Abstract
Healthy aging is associated with a progressive decline across a range of cognitive functions. An important factor underlying this decline may be the age‐related impairment in stimulus–reward processing. Several studies have investigated age‐related effects, but compared young versus old subjects. This is the first study to investigate the effect of aging on brain activation during reward processing within a continuous segment of the adult life span. We scanned 49 healthy adults aged 40–70 years, using functional MRI. We adopted a simple reward task, which allowed separate evaluation of neural responses to reward anticipation and receipt. The effect of reward on performance accuracy and speed was not related to age, indicating that all subjects could perform the task correctly. We identified a whole‐brain significant age‐related decline of ventral striatum activation during reward anticipation as compared to neutral anticipation. Importantly, the specificity of this finding was underscored by the observation that there was no general decline in activation during anticipation. Activation in the ventral striatum increased with age during reward receipt as compared to receiving neutral outcome. Finally, activation in the ventromedial prefrontal cortex during outcome was not affected by age. Our data demonstrate that the typical shift in striatal activation from reward receipt to reward anticipation in young adults disappears with healthy aging. These changes are consistent the well‐ocumented age‐related decline of striatal dopamine availability, and may provide a stepping stone for further research of age‐related neurodegenerative diseases. Hum Brain Mapp 36:2305–2317, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: aging, ventral striatum, ventromedial prefrontal cortex, reward, fMRI
INTRODUCTION
Advancing age is associated with a decline across a range of cognitive functions, including decision making, learning, and attentional control [Coubard et al., 2011; Denburg et al., 2005; Rhodes, 2004; Ridderinkhof et al., 2002]. It has been suggested that this is the result of an age‐related attenuation in reward‐based learning [Eppinger et al., 2009, 2011; Mell et al., 2003, 2009]. Reward‐based learning is the ability to associate environmental stimuli and actions with subsequent reward receipt, using both positive and negative feedback. As such, it is crucial for the construction of goal‐directed behavior [Ridderinkhof et al., 2004].
Stimulus–reward processing is thought to be modulated by the ventral striatum, an area innervated by dopaminergic projections from the midbrain [Bijleveld et al., 2014; Schultz, 2007; Schultz et al., 1997; Vink et al., 2013]. Neurophysiological studies in monkeys and humans have consistently shown that activation in the ventral striatum signals the receipt of unexpected rewards as well as the anticipation of expected rewards and associated prediction errors [Haber and Knutson, 2010; Knutson et al., 2001a,b; Schultz et al., 1997]. Learning stimulus–reward associations is characterized by a temporal shift in striatal activation from actual reward receipt to the anticipation of the reward [Schultz et al., 1997]. In other words, if a reward can be predicted using a cue, then ventral striatum activation will increase in response to that cue and no longer as a reaction to receiving of the reward. We and others have shown that this shift develops throughout adolescence [Bjork et al., 2010; Ernst et al., 2005; Hoogendam et al., 2013] and is paralleled by frontostriatal maturation [May et al., 2014; Vink et al., 2014]. Another key structure in the reward circuitry is the ventromedial prefrontal cortex (vmPFC), which is known to attribute subjective value to a reward and monitor outcome of current choices during decision making [Boorman et al., 2009; Figee et al., 2011; Haber and Knutson, 2010; Levy and Glimcher, 2012; Liu et al., 2011]. The vmPFC is believed to have a rather specific function during reward outcome, while its role during reward anticipation is more passive [Diekhof et al., 2012].
To date, only a few studies have investigated reward processing in the aging brain. These studies typically compare brain activation in older subjects (aged 62–80 years) with that of young adults (aged 19–28 years). Results for age‐related changes in reward anticipation are inconsistent across studies, with some studies reporting reduced striatal activation during reward anticipation in older versus younger subjects [Dreher et al., 2008; Schott et al., 2007], whereas others do not find such a decrease [Lorenz et al., in press; Rademacher et al., 2013; Samanez‐Larkin et al., 2007]. In contrast, striatal activation at reward receipt may be increased in older compared to younger subjects [Schott et al., 2007]. These divergent patterns across the various components of reward processing indicate that striatal activation levels do not simply increase or decrease with aging. Rather, striatal activation during reward anticipation and reward receipt may be impacted in an opposite direction. More specifically, these data suggest that the characteristic shift in striatal activation from reward receipt to reward anticipation may no longer occur in healthy aging. To date, no age‐related changes have been reported in the vmPFC during reward receipt. Previous studies identified age‐related changes in reward processing by comparing groups of adolescent versus elderly subjects (subjects older than 60 years). However, the relation between reward processing in middle‐aged subjects relative to elderly subjects has not yet been explored. Therefore, investigating age effects within a continuous segment of the adult life span could provide additional information.
Here, we investigate the alterations in reward processing during middle age in a sample of 49 healthy adults aged 40–70 years using a cross‐sectional design. Subjects performed a reward task based on the Monetary Incentive Delay task [Knutson et al., 2001a,b], designed to optimally evaluate brain activation during reward anticipation and receipt separately [Figee et al., 2011; Van Hell et al., 2010], while being scanned with functional MRI. We examine age‐related changes in performance and brain activation in two ways: (a) regression‐analyses with age as a continuous factor and (b) analyses across three age‐groups, representing middle age (40–50 years), late middle age (50–60 years), and old age (60–70 years). Age‐related changes in activation are analyzed using a whole brain approach and using two predefined anatomical Regions of Interest (ROIs) which are the two key areas involved in reward processing: the bilateral ventral striatum and vmPFC [Haber and Knutson, 2010; Hoogendam et al., 2013; Liu et al., 2011; Schultz, 2000].
Since behavioral research has shown that reward‐based learning is subject to an age‐related decline, we hypothesize that increasing age will impair those characteristic elements of adult reward processing [Schultz et al., 1997]. Specifically, we hypothesize that with age, striatal activation during the anticipation of reward will decline, while striatal activation during reward receipt will increase. If true, this would support the notion that with increasing age the shift from reward receipt to reward anticipation, which is characteristic of adult reward processing [Schultz et al., 1997], no longer occurs. We will not investigate age‐effects in the vmPFC during reward anticipation, since it appears to have a mainly passive role in this part of reward processing [Diekhof et al., 2012]. Data to identify age effects in the vmPFC during reward receipt are generally lacking. Therefore, the effect of aging on the processing of reward receipt in the vmPFC is difficult to predict.
MATERIALS AND METHODS
Participants
Fifty‐seven healthy subjects aged 40–70 years (mean age 54.89 years; standard deviation (SD) 6.96; 23 males) participated in the study. Data were screened for outliers (>2 SD from the group mean) and tested for highly influential observations using Cook's distance and this resulted in the removal of two subjects (2 males aged 55.8 and 60.4 years) based on behavioral performance and six subjects (2 males aged 64.6 and 66.2 years; 4 females aged 41.2, 48.3, 49.7, and 56.2) based on activation data. Therefore, all analyses were performed on the remaining 49 adults. To perform additional analyses, we defined three age groups of 10 years each, representing middle age (40–50 years, n = 11, 3 males), late middle age (50–60 years, n = 28, 9 males), and old age (60–70 years, n = 10, 7 males).
The study protocol was approved by the Medical Ethics Committee of the University Medical Center Utrecht and all participants gave written informed consent. Subjects received monetary compensation consisting of a fixed amount for participation and an additional variable amount based on their task earnings.
All subjects were right‐handed, not colorblind, did not report a history of neurological or psychiatric illness, nor did they use psychotropic medication or medication known to influence the blood‐oxygen‐level‐dependent (BOLD) signal.
Reward Task
Participants performed a reward task [Fig. 1, De Leeuw et al., 2015; Figee et al., 2011; Hoogendam et al., 2013; Van Hell et al., 2010] based on the Monetary Incentive Delay task [Knutson et al., 2001a,b]. This task allows the investigation of anticipation and receipt of reward, separately. At the beginning of each trial, a cue was presented for 750 ms signaling whether the subject could win money (potentially rewarding trial) or not (nonrewarding trial). For the potentially rewarding trials, this cue was a smiling face and for the nonrewarding trials a neutral face. Immediately after the cue, a fixation star was presented (mean duration 3,286 ms, range 779–6,729 ms). Next, subjects had to respond as fast as possible, by pressing a button, when a target stimulus (exclamation mark) appeared on the screen. Subsequent feedback notified participants of their performance, indicating if they earned money on that trial, as well as their cumulative total at that moment. Subjects could win €1 during a potentially rewarding trial.
Figure 1.
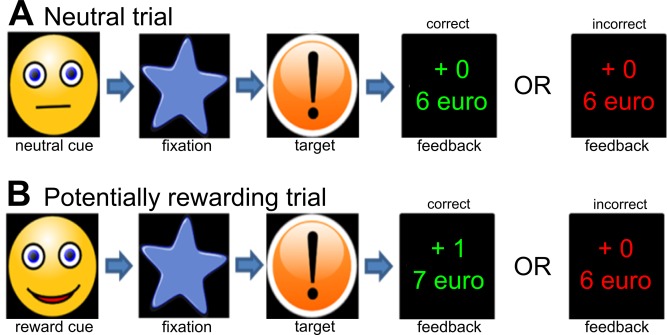
Schematic representation of the reward task. There were two types of trials: neutral trials (A) and potentially rewarding trials (B) as indicated by the cue (neutral face for a neutral trial and a smiling face for a potentially rewarding trial). Subjects had to press a button as fast as possible when the target stimulus appeared. The fixation time between cue and target was varied. Feedback was given after the response and indicated via color if the response was given within the time limit (green) or not (red). Also the amount of money won in that trial was presented (either +1 or +0). Finally, the cumulative amount of money won was presented. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
For both potentially rewarding and nonrewarding trials, subjects had to respond to the target stimulus within a certain time limit, that is, target duration. Responses were considered correct (correct feedback) if subjects responded within this time limit. Responses given after the time limit were considered incorrect (incorrect feedback).
The time limit was individually adjusted to ensure that each participant succeeds in 50% of the trials. This adjustment was based on 20 practice trials, which were presented prior to the start of the experiment (when subjects were already in the scanner). From these practice data, the shortest reaction time to the target was used to determine the individual time limit for responses to the target. In 50% of the trials, 200 ms was added to the duration of the individual time limit, enabling participants to be successful in these trials. In the remaining trials, 150 ms was subtracted from the time limit, to make sure that participants could not respond in time. This procedure resulted in about 50% correct feedback for both rewarding and nonrewarding trials, separately. Participants were told that they would receive the cumulative total amount of reward of the actual experiment in addition to the standard compensation for participation. The task consisted of 60 trials with a mean duration of 9,571 ms (range 4,946–16,107 ms), resulting in a total task duration of 9 min 35 s.
fMRI Data Acquisition
The experiment was performed on a 3.0 T Philips Achieva MRI scanner (Philips Medical Systems, Best, the Netherlands) at the University Medical Center Utrecht. Images were acquired using an eight‐channel sensitivity‐encoding (SENSE) parallel‐imaging head coil. Whole‐brain T2*‐weighted echo planar images (EPI) with BOLD contrast, oriented in a transverse plane tilted 20° over the left‐right axis, were acquired in a single run (372 volumes; 30 slices per volume; interleaved acquisition; repetition time, 1,600 ms; echo time, 23 ms; field of view: 208 × 120 × 256 mm; flip angle = 72.5°; 64 × 64 matrix; 4 × 4 mm in‐plane resolution; 4‐mm slice thickness; SENSE‐factor, 2.4 [anterior–posterior]). A whole‐brain 3D fast field echo T1‐weighted scan (185 slices; repetition time = 8.4 ms; echo time = 3.8 ms; flip angle = 8°; field of view, 252 × 288 × 185 mm; voxelsize: 1 mm isotropic) was obtained for within‐subject registration purposes.
Preprocessing and Individual Subject Analysis
Image data were preprocessed and analyzed using SPM software (http://www.fil.ion.ucl.ac.uk/spm/software/spm/). After realignment of the functional scans, the anatomical image was coregistered to the mean functional image. This image was segmented and normalization parameters were estimated. Using these parameters, the functional and anatomical images were matched to the Montreal Neurological Institute (MNI) T1‐template brain. Functional images were spatially smoothed using an 8‐mm full‐width at half‐maximum Gaussian kernel. Each participant's translation and rotation corrections were examined to ensure there was no excessive head motion [>3 mm in any direction between subsequent scans; Van Dijk et al., 2012].
The preprocessed time‐series data for each individual were analyzed using a general linear model regression analysis. The regression model consisted of six factors, representing hemodynamic changes which were event‐related to (1) anticipation during and after the presentation of the reward cue (Anticipation Reward), (2) anticipation during and after the neutral cue (Anticipation Neutral), (3) feedback reflecting monetary reward (Feedback Reward), (4) feedback reflecting a missed reward in a potentially rewarding trial (Feedback No Reward), (5) feedback reflecting a correct response in a neutral trial (Feedback Correct Neutral), and (6) feedback reflecting an incorrect response in a neutral trial (Feedback Incorrect Neutral). The onset of the factors modeling anticipation (duration range 1,529–7,479 ms) was at the presentation of the cue, while the onset of the factors modeling feedback (duration 2,000 ms) was at the presentation of the target, including the button press to the target and the subsequent feedback. To take residual head motion effects into account, motion parameters from the realignment procedure were included as regressors of no interest. Low frequency drifts were removed from the signal by applying a high‐pass filter with a cut‐off frequency of 1/128 Hz.
For each participant, statistical maps were generated for the contrasts (1) Anticipation Reward versus Anticipation Neutral (hereafter referred to as Reward Anticipation), (2) Feedback Reward versus Feedback Correct Neutral (Reward Receipt), and (3) Feedback No Reward versus Feedback Incorrect Neutral (No‐Reward Receipt).
Whole‐Brain Analysis
Individual statistical maps were used for the whole‐brain group‐analyses investigating the relation between age and brain activation. These maps were tested for significance at a familywise error (FWE) corrected cluster level of P = 0.05 (cluster‐defining threshold of P = 0.001, cluster size of 36 voxels). These parameters were determined using SPM (version 5) and a script (CorrClusTh.m, to be found on http://www2.warwick.ac.uk/fac/sci/statistics/staff/academic-research/nichols/scripts/spm), which uses estimated smoothness (estimated full width at half maximum: 3.56 × 3.65 × 3.46 voxels) and Random Field Theory to find these corrected thresholds.
Region of Interest Analysis
A ROI analysis was applied to investigate the relation between age and brain activation levels. Two bilateral anatomical ROIs were a priori selected, based on their known involvement in the anticipation and outcome of reward [Haber and Knutson, 2010; Knutson et al., 2001a,b]: the ventral striatum and vmPFC. ROIs were based on definitions of the Anatomic Automatic Labeling atlas [Tzourio‐Mazoyer et al., 2002] and created using the WFU PickAtlas Toolbox implemented in SPM. The ventral striatum was defined as that part of the caudate nucleus below the z‐coordinate of 0 mm. The vmPFC ROI consisted of the medial part of the orbitofrontal cortex, entailing the bilateral gyrus rectus and medial orbital gyrus [Zald and Andreotti, 2010]. We subsequently created an additional ROI for the anterior vmPFC, defined as the ventral half of the vmPFC ROI. This anterior part of the vmPFC is particularly relevant to our study, since it is believed to be predominantly sensitive to monetary reward outcome, whereas the posterior vmPFC more sensitive to primary reward, such as taste or tactile rewards [Haber and Knutson, 2010].
For each participant, the mean activation level (expressed as percent signal change) during the three contrasts of interest (Reward Anticipation, Reward Receipt, and No‐Reward Receipt) was calculated over all voxels in each ROI. Regression analyses were then performed for each ROI separately with activation level as dependent variable and age as predictor. We defined a primary significance level of P ≤ 0.05 for all ROI analyses. We calculated an additional Bonferroni‐corrected significance level of P ≤ 0.0125, since we investigated two bilateral ROI's (4 ROI's in total).
RESULTS
Behavioral Data
Behavioral data are presented in Figure 2 and Supporting Information, Figure 1. Regression analyses showed that, as expected, subjects responded faster to the target on potentially rewarding trials relative to neutral trials (reward: 301 ± 34 ms, neutral: 315 ± 31 ms, F(1,48) = 19.40; P < 0.001; Supporting Information, Fig. 1). Figure 2 shows that the effect of reward (difference between potentially rewarding trials and neutral trials) on reaction times was not affected by age (F(1,48) = 0.52; r = 0.10; P = 0.47). Moreover, there was no effect of age on overall reaction times (F(1,48) = 2.34; r = 0.22; P = 0.13), nor for potentially rewarding trials (F(1,48) = 1.3; r = 0.17; P = 0.25) or neutral trials (F(1,48) = 3.23; r = 0.25; P = 0.08) separately. Next, we performed a repeated‐measures analysis with group (three levels) and condition (reward cue, neutral cue). Similar to the regression analysis results, this analysis revealed a main effect of condition (F(1,46) = 19.21; P < 0.001). However, there was no group by condition interaction (F(2,46) = 0.76; P = 0.47), nor did the main effect of group reach significance (F(2,46) = 1.63; P = 0.21). These results indicate that older subjects (60–70 years) show a similar reward‐induced speeding effect on reaction times as do middle age (40–50 years) and late middle age adults (50–60 years).
Figure 2.
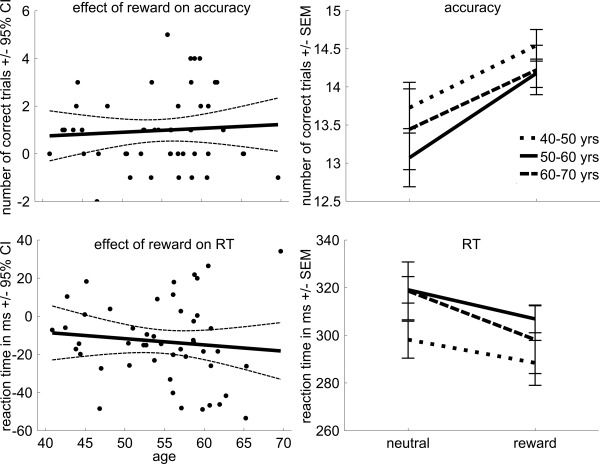
Behavioral data. Scatter plot of the reward effect (reward anticipation vs. neutral anticipation) on accuracy (indicated by the difference in number of correct trials) and reaction time as a function of age (with linear trend line and 95% confidence interval), and line plots for the three age groups (±standard error of the mean) for neutral trials and potentially rewarding trials.
Subjects made more correct responses (i.e., responses within the time limit, see Materials and Methods section) in potentially rewarding trials relative to neutral trials (reward: 14.25 out of 30 trials correct: 47.5 ± 6.8%, neutral: 13.2 out of 30 trials correct: 44 ± 19%, F(1,48) = 20.25; P < 0.001; Supporting Information Fig. 1). Figure 2 shows that the effect of reward (difference between potentially rewarding trials and neutral trials) on accuracy was not affected by age (F(1,48) = 0.56; r = 0.11; P = 0.46). Moreover, there was no effect of age on overall accuracy (F(1,48) = 1.34, r = −0.17, P = 0.25), nor for potentially rewarding trials (F(1,48) = 0.91; r = −0.14; P = 0.35) or neutral trials (F(1,48) = 1.19; r = −0.16; P = 0.28) separately. Next, we performed a repeated‐measures analysis similar to that for reaction times. This analysis (Fig. 2) revealed a main effect of condition (F(1,46) = 19.54; P < 0.001), but not of group (F(2,46) = 1.0; P = 0.37) nor a group by condition interaction (F(2,46) = 0.16; P = 0.85). So, accuracy approached 50% and task performance was not affected by age, as was expected from the individual adaptation of the time limit for a correct response (see Materials and Methods section).
Whole‐Brain Analyses
Reward anticipation
Whole‐brain results are presented in Figure 3, Supporting Information, Figure 2 and Table 1. A whole‐brain analysis contrasting anticipation of reward and neutral anticipation revealed a well‐documented pattern of activation that includes parts of the reward network, comprising the ventral striatum, thalamus, dorsal caudate, cingulate cortex, and putamen, as well as areas associated with task performance, comprising the primary motor cortex and supplementary motor area (Supporting Information, Fig. 2 and Table 1).
Figure 3.
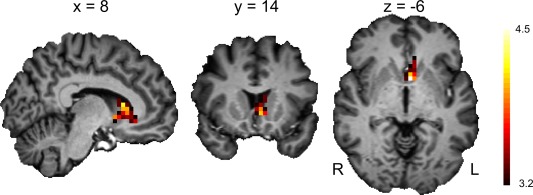
Whole‐brain effect of age on brain activation during reward anticipation versus neutral anticipation. Activation was tested for significance at a familywise error (FWE) corrected cluster level of P = 0.05 (cluster‐defining threshold of P = 0.001, cluster size of 36 voxels). L = left, R = right. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Importantly, we found an effect of age on activation in the left ventral striatum (cluster center x = −4, y = 8, z = −4; FWE‐corrected threshold of P = 0.05 and cluster size of 36 voxels), with older subjects showing less activation (Fig. 3).
Reward receipt
As presented in Supporting Information, Figure 2, whole‐brain analysis contrasting receipt of reward and correct neutral outcome activated the vmPFC, dorsal caudate, posterior cingulate cortex, and bilateral parahippocampal cortex. We found no regions showing age‐related changes in activation levels.
Region of Interest analyses
Reward anticipation
Reward anticipation results are presented in Figure 4 and Supporting Information, Figure 3. Regression analyses showed activation in the ventral striatum to be significantly increased during reward anticipation compared to neutral anticipation (left: F(1,48) = 25.29; P < 0.001, right: F(1,48) = 43.82; P < 0.001; Fig. 4). Importantly, this difference was modulated by age (left: F(1,48) = 14.18; r = −0.48; P < 0.001, right: F(1,48) = 6.67; r = −0.35; P = 0.013), with older subjects showing reduced levels of ventral striatum activation during reward anticipation. Only the age effect in the left ventral striatum survived Bonferroni correction. Finally, there was no main effect of age (left: F(1,48) = 0.03; r = −0.02; P = 0.88, right: F(1,48) = 0.02; r = −0.02; P = 0.89), indicating that there was no general decline in activation levels with age. Rather, activation patterns during reward anticipation and neutral anticipation became more similar with age, with declining activation during reward anticipation (left: F(1,48) = 1.00; r = −0.14; P = 0.32, right: F(1,48) = 1.38; r = −0.17; P = 0.24), and activation during neutral anticipation increasing with age numerically, but not significantly (left: F(1,48) = 1.11; r = 0.16; P = 0.29, right: F(1,48) = 0.93; r = 0.14; P = 0.34) (Supporting Information, Fig. 3).
Figure 4.
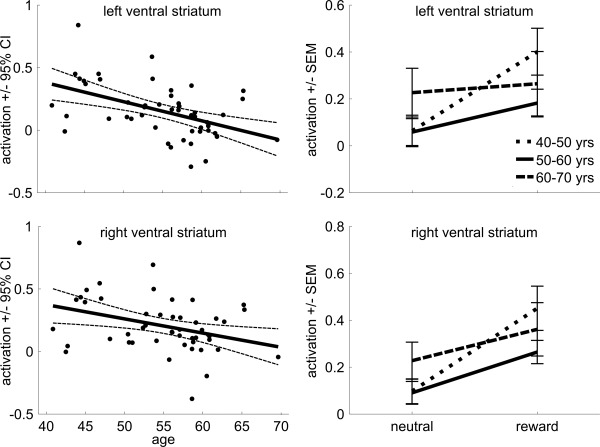
Reward anticipation. Scatter plot of the reward effect (reward anticipation vs. neutral anticipation) on brain activation in the left and right ventral striatum as a function of age (with linear trend line and 95% confidence interval), and line plots for the three age groups (±standard error of the mean) for neutral anticipation and reward anticipation.
Next, we performed a repeated‐measures analysis with group (three levels) and condition (reward cue, neutral cue). This revealed a significant main effect of condition (left: F(1,46) = 31.91; P < 0.001, right: F(1,46) = 48.88; P < 0.001), with subjects showing more activation during reward anticipation as compared to neutral anticipation (Fig. 4). More importantly, the group by condition interaction was significant (left: F(2,46) = 7.27; P = 0.001, right: F(2,46) = 3.7724; P = 0.03), with a smaller effect of reward in the oldest group. Indeed, post‐hoc t‐tests showed that ventral striatum activation was significantly higher when anticipating reward compared to neutral anticipation in the group aged 40–50 years compared to subjects aged 50–60 years (left: t(37) = 3.04; P = 0.004, right: t(37) = 2.37; P = 0.022) and 60–70 years (left: t(19) = 3.38; P = 0.003, right: t(19) = 2.27; P = 0.035). Finally, there was no main effect of age (left: F(2,46) = 0.91; P = 0.41, right: F(2,46) = 1.12; P = 0.34), indicating that there was no general decline in activation.
Reward receipt
Reward receipt results are presented in Figure 5 and Supporting Information, Figure 4. Regression analyses showed significantly more activation in the ventral striatum during reward receipt as compared to neutral outcome (left: F(1,48) = 8.54; P = 0.01, right: F(1,48) = 7.42; P = 0.009; Fig. 5). Importantly, this difference was modulated by age (left: F(1,48) = 6.81; r = 0.36; P = 0.01, right: F(1,48) = 4.58; r = 0.29; P = 0.04), with older subjects showing an increased effect of reward receipt on ventral striatum activation. Only the age effect in the left ventral striatum survived Bonferoni correction. Finally, there was no main effect of age (left: F(1,48) = 1.94; r = 0.19; P = 0.17, right: F(1,48) = 1.84; r = 0.19; P = 0.18), indicating that there was no general change in activation levels with age. Rather, activation during reward receipt and neutral outcome became more similar with age, with activation during reward receipt significantly increasing (left: F(1,48) = 4.04; r = 0.28; P = 0.05, right: F(1,48) = 4.20; r = 0.29; P = 0.046), while activation during neutral anticipation remained the same with age (left: F(1,48) = 0.29; r = 0.08; P = 0.59, right: F(1,48) = 0.07; r = 0.04; P = 0.80; Supporting Information, Fig. 4).
Figure 5.
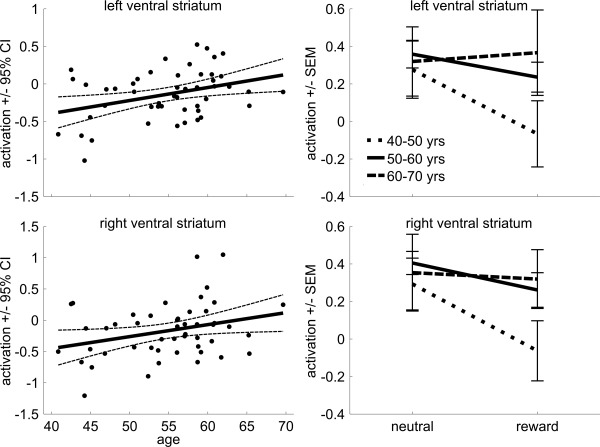
Reward receipt. Scatter plot of the reward effect (reward receipt vs. correct neutral outcome) on brain activation in the left and right ventral striatum as a function of age (with linear trend line and 95% confidence interval), and line plots for the three age groups (±standard error of the mean) for correct neutral outcome and reward receipt.
A similar analysis of activation in the vmPFC yielded a significant effect of condition (left: F(1,48) = 37.1; P < 0.001, right: F(1,48) = 24.39; P < 0.001). This was expected as this region is known to be involved in reward receipt. However, none of the age‐related effects were significant (all F < 1; Supporting Information, Fig. 5).
Next, we performed a repeated‐measures analysis with group (three levels) and condition (reward cue, neutral cue) on activation in the ventral striatum. This revealed a significant main effect of condition (left: F(1,46) = 9.69; P = 0.003, right: F(1,46) = 7.59; P = 0.008), with subjects showing less activation during reward receipt as compared to neutral outcome (Fig. 5). More importantly, the group by condition interaction was significant in the left ventral striatum (F(2,46) = 4.23; P = 0.02), with a smaller effect of reward in the oldest group (60–70 years) compared to the youngest group (40–50 years; t(19) = −2.75, P = 0.01). The interaction did not reach significance in the right ventral striatum (F(2,46) = 1.58; P = 0.22). Finally, there was no main effect of age (left: F(2,46) = 0.84; P = 0.44, right: F(2,46) = 1.18; P = 0.32), indicating that there was no general decline in activation.
As there was no effect of age on activation in the vmPFC, we did not perform repeated‐measures analyses on activation in that region.
Effect of age on activation shift from reward receipt to anticipation
Activation shift results are presented in Figure 6. We directly compared the effect of aging on striatal activation during reward anticipation (vs. neutral anticipation) and reward receipt (compared to neutral outcome). This analysis revealed a significant main effect of condition (left: F(1,48) = 17.40; P < 0.001, right: F(1,48) = 19.75; P < 0.001), with more ventral striatum activation during reward anticipation compared to receipt of reward. This effect was modulated by age (left: F(1,48) = 11.96; r = −0.45; P = 0.001, right: F(1,48) = 6.5234; r = −0.34; P = 0.01), with the difference between reward anticipation and reward receipt diminishing with age. This suggests that the typical temporal shift in activation from reward receipt to reward anticipation no longer occurs in older subjects. Finally, there was no main effect of age (left: F(1,48) = 0.13; r = 0.05; P = 0.72, right: F(1,48) = 1.09; r = 0.15; P = 0.30), indicating there was no general effect of age on activation.
Figure 6.
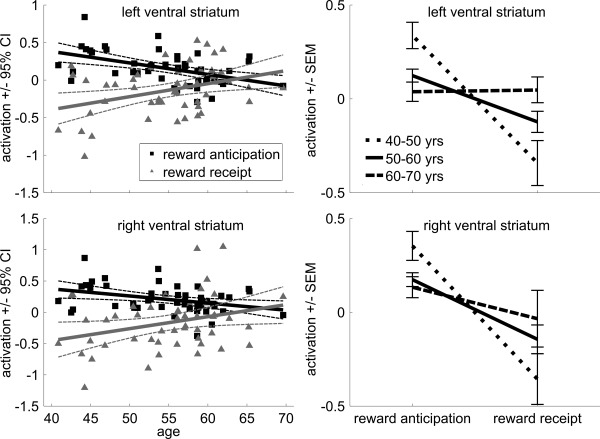
Activation shift from receipt to anticipation. Scatter plot of the shift in activation from reward receipt to reward anticipation on brain activation in the left and right ventral striatum as a function of age (with linear trend line and 95% confidence interval), and line plots for the three age groups (±standard error of the mean) for reward anticipation and reward receipt.
Next, we performed a repeated‐measures analysis with group (three levels) and condition (reward anticipation vs. neutral, reward receipt vs. neutral) on activation in the ventral striatum. This revealed a significant main effect of condition (left: F(1,46) = 21.59; P < 0.001, right: F(1,46) = 21.14; P < 0.001), with subjects showing more activation during reward anticipation as compared to reward receipt. More importantly, the group by condition interaction was significant in the left ventral striatum (F(2,46) = 6.79; P = 0.003), with a smaller difference between reward anticipation and reward outcome in the oldest group (60–70 years) compared to the youngest group (40–50 years) (t(19) = −2.75, P = 0.01). The interaction did not reach significance in the right ventral striatum (F(2,46) = 2.69; P = 0.08). Finally, there was no main effect of age (left: F(2,46) = 0.41; P = 0.67, right: F(2,46) = 0.22; P = 0.79), indicating there was no general effect of age on activation.
DISCUSSION
Here, we investigated age‐related changes in reward processing in a cross‐sectional sample of 49 healthy adults aged 40–70 years. We observed no age‐effects on task accuracy or response speed, indicating adequate task performance in all subjects. Whole‐brain analyses revealed a significant age‐related decrease in ventral striatum activation during the anticipation of reward compared to neutral anticipation. ROI analyses showed that this decline was not caused by a general reduction in ventral striatum activation levels, but rather indicates a loss of discrimination between the processing of reward and neutral cues. Ventral striatum activation during reward receipt, as compared to a correct neutral outcome, increased with age. No effect of age was found in the vmPFC, a region commonly associated with reward receipt [Diekhof et al., 2012; Figee et al., 2011; Liu et al., 2011; Haber and Knutson, 2010]. Our data provide further evidence for the notion that healthy aging is associated with changes in stimulus–reward processing. Increased ventral striatum activation during reward receipt could potentially be a consequence of decreased reward anticipation. Specifically, the typical activation shift in the ventral striatum from reward receipt to reward anticipation no longer occurs in older subjects.
Reward Anticipation
Our finding of decreased anticipatory ventral striatum activation in healthy aging is in part consistent with the results of Schott et al. [2007] and Dreher et al. [2008]. Schott et al. [2007] reported reduced levels of activation in a cluster of 5 voxels in the ventral striatum ([9 9 −3]) in old subjects (n = 19, mean age 69.0, range 62–78) versus young subjects (n = 18, mean age 23.3, range 19–28). However, their results did not survive whole‐brain corrections for multiple comparisons. Dreher et al. [2008] compared young subjects (n = 20, mean age 25, SD 3.7 years) with aging subjects (n = 13, mean age 66, SD 5 years) and reported reduced activation in dorsal but not ventral striatum (peak voxel [8 4 15]). The current findings extend these studies by showing a whole‐brain significant effect of aging on ventral striatum activation during reward anticipation. Moreover, we found this decline to occur gradually across subjects aged 40–70, with the youngest subjects in our study being older than those in previous studies. Using this sample, we could show for the first time that ventral striatum activation during reward anticipation declines between the ages of 40 and 70. In fact, our repeated‐measures ANOVA analyses suggest that this decline sets in after the age of 50. This is consistent with recent insights in age‐related structural brain changes, with diffusion tensor imaging (DTI) studies showing white‐matter disturbances begin to impact brain function at middle age [for a review, see Kohama et al., 2012]. Indeed, a meta‐analysis combining functional MRI and DTI measures showed a negative relation between brain activation levels and white‐matter integrity in aging subjects [Bennett and Rypma, 2013].
Our results seem to be less consistent with results from studies by Samanez‐Larkin et al. [2007], Rademacher et al. [2014], and Lorenz et al. [in press]. Samanez‐Larkin et al. [2007] compared brain activation of a small sample of young (n = 12, age 19–27) and aging subjects (n = 12, age 65–81). They failed to detect an age‐related effect on ventral striatum activation during reward anticipation using whole‐brain analyses, and subsequent volume of interest analyses. However, they do report an age‐related reduction of striatal activation during reward anticipation, which was paralleled by an increase of activation in the parietal cortex. Rademacher et al. [2014] compared brain activation in young subjects (n = 24, age 20–28) and aging subjects (n = 24, age 60–78) while they performed a monetary incentive delay task offering monetary or social rewards. They did not find any effect of age on ventral striatum activation during anticipation of either monetary or social reward (both whole‐brain and in a ventral striatum region‐of‐interest). However, in their earlier work [Spreckelmeyer et al., 2009], they found a strong effect of both monetary and social reward on anticipation‐related ventral striatum activation in a sample of adults (n = 32, mean age 29.0, range 20–48). Taken the data from both studies together, the failure to find an overall effect of anticipation on ventral striatum activation may in fact be due to the older subjects being added to the sample in the 2014 paper. Alternatively, this inconsistency between their papers could be due to the relatively long echo time (50 ms), which makes it difficult to reliably detect activation in regions such as the ventral striatum [Figee et al., 2013]. Finally, Lorenz et al. [in press] acquired brain activation data from a sample of adolescents (n = 34, mean age 14.9, range 13–16), young adults (n = 34, mean age 26, range 19–35), and aging subjects (n = 34, mean age 67.5, range 61–80), while performing a slot machine task. The authors report no difference in ventral striatum activation during reward anticipation between the young adults and aging subjects. Remarkably, the adolescents show significantly more activation in the ventral striatum compared to both age groups. This is in direct opposition with the majority of literature on reward processing in adolescents, which agrees on the fact that reward anticipation is reduced in adolescents compared to adults [Bjork et al., 2004, 2010; Geier et al., 2010; Hoogendam et al., 2013]. This inconsistency may be caused by the type of task is being used (slot machine versus monetary incentive delay task), and therefore, it is difficult to relate their findings to those we present here.
Reward Receipt
We found that activation in the ventral striatum increased with aging during receipt of reward compared to a neutral outcome. This is in agreement with the results of Schott et al. [2007], who reported significantly higher ventral striatum activation during reward outcome in older compared to younger subjects. Samanez‐Larkin et al. [2007] and Dreher et al. [2008] reported on reward receipt, but they did not identify age‐related changes in the ventral striatum. Rademacher et al. [2014] and Lorenz et al. [in press] did not report outcome data.
We found no effect of aging in the vmPFC during reward receipt. The vmPFC is commonly associated with the outcome component of reward processing, and is believed to process reward magnitude [Diekhof et al., 2012]. The anterior part of the vmPFC is thought to be most sensitive to monetary reward [Haber and Knutson, 2010]. Indeed, we did find the vmPFC to be activated in response to reward receipt across the entire sample.
Effect of Age on Activation Shift from Receipt to Anticipation
Taken together, our results indicate that with healthy aging, the shift in activation from outcome phase to anticipation phase no longer occurs. This shift is characteristic for normal young–adulthood, and is thought to arise during adolescent development [Hoogendam et al., 2013; May et al., 2014]. This failure to shift in old age is also identified in more elaborate reversal learning tasks. For example, Mell et al. [2009] showed that younger subjects (n = 14, mean age 26.5, SD 3.9 years) activate the ventral striatum primarily during “learned” trials, when rewards are expected and the ventral striatum signals anticipation. Contrastingly, elderly subjects (n = 14, mean age 67.8, SD 5.0 years) activate mainly in “search” trials, when rewards are given at chance and the ventral striatum signals reward receipt. Interestingly, these findings are in direct opposition to adolescent development (10–25 years), when striatal reward processing shifts from being primarily outcome driven to being primarily anticipation driven [Hoogendam et al., 2013].
The failure to shift activation from outcome phase to anticipation phase may reflect diminished reward‐based learning in healthy aging. Our findings could represent the neurofunctional underpinning of the age‐related cognitive decline that is identified in prior behavioral research [Chowdhury et al., 2013; Mell et al., 2003]. An important factor supporting the hypothesis of decreased stimulus–reward coupling in old age is the well‐documented age‐related decline of striatal dopamine availability [Bäckman and Farde, 2005; Bäckman et al., 2006; Dreher et al., 2008; Marschner et al., 2005]. Efficient reward processing depends critically upon dopamine availability [Düzel et al., 2010; Waelti et al., 2001]. The notion of dopamine loss underlying the age‐related alterations in stimulus–reward coupling is further supported by the results of Chowdhury et al. [2013], who found that learning abilities of healthy older adults increased to the level of young adults after administration of the dopamine precursor levodopa (l‐DOPA). The age‐related decrease in striatal dopamine availability has also been suggested to increase neural noise, which diminishes distinctive signalling [Li et al., 2001]. This seems to be in accordance with the overall age‐related decrease in reward–neutral discrimination observed in our data.
However, our data could also reflect a reorganization of reward processing within the elderly brain. In elderly subjects reward processing could be executed by a different combination of brain regions than in younger subjects, without this affecting subsequent stages of processing or behavior. This seems in line with our results, since we did not observe age‐related changes in response times and trial accuracy. However, these results are inconsistent with some prior studies [Park and Reuter‐Lorenz, 2009; Schott et al., 2007]. It should be noted that we applied a relatively simple motor task and adapted target speeds individually to insure a trial accuracy of 50% in all subjects [Figee et al., 2011; Hoogendam et al., 2013; Van Hell et al., 2010]. Age‐related declines in performance are generally observed using more demanding reward‐related tasks [Chowdhury et al., 2013; Mell et al., 2003]. Senescence is found to particularly affect the ability to adapt flexibly to changed stimulus‐reward associations (reversal learning), which was not required in our task [Eppinger et al., 2011].
Alternatively, reduced ventral striatum activation during reward anticipation with aging may be a consequence of an age‐related decline in gain probability. With decreased probability of reward receipt, the stimulus–reward association will be less profound [Fiorillo et al., 2003]. However, our task design ensured that all subjects would win the same amount of money. Indeed, we did not find an effect of age on reward accuracy. However, the fact that there was no objective difference in reward probability does not exclude the possibility that age affects the subjective estimation of reward probability, resulting in lower gain expectancies for the same reward probabilities in older relative to younger subjects [Frank et al., 2004]. Finally, Rademacher et al. [2013] suggested that with increasing age people tend to devaluate monetary rewards. If true, then the performance in elderly subjects should not be affected by potential rewards. However, our behavioral data show that older subjects, like younger subjects, respond faster when they can win money. Moreover, older subjects show increased activation during reward receipt, suggesting that they do evaluate rewards differently from a neutral outcome. Also, we found reward responsiveness in the vmPFC to be unaffected by age, further supporting the notion that reward incentive remains relatively equal with aging.
This study has several limitations. First, the group sizes for the ages of 40–50 years and 60–70 years were smaller than those of the middle group, which may have influenced between‐group analyses. Second, although we find clear evidence in support of diminishing reward processing after the age of 40, we cannot make inferences about the trajectory of changes prior to the age of 40. Although we do extend studies using only young subjects and old subjects, it remains unclear what happens in reward processing during young adulthood and the age of 40.
Finally, using functional magnetic resonance imaging (fMRI), we measured cerebral activity indirectly, through the BOLD response. The BOLD‐response is sensitive to hemodynamic changes in the brain. Old age is associated with an increased prevalence of hypertension, hyperlipidemia, and clinically silent cerebral or vascular pathology, which could hypothetically alter the BOLD‐response [Raemaekers et al., 2006]. An age‐related decrease in BOLD‐response is generally reported [Ances et al., 2009; Buckner et al., 2000], although some studies report no age‐effect [Aizenstein et al., 2004; Huettel et al., 2001]. Since our data show age‐related increases as well as decreases in ventral striatal activation, the bias presented by age‐related hemodynamic changes appears limited.
CONCLUSION
We identified a whole‐brain significant age‐related decrease of ventral striatum activation during reward anticipation. In contrast, we found activation in the ventral striatum to increase with age during reward receipt. Activation in the vmPFC was not affected by age. These data demonstrate that the typical temporal shift from striatal activation being primarily outcome driven toward being increasingly anticipation driven no longer occurs in healthy aging. Taken together, our findings indicate an age‐related deterioration of stimulus–reward processing. This study expands our knowledge of the effects of healthy aging on reward processing, and can serve as a stepping stone for further research of the impact of neurodegenerative diseases typically associated with aging on reward‐related processing. Altered stimulus–reward processing is a common symptom of neurodegenerative disease [Perry and Kramer, in press; Rutledge et al., 2009]. Understanding the interplay between age‐related changes in brain functionality and cognitive processing, particularly reward processing, may be crucial for future models of healthy aging and age‐related psychopathology.
Supporting information
Supplementary Information Figure 1
Supplementary Information Figure 2
Supplementary Information Figure 3
Supplementary Information Figure 4
Supplementary Information Figure 5
Iris Kleerekooper is a medical student participating in the Honours program of the Faculty of Medicine, UMC Utrecht.
REFERENCES
- Aizenstein HJ, Clark K A, Butters MA, Cochran J, Stenger VA, Meltzer CC, Reynolds CF, Carter CS (2004): The BOLD hemodynamic response in healthy aging. J Cogn Neurosci 16:786–793. [DOI] [PubMed] [Google Scholar]
- Ances BM, Liang CL, Leontiev O, Perthen JE, Fleisher AS, Lansing AE, Buxton RB (2009): Effects of aging on cerebral blood flow, oxygen metabolism, and blood oxygenation level dependent responses to visual stimulation. Hum Brain Mapp 30:1120–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckman L, Farde L (2005): The role of dopamine systems in cognitive aging In: Cabeza R, Nyberg L, Park DC, editors. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. New York: Oxford University Press; pp 58–88. [Google Scholar]
- Bäckman L, Nyberg L, Lindenberger U, Li S, Farde L (2006): The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neurosci Biobehav Rev 30:791–807. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Rypma B (2013): Advances in functional neuroanatomy: A review of combined DTI and fMRI studies in healthy younger and older adults. Neurosci Biobehav Rev 37:1201–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijleveld E, Custers R, Van der Stigchel S, Aarts H, Pas P, Vink M. Distinct neural responses to conscious versus unconscious monetary reward cues. Hum Brain Mapp 2014;35:5578–5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW (2004): Incentive‐elicited brain activation in adolescents: Similarities and differences from young adults. J Neurosci 24:1793–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork M, Smith AR, Chen G, Hommer DW (2010): Adolescents, adults and rewards: Comparing motivational neurocircuitry recruitment using fMRI. PLoS One 5:e11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman ED, Behrens TE, Woolrich MW, Rushworth MF (2009) How green is the grass on the other side? Frontopolar cortex and the evidence in favor of alternative courses of action. Neuron 62:733–743. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Sanders AL, Raichle ME, Morris JC (2000): Functional brain imaging of young, nondemented, and demented older adults. J Cogn Neurosci 12:24–34. [DOI] [PubMed] [Google Scholar]
- Chowdhury R, Guitart‐Masip M, Lambert C, Dayan P, Huys Q, Düzel E, Dolan RJ (2013): Dopamine restores reward prediction errors in old age. Nat Neurosci 16:648–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coubard OA, Ferrufino L, Boura M, Gripon A, Renaud M, Bherer L (2011): Attentional control in normal aging and Alzheimer's disease. Neuropsychology 25:353–367. [DOI] [PubMed] [Google Scholar]
- de Leeuw M, Kahn RS, Vink M. Fronto‐striatal dysfunction during reward processing in unaffected siblings of schizophrenia patients. Schizophr Bull 2015:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denburg NL, Tranel D, Bechara A (2005): The ability to decide advantageously declines prematurely in some normal older persons. Neuropsychologia 43:1099–1106. [DOI] [PubMed] [Google Scholar]
- Diekhof D, Kaps L, Falkai P, Gruber O (2012): The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude—An activation likelihood estimation meta‐analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia 50:1252–1266. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Meyer‐Lindenberg A, Kohn P, Berman KF (2008): Age‐related changes in midbrain dopaminergic regulation of the human reward system. Proc Natl Aacd Sci USA 105:15106–15111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Düzel E, Bunzeck N, Guitart‐Masip M, Düzel S (2010): Novelty‐related motivation of anticipation and exploration by dopamine (NOMAD): implications for healthy aging. Neurosci Biobehav Rev 34:660–669. [DOI] [PubMed] [Google Scholar]
- Eppinger B, Herbert M, Kray J (2009): We remember the good things: Age differences in learning and memory. Neurobiol Learn Mem 93:515–521. [DOI] [PubMed] [Google Scholar]
- Eppinger B, Hämmerer D, Li S (2011): Neuromodulation of reward‐based learning and decision making in human aging. Ann N Y Acad Sci 1235:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppinger B, Schuck NW, Nystrom LE, Cohen JD (2013): Reduced striatal responses to reward prediction errors in older compared with younger adults. J Neurosci 33:9905–9912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, Pine DS (2005): Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage 25:1279–1291. [DOI] [PubMed] [Google Scholar]
- Fera F, Passamonti L, Herzallah MM, Myers CE, Veltri P, Morganti G, Quattrone A, Gluck MA (2014): Hippocompal BOLD response during category learning predicts subsequent performance on transfer generalization. Hum Brain Mapp 35:3122–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figee M, Vink M, De Geus F, Vulink N, Veltman DJ, Westenberg H, Denys D (2011): Dysfunctional reward circuitry in obsessive‐compulsive disorder. Biol Psychiatry 69:867–874. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W (2003): Discrete coding of reward probability and uncertainty by dopamine neurons. Science 299:1898–1902. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Seeberger LC, O'Reilly RC (2004): By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science 306:1940–1943. [DOI] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B (2010): Immaturities in reward processing and its influence on inhibitory control in adolescence. 20:1613–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B (2010): The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 35:4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogendam JM, Kahn RS, Hillegers MH, van Buuren M, Vink, M (2013): Different developmental trajectories for anticipation and receipt of reward during adolescence. Dev cogn neurosci 6:113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel SA, Singerman JD, McCarthy D (2001): The effects of aging upon the hemodynamic response measured by functional MRI. Neuroimage 13:161–175. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, and Hommer D (2001a): Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 21:RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D (2001b): Dissociation of reward anticipation and outcome with event‐related fMRI. Neuroreport 12:3683–3687. [DOI] [PubMed] [Google Scholar]
- Kohama SG, Rosene DL, Sherman LS (2012): Age‐related changes in human and non‐human primate white matter: from myelination disturbances to cognitive decline. Age (Dordr) 34:1093–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DJ, Glimcher PW (2012): The root of all value: a neural common currency for choice. Curr Opin Neurobiol 22:1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SC, Lindenberger U, Sikström S (2001): Aging cognition: From neuro‐modulation to representation. Trends Cogn Sci 5:479–486. [DOI] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J (2011): Common and distinct networks underlying reward valence and processing stages: A meta‐analysis of functional neuroimaging studies. Neurosci Biobehav Rev 35:1219–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz RC, Gleich T, Beck A, Pöhland L, Raufelder D, Sommer W, Rapp MA, Kühn S, Gallinat J (2014): Reward anticipation in the adolescent and aging brain Hum Brain Mapp 35:5153–5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Rao V, Lu B, Saletin JM, Lindquist JR, Ancoli‐Israel S, Jagust W, Walker MP (2013): Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal‐dependent memory in aging. Nat Neurosci 16:357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner A, Mell T, Wartenburger I, Villringer A, Reischies FM, Heekeren HR (2005): Reward‐based decision‐making and aging. Brain Res Bull 67:382–390. [DOI] [PubMed] [Google Scholar]
- May AC, Stewart JL, Tapert SF, Paulus MP (2014): The effect of age on neural processing of pleasant soft touch stimuli. Front Behav Neurosci 8:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mell T, Heekeren HR, Marschner A, Wartenburger I, Villringer A, Reischies FM (2003): Effect of aging on stimulus‐reward association learning. Neuropsychologia 43:554–563. [DOI] [PubMed] [Google Scholar]
- Mell T, Wartenburger I, Marschner A, Villringer A, Reischies FM Heekeren HR (2009): Altered function of ventral striatum during reward‐based decision making in old age. Front Hum Neurosci 3:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Reuter‐Lorenz P (2009) The adaptive brain: Aging and neurocognitive scaffolding. Annu Rev Psychol 60:173–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry DC, Kramer JH (2015): Reward processing in neurodegenerative disease. Neurocase 21:120–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher L, Salama A, Grunder G, Spreckelmeyer KN (2014): Differential patterns of nucleus accumbens activation during anticipation of monetary and social reward in young and older adults. Soc Cogn Affect Neurosci 9:825–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raemaekers M, Vink M, Van Den Heuvel MP, Kahn RS, Ramsey NF (2006): Effects of aging on BOLD fMRI during prosaccades and antisaccades. J Cogn Neurosci 18:594–603. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM (2006): Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev 30:730–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes MG (2004): Age‐related differences in performance on the Wisconsin Card Sorting Test: a meta‐analytic review. Psychol Aging 19:482–494. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Span MM, Van Der Molen MW (2002): Perseverative behavior and adaptive control in older adults: performance monitoring, rule induction, and set shifting. Brain Cogn 49:382–401. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Van Den Wildenberg WP, Segalowitz SJ, Carter CS (2004): Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward‐based learning. Brain Cogn 56:129–140. [DOI] [PubMed] [Google Scholar]
- Rutledge RB, Lazzaro SC, Lau B, Myers CE, Gluck MA, Glimcher PW (2009): Dopaminergic drugs modulate learning rates and perseveration in Parkinson's patients in a dynamic foraging task. J Neurosci 29:15104–15114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez‐Larkin GR, Gibbs SE, Khanna K, Nielsen L, Carstensen LL, Knutson B (2007): Anticipation of monetary gain but not loss in healthy older adults. Nat Neurosci 10:787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez‐Larkin GR, Levens SM, Perry LM, Dougherty RF, Knutson B (2011): Frontostriatal white‐matter integrity mediates adult age differences in probabilistic reward learning. J Neurosci 32:5333–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott BH, Niehaus L, Wittmann BC, Schutze H, Seidenbecher CI, Heinze HJ, Düzel E (2007): Ageing and early‐stage Parkinson's disease affect separable neural mechanisms of mesolimbic reward processing. Brain 130:2412–2424. [DOI] [PubMed] [Google Scholar]
- Schultz W (2000): Multiple reward signals in the brain. Nat Rev Neurosci 1:199–207. [DOI] [PubMed] [Google Scholar]
- Schultz W (2007): Multiple dopamine functions at different time courses. Annu Rev Neurosci 30:259–288. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR (1997): A neural substrate of prediction and reward. Science 275:1593–1599. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labelling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL (2012): The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 59:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hell HH, Vink M, Ossewaarde L, Jager G, Kahn RS, Ramsey NF (2010): Chronic effects of cannabis use on the human reward system: an fMRI study. Eur Neuropsychopharmacol 20:153–163. [DOI] [PubMed] [Google Scholar]
- Vink M, Pas P, Bijleverd E, Custers R, Gladwin TE (2013): Ventral striatum is related to within‐subject learning performance. Neuroscience 250:408–416. [DOI] [PubMed] [Google Scholar]
- Vink M, Zandbelt BB, Gladwin T, Hillegers M, Hoogendam JM, van den Wildenberg WP, Du Plessis S, Kahn RS (2014): Frontostriatal activity and connectivity increase during proactive inhibition across adolescence and early adulthood. Hum Brain Mapp 35:4415–4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waelti P, Dickinson A, Schultz W (2001): Dopamine responses comply with basic assumptions of formal learning theory. Nature 412:43–48. [DOI] [PubMed] [Google Scholar]
- Zald D, Andreotti C (2010): Neuropsychological assessment of the orbital and ventromedial prefrontal cortex. Neuropsychologia 48:3377–3391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information Figure 1
Supplementary Information Figure 2
Supplementary Information Figure 3
Supplementary Information Figure 4
Supplementary Information Figure 5


