Abstract
The development of language, social interaction, and communicative skills are remarkably different in the child with autism spectrum disorder (ASD). Atypical brain connectivity has frequently been reported in this patient population. However, the interplay between their brain connectivity and language performance remains largely understudied. Using diffusion tensor imaging tractography and resting‐state fMRI, the authors explored the structural and functional connectivity of the language network and its relation to the language profile in a group of healthy control subjects (N = 25) and a group of children with ASD (N = 17). The authors hypothesized that in children with ASD, a neural connectivity deficit of the language network can be related to the observed abnormal language function. They found an absence of the right‐hemispheric arcuate fascicle (AF) in 28% (7/25) of the healthy control children and in 59% (10/17) of the children with ASD. In contrast to healthy control children, the absence of the right‐hemispheric AF in children with autism was related to a lower language performance as indicated by a lower verbal IQ, lower scores on the Peabody Picture Vocabulary Test, and lower language scores on the Dutch version of the Clinical Evaluation of Language Fundamentals (CELF‐4NL). In addition, through iterative fMRI data analyses, the language impairment of children with ASD could be linked to a marked loss of intrahemispheric functional connectivity between inferior frontal and superior temporal regions, known as the cortical language network. Both structural and functional underconnectivity patterns coincide and are related to an abnormal language function in children with ASD. Hum Brain Mapp 35:3602–3615, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: autism spectrum disorder, diffusion tensor imaging, language impairment, arcuate fascicle, functional MRI
INTRODUCTION
Autism spectrum disorder (ASD) is a pervasive neurodevelopmental disorder characterized by severe difficulties in reciprocal social interaction, stereotyped patterns of behavior, and profound impairments in verbal and nonverbal communication. The ability to communicate verbally is considered to be a positive prognostic indicator for children with ASD [Groen et al., 2008; Wan et al., 2012; Williams et al., 2008]. Detailed linguistic studies have shown that for some children with ASD, the linguistic abilities can be altered in pragmatic, semantic, syntactic, and phonological domains extending to both receptive and expressive aspects [Groen et al., 2008; Verhoeven et al., 2010; Williams et al., 2008].
The notion that ASD is a disorder of brain development is widely acknowledged, and an increasing number of functional [Herbert et al., 2005; Just et al., 2004; Wicker et al., 2008] and structural [Boddaert et al., 2004; Carper and Courchesne, 2000; Herbert et al., 2003, 2005] neuroimaging studies have been conducted to explore the neurobiological origin of ASD. Structural and functional neuroimaging are promising methods for investigating the neural correlates underlying the linguistic deficits in autism [Courchesne et al., 2007; Verhoeven et al., 2010]. Findings to date indicate that children with autism activate alternative and possibly less flexible networks during phonetic, semantic, syntactic, and pragmatic language processing [Groen et al., 2008]. This highlights the importance of the investigation of white matter (WM) structures and interregional connectivity necessary for the efficient completion of higher cognitive functions such as language. WM structures can be measured with diffusion tensor imaging (DTI), which is a modification of the MRI technique that is sensitive to the Brownian motion and the constraint of the free motion of water molecules in different directions in biological tissues [Basser et al., 1994]. DTI tractography is currently the golden standard analysis method for exploring WM organization and maturation at a microstructural level in children [Dubois et al., 2009]. It is one of the most powerful and noninvasive methods providing insight in the orientation and integrity of WM fibers in vivo both qualitatively and quantitatively. Fibers depicted with tractography do not represent individual axons or nerve fibers, but should be viewed in physical terms as lines of fast diffusion reflecting axonal architecture. Different algorithms have been developed for fiber tractography, and delineation protocols to reconstruct WM tracts have been published and tested on their reproducibility [Catani et al., 2005; Verhoeven et al., 2012; Wakana et al., 2007]. Using tractography, DTI parameters such as fractional anisotropy (FA) and mean apparent diffusion coefficient (ADC), which are indirect measures of myelination and/or axonal density within WM, can be measured along specific WM tracts [Beaulieu, 2002]. ADC is a measure of the rate of diffusion in each image voxel, whereas FA measures the extent to which that diffusion is directionally restricted [Correia et al., 2008]. Confusion can arise from the often interchangeable use of mean diffusivity and ADC in literature. DTI is the imaging tool of choice to analyze structural connectivity and to explore WM abnormalities at a microstructural level.
To study functional connectivity of the brain, there has been an increase in the use of fMRI in recent years. fMRI measures local changes in magnetic susceptibility caused by fluctuations in the capillary concentration of deoxyhemoglobin due to blood flow and blood volume increases in response to neuronal activation [Deco and Corbetta, 2011]. Even at rest, low‐frequency (≤0.1 Hz) fluctuations in the blood‐oxygenation level‐dependent (BOLD) signal can be observed. These spontaneous, not stimulus or task‐driven, fluctuations do not occur randomly, but represent neuronal activity organized into highly structured spatiotemporal profiles reflecting the functional architecture of the brain [Deco and Corbetta, 2011]. Resting‐state fMRI (rfMRI) studies examine the level of coactivation between the functional time series of anatomically separated brain regions during rest, believed to reflect functional communication between them [Biswal et al., 1995; Damoiseaux et al., 2006; Greicius et al., 2003]. As the results are not dependent on task performance and no specific cooperation is required, this technique is well suited to study functional connectivity patterns and the potential disruption of connections in young or noncooperative patients.
In contrast to the variability for most of the observations made in ASD research, the theory of a disorganized brain seems to be very consistent. This theory links the core symptoms observed in ASD to a deficient integration and synchronization of brain regions preferentially affecting long‐range connections [Courchesne et al., 2007]. Previous studies reported an aberrant functioning between cortical areas on a range of language tasks [Harris et al., 2006; Just et al., 2004; Kana et al., 2006; Knaus et al., 2008], suggesting that alterations in cortical connectivity and the deficient communication among cortical regions may be part of the language difficulties seen in ASD [Courchesne, 2004; Courchesne et al., 2003]. Further evidence for atypical brain connectivity of the language network in ASD has been corroborated by studies using DTI [Barnea‐Goraly et al., 2004; Fletcher et al., 2010; Keller et al., 2007; Nagae et al., 2012]. These studies pointed toward abnormal DTI parameters that may underlie the behavioral pattern observed in autism. Knaus et al. [2010] combined structural and functional imaging in a group of 14 adolescent boys with ASD to unravel the relationship between language function, language laterality, and anatomical measures. Their findings suggested that language laterality rather than diagnosis would be strongly associated with differences in anatomy and behavior. Although a diagnostic imaging marker is at present still lacking, recent functional and diffusion imaging techniques seem to reveal subtle, but important, functional and/or microstructural changes in the brains of autistic children and adults.
By applying advanced neuroimaging techniques, the current study aims first to find a neurobiological substrate underlying the language difficulties seen in a subgroup of children with ASD. Our main focus was to examine the structural connectivity of the superior longitudinal fascicle (SLF) in a group of children with ASD and to relate the structural connectivity measures to their language profile. The SLF is a major association WM tract that can be considered as one of the key language tracts connecting Broca's area and Wernicke's area [Friederici et al., 2006; Makris et al., 2005]. First identified by Reil and Burdach (1819–1826) and subsequently Dejerine (1895) described this fiber system as one bundle of tracts connecting the posterior temporal lobe with the frontal lobe, arching around the Sylvian fissure in each hemisphere, using interchangeably the terms “Superior Longitudinal Fascicle” or “Arcuate Fascicle” in their descriptions [Catani and Mesulam, 2008]. Catani et al. [2005] proposed a greater complexity of the SLF connecting frontal and temporal language regions and described three important parts (instead of one bundle) based on tractography. First, they detected a direct longitudinal segment that corresponds to the previously called arcuate fascicle (AF) connecting the two most language‐relevant cortical regions in the human brain, that is, Wernicke's territory in the left temporal lobe and Broca's territory in the left frontal lobe (denoted in blue in Fig. 1). In addition, they found an indirect pathway consisting of two other segments: an anterior lateral segment linking Broca's territory with the inferior parietal lobe (Geschwind's area; denoted in yellow in Fig. 1) and a posterior lateral segment linking Geschwind's area with Wernicke's territory (denoted in red in Fig. 1). Although a significant relationship has been shown between interhemispheric asymmetry of the language network and specific cognitive abilities, the specific functions of the different segments remain poorly understood [Lebel and Beaulieu, 2009]. Studies using DTI tractography to explore the WM microstructural properties of the different segments of the SLF and its relation to the language profile in a group of children with ASD are limited. Recently, Nagae et al. [2012] reported abnormal DTI parameters of the SLF appearing to be associated with language impairment in ASD. Significantly elevated ADC values were detected in children with ASD and co‐occurring language impairment and were most pronounced in the left cerebral hemisphere, specifically in the temporal portion of the SLF [Nagae et al., 2012]. In contrast, Verhoeven et al. [2012] showed no significant differences in mean FA and mean ADC values for the SLF between ASD children with co‐occurring language impairment and healthy control children. Based on our structural connectivity results, our second main focus was to explore the functional connectivity of the language network in the same group of children with ASD using rfMRI. To our knowledge, no previous studies have placed language function in autism in the context of its structural connectivity, laterality, functional connectivity, and dynamics using DTI and rfMRI. In the final step, we aimed to relate the functional connectivity measures to the language function of our children with ASD. We hypothesized that in children with ASD, a neural connectivity deficit of the language network can be related to the observed abnormal language function.
Figure 1.
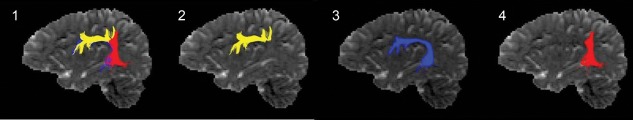
Reconstruction of the left‐hemispheric perisylvian language network and the subcomponents according to the reconstruction protocol of Catani in a healthy control subject: (1) superior longitudinal fascicle; (2) anterior segment of the SLF (denoted in yellow); (3) longitudinal segment of the SLF according to the arcuate fascicle (denoted in blue); and (4) posterior segment of the SLF (denoted in red).
METHODS
Participants
Twenty‐one children with ASD (mean age 13.77 ± 1.5 years; 18 males and 3 females) and 26 age‐matched controls (CO; mean age 14.33 ± 1.25 years; 19 males and 7 females) were included. All participants were right‐handed, native Dutch speakers with normal hearing. All the included children had a normal intelligence with a performance or full‐scale IQ above 80. Inclusion criteria for the ASD group were as follows: (1) a diagnosis of autistic disorder or pervasive developmental disorder—not otherwise specified according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition, Text Revision (DSM‐IV‐TR) criteria [American Psychiatric Association, 2000]; (2) raw scores equal to or greater than 15 on the Social Communication Questionnaire (SCQ) [Rutter et al., 2003]; and/or (3) raw scores above 60 on the Social Responsiveness Scale (SRS) [Constantino et al., 2003]. Participants were selected from a clinical sample of children with previous diagnosis made by a multidisciplinary team including a pediatric neurologist/psychiatrist and based on the DSM‐IV‐TR criteria. SCQ and SRS were used to ensure the current presence of substantial ASD symptoms. Participants were excluded if there was a chronic medical illness, a metabolic disorder, or an abnormal neurological examination, if ASD was associated with a genetic syndrome or if conventional MRI was found to be abnormal.
Healthy volunteers were actively recruited. None of them had a history of neurological or psychiatric conditions or a current medical, developmental, or psychiatric diagnosis. They did not report any language problems. The parents of the healthy volunteers completed the SCQ and SRS questionnaires to exclude the presence of substantial ASD symptoms.
This study protocol was approved by the ethical board of the University Hospitals Leuven, Belgium. Parents and children were informed about the experiment; informed consent was obtained from all parents/guardians according to the Declaration of Helsinki, with additional assent from all participating children.
Neuropsychological and Language Assessment
Participants were assessed with an abbreviated version of the Dutch Wechsler Intelligence Scale for Children, Third Edition, to estimate IQ [Kort et al., 2005]. The abbreviated intelligence test involved the subtests Block Design and Picture Completion to estimate a performance IQ (PIQ) and the subtests Vocabulary and Similarities to estimate verbal IQ (VIQ). The abbreviated version has been found to correlate well with a full IQ battery and has been used in other studies of cognitive ability and language [Hohnen and Stevenson, 1999]. The IQ scores were used to confirm normal intelligence in all participants. Handedness was assessed with the Dutch version of the Oldfield Handedness Questionnaire [Oldfield, 1971] in all participants. Language skills of our study population were assessed with the Dutch version of the Clinical Evaluation of Language Fundamentals (CELF‐4NL) [Kort et al., 2008]. To assess language performance of all language domains (e.g., semantics, phonology, morphology, syntax, and pragmatics) in an expressive and receptive way, the following subtests of the CELF‐4NL were used: Concepts and Following Directions (CFD), Sentence Formulation (SF), Sentence Assembly (SA), Word Definitions (WD), Word Classes Expressive (WC‐E), Word Classes Receptive (WC‐R), Text Comprehension (TC), Semantic Relations (SR), and Word Associations (WA). The Peabody Picture Vocabulary Test (PPVT) [Lloyd and Leota, 2005] was used to assess receptive vocabulary. IQ scores and language skills were evaluated on the day of scanning or in a time interval of less than 1 month prior to scanning. The total duration of testing was on average 2.5 h.
Imaging Acquisition
All acquisitions were performed on the same 3T whole‐body scanner (Philips, Best, The Netherlands) using an eight‐channel head coil. Anatomical imaging consisted of a high‐resolution structural volume acquired using a coronal 3D turbo field echo T1‐weighted images sequence with the following parameters: 182 contiguous coronal slices covering the whole brain and brainstem, slice thickness = 1.2 mm, repetition time (TR) = 9.7 ms, echo time (TE) = 4.6 ms, field of view (FOV) = 250 × 250 mm2, matrix size = 256 × 256, in‐plane pixel size = 0.98 × 1.20 mm2, and acquisition time = 6 min, 38 s.
DTI data were acquired using an optimized single‐shot‐spin‐echo, echo planar imaging sequence with the following parameters [Leemans and Jones, 2009]: 68 contiguous sagittal slices, slice thickness = 2.2 mm, TR = 11.043 s, TE =55 ms, FOV = 220 × 220 mm2, matrix size = 112 × 109, in‐plane pixel size = 1.96 × 2.00 mm2, acquisition time = 10 min, 34 s. Diffusion gradients were applied in 45 noncollinear directions (b = 800 s/mm2), and one nondiffusion‐weighted image was acquired. Two identical DTI datasets were consecutively acquired per subject to improve the reliability of the estimated diffusion measures, bringing the total acquisition time to 21 min, 8 s.
For the task‐related fMRI session, a T2*‐weighted GE‐EPI sequence was used with the following parameters: TR = 3,000 ms; TE = 33 ms; matrix size = 80 × 80; FOV = 230 mm; flip angle = 90°; slice thickness = 4 mm; no gap; axial slices = 35. A total of 80 functional volumes per subject were collected. To localize cortical regions involved in language function, a relatively easy‐to‐perform verb‐generation task was used. During the task, a noun was visually displayed on a screen outside the magnet bore, and the participant was instructed to covertly generate one or more verbs associated with it (e.g., “coffee” → “drink”). Stimuli were presented in a block design. Four task epochs (30 s each, 3 s for a single noun) are alternated with four periods of rest (30 s each). During rest epochs, participants passively viewed a series of unpronounceable scrambled visual symbols at the center of the screen (e.g., #/°$‐). Using Presentation software, Version 14.1 (Neurobehavioral Systems, CA) visual stimuli were projected and viewed with a mirror placed above the head coil. To ensure that all participants were able to generate correct verbs in response to the presented nouns, task performance was assessed outside the scanner before the scan session. Response times outside the scanner were 1.5 s, suggesting that they had enough time in the scanner to complete each trial within the time given (TR = 3 s).
For the task‐independent rfMRI, data were acquired using a T2*‐weighted GE‐EPI sequence with the following parameters: TR = 1,700 ms; TE = 33 ms; matrix size = 64 × 64; FOV = 230 mm; flip angle = 90°; slice thickness = 4 mm; no gap; axial slices = 32. Two hundred fifty functional volumes were obtained in 7 min. All participants were instructed to relax (but not sleep), keep their eyes closed, and think of nothing in particular during the rfMRI scanning.
Imaging Processing
DTI data analysis
Raw diffusion MR data were transferred to an offline workstation. All the images were first visually inspected for the presence of apparent artifacts. MRI data were preprocessed and postprocessed using ExploreDTI [Leemans et al., 2009]. Motion and eddy current correction of the diffusion‐weighted images was performed. During this preprocessing step, the b‐matrix was corrected for the rotational component of subject motion to ensure that deviations in the diffusion weighting originating from these rotations would be taken into account [Leemans and Jones, 2009]. Based on visual inspection of the acquired native data and of the data‐quality‐summary report as implemented in ExploreDTI, those subjects with apparent motion artifacts (signal drop outs, smearing, etc.) were excluded.
Subsequently, the diffusion tensors (DTs) were estimated using nonlinear least squares fitting (note that this was performed on the concatenation, not the average, of both DTI data sets to improve the reliability of the estimated diffusion measures). Whole‐brain fiber tractography was calculated for each DTI data set using a uniform 2‐mm seed point resolution and the following thresholds: FA termination threshold of 0.2, angle threshold of 30°, and a minimal fiber tract length threshold of 50 mm. A good reliability for these algorithm settings was confirmed in a previous study [Verhoeven et al., 2012]. Region of interest (ROI) delineation for the SLF was done manually and according to the ROI definition protocols of Catani et al. [2002], who showed a high reproducibility and reliability of their tract reconstruction protocols.
Mean number of streamlines (N°), mean FA, and mean ADC were entered as dependent variables in two‐way (2 × 2), mixed analysis of variance (ANOVA) models using PROC MIXED in SAS 9.3 (SAS Institute, Cary, NC). Hemisphere (left vs. right) and participant group (CO vs. ASD) were entered as within‐subject and between‐subject independent variables, respectively, as well as a group × hemisphere interaction effect. The Kolmogorov‐Smirnov test was performed to confirm normal distribution of the dependent variables, and normal distribution of the residuals was confirmed after performing the analysis. The significance threshold was set at P < 0.05, and post hoc t‐tests with Bonferroni correction for multiple testing were applied when appropriate (i.e., in case of a significant group × hemisphere interaction effect).
Structural connectivity linked to behavioral parameters
To detect neuroanatomical related differences in language performance, all children receiving language assessments were divided into four groups. Control groups and clinical groups were labeled as CO+ and CO−, ASD+ and ASD−, referring to the presence (+) or absence (−) of the right‐hemispheric AF, respectively. A series of ANOVA was performed to characterize the main effects of group (ASD vs. CO) and/or AF (presence vs. absence of the right‐hemispheric AF) and an interaction effect between both independent variables (group × AF), with each behavioral parameter as the dependent variable of a separate model. The Kolmogorov‐Smirnov test was performed to confirm normal distribution of the dependent variables. The significance threshold was set at P < 0.05, and post hoc t‐tests with Bonferroni correction for multiple testing were applied when appropriate (i.e., in case of a significant group × AF interaction effect).
Blocked design language‐related fMRI data analysis
SPM8 was used for image preprocessing and statistical analysis [Friston et al., 1999]. Single‐subject functional image time series were realigned to each other using a two‐pass procedure, registering the images to the mean image after a first realignment. Next, the structural image was coregistered with the mean of the functional time series. Finally, the images were normalized to standard Montreal Neurological Institute (MNI) EPI space, resliced to a voxel size of 2 mm × 2 mm × 2 mm and smoothed with a full width at half‐maximum (FWHM) Gaussian Smoothing Kernel of 8 mm.
After preprocessing, the following analyses were carried out. First, for each subject, a general linear model was used to regress the time course of verb generation versus rest as predictors and with the realignment parameters as regressors of no interest. A high‐pass filter with a cutoff of 128 s was used to remove slow signal drifts. Voxel‐wise T‐maps were constructed for each subject. Second, the contrast maps were carried to a second‐level analysis to test for significant group effects using a two‐level, one‐way ANOVA. Third, a conjunction analysis was performed starting from the single‐group fMRI statistical activation maps to select those voxels active in both subject groups during the execution of the language task. Conjunctions were assessed with the standard global null hypothesis using SPM software. This method compares the minimum t statistic to the null distribution of a minimum t statistic. This null distribution assumes that there is no effect for the first group (ASD) and that there is no effect for the second group (CO). In general, this method tests against the null hypothesis of no effect in any of the comparisons (none of the comparisons have activated). The conjunction maps were thresholded at P = 0.001 uncorrected for multiple comparisons and 10 voxels cluster size. At each peak of activation, a seed was selected, and the volume of the seed was extended to a maximum of ∼200 voxels, which corresponds to a sphere of 11 mm. The selected seeds are assumed to encompass the language network (see below). MNI coordinates of the peak activation (center of the seed), the T‐value of the peak, and cluster size are given in Table 1.
Table 1.
Seed definitions
| Language‐related areas | |||||
|---|---|---|---|---|---|
| Region of interest | MNI coordinates of peak activation | Cluster size | T‐value of the peak | ||
| x | y | z | |||
| Left Broca | −52 | 14 | −2 | 145 | 5.89 |
| Right Broca | 48 | 14 | −2 | 132 | 3.93 |
| Left Wernicke | −58 | −42 | 2 | 117 | 3.88 |
| Right Wernicke | 54 | −34 | −6 | 107 | 3.76 |
Resting‐state fMRI data analysis
Functional connectivity analysis of the language network was performed using in‐house developed software [Ebisch et al., 2011]. To check for potential differences in head movement between participants of both control groups and ASD groups, we compared both subgroups with respect to motion parameters using the root‐mean‐squared variance (RMS variance) [Church et al., 2009]. Preprocessing of the rfMRI data was very similar to the preprocessing of the task‐related fMRI time series, comprising realignment to the mean, followed by a coregistration to the structural image and normalization into standard MNI EPI space. Images were resliced to a 3 mm × 3 mm × 3 mm voxel size and smoothed with a FWHM Gaussian Smoothing Kernel of 8 mm. Further preprocessing included (1) band‐pass filtering between 0.009 and 0.08 Hz, (2) regression of the WM and cerebrospinal fluid signals based on the MNI WM and the ventricular segmentation mask, and (3) regression of the 3D motion parameters. To avoid the introduction of false‐negative correlations, the global signal was not regressed out [Weissenbacher et al., 2009]. In addition, we used scrubbing [Power et al., 2012] to control for movement‐induced artifacts, with a framewise displacement threshold of 0.5 mm and a threshold for the derivative of the variance across brain voxels equal to 0.5% signal change (identical algorithms and thresholds were used by Power et al. [2012]). For each ROI, a representative BOLD time course was obtained by averaging the signal of all the voxels within the ROI. To assess functional connectivity, we first calculated the Pearson's correlation coefficient between the mean signal intensity time courses of each ROI pair. A Fisher's r‐to‐z transformation was applied to each correlation map to obtain an approximately normal distribution of the functional connectivity values and to accordingly apply parametric statistics. A random‐effect analysis was performed independently for each group of participants to reveal coherent functional connectivity patterns between ROI that were consistent across participants. Statistical significance was assessed using a threshold of P < 0.001 uncorrected. Using the results from the random‐effect analysis, we used an independent samples t‐test to calculate direct contrasts between the participant groups with respect to the different ROI. Here, we applied a statistical threshold of P < 0.05 uncorrected.
Functional connectivity linked to behavioral parameters
In the final step, we calculated Pearson's correlations between functional connectivity values and behavioral measures, focusing on language performance [VIQ and scores on the PPVT and the Mean Language Score on the CELF‐4NL], for both control groups and ASD groups.
RESULTS
Demographics
An overview of the group characteristics is presented in Table 2. Originally 21 right‐handed children with ASD and 26 age‐matched right‐handed control children were included in the study; 4 children with ASD and one healthy CO child were excluded due to excessive motion artefacts. For final analysis, data of 17 children with ASD and 25 CO children were used. There were no age differences between the ASD and CO group. No significant age differences are detected between the ASD+ and ASD‐ (P = 0.226) group and the CO+ and CO‐ group (P = 0.307). The VIQ of the ASD group was significantly lower than the control group, reflecting possible language problems in all or a sub‐group of children with ASD. A trend was observed in the PIQ being lower in the ASD group. All control children scored below the risk value for ASD on the SRS and SCQ questionnaires, whereas all children with a diagnosis of ASD presented with SRS and/or SCQ values above the set risk value.
Table 2.
Participant characteristics
| Subject characteristics per group | P value between study groups | ||||
|---|---|---|---|---|---|
| ASD (N = 17) | CO (N = 25) | ||||
| Mean ± SD | Range | Mean ± SD | Range | ||
| Age (months) | 167.41 ± 16.18 | 145–202 | 173.72 ± 15.89 | 146–203 | n.s. |
| Gender (male:female) | 14:3 | 19:6 | |||
| VIQ | 88.24 ± 19.20 | 50–124 | 112.32 ± 13.77 | 86–130 | <0.001a |
| PIQ | 98.00 ± 14.94 | 77–132 | 105.36 ± 8.13 | 89–126 | n.s. |
| SRS | 92.00 ± 32.14 | 27–139 | 17.92 ± 14.62 | 2–53 | <0.001a |
| SCQ | 20.12 ± 8.59 | 5–37 | 3.64 ± 3.65 | 0–15 | <0.001a |
Note: An overview of the participant characteristics age, gender, Verbal IQ (VIQ), Performance IQ (PIQ), and scores on the Social Responsiveness Scale (SRS) and Social Communication Questionnaire (SCQ) for the autism group (ASD; N = 17) and their respective age‐matched controls (CO; N = 25) is given. The mean and standard deviation (SD) are given for each parameter. P values are given for significant between‐group differences (P < 0.05).
Values obtained using a nonparametric test (Mann‐Whitney test).
Results of DTI Analysis
Results of the DTI analysis are presented in Figure 2. In all children the entire left‐hemispheric and right‐hemispheric language network (further called SLF) could be reconstructed. A main effect of hemisphere (left vs. right) was found for mean number of streamlines (N°), mean FA and mean ADC. An overall interhemispheric leftward asymmetry was found across both study groups: number of steamlines, FA and ADC were significantly lower on the right side compared to the left. No main effect of group (ASD vs. CO) was found for mean number of streamlines, mean FA or mean ADC. For both left‐ and right‐hemispheric SLF, no significant differences were found for mean number of streamlines, mean FA and mean ADC between the CO subjects and ASD participants. No interaction effects (group × hemisphere) were found for mean number of streamlines, mean FA and mean ADC.
Figure 2.
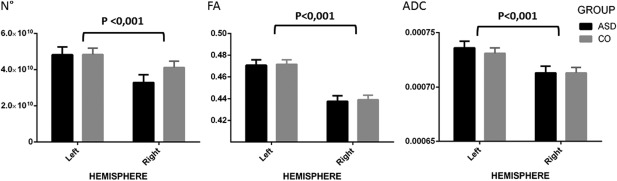
Bar graph showing the mean number of streamlines (N°), mean fractional anisotropy (FA), and mean apparent diffusion coefficient (ADC) in controls and children with ASD. The T‐bars that extend from the boxes represent the standard deviation (SD). Main effects of hemisphere are indicated above the bar graphs (***P < 0.001).
After reconstruction of the SLF, virtual dissections of the three segments of the perisylvian network (anterior SLF, posterior SLF, and longitudinal AF) were made according to the delineation protocol of Catani et al. [2005]. Following the predefined algorithm settings (see Methods section), no significant differences were found for mean FA and mean ADC between CO children and ASD children with a detectable anterior SLF, posterior SLF and AF. However, a significantly higher number of streamlines was found for the left posterior SLF in the ASD group (P = 0.028).
Using the predefined tractography methods (see Methods section), reconstruction of the right‐hemispheric AF, which directly connects the two most relevant language‐related cortical areas, revealed no detectable right‐hemispheric AF in 28% (7/25) of the healthy control children and in 59% (10/17) of the children with ASD. The right‐hemispheric AF was significantly more absent in children with ASD when compared with healthy control children (X 2 = 3.990; df = 1; P = 0.046). In participants with a unilateral left‐hemispheric AF, no overconnectivity was found on the ipsilateral side: no higher number of streamlines, FA, or ADC was found leftsided when compared with children with a bilateral AF.
Structural Connectivity Linked to Behavioral Parameters
All children who underwent language assessments (N = 42) were divided into four groups according to the bilateral presence (+) or absence (−) of the AF. These groups were labeled as follows: ASD+ (N = 7); ASD− (N = 10); CO+ (N = 18); and CO− (N = 7). To detect neuroanatomical related differences in language, the age‐standardized language scores were compared amongst these groups. These results are presented in Figure 3 and Table 3. When comparing ASD children with CO children, we found an overall group difference for the scores of the SRS, SCQ, VIQ, PPVT, and every language subtest of the CELF‐4NL, except for the subtest WA. A main effect of AF was found for language scores on the PPVT: the absence of the right‐hemispheric AF was related to a better performance on the PPVT in both CO children and ASD children. We observed an interaction effect (group × AF) between the presence versus absence of the right‐hemispheric AF and the CO versus ASD group on VIQ, scores on the PPVT, and the following subtests of the CELF‐4NL: CFD, SF, WC‐R, WC‐E, WD, SA, and SR. In addition, this relationship was found for the mean Language Score, defined as the average of the scores on all subtests of the CELF‐4NL. This indicates that a difference in language performance between control children and children with ASD is driven by the absence of the right‐hemispheric AF.
Figure 3.
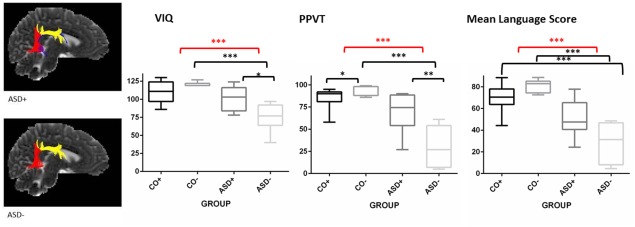
A boxplot representation of the VIQ, scores on the PPVT, and mean language score on the CELF‐4NL in bilateral control (CO+) and ASD (ASD+) children and left‐hemispheric unilateral control (CO−) and ASD children (ASD−). The central line of the box represents the median value for each subject group. The T‐bars that extend from the boxes relate to the minimum and maximum values. The red line represents a significant interaction effect (group × AF). The black lines show post hoc t‐tests with Bonferroni correction (*P < 0.05; **P < 0.01; ***P < 0.001).
Table 3.
Structural connectivity linked to behavioral parameters
| P value | |||
|---|---|---|---|
| Main effect GROUP | Main effect AF | Interaction effect (GROUP × AF) | |
| SRS | <0.001 | n.s. | n.s |
| SCQ | <0.001 | n.s. | n.s. |
| VIQ | <0.001 | n.s. | 0.001 |
| PIQ | 0.064 | n.s. | n.s. |
| Pragmatieklijst | <0.001 | n.s. | n.s. |
| PPVT | <0.001 | 0.034 | <0.001 |
| CFD | <0.001 | n.s. | 0.047 |
| SF | <0.001 | n.s. | 0.013 |
| WC‐R | <0.001 | n.s. | 0.003 |
| WC‐E | <0.001 | n.s. | 0.001 |
| WD | <0.001 | n.s. | 0.008 |
| TC | 0.004 | n.s. | n.s. |
| SA | <0.001 | n.s. | 0.015 |
| SR | <0.001 | n.s. | 0.034 |
| WA | n.s. | n.s. | n.s. |
| Mean language score | <0.001 | n.s. | 0.001 |
Note: A series of ANOVA analyses were performed to characterize the main effect of GROUP and/or AF and an interaction effect for scores on the Social Responsiveness Scale (SRS), Social Communication Questionnaire (SCQ), Verbal IQ (VIQ), Performance IQ (PIQ), and all subtests of the CELF‐4NL. The results of the ANOVA analyses with GROUP (ASD or CO) and AF (+ or −) as between‐subject factors are given. P values are given for significant main and/or interaction effects (P < 0.05) or when a statistical trend was observed (P < 0.065). Selected results are graphically shown in Figure 3.
Post hoc t‐tests with Bonferroni correction for multiple testing were applied when appropriate (i.e., in case of a significant group × AF interaction effect). These results are presented in Figure 3 and Supporting Information Table SIV. Healthy control children with a unilateral left‐hemispheric AF presented with a significantly higher score on the PPVT and the subtest WC‐E of the CELF‐4NL when compared with control children with a bilateral AF. The ASD group with bilateral presentation of the AF did not differ from the bilateral healthy control group for language performance. This is despite the fact that pragmatic language skills in the ASD+ group are significantly lower than the control group. On the other hand, children with ASD and a unilateral leftsided presentation of the AF had significantly lower scores on the PPVT and all subtests of the CELF‐4NL when compared with the CO− group. Children with ASD and a bilateral AF performed significantly better on VIQ, the PPVT, and the subtests WC‐E and SA of the CELF‐4NL when compared with unilateral children with ASD.
Results of Functional Connectivity Analysis
Subsequently, we evaluated the relationship between the absence of the right‐hemispheric AF and the functional connectivity of the language network in both control groups (CO+ and CO−) and clinical groups (ASD+ and ASD−). To control for differences in head motion between both patient groups and control groups, statistical comparison of the amount of head movement, as seen on fMRI, was performed. The average subject RMS variance [Church et al., 2009] of motion was 0.077 mm (SD = 0.023) in the CO+ group, 0.095 mm (SD = 0.028) in the CO− group, 0.092 mm (SD = 0.041) in the ASD+ group, and 0.010 mm (SD = 0.040) in the ASD− group. No significant differences in motion could be detected between healthy CO children (P = 0.243) and ASD children and between both CO groups (P = 0.101) and both ASD groups (P = 0.703). Functional connectivity in the language network was evaluated by correlating the mean signal intensity time courses for each ROI pair combination in the four cortical language network components: left Broca (LBroca), left Wernicke (LWernicke), right Broca (RBroca), and right Wernicke (RWernicke). In the CO+, CO−, and ASD+ group, significant connectivity (P < 0.001) was identified in five of six functional connectivity links (Fig. 4A–C). In these groups, no statistical significance was found between right Broca and left Wernicke. In the ASD− group, only two of six functional connectivity links were significant (P < 0.001; Fig. 4D). Note the preserved connectivity between the left and right inferior frontal gyrus (Broca) and the left and right superior temporal gyrus (Wernicke) and the profound loss of intrahemispheric connectivity in the ASD− group. Direct contrasts between both CO groups and both ASD groups were calculated (P < 0.05 for between group comparisons). In Supporting Information Figure S5, the comparison of functional connectivity of the language network between both control groups (Supporting Information Fig. S5A) and both ASD groups (Supporting Information Fig. S5B) is shown. A higher functional connectivity of the language network could be detected in three of six functional connectivity links for healthy CO children with a unilateral structural language network when compared with bilateral CO children. However, these functional connectivity links are still significant in both CO groups. In the ASD− group, a significantly lower functional connectivity could be detected in two of six functional connectivity links being the left Broca–left Wernicke and left Broca–right Wernicke connections.
Figure 4.
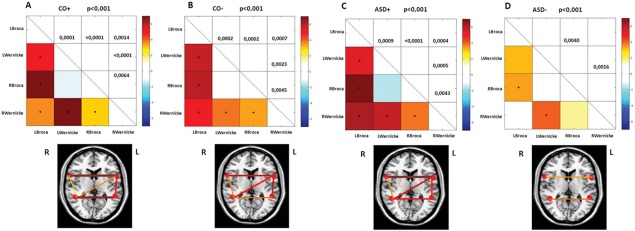
Correlation matrices representing functional connectivity links of the language network for both CO groups and ASD groups. The color represents the T‐value of connectivity between two connected brain regions. Significant correlations at P < 0.001 uncorrected are indicated with a dot at the center of the matrix square. P values are given for the significant correlations. Below each correlation matrix, significant correlations are schematically represented as lines on an axial slice. Line thickness and color vary according to the scale above. LBroca = Left Broca; RBroca = Right Broca; LWernicke = Left Wernicke; RWernicke = Right Wernicke.
Functional Connectivity Linked to Behavioral Parameters
For all participants, a significant correlation was found between VIQ and the intrahemispherical left Broca–left Wernicke (r = 0.505; P = 0.001) and interhemispherical left Broca–right Wernicke (r = 0.346; P = 0.003) connections. Scores on the PPVT were significantly correlated with the left Broca−left Wernicke (r = 0.515; P = 0.002), left Broca−right Wernicke (r = 0.482; P = 0.003), and right Broca−right Wernicke (r = 0.381; P = 0.024) connections. For healthy control children, a significant correlation was found between scores on the PPVT and the left Broca−left Wernicke connection (r = 0.563; P = 0.006), the left Broca−right Wernicke connection (r = 0.468; P = 0.028), and the right Broca−right Wernicke connection (r = 0.561; P = 0.007). In contrast, for the ASD group, a significant correlation was found between VIQ and the direct left Broca−left Wernicke connections (r = 0.580; P = 0.023) and between scores on the PPVT and the right Broca−right Wernicke connections (r = 0.593; P = 0.032).
DISCUSSION
The development of language, social interaction, and communicative skills are remarkably different in the child with autism. Although these skills result from long‐distance connections between diverse brain regions, such alterations in brain architecture could mediate the early clinical manifestations of autism [Courchesne et al., 2007]. Evidence from structural and functional neuroimaging studies suggests the frontal and temporal lobes as the cortical areas most heavily affected in ASD [Courchesne and Pierce, 2005; Courchesne et al., 2007]. The current study investigated the microstructural properties of the language network in ASD using DTI. Second, these neurostructural findings, assessed with DTI, were linked to the functional connectivity of the language network in our study population using rfMRI. The language network could be confidently identified using a well‐established verb‐generation task, which has proven to consistently activate the regions involved in language processing [Benson et al., 1999; Petersen et al., 1988]. Three important findings emerge from this study. First, the WM anatomy of the perisylvian language network varies within a group of children with autism. Second, in contrast to healthy control children, a bilateral representation of the AF is associated with a better verbal outcome in children with autism. Third, functional underconnectivity between frontal and temporal brain areas is related to a lower language function in children with autism.
First, we detected heterogenous WM asymmetry of the AF in our group of children with ASD. Most interestingly, we found an absence of the right‐hemispheric AF in 59% of children with ASD. This finding is pertinent to autism as it supports the underconnectivity hypothesis in autism stating that underconnectivity of association fibers is a consequence of early brain overgrowth, leading to widespread alterations in synapse elimination and/or formation [Courchesne et al., 2005]. The brain overgrowth occurs postnatally within the first 6–14 months, coinciding with what is normally a critical period of postnatal exuberant synaptogenesis, dendritic arborization, and ongoing myelination for the typically developing toddler [Belmonte et al., 2004; Courchesne et al., 2003]. It has been argued that within the frontal lobe, the dorsolateral convexity shows significant overgrowth, whereas the precentral gyrus and orbital cortex are not robustly affected [Belmonte et al., 2004]. Interestingly, we found a well‐developed bilateral indirect anterior SLF reaching the precentral gyrus in all children with ASD, except in two children who did not show an indirect left anterior component.
Second, previous studies have reported severe linguistic deficits, including morphologic, syntactic, and semantic deficits in a subgroup of subjects with ASD [Groen et al., 2008; Kjelgaard and Tager‐Flusberg, 2001; Williams et al., 2008]. This clustering of symptoms may suggest that common causes underlie these language deficits in autism. However, a clear relationship between the linguistic categories and cortical function is currently lacking. The linguistic phenotype observed in our subgroup of subjects with ASD indicates a novel underlying neuroanatomical substrate of language function in ASD: bilateral intrahemispheric connections between the inferior frontal and posterior temporal regions reveal an advantage for the language performance in children with ASD (Fig. 3). In other words, in children with autism, the absence of the right‐hemispheric AF is found to be a negative prognostic marker for their language performance. Similar to the findings of Fine et al. [1994], we found that language abilities of ASD children with both a left‐ and right‐hemispheric AF are within the normal range of functioning, although their social communication abilities remain impaired.
Our study shows a significantly higher number of streamlines, FA, and ADC in the left hemisphere when compared with the right hemisphere in both control children and children with ASD. This is consistent with previous DTI studies reporting a leftward asymmetry in healthy adult and pediatric populations [Glasser and Rilling, 2008; Makris et al., 2005; Parker et al., 2005; Powell et al., 2006; Vernooij et al., 2007]. A unilateral left AF was observed in our healthy control children in 28% being within the range of previous findings [Eluvathingal et al., 2007; Lebel and Beaulieu, 2009]. Our data show that in contrast to children with ASD, healthy control children do not seem to benefit from a bilateral representation. Healthy control children with a unilateral language network presented with higher scores on the PPVT and the Expressive Word Class subtest of the CELF‐4NL when compared with bilateral control children (Fig. 3). These findings are consistent with the studies of Lebel and Beaulieu [2009] who showed that a unilateral representation of the AF was associated with better scores on the PPVT. Future studies on different populations will help to further elucidate the relationship between language function and lateralization of language‐related WM tracts.
Third, in the patients with ASD without right‐hemispheric AF, we found a marked loss of intrahemispheric functional connectivity between the cortical language centers (Fig. 4). Functional underconnectivity between frontal and posterior brain regions was first detected using a sentence comprehension task in children with high‐functioning autism [Just et al., 2004]. Reduced interregional collaboration was also shown in other studies using semantic processing tasks [Harris et al., 2006; Kana et al., 2006]. In contrast to this task‐dependent functional connectivity, other studies focused on spontaneous low‐frequency fluctuations in BOLD signal intensity by low‐pass filtering and by removing task‐dependent effects from the time series data before calculating functional connectivity links. Both reduced [Jones et al., 2010; Villalobos et al., 2005] and increased [Mizuno et al., 2006; Noonan et al., 2009; Shih et al., 2010; Turner et al., 2006; Weng et al., 2010] task‐free (or intrinsic) functional connectivity in autism has been described, reflecting abnormal information integration and synchronization. Using rfMRI, our results are not influenced by potential differences in task performance and provide further evidence of cortical anterior–posterior underconnectivity in autism expanding to the resting state of the brain [Cherkassky et al., 2006; Kennedy and Courchesne, 2008; Weng et al., 2010]. However, most interestingly, in our control group without right‐hemispheric AF studied, we detected preserved functional connectivity of their language network. This indicates as previously suggested by Deco and Corbetta [2011] in a general adult population that indirect functional connectivity maps do not only reflect direct but also indirect anatomical connections, either through other cortical or subcortical regions. In all children, the existence of a well‐developed intrahemispherical and interhemispherical functional language network was associated with better verbal language outcomes. In our subgroup of children with autism and a bilateral structural language network, a significant correlation was found between scores on the PPVT and the right Broca–right Wernicke functional connections, suggesting that a right‐hemispheric compensation mechanism might cause a better verbal performance in those ASD children. However, the nature of this structure–function relationship is only beginning to be understood. To further unravel this enigma of neuroplasticity, new preprocessing and postprocessing techniques examining joint information of both structural and functional imaging data can help future studies to find hidden traits in complex neurodevelopmental disorders such as autism.
This study has limitations, some of which relate to the technical aspects of DTI tractography and fMRI and other to the sample size of our study population. DTI tractography only provides indirect indices of tissue properties and therefore uncertainty exists about the correspondence between tractography measures and underlying biological factors (number of streamlines, degree of myelination, etc.) [Catani et al., 2007]. Furthermore, partial volume effects and complex multiple fiber orientations within a single voxel detract from the accuracy of DTI‐based fiber tracking [Vos et al., 2011; Wedeen et al., 2008]. Such challenges are being addressed by other techniques such as Q‐ball and Q‐space imaging and constrained spherical deconvolution, which hold promise for future work [Jeurissen et al., 2011; Tournier et al., 2008]. Although these recently developed techniques might provide more accurate and unambiguous results, the more complex data processing, long imaging times, and strong demands on the magnetic field gradient hardware still impede practical application in a clinical setting. Because we were studying an autistic and pediatric population, we were not able to perform longer imaging times. In terms of the DTI analysis, the extent and geometry of tracking results is influenced by the tracking parameters used. To satisfy ourselves that the findings we describe in this study are not an artifact of these parameters, we attempted to track the right AF using new thresholds (FA termination threshold of 0.1, angle threshold of 45°, and a minimal fiber tract length threshold of 20 mm) in ASD− and CO− groups. The right‐hemispheric AF, however, remained absent, increasing our confidence in the reported results. Furthermore, it is important to acknowledge the general limitations of rfMRI. First, using a task‐based fMRI in children with autism may have its limitations because of the potential influence of subject performance on fMRI activity. Interpretation becomes complicated when performance on the well‐known and well‐validated language task is not matched. More specifically, in such cases, the abnormal fMRI response may be either the result or the cause of the performance deficit. Therefore, drawing extended conclusions may not be preferable. We did use the task‐based fMRI to identify the language system in both patients and controls. Activation peaks were used as seeds for the rfMRI analysis. Second, resting‐state connectivity is vulnerable to noise and physiological artifacts. Although we ruled out the differences due to head motion and regressed out the physiological variations of the time series data, it might be of additional value to perform cardiac gating and to synchronize the respiratory signal. Because we were working with an autistic and young population, we were not able to perform this extra monitoring. Finally, the relatively small sample size limits the conclusions that can be drawn regarding the findings of this study. Therefore, the specificity of our findings to autism requires further investigation using comparison groups in larger scale studies. We believe that the application of advanced neuroimaging to ASD is able to provide new insights into the microstructural and microfunctional organization of the diseased brain. For inferences on the whole ASD population, refinement of the neurobiological properties of the language network in younger children with ASD needs further study and is beyond the aims of this study.
CONCLUSION
In a group of children with ASD, both structural and functional connectivity of the language network was related to language function. DTI tractography revealed a pattern of extreme leftward asymmetry in a subgroup of children with ASD. In this subgroup, the absence of the right‐hemispheric AF was related to their co‐occurring language impairment. Our rfMRI data link the language impairment in ASD to a marked loss of functional connectivity between inferior frontal and superior temporal regions, reflecting the cortical language network. Both structural and functional underconnectivity patterns are found to be negative prognostic markers for the language performance in children with ASD. By combining the analysis of structural and functional connectivity, this study allows a better understanding of the neurobiology of language problems in ASD.
Supporting information
Supporting Information Figure
Supporting Information Table
ACKNOWLEDGMENTS
The authors are grateful to the participants and healthy volunteers who made this research possible.
REFERENCES
- American Psychiatric Association (2000): Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association. [Google Scholar]
- Barnea‐Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. (2004): White matter structure in autism: Preliminary evidence from diffusion tensor imaging. Biol Psychiatry 55:323–326. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D (1994): MR diffusion tensor spectroscopy and imaging. Biophys J 66:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C (2002): The basis of anisotropic water diffusion in the nervous system—A technical review. NMR Biomed 15:435–455. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel‐Mitchener A, Boulanger LM, Carper RA, Webb SJ (2004): Autism and abnormal development of brain connectivity. J Neurosci 24:9228–9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson RR, FitzGerald DB, LeSueur LL, Kennedy DN, Kwong KK, Buchbinder BR, Davis TL, Weisskoff RM, Talavage TM, Logan WJ, Cosgrove GR, Belliveau JW, Rosen BR (1999): Language dominance determined by whole brain functional MRI in patients with brain lesions. Neurology 52:798–809. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34:537–541. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Chabane N, Gervais H, Good CD, Bourgeois M, Plumet MH, Barthélémy C, Mouren MC, Artiges E, Samson Y, Brunelle F, Frackowiak RS, Zilbovicius M (2004): Superior temporal sulcus anatomical abnormalities in childhood autism: A voxel‐based morphometry MRI study. Neuroimage 23:364–369. [DOI] [PubMed] [Google Scholar]
- Carper RA, Courchesne E (2000): Inverse correlation between frontal lobe and cerebellum sizes in children with autism. Brain 123 (Part 4):836–844. [DOI] [PubMed] [Google Scholar]
- Catani M, Mesulam M (2008): The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex 44:953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK (2002): Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage 17:77–94. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Ffytche DH (2005): Perisylvian language networks of the human brain. Ann Neurol 57:8–16. [DOI] [PubMed] [Google Scholar]
- Catani M, Allin MP, Husain M, Pugliese L, Mesulam MM, Murray RM, Jones DK (2007): Symmetries in human brain language pathways correlate with verbal recall. Proc Natl Acad Sci USA 104:17163–17168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. (2006): Functional connectivity in a baseline resting‐state network in autism. Neuroreport 17:1687–1690. [DOI] [PubMed] [Google Scholar]
- Church JA, Fair DA, Dosenbach NU, Cohen AL, Miezin FM, Petersen SE, Schlaggar BL (2009): Control networks in paediatric Tourette syndrome show immature and anomalous patterns of functional connectivity. Brain 132 (Part 1):225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, Metzger LM, Shoushtari CS, Splinter R, Reich W (2003): Validation of a brief quantitative measure of autistic traits: Comparison of the social responsiveness scale with the autism diagnostic interview‐revised. J Autism Dev Disord 33:427–433. [DOI] [PubMed] [Google Scholar]
- Correia S, Lee SY, Voorn T, Tate DF, Paul RH, Zhang S, Salloway SP, Malloy PF, Laidlaw DH (2008): Quantitative tractography metrics of white matter integrity in diffusion‐tensor MRI. Neuroimage 42:568–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E (2004): Brain development in autism: Early overgrowth followed by premature arrest of growth. Ment Retard Dev Disabil Res Rev 10:106–111. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K (2005): Why the frontal cortex in autism might be talking only to itself: Local over‐connectivity but long‐distance disconnection. Curr Opin Neurobiol 15:225–230. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Carper R, Akshoomoff N (2003): Evidence of brain overgrowth in the first year of life in autism. JAMA 290:337–344. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Redcay E, Morgan JT, Kennedy DP (2005): Autism at the beginning: Microstructural and growth abnormalities underlying the cognitive and behavioral phenotype of autism. Dev Psychopathol 17:577–597. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, Morgan J. (2007): Mapping early brain development in autism. Neuron 56:399–413. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. (2006): Consistent resting‐state networks across healthy subjects. Proc Natl Acad Sci USA 103:13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Corbetta M (2011): The dynamical balance of the brain at rest. Neuroscientist 17:107–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J, Hertz‐Pannier L, Cachia A, Mangin JF, Le Bihan D, Dehaene‐Lambertz G (2009): Structural asymmetries in the infant language and sensori‐motor networks. Cereb Cortex 19:414–423. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn ML, editors (2005): Peabody Picture Vocabulary Test‐III‐NL. Amsterdam: Pearson Assessment and Information B.V. [Google Scholar]
- Ebisch SJ, Gallese V, Willems RM, Mantini D, Groen WB, Romani GL, Buitelaar JK, Bekkering H (2011): Altered intrinsic functional connectivity of anterior and posterior insula regions in high‐functioning participants with autism spectrum disorder. Hum Brain Mapp 32:1013–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eluvathingal TJ, Hasan KM, Kramer L, Fletcher JM, Ewing‐Cobbs L (2007): Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb Cortex 17:2760–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine J, Bartolucci G, Szatmari P, Ginsberg G (1994): Cohesive discourse in pervasive developmental disorders. J Autism Dev Disord 24:315–329. [DOI] [PubMed] [Google Scholar]
- Fletcher PT, Whitaker RT, Tao R, DuBray MB, Froehlich A, Ravichandran C, Alexander AL, Bigler ED, Lange N, Lainhart JE (2010): Microstructural connectivity of the arcuate fasciculus in adolescents with high‐functioning autism. Neuroimage 51:1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD, Fiebach CJ, Schlesewsky M, Bornkessel ID, von Cramon DY (2006): Processing linguistic complexity and grammaticality in the left frontal cortex. Cereb Cortex 16:1709–1717. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Büchel C, Worsley KJ (1999): Multisubject fMRI studies and conjunction analyses. Neuroimage 10:385–396. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Rilling JK (2008): DTI tractography of the human brain's language pathways. Cereb Cortex 18:2471–2482. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V (2003): Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen WB, Zwiers MP, van der Gaag RJ, Buitelaar JK (2008): The phenotype and neural correlates of language in autism: An integrative review. Neurosci Biobehav Rev 32:1416–1425. [DOI] [PubMed] [Google Scholar]
- Harris GJ, Chabris CF, Clark J, Urban T, Aharon I, Steele S, McGrath L, Condouris K, Tager‐Flusberg H (2006): Brain activation during semantic processing in autism spectrum disorders via functional magnetic resonance imaging. Brain Cogn 61:54–68. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O'Brien LM, Lange N, Bakardjiev A, Hodgson J, Adrien KT, Steele S, Makris N, Kennedy D, Harris GJ, Caviness VS Jr (2003): Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain 126 (Part 5):1182–1192. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O'Brien LM, Kennedy DN, Filipek PA, Bakardjiev AI, Hodgson J, Takeoka M, Makris N, Caviness VS Jr (2005): Brain asymmetries in autism and developmental language disorder: A nested whole‐brain analysis. Brain 128 (Part 1):213–226. [DOI] [PubMed] [Google Scholar]
- Hohnen B, Stevenson J (1999): The structure of genetic influences on general cognitive, language, phonological, and reading abilities. Dev Psychol 35:590–603. [DOI] [PubMed] [Google Scholar]
- Jeurissen B, Leemans A, Jones DK, Tournier JD, Sijbers J (2011): Probabilistic fiber tracking using the residual bootstrap with constrained spherical deconvolution. Hum Brain Mapp 32:461–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TB, Bandettini PA, Kenworthy L, Case LK, Milleville SC, Martin A, Birn RM (2010): Sources of group differences in functional connectivity: An investigation applied to autism spectrum disorder. Neuroimage 49:401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ (2004): Cortical activation and synchronization during sentence comprehension in high‐functioning autism: Evidence of underconnectivity. Brain 127 (Part 8):1811–1821. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA (2006): Sentence comprehension in autism: Thinking in pictures with decreased functional connectivity. Brain 129 (Part 9):2484–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller TA, Kana RK, Just MA (2007): A developmental study of the structural integrity of white matter in autism. Neuroreport 18:23–27. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E (2008): The intrinsic functional organization of the brain is altered in autism. Neuroimage 39:1877–1885. [DOI] [PubMed] [Google Scholar]
- Kjelgaard MM, Tager‐Flusberg H (2001): An investigation of language impairment in autism: Implications for genetic subgroups. Lang Cogn Process 16:287–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus TA, Silver AM, Lindgren KA, Hadjikhani N, Tager‐Flusberg H (2008): fMRI activation during a language task in adolescents with ASD. J Int Neuropsychol Soc 14:967–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus TA, Silver AM, Kennedy M, Lindgren KA, Dominick KC, Siegel J, Tager‐Flusberg H (2010): Language laterality in autism spectrum disorder and typical controls: A functional, volumetric, and diffusion tensor MRI study. Brain Lang 112:113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kort W, Schittekatte M, Bosmans M, Compaan EL, Dekker PH, Vermeir G, Verhaeghe P, editors (2005): Handleiding en Verantwoording. Wechsler Intelligence Scale for Children. Amsterdam: Harcourt Publishers. [Google Scholar]
- Kort W, Schittekatte M, Compaan E, editors (2008): CELF‐4‐NL: Clinical Evaluation of Language Fundamentals. Amsterdam: Pearson Assessment and Information B.V. [Google Scholar]
- Lebel C, Beaulieu C (2009): Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children. Hum Brain Mapp 30:3563–3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans A, Jones DK (2009): The B‐matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med 61:13. [DOI] [PubMed] [Google Scholar]
- Leemans A, Jeurissen B, Sijbers J, Jones DK (2009):ExploreDTI: A graphical toolbox for processing, analyzing and visualizing diffusion MR data. In: Paper presented at the 17th Annual Meeting of Proceedings of the International Society for Magnetic Resonance in Medicine, Hawaii, USA.
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS Jr, Pandya DN (2005): Segmentation of subcomponents within the superior longitudinal fascicle in humans: A quantitative, in vivo, DT‐MRI study. Cereb Cortex 15:854–869. [DOI] [PubMed] [Google Scholar]
- Mizuno A, Villalobos ME, Davies MM, Dahl BC, Müller RA (2006): Partially enhanced thalamocortical functional connectivity in autism. Brain Res 1104:160–174. [DOI] [PubMed] [Google Scholar]
- Nagae LM, Zarnow DM, Blaskey L, Dell J, Khan SY, Qasmieh S, Levy SE, Roberts TP (2012): Elevated mean diffusivity in the left hemisphere superior longitudinal fasciculus in autism spectrum disorders increases with more profound language impairment. AJNR Am J Neuroradiol 33:1720–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan SK, Haist F, Müller RA (2009): Aberrant functional connectivity in autism: Evidence from low‐frequency BOLD signal fluctuations. Brain Res 1262:48–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Parker GJ, Luzzi S, Alexander DC, Wheeler‐Kingshott CA, Ciccarelli O, Lambon Ralph MA. (2005): Lateralization of ventral and dorsal auditory‐language pathways in the human brain. Neuroimage 24:656–666. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME (1988): Positron emission tomographic studies of the cortical anatomy of single‐word processing. Nature 331:585–589. [DOI] [PubMed] [Google Scholar]
- Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, Wheeler‐Kingshott CA, Barker GJ, Noppeney U, Koepp MJ, Duncan JS (2006): Hemispheric asymmetries in language‐related pathways: A combined functional MRI and tractography study. Neuroimage 32:388–399. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012): Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, Lord C (2003): Social Communication Questionnaire. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Shih P, Shen M, Ottl B, Keehn B, Gaffrey MS, Müller RA (2010): Atypical network connectivity for imitation in autism spectrum disorder. Neuropsychologia 48:2931–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier JD, Yeh CH, Calamante F, Cho KH, Connelly A, Lin CP (2008): Resolving crossing fibres using constrained spherical deconvolution: Validation using diffusion‐weighted imaging phantom data. Neuroimage 42:617–625. [DOI] [PubMed] [Google Scholar]
- Turner KC, Frost L, Linsenbardt D, McIlroy JR, Müller RA (2006): Atypically diffuse functional connectivity between caudate nuclei and cerebral cortex in autism. Behav Brain Funct 2:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven JS, De Cock P, Lagae L, Sunaert S (2010): Neuroimaging of autism. Neuroradiology 52:3–14. [DOI] [PubMed] [Google Scholar]
- Verhoeven JS, Rommel N, Prodi E, Leemans A, Zink I, Vandewalle E, Noens I, Wagemans J, Steyaert J, Boets B, Van de Winckel A, De Cock P, Lagae L, Sunaert S (2012): Is there a common neuroanatomical substrate of language deficit between autism spectrum disorder and specific language impairment? Cereb Cortex 22:2263–2271. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, Smits M, Wielopolski PA, Houston GC, Krestin GP, van der Lugt A (2007): Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right‐ and left‐handed healthy subjects: A combined fMRI and DTI study. Neuroimage 35:1064–1076. [DOI] [PubMed] [Google Scholar]
- Villalobos ME, Mizuno A, Dahl BC, Kemmotsu N, Müller RA (2005): Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. Neuroimage 25:916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos SB, Jones DK, Viergever MA, Leemans A (2011): Partial volume effect as a hidden covariate in DTI analyses. Neuroimage 55:1566–1576. [DOI] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, Blitz A, van Zijl P, Mori S (2007): Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 36:630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan CY, Marchina S, Norton A, Schlaug G (2012): Atypical hemispheric asymmetry in the arcuate fasciculus of completely nonverbal children with autism. Ann NY Acad Sci 1252:332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WY, Dai G, Pandya DN, Hagmann P, D'Arceuil H, de Crespigny AJ (2008): Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage 41:1267–1277. [DOI] [PubMed] [Google Scholar]
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C (2009): Correlations and anticorrelations in resting‐state functional connectivity MRI: A quantitative comparison of preprocessing strategies. Neuroimage 47:1408–1416. [DOI] [PubMed] [Google Scholar]
- Weng SJ, Wiggins JL, Peltier SJ, Carrasco M, Risi S, Lord C, Monk CS (2010): Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Res 1313:202–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker B, Fonlupt P, Hubert B, Tardif C, Gepner B, Deruelle C (2008): Abnormal cerebral effective connectivity during explicit emotional processing in adults with autism spectrum disorder. Soc Cogn Affect Neurosci 3:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D, Botting N, Boucher J (2008): Language in autism and specific language impairment: Where are the links? Psychol Bull 134:944–963. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure
Supporting Information Table


