Abstract
Pharmacological and anatomical evidence implicates striatal dopamine receptors in Tourette syndrome (TS). Nevertheless, results of positron emission tomography (PET) studies of the dopamine system in TS have been inconsistent. We investigated striatal D2/3 dopamine receptors in TS using the radioligands [11C]raclopride and [11C]‐(+)‐PHNO, an agonist that binds preferentially to D3 receptors, thus allowing higher sensitivity and measurement of receptors in a high affinity state. Eleven adults with TS and 11 matched healthy control (HC) participants underwent [11C]raclopride and [11C]‐(+)‐PHNO PET scans. General linear model was used for voxelwise contrasts of striatal binding potentials (BPND) between TS and HC participants. Analysis of variance was performed to investigate main effect of radioligand. In addition, BPND values were extracted for ventral, motor, and associative striatum. Finally, we examined the relationship between BPND measures and symptom severity in TS participants. Main effects analyses showed that [11C]‐(+)‐PHNO BPND was higher in ventral striatum, whereas [11C]raclopride BPND was higher in motor and associative striatum. There were no significant group differences between TS and HC. Furthermore, TS and HC participants had similar [11C]‐(+)‐PHNO and [11C]raclopride BPND in the three striatal subregions. Moreover, there was no significant correlation between BPND and symptom severity. TS and HC participants had similar striatal D2/3 receptor availability measures. These results challenge the assumption that striatal dopamine receptors have a major role in the pathophysiology of TS. Consistent with previous findings, [11C]‐(+)‐PHNO localized preferentially to ventral striatal, D3 receptor‐rich regions, in contrast to [11C]raclopride, which localized preferentially in the dorsal striatum. Hum Brain Mapp 36:2592–2601, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: basal ganglia; corpus striatum; neostriatum; dopamine; receptors, dopamine; receptors, dopamine D2; positron‐emission tomography; raclopride; Tourette syndrome
INTRODUCTION
Tourette syndrome (TS) is a developmental neuropsychiatric disorder defined by motor and vocal tics, with an estimated prevalence of up to 1% (Abi‐Jaoude et al., 2009; McNaught and Mink, 2011). Common comorbidities include obsessive‐compulsive disorder (OCD) and attention deficit/hyperactivity disorder (ADHD). There has been much interest in the role of dopamine in TS (Buse et al., 2013; Rickards, 2009; Segura and Strafella, 2013). Over half a century ago, high potency dopamine D2 receptor (D2R) blockers were found to be effective in reducing tics (Abi‐Jaoude et al., 2009), and these remain as the agents with the most evidence for efficacy in the pharmacological treatment of tics (Pringsheim et al., 2012). Further, cerebrospinal fluid analysis and human postmortem studies have implicated the dopamine system in TS (Buse et al., 2013). In addition, striatal dopamine is known to play a role in habit formation (Graybiel, 2008), and in animal models of TS (Macrì et al., 2013). Finally, dopamine is involved in other movement disorders, such as Parkinson's Disease and Huntingon's Chorea (Buse et al., 2013). It is thus not surprising that the first and most widely studied ligands in receptor nuclear imaging studies in TS have targeted the dopamine system (Rickards, 2009).
However, human positron emission tomography (PET) studies in TS patients have been far from conclusive (Buse et al., 2013; Rickards, 2009; Segura and Strafella, 2013; Steeves et al., 2010). Such studies have investigated striatal dopamine innervation, dopamine release, presynaptic, and postsynaptic dopamine receptors, but these have yielded inconsistent results. Striatal dopamine receptors are of particular interest, based on their role in habit formation, as well as evidence implicating the striatum in TS (Ganos et al., 2013).
Four single‐photon emission computed tomography (SPECT) studies have investigated striatal D2Rs in TS using the D2R antagonist [123I]iodobenzamide ([123I]‐IBZM). In the first of these, investigators found decreased ligand binding in the basal ganglia of the seven medicated but no difference in the eight unmedicated TS subjects in comparison to six controls (George et al., 1994). These findings are consistent with those from another [123I]‐IBZM SPECT study which found reduced striatal ligand binding in the seven medicated compared with the ten unmedicated patients and to the seven healthy controls (HCs), but no difference between the unmedicated patients and the controls (Müller‐Vahl et al., 2000). The most recent TS report using [123I]‐IBZM found no difference in ligand uptake between TS patients and controls (Hwang et al., 2008). Finally, an interesting [123I]‐IBZM SPECT investigation in five monozygotic twin pairs with TS found higher binding in the caudate of the more severely affected twin; further, the within pair difference in binding correlated positively with within pair differences in tic severity (Wolf et al., 1996).
Several studies investigating striatal dopamine receptors in TS have used PET imaging, which has higher spatial resolution compared with SPECT. In most of these studies, subjects were medication free when they were scanned. An early small PET study using the D2 and D3 receptor antagonist [11C]raclopride in five adult patients with TS found no differences in comparison with HCs (Turjanski et al., 1994). A larger study of 29 subjects focusing specifically on the caudate and using the D2R antagonist [11C]methylspiperone did not find differences compared with controls (Wong et al., 1997). A subsequent study with [11C]raclopride in seven TS patients again showed no baseline difference in D2/D3 striatal receptor availability (Singer et al., 2002). A more recent study investigating various neurotransmitter measures found no baseline differences in [11C]raclopride binding potential (BP) between the 12 TS subjects and 3 HCs with complete data (Wong et al., 2008). Interestingly, using high‐ and low‐specific activity [11C]raclopride scans, the investigators estimated D2R affinity to be higher in the anterior putamen in TS subjects relative to the controls. Of note, dopamine receptor “supersensitivity” has been hypothesized to play a role in TS in a biochemical study over 30 years ago (Singer et al., 1982). Finally, in the most recent PET study of its kind, using the radioligand [11C]raclopride, Denys et al. found lower D2/D3 striatal receptor availability in the putamen of 12 TS participants compared with 12 HCs (Denys et al., 2013).
Altogether, the findings regarding striatal dopamine receptors in TS have been inconsistent. Moreover, the literature has been characterized by several limitations, including small sample sizes, confounders such as age differences between comparison groups, medication effects, and low spatial resolution in the case of SPECT studies. Furthermore, while dopamine receptor “supersensitivity” has been hypothesized as an underlying pathophysiological mechanism in TS (Buse et al., 2013; Segura and Strafella, 2013; Singer et al., 1982), only one group has actually investigated this question (Wong et al., 2008).
In this article, we report findings from our investigation of striatal dopamine receptors in TS using two different ligands, the D2/3 receptor antagonist [11C]raclopride, as well as [11C]‐(+)‐Propyl‐Hexahydro‐Naphtho‐Oxazin ([11C]‐(+)‐PHNO), an agonist with preferential binding to D3 dopamine receptors. This unique binding profile allows the evaluation of differences in D2 versus D3 receptors, which would not be possible with [11C]raclopride alone. Furthermore, because [11C]‐(+)‐PHNO is an agonist, it can permit the measurement of dopamine receptors in their high affinity state, thus providing an opportunity to interrogate whether striatal dopamine receptor affinity is involved in the pathophysiology of TS (Sibley, De Lean, and Creese, 1982; Ginovart et al., 2006; Willeit et al., 2006). To our knowledge, this is the first study using the [11C]‐(+)‐PHNO ligand in TS.
MATERIALS AND METHODS
Participants
A total of 22 adult subjects, 11 with TS and 11 matched HCs participated in the study. The participants with TS were recruited through the TS Neurodevelopmental Clinic at the Toronto Western Hospital, Toronto, Canada. HC participants were recruited through postings and web advertisements. The groups were matched for age and sex. The group mean age and standard deviation was 34.0 ± 7.9 for the HC, and 32.2 ± 10.1 for the TS subjects.
The subject assessments, neuroimaging scans and data analysis were performed at the PET Center, Research Imaging Center at the Center for Addiction and Mental Health, Toronto, Canada. The study was approved by the relevant institutional research ethics boards. The study was conducted in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and the standards established by the relevant Institutional Review Board and granting agencies. All participants received financial reimbursement. After complete description of the study to the subjects, written informed consent was obtained from all study participants prior to any procedures.
Clinical Measures
Subjects underwent a neuropsychiatric assessment by a psychiatrist experienced in TS (EA‐J). Diagnoses of TS and other comorbidities including OCD and ADHD were made according to criteria in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision (DSM‐IV‐TR; American Psychiatric Association, 2000). Tic severity scores were measured with the Yale Global Tic Severity Scale‐Total Tic Score (YGTSS‐TTS; Leckman et al., 1989), and obsessive‐compulsive symptoms were measured using the Yale‐Brown Obsessive Compulsive Scale (Y‐BOCS; Goodman et al., 1989a,b). A detailed medication history was obtained from patient interview and chart review.
Image Acquisition
Each study subject underwent two PET scans on separate days, and one magnetic resonance imaging (MRI) scan. PET scans were performed with a high‐resolution PET/CT, Siemens‐Biograph HiRez XVI (Siemens Molecular Imaging, Knoxville, TN) operating in three‐dimensional (3D) mode with an intrinsic in‐plane resolution of ∼4.6 mm full width at half‐maximum (FWHM). To minimize head motion, subjects were fitted with a custom‐made thermoplastic facemask that was secured to the scanner platform (Tru‐Scan Imaging, Annapolis). Prior to each emission scan, a scout view was used to verify accurate subject head positioning, and a low dose (0.2 mSv) CT scan was acquired to correct for attenuation.
Radioligands were injected into the left antecubital vein. [11C]raclopride (mean ± standard deviation; mean dose 10.0 ± 0.7 mCi; specific activity 1,924 ± 582 mCi/µmol; mass 2.0 ± 0.6 µg) emission data were acquired over 60 min and subsequently redefined into 28 frames of progressively increasing duration (five 1‐min frames, 20 2‐min frames, and three 5‐min frames). [11C]‐(+)‐PHNO (mean dose 9.6 ± 1.4 mCi; specific activity 1,286 ± 388 mCi/µmol; mass 2.0 ± 0.4 µg) emission data were acquired over 90 min and subsequently redefined into 30 frames of progressively increasing duration (fifteen 1‐min frames and fifteen 5‐min frames). The radiosynthesis of [11C]‐(+)‐PHNO has been described in detail elsewhere (Wilson et al., 2005). For each 3D sinogram, data was normalized for attenuation and scatter corrected before applying fourier rebinning to convert the 3D sinograms into two‐dimensional (2D) sinograms. The 2D sinograms were then reconstructed into image space using a 2D filtered back projection algorithm, with a ramp filter at Nyquist cut‐off frequency. After reconstruction, a Gaussian filter with a 5 mm FWHM was applied.
MRI scans were done to rule out structural brain abnormalities and to provide anatomical reference for the image analyses. A T1‐weighted MRI image was obtained for each subject using a high‐resolution MRI (GE Discovery MR750 3T, T1‐weighted images, FSPGR with repletion time = 6.7 ms, echo time = 3.0 ms, flip angle = 8 mm, slice thickness = 1 mm, NEX = 1, matrix size = 256 × 192).
Image Analysis
PET imaging analysis was performed in MATLAB version 7.4 (Mathworks, Natick, MA) using an in‐house image analysis platform (Gunn et al., 1997; Lammertsma and Hume, 1996). After frame realignment for motion correction in SPM2 (Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm), motion‐corrected PET frames were summed, coregistered to the corresponding MRI and transformed into MNI standardized stereotaxic space (Collins et al., 1994) using the transformation parameters of the individual structural MRIs. Voxelwise nondisplaceable parametric binding potentials (BPND) were calculated using a simplified reference tissue (cerebellum) method (Gunn et al., 1997). Subsequent to calculation of BPND, parametric BPND images were smoothed in SPM8 (Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm) with a Gaussian function at 4 mm FWHM. Statistical parametric analysis was performed in SPM8 to obtain voxelwise general linear model contrasts comparing striatal BPND between the TS and HC groups for [11C]raclopride and [11C]‐(+)‐PHNO. In addition, a 2 × 2 repeated measures analysis of variance (ANOVA), with radioligand ([11C]raclopride and [11C]‐(+)‐PHNO) and group (HC and TS) included as factors was performed to obtain a main effect of radioligand. Statistical map thresholds were set at P < 0.05, familywise error‐corrected, with an extent threshold k = 5 voxels. Furthermore, we conducted region of interest (ROI) analysis using probabilistic ROI masks, created manually as previously described (Martinez et al., 2003; Mawlawi et al., 2001), using a histological‐based basal ganglia brain atlas (Chakravarty et al., 2006). BPND values were extracted for ventral, motor, and associative striatum for each subject with the MarsBaR ROI toolbox (Brett et al., 2002). We then used SPSS (Version 16.0) to compare the extracted BP values between HC and TS for each of the striatal regions, using 2‐tailed student t‐tests and a P‐value threshold of 0.05. Moreover, Pearson correlation coefficients were calculated between [11C]raclopride and [11C]‐(+)‐PHNO BPND on the one hand, and YGTSS‐TTS and Y‐BOCS severity rating total scores on the other. Mean BP images were visualized using MRIcro (Rorden and Brett, 2000), and BPND voxelwise contrasts were visualized using the xjView toolbox (http://www.alivelearn.net/xjview).
RESULTS
Demographic and Clinical Characteristics
Table 1 shows demographic and clinical characteristics of TS study participants. Six of the TS subjects had no comorbidities. Three TS subjects had OCD, four had ADHD, and two had a past history of substance abuse. Of the three patients with comorbid OCD, one was female, two had a past history of substance abuse, and their mean age 31.7 years (±5.5), which was not significantly different from that of the rest of the TS participants, or the HC group. Based on YGTSS‐TTS, tic symptoms ranged from mild to severe. With the exception of one TS subject who had started a low dose of clonidine one month prior to participating, none of the subjects were on psychotropic medication when they took part in the study. Three patients had been on medication up to 3 months prior to their participation in the study: dextroamphetamine/amphetamine for 3 months (discontinued 3 months prior to scanning); clonidine for 1 year (discontinued 3 months prior to scanning); SSRI for 3 weeks (discontinued 4 months prior to scanning). For three other patients that had been on psychotropic medication, these had been discontinued at least 6 months prior: antipsychotic for 1 month (discontinued 6 months prior to scanning); antipsychotic (discontinued 10 months prior to scanning); remote brief trial of methylphenidate (16 years prior to the study). Four of the 11 TS participants were medication naïve. Further, six of the TS participants were naïve to dopaminergic medication, seven if the participant with a brief remote trial of methylphenidate is included. As detailed above, one patient had been on dextroamphetamine/amphetamine up to 3 month prior to scanning, and three had been on dopamine antagonist and/or agonist medication at least 6 months prior to scanning (see Table 1 for further details). The mean age of participants in the TS group was 32.2 years (standard deviation 10.1), and in the HC group 34.0 years (7.9). There were two females and nine males in each of the TS and HC groups. The HC and TS groups each received similar amounts of radioligand for the [11C]raclopride (mean ± standard deviation; HC: mean dose 10.1 ± 0.5 mCi, specific activity 1,843 ± 480 mCi/µmol, mass 2.0 ± 0.6 µg; TS: mean dose 9.9 ± 0.9 mCi, specific activity 2,004 ± 684 mCi/µmol, mass 1.9 ± 0.6 µg; P > 0.5) and for the [11C]‐(+)‐PHNO (HC: mean dose 9.4 ±1.5 mCi, specific activity 1,262 ± 373 mCi/µmol, mass 2.0 ± 0.4 µg; TS: mean dose 9.7 ± 1.5 mCi, specific activity 1,310 ± 419 mCi/µmol, mass 2.0 ± 0.5 µg; P > 0.5) scans.
Table 1.
Demographic and clinical characteristics for TS participants
| Participant number | Age | Sex | Education | Comorbid diagnoses | YGTSS‐TTS | Y‐BOCS | Medications at time of scan | Past medications (listed in reverse chronological order) |
|---|---|---|---|---|---|---|---|---|
| 1 | 28 | M | College | None | 30 | 12 | Nil | 6 months prior to scan, 1 month trial of aripiprazole 4 mg daily; >3 years prior to scan: clonazepam, ziprasidone, tetrabenazine, ropinirole, pergolide, risperidone, carbidopa/levodopa, clonidine, buproprion, donepezil, quetiapine, pimozide, haloperidol, nitrazepam |
| 2 | 43 | M | University | None, OCS | 13 | 13 | Nil | Nil |
| 3 | 29 | M | High school | OCS, ADHD | 38 | 12 | Nil | 10 months prior to scan: ziprasidone, aripiprazole, buproprion, methylphenidate, haloperidol |
| 4 | 33 | F | University | None | 8 | 0 | Nil | Nil |
| 5 | 55 | M | University | None | 26 | 6 | Nil | Nil |
| 6 | 23 | M | University | ADHD, LD | 9 | 11 | Nil | Methylphenidate brief trial at age 7 |
| 7 | 18 | M | University | None | 31 | 0 | Nil | Clonidine × 1 year, discontinued 3 months prior to scan |
| 8 | 38 | M | College | OCD, ADHD, substance abuse (past) | 9 | 19 | Clonidine | Clonidine 0.15 mg daily, started 1 month prior to scan |
| 9 | 30 | M | University | None, mild OCS | 16 | 0 | Nil | Nil |
| 10 | 29 | M | University | OCD, MDD (past), substance abuse (past) | 23 | 19 | Nil | Dextroamphetamine/amphetamine 20 mg for 3 months, discontinued 3 months prior to scan; paroxetine >2 years prior to scan |
| 11 | 28 | F | High school | OCD, ADHD, asthma, subclinical hyperthyroidism | 16 | 26 | Albuterol, fluticasone, acetaminophen/codeine | 4 months prior to scan: escitalopram 10 mg × 3 weeks; prior to this: sertraline, mirtazapine, melatonin, clonidine, methylphenidate, carbidopa/levodopa, trazodone, zopiclone, domperidone, imipramine, risperidone |
M, male; F, female; OCS, obsessive‐compulsive symptoms; ADHD, attention deficit/hyperactivity disorder; LD, learning disability; OCD, obsessive‐compulsive disorder; MDD, major depressive disorder; YGTSS‐TTS, Yale Global Tic Severity Scale‐Total Tic Score; Y‐BOCS, Yale‐Brown Obsessive Compulsive Scale.
Striatal Dopamine Receptor Radioligand Binding
Mean striatal BP images are displayed in Figure 1, which shows similar radioligand binding for the TS and HC groups. Statistical parametric maps of voxelwise contrasts comparing BPND in the TS and HC groups showed no significant voxels with either [11C]raclopride or [11C]‐(+)‐PHNO. Individual participant BPND for each of the ligands, across motor, associative and ventral subregions of the striatum are shown in Figure 2. There were no group differences in BPND in any of the striatal subregions with either radioligand, nor was there a trend in either direction. We also extracted group mean [11C]raclopride and [11C]‐(+)‐PHNO BPND values separately for right and left striatal subregions, and calculated group difference 95% confidence intervals. There were no notable trends and most confidence intervals were fairly symmetrical with respect to zero and showed good precision (Table 2). Secondary analyses comparing the three patients with comorbid OCD to the HC group did not reveal any significant differences in BPND in any of the striatal subregions with either of the radioligands. We also tested the correlations between BPND of striatal subregions and YGTSS‐TTS for [11C]raclopride (Fig. 3A) and [11C]‐(+)‐PHNO BPND (Fig. 3B) in TS participants. Although there was a positive correlation between [11C]raclopride BPND and YGTSS‐TTS in the ventral striatum (r = 0.62, P‐value = 0.044), this would not survive a correction for multiple comparisons. Otherwise, none of the correlations were significant. Likewise, there were no significant correlations between either radioligand BPND and Y‐BOCS severity rating total scores (data not shown). Radioligand main effects analysis from the ANOVA showed that [11C]‐(+)‐PHNO BPND was higher in ventral striatum, whereas [11C]raclopride BPND was higher in motor and associative striatum (Fig. 4; see also Fig. 2).
Figure 1.
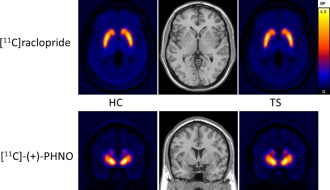
HC (N = 11) and TS (N = 11) group [11C]raclopride (transverse view) and [11C]‐(+)‐PHNO (coronal view) mean BP images. HC, healthy controls; TS, Tourette syndrome; BP, binding potential.
Figure 2.
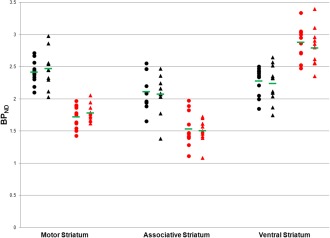
Individual participant and group average (green horizontal bar) [11C]raclopride (black) and [11C]‐(+)‐PHNO (red) BPND for HC (circles, N = 11) and TS (triangles, N = 11) across striatal subregions. BPND, Nondisplaceable binding potential; HC, healthy control; TS, Tourette syndrome.
Table 2.
[11C]raclopride and [11C]‐(+)‐PHNO BPND for TS and HC subjects, with group difference 95% confidence intervals across striatum subregions
| [11C]Raclopride | [11C]‐(+)‐PHNO | ||||
|---|---|---|---|---|---|
| HC N = 11 | TS N = 11 | HC N = 11 | TS N = 11 | ||
| Motor striatum | Left | 2.35 | 2.40 | 1.71 | 1.77 |
| (−0.26, 0.16) | (−0.21, 0.08) | ||||
| Right | 2.48 | 2.54 | 1.72 | 1.79 | |
| (−0.31, 0.19) | (−0.23, 0.10) | ||||
| Associative striatum | Left | 2.18 | 2.10 | 1.61 | 1.57 |
| (−0.17, 0.34) | (−0.16, 0.25) | ||||
| Right | 2.04 | 2.04 | 1.45 | 1.45 | |
| (−0.27, 0.26) | (−0.20, 0.21) | ||||
| Ventral striatum | Left | 2.24 | 2.16 | 2.96 | 2.90 |
| (−0.16, 0.32) | (−0.19, 0.32) | ||||
| Right | 2.31 | 2.31 | 2.81 | 2.69 | |
| (−0.23, 0.24) | (−0.14, 0.38) | ||||
BPND, nondisplaceable binding potential; HC, healthy control; TS, Tourette syndrome.
Figure 3.
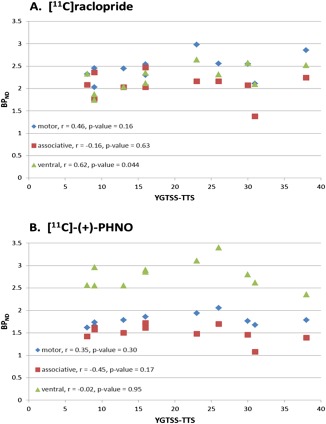
Radioligand striatal subregion BPND correlations with YGTSS‐TTS. BPND, Nondisplaceable binding potential; YGTSS‐TTS, Yale Global Tic Severity Scale‐Total Tic Score.
Figure 4.
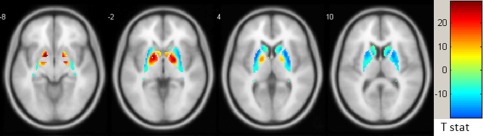
Transverse brain slices of statistical parametric map of radioligand main effect with ANOVA (2 × 2, repeated measures): Areas with higher [11C]raclopride BPND are shown in cool colors, and those with higher [11C]‐(+)‐PHNO BPND in warm colors (FWE‐corrected P‐value > 0.05). BPND, Nondisplaceable binding potential; FEW, familywise error.
DISCUSSION
This is the first study using [11C]‐(+)‐PHNO to investigate D2/3 receptors in TS. Consistent with previous findings, we showed that [11C]‐(+)‐PHNO BPND was higher in ventral striatum, and [11C]raclopride BPND was higher in motor and associative striatum. However, we did not find any differences in [11C]‐(+)‐PHNO or [11C]raclopride BPND between TS and HC groups. Further, there was no relationship between symptom severity scores and BPND for either radioligand. In addition, we found no significant differences in the three patients with comorbid OCD, though this is limited by the small number of participants in this subgroup. Our TS and HC groups were well‐matched, and most of our TS participants did not have a history of significant exposure to medications that directly influence dopamine transmission. Moreover, our TS participants are a good representation of the TS population in terms of comorbidities and range of symptom severity. These findings do not support a role for changes in striatal D2 or D3 receptor availability or affinity in the pathophysiology of TS.
A number of possible explanations should be considered in interpreting our findings. Radioligand binding in vivo is influenced by the number of available receptors, endogenous dopamine levels, and receptor affinity. It is thus conceivable that opposing processes—for example, increased dopamine receptor affinity but also increased binding competition from endogenous dopamine—may cancel out each other's effects on radioligand binding such that overall receptor availability as measured by BPND remains unchanged. As we do not have an estimate of endogenous dopamine levels, we cannot rule out this possibility with our study. Moreover, it is possible that striatal dopamine changes in TS are limited to specific micro areas that are beyond the reach of the spatial resolution of current in vivo imaging. In addition, it is likely that the pathophysiology of TS is variable across individuals, and as such, it is possible that striatal dopamine receptors are involved in only a subset of TS patients. Such a possibility cannot be tested in a reliable fashion with typical sample sizes of most PET studies, including ours. One might wonder whether increasing our sample size could result in significant differences between TS and HC groups; however, our data do not suggest any trend, in either direction. Indeed, our effect sizes were all fairly small; further, our confidence intervals were almost all symmetrical around the null (see Table 2), and were fairly narrow, comparable to our standard deviations. Our sample size of 11 HC and 11 TS participants was larger or comparable to that of other studies of striatal dopamine receptors in TS. Importantly, based on our effect sizes, P‐values and confidence intervals, there is no trend in our data to suggest that increasing sample size would result in a positive finding. This is most clearly illustrated by the individual participant BPND values plotted in Figure 2.
Although striatal dopamine receptors have been believed to be involved in the pathophysiology of TS (Buse et al., 2013), the literature is far from consistent, and studies reported as positive are often limited by methodological issues. The [123I]‐IBZM SPECT studies reviewed above found decreased binding only in medicated patients (George et al., 1994; Müller‐Vahl et al., 2000). This is likely the result of competitive dopamine receptor binding by antipsychotic medications. In one study, five unmedicated patients with a disease duration of 15 years and greater had lower binding compared with the controls, and there was an inverse relationship between ligand uptake and disease duration in the 10 unmedicated patients (Müller‐Vahl et al., 2000); however, this finding did not adequately account for age, which differed between the groups and was also inversely related to ligand uptake. Furthermore, there was no relationship between ligand uptake and symptom severity (Müller‐Vahl et al., 2000). The study showing higher [123I]‐IBZM caudate binding in the more severely affected twin among five twin pairs with TS (Wolf et al., 1996) is interesting but has not been replicated in a larger sample. Initial PET investigations did not identify differences in striatal dopamine receptor availability between TS and HC participants (Singer et al., 2002; Turjanski et al., 1994; Wong et al., 1997). The study by Wong et al. (2008) estimated D2R affinity to be higher in the anterior putamen in 12 TS subjects; however, the control group was comprised of only the three subjects with complete data. Moreover, despite numerous tests in that study, there was no correction for multiple comparisons. Of note, consistent with our findings, there were no group differences in their primary outcome measure of striatal D2/3 receptor BP. The [11C]raclopride study by Denys et al. found lower D2/D3 striatal receptor availability in the putamen of 12 TS participants, most of whom were medication naive (Denys et al., 2013). The discrepant results relative to our findings may be related to one or more of the following factors in that study: the groups were not matched for gender; there were higher depression and anxiety scores in the TS group; there was no information about comorbid ADHD.
Some authors have suggested that disturbances in the dopamine system in TS may be more related to changes in striatal dopamine innervation or dopamine release rather than dysfunction of dopamine receptors (Buse et al., 2013; Segura and Strafella, 2013). In the [11C]raclopride study by Singer et al. (2002), there was no baseline difference in D2/D3 striatal receptor availability, but an amphetamine challenge resulted in increased dopamine release in TS subjects in the putamen relative to controls. However, while the result was statistically significant (P‐value = 0.04), there were four tests performed (two regions and two analytical methods) without correction, and the study included only seven TS and five HC participants (Singer et al., 2002). In the study by Wong et al. (2008), there was a robust increase in amphetamine‐induced dopamine release in the right ventral striatum in the TS group relative to the HC group. However, this result should be interpreted bearing in mind the numerous uncorrected tests in that study including: 10 striatum subdivisions, 7 ligand measures, 2 analysis methods, and various neuropsychiatric and neuropsychological measures used for correlations (only two of which were reported, neither related to tic symptom severity measures). Moreover, the groups were not matched for gender. Showing the opposite effect, the recent study by Denys et al. found amphetamine‐induced striatal dopamine release to be decreased in 12 TS participants relative to HC subjects (though the differences disappeared after the investigators controlled for baseline binding; Denys et al., 2013). Conversely, a study of extrastriatal cortical and subcortical D2/3 receptors using the radiotracer [11C]FLB 457 found differences in amphetamine‐induced dopamine release between eight medication naïve TS and eight HC participants, with some areas being significantly increased in TS, while the opposite was seen for other areas (Steeves et al., 2010). There has not been another study of extrastriatal cortical and subcortical amphetamine‐induced dopamine release in TS. Overall, neuroimaging investigations of dopamine in TS have resulted in a heterogeneous literature.
CONCLUSION
We have shown similar striatal D2/D3 dopamine receptor availability in adults with TS compared with HC using the radioligands [11C]‐(+)‐PHNO and [11C]raclopride. Our results challenge the widely assumed role of striatal dopamine receptors in the pathophysiology of TS. Although dopamine has long been believed to underlie the pathophysiology of TS, decades of investigation have yielded inconsistent results. Interestingly, in a case series of four patients with comorbid TS and Parkinson's disease, there was improvement in parkinsonism without worsening of tics with treatment with levodopa, and there was a lack of a consistent relationship between “on” and “off” states and tic symptom severity (Kumar and Lang, 1997). More recently, the γ‐aminobutyric acid‐ergic system has been implicated in TS based on two post‐mortem histologic studies (Kalanithi et al., 2005; Kataoka et al., 2010), a PET study (Lerner et al., 2012), a recent magnetic resonance spectroscopy/magnetoencephalography investigation (Tinaz et al., 2014), and a recent basal ganglia transcriptome analysis (Lennington et al., in press). These suggest new avenues that are worth pursuing further as part of investigations to elucidate the underlying pathophysiology of TS. Nevertheless, it is likely that the causes and neural mechanisms involved in TS are complex, varied, and may involve interactions among different systems. As such, the field would benefit from concerted efforts and collaborations to carry out multimodal studies with large samples and longitudinal design.
ACKNOWLEDGMENTS
The authors thank the following individuals: Alvina Ng, Laura Nguyen, and Anusha Ravichandran for technical assistance; Kelly Aminian for administrative assistance; Ann Feng and Dr. Ariel Graff for help with healthy control subject recruitment; Dr. Adrian Crawley for helpful discussion regarding the analysis; and Dr. Donna Stewart for valuable feedback on the manuscript. The authors are also grateful to all Tourette syndrome and healthy control study participants.
Financial Disclosures
Dr. Anthony Lang has served as an advisor for Abbvie, Allon Therapeutics, Avanir Pharmaceuticals, Biogen Idec, Boerhinger‐Ingelheim, Ceregene, Lilly, Medtronic, Merck, Novartis, NeuroPhage Pharmaceuticals, Teva and UCB; received honoraria fromTeva, UCB, AbbVie; received grants from Brain Canada, Canadian Institutes of Health Research, Edmond J Safra Philanthropic Foundation, Michael J. Fox Foundation, the Ontario Brain Institute, National Parkinson Foundation, Parkinson Society Canada, TS Association, W. Garfield Weston Foundation; received publishing royalties from Saunders, Wiley‐Blackwell, Johns Hopkins Press, and Cambridge University Press; and has served as an expert witness in cases related to the welding industry. Dr. Paul Sandor has received unrestricted grants in support of conferences from Purdue, Shire, CME speaker fees from Purdue, clinical trial support from Otsuka, and has been on the data safety monitoring committee for Psyadon. Drs. Elia Abi‐Jaoude, Barbara Segura, Ignacio Obeso, Sang Soo Cho, Pablo Rusjan, and Antonio Strafella report no financial relationships with commercial interests.
This paper was presented as a poster at the International Symposium on Synaptic Plasticity and Brain Disorders on April 8, 2014, in Toronto, Ontario, Canada.
The work was done at the Research Imaging Centre, Centre for Addiction and Mental Health, University of Toronto, Toronto, Ontario, Canada
REFERENCES
- Abi‐Jaoude E, Kideckel D, Stephens R, Lafreniere‐Roula M, Deutsch J, Sandor P (2009): Tourette syndrome: A model of integration In: Carlstedt RA, editor. Handbook of Integrative Clinical Psychology, Psychiatry and Behavioral Medicine: Perspectives, Practices and Research. New York: Springer Publishing Company; pp 549–588. [Google Scholar]
- American Psychiatric Association (2000): Diagnostic and Statistical Manual of Mental Disorders: DSM‐IV‐TR. Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Brett M, Anton J‐L, Valabregue R, Poline J‐P (2002): Region of interest analysis using an SPM toolbox [abstract] Presented at the 8th International Conference on Functional Mapping of the Human Brain, June 2–6, 2002, Vol. 16, No. 2, Sendai, Japan. Available on CD‐ROM in NeuroImage.
- Buse J, Schoenefeld K, Münchau A, Roessner V (2013): Neuromodulation in Tourette syndrome: Dopamine and beyond. Neurosci Biobehav Rev 37:1069–1084. [DOI] [PubMed] [Google Scholar]
- Chakravarty MM, Bertrand G, Hodge CP, Sadikot AF, Collins DL (2006): The creation of a brain atlas for image guided neurosurgery using serial histological data. Neuroimage 30:359–376. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC (1994): Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18:192–205. [PubMed] [Google Scholar]
- Denys D, de Vries F, Cath D, Figee M, Vulink N, Veltman DJ, van der Doef TF, Boellaard R, Westenberg H, van Balkom A, Lammertsma AA, van Berckel BNM (2013): Dopaminergic activity in Tourette syndrome and obsessive‐compulsive disorder. Eur Neuropsychopharmacol 23:1423–1431. [DOI] [PubMed] [Google Scholar]
- Ganos C, Roessner V, Münchau A (2013): The functional anatomy of Gilles de la Tourette syndrome. Neurosci Biobehav Rev 37:1050–1062. [DOI] [PubMed] [Google Scholar]
- George MS, Robertson MM, Costa DC, Ell PJ, Trimble MR, Pilowsky L, Verhoeff NP (1994): Dopamine receptor availability in Tourette's syndrome. Psychiatry Res 55:193–203. [DOI] [PubMed] [Google Scholar]
- Ginovart N, Galineau L, Willeit M, Mizrahi R, Bloomfield PM, Seeman P, Houle S, Kapur S, Wilson AA (2006): Binding characteristics and sensitivity to endogenous dopamine of [11C]‐(+)‐PHNO, a new agonist radiotracer for imaging the high‐affinity state of D2 receptors in vivo using positron emission tomography. J Neurochem 97:1089–1103. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS (1989a): The Yale‐brown obsessive compulsive scale. I. Development, use, and reliability. Arch Gen Psychiatry 46:1006–1011. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, Charney DS (1989b): The Yale‐brown obsessive compulsive scale. II. Validity. Arch Gen Psychiatry 46:1012–1016. [DOI] [PubMed] [Google Scholar]
- Graybiel AM (2008): Habits, rituals, and the evaluative brain. Annu Rev Neurosci 31:359–387. [DOI] [PubMed] [Google Scholar]
- Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ (1997): Parametric imaging of ligand‐receptor binding in PET using a simplified reference region model. Neuroimage 6:279–287. [DOI] [PubMed] [Google Scholar]
- Hwang W‐J, Yao W‐J, Fu Y‐K, Yang A‐S (2008): [99mTc]TRODAT‐1/[123I]IBZM SPECT studies of the dopaminergic system in Tourette syndrome. Psychiatry Res 162:159–166. [DOI] [PubMed] [Google Scholar]
- Kalanithi PSA, Zheng W, Kataoka Y, DiFiglia M, Grantz H, Saper CB, Schwartz ML, Leckman JF, Vaccarino FM (2005): Altered parvalbumin‐positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc Natl Acad Sci USA 102:13307–13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka Y, Kalanithi PSA, Grantz H, Schwartz ML, Saper C, Leckman JF, Vaccarino FM (2010): Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J Comp Neurol 518:277–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Lang AE (1997): Coexistence of tics and parkinsonism: Evidence for non‐dopaminergic mechanisms in tic pathogenesis. Neurology 49:1699–1701. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP (1996): Simplified reference tissue model for PET receptor studies. Neuroimage 4:153–158. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, Cohen DJ (1989): The Yale global tic severity scale: Initial testing of a clinician‐rated scale of tic severity. J Am Acad Child Adolesc Psychiatry 28:566–573. [DOI] [PubMed] [Google Scholar]
- Lennington JB, Coppola G, Kataoka‐Sasaki Y, Fernandez TV, Palejev D, Li Y, Huttner A, Pletikos M, Šestan N, Leckman JF, Vaccarino FM: Transcriptome analysis of the human striatum in Tourette syndrome (in press). doi: 10.1016/j.biopsych.2014.07.018. [DOI] [PMC free article] [PubMed]
- Lerner A, Bagic A, Simmons JM, Mari Z, Bonne O, Xu B, Kazuba D, Herscovitch P, Carson RE, Murphy DL, Drevets WC, Hallett M (2012): Widespread abnormality of the γ‐aminobutyric acid‐ergic system in Tourette syndrome. Brain 135:1926–1936.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrì S, Onori MP, Roessner V, Laviola G (2013): Animal models recapitulating the multifactorial origin of Tourette syndrome. Int Rev Neurobiol 112:211–237. [DOI] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang D‐R, Huang Y, Cooper T, Kegeles L, Zarahn E, Abi‐Dargham A, Haber SN, Laruelle M (2003): Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: Amphetamine‐induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab 23:285–300. [DOI] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang D‐R, Huang Y, Simpson N, Ngo K, Van Heertum R, Laruelle M (2001): Imaging Human Mesolimbic Dopamine Transmission With Positron Emission Tomography: I. Accuracy and Precision of D2 Receptor Parameter Measurements in Ventral Striatum. J Cereb Blood Flow Metab 21:1034–1057. [DOI] [PubMed] [Google Scholar]
- McNaught KSP, Mink JW (2011): Advances in understanding and treatment of Tourette syndrome. Nat Rev Neurol 7:667–676. [DOI] [PubMed] [Google Scholar]
- Müller‐Vahl KR, Berding G, Kolbe H, Meyer GJ, Hundeshagen H, Dengler R, Knapp WH, Emrich HM (2000): Dopamine D2 receptor imaging inGilles de la Tourette syndrome. Acta Neurol Scand 101:165–171. [DOI] [PubMed] [Google Scholar]
- Pringsheim T, Doja A, Gorman D, McKinlay D, Day L, Billinghurst L, Carroll A, Dion Y, Luscombe S, Steeves T, Sandor P (2012): Canadian guidelines for the evidence‐based treatment of tic disorders: Pharmacotherapy. Can J Psychiatry 57:133–143. [DOI] [PubMed] [Google Scholar]
- Rickards H (2009): Functional neuroimaging in Tourette syndrome. J Psychosom Res 67:575–584. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M (2000): Stereotaxic display of brain lesions. Behav Neurol 12:191–200. [DOI] [PubMed] [Google Scholar]
- Segura B, Strafella AP (2013): Functional imaging of dopaminergic neurotransmission in Tourette syndrome. Int Rev Neurobiol 112:73–93. [DOI] [PubMed] [Google Scholar]
- Sibley DR, De Lean A, Creese I (1982): Anterior pituitary dopamine receptors. Demonstration of interconvertible high and low affinity states of the D‐2 dopamine receptor. J Biol Chem 257:6351–6361. [PubMed] [Google Scholar]
- Singer HS, Butler IJ, Tune LE, Seifert WE Jr, Coyle JT (1982): Dopaminergic dsyfunction in Tourette syndrome. Ann Neurol 12:361–366. [DOI] [PubMed] [Google Scholar]
- Singer HS, Szymanski S, Giuliano J, Yokoi F, Dogan AS, Brasic JR, Zhou Y, Grace AA, Wong DF (2002): Elevated intrasynaptic dopamine release in Tourette's syndrome measured by PET. Am J Psychiatry 159:1329–1336. [DOI] [PubMed] [Google Scholar]
- Steeves TDL, Ko JH, Kideckel DM, Rusjan P, Houle S, Sandor P, Lang AE, Strafella AP (2010): Extrastriatal dopaminergic dysfunction in Tourette syndrome. Ann Neurol 67:170–181. [DOI] [PubMed] [Google Scholar]
- Tinaz S, Belluscio BA, Malone P, van der Veen JW, Hallett, M , Horovitz SG (2014): Role of the sensorimotor cortex in Tourette syndrome using multimodal imaging. Hum Brain Mapp 35:5834–5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turjanski N, Sawle GV, Playford ED, Weeks R, Lammerstma AA, Lees AJ, Brooks DJ (1994): PET studies of the presynaptic and postsynaptic dopaminergic system in Tourette's syndrome. J Neurol Neurosurg Psychiatry 57:688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willeit M, Ginovart N, Kapur S, Houle S, Hussey D, Seeman P, Wilson AA (2006): High‐affinity states of human brain dopamine D2/3 receptors imaged by the agonist [11C]‐(+)‐PHNO. Biol Psychiatry 59:389–394. [DOI] [PubMed] [Google Scholar]
- Wilson AA, McCormick P, Kapur S, Willeit M, Garcia A, Hussey D, Houle S, Seeman P, Ginovart N (2005): Radiosynthesis and evaluation of [11C]‐(+)−4‐propyl‐3,4,4a,5,6,10b‐hexahydro‐2H‐naphtho[1,2‐b][1,4]oxazin‐9‐ol as a potential radiotracer for in vivo imaging of the dopamine D2 high‐affinity state with positron emission tomography. J Med Chem 48:4153–4160. [DOI] [PubMed] [Google Scholar]
- Wolf SS, Jones DW, Knable MB, Gorey JG, Lee KS, Hyde TM, Coppola R, Weinberger DR (1996): Tourette syndrome: Prediction of phenotypic variation in monozygotic twins by caudate nucleas D2 receptor binding. Science 273:1225. [DOI] [PubMed] [Google Scholar]
- Wong DF, Singer HS, Brandt J, Shaya E, Chen C, Brown J, Kimball AW, Gjedde A, Dannals RF, Ravert HT, Wilson PD, Wagner HN Jr (1997): D2‐Like Dopamine Receptor Density in Tourette Syndrome Measured by PET. J Nucl Med 38:1243–1247. [PubMed] [Google Scholar]
- Wong DF, Brašić JR, Singer HS, Schretlen DJ, Kuwabara H, Zhou Y, Nandi A, Maris MA, Alexander M, Ye W, Rousset O, Kumar A, Szabo Z, Gjedde A, Grace AA (2008): Mechanisms of dopaminergic and serotonergic neurotransmission in Tourette syndrome: Clues from an in vivo neurochemistry study with PET. Neuropsychopharmacology 33:1239–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]


