Abstract
Migraine sufferers with aura often report photosensitivity and visual discomfort outside of attacks and many consider bright or flickering light an attack‐precipitating factor. The nature of this visual hypersensitivity and its relation to the underlying pathophysiology of the migraine aura is unknown. Using fMRI measurements during visual stimulation we examined the visual cortical responsiveness of patients with migraine with aura. We applied a within‐patient design by assessing functional interhemispheric differences in patients consistently experiencing visual aura in the same visual hemifield. We recruited 20 patients with frequent side‐fixed visual aura attacks (≥90% of auras occurring in the same visual hemifield) and 20 age and sex matched healthy controls and compared the fMRI blood oxygenation level dependent (BOLD) responses to visual stimulation between symptomatic and asymptomatic hemispheres during the interictal phase and between migraine patients and controls. BOLD responses were selectively increased in the symptomatic hemispheres. This was found in the inferior parietal lobule (P = 0.002), the inferior frontal gyrus (P = 0.003), and the superior parietal lobule (P = 0.017). The affected cortical areas comprise a visually driven functional network involved in oculomotor control, guidance of movement, motion perception, visual attention, and visual spatial memory. The patients also had significantly increased response in the same cortical areas when compared to controls (P < 0.05). We discovered a lateralized alteration of a visually driven functional network in patients with side‐fixed aura. These findings suggest a hyperexcitability of the visual system in the interictal phase of migraine with visual aura. Hum Brain Mapp 35:2714–2723, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: migraine, aura, headache, functional MRI, cortical spreading depression
INTRODUCTION
Migraine is the most prevalent neurological disorder with a 1‐year prevalence of ∼10% in the general population [Stovner et al., 2007]. About one third of migraine patients experience an aura, i.e., gradually developing, transient neurological symptoms [Russell et al., 1995], most commonly in the form of homonymous visual disturbances [Russell and Olesen, 1996].
The migraine aura clearly reflects a cortical process; most likely cortical spreading depression (CSD) [Lauritzen, 1994], characterized by a slowly spreading wave of depolarization in grey matter followed by suppression of neuronal activity [Leao, 1944]. It has been suggested that an increased responsiveness especially to visual stimuli in the interictal state predisposes migraine patients to developing attacks, possibly by triggering CSD (reviewed in [Aurora and Wilkinson, 2007; Welch et al., 1990]). Indeed many patients consider bright or flickering light an attack‐precipitating factor [Hauge et al., 2010; Kelman, 2007]. Furthermore, psychophysical studies have demonstrated increased photosensitivity [Drummond, 1986; Main et al., 1997] and aversion to the exposure to certain visual patterns [Haigh et al., 2012] outside of attacks. Numerous studies of visual evoked potentials (VEPs) have shown increased amplitudes, however not consistently [Nguyen et al., 2012], and lack of habituation (reviewed in [Ambrosini et al., 2003]). Transcranial magnetic stimulation (TMS) studies have yielded conflicting results (reviewed in [Brigo et al., 2012]) but generally they found lower thresholds for the induction of visual perception (phospenes) in migraine with aura (MA) compared to controls [Brigo et al., 2012]. Functional magnetic resonance imaging (fMRI) has the potential not only to detect, but also to localize hypersensitive cortex. There are only very few fMRI studies of cortical response to visual stimulation during the interictal state of migraine with aura and results are inconsistent [Huang et al., 2003, 2011; Vincent et al., 2003].
Aura symptoms are mostly half‐sided [Russell and Olesen, 1996] but the symptoms rarely occur consistently on the same side. A powerful study design is to compare symptomatic and nonsymptomatic cortical responsiveness in MA patients who always experience aura symptoms on the same side (fixed side of aura). This method is very challenging since MA patients with fixed side aura and frequent attacks are rare [Russell and Olesen, 1996]. In this report, we present the first application of this approach in an fMRI investigation of the blood oxygenation level dependent (BOLD) responses to visual stimulation in MA patients outside of attacks. We compared the symptomatic and the nonsymptomatic cerebral hemisphere of patients with side‐fixed aura. On the basis of the previous neurophysiological and psychophysical findings, we hypothesized that a greater BOLD signal amplitude would be seen in symptomatic hemispheres compared to asymptomatic hemispheres. The response to stimulation in the patients was also compared to age and sex matched healthy controls.
MATERIALS AND METHODS
We recruited 20 patients (15 F, 5 M, mean age 35.0 [range 20.7–55.0]) suffering from migraine with typical aura (MA) according to the second edition of The International Classification of Headache Disorders (‘Headache Classification Subcommittee of the International Headache Society.’, 2004). For patient clinical characteristics see Table 1. Inclusion criteria were: Unilateral, homonymous visual aura occurring on the same side (either the left or right hemifield) in 90% of attacks or more and an attack frequency of one attack per month or more. We also included 20 healthy control subjects (15 F, 5 M, mean age 35.1 [range 20.6–54.7]) who were individually age and sex matched to the migraine patients. Exclusion criteria for both groups were: Any other type of headache (except for tension‐type headache 3 days per month or less), serious somatic or psychiatric conditions, or intake of daily medication including prophylactic migraine treatment. For healthy controls specifically: a history of any type of migraine or first‐degree relatives with a history of any type of migraine. Patients and controls had a general medical history taken and underwent a neurological examination. All participants reported normal or corrected to normal vision.
Table 1.
Patient characteristics
| Patient no. | Sex | Age at inclusion (years) | Visual aura, affected visual field | Headache location | Attack frequency (attacks/month) | Duration of MA history (years) |
|---|---|---|---|---|---|---|
| 01 | Female | 22,6 | Left | Right | 8 | 20 |
| 02 | Female | 41,7 | Right | Left | 1 | 30 |
| 03 | Male | 41,9 | Right | Right | 2 | 21 |
| 04 | Female | 46,6 | Right | Bilateral | 3 | 34 |
| 05 | Female | 33,0 | Left | Bilateral | 1 | 1 |
| 06 | Male | 48,0 | Left | Left | 1 | 10 |
| 07 | Female | 30,7 | Left | Bilateral | 3 | 23 |
| 08 | Female | 20,7 | Right | Left | 3 | 16 |
| 09 | Male | 53,1 | Right | Left | 1 | 43 |
| 10 | Female | 55,0 | Left | Right | 1 | 39 |
| 11 | Male | 24,6 | Right | Bilat | 2 | 18 |
| 12 | Female | 51,7 | Right | Left | 1 | 35 |
| 13 | Female | 26,4 | Right | Right | 2 | 11 |
| 14 | Female | 33,1 | Left | Bilateral | 1 | 31 |
| 15 | Male | 44,1 | Left | Right | 1 | 22 |
| 16 | Female | 27,6 | Left | Right | 2 | 6 |
| 17 | Female | 25,8 | Left | Right | 8 | 21 |
| 18 | Female | 22,5 | Right | Bilateral | 2 | 6 |
| 19 | Female | 23,0 | Right | Bilateral | 2 | 11 |
| 20 | Female | 27,5 | Left | Right | 2 | 19 |
The median MA attack frequency was two attacks per month [range 1–8 attacks/month]. Prior to inclusion, patients gave a very detailed description of their aura symptoms. All patients experienced visual symptoms. In some patients visual aura was followed by sensory (n = 11) and aphasic (n = 5) symptoms. Patients reporting more types of aura experienced visual aura in every attack.
The Ethics Committee of the County of Copenhagen (H‐KA‐20060083) approved the study, which was undertaken in accordance with the Helsinki Declaration of 1964, as revised in 2008. The study was carried out at Glostrup Hospital, Copenhagen Area, Denmark from April 2011 to February 2012. All subjects gave informed consent to participate in the study.
MRI Procedure
MRI was performed on a 3.0T Philips Intera Achieva scanner (Philips Medical Systems, Best, The Netherlands) using a 32‐element phased‐array receive head coil. Anatomical images were acquired using a T1‐weighted three‐dimensional turbo field‐echo sequence (170 sagittal slices of 1‐mm thickness; in‐plane resolution 1 × 1 mm2; repetition time 9.9 s; echo time 4.6 ms; and flip angle 81°).
Functional imaging used a gradient‐echo planar imaging sequence (32 slices of 4.0‐mm thickness; slice gap 0.1 mm; field of view 230 × 230 mm2; in‐plane acquired resolution 2.9 × 2.9 mm2; repetition time 3.0 s; echo time 35 ms; flip angle 90°; and SENSE (SENSitivity Encoding factor 2). Dummy scans (two volumes) were applied to ensure steady‐state longitudinal magnetization. Heart rate, respiratory frequency and end‐tidal carbon dioxide were monitored during the scanning procedure. The lighting conditions in the scanner room were identical during each scan. Visual stimulation was presented using OLED video goggles (NordicNeuroLab, Bergen, Norway; SVGA, 800 × 600 pixels, refresh rate 85 Hz, FOV 30° horizontal, 23° vertical, stimulus luminance: 70–110 cd m−2). A fiber optic cable connected the system to a control computer outside the scanner room. The block‐design stimulation paradigm consisted of an alternation of stimulation and rest blocks each comprising 18 s. A salient high contrast motion stimulus was used to drive large expanses of visual cortex [Winawer et al., 2010], i.e., a moving black and white dartboard pattern [diameter: 22° (circular aperture); ring width: 0.6°; spoke width: 15°; patterns in each spoke moved in opposite directions, alternately inward and outward, with random changes of the motion direction approx. every 2–3 s]. The stimulus was generated using freely available Matlab‐based software (http://vistalab.stanford.edu/software). A complete scan comprised thirty‐two 18‐s blocks and lasted 576 s. The subjects were instructed to fixate on a central fixation point during the entire scan. They performed no additional stimulus driven task (e.g., button press) to avoid task related effects on response lateralization [Wolynski et al., 2009]. The onset of visual stimulation was triggered by the scan acquisition.
Data Analysis
Functional activation of the symptomatic hemispheres (i.e., contralateral to the visual symptoms) of the patients was compared to the activation level of their contralateral asymptomatic hemispheres (Fig. 1). Because right‐sided and left‐sided symptoms were reported by an equal number of patients, an equal number of right and left symptomatic hemispheres were analyzed. Thus, any differences between right and left hemispheres (e.g., caused by physiological left/right bias, asymmetry of the visual stimulation or magnetic field inhomogeneity of the scanner) would be expected to cancel each other out in the analysis. The symptomatic hemispheres were also compared to the corresponding hemispheres in age and sex‐matched healthy controls, as were the asymptomatic hemispheres (e.g., for a patient with left‐sided symptoms, the symptomatic right hemisphere was compared to a right hemisphere in a healthy control of same age and sex). The functional activation in response to visual stimulation was assessed using a voxel‐wise analysis and a Region of Interest (ROI)‐based analysis.
Figure 1.
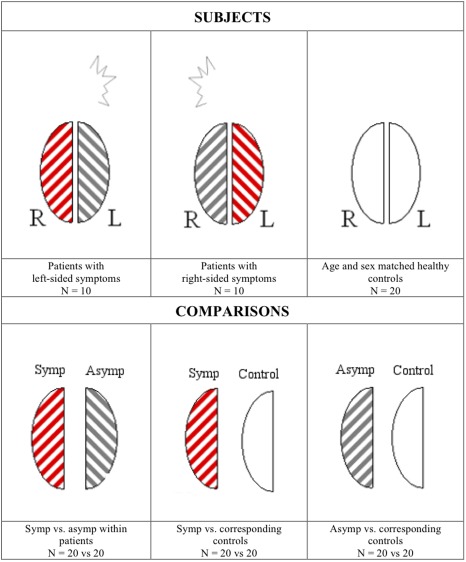
General principle of the hemisphere comparison. Hemispheres of patients with visual aura symptoms consistently originating from either the right (N = 10) or the left (N = 10) hemisphere are examined. Symptomatic (symp) hemispheres (red stripes) of the patients (N = 20) are compared to their contralateral asymptomatic (asymp) hemispheres (gray stripes, N = 20). Subsequently, the symptomatic and asymptomatic patient hemispheres are compared to hemispheres of matched healthy control subjects (white). Left patient hemispheres are compared to left control hemispheres and vice versa. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Voxel‐Wise Analysis
The objective of this analysis was the direct comparison of voxels in symptomatic hemispheres to corresponding voxels in asymptomatic hemispheres by comparing fMRI data in the radiological convention to mirrored data (i.e., flipped horizontally in the left‐right direction). This approach for interhemispheric comparison has previously been validated for assessing language dominance [Baciu et al., 2005].
Analysis of the fMRI images to identify regions exhibiting significant stimulus‐correlated changes in blood oxygen level dependent (BOLD) signal was carried out in a multi‐stage process using FMRIB's Software Library (FSL) ver. 4.1.19 [Jenkinson et al., 2012].
Mirror images of the acquired functional and anatomical data were created for each subject. To avoid left‐right bias from registration to an asymmetrical standard space, a symmetrical version of the Montreal Neurological Institute (MNI) 152 template was created by adding a mirrored version of the template to the original version [Baciu et al., 2005]. Functional data were registered to the brain extracted T1‐weighted high‐resolution scans and to the symmetrical MNI152 template using FSL FLIRT linear registration. Registration from high resolution structural to standard space was further refined using FSL FNIRT nonlinear registration. Mirrored functional images were registered to the corresponding mirrored T1‐weighted images. Functional data were also registered to the standard MNI152 template in a separate analysis for use in the ROI‐based analyses (see below).
First‐level analysis was carried out using FSL FEAT (FMRI EXPERT Analysis Tool) ver. 5.98. Preprocessing included slice time correction, spatial smoothing (FWHM 5 mm), high pass filtering (cut‐off 36 s), head motion correction using FSL MCFLIRT and brain extraction of functional and anatomical images using FSL BET.
After preprocessing, a voxel‐based analysis was performed using a general linear modeling approach, with local autocorrelation correction, of seven regressors (main stimulus (box‐car convolved with a canonical single gamma hemodynamic response function), a temporal derivative and six motion regressors). To compare activation between hemispheres, a paired voxel‐wise group comparison of the first‐level results using FSL FLAME1+2 [Woolrich et al., 2009] was carried out. Differences between symptomatic and asymptomatic hemispheres were assessed by comparing original data of patients with right‐sided symptoms and mirrored data of patients with left‐sided symptoms to the corresponding mirrored data of patients with left‐sided symptoms and original data of patients with right‐sided symptoms. Similarly, symptomatic hemispheres and asymptomatic hemispheres of patients were compared to the corresponding hemispheres of matched controls (see Fig. 1). Z (Gaussianized T) statistic images were thresholded using clusters determined by Z > 2.3 and a corrected cluster significance threshold of P < 0.05 (using a distribution based on Gaussian Random Field Theory).
Region of Interest‐Based Analysis
Values from ROIs were extracted from the previously calculated FSL‐FEAT results using featquery (a part of the FSL software package). ROIs were mask images derived from the results of the voxel‐wise analyses, from Freesurfer segmentations (see below) or ROIs from the Jülich histological (cyto‐ and myelo‐architectonic) atlas [Eickhoff et al., 2006]. The values used for calculations were the median percentual signal changes during activation.
For ROIs derived from the voxel‐wise analysis values were extracted from functional data registered to the symmetrical version of the MNI‐template. For all other ROIs, values were extracted from functional data registered to the standard MNI152 2‐mm template. Statistical calculations were carried out using R ver. 2.14.1 for MacOS X. Values of ROIs were compared using paired T tests corrected for multiple comparison using the sequential Bonferroni correction after Holm [Holm, 1979].
Individual visual area ROIs were created for each subject by cortical reconstruction and volumetric segmentation of the acquired high‐resolution T1‐weighted images using the Freesurfer image analysis suite [Dale et al., 1999]. In this procedure spatial probability maps of different Brodmann areas were created. An accurate prediction of primary visual cortex (V1) based on cortical folds [Hinds et al., 2008] was furthermore carried out. The latter method has a very high agreement with fMRI retinotopic mapping for the identification of V1 [Benson et al., 2011]. In Freesurfer only the following visual area definitions are provided: Primary visual cortex (V1), secondary visual cortex (V2) and visual area V5/MT. To expand the analysis to other areas, we additionally evaluated ROIs provided by the Jülich atlas [Eickhoff et al., 2006] i.e., visual areas V3 and V4 and the lateral geniculate nucleus (LGN).
RESULTS
All subjects completed the study and complied well with the study procedures. No patients reported aura or headache during or following visual stimulation. The T1‐weighted images were reviewed by an experienced neuroradiologist (VAL) who did not find structural abnormalities in any of the subjects.
Within‐Patient Comparisons
The voxel‐wise analysis revealed multiple areas of significantly higher activation levels in the symptomatic compared to the contralateral asymptomatic hemispheres. Significantly lower activation was not detected anywhere in the symptomatic hemispheres. Figure 2A shows “glass brain” maximum intensity projections of these voxel‐wise results. The most significant voxel clusters were in the inferior frontal gyrus (561 voxels, most significant voxel MNI coordinates (x,y,z)) = (−38, 36, 16), P = 8.5 × 10−6), in the superior parietal lobule (SPL)/intra‐parietal sulcus (IPS) (324 voxels, (−18, −62, 50), P = 0.0013), in the inferior parietal lobule (IPL) (234 voxels, (−60, −40, 38), P = 0.011) and in a separate cluster of the inferior frontal gyrus, possibly corresponding to the pars opercularis (195 voxels, (−46, 10, 16), P = 0.03). A detailed account of the individual results for these areas is given in Figure 3. In this quantitative ROI analysis the largest signal increases in the symptomatic hemisphere were evident for the cluster superior parietal lobule/intra‐parietal sulcus. In the asymptomatic hemisphere median percentual signal changes are negative for some of the clusters analyzed. This signal decrease is most pronounced for the cluster inferior parietal lobule/supramarginal gyrus. No significant differences were found for the occipital lobe or LGN ROIs. Comparison of mean instead of median percentual signal changes provided the same statistical conclusions for the paired T test and the sign test.
Figure 2.
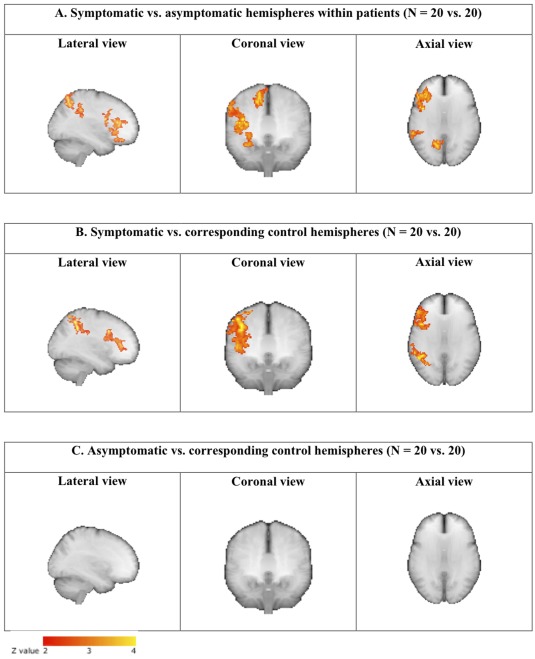
Maximum intensity projections (“Glass brain”) from the voxel‐wise hemisphere comparisons of the fMRI‐BOLD responses to visual stimulation. The significantly activated three‐dimensional areas are projected onto different two‐dimensional planes: lateral, coronal and axial. Symptomatic hemispheres of the patients are compared to their contralateral asymptomatic hemispheres (A). Then the symptomatic and asymptomatic patient hemispheres are compared to hemispheres of matched healthy controls (B and C). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 3.
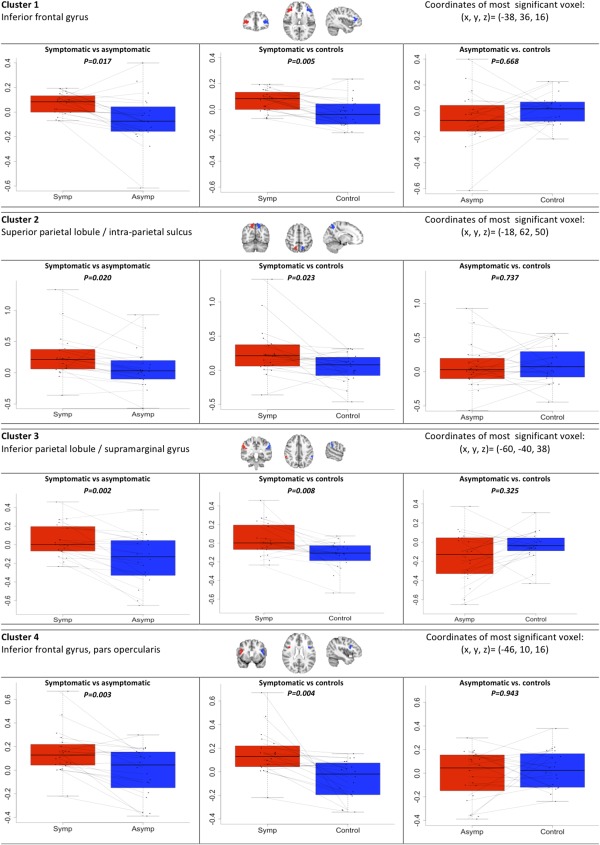
ROI‐based results from extracted clusters. The fMRI‐BOLD activation levels in the four significant clusters of activation from the voxel‐wise comparison of symptomatic and asymptomatic patient hemispheres. For each cluster a comparison of symptomatic hemispheres (symp) to the contralateral asymptomatic hemispheres (asymp) within patients is shown. Also shown are comparisons of the symptomatic and asymptomatic patient hemispheres to hemispheres of matched healthy control subjects. Activation levels were compared using paired T tests corrected for multiple comparison using the sequential Bonferroni correction after Holm. Values on y‐axes are median percentual BOLD signal changes during activation. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
We did not find correlations between the level and direction of asymmetry and the attack frequency or disease duration.
Comparison of Patients to Healthy Controls
In the voxel‐wise comparison of symptomatic hemispheres in patients to corresponding hemispheres in controls (Fig. 2B), we found an activation pattern similar to that of the within‐patient analysis, and no differences when comparing asymptomatic hemispheres to corresponding controls (Fig. 2C). No voxels had significantly higher activity in hemispheres of controls than in hemispheres of patients. Accordingly, the individual results indicate significantly higher activations in symptomatic than in corresponding control hemispheres for all four clusters identified above, but no differences between asymptomatic patient hemispheres and controls in any clusters (Fig. 3). No significant differences were found for the occipital lobe or LGN ROIs. No statistical voxel‐wise differences were found in a whole‐brain comparison of patients versus controls with images from both groups in the original orientation.
DISCUSSION
The major result of the present study was that fMRI‐BOLD signals to visual stimulation were increased in several nonoccipital cortical areas (inferior frontal gyrus, SPL, IPL, and IPS) of the symptomatic hemispheres. This hyperresponsiveness was also seen in the symptomatic hemispheres of patients when compared to age and sex matched healthy controls.
fMRI Studies in Migraine With Aura
There are only few previous fMRI studies addressing visually driven activity in MA. They are heterogeneous in terms of the visual stimulation schemes applied and fail to provide a coherent picture of the activation patterns in MA [Huang et al., 2003; 2006; 2011; Vincent et al., 2003]. Huang et al., in a small pilot study of MA patients (n = 6), reported increased BOLD response in the primary visual cortex [Huang et al., 2003], but this was not reproduced in a recent follow‐up study with a larger sample size (n = 11) [Huang et al., 2011]. Vincent et al. [2003] reported increased BOLD responses to presentation of pattern discontinuities in the striate and extra‐striate visual cortex of MA patients (n = 5). Normal impact of visual masking on BOLD responses was found (n = 10) [Huang et al., 2006]. In a recent study, applying a method very similar to that of the present study, Datta et al. also reported increased BOLD signals in MA patients compared to patients with migraine without aura and to healthy controls [Datta et al., 2013], but these were located in V1 and LGN. The stimulation used by Datta et al. was designed to maximize V1 responses, while we here used a larger stimulus designed to activate V1 and large expanses of extra‐striate cortex. It is possible that the differences in the visual stimulation paradigms account for this discrepancy.
Functional Relevance of Hyper‐Responsive Cortex
The cortical areas with higher responsiveness are part of a distributed network associated with advanced visual processing [Van Essen and Gallant, 1994]. This network covers areas of the dorsal processing stream and fronto‐lateral regions and is particularly relevant for spatial tasks, i.e., localization of objects in space and guidance of actions, eye‐movements and shifts of spatial attention, as has been demonstrated by numerous studies in human and nonhuman primates [Perani et al., 1987; Rizzolatti and Matelli, 2003; Swisher et al., 2007]. The frontal regions with hyper‐responsiveness overlap dorso‐lateral prefrontal areas that are engaged in memory‐guided saccades, spatial working memory and the executive control of spatial attention [Seeck et al., 1995; Walker et al., 1998]. Taken together, the hyper‐responsive areas found in MA hemispheres comprise a functional network involved in oculomotor control, guidance of movement, motion perception, visual attention, and visual spatial memory. The present study thus highlights a very specific and lateralized alteration of this cortical network in migraine with unilateral aura.
Some previous studies demonstrated that parts of visual function supported by the above network are impaired in MA patients. They perform slower on tests of visual attention and visual memory outside of attacks compared to MO patients and healthy controls [Mulder et al., 2009]. Deficits of motion perception and orientation discrimination were also found in MA interictally [McKendrick et al., 2001]. MA patients with side‐fixed aura experience visual illusions of motion and orientation in the affected hemifield when viewing stripe patterns [Khalil et al., 2011]. MA patients find these patterns more unpleasant than MO patients and controls [Chronicle et al., 1995]. Dysfunction of saccadic eye movements in migraine has been reported [Cambron et al., 2011; Chandna et al., 2012], although not consistently [Wilkinson et al., 2006]. It is possible that these abnormalities of visual function in migraine patients are correlated to the hyper‐responsiveness of the cortical visual areas found in the present study and, thus, that the altered function of this network may explain visual dysfunction in MA.
Abnormalities of motion processing in migraine has previously been related to anatomical alterations of cortical areas MT/V5 and V3A [Granziera et al., 2006]. In the present study we specifically studied the activation of these areas in the ROI based analysis and found no interhemispheric functional differences. While MT/V5 plays an integral role in motion perception, prefrontal and parietal areas are probably equally important at least for some types of motion perception [Billino et al., 2009; Bremmer et al., 2001].
It should be noted that since the BOLD response primarily reflects input and local processing of neuronal information rather than the output [Logothetis and Wandell, 2004] it is theoretically possible that the increased activity reflect hyperresponsiveness of structures that provide efferent input to these areas. The interhemispheric differences in the patients could reflect inhibition of the asymptomatic hemispheres but since we found no difference between asymptomatic hemispheres and healthy controls this effect is not likely. Our findings suggest that MA patients with alternating aura sides or bilateral auras would have no or little interhemispheric differences while still being hyperresponsive compared to healthy controls. This should however be confirmed in future studies and at present our conclusions directly apply to patients with side‐fixed aura only.
Cortical Hyperresponsiveness and Cortical Spreading Depression
The relation of hyperresponsiveness of the above network to CSD is not obvious. Because this was a cross‐sectional study we cannot determine whether this functional abnormality is a cause or a consequence of the disease. Olesen et al. observed frontal or parietal hyperemia in some cases very shortly before the occurrence of occipital spreading oligemia in a study of regional cerebral blood flow (rCBF) during attacks of MA using the intra‐arterial xenon injection method [Olesen et al., 1981]. In a later study by Lauritzen et al., using xenon single‐photon emission tomography (SPECT), a marked hypoperfusion of the frontal, temporal and parietal, but not occipital lobes of the hemisphere contralateral to visual aura symptoms was observed in a patient who was examined early in an MA attack [Lauritzen and Olesen, 1984]. In a more recent case study investigating the occipital lobe with fMRI, a spreading decrease of the BOLD signal starting in visual area V3a was seen during an MA attack [Hadjikhani et al., 2001]. Contrary to the common notion that visual aura symptoms originate from the primary visual cortex [Lauritzen, 2001], it is thus plausible that the pathophysiological process is initiated in associated higher level visual cortex. There is also evidence that higher level visual areas project back to V1 [Lauritzen et al., 2009] but this has previously not been related to setting the threshold for spreading depression.
In conclusion, applying fMRI measurements during visual stimulation to patients with side‐fixed visual aura we found higher responsiveness of a visually driven functional network in the frontal and parietal cortex contralateral to the visual aura symptoms. These findings suggest a hyperexcitability of the visual system in the interictal phase of migraine with visual aura. In addition, they may explain previous findings of visual dysfunction in migraine with aura and they emphasize the importance of cortex outside of the primary visual area for this condition.
Conflicts of Interest: Dr. Hougaard reports no conflicts of interest. Dr. Amin has received honoraria for lecturing from Allergan. Dr. Hoffmann reports no conflicts of interest. Dr. Rostrup reports no conflicts of interest. Dr. Larsson reports no conflicts of interest. Dr. Asghar reports no conflicts of interest. Dr. Larsen reports no conflicts of interest. Dr. Olesen has received grants and/or research support from, has been a consultant and/or scientific adviser for, and has been in the speakers' bureau of Allergan Inc, AstraZeneca Pharmaceuticals LP, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Janssen Pharmaceutical Products, Lundbeck, Merck and Pfizer. Dr. Ashina has received grant support and honoraria for lecturing from Merck, and honoraria for lecturing from Pfizer, GlaxoSmithKline, Norpharma and AstraZeneca, and he is a consultant and/or scientific adviser for Allergan, Amgen and Alder.
REFERENCES
- Ambrosini A, de Noordhout AM, Sándor PS, Schoenen J (2003): Electrophysiological studies in migraine: A comprehensive review of their interest and limitations. Cephalalgia 23(Suppl 1):13–31. [DOI] [PubMed] [Google Scholar]
- Aurora SK, Wilkinson F (2007). The brain is hyperexcitable in migraine. Cephalalgia 27:1442–1453. [DOI] [PubMed] [Google Scholar]
- Baciu M, Juphard A, Cousin E, Bas JFL (2005): Evaluating fMRI methods for assessing hemispheric language dominance in healthy subjects. Eur J Radiol 55:209–218. [DOI] [PubMed] [Google Scholar]
- Benson NC, Butt OH, Datta R, Brainard DH, Aguirre GK (2011). A universal retinotopic mapping of V1 with respect to anatomy. J Vis 11:1067–1067. [Google Scholar]
- Billino J, Braun DI, Böhm K‐D, Bremmer F, Gegenfurtner KR (2009): Cortical networks for motion processing: Effects of focal brain lesions on perception of different motion types. Neuropsychologia 47:2133–2144. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Schlack A, Shah NJ, Zafiris O, Kubischik M, Hoffmann K, Zilles K, Fink GR (2001): Polymodal motion processing in posterior parietal and premotor cortex: A human fMRI study strongly implies equivalencies between humans and monkeys. Neuron 29:287–296. [DOI] [PubMed] [Google Scholar]
- Brigo F, Storti M, Nardone R, Fiaschi A, Bongiovanni LG, Tezzon F, Manganotti P (2012): Transcranial magnetic stimulation of visual cortex in migraine patients: A systematic review with meta‐analysis. J Headache Pain 13:339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambron M, Anseeuw S, Paemeleire K, Crevits L (2011): Saccade behavior in migraine patients. Cephalalgia 31:1005–1014. [DOI] [PubMed] [Google Scholar]
- Chandna A, Chandrasekharan DP, Ramesh AV, Carpenter R (2012): Altered interictal saccadic reaction time in migraine: A cross‐sectional study. Cephalalgia 32:473–480. [DOI] [PubMed] [Google Scholar]
- Chronicle EP, Wilkins AJ, Coleston DM (1995): Thresholds for detection of a target against a background grating suggest visual dysfunction in migraine with aura but not migraine without aura. Cephalalgia 15:117–122. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI (1999). Cortical surface‐based analysis. I. Segmentation and surface reconstruction. Neuroimage 9:179–194. [DOI] [PubMed] [Google Scholar]
- Datta R, Aguirre GK, Hu S, Detre JA, Cucchiara B (2013): Interictal cortical hyperresponsiveness in migraine is directly related to the presence of aura. Cephalalgia 33:365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond PD (1986): A quantitative assessment of photophobia in migraine and tension headache. Headache 26:465–469. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K (2006): Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage 32:570–582. [DOI] [PubMed] [Google Scholar]
- Granziera C, DaSilva AFM, Snyder J, Tuch DS, Hadjikhani N (2006): Anatomical alterations of the visual motion processing network in migraine with and without aura. Plos Med 3:e402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, Sanchez del Rio M, Wu O, Schwartz D, Bakker D, Fischl B, Kwong KK, Cutrer FM, Rosen BR, Tootell RB, Sorensen AG, Moskowitz MA (2001): Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci USA 98:4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigh S, Karanovic O, Wilkinson F, Wilkins A (2012): Cortical hyperexcitability in migraine and aversion to patterns. Cephalalgia 32:236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauge AW, Kirchmann M, Olesen J (2010): Trigger factors in migraine with aura. Cephalalgia 30:346–353. [DOI] [PubMed] [Google Scholar]
- Hinds OP, Rajendran N, Polimeni JR, Augustinack JC, Wiggins G, Wald LL, Diana Rosas H, Potthast A, Schwart EL, Fischl B (2008): Accurate prediction of V1 location from cortical folds in a surface coordinate system. Neuroimage 39:1585–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S (1979): A simple sequentially rejective multiple test procedure. Scand J Stat 1979:65–70. [Google Scholar]
- Huang J, Cooper TG, Satana B, Kaufman DI, Cao Y (2003). Visual distortion provoked by a stimulus in migraine associated with hyperneuronal activity. Headache 43:664–671. [DOI] [PubMed] [Google Scholar]
- Huang J, DeLano M, Cao Y (2006): Visual cortical inhibitory function in migraine is not generally impaired: evidence from a combined psychophysical test with an fMRI study. Cephalalgia 26:554–560. [DOI] [PubMed] [Google Scholar]
- Huang J, Zong X, Wilkins A, Jenkins B, Bozoki A, Cao Y (2011): fMRI evidence that precision ophthalmic tints reduce cortical hyperactivation in migraine. Cephalalgia 31:925–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM (2012): FSL. Neuroimage 62:782–790. [DOI] [PubMed] [Google Scholar]
- Kelman L (2007): The triggers or precipitants of the acute migraine attack. Cephalalgia 27:394–402. [DOI] [PubMed] [Google Scholar]
- Khalil NM, Nicotra A, Wilkins AJ (2011): Asymmetry of visual function in migraine with aura: Correlation with lateralization of headache and aura. Cephalalgia 31:213–221. [DOI] [PubMed] [Google Scholar]
- Lauritzen M (1994): Pathophysiology of the migraine aura. Brain 117:199–210. [DOI] [PubMed] [Google Scholar]
- Lauritzen M (2001): Cortical spreading depression in migraine. Cephalalgia 21:757–760. [DOI] [PubMed] [Google Scholar]
- Lauritzen M, Olesen J (1984): Regional cerebral blood flow during migraine attacks by Xenon‐133 inhalation and emission tomography. Brain 107 (Part 2):447–461. [DOI] [PubMed] [Google Scholar]
- Lauritzen TZ, D'Esposito M, Heeger DJ, Silver MA (2009): Top‐down flow of visual spatial attention signals from parietal to occipital cortex. J Vis 9:18–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leao AAP (1944): Spreading depression of activity in the cerebral cortex. J Neurophysiol 7:359–390. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Wandell BA (2004): Interpreting the BOLD signal. Annu Rev Physiol 66:735–769. [DOI] [PubMed] [Google Scholar]
- Main A, Dowson A, Gross M (1997): Photophobia and phonophobia in migraineurs between attacks. Headache 37:492–495. [DOI] [PubMed] [Google Scholar]
- McKendrick AM, Vingrys AJ, Badcock DR, Heywood JT (2001): Visual dysfunction between migraine events. Investig Ophthalmol Vis Sci 42:626–633. [PubMed] [Google Scholar]
- Mulder E, Linssen W, Passchier J, Orlebeke JF, Geus EJC (2009): Interictal and postictal cognitive changes in migraine. Cephalalgia 19:557–565. [DOI] [PubMed] [Google Scholar]
- Nguyen BN, McKendrick AM, Vingrys AJ (2012): Simultaneous retinal and cortical visually evoked electrophysiological responses in between migraine attacks. Cephalalgia 32:896–907. [DOI] [PubMed] [Google Scholar]
- Olesen J, Larsen B, Lauritzen M (1981): Focal hyperemia followed by spreading oligemia and impaired activation of rCBF in classic migraine. Ann Neurol 9:344–352. [DOI] [PubMed] [Google Scholar]
- Perani D, Vallar G, Cappa S, Messa C, Fazio F (1987): Aphasia and neglect after subcortical stroke. A clinical/cerebral perfusion correlation study. Brain 110 (Part 5):1211–1229. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Matelli M (2003): Two different streams form the dorsal visual system: Anatomy and functions. Exp Brain Res 153:146–157. [DOI] [PubMed] [Google Scholar]
- Russell M, Olesen J (1996): A nosographic analysis of the migraine aura in a general population. Brain 119:355–361. [DOI] [PubMed] [Google Scholar]
- Russell MB, Rasmussen BK, Thorvaldsen P, Olesen J (1995): Prevalence and sex‐ratio of the subtypes of migraine. Int J Epidemiol 24:612–618. [DOI] [PubMed] [Google Scholar]
- Seeck M, Schomer D, Mainwaring N, Ives J, Dubuisson D, Blume H, Cosgrove R, Ransil BJ, Mesulam MM (1995): Selectively distributed processing of visual object recognition in the temporal and frontal lobes of the human brain. Ann Neurol 37:538–545. [DOI] [PubMed] [Google Scholar]
- Stovner LJ, Hagen K, Jensen R, Katsarava Z, Lipton RB, Scher AI, Steiner T, Zwart JA, (2007): The global burden of headache: A documentation of headache prevalence and disability worldwide. Cephalalgia 27:193–210. [DOI] [PubMed] [Google Scholar]
- Swisher JD, Halko MA, Merabet LB, McMains SA, Somers DC (2007): Visual topography of human intraparietal sulcus. J Neurosci 27:5326–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Gallant JL (1994): Neural mechanisms of form and motion processing in the primate visual system. Neuron 13:1–10. [DOI] [PubMed] [Google Scholar]
- Vincent M, Pedra E, Mourão‐Miranda J, Bramati IE, Henrique AR, Moll J (2003): Enhanced interictal responsiveness of the migraineous visual cortex to incongruent bar stimulation: A functional MRI visual activation study. Cephalalgia 23:860–868. [DOI] [PubMed] [Google Scholar]
- Walker R, Husain M, Hodgson TL, Harrison J (1998): Saccadic eye movement and working memory deficits following damage to human prefrontal cortex. Neuropsychologia 36:1141–1159. [DOI] [PubMed] [Google Scholar]
- Welch KM, D'Andrea G, Tepley N, Barkley G, Ramadan NM (1990): The concept of migraine as a state of central neuronal hyperexcitability. Neurol Clin NA 8:817–828. [PubMed] [Google Scholar]
- Wilkinson F, Karanovic O, Ross EC, Lillakas L, Steinbach MJ (2006): Ocular motor measures in migraine with and without aura. Cephalalgia 26:660–671. [DOI] [PubMed] [Google Scholar]
- Winawer J, Horiguchi H, Sayres RA, Amano K, Wandell BA (2010): Mapping hV4 and ventral occipital cortex: The venous eclipse. J Vis 2010;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolynski B, Schott BH, Kanowski M, Hoffmann MB (2009): Visuo‐motor integration in humans: Cortical patterns of response lateralization and functional connectivity. Neuropsychologia 47:1313–1322. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM (2009): Bayesian analysis of neuroimaging data in FSL. Neuroimage 45:S173–S186. [DOI] [PubMed] [Google Scholar]
- Headache Classification Subcommittee of the International Headache Society. (2004): The International Classification of Headache Disorders, 2nd ed. Cephalalgia 2004;24:1–160. [DOI] [PubMed] [Google Scholar]


