Abstract
Evolution has provided us with a highly flexible neuroendocrine threat system which, depending on threat imminence, switches between active escape and passive freezing. Cortisol, the “stress‐hormone”, is thought to play an important role in both fear behaviors, but the exact mechanisms are not understood. Using pharmacological functional magnetic resonance imaging we investigated how cortisol modulates the brain's fear systems when humans are under virtual‐predator attack. We show dissociated neural effects of cortisol depending on whether escape from threat is possible. During inescapable threat cortisol reduces fear‐related midbrain activity, whereas in anticipation of active escape cortisol boosts activity in the frontal salience network (insula and anterior cingulate cortex), which is involved in autonomic control, visceral perception and motivated action. Our findings suggest that cortisol adjusts the human neural threat system from passive fear to active escape, which illuminates the hormone's crucial role in the adaptive flexibility of fear behaviors. Hum Brain Mapp 36:4304–4316, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: cortisol, threat escape, salience network
INTRODUCTION
When a predator attacks, flexible action from the prey is crucial for its survival. The prey can passively freeze or actively escape, behaviors that gain efficiency over repeated attacks. Evolution thus provided for a neuroendocrine threat system that can flexibly switch between active escape and passive defence behaviors [Blanchard et al., 2001; Koolhaas et al., 2010]. Rapid surges of catecholamines, that initially drive this threat system, are followed by a slower hormonal cascade of the hypothalamic‐pituitary‐adrenal (HPA) axis. This cascade produces glucocorticoids (cortisol in humans), which have been argued to underlie system normalization after threat exposure, but also the progression of efficiency in fear responses to the next threat [de Kloet et al., 2005; Karatsoreos and McEwen, 2011; Sapolsky et al., 2000].
Acute glucocorticoids can indeed have fear‐reducing effects in humans [Buchanan et al., 2001; Putman et al., 2007a,b; Sapolsky et al., 2000; Soravia, 2006; Soravia et al., 2013], and in animals they particularly seem to reduce fear behavior, i.e. freezing [Skorzewska et al., 2006, 2007]. In contrast, acute glucocorticoids can also increase fear [Grillon et al., 2011; Mitra and Sapolsky, 2008]. These seemingly opposing effects might reflect context‐dependency as glucocorticoids can for example increase responsivity to unpredictable threat (e.g. anxiety) without affecting the acute, short‐term threat response (i.e. fear) [Grillon et al., 2011]. Moreover, evidence showing that glucocorticoids induce approach motivation [Putman et al., 2007b, 2010a], aggression [Böhnke et al., 2010] and active stress‐behaviors [Thaker et al., 2009], suggests that glucocorticoids particularly drive active fight‐flight behaviors and might not be anxiolytic per se.
Cortisol's effects on the human brain's threat systems have however only been studied using passive threat paradigms wherein no active escape was possible [Henckens et al., 2010; Merz et al., 2010]. The question therefore arises whether cortisol can exert dissociating effects on the brain's threat systems depending on active (escape) and passive (fear) threat conditions.
The brain's salience network is the likely candidate for such cortisol action as it not only consists of areas involved in fear, such as the midbrain [Seeley et al., 2007], but also areas dedicated to visceral perception, autonomic control, and motivated action such as anterior insula (AIC) and dorsal anterior cingulate cortices (dACC) [Craig, 2009; Neta et al., 2014]. Importantly, the salience network seems indeed to be controlled by glucocorticoids after stress [Hermans et al., 2014]. In support of cortisol's system normalization function after stress, acute threat‐responsivity within the brain's salience network is unaffected by cortisol synthesis inhibition [Hermans et al., 2011]. The delayed cortisol response following a stressor has instead been argued to attenuate threat‐responsivity later in time [Hermans et al., 2014]. Combined with the evidence that glucocorticoids reduce freezing, it might be expected that cortisol administration suppresses the salience network, and the midbrain fear‐response in particular, when threats are inescapable [McNaughton and Corr, 2004; Mobbs et al., 2007, 2009]. When escape is possible, however, cortisol's promotion of active fight‐flight mechanisms might come into play and boost activity in the frontal salience network.
MATERIALS AND METHODS
Subjects
Twenty healthy, right‐handed young men (age range 19–30, mean age 22.6, SD 2.7) provided informed consent to participate in this study, which was approved by the medical ethical committee of the University Medical Center Utrecht. One subject was excluded from all data‐analyses because his button‐box device broke during the task (i.e., n = 19), and one subject forgot to fill in the post‐scan questionnaires. We chose not to include females because their response to cortisol administration is dependent on contraceptive‐use and menstrual cycle phase [Merz et al., 2012]. Further exclusion criteria were physical or mental illness, abuse of alcohol and drugs, and regular smoking.
Fear‐and‐Escape Task (FAET)
We used a newly developed functional magnetic resonance imaging (fMRI) design that provides for an environment wherein threat and escape possibilities are dynamically changing; the fear‐and‐escape task (FAET). Participants in the FAET repeatedly experience the circa‐strike phase of a virtual‐predator attack [Mobbs et al., 2007, 2009]. This is achieved through presentation of visual stimuli that rapidly approach the participant culminating in the presentation of a highly aversive noise (AN) when the stimuli reach full‐size. The participants can escape these virtual‐predators by pressing a button, and the predator is manipulated to be escapable, imminent (escapable at chance‐level), or inescapable (see Fig. 1). Brain activity during the anticipation of these attacks is compared with a safe‐context control condition using exactly the same procedure, but with a visual stimulus that is not linked to AN exposure. This design not only allows us to investigate and compare the effects of anticipated attack from escapable and imminent predators, but can also compare active escape anticipation with passive fear conditions as used in previous studies [Grupe and Nitschke, 2013; Klumpers et al., 2010]. As such, the FAET can elucidate new insights into the neural differences between anticipation of active escape and passive fear, as well as test differential modulation by cortisol.
Figure 1.
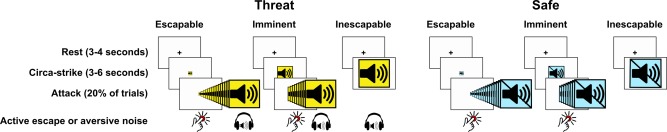
Outline of the fear‐and‐escape task. Participants are repeatedly attacked by rapidly approaching pictures. Participants can escape by pressing a button, but when they fail to do so they will be presented with a highly aversive noise (AN). The pictures are manipulated to be escapable, imminent (escapable at chance‐level) or inescapable, and all conditions are compared with an equivalent safe‐context control condition involving exactly the same procedure but without the threat of AN exposure. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
An overview of the events in the FAET is depicted in Figure 1. Each trial in the FAET commenced with a rest‐phase of 3 or 4 s (randomized and counterbalanced across conditions) consisting of a black fixation‐cross on a white background. Next, an image appeared that either represented the THREAT (a sound pictogram on a yellow background) or SAFE (a crossed‐out sound pictogram on an equiluminant blue background) condition. These pictures appeared either full‐screen (inescapable condition; IE), full‐screen divided by 2 (imminent condition; I) or full‐screen divided by 16 (escapable condition; E), and were presented for 3, 4.5, or 6 s (randomized and counterbalanced across conditions).
Except for the SAFE/IE condition the pictures could “attack” the participant. An attack in the THREAT/IE condition consisted of the presentation of an aversive noise (AN) through MR‐compatible headphones. The AN was a loud (110 dB) 1‐s female scream, which compared with electric shock stimuli evokes similar peripheral fear‐potentiated physiological responses [Glenn et al., 2012.
An attack in the E and I conditions consisted of a rapid increase in size of the pictures, which could be stopped by the participant with a button press (escape). When the participant failed to escape, and the pictures reached full‐screen size, they were followed by the AN, but only in the THREAT condition. Immediately following this sequence of events the next trial commenced.
The FAET is particularly designed to measure neural responses during attack anticipation. Furthermore, repeated exposure to the AN can result in habituation effects, and might be considered un‐ethical. For these reasons only 20% of the trials did involve an actual attack. Moreover, to ensure an even threat‐level throughout the scan‐session, the attack trials were evenly distributed over time. This was achieved by presenting the trials in three blocks. Each block consisted of four trials of each condition without attack, and one with attack (excluding the SAFE/IE condition). Thus, the number of trials in each block was 29, making a total of 87 trials, and within each block trial‐order was fully randomized.
Speed of the attacking pictures was adjusted individually to ensure that each participant could escape the THREAT/I condition at chance‐level. To this end participants engaged in an elaborate practice session preceding drug administration. Participants first performed 20 trials of a reaction time task (RTT) wherein they were instructed to press a button as fast as possible when a grey rectangle (similar in size to the E condition) started to grow in size. Reaction times were recorded and averaged. Next, after a thorough explanation of the FAET the participants engaged in a 36‐trial practice session with visual feedback (“SCREAM!!!”) instead of AN presentation, and a 100% attack rate. Average reaction time from the RTT was used as baseline duration of the imminent attacks (DIA). Whenever the participant failed to escape an imminent attack (button press too late) DIA was adjusted by adding 33ms (two frames at a display‐refresh rate of 60 Hz). Whenever the participant did successfully escape an imminent attack DIA was adjusted by subtracting 33 ms. Duration of escapable attacks was always set at DIA × 4.
Finally, participants engaged in a 24‐trial practice session that did include AN‐presentation, but at low (nonaversive) volume, and with a similar percentage of attacks (20%) as in the actual FAET. DIA was adjusted as before and the resulting DIA was entered as baseline DIA in the actual MRI‐task. During the MRI‐session DIA‐adjustment continued, but now based on threat trials only to ensure that reduced motivation to press the button during the safe condition would not result in slower attacks. Furthermore, in order to keep anticipation for motor responses in THREAT and SAFE conditions comparable, participants were explicitly instructed to also initiate escape during the SAFE trials.
fMRI Data Collection and Analyses
Preprocessing
Scanning was performed on a 3 T Philips Achieva MRI scanner (Philips Medical Systems, Best, The Netherlands). Before the functional scans, a high resolution anatomical T1‐weighted scan with the following parameters was obtained for co‐registration and normalization purposes: 3.8 ms echo time, 8.4 ms repetition time, 288 × 288 × 175 mm field of view, 175 sagittal slices, flip angle of 8.0°, voxelsize 1.0 mm isotropic. Blood oxygen level dependent (BOLD‐) response was measured with functional T2*‐weighted axial whole‐brain images, of which 550 were obtained throughout the task. The 2D‐EPI‐SENSE sequence had the following parameters: echo time 23 ms, repetition time 1.4 s, 208 × 119 × 256 mm field of view, 30 slices, flip angle of 70°, SENSE‐factor R = 2.4 (anterior‐posterior), voxelsize 4.0 mm isotropic.
Preprocessing and subsequent analyses were performed with SPM8 (http://www.fil.ion.ucl.ac.uk/spm). Functional scans of both sessions were motion corrected to the first dynamic scan and slice‐time corrected to the middle slice. The anatomical scan was then coregistered to the mean functional scan. Subsequently, using unified segmentation, the structural scan was segmented and normalization parameters were estimated. Using these normalization parameters, all volumes were normalized to a standard brain template (MNI) and resliced at 2.0 mm isotropic voxelsize. Smoothing with an 8.0 mm full width at half maximum Gaussian kernel was applied to the normalized functional volumes.
Statistical analyses
The effects of cortisol on brain activity related to threat anticipation were investigated within general linear models (GLM). The FAET was designed to measure BOLD‐responses during the anticipation phase of passive fear for, or active escape from, an aversive stimulus. Therefore, trials without actual attacks (12 trials for each condition) were of main interest, whereas trials with attacks (three trials for each condition excluding safe/inescapable) were treated as separate variables in the model, which ensures that the effects of motion related artefacts due to button‐presses and AN presentation do not affect our measure of interest. Thereto, in the first‐level GLM for each test‐session, we used 12 regressors for our trials of interest: Six for the trial‐onsets (box‐car function for stimulus‐duration, 3–6 s), and six for the trial‐offsets (delta function). Trial‐offset regressors were included based on a previous study into threat‐offset effects [Klumpers et al., 2010], but considered of no‐interest for the current study. Furthermore, 10 other nuisance regressors were defined: Five for the trial‐onsets for stimuli that actually attacked, four for the attack‐onset (box‐car function for attack‐duration), and one for the AN‐onset (box‐car function for AN‐duration, 1 s).
These regressors were all convolved with the hemodynamic response function as implemented in the SPM8 software. In addition, realignment parameters and a discrete cosine transform high‐pass filter with a 1/128 Hz cut‐off frequency were entered into the analyses to reduce variance due to nuisance factors such as movement and drifts in the signal. Thus, in total 29 regressors were entered in the first level statistical analysis. For each subject and session we computed contrast maps for onset of escapable, imminent and inescapable threat and safe cues versus baseline.
For the second level analysis, the contrast maps of threat onset from the placebo condition were entered in a full‐factorial 2 × 3 ANOVA design with threat (threat and safe) and condition (escapable, imminent, inescapable) as within‐subjects factors. We looked within the placebo condition only, since analysing the data of both conditions together will confound the effects of the FAET with possible effects of cortisol. For investigating the effects of drug, we ran a full‐factorial 2 × 2 × 3 ANOVA designs with drug (cortisol and placebo), threat (threat and safe) and condition (escapable, imminent, inescapable) as within‐subjects factors.
All calculated linear contrasts were thresholded at P < 0.05, family‐wise error (FWE) ‐corrected at the voxel‐level. For regions of interest (ROIs) (see below), we applied small volume corrections (s.v.c., FWE; P < 0.05 at the voxel‐level). To link activation patterns to anatomy, the significant clusters were inspected with the Anatomy Toolbox for SPM [Eickhoff et al., 2007] or the automated anatomical AAL template [Tzourio‐Mazoyer et al., 2002] if the region was not included in the probabilistic cytoarchitectonic maps of the Anatomy Toolbox.
Finally, to test for differential cortisol effects within and between the brain structures of interest we extracted parameter estimates, using the SPM8 toolbox Marsbar, from functionally and anatomically defined ROIs (see below), which were further tested using repeated measures ANOVAs. Greenhouse‐Geisser corrections were applied if the assumption of sphericity was violated.
ROI masks
We used ROI masks of the salience network structures (AIC, dACC, amygdala, midbrain). The masks of the AIC and dACC were based on a mask of the anterior salience network (downloaded from http://findlab.stanford.edu/functional_ROIs.html, for a description see Shirer et al. [2012]). The AIC (volume left 1,568 mm3, volume right 1,856 mm3) and dACC (volume 7,984 mm3) masks were restricted to the bilateral entire insula and ACC plus MCC respectively as defined in AAL atlas implemented in SPM8 to ensure that these masks cover only the insula and cingulate cortices. The amygdala mask was also taken from the AAL atlas. For the midbrain ROI we used the bilateral midbrain mask from the TD Lobes atlas from the WFU Pickatlas Toolbox (implemented in SPM8) [Maldjian et al., 2003].
In addition, because the subgenual ACC (sgACC) is specifically linked to escapable threat [Amat et al., 2005; Jahn et al., 2010; Mobbs et al., 2007, 2009], but is not included in the salience network mask, we included a separate mask for this region. We used the mask of Brodmann area 25 from the WFU Pickatlas as it corresponds to this region. Finally, to investigate deactivations in the default mode network during threat anticipation we used the mask of the default mode network (medial prefrontal cortex and posterior cingulate cortex) from (http://findlab.stanford.edu/functional_ROIs.html, for a description see Shirer et al. [ 2012]).
Questionnaires and hormone measurements
To obtain a subjective measure of fear, we asked participants to indicate how afraid they were for the scream. Subjects provided their ratings using a visual analogue scale (VAS) ranging from −100 to +100. The same VAS was used to measure participant's mood state using the Profile of Mood States (POMS) – Short Version questionnaire [Shacham, 1983]. The POMS has six subscales (anger, anxiety, depression, tension, fatigue, vigour) and was administered before pill‐intake and at the end of the experiment to investigate if cortisol administration resulted in altered mood. We computed difference scores of the pre‐ and postdrug intake measures of each of the subscales of the POMS questionnaire, and tested these difference scores in placebo condition versus cortisol condition.
Saliva samples were taken on both days before drug‐intake to assess endogenous cortisol levels. Subjects were asked to refrain from eating and drinking anything else but water 1 h before providing the saliva sample.
Cortisol administration and procedure of the experiment
This experiment was part of a larger study with two other fMRI tasks [Bos et al., 2014; Montoya et al., 2014]. The FAET was always the last task of the fMRI protocol. The experiment took place between 02.00 and 09.00 PM when endogenous cortisol levels are low [Lupien et al., 2007]. To control for diurnal fluctuations of cortisol the time of the day when testing took place was kept similar within subjects. Furthermore, there was always at least one week between the two sessions (cortisol and placebo).
Subjects arrived at the lab to fill in questionnaires and provided a saliva sample (5 ml). Subsequently, the drug was administered under supervision of the experimenter. Cortisol was orally administered in a capsule, containing 40 mg hydrocortisone + 320 mg Primogel, in a double‐blind, placebo‐controlled crossover design. The placebo consisted of 360 mg Primogel. Order of drug administration was counterbalanced across subjects.
Forty minutes postdrug‐intake participants were placed in the MRI scanner and an anatomical and two functional scans were made. Following these scans, the participants did the FAET that started approximately 80 min after drug‐intake. Before the actual task started, participants again practiced 12 trials (six threat and six safe cues, 2× escapable, 2× imminent, 2× inescapable) to make sure they fully understood the task, and to accustom to the scanner button‐box. Furthermore, the inclusion of an inescapable threat trial assured that the scream was presented at full volume at least once before the actual scanning commenced. This ascertains that the participants will know what to expect of the scream, which minimizes any ambiguity in threat‐level between participants and sessions by diminishing expectancy effects.
The dosage of cortisol, and the time of the testing relative to the time of administration that we employed is similar to earlier behavioral studies that were conducted in our lab [Putman et al., 2007a, 2010b]. Similar dosages (30 and 35 mg) timing between administration and testing (∼60 min) have also been employed by other research groups, showing behavioral and physiological effects of cortisol administration [Merz et al., 2012; Tops et al., 2006]. Following scanning, subjects had to fill in questionnaires, were debriefed and received forty euros as payment, and an additional amount (10 euros on average) that they could win during a task they performed earlier.
RESULTS
Subjective and Behavioral Data
Nineteen males participated in the FAET in two separate counterbalanced sessions following cortisol and placebo administration. Subjective ratings (scale: −100 = “not at all fearful”, 0 = “fearful”, 100 = “very fearful”) after each session were checked for normality and analyzed. The fear ratings provided in the placebo were normally distributed (Kolmogorov‐Smirnov test, P = 0.200), whereas they were not normally distributed for the cortisol condition (Kolmogorov‐Smirnov test, P = 0.014). Nonparametric tests confirmed that the participants were substantially afraid of the AN, which was not different over the two sessions (first session: M = 21.31, SD = 48.80, second session: M = 13.58, SD = 48.10, Z = −0.588, P = 0.557), nor between cortisol and placebo (Z = −0.653, P = 0.514, see Table 1).
Table 1.
Mean (and standard deviations) for salivary hormone levels and fear ratings for the AN (the scream) for cortisol and placebo conditions
| Placebo | Cortisol | |
|---|---|---|
| Cortisol (nmol/l) | 10.06 (5.68) | 12.23 (7.35) |
| Fear rating | 22.94 (42.58) | 13.39 (55.89) |
The hormone levels were measured at baseline. Statistical tests are reported in text.
Behavioral data from the FAET (see Table 2) confirmed that participants attempted to escape consistently in all conditions, succeeded in escape more often in the I/THREAT compared with I/SAFE condition (P < 0.001), but the DIA‐adjustment ensured that escape success in the I/THREAT condition was not different from chance (P = 0.40). Average reaction time measures, although based on very few trials, showed a similar pattern. Escape from threat compared with safe conditions was significantly faster (P = 0.019), which was also the case for escape from imminent compared with escapable conditions (P = 0.014). No significant differences were observed between cortisol and placebo conditions on any on the behavioral measures (paired samples t‐tests, all Ps > 0.3).
Table 2.
Behavioral data from the FAET
| Condition | Attempted escapes (SD) | Succeeded escapes (SD) | Reaction time (SD) | |
|---|---|---|---|---|
| Escapable | Threat | 100% (0) | 100% (0) | 359 ms (50) |
| Safe | 100% (0) | 100% (0) | 391 ms (76) | |
| Imminent | Threat | 95% (15) | 54% (25) | 317 ms (55) |
| Safe | 95% (12) | 32% (29) | 366 ms (116) |
fMRI Data
As expected threat versus safe conditions recruited the salience network. Particularly the bilateral AIC (left: x = −30, y = 26, z = 2, and right extending from the inferior orbital cortex to the AIC: x = 34, y = 24, z = 6) together with right‐sided midbrain (x = 6, y = −16, z = −12) and bilateral dACC (left: x = −6 y = 8, z = 43, right: x = 8, y = 10, z = −44) [Seeley et al., 2007]. Furthermore, the bilateral supplementary motor area was activated (see Fig. 2 and Table 3). This activity originated mostly from inescapable threat conditions (see Fig. 3 and Table 3), and in line with the work of Mobbs and colleagues [Mobbs et al., 2007, 2009], we also observed a shift from parietal and prefrontal regions towards midbrain depending on threat imminence. More specifically, a set of dorsal regions belonging to the default mode network, medial prefrontal cortex, and posterior cingulate cortex [Raichle et al., 2001], was deactivated during inescapable compared with escapable threat. This prefrontal deactivation, together with deactivation in middle temporal cortex, was also present during imminent threat when compared with escapable threat (see Fig. 3 and Table 3). These findings are in inline with the influential model of McNaughton and Corr [2004] stating that cortical functions are down regulated during acute panic and freezing reactions from subcortical structures.
Figure 2.
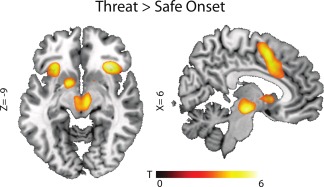
Effects of threat anticipation within the placebo condition. Threat activated the anterior salience network, consisting of anterior insular cortices and dorsal anterior cingulate cortices together with supplementary motor cortex and midbrain. Statistical map is overlaid on a template brain in MNI‐space and thresholded at P < 0.001 uncorrected (T‐threshold is T = 3.17) for illustrative purposes only (for statistical analyses see Table 3). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 3.
Threat onset—placebo condition
| Contrast/region | Side | x | y | z | n voxels | Peak, T | P |
|---|---|---|---|---|---|---|---|
| Main effect of threat onset | |||||||
| Threat Onset > Safe Onset | |||||||
| AIC | L | −30 | 26 | 2 | 253 | 6.42 | <0.001 |
| −34 | 20 | 6 | s.c. | 6.21 | <0.001 | ||
| Frontal inferior orbital cortex | R | 32 | 26 | −6 | 232 | 5.97 | 0.001 |
| AIC | 34 | 24 | 6 | s.c. | 5.67 | 0.002 | |
| Midbrain | R | 6 | −16 | −12 | 18 | 5.39 | 0.006 |
| Supplementary motor area | L | −8 | 6 | 46 | 18 | 5.29 | 0.009 |
| Putamen | L | −16 | 6 | −8 | 1 | 5.03 | 0.022 |
| Supplementary motor area | R | 4 | 6 | 52 | 5 | 4.87 | 0.039 |
| dACC | R | 8 | 10 | −44 | 231 | 4.69 | 0.001a |
| L | −6 | 8 | 42 | s.c. | 4.32 | 0.002a | |
| −6 | 20 | 30 | s.c. | 3.57 | 0.022a | ||
| sgACC (BA 25) | L | −2 | 10 | −4 | 3 | 3.38 | 0.034a |
| Safe Onset > Threat onset | |||||||
| Middle temporal cortex | L | −60 | −10 | −12 | 20 | 5.00 | 0.007 |
| L | −58 | 0 | −20 | 16 | 5.05 | 0.020 | |
| Middle OFC | L | −34 | 38 | 12 | 7 | 4.90 | 0.035 |
| Interactions: Threat × Distance | |||||||
| Threat [Escapable > Imminent] > Safe [Imminent > Escapable] | |||||||
| Dorsomedial prefrontal cortex (Brodmann area 8) | R | 14 | 36 | 56 | 31 | 5.15 | 0.014 |
| Middle temporal cortex | L | −66 | −52 | −4 | 10 | 5.12 | 0.016 |
| L | −64 | −6 | −20 | 1 | 4.81 | 0.049 | |
| Threat [Escape > Inescapable] > Safe [Inescapable > Escapable] | |||||||
| Superior frontal cortex (Brodmann area 9) | R | 16 | 40 | 50 | 24 | 5.20 | 0.012 |
| Posterior cingulate cortex (DMN mask) | L | −10 | −46 | 26 | 228 | 4.69 | 0.004a |
| R | 4 | −56 | 28 | s.c. | 4.56 | 0.006a | |
| L | −4 | −54 | 30 | s.c. | 4.36 | 0.012a | |
| Threat [Inescapable > Escapable] > Safe [Escapable > Inescapable] | |||||||
| AIC | L | −34 | 24 | 4 | 70 | 5.85 | 0.001 |
| dACC | L | −4 | 24 | 26 | 47 | 3.67 | 0.016a |
| Simple effects [Threat > Safe] for the different escape conditions | |||||||
| Escapable: no suprathreshold voxels | |||||||
| Imminent | |||||||
| AIC | R | 32 | 22 | 6 | 1 | 3.14 | 0.043a |
| Midbrain | R | 8 | −14 | −12 | 1 | 3.61 | 0.035a |
| Inescapable | |||||||
| AIC | L | −34 | 24 | 4 | 508 | 7.82 | <0.001 |
| R | 34 | 24 | −8 | 434 | 6.66 | <0.001 | |
| Supplementary motor area (Brodmann area 6) | R | 8 | 4 | 60 | 39 | 5.08 | 0.018 |
| 4 | 8 | 54 | s.c. | 4.94 | 0.030 | ||
| Midbrain | R | 2 | −20 | −12 | 127 | 4.37 | 0.003a |
| dACC | R | 4 | 12 | 44 | 520 | 4.27 | 0.003a |
| L | −2 | 24 | 28 | s.c. | 4.24 | 0.003a | |
| R | 4 | 18 | 36 | s.c. | 4.20 | 0.003a | |
Table shows anatomical region, MNI coordinates and T‐values for the reported contrasts of the FAET threat onset from the placebo condition. All analyses are conducted at the voxel‐level, whole‐brain P < 0.05, FWE‐corrected.
Small volume corrected at P < 0.05, FWE‐corrected. s.c., same cluster.
Figure 3.
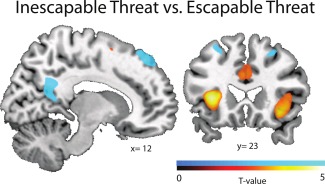
Effects of inescapable versus escapable threat in contrast to the safe conditions ([escapable threat > escapabale safe] > [inescapable threat > inescapable safe]). Modulation of the salience and default mode networks of the brain when threat was inescapable compared with escapable. Increased activity in the anterior salience network: the anterior insular cortex (AIC) and dorsal anterior cingulate (dACC) (shown in red), and decreased activity in the default mode network: posterior cingulate, prefrontal and parietal cortices (shown in blue). Statistical maps are overlaid on a template brain in MNI‐space and based on placebo condition and thresholded at P < 0.001 uncorrected (T‐threshold is T = 3.17) for illustrative purposes only (for statistical analyses see Table 3). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Next, we investigated how cortisol modulated these networks. Crucially, all three activated salience network structures (midbrain, AIC and dACC) showed differential modulation by cortisol based on whether the threat was escapable or not (see Fig. 4A and Table 4). Firstly, in line with its fear‐reducing functions, cortisol reduced the midbrain response to inescapable threat. Furthermore, we observed an intriguing diametric modulation of the frontal salience network as cortisol reduced activity in the AIC during inescapable threat anticipation, but increased activity in the AIC as well as in the dACC, during escapable threat (see Fig. 4B,C).
Figure 4.
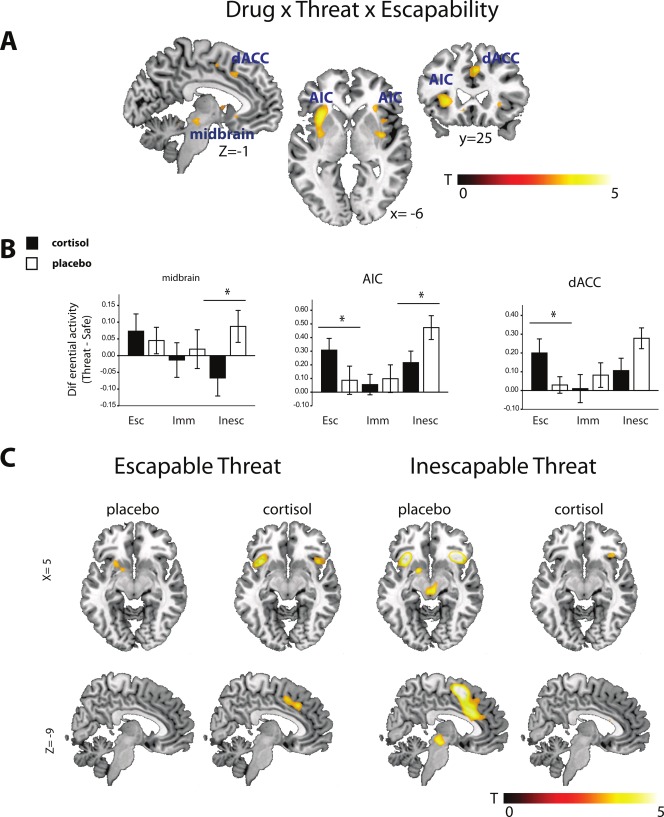
Effects of cortisol. (A) All three salience structures were modulated by cortisol depending on threat escapability. Statistical map is overlaid on a template brain in MNI‐space and thresholded at P< 0.001 uncorrected (T‐threshold is T = 3.17) for illustrative purposes only (for statistical analyses see Table 4). (B) Plots of bilateral threat‐specific parameter estimates (threat minus safe) from the midbrain, anterior insular cortex (AIC) and dorsal anterior cingulate cortex (dACC). Cortisol attenuated midbrain and AIC activity when threat was inescapable, and upregulated dACC and AIC when threat was escapable. Error bars depict standard errors. (C) Threat versus safe contrast maps for the inescapable and escapable conditions in cortisol and placebo conditions separately show cortisol's diametric modulation of AIC, the upregulation of dACC during escapable threat and the attenuation of midbrain activation during escapable threat. Statistical map is overlaid on a template brain in MNI‐space and thresholded at P < 0.001 uncorrected (T‐threshold is T = 3.17) for illustrative purposes only (for statistical analyses see Table 4). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 4.
Drug effects
| Contrast/region | Side | x | y | z | cluster size | Peak T | P |
|---|---|---|---|---|---|---|---|
| Main effect of drug | |||||||
| Cortisol > placebo | |||||||
| Midbrain | L | −8 | −12 | 10 | 2 | 3.47 | 0.044 |
| Interaction: Drug × Threat × Distance | |||||||
| Cortisol [Threat[Escapable > Inescapable] > Safe[Escapable > Inescapable]] × Placebo [Threat[Inescapable > Escapable]] >Safe [Inescapable > Escapable]] | |||||||
| dACC | R | 6 | 26 | 38 | 51 | 3.73 | 0.011a |
| AIC | L | −36 | 16 | −10 | 119 | 4.55 | <0.001a |
| R | 38 | 18 | −10 | 43 | 3.39 | <0.001a | |
| Midbrain | L | −6 | −24 | −10 | 1 | 3.51 | 0.039a |
| Placebo: Threat [Inescapable] > Safe [Inescapable] | |||||||
| AIC | L | −32 | 24 | 2 | 628 | 7.61 | <0.001 |
| R | 36 | 24 | −6 | 497 | 6.61 | <0.001 | |
| Supplementary Motor Area | R | 8 | 4 | 60 | 266 | 5.67 | 0.017 |
| dACC | R | 6 | 22 | 30 | 541 | 4.55 | 0.001a |
| L | −2 | 24 | 28 | s.c. | 4.50 | 0.001a | |
| R | 8 | 12 | 44 | s.c. | 4.47 | 0.001a | |
| Midbrain | R | 0 | −24 | −8 | 102 | 4.05 | 0.007a |
| Cortisol: Threat [Inescapable] > Safe [Inescapable] | |||||||
| AIC | R | 36 | 22 | 4 | 22 | 4.11 | 0.002a |
| L | −34 | 22 | 4 | 19 | 3.61 | 0.009a | |
| Placebo: Threat [Escapable] > Safe [Escapable] | |||||||
| No Suprathreshold Clusters | |||||||
| Cortisol: Threat [Escapable] > Safe [Escapable] | |||||||
| dACC | L | −8 | 12 | 40 | 73 | 3.86 | 0.007a |
| R | 6 | 12 | 40 | s.c. | 3.69 | 0.013a | |
| R | 6 | 26 | 40 | 54 | 3.63 | 0.012a | |
| AIC | L | −38 | 16 | −10 | 130 | 4.47 | <0.001a |
| L | −34 | 16 | −2 | s.c. | 4.10 | 0.002a | |
| L | −30 | 22 | 4 | s.c. | 4.07 | 0.002a | |
| R | 34 | 22 | 4 | 172 | 4.21 | 0.001a | |
| R | 40 | 18 | −8 | s.c. | 3.75 | 0.006a | |
| Cortisol: Threat [Imminent] > Safe [Imminent] | |||||||
| Nu Suprathreshold Clusters | |||||||
| Placebo: Threat [Imminent] > Safe [Imminent] | |||||||
| AIC | L | −34 | 20 | 6 | 5 | 3.37 | 0.020a |
Table shows anatomical region, MNI coordinates and peak T‐values for the reported contrasts of the drug effects. All analyses are conducted at the voxel‐level, whole‐brain P < 0.05, FWE‐corrected.
Small volume corrected at P < 0.05, FWE‐corrected. s.c., same cluster.
We investigated this relation further by extracting the average signal for each task condition from the three salience network structures and entered these in a 3 × 2 × 3 × 2 repeated measures ANOVA with ROI (midbrain, AIC, dACC), THREAT (threat, safe), CONDITION (escapable, imminent, inescapable), and DRUG (cortisol, placebo) as within‐subjects factors, respectively. The crucial three‐way (DRUG × THREAT × CONDITION, F (1.5,26.9) = 4.50, P = 0.030, η 2 p = 0.20) and four‐way (ROI × DRUG × THREAT × CONDITION, F (4,72) = 2.74, P = 0.035, η 2 p = 0.13) interactions were significant, indicating differential modulation of task effects by cortisol administration, with significant differences between the ROIs, thus next we turned to separate investigations of the three ROIs.
A 2 × 3 × 2 repeated measures ANOVA on the extracted midbrain signal revealed a significant DRUG × THREAT interaction (F (1,18) = 4.86, P = 0.041, η 2 p = 0.21), but no other main or interactions effects (all Ps > 0.2). This interaction was due to a significant THREAT effect after placebo (F (1,18) = 4.75, P = 0.043, η 2 p = 0.21), which disappeared after cortisol administration (F (1,18) = 0.01, P = 0.941, η 2 p = 0.00). This indicates an overall decrease of midbrain reactivity to threat after cortisol administration, and follow‐up paired t‐tests showed that this attenuation was largely driven by the inescapable condition (t (18) = −2.23, P = 0.039), and not the escapable and imminent conditions (Ps > 0.6, see also Fig. 4).
A 2 × 3 × 2 repeated measures ANOVA on the extracted AIC signal revealed a significant DRUG × THREAT × CONDITION interaction (F (2,36) = 5.03, P = 0.012, η 2 p = 0.22). Other significant effects included THREAT (F (1,18) = 19.16, P < 0.001, η 2 p = 0.52), CONDITION (F (2,36) = 8.42, P = 0.001, η 2 p = 0.32), and their interaction (F (2,36) = 5.52, P = 0.008, η 2 p = 0.24) (all other Ps > 0.2). Further investigation of the three‐way interaction revealed significant DRUG × THREAT (F (1,18) = 8.21, P = 0.010, η 2 p = 0.31) and THREAT (F (1,18) = 10.30, P = 0.005, η 2 p = 0.36) effects in the escapable condition, significant DRUG × THREAT (F (1,18) = 4.64, P = 0.045, η 2 p = 0.21) and THREAT (F (1,18) = 30.91, P < 0.001, η 2 p = 0.63) effects in the inescapable condition, but no significant effects in the imminent condition (all Ps > 0.2). These effects thus indicate that cortisol attenuated AIC responsivity to inescapable threat anticipation, and boosted AIC responsivity to escapable threat anticipation (see also Fig. 4).
A 2 × 3 × 2 repeated measures ANOVA on the extracted dACC signal also revealed a significant DRUG × THREAT × CONDITION interaction (F (2,36) = 4.53, P = 0.018, η 2 p = 0.20). Main effect of THREAT was also significant (F (1,18) = 12.13, P = 0.003, η 2 p = 0.40), and the interaction of THREAT and CONDITION reached trend‐level significance (F (2,36) = 2.73, P = 0.078, η 2 p = 0.13) (all other Ps > 0.1). Further investigation of the three‐way interaction revealed significant DRUG × THREAT (F (1,18) = 5.44, P = 0.031, η 2 p = 0.23) and THREAT (F (1,18) = 5.40, P = 0.032, η 2 p = 0.23) effects in the escapable condition, trend‐level significant DRUG × THREAT (F (1,18) = 3.86, P = 0.065, η 2 p = 0.18) and significant THREAT (F (1,18) = 20.62, P < 0.001, η 2 p = 0.53) effects in the inescapable condition, but no significant effects in the imminent condition (all Ps > 0.4). These effects thus indicate that cortisol boosted dACC responsivity to escapable threat anticipation, and marginally attenuated dACC responsivity to inescapable threat anticipation (see also Fig. 4).
In the imminent condition we observed no effect of cortisol on salience activity, which is arguably related to the anticipation of effortful rapid reaction within this condition, rendering modulation by cortisol less likely. In a recent article by Neta et al. (2014) it was shown that activation of the frontal salience network, consisting of AIC and dACC, has a strong positive relation with reaction time. In this respect it is important to note that the timing of the FAET was adjusted online to individual performance in such a way that the imminent trials remained escapable at chance level. In other words, during imminent trials, independent from threat or safe conditions, the participants were anticipating to press the button as fast as they could. Indeed, the behavioral data (see Table 2) show that escape was attempted in imminent threat and safe conditions equally often. Moreover, although escape success was higher for the imminent threat trials, which was also reflected in a reaction time difference, it is important to note that the average reaction time between escapable threat and imminent safe conditions was not significantly different (F (1,18) = 0.75, P = 0.787). This suggests that activity in the imminent condition is more strongly affected by effortful rapid reaction anticipation than the other conditions. Although Neta et al. (2014) showed that salience network activity could reflect other processes above the reaction time related activity, they did not investigate such effortful conditions as investigated here. Therefore it might be argued that the salience network activity in the imminent condition of the FAET is relatively more driven by effortful rapid reaction anticipation than the threat versus safe manipulation, rendering modulation by cortisol less likely. This possibility is furthermore reflected by a significant engagement of the anterior salience network (bilateral dACC; −6, 8, 42, t = 3.78; 6, 12, 44, t = 3.65, both P < 0.05, s.v.c.; bilateral AIC; 36, 22, 4, t = 4.46; −34, 18, 6, t = 5.58, both P < 0.05, s.v.c), during the safe imminent condition, whereas the safe escapable condition recruited only AIC (bilateral AIC; 36, 22, 4, t = 4.06, and −34, 18, 6, t = 4.05, both P < 0.05, s.v.c) and the safe inescapable condition did not recruit anterior salience network activity. In sum, the lack of modulation of anterior salience network reactivity by cortisol is most likely due to the high demands on reaction time of the imminent threat as well as safe conditions.
Mood and Hormone Measurements
Cortisol did not significantly affect any of the mood scales (all P's > 0.15). Furthermore, subjects could not correctly guess in which condition they were (binomial, P = 1.00) which suggests that they did not subjectively experience the effects of cortisol administration.
The baseline cortisol levels measured from saliva in both conditions were positively skewed (cortisol condition, skewness = 1.444, placebo condition, skewness = 1.960, P's < 0.05), therefore we used a non‐parametric test that showed hormone levels did not differ significantly between conditions (Z = −0.966, P = 0.334) (see Table 1).
In sum, the effects that we found of cortisol cannot readily be explained by alterations in mood caused by the hormone, conscious awareness of drug administration, or differences in baseline cortisol levels between the two conditions.
DISCUSSION
Our main research aim was to investigate the effects of the stress‐hormone cortisol on brain functions involved in active escape and passive threat anticipation. We show that cortisol has differential effects on the human brain's salience system depending on whether a threat is escapable or not. Cortisol administration attenuated the midbrain and AIC response to passive fear conditions, while increasing activity in AIC and dACC when active escape was possible. These results reveal context‐dependent neural mechanisms of cortisol in humans, and suggest that cortisol adjusts the human neural threat system from passive fear to active escape via the salience network.
Using a newly developed paradigm we show that inescapable nearby threat activates a set of brain regions known as the salience network, consisting of midbrain, dACC, and AIC [Seeley et al., 2007]. When contrasted with inescapable threat, we find that distant and escapable threat generates a higher BOLD response in prefrontal, posterior cingulate and temporal brain regions. This shift from higher brain regions towards lower brain regions depending on threat distance is an important mechanism in the influential model of McNaughton and Corr [2004]. In this model, fear responses to immediate threat (flight‐fight‐freeze) are generated by lower brain regions such as amygdala and midbrain, which together have also been proposed as a core fear system across species [Panksepp, 2011]. Anxiety in response to distant threat on the other hand, leads to prefrontal cortex activation, which mediates approach behaviors such as risk assessment [McNaughton and Corr, 2004]. This prefrontal to midbrain shift depending on threat distance has also been shown in humans by Mobbs et al. [2007, 2009]. The present data are thus in line with influential theoretical frameworks [McNaughton and Corr, 2004; Panksepp, 2011] and previous experimental data [Mobbs et al., 2007, 2009], as during acute fear conditions midbrain was activated, whereas during conditions related to anxiety, there was a relative stronger contribution of prefrontal areas.
The midbrain is specifically linked to freezing responses to inescapable threat [Hagenaars et al., 2014; Hermans et al., 2013; Mobbs et al., 2007, 2009]. Our observation that cortisol suppresses midbrain reactivity to inescapable threat suggests therefore that, similar to what has been observed in rodents [Skorzewska et al., 2006, 2007], cortisol attenuates freezing in humans. Moreover, cortisol's fear‐reducing effects in humans [Putman et al., 2007b, 2010b; Soravia, 2006; Soravia et al., 2013] have previously been associated with suppression of physiological arousal and limbic reactivity to threat [Buchanan et al., 2001; Henckens et al., 2010; Merz et al., 2010]. Recently, Hermans et al. [2014] suggested that cortisol's fear‐reducing actions involve down regulation of acute threat‐reactivity in the salience network which sub‐serves system normalization in the aftermath of stress [de Kloet et al., 2005; Karatsoreos and McEwen, 2011; Sapolsky et al., 2000]. Indeed, we also observed that under inescapable threat the AIC, an important node of the frontal salience network involved in visceral perception [Craig, 2009], was down regulated by cortisol. Intriguingly, this effect was reversed during active escape anticipation, wherein cortisol not only boosted AIC, but also dACC activity. Since the dACC is particularly involved in autonomic control and motivated action selection [Craig, 2009], this combination of effects suggests that cortisol not only suppresses passive fear responsivity, but also promotes active escape.
We also found sgACC activation for Threat > Safe, which is in line with research showing the importance of this region in controllable stress and monitoring escape possibilities. In animals, sgACC inhibits stress‐induced brainstem responses during controllable fear [Amat et al., 2005] and in humans this region is more activated when threat is distant compared with imminent [Mobbs et al., 2007, 2009]. Moreover, sgACC also underlies cortisol reactivity during stress [Jahn et al., 2010]. In the present study sgACC was activated over all threat conditions and not specifically on escapable conditions, which might be due to the different task demands of the FAET in comparison with the task used in Mobbs et al. [2007, 2009] where subjects had to strategically navigate through a maze to escape from a virtual predator.
In sum, with the FAET we found similar threat systems as have been hypothesized and found by others using different levels of threat distance and escape [Amat et al., 2005; McNaughton and Corr, 2004; Mobbs et al., 2007, 2009] and we find that cortisol increases salience network activation to escapable threat and decreases midbrain and AIC activation for inescapable threat. Of note is the absence of a down regulation of amygdala threat reactivity after cortisol administration. Such effects have been reported previously, but predominantly when using indirect threats, like facial expressions [Henckens et al., 2010]. In line with the present findings, the amygdala has however been shown to be relatively unresponsive to a direct threat, that is threat‐of‐shock [Klumpers et al., 2010]. Although cortisol might also have down regulated amygdala reactivity in our study, the lack of general amygdala reactivity to the FAET might have obscured these effects.
After stress, cortisol is released with a delay of approximately thirty minutes, whereas catecholamines are released immediately [de Kloet et al., 2005]. The catecholamine response has been causally linked to upregulation of salience activity during inescapable threat, whereas such a relationship has not been found for cortisol [Henckens et al., 2010; Hermans et al., 2011]. The present findings however imply that glucocorticoids can also increase salience activity, but in a context‐dependent manner. That is, cortisol increased salience activity when escape from threat is possible but decreased it when escape is impossible. In other words, during an encounter with a threat, the delayed cortisol response will decrease fear responses, but will increase the saliency of the next threat, presumably so that humans will become more successful in escaping and avoiding future threat. This notion is in line with the preparative role for efficient threat behavior ascribed to glucocorticoids [Sapolsky et al., 2000].
Cortisol has many neurobiological routes that could be at play in the neural effects that we found, such as inhibition of corticotrophin‐releasing hormone (CRH) [Handa and Weiser, 2014], interaction with catecholamines [Roozendaal et al., 2009], and non‐genomic and genomic actions at the mineralocorticoid receptor (MR) and glucocorticoid receptor (GR), respectively [de Kloet et al., 2008]. This latter route seems highly flexible as effects via these receptors are functionally different (i.e. excitatory and inhibitory) and the balance between MR and GR differs per brain region [de Kloet, 1991; de Kloet et al., 2008; Groeneweg et al., 2011]. Importantly, our time interval of 1 h likely targets fast, non‐genomic effects, but cannot exclude the slow, genomic effects [Oitzl et al., 2010]. The variety of possible neurobiological routes might allow for the flexible (i.e. context‐dependent) effects of cortisol that we observed in the AIC and dACC. Arguably, escapable and inescapable conditions may by themselves induce different levels of catecholamine release leading to different effects of cortisol administration.
Lastly, as our data shows that the salience network is affected differentially by cortisol under escapable and escapable threat, an important venue for further investigation is if cortisol affects escape behavior in humans. It is known that elevation of glucocorticoids is important for preparation for coping with future stressors in animals, and these changes also affect active motor behavior [Sapolsky et al., 2000]. Notably, animal research shows that exogenous corticosterone modulates antipredator behavior such that animals react faster and hide longer from a predator [Thaker et al., 2009]. Furthermore, inhibition of the glucocorticoid response by metyrapone during an acute encounter with a predator decreases the escape behavior of the prey and negatively impacts aversive learning in future predator encounters [Thaker et al., 2010]. Thus, glucocorticoids seem indeed to promote more active responses to threat in animals, but further studies need to be done to confirm this in humans.
CONCLUSION
Using a novel active escape paradigm we show that, in tune with an environment where threat and escape possibilities are dynamically changing, cortisol combines its reduction of midbrain activity in situations of passive fear with a flexible modulation of frontal salience activity. These findings not only underscore cortisol's function in system normalization, but also show how this hormone might prepare our active survival mechanisms.
ACKNOWLEDGMENTS
The authors thank Lisa A. Rosenberger for help in collecting the data.
REFERENCES
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF (2005): Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci 8:365–371. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ (2001): Mouse defensive behaviors: pharmacological and behavioral assays for anxiety and panic. Neurosci Biobehav Rev 25:205–218. [DOI] [PubMed] [Google Scholar]
- Böhnke R, Bertsch K, Kruk MR, Richter S, Naumann E (2010): Exogenous cortisol enhances aggressive behavior in females, but not in males. Psychoneuroendocrinology 35:1034–1044. [DOI] [PubMed] [Google Scholar]
- Bos PA, Montoya ER, Terburg D, van Honk J (2014): Cortisol administration increases hippocampal activation to infant crying in males depending on childhood neglect. Hum Brain Mapp 35:5116–5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan TW, Brechtel A, Sollers JJ, Lovallo WR (2001): Exogenous cortisol exerts effects on the startle reflex independent of emotional modulation. Pharmacol Biochem Behav 68:203–210. [DOI] [PubMed] [Google Scholar]
- Craig AD (2009): How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci 10:59–70. [DOI] [PubMed] [Google Scholar]
- de Kloet ER (1991): Brain corticosteroid receptor balance and homeostatic control. Front Neuroendocrinol 12:95–164. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joëls M, Holsboer F (2005): Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6:463–475. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Karst H, Joels M (2008): Corticosteroid hormones in the central stress response: Quick‐and‐slow. Front Neuroendocrinol 29:268–272. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amunts K (2007): Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage 36:511–521. [DOI] [PubMed] [Google Scholar]
- Glenn CR, Lieberman L, Hajcak G (2012): Comparing electric shock and a fearful screaming face as unconditioned stimuli for fear learning. Int J Psychophysiol 86:214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Heller R, Hirschhorn E, Kling MA, Pine DS, Schulkin J, Vythilingam M (2011): Acute hydrocortisone treatment increases anxiety but not fear in healthy volunteers: A fear‐potentiated startle study. Biol Psychiatry 69:549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneweg FL, Karst H, de Kloet ER, Joels M (2011): Rapid non‐genomic effects of corticosteroids and their role in the central stress response. J Endocrinol 209:153–167. [DOI] [PubMed] [Google Scholar]
- Grupe DW, Nitschke JB (2013): Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nat Rev Neurosci 14:488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenaars MA, Oitzl M, Roelofs K (2014): Updating freeze: Aligning animal and human research. Neurosci Biobehav Rev 47:165–176. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Weiser MJ (2014): Gonadal steroid hormones and the hypothalamo‐pituitary‐adrenal axis. Front Neuroendocrinol 35:197–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens MJ, van Wingen GA, Joels M, Fernandez G (2010): Time‐dependent effects of corticosteroids on human amygdala processing. J Neurosci 30:12725–12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans EJ, van Marle HJ, Ossewaarde L, Henckens MJ, Qin S, van Kesteren MT, Schoots VC, Cousijn H, Rijpkema M, Oostenveld R, Fernandez G (2011): Stress‐related noradrenergic activity prompts large‐scale neural network reconfiguration. Science 334:1151–1153. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, Henckens MJ, Roelofs K, Fernandez G (2013): Fear bradycardia and activation of the human periaqueductal grey. Neuroimage 66:278–287. [DOI] [PubMed] [Google Scholar]
- Hermans E, Henckens MJ, Joels M, Fernandez G (2014): Dynamic adaptation of large‐scale brain networks in response to acute stressors. Trends Neurosci 37: 304–314. [DOI] [PubMed] [Google Scholar]
- Jahn AL, Fox AS, Abercrombie HC, Shelton SE, Oakes TR, Davidson RJ, Kalin NH (2010): Subgenual prefrontal cortex activity predicts individual differences in hypothalamic‐pituitary‐adrenal activity across different contexts. Biol Psychiatry 67:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatsoreos IN, McEwen BS (2011): Psychobiological allostasis: Resistance, resilience and vulnerability. Trends Cogn Sci 15:576–584. [DOI] [PubMed] [Google Scholar]
- Klumpers F, Raemaekers MA, Ruigrok AN, Hermans EJ, Kenemans JL, Baas JM (2010): Prefrontal mechanisms of fear reduction after threat offset. Biol Psychiatry 68:1031–1038. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, de Boer SF, Coppens CM, Buwalda B (2010): Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front Neuroendocrinol 31:307–321. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE (2007): The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cognit 65:209–237. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage 19:1233–1239. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Corr PJ (2004): A two‐dimensional neuropsychology of defense: Fear/anxiety and defensive distance. Neurosci Biobehav Rev 28:285–305. [DOI] [PubMed] [Google Scholar]
- Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, Wolf OT (2010): Investigating the impact of sex and cortisol on implicit fear conditioning with fMRI. Psychoneuroendocrinology 35:33–46. [DOI] [PubMed] [Google Scholar]
- Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, Wolf OT (2012): Neuronal correlates of extinction learning are modulated by sex hormones. Soc Cogn Affect Neurosci 7:819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, Sapolsky RM (2008): Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci USA 105:5573–5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, Dolan RJ, Frith CD (2007): When fear is near: Threat imminence elicits prefrontal‐periaqueductal gray shifts in humans. Science 317:1079–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Marchant JL, Hassabis D, Seymour B, Tan G, Gray M, Petrovic P, Dolan RJ, Frith CD (2009): From threat to fear: The neural organization of defensive fear systems in humans. J Neurosci 29:12236–12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya ER, Bos PA, Terburg D, Rosenberger LA, van Honk J (2014): Cortisol administration induces global down‐regulation of the brain's reward circuitry. Psychoneuroendocrinology 47:31–42. [DOI] [PubMed] [Google Scholar]
- Neta M, Schlaggar BL, Petersen SE (2014): Separable responses to error, ambiguity, and reaction time in cingulo‐opercular task control regions. Neuroimage 99:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oitzl MS, Champagne DL, van der Veen R, de Kloet ER (2010): Brain development under stress: Hypotheses of glucocorticoid actions revisited. Neurosci Biobehav Rev 34:853–866. [DOI] [PubMed] [Google Scholar]
- Panksepp J (2011): Cross‐species affective neuroscience decoding of the primal affective experiences of humans and related animals. PLoS One 6: e21236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putman P, Hermans E, Koppeschaar H, Vanschijndel A, Vanhonk J (2007a): A single administration of cortisol acutely reduces preconscious attention for fear in anxious young men. Psychoneuroendocrinology 32:793–802. [DOI] [PubMed] [Google Scholar]
- Putman P, Hermans E, Vanhonk J (2007b): Exogenous cortisol shifts a motivated bias from fear to anger in spatial working memory for facial expressions. Psychoneuroendocrinology 32:14–21. [DOI] [PubMed] [Google Scholar]
- Putman P, Antypa N, Crysovergi P, van der Does WA (2010a): Exogenous cortisol acutely influences motivated decision making in healthy young men. Psychopharmacology 208:257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putman P, Hermans EJ, van Honk J (2010b): Cortisol administration acutely reduces threat‐selective spatial attention in healthy young men. Physiol Behav 99:294–300. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci USA 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S (2009): Stress, memory and the amygdala. Nat Rev Neurosci 10:423–433. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU (2000): How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55–89. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shacham S (1983): A shortened version of the profile of mood states. J Pers Assess 47:305 –3306. [DOI] [PubMed] [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD (2012): Decoding subject‐driven cognitive states with whole‐brain connectivity patterns. Cereb Cortex 22:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorzewska A, Bidzinski A, Lehner M, Turzynska D, Wislowska‐Stanek A, Sobolewska A, Szyndler J, Maciejak P, Taracha E, Plaznik A (2006): The effects of acute and chronic administration of corticosterone on rat behavior in two models of fear responses, plasma corticosterone concentration, and c‐Fos expression in the brain structures. Pharmacol Biochem Behav 85:522–534. [DOI] [PubMed] [Google Scholar]
- Skorzewska A, Bidzinski A, Lehner M, Turzynska D, Sobolewska A, Hamed A, Szyndler J, Maciejak P, Plaznik A (2007): The effects of acute corticosterone administration on anxiety, endogenous corticosterone, and c‐Fos expression in the rat brain. Horm Behav 52:317–325. [DOI] [PubMed] [Google Scholar]
- Soravia LM (2006): Glucocorticoids reduce phobic fear in humans. Proc Natl Acad Sci USA 103:5585–5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soravia LM, Heinrichs M, Winzeler L, Fisler M, Schmitt W, Horn H, Dierks T, Strik W, Hofmann SG, de Quervain DJ (2013): Glucocorticoids enhance in vivo exposure‐based therapy of spider phobia. Depress Anxiety: 429–435. [DOI] [PubMed] [Google Scholar]
- Thaker M, Lima SL, Hews DK (2009): Acute corticosterone elevation enhances antipredator behaviors in male tree lizard morphs. Horm Behav 56:51–57. [DOI] [PubMed] [Google Scholar]
- Thaker M, Vanak AT, Lima SL, Hews DK (2010): Stress and aversive learning in a wild vertebrate: The role of corticosterone in mediating escape from a novel stressor. Am Nat 175:50–60. [DOI] [PubMed] [Google Scholar]
- Tops M, Wijers A, Koch T, Korf J (2006): Modulation of rotational behavior in healthy volunteers by cortisol administration. Biol Psychol 71:240–243. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]


