Abstract
In previous work, smokers showed steeper devaluation of delayed rewards than non‐smokers. While the neural correlates of this link between nicotine dependence and delay of discounting are not established, altered activity in executive networks may relate to impaired delayed gratification. The goal of this study was to examine neural correlates of discounting and their relation to nicotine dependence. Thirty‐nine smokers and 33 non‐smokers completed a functional magnetic resonance imaging (fMRI) intertemporal choice task in which they made individualized Hard (similarly valued), easy (dissimilarly valued), and control monetary choices. FMRI data were analyzed using a group independent component analysis and dual regression. Smokers discounted more steeply than non‐smokers, although this difference was only significant among severely dependent smokers. Intertemporal choices recruited distinct left‐ and right‐lateralized fronto‐parietal networks. A group‐by‐difficulty interaction indicated that smokers, relative to non‐smokers, exhibited less difficulty‐sensitivity in the right fronto‐parietal network. In contrast, smokers showed greater functional connectivity between the left fronto‐parietal network and the left fronto‐insular cortex. Moreover, the degree of functional connectivity between the left fronto‐parietal network and left fronto‐insular cortex was significantly correlated with individual differences in discounting. Thus, greater functional coupling between the anterior insula and left fronto‐parietal network is a candidate neural substrate linking smoking and impulsivity. Given the anterior insula's role in interfacing cognitive and interoceptive processing, this altered functional connectivity may relate to an addiction‐related bias towards immediate rewards. Hum Brain Mapp 35:3774–3787, 2014. © 2014 Wiley Periodicals, Inc.
Keywords: delay discounting, nicotine dependence, fMRI, independent component analysis, dual regression
INTRODUCTION
Delay discounting involves the devaluation of expected outcomes as a function of their anticipated delay. The desire to take an immediate reward rather than waiting for a larger one reflects fundamental issues of self‐control. Thus, a logical hypothesis is that people who discount more steeply should be at greater risk for developing problems related to self‐control, including addiction (Ainslie and Haendel, 1983; Bickel and Johnson, 2003). Consistent with this hypothesis, greater delay discounting has repeatedly been associated with addictive behaviors, including gambling (Alessi and Petry, 2003), illicit drug use (Bickel et al., 2001; Kirby et al., 1999; Kirby and Petry, 2004), and cigarette smoking (Bickel et al., 1999; Mitchell, 1999; Odum et al., 2002; Reynolds et al., 2004; Reynolds, 2006). Moreover, among smokers, there is evidence of an association between steeper discounting and more severe dependence (Heyman and Gibb, 2006; Ohmura et al., 2005; Sweitzer et al., 2008) and more difficulty quitting (Krishnan‐Sarin et al., 2007; Yoon et al., 2007). These findings, along with evidence that steep discounting predicts subsequent initiation of smoking among adolescents (Krishnan‐Sarin et al., 2007), are consistent with the possibility that low valuation of delayed expected outcomes relative to immediate expected outcomes may contribute causally to nicotine dependence.
The well‐documented connections between delay discounting and smoking, as well as other addictive behaviors, presents an opportunity for systems‐level neuroscience research on addiction. It is not currently practical to study brain function during the moments when individuals initiate, escalate, and relapse to drug use. However, the neural correlates of intertemporal decision‐making can be studied. If intertemporal choice tasks capture aspects of decision‐making relevant to drug use, then they can serve as models for some aspects of drug‐related decision making. Because of this perceived potential as a model task, many recent studies have paired delay discounting with functional magnetic resonance imaging (fMRI). Several brain regions have been implicated in these paradigms, including sectors of lateral prefrontal cortex, dorsal parietal cortex, insular cortex, ventral striatum (VS), posterior cingulate cortex (PCC), and medial prefrontal cortex (mPFC; Carter et al., 2010; Monterosso and Luo, 2010; Peters and Buchel, 2011; Scheres et al., 2013). The activity in the VS, PCC, and mPFC are hypothesized to track value during intertemporal choice, although there is no consensus on the specific roles these regions play in delay discounting (Kable and Glimcher, 2007; McClure et al., 2004; McClure et al., 2007). Numerous studies have shown that the lateral sector of prefrontal cortex and other regions implicated in executive control, such as the anterior cingulate cortex (ACC) and parietal cortex, are recruited during difficult intertemporal decisions (Hoffman 2008; McClure, 2004; Meade et al., 2011; Monterosso, 2007). Furthermore, there is some evidence that these brain regions tend to be more active when more patient choices are made (Luo et al., 2012; McClure et al., 2004; Rubia et al., 2009; Weber and Huettel, 2008; Wittmann et al., 2007), which could signify top‐down modulation of value signaling in reward‐processing structures, such as the mPFC (Hare et al., 2009).
Neuroimaging work addressing neural correlates of steep delay discounting in both clinical and healthy populations have suggested at least two markers of problematic intertemporal decision‐making processes: (1) hyper‐activation in response to expected imminent reward within reward and value‐tracking regions (Hariri et al., 2006; MacKillop et al., 2012), and (2) hypo‐activation during intertemporal decision‐making within brain regions implicated in deliberative decision‐making and cognitive control (Boettiger et al., 2007; Monterosso et al., 2007; MacKillop et al., 2012; Stoeckel et al., 2013; Wittmann et al., 2007). Consistent with the idea that these patterns characterize pathological decision processes, clinical populations with disorders linked to impulsivity (e.g., pathological gamblers, attention deficit hyperactive disorder patients, methamphetamine abusers, HIV‐positive cocaine users) have evidenced either high activation in the VS and mPFC during intertemporal choices (Miedl et al., 2012), or low recruitment of the lateral prefrontal cortex, parietal cortex or ACC during far‐sighted choices (Rubia et al., 2009) or difficult intertemporal choices (Hoffman et al., 2008; Meade et al., 2011; Monterosso et al., 2007).
To date, the majority of delay‐discounting fMRI studies have described a constellation of regions and it is not clear whether the identified regions represent one, or perhaps more than one, coherent functional network. An increasing number of studies have shifted from nodes to networks as the primary unit of analysis in mapping neural correlates of decision‐making (Laird et al., 2011) and inhibitory control (Congdon et al., 2010). These functional brain networks are defined as sets of regions with activity that covaries over time, and have been reliably identified in both resting‐state and task‐related studies using seed‐based correlations (Dosenbach et al., 2007) or independent component analyses (ICA; Allen et al., 2011; Biswal et al., 2010; Laird et al., 2011; Leech et al., 2011; Smith et al., 2009). An ICA, in particular, holds an important advantage over mean subtraction analyses in that it disentangles distinct functional activation timecourses that are intermingled within a given voxel. As a result, an ICA can dissociate patterns of activity within partially overlapping networks, which would be indistinguishable in a conventional mean subtraction (Xu et al., 2013a, b).
Recent findings suggest that several functional networks may play an important role in delay discounting (Laird et al., 2011; Li et al., 2013). For example, Li et al. (2013) determined that activity in a left fronto‐parietal network, fronto‐striatal network, and a cingulo‐opercular/right fronto‐parietal network were associated with the timing, value, and choice aspects of delay discounting, respectively. Moreover, the strength of resting‐state functional connectivity within these networks predicted individual differences in discounting rate. Consistent with these findings, a recent meta‐analysis linked delay discounting to activity in the fronto‐striatal network (FSN), default mode network (DMN), right/left fronto‐parietal networks, and a thalamic network by matching metadata clusters from the BrainMap database (http://www.brainmap.org) to brain network templates acquired at rest (Laird et al., 2011). Notably, the fronto‐parietal networks identified in these studies also coincide with delay‐discounting regions uncovered in earlier studies using mean subtraction methods (McClure et al., 2004). For example, one fMRI study showed that fronto‐parietal regions were preferentially engaged when participants discounted future gains and losses, whereas the DMN and FSN were preferentially engaged during the processing of immediate rewards (Xu et al., 2009). Relatedly, the strength of functional coupling between the ACC and dlPFC—primary nodes within fronto‐parietal networks—has been associated with steepness of discounting and tends to be higher in cocaine users (Camchong et al., 2011). Together these findings suggest that the fronto‐parietal networks are an important substrate of the common discounting process and support higher cognitive aspects of decision making, such as farsightedness and a more deliberative deferral of gratification (Miller and Cohen, 2001; Smith and Jonides, 1999).
Numerous neuroimaging studies have also shown that smoking influences large‐scale network dynamics. For example, smoking has been shown to alter resting‐state functional connectivity in the DMN and two executive networks, including the cingulo‐opercular network and fronto‐parietal network (Ding et al., 2013; Cole et al., 2010). More recently, functional connectivity analyses have not only been used to identify smoking‐related network changes, but also to examine how these network differences are modulated by the severity of nicotine dependence (for a review, see Sutherland et al., 2012). For example, increased functional connectivity between the dorsal ACC and lateral parietal cortex—two nodes typically attributed to fronto‐parietal networks—has been reported following acute nicotine administration (Hong et al., 2009). Given evidence that fronto‐parietal networks are also involved in delay discounting, it is possible that altered executive network activity may contribute to behavioral differences between smokers and non‐smokers.
Despite the promise of network approaches in identifying the neural correlates of nicotine addiction, functional connectivity methods have not, to our knowledge, been used to examine the neural bases of the steep discounting observed in cigarette smokers. Thus, the aim of the present study was to determine the neural correlates of abnormal delay discounting observed among cigarette smokers. To provide a novel network‐level account of brain function associated with intertemporal choice, we first used a group ICA to identify task‐relevant functional networks. Next, the timecourses of these task‐related networks were analyzed in a general linear model (GLM) to determine how the degree of network‐engagement differed according to intertemporal choice difficulty and between smokers and non‐smokers. Finally, we used a dual regression approach to determine how functional connectivity within intertemporal choice‐related networks related to smoking behavior and delay discounting.
METHODS
Participants
Recruitment was done through postings on http://Craigslist.com. Three hundred and thirty‐eight individuals signed informed consent to be interviewed for inclusion in the study. Two hundred and twenty‐nine self‐identified as cigarette smokers and one hundred and nine self‐identified as non‐smokers. From this original pool, 52 smokers and 50 non‐smokers met all inclusion/exclusion criteria and completed the delay‐discounting neuroimaging task. However, 30 of the 102 (29.4%) were not included in this report, due to either artifact in neuroimaging data related to head motion (n = 8) or because performance indicated that the individualization of the intertemporal choice task was not successful (n = 22). Individuation of the choice task was considered unsuccessful if either the participant did not choose an alternative generated to be of substantially greater present value on ≥15% of trials, or the participant chose the same alternative on ≥85% of the trials intended to be present‐value equivalent (e.g., always or nearly always chose the delayed option or the immediate option on trials in which the alternatives were individualized to be equally attractive). This report is based on the remaining 72 participants (33 non‐smokers and 39 smokers). All smokers were nicotine‐dependent according to DSM‐IV criteria, as assessed using the M.I.N.I. (Sheehan et al., 1998). Both groups had no history of Axis I or II pathology or neurological disorders. Additionally, smoker inclusion required (1) self‐reported smoking of at least 15 cigarettes per day for at least 2 years, and (2) biochemical confirmation of smoking by either carbon monoxide in expired breath during a baseline visit of at least 15 ppm, or a positive cotinine urinalysis (cutoff level = 200 ng/ml). The two groups did not differ significantly in gender or race, however, as can be seen in Table 1, the smokers in this sample were significantly older and less educated than the non‐smokers (ps < 0.01). Among smokers, Fagerström scores (Heatherton et al., 1991) ranged from 1 to 9, with a mean of 4.97 ± 1.90, and the group smoked on average 19.4 ± 4.3 cigarettes per day (Table 1).
Table 1.
Demographics data
| Smokers | Non‐smokers | Significance | |
|---|---|---|---|
| Gender | 33.3% female | 46.9% female | c2 (1) = 2.18, P = 0.14 |
| Race | 59.0% European ancestry, 28.2% African ancestry, 2.6% Hispanic, 11.2% other | 59.3% European ancestry, 11.1% African ancestry, 14.8% Hispanic, 14.8% other | c2 (3) = 5.63, P = 0.13 |
| Age | 35.9 ± 8.7 | 30.1 ± 7.2 | t(70) = 3.0, P < 0.01 |
| Education (years) | 14.3 ± 1.8 | 15.6 ± 1.5 | t(70) = 3.3, P < 0.01 |
| Fagerström | 4.97 ± 1.90 | NA | NA |
| Cigarettes per day | 19.4 ± 4.3 | NA | NA |
| Smoking duration (years) | 16.7 ± 9.1 | NA | NA |
Behavioral Assessment of Delay Discounting
Prior to neuroimaging, a computerized version of the Monetary‐Choice Questionnaire developed by Kirby et al. (1999) was used to assess individual level of delay discounting. Participants were instructed to select alternatives they preferred between smaller immediate rewards (ranging from $11 to $80) and larger delayed rewards (ranging in amount from $20 to $85 and in delay from 7 to 186 days). Participants' delay discounting level was estimated by fitting data to the discount function equation: [Eq. (1)] V = A/(1 + kD), in which V is the value of the amount A at delay D (in days), and the best‐fit value for parameter k is the index of delay discounting (Ainslie and Haendel, 1983; Kirby et al., 1999; Mazur, 1987). Participants were instructed that one choice made during their session would be randomly selected for payout using gift cards, and if the item selected had an associated delay, that the gift card would be activated on the corresponding day. The participant was assured that a replacement would be provided if a gift card were lost. The detailed description of the method for computing the best‐fit parameter is included in Monterosso et al. (2007). The overall level of delay discounting as captured by the fit parameter ‘k’ was transformed by natural log prior to parametric analyses. Group differences in discounting behavior were analyzed by an independent samples t test, and by one‐way analysis of covariance (ANCOVA) adjusting for age and education.
With regard to smoking severity, prior work examining the association between discounting and severity of smoking has operationalized the latter as either reported cigarettes per day, or as score on the Fagerström Test for Nicotine Dependence (Heyman and Gibb, 2006; Ohmura et al., 2005; Sweitzer et al., 2008). Thus, we included both measures in our initial investigation of the severity‐discounting association. However, it should be noted that at least one study that looked at cigarettes per day did not find an association to discounting (Johnson et al., 2007) and the only study of which we are aware that separated these constructs (i.e., cigarettes per day vs. Fagerström Test for Nicotine Dependence) found Fagerström scores to be more robustly associated with steepness of discounting (Sweitzer et al., 2008).
Delay Discounting Task Paired With fMRI
The fMRI delay discounting choice task utilized a block design. The task consisted of two runs, each of which contained four “Hard choice” blocks, four “Easy choice” blocks, and four “Control choice” blocks. Hard choices presented participants with a smaller but immediate alternative and a larger but delayed alternative ranging from $21 to $60, and delayed by 90 to 150 days. The amount of the immediate alternative was computer‐generated based on the model fit [Eq. (1)] of participants' responses during the initial behavioral assessment of individual delay discounting. For alternatives in the Hard choice blocks, the immediate alternative was the amount that, given the individual's k‐parameter fit, was expected to be equally valued to the delayed alternative (rounded to the nearest dollar). For Easy choice blocks, the same procedure was implemented except the k value used to generate the immediate alternative was either one log10 step larger (50%) or one log10 step smaller (50%) than the participant's k parameter fit. This was intended to produce blocks of intertemporal choices with a clear preferred alternative, i.e., the immediate when a log step smaller k value was used, and the delayed when a log step larger k value was used. Control trials did not include an intertemporal component. Alternatives in Control trials did not differ in immediacy but did differ in amount by as much as a factor of 2. For example, for a participant whose behavior is characterized by a hyperbolic discount function [Eq. (1)] with a parameter fit of k = 0.01, $25 Today vs. $50 in 100 days would be present‐value equivalent (a Hard Choice), $5 Today vs. $50 in 100 days (corresponding to k = 0.1) or $45 Today vs. $50 (corresponding to k = 0.001) could be Easy trials, and $50 in 100 days vs. $25 in 100 days could be a Control Trial. Each trial lasted 8 s, during which time, the two alternatives were presented on either side of the screen separated by a line (the side was randomized) and participants were free to indicate their preferences at any time by pressing the button that corresponds to the preferred option. After 5 s, if a response has not been made, the instruction “Please Respond” appeared at the bottom of the screen. The text of selected alternative was changed from white to yellow, and the screen turned blank until next trial. Trials were distributed in blocks, each composed of three trials of the same type (Hard Choice, Easy Choice, or Control Choice). Each participant performed two consecutive task runs (each run lasted 4 min and 48 s), separated by a 4‐min structural image acquisition.
MRI Acquisition
MRI scanning was performed in the Dana and David Dornsife Cognitive Neuroscience Imaging Center at USC using 3T Siemens MAGNETOM Tim/Trio system with a standard 12‐channel birdcage head‐coil. Participants laid supine on a scanner bed, viewing stimuli through a mirror mounted on the head coil. Blood oxygen level‐dependent signal was acquired by echo planar imaging (EPI) sequence with prospective acquisition correction (sequence parameters included TR = 2 s, TE = 30 ms, flip angle = 90°, FOV = 192, in‐plane resolution = 64 × 64). A total of 32 axial slices were used to cover the whole brain with no gap. A high‐resolution 3D magnetization prepared rapid gradient echo sequence (TR = 2,530 ms; TE = 2.62 ms; bandwidth = 240 Hz/pixel; flip angle = 90°; slice thickness = 1 mm; field of view = 256 × 256 mm2) was used to acquire anatomical images.
fMRI Preprocessing and GLM Set‐up
The fMRI data were first analyzed using a whole‐brain GLM. All fMRI data were processed using fMRI Expert Analysis Tool version 5.98, part of the Oxford University Centre for Functional MRI of the Brain Software Library (http://www.fmrib.ox.ac.uk/fsl). A total of eight functional volumes (4 TRs) were discarded in order to account for magnetic saturation effects. The fMRI data were preprocessed with a high‐pass filter = 100 s and spatially smoothed with a Gaussian kernel of full‐width at half‐maximum = 5 mm. The functional volumes were realigned to each participant's respective T1‐weighted anatomical image, and then normalized into standard space [Montreal Neurological Institute (MNI)] using affine transformation (Jenkinson and Smith, 2001).
A GLM was used to analyze task‐related modulation of the mean functional network timeseries across the task. In the GLM, we included three task regressors representing Hard choice blocks, Easy choice blocks, and Control choice blocks. A set of contrasts were used to examine temporal fluctuations in network activity that were associated with intertemporal choices in general ([Hard + Easy] − Control) and varied according to intertemporal choice difficulty (Hard‐Control and Easy‐Control) using one‐sample t tests.
Group ICA
To identify functional networks with significant variance contributions to the dataset, we conducted a group ICA using the MELODIC tool in FSL. An ICA is a data‐driven approach that decomposes multivariate signal into spatially and temporally distinct components. The preprocessed fMRI data from the 144 runs (2 per participant) were temporally concatenated into a single 4D file. Spatially independent components were variance normalized and thresholded using an alternative hypothesis test fitting a Gaussian/Gamma mixture model to a histogram of intensity values, with a significance level of P < 0.05. The group ICA produced a 153 × 145 matrix for each component, with each row representing the component's temporal responses per TR. The first column of the matrix represented the first eigenvector of the temporal responses from all of the different fMRI runs, whereas the remaining columns corresponded to each fMRI run's temporal responses. For the subsequent temporal regression, this group‐level matrix was divided into single‐column text files containing run‐specific temporal responses (two per participant) for each group independent component.
Following the ICA, the mean functional image from the concatenated dataset was affine registered to the MNI152 2 mm standard brain using 12 degrees of freedom. This functional‐to‐standard space transformation was then used to up‐sample the component maps into 2 mm standard space to aid in template matching. The normalized component maps were spatially cross‐correlated with 10 intrinsic brain network templates acquired by Smith et al. (2009) to distinguish brain networks from artifactual components and to identify canonical networks corresponding to identified components.
Intertemporal Choice‐Related Changes in Functional Network Activity
To determine how functional network engagement differed by Group and intertemporal choice Difficulty, the run‐specific temporal responses, that is activity timecourses, of each component were fit to each run's respective GLM design matrices. This temporal regression approach enabled us to identify components as task‐relevant by comparing how effectively each regressor could account for within‐subject variability in component activity across the task. Specifically, using one‐sample t tests, we identified group components that were significantly more active during intertemporal choice blocks ([(Hard + Easy) − Control]). These components were considered task‐relevant and subjected to further analyses. The components that did not meet this criterion were not included in subsequent analyses, thereby limiting the number of statistical tests that were carried out. For each task‐relevant component and participant, a fixed‐effects analysis was conducted by concatenating the component's temporal responses and the design matrices from each participant's two fMRI runs. These temporal regressions resulted in a set of beta weights for each participant and contrast (i.e., Hard‐Control and Easy‐Control) that indicated the degree to which functional component activity was modulated by intertemporal choice Difficulty. These contrast estimates were entered into a 2 × 2 repeated‐measures ANCOVA with Difficulty (Easy or Hard) and Group (Smoker or Non‐Smoker) modeled as within‐ and between‐subjects factors, respectively, and age and education modeled as nuisance covariates.
Spatial Integration Within Intertemporal Choice‐Related Functional Networks
In order to explore possible spatial group differences in networks related to intertemporal choice, we used dual regression to derive participant‐specific timecourses and spatial maps corresponding to each component (Beckmann et al., 2009). This method has been shown to be superior to component template matching and has high reproducibility and test‐retest reliability (Zuo et al., 2010). The dual regression procedure involved four steps: (1) The unthresholded group component spatial maps were linearly regressed against the individual preprocessed datasets to produce run‐specific mean timecourses for each component; (2) The run‐specific timecourses were variance‐normalized and used as predictors in a temporal regression to create corresponding run‐specific spatial maps for each group component; notably, this method holds an advantage over seed‐based functional connectivity approaches in that it regresses out the influence of other nuisance signals (every other component); (3) each participant's two run‐specific spatial maps were averaged to produce participant‐specific components; and (4) All of the participant‐specific spatial maps for each task‐relevant group‐level component were merged into separate 4D files, with each 3D volume representing a single participant's spatial map for that component. These 4D component files served as inputs for cross‐participant statistics.
To determine whether the spatial characteristics (i.e., voxel‐wise functional connectivity) of the task‐relevant networks differed between smokers and non‐smokers, we performed a masked voxel‐wise regression using the group‐level spatial component maps produced by the ICA. Multiple linear regressions were constrained to voxels within a binarized mask of the task‐relevant components (z > 2.3) in order to compare smoker vs. non‐smoker differences in intra‐network functional connectivity. Non‐parametric tests were conducted voxel‐wise via the randomize tool in FSL and group differences in network functional connectivity were analyzed using a two‐sample unpaired t test. A total of 5,000 permutations were carried out and significant clusters were corrected for multiple comparisons using threshold‐free cluster enhancement (Smith and Nichols, 2009). Results for the network‐masked regressions are reported at P < 0.05, corrected.
Functional Network Integration Scores and Discounting
An ROI analysis was performed in order to determine whether smoker vs. non‐smoker differences in intra‐network functional connectivity were associated with severity of nicotine dependence and discounting behavior [parameter‐fit k in Eq. (1)]. Beta weights, or integration scores, for regions showing spatial differences between smokers and non‐smokers were extracted from each participant's task‐related network functional connectivity maps output by the dual regression. These integration scores indexed the degree of functional integration between the ROIs and their respective networks across the entire inter‐temporal choice task. Pearson's partial correlations were performed between the functional integration and discounting scores to determine whether differences in integration scores mediated the group differences observed in discounting behavior. In addition, among smokers, we examined the association between the integration scores and individual differences in smoking severity.
RESULTS
Behavioral Findings
The median k‐value parameter was 0.025 for smokers, and 0.010 for non‐smokers. As points of reference, relative to $100 in 100 days, these median best‐fit parameters correspond to an indifference immediate amount of $28 and $50, respectively. For statistical comparisons, these parameter estimates were first normalized using a natural log transformation and then subjected to an ANCOVA with age and years of education included as covariates, which did not indicate a significant group difference in discounting, F(68,1) = 2.39, P = 0.13. It is noteworthy that reported years of education, which was lower among smokers, was associated with delay discounting across the entire sample, r(72) = −0.25, P = 0.04. Indeed, without adjusting for education, the group statistic in the model was significant, F(1,69) = 4.82, P = 0.03.
Since Fagerström scores were ordinal, their relationship to discounting was assessed using the rank‐based Spearman's Rho test. Severity of dependence was significantly positively correlated with steepness of discounting, r(39) = 0.33, P = 0.04. No relation was observed between delay discounting and cigarettes smoked per day, r(39) = 0.03, P = 0.86. Therefore, Fagerström scores were used as the primary index of smoking severity in neuroimaging analyses. In a post hoc analysis, we reconsidered the comparison of discounting between smokers and non‐smokers, including only smokers with Fagerström scores in the top half of our distribution (≥5), which corresponds to the scale's recommended cut‐off score for “High Dependence” (http://www.health.wa.gov.au/smokefree/docs/Fgerstrom_Test.pdf). Among this group of severely dependent smokers, discounting was steeper than among non‐smokers, even after adjusting for education and age, F(1,53) = 4.4, P = 0.04.
Due to a programming error, reaction time data were not recorded on fourteen participants. For the remaining 58 participants, a repeated‐measures analysis was performed examining reaction time as a function of Difficulty (a within‐subjects factor) and Group (a between‐subjects factor). Responses differed by Difficulty, F(2,55) = 33.20, P < 0.001. The source of this interaction was significant slowing during the Hard choice condition (mean RT = 2.16 ± 0.58 for Hard, 1.70 ± 0.28 for Easy, and 1.72 ± 0.28 for Control trials). Choice RT did not differ as a main effect of Group, F(1,56) = 0.01, P = 0.93, nor as a function of Group × Difficulty, F(2,55) = 0.84, P = 0.43.
ICA Results
The group ICA produced a total of 22 independent components in the dataset. To identify the functional significance of these networks, each component was spatially cross‐correlated with 10 intrinsic brain network templates acquired by Smith et al. (2009). Each of these 10 canonical networks were identified in the dataset: (1–3) Primary (V1; r = 0.53), secondary (occipital pole: r = 0.40), and tertiary (ventral lateral occipital; r = 0.27) visual networks; (4) the DMN (r = 0.62); (5) a cerebellar network (r = 0.3); (6) a sensorimotor network (r = 0.48); (7) an auditory network (r = 0.54); (8) an executive control or salience network (r = 0.49); (9) a right‐lateralized fronto‐parietal network (r = 0.48); and (10) a left‐lateralized fronto‐parietal network (r = 0.4). Though these components were identified as the best spatially‐matched components to the 10 canonical network templates in Smith et al. (2009), seven other components also demonstrated high correspondence. Specifically, these components were good matches for the secondary visual network (r = 0.24), auditory network (r = 0.42 and r = 0.28), right‐lateralized fronto‐parietal network (r = 0.34), and salience network (r = 0.4, r = 0.28, and r = 0.26). The five remaining group components were deemed artifactual (e.g., physiological) due to predominant activation in white matter, ventricles, or vasculature, head movement, or signal dropout.
Group Independent Components Related to Intertemporal Choices
Of the 17 components identified as brain networks, only two satisfied a priori criterion for task relevance (greater recruitment during intertemporal choices than during Control blocks): the left fronto‐parietal network and right fronto‐parietal network, t(71) = 2.72, P = 0.008 and t(71) = 2.88, P = 0.005, respectively. Thus, only these two networks were carried forward for subsequent analysis.
Comparison of Intertemporal Choice‐Related Functional Networks Between Smokers and Non‐smokers
To determine whether activity in the left and right fronto‐parietal networks differed as a function of choice difficulty or group, we performed a 2 × 2 × 2 repeated‐measures ANCOVA with Group as a between‐subjects factor and Network and Difficulty as within‐subjects factors. After controlling for age and education, we found a significant Group × Difficulty × Network interaction effect, F(1,68) = 4.43, P = 0.039. To better interpret the directionality of this interaction, we plotted the mean beta weights by Group and Difficulty for the two networks, separately (Fig. 1). A follow‐up 2 × 2 ANCOVA for just the right fronto‐parietal network revealed a significant Group × Difficulty interaction effect, F(1,68) = 8.05, P = 0.006. An independent sample t test confirmed that the interaction effect in right fronto‐parietal network activity was driven by greater differences between smokers and non‐smokers during the Hard than Easy condition, t(70) = 2.1, P < 0.04.
Figure 1.
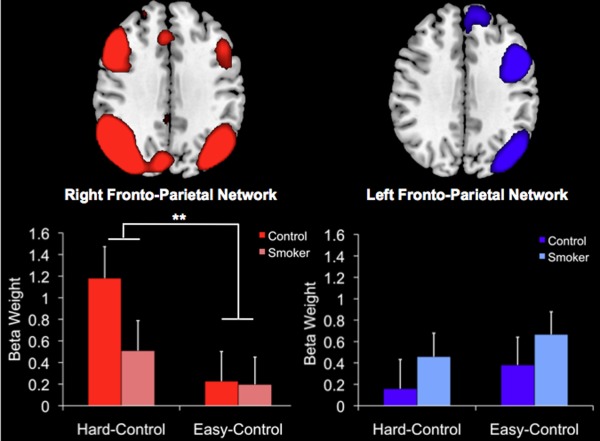
Comparison of mean parameter estimates from the two task‐related functional networks identified by the ICA, the right‐lateralized fronto‐parietal network (red) and left‐lateralized fronto‐parietal network (blue). The means are separated by Group (Smoker vs. Non‐Smoker) and Difficulty (Hard‐Control vs. Easy‐Control). The difference in right fronto‐parietal network activity between Hard‐Control vs. Easy‐Control choices was significantly greater in non‐smokers than smokers. Bars represent standard errors of the means. **P < 0.005. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Since smokers exhibited less difficulty‐sensitivity in right fronto‐parietal network activity, we next examined whether the difference score for Hard–Easy blocks was related to discounting behavior and smoking severity. Across the sample, we did not observe a relationship between the magnitude of difficulty‐differentiation in the right fronto‐parietal network and discounting, r(72) = 0.13, P = 0.29. We also did not observe a relationship between the same difficulty differentiation score and smoking severity among the smokers, r(39) = −0.16, P = 0.34.
Spatial Differences in Task‐Related Network Functional Connectivity
To investigate smoker vs. non‐smoker differences in intra‐network functional connectivity for the left and right fronto‐parietal networks, we conducted voxelwise linear regressions using non‐parametric permutation testing. The regression analyses revealed a small cluster in the left fronto‐insular cortex that was significantly more functionally coupled with the left fronto‐parietal network in smokers than non‐smokers (P < 0.05). No significant group differences were observed in the right fronto‐parietal network.
Since altered left fronto‐parietal network functional connectivity was associated with greater FIC integration in smokers than non‐smokers, we explored the possibility that these group differences would be greater when comparing non‐smokers with a subset of smokers who were severely nicotine‐dependent. As in the behavioral analysis above, this sub‐group (n = 24) was defined as smokers who scored a 5 or above on the Faegerström Scale. Confirming our hypothesis, the highly nicotine‐dependent sub‐group showed greater functional integration between the left fronto‐parietal network and left fronto‐insular cortex than the non‐smokers (P = 0.002). Furthermore, the size and extent of the fronto‐insular cluster was larger and encompassed the posterior lateral orbitofrontal cortex (Fig. 2).
Figure 2.
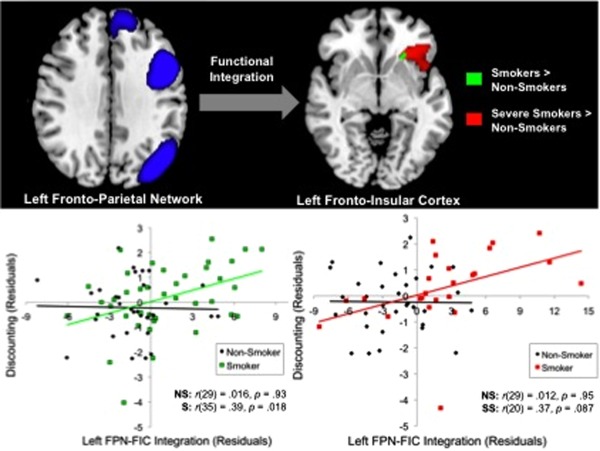
Significant mean functional connectivity differences in the left fronto‐parietal network (FPN) between smokers and non‐smokers. A cluster within the left fronto‐insular cortex (FIC) was significantly more functionally coupled with the left FPN in all smokers than non‐smokers (green). The size and strength of this relationship increased when examining only the most severe smokers, that is, individuals with Faegerström scores ≥5, vs. non‐smokers (red). The degree of left FPN‐FIC integration significantly predicted discounting within the entire smoker group, but not within the non‐smoker group for both comparisons. Pearson's partial correlation coefficients indicate the strength of the association between group and discounting after controlling for age and education. NS = non‐smoker, S = smoker, SS = severe smoker. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
To determine whether functional integration (i.e., synchronous activity) between the left fronto‐parietal network and left fronto‐insular cortex was associated with discounting behavior, we performed Pearson's partial correlations while controlling for the influence of age and education. The degree of left fronto‐parietal network‐fronto‐insular cortex integration was significantly positively correlated with the steepness of discounting, r(68) = 0.29, P = 0.016. This brain–behavior relationship was not significant within non‐smokers, r(29) = 0.016, P = 0.93, but was significant in the smoker sub‐group, r(35) = 0.39, P = 0.018 (Fig. 2).
Based on our finding that steepness of discounting varied as a function of smoking severity, we conducted separate post hoc partial correlations within the non‐smokers 33 non‐smokers and 24 severe smokers. Since the whole‐brain severe smoker > non‐smoker cluster was larger than the cluster from the comparison with all smokers, this new cluster (Fig. 2) was used to extract mean left fronto‐parietal network‐fronto‐insular cortex integration scores. As illustrated in Figure 2, the partial correlation analysis revealed that discounting was not significantly associated with degree of functional coupling between the left fronto‐parietal network and left fronto‐insular cortex in the non‐smokers, r(29) = 0.012, P = 0.95. In contrast, smokers demonstrated a marginally significant relationship between steeper discounting and left fronto‐parietal network functional integration of the left fronto‐insular cortex, r(20) = 0.37, P = 0.087.
Functional Coupling Between the Left Fronto‐Parietal Network and Left Fronto‐Insular Cortex as a Mediator of the Influence of Smoking Severity on Discounting
Within severe smokers, our partial correlation analyses revealed an association between the left fronto‐parietal network‐fronto‐insular cortex integration predictor and our primary behavioral measure, discounting (parameterized as k). To examine the possibility that anterior insula integration with the left fronto‐parietal network might mediate the observed effect of group membership on discounting behavior, we performed a Sobel test. While the Sobel test indicated that this mediation effect was not significant, t = 1.46, P = 0.14, the trend did suggest that there may have been partial mediation (Fig. 3). It is noteworthy that one severe smoker's log‐transformed discounting scores (k = −7.32) was three standard deviations below the sample mean for severe smokers (M = −3.40; SD = 1.31; refer to right panel of Fig. 2). In a post hoc exploratory analysis with this participant excluded, the mediation effect was significant, t = 2.04, P = 0.042; one‐tailed: P = 0.021.
Figure 3.
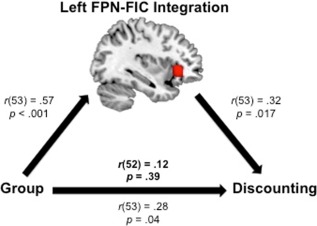
Functional connectivity between the left fronto‐parietal network (FPN) and left fronto‐insular cortex (FIC) partially mediated the influence of smoker group (coded as: 1 = non‐smoker and 2 = highly nicotine‐dependent smoker) on discounting. The red cluster represents the mean group difference in left FPN‐FIC integration from the severe smoker vs. non‐smoker contrast. Each path (arrow) represents the Pearson's partial correlation coefficient and P value from pairwise variable comparisons after controlling for age and education. The bold statistics located above the bottom arrow indicate the mediation effect when left FPN‐FIC integration scores were modeled in the partial correlation between group and discounting. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
The goal of the present study was to identify the neural correlates underlying nicotine‐dependent smoker's tendency to steeply discount expected rewards. Using ICA we distinguished functional network‐level contributions to intertemporal choices, and provided evidence that, among smokers, greater integration of the left fronto‐insular cortex with the left fronto‐parietal network is associated with steeper discounting. It has been shown that intertemporal choices recruit activity within a network of frontal and parietal brain regions (McClure et al., 2004). However, mean subtraction analyses only assess how tasks affect mean activity across the brain and so cannot discern how tasks influence the coordination of activity across different brain regions. To address this issue, we used ICA and observed altered fronto‐parietal network activity during intertemporal choices in smokers.
Our behavioral findings were generally consistent with prior reports, though with one important caveat. While smokers in our sample discounted more steeply than non‐smokers, the difference was not significant when correcting for age and education differences between groups. Given that smoking in the US is strongly associated with lower education (Hu et al., 2006) and that lower education is associated with steeper discounting behavior (Reimers et al., 2009), this is an important consideration with regard to interpreting prior smoker vs. non‐smoker comparisons of discounting behavior. This observation notwithstanding, we did find evidence linking discounting and smoking, even when adjusting for age and education. Among smokers, the severity of nicotine dependence, as assessed by the Fagerström Test for Nicotine Dependence, was positively associated with steepness of discounting. Furthermore, when comparing discounting between smokers that scored five or above (the top half in severity among our participants) and non‐smokers, the difference in discounting was significant even when adjusting for education and age. These behavioral data align with previous reports (Heyman and Gibb, 2006; Ohmura et al., 2005; Sweitzer et al., 2008) and suggest that severity of dependence and differences in education may be sources of the high variability in effect‐sizes observed across studies comparing discounting in drug‐using and non‐using populations (MacKillop et al., 2011).
Our fMRI results indicate that brain regions implicated in intertemporal choice segregate into distinct left‐ and right‐lateralized fronto‐parietal networks. Among smokers, task‐related right fronto‐parietal network activity differed less as a function of the difficulty of the intertemporal choices than it did among non‐smokers. This pattern of low difficulty‐sensitivity in smokers vs. non‐smokers during intertemporal choices is qualitatively similar to three prior studies of addicted populations (Hoffman et al., 2008; Meade et al., 2011; Monterosso et al., 2007). Furthermore, we expand upon previous findings by showing that the non‐smokers' difficulty sensitivity was lateralized to the right fronto‐parietal network. Previous research has indicated that the fronto‐parietal executive networks are involved in higher‐order cognitive control aspects of intertemporal decision‐making, and that these networks are also relevant to nicotine dependence (Ding et al., 2013; Hong et al., 2009; Sutherland et al., 2012). For instance, this network has been associated with discounting of gains and losses (Xu et al., 2009), choice difficulty (Hoffman, 2008; Meade et al., 2011; Monterosso et al., 2007), and preference for later‐larger (LL) rewards (Luo et al., 2012; McClure et al., 2004). Hard choices and LL choices both involve deployment of cognitive resources, suggesting that lower right fronto‐parietal network‐engagement among smokers during difficult intertemporal choices represents a deficit in executive control. Consistent with this suggestion, the same data pattern has been observed in drug abusers performing tasks requiring executive control, such as the Stroop task and Go/No‐Go task (Barros‐Loscertales et al., 2011; Garavan et al., 2008). Given that activity in the right fronto‐parietal network differed by choice difficulty, it may be regarded as surprising that we did not observe functional connectivity differences in this network across the task. This may be due to Type 2 error, or alternatively, it may be the case that the interaction with difficulty does not entail altered connectivity between specific sub‐nodes of the network (e.g., dorsolateral prefrontal cortex) but rather in coherent network connectivity as a whole. Among smokers and non‐smokers, we did not observe evidence linking lower difficulty‐related brain activity during Hard vs. Easy intertemporal choices to discounting behavior. Since our study taps aspects of higher‐order cognitive function, investigating the degree to which these choice‐related functional connectivity patterns are related to IQ or perhaps some specific cognitive capacity should also be considered in future studies.
A second observed group difference in brain activity was, however, related to steep discounting. Across the entire task, smokers, and especially severely dependent smokers, exhibited greater functional connectivity between the left fronto‐insular cortex and left fronto‐parietal network. Moreover, individual differences in this effect predicted discounting behavior (both of the entire group, as well as among smokers). That is, in the same anterior insula cluster, those smokers that had greatest functional connectivity discounted most steeply. Additionally, although not statistically significant, there was suggestive evidence that integration of this anterior insula cluster with the left fronto‐parietal network partially mediated behavioral differences in discounting. Therefore, we believe that enhanced functional integration of the left anterior insula with the left fronto‐parietal network should be considered as a candidate neural contributor to links between addiction and delay discounting.
While our data are correlational, there is a priori plausibility to this. Although no previous studies have examined the relationship between greater left fronto‐parietal network integration of the anterior insula and discounting behavior, intertemporal choice related activity has been repeatedly observed in the insula (Bickel et al., 2009; Carter et al., 2010; Hoffman et al., 2008; Luo et al., 2009; Xu et al., 2009). A well‐established role of anterior insula is to map internal bodily states that influence feelings (Damasio, 1994), which in turn causally contributes to action. It is possible that in the context of our decision‐making task (where explicit or implicit rules related to monetary choices may compete with emotion signaling related to the possible outcomes, see Luo et al 2009) polymodal sensory and affective integration may have biased preference towards immediate alternatives.
Increasing attention has been directed towards the anterior insula as a critical neural substrate of nicotine addiction (Naqvi and Bechara, 2009, 2010). Numerous studies have demonstrated that addicts show activation of the insula during exposure to drug cues, and it has been shown that smokers with insular damage have a higher likelihood of quitting smoking than smokers without (Naqvi et al., 2007). A recent structural MRI study further supports the insula's involvement in addiction, showing that smokers had greater gray matter density in the anterior insula than non‐smokers (Zhang et al., 2010). One explanation for the key role of the anterior insula in addiction is that it forms a critical junction between executive and internally directed networks (Menon and Uddin, 2010), and that addiction is a manifestation of imbalanced recruitment of the insula that shifts decision‐making towards more impulsive choices (Naqvi and Bechara, 2010). As a critical node within a brain “Salience Network” (Seeley et al., 2007), the manner in which the anterior insula coactivates with other large‐scale networks during decision‐making tasks could signify the salience of different choice alternatives. Among smokers, these dynamic task‐related interactions between the Salience and executive networks might therefore be a biomarker of deficits in farsighted choices. However, previous investigations of the Salience Network favor activity in the right hemisphere, whereas our finding was localized to the left hemisphere. Indeed, when we explored brain activity patterns at uncorrected statistical thresholds, the right‐lateralized fronto‐parietal network did not integrate the right fronto‐insular cortex, thereby limiting our interpretation of the Salience Network's involvement in steeper discounting in severe smokers. Taken together, the enhanced left fronto‐parietal network‐fronto‐insular cortex functional integration we observed among smokers may be a contributor to actions that achieve more immediate rewards, whether in the form of drug cues or money.
Several limitations should be noted. Whereas previous fMRI delay discounting studies focus on competition between impulse‐based value networks (e.g., DMN) and more executive brain networks associated with deliberative decision‐making, our blocked design did not allow us to examine the neural correlates of value. This was due to the fact that the value of the choice alternatives did not significantly differ between intertemporal and control choice trials. Relatedly, we did not observe task‐related activation in some brain regions generally associated with value‐tracking, such as the mPFC and VS (Carter et al., 2010) that are central to some speculation regarding intertemporal choice behavior (McClure et al., 2004) and its link to substance use (Hariri et al., 2006; for reviews, see Bickel and Marsch 2001; Sutherland et al., 2012). This discrepancy is not surprising given the absence of value difference between our task conditions.
In addition, the discount model we used ignores diminishing marginal utility. We have assumed that the individual variation in intertemporal choice trade‐offs reflects differences in discounting, but they could also reflect differences in the form of the utility function. Individuals unwilling to wait 4 months for an amount twice that of an available immediate reward may do so because they discount steeply. Alternatively, they may discount modestly, but exhibit steep diminishing marginal utility (Ho et al., 1999; Pine et al., 2009, 2010). Both of these interpretations warrant consideration (Andersen et al., 2008).
Finally, while participants in our study were allowed to smoke 15 min before scanning (approximately 30 min before task data acquisition), they were not required to do so. It is therefore possible that our neuroimaging findings were related to early withdrawal symptoms manifesting during the task. Supporting this idea, one effective connectivity study showed that the anterior insula modulated activity in nodes of executive networks, such as the fronto‐parietal network, during nicotine abstinence, suggesting that its functional integration varies as a function of nicotine levels (Ding et al., 2013). Similarly, nicotine administration has been shown to alter dynamic interactions between fronto‐parietal networks and other task‐related attention networks (Cole et al., 2010). These findings are particularly interesting in light of evidence of state‐dependent increases in delay discounting during nicotine withdrawal (Field et al., 2006), opiate withdrawal (Giordano et al., 2002), and other strong appetitive states (Wilson and Daly, 2004). Thus, future studies could use fMRI to examine the effects of nicotine administration on delay discounting to further elucidate the link between physiological states, brain network function, and decision‐making.
CONCLUSION
Consistent with previous delay discounting studies, we showed that nicotine dependence is associated with greater discounting of future rewards. Using a group ICA, we found that difficult intertemporal choices corresponded to greater engagement of a right‐lateralized fronto‐parietal network, and that this pattern of brain activity was diminished in smokers. In addition, a dual regression analysis revealed that smokers showed greater functional connectivity between left fronto‐insular cortex and the left‐lateralized fronto‐parietal network, which was in turn related to steeper discounting. We speculate that higher functional coupling between the left fronto‐parietal network fronto‐insular cortex in smokers may result in a decision bias towards immediate rewards.
ACKNOWLEDGMENTS
The authors would like to thank Lisa Giragosian for assistance with data collection and Jiancheng Zhuang, PhD, for assistance with MRI operations.
David Clewett and Shan Luo contributed equally to this article.
This work was supported by the National Institute of Health R01DA023176 (JM).
REFERENCES
- Ainslie G, Haendel V (1983): The motives of the will In: Gottheil E, Druley K, Skodola T, Waxman H, editors. Etiology Aspects of Alcohol and Drug Abuse. Springfield, IL: Charles C. Thomas: 119–140. [Google Scholar]
- Alessi SM, Petry NM (2003): Pathological gambling severity is associated with impulsivity in a delay discounting procedure. Behav Process 64:345–354. [DOI] [PubMed] [Google Scholar]
- Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, Silva RF, Calhoun VD (2011): A baseline for the multivariate comparison of resting‐state networks. Front Syst Neurosci 5:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrós‐Loscertales A, Bustamante JC, Ventura‐Campos N, Llopis JJ, Parcet MA, Ávila C (2011): Lower activation in the right frontoparietal network during a counting stroop task in a cocaine‐dependent group. Psychiatry Res 194:111–118. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Mackay CE, Filippini N, Smith SM (2009): Group comparison of resting‐state FMRI data using multi‐subject ICA and dual regression. Neuroimage 47:S148. [Google Scholar]
- Bickel WK, Odum AL, Madden GJ (1999): Impulsivity and cigarette smoking: Delay discounting in current, never, and ex‐smokers. Psychopharmacology 146:447–454. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA (2001): Toward a behavioral economic understanding of drug dependence: Delay discounting processes. Addiction 96:73–86. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Johnson MW (2003): Delay discounting: A fundamental behavioral process of drug dependence. In: Time and decision: Economic and psychological perspectives on intertemporal choice, pp 419–440.
- Bickel WK, Pitcock JA, Yi R, Angtuaco EJ (2009): Congruence of BOLD response across intertemporal choice conditions: fictive and real money gains and losses. J Neurosci 29:8839–8846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Windischberger C (2010): Toward discovery science of human brain function. Proc Natl Acad Sci 107:4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger CA, Mitchell JM, Tavares VC, Robertson M, Joslyn G, D'Esposito M, Fields HL (2007): Immediate reward bias in humans: Fronto‐parietal networks and a role for the catechol‐O‐methyltransferase 158Val/Val genotype. J Neurosci 27:14383–14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RM, Meyer JR, Huettel SA (2010): Functional neuroimaging of intertemporal choice models: A review. J Neurosci Psychol Econ 3:27–45. [Google Scholar]
- Cole DM, Beckmann CF, Long CJ, Matthews PM, Durcan MJ, Beaver JD (2010): Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. Neuroimage 52:590–599. [DOI] [PubMed] [Google Scholar]
- Congdon E, Mumford JA, Cohen JR, Galvan A, Aron AR, Xue G, Poldrack RA (2010): Engagement of large‐scale networks is related to individual differences in inhibitory control. Neuroimage 53:653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio A (1994): Descates' Error. London: Papermac/Macmillan. [Google Scholar]
- Ding X, Lee SW (2013): Changes of functional and effective connectivity in smoking replenishment on deprived heavy smokers: A resting‐state fMRI study. PloS One 8:e59331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Petersen SE (2007): Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci 104:11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Kaufman JN, Hester R (2008): Acute effects of cocaine on the neurobiology of cognitive control. Philos Trans Roy Soc B Biol Sci 363:3267–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A (2009): Self‐control in decision‐making involves modulation of the vmPFC valuation system. Science 324:646–648. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB (2006): Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J Neurosci 26:13213–13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman GM, Gibb SP (2006): Delay discounting in college cigarette chippers. Behav Pharmacol 17:669–679. [DOI] [PubMed] [Google Scholar]
- Hoffman W, Schwartz D, Huckans M, McFarland B, Meiri G, Stevens A, Mitchell S (2008): Cortical activation during delay discounting in abstinent methamphetamine dependent individuals. Psychopharmacology 201:183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M‐Y, Mobini S, Chiang T‐J, Bradshaw CM, Szabadi E (1999): Theory and method in the quantitative analysis of “impulsive choice” behaviour: Implications for psychopharmacology. Psychopharmacology 146:362–372. [DOI] [PubMed] [Google Scholar]
- Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, Stein EA (2009) Association of nicotine addiction and nicotine's actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry 66:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Smith S (2001): A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK, Baker F (2007) Moderate drug use and delay discounting: a comparison of heavy, light, and never smokers. Exp Clin Psychopharmacol 15:187. [DOI] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW (2007): The neural correlates of subjective value during intertemporal choice. Nat Neurosci 10:1625–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK (1999): Heroin addicts have higher discount rates for delayed rewards than non‐drug‐using controls. J Exp Psychol Gen 128:78. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM (2004): Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non drug using controls. Addiction 99:461–471. [DOI] [PubMed] [Google Scholar]
- Krishnan‐Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A, Potenza MN (2007): Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug Alcohol Depend 88:79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein G, Read D, Baumeister R. F. (Eds.). (2003): Time and decision: Economic and psychological perspectives on intertemporal choice. Russell Sage Foundation. [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, Fox PT (2011): Behavioral interpretations of intrinsic connectivity networks. J Cogn Neurosci 23:4022–4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Kamourieh S, Beckmann CF, Sharp DJ (2011): Fractionating the default mode network: Distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci 31:3217–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Ainslie G, Giragosian L, Monterosso JR (2009): Behavioral and neural evidence of incentive bias for immediate rewards relative to preference‐matched delayed rewards. J Neurosci 29:14820–14827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Ainslie G, Pollini D, Giragosian L, Monterosso JR (2012): Moderators of the association between brain activation and farsighted choice. NeuroImage 59:1469–1477. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Wier L, David SP, Ray LA, Bickel WK, Sweet LH (2012): The neuroeconomics of nicotine dependence: a preliminary study of delay discounting of monetary and cigarette rewards in smokers using fMRI. Psychiatry Res 202:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE (1987): An adjusting procedure for studying delayed reinforcement. Quant Anal Behav 5:55–73. [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD (2004): Separate neural systems value immediate and delayed monetary rewards. Science 306:503–507. [DOI] [PubMed] [Google Scholar]
- McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD (2007): Time discounting for primary rewards. J Neurosci 27:5796–5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade CS, Lowen SB, MacLean RR, Key MD, Lukas SE (2011): fMRI brain activation during a delay discounting task in HIV‐positive adults with and without cocaine dependence. Psychiatry Res 192:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ (2010): Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct 214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedl SF, Peters J, Buchel C (2012): Altered neural reward representations in pathological gamblers revealed by delay and probability discounting. Arch Gen Psychiatry 69:177. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD (2001): An integrative theory of prefrontal cortex function. Ann Rev Neurosci 24:167–202. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED (2007): Frontoparietal cortical activity of methamphetamine‐dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp 28:383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso JR, Luo S (2010): An argument against dual valuation system competition: Cognitive capacities supporting future orientation mediate rather than compete with visceral motivations. J Neurosci Psychol Econ 3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A (2007): Damage to the insula disrupts addiction to cigarette smoking. Science 315:531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A (2009): The hidden island of addiction: The insula. Trends Neurosci 32:56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A (2010): The insula and drug addiction: an interoceptive view of pleasure, urges, and decision‐making. Brain Struct Funct 214:435–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum AL, Madden GJ, Bickel WK (2002): Discounting of delayed health gains and losses by current, never‐and ex‐smokers of cigarettes. Nicotine Tobacco Res 4:295–303. [DOI] [PubMed] [Google Scholar]
- Ohmura Y, Takahashi T, Kitamura N (2005): Discounting delayed and probabilistic monetary gains and losses by smokers of cigarettes. Psychopharmacology 182:508–515. [DOI] [PubMed] [Google Scholar]
- Peters J, Büchel C (2011) The neural mechanisms of inter‐temporal decision‐making: Understanding variability. Trends Cogn Sci 15:227–239. [DOI] [PubMed] [Google Scholar]
- Pine A, Seymour B, Roiser JP, Bossaerts P, Friston KJ, Curran HV, Dolan RJ (2009) Encoding of marginal utility across time in the human brain. J Neurosci 29:9575–9581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine A, Shiner T, Seymour B, Dolan RJ (2010): Dopamine, time, and impulsivity in humans. J Neurosci 30:8888–8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B, Richards JB, Horn K, Karraker K (2004): Delay discounting and probability discounting as related to cigarette smoking status in adults. Behav Process 65:35–42. [DOI] [PubMed] [Google Scholar]
- Reynolds B (2006): The experiential discounting task is sensitive to cigarette‐smoking status and correlates with a measure of delay discounting. Behav Pharmacol 17:133–142. [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Christakou A, Taylor E (2009): Impulsiveness as a timing disturbance: Neurocognitive abnormalities in attention‐deficit hyperactivity disorder during temporal processes and normalization with methylphenidate. Philos Trans Roy Soc B Biol Sci 364:1919–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres A, De Water E, Mies GW (2013): The neural correlates of temporal reward discounting. Wiley Interdiscip Rev Cogn Sci 4(5):523–545. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Greicius MD (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Beckmann CF (2009): Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci 106:13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Nichols TE (2009): Threshold‐free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44:83–98. [DOI] [PubMed] [Google Scholar]
- Stoeckel LE, Murdaugh DL, Cox JE, Cook EW, III , Weller RE (2013). Greater impulsivity is associated with decreased brain activation in obese women during a delay discounting task. Brain Imag Behav 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA (2012): Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage 62:2281–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer MM, Donny EC, Dierker LC, Flory JD, Manuck SB (2008): Delay discounting and smoking: Association with the Fagerström Test for Nicotine Dependence but not cigarettes smoked per day. Nicotine Tobacco Res 10:1571–1575. [DOI] [PubMed] [Google Scholar]
- Weber BJ, Huettel SA (2008): The neural substrates of probabilistic and intertemporal decision making. Brain Res 1234:104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M, Daly M (2004): Do pretty women inspire men to discount the future? Proc Roy Soc B Biol Sci 271(Suppl 4):S177–S179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Leland D, Paulus M (2007): Time and decision making: differential contribution of the posterior insular cortex and the striatum during a delay discounting task. Exp Brain Res 179:643–653. [DOI] [PubMed] [Google Scholar]
- Xu L, Liang ZY, Wang K, Li S, Jiang T (2009): Neural mechanism of intertemporal choice: From discounting future gains to future losses. Brain Res 1261:65–74. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhang S, Calhoun VD, Monterosso J, Li CSR, Worhunsky PD, Potenza MN (2013a): Task‐related concurrent but opposite modulations of overlapping functional networks as revealed by spatial ICA. NeuroImage 79:62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Potenza MN, Calhoun VD (2013b): Spatial ICA reveals functional activity hidden from traditional fMRI GLM‐based analyses. Front Neurosc 7:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Higgins ST, Heil SH, Sugarbaker RJ, Thomas CS, Badger GJ (2007): Delay discounting predicts postpartum relapse to cigarette smoking among pregnant women. Exp Clin Psychopharmacol 15:176. [DOI] [PubMed] [Google Scholar]
- Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP (2010): Reliable intrinsic connectivity networks: Test–retest evaluation using ICA and dual regression approach. Neuroimage 49:2163–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Salmeron BJ, Ross TJ, Geng X, Yang Y, Stein EA (2011): Factors underlying prefrontal and insula structural alterations in smokers. Neuroimage 54:42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]


