Abstract
Insulin and cortisol play a key role in the regulation of energy homeostasis, appetite, and satiety. Little is known about the action and interaction of both hormones in brain structures controlling food intake and the processing of neurovisceral signals from the gastrointestinal tract. In this study, we assessed the impact of single and combined application of insulin and cortisol on resting regional cerebral blood flow (rCBF) in the insular cortex. After standardized periods of food restriction, 48 male volunteers were randomly assigned to receive either 40 IU intranasal insulin, 30 mg oral cortisol, both, or neither (placebo). Continuous arterial spin labeling (CASL) sequences were acquired before and after pharmacological treatment. We observed a bilateral, locally distinct rCBF increase after insulin administration in the insular cortex and the putamen. Insulin effects on rCBF were present regardless of whether participants had received cortisol or not. Our results indicate that insulin, but not cortisol, affects blood flow in human brain structures involved in the regulation of eating behavior. Hum Brain Mapp 35:1944–1956, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: cerebral cortex, basal ganglia, hippocampus, glucocorticoids, pancreatic hormones, metabolism
INTRODUCTION
Adrenal glucocorticoids and the pancreatic peptide insulin jointly play key roles in energy homeostasis, co‐dependably regulating food intake and metabolism [Dallman et al., 2007; Peters et al., 2004]. Previous research has shown that a disruption in the normal functioning of both these hormones might be crucially involved in the development of obesity and the metabolic syndrome [la Fleur et al., 2004; Rosmond, 2005]. Under normal physiological circumstances, insulin and glucocorticoids have opposite effects on the metabolism of peripheral tissues [Dallman et al., 1993]. Additionally, both hormones cross the blood–brain barrier and act on the central nervous system [Joels, 1997; Plum et al., 2005] but the action and interaction of these hormones at the central level still remain issues of investigation.
Insulin is known to affect food‐associated behavior in rodents and humans independently of systemic hypoglycemia [Dallman et al., 2007; Hallschmid et al., 2004a]. Peripheral insulin accessing the brain communicates the size of adipose stores to the central nervous system [Figlewicz and Sipols, 2010], acting along with leptin as an “adiposity signal” that exerts negative feedback on brain structures that regulate feeding behavior, and maintaining adipose mass at constant levels [Morton et al., 2006]. Accordingly, direct infusion of insulin into the brain has an anorexigenic effect, provoking inhibition of food intake, decreased body weight, and improvement in insulin sensitivity in the periphery. In contrast, if insulin signaling is inhibited, there is an increase in food intake and peripheral insulin resistance [Plum et al., 2005]. In line with these observations, it has been shown that intravenous insulin application increases visceral sensitivity without influencing the somatic sensory functions [Softeland et al., 2011] and that central nervous administration of insulin via the intranasal pathway increases satiety and reduces food intake in humans [Hallschmid et al., 2012]. Recently, it has been demonstrated that intranasal insulin increases cerebral high‐energy phosphate content which further highlights the crucial importance of insulin in the human brain for metabolic homoeostasis [Jauch‐Chara et al., 2012].
Stress and stress hormones, such as adrenal corticosteroids, are also known to influence eating behavior, appetite, and satiety. Humans respond to stress with an increased intake of highly palatable food, i.e., “comfort food” [Born et al., 2010; la Fleur, 2006]. Adam and Epel [2007] proposed a theoretical model of Reward Based Stress Eating, where cortisol effects on central reward circuitry may motivate calorically dense food intake. The effect of “comfort food” in stress dampening might be mediated by insulin signaling to the brain [Warne, 2009], and it was shown that intranasal insulin administration reduces stress reactivity [Bohringer et al., 2008]. Corticosteroid blood levels are positively correlated with insulin levels, and some types of obesity, such as visceral obesity, might be a direct result of elevated corticosteroids, insulin resistance, and increases in insulin [Rosmond, 2005]. Low doses of corticosteroids stimulate food and energy intake while high doses inhibit it, and the latter effect might be mediated by high levels of insulin [Dallman et al., 1993]. In general, corticosteroids guarantee energy supply and stimulate food intake [Dallman et al., 2007]. Insulin might interact with this effect by determining the type of nutrients chosen [la Fleur, 2006].
Insulin and glucocorticoid receptors are found throughout the central nervous system, although not uniformly. Receptors for both hormones show high densities in the cerebral cortex [Lee et al., 2000; Schulingkamp et al., 2000; Werther et al., 1987]. Recently, it has been demonstrated that insulin facilitates repetitive spike firing in the rat insular cortex [Takei et al., 2010], a region containing the primary gustatory cortex in humans [Small, 2010]. The insular cortex contains a topographically organized visceral sensory representation, integrates information from other sensory modalities to control for feeding behavior [Small, 2010], is sensitive to a large number of food cues such as taste, texture [De Araujo and Rolls, 2004] and visual appearance [Ohla et al., 2012] and has been linked to insulin and satiety [Tataranni et al., 1999]. We assumed that the insular cortex might be particularly sensitive to insulin's regulatory impact on eating behavior.
We studied the effects of oral cortisol and intranasal insulin on resting regional cerebral blood flow (rCBF). Hormones were applied separately and combined. The intranasal application of insulin was chosen since it allows the peptide to reach the cerebrospinal fluid (CSF) without relevant blood absorption, and consequently without peripheral side‐effects such as hypoglycemia [Born et al., 2002; Kern et al., 1999]. We measured rCBF in the putamen, hippocampus, insular, and visual cortex, but we assumed that the insular cortex would be the main target region for the effects of both hormones.
METHODS
Participants
Forty‐eight right‐handed healthy male volunteers (mean age: 23.98, SD: 3.4) participated in the study and received a monetary reward for participation. Exclusion criteria were any acute or chronic somatic or psychiatric illness, any history of psychiatric disorders, glaucoma, any family history of epilepsy or aneurysms, a body mass index lower than 20 or greater than 25, any disfavor toward certain kinds of food (including vegetarianism), smoking, caffeine consumption exceeding five cups of coffee per day, or any illicit drug intake in the last 6 months. In addition participants had to fulfill general magnetic resonance imaging (MRI) safety standards [Kanal et al., 2002] such as lack of any ferromagnetic objects in their body and to be negative of any history of claustrophobia or uneasiness toward medical settings or procedures. Participants gave their informed written consent and were told that they had the right to stop the experiment at any time they wanted. The study was approved by the Ethical Committee of the State's Medical Association (Landesärztekammer Rheinland‐Pfalz) and was in accordance with the latest revision of the Declaration of Helsinki.
Experimental Design and Pharmaceutical Materials
All participants received oral and intranasal treatment. According to a double‐blinded, two by two between‐subject design, participants were randomly assigned to receive either oral cortisol versus oral placebo and intranasal insulin versus intranasal placebo, resulting in four groups (“insulin and cortisol,” “insulin and oral placebo,” “cortisol and intranasal placebo,” and “oral and intranasal placebo”). A total of 30 mg cortisol (Hydroson® Tabl., Dermapharm, Grünwald, Germany) was administered in three doses of 10 mg over a period of 30 min (15 min between administrations) to assure heightened “steady‐state like” cortisol plasma levels throughout the whole experiment. Participants received 40 units of insulin intranasally (Insulin Human Actrapid Penfill® 100 I.E./ml; Novo Nordisk, Mainz, Germany) or placebo (dilution buffer) by applying two 0.1 ml puffs into each nostril via a high precision medical nose pump (kindly provided by Aero Pump, Hochheim, Germany). Intranasal insulin versus intranasal placebo was administered together with the second dose of cortisol. Blood glucose was determined by an Accu‐Check device (Aviva, Roche Diagnostics Deutschland, Mannheim, Germany).
Procedure
Scanning took place between 8 am and 12 pm or 12 pm and 4 pm. Participants of all four groups were distributed to morning or afternoon in a pseudo randomized, balanced fashion. All participants were instructed to have the last meal at 10 pm of the previous day, afternoon participants were instructed to have one extra slice of bread with butter or marmalade at 8 am of the same day. Upon arrival participants had a short medical check‐up, filled out some questionnaires, were familiarized with the scanner environment, and then entered the scanner for the first scan. Participants were instructed to move as little as possible, to keep their eyes open and to not fall asleep. The first scan consisted of a functional measurement‐block (Baseline; duration 6:50 min) which was later used as a baseline reference for the following functional measurement‐blocks. At the end of the first scan, participants left the scanner, received the assigned pharmacological treatment and reentered the scanner. At the beginning of the second scan an anatomical T1‐image (duration 13:22 min) was acquired. This was followed by five independent but subsequent functional measurement‐blocks (Block 1–5; duration of 6:50 min each, no pause between blocks) to assess the change of regional cerebral blood flow over time. Intranasal insulin is known to show its peak concentration in the CSF around 30 min after administration [Born et al., 2002]. The functional measurement‐blocks therefore covered a time span of approximately 35 min, lasting 30 to 65 min after intranasal insulin administration. After the last functional measurement‐block, a T2‐weighted anatomical image of the whole brain was acquired. T2‐images of all participants were later checked by a board‐certified radiologist for anatomical abnormalities. Last, an M0‐measurement (equilibrium magnetization) was acquired. Total duration of the second scan was approximately 55 min including entering and leaving the scanner. In the end of the experiment, participants filled out some questionnaires and received a snack. After eating, following directions of the Ethical Committee blood glucose was assessed to reassure normal levels in blood. Also, the monetary reward and a letter disclosing the experimental condition were handed out. See also Figure 1 for an overview on the experimental procedure.
Figure 1.
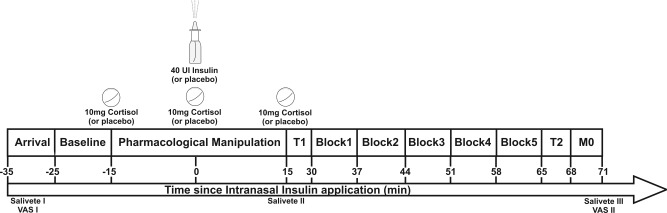
Flowchart of the experiment. After arrival participants entered the scanner for scan 1 (baseline). The pharmacological treatment was given out of the scanner. The second scan started by recording an anatomical T1‐scan before five functional‐blocks, an anatomical T2‐scan and a M0‐scan were recorded. Salivary cortisol was assessed three times during the experiment (“Salivette”), mood, and hunger ratings were assessed two times via a visual‐analog‐scale (“VAS”). Also, blood sugar levels were taken at the end of the experiment.
Measurements
Assessment of salivary cortisol
Salivary morning cortisol on the experimental day was assessed for all participants 0, 30, 45, and 60 min after awakening with devices for saliva sampling (Salivettes®, Sarstedt, Nümbrecht, Germany). This was done in order to check for preexisting differences in basal cortisol levels between the four groups.
We also measured salivary cortisol levels during the experimental session to check whether the pharmacological cortisol manipulation was successful. Salivary cortisol was acquired three times during the experiment. The first sample was taken immediately after arriving, before any pharmacological manipulation. The second sample was acquired after the pharmacological manipulation, immediately before participants entered the scanner for the second scan. The third sample was taken at the end of the second scan, right after leaving the scanner. Salivary cortisol was analyzed by an immunoassay with fluorescence detection [Dressendorfer et al., 1992] with a concentration detection limit of 100 nmol/l. Salivary cortisol levels exceeding 100 nmol/l were marked as above 100 without stating the exact value.
Subjective mood and hunger ratings
Mood and hunger ratings were assessed upon arrival and after the second scan with the help of a visual‐analog scale. This was done to check whether participants of all four groups were in a comparable subjective state during the experiment. The mood rating comprised the subscales stress and arousal.
MRI‐measurements
Continuous arterial spin labeling (CASL)
Cerebral blood flow (CBF) was assessed using continuous arterial spin labeling (CASL) sequences. CASL allows the quantification of cerebral blood flow values in absolute physiological values in ml/100 g brain tissue/min. It has a high specificity to tissue, can be repeated many times in short intervals, and shows no sensitivity to baseline drifts which makes it an ideal method to assess the change of blood flow over time following a pharmacological manipulation [Detre and Alsop, 1999; Wang et al., 2008].
Data acquisition
Imaging was performed on a 1.5 T scanner (Intera; Philips Medical Systems) with interleaved label and control images acquisitions, using a single‐shot spin echo EPI sequence [Hermes et al., 2007]. Thirteen slices covering the whole brain were acquired from inferior to superior (FOV, 230 mm; matrix, 64 × 64; slice thickness, 8 mm; 1 mm gap; bandwidth, 78.4 kHz, flip angle 90°; TR, 4,125 ms; TE, 42 ms) and reconstructed on a 128 × 128 matrix. Labeling was achieved with a flow driven adiabatic inversion technique [Alsop and Detre, 1996]. In order to control for magnetization transfer effects an amplitude modulated version of the CASL technique was used [Alsop and Detre, 1998].The labeling plane was placed 60 mm beneath the center of the imaging slices (labeling duration, 2.2 s; labeling amplitude, 35 mg; labeling gradient, 0.25 g/cm; post‐labeling delay, 0.8–1.8 s). The post‐labeling delay varied from 0.8 to 1.8 s between slices because each slice was acquired at a slightly different time relative to the labeling pulse. CBF was measured in six measurement‐blocks. Each of the six measurement‐blocks (6:50 min) consisted of 46 pairs of label and control images. A T1‐weighted sequence (T1‐weighted gradient recalled echo (fast field echo); 160 slices; FOV, 256 × 192 mm; matrix, 256 × 256; slice thickness, 1 mm; TR, 11.9 ms; TE, 3.3 ms; duration, 13:22 min) was acquired at the beginning of the second scan. At the end of the second scan a series of 46 M0‐images was acquired to determine the equilibrium magnetization (scan duration 3:25 min). Additionally, a T2‐weighted sequence was recorded (scan duration 2:57 min). Spatial realignment of data from the first and second scan was facilitated by using the Philips Smart Brain procedure (Intera; Philips Medical Systems) which automatically detects landmarks on the participant's brain anatomy, uses them to realign separately acquired functional or anatomical images, and thereby supports comparison of functional imaging data which are acquired in different scans.
Data preprocessing
Preprocessing of functional CASL‐images and structural T1‐images was done using SPM8 (Welcome Department of Imaging Neuroscience, London, UK) implemented on a MATLAB System (Version 2011a, Mathworks, Natick, MA) as reported elsewhere [Hermes et al., 2007; Strelzyk et al., 2012]. First, label‐, control‐, and M0 images were spatially realigned and coregistered to the anatomical T1‐image and then normalized to the MNI 152 average brain [Montreal Neurological Institute; Mazziotta et al., 1995]. Next, label‐, and control‐images were averaged separately and separated for each measurement‐block. The M0‐images were also averaged. We estimated global CBF values separately for each measurement‐block using label, control, and M0 images following a formula described by Alsop and Detre [1996], and using the parameter values described in detail by Hermes et al. [2007]. The blood–brain partition coefficient of water was set to λ = 0.98 for gray matter quantification [Herscovitch and Raichle, 1985], the T1 for arterial blood to 1.4 s and the labeling efficiency to α = 0.71. The value for the post‐labeling delay in the quantification formula was different for each slice to adjust for slice timing delays during image acquisition (varying between 0.8 and 1.8 s). T1‐images were segmented and normalized to the MNI 152 average brain and the resulting tissue probability maps converted into dichotomous gray matter mask. These gray matter masks were multiplied with the CBF images resulting in gray matter CBF maps. We defined templates for regions of Interest (ROI) based on published templates [Tzourio‐Mazoyer et al., 2002] with the help of the MARINA‐toolbox [Walter et al., 2003]. Additionally, images for the voxel‐based result presentations were generated by spatially smoothing the unsegmented CBF images of each measurement‐block with a 6 × 6 × 12 mm full width at half maximum kernel.
Selection of region of interest
Receptors for insulin and cortisol have been reported in the entire cortex as well as in subcortical structures [Sanchez et al., 2000; Sara et al., 1982; Schulingkamp et al., 2000; Unger et al., 1991; Werther et al., 1987]. According to our hypotheses we defined the left and right insular cortices to be our primary ROI. This primary ROI was contrasted with the calcarine fissure and surrounding cortex, which forms part of the primary visual cortex. We chose this region as a control ROI because it has recently been shown that intranasal insulin does not affect rCBF in the primary visual cortex [Grichisch et al., 2012]. In addition to the primary and control ROI two secondary ROI were included. We selected the hippocampus as a secondary ROI because effects of insulin and cortisol on hippocampus‐dependent declarative memory systems have frequently been reported [Benedict et al., 2007; Wolf, 2009] and the hippocampus is a region rich in both cortisol and insulin receptors [Sanchez et al., 2000; Schulingkamp et al., 2000]. Since insulin is implicated in food reward behavior [Figlewicz and Sipols, 2010] and a positive relation between fasting insulin levels and functional connectivity in the putamen has been reported [Kullmann et al., 2012], we also chose the putamen as secondary ROI in this study.
Statistical Analysis
All statistical analyses were conducted with SPSS 19 (IBM SPSS statistics). For each model an alpha symbol‐level value of P = 0.05 was selected and a Greenhouse‐Geisser correction for degrees of freedom was applied whenever appropriate. Interactions and main effects were further decomposed using Dunn's multiple comparison procedure [Dunn, 1961]: the critical value (ψ) and the number of comparisons (C) are stated. Two participants were excluded after data acquisition from further statistical analysis. The first exclusion was the result of a technical failure during scanning which led to data loss. The second exclusion was necessary due to an abnormal sleeping pattern in the night before the experiment (participant slept less than 2½ h). A total of 46 datasets were entered in the final statistical analysis (“insulin and cortisol” = 11, “insulin and oral placebo” = 11, “cortisol and intranasal placebo” = 12, and “oral placebo and intranasal placebo” = 12).
Salivary morning cortisol
Salivary morning cortisol of the experimental day was calculated as the area under the curve with respect to ground (AUCg) following a formula described by Pruessner et al. [2003]. Groups were compared using a one‐way ANOVA.
Manipulation check
The success of the pharmacological cortisol manipulation was assessed using the three saliva samples acquired during the experiment. Since we did not asses the exact value for salivary cortisol levels exceeding 100 nmol/l only descriptive data are shown.
Subjective mood and hunger ratings
Subjective mood and hunger ratings were analyzed employing a 2(insulin) × 2(cortisol) × 2(point of measurement) ANOVA model for each of the dependent variables hunger, stress, and arousal.
Continuous arterial spin labeling analysis
Primary ROI
Regional cerebral blood flow values of the primary and control ROI were used for further statistical analysis. Data of both ROI were entered in one model employing a 2(“insulin”) × 2(“cortisol”) × 2(“ROI”) × 2(“hemisphere”) × 6(“measurement‐block”) ANOVA in order to assess whether the impact of the pharmacological manipulation on the change in rCBF over time was different in one ROI from the other.
Secondary ROI
For both secondary ROI “hippocampus” and “putamen” separate ANOVA models were calculated, entering the same factors as described above except for the factor “ROI.”
Voxel‐based exploration
An open, non‐hypothesis driven, whole brain voxel‐based exploration was conducted within the framework of the general linear model as implemented in SPM8. The unsegmented and smoothed mean CBF‐images were employed in a full‐factorial 4(“group”) × 6(“measurement‐block”) model and statistical parametric maps (SPMs) were calculated [Friston et al., 1991]. We set up normalized contrast vectors for the interactions “insulin” × “measurement‐block,” “cortisol” × “measurement‐blocks,” and “insulin” × “cortisol” × “measurement‐block,” contrasting all five post‐intervention measurement blocks with the baseline block. Further, the respective “treatment” group (receiving insulin, cortisol, or both) was contrasted with the corresponding placebo group. By including the baseline values only the differences between conditions in CBF changes from the baseline to post‐intervention measurement blocks were contrasted and possible baseline differences, which might have preexisted independently of the pharmacological manipulation, were eliminated. Altogether, six different SPMs were calculated (interaction “insulin” × “measurement‐block”; “CBF increase/decrease after insulin intake”; interaction “cortisol” × “measurement‐block”; “CBF increase/decrease after cortisol intake”; interaction “insulin” × “cortisol” × “measurement‐block”; “CBF increase/decrease after intake of insulin and cortisol”). Each SPM spanned the time interval of 30 to 65 min after intranasal insulin intake, showing either the baseline corrected CBF increases or decreases after insulin or cortisol or insulin and cortisol intake. The height threshold at the voxel level was set to a liberal criterion of P < 0.001 (uncorrected). Significant clusters [Forman et al., 1995] were identified at a family‐wise‐error‐corrected extent threshold level of P < 0.05 (FWE‐corrected). No covariates were entered. We applied no predefined template or masking and therefore the statistical parametric maps comprised the whole brain as it was covered during image acquisition. For visualization purposes the SPMs were saved and overlaid on the Ch2bet brain template implemented in MRIcron (available at: http://www.mccauslandcenter.sc.edu/ricro/mricron/).
RESULTS
Salivary Morning Cortisol
Participants of the four groups had comparable salivary morning cortisol levels, calculated as AUCg (F (3,42) = 0.51, P = 0.68). Mean and standard error of means for each group were as follows: “insulin and cortisol” M = 665 ± 89; “insulin and oral placebo” M = 543 ± 87, “cortisol and intranasal placebo,” M = 562 ± 51, “oral and intranasal placebo” M = 633 ± 89.
Manipulation‐Check
Participants receiving oral cortisol but not those receiving oral placebo showed increased salivary cortisol levels at measurement 2 (cortisol condition M = 77.38 nmol/l, placebo condition M = 7.75 nmol/l) and measurement 3 (cortisol condition M = 87.53 nmol/l, placebo condition M = 10.06 nmol/l). The pharmacological manipulation with cortisol was, therefore, successful.
Subjective Mood and Hunger Ratings
Participants did not differ regarding their subjective mood or hunger ratings. We found neither a main effect of “insulin” or “cortisol” nor an interaction between the two hormones, nor an interaction with the factor “point of measurement” in any of the dependent variables (all P > 0.05) (Fig. 2).
Figure 2.
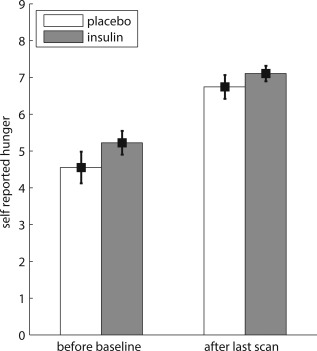
Values of self‐reported hunger of participants receiving insulin (N = 22) compared with participants receiving placebo (N = 24) before baseline and after the last scan. Data present mean and standard error of the mean.
ROI‐Analysis
Primary versus control ROI
We observed a three‐way interaction “insulin” × “ROI” × “measurement‐block” (F (5,210) = 2.804, P = 0.018, η p 2 = 0.063). The influence of insulin on the change in rCBF over time was different in the primary ROI compared with the control ROI. Dunn's multiple comparison procedure (ψ 5% = 5.00 ml/100 g brain tissue/min, C = 20) showed that this effect was due to an increase in rCBF in the insular cortex relative to baseline in participants receiving insulin. Increased rCBF compared with baseline values was observed in block 2 (mean increase compared with baseline and standard error: 6.68 ± 1.89 ml/100 g/min), block 3 (7.51 ± 2.03 ml/100 g/min) and block 4 (6.79 ± 1.99 ml/100 g/min) after the pharmacological manipulation. This corresponded to a time interval of about 37 to 58 min after intranasal insulin administration. No change in rCBF in the insular cortex was observed for participants receiving placebo (all differences below the critical Dunn's value of ψ 5% = 5.00 ml/100 g brain tissue/min). Also, insulin did not have an influence on rCBF in the calcarine fissure and surrounding cortex (see Fig. 3). Both higher order interactions including the factors “insulin,” “cortisol,” “ROI,” and “measurement‐block” failed to reach significance (“insulin” × “cortisol” × “ROI” × “measurement‐block” (F (5,210) = 0.66, P = 0.65) and “insulin” × “cortisol” × “ROI” × “hemisphere” × “measurement‐block” (F (5,210) = 0.78, P = 0.56). Also, no interaction including the factors “cortisol” and “measurement‐block” was found (“cortisol” × “ROI” × “measurement‐block” (F (5,210) = 0.55, P = 0.53); “cortisol” × “ROI” × “hemisphere” × “measurement‐block” (F (5,210) = 0.72, P = 0.61)).
Figure 3.
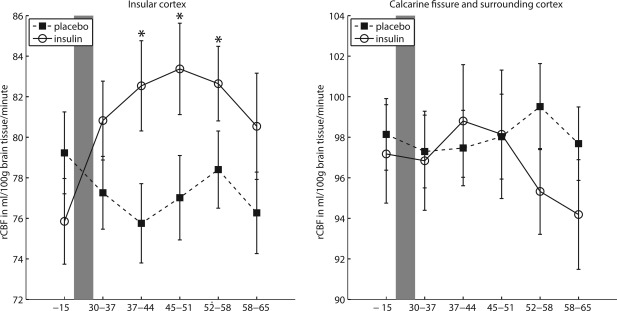
Regional cerebral blood flow (rCBF) over time for participants receiving insulin (N = 22) versus participants receiving placebo (N = 24); left panel: in the insular cortex; right panel in the calcarine fissure and surrounding cortex. X‐axis values are in minutes relative to the application of the intranasal insulin versus placebo. Grey‐shaded area indicates pharmacological treatment. Data present mean and standard error of the mean. Post‐intervention measurement‐blocks which differ significantly from their corresponding baseline are marked with an “*” (according to Dunn's multiple comparison test, ψ5% = 5.00 ml/100 g brain tissue/minute, C = 20).
Secondary ROI
We observed a two‐way interaction “insulin” × “measurement‐block” in the secondary ROI putamen (F (5,210) = 4.087, P = 0.001, η p 2 = 0.089). Dunn's post hoc procedure (ψ 5% = 5.90 ml/100 g brain tissue/min, C = 10) showed that rCBF was elevated compared with baseline values in the post‐intervention measurement‐blocks 1 to 4 (mean increase compared with baseline and standard error in block 1: 6.28 ± 1.94, block 2: 6.58 ± 2.09, block 3: 6.76 ± 2.53, block 4: 6.55 ± 2.21, block 5: 5.83 ± 2.32 ml/100 g/min) in participants receiving insulin but not in those receiving placebo. We did not observe any interaction between “measurement‐block” and “insulin,” or “cortisol” in the secondary ROI hippocampus (all P > 0.05).
Voxel‐based exploration
The voxel‐based exploration revealed CBF increases after intranasal insulin intake, lasting throughout all five post‐intervention measurement‐blocks (interaction “insulin” × “measurement‐block”; SPM “CBF increases after insulin intake”). Clusters of significant activation were found bilaterally in the insular cortex as well as unilaterally in left putamen and left caudate nucleus (see Fig. 4 and Table 1). Moreover, a bilateral CBF increase in the opercular part of the inferior frontal gyrus was observed. No CBF changes were observed in any of the other SPMs calculated. There were no CBF decreases after insulin intake, neither CBF increases nor decreases after cortisol intake, and no interaction between insulin and cortisol on CBF.
Figure 4.
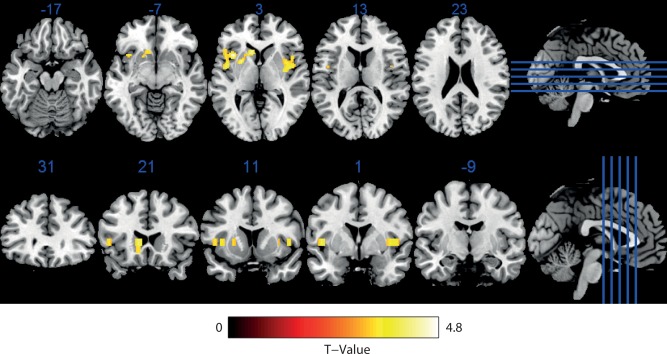
Baseline corrected CBF‐increases in participants receiving intranasal insulin compared with participants receiving placebo in all five post‐intervention measurement‐blocks, corresponding to a time interval of 30 to 65 min after the pharmacological manipulation. No CBF‐decreases were observed. Functional image overlaid on the Ch2bet template implemented in MRIcron and then multislized in the axial and coronar plane. Statistical parametric mapping of the normalized contrast vector of the interaction “insulin” × “measurement‐block” contrasting the post‐intervention measurement‐blocks 1 to 5 with the baseline block and participants receiving insulin with participants receiving placebo (full‐factorial 4(“group”) × 6(“measurement‐block”) model), peak level P < 0.001 (uncorrected), cluster level P < 0.05 (FWE‐corrected), no covariates). See also Table 1 for more information on cluster significance values and peak Z‐scores. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 1.
Clusters of significant activation in the voxel‐based exploration in participants receiving intranasal insulin
| Anatomical regiona | Cluster‐level | Peak‐level | ||||
|---|---|---|---|---|---|---|
| PFWE‐corr b | k E c | Z‐Value | MNId coordinates (mm) | |||
| X | Y | Z | ||||
| Insula and opercular part of inferior frontal gyrus, left | 0.01 | 98 | 4.69 | −37 | 14 | 4 |
| 3.95 | −46 | 0 | 4 | |||
| 3.39 | −46 | 10 | 4 | |||
| Putamen and caudate nucleus, left | 0.03 | 69 | 4.30 | −26 | 7 | 4 |
| 4.20 | −10 | 19 | 4 | |||
| 3.37 | −17 | 14 | −5 | |||
| Insula and putamen, opercular part of inferior frontal gyrus, right | 0.02 | 81 | 3.85 | 41 | −2 | 4 |
| 3.51 | 37 | 5 | 4 | |||
| 3.42 | 46 | 10 | 4 | |||
Baseline corrected CBF‐increases in participants receiving intranasal insulin compared with participants receiving placebo in all five post‐intervention measurement blocks, corresponding to a time interval of 30 to 65 min after the pharmacological manipulation. Table shows local maxima more than 8.0 mm apart and corresponds to Figure 4 (normalized contrast vector of the interaction “insulin” × “measurement‐block” contrasting the post‐intervention measurement‐blocks 1 to 5 with the baseline block and participants receiving insulin with participants receiving placebo, employed in a full‐factorial 4(“group”) × 6(“measurement‐block”) model, peak level P < 0.001 uncorrected, cluster level P < 0.05 (FWE‐corrected), no covariates).
Based on the AAL template implemented in MRIcron.
Family‐wise error corrected value for cluster.
Number of active voxels per cluster.
Montreal Neurological Institute.
DISCUSSION
The aim of the present experiment was to assess the effect of single and combined administration of intranasal insulin and oral cortisol on neuronal activity of the brain. Our main purpose was to investigate the impact of both substances on rCBF in the insular cortex, a region containing the primary gustatory cortex in humans and crucially involved in the processing of gustatory information and interoceptive signals from the gastrointestinal tract. Changes in rCBF in the insular cortex were contrasted with blood flow values in the primary visual cortex, a region proven to be unaffected by intranasal insulin in previous research [Grichisch et al., 2012]. We observed a bilateral increase in rCBF in the insular cortex 37 to 58 min after intranasal insulin administration. As expected, no effect of insulin in the primary visual cortex was found. Also, we did not observe an interaction of insulin × cortisol in the primary or the control ROI, indicating that central insulin pathways in the insular cortex are not modulated by cortisol at rest.
Cerebral blood flow is linked to neuronal signaling presumably due to increased energy demand of active neurons which in turn leads to vasodilatation around the active area [Attwell and Iadecola, 2002]. Hence, by assessing rCBF, an indirect inference of neuronal activity in a certain brain area can be made. In our study we used CASL‐sequences, which allow the quantification of rCBF in absolute physiological values in ml/100 g brain tissue/min. In CASL, cerebral perfusion is estimated using magnetically labeled blood as an endogenous tracer by subtracting an image with magnetically labeled blood from an image without labeling [Alsop and Detre, 1996; Hermes et al., 2007]. This is a major advantage compared with positron emission tomography (PET), which exposes participants to radioactively labeled tracer substances. Also, unlike in PET, the endogenous tracer used in CASL has a very short decay rate (it relaxes with the T1 of arterial blood) which allows a fast repetition of the measurement. Therefore, CASL presents a noninvasive, precise quantification of rCBF in short intervals over a long period of time, making it an ideal method to assess changes in cerebral blood flow following a pharmacological manipulation.
In our experiment, insulin was administered intranasally. Compared with the peripheral administration, the intranasal route of insulin application has the advantage of impacting neither peripheral blood glucose levels [Born et al., 2002] nor peripheral insulin levels [Kern et al., 1999]. Intranasal application of insulin is, therefore, the method of choice to investigate insulin effects on the brain. The oral cortisol administration enabled a fast and safe elevation of blood cortisol levels, leading to an elevated steady‐state‐like cortisol level. Although the oral route of cortisol administration does not allow distinguishing between fast non‐genomic and slow genomic cortisol effects, it has, compared with an intravenous (IV) application, the major advantage of not being invasive and stressful by itself.
This experiment is the first study showing a direct impact of central insulin on rCBF in the insular cortex in a double‐blind placebo controlled experiment. The impact of intranasal insulin on rCBF in the insular cortex is functionally plausible. The insular cortex contains the primary gustatory and visceral cortex in humans [Frey and Petrides, 1999; Small, 2010; Veldhuizen et al., 2011]. Activity in the insular cortex is known to be modulated by subjective appetite ratings [Porubska et al., 2006] and seems to be strongly related to eating behavior [Stoeckel et al., 2008; Wagner et al., 2008]. We are not the first to report an association between insulin and neuronal functioning in the insular cortex. In rats insulin was found to facilitate repetitive spike firing in the insular cortex, which is in line with our result [Takei et al., 2010]. Moreover, Tataranni et al. [1999] found an inverse correlation between rCBF in the insular cortex and blood plasma insulin levels after food intake in humans. Although this result also emphasizes a functional connection between insulin and rCBF in the insular cortex, it contrasts with our finding of increased rCBF after intranasal insulin administration. Since the insular cortex is characterized by high insulin receptor density [Schulingkamp et al., 2000] it may be speculated that a direct receptor‐mediated mechanism is responsible for the observed increase in perfusion. However, the insula has functional connections to other central and peripheral regions and is highly sensitive to visceral feedback. Therefore, something other than a direct receptor mediated mechanism may underlie the observed effect. This could explain the discrepancy with other studies, such as Tataranni et al. [1999], who found a negative association of plasma insulin levels and insular perfusion during a postprandial state. The experiment presented here is neither able to clarify the mechanisms behind the described effect nor its functional implications, and therefore further research is necessary to clarify the opposite results. However, the experimental setting used by Tataranni et al. [1999] differs from our in several aspects such as the time of fasting before the experiment. Hence, the results of both studies might not be directly comparable.
Aside from the intranasal application, effects of systemically administered insulin on neuronal functioning have also been investigated. Kennan et al. [2005] administered IV‐insulin in order to reach a controlled hypoglycemic state and reported increased rCBF in the motor cortex after insulin administration. Although the effect was observed in a different brain area and insulin was administered systemically, it parallels our finding in that insulin increases blood flow in the human cortex. Seaquist et al. [2007] examined the effect of IV‐insulin application on the fMRI blood oxygen level‐dependent (BOLD) signal in the visual cortex and reported a lower BOLD response in the high insulin state, but no effect of insulin on a P100 visually evoked potential. The authors suggest that this effect could be due to an unspecific effect of insulin on blood vessels resulting in a reduced BOLD signal. In the periphery, insulin is known to enhance blood flow in muscle tissue [Baron et al., 1995]. Most recently, intranasal insulin has been shown to not affect rCBF or the BOLD signal in the primary visual cortex [Grichisch et al., 2012] and to increase the cerebral high‐energy phosphate content [Jauch‐Chara et al., 2012]. We therefore suggest that the increased blood flow in the insular cortex and putamen observed in our study reflect locally distinct flow enhancements due to an increase in neuronal activity.
In addition to the results observed in the insular cortex, effects of insulin on rCBF were also evident in the putamen. Intranasal insulin increased rCBF in the putamen in all but the fifth post‐intervention measurement block, corresponding to the time period of 30 to 58 min after insulin administration. Like the insular cortex the putamen shows increased rCBF in a hungry compared with a satiated state [Tataranni et al., 1999] and is positively modulated by subjective appetite [Porubska et al., 2006]. Although effects of insulin on hippocampus‐dependent declarative memory have often been reported [Benedict et al., 2007], we did not observe an effect of insulin on rCBF in the hippocampus. This suggests that either insulin effects on memory are not reflected in a fast change in rCBF in the hippocampus, or that these changes are context dependent and task‐specific.
The voxel‐based analysis revealed that the insulin‐induced CBF increases were not restricted to the insular cortex and the putamen, but extended into the opercular part of the inferior frontal gyrus and into the caudate nucleus. Interestingly, like the insular cortex, the frontal operculum is considered being a part of the human gustatory cortex [Veldhuizen et al., 2011]. Additionally, both structures are connected to each other. Therefore, the result of our voxel‐based analysis suggests that insulin affects different regions in a cortical network involved in the perception of gustatory information. As the voxel based exploration covered all five post‐intervention measurement blocks in one contrast and CBF increases after insulin intake were visible for the entire period, it is possible that insulin effects on the brain might start as early as 30 min after insulin admission and last until 65 min after. The time span of insulin effects observed in the ROI analysis of 37 to 58 min after insulin admission might, therefore, represent the peak time of insulin effects in the brain.
Since participants were restricted from eating from 10 pm of the previous day if scanned in the morning or from 8 am if scanned in the afternoon, a possible explanation of the increased blood flow in the insular cortex and the putamen could be different levels of hunger or distress. However, participants of all four groups did not differ regarding their hunger or subjective mood feelings. We can therefore exclude different levels of hunger, stress, or arousal as a possible explanation for the differences in rCBF observed in our study. The lack of differences in hunger ratings is according to previous research where hunger was assessed after acute application of intranasal insulin [Benedict et al., 2008; Hallschmid et al., 2004b; Jauch‐Chara et al., 2012]. Although intranasal insulin provokes a decrease in food intake in men, hunger ratings do not seem to reflect this tendency, suggesting that the acute anorexigenic effects of central insulin are mediated by satiation signals but not by motivation to eat or a conscious behavioral process [Benedict et al., 2008].
Intranasal insulin effects on rCBF in the insular cortex were present regardless of whether participants received oral cortisol or oral placebo. This may emphasize the fact that insulin‐dependent pathways in the human insular cortex are not influenced by circulating cortisol. In line with this, a recent study showed that while stress modulates orbitofrontal cortex activity in response to highly palatable foods, an insular cortex response to this kind of stimuli was present independent of stress [Rudenga et al., 2012]. Nevertheless, since we administered oral cortisol in order to reach steady‐state cortisol plasma levels, we cannot exclude the possibility that fast non‐genomic cortisol effects would not affect insulin‐dependent signal pathways. Direct IV‐administration of cortisol into the human blood exerts non‐genomic effects on the human brain, as previously described by our group [Strelzyk et al., 2012], and therefore could also interact with the effects of intranasal insulin.
An interaction between insulin and cortisol is suggested to play a role in the development of the metabolic syndrome and obesity. Intranasal insulin has been shown to reduce the hypothalamic‐pituitary‐adrenalcortical (HPA) axis response to psychosocial stress in healthy individuals [Bohringer et al., 2008]. The metabolic syndrome is characterized by an increased HPA‐axis reactivity to an oral glucose tolerance test and indices of central adiposity are negatively associated with the cortisol and adrenocorticotropic hormone (ACTH) responses to dexamethasone [Kazakou et al., 2012; Tyrka et al., 2012]. Also, obese men were found to show reduced cerebral insulin suppression to psychosocial stress [Kubera et al., 2012]. Furthermore, there is accumulating evidence that Alzheimer's disease is accompanied by central insulin resistance [Craft, 2005], and a positive association between blood and CSF cortisol levels and the severity and progression of Alzheimer's disease has been reported [Czech et al., 2012; Davis et al., 1986; Laske et al., 2009; Popp et al., 2009]. We did not find any interaction between insulin and cortisol in any ROI investigated. Therefore, on a basic physiological level, insulin‐dependent signal pathways were not modulated by genomic cortisol effects in this study. Nevertheless, an interaction between both hormones associated with the development of the above‐mentioned disorders may not be reflected in changes in rCBF because such an interaction could emerge in a much longer timeframe, on a molecular level, or could be seen only during specific tasks.
We observed increased rCBF in the insular cortex 37 to 58 min after intranasal insulin administration. This effect was independent of whether participants received oral cortisol or not. Our results indicate that insulin plays a central role in metabolism by modulating effects in the gustatory centers. However, this impact seems to be not affected by glucocorticoids, which might come into play only during cognitive tasks and through different pathways.
ACKNOWLEDGMENTS
The authors thank Manfred Reifer, Hermann Kirschhöfer, and Birgit Bungert for their excellent help in data acquisition. The authors also thank Dr. Michael Hermes for his very helpful advice on data analysis and technical questions.
REFERENCES
- Adam TC, Epel ES (2007): Stress, eating and the reward system. Physiol Behav 91:449–458. [DOI] [PubMed] [Google Scholar]
- Alsop DC, Detre JA (1996): Reduced transit‐time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. J Cereb Blood Flow Metab 16:1236–1249. [DOI] [PubMed] [Google Scholar]
- Alsop DC, Detre JA (1998): Multisection cerebral blood flow MR imaging with continuous arterial spin labeling. Radiology 208:410–416. [DOI] [PubMed] [Google Scholar]
- Attwell D, Iadecola C (2002): The neural basis of functional brain imaging signals. Trends Neurosci 25:621–625. [DOI] [PubMed] [Google Scholar]
- Baron AD, Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G (1995): Insulin‐mediated skeletal muscle vasodilation contributes to both insulin sensitivity and responsiveness in lean humans. J Clin Invest 96:786–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict C, Hallschmid M, Schultes B, Born J, Kern W (2007): Intranasal insulin to improve memory function in humans. Neuroendocrinology 86:136–142. [DOI] [PubMed] [Google Scholar]
- Benedict C, Kern W, Schultes B, Born J, Hallschmid M (2008): Differential sensitivity of men and women to anorexigenic and memory‐improving effects of intranasal insulin. J Clin Endocrinol Metab 93:1339–1344. [DOI] [PubMed] [Google Scholar]
- Bohringer A, Schwabe L, Richter S, Schachinger H (2008): Intranasal insulin attenuates the hypothalamic‐pituitary‐adrenal axis response to psychosocial stress. Psychoneuroendocrinology 33:1394–1400. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. (2002): Sniffing neuropeptides: A transnasal approach to the human brain. Nat Neurosci 5:514–516. [DOI] [PubMed] [Google Scholar]
- Born JM, Lemmens SG, Rutters F, Nieuwenhuizen AG, Formisano E, Goebel R, Westerterp‐Plantenga MS (2010): Acute stress and food‐related reward activation in the brain during food choice during eating in the absence of hunger. Int J Obes (Lond) 34:172–181. [DOI] [PubMed] [Google Scholar]
- Craft S (2005): Insulin resistance syndrome and Alzheimer's disease: Age‐ and obesity‐related effects on memory, amyloid, and inflammation. Neurobiol Aging 26(Suppl 1):65–69. [DOI] [PubMed] [Google Scholar]
- Czech C, Berndt P, Busch K, Schmitz O, Wiemer J, Most V, Hampel H, Kastler J, Senn H (2012): Metabolite profiling of Alzheimer's disease cerebrospinal fluid. PLoS One 7:e31501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Strack AM, Akana SF, Bradbury MJ, Hanson ES, Scribner KA, Smith M (1993): Feast and famine: Critical role of glucocorticoids with insulin in daily energy flow. Front Neuroendocrinol 14:303–347. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Warne JP, Foster MT, Pecoraro NC (2007): Glucocorticoids and insulin both modulate caloric intake through actions on the brain. J Physiol 583:431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KL, Davis BM, Greenwald BS, Mohs RC, Mathe AA, Johns CA, Horvath TB (1986): Cortisol and Alzheimer's disease. I: Basal studies. Am J Psychiatry 143:300–305. [DOI] [PubMed] [Google Scholar]
- De Araujo IE, Rolls ET (2004): Representation in the human brain of food texture and oral fat. J Neurosci 24:3086–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detre JA, Alsop DC (1999): Perfusion magnetic resonance imaging with continuous arterial spin labeling: Methods and clinical applications in the central nervous system. Eur J Radiol 30:115–124. [DOI] [PubMed] [Google Scholar]
- Dressendorfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ (1992): Synthesis of a cortisol‐biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J Steroid Biochem Mol Biol 43:683–692. [DOI] [PubMed] [Google Scholar]
- Dunn OJ (1961): Multiple comparisons among means. J Am Statist Assoc 56:52–64. [Google Scholar]
- Figlewicz DP, Sipols AJ (2010): Energy regulatory signals and food reward. Pharmacol Biochem Behav 97:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC (1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster‐size threshold. Magn Reson Med 33:636–647. [DOI] [PubMed] [Google Scholar]
- Frey S, Petrides M (1999): Re‐examination of the human taste region: A positron emission tomography study. Eur J Neurosci 11:2985–2988. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS (1991): Comparing functional (PET) images: The assessment of significant change. J Cereb Blood Flow Metab 11:690–699. [DOI] [PubMed] [Google Scholar]
- Grichisch Y, Cavusoglu M, Preissl H, Uludag K, Hallschmid M, Birbaumer N, Haring HU, Fritsche A, Veit R (2012): Differential effects of intranasal insulin and caffeine on cerebral blood flow. Hum Brain Mapp 33:280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallschmid M, Benedict C, Born J, Fehm HL, Kern W (2004a): Manipulating central nervous mechanisms of food intake and body weight regulation by intranasal administration of neuropeptides in man. Physiol Behav 83:55–64. [DOI] [PubMed] [Google Scholar]
- Hallschmid M, Benedict C, Schultes B, Fehm HL, Born J, Kern W (2004b): Intranasal insulin reduces body fat in men but not in women. Diabetes 53:3024–3029. [DOI] [PubMed] [Google Scholar]
- Hallschmid M, Higgs S, Thienel M, Ott V, Lehnert H (2012): Postprandial administration of intranasal insulin intensifies satiety and reduces intake of palatable snacks in women. Diabetes 61:782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes M, Hagemann D, Britz P, Lieser S, Rock J, Naumann E, Walter C (2007): Reproducibility of continuous arterial spin labeling perfusion MRI after 7 weeks. MAGMA 20:103–115. [DOI] [PubMed] [Google Scholar]
- Herscovitch P, Raichle ME (1985): What is the correct value for the brain–blood partition coefficient for water? J Cereb Blood Flow Metab 5:65–69. [DOI] [PubMed] [Google Scholar]
- Jauch‐Chara K, Friedrich A, Rezmer M, Melchert UH, G Scholand‐Engler H, Hallschmid M, Oltmanns KM (2012): Intranasal insulin suppresses food intake via enhancement of brain energy levels in humans. Diabetes 61:2261–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M (1997): Steroid hormones and excitability in the mammalian brain. Front Neuroendocrinol 18:2–48. [DOI] [PubMed] [Google Scholar]
- Kanal E, Borgstede JP, Barkovich AJ, Bell C, Bradley WG, Felmlee JP, Froelich JW, Kaminski EM, Keeler EK, Lester JW, Scoumis EA, Zaremba LA, Zinninger MD; American College of Radiology (2002): American college of radiology white paper on MR safety. AJR Am J Roentgenol 178:1335–1347. [DOI] [PubMed] [Google Scholar]
- Kazakou P, Kyriazopoulou V, Michalaki M, Ierodiakonou V, Psyrogiannis A, Habeos I (2012): Activated hypothalamic pituitary adrenal axis in patients with metabolic syndrome. Horm Metab Res 44:839–844. [DOI] [PubMed] [Google Scholar]
- Kennan RP, Takahashi K, Pan C, Shamoon H, Pan JW (2005): Human cerebral blood flow and metabolism in acute insulin‐induced hypoglycemia. J Cereb Blood Flow Metab 25:527–534. [DOI] [PubMed] [Google Scholar]
- Kern W, Born J, Schreiber H, Fehm HL (1999): Central nervous system effects of intranasally administered insulin during euglycemia in men. Diabetes 48:557–563. [DOI] [PubMed] [Google Scholar]
- Kubera B, Hubold C, Zug S, Wischnath H, Wilhelm I, Hallschmid M, Entringer S, Langemann D, Peters A (2012): The brain's supply and demand in obesity. Front Neuroenergetics 4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann S, Heni M, Veit R, Ketterer C, Schick F, Haring HU, Fritsche A, Preissl H (2012): The obese brain: Association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum Brain Mapp 33:1052–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- la Fleur SE (2006): The effects of glucocorticoids on feeding behavior in rats. Physiol Behav 89:110–114. [DOI] [PubMed] [Google Scholar]
- la Fleur SE, Akana SF, Manalo SL, Dallman MF (2004): Interaction between corticosterone and insulin in obesity: Regulation of lard intake and fat stores. Endocrinology 145:2174–2185. [DOI] [PubMed] [Google Scholar]
- Laske C, Stransky E, Fritsche A, Eschweiler GW, Leyhe T (2009): Inverse association of cortisol serum levels with T‐tau, P‐tau 181 and P‐tau 231 peptide levels and T‐tau/Abeta 1–42 ratios in CSF in patients with mild Alzheimer's disease dementia. Eur Arch Psychiatry Clin Neurosci 259:80–85. [DOI] [PubMed] [Google Scholar]
- Lee J, Herman JP, Mattson MP (2000): Dietary restriction selectively decreases glucocorticoid receptor expression in the hippocampus and cerebral cortex of rats. Exp Neurol 166:435–441. [DOI] [PubMed] [Google Scholar]
- Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J (1995): A probabilistic atlas of the human brain: Theory and rationale for its development. The International Consortium for Brain Mapping (ICBM). Neuroimage 2:89–101. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW (2006): Central nervous system control of food intake and body weight. Nature 443:289–295. [DOI] [PubMed] [Google Scholar]
- Ohla K, Toepel U, le Coutre J, Hudry J (2012): Visual‐gustatory interaction: Orbitofrontal and insular cortices mediate the effect of high‐calorie visual food cues on taste pleasantness. PLoS One 7:e32434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Schweiger U, Pellerin L, Hubold C, Oltmanns KM, Conrad M, Schultes B, Born J, Fehm HL (2004): The selfish brain: Competition for energy resources. Neurosci Biobehav Rev 28:143–180. [DOI] [PubMed] [Google Scholar]
- Plum L, Schubert M, Bruning JC (2005): The role of insulin receptor signaling in the brain. Trends Endocrinol Metab 16:59–65. [DOI] [PubMed] [Google Scholar]
- Popp J, Schaper K, Kolsch H, Cvetanovska G, Rommel F, Klingmuller D, Dodel R, Wullner U, Jessen F (2009): CSF cortisol in Alzheimer's disease and mild cognitive impairment. Neurobiol Aging 30:498–500. [DOI] [PubMed] [Google Scholar]
- Porubska K, Veit R, Preissl H, Fritsche A, Birbaumer N (2006): Subjective feeling of appetite modulates brain activity: An fMRI study. Neuroimage 32:1273–1280. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH (2003): Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time‐dependent change. Psychoneuroendocrinology 28:916–931. [DOI] [PubMed] [Google Scholar]
- Rosmond R (2005): Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology 30:1–10. [DOI] [PubMed] [Google Scholar]
- Rudenga KJ, Sinha R, Small DM (2012): Acute stress potentiates brain response to milkshake as a function of body weight and chronic stress. Int J Obes (Lond) 37:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR (2000): Distribution of corticosteroid receptors in the rhesus brain: Relative absence of glucocorticoid receptors in the hippocampal formation. J Neurosci 20:4657–4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara VR, Hall K, Von Holtz H, Humbel R, Sjogren B, Wetterberg L (1982): Evidence for the presence of specific receptors for insulin‐like growth factors 1 (IGE‐1) and 2 (IGF‐2) and insulin throughout the adult human brain. Neurosci Lett 34:39–44. [DOI] [PubMed] [Google Scholar]
- Schulingkamp RJ, Pagano TC, Hung D, Raffa RB (2000): Insulin receptors and insulin action in the brain: Review and clinical implications. Neurosci Biobehav Rev 24:855–872. [DOI] [PubMed] [Google Scholar]
- Seaquist ER, Chen W, Benedict LE, Ugurbil K, Kwag JH, Zhu XH, Nelson CA (2007): Insulin reduces the BOLD response but is without effect on the VEP during presentation of a visual task in humans. J Cereb Blood Flow Metab 27:154–160. [DOI] [PubMed] [Google Scholar]
- Small DM (2010): Taste representation in the human insula. Brain Struct Funct 214:551–561. [DOI] [PubMed] [Google Scholar]
- Softeland E, Dimcevski G, Graversen C, Nedrebo BG, Drewes AM, Frokjaer JB (2011): Effects of isolated hyperinsulinaemia on sensory function in healthy adults. Exp Clin Endocrinol Diabetes 119:604–609. [DOI] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW III, Twieg DB, Knowlton RC, Cox JE (2008): Widespread reward‐system activation in obese women in response to pictures of high‐calorie foods. Neuroimage 41:636–647. [DOI] [PubMed] [Google Scholar]
- Strelzyk F, Hermes M, Naumann E, Oitzl M, Walter C, Busch HP, Richter S, Schachinger H (2012): Tune it down to live it up? Rapid, nongenomic effects of cortisol on the human brain. J Neurosci 32:616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei H, Fujita S, Shirakawa T, Koshikawa N, Kobayashi M (2010): Insulin facilitates repetitive spike firing in rat insular cortex via phosphoinositide 3‐kinase but not mitogen activated protein kinase cascade. Neuroscience 170:1199–1208. [DOI] [PubMed] [Google Scholar]
- Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, Pratley RE, Lawson M, Reiman EM, Ravussin E (1999): Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci USA 96:4569–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Walters OC, Price LH, Anderson GM, Carpenter LL (2012): Altered response to neuroendocrine challenge linked to indices of the metabolic syndrome in healthy adults. Horm Metab Res 44:543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Unger JW, Livingston JN, Moss AM (1991): Insulin receptors in the central nervous system: Localization, signalling mechanisms and functional aspects. Prog Neurobiol 36:343–362. [DOI] [PubMed] [Google Scholar]
- Veldhuizen MG, Albrecht J, Zelano C, Boesveldt S, Breslin P, Lundstrom JN (2011): Identification of human gustatory cortex by activation likelihood estimation. Hum Brain Mapp 32:2256–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A, Aizenstein H, Mazurkewicz L, Fudge J, Frank GK, Putnam K, Bailer UF, Fischer L, Kaye WH (2008): Altered insula response to taste stimuli in individuals recovered from restricting‐type anorexia nervosa. Neuropsychopharmacology 33:513–523. [DOI] [PubMed] [Google Scholar]
- Walter B, Blecker C, Kirsch P, Sammer G, Schienle A, Stark R, Vaitl D (2003): MARINA: An easy to use tool for the creation of MAsks for Region of INterest Analyses [abstract]. Presented at the 9th International Conference on Functional Mapping of the Human Brain, June 19–22, 2003, New York, NY. [Google Scholar]
- Wang Z, Aguirre GK, Rao H, Wang J, Fernandez‐Seara MA, Childress AR, Detre JA (2008): Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging 26:261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warne JP (2009): Shaping the stress response: Interplay of palatable food choices, glucocorticoids, insulin and abdominal obesity. Mol Cell Endocrinol 300:137–146. [DOI] [PubMed] [Google Scholar]
- Werther GA, Hogg A, Oldfield BJ, McKinley MJ, Figdor R, Allen AM, Mendelsohn FA (1987): Localization and characterization of insulin receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry. Endocrinology 121:1562–1570. [DOI] [PubMed] [Google Scholar]
- Wolf OT (2009): Stress and memory in humans: twelve years of progress? Brain Res 1293:142–154. [DOI] [PubMed] [Google Scholar]


