Abstract
While task‐based neuroimaging studies have identified alterations in neural circuitry underlying language processing in children with autism spectrum disorders [ASD], resting state functional magnetic resonance imaging [rsfMRI] is a promising alternative to the constraints posed by task‐based fMRI. This study used rsfMRI, in a longitudinal design, to study the impact of a reading intervention on connectivity of the brain regions involved in reading comprehension in children with ASD. Functional connectivity was examined using group independent component analysis (GICA) and seed‐based correlation analysis of Broca's and Wernicke's areas, in three groups of participants: an experimental group of ASD children (ASD‐EXP), a wait list control group of ASD children (ASD‐WLC), and a group of typically developing (TD) control children. Both GICA and seed‐based analyses revealed stronger functional connectivity of Broca's and Wernicke's areas in the ASD‐EXP group postintervention. Additionally, improvement in reading comprehension in the ASD‐EXP group was correlated with greater connectivity in both Broca's and Wernicke's area in the GICA identified reading network component. In addition, increased connectivity between the Broca's area and right postcentral and right STG, and the Wernicke's area and LIFG, were also correlated with greater improvement in reading comprehension. Overall, this study revealed widespread changes in functional connectivity of the brain's reading network as a result of intervention in children with ASD. These novel findings provide valuable insights into the neuroplasticity of brain areas underlying reading and the impact of intensive intervention in modifying them in children with ASD. Hum Brain Mapp 36:2965–2979, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: autism, resting state fMRI, functional connectivity, reading intervention, visualizing and verbalizing, reading network
INTRODUCTION
Autism spectrum disorder (ASD) is characterized by profound impairments in verbal and nonverbal communication [American Psychiatric Association, 2013]. With the rising prevalence in ASD [currently 1 in 68 children; Center for Disease Control, 2014], there is a growing need for improved understanding of the neurobiology of this disorder which can lead to developing targeted interventions. Both behavioral and neuroimaging studies have reported that high‐functioning children with ASD struggle with different aspects of oral and reading comprehension, including pragmatics, semantics, and phonological processes [Groen et al., 2008, 2010; Williams et al., 2008], while their decoding and word identification skills remain relatively intact [Nation et al., 2006; Newman et al., 2007; Norbury and Nation, 2011]. Concurrently, neuroimaging research has demonstrated alterations in the synchronization of brain activity (underconnectivity of frontal and temporal language regions) underlying language comprehension, including semantics and integration of social information [Groen et al., 2010], lexical over thematic processing [Just et al., 2004], and pragmatics and syntax [Groen et al., 2008]. In typically developing (TD) individuals, the language comprehension network is left‐hemisphere (LH) dominant, consisting of the left posterior middle temporal gyrus (LMTG), superior temporal gyrus (LSTG), superior temporal sulcus (LSTS), inferior frontal gyrus (LIFG), and middle frontal gyrus (LMFG) [Turken and Dronkers, 2011; Tomasi and Volkow, 2012]. Individuals with ASD, conversely, tend to recruit additional right‐hemisphere (RH) regions and appear to favor local connectivity between parietal and occipital regions during language comprehension [Kana et al., 2006; Kana and Wadsworth, 2012; Mason et al., 2008] at the expense of more long‐range frontal to posterior connections [Courchesne et al., 2007; Minshew and Keller, 2010; Sahyoun et al., 2010]. These results are consistent with the cortical underconnectivity hypothesis which posits that complex cognitive functions such as language are dependent on the coordination of brain areas which may be compromised in individuals with ASD [Just et al., 2004; Kana et al., 2006].
While task‐based neuroimaging studies (e.g., sentence comprehension, theory‐of‐mind, problem‐solving) are important in understanding the neural mechanisms of cognitive processes, they are also constrained by the level of task difficulty, response time, and participant performance. Such demands make pediatric neuroimaging and imaging children with neurodevelopmental disorders particularly challenging. The advent of resting state functional MRI (rsfMRI) has marked a paradigm shift in the field of neuroimaging [Raichle, 2009] and has opened new doors for understanding the neurobiology of disorders like autism. Neuroimaging studies have applied rsfMRI as a task‐independent method to identify low frequency (<0.1 Hz) fluctuations of spontaneous brain activity that represent structured and organized brain networks that closely relate to functional patterns of connectivity observed during task performance [Deco and Corbetta, 2011; Smith et al., 2009; Toro et al., 2008]. More recently, studies have used rsfMRI to assess the neural networks underlying language comprehension, specifically reading ability in TD individuals [Hampson et al., 2006; Koyama et al., 2010, 2011; Tomasi & Volkow, 2012].
Using rsfMRI brain activity to study language comprehension, particularly reading comprehension, a basic network can be identified that acts as a framework for language processing regardless of task [Lohmann et al., 2010]. This is especially important for children with ASD, where developing language tasks that can address the wide spectrum of comprehension abilities is challenging. The language comprehension network in TD children has been successfully mapped in previous studies using rsfMRI brain activity. In addition, reading performance of TD children was positively correlated with resting state connectivity between posterior superior temporal (Wernicke's area) and inferior frontal (Broca's area) cortical areas [Koyama et al., 2011]. To our knowledge, only one study, so far, has assessed the language comprehension network using rsfMRI in children with ASD [Verly et al, 2014] finding intact connectivity between Wernicke's and Broca's area. However, underconnectivity was identified between Broca's area and supplemental motor area (SMA) and dorsolateral prefrontal cortex (DLPFC) as well as between frontal and cerebellar regions [Verly et al., 2014]. Thus, recent research indicates the potential of rsfMRI to characterize language comprehension networks, including the reading network, during task‐free resting state. While such characterization is vital in understanding ASD further, an equally or perhaps more important next step is to determine the plasticity of this intrinsically organized network, and to test whether intervention can effect changes in rsfMRI brain connectivity. The main goal of the present study is to address this question in children with ASD with the use of an intense, behaviorally tested, and comprehensive reading intervention.
While all previous studies have assessed the connectivity between brain regions associated with language comprehension during rest using a seed‐based method [Hampson et al., 2006; Koyama et al., 2010, 2011; Tomasi et al., 2012; Verly et al., 2014], this study uses a purely data‐driven independent component analysis (ICA) approach to identify spatially distinct, temporally correlated brain networks [Calhoun et al., 2001]. Additionally, we used a seed‐based approach to assess the connectivity of both Broca's and Wernicke's area with the rest of the brain [Tomasi and Volkow, 2012]. Seed‐based methods are advantageous in analyzing correlations in rsfMRI between specific regions of interest (ROI), or seeds, with other brain regions in networks that have been previously well defined; whereas, the benefit of the ICA approach is that all significantly weighted voxels within an independent component are highly correlated, and can be considered a functionally connected network. In ICA, the time series of all of the significant voxels can be assumed to share commonality, and can be considered to be functionally connected with one another within the network [Erhardt et al., 2011]. Given the relatively novel nature of using rsfMRI to better understand the language comprehension network, both ICA and seed‐based approaches can provide valuable information in identifying specific regions encompassed within this network that are altered in children with ASD, and additionally increase the specificity to determine intervention‐related changes in functional connectivity.
We used rsfMRI in a longitudinal design before and after a reading remediation program to assess changes in brain connectivity of brain regions involved in reading comprehension in children with ASD. In addition, we assessed differences in rsfMRI connectivity between children with ASD and TD children, to further evaluate differences between children with and without reading comprehension difficulties. We expect that TD children will have neural differences distinct from our ASD children based on previous literature [e.g., Verly et al, 2014]. The intervention used in this study [Visualizing and Verbalizing for Language Comprehension and Thinking (V/V)] has been found to be successful in children with reading disorders, but has never been applied to study children with ASD. The V/V intervention has significant implications for autism as it relies primarily on visual nonverbal methods, a relative strength in individuals with ASD, to aid in developing both oral and reading comprehension [Bell, 1991a, 1991b; Torgesen et al., 2001]. This intervention is a practical application of the principles of dual coding theory which posits that cognition involves the activity of two distinct subsystems, a verbal system specialized for dealing directly with language, and a nonverbal (imagery) system specialized for dealing with nonlinguistic objects and events [Paivio, 2007; Sadoski and Paivio, 2001]. We applied two connectivity analysis approaches in this study, data‐driven ICA and seed‐based correlation analysis, to evaluate the functional integrity of the reading network during resting state and to delineate changes in functional connectivity due to targeted intervention in children with ASD. We hypothesize that children with ASD who participated in the reading intervention would show strengthened functional connectivity within the identified reading network after intervention as well as would recruit additional brain regions as compensatory resource for reading comprehension. This study is novel in its translational neuroimaging focus and the findings will have a significant impact on understanding and in applying targeted behavioral interventions to children with ASD.
METHOD
Participants
Participants were 31 children with ASD (mean age = 10.6) and 22 age and IQ‐matched TD children (mean age = 10.4; see Table 1). The children with ASD underwent two fMRI sessions, 10 weeks apart; the TD children only participated in one fMRI session. The children with ASD were randomly assigned to participate in the V/V Intervention Program either between imaging sessions (experimental group; ASD‐EXP; n = 16) or after completing both imaging sessions (wait‐list control group; ASD‐WLC; n = 15). The TD control group did not participate in the intervention. Children were determined to have an ASD diagnosis by either being diagnosed by a licensed clinical psychologist using the Autism Diagnostic Observation Schedule [ADOS; Lord et al, 2000] and/or the Autism Diagnostic Interview‐Revised [ADI; Lord et al., 1994] or being diagnosed by a licensed clinical psychologist and a research diagnosis using the ADOS by trained research‐reliable personnel. No statistically significant differences between the ASD group and the TD group were observed for age [t(51) = 0.427, P = 0.671] or Full‐Scale IQ [mean ASD = 95.8; mean TD = 97.6; t(51)= 0.497, P = 0.621], although there was a marginally significant difference in verbal IQ [mean ASD = 91.6; mean TD = 98.9; t(51) = 2.04, P = 0.048]. In addition, the ASD‐EXP and ASD‐WLC ASD groups did not differ on reading comprehension level prior to the first fMRI session, as measured by the Gray Oral Reading Test (GORT‐4): Comprehension Score [mean ASD‐EXP = 76.5; mean ASD‐WLC = 84.2; t(29) = 1.59, P = 0.062]. Among the 31 children with ASD, 7 were female (4 in the ASD‐EXP group and 3 in the ASD‐WLC group), and all were right‐handed. In the TD group, 6 were female, and all were right‐handed.
Table 1.
Participant Demographics
| Characteristic | ASD‐EXP Grou (n = 16) | ASD‐WLC Group (n =15) | TD Group (n = 22) |
|---|---|---|---|
| Agea | 10.3 ± 1.49 | 11.0 ± 1.14 | 10.4 ± 1.56 |
| Gender | |||
| Male | 12 | 12 | 16 |
| Female | 4 | 3 | 6 |
| Self‐Identify | |||
| Caucasian | 10 | 9 | 10 |
| Black | 1 | 1 | 9 |
| Asian | 3 | 4 | |
| Hispanic | 1 | ||
| WASI FSIQb | 94.7 ± 12.98 | 97.2 ± 15.53 | 97.6 ± 12.08 |
| WASI VIQc | 91.3 ± 9.19 | 92.1 ± 13.21 | 98.9 ± 15.50 |
| GORT‐4 Comprehensiond | 76.7 ± 11.56 | 84.2 ± 9.76 | 105.7 ± 19.18 |
| SORT‐R Reading Scoree | 107.5 ± 7.78 | 105.5 ± 8.18 | 109.2 ± 9.08 |
Note: Value ± standard deviation.
Age in decimal years at first imaging session.
Wechsler Abbreviated Scale of Intelligence, Full Scale Intelligence Quotient.
Wechsler Abbreviated Scale of Intelligence, Verbal Intelligence Quotient.
Gray Oral Reading Test—Forth Edition Comprehension subtest at the first imaging session in standard scores.
Slosson Oral Reading Test—Revised (SORT‐R) reading score at the first imaging session in standard scores.
All participants with ASD were recruited through multiple sources, such as the Civitan‐Sparks Clinic at UAB, Mitchell's Place for Autism in Birmingham, the Autism Spectrum Disorders Clinic at the University of Alabama, through the Alabama Autism Society, from the greater Birmingham area, and from nearby cities, such as Montgomery, Mobile, Huntsville, and Tuscaloosa. In addition, the Lindamood–Bell Learning centers recruited potential participants at their centers, and sent those families to UAB for eligibility testing. The TD participants and their families were recruited by advertisements in local newspapers and in UAB Reporter, and by flyers posted on UAB campus. All participants with ASD met the following inclusion criteria: ages from 8 to 13 years, current diagnosis of ASD as specified above, right‐handed, and be recommended for the V/V intervention, indexed by being a native English speaker, having a Slosson Oral Reading Test ‐ Revised (SORT‐R) reading score of at least 37th percentile and/or a Gray Oral Reading Test – Forth Edition (GORT‐4) accuracy score of at least 25th percentile, a GORT‐4 comprehension score below 37th percentile, and a Verbal IQ score of at least 75, as measured by the Wechsler Abbreviated Scale of Intelligence (WASI). The inclusion criteria of TD participants included: ages from 8 to 13, no diagnosis of an autism spectrum disorder or a language disorder, and an average (greater than the 25th percentile) oral reading and reading comprehension, as measured by the SORT‐R and GORT‐4. Participants failing to meet any of the inclusion criteria and participants currently taking beta‐blockers or vasodilators, having a history of ferromagnetic material in the body, or neurostimulators, being claustrophobic, or history of kidney disease, seizure disorder, diabetes, hypertension, anemia, or sickle cell disease were excluded from the study. All participants were off medication at the time of their imaging session. All participants’ legal guardians gave written informed consent and all participants gave written informed assent, approved by the UAB Institutional Review Board, to participate in the study and were compensated for their participation.
Reading Intervention Program
The Visualizing and Verbalizing for Language Comprehension and Thinking (V/V) Intervention is based on the use of nonverbal sensory input, in the form of imaged gestalts, to develop oral and written language comprehension, establish vocabulary, and develop higher order thinking skills [Bell, 1991a, 1991b; Lindamood and Bell, 1997; Johnson‐Glenberg, 2000]. The V/V program is designed to teach children to form imaged gestalts, or concept imagery, as they read and hear language. Through the sequential teaching methods of the program, the imaged gestalt helps develop the imagery‐language connection and improve oral and reading comprehension. This is based on the dual coding theory which posits that cognition involves the activity of two distinct subsystems, a verbal system specialized for dealing directly with language, and a nonverbal (imagery) system specialized for dealing with nonlinguistic objects and events [Paivio, 2007; Sadoski and Paivio, 2001]. The student progresses in the program by beginning with word imagery and then extending their understanding to sentence, paragraph, and page imagery. The ultimate goal of this intervention is to apply nonverbal imagery to language comprehension to improve children's reading and listening comprehension, communication skills, and critical thinking skills. The clinical teaching is what the program describes as a process‐based approach, “responding to the response,” leading students to meta‐cognitively “see” the meaning of what they are reading or listening to and then verbalize what they have visualized. Research has shown this intervention to be effective for children with other learning disabilities specific to reading, including dyslexia and hyperlexia [Bell, 1991a; Johnson‐Glenberg, 2000].
The V/V intervention the children with ASD received in the current study was intensive, taking place in 4‐hour sessions per day, 5 days a week for 10 weeks. The intervention was conducted at the Lindamood–Bell Learning Processes center closest and/or convenient to where each participant's family lives. Trained clinicians administered the intervention in a standardized manner, and were monitored by an experienced supervisor who gives constant feedback to the clinicians. Implementation of V/V instruction to the participants was done one‐on‐one in a distraction‐free setting, with clinicians rotating every hour.
fMRI Data Acquisition
The MRI data were collected using a Siemens 3.0 Tesla Allegra head‐only Scanner (Siemens Medical, Erlangen, Germany) located at the UAB Civitan Functional Neuroimaging Laboratory (CFNL). Each session consisted of the following scans: (1) 3‐plane localizer scan; (2) SENSE calibration scan; (3) 3‐D high‐resolution anatomical scan; (4) fMRI scans of activation to the resting state scan. For the high resolution anatomical scan, T1‐weighted scans were acquired using a 160‐slice 3D MPRAGE (Magnetization Prepared Rapid Gradient Echo) volume scan with repetition time (TR) = 200 ms, echo time (TE) = 3.34 ms, flip angle = 7, FOV = 25.6 cm, 256 × 256 matrix size, and 1 mm slice thickness. Functional MR images were acquired using a single‐shot T2*‐weighted gradient‐echo EPI pulse sequence. We used TR = 1000 ms, TE = 30 ms, and a 60° flip angle for 17 oblique axial slices 5 mm slice thickness with a 1 mm slice gap, a 24 × 24 cm field of view (FOV), and a 64 × 64 matrix, resulting in an in‐plane resolution of 3.75 × 3.75 × 5 mm3. For the resting state scan, a total of 419 volumes were acquired for a total scan time of 6 min 59 s, with a TR of 1000 ms.
Data Preprocessing
Functional images were processed using the Statistical Parametric Mapping (SPM8) software (Wellcome Department of Cognitive Neurology, London, UK) and Analysis of Functional NeuroImages AFNI software [Cox, 1996]. Functional images were corrected for slice acquisition timing, motion‐correction by registering each functional volume to the first time point of the scan, normalized to the MNI space, resampled (3mm isotropic) and smoothed with an 8 mm Gaussian kernel. The normalized and smoothed images were then low bandpass filtered (0.008 < f < 0.08 Hz).
Correction for Head Motion
Because head motion can impact functional connectivity analyses, the following precautions were taken [Satterthwaite et al., 2013; Van Dijk et al., 2012]. Head motion was quantified as the Euclidean distance calculated from the six rigid‐body motion parameters for two consecutive time points. For any instance >2mm, considered excessive motion, the time point as well as the immediately preceding and subsequent time points were censored, or “scrubbed” [Power et al., 2012]. If two censored time points occurred within ten time points of each other, all time points between them were also censored. All participants retained >90% of their time points after censoring. Average head motion over each participant's session was defined as the root mean square of displacement (RMSD) and did not significantly differ between all groups (all P values not significant from two‐ and paired‐sample t‐tests). We additionally computed correlations between RMSD and functional connectivity values and found no significant correlations in the TD and ASD groups. Three subjects with ASD (2 in the ASD‐EXP group and 1 on the ASD‐WLC group) and three TD subjects had to be excluded because of excessive head motion in which too many time points would have had to be censored.
Group Independent Component Analysis
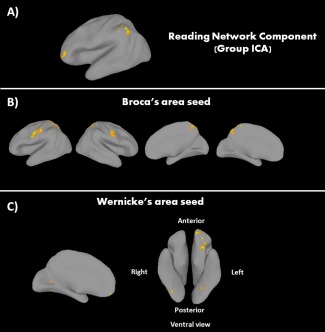
Group independent component analysis (GICA) was conducted on all 53 participants using the fMRI Group ICA Toolbox (GIFT; http://icatb.sourceforge.net, version 1.3e). The GICA was carried out twice, with all subjects at the first imaging session (n = 53), and the ASD groups (EXP and WLC) at the second imaging session (n = 31). For both GICA, independent components were estimated using the minimum description length criteria, modified to account for spatial correlation, [Li et al., 2007], with 34 independent components being estimated at the first session and 21 independent components being estimated for the second session. The GIFT toolbox organizes the data into three main batch steps: data reduction using principal component analysis (PCA), ICA and back reconstruction. Two data reduction steps are used in which each subject's data is reduced by PCA, which helped to reduce the impact of noise and made the estimation computationally tractable. ICA was then applied to the data set, and then back reconstruction of subjects’ time courses and spatial maps are generated such that each subject has a specific spatial map and time course for each estimated component [Calhoun et al., 2001, 2008]. Each component was visually inspected for artifacts, such as activation primarily in white matter or ventricles, or components suggestive of eye movements, head motion or cardiac‐inducted pulsatile artifact at the base of the brain. This resulted in 23 components at the first session and 17 components at the second session that were selected for further analysis.
Based on our specific hypotheses of intervention related changes to the reading network, we performed a spatial correlation analyses with the surviving components to determine which components had a high spatial overlap with this network. For the reading network we created a spatial map based of the anatomical regions identified during rest from a meta‐analysis of core language regions involved in reading in children conducted by Koyama et al. [2011]. These bilateral seeds included: inferior occipital gyrus (IOG), fusiform gyrus (FFG), superior temporal gyrus (STG), precentral gyrus (PrCG), intraparietal sulcus (IPS), supplementary motor area (SMA), inferior frontal gyrus triangularis (IFGtr), middle frontal gyrus (MFG), and thalamus (THAL). All regions were defined structurally using templates from the WFU Pickatlas toolbox within SPM8 using the AAL or Talairach Daemon atlases [Lancaster et al., 2000; Maidjian et al., 2004]. The exception was the intraparietal sulcus, for which we used a 6 mm predefined sphere identified by Koyama et al. [2011]. This anatomical mask, referred to as the Koyama reading network, was used specifically as an a priori mask to aid in selecting an independent component that best represented the reading network. Five components were identified as having a correlation value over 0.1 with the Koyama reading network, and visual inspection of these components confirmed that they included regions from our spatial map of the Koyama reading network. The component with the highest correlational value (Pearson's r = 0.272), which we will refer to throughout the rest of the paper as the reading network component, was selected for the between‐group analyses (described below). It should be noted that this corresponding network had the highest spatial correlation with the Koyama reading network mask at both the first and second imaging session.
GICA Component Identification
Of the five components with relatively moderate correlation (Pearson's correlation coefficient range = 0.117–0.272) with our a priori spatial map of the Koyama reading network, only one component had a significant correlation value. This component we identified as the reading network component, which included bilateral IFG—triangularis (IFGtr; BA45), right IFG—opercularis (RIFGop), left IFG—orbital (LIFGor), bilateral middle temporal gyrus (MTG), bilateral FFG, LIPS, bilateral medial prefrontal cortex (MPFC), and right cuneus (see Fig. 1A; Supporting Information Table S1). One‐sample t‐tests of the regions included in this component for each group for each imaging session demonstrated the same regions were represented within this component across groups and time points. Additionally, we assessed the other components (n = 18) derived from group ICA that were not correlated with the Koyama reading network to determine if any additional components showed intervention‐related changes outside of the reading network regions. We found no additional brain region pairs that increased their magnitude of connectivity from preintervention to postintervention that were not encompassed in the reading network component as identified above.
Figure 1.
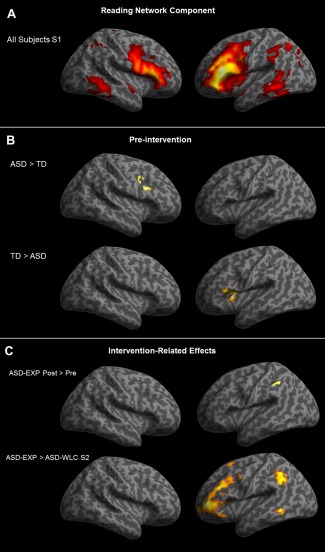
(A) The independent component with the highest spatial correlation with our reading network mask derived from all subjects combined (TD + ASD‐EXP + ASD‐WLC groups; n = 53) at the first imaging session (FWE corrected at P < 0.05). (B) Between‐group differences in within‐network connectivity for all ASD combined at the first imaging session (ASD‐EXP + ASD‐WLC groups) versus TD group (P < 0.001, FDR corrected). (C) Intervention related effects: Top panel: Between‐group analysis showing greater network connectivity at postintervention than perintervention for the ASD‐EXP group; bottom panel: Between group analysis showing greater network connectivity in the ASD‐EXP group compared to the ASD‐WLC group at the second imaging session. Maps are thresholded at P < 0.001, FDR corrected. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Statistical Analysis of Reading Network Component
For each subject, the reading network component was transformed to z‐scores and were entered into SPM8 for a series of two‐sample tests, with the TD children contrasted with the children with ASD (ASD‐EXP + ASD‐WLC groups) at the first imaging session, and the ASD‐EXP group contrasted with the ASD‐WLC group at the second imaging session. In addition, a paired t‐test was conducted to assess differences preintervention to postintervention in the ASD‐EXP group. All results were masked with all subjects’ (TD, ASD‐EXP, and ASD‐WLC groups) averaged independent component thresholded at FWE corrected P < 0.05 at either the first or second imaging session to assess only within network differences. In addition, for the ASD‐EXP group, we also assessed for differences outside the masked average component at both the first and second imaging sessions to better identify differences based on the intervention. For regions outside the masked average component, we corrected for multiple comparisons using a FDR cluster correction of P < 0.05.
Seed‐Based whole‐Brain Analysis
The MNI coordinates for Broca's area (−51, 27, 18), and Wernicke's area (−51, −51, 30) were selected based on a previous study involving TD population [Tomasi and Volkow, 2012]. Seeds were created using spherical binary masks (6 mm‐radius) and residual time‐series were extracted for each seed and correlated with every other voxel in the brain for every participant. A Fisher's r to z transformation was applied to the correlation maps for each participant before averaging and computing statistical maps for each seed. Group comparisons for each seed connectivity map included two‐sample t‐tests to compare: (1) TD vs. all ASD participants (ASD‐EXP + ASD‐WLC) at first imaging session, (2) ASD‐EXP vs. ASD‐WLC at second imaging session, and (3) ASD‐EXP vs. ASD‐WLC at second imaging session while using the first imaging session connectivity maps as covariates; and paired‐sample t‐tests to compare: (4) ASD‐WLC first imaging session vs. second imaging session, and (5) ASD‐EXP preintervention vs. postintervention. To correct for multiple comparisons, 10,000 Monte Carlo simulations were computed to obtain a cluster‐size‐corrected FWE threshold of P < 0.05. To account for the signal from cerebral white matter and lateral ventricles, masks were defined by anatomical masks using the WFU PickAtlas [Maldjian et al., 2003]. Masks were trimmed to avoid partial‐volume effects, and an average time‐series for each region was extracted. Derivatives for head motion parameters, white matter and ventricular time series were computed. Sources of noise (head motion, white matter, and lateral ventricles plus derivatives) were low bandpass filtered (0.008 < f < 0.08 Hz), modeled, and removed using a general linear model, and residuals were used for functional connectivity analysis.
Correlating Improvement in Reading Comprehension with Connectivity
We further examined the relationship between changes in functional connectivity in the ASD‐EXP group and changes in reading performance measured by the Gray Oral Reading Test (GORT, 4th edition). The percent change GORT‐4 scores from preintervention to postintervention and z‐score maps of each whole‐brain analysis (Wernicke's and Broca's area) were examined in a voxel‐wise manner through simple regression analyses. This generated a whole‐brain correlation map showing which regions in the brain were correlated with GORT‐4 scores, and cluster correction was applied as described above. In addition, changes in GORT‐4 scores were correlated with the reading network component identified by GICA for the ASD‐EXP group postintervention. For all analyses, verbal IQ was included as a covariate.
RESULTS
Overview
The key findings of this study are: (1) The ASD‐EXP group showed a significant increase in their reading comprehension abilities that were not seen in the ASD‐WLC group; (2) ICA‐based connectivity analysis revealed a strengthening of within‐network functional connectivity of both Broca's (LIFG) and Wernicke's (LSTG) areas in the ASD‐EXP group postintervention; (3) Seed‐based functional connectivity results revealed the ASD‐EXP group showing increased connectivity post‐intervention between Broca's area and motor regions (e.g., SMA and precentral gyrus) and supramarginal gyrus, and between Wernicke's area and LH language regions (e.g., LIFG and LMTG); and (4) Lastly, greater connectivity in both Broca's and Wernicke's area was correlated with greater improvement in reading comprehension in the ASD‐EXP group.
Behavioral Results
The ASD‐EXP group showed significantly greater improvement in reading comprehension (from preintervention to postintervention) as measured by the GORT‐4 Comprehension subtest [paired t‐test, t(15) = 5.01, P < 0.0001].Conversely, the ASD‐WLC group did not have a significant change in reading comprehension from the first to second imaging session [paired t‐test, t(14) = 1.29, P = 0.218]. In addition, the ASD‐EXP group significantly improved their reading comprehension scores, indexed by percent change from first to second session in GORT‐4 scores, compared to the ASD‐WLC group [ASD‐WLC mean: 2.6%, ASD‐EXP mean: 16.4%, t(29) = 2.92, P = 0.006]. The TD group had significantly higher reading comprehension scores than the children with ASD at the one session they were tested [ASD‐EXP + ASD‐WLC groups versus TD; t(51) = 5.91, P < 0.0001].
Reading Network Component Connectivity: Independent Component Analysis
When the reading network component was compared between the TD children and the children with ASD (ASD‐EXP + ASD‐WLC) at the first imaging session, there was significantly reduced functional connectivity in the ASD groups compared to the TD group (ASD < TD) in the left insula (x= −32, y = 12, z = 10) and LIFG (Broca's area; x = −52, y = 20, z = 12), and overconnectivity (ASD > TD) in the RIFGop. There were no differences between the ASD‐EXP and ASD‐WLC group at the first imaging session. In a second set of comparisons, we assessed intervention effects in two different analyses: (1) The ASD‐EXP group revealed an increase in functional connectivity from preintervention to postintervention within the reading network component in Wernicke's area (pSTS; BA39/40; x = −58, y = −58, z = 44), and a decrease in connectivity from preintervention to postintervention within the RIFG ‐ orbital (IFGor; BA 47; see Fig. 1); (2) The ASD‐EXP group vs. ASD‐WLC group comparison at the second imaging session revealed greater functional connectivity in the ASD‐EXP group after intervention within the reading network component in bilateral IFG (x = −38, y = 50, z = 4; x = 52, y = 50, z= −4), LMFG (x = −44, x = 30, x = 22), LMTG (x = −58, y = −50, z = −8), and Wernicke's area (BA40, x = −46, y = −52, z = 44). There were no regions with greater connectivity in the ASD‐WLC group compared to the ASD‐EXP group at the second imaging session. A secondary analysis was conducted specifically to determine if there were regions outside of the reading network that also showed intervention related changes. Outside the reading network, we found additional recruitment of LSFG (x = −30, y = −62, z = −4) and RMFG (x = 52, y = 50, z = −4) at postintervention that was not seen in the reading network component at preintervention in the ASD‐EXP group.
Functional Connectivity of Broca's Area
An examination of all within‐group results in this study revealed strong functional connectivity between Broca's area with bilateral IFG and IPL with some additional connectivity, which varied across analysis, in regions such as SMA, subcortical regions, and temporal lobe regions (see Supporting Information Table S2). Analysis of group differences comparing TD children with all children with ASD (ASD‐EXP + ASD‐WLC groups) at the first imaging session showed overconnectivity (ASD > TD) between Broca's area with LIFG, bilateral MFG, and LMOG, along with underconnectivity in one cluster (TD > ASD) with LFFG (see Table 2).
Table 2.
Between‐group differences in connectivity with Broca's and Wernicke's seeds in the ASD group (ASD‐EXP + ASD‐WLC groups; n = 31) at the first imaging session and the TD group (n = 22) (P < 0.05, FWE corrected)
| Seed | Direction | Region | Hemisphere | Volume (# of voxels) | Peak coordinates | Peak | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | t | |||||
| Broca | ASD > TD | Middle frontal gyrus | R | 1049 | 52 | 28 | 13 | 3.5 |
| Middle frontal gyrus | L | 612 | −54 | 34 | 10 | 3.8 | ||
| Inferior frontal gyrus | L | 221 | −67 | 42 | 6 | 4.5 | ||
| Middle occipital gyrus | L | 127 | −52 | −42 | 49 | 4.0 | ||
| TD > ASD | Fusiform gyrus | L | 112 | −63 | −33 | −10 | −3.6 | |
| Wernicke | ASD > TD | Calcarine gyrus | L | 103 | −13 | −78 | −13 | 2.9 |
The second set of analyses was specifically aimed at assessing intervention‐related changes by comparing (1) ASD‐WLC and ASD‐EXP group at the second imaging session, while controlling for connectivity at the first imaging session. This revealed an overall shift in connectivity such that the ASD‐EXP group showed enhanced connectivity of Broca's area with right middle cingulate, RSMA, right precentral gyrus (RPrCG) and right supramarginal gyrus (RSMG), and weaker connectivity with the RSPL and right precuneus (see Fig. 2; Table 3) and (2) Additionally, the ASD‐EXP group preintervention to postintervention showed an overall shift in connectivity involving stronger connections of Broca's area with RMFG, RSTG, LSMG, and right caudate postintervention (see Fig. 3; Table 3). This was not seen in the ASD‐WLC group, who showed an overall reduction in connectivity between Broca's area with RSMA, and bilateral occipital, temporal, and subcortical regions from first to second imaging session. (see Supporting Information Table S3).
Figure 2.
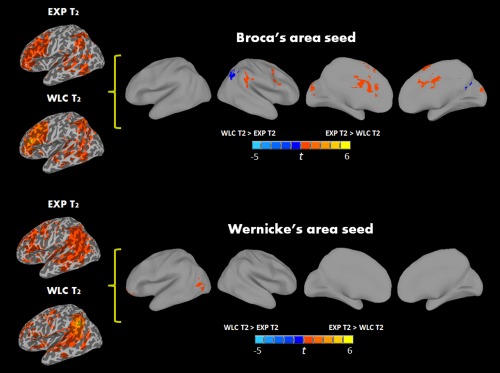
Areas of greater connectivity with Broca's and Wernicke's seeds in the ASD‐EXP group than the ASD‐WLC group postintervention, while controlling for within‐group connectivity at the first imaging session (P < 0.05, FWE corrected). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 3.
Intervention related effects in whole‐brain functional connectivity with both Broca's and Wernicke's seed (P < 0.05, FWE corrected)
| Seed | Effect | Region | Hemisphere | Volume (# of voxels) | Peak coordinates | Peak | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | t | |||||
| ASD‐EXP vs. ASD‐WLC at second imaging session | ||||||||
| Broca | WLC > EXP | Superior parietal lobule | R | 166 | 36 | −75 | 55 | 5.3 |
| WLC > EXP | Precuneus | R | 116 | 18 | −42 | 37 | 5.6 | |
| EXP > WLC | Middle cingulate | R | 464 | 3 | 6 | 37 | −5.2 | |
| EXP > WLC | SMA | R | 162 | 12 | 10 | 70 | −4.5 | |
| EXP > WLC | Precentral gyrus | R | 112 | 48 | −4 | 22 | −4.7 | |
| EXP > WLC | Supramarginal gyrus | R | 111 | 57 | −42 | 31 | −4.1 | |
| Wernicke | EXP > WLC | Cerebellum | R | 313 | 7 | −60 | −9 | −4.1 |
| EXP > WLC | Inferior frontal gyrus | L | 187 | −19 | 64 | 15 | −2.1 | |
| EXP > WLC | Middle temporal gyrus | L | 109 | −58 | −78 | −4 | −3.5 | |
| ASD‐EXP Preintervention vs. Postintervention | ||||||||
| Broca | Pre > Post | Middle occipital gyrus | R | 152 | 42 | −75 | 34 | −4.7 |
| Posterior cingulate cortex | R | 113 | 9 | −48 | 28 | −4.6 | ||
| Post > Pre | Middle frontal gyrus | R | 150 | 33 | 49 | 25 | 5.0 | |
| Supramarginal gyrus | L | 105 | −58 | −48 | 25 | 5.0 | ||
| Caudate nucleus | R | 100 | 21 | 16 | 16 | 4.9 | ||
| Superior temporal gyrus | R | 100 | 69 | −45 | 13 | 5.4 | ||
| Wernicke | Post > Pre | Anterior cingulate | R | 505 | 3 | 31 | 10 | 4.8 |
| Middle orbital gyrus | L | 318 | −34 | 61 | −9 | 4.6 | ||
| Inferior frontal gyrus | R | 211 | 51 | 19 | 12 | 4.5 | ||
| Inferior frontal gyrus | L | 205 | −34 | 16 | 22 | 4.9 | ||
| Middle frontal gyrus | R | 149 | 33 | 52 | 37 | 4.7 | ||
| Middle cingulate | R | 131 | 9 | 24 | 25 | 5.8 | ||
| Precentral gyrus | R | 103 | 36 | −24 | 55 | 4.2 | ||
Figure 3.
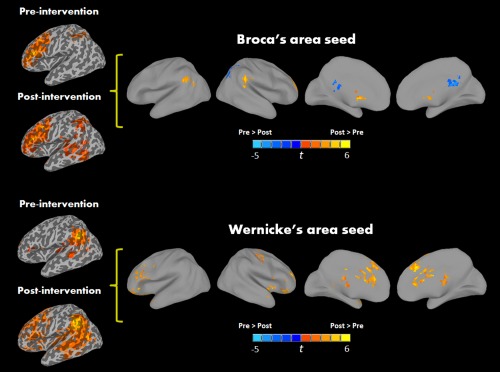
Areas of greater connectivity with Broca's and Wernicke's seeds postintervention than preintervention in the ASD‐ EXP group (P < 0.05, FWE corrected). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Functional Connectivity of Wernicke's Area
Functional connectivity of Wernicke's area with the rest of the brain in all of our groups showed strong time‐series correlations between this seed and IFG, temporal lobe regions, and with midline cortical structures, such as middle cingulate and precuneus in different analyses (see Supporting Information Table S2). Group difference analysis involving TD children and all children with ASD, at the first imaging session, revealed overconnectivity in the ASD group between Wernicke's area and calcarine sulcus (see Table 2). Our second analyses specific to intervention‐related changes compared 1) the ASD‐EXP and ASD‐WLC group at the second imaging session while controlling for connectivity at the first imaging session. The ASD‐EXP group showed enhanced connections postintervention with LIFG and LMTG and bilateral cerebellum when compared the ASD‐WLC group (see Fig. 2; Table 3); 2) the ASD‐EXP group preintervention to postintervention showed an overall shift in connectivity such that stronger connections of Wernicke's area with bilateral IFG, bilateral MFG, RPrCG, and cingulate cortex were detected postintervention (see Fig. 3; Table 3). Lastly, we again found that the ASD‐WLC group from first to second session showed an overall reduction in connectivity between Wernicke's area and LFFG and RIPL (see Supporting Information Table S3).
Relationship between fcMRI and Percent Change in Reading Comprehension Scores
Multiple regression analyses were performed to determine whether improvement in reading comprehension due to intervention, as measured by the GORT‐4 Comprehension subtest (controlling for verbal IQ), was correlated with functional connectivity in either our GICA or seed‐based analyses in the ASD‐EXP group. A significant positive correlation was found between percent change in GORT‐4 comprehension scores and connectivity of the reading network component postintervention for Wernicke's area (BA40) and LIFGor. There were no significant negative correlations with the reading network component (see Fig. 4a). For the seed‐based whole brain analyses involving Broca's area seed, a significant positive correlation between percent change in GORT‐4 scores and increase in connectivity from preintervention to postintervention was found in the bilateral postcentral gyrus (PoCG) and RSPL (see Fig. 4b). From Wernicke's area seed, a significant positive correlation between change in GORT‐4 scores and increase in connectivity from preintervention to postintervention was found in LIFGor, right cerebellum, and LMOG/SOG (see Fig. 4c). There were no significant negative correlations with the Broca's or Wernicke's seed.
Figure 4.
Positive correlations with percent change in reading comprehension scores (GORT‐4), controlling for verbal IQ, and functional connectivity from pre‐ to postintervention in the ASD‐EXP group: (A) Greater within‐network connectivity for our GICA identified reading component postintervention was correlated with greater reading improvement; (B) Greater connectivity in brain regions with Broca's seed was correlated with greater reading improvement; and (C) Greater connectivity in brain regions with Wernicke's seed was correlated with greater reading improvement. Maps are thresholded at P < 0.05, FWE corrected. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
This study identified low frequency fluctuations of spontaneous brain activity using rsfMRI to characterize the functional integrity of the language comprehension network in children with ASD. More importantly, we demonstrate the plasticity of functional connectivity across brain regions in children with ASD who received the V/V intervention, resulting in stronger connectivity of the reading network postintervention. It should also be noted that the improvement in reading comprehension in children with ASD was correlated with increase in functional connectivity in the reading network component identified by GICA (LIFG and LSTG) and between Broca's area and RSTG and Wernicke's area and LIFG in our seed‐based connectivity analyses. Moreover, children with ASD who received the intervention also showed additional recruitment of regions outside of the reading network postintervention.
Both the GICA and seed‐based approaches were sensitive in showing intervention‐related changes in our ASD‐EXP group at the second imaging session. In addition to both types of analyses showing stronger functional connectivity between Broca's and Wernicke's area as a result of intervention, we also found strengthening of connectivity of these regions with motor regions (SMA and PrCG). Moreover, greater improvement in reading comprehension was correlated with increased connectivity between Broca's area and PoCG. The SMA and PrCG/PoCG have been shown to be activated during different aspects of language processing, such as processing abstract sentences [Sakreida et al., 2013], mental rotation and mental imagery [Kosslyn et al., 2001; Tomasino and Rumiati, 2013], imagining concrete words [Postle et al., 2008; Pulvermuller and Hank, 2006], and lexical decision making [Carreiras et al., 2006; Tomasino et al., 2010]. Resting state studies of the language comprehension network also have consistently found connectivity between Broca's and Wernicke's areas and premotor and motor regions [Koyama et al., 2010, 2011; Tomasi et al., 2012; Verly et al., 2014]. For example, more effortful lexical processing (differentiating between words and pseudowords) elicited greater recruitment of motor regions, specifically SMA [Carreiras et al., 2006]. Similarly, Koyama et al., [2011] found that increased functional connectivity between Broca's and Wernicke's areas and PrCG and PoCG, in children, but not adults, was correlated with increased reading competence, and may reflect more effortful sight reading. In children with ASD, loss of connectivity between Broca's area and SMA was found to be correlated with lower level of language skills, including word and sentence comprehension [Verly et al., 2014]. Increased connectivity between language and motor regions in our ASD‐EXP group is of especial interest in the context of Tomasino and Rumiati's [2013] account of the utility of motor activation in language comprehension. They proposed that, given the large number of language studies that find sensorimotor activity independent of action related stimuli, activation of these areas are related to a selective cognitive strategey (whether explicitly or automatically) of utilizing mental imagery to enhance reading comprehension across linguistic tasks [Tomasino and Rumiati, 2013]. These findings are also in support of the dual coding theory of cognition which posits that all modality‐specific mental representations derive from sensory experience and can be classified as either verbal or nonverbal [Paivio, 2007; Sadoski and Paivio, 2001]. This is especially pertinent given the V/V intervention the children with ASD received in our study focused on imagery to enhance oral and written language comprehension.
Another specific difference we observed in intrinsic connectivity was an increase in functional connectivity unique to the ASD‐EXP group between Broca's area and bilateral SMG. The SMG has been found to have an active role in language comprehension, specific to phonological processes [Hartwigsen et al., 2010; Sliwinska et al., 2012; Turkeltaub et al. 2003]. The SMG has also been found to be involved in mentally sounding out words [Stoeckel et al., 2009], during visual word recognition [Carreiras et al., 2009; Stoeckel et al., 2009], in lexical processing [Osipowicz et al., 2011], and in verbal working memory [Acheson et al., 2011; Deschamps et al., 2014]. Additionally, greater activation of bilateral SMG has been correlated with greater accuracy and efficiency in phonological decision making [Harwigsen et al., 2010]. Interestingly, a recent diffusion tensor imaging (DTI) study by Li et al. [2014] found that greater white matter connectivity associated with the SMG was correlated with increased reading comprehension (using the same GORT‐4 measure as used in our study), but only in the ASD group. It is noteworthy that strengthening of RSMG connectivity with motor regions [PrCG and PoCG; Li et al., 2014] was also found in our study, specifically, increased connectivity with both Broca's area and PrCG and PoCG as well as SMG. This suggests compensatory mechanisms in gaining proficiency in reading comprehension that involves both the SMG and motor regions that may underlie unique strategies in children with ASD.
Intervention‐related effects were also seen with stronger connectivity in the caudate and cerebellum, regions that have only recently begun to be identified as having a role in reading comprehension. Additionally, improvement in reading comprehension after intervention was correlated with increased connectivity between Wernicke's area and right cerebellum. The caudate has been shown to be functionally connected to Broca's and Wernicke's areas in typical adults [Tomasi et al., 2012]. Recent studies have found caudate to be involved in accuracy of phonological processing [Tettamanti et al., 2005] and suppressing word interference [Ali et al., 2010]. Indeed the frontal‐caudate loop returns back to Broca's area [Middleton and Strick, 1994], and lesions of the caudate show similar cognitive deficts as lesions to Broca's area including semantic and phonological processing and memory retrieval for fluent reading [Abdullaev et al., 1998]. Along with IFG, the cerebellum has been found to be involved in processing semantic word association [Frings et al., 2006; Xiang et al., 2003]. Moreover, cerebellar lesion studies have found specific deficits in higher‐order language processes, such as verbal fluency, syntax, word retrieval, and reading and writing [Marien et al., 2001; Schmahmann and Sherman, 1998; Silveri et al., 1994]. In ASD, abnormal function of the right cerebellum has been shown to be related to deficits in language functioning [Hodge et al. 2010; Verly et al., 2014].
Interestingly, there was a distinct hemispheric difference in the improvement in connectivity in the Broca's and Wernicke's seed regions when the ASD‐EXP group was compared to the ASD‐WLC group postintervention. The ASD‐EXP group who received intervention showed increased connectivity between Broca's area and RH language‐analogous regions, such as the SMA, PrCG, and SMG. Wernicke's area, conversely, showed strengthening of connectivity with LH language regions, including LIFG and LMTG. It has been well documented that individuals with ASD tend to rely heavily on posterior parietal and occipital regions when engaged in a language task, at the expense of recruiting left frontal regions [Kana et al., 2006; Kana and Wadsworth, 2012; Mason et al., 2008]. In addition, recent studies have demonstrated that even young children with ASD fail to activate LH regions in response to auditory and verbal language tasks [Eyler et al., 2012; Groen et al., 2010; Sahyoun et al. 2010]. This suggests that the children with ASD who received the intervention may have difficulties modulating Broca's area with other LH regions, and are compensating by increasing functional connectivity between Broca's area and RH regions. Conversely, the posterior parietal regions in our ASD participants may help in increasing connectivity between Wernicke's area and more traditional LH language regions. Indeed, in the reading network component identified by GICA, the ASD‐EXP group strengthened the connectivity of LSTG while decreasing reliance on RIFG. This is further supported by the fact that increased connectivity across different regions was correlated with greater improvement in reading comprehension. Additionally, while failure to activate LH regions in response to language has been a characteristic feature of ASD [Herbert et al., 2005], recent studies assessing the language comprehension network in TD individuals have found RH involvement specific to RMTG and RSMG when interpreting higher‐order language, such as idiomatic sentences [Proverbio et al., 2009] and phonological interpretation [Hartwigsen et al., 2010]. Lexical decoding also has been shown to be associated with activation of RH regions, specifically RMFG, RSMG, and bilateral cerebellum, as a function of skilled reading ability [Osipowicz et al., 2011]. Thus, it is also possible that the ASD‐EXP group may be strengthening a left‐right connection between Broca's area and MFG, SMG and MTG, which already exists in unimpaired adult readers [Tomasi et al., 2012].
One interesting result that deserves mention is a significantly greater connectivity in ASD children, relative to TD children, between Broca's and left frontal regions, and Wernicke's area and calcarine gyrus. While initial literature in intrinsic connecitivity had focused on adults with ASD [e.g., Assaf et al., 2010; Kennedy and Courchesne, 2008; Monk et al., 2009; von dem Hagen et al., 2012], studies investigating intrinsitc connecitivity in children with ASD have consistently shown hyperconnecitivy compared to TD children [Supekar et al., 2013; Di Martino et al., 2011; Uddin et al., 2013]. This may suggest a developmental shift from hyperconnectivity to hypoconnectivity as individuals with ASD mature into adulthood. In addition, the underconnectivity account emphasizes weaker connectivity of long‐range cortical connections in favor of local connectivity [e.g., Just et al., 2004; Muller et al., 2011; Courchesne et al., 2007]. This is consistent with what we observed in our study, with hyperconnectivity in ASD between Broca's and spacially adjacent frontal regions, and between Wernicke's and calcarine regions, when compared to TD controls. Indeed, greater connectivity in the TD group was seen only for more long‐range connections, between Broca's area and FFG. Interestingly, Li et al. [2014] found hyperconnectivity differences specific to local connectivity in children with ASD compared to TD children, and that greater local connectivity in the ASD group, but not the TD group, was postively correlated with reading ability. However, it is important to note that our ASD‐EXP group was able to strengthen frontal to posterior connections in reading related brain regions through intervention. This has larger implications by emphasizing the need for targeted intervention in children with ASD. Morever, in recent studies of functional connectivity and language in TD individuals, the differences that have been observed in connectivity due to development have only been assessed via cross‐sectional studies [Church et al., 2008; Vogel et al., 2013], whereas our pretest/post‐test design allowed us to examine the developmental trajectories and intervention effects in children with ASD.
While we have carefully controlled and matched the different groups of participants, a potential limitation pertains to the selection of children with ASD with a specific reading profile that would be most likely to benefit from the intervention, namely adequate decoding skills coupled with statistically poorer reading comprehension. As such, the results of this study may not be generalized to different subtypes of ASD. Additionally, even in high‐functioning individuals, it has been observed that individuals with lower or higher symptom severity tend to reflect different connectivity profiles [Fishman et al., 2014; Keown et al., 2013]. This may result in differences in improvement due to the intervention, i.e., different connectivity routes/regions for some individuals. Future studies should assess whether reading interventions targeting other deficits in ASD show similar patterns of change in functional connectivity. Lastly, it is unclear whether the changes in functional connectivity seen in this study may be contributed by anatomical changes in the regions involved. Future work should assess structural changes of the reading network in children with ASD after intervention and its relation with functional connectivity. To our knowledge, there have been no translational studies involving intervention that have assessed structural changes of the reading network in children, which would be the next logical step.
In summary, we found that improvement in reading comprehension due to reading intervention in children with ASD was associated with strengthening of functional connectivity in Wernicke's area and LIFGor and other premotor and language regions. We also found increased connectivity specific to the children with ASD who participated in the reading intervention between Wernicke's area and posterior language and visual brain regions, increased compensatory mechanisms for language comprehension in RH connections with Broca's area, strengthening of connections with SMG, and strengthening of connectivity of regions outside to conventional language network, including caudate and cerebellum. Furthermore, our study utilized intrinsic resting state data to accurately identify the reading network and determine functional connectivity differences independent of the constraints of task. The findings of this study emphasize the importance of targeted interventions for children with ASD, and the neuroplasticity in ASD is encouraging for future studies to continue to assess intervention‐related changes in brain circuitry.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors would like to thank Lindamood–Bell Learning Processes for providing financial support for this study. No representatives of the company were involved in data analysis or development of this report, nor did the company exert any control or restrictions with regard to these activities. Thus, there are no conflicts of interest to be declared. Authors would also like to thank Amy Lemelman, Miranda Morris, Claire Crider, Hrishikesh Deshpande, Soumya Sivaraman, David Knight, Kristina Visscher, Edwin Cook, Sarah O'Kelley, Dave Hungerford, Liz Szporn, Lynn Flowers, Nick Eardley, Nanci Bell, Paul Worthington, and Nancy Minshew for their help with different aspects of this study. Finally, we would like to extend our sincerest appreciation to the participants and families who gave generously of their time and courage to participate in this intensive neuroimaging study.
REFERENCES
- Abdullaev YG, Bechtereva NP, Melnichuk KV (1998): Neuronal activity of human caudate nucleus and prefrontal cortex in cognitive tasks. Behav Brain Res 97:159–177. [DOI] [PubMed] [Google Scholar]
- Acheson DJ, Hamidi M, Binder JR, Postle BR (2011): A common neural substrate for language production and verbal working memory. J Cogn Neurosci 23:1358–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali N, Green DW, Kherif F, Devlin JT, Price CJ (2010): The role of the left head of caudate in suppressing irrelevant words. J Cogn Neurosci 22:2369–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013): Diagnostic and statistical manual of mental disorders (5th ed). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Assaf M, Jagannathan K, Calhoun VD, Miller L, Stevens MC, Sahl R, O'Boyle JG, Pearlson GD (2010): Abnormal functional connectivity of default mode sub‐networks in autism spectrum disorder patients. Neuroimage 15:247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell N (1991a): Gestalt imagery: A critical factor in language comprehension. Ann Dyslexia 41:246–260. [DOI] [PubMed] [Google Scholar]
- Bell N. (1991b). Visualizing and Verbalizing for Language Comprehension and Thinking. Paso Robles: Academy of Reading Publications. [Google Scholar]
- Carreiras M, Mechelli A, Price CJ (2006): Effect of word and syllable frequency on activation during lexical decision and reading aloud. Hum Brain Mapp 27:963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreiras M, Seghier M, Baquero S, Este´vez A, Lozano A, Devlin J, Price CJ (2009): An anatomical signature for literacy. Nature 461:983–986. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) (2014. ): Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network , 11 Sites, United States, 2010. MMWR Surveillance Summary, 63, 1–21. [PubMed]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ (2001): Spatial and temporal independent component analysis of functional MRI data containing a pair of task‐related waveforms. Hum Brain Mapp 13:43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Kiehl KA, Pearlson GD (2008): Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum Brain Mapp 29:828–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church JA, Coalson RS, Lugar HM, Petersen SE, Schlaggar BL (2008): A developmental fMRI study of reading and repetition reveals changes in phonological and visual mechanisms over age. Cereb Cortex 18:2054–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, Morgan J (2007): Mapping early brain development in autism. Neuron 56:399–413. [DOI] [PubMed] [Google Scholar]
- Cox RW (1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173. [DOI] [PubMed] [Google Scholar]
- Deco G, Corbetta M (2011): The dynamical balance of the brain at rest. Neuroscientist 17:107–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps I, Baum SR, Gracco VL (2014): On the role of the supramarginal gyrus in phonological processing and verbal working memory: Evidence from rTMS studies. Neuropsychologia 53:39–46. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Kelly C, Grzadzinski R, Zuo XN, Mennes M, Mairena MA, Lord C, Milham MP (2011): Aberrant striatal functional connectivity in children with autism. Biol Psychiatry 69:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt EB, Allen EA, Damaraju E, Calhoun VD (2011): On network derivation, 753 classification, and visualization: A response to habeck and moeller. Brain Connect 754: 1–19. [PMC free article] [PubMed] [Google Scholar]
- Eyler LT, Pierce K, Courchesne E (2012): A failure of left temporal cortex to specialize for language is an early emerging and fundamental property of autism. Brain 135:949–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman I, Keown CL, Lincoln AJ, Pineda JA, Muller RA (2014): Atypical cross talk between mentalizing and mirror neuron networks in autism spectrum disorder. JAMA Psychiatry 71:751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen WB, Zwiers MP, van der Gaag RJ, Buitelaar JK (2008): The phenotype and neural correlates of language in autism: An integrative review. Neurosci. Biobehav Rev 32:1416–1425. [DOI] [PubMed] [Google Scholar]
- Groen WB, Tesink C, Petersson KM, van Berkum J, van der Gaag RJ, Hagoort P, Buitelaar JK (2010): Semantic, factual, and social language comprehension in adolescents with autism: An fMRI study. Cereb Cortex 20:1937–1945. [DOI] [PubMed] [Google Scholar]
- Hampson M, Tokoglu F, Sun Z, Schafer RJ, Skudlarski P, Gore JC, Constable RT, (2006): Connectivity‐behavior analysis reveals that functional connectivity between left ba39 and broca's area varies with reading ability. Neuroimage 31:513–519. [DOI] [PubMed] [Google Scholar]
- Hartwigsen G, Baumgaertner A, Price CJ, Koehnke M, Ulmer S, Siebner H (2010): Phonological decisions require both the left and right supramarginal gyri. Proc Natl Acad Sci USA 107:16494–16499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert M, Ziegler D, Deutsch C, O'Brien L, Kennedy D, Filipek P, Bakardjiev AI, Caviness VS Jr. (2005): Brain asymmetries in autism and developmental language disorder: A nested whole‐brain analysis. Brain 128:213–226. [DOI] [PubMed] [Google Scholar]
- Hodge SM, Makris N, Kennedy DN, Caviness VS, Jr. , Howard J, McGrath L, Steele S, Frazier JA, Tager‐Flusberg H, Harris GJ (2010): Cerebellum, language, and cognition in autism and specific language impairment. J Autism Dev Disord 40:300–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson‐Glenberg MC (2000): Training reading comprehension in adequate decoders/poor comprehenders: Verbal versus visual strategies. J Educa Psychol 92:772–782. [Google Scholar]
- MA Just, VL Cherkassky, TA Keller, NJ Minshew (2004): Cortical activation and synchronization during sentence comprehension in high‐functioning autism: evidence of underconnectivity. Brain 127:1811–1821. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VA, Minshew NJ, Just MA (2006): Sentence comprehension in autism: Thinking in pictures with decreased functional connectivity. Brain: J Neurol 129:2484–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Wadsworth HM (2012): The archeologist's career ended in ruins: Hemispheric differences in pun comprehension in autism. Neuroimage 62:77–86. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E (2008): The intrinsic functional organization of the brain is altered in autism. Neuroimage 39:1877–1885. [DOI] [PubMed] [Google Scholar]
- Keown CL, Shih P, Nair A, Peterson N, Mulvey ME, Muller RA (2013): Local functional overconnectivity in posterior brain regions is associated with symptom severity in autism spectrum disorders. Cell Rep 5:567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslyn SM, Thompson WL, Wraga M, Alpert NM (2001): Imagining rotation by endogenous versus exogenous forces: Distinct neural mechanisms. Neuroreport 12:2519–2525. [DOI] [PubMed] [Google Scholar]
- Koyama MS, Kelly C, Shehzad Z, Penesetti D, Castellanos FX, Milham MP (2010): Reading networks at rest. Cereb Cortex 20:2549–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama MS, Di Martino A, Zuo XN, Kelly C, Mennes M, Jutagir DR, Milham MP (2011): Resting‐state functional connectivity indexes reading competence in children and adults. J Neurosci 31:8617–8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Fox PT (2000): Automated talairach atlas labels for functional brain mapping. Hum Brain Mapp 10:120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YO, Adali T, Calhoun VD (2007): Estimating the number of independent components for functional magnetic resonance imaging data. Hum Brain Mapp 28:1251–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Xue Z, Ellmore TM, Frye RE, Wong ST (2014): Network‐based analysis reveals stronger local diffusion‐based connectivity and different correlations with oral language skills in brains of children with high functioning autism spectrum disorders. Hum Brain Mapp 35:396–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann G, Hoehl S, Brauer J, Danielmeier C, Bornkessel‐Schlesewsky I, Bahlmann J, Turner R, Friederici A (2010): Setting the frame: The human brain activates a basic low‐frequency network for language processing. Cereb Cortex 20:1286–1292. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A (1994): Autism diagnostic Interview‐revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 24:659–685. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook Jr, EH , Leventhal BL, DiLavore PC, Pickles A, Rutter M (2000): The autism diagnostic observation schedule‐generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 30:205–223. [PubMed] [Google Scholar]
- Lindamood P, Bell N (1997): Sensory‐cognitive factors in the controversy over reading instruction. J Dev Learn Disorders 1:143–182. [Google Scholar]
- Mason RA, Williams DL, Kana RK, Minshew NJ, Just MA (2008): Theory of mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia 46:269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage, 19:1233–1239. [DOI] [PubMed] [Google Scholar]
- Maidjian JA, Laurienti PJ, Burdette JH (2004): Precentral gyrus discrepancy in electronic versions of the talairach atlas. Neuroimage 21:450–455. [DOI] [PubMed] [Google Scholar]
- Marien P, Engelborghs S, Fabbro F, De Deyn PP (2001): The lateralized linguistic cerebellum: A review and a new hypothesis. Brain Lang 79:580–600. [DOI] [PubMed] [Google Scholar]
- Mason RA, Williams DL, Kana RK, Minshew NJ, Just MA (2008): Theory of mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia 46:269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Peltier SJ, Wiggins JL, Weng SJ, Carrasco M, Risi S, Lord C (2009): Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage 47:764–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL (1994): Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science 266:458–461. [DOI] [PubMed] [Google Scholar]
- R‐A Müller, P Shih, B Keehn, JR Deyoe, KM Leyden, DK Shukla (2011): Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cereb Cortex 21:2233–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frings M, Dimitrova A, Schorn CF, Elles HG, Hein‐Kropp C, Gizewski ER, Diener HC, Timmann D (2006): Cerebellar involvement in verb generation: An fMRI study. Neurosci Lett 409:19–23. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Keller TA (2010): The nature of brain dysfunction in autism: Functional brain imaging studies. Curr Opin Neurol 23:124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nation K, Clarke P, Wright B, Williams C (2006): Patterns of reading ability in children with autism spectrum disorder. J Autism Dev Disorders 36:911–919. [DOI] [PubMed] [Google Scholar]
- Newman TM, Macomber D, Naples AJ, Babitz T, Volkmar F, Grigorenko EL (2007): Hyperlexia in children with autism spectrum disorders. J Autism Dev Disorders 37:760–774. [DOI] [PubMed] [Google Scholar]
- Norbury C, Nation K (2011): Understanding variability in reading comprehension in adolescents with autism spectrum disorders: Interactions with language status and decoding skill. Sci Studies Read 15:191–210. [Google Scholar]
- Osipowicz K, Rickards T, Shah A, Sharan A, Sperling M, Kahn W, Tracy J (2011): A test of the role of the medial temporal lobe in single‐word decoding. Neuroimage 54:1455–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paivio A. (2007). Mind and its evolution: A dual coding theoretical approach. Mahwah, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Postle N, McMahon KL, Ashton R, Meredith M, de Zubicaray GI (2008): Action word meaning representations in cytoarchitectonially defined primary and premotor cortices. Neuroimage 43:634–644. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012): Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proverbio A, Crotti N, Zani A, Adorni R (2009): The role of left and right hemispheres in the comprehension of idiomatic language: An electrical neuroimaging study. BMC Neurosci 10:116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvermuller F, Hauk O (2006): Category‐specific conceptual processing of color and form in left fronto‐temporal cortex. Cereb Cortex 16:1193–1201. [DOI] [PubMed] [Google Scholar]
- Raichle ME (2009): A paradigm shift in Functional brain imaging. J Neurosci 29:12729–12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoski M, Paivio A. Imagery and text: A dual coding theory of reading and writing. Mahwah, NJ: Lawrence Erlbaum Associates; 2001. [Google Scholar]
- Sahyoun CP, Belliveau JW, Soulières I, Schwartz S, Mody M (2010): Neuroimaging of the functional and structural networks underlying visuospatial vs. Linguistic reasoning in highfunctioning autism. Neuropsychologia 48:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakreida K, Scorolli C, Menz MM, Heim S, Borghi AM, Binkofski F (2013): Are abstract action words embodied? An fMRI investigation at the interface between language and motor cognition. Front Hum Neurosci 9:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Wolf DH (2013): An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting‐state functional connectivity data. Neuroimage 64:240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC (1998): The cerebellar cognitive affective syndrome. Brain 121:561–579. [DOI] [PubMed] [Google Scholar]
- Sliwinska MW, Khadilkar M, Campbell‐Ratcliffe J, Quevenco F, Devlin JT (2012): Early and sustained supramarginal gyrus contributions to phonological processing. Front Psychol 3:161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MC, Leggio MG, Molinari M (1994): The cerebellum contributes to linguistic production: A case of agrammatic speech following a right cerebellar lesion. Neurology 44:2047–2050. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Beckmann CF (2009): Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci USA 106:13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel C, Gough PM, Watkins KE, Devlin JT (2009): Supramarginal gyrus involvement in visual word recognition. Cortex 45:1091–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Uddin LQ, Khouzam A, Phillips J, Gaillard WD, Kenworthy LE, Yerys BE, Menon V (2013): Brain hyperconnectivity in children with autism and its links to social deficits. Cell Rep 5:738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tettamanti M, Moro A, Messa C, Moresco RM, Rizzo G, Carpinelli A, Matarrese M, Perani D (2005): Basal ganglia and language: Phonology modulates dopaminergic release. Neuroreport 16:397–401. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND (2012): Resting functional connectivity of language networks: Characterization and reproducibility. Mol Psychiatry 17:841–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasino B, Rumiati RI (2013): At the mercy of strategies: The role of motor representations in language understanding. Front Psychol 4:24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasino B, Weiss PH, Fink GR (2010): To move or not to move: Imperatives modulate action‐related verb processing in the motor system. Neuroscience 169:246–258. [DOI] [PubMed] [Google Scholar]
- Torgesen JK, Alexander AW, Wagner RK, Rashotte CA, Voeller KK, Conway T (2001): Intensive remedial instruction for children with severe reading disabilities: Immediate and long‐term outcomes from two instructional approaches. J Learn Disorders 34:33–58. [DOI] [PubMed] [Google Scholar]
- Toro R, Fox PT, Paus T (2008): Functional coactivation map of the human brain. Cereb Cortex 18:2553–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF (2003): Development of neural mechanisms for reading. Nat Neurosci 6:767–773. [DOI] [PubMed] [Google Scholar]
- AU Turken, NF Dronkers (2011): The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Front Syst Neurosci 5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V (2013): Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front Hum Neurosci 7:458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL (2012): The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 59:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verly M, Verhoeven J, Zink I, Mantini D, Peeters R, Deprez S L Emsell, B Boets, I Noens, J Steyaert, L Lagae, P De Cock, N Rommel, S Sunaert (2014): Altered functional connectivity of the language network in ASD: Role of classical language areas and cerebellum. Neuroimage Clin 4:374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel AC, Church JA, Power JD, Miezin FM, Petersen SE, Schlaggar BL (2013): Functional network architecture of reading‐related regions across development. Brain Lang 125:231–243. doi: 10.1016/j.bandl.2012.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von dem Hagen EA, Stoyanova RS, Baron‐Cohen S, Calder AJ (2012): Reduced functional connectivity within and between 'social' resting state networks in autism spectrum conditions. Soc Cogn Affect Neurosci 8:694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D, Botting N, Boucher J (2008): Language in autism and specific language impairment: Where are the links? Psychol Bull 134:944–963. [DOI] [PubMed] [Google Scholar]
- Xiang H, Lin C, Ma X, Zhang Z, Bower JM, Weng X, Gao JH (2003): Involvement of the cerebellum in semantic discrimination: An fMRI study. Hum Brain Mapp 18:208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


