Abstract
Dual‐process theories have dominated the study of risk perception and risk‐taking over the last two decades. However, there is a lack of objective brain‐level evidence supporting the two systems of processing in every‐day risky behavior. To address this issue, we propose the dissociation between evaluative and urgent behaviors as evidence of dual processing in risky driving situations. Our findings show a dissociation of evaluative and urgent behavior both at the behavioral and neural level. fMRI data showed an increase of activation in areas implicated in motor programming, emotional processing, and visuomotor integration in urgent behavior compared to evaluative behavior. These results support a more automatic processing of risk in urgent tasks, relying mainly on heuristics and experiential appraisal. The urgent task, which is characterized by strong time pressure and the possibility for negative consequences among others factors, creates a suitable context for the experiential‐affective system to guide the decision‐making process. Moreover, we observed greater frontal activation in the urgent task, suggesting the participation of cognitive control in safe behaviors. The findings of this research are relevant for the study of the neural mechanisms underlying dual process models in risky perception and decision‐making, especially because of their proximity to everyday activities. Hum Brain Mapp 36:2853–2864, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: Automatic Processing, Driving, fMRI, Risk perception, Traffic Psychology, Urgent behavior
INTRODUCTION
Dual‐process theories explaining human decision‐making have boomed over the two last decades. A number of models have emerged trying to explain decision‐making based on two different processing routes or systems [Damasio, 1994; Epstein, 1994; Kahneman and Frederick, 2005; Lieberman et al. 2002; Slovic et al. 2007, among others]. Diverse terminologies have been used to characterize the two systems and the proposed models include different features [Evans, 2008]. However, researchers generally agree that System 1 comprises processes of an experiential‐affective nature: predominantly automatic, associative, rapid, and undemanding. System 2, conversely, is of a rational‐analytic nature: controlled, deliberative, rule‐based, slow, and conscious [Evans, 2008; Kahneman and Frederick, 2005; Sloman, 1996].
The continuum of automatic and controlled processing has also dominated the study of risk perception and risky decision making [Loewenstein et al. 2001; Slovic et al. 2007]. An outstanding amount of literature dealing with dual processes has demonstrated that risk behaviors are hardly explicable from a rational and deliberative point of view. However, there is a lack of objective brain‐level evidence confirming the presence of two systems of processing in risk behavior. The evidence that exists on this issue comes from artificial tasks which have largely focused on gambling [Keren and Schul, 2009]. Little research has addressed the brain mechanisms involved in dual processing pathways in everyday tasks.
To address this issue, Megías et al. [2013, 2011] proposed the dissociation between urgent and evaluative behaviors as evidence of dual processing in every‐day risk behavior. Urgent behaviors are performed under time pressure, triggered by a stimulus, and can lead to negative consequences if the action is unsuccessful. Evaluative behaviors simply consist of an evaluation of the situation, where a response is not imperative and does not involve actual negative consequences. The features characterizing tasks requiring urgent behavior create appropriate conditions for automated processes based on previous experience, determined by stimulus–response connections and enabling fast responses to hazards. Conversely, tasks requiring evaluative behavior would activate a more controlled mode of processing, carrying out a deeper evaluation of the situation and basing its results on logical rules. For instance, if you are driving and suddenly a ball appears on the road between two stationary cars, what would your reaction be? This scenario creates a hazardous situation for the driver because a child may come chasing the ball. In this case, experienced drivers most likely do not conduct a slow rational evaluation. Rather, an automated urgent braking behavior will be triggered by the risk stimulus, to avoid hitting a child.
The main aim of the current research was to investigate the brain‐level mechanisms underlying urgent and evaluative behavior in the context of driving by means of using functional magnetic resonance imaging (fMRI). The study of these behaviors can provide supporting evidence for the existence of two‐way processing in risk decision making. Further, it can increase our understanding of the brain systems involved in risk perception and behavior.
METHODS
Participants had to perform two different tasks: an evaluative and an urgent task. Both tasks were identical except for the requested response from the participants. The evaluative task required participants to evaluate whether the displayed traffic situation entailed risk or not. The urgent task required participants to brake or not in the given traffic situation.
Following previous studies by Megías et al. [2013, 2011], the simulated braking task may be regarded as urgent because even a very short delay in braking during an actual risky situation increases the likelihood of having an accident (creating time pressure and a potential negative outcome, the two main features characterizing urgent behavior). In contrast, evaluating the risk in a driving situation in which participants act as mere observers is less compelling than braking. Participants simply assign a value to the situation (to categorize it as risky or not). Thus, the response would not involve negative consequences for the observer, and would not be imperative.
However, as our urgent task did not actually include time pressure/negative outcomes, and traffic situations and response buttons were common to both tasks, it would be possible to assume that the urgent behavior is only an evaluative one with stronger motor components. Thus, before embarking on the fMRI main experiment, a behavioral study was conducted to test whether our simulated braking and risk evaluation tasks conformed to the characteristics of urgent and evaluative behavior, respectively.
Experiment 1a
A behavioral study with 38 participants (all of them held valid driver's license and had similar socio‐demographic characteristics of those of main experiment) was carried out to determine whether the factors that characterize urgent behaviors—namely time pressure and probability of negative consequences with high emotional value—can explain the differences observed between the simulated braking task (urgent behavior) and the risk evaluation task (evaluative behavior) [Megías et al., 2011]. We added the main features of the urgent task to the evaluative task with the aim to close the gap between evaluative and urgent behavior.
Method, results, and discussion
Participants viewed 100 traffic images and performed five different tasks. Two of the tasks were the original urgent (whether to brake or not) and evaluative tasks (evaluate as risky or not). The remaining three tasks were modified versions of the original evaluative task (modified evaluative tasks): (1) ETP: evaluative with time pressure, (2) EEO: evaluative with emotional outcome, and (3) ETO: evaluative with both time pressure and emotional outcomes. In particular, in the ETP task, participants had to evaluate the situation for a maximum response time of 850 ms. In the EEO task, participants had to imagine that their responses would be used to remove road black spots to prevent accidents. In the ETO task, participants were given the same instructions as in EEO, but they were additionally required to evaluate the situation for a maximum response time of 850 ms. Participants completed the tasks in a counterbalanced order.
A single‐factor repeated measures ANOVA with Task (5 levels: urgent, evalutive, ETP, EEO, and ETO) as within‐subject variable on the averages of the probability of “brake/risk” showed a significant effect of Task, F (4, 148) = 13.63, MSE = 0.1049, P < 0.05. As we were interested in maximizing the likelihood of detecting differences between the evaluative and modified evaluative tasks, we used LSD post hoc analysis. Note that Bonferroni corrected P‐value will be <0.005. The probability of braking (0.57) was higher than the probability of evaluating risk (0.43) (P < 0.0001). Out of the remaining comparisons, only the EEO (0.49) vs. evaluative was significant (P < 0.05). For reaction times, the single‐factor repeated measures ANOVA yielded a significant effect of Task, F (4, 148) = 47.99, MSE = 465449, P < 0.0001. Post hoc analysis showed faster reaction times in the urgent condition (752 ms) than in the evaluative one (789 ms) (P < 0.05). As expected, time pressure had a huge impact on reaction times in ETP (587 ms) and ETO (589 ms), the reaction times were significantly faster than those in the urgent, evaluative and EEO tasks, all P < 0.0001.
Signal Detection Theory response bias (c) and sensitivity indices (d′) were used to evaluate the response performance [Macmillan and Creelman, 2005]. Risk was considered the signal to be discriminated from noise (nonrisky situations) for Signal Detection Theory Analysis. The ANOVA on the averages of response bias (c) and discriminability indices (d′) showed a main effect of Task. There was lower response bias in the urgent condition (0.24) than in evaluative one (−0.29) (P < 0.05). A better discriminability and higher response bias was observed in the evaluative (d′ = 2.04; c = −0.29) than in ETO (d′ = 1.67; c = −0.07) (P < 0.05).
Taken together, these results support the hypothesis that urgent responses (braking) are not always linked to the evaluation of risk [Megías et al., 2011]. The differences observed between the two tasks seem to stem from the joint effect of the two main features which distinguish urgent and evaluative behavior: time pressure and emotional outcomes. Thereby, our simulated braking task and the risk evaluation task adequately capture the main features of urgent and evaluative behavior and can thus be a valid measure of these. At this point, we are in a position to study the neural mechanism involved in urgent and evaluative behaviors.
MAIN EXPERIMENT
Participants
Fifty seven volunteers from the University of Granada (M age = 22.24 years old, SD age = 2.7; 39 women) participated in the study in exchange for course credits. All of them had a valid driver's license (M number of months = 52 months, SD = 30) and normal or corrected‐to‐normal vision. The study was conducted in conformity with the declaration of Helsinki [World Medical Association, 2008] and was approved by the Ethical Committee on Human Research of the University of Granada. All participants provided written consent.
Stimulus Material
The stimuli employed in this experiment comprised 140 real traffic pictures taken from the driver's perspective. The pictures were selected from a large and detailed image database depicting risky driving situations. All images met certain statistical criteria aimed to reduce interpersonal variability in the interpretation of the traffic situation and the estimated speed at which a vehicle is traveling in static traffic scenes [Vlakveld, 2011]. In particular, all images were evaluated by 40 driving instructors. The selected images were those with standard deviation of speed perception lower than 25% of the average speed perception, and where the best option to avoid the hazard was always to brake for at least 70% of the driving instructors. In addition, images were evaluated in relation to the level of risk judged by a nonexpert population (40 participants with driving license). The final set of pictures included 70 pictures representing road situations with low risk (average risk score = 1.92; where 0 = no risk, and 7 = high risk) and 70 pictures with medium‐high risk (average = 4.34) (see Supporting Information Appendix 1). The risk level of the images was also corroborated a posteriori by the participants of the current study (low risk average: 2.28; medium‐high risk: 4.40).
The stimuli displayed on a screen were visible through an angled mirror mounted on the fMRI head‐coil. The task was developed and controlled by E‐Prime software [Schneider et al., 2002].
Procedure
As we have previously described, all participants performed an urgent task (to brake or not in a given traffic situation) and an evaluative task (to evaluate whether the traffic situation entailed risk or not) during the experiment. The only difference between both tasks was the required response from the participants. The order of tasks was counterbalanced between participants.
Each task comprised 140 trials (70 risky situations and 70 nonrisky situations). Participants saw the trials in a random order. Every trial had the following sequence: after a fixation point (750 ms), the traffic situation was presented and the participant was asked to press the button of the MR response pad with his index finger if he thought that the situation entailed risk (vs. no risk in the evaluative task) or decided to brake (vs. not to brake in the urgent task). After 2000 ms or response execution, a black screen was shown for 3500 ms (see Fig. 1). The experiment lasted approximately 30 minutes (15 min each task).
Figure 1.
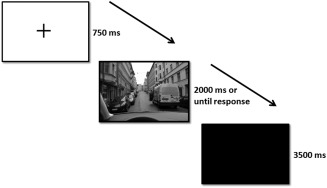
Scheme of the experimental procedure. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
fMRI Data Acquisition
Images were acquired on a Siemens 3T TRIO system at the Mind, Brain and Behavior Research Center, University of Granada, equipped with a 32‐channel headcoil. High‐resolution structural images were obtained using a T1‐weighted MPRAGE sequence (TR = 1900 ms; TE = 2.52 ms; flip angle = 9°). For each volume 176 slices of 1 mm thickness were obtained providing whole brain coverage (voxel size = 1 × 1 × 1 mm3; FOV = 256 mm; 256 × 256 data acquisition matrix). Functional images were recorded using a T2*‐weighted echo‐planar sequence with 35 noncontiguous axial slices of 3.5 mm thickness providing whole brain coverage (TR = 2000 ms, TE = 25 ms, flip angle = 80°; voxel size: 3.5 × 3.5 × 3.5 mm3; FOV = 238 mm; 68 × 68 data acquisition matrix).
In a single session, two functional runs were obtained for each participant, one per experimental task (450 volumes each). The two tasks were only separated by the instructions screen of the second task; the subjects did not leave the scanner or move position between tasks. Thus, a one‐block design fMRI paradigm was used to compare the two tasks (urgent‐evaluative) combining both runs. We used a nonstandard fMRI block design, consisting of only one block with two conditions, following the results from previous behavioral experiments (pilot studies). The use of more than one block impeded the formation of the specific mental set for each task in the subsequent blocks, indicated by reduced behavioral effects. The use of a one‐block design maximizes the power to detect activation in fMRI data; however, it has the limitation that is less robust in nuisance control terms (e.g., head motion artifact) [Liu, 2004]. Thus, special attention was placed on controlling artifacts. Participants were instructed and reminded not to move any part of their body, except their index finger, during the whole experiment. Head restraint and foam padding around the head were used to minimize head motion, and functional volumes were corrected for head movement by SPM12 software. Moreover, a large number of subjects were recruited in this study to reduce noise and improve signal detection.
fMRI Data Analysis
PREPROCESSING and statistical analysis of the fMRI data were carried out using SPM12 (Wellcome Trust Center for Neuroimaging, London, UK; http://www.fil.ion.ucl.ac.uk/spm/)
The first 5 EPI volumes of each run were discarded to allow magnetization to reach equilibrium. The last volumes of each run after task completion were also discarded. The remaining volumes were motion corrected to the average. The anatomical scans were coregistered to the mean EPI volume using linear rigid body transformation, and segmented to estimate the normalization parameters. The transformation parameters were applied to the set of functional volumes for spatial normalization to the MNI space. Next, functional volumes were resampled to a resolution of 3 × 3 × 3 mm and spatially smoothed with an isotropic Gaussian kernel of 8 mm full‐width‐at‐half‐maximum (FWHM). Before the statistical analysis, functional images were high‐pass filtered (128 s).
A composition of two gamma functions was used to model the hemodynamic response function, and robust weighted least squares (rWLS) estimation [Diedrichsen and Shadmehr, 2005] was performed in the first level models. Two whole‐brain contrasts were defined: urgent > evaluative and urgent < evaluative. The resulting individual contrast maps were submitted to a single sample t‐test analysis to determine locations showing larger activation for the urgent than for the evaluative task, and vice versa. To determine an appropriate level of statistical significance, the results were corrected for multiple comparisons using AlphaSim correction [Ward, 2000, for more detail). AlphaSim correction uses individual voxel probability thresholding in combination with minimum cluster size thresholding to estimate a specified significance level by Monte Carlo simulation. AlphaSim (10,000 runs in Monte Carlo simulation) set up the following statistical criterion: P < 0.001 with at least 132 contiguous voxels, considering the whole brain as the volume of interest, (which resulted in corrected P‐value < 0.05). Sex, age, handedness, and the order of the tasks were included as nuisances.
RESULTS
Behavioral Results
The probability of positive response (brake or risk), reaction times, and performance indices (d′ and c) were submitted to paired t‐test analyses with Task (urgent vs. evaluative) as within‐subject factor. Kolmogorov‐Smirnov and Levene's tests were used to test for normality and homogeneity of variances for each dependent variable. The significance level was set at 0.05 for all statistical decisions.
Averages of the probability of positive response (brake or risk) showed a main effect of Task, t(56) = 6.93, P < 0.0001. The probability of braking (0.50) was higher than the probability of evaluating risk (0.42). Reaction times analysis also showed a main effect of Task, t(56) = 6.06, P < 0.0001. Reaction times were shorter in the urgent task (969 ms) than in the evaluative task (1047 ms) (Fig. 2).
Figure 2.
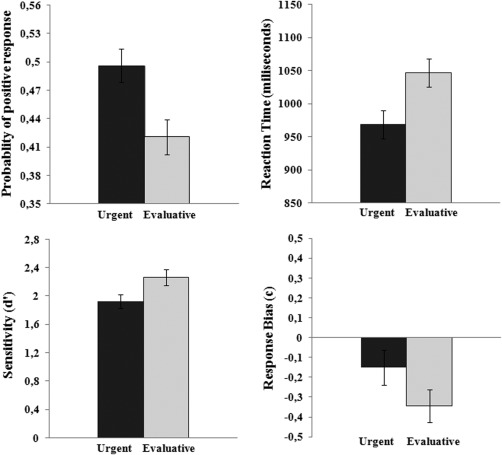
Top panels: average probability of positive response (to brake or to evaluate risk) and reaction times for each task. Bottom panels: average sensitivity index (d′) and response bias (c) for each task.
Significant effects of Task were observed for both the discrimination index (d′) and the response bias (c): t(56) = 2.44, P = 0.0176, and t(56) = 3.48, P = 0.0009, respectively. Risk discrimination was lower in the urgent task (1.92) than in the evaluative task (2.26); there was a response bias toward more cautious responses in the urgent task (−0.15) than in evaluative task (−0.35) (Fig. 2).
Imaging Results
Brain areas in which there was a significant modulation of BOLD signal by Task (urgent vs. evaluative) are displayed in Table 1 (significance threshold: P < 0.001; extended threshold: k > 132 voxels).
Table 1.
Regions belonging to significant clusters (P < 0.0001) with a cluster size of more than 132 contiguous voxels for task variable (urgent vs. evaluative)
| Region | Hemisphere | MNI‐coordinates | T | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Urgent > Evaluative | |||||
| Cluster 1 (k = 1263) | |||||
| Postcentral Gyrus (BA4) | R | 42 | −19 | 46 | 6.25 |
| Superior Temporal Gyrus (BA 22) | R | 45 | −19 | 4 | 5.70 |
| Precentral Gyrus | R | 54 | −4 | 16 | 5.52 |
| Inferior Frontal Gyrus | R | 57 | 5 | 25 | 5.42 |
| Superior Temporal Gyrus | R | 57 | −7 | 4 | 5.31 |
| Precentral Gyrus (BA 6) | R | 57 | −7 | 31 | 4.80 |
| Subgyral (frontal lobe) | R | 21 | −13 | 52 | 4.52 |
| Medial Frontal Gyrus (BA 6)Supp. motor area | R | 12 | −16 | 55 | 4.11 |
| Superior Temporal Gyrus | R | 39 | −34 | 16 | 3.76 |
| Cluster 2 (k = 1809) | |||||
| Precentral Gyrus | L | −51 | −1 | 25 | 5.91 |
| Postcentral Gyrus (BA 43) | L | −54 | −7 | 16 | 5.79 |
| Superior Temporal Gyrus | L | −54 | −13 | 7 | 5.54 |
| Sub‐Gyral (frontal lobe) | L | −18 | −19 | 46 | 5.06 |
| Medial Frontal GyrusSupp_Motor_Area_L | L | −12 | −19 | 52 | 4.99 |
| Precentral Gyrus (BA4) | L | −15 | −31 | 61 | 4.99 |
| Medial Frontal Gyrus | L | −12 | 20 | 52 | 4.94 |
| Cingulate Gyrus | L | −15 | −34 | 25 | 4.76 |
| Superior Frontal Gyrus (BA 9) | L | −12 | 50 | 28 | 4.44 |
| Cluster 3 (k = 246) | |||||
| Superior Frontal Gyrus | R | 12 | 29 | 52 | 4.32 |
| Medial Frontal Gyrus | R | 18 | 32 | 34 | 4.10 |
| SubGyral (frontal lobe) | R | 27 | 14 | 37 | 3.96 |
Three significant activation clusters were observed for the contrast urgent > evaluative. The first cluster (k = 1263 voxels) peak was located in the right precentral gyrus [t(48) = 6.25, P < 0.0001, MNI coordinates x: 42, y: −19, z: 46]. This cluster encompassed several areas of the right hemisphere: inferior and medial frontal gyrus, subgyral frontal lobe, postcentral gyrus, supplementary motor area, superior temporal gyrus, and insula. The second cluster (k: 1809 voxels) peak was located in the left postcentral gyrus [t(48) = 5.91, P < 0.0001, MNI coordinates x: −51, y: −1, z: 25], and encompassed several left hemisphere areas including portions of the inferior, medial, and superior frontal gyrus, subgyral frontal lobe, precentral gyrus, supplementary motor area, middle temporal gyrus, superior temporal gyrus, insula, cingulate gyrus, anterior cingulate. The third cluster [k = 246 voxels; peak voxel: (t(48) = 4.32, P < 0.0001; MNI coordinates x: 12, y: 29, z: 52] showed higher activation in the superior and medial frontal gyrus, subgyral frontal lobe, and anterior cingulate. Finally, when an uncorrected P‐value was set up we observed a fourth cluster [k = 65, peak at: x: −9, y: −55, z: −11, t(48) = 4.33, P < 0.0001] comprising parts of the culmen and cerebellar lingual in the left anterior lobe of the cerebellum (see Fig. 3).
Figure 3.
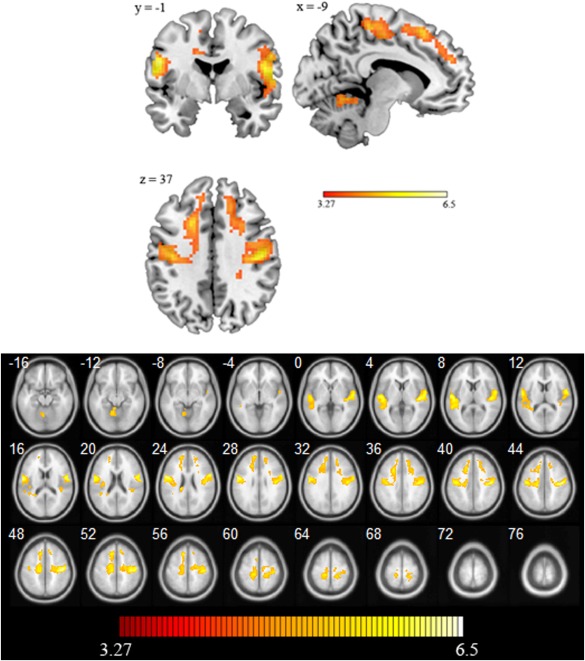
Top panel: Activation map (urgent task > evaluative task) in orthogonal projection. Bottom panel: Statistical parametric maps showing enhanced neural activity for the urgent than for the evaluative task. Numbers indicate the z coordinate. The color scale showed the range of t‐test values.
There were no significant clusters in the reverse contrast (evaluative > urgent) at the significance threshold. However, using an uncorrected P < 0.0001, a smaller cluster (k = 76 voxels) was found in the occipital lobe [t(48) = 4.33; MNI coordinates x: −9, y: −55, z: −11] including portions of the fusiform and lingual gyrus.
DISCUSSION
The main purpose of this study was to uncover the brain areas differentially involved in evaluative and urgent behaviors in risky driving. First, we replicated the behavioral findings reported by Megías et al.'s [2011]. Compared to evaluative behavior, urgent behavior showed a higher probability of positive response (brake or risk), shorter reaction times, worse sensitivity to risk, and a more cautious response bias.
This behavioral difference was related to the differential activation of a set of brain areas commonly linked to behavioral control and motor planning and performance. Our fMRI data showed an increase of activation of multiple brain regions when participants engaged in an urgent behavior in contrast to an evaluative one (urgent > evaluative contrast). Enhanced neural activity was observed bilaterally in the precentral gyrus, supplementary motor area, postcentral gyrus, superior frontal gyrus, medial frontal gyrus, inferior frontal gyrus, subgyral frontal lobe, cingulate gyrus, anterior cingulate cortex, insula, and superior temporal gyrus. In the left hemisphere there was higher activation of the middle temporal gyrus, culmen and cerebellar lingual. The evaluative > urgent contrast showed differences in the occipital lobe (fusiform gyrus and lingual gyrus).
Below, we discuss the neural mechanisms involved in urgent and evaluative behaviors in three sections: motor programming and visuomotor integration, emotional components, and involvement of the frontal lobe.
Motor programming and visuomotor integration of the response to the risk
Several neuroimaging studies investigated the neural processes involved in driving [e.g., Calhoun, 2007; Callan et al., 2009; Graydon et al., 2004; Lei, 2011; Uchiyama et al., 2003]. Some of these have compared brain activity during driving with brain activity under resting conditions or passive viewing of driving. The latter condition seems similar to our evaluative task, except that no response is required when viewing driving passively [Horikawa et al., 2005; Calhoun et al., 2002; Walter et al., 2001]. These studies demonstrated that, compared to resting periods, driving is associated with an increase of activation in the temporo‐parietal, parieto‐occipital, and cerebellum areas due to higher demands on visual and motor skills and visuomotor integration. Nevertheless, a comparison between active vs. passive driving revealed that cortical activation associated with visuomotor coordination was shared by both conditions. The differences between active and passive driving were located in the cerebellum, sensorimotor cortex, and precentral gyrus.
In addition, Spiers and Maguire [2007] examined brain activity associated with the driver's specific actions. They found that there was an increased activity in supplementary motor area, parietal, and cerebellar regions while drivers were performing both prepared actions (e.g., starting the car) and unprepared actions (e.g., braking or swerving to avoid a hazard). These findings are consistent with the results of the studies discussed above, suggesting that the cerebellum as well as the premotor areas play an important role in the execution of driving actions to generate appropriate motor outputs.
The results of our research share with this previous set of experiments the activation of motor areas and anterior parietal areas. Precentral gyrus, supplementary motor area, and cerebellum exhibited more activity in the urgent task than in evaluative task. Considering that the only difference between the tasks was the type of response required by the participants—an urgent behavioral decision (to brake) vs. an evaluative judgment (to evaluate risk)—this can suggest that there is a more active “driving” present in the context of the urgent task compared to the evaluative task. Thus, motor programming to avoid a hazard would be more activated in the urgent task, even though the participants responded by pressing the same button in both tasks. Moreover, the changes in neural activity in the postcentral gyrus and occipital lobe may reflect differences in the visuomotor integration and visual exploration of the environment. These last results are also in line with the differences in discriminability (d′) found in the behavioral data.
Emotional Components Associated with Hazard
The anterior cingulate and insula showed stronger activity for the urgent than for the evaluative task. These brain areas are commonly related to emotional processes [Damasio et al., 2000; Phan et al., 2002]. Increased activation in both cortices is associated with visceral arousal by emotive stimuli (e.g. threats or hazards) [Critchley, 2005]. Anterior cingulate and anterior insula are essential in the bottom‐up detection of salient events [Menon and Uddin, 2010]. One example is detecting deviant cues in a stream of continuous stimuli [Crottaz‐Herbette and Menon, 2006]. Therefore, when drivers suddenly confront road hazards (emotive stimuli) during their driving (which itself comprises a continuous stream of stimuli), these brain areas should show more activity (see Vlakveld, 2011]. Neuroimaging research studying hazard detection in driving is only limited; however, several studies indicated that the insula and the anterior cingulate may be associated with arousal in urgent events. For example, Spiers and Maguire [2007] showed that both brain areas were recruited in actions directed at avoiding collisions. In Callan et al.'s [2009] study, drivers had to anticipate a possible hazard when the view of oncoming traffic was occluded by a truck. Results showed a greater activation in a set of brain areas, including the insula and the anterior cingulate, when the driver had to anticipate the hazard compared to when the uncertainty of the risk had been resolved.
In our experiment, the same hazardous stimuli were displayed in both tasks (urgent and evaluative). However, the urgent rather than the evaluative task exerted stronger time pressure on respondents and entailed the possibility of suffering negative consequences if the hazard was not avoided [Megías et al., 2011]. Thus, it is logical to suggest that hazardous stimuli on the road entail a stronger emotional component in the context of urgency, which could explain the differences of activation in the insula and the anterior cingulate.
Involvement of Frontal Brain Areas in Driving
Compared to evaluative behavior, urgent behavior also increased the activity of frontal areas. Neuroscience research has established the involvement of regions of the frontal lobe in risky decision making. Ventromedial prefrontal cortex, dorsolateral prefrontal cortex, orbitofrontal cortex, and inferior and medial frontal gyrus are commonly associated with risky decision‐making [e.g., Bjork et al., 2007; Ernst et al., 2002; Minati et al., 2012; Vorhold et al., 2007]. Evidence about the functions of these frontal areas has been obtained from different contexts, but mainly using gambling tasks [Bechara et al., 1994]. It is expected that distinct risky behaviors share neural networks; however, driving is a more complex activity involving multiple cognitive functions which are absent in gambling tasks [Groeger, 2000]. Focusing on driving, Hirth et al. [2007] explored the brain areas recruited for hazard detection by displaying videos with hazardous stimuli versus uneventful driving videos. Their results showed that identification of hazards was linked to activation of the right prefrontal cortex. In another interesting study, Beeli et al. [2008] demonstrated that the external excitation of the dorsolateral prefrontal cortex (by a transcranial Direct Current Stimulation [tDCS]) led to less risky driving style. In the anodal stimulation phase participants kept at a greater distance from the car ahead, made fewer speed violations, and reduced their speed and the revolutions per minute of the car engine. A decrease of neural activation in the right lateral prefrontal cortex related to fast driving was also showed by Jancke et al. [2008] using realistic virtual driving scenarios with EEG recording. Accordingly, the prefrontal cortex plays a significant role in the control of risk‐taking and inhibitory behavior [Aron et al., 2003; Fecteau et al., 2007].
At the behavioral level, the urgent task resulted in fewer risky decisions than the evaluative task in risk evaluations (i.e., participants more often braked than evaluated the situation as risky). Based on the experiments described above, this more cautious attitude in the urgent task could be linked to the increased activation in certain frontal regions. Additionally, higher activity in the medial prefrontal cortex could also be implicated in cognitive aspects of emotional processing of hazardous stimuli or threats [Pessoa, 2009; Phan et al., 2002]. In any case, understanding of the function of the frontal lobe in urgent driving behavior needs further specific research.
Urgent and Evaluative Neural Mechanisms as a Function of the Task Features
Taken together, evaluative and urgent behaviors seem to depend on different neural mechanisms. Our findings show a larger involvement of emotional and motor areas in the urgent task. Moreover, we observed greater frontal activation in the urgent task; this could be related to a more cautious response bias (suggesting the participation of cognitive control in safe behavior). Overall, these results could be partially explained by the task features of the urgent task. Situations with temporal pressure, where strong emotional consequences are possible, are characteristic of risky driving situations. In such urgent contexts, a more automated process relying on heuristics and experiential appraisal (bottom‐up processing) would be more advantageous than a more demanding and slow analytical appraisal of risk (top‐down) [Kinnear et al., 2008; Slovic et al., 2007]. Thus, the limited response time to avoid negative consequences would force the participants to carry out more intuitive decisions in the urgent task than in the evaluative task. According to dual process models, intuitive decision‐making is largely based on System 1 (experiential‐affective), whereas reflective decision‐making is closer to System 2 (rational‐analytic) [Epstein, 1994; Kahneman and Frederick, 2005]. From this theoretical perspective, the differences between urgent and evaluative behavior would be in line with the distinction between both systems of processing [Megías et al., 2011].
We suggest that risky decision‐making in an urgent context is more automatic, guided by the emotions or feelings associated with the environmental stimuli (i.e., somatic markers). This process is reflected in an increased activation of the insula and the anterior cingulate cortex (areas implicated in the neural network of somatic markers [Bechara and Damasio, 2005]). The emotional component automatically triggers motor patterns to avoid a hazard. This interpretation is further supported by the neural motor programming and the lower reaction times found in the urgent task. This view of driving from an emotional approach is in line with the most current motivational theories of driving. According to these theories, the risk is processed from both an analytical (risk as analysis) and emotional (risk as feeling) point of view during driving [Fuller, 2011; Kinnear et al., 2013; Summala, 2007; Vaa, 2007]. Thus, risky decision making in urgent contexts is not only a result of deliberated reasoning mechanisms, but also relies on more automatic mechanisms (experiential‐affective system or somatic markers), led by emotional warning signals [Vorhold et al., 2007].
Finally, there are some controversial aspects worth mentioning. More automatic processing (e.g., in well practiced behaviors) is generally associated with decreased brain activity [Garavan et al., 2000; Jansma et al., 2001]. Our results seem to contradict this idea. However, recent research on the automaticity of higher cognitive processes [Bargh et al., 2012] has shown that automatic nonconscious behaviors requested executive processes similarly to conscious process [Dijksterhuis and Aarts, 2010, for review). In this vein, Marien et al. [2012] demonstrated in a series of six experiments that unconscious goals affect executive functions, as they interfere with working memory performance, and error detection, especially important and valuable goals. Importantly, the results of Marien et al.'s study indicated that interference produced by nonconscious goals was similar to that of conscious ones. Activation of executive areas, including right dorsolateral and ventrolateral prefrontal cortex and left inferior operculum have been observed in unconscious decision‐making [Creswell et al., 2013]. It seems plausible that braking in a risky traffic situation, like other reactive behaviors [Braver, 2012, Stuphorn and Emeric, 2012], may exhibit a greater level of activity in extended regions of the lateral and medial prefrontal cortex. Moreover, urgent tasks, which are characterized by strong time pressure and potential negative consequences, create a suitable context for the experiential‐affective system to control behavior. The change of mindset generated by the instructions of each task [Megías et al., 2011] may include differences in the associated emotional component to the hazard, and in motor processes related to the simulated brake response.
It could also be argued that the increase of activation observed predominantly in the urgent > evaluative contrast could reflect the extent to which participants actively performed the tasks, and that both tasks involve almost the same brain areas. To remove the effects of changes in global brain activation between conditions, scaling global normalization was performed for both the urgent and the evaluative task. This normalization scales the intensity for each voxel of each scan to the global mean of that scan [Ashburner et al., 2012]. A t‐test analysis was conducted to determine significant activations compared to the implicit baseline in each task (with the purpose of ruling out an effect of global brain activation the statistical criterion was set at P < 0.05), see Figure 4: top panel. Conjunction analysis of the significant activations for the urgent and the evaluative task (compared to the baseline) showed shared significant activity in the superior temporal gyrus, precentral gyrus, and postcentral gyrus, see Figure 4: bottom panel. Activations of the two tasks compared to baseline were different at whole brain level, resulting in only a partial overlap. Therefore, it does not seem plausible that the observed differences between the urgent and the evaluative task merely reflect greater overall brain activation in the urgent task. However, this issue remains an open question to be investigated by further research.
Figure 4.
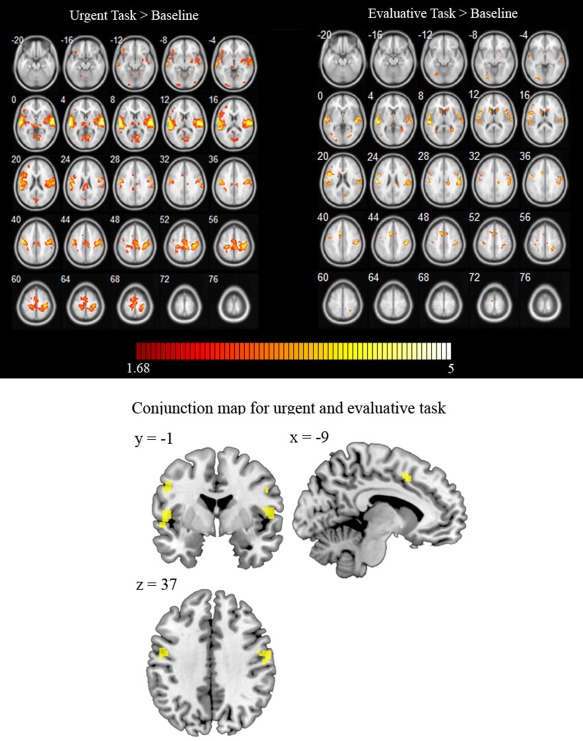
Top panel: Activation map for the “urgent task > baseline” and the “evaluative task > baseline” contrasts in orthogonal projection. Bottom panel: Brain regions with common activation for both the urgent and the evaluative task compared to the implicit baseline.
Future research should investigate in greater detail the circuits which urgent and evaluative behaviors rely upon and their interaction with the level of risk with designs suitable for this purpose. Further studies investigating whether both tasks share a similar functional anatomical representation differing in the level of activation or whether they are associated with different underlying neuronal networks would help to address some limitations of this research.
CONCLUSION
This research aimed to investigate the brain‐level mechanisms underlying urgent and evaluative behavior in driving. Our findings showed a dissociation of urgent and evaluative behavior both at the behavioral and at the neural level. Our results support a more automatic processing of risk in urgent tasks, based on previous experience and guided mainly by an emotional component. Compared to the evaluative task, the urgent task is characterized by more time pressure and the possibility for negative consequences [Megías et al., 2011]. These task features create a suitable context for the experiential‐affective system to guide the decision‐making process. The findings of this research are relevant for the study of dual process models, especially because of their greater proximity to every‐day activities in comparison to previous neuroimaging studies about risky decision‐making. Finally, they offer support for models of driving behavior that consider emotion as a fundamental mechanism in driving decision‐making.
Supporting information
Supplementary Information
ACKNOWLEDGMENTS
The authors thank the anonymous reviewers for their help in improving the manuscript quality.
REFERENCES
- J Ashburner, G Barnes, C Chen, J Daunizeau, G Flandin, K Friston., S Kiebel, J Kilner, V Litvak, R Moran, W Penny, K Stephan, D Gitelman, R Henson, C Hutton, V Glauche, H Mattout, C Phillips (2012): SPM8 manual. London: Functional Imaging Laboratory, Institute of Neurology. [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW (2003): Stop‐signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci 6:115–116. [DOI] [PubMed] [Google Scholar]
- Bargh JA, Schwader KL, Haile SE, Dyer RL, Boothby EJ (2012): Automaticity in social‐cognitive processes. Trends Cogn Sci 16:593–605. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR (2005): The somatic marker hypothesis: A neural theory of ‐economic decision. Games Econ Behav 52:336–372. [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson, SW (1994): Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 50:7–15. [DOI] [PubMed] [Google Scholar]
- Beeli G, Koeneke S, Gasser, K , Jancke L (2008): Brain stimulation modulates driving behavior. Behav Brain Functions 4:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Danube CL, Hommer DW (2007): Developmental differences in posterior mesofrontal cortex recruitment by risky rewards. J Neurosci 27:4839–4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS (2012): The variable nature of cognitive control: A dual mechanisms framework. Trends Cogn Sci 16:106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD (2007): Functional magnetic resonance imaging (fMRI): Advanced methods and applications to driving In: Parasuraman R, Rizzo M, editors. Neuroergonomics: The Brain at Work. New York: Oxford University Press; pp 51–64. [Google Scholar]
- Calhoun VD, Pekar JJ, McGinty VB, Adali T, Watson TD, Godfrey GD (2002): Different activation dynamics in multiple neural systems during simulated driving. Human Brain Mapp 16:158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callan AM, Osu R, Yamagishi Y, Callan DE, Inoue, N (2009): Neural correlates of resolving uncertainty in driver's decision making. Human Brain Mapp 30:2804–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell JD, Bursley JK, Satpute AB (2013): Neural reactivation links unconscious thought to decision making performance. Soc Cogn Affective Neurosci 8:863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD (2005): Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol 493:154–166. [DOI] [PubMed] [Google Scholar]
- Crottaz‐Herbette S, Menon V (2006): Where and when the anterior cingulate cortex modulates attentional response: Combined fMRI and ERP evidence. J Cogn Neurosci 18:766–780. [DOI] [PubMed] [Google Scholar]
- Damasio AR. (1994): Descartes error: Emotion, reason, and the human brain. New York: Avon. [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LLB, Parvizi J, Hichwa RD (2000): Subcortical and cortical brain activity during the feeling of self‐generated emotions. Nat Neurosci 3:1049–1056. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Shadmehr R (2005): Detecting and adjusting for artifacts in fMRI time series data. Neuroimage 27:624–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijksterhuis A, Aarts H. (2010): Goals, attention, and (un) consciousness. Ann Rev Psychol 61:467–490. [DOI] [PubMed] [Google Scholar]
- M Ernst, K Bolla, M Mouratidis, C Contoreggi, JA Matochik, V Kurian, JL Cadet, AS Kimes, ED London (2002): Decision‐making in a risk‐taking task: A PET study. Neuropsychopharmacology 26:682–691. [DOI] [PubMed] [Google Scholar]
- Epstein S (1994): Integration of the cognitive and psychodynamic unconscious. Am Psychol 49:709–24. [DOI] [PubMed] [Google Scholar]
- Evans J (2008): Dual‐processing accounts of reasoning, judgment and social cognition. Ann Rev Psychol 59:255–278. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Knoch D, Fregni F, Sultani N, Boggio P, Pascual‐Leone A (2007): Diminishing risk‐taking behavior by modulating activity in the prefrontal cortex: A direct current stimulation study. J Neurosci 27:12500–12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R (2011): Driver control theory: From task difficulty homeostasis to risk allostasis In: Porter BE, editor. Handbook of Traffic Psychology. Amsterdam: Elsevier; pp 13–26. [Google Scholar]
- Garavan H, Kelley D, Rosen A, Rao SM, Stein, EA (2000): Practice‐related functional activation changes in a working memory task. Microscopy Res Technol 51:54–63. [DOI] [PubMed] [Google Scholar]
- Graydon FX, Young R, Benton MD, Genik RJ, Posse S, Hsieh L, Green CF (2004): Visual event detection during simulated driving: Identifying the neural correlates with functional neuroimaging, Transport Res Part F: Psychol Behav 7:271–286. [Google Scholar]
- Groeger J. 2000. Understanding Driving: Applying Cognitive Psychology to a Complex Everyday Task. New York: Psychology Press. [Google Scholar]
- Hirth VA, Davis B, Fridriksson J, Rorden C, Bonilha L (2007): Cognitive performance and neural correlates of detecting driving hazards in healthy older adults. Dementia Geriatric Cogn Disorders 24:335–342. [DOI] [PubMed] [Google Scholar]
- E Horikawa, N Okamura, M Tashiro, Y Sakurada, M Maruyama, H Arai, K Yamaguchi, H Sasaki, K Yanai, M Itoh (2005): The neural correlates of driving performance identified using positron emission tomography. Brain Cogn 58:166–171. [DOI] [PubMed] [Google Scholar]
- Jansma JM, Ramsey NF, Slagter HA, Kahn RS (2001): Functional anatomical correlates of controlled and automatic processing. J Cogn Neurosci 13:730–743. [DOI] [PubMed] [Google Scholar]
- Jancke L, Brunner B, Esslen M (2008): Brain activation during fast driving in a driving simulator: The role of the lateral prefrontal cortex. Neuroreport 19:1127–1130. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Frederick S (2005): A model of heuristic judgment In: Holyoak KJ. Morrison RG, editors. The Cambridge Handbook of Thinking and Reasoning. New York: Cambridge University Press; pp 267–293. [Google Scholar]
- Keren G, Schul Y (2009): Two is not always better than one: A critical evaluation of two‐system theories. Perspect Psychol Sci 4:533–550. [DOI] [PubMed] [Google Scholar]
- N Kinnear, S Stradling, C McVey (2008): Do we really drive by the seat of our pants? In: Dorn L, editor. Driver behaviour and training. Aldershot, UK: Ashgate; pp 349–365. [Google Scholar]
- Kinnear N, Kelly SW, Stradling S, Thomson, J (2013): Understanding how drivers learn to anticipate risk on the road: A laboratory experiment of affective anticipation of road hazards. Accident Anal Prev 50:1025–1033. [DOI] [PubMed] [Google Scholar]
- Lei S (2011): Driver mental states monitoring based on brain signals (Doctoral Thesis). Berlin: Berlin Institute of Technology. [Google Scholar]
- Lieberman MD, Gaunt R, Gilbert DT, Trope Y (2002): Reflection and reflexion: A social cognitive neuroscience approach to attributional inference In: Zanna M, editor. Advances in Experimental Social Psychology. San Diego, CA: Academic Press; pp 199–249. [Google Scholar]
- Liu TT (2004): Efficiency, power, and entropy in event‐related fMRI with multiple trial types: Part II: Design of experiments. NeuroImage 21:401–413. [DOI] [PubMed] [Google Scholar]
- Loewenstein G, Weber E, Hsee C, Welch S (2001): Risk as feelings. Psychol Bull 127:267–286. [DOI] [PubMed] [Google Scholar]
- Marien H, Custers R, Hassin RR, Aarts H (2012): Unconscious goal activation and the hijacking of the executive function. J Personality Soc Psychol 103:399–415. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman, CD (2005): Detection Theory: A User's Guide. (2nd Ed.). Mahwah, NJ: Erlbaum. [Google Scholar]
- Megías A, López‐Riañez M, Cándido A (2013): Conductas urgentes y evaluativas en función del nivel de riesgo en situaciones de conducción. Anales de Psicología 29:1032–1037. [Google Scholar]
- Megías A, Maldonado A, Cándido A, Catena A (2011): Emotional modulation of urgent and evaluative behaviors in risky driving scenarios. Accident Anal Prevent 43:813–817. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin L (2010): Saliency, switching, attention and control: A network model of insula function. Brain Struct Function 214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minati L, Grisoli M, Seth AK, Critchley HD (2012): Decision‐making under risk: A graph‐based network analysis using functional MRI. NeuroImage 60:2191–2205. [DOI] [PubMed] [Google Scholar]
- Pessoa L (2009): How do emotion and motivation direct executive control? Trends Cogn Sci 13:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I (2002): Functional neuroanatomy of emotion: A meta‐analysis of emotion activation studies in PET and fMRI. Neuroimage 16:331–348. [DOI] [PubMed] [Google Scholar]
- Sloman SA (1996): The empirical case for two systems of reasoning. Psychol Bull 119:3–22. [Google Scholar]
- Slovic P, Finucane M, Peters E, MacGregor D (2007): The affect heuristic. Eur J Oper Res 177:1333–1352. [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A (2002): E‐Prime reference guide. Pittsburgh: Psychology Software Tools. [Google Scholar]
- Spiers HJ, Maguire EA (2007): Neural substrates of driving behaviour. Neuroimage 36:245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuphorn V, Emeric E (2012): Proactive and reactive control by the medial frontal cortex. Front Neuroeng 5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summala H (2007): Towards understanding motivational and emotional factors in driver behaviour: Comfort through satisfaction. In Cacciabue PC, editor. Modelling Driver Behaviour in Automotive Environments: Critical Issues in Driver Interactions with Intelligent Transport Systems. London: Springer‐Verlag; pp 189–207. [Google Scholar]
- Uchiyama Y, Ebe K, Kozato A, Okada T, Sadato N (2003): The neural substrates of driving at a safe distance: A functional MRI study. Neurosci Lett 352:199–202. [DOI] [PubMed] [Google Scholar]
- Vaa T (2007): Modelling driver behaviour on basis of emotions and feelings: Intelligent transport systems and behavioural adaptations. In: Cacciabue P, editor. Modelling Driver Behaviour in Automotive Environments: Critical Issues in Driver Interactions with Intelligent Transport Systems. London: Springer‐Verlag; pp 208–232. [Google Scholar]
- Vlakveld WP (2011): Hazard anticipation of young novice drivers (Doctoral Dissertation). Leidschendam, The Netherlands: SWOV Institute for Road Safety Research. [Google Scholar]
- Vorhold V, Giessing C, Wiedemann PM, Schütz H, Gauggel S, Fink GR (2007): The neural basis of risk ratings: Evidence from a functional magnetic resonance imaging (fMRI) study. Neuropsychologia 45:3242–3250. [DOI] [PubMed] [Google Scholar]
- Walter H, Vetter SC, Grothe J, Wunderlich AP, Hahn S, Spitzer M (2001): The neural correlates of driving. Neuroreport 12:1763–1767. [DOI] [PubMed] [Google Scholar]
- Ward BD. 2000. Simultaneous Inference for fMRI Data. Milwaukee, Wisconsin: Medical College of Wisconsin. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


