mapping are currently emerging [Mah et al., 2014]. These new methods seek to resolve the above raised issues regarding the problem of identical origins by constructing “models using high‐dimensional interference that captures the multivariate lesion distribution, explicitly modeling the voxel‐voxel associations that are the source of the error” [Mah et al., 2014]. However, such models require hundreds to thousands of cases. As such, these new and more sophisticated methods could unfortunately not be applied in this study. Fourth, we used both CT and MRI scans for lesion segmentation, which is not uncommon in lesion‐symptom mapping studies [Karnath et al., 2004; Robinson et al., 2012; Schwartz et al., 2009; Thothathiri et al., 2012; Theys et al., 2013]. Both modalities allow for accurate detection of the location on the ischemic lesion. However, the boundary of the lesion might be drawn differently between modalities. In addition, this boundary is also influenced by the elapsed time between stroke onset and CT/MRI scan acquisition. The variability in lesion segmentation could be minimized by applying a single scan modality in a certain time window (e.g., MRI acquired 48–72 h after stroke onset). However, we chose for a robust design including as many patients as possible (with either CT or MRI scans) to optimize statistical power while accepting some heterogeneity in scan acquisition. It should be noted that the marked differences in anatomical correlates of discriminability and criterion setting cannot be attributed to slight variability in the segmentation of lesion boundaries. Finally, it should be noted that the precision of the VLSM results is determined by the resolution of the original CT and MRI scans. The in‐plane voxel‐size (i.e., along x‐ and y‐axis) of the original CT and MRI scans was less than 1 mm in all cases, but slice thickness (i.e., voxel‐size along the z‐axis) ranged from 4–6 mm, resulting in lower precision in that direction.
There are some considerations that should be taken into account when interpreting the findings of the current study. First, it should be noted that due to the applied inclusion criteria, the prevalence of cognitive impairment in the study group might not reflect the prevalence in the overall stroke population. However, our aim was not to assess the prevalence of disturbances in recognition memory following acute ischemic stroke but to determine the anatomical correlates of recognition memory by relating lesion location to variance in performance on recognition tasks within the study group. Importantly, the variance in discriminability and response bias in the patient group was high, which increases the power for correlational analysis (Table 1). Second, a further distinction in processes that underlie discriminability has been proposed based on the introspective awareness state of the memory holder, contrasting conscious recollection with familiarity [Berry et al., 2008]. Since we have no data on whether our recognition responses were based on recollection or familiarity, we could not address this distinction here. Finally, when comparing the findings of our study with prior work, it should be kept in mind that we studied patients in the acute phase of ischemic stroke. Neuropsychological examination was performed within 30 days after ischemic stroke and the majority of scans were acquired in the acute phase as well. Therefore, the observed relations between lesion location and measures of recognition memory apply to the acute phase. In some cases, the extent of ischemic lesions on diffusion weighted imaging might be overestimated in the acute phase compared to imaging in the chronic phase [Jauch et al., 2013]. Furthermore, a prior study in which the prevalence of post‐stroke cognitive impairment was assessed has shown that more than half of the patients with verbal memory impairments in the acute phase after stroke (within 3 weeks) will have recovered in the chronic phase (after 6–10 months) [Nys et al., 2005]. As such, it is possible that the anatomical correlates of discriminability and criterion setting might change in more chronic stages.
In conclusion, this study demonstrated a crucial role of left hemispheric medial temporal and temporo‐occipital regions, both thalami, and the right hippocampus in discriminability in verbal recognition memory while criterion setting depends on the inferior frontal gyrus. Lesions in the right inferior frontal gyrus are associated with liberal response bias but not with impaired discriminability. These findings indicate that discriminability and criterion setting depend on distinct anatomical structures and provide new insights in the anatomical correlates of these cognitive processes that underlie verbal recognition memory.
Supporting information
Supplementary Information
Supplementary Information Figures.
Supplementary Information Table1.
ACKNOWLEDGMENTS
We gratefully acknowledge Nick A. Weaver, and Pieter C. Vos for their help in generating the lesion maps. The authors thank Marco Duering, from the Institute for Stroke and Dementia Research of the Klinikum der Universität München, for his advice on the analytical approach. Furthermore, we would like to acknowledge Nathan Van der Stoep, Haike van Stralen, and Sophie Heringa for performing the neuropsychological examinations, and the members of the Vascular Cognitive Impairment Study group of the University Medical Center Utrecht. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- Berry CJ, Shanks DR, Henson RN (2008): A unitary signal‐detection model of implicit and explicit memory. Trends Cogn Sci 12:367–373. [DOI] [PubMed] [Google Scholar]
- Biesbroek JM, Kuijf HJ, van der Graaf Y, Vincken KL, Postma A, Mali WP, et al, (2013): Association between subcortical vascular lesion location and cognition: a voxel‐based and tract‐based lesion‐symptom mapping study. The SMART‐MR study. PLoS One 8:e60541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand N, Jolles J (1985): Learning and retrieval rate of words presented auditorily and visually. J Gen Psychol 112:201–210. [DOI] [PubMed] [Google Scholar]
- Brett M, Leff AP, Rorden C, Ashburner J (2001): Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage 14:486–500. [DOI] [PubMed] [Google Scholar]
- Brown MW, Warburton EC, Aggleton JP (2010): Recognition memory: Material, processes, and substrates. Hippocampus 20:1228–1244. [DOI] [PubMed] [Google Scholar]
- Donaldson W (1992): Measuring recognition memory. J Exp Psychol Gen 121:275–278. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA (1987): MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol 149:351–356. [DOI] [PubMed] [Google Scholar]
- Fonov V, Evans A, McKinstry R, Almli C, Collins D (2009): Unbiased nonlinear average age‐appropriate brain templates from birth to adulthood. Neuroimage 47:S102. [Google Scholar]
- Haramati S, Soroker N, Dudai Y, Levy DA (2008): The posterior parietal cortex in recognition memory: A neuropsychological study. Neuropsychologia 46:1756–1766. [DOI] [PubMed] [Google Scholar]
- Jacoby LL, Shimizu Y, Daniels KA, Rhodes MG (2005): Modes of cognitive control in recognition and source memory: Depth of retrieval. Psychon Bull Rev 12:852–857. [DOI] [PubMed] [Google Scholar]
- Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, et al, (2013): Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 44:870–947. [DOI] [PubMed] [Google Scholar]
- Kantner J, Lindsay DS (2012): Response bias in recognition memory as a cognitive trait. Mem Cogn 40:1163–1177. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Berger MF, Kueker W, Rorden C (2004): The anatomy of spatial neglect based on voxelwise statistical analysis: A study of 140 patients. Cereb Cortex 14:1164–1172. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, Coslett HB, Schwartz MF (2007): Power in voxel‐based lesion‐symptom mapping. J Cogn Neurosci 19:1067–1080. [DOI] [PubMed] [Google Scholar]
- Klein S, Staring M, Murphy K, Viergever MA, Pluim JP (2010): Elastix: A toolbox for intensity‐based medical image registration. IEEE Transactions on Medical Imaging 29:196–205. [DOI] [PubMed] [Google Scholar]
- Knutson KM, Monte OD, Raymont V, Wassermann EV, Krueger F, Grafman J (2014): Neural correlates of apathy revealed by lesion mapping in participants with traumatic brain injuries. Hum Brain Mapp 35:943–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijf HJ, Biesbroek JM, Viergever MA, Biessels GJ, Vincken KL (2013): Registration of brain CT images to an MRI template for the purpose of lesion‐symptom mapping. In Multimodal Brain Image Analysis, Lecture Notes in Computer Science. Springer International Publishing, Vol. 8159. pp 119–128.
- Mah YH, Husain M, Rees G, Nachev P (2014): Human brain lesion‐deficit inference remapped. Brain 137: 2522–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitsch HJ. 2008. Anterograde amnesia In: Goldenberg G, Miller B, editors. Handbook of Clinical Neurology. Amsterdam: Elsevier Press; pp 160–162. [Google Scholar]
- McNally KA, Schefft BK, Szaflarski JP, Howe SR, Yeh HS, Privitera MD (2009): Application of signal detection theory to verbal memory testing to distinguish patients with psychogenic nonepileptic seizures from patients with epileptic seizures. Epilepsy Behav 14:597–603. [DOI] [PubMed] [Google Scholar]
- Melo B, Winocur G, Moscovitch M (1999): False recall and false recognition: An examination of the effects of selective and combined lesions to the medial temporal lobe/diencephalon and frontal lobe structures. Cogn Neuropsychol 16:343–359. [Google Scholar]
- Molenberghs P, Sale MV (2011): Testing for spatial neglect with line bisection and star cancellation: Are both tasks really unrelated? PLoS One 6:e23017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nys GM, van Zandvoort MJ, de Kort PL, van der Worp HB, Jansen BP, Algra A, de Haan EH, Kappelle LJ (2005): The prognostic value of domain‐specific cognitive abilities in acute first‐ever stroke. Neurology 64:821–827. [DOI] [PubMed] [Google Scholar]
- Parkin AJ, Blindschaedler C, Harsent L, Metzler C (1996): Pathological false alarm rates following damage to the left frontal cortex. Brain Cogn 32:14–27. [DOI] [PubMed] [Google Scholar]
- Rey A (1958): L'examin clinique en psychologie. Paris, France: Presses Universitaires de France. [Google Scholar]
- Robinson G, Shallice T, Bozzali M, Cipolotti L (2012): The differing roles of the frontal cortex in fluency tests. Brain 135:2202–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Karnath HO (2004): Using human brain lesion to infer function: A relic from a past era in the fMRI age? Nat Rev Neurosci 5:813–819. [DOI] [PubMed] [Google Scholar]
- Rorden C, Bonilha L, Nichols TE (2007): Rank‐order versus mean based statistics for neuroimaging. Neuroimage 35:1531–1537. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Viskontas IV (2008): Cortical neuroanatomy and cognition In: Goldenberg G, Miller B, editors. Handbook of Clinical Neurology. Amsterdam: Elsevier Press; pp 48–50. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Curran T, Galluccio L, Milberg WP, Bates JF (1996): False recognition and the right frontal lobe: A case study. Neuropsychologia 34:793–808. [DOI] [PubMed] [Google Scholar]
- Schoo LA, van Zandvoort MJ, Biessels GJ, Kappelle LJ, Postma A, de Haan EH (2011): The posterior parietal paradox: Why do functional magnetic resonance imaging and lesion studies on episodic memory produce conflicting results? J Neuropsychol 5:15–38. [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Kimberg DY, Walker GM, Faseyitan O, Brecher A, Dell GS, Coslett HB (2009): Anterior temporal involvement in semantic word retrieval: Voxel‐based lesion‐symptom mapping evidence from aphasia. Brain 132:3411–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass JG, Levy‐Berger G, Haydon M (1985): Human Experimental Psychology. New York: Oxford University Press. [Google Scholar]
- Stanislaw H, Todorov N (1999): Calculation of signal detection theory measures. Behav Res Methods Instrum Comput 31:137–149. [DOI] [PubMed] [Google Scholar]
- Theys C, De Nil L, Thijs V, van Wieringen A, Sunaert S (2013): A crucial role for the cortico‐striato‐cortical loop in the pathogenesis of stroke‐related neurogenic stuttering. Hum Brain Mapp 34:2103–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thothathiri M, Kimberg DY, Schwartz MF (2012): The neural basis of reversible sentence comprehension: Evidence from voxel‐based lesion‐symptom mapping in aphasia. J Cogn Neurosci 24:212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Van der Elst W, van Boxtel MP, van Breukelen GJ, Jolles J (2005): Rey's verbal learning test: Normative data for 1855 healthy participants aged 24–81 years and the influence of age, sex, education, and mode of presentation. J Int Neuropsychol Soc 11:290–302. [DOI] [PubMed] [Google Scholar]
- van Strien NM, Cappaert NL, Witter MP (2009): The anatomy of memory: An interactive overview of the parahippocampal‐hippocampal network. Nat Rev Neurosci 10:272–282. [DOI] [PubMed] [Google Scholar]
- Vann SD, Tsivilis D, Denby CE, Quamme JR, Yonelinas AP, Aggleton JP, Montaldi D, Mayes AR (2009): Impaired recollection but spared familiarity in patients with extended hippocampal system damage revealed by 3 convergent methods. Proc Natl Acad Sci USA 106:5442–5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verfaellie M, Rapcsak SZ, Keane MM, Alexander MP (2004): Elevated false recognition in patients with frontal lobe damage is neither a general nor a unitary phenomenon. Neuropsychology 18:94–103. [DOI] [PubMed] [Google Scholar]
- Verhage F (1964): Intelligence and age (in Dutch). Assen: Van Gorcum. [Google Scholar]
- Vilberg KL, Rugg MD (2008): Memory retrieval and the parietal cortex: A review of evidence from a dual‐process perspective. Neuropsychologia 46:1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wixted JT (2007): Dual‐process theory and signal‐detection theory of recognition memory. Psychol Rev 114:152–176. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Squire LR (2010): The role of the human hippocampus in familiarity‐based and recollection‐based recognition memory. Behav Brain Res 215:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP (2002): The nature of recollection and familiarity: A review of 30 years of research. J Mem Lang 46:441–517. [Google Scholar]
- Yonelinas AP, Aly M, Wang WC, Koen JD (2010): Recollection and familiarity: Examining controversial assumptions and new directions. Hippocampus 20:1178–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Information Figures.
Supplementary Information Table1.


