Abstract
Recent neuroimaging studies have investigated the neural substrates involved in the valuation of supraliminally presented targets and the subsequent preference decisions. However, the neural mechanisms of the valuation of subliminally presented targets, which can guide subsequent preference decisions, remain to be explored. In the present study, we determined whether the neural systems associated with the valuation of supraliminally presented faces are involved in the valuation of subliminally presented faces. The subjects were supraliminally and subliminally presented with faces during functional magnetic resonance imaging (fMRI). Following fMRI, the subjects were presented with pairs of faces and were asked to choose which face they preferred. We analyzed brain activation by back‐sorting the fMRI data according to the subjects' choices. The present study yielded two main findings. First, the ventral striatum and the ventromedial prefrontal cortex predict preferences only for supraliminally presented faces. Second, the dorsomedial prefrontal cortex may predict preferences for subliminally presented faces. These findings indicate that neural correlates of the preference‐related valuation of faces are dissociable, contingent upon whether the subjects consciously perceive the faces. Hum Brain Mapp 36:2865–2877, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: preference, value, subliminal, ventral striatum, ventromedial prefrontal cortex, dorsomedial prefrontal cortex
INTRODUCTION
Recent neuroeconomic evidence regarding making preferential choices has suggested that value signals are first assigned to each option and that these value signals are subsequently compared [Glimcher, 2009; Kable and Glimcher, 2009]. This value‐related processing is supported by the ventral striatum (VS) and the ventromedial prefrontal cortex (vmPFC) [Bartra et al., 2013; Camille et al., 2011; Chib et al., 2009; Hare et al., 2008; Knutson et al., 2007, 2005; Lebreton et al., 2009; McNamee et al., 2013; Pessiglione et al., 2008; Wunderlich et al., 2012]. These brain areas are thought to represent the value signals of the available options that predict subsequent preferences, regardless of the different stimulus types. For example, many previous studies have explored the neural mechanisms that underlie value‐related processing associated with facial attractiveness [Aharon et al., 2001; Bray and O'Doherty, 2007; Cloutier et al., 2008; Ishai, 2007; Kampe et al., 2001; Kranz and Ishai, 2006; O'Doherty et al., 2003; Oikawa et al., 2012; Scheele et al., 2013; Tsukiura and Cabeza, 2011; Ueno et al., 2014]. Other studies have used various behavioral measures such as pleasantness [Kim et al., 2011; Lebreton et al., 2009], likeableness [Izuma and Adolphs, 2013; Izuma et al., 2010; Kitayama et al., 2013; Plassmann et al., 2008], desirability [Lebreton et al., 2012], and willingness‐to‐pay (WTP) [Chib et al., 2009; Plassmann et al., 2007, 2010] to calculate the subjective value that subjects assign to their preferences. Despite differences in the types of behavioral measures, these studies have repeatedly demonstrated a domain‐general contribution of the VS and vmPFC to the representation of subjective value.
Notably, these two brain areas engage in value‐related processing even when subjects perform tasks that are irrelevant to the valuation [Kim et al., 2007; Lebreton et al., 2009]. For example, Lebreton et al. [2009] demonstrated that the VS and vmPFC encode value signals associated with subsequent preference decisions not only when subjects perform a valuation task but also when subjects perform an age‐rating task. These findings indicate the existence of an “automatic brain valuation system,” that is, a system that is automatically engaged in valuation and guides subsequent preferences regardless of whether the subjects are required to make a value judgment.
In addition to the VS and vmPFC, more recent studies have extended the role of the automatic brain valuation system and have identified the critical contribution of the dorsomedial prefrontal cortex (dmPFC) using a passive exposure paradigm [Levy et al., 2011]. The dmPFC automatically performs the preference‐related valuations even when participants passively view consumer goods [Levy et al., 2011] and their attention to the targets is distracted [Tusche et al., 2010]. Thus, the VS, vmPFC, and dmPFC might encode target value signals, which are then available for subsequent preference decisions even if no active valuation task is required for the targets.
From a psychological perspective, our preferences can be affected by subliminally presented targets [Karremans et al., 2006; Kunst‐Wilson and Zajonc, 1980; Murphy et al., 1995]. For example, in a study by Karremans et al. [2006], participants engaged in a visual detection task in which they were asked to search for a small “b” among a string of capital “B”s while being subliminally presented with drink brand names. After the task, the subjects performed a binary choice task for the previous subliminally presented drink brand names. The subjects who were thirsty chose the drink brand that was subliminally presented more frequently rather than the alternative drink brands that were not presented. Moreover, other psychological studies have demonstrated that multiple repetitions of the subliminal presentation of targets such as irregular octagons and Chinese ideographs enhance the likeability of targets (i.e., the subliminal mere exposure effect) [Kunst‐Wilson and Zajonc, 1980; Monahan et al., 2000]. Thus, specific neural mechanisms are likely involved in the valuation of subliminal targets; furthermore, these mechanisms guide preference decisions.
One potentially critical player in the process of representing value signals and guiding preference decisions for subliminal targets may be the VS. In fact, recent neuroimaging studies have implicated the VS in the reward‐related processing of subliminal stimuli [Oei et al., 2012; Pessiglione et al., 2008]. In a study by Pessiglione et al. [2008], subjects performed a subliminal instrumental conditioning task during functional magnetic resonance imaging (fMRI) in which they were randomly presented with masked stimuli followed by a monetary reward and other masked stimuli followed by a monetary loss. The subjects subsequently exhibited a preference for the masked stimuli followed by a monetary reward. Additionally, VS activity was increased for the masked stimuli followed by a monetary reward compared with the masked stimuli followed by a monetary loss. Therefore, the VS encodes the subliminally presented stimuli values. Oei et al. [2012] used a backward‐masking procedure in which subjects were subliminally presented with a sexual stimulus or a neutral stimulus followed by a backward mask. The authors identified significant VS activation during the presentation of sexual stimuli compared with neutral stimuli.
The second candidate region in the process of representing value signals and guiding preference decisions for subliminal targets is the dmPFC. Tusche et al. [2010] investigated the neural responses to unattended products and demonstrated that the dmPFC activity patterns predicted consumer choice even when the subjects did not pay attention to the products, suggesting the important role of the dmPFC in implicit value processing, which predicts the subject's choice. Gillath and Canterberry [2012] sought to more directly identify the neural systems associated with the processing of supraliminally and subliminally presented sexual stimuli using a sandwich‐masking paradigm. They identified significant activation in the frontal regions, including the dmPFC, following the subliminal presentation of sexual stimuli compared with neutral stimuli; however, whether dmPFC activity can predict subsequent preference remains unclear.
Thus, the main purpose of the present study was to clarify the specific brain regions responsible for the valuation of subliminal and supraliminal targets. Specifically, we aimed to determine whether the VS, vmPFC, and dmPFC are associated not only with the valuation of supraliminally presented faces but also with the valuation of subliminally presented faces. The second purpose was to determine whether the activity in these regions could predict preference decisions. Although the previously cited studies have demonstrated that the VS and dmPFC can represent the value signals of imperceptible targets, whether these regions directly contribute to subsequent preference decisions remains unclear. To address these issues, we used fMRI and a binary forced‐choice paradigm to more clearly identify the neural correlates of the preference‐related valuations of supraliminally and subliminally presented faces (Fig. 1).
Figure 1.
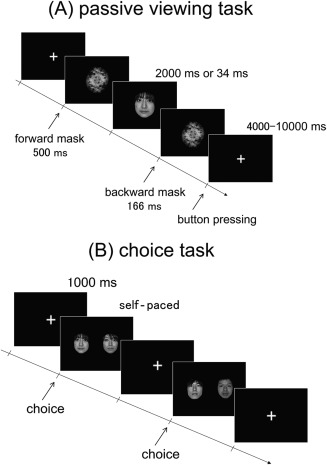
Illustration of the passive viewing task during fMRI scanning and the choice task following fMRI. (A) In the passive viewing task, each trial comprised the sequential presentation of a fixation cross, a forward mask (500 ms), a face photograph (2000 or 34 ms), and a backward mask (166 ms). The subjects were asked to fixate on the center of the screen and to press the button as soon as possible when the fixation cross appeared following the backward mask. (B) In the choice task, the subjects were presented with pairs of face photographs that had been presented during the passive viewing task and were asked to choose the face photograph that they preferred.
MATERIALS AND METHODS
Participants
Twenty‐eight healthy, young volunteers (14 females and 14 males; age range, 20–23 years; mean age, 21.6 years) with no history of neurological or psychiatric disease were compensated for their participation in this study. No pathological findings in the participants' brains were identified by MRI. All subjects were right‐handed, as assessed by the Edinburgh Handedness Inventory [Oldfield, 1971], and had normal or corrected‐to‐normal vision. After being provided a detailed description of the study, the subjects provided their written informed consent in accordance with the Declaration of Helsinki and the guidelines approved by the Ethical Committee of Tohoku Fukushi University.
Stimuli
We recruited 120 male and 120 female volunteers to pose for headshot photographs. To avoid a situation in which the subjects who participated in the fMRI experiment were acquainted with the individuals whose faces were used as the experimental stimuli, we collected these photographs in the Hokkaido prefecture, which is distant from our laboratory. The volunteers were informed that the pictures would only be used for research purposes, and all volunteers provided their written informed consent. The photographs were captured using a DMC‐LX2 digital camera (Panasonic, Japan) with flash and a resolution of 1920 × 1080 pixels. All volunteers were asked to present a neutral facial expression and to look directly into the camera. All images were subsequently downloaded onto a computer and edited using Adobe Photoshop CS 5.1 and Adobe Illustrator CS 5.1 to produce greater uniformity across the photographs. The images were then transformed into grayscale. The mask image was constructed from the overlays of six upright and six inverted images of three males and three females whose photographs were not used for the experimental stimuli. A separate group of 14 healthy volunteers (7 females and 7 males; age range, 18–20 years; mean age, 19.0 years) who did not participate in the fMRI study used a 10‐point scale to rate the 240 grayscale face photographs regarding pleasantness, emotional valence, arousal, and age. Prior to the initiation of the fMRI experiment, only the pleasantness score was used for the stimuli selection: the mean scores for pleasantness were independently ranked for the male and female photographs. For each group, the photograph ranked “n” (n = 1–60) was paired with the photograph ranked “n + 60.” These 60 pairs of male and 60 pairs of female photographs were used in a choice task following fMRI. The other rating scores were used to examine the characteristics of the stimuli for each experimental condition.
Experimental Design
The experiment comprised three tasks: a passive viewing task during fMRI (Fig. 1A), a choice task following fMRI (Fig. 1B), and a gender‐discrimination task after the choice task. For the passive viewing task during fMRI, all 240 face photographs were individually presented in a random order (Fig. 1A). Following a fixation cross, a forward mask, a face stimulus, and a backward mask were presented in sequence. The stimuli were presented using MATLAB (MathWorks, Natick, MA, USA) and Cogent Graphics (http://www.vislab.ucl.ac.uk/cogent.php) software packages. The forward and backward masks were presented for 500 and 166 ms, respectively. The presentation duration of the face stimulus was 2,000 ms for half of the stimuli (“supraliminal” condition) and 34 ms for the other half of the stimuli (“subliminal” condition). The stimuli assigned to each condition were counterbalanced across the subjects. The interstimulus interval, during which the cross‐fixation was constantly presented, ranged from 4000 to 10,000 ms to maximize the efficiency of the event‐related design [Dale, 1999]. The passive viewing task was divided into three consecutive runs, each of which lasted approximately 11 min. The participants were asked to fixate on the center of the screen and to press the button as soon as possible after the appearance of the fixation cross, which followed the backward mask.
During the choice task following fMRI, the subjects were presented with the 120 pairs of face photographs side by side and were asked to choose the face photograph that they preferred by pressing one of two buttons (Fig. 1B). The subjects performed this choice task while inside of the scanner to maintain the conditions associated with viewing the face stimuli. The face stimulus that was ranked “n” and was presented for 2000 ms in the passive viewing task was paired with the face stimulus that was ranked “n + 60” and was presented for 2000 ms in the passive viewing task. Similarly, the face stimulus that was ranked “n” and was presented for 34 ms in the passive viewing task was paired with the face stimulus that was ranked “n + 60” and was presented for 34 ms in the passive viewing task. Although the two photographs selected for comparison as pairs were fixed, the pair presentation order was randomized across the subjects. The positions of the two photographs on the screen were counterbalanced across the subjects.
To confirm that the subjects could not perceive the identity of the face stimulus presented for 34 ms in the passive viewing task, they were asked to perform a gender‐discrimination task after the choice task. This gender‐discrimination task was performed in a self‐paced manner while inside of the scanner. In this task, 120 face photographs were individually presented in a random order. Similar to the passive viewing task, following the fixation cross, the forward mask, face stimulus, and backward mask were presented in sequential order. The forward and backward masks were presented for 500 and 166 ms, respectively. The duration of face stimulus presentation was the same as for the subliminal condition of the passive viewing task (34 ms). After the backward mask, the subjects were asked to determine whether the face represented a female or male.
Image Acquisition
Whole‐brain imaging was performed using a 3.0‐Tesla MRI scanner (MAGNETOM Verio, Siemens, Germany) equipped with a 12‐channel head coil array for signal reception. A T2*‐weighted echo planar imaging (EPI) sequence sensitive to blood oxygen level‐dependent (BOLD) contrast was used for functional imaging with the following parameters: repetition time (TR) = 2500 ms; echo time (TE) = 30 ms; flip angle = 90°; 80 × 80 acquisition matrix; field of view (FOV) = 240 mm; in‐plane resolution = 3 × 3 mm; and 43 axial slices, with a slice thickness of 3 mm and an interslice gap of 0.5 mm. We used a tilted acquisition sequence at 30° to the anterior commissure‐posterior commissure (AC‐PC) line to recover the magnetic susceptibility‐induced signal losses that resulted from the sinus cavities [Deichmann et al., 2003]. A high‐resolution (spatial resolution 1 × 1 × 1 mm) structural image was also acquired using a T1‐weighted, magnetization‐prepared rapid‐acquisition gradient echo (MP‐RAGE) pulse sequence. Each subject's head motion was restricted using firm padding surrounding the head. The visual stimuli were presented on a screen mounted on a head coil through a projector outside the scanner room. The subject's responses were collected using a magnet‐compatible response box. The EPI images were acquired in three consecutive runs. The first four scans in each run were discarded to allow for T1 equilibration effects.
Image Preprocessing
Data preprocessing and statistical analyses were performed using Statistical Parametric Mapping (SPM) 8 software (Wellcome Department of Imaging Neuroscience, London, UK). All volumes acquired from each subject were realigned to correct for small movements that occurred between scans. This process generated an aligned set of images and a mean image per subject. The realigned images were subsequently corrected for the different slice acquisition times. Each participant's T1‐weighted structural MRI was coregistered to the mean of the realigned EPI images and segmented to separate the gray matter, which was normalized to the gray matter in a template image based on the Montreal Neurological Institute (MNI) reference brain (resampled voxel size 3 × 3 × 3 mm). Using the parameters from this normalization process, the EPI images were subsequently normalized to the MNI template and smoothed using an 8‐mm full‐width, half‐maximum Gaussian kernel.
Statistical Analyses
The fMRI data were analyzed using an event‐related model. For each participant and on a voxel‐by‐voxel basis, the hemodynamic response to the stimulus onset for each event type was modeled via convolution using a canonical hemodynamic response function with temporal derivatives. We analyzed brain activity patterns during the passive viewing task by back‐sorting the fMRI data according to the subjects' responses during the choice task. The experimental conditions comprised the following: (1) the male‐supraliminal‐preferred condition, in which the male face photographs were presented for 2000 ms in the passive viewing task and were chosen in the choice task following fMRI; (2) the male‐supraliminal‐not‐preferred condition, in which the male face photographs were presented for 2000 ms in the passive viewing task and were not chosen in the choice task; (3) the female‐supraliminal‐preferred condition, in which the female face photographs were presented for 2000 ms in the passive viewing task and were chosen in the choice task; (4) the female‐supraliminal‐not‐preferred condition, in which the female face photographs were presented for 2000 ms in the passive viewing task and were not chosen in the choice task; (5) the male‐subliminal‐preferred condition, in which the male face photographs were presented for 34 ms in the passive viewing task and were chosen in the choice task; (6) the male‐subliminal‐not‐preferred condition, in which the male face photographs were presented for 34 ms in the passive viewing task and were not chosen in the choice task; (7) the female‐subliminal‐preferred condition, in which the female face photographs were presented for 34 ms in the passive viewing task and were chosen in the choice task; and (8) the female‐subliminal‐not‐preferred condition, in which the female face photographs were presented for 34 ms in the passive viewing task and were not chosen in the choice task. The events for which no response was provided were modeled as events of no interest. Because our primary purpose was to identify the brain regions associated with the valuation of supraliminally or subliminally presented faces, we collapsed the results across the gender of the face stimuli in our main analysis. Thus, we analyzed the following four conditions: the supraliminal‐preferred condition, in which (1) and (3) were combined; the supraliminal‐not‐preferred condition, in which (2) and (4) were combined; the subliminal‐preferred condition, in which (5) and (7) were combined; and the subliminal‐not‐preferred condition, in which (6) and (8) were combined. A high‐pass filter of 1/128 Hz was used to remove low‐frequency noise, and an autoregressive (AR) (1) model was used to correct for temporal auto‐correlations.
ROI Analyses
To assess whether the previously discussed brain regions are associated with the valuation of both supraliminally and subliminally presented faces, we performed region of interest (ROI) analyses for the VS, vmPFC, and dmPFC. ROIs of the VS (i.e., the nucleus accumbens) and the vmPFC (i.e., the medial front‐orbital gyrus) were anatomically defined and derived from Individual Brain Atlases using SPM software (IBASPM) [Alemán‐Gómez et al., 2006], which was implemented in the WFU PickAtlas (Wake Forest University, Winston‐Salem, NC) [Maldjian et al., 2004, 2003]. Because the IBASPM atlas does not include the ROI representing the dmPFC, we manually created the anatomical ROI of the dmPFC. Thus, we used the method reported by Falk et al. [2014] in which the ROI of the dmPFC was defined as all voxels within Brodmann areas 8 and 9 intersected with a box‐shaped mask centered at x = 0, y = 52, and z = 50 and extending 40, 44, and 48 mm along the x‐, y‐, and z‐axes, respectively [Falk et al., 2014]. We used MarsBaR software [Brett et al., 2002] to extract the signal changes in the ROI analyses. For the percentage of signal changes of each ROI, we performed two‐way repeated‐measures analysis of variance (ANOVA) using the face presentation duration (supraliminal and subliminal) and the subject's preference (preferred and not‐preferred) as factors. In a separate analysis, we also performed three‐way ANOVA for each ROI to examine the interaction effect among the subject's preference (preferred or not‐preferred), the gender of the stimuli (same gender or opposite gender), and the gender of the subjects (male or female).
Whole‐Brain Subtraction Analyses
We subsequently performed whole‐brain subtraction analyses to explore the brain regions associated with the preference‐related valuation of supraliminally and subliminally presented faces. Contrast images for all subjects were entered in a series of one‐sample t‐tests. This procedure permitted statistical inference at the population level (random‐effects analysis). The threshold of significance was set at P < 0.001 at the voxel level (uncorrected for multiple comparisons), with an extent threshold of 10 contiguous voxels unless otherwise specified.
RESULTS
Behavioral Data
The mean reaction time during the passive viewing task was 386 ms (standard deviation [SD] = 83) for the supraliminal‐preferred condition, 407 ms (SD = 103) for the supraliminal‐not‐preferred condition, 404 ms (SD = 88) for the subliminal‐preferred condition, and 424 ms (SD = 98) for the subliminal‐not‐preferred condition. The data were analyzed using two‐way repeated‐measures ANOVA with the face presentation duration (supraliminal and subliminal) and the subject's preference (preferred and not‐preferred) as factors. The ANOVA identified a significant main effect of the face presentation duration (F[1,27] = 7.005, P < 0.05). There was no significant main effect of the subject's preference (F[1,27] = 1.782, P = 0.193), nor was there an interaction between the two factors (F[1,27] = 0.068, P = 0.797). The percentage of correct responses in the gender discrimination task was 51.3 (SD = 0.11), which was not significantly different from chance (P > 0.05, one‐sample t‐test).
We subsequently examined whether the characteristics of the stimuli (emotional valence, arousal, and age) influenced preference judgments using the data from the separate group of 14 individuals. Specifically, we classified the ratings of these characteristics based on the results of a choice task for the individuals who participated in the fMRI experiment. For each characteristic of the face stimuli, we conducted two‐way repeated‐measures ANOVA with the face presentation duration (supraliminal and subliminal) and the subject's preference (preferred and not‐preferred) as factors. The results for emotional valence revealed a significant main effect of the subject's preference (F[1,27] = 272.943, P < 0.001) and a marginally significant interaction between the two factors (F[1,27] = 3.534, P = 0.071) but no significant main effect of the face presentation duration (F[1,27] = 0.993, P = 0.328). The results for arousal revealed a significant main effect of preference (F[1,27] = 241.411, P < 0.001) but no significant main effect of the face presentation duration (F[1,27] = 0.055, P = 0.817) and no significant interaction (F[1,27] = 0.561, P = 0.460). The results for age revealed a significant main effect of the subject's preference (F[1,27] = 300.758, P < 0.001) but no significant main effect of face presentation duration (F[1,27] = 0.144, P = 0.707) and no significant interaction (F[1,27] = 0.436, P = 0.515). Taken together, these results suggest that positive emotion, youthfulness, and higher arousal of the faces were associated with preferential choices.
ROI Analyses
We first investigated the overall activity patterns of the VS, vmPFC, and dmPFC for each condition. We performed two‐way repeated‐measures ANOVA with the face presentation duration (supraliminal and subliminal) and the subject's preference (preferred and not‐preferred) as factors for each anatomical ROI.
The results of ANOVA for the left VS showed significant main effects of the face presentation duration (F[1,27] = 19.750, P < 0.001) and the subject's preference (F[1,27] = 5.833, P < 0.05) but no significant interaction between the two factors (F[1,27] = 2.488, P = 0.126) (Fig. 2A). Although no significant interaction was identified, we conducted planned t‐tests between the supraliminal‐preferred and supraliminal‐not‐preferred conditions and between the subliminal‐preferred and subliminal‐not‐preferred conditions. The signal change in the supraliminal‐preferred condition was significantly greater than in the supraliminal‐not‐preferred condition (t[27] = 2.556, P < 0.05), whereas no significant difference was observed in the signal changes between the subliminal‐preferred and subliminal‐not‐preferred conditions (t[27] = 0.322, P = 0.750). The results of ANOVA for the right VS showed significant main effects of the face presentation duration (F[1,27] = 8.769, P < 0.01) and the subject's preference (F[1,27] = 6.776, P < 0.05) but no significant interaction between the two factors (F[1,27] = 1.852, P = 0.185) (Fig. 2B). The planned t‐tests showed that the signal change in the supraliminal‐preferred condition was significantly greater than in the supraliminal‐not‐preferred condition (t[27] = 2.553, P < 0.05), whereas no significant difference was found in the signal change between the subliminal‐preferred and subliminal‐not‐preferred conditions (t[27] = 0.783, P = 0.440).
Figure 2.
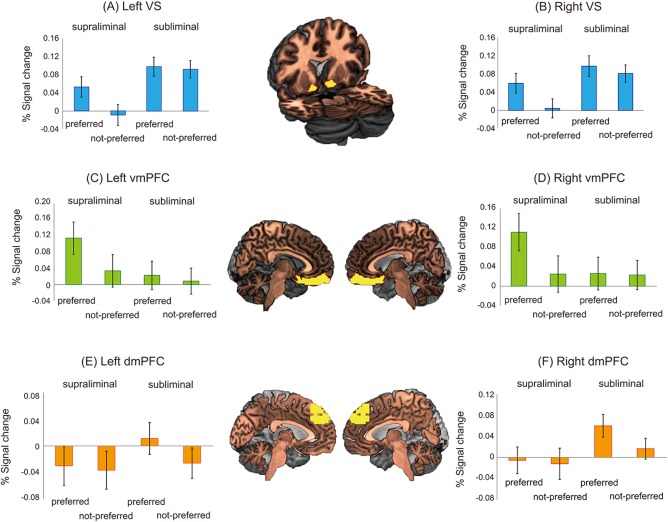
Percentage of signal change in the VS, vmPFC, and dmPFC for each condition. The error bars indicate the standard error. The yellow areas represent the anatomically defined ROIs superimposed on a standard brain. VS, ventral striatum; vmPFC, ventromedial prefrontal cortex; dmPFC, dorsomedial prefrontal cortex.
The results of ANOVA for the left vmPFC showed marginally significant main effects of the face presentation duration (F[1,27] = 3.854, P = 0.060) and the subject's preference (F[1,27] = 3.497, P = 0.072) as well as a significant interaction between the two factors (F[1,27] = 4.236, P < 0.05) (Fig. 2C). Post hoc t‐tests demonstrated that the signal change in the supraliminal‐preferred condition was greater than that in the supraliminal‐not‐preferred condition (t[27] = 2.567, P < 0.05); however, no significant difference was found in the signal changes between the subliminal‐preferred and subliminal‐not‐preferred conditions (t[27] = 0.523, P = 0.605). The results of ANOVA for the right vmPFC showed a marginally significant main effect of the subject's preference (F[1,27] = 3.384, P = 0.077) and a significant interaction between the two factors (F[1,27] = 8.225, P < 0.01); however, no significant main effect of the face presentation duration (F[1,27] = 1.814, P = 0.189) was found (Fig. 2D). Post hoc t‐tests demonstrated that the signal change in the supraliminal‐preferred condition was greater than that in the supraliminal‐not‐preferred condition (t[27] = 2.892, P < 0.01); however, no significant difference was found in the signal changes between the subliminal‐preferred and subliminal‐not‐preferred conditions (t[27] = 0.128, P = 0.899).
The results of ANOVA for the left dmPFC showed no significant main effects of the face presentation duration (F[1,27] = 1.651, P = 0.210) or the subject's preference (F[1,27] = 2.409, P = 0.132) and no significant interaction between the two factors (F[1,27] = 1.505, P = 0.231) (Fig. 2E). Although no significant interaction was identified, we conducted planned t‐tests between the supraliminal‐preferred and supraliminal‐not‐preferred conditions and between the subliminal‐preferred and subliminal‐not‐preferred conditions. No significant difference was found in the signal change between the supraliminal‐preferred and supraliminal‐not‐preferred conditions (t[27] = 0.305, P = 0.763), whereas the signal change in the subliminal‐preferred condition was greater than the subliminal‐not‐preferred condition (t[27] = 2.406, P < 0.05). The results of ANOVA for the right dmPFC showed a significant main effect of the face presentation duration (F[1,27] = 6.710, P < 0.05) and a marginally significant main effect of the subject's preference (F[1,27] = 4.131, P = 0.052); however, no significant interaction was found between the two factors (F[1,27] = 2.823, P = 0.104) (Fig. 2F). The results of planned t‐test demonstrated that no significant difference in the signal change between the supraliminal‐preferred and supraliminal‐not‐preferred conditions (t[27] = 0.392, P = 0.698), whereas the signal change in the subliminal‐preferred condition was greater than that in the subliminal‐not‐preferred condition (t[27] = 2.774, P < 0.01).
For the ROIs of the VS, vmPFC, and dmPFC, we subsequently examined the interaction of the subject's preference, the gender of the stimuli, and the gender of the subjects. We conducted three‐way ANOVAs using the subject's preference (preferred or not‐preferred) and the gender of the stimuli (same gender or opposite gender) as the within‐subject factors and using the gender of the subjects (male or female) as the between‐subject factor. For the left and right VS, a significant main effect of the subject's preference (F[1,26] = 5.622, P < 0.05 for the left VS; F[1,26] = 6.675, P < 0.05 for the right VS) was identified, but no significant main effects of the gender of the stimuli (F[1,26] = 0.001, P = 0.976 for the left VS; F[1,26] = 0.348, P = 0.560 for the right VS) or the gender of the subjects (F[1,26] = 2.416, P = 0.132 for the left VS; F[1,26] = 0.00007, P = 0.993 for the right VS) and no significant interactions were identified (all P‐values > 0.1) (Figs. 3A,B). For the left and right vmPFC, marginally significant main effects of the subject's preference (F[1,26] = 3.371, P = 0.078 for the left vmPFC; F[1,26] = 3.322, P = 0.080 for the right vmPFC) and the gender of the stimuli (F[1,26] = 3.218, P = 0.084 for the left vmPFC; F[1,26] = 3.758, P = 0.063 for the right vmPFC) but no significant main effect of the gender of the subjects (F[1,26] = 0.060, P = 0.809 for the left vmPFC; F[1,26] = 0.245, P = 0.624 for the right vmPFC) and no significant interactions were identified (all P‐values >0.1) (Figs. 3C,D). For the left dmPFC, a significant main effect of the gender of the stimuli (F[1,26] = 11.064, P < 0.005) was found; however, no significant main effects of the subject's preference (F[1,26] = 2.334, P = 0.139) or the gender of the subjects (F[1,26] = 2.514, P = 0.125) and no significant interactions were identified (all P‐values > 0.1) (Fig. 3E). For the right dmPFC, marginally significant main effects of the subject's preference (F[1,26] = 3.991, P = 0.056) and the gender of the stimuli (F[1,26] = 3.861, P = 0.060) were identified, but no significant main effect of the gender of the subjects (F[1,26] = 2.037, P = 0.165) and no interactions were identified (all P‐values >0.1) (Fig. 3F). Thus, our results demonstrated that only the left dmPFC exhibited greater activity for faces of the same gender compared with the opposite gender, irrespective of the gender of the subjects. Because these results were not a priori hypothesized, we do not discuss these results further in the present study.
Figure 3.
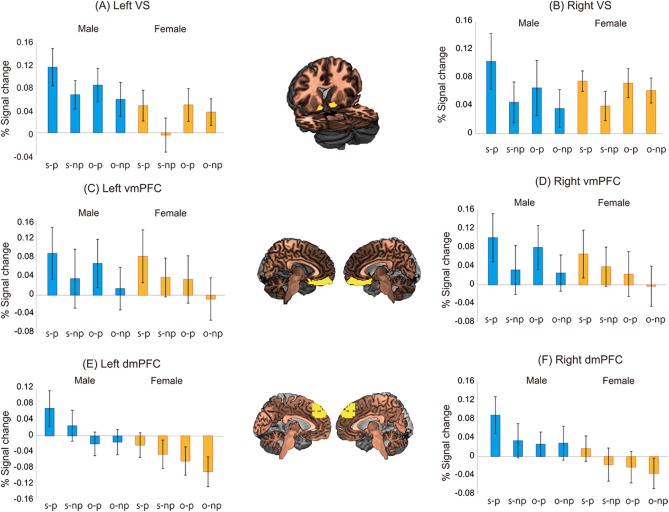
Gender differences in the percentage of signal change in the VS, vmPFC, and dmPFC for the faces of the same gender that the participants preferred (s‐p), the faces of the same gender that the participants did not prefer (s‐np), the faces of the opposite gender that the participants preferred (o‐p), and the faces of the opposite gender that the participants did not prefer (o‐np). The error bars indicate the standard error. VS, ventral striatum; vmPFC, ventromedial prefrontal cortex; dmPFC, dorsomedial prefrontal cortex.
We then tested for correlations between the signal changes in the VS and vmPFC for each condition based on the previous finding that reported functional coupling in these two regions during valuation tasks [Salimpoor et al., 2013]. For this analysis, we calculated the bilaterally averaged signal changes of the VS and the vmPFC. Regarding the correlations between the bilaterally averaged signal changes in the VS and vmPFC, we identified significant correlations in the supraliminal‐preferred (r = 0.376, P < 0.05) and supraliminal‐not‐preferred (r = 0.446, P < 0.05) conditions. However, we did not identify significant correlations in the subliminal‐preferred (r = 0.302, P = 0.118) or subliminal‐not‐preferred (r = 0.101, P = 0.609) conditions. At this stage of the analysis, we examined differences in the correlations between the supraliminal‐preferred and subliminal‐preferred conditions and between the supraliminal‐not‐preferred and subliminal‐not‐preferred conditions. We did not identify a significant difference between either the supraliminal‐preferred and subliminal‐preferred conditions or the supraliminal‐not‐preferred and subliminal‐not‐preferred conditions (all P‐values >0.05).
Whole‐Brain Subtraction Analyses
The results are summarized in Table 1. For the supraliminal conditions, we identified significant activations in the left vmPFC, right VS, and right superior temporal gyrus when the preferred faces were compared with the not‐preferred faces (Fig. 4A). For the subliminal conditions, we identified significant activations in the bilateral dmPFC, left ventrolateral prefrontal cortex (vlPFC), and left dorsolateral prefrontal cortex (dlPFC) when the preferred faces were compared with the not‐preferred faces (Fig. 4B). No overlapping activations were identified between the supraliminal and subliminal conditions.
Table 1.
Brain regions that exhibited significant activation
| Region (Brodmann's area) | Coordinates | Z‐value | Cluster size | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Supraliminal condition | |||||
| Preferred vs. Not‐preferred | |||||
| Left ventromedial prefrontal cortex (11) | −18 | 44 | −8 | 4.52 | 16 |
| Right ventral striatum | 6 | 14 | −11 | 4.03 | 11 |
| Right superior temporal gyrus (38) | 51 | 2 | −14 | 3.70 | 13 |
| Not‐preferred vs. Preferred | |||||
| No significant activation | |||||
| Subliminal condition | |||||
| Preferred vs. Not‐preferred | |||||
| Bilateral dorsomedial prefrontal cortex (9) | 0 | 56 | 37 | 3.53 | 15 |
| Left ventrolateral prefrontal cortex (47) | −45 | 32 | −5 | 3.99 | 23 |
| Left dorsolateral prefrontal cortex (46) | −36 | 26 | 40 | 3.91 | 31 |
| Not‐preferred vs. Preferred | |||||
| No significant activation | |||||
The threshold of significance was set at P < 0.001 at the voxel level (uncorrected for multiple comparisons) with an extent threshold of 10 contiguous voxels.
Figure 4.
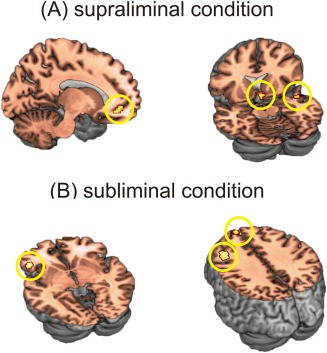
Results of whole‐brain subtraction analyses. (A) The left vmPFC, right VS, and right superior temporal gyrus exhibited greater activity for the supraliminally presented faces that the subjects preferred compared with the supraliminally presented faces that they did not prefer. (B) The bilateral dmPFC, left vlPFC, and left dlPFC exhibited greater activity for the subliminally presented faces that the subjects preferred compared with the subliminally presented faces that they did not prefer. VS, ventral striatum; vmPFC, ventromedial prefrontal cortex; dmPFC, dorsomedial prefrontal cortex; vlPFC, ventrolateral prefrontal cortex; dlPFC, dorsolateral prefrontal cortex.
To explicitly identify the voxels in which the effects were specific to the supraliminal or subliminal conditions, we conducted two‐sample t‐tests using the resulting t‐maps from comparing the supraliminal‐preferred vs. supraliminal‐not‐preferred and the subliminal‐preferred vs. subliminal‐not‐preferred conditions. When the supraliminal condition was compared to the subliminal condition (P < 0.001 at the voxel level (uncorrected for multiple comparisons) with an extent threshold of 10 contiguous voxels), we did not identify a significant activation in any brain region. When the subliminal condition was compared with the supraliminal condition, we identified a significant activation only in the left dlPFC (coordinates, −36, 23, 31; Z‐value = 3.42; cluster size = 11).
Because the previously described two‐sample t‐tests failed to demonstrate significant effects specific to the supraliminal or subliminal conditions in the hypothesized regions, we utilized another method to determine whether the VS and vmPFC were specific to the supraliminal‐preferred condition and whether the dmPFC was specific to the subliminal‐preferred condition. For the signal changes in the clusters of the right VS, left vmPFC, and bilateral dmPFC, in which activation was identified in the whole‐brain subtraction analyses (i.e., functional ROIs), we conducted two‐way repeated‐measures ANOVA with the face presentation duration (supraliminal and subliminal) and the subject's preference (preferred and not‐preferred) as factors. Here, the critical test is determining whether the interaction effects are significant. These analyses to identify interaction effects (not the main effects) are independent of the SPM subtraction analyses to determine the functional ROIs. Our analysis has therefore avoided the issues of the “double dipping” phenomenon [Kriegeskorte et al., 2009]. The results of ANOVA showed a significant interaction between the two factors for the right VS (F[1,27] = 10.866, P < 0.01) and the left vmPFC (F[1,27] = 8.012, P < 0.01) and a marginally significant interaction between the two factors for the bilateral dmPFC (F[1,27] = 3.485, P = 0.073). These results suggest that the brain activations associated with the supraliminal and subliminal conditions are dissociable.
Group Preference
Based on a previous study by Kim et al. [2007], we performed an additional analysis to assess whether the VS is associated with the group preference. We first calculated how often each face was preferred by the 28 subjects using data from the choice session. Based on the results of the choice session, we separated the faces into three categories: (a) preferred‐by‐group faces, which represented the faces chosen by 15 or more participants (i.e., chosen by more than 50% of the subjects); (b) not‐preferred‐by‐group faces, which represented the faces chosen by 13 or fewer participants (i.e., chosen by fewer than 50% of the subjects); and (c) no‐preference faces, which represented the faces preferred by 14 participants (i.e., chosen by exactly 50% of the subjects). The number of faces in each category was 118 for preferred faces, 118 for not‐preferred faces, and 4 for no‐preference faces. We analyzed the brain activity patterns during the passive viewing task by retrospectively classifying the fMRI data according to the results of the previously discussed classification. Specifically, we compared the BOLD signals associated with the preferred‐by‐group and not‐preferred‐by‐group faces. The events that corresponded to the no‐preference faces were modeled as events of no interest. We did not identify a significant activation when the original threshold (P < 0.001 at the voxel level (uncorrected for multiple comparisons) with an extent threshold of 10 contiguous voxels) was used. However, using a lenient threshold (P < 0.005 at the voxel level (uncorrected for multiple comparisons) with an extent threshold of 10 contiguous voxels), which was a joint thresholding procedure that balances the risk of type I and II errors [Lieberman and Cunningham, 2009], we identified significant activation in the left VS (coordinates, −24, 8, −11; Z‐value = 3.49; cluster size = 12) and the right VS (coordinates, 12, 2, −11; Z‐value = 3.47; cluster size = 22).
DISCUSSION
We used fMRI and a passive viewing paradigm with sandwich masking to more clearly identify the neural correlates of the preference‐related valuation of supraliminally and subliminally presented faces. The results demonstrate that the VS and vmPFC are engaged in the preference‐related valuation specifically for supraliminally presented faces. Moreover, the activities of the VS and vmPFC were significantly correlated during conditions in which the faces were presented supraliminally. The results also demonstrate that the dmPFC may be associated with the preference‐related valuation for subliminally presented faces. Taken together, these results suggest that dissociable neural systems are engaged in the preference‐related valuation of supraliminally and subliminally presented faces.
ROI analysis of the VS and vmPFC highlighted the important roles of these areas in the valuation of supraliminally presented faces. These areas exhibited significant activation for the preferred faces compared with the not‐preferred faces only when the faces were supraliminally presented. Moreover, although preliminary, we identified significant correlations between the activities of the VS and vmPFC only when the subjects perceived the identity of the faces. These results suggest that these two regions interacted with each other to calculate the facial value signal when the subjects identified the faces. Consistent with this idea, in the context of reinforcement learning, a functional coupling between the basal ganglia and the vmPFC has been reported in strategic choice processes [Wunderlich et al., 2012]. A recent neuroimaging study using functional connectivity analysis also identified increased connectivity between the VS and the vmPFC when subjects heard music that they considered highly desirable compared with music that they did not want to hear again [Salimpoor et al., 2013]. Moreover, anatomical studies have demonstrated that the vmPFC possesses a substantial connection to the VS [Haber et al., 1995; Rigoard et al., 2011]. Dopaminergic neurons may be key to the functional coupling between these two regions.
The VS also exhibited significant activation associated with the group preference for faces. In a previous study, Kim et al. [2007] demonstrated that the VS (more precisely, the nucleus accumbens) activity was better correlated with preference judgments averaged across the entire subject group than with individual preference judgments [Kim et al., 2007]. The authors concluded that the VS activity reflects a relatively automatic and rapid preference process that is shared across subjects. Although the present results are based on a relatively lower threshold (P < 0.005 at the voxel level (uncorrected for multiple comparisons) with an extent threshold of 10 contiguous voxels), our results of the VS activity can be interpreted as a neural correlate of the preference process shared across participants.
There was no significant activation in the VS for the subliminal‐preferred condition compared with the subliminal‐not‐preferred condition. This negative finding is inconsistent with some previous studies [Oei et al., 2012; Pessiglione et al., 2008]. One potential explanation for this inconsistency is the difference in the experimental stimuli used; the VS might be unable to sufficiently represent the subliminal facial stimuli values that predict subsequent preference decisions. Nevertheless, the VS is not necessarily never associated with any process of subconscious preference‐related valuation. Other experimental paradigms may be better at detecting the role of the VS in the valuation of subliminally presented targets.
The VS was not identified as a region responsible for the valuation of subliminal targets. However, the dmPFC is involved in the preference‐related valuation of subliminally presented faces. In parallel to the present results, Gillath and Canterberry [2012] identified significant dmPFC activation when sexual stimuli, relative to neutral stimuli, were subliminally presented; however, the association between dmPFC activity and the subsequent preference remained unclear. To the best of our knowledge, our results are the first to provide direct evidence that dmPFC activity can predict preference decisions for subliminally presented faces. Consistent with this idea, the dmPFC is involved in the preference‐related valuation even when a subject's attention is diverted from the target stimuli [Tusche et al., 2010]. Regarding the lack of active processing of target stimuli, this experimental situation was similar to the present study in which the subjects could not perceive the identity of the face stimulus.
Our results also demonstrated that the VS activity was increased for subliminally presented faces compared with supraliminally presented faces, indicating that the VS is more sensitive to subliminally presented stimuli than supraliminally presented stimuli. One potential explanation for this finding is that this main effect reflects an increased cognitive load, which is indicated by the longer reaction time in the subliminal condition. The short duration of stimulus presentation in the subliminal condition might make rapidly pressing a button more difficult compared with the supraliminal condition. Alternatively, this effect might reflect the difference in the expected reward value. Because the subliminally presented faces cannot be consciously processed, this uncertainty might cause an excessive expectation of the reward values.
The results of the present study have implications that might help to explain the previously reported mechanisms of preference change for supraliminally and subliminally presented targets. For example, preference can be consciously modulated using the manipulation of perceptual factors such as the gaze duration and frequency [Park et al., 2010; Shimojo et al., 2003; Zajonc, 1968]. Preference change can also be caused by the manipulation of social factors such as social norms or the opinions of other individuals [Izuma and Adolphs, 2013; Zaki et al., 2011]. Because these phenomena require perceptions of the target and the environment, both the VS and vmPFC might play key roles in these types of preference changes. In fact, the activities of the VS and vmPFC have been modulated by social manipulation [Zaki et al., 2011]. In contrast, the subliminal mere exposure effect [Kunst‐Wilson and Zajonc, 1980] and subliminal priming [Karremans et al., 2006] occur even without the subject's perception of the target; these phenomena may be supported by the dmPFC. Although we speculate that different neural mechanisms are involved in the preference changes for supraliminally and subliminally presented targets, future studies are required to explore these unanswered questions.
We believe that our methodology successfully avoids critical issues regarding the subject's perception of the face stimulus. In the gender‐discrimination task that followed the fMRI experiment, the subjects could not distinguish the gender of the face stimulus. Thus, the dmPFC activity for subliminally presented faces cannot be attributed to the subject's perception of the targets. Additionally, although a previous psychological study demonstrated that manipulating the gaze duration (not only exposure duration) can affect preference formation [Shimojo et al., 2003], the duration of the subliminal stimulus presentation in the present study was too short (i.e., 34 ms) to induce eye movements for the perception of face stimuli. Thus, the dmPFC activity patterns cannot be attributed to gazing.
In summary, dissociable neural underpinnings are associated with supraliminal and subliminal preference‐related valuation for faces. Three further limitations of the present study should be considered. First, the interaction effect in the dmPFC did not reach significance. Accordingly, interpretations of the role of the dmPFC are based on the marginal effect, and we therefore cannot draw definitive conclusions regarding the role of the dmPFC in the preference‐related valuation for subliminally presented faces. Second, the rating scores of emotional valence, arousal, and age of the faces were associated with the preference for faces. The present experimental paradigm thus cannot dissociate the brain activation associated with preferred faces from that associated with positive emotion, youthfulness, and higher arousal. Finally, a marginally significant interaction of the face presentation duration and the subject's preference for emotional valence of the faces might affect the pattern of fMRI results. We therefore report the present results with great caution, and further studies are required to demonstrate whether some or all of the results can be replicated. Despite these limitations, our results provide novel insights into how our preference decisions are affected by both supraliminal and subliminal processes and their underlying neural mechanisms.
ACKNOWLEDGMENTS
The authors thank Yul‐Wan Sung, Satoshi Sakuraba, Ayumi Seo, and Yui Murakami for their assistance in collecting the data. This experiment was implemented using Cogent Graphics, developed by John Romaya at the LON at the Wellcome Department of Imaging Neuroscience.
REFERENCES
- Aharon I, Etcoff N, Ariely D, Chabris CF, O'Connor E, Breiter HC (2001): Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron 32:537–551. [DOI] [PubMed] [Google Scholar]
- Alemán‐Gómez Y, Melie‐García L, Valdés‐Hernandez P (2006): IBASPM: Toolbox for automatic parcellation of brain structures. In: Presented at the 12th Annual Meeting of the Organization for Human Brain Mapping, June 11–15, Florence, Italy, Available on CD‐Rom in Neuroimage.
- Bartra O, McGuire JT, Kable JW (2013): The valuation system: a coordinate‐based meta‐analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage 76:412–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray S, O'Doherty J (2007): Neural coding of reward‐prediction error signals during classical conditioning with attractive faces. J Neurophysiol 97:3036–3045. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB (2002): Region of interest analysis using an SPM toolbox [abstract] Presented at the 8th International Conference on Functional Mapping of the Human Brain, Vol. 16, No 2, June 2–6, Sendai, Japan. Available on CD‐ROM in NeuroImage.
- Camille N, Griffiths CA, Vo K, Fellows LK, Kable JW (2011): Ventromedial frontal lobe damage disrupts value maximization in humans. J Neurosci 31:7527–7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chib VS, Rangel A, Shimojo S, O'Doherty JP (2009): Evidence for a common representation of decision values for dissimilar goods in human ventromedial prefrontal cortex. J Neurosci 29:12315–12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier J, Heatherton TF, Whalen PJ, Kelley WM (2008): Are attractive people rewarding? Sex differences in the neural substrates of facial attractiveness. J Cogn Neurosci 20:941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM (1999): Optimal experimental design for event‐related fMRI. Hum Brain Mapp 8:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deichmann R, Gottfried JA, Hutton C, Turner R (2003): Optimized EPI for fMRI studies of the orbitofrontal cortex. NeuroImage 19:430–441. [DOI] [PubMed] [Google Scholar]
- Falk EB, Cascio CN, O'Donnell MB, Carp J, Tinney FJ, Jr. , Bingham CR, Shope JT, Ouimet MC, Pradhan AK, Simons‐Morton BG (2014): Neural responses to exclusion predict susceptibility to social influence. J Adolesc Health 54:S22–S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillath O, Canterberry M. (2012): Neural correlates of exposure to subliminal and supraliminal sexual cues. Soc Cogn Affect Neurosci 7:924–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher PW (2009): Choice: Towards a standard back‐pocket model In: Glimcher PW, Camerer CF, Fehr E, Poldrack RA, editor. Neuroeconomics: Decision Making and the Brain. New York: Academic; pp 503–521. [Google Scholar]
- Haber SN, Kunishio K, Mizobuchi M, Lyndbalta E (1995): The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci 15:4851–4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, O'Doherty J, Camerer CF, Schultz W, Rangel A (2008): Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci 28:5623–5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai A (2007): Sex, beauty and the orbitofrontal cortex. Int J Psychophysiol 63:181–185. [DOI] [PubMed] [Google Scholar]
- Izuma K, Adolphs R (2013): Social manipulation of preference in the human brain. Neuron 78:563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma K, Matsumoto M, Murayama K, Samejima K, Sadato N, Matsumoto K (2010): Neural correlates of cognitive dissonance and choice‐induced preference change. Proc Natl Acad Sci USA 107:22014–22019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW (2009): The neurobiology of decision: Consensus and controversy. Neuron 63:733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampe KK, Frith CD, Dolan RJ, Frith U (2001): Reward value of attractiveness and gaze. Nature 413:589. [DOI] [PubMed] [Google Scholar]
- Karremans JC, Stroebe W, Claus J (2006): Beyond Vicary's fantasies: The impact of subliminal priming and brand choice. J Exp Soc Psychol 42:792–798. [Google Scholar]
- Kim H, Adolphs R, O'Doherty JP, Shimojo S (2007): Temporal isolation of neural processes underlying face preference decisions. Proc Natl Acad Sci USA 104:18253–18258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Shimojo S, O'Doherty JP (2011): Overlapping responses for the expectation of juice and money rewards in human ventromedial prefrontal cortex. Cereb Cortex 21:769–776. [DOI] [PubMed] [Google Scholar]
- Kitayama S, Chua HF, Tompson S, Han S (2013): Neural mechanisms of dissonance: an fMRI investigation of choice justification. NeuroImage 69:206–212. [DOI] [PubMed] [Google Scholar]
- Knutson B, Rick S, Wimmer GE, Prelec D, Loewenstein G (2007): Neural predictors of purchases. Neuron 53:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G (2005): Distributed neural representation of expected value. J Neurosci 25:4806–4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz F, Ishai A (2006): Face perception is modulated by sexual preference. Curr Biol 16:63–68. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI (2009): Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci 12:535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunst‐Wilson WR, Zajonc RB (1980): Affective discrimination of stimuli that cannot be recognized. Science 207:557–558. [DOI] [PubMed] [Google Scholar]
- Lebreton M, Jorge S, Michel V, Thirion B, Pessiglione M (2009): An automatic valuation system in the human brain: evidence from functional neuroimaging. Neuron 64:431–439. [DOI] [PubMed] [Google Scholar]
- Lebreton M, Kawa S, Forgeot d'Arc B, Daunizeau J, Pessiglione M (2012): Your goal is mine: unraveling mimetic desires in the human brain. J Neurosci 32:7146–7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy I, Lazzaro SC, Rutledge RB, Glimcher PW (2011): Choice from non‐choice: predicting consumer preferences from blood oxygenation level‐dependent signals obtained during passive viewing. J Neurosci 31:118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA (2009): Type I and Type II error concerns in fMRI research: re‐balancing the scale. Soc Cogn Affect Neurosci 4:423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH (2004): Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage 21:450–455. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. NeuroImage 19:1233–1239. [DOI] [PubMed] [Google Scholar]
- McNamee D, Rangel A, O'Doherty JP (2013): Category‐dependent and category‐independent goal‐value codes in human ventromedial prefrontal cortex. Nat Neurosci 16:479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan JL, Murphy ST, Zajonc RB (2000): Subliminal mere exposure: specific, general, and diffuse effects. Psychol Sci 11:462–476. [DOI] [PubMed] [Google Scholar]
- Murphy ST, Zajonc RB, Monahan JL (1995): Additivity of nonconscious affect: combined effects of priming and exposure. J Pers Soc Psychol 69:589–602. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Winston J, Critchley H, Perrett D, Burt DM, Dolan RJ (2003): Beauty in a smile: the role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia 41:147–55. [DOI] [PubMed] [Google Scholar]
- Oei NY, Rombouts SA, Soeter RP, van Gerven JM, Both S (2012): Dopamine modulates reward system activity during subconscious processing of sexual stimuli. Neuropsychopharmacology 37:1729–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa H, Sugiura M, Sekiguchi A, Tsukiura T, Miyauchi CM, Hashimoto T, Takano‐Yamamoto T, Kawashima R (2012): Self‐face evaluation and self‐esteem in young females: an fMRI study using contrast effect. NeuroImage 59:3668–3676. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Park J, Shimojo E, Shimojo S (2010): Roles of familiarity and novelty in visual preference judgments are segregated across object categories. Proc Natl Acad Sci USA 107:14552–14555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessiglione M, Petrovic P, Daunizeau J, Palminteri S, Dolan RJ, Frith CD (2008): Subliminal instrumental conditioning demonstrated in the human brain. Neuron 59:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassmann H, O'Doherty J, Rangel A (2007): Orbitofrontal cortex encodes willingness to pay in everyday economic transactions. J Neurosci 27:9984–9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassmann H, O'Doherty J, Shiv B, Rangel A (2008): Marketing actions can modulate neural representations of experienced pleasantness. Proc Natl Acad Sci USA 105:1050–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassmann H, O'Doherty JP, Rangel A (2010): Appetitive and aversive goal values are encoded in the medial orbitofrontal cortex at the time of decision making. J Neurosci 30:10799–10808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigoard P, Buffenoir K, Jaafari N, Giot JP, Houeto JL, Mertens P, Velut S, Bataille B (2011): The accumbofrontal fasciculus in the human brain: a microsurgical anatomical study. Neurosurgery 68:1102–1111. [DOI] [PubMed] [Google Scholar]
- Salimpoor VN, van den Bosch I, Kovacevic N, McIntosh AR, Dagher A, Zatorre RJ (2013): Interactions between the nucleus accumbens and auditory cortices predict music reward value. Science 340:216–219. [DOI] [PubMed] [Google Scholar]
- Scheele D, Wille A, Kendrick KM, Stoffel‐Wagner B, Becker B, Gunturkun O, Maier W, Hurlemann R (2013): Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proc Natl Acad Sci USA 110:20308–20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojo S, Simion C, Shimojo E, Scheier C (2003): Gaze bias both reflects and influences preference. Nat Neurosci 6:1317–1322. [DOI] [PubMed] [Google Scholar]
- Tsukiura T, Cabeza R (2011): Shared brain activity for aesthetic and moral judgments: implications for the Beauty‐is‐Good stereotype. Soc Cogn Affect Neurosci 6:138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusche A, Bode S, Haynes JD (2010): Neural responses to unattended products predict later consumer choices. J Neurosci 30:8024–8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno A, Ito A, Kawasaki I, Kawachi Y, Yoshida K, Murakami Y, Sakai S, Iijima T, Matsue Y, Fujii T (2014): Neural activity associated with enhanced facial attractiveness by cosmetics use. Neurosci Lett 566:142–146. [DOI] [PubMed] [Google Scholar]
- Wunderlich K, Dayan P, Dolan RJ (2012): Mapping value based planning and extensively trained choice in the human brain. Nat Neurosci 15:786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajonc RB (1968): Attitudinal effects of mere exposure. J Pers Soc Psychol 9:1–27. 5667435 [Google Scholar]
- Zaki J, Schirmer J, Mitchell JP (2011): Social influence modulates the neural computation of value. Psychol Sci 22:894–900. [DOI] [PubMed] [Google Scholar]


