Abstract
Perceptual rivalry—the experience of alternation between two mutually exclusive interpretations of an ambiguous image—provides powerful opportunities to study conscious awareness. It is known that individual subjects experience perceptual alternations for various types of bistable stimuli at distinct rates, and this a stable, heritable trait. Also stable and heritable is the peak frequency of induced gamma‐band (30–100 Hz) oscillation of a population‐level response in occipital cortex to simple visual patterns, which has been established as a neural correlate of conscious processing. Interestingly, models for rivalry alternation rate and for the frequency of population‐level oscillation have both cited inhibitory connections in cortex as crucial determinants of individual differences, and yet the relationship between these two variables has not yet been investigated. Here, we used magnetoencephalography to compare differences in alternation rate for binocular and monocular types of perceptual rivalry to differences in evoked and induced gamma‐band frequency of neuromagnetic brain responses to simple nonrivalrous grating stimuli. For both types of bistable images, alternation rate was inversely correlated with the peak frequency of late evoked gamma activity in primary visual cortex (200–400 ms latency). Our results advance models of inhibition that account for subtle variation in normal visual cortex, and shed light on how small differences in anatomy and physiology relate to individual cognition and performance. Hum Brain Mapp 36:566–576, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: binocular rivalry, bistability, gamma‐band, individual differences, magnetoencephalography
INTRODUCTION
Perceptual rivalry refers to the tendency of certain ambiguous images to induce conscious shifts between two mutually exclusive perceptual solutions. As such bistable images can elicit ongoing perceptual changes with no stimulus modulation, they are often used to investigate the neural substrates of conscious vision [Rees et al., 2002]. One special case of perceptual bistability is binocular rivalry (BR), which arises from incompatible images presented dichoptically to each eye. Binocular rivalry is one of the leading paradigms for the study of consciousness [Engel et al., 1999; Rees et al., 2002], as well as for interocular neural dynamics [Baker et al., 2008; Blake and Wilson, 2011]. This can be contrasted with “monocular” or “pattern” rivalry, which does not require dichoptic presentation to induce alternations in consciously perceived patterns [Blake and Logothetis, 2002; Buckthought et al., 2011; Maier et al., 2005].
With regard to neurophysiology, a well‐established correlate of conscious perception is the synchronous oscillation of neuronal populations occurring at frequencies in the gamma band, or 30–100 Hz [Fries, 2009]. It has been proposed that neuronal synchronization at such high frequencies is necessary for the binding of information across the regions of the brain (Fries, 2005; Melloni et al., 2007; Singer, 1999]. Gamma‐band activity has been linked to many aspects of conscious experience, including perception [Martinovic and Busch, 2011], ocular‐motor responses [Yuval‐Greenberg et al., 2008], and cognitive processing [Herrmann et al., 2010]. As for visual perception, the onset or change of a stimulus is known to evoke a transient phase‐locked gamma response in occipital regions [Tallon‐Baudry et al., 1996]. This transient evoked gamma activity is thought to correspond to early processing of physical stimulus properties, while later, nonphase‐locked “induced” gamma activity is thought to reflect integrative processes [Basar‐Eroglu et al., 1996a; Tallon‐Baudry and Bertrand, 1999]. However, it has since been demonstrated that the properties of the transient evoked gamma response can also be affected by cognitive factors such as expectation, attention, and memory [Busch et al., 2008; Debener et al., 2003; Haenschel et al., 2000; Herrmann et al., 2004; Oppermann et al., 2012]. In the case of rivalry, where a perceived pattern changes while the stimulus does not, several studies have reported gamma‐band synchronization in frontal scalp regions preceding the perceptual transition, which may reflect top‐down attentional processing (Basar‐Eroglu et al., 1996b; Doesburg et al., 2005; Ehm et al., 2011; Freeman and Rogers, 2002].
Another possible way in which gamma‐band activity can further our understanding of the brain's implementation of perceptual rivalry is as an index of cortical inhibition. With respect to the generation of gamma‐band oscillations among cortical neuron populations, a recent model cited the ratio of excitation‐to‐inhibition in cortex as crucial for determining the exact frequency of gamma‐band oscillations [Brunel and Wang, 2003]. Consistent with this, individual differences in the peak frequency of induced gamma‐band responses in early visual cortex to the onset of simple grating stimuli have been shown to predict individual resting‐state levels of gamma‐aminobutyric acid (GABA), the neurotransmitter that enables the inhibitory functions of interneurons [Edden et al., 2009; Muthukumaraswamy et al., 2009; but see Cousijn et al., 2014], such that higher peak frequencies predicted greater GABA levels. Also consistent with Brunel and Wang [2003]'s model is the finding by Lally et al. [2014] that higher resting levels of glutamate, an excitatory neurotransmitter, predicted lower peak frequencies of an individual's evoked gamma responses.
With respect to perceptual rivalry, the alternations have long been understood to be driven by mutual inhibition between separate pools of neurons, in addition to neural adaptation [Blake, 1989; Kang and Blake, 2010; Kang et al., 2010; Tong et al., 2006; Wilson, 2003]. It is also known that individuals differ reliably in their rate of perceptual switching for both binocular and pattern rivalry (PR) [Carter and Pettigrew, 2003; Kleinschmidt et al., 2012], and this is a stable, heritable trait [Miller et al., 2010]. The causal role of GABA in visual cortex on switch rate in perceptual rivalry has been recently been demonstrated by Van Loon et al. [2013], where greater levels of GABAA corresponded to slower switching in rivalry. Interestingly, a study evaluating the causal role of GABA for peak frequency of gamma‐band activity found that transient evoked gamma responses were affected by endogenous levels of GABA, while the induced responses were not [Muthukumaraswamy et al., 2013]. Given the findings of these studies, we sought to formally assess the relationship between individual differences in peak frequency of evoked as well as induced gamma‐band activity and in perceptual rivalry switch rate.
We used magnetoencephalography (MEG) technology to measure neuromagnetic responses to a simple grating stimulus, and then compared the peak frequency of evoked and induced gamma‐band activity to participant switch rates for binocular and PR tasks. Given that the extent of inhibitory connections in cortex may be positively correlated with peak gamma oscillation frequency [Brunel and Wang, 2003; Muthukumaraswamy et al., 2009], and that the dynamics of rivalry are thought to depend upon durations of suppression [Van Loon et al., 2013; Yamashiro et al., 2014], we predicted that peak frequency would here be inversely correlated with alternation rate of perceptual rivalry. We also predicted that the effect would be stronger in V1 than in V2 [Lee et al., 2005; Kamphuisen et al., 2008; Tong and Engel, 2001], and might be stronger for BR than for PR [Wilson, 2003; Bhardwaj et al., 2008]. Finally, a right hemisphere bias has been noted repeatedly in the rivalry literature [Buckthought et al., 2011; Kanai et al., 2011; Lumer et al., 1998], so the possibility of laterality effects was expected.
MATERIALS AND METHODS
Subjects
Twelve healthy adults (6 female, mean age: 25 years) were recruited via McGill University's online classified section and internal listservs, and were paid for two separate visits. All participants had normal or corrected–to‐normal vision, as determined by a Snellen Optotype Acuity test, and a stereoacuity test. Participants were also screened for magnetic resonance imaging (MRI) safety, and for potential MEG artifacts due to jewelry or dental work before partaking in the experiment. During the consent procedure, participants were introduced to a preliminary run of the PR task, performed on a laptop. This procedure was included to demonstrate the nature of rivalry tasks to participants for the sake of informed consent, and also to ensure that they were comfortable with the task before any recording was done. As such, no data from these practice runs were recorded.
Image Fusion
In contrast to the practice run, all stimuli in our experiment were presented as two images to be fused via prism lenses (diopter of 12Δ) worn by the participant. The setup was designed for the BR task, but we used prisms for the PR and event detection (ED) tasks as well, to ensure identical viewing conditions throughout the recording session. To assist image fusion, we provided prominent fixation marks at the center of each image consisting of a white diamond within a larger black diamond (Fig. 1). We also provided a reference border surrounding each image to help with initial image alignment. A preparatory alignment screen with fusible images (distinct from the experimental stimuli) was shown during set‐up to confirm image fusion. Head alignment and stabilization was assisted by a black divider that was placed in between the participant's nasion and the center of the screen. The divider was also employed to control for distracting image duplicates that can result from the prisms. The stimuli were presented via Psychtoolbox (version 3.0.10) running on Matlab (version R2012b) on a Dell Precision T1650 computer. The image was projected into the recording chamber via a Sanyo projector, and was reflected by mirrors onto a screen at 42 cm viewing distance.
Figure 1.
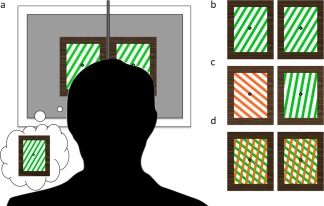
Experimental stimuli. (a) All stimuli were presented as image pairs to be fused via prisms. (b) For the Event‐Detection task, participants were presented with either a red or green square‐wave grating stimulus, with orientation randomly varied throughout the trials. (c) For the Binocular Rivalry task, red and green gratings with an orientation difference of 60° were presented dichoptically to induce alternations of perceptual dominance. (d) For the Pattern Rivalry task, a stimulus composed of two overlaid gratings (also Δ60° orientation) was presented to induce perceptual dominance alternations without interocular competition.
Stimuli
Event detection task
For the ED task, two identical oblique square‐wave gratings were presented for fusion via the prism lenses. Once fused, the grating subtended 9.5° × 13° of visual angle from the center of the screen. For the sake of compatibility with the PR task, which employed chromatic stimuli to enhance the experience of pattern rivalry, we used chromatic gratings for the ED task as well. As displayed by the facility projector, the green gratings (with CIE 1931 xy coordinates of: 0.33875, 0.60425) had a contrast of 42% against the white components of the stimuli (luminance: 657.55 c/m2), and the red gratings (CIEx , y: 0.5337, 0.42175) had a similar contrast of 47%. Event detection trials consisted of either red‐white or green‐white gratings against a mean luminance gray background, and inter‐trial intervals (in which a grating was absent) consisted only of the fusible fixation marks against the gray background. Also, to minimize effects of adaptation, the orientation of the grating stripes varied pseudorandomly across trials from 30°, 50°, 330°, and 350°.
Rivalry tasks
Stimuli for the BR task were identical to those of the ED task, except that the two images presented for fusion via the prisms were gratings of differing color and orientation, to elicit rivalry (Fig. 1). The gratings were presented in pairs that differed 60° in orientation: 50°/350°, and 30°/330°, with the color of each orientation and left‐versus‐right eye position counterbalanced and changing every trial, to prevent color and classical adaptation. Stimuli for the PR task consisted of two overlaid gratings, consisting of 50°/350°, and 30°/330° orientation pairs. The gratings were red versus green against a white background. The color of the areas at which the gratings intersected was defined as the product of the red and green color values. The orientation pair of the PR gratings was changed every trial, to prevent color and classical adaptation.
Procedure
Event detection task
Before the ED task began, image fusion was confirmed via a preparatory alignment screen. The participant would initiate the start of a recording block once he or she felt comfortable and had reliable image fusion. The ED recording block consisted of 90 trials in which a fused grating appeared on the screen, and participants were instructed to press a button when the grating disappeared. Trial duration and interstimulus interval each varied randomly across trials from 1.5–3 s. The grating color changed randomly from red to green across the trials, and participants were instructed to press a button with their left hand for the offset of green stimuli, and with their right hand for red stimuli. Although the orientation of the grating also changed across trials to control for adaptation, this was unrelated to the task.
Rivalry tasks
Like the ED task, the BR and PR recording blocks were preceded by a preparatory alignment screen, and were initiated by the participant via a button press once image fusion was stable. For each participant session, there were two blocks of six 60‐s trials recorded for both BR and PR tasks. In one of the blocks for each rivalry type, we instructed participants to press the left button whenever the green grating appeared to take up at least two‐thirds of the image space, and to press the right button for all other percepts. For the second block, we gave opposite instructions: the right button was to be pressed whenever the red grating took two‐thirds of the image, and the left button was to be pressed for all other percepts. This was to control against mixed percepts by making them approximately counterbalanced across the two blocks. After each trial, an alignment screen was presented to ensure that fusion was stable before a new trial was initiated. The participants were encouraged to rest their eyes after each trial, and to stretch after every recording block.
MEG Recording and Analysis
Before entering the recording chamber, participants had localizer sensors fastened to three fiducial points on their head. We used a Fastrak localizer (Polhemus, Vermont) to acquire three‐dimensional coordinates of the fiducials, as well as about 100 points freely recorded across each participant's scalp using a stylus. Skin electrodes (In Vivo Metric, CA) were also placed above and below the left eye, and next to the outer canthi of each eye for electrooculography (EOG) recording, and sensors were placed across the torso for electrocardiogram (ECG) recording. A ground sensor was placed on the left shoulder. All electrode placement regions were first prepped with alcohol wipes, and the electrodes were placed on the skin with Elefix EEG paste (Nihon‐Kohden, Tokyo, Japan) to lower electrical impedance.
MEG activity was recorded via a full‐head axial gradiometer system (CTF/VSM MedTech, Coquitlam, British Columbia, Canada) with 275 channels, sampled at 2,400 Hz. The fiducial sensors were used during recording sessions to monitor participant head movements, and the EOG sensors were used to monitor for excessive eye movements or blinks during trials.
In a separate session, anatomical MRI scans were acquired for all participants using a 3T Siemens Trio scanner. We used a magnetization‐prepared rapid gradient echo sequence optimized for gray/white matter contrast (176 slices, TR: 2300 ms, TE: 2.98 ms, FOV: 256, voxel size: 1 × 1 × 1 mm). Three scans were acquired per participant for later averaging, cortical reconstruction, and volumetric segmentation using FreeSurfer software. This software is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/), and the details of the procedures performed by Freesurfer's auto‐reconstruction tool are available in previous publications [Dale et al., 1999; Fischl et al., 2004].
All offline preprocessing and analysis of the MEG data was performed with Brainstorm [Tadel et al., 2011], a software package that is documented and freely available for download online under the GNU general public license (http://neuroimage.usc.edu/brainstorm). All raw data was preprocessed with a 0.1 Hz high‐pass filter, and a notch filter to remove noise at 60, 120, 180, and 240 Hz. Artifacts due to EOG and ECG were extracted from the raw data using a principle components analysis tool, and components explaining 15% or more of the variance were removed. Raw data from the ED task trials were divided into epochs starting 100 ms before event onset, and ending 1000 ms after onset. Cortical sources of the event‐related responses were modeled using a Tikhonov‐regularized minimum‐norm estimation [Baillet et al., 2001] fitted to a surface mesh of each participant's anatomical data, and using a head model of 275 overlapping spheres [Huang et al., 1999]. Regions of interest were determined via two recently created anatomical parcellation atlases imported from Freesurfer [Hinds et al., 2008; Destrieux et al., 2010]. From these atlases, labels for V1, V2, and MT were selected [Destrieux et al., 2010], as well as the boundaries of the occipital pole [Hinds et al., 2008], overlapping with central visual field eccentricities stimulated in this study. Using these label boundaries as a reference, new ROI labels for left and right V1 and V2 limited to the occipital pole were created for each subject (see Fig. 2).
Figure 2.
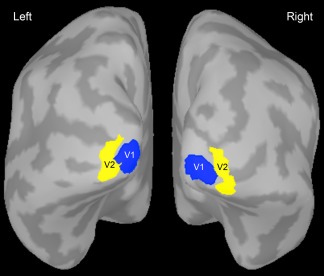
Inflated surface mesh of one participant's cortex showing the regions of interest used for time‐frequency analyses. The V1 and V2 ROIs were both limited to the occipital pole. The regions of interest were defined for each participant's anatomical data using parcellation atlases from FreeSurfer.
For the evoked gamma activity, we used a conventional protocol for event‐related activity: averaging the source data across trials, then computing Morlet wavelet analyses (central frequency: 1 Hz; FWHM: 3s) for the vertices of the four ROIs, which were then averaged per ROI. For the induced gamma activity, we used an analysis protocol that is better suited to capture activity that is not phase‐locked to the stimulus (see Tallon‐Baudry et al., 1996]. This consisted of a Morlet analysis (same as above) for the vertices of the four ROIs individually for each trial, which was then averaged across trials per ROI. All time‐frequency data was normalized via event‐related spectral perturbation (ERSP) analysis, and using the 100 ms of data before event onset as a baseline reference for the activity after event onset. The ERSP analysis yielded values indicating the percent signal increase or decrease relative to the baseline.
Results in the time‐frequency domain were used to determine the magnitude per frequency bin of the neuromagnetic oscillations of 30–100 Hz, within three latency ranges (see Fig. 3). The time‐frequency plots for evoked gamma featured prominent activity around 200–500 ms after stimulus onset, well outside of the range for the classically defined transient evoked gamma. As differential task effects have been observed across gamma activity at differing latencies—both early induced [Haenschel et al., 2000] and late evoked [Gallinat et al., 2004]—we decided to include this late evoked gamma as a third latency range in our analysis. Our “early evoked” range was 10–150 ms; the “late evoked” range was 200–450 ms; and the “induced” range was 500–1000 ms. Figure 4 depicts representative time‐frequency plots for an individual subject in one ROI. The peak frequency within each latency range was then defined as the median frequency of activity with the highest spectral magnitude value per latency range. Peak frequency and magnitude were used as dependent variables for separate statistical analyses.
Figure 3.
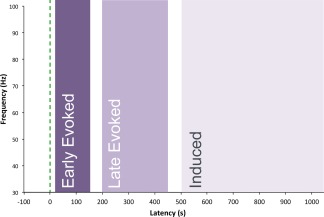
Latency ranges tested for gamma‐band responses to stimulus onset. The green dashed line indicates the time of onset of the grating stimulus. Peak frequency was observed for each range, as was the spectral magnitude of activity at that frequency.
Figure 4.
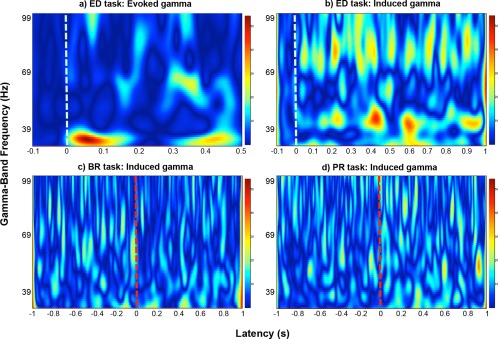
Time‐frequency plots showing normalized spectral magnitude of activity within the gamma band (30–100 Hz). The data shown is from an exemplar participant for one region of interest—left V1. The values indicate the percent signal change relative to baseline. (a) Shows a TF plot from the analysis pipeline for evoked gamma responses during the event detection (ED) task; (b) shows a plot for induced gamma activity for the same condition. The white dashed lines mark the stimulus onset. (c) Shows gamma activity for binocular rivalry (BR) trials, and (d) shows gamma activity for pattern rivalry (PR) trials. The red dashed lines mark the button press for the trial, which indicates a perceptual switch.
Rivalry sessions
To test for similar effects in the gamma band during the rivalry sessions themselves, we ran analogous analyses for these data. The patterns used for the rivalry tasks remained unchanged during each trial, and so the resulting MEG gamma activity recorded during these trials would not be expected to be phase‐locked to the stimulus. Because of this, we did not consider the same latency windows as for the responses to the transient grating stimuli, and instead only employed the pipeline described above for induced gamma to analyze the rivalry data. The epochs were 1,000 ms before and after each button press that indicated a switch in percept. Although rivalry rate did vary across participants (discussed in the Results section), the duration data per individual indicated that dominance phases almost always lasted longer than 1,000 ms for all individuals. As there was no proper baseline for the rivalry sessions, the ERSP normalization was run using an average of the entire 2,000 ms of the trial as a baseline. With the resulting normalized time‐frequency maps, we noted the peak gamma‐band frequency for activity preceding a button press, as well as after the press.
RESULTS
Behavioral Data
All responses were recorded via LUMItouch optical response keypads for the left and right hand (Photon Control, British Columbia, Canada) interfacing via USB with the stimulus computer. For the ED task, response accuracy for the red and green grating trials was 100% for all subjects.
For the BR and PR responses, each subject's mean switch rate (button presses per second, first press not included) was calculated per 60‐s trial, and then averaged across the trials of the two blocks. Individual switch rates for BR and PR were significantly correlated with one another [r(10) = 0.73, P < 0.01], although Welch two sample t‐tests showed significantly faster switch rates for BR than for PR [t(20.27) = 4.56, P < 0.01]. This difference might be expected, as switch rates for BR versus PR tasks are differentially affected by stimulus contrast (O'Shea et al., 2009] and we used fairly high‐contrast gratings for our experiment to maximize induced gamma responses [Muthukumaraswamy et al., 2009; Schwarzkopf et al., 2012]. With respect to the duration of perceptual dominance, the mean duration across participants was 2.14 s (STD = 0.73) for BR, and 4.5 s (STD = 1.2) for PR. For the BR task, dominance durations for red patterns were significantly longer than durations for green [BR: t(20.14) = −2.50, P < 0.05], although this trend was reversed for PR [t(16.55) = 3.37, P < 0.01]. The mean percentage of time viewing mixed percepts was 7% for BR and 13% for PR, and their difference was not significant.
Rivalry Switch Rate and Peak Gamma Frequency
Given, our a priori hypotheses regarding the effects on peak gamma frequency of alternation rate (two levels: high and low), rivalry type (two levels: BR and PR), visual area (two levels: V1 and V2), cortical hemisphere (two levels: left and right), and latency (three levels: early evoked, late evoked, and induced) we first assessed these influences with a 5‐way analysis of variance (ANOVA). A median split was used to divide the participant alternation rates into the two levels used for the analysis. There was a significant main effect of switch rate [F(1,240) = 10.29, P < 0.01], and importantly there were significant interactions between region and latency [F(2, 240) = 4.61, P < 0.05], hemisphere and latency [F(2, 240) = 4.14, P < 0.05], and hemisphere and switch rate [(F(1, 240) = 4.93, P < 0.05]. Accordingly, we tested for the individual subject correlations between alternation rate and peak frequency, for left and right V1 and V2 for the three latencies (next section). Finally, we also ran an analogous multifactorial ANOVA with MEG signal magnitude as the dependent variable. However, in this case the only effect observed was the main effect of latency [F(2, 240) = 25.84, P < 0.001], and so we did not consider this variable for further analysis.
Pearson product moment correlations were run to test for relationships between each subject's peak gamma frequency in V1, and V2 for early evoked (10–150 ms), late evoked (200–450 ms), and induced (500–1000 ms) gamma activity for the ED task and their respective mean switch rate for BR and PR (see Table 1). As Table 1 indicates, no correlations were significant for the early evoked or induced gamma ranges. However, for late evoked gamma, our results show significant inverse correlations for BR switch rate and peak gamma frequencies in left and right V1 (Fig. 5). We found similar effects for PR, although the correlation in right V1 did not reach statistical significance. The effects in V2 were weak and inconsistent in direction.
Table 1.
Correlations for BR and PR switch rates and peak gamma frequency in four ROIs, and three latency ranges
| Early Evoked | Late Evoked | Induced | ||||
|---|---|---|---|---|---|---|
| R | P‐value | R | P‐value | R | P‐value | |
| BR | ||||||
| V1L | 0.054 | 0.867 | −0.618 | 0.032 | 0.262 | 0.411 |
| V1R | −0.244 | 0.44 | −0.649 | 0.022 | 0.301 | 0.341 |
| V2L | −0.040 | 0.903 | −0.282 | 0.375 | 0.340 | 0.280 |
| V2R | 0.277 | 0.392 | 0.446 | 0.146 | −0.169 | 0.599 |
| PR | ||||||
| V1L | 0.011 | 0.972 | −0.764 | 0.004 | 0.433 | 0.160 |
| V1R | −0.478 | 0.116 | −0.527 | 0.078 | 0.439 | 0.153 |
| V2L | 0.108 | 0.738 | 0.312 | 0.324 | 0.453 | 0.139 |
| V2R | 0.294 | 0.354 | 0.263 | 0.409 | −0.041 | 0.899 |
Significant correlations were observed only in V1 for late evoked gamma activity (200–450 ms).
Figure 5.
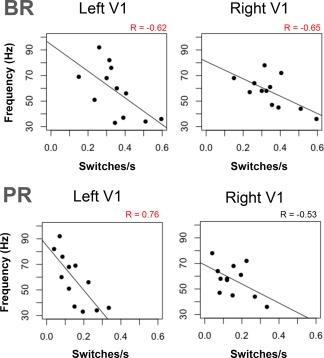
Individual response rates for binocular (BR) and pattern rivalry (PR) tasks plotted against individual peak frequency of late evoked gamma activity (200–450 ms) observed in V1 in response to stimulus onset. R values printed in red indicate statistically significant correlations (P < 0.05).
To assess if the peak gamma effects observed for the ED task might generalize to the rivalry tasks, we also ran a 5‐way ANOVA to test for effects upon the gamma frequency data from the rivalry sessions (using the same variables as before, though with only two levels for latency: pre and post). In this case, we observed a main effect of hemisphere [F(1,160) = 5.21, P < 0.05] and an interaction between region of interest and latency [F(1,160) = 4.20, P < 0.05]. Overall, the peak frequencies in the left hemisphere were higher than those in the right (mean for left hemisphere: 62.58 Hz; mean for right hemisphere: 57.77 Hz). As for region of interest and latency, Figure 6 depicts the nature of the interaction. The mean peak gamma frequency in V1 before a button press was significantly higher than for the activity following a press, while this difference of mean frequency across the two time windows was negligible for V2. Interestingly, this effect in V1 might be taken as an indication of greater inhibition immediately before a switch than after, and will be discussed further in the next section. As there were no further effects observed from the ANOVA, and none concerning switch rate and peak frequency, further correlation testing at the individual level was not pursued.
Figure 6.
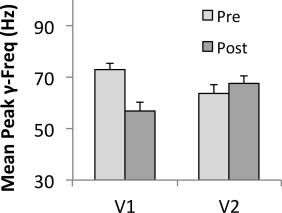
Bar chart showing mean peak frequencies in V1 and V2 before and after a button press during perceptual rivalry. The plot shows the interaction between region of interest and time of activity relative to the button press: while peak frequencies in V1 are higher before a button press compared to after the press, there is no such difference observed in V2.
DISCUSSION
Perceptual rivalry and measurements of cortical gamma‐band activity have both proven to be popular in the investigation of conscious visual perception [Alais, 2012; Fries, 2009; Martinovic and Busch, 2011], and so it is not surprising that the two would be investigated together (Basar‐Eroglu et al., 1996b; Doesburg et al. 2005, 2009; Ehm et al., 2011; Engel et al., 1999; Fairhall et al., 2008; Freeman and Rogers, 2002; Fries et al., 2002]. The synchronization of neuronal populations at these high frequencies is thought to enable conscious cognitive and perceptual experiences, including pattern dominance during perceptual rivalry, to occur at lower frequencies such as the theta band [Doesburg et al., 2009; Fries, 2009]. In one proposal particularly relevant to the competitive dynamics of perceptual rivalry, Fries et al. [2005] suggest that inter‐regional gamma‐band synchronization contributes to a winner‐take‐all dynamic of competition between neural populations with distinct oscillation frequencies. As cells are recruited into a population synchronized with certain other populations, but not with those resonating at incompatible frequencies, their collective inhibition may reinforce this dynamic. Interestingly, such inter‐region (global) phase locking of electroencephalographic (EEG) activity in the gamma band has been found to predict a subsequent change in perceptual state during BR [Doesburg et al., 2005], while local gamma synchronization of activity in occipital channels did not (see also Fairhall et al., 2008].
Rather than predict an alternation, local gamma‐band synchronization has been more compellingly linked to rivalry as an index of resting‐state inhibition in visual cortex. A recent model of gamma oscillation frequency in cortex cites differences in the extent of inhibition relative to excitation as an important determining factor of peak frequency [Brunel and Wang, 2003], and some experiments have indicated that individual differences in peak gamma frequency correspond to differences in levels of GABA [Edden et al., 2009; Muthukumaraswamy et al., 2009]. As mutual inhibition between alternative perceptual representations is considered to be an important contributor to the alternation dynamics in rivalry [Wilson, 2003], and because rivalry switch rate and perceptual suppression duration are both known to vary reliably across individuals [Carter and Pettigrew, 2003; Miller et al., 2010; Van Loon et al., 2013; Yamashiro et al., 2014], we sought to assess the predictive relationship between individual switch rates in rivalry and the peak gamma frequency of neuromagnetic responses to a visual stimulus.
We found that the peak frequency of late evoked gamma in V1 was indeed inversely correlated with switch rate for rivalry, meaning that participants with slower alternation rates exhibited higher peak gamma frequencies, and by extension from the previous models [Atallah and Scanziani, 2009; Brunel and Wang, 2003], greater extents of inhibition. Compatible with this is a recent study comparing resting‐state GABA levels and switch rate for BR, in which greater levels of GABA predicted slower switch rates [Van Loon et al., 2013]. This provides further support for the link between the extent of inhibitory connections in cortex and perceptual alternation, and is compatible with our observed link between switch rate and peak induced gamma frequency. Several models of perceptual bistability cite mutual inhibition as an important factor [Blake, 1989; Seely and Chow, 2011; Wilson, 2003]. Accordingly, we argue that individual differences in perceptual switch rate can serve as an index of a subject's inhibitory connections in visual cortex, and that the strength or extent of rivalry‐related mutual inhibition likely also contributes to the excitation/inhibition ratio that determines peak gamma oscillation frequency. It stands to reason that the other key component of many models of rivalry, local neural adaptation, is a distinct factor not measured here.
In addition, with regard to BR, recent models include two stages of competition: interocular rivalry occurring in V1, and higher‐level perceptual rivalry occurring across the visual cortex [Lee et al., 2007; Wilson, 2003]. Interestingly, our results show clear predictive trends in V1, with more mixed results in extrastriate cortex. Nevertheless, given the fact that we observed similar effects for both rivalry types, it can be assumed that our observed link between switch rate and peak gamma frequency indexes inhibitory properties that are relevant for higher‐level rivalry, not confined to interocular rivalry only. A common oscillation mechanism has been proposed to explain individual differences in alternation rates across different types of perceptual rivalry [Carter and Pettigrew, 2003], and is thought to have a strong heritable component [Miller et al., 2010]. The only other known behavioral correlate of peak gamma frequency, orientation discrimination, is also unlikely to rely only on interocular connections [Edden et al., 2009].
Also compatible with previous research is the lack of correspondence between rivalry switch rate and the magnitude of the peak activity found here. Muthukumaraswamy et al. [2009] found that, despite correlating with blood oxygen level dependent (BOLD) response magnitude, resting levels of GABA did not predict the signal magnitude or power of the stimulus induced neuromagnetic response. Conversely, Lally et al. [2014] found that individual levels of glutamate did reliably predict MEG signal magnitude. Given this, our observation of no effect of switch rate on MEG signal magnitude seems to indicate that rivalry switch rate does indeed reflect differences in individual levels of inhibition in visual cortex, rather than both inhibition and excitation. Clearly the possibility that fMRI and MEG magnitude measures have differing sensitivities to resting GABA is important and worthy of further study.
Additionally, our data indicate that the connection between switch rate and peak frequency of gamma‐band activity depends upon latency, with the late‐evoked, but not induced gamma range yielding significance. Evoked gamma activity is often thought to simply reflect early processing of stimulus properties, while induced gamma reflects more integrative processes [Tallon‐Baudry and Bertrand, 1999], yet there is evidence that the distinction may be more subtle than this traditional interpretation. For instance, several studies have demonstrated task effects related to cognition and memory on evoked gamma responses properties [Busch et al., 2008; Debener et al., 2003; Haenschel et al., 2000; Herrmann et al., 2004; Oppermann et al., 2012]. Moreover, with regard to Brunel and Wang [2003]'s model of oscillation frequency among neuronal populations, both evoked [Lally et al., 2014] and induced [Edden et al., 2009; Muthukumaraswamy et al. 2009] gamma response data have successfully predicted individual physiology of visual cortex. In fact, while Muthukumaraswamy et al. [2009] and Edden et al. [2009] initially focused on induced gamma‐band activity for their correlations with resting level GABA, the researchers recently reported that increasing participants' levels of endogenous GABA via GAT‐1 blockade affected evoked but not induced gamma activity [Muthukumaraswamy et al., 2013]. Additionally, a recent attempt to replicate the findings of Muthukumaraswamy et al. [2009]'s study reported no significant link between peak induced gamma frequency and resting state GABA measured by MR spectroscopy [Cousijn et al., 2014]. The issue of how the different response types relate to one another certainly requires further exploration.
As suggested by earlier studies [Gallinat et al., 2004; Haenschel et al., 2000] and supported by our data here, future exploration of the gamma band response could benefit from a focus on response latencies beyond the conventional dichotomy of evoked and induced ranges. For instance, Haenschel et al. [2000] noted differential effects of a learning task for early versus late induced gamma activity, and the latency range for their “early induced” gamma activity was similar to that for our observed “late evoked” gamma activity. Another study by Gallinat et al. [2004] found reduced power in late evoked gamma activity for unmedicated schizophrenic patients, which covered a similar latency range. The tasks, recording modalities, and regions of interest of these studies were all distinct from ours, and so the activity reported likely reflected different processes from what we observed here, yet all of the studies suggest that a finer discrimination of evoked and induced gamma activity at different latencies may improve our understanding of the various ways in which gamma‐band activity is implicated in perceptual and cognitive processing.
Finally, to test the generality of the link between switch rate and peak gamma frequency, we also compared the switch rates to the peak gamma frequencies of activity during the rivalry sessions. With respect to group‐level effects, the higher peak frequency for V1 activity preceding the button press versus activity following the button press is intriguing, and perhaps could be interpreted as showing increased inhibition necessary for perceptual suppression of the dominant percept at the end of each switch. This could be tested for in future studies, and in fact a specific prediction would be a steeper slope of inhibition increase for shorter alternations [e.g., Alais et al., 2010]. However, the suitability of the continuous rivalry tasks to yield peak gamma activity is unclear, and so the extent to which the properties of these data can be compared with those from more conventional event‐related designs is not known.
With regard to study limitations, our sample of 12 participants may not be ideal for exploring small population effects in a correlation study. However, the fact that similar effects were found for two types of rivalry suggests that the observed predictive relationship is real. Of course, a larger sample could perhaps reveal effects for the more variable data, such as the induced gamma activity, or the responses from V2. It also should be stressed that correlational relationships do not necessarily indicate causation. For instance, the surface area of V1 has been shown to vary in size threefold across individuals, and to correlate positively with peak induced gamma [Schwarzkopf et al., 2012], and so this could be considered an additional relevant covariate for all such correlational studies, although the relationship between V1 surface area and peak evoked gamma frequency remains to be investigated. Finally, it is important to note that the link proposed by Muthukumaraswamy et al. [2009] and Edden et al. [2009] between resting state GABA as measured by voxel‐wise magnetic resonance spectroscopy and actual inhibitory functions remains a contentious issue [Cousijn et al., 2014], and multiple technical issues may be relevant. Overall, however, there is theoretical support for our interpretation of the data, including evidence for a causal role of GABAA in perceptual rivalry dynamics [Van Loon et al., 2013], and it is easily accommodated by a framework for cortical gamma oscillation suggested by Schwarzkopf et al. [2012].
In conclusion, we observed a predictive link between individual peak gamma‐band frequency in V1 and in alternation rate for two perceptual tasks. Our findings support models that propose that inhibitory connections in visual cortex are crucial for the tuning of both of these variables. The study demonstrates that subtle variations of behavior within a normal population can directly advance our understanding of brain function by requiring modeling at increasingly finer scales.
ACKNOWLEDGMENTS
The authors thank Sylvain Baillet and the staff at the MNI MEG center for their support.
Conflict of interest: The authors have no conflict of interest with respect to the research reported here.
REFERENCES
- Alais D (2012): Binocular rivalry: Competition and inhibition in visual perception. WIREs: Cogn Sci 3:87–103. [DOI] [PubMed] [Google Scholar]
- Alais D, Cass J, O'Shea RP, Blake R (2010): Visual sensitivity underlying changes in visual consciousness. Curr Bio 20:1362–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atallah BV, Scanziani M (2009): Instantaneous modulation of gamma oscillation frequency by balancing excitation with inhibition. Neuron 62:566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillet S, Mosher JC, Leahy RM (2001): Electromagnetic brain mapping. IEEE Sig Proc 18:14–30. [Google Scholar]
- Baker DH, Meese TS, Hess RF (2008): Contrast masking in strabismic amblyopia: Attenuation, noise, interocular suppression, and binocular summation. Vis Res 48:1625–1640. [DOI] [PubMed] [Google Scholar]
- Başar‐Eroglu C, Strüber D, Kruse P, Başar E, Stadler M (1996a): Frontal gamma‐band enhancement during multistable visual perception. Intl J Psychophys 24:113–25. [DOI] [PubMed] [Google Scholar]
- Başar‐Eroglu C, Strüber D, Schürmann M, Stadler M, Başar E (1996b): Gamma‐band responses in the brain: A short review of psychophysiological correlates and functional significance. Intl J Psychophys 24:101–12. [DOI] [PubMed] [Google Scholar]
- Bhardwaj R, O'Shea RP, Alais D, Parker A (2008): Probing visual consciousness: Rivalry between eyes and images. J Vis 8:1–13. [DOI] [PubMed] [Google Scholar]
- Blake R (1989): A neural theory of binocular rivalry. Psych Rev 96:145–67. [DOI] [PubMed] [Google Scholar]
- Blake R, Logothetis N (2002): Visual competition. Nat Rev Neurosci 3:13–21. [DOI] [PubMed] [Google Scholar]
- Blake R, Wilson H (2011): Binocular vision. Vis Res 51:745–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunel N, Wang X (2003): What determines the frequency of fast network oscillations with irregular neural discharges? I. Synaptic dynamics and excitation‐inhibition balance. J Neurophys 90:415–430. [DOI] [PubMed] [Google Scholar]
- Buckthought A, Jessula S, Mendola JD (2011): Bistable percepts in the brain: fMRI contrasts monocular pattern rivalry and binocular rivalry. PLoS One 6:e20367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch NA, Groh‐Bordin C, Zimmer HD, Herrmann CS (2008): Modes of memory: Early electrophysiological markers of repetition suppression and recognition enhancement predict behavioral performance. Psychophys 45:25–35. [DOI] [PubMed] [Google Scholar]
- Carter OL, Pettigrew JD (2003): A common oscillator for perceptual rivalries? Perception 32:295–305. [DOI] [PubMed] [Google Scholar]
- Cousijn H, Haegens S, Wallis G, Near J, Stokes MG, Harrison PJ, Nobre AC (2014): Resting GABA and glutamate concentrations do not predict visual gamma frequency or amplitude. Proc Natl Acad Sci USA 111:9301–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI (1999): Cortical surface‐based analysis: I. Segmentation and surface reconstruction. Neuroimage 9:179–194. [DOI] [PubMed] [Google Scholar]
- Debener S, Herrmann CS, Kranczioch C, Gembris D, Engel AK (2003): Top‐down attentional processing enhances auditory evoked gamma band activity. NeuroReport 14:683–686. [DOI] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E (2010): Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 53:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doesburg SM, Kitajo, K , Ward, LM (2005): Increased gamma‐band synchrony precedes switching of conscious perceptual objects in binocular rivalry. NeuroReport 16:1139–1142. [DOI] [PubMed] [Google Scholar]
- Doesburg SM, Green JJ, McDonald JJ, Ward LM (2009): Rhythms of consciousness: Binocular rivalry reveals large‐scale oscillatory network dynamics mediating visual perception. PLoS One 4:e6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden RAE, Muthukumaraswamy SD, Freeman TCA, Singh KD (2009): Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J Neurosci 29:19721–19726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehm W, Bach M, Kornmeier J (2011): Ambiguous figures and binding: EEG frequency modulations during multistable perception. Psychophysiology 48:547–558. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, Konig P, Brecht M, Singer W (1999): Temporal binding, binocular rivalry, and consciousness. Consci Cogn 8:128–151. [DOI] [PubMed] [Google Scholar]
- Fairhall SL, Hamm JP, Kirk IJ (2008): Binocular rivalry reveals a dissociation between the subjective experience and induced gamma oscillations. Eur J Neurosci 27:213–216. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, DH Salat, E Busa, LJ Seidman, J Goldstein, D Kennedy, V Caviness, N Makris, B Rosen, AM Dale (2004): Automatically parcellating the human cerebral cortex. Cereb Cortex 14:11–22. [DOI] [PubMed] [Google Scholar]
- Freeman WJ, Rogers LJ (2002): Fine temporal resolution of analytic phase reveals episodic synchronization by state transitions in gamma EEGs. J Neurophys 87:937–945. [DOI] [PubMed] [Google Scholar]
- Fries P (2005): A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends Cogn. Sci 9:474–480. [DOI] [PubMed] [Google Scholar]
- Fries P (2009): Neuronal gamma‐band synchronization as a fundamental process in cortical computation. Ann Rev Neurosci 32:209–224. [DOI] [PubMed] [Google Scholar]
- P Fries, J‐H Schröder, PR Roelfsema, W Singer, AK Engel (2002): Oscillatory neuronal synchronization in primary visual cortex as a correlate of stimulus selection. J Neurosci 22:3739–3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat J, Winterer G, Herrmann CS, Senkowski D (2004): Reduced oscillatory gamma‐band responses in unmedicated schizophrenic patients indicate impaired frontal network processing. Clin Neurophys 115:1863–1874. [DOI] [PubMed] [Google Scholar]
- Haenschel C, Baldeweg T, Croft RJ, Whittington M, Gruzelier J (2000): Gamma and beta frequency oscillations in response to novel auditory stimuli: A comparison of human electroencephalogram (EEG) data with in vitro models. Proc Nat Acad Sci 97:7645–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann CS, Fründ I, Lenz D (2010): Human gamma‐band activity: a review on cognitive and behavioral correlates and network models. Neurosci Biobehav Rev 34:981–992. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Munk MHJ, Engel AK (2004): Cognitive functions of gamma‐band activity: Memory match and utilization. Trends Cogn Sci 8:347–355. [DOI] [PubMed] [Google Scholar]
- Hinds OP, Rajendran N, Polimeni JR, Augustinack JC, Wiggins G, LL Wald, HD Rosas, A Potthast, EL Schwartz, B Fischl (2008): Accurate prediction of V1 location from cortical folds in a surface coordinate system. Cereb Cortex 39:1585–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MX, Mosher JC, Leahy RM (1999): A sensor‐weighted overlapping‐sphere head model and exhaustive head model comparison for MEG. Phys. Med Biol 44:423–440. [DOI] [PubMed] [Google Scholar]
- Kamphuisen A, Bauer M, Ee R Van (2008): No evidence for widespread synchronized networks in binocular rivalry: MEG frequency tagging entrains primarily early visual cortex. J Vis 8:1–8. [DOI] [PubMed] [Google Scholar]
- Kanai R, Carmel D, Bahrami B, Rees G (2011): Structural and functional fractionation of right superior parietal cortex in bistable perception. Curr Biol 21:R106–R107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M‐S, Blake R (2010): What causes alternations in dominance during binocular rivalry? Atten Percept Psychophys 72:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M, Lee S, Heeger D, Blake R (2010): Modulation of spatiotemporal dynamics of binocular rivalry by collinear facilitation and pattern‐dependent adaptation, J Vis 10:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt A, Sterzer P, Rees G (2012): Variability of perceptual multistability: From brain state to individual trait. Philos Trans R Soc Lond Series B Bio Sci 367:988–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally N, Mullins PG, Roberts MV, Price D, Gruber T, Haenschel C (2014): Glutamatergic correlates of gamma‐band oscillatory activity during cognition: A concurrent ER‐MRS and EEG study. NeuroImage 85(Pt 2):823–833. [DOI] [PubMed] [Google Scholar]
- Lee S‐H, Blake R, Heeger, DJ (2005): Traveling waves of activity in primary visual cortex during binocular rivalry. Nat Neurosci 8:22–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S‐H, Blake R, Heeger DJ (2007): Hierarchy of cortical responses underlying binocular rivalry. Nat Neurosci 10:1048–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumer ED, Friston KJ, Rees G (1998): Neural correlates of perceptual rivalry in the human brain. Science 280:1930–1934. [DOI] [PubMed] [Google Scholar]
- Maier A, Logothetis N, Leopold DA (2005): Global competition dictates local suppression in pattern rivalry. J Vis 5:668–677. [DOI] [PubMed] [Google Scholar]
- Martinovic J, Busch NA (2011): High frequency oscillations as a correlate of visual perception. Intl J Psychophys 79:32–38. [DOI] [PubMed] [Google Scholar]
- Melloni L, Molina C, Pena M, Torres D, Singer W, Rodriguez E (2007): Synchronization of neural activity across cortical areas correlates with conscious perception. J Neurosci 27:2858–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SM, Hansell NK, Ngo TT, Liu GB, Pettigrew JD, NG Martin, MJ Wright (2010): Genetic contribution to individual variation in binocular rivalry rate. Proc Nat Acad Sci 107:2664–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy S, Edden RAE, Jones DK, Swettenham JB, Singh KD (2009): Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Nat Acad Sci 106:8356–8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Myers JFM, Wilson SJ, Nutt DJ, Hamandi K, Lingford‐Hughes A Singh, KD (2013): Elevating endogenous GABA levels with GAT‐1 blockade modulates evoked but not induced responses in human visual cortex. Neuropsychopharmacology 38:1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppermann F, Hassler U, Jescheniak JD, Gruber T (2012): The rapid extraction of gist‐early neural correlates of high‐level visual processing. J Cogn Neurosci 24:521–529. [DOI] [PubMed] [Google Scholar]
- O'Shea RP, Parker A, La Rooy D, Alais D (2009): Monocular rivalry exhibits three hallmarks of binocular rivalry. Vis Res 49:671–681. [DOI] [PubMed] [Google Scholar]
- Rees G, Kreiman G, Koch C (2002): Neural correlates of consciousness in humans. Nat Rev Neurosci 3:261–270. [DOI] [PubMed] [Google Scholar]
- Schwarzkopf DS, Robertson DJ, Song C, Barnes GR, Rees G (2012): The frequency of visually induced ©‐band oscillations depends on the size of early human visual cortex. J Neurosci 32:1507–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely J, Chow CC (2011): Role of mutual inhibition in binocular rivalry. J Neurophys 106:2136–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W (1999): Neural synchrony: A versatile code for the definition of relations? Neuron 24:49–65. [DOI] [PubMed] [Google Scholar]
- Tadel F, Baillet S, Mosher JC, Pantazis D, Leahy RM (2011): Brainstorm: A user‐friendly application for MEG/EEG analysis. Comput Intell Neurosci 2011, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon‐Baudry C, Bertrand O (1999): Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci 3:151–162. [DOI] [PubMed] [Google Scholar]
- Tallon‐Baudry C, Bertrand O, Delpuech C, Pernier J (1996): Stimulus specificity of phase‐locked and non‐phase‐locked 40 Hz visual responses in human. J Neurosci 16:4240–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong F, Engel SA (2001): Interocular rivalry revealed in the human cortical blind‐spot representation. Nature 411:195–199. [DOI] [PubMed] [Google Scholar]
- Tong F, Meng M, Blake R (2006): Neural bases of binocular rivalry. Trends Cogn Sci 10:502–511. [DOI] [PubMed] [Google Scholar]
- Van Loon AM, Knapen T, Scholte HS, St John‐Saaltink E, Donner TH, Lamme VAF (2013): GABA shapes the dynamics of bistable perception. Curr Biol 23:823–827. [DOI] [PubMed] [Google Scholar]
- Wilson HR (2003): Computational evidence for a rivalry hierarchy in vision. Proc Nat Acad Sci 100:14499–14503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro H, Yamamoto H, Mano H, Umeda M, Higuchi T, Saiki J (2014): Activity in early visual areas predicts interindividual differences in binocular rivalry dynamics. J Neurophysiol 111:1190–202. Doi: 10.1152/jn.00509.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuval‐Greenberg S, Tomer O, Keren AS, Nelken I, Deouell LY (2008): Transient induced gamma‐band response in EEG as a manifestation of miniature saccades. Neuron 58:429–41. [DOI] [PubMed] [Google Scholar]


