Abstract
Very preterm (PT) birth (≤32 weeks of gestation) carries a high risk for an adverse neurodevelopmental outcome. In recent years, the importance of neurocognitive deficits in the language domain has been increasingly recognized, which can be well‐characterized using neuropsychological testing and noninvasive imaging approaches. We compared former early PT born children and adolescents (PT, n = 29, 20M) and typically developing children (TD, n = 19, 7M), using conventional fMRI group analyses as well as functional connectivity analyses. We found only small regions with significantly different group activation (PT > TD) but significantly stronger connectivity between superior temporal lobe (STL) language regions in TD participants. There were also significant differences in local and global network efficiency (TD > PT). Surprisingly, there was a stronger connectivity of STL regions with non‐STL regions both intrahemispherically and interhemispherically in PT participants, suggesting the coexistence of reduced and increased connectivity in the language network of former PTs. Very similar results were obtained when using task‐based versus resting state functional connectivity approaches. Finally, lateralization of functional connectivity correlated with verbal comprehension abilities, suggesting that a more bilateral language comprehension representation is associated with better performance. Our results underline the importance of interhemispheric crosstalk for language comprehension. Hum Brain Mapp 35:3372–3384, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: prematurity, language impairment, functional magnetic resonance imaging, task‐based functional connectivity, resting state functional connectivity
INTRODUCTION
Prematurity, that is, birth before the 37th week of gestation, is associated with a high risk of an unfavorable neurodevelopmental outcome. This is particularly true for children born very preterm (PT), or with very low birthweight (≤32 weeks of gestation, ≤1500 g birth weight). In this group in particular, the relevance of neurocognitive impairments (in addition to more overt neurological impairment such as cerebral palsy) is increasingly recognized: they contribute significantly to the long‐term prognosis of the former PTs [Allen, 2008; Doyle and Anderson, 2010; Latal, 2009; Marlow, 2004; SCPE, 2000; Zwicker and Harris, 2008].
Language is increasingly recognized to feature consistently among the cognitive domains impaired in former early PTs, with both expressive and receptive language being affected. As a complex cognitive process, understanding and producing language is represented not only in distinct nodes, but in complex networks in the brain. These nodes and networks are increasingly well‐described, not least because language can be assessed comprehensively using both neuropsychological testing and non‐invasive imaging approaches; language may therefore serve as a model to further understand early adverse interference with normal brain development. Language networks are known to undergo developmental changes, and an impairment of the connections underlying these networks must be expected to underlie at least some of the observable difficulties in language disorders [Barre et al., 2011; Bitan et al., 2010; Friederici et al., 2011; Luu et al., 2011; Reidy et al., 2013; Staudt, 2010]. Using functional imaging, previous studies have shown differences in connectivity between distinct language regions between former PTs and typically developing (TD) children. In these studies, increases in functional connectivity (i.e., higher temporal coherence in timecourses between brain regions) were found between distinct language regions in former PTs, interpreted to reflect a compensatory mechanism [Constable et al., 2013; Gozzo et al., 2009]. In contrast to this, a recent study investigated the structural connectivity between temporal language regions in a large cohort of former early PTs and TD children, using diffusion MRI. This group reported decreased structural connectivity between both hemispheres in former PTs, which was predicted by the individual degree of language impairment [Northam et al., 2012]. Previous studies also support the notion of altered structural connectivity in PT‐born adolescents [Mullen et al., 2011]. These seemingly contradictory findings (increased functional, but decreased structural connectivity) are at present difficult to reconcile.
In this study, we therefore aimed to assess functional connectivity between language regions in former very early PTs and TD children and adolescents. Specifically, we were interested in investigating whether evidence for both increased and decreased connectivity can be found, and whether possible group differences are detectable using traditional task‐based as well as resting state functional magnetic resonance imaging (fMRI) data for connectivity analyses.
SUBJECTS AND METHODS
Former very early PT were recruited as a follow‐up from previous studies on the effect of early postnatal human cytomegalovirus infection [Bevot et al., 2012; Goelz et al., 2013; Maschmann et al., 2001; Neuberger et al., 2006]. As such, all were born in or before the 32nd week of pregnancy, constituting early prematurity, and all but 2 were born at <1500 g, constituting very‐low birthweight. TD children and adolescents were recruited by public announcements; in the course of the study, a small number of participants were recruited by word of mouth. For both groups, standard MR contraindications applied; in addition, TD participants were excluded if there was a personal history of neurological/psychiatric disorders, perinatal infection, hearing or vision deficits (other than refraction anomalies), or prematurity. Overall, data from 56 subjects was acquired, but imaging data from 8 participants had to be discarded due to subject motion (see below). This left data from 48 children and adolescents for analyses, 19 TD (12F; mean age = 12.84 ± 2.03 years) and 29 PT (9F; mean age = 14.66 ± 1.24 years). Further demographic data of these children are listed in Table 1. Parents of all participants provided written informed consent, and all participants verbally assented. Procedures were in accordance with the ethical regulations set forth in the declaration of Helsinki in its latest version, and the ethics committee of the medical faculty of the Eberhard Karls University Tübingen approved the study.
Table 1.
Demographic data from all participants
| TD participants | Early PT born participants | P‐value | |
|---|---|---|---|
| Age (years) | 12.84 ± 2.03 | 14.66 ± 1.24 | 0.002a |
| Gender composition | 7 M, 12 F | 20 M, 9 F | 0.028b |
| Handedness Score (EHI) | 0.66 ± 0.55 | 0.55 ± 0.6 | n.s.a |
| MEL (years) | 14.16 ± 3.33 | 12.62 ± 2.72 | n.s.a |
| Verbal comprehension | 112.53 ± 10.43 | 100.1 ± 19.4 | 0.015c |
| Gestational age (weeks) | – | 28.27 ± 1.97 | – |
| Birth weight (g) | – | 1130 ± 303 | – |
| Postnatal hCMV | – | 11/29 | – |
−, Data not available or test not meaningful; EHI, Edinburgh handedness inventory score.
Wilcoxon signed rank test.
Chi‐square test.
Student's t‐test.
Subject Characterization
All TD participants were born at term (>37 weeks of gestation); for the PT participants, gestational age, and birth weight were drawn from clinical charts. For assessing receptive language abilities, the verbal comprehension subscale of the HAWIK‐IV [Petermann and Petermann, 2007], the German version of the Wechsler Intelligence Scale for Children, was used. Subject handedness was assessed using the Edinburgh Handedness Inventory (EHI) [Oldfield, 1971], which is well‐applicable also in children [Wilke et al., 2008]. PT subjects were assessed regarding the presence of cerebral palsy [SCPE, 2000] and characterized using the bimanual fine motor function rating scale (BMFM) [Beckung and Hagenberg, 2002] and the gross motor function classification system (GMFCS) [Palisano et al., 1997]. Both scales range from 0 (no impairment) to V (severe impairment); any occurrence of a score of I–V was considered pathological. Finally, maternal education level (MEL) was assessed in years of maternal education, as this was shown to be of substantial relevance for the neurocognitive outcome in former PTs [Voss et al., 2012].
MR‐Imaging and Data Preprocessing
Children were imaged on a 1.5 T MR‐scanner (Siemens Avanto, Erlangen, Germany), using a 12‐channel head coil. For functional series, echoplanar imaging (EPI, 40 axial slices of 3 mm thickness, no gap, matrix = 64 × 64, yielding a voxel size of 3 × 3 × 3 mm3 with TR/TE = 3000/40 ms) was used to acquire 110 volumes in 5:30 min in an interleaved fashion. From all studies, the first ten image volumes were removed to allow for the stabilization of longitudinal magnetization, leaving 100 images for analyses. For data processing, a structural T1‐weighted 3D‐data set was also acquired (176 sagittal slices of 1 mm thickness, no gap, matrix = 256 × 256, yielding a voxel size of 1 × 1× 1 mm3 with TR/TE = 1300/2.92 ms). All images were read by an experienced pediatric neuroradiologist for incidental findings as well as for signs of early brain injury (such as enlarged ventricles or white matter abnormalities indicative of periventricular leucomalacia) [Volpe, 2009]. Additionally, a gradient‐echo B0‐fieldmap was acquired (with the same resolution and slice prescription as the functional series and with TR/TE1|2 = 546/5.19|9.95 ms).
All processing and analysis steps were done employing functionality available within SPM8 (Wellcome Trust Centre for Neuroimaging, University College London, UK) or using custom scripts and functions, running within Matlab (The Mathworks, Natick). To minimize interpolation artifacts [Grootoonk et al., 2000], seventh degree B‐spline interpolation [Unser, 1999] was used whenever possible. Initially, slice timing was performed to correct for timing delays introduced as part of the acquisition scheme [Sladky et al., 2011]. Thereafter, functional images were realigned [Friston et al., 1996] to correct for subject motion, with the “quality” flag set to maximum. From these rigid‐body translations, total displacement at average cortical distance (TDavg) was computed by combining shifts and rotations along/around each principal axis; this is a single, comprehensive feature describing subject motion [Wilke, 2012]. “Excessive motion” was defined as TDavg exceeding voxel size (3 mm) at more than one time point; subjects with one dataset fulfilling this criterion were rejected. Realignment over both sessions ensures that both sessions are coregistered to each other; they were also coregistered to the anatomical image. Finally, global signal drifts were removed [Macey et al., 2004]. For statistical analyses, images from the functional series were spatially smoothed with a Gaussian filter of FWHM = 6 mm. The anatomical dataset of each subject was segmented into tissue classes [Ashburner and Friston, 2005], employing the so‐called priorless segmentation functionality available within the VBM8‐toolbox [Gaser, 2012] and using custom‐made anatomical priors for spatial normalization [Wilke et al., 2008]. These normalization parameters were then applied to the native‐space statistical parametrical maps (see below); following inversion, they were also used to back‐transform the normalized‐space masks into the individual's native space (see below). It should be noted that the usage of customized priors for spatial normalization of MRI data in children precludes reporting coordinates in standard space.
Functional Series
From each subject, two functional series were acquired. First, a beep story dataset (BSD) was acquired, using a modified story‐listening task [Wilke et al., 2005]. In the active condition, subjects listen to simple children's stories of 30 s duration. From each story, 6–8 key words were removed and replaced by a sinus tone (a “beep”), which makes the story harder to follow and induces a stronger left‐inferior frontal involvement [Wilke et al., 2005]. In the control condition, subjects listen to different sinus tones in the range of human language. Participants are instructed to pay close attention to the stories and are quizzed after the exam with a set of six control questions, one for each story. Performance on this test below chance level was considered grounds to reject the dataset. Second, a resting state dataset (RSD) was acquired, where participants were instructed to “lie still with eyes closed.” The order of both tasks was always the same, but other series (including the fieldmap) were acquired in the intermediate.
Conventional Group Analyses (BSD)
Functional MRI data from the BSD was analyzed using the framework of the general linear model [Friston et al., 1995] on the first (individual subject) level, including the motion fingerprint (three traces and their shifted versions) [Wilke, 2012] as covariates of no interest. Following transformation of the parameter maps to normalized space, second‐level random effects group analyses [McGonigle et al., 2000] were performed (see below).
Connectivity Between Language Regions
Connectivity was assessed in both functional datasets, using the group activation results from the BSD as a functional localizer. In essence, the superior temporal lobe (STL) activation clusters from the second‐level group analyses (see below) were back‐transformed into each individual's native space by inverting the spatial normalization deformation field. From these native‐space regions, all timecourses were extracted. This results in different numbers of timecourses stemming from noncorresponding brain regions, consequently requiring standardization prior to comparisons. This was achieved by sorting them along an anterior‐posterior gradient and by condensing them into 50 time courses, now representing 50 anterior‐to‐posterior subregions along each subject's STL. Prior to analyses, motion effects and linear trends as well as global signal changes observable in the CSF class were removed from the data [Birn, 2012; Power et al., 2012; Schwarz and McGonigle, 2009] using functionality available within the REST toolbox [Xiao‐Wei et al., 2011], and minimal robust smoothing in the time domain was applied to safeguard against outliers [Garcia, 2010]. Time courses from subregions in either hemisphere were then compared with those from the opposite hemisphere, resulting in a 50 × 50 correlation matrix for each subject, reflecting interhemispheric functional connectivity between STL language areas. For each subregion, the average correlations with all subregions in the other hemisphere was calculated (allowing for a regional assessment of interhemispheric connectivity between language regions), and the area under the curve of these regional connectivity measures was calculated (allowing for a global assessment of interhemispheric connectivity between language regions). This was done for both functional series, assessing both task‐based as well as non task‐based functional connectivity [Rehme et al., 2013]. The main results shown are those from the task‐based connectivity analyses; the (very similar) results from the non task based connectivity analyses are described below and are shown in more detail in Supporting Information figures (see below).
Network Analyses
Connectivity between STL language regions was also assessed in terms of network efficiency on the local as well as the global level. For the former, the sum of significant connections is related to the sum of possible connections from each of the 50 subregions; for the latter, the inverse of the path length of significant connections between subregions is calculated [Bullmore and Sporns, 2012; Power et al., 2010]. For all analyses, a group threshold of 60% was enforced as recently suggested, ensuring that spurious results stemming from only a small number of participants are ruled out [de Reus and van den Heuvel, 2013].
Connectivity of STL Language Regions with Non‐STL Regions
To rule out nonspecific effects secondary to a globally altered functional connectivity, we also assessed connectivity on the hemispheric level. To this effect, connectivity was assessed from each language region (left or right STL) to the rest of the ipsilateral and contralateral hemisphere (exclusive of the STL). To investigate laterality effects, a lateralization index [Wilke and Lidzba, 2007] was computed from these values, reflecting the lateralization of functional connectivity from each region. The LI is a composite measure that allows assessing the balance between two contributing sides; therefore, a change in this value may be brought about by both an increase on the one as well as a decrease on the other side. It is one of the most‐used parameters for hemispheric specialization in language research [Wilke and Lidzba, 2007] and is computed by dividing the difference between both sides by its sum; it is therefore normalized to itself and may take on values ranging from −1 (completely right) to 1 (completely left).
Statistics
For the conventional group analyses of the BSD, a second‐level random effects group analysis was performed [McGonigle et al., 2000], using an ANOVA model with subject age, gender, handedness, and postnatal hCMV‐infection status as covariates of no interest, and prematurity status as grouping variable. Age (in months) and handedness (as EHI scores) were provided as continuous variables, while gender and postnatal hCMV infection status were binary variables. For whole‐brain analyses, results were thresholded at P ≤ 0.01, FWE‐corrected for multiple comparisons. For assessing group differences within the global activation pattern, activation differences were thresholded at P ≤ 0.001, uncorrected on the voxel level. In both cases, an additional extent threshold of k = 10 voxels was used to safeguard against spurious activation.
All other statistical analyses were carried out using functionality from Matlab's statistics toolbox (V8.1). Intrasubject connectivity analyses (testing similarities between timecourses) were performed using Pearson's correlation coefficient [Rehme et al., 2013]. Where significance was derived directly from this data, significance was assumed at r = 0.324 (corresponding to P ≤ 0.001 with n = 100 data points). Demographic variables were compared using chi‐square tests for categorical variables (or, in the case of low frequency of occurrence [<5], Fisher's exact Test) and Student's t‐tests for continuous variables. Derivative values were analyzed using an ANOVA (for group differences) or a partial regression model (for correlations), again using subject age, gender, handedness, and postnatal hCMV‐infection status as covariates of no interest. Significance was assumed at P ≤ 0.05. A Kolmogorov‐Smirnoff‐Lilliefors test, a 2‐sided goodness‐of‐fit test, was used to ensure a normal distribution; if this condition was not met, the nonparametrical Wilcoxon's rank sum test was used, data was rank‐transformed prior to submitting it to an ANOVA (ANOVAR), or the nonparametrical Spearman's rank correlation was used within the partial correlation framework.
RESULTS
Demographic Details
There was a significant difference in gender composition (Chi‐square, P = 0.028), age (Wilcoxon, P = 0.002) and language comprehension (t‐test, P = 0.015) between the groups. There was no significant difference in MEL or handedness score between the groups (see Table 1 for further demographic details). MRI changes compatible with early white matter lesions were seen in 7 PT participants, in the form of slight ventricular enlargements. No cerebral abnormalities were seen in TD participants; the difference between groups was significant (Fisher's exact test, P = 0.032). One child in the PT group was found to have bilateral spastic cerebral palsy (with a BMFM‐score of 2 and a GMFCS‐score of 3). No neurological abnormalities were seen in TD participants; the difference between groups was not significant (Fisher's exact test).
Conventional Group Analyses (BSD)
Over the whole group, the expected activation pattern of bilateral STL and left inferior frontal activation was seen (Fig. 1, top panels). Additionally, bilateral cerebellar co‐activation was seen. Within these regions, there were no regions where TD participants showed stronger activation than PT participants. However, there were distinct clusters in both temporal lobes and in the left cerebellum where PT participants showed stronger activation than TD participants (Fig. 1, bottom panels).
Figure 1.
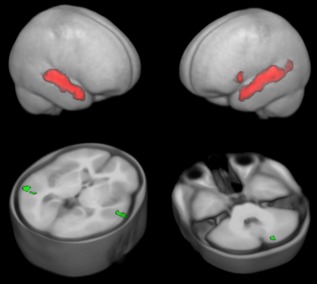
BSD: top panels: whole‐group activation pattern in conventional, second‐level random effects analyses, rendered on the custom‐made gray matter prior, thresholded at P = 0.01, FWE‐corrected; bottom panels: activation difference (PT > TD), overlaid on the custom‐made T1 reference dataset, thresholded at P = 0.001, uncorrected. Note only minor group activation differences. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Connectivity Between Language Regions
In a first step, it was ensured that the extracted native‐space brain regions did not differ in size between the groups, which was not the case for the left (mean number of voxels = 797.75, SD = 147.53) or the right side (mean number of voxels = 678.77, SD = 147.31; ANOVAR).
For the task‐based functional connectivity derived from the BSD, a roughly symmetrical pattern of interhemispheric connectivity between subregions within both STL language regions was found, with an apparently more pronounced connectivity between midposterior subregions (Fig. 2, left). This pattern was similar, but generally, less pronounced in the PT participants, with the strongest differences in connectivity between left‐anterior to right‐middle subregions (Fig. 2, right). The overall strength of connectivity between language regions in both hemispheres was significantly stronger in TD participants (ANOVA, P = 0.032 and P = 0.035). For the non task‐based functional connectivity derived from the RSD, a similar pattern could be observed, although with a lower level of connectivity (Supporting Information Fig. S1). Again, the overall strength of connectivity between STL language regions was significantly stronger in TD participants (ANOVA, P = 0.019 and P = 0.018).
Figure 2.
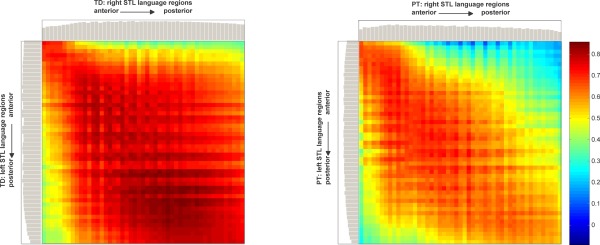
BSD: functional connectivity matrix between right and left STL language regions, for TD (left) and early PT born (right) participants. Color indicates strengths of correlation between regions. Gray bars: average connectivity for each region. Note overall significantly stronger connectivity in TD participants as compared to PT participants, pronounced in middle and posterior regions; cf. Supporting Information Figure S1 and see text for details. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Network Analyses
When assessing network efficiency, local efficiency was significantly lower in PT participants than in TD participants, in both STL language regions and both datasets (ANOVA, P = 0.016 and P = 0.013 [BSD] and P = 0.008 and P = 0.008 [RSD]). There was a significant group difference in global efficiency in the left STL in the BSD only (ANOVAR, P = 0.014); there were no differences in global efficiency in either region in the RSD (Fig. 3 and Supporting Information Fig. S2).
Figure 3.
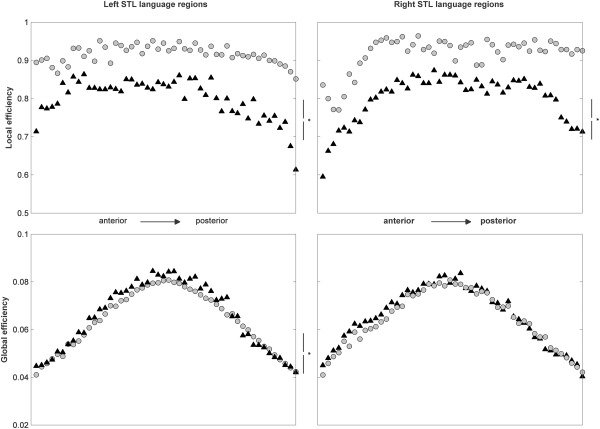
BSD: network analyses, assessing local (top panels) and global (bottom panels) network efficiency of left (left panels) and right (right panels) STL language regions, for TD (gray circles) and early PT born (black triangles) participants. * indicates significant difference in an ANOVA. Note consistently higher local network efficiency in TD participants as compared to PT participants, but only minor differences in left STL global efficiency; cf. Supporting Information Figure S2 and see text for details.
Connectivity of STL Language Regions with Non‐STL Regions
When assessing connectivity between left and right STL language regions and the rest of the ipsilateral and contralateral hemisphere, there was evidence for a significant increase in connectivity in all connections in former PT participants in the task‐based functional connectivity analyses (BSD; ANOVA, all P < 0.05). In the non task‐based functional connectivity analyses, only the group difference in connectivity between the left STL and the contralateral hemisphere remained significant (RSD; ANOVAR, P = 0.005); all other differences between groups did not reach significance (Fig. 4 and Supporting Information Fig. S3). When calculating a lateralization index from the connectivity to left‐ versus right‐sided brain regions, there was a strong correlation in the BSD of the resulting LIs with the verbal comprehension subscale (partial Spearman's rho = −0.445, P = 0.002), indicating that stronger laterality (i.e., weaker bilaterality) is associated with a lower language performance. There was no correlation of this index with verbal comprehension in the RSD.
Figure 4.
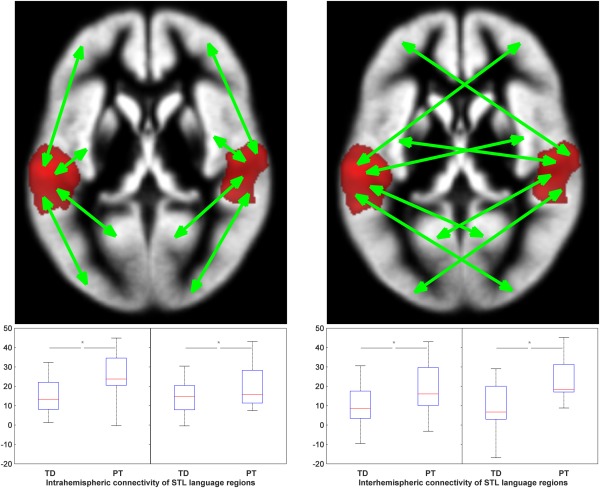
BSD: intrahemispheric (left) and interhemispheric (right) connectivity of left and right STL language regions, for TD and early PT born (PT) participants. * indicates significant difference in an ANOVA. Note consistently higher connectivity of STL language regions with non‐STL language regions both intrahemispherically and interhemispherically, in PT participants; cf. Supporting Information Figure S3 and see text for details. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
In summary, we found evidence for an impairment of interhemispheric functional connectivity between STL language regions in former early PTs, in both task‐based and non task‐based functional connectivity analyses, despite only small group activation differences in conventional fMRI group analyses. This is not a nonspecific effect as global connectivity was increased, rather than reduced, between STL language regions and non‐STL regions in PT subjects, both intrahemispherically and interhemispherically. Finally, lateralization of connectivity between STL language regions correlated strongly with verbal comprehension measures, indicating that a more bilateral pattern of connectivity is associated with higher language abilities. Our results are summarized in Table 2.
Table 2.
Summary of conducted analyses and significant results
| Dataset | Analysis | Cf. Figure | Outcome |
|---|---|---|---|
| BSD | Second Level Random Effects Analysis | 1 | PT > TD |
| Connectivity between STL language regions | 2 | TD > PT | |
| Local network efficiency in STL | 3 | TD > PT (L, R) | |
| Global network efficiency in STL | TD > PT (L) | ||
| Intrahemispheric connectivity | 4 | PT > TD (L, R) | |
| Interhemispheric connectivity | PT > TD (L, R) | ||
| Lateralization of connectivity | NA | Correlation with verbal comprehension | |
| RSD | Connectivity between STL language regions | S1 | TD > PT |
| Local network efficiency in STL | S2 | TD > PT (L, R) | |
| Global network efficiency in STL | n.s. | ||
| Intrahemispheric connectivity | S3 | n.s. | |
| Interhemispheric connectivity | PT > TD (L) | ||
| Lateralization of connectivity | NA | n.s. |
Note: strong overlap of results between the two datasets (i.e., task‐based [BSD] vs. non task‐based [RSD] approach); see text for details. STL, superior temporal lobe; PT, former PT born participants; TD; typically developing participants; L, left; R, right; NA, not available; n.s., not significant; >, significantly stronger or better.
Task‐Based fMRI Results
When performing conventional second‐level random effects analyses [McGonigle et al., 2000], the expected pattern of activation [Wilke et al., 2005] in STL language regions was seen over the whole group. There are distinct, but not overly large areas of activation differences within language regions between both groups (PT > TD, Fig. 1), although it should be noted that no single region showed stronger activation in the TD > PT contrast. It is also interesting to note the activation difference in the left cerebellum, substantiating the recently reported role of this structure in pathological language processing in former PT children [Constable et al., 2013]. However, these results suggest that the overall pattern of task‐based activation is not dramatically different between former PTs and TD participants, when appropriate covariates are included in the statistical model. In our cases, this included motion parameters [Wilke, 2012] on the first and age, gender, handedness, and postnatal hCMV‐infection status on the second level. While the influence of age, gender, and handedness on fMRI language activation patterns is well‐documented [Bitan et al., 2010; Lidzba et al., 2011; Plante et al., 2006; Szaflarski et al., 2012], the impact of the latter has yet to be described in full [Bevot et al., 2012; Goelz et al., 2013; Vollmer et al., 2004]. While the detailed exploration of this effect was not the aim of this study, the importance of postnatal inflammatory processes in the etiology of PT brain damage is increasingly recognized [Leviton et al., 2013; Volpe, 2009], and such an early infection in only some participants must consequently be considered a potential bias. We therefore opted to account for this effect by including it as a covariate of no interest.
Functional Connectivity Analyses
The lack of substantial group differences in the conventional fMRI group analyses suggests that the observable differences in language performance (cf. Table 1) is more likely due to alterations in the interaction between regions. A reduced structural connectivity between temporal lobe language regions was recently reported in former early PTs [Northam et al., 2012]. In particular, fibers traversing the posterior part of the corpus callosum were reduced, whereas fibers connecting anterior parts of the temporal lobe via the anterior commissure were still preserved in those children with good language skills, conforming and extending previous results [Mullen et al., 2011]. Based on these earlier reports and the fact that bilateral STL language activation can robustly be achieved using our child‐friendly beep story task [Wilke et al., 2005], we decided to focus on these regions in this study. Abnormalities in the corpus callosum are among the more widely reported abnormalities in former early PTs [Hart et al., 2008; Nosarti et al., 2004; Thompson et al., 2012], very likely as a consequence of the (primarily posterior) white matter pathology occurring in the complex “encephalopathy of prematurity” now known to affect the very immature brain in several ways [Volpe, 2009]. This large white matter structure is among the main pathways that connect the two hemispheres, and an early onset, specific impairment must be expected to affect language network formation, language acquisition and, ultimately, performance [Bitan et al., 2010; Friederici, 2011; Friederici et al., 2011; Hinkley et al., 2012]. Indeed, our results are in agreement with these recent findings in that we detect a decreased interhemispheric connectivity between STL language regions in former PTs, with a more pronounced reduction in mid‐ to posterior‐temporal brain regions (Fig. 2). When assessing network properties such as local or global efficiency, the PT group scores significantly worse than the TD participants (Fig. 3), again suggesting that the interaction between both STL language regions is substantially altered. Interestingly, these differences were detectable not only in the task‐based analysis of the BSD but very similarly in the analysis of non task‐based functional connectivity from the RSD (Supporting Information Figs. S1 and S2). This is remarkable in so far as the brain was not engaged in an overt language task during the acquisition of the latter dataset, arguing for a more persistent, not wholly task‐dependent impairment. Although a certain “spill‐over” effect from previous tasks must be expected [Gordon et al., 2013; Waites et al., 2005], the RSD was not acquired directly after the BSD but, in most cases, following either a diffusion MRI sequence or the vowel identification task, a productive language/visuospatial task with visual stimulation [Ebner et al., 2011; Wilke et al., 2006]. Hence, a task involving auditory stimulation or language perception in no case preceded the second dataset, making such a contamination less likely. For an additional discussion regarding the difference between the here‐used non task‐based functional connectivity and typical resting state analyses, see below.
It is important to delineate whether observed connectivity differences between brain regions are specific (i.e., attributable to the region and/or domain under study) or unspecific (i.e., attributable to global differences in connectivity). From our further analyses, it is apparent that connectivity between both STL language regions was not unspecifically reduced in PT participants. Indeed, when assessing the connectivity of these regions with all other regions in the ipsilateral or contralateral hemisphere, PT participants show a consistently and significantly stronger overall connectivity in the analyses of the BSD. In the RSD analyses, this remains significant for the connectivity of the left temporal lobe with the right hemisphere (Fig. 4 and Supporting Information Fig. S3). These results are very interesting not only because they now allow to classify the above‐identified connectivity differences between the language regions as a specific impairment, but also because they allow to reconcile seemingly contradictory results from previous studies, namely reports on decreased structural [Mullen et al., 2011; Northam et al., 2012] and increased functional [Constable et al., 2013; Gozzo et al., 2009] connectivity in former PTs. Although there are important differences between our current and previous studies (such as examining older children and adolescents, and using functional instead of anatomical localizers), our results demonstrate that it is well possible that both weaker and stronger connectivity may be present within the language network. Taken together, the accumulated body of evidence suggests that the former may be lesion‐driven (e.g., due to an alteration of the posterior corpus callosum), while the latter may be part of a compensatory mechanism (e.g., via alternative pathways such as the anterior commissure, or via a stronger intrahemispheric connectivity). However, it must be pointed out that prematurity status and language comprehension impairment are so inherently (and significantly) linked [Barre et al., 2011; Reidy et al., 2013] that separating their influence is statistically challenging, if not impossible. If separate, large‐enough groups were available that do or do not not show impairment in language functions following prematurity, it would be easier to ascribe the observable group differences to one factor only (particularly if language impairment changes with age) [Luu et al., 2011]. As it is, it is difficult to tease apart independent contributions of prematurity and impaired language comprehension. This distinction may seem academic, as it is clear that it is prematurity that leads to language impairment and not vice versa. However, atypical lateralization of language has also been described in specific language impairment [Bernal and Altman, 2003; Whitehouse and Bishop, 2008]; to ascertain that the effects are specifically ascribable to prematurity, not unspecifically to “language problems,” it would therefore require a group with comparable difficulties in language comprehension who were not born prematurely (in addition to term‐born children with no impairment). Only such a 2 × 2 design (modeling both language impairment and prematurity) would ultimately allow disentangling the nature of the here‐observed group differences.
Task‐Based Versus Non task‐Based Connectivity
For our connectivity analyses, we used both task‐based (BSD) and non task‐based (RSD) analyses. While the latter is increasingly used to assess low‐frequency fluctuations within the so‐called resting state networks [Biswal et al., 1995], the former is employed to assess connectivity between brain regions engaged in an active task [Newton et al., 2007]. It was recently suggested that both approaches are complementary and that they may reflect slightly different aspects of connectivity [Kellermann et al., 2013; Rehme et al., 2013]. There are several interesting points that can be made when comparing the results from both analyses: first, there is a uniformly greater level of connectivity between STL language regions in the BSD analyses, on all metrics (cf. Fig. 2 and Supporting Information S1, Fig. 3 and Supporting Information S2, and Fig. 4 and Supporting Information S3). This in itself is to be expected, given that language regions should be more active and therefore (ideally) interacting more, when performing a language task. At first, the striking similarity of the results obtained from the RSD with those obtained while performing an explicit language task (BSD) seems surprising: most of the metrics used to describe both populations are similarly (significantly) different between them. However, these results are well in line with previous observations that the brain employs very similar networks during “rest” (once ironically termed “random episodic silent thinking”) [Andreasen et al., 1995] and activation during a task [Smith et al., 2009], and only recently, individual language networks were identified from resting‐state data [Tie et al., 2013]. Similar amplitude of signal fluctuations were seen [Damoiseaux et al., 2006], such that a lower coherence of the fluctuations might explain the weaker pattern of connectivity when comparing our BSD and RSD results. Further analyses regarding the identification of networks and network nodes from resting state analyses in children seem warranted, as the outlook of substituting complex task execution with a much more simple “lying in the scanner” task is promising for children in particular [Church et al., 2010; Vogel et al., 2010]. However, the issue of motion, a substantial confounding factor in resting state analyses [Birn, 2012; Power et al., 2012], must always be considered as the absence of visual stimuli is associated with more motion in children [Yuan et al., 2009].
Hemispheric Lateralization of Connectivity and Language Abilities
The analysis of connectivity has been suggested to be particularly revealing when assessing hemispheric specialization [Stephan et al., 2007], and the transfer of information may be one of the decisive factors for hemispheric lateralization [Seghier et al., 2010]. We therefore calculated a lateralization index from our connectivity results, relating intrahemispheric to interhemispheric connectivity. We found a strong correlation of this metric with impaired verbal comprehension, such that stronger laterality (i.e., weaker bilaterality) is associated with a lower language performance. This is particularly interesting, for several reasons. First, this observation is well in line with the impaired connectivity on the structural level, also correlating with language impairment [Northam et al., 2012]. Further, we previously reported a correlation of a less‐lateralized activation when listening to beep‐stories and higher scores on language tests in an independent sample of healthy children [Lidzba et al., 2011], hypothesizing that higher language abilities in children are reflected in a more bilateral involvement of temporal language cortices. Of note, while the significant correlation (r = −0.445) observed here includes the whole sample, it was even stronger (r = −0.485) in the PT participants alone and similarly strong in the TD group (r = −0.386), where it fails to reach significance only due to the smaller sample size. Also, while PT participants scored significantly lower than TD participants in the language comprehension subscale, the mean of the PT participants was still within the low‐normal range (cf. Table 1), in line with previous results [Barre et al., 2011]. This suggests that a continuum exists with regard to the relation of lateralized language perception and language abilities, including both the normal and the abnormal range of language functions. All of these results point towards a major role of interhemispheric connectivity for the development of receptive language functions in the developing brain. This is also suggested by the observation that interhemispheric connectivity between posterior language regions positively correlates with performance [Schmithorst and Holland, 2007] and is impaired by early white matter lesions in PT‐born children [Reidy et al., 2013]. In this context, it is interesting to remember that the developing brain was suggested to generally rely more on interhemispheric connectivity than the adult brain, as long‐reaching intrahemispheric connections only develop later [Power et al., 2010]. This was also shown to be relevant for the maturation of the language network [Friederici et al., 2011] and nicely fits in with observations that, in healthy children, hemispheric dominance for language increases with age, as observable using fMRI [Szaflarski et al., 2012], MEG [Ressel et al., 2008], or diffusion MRI [Brauer et al., 2011]. A stronger role of these intrahemsipheric connections in PT participants would be compatible with our findings of stronger intrahemispheric connectivity (Fig. 4 and Supporting Information S3). Such later‐developing, intrahemispheric connections (not relying as much on interhemispheric crosstalk) may potentially also explain the catch‐up of (particularly receptive) language abilities observable in late childhood in some former PTs [Luu et al., 2011].
Methodological Considerations
Our PT participants were recruited following up on an observational study aimed at assessing transmission rate of early postnatal, vertical hCMV infection [Maschmann et al., 2001; Neuberger et al., 2006]. This study was open to all neonates at Tübingen University Children's Hospital who were born at ≤32 weeks of gestation. However, whenever children formerly born very PT are followed up, there is the potential issue of a recruitment bias [Stang, 2003]: if only those subjects with less impairment are willing to participate, this will skew comparisons relying on the assumption that a representative sample was investigated [Callanan et al., 2001]. For example, the low prevalence of cerebral palsy (n = 1) in our patient group was surprising. However, this study was not aimed at providing epidemiological information about the prevalence of (e.g.) language impairment, and a potentially skewed recruitment as described above would only decrease, not inflate, effect sizes. Further, when comparing the major neonatological parameters (gestational age, birth weight, postnatal hCMV infection, and gender) of the PT participants who took part in our study with those who did not (i.e., were approached and declined [n = 21], were approached and excluded due to contraindications [n = 10], or were lost to follow up [n = 24]), neither their gestational age (28.45 ±2.33 weeks), birth weight (1108 ± 310 g), or postnatal positive hCMV infection status (24/55), nor their gender composition (41 M, 14 F) was significantly different from our final sample (c.f. Table 1). This indicates that we were able to investigate a subgroup that was representative of the whole group of very early PTs potentially available for inclusion.
It should also be remembered that we examined two groups that were not ideally matched. However, this should only reduce power in analyses where age and gender were included as covariates (as they will invariably explain some of the “main” group differences); our result are therefore most likely an under‐, not overestimation of a “true” group difference [Zuur et al., 2010]. Further, not to investigate too small a brain region, we abstained from assessing connectivity to, for example, the cerebellar activation clusters [Constable et al., 2013], as the signal‐to‐noise ratio of such a small region near the edge of the field of view was considered too limited. As functional localizers for frontal language functions [Ebner et al., 2011; Wilke et al., 2006] were not available for all children, we abstained from performing further analyses on additional brain regions. Finally, although we assessed resting state MRI data, our results were not restricted to the frequency range conventionally considered to be reflecting resting state networks, that is, 0‐0.1 Hz [Birn, 2012; Biswal et al., 1995] to ascertain comparability of results between task‐based and non task‐based functional connectivity. Hence, our results do not reflect resting state connectivity per se, they rather reflect correlated fluctuations over time in a broader frequency spectrum (although previous simulations suggest that the lower frequencies assessed in classical resting state analyses will also dominate results when not introducing a hard cutoff) [Cordes et al., 2001]. Similarly, to resting state data processing, however, we removed global as well as motion confounds from all our time series [Birn, 2012; Power et al., 2012; Xiao‐Wei et al., 2011], these should therefore not unduly influence our findings.
CONCLUSIONS
To conclude, we provide evidence for a substantial impairment of functional connectivity, which was specific to STL language regions in former early PTs, in the presence of only insubstantial group differences in a conventional fMRI group analyses. Further, connectivity with other brain regions was increased, not reduced, in PT participants, suggesting the presence of both stronger and weaker connectivity within the language network. Finally, a more bilateral pattern of functional connectivity between STL language regions was associated with better language comprehension scores, underlining the relevance of interhemispheric connectivity for language comprehension.
ACKNOWLEDGMENTS
The authors would like to gratefully acknowledge the willingness of our subjects and their families to contribute to this study. They would also like to thank Kathina Ebner and Maik Dorn for help with data acquisition, Andrea Bevot, and Rangmar Goelz for help with demographic data collection, and Ulrike Ernemann for continued support.
Supporting information
Supporting Information
Supporting Information
REFERENCES
- Allen MC (2008): Neurodevelopmental outcomes of preterm infants. Curr Opin Neurol 21:123‐128. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O'Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, Ponto LL, Hichwa RD (1995): Remembering the past: Two facets of episodic memory explored with positron emission tomography. Am J Psychiatry 152:1576‐1585. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005): Unified segmentation. Neuroimage 26:839‐851. [DOI] [PubMed] [Google Scholar]
- Barre N, Morgan A, Doyle LW, Anderson PJ (2011): Language abilities in children who were very preterm and/or very low birth weight: A meta‐analysis. J Pediatr 158:766‐774. [DOI] [PubMed] [Google Scholar]
- Beckung E, Hagenberg G (2002): Neuroimpairments, activity limitations and participation restrictions in children with cerebral palsy. Dev Med Child Neurol 44:309–316. [DOI] [PubMed] [Google Scholar]
- Bernal B, Altman NR (2003): Speech delay in children: A functional MR imaging study. Radiology 229:651‐658. [DOI] [PubMed] [Google Scholar]
- Bevot A, Hamprecht K, Krägeloh‐Mann I, Brosch S, Goelz R, Vollmer B (2012): Long‐term outcome in preterm children with human cytomegalovirus infection transmitted via breast milk. Acta Paediatr 101:e167–e172. [DOI] [PubMed] [Google Scholar]
- Birn RM (2012): The role of physiological noise in resting‐state functional connectivity. Neuroimage 62:864‐870. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MR imaging. Magn Reson Med 34:537–541. [DOI] [PubMed] [Google Scholar]
- Bitan T, Lifshitz A, Breznitz Z, Booth JR (2010): Bidirectional connectivity between hemispheres occurs at multiple levels in language processing but depends on sex. J Neurosci 30:11576‐11585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer J, Anwander A, Friederici AD (2011): Neuroanatomical prerequisites for language functions in the maturing brain. Cereb Cortex 21:459‐466. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O (2012): The economy of brain network organization. Nat Rev Neurosci 13:336‐349. [DOI] [PubMed] [Google Scholar]
- Callanan C, Doyle LW, Rickards AL, Kelly EA, Ford GW, Davis NM (2001): Children followed with difficulty: How do they differ? J Paediatr Child Health 37:152–156. [DOI] [PubMed] [Google Scholar]
- Church JA, Petersen SE, Schlaggar BL (2010): The “Task B problem” and other considerations in developmental functional neuroimaging. Hum Brain Mapp 31:852‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constable RT, Vohr BR, Scheinost D, Benjamin JR, Fulbright RK, Lacadie C, Schneider KC, Katz KH, Zhang H, Papademetris X, Ment LR (2013): A left cerebellar pathway mediates language in prematurely‐born young adults. Neuroimage 64:371‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME (2001): Frequencies contributing to functional connectivity in the cerebral cortex in “resting‐state” data. AJNR Am J Neuroradiol 22:1326‐1333. [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF (2006): Consistent resting‐state networks across healthy subjects. Proc Natl Acad Sci USA 103:13848‐13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Reus MA, van den Heuvel MP (2013): Estimating false positives and negatives in brain networks. Neuroimage 70:402‐429. [DOI] [PubMed] [Google Scholar]
- Doyle LW, Anderson PJ (2010): Adult outcome of extremely preterm infants. Pediatrics 126:342‐351. [DOI] [PubMed] [Google Scholar]
- Ebner K, Lidzba K, Hauser TK, Wilke M (2011): Assessing language and visuospatial functions with one task: A “dual use” approach to performing fMRI in children. Neuroimage 58:923‐929. [DOI] [PubMed] [Google Scholar]
- Friederici AD (2011): The brain basis of language processing: from structure to function. Physiol Rev 91:1357‐1392. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Brauer J, Lohmann G (2011): Maturation of the language network: From inter‐ to intrahemispheric connectivities. PLoS One 6: e20726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiack RS (1995): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2:189–210. [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R (1996): Movement‐related effects in fMRI time‐series. Magn Reson Med 35:346‐355. [DOI] [PubMed] [Google Scholar]
- Garcia D (2010): Robust smoothing of gridded data in one and higher dimensions with missing values. Comp Stat Data Anal 54:1167–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaser C. VBM8‐toolbox, r435. Available at: http://dbm.neuro.uni-jena.de/vbm/. Accessed on 12/10/2012.
- Goelz R, Meisner C, Bevot A, Hamprecht K, Kraegeloh‐Mann I, Poets CF: Long‐term cognitive and neurological outcome of preterm infants with postnatally acquired CMV infection through breast milk. Arch Dis Child Fetal Neonatal Ed (in press). [DOI] [PubMed] [Google Scholar]
- Gordon EM, Breeden AL, Bean SE, Vaidya CJ: Working memory‐related changes in functional connectivity persist beyond task disengagement. Hum Brain Mapp(in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzo Y, Vohr B, Lacadie C, Hampson M, Katz KH, Maller‐Kesselman J, Schneider KC, Peterson BS, Rajeevan N, Makuch RW, Constable RT, Ment LR (2009): Alterations in neural connectivity in preterm children at school age. Neuroimage 48:458‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootoonk S, Hutton C, Ashburner J, Howseman AM, Josephs O, Rees G, Friston KJ, Turner R (2000): Characterization and correction of interpolation effects in the realignment of fMRI time series. Neuroimage 11:49‐57. [DOI] [PubMed] [Google Scholar]
- Hart AR, Whitby EW, Griffiths PD, Smith MF (2008): Magnetic resonance imaging and developmental outcome following preterm birth: Review of current evidence. Dev Med Child Neurol 50:655‐663. [DOI] [PubMed] [Google Scholar]
- Hinkley LB, Marco EJ, Findlay AM, Honma S, Jeremy RJ, Strominger Z, Bukshpun P, Wakahiro M, Brown WS, Paul LK, Barkovich AJ, Mukherjee P, Nagarajan SS, Sherr EH (2012): The role of corpus callosum development in functional connectivity and cognitive processing. PLoS One 7:e39804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermann TS, Caspers S, Fox PT, Zilles K, Roski C, Laird AC, Turetsky BI, Eickhoff SB (2013): Task‐ and resting‐state functional connectivity of brain regions related to affection and susceptible to concurrent cognitive demand. Neuroimage 72:69‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latal B (2009): Prediction of neurodevelopmental outcome after preterm birth. Pediatr Neurol 40:413‐419. [DOI] [PubMed] [Google Scholar]
- Leviton A, Fichorova RN, O'Shea TM, Kuban K, Paneth N, Dammann O, Allred EN (2013): Two‐hit model of brain damage in the very preterm newborn: small for gestational age and postnatal systemic inflammation. Pediatr Res 73:362‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidzba K, Schwilling E, Grodd W, Krägeloh‐Mann I, Wilke M (2011): Language comprehension vs. language production: Age effects on fMRI activation. Brain Lang 119:6‐15. [DOI] [PubMed] [Google Scholar]
- Luu TM, Vohr BR, Allan W, Schneider KC, Ment LR (2011): Evidence for catch‐up in cognition and receptive vocabulary among adolescents born very preterm. Pediatrics 128:313‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Macey KE, Kumar R, Harper RM (2004): A method for removal of global effects from fMRI time series. Neuroimage 22:360‐366. [DOI] [PubMed] [Google Scholar]
- Marlow N (2004): Neurocognitive outcome after very preterm birth. Arch Dis Child Fetal Neonatal Ed 89:224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maschmann J, Hamprecht K, Dietz K, Jahn G, Speer CP (2001): Cytomegalovirus infection of extremely low‐birth weight infants via breast milk. Clin Infect Dis 33:1998‐2003. [DOI] [PubMed] [Google Scholar]
- McGonigle DJ, Howseman AM, Athwal BS, Friston KJ, Frackowiak RS, Holmes AP (2000): Variability in fMRI: an examination of intersession differences. Neuroimage 11:708‐734. [DOI] [PubMed] [Google Scholar]
- Mullen KM, Vohr BR, Katz KH, Schneider KC, Lacadie C, Hampson M, Makuch RW, Reiss AL, Constable RT, Ment LR (2011): Preterm birth results in alterations in neural connectivity at age 16 years. Neuroimage 54:2563‐2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger P, Hamprecht K, Vochem M, Maschmann J, Speer CP, Jahn G, Poets CF, Goelz R (2006): Case‐control study of symptoms and neonatal outcome of human milk‐transmitted cytomegalovirus infection in premature infants. J Pediatr 148:326‐331. [DOI] [PubMed] [Google Scholar]
- Newton AT, Morgan VL, Gore JC (2007): Task demand modulation of steady‐state functional connectivity to primary motor cortex. Hum Brain Mapp 28:663‐672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northam GB, Liégeois F, Tournier JD, Croft LJ, Johns PN, Chong WK, Wyatt JS, Baldeweg T (2012): Interhemispheric temporal lobe connectivity predicts language impairment in adolescents born preterm. Brain 135:3781‐3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosarti C, Rushe TM, Woodruff PW, Stewart AL, Rifkin L, Murray RM (2004): Corpus callosum size and very preterm birth: Relationship to neuropsychological outcome. Brain 127:2080‐2089. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97‐113. [DOI] [PubMed] [Google Scholar]
- Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B (1997): Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 39:214‐223. [DOI] [PubMed] [Google Scholar]
- Petermann F, Petermann U (2007): Hamburg‐Wechsler Intelligenztest für Kinder – IV (HAWIK‐IV). Bern: Huber‐Verlag. [Google Scholar]
- Plante E, Schmithorst VJ, Holland SK, Byars AW (2006): Sex differences in the activation of language cortex during childhood. Neuropsychologia 44:1210‐1221. [DOI] [PubMed] [Google Scholar]
- Power JD, Fair DA, Schlaggar BL, Petersen SE (2010): The development of human functional brain networks. Neuron 67:735‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012): Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142‐2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehme AK, Eickhoff SB, Grefkes C (2013): State‐dependent differences between functional and effective connectivity of the human cortical motor system. Neuroimage 67:237‐246. [DOI] [PubMed] [Google Scholar]
- Reidy N, Morgan A, Thompson DK, Inder TE, Doyle LW, Anderson PJ (2013): Impaired language abilities and white matter abnormalities in children born very preterm and/or very low birth weight. J Pediatr 162:719‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressel V, Wilke M, Lidzba K, Lutzenberger W, Krägeloh‐Mann I (2008): Increases in language lateralization in normal children as observed using magnetoencephalography. Brain Lang 106:167‐176. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK (2007). Sex differences in the development of neuroanatomical functional connectivity underlying intelligence found using Bayesian connectivity analysis. Neuroimage 35:406‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz AJ, McGonigle J (2011): Negative edges and soft thresholding in complex network analysis of resting state functional connectivity data. Neuroimage 55:1132‐1146. [DOI] [PubMed] [Google Scholar]
- Seghier ML, Josse G, Leff AP, Price CJ (2010): Lateralization is predicted by reduced coupling from the left to right prefrontal cortex during semantic decisions on written words. Cereb Cortex 21:1519‐1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCPE (2000): Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Dev Med Child Neurol 42:816‐824. [DOI] [PubMed] [Google Scholar]
- Sladky R, Friston KJ, Tröstl J, Cunnington R, Moser E, Windischberger C (2011): Slice‐timing effects and their correction in functional MRI. Neuroimage 58:588‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF (2009): Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci USA 106:13040‐13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stang A (2003): Nonresponse research‐an underdeveloped field in epidemiology. Eur J Epidemiol 18:929‐931. [DOI] [PubMed] [Google Scholar]
- Staudt M (2010): Reorganization after pre‐ and perinatal brain lesions. J Anat 217:469‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Fink GR, Marshall JC (2007): Mechanisms of hemispheric specialization: insights from analyses of connectivity. Neuropsychologia 45:209‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Rajagopal A, Altaye M, Byars AW, Jacola L, Schmithorst VJ, Schapiro MB, Plante E, Holland SK (2012): Left‐handedness and language lateralization in children. Brain Res 1433:85‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DK, Inder TE, Faggian N, Warfield SK, Anderson PJ, Doyle LW, Egan GF (2012): Corpus callosum alterations in very preterm infants: Perinatal correlates and 2 year neurodevelopmental outcomes. Neuroimage 59:3571‐3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tie Y, Rigolo L, Norton IH, Huang RY, Wu W, Orringer D, Mukundan S, Golby AJ: Defining language networks from resting‐state fMRI for surgical planning‐a feasibility study. Hum Brain Mapp (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unser M (1999): Splines: A perfect fit for signal and image processing. IEEE Signal Process Mag 16:22‐38. [Google Scholar]
- Vogel AC, Power JD, Petersen SE, Schlaggar BL (2010): Development of the brain's functional network architecture. Neuropsychol Rev 20:362‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer B, Seibold‐Weiger K, Schmitz‐Salue C, Hamprecht K, Goelz R, Krageloh‐Mann I, Speer CP (2004): Postnatally acquired cytomegalovirus infection via breast milk: Effects on hearing and development in preterm infants. Pediatr Infect Dis J 23:322‐327. [DOI] [PubMed] [Google Scholar]
- Volpe JJ (2009): Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 8:110‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss W, Jungmann T, Wachtendorf M, Neubauer AP (2012): Long‐term cognitive outcomes of extremely low‐birth‐weight infants: The influence of the maternal educational background. Acta Paediatr 101:569‐573. [DOI] [PubMed] [Google Scholar]
- Waites AB, Stanislavsky A, Abbott DF, Jackson GD (2005): Effect of prior cognitive state on resting state networks measured with functional connectivity. Hum Brain Mapp 24:59‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse AJ, Bishop DV (2008): Cerebral dominance for language function in adults with specific language impairment or autism. Brain 131:3193‐3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Lidzba K (2007): LI‐tool: A new toolbox to assess lateralization in functional MR‐data. J Neurosci Methods 163:128‐136. [DOI] [PubMed] [Google Scholar]
- Wilke M, Lidzba K, Staudt M, Buchenau K, Grodd W, Krägeloh‐Mann I (2005): Comprehensive language mapping in children, using functional magnetic resonance imaging: what's missing counts. Neuroreport 16:915‐919. [DOI] [PubMed] [Google Scholar]
- Wilke M, Lidzba K, Staudt M, Buchenau K, Grodd W, Krägeloh‐Mann I (2006): An fMRI task battery for assessing hemispheric language dominance in children. Neuroimage 32:400‐410. [DOI] [PubMed] [Google Scholar]
- Wilke M, Holland SK, Altaye M, Gaser C (2008): Template‐O‐Matic: A toolbox for creating customized pediatric templates. Neuroimage 41:903‐913. [DOI] [PubMed] [Google Scholar]
- Wilke M (2012): An alternative approach towards assessing and accounting for individual motion in fMRI timeseries. Neuroimage 59:2062‐2072. [DOI] [PubMed] [Google Scholar]
- Xiao‐Wei S, Zhang‐Ye D, Xiang‐Yu L, Su‐Fang L, Xi‐Nian Z, Chao‐Zhe Z, Yong H, Chao‐Gan Y, Yu‐Feng Z (2011): REST: A toolkit for resting‐state functional magnetic resonance imaging data processing. PLoS One 6:e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Altaye M, Ret J, Schmithorst V, Byars AW, Plante E, Holland SK (2009): Quantification of head motion in children during various fMRI language tasks. Hum Brain Mapp 30:1481‐1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuur AF, Ieno EN, Elphick CS (2010): A protocol for data exploration to avoid common statistical problems. Meth Ecol Evol 1:3‐14. [Google Scholar]
- Zwicker JG, Harris SR (2008): Quality of life of formerly preterm and very low birth weight infants from preschool age to adulthood: a systematic review. Pediatrics 121:366‐376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


