Abstract
Rostrolateral prefrontal cortex (RLPFC) is part of a frontoparietal network of regions involved in relational reasoning, the mental process of working with relationships between multiple mental representations. RLPFC has shown functional and structural changes with age, with increasing specificity of left RLPFC activation for relational integration during development. Here, we used dynamic causal modeling (DCM) to investigate changes in effective connectivity during a relational reasoning task through the transition from adolescence into adulthood. We examined fMRI data of 37 healthy female participants (11–30 years old) performing a relational reasoning paradigm. Comparing relational integration to the manipulation of single relations revealed activation in five regions: the RLPFC, anterior insula, dorsolateral PFC, inferior parietal lobe, and medial superior frontal gyrus. We used a new exhaustive search approach and identified a full DCM model, which included all reciprocal connections between the five clusters in the left hemisphere, as the optimal model. In line with previous resting state fMRI results, we showed distinct developmental effects on the strength of long‐range frontoparietal versus frontoinsular short‐range fixed connections. The modulatory connections associated with relational integration increased with age. Gray matter volume in left RLPFC, which decreased with age, partly accounted for changes in fixed PFC connectivity. Finally, improvements in relational integration performance were associated with greater modulatory and weaker fixed PFC connectivity. This pattern provides further evidence of increasing specificity of left PFC function for relational integration compared to the manipulation of single relations, and demonstrates an association between effective connectivity and performance during development. Hum Brain Mapp 35:3262–3276, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: rostral prefrontal cortex, adolescence, relational integration, DCM, reasoning, gray matter
INTRODUCTION
Adolescence is a period of mental change both in the social cognition [Burnett et al., 2011] and the cognitive control and reasoning domains [Ferrer et al., 2009; Luna et al., 2010]. Relational reasoning is the mental process of working on the relationships between multiple mental representations. It is a critical component of fluid reasoning, the capacity to think logically and solve problems in novel situations [Ferrer et al., 2009]. Relational reasoning is supported by a network of frontoparietal regions including the rostrolateral prefrontal cortex (RLPFC) [Bunge et al., 2009; Christoff et al., 2001; Kroger et al., 2002; Wendelken et al., 2008], a brain region which changes structurally and functionally during late childhood and adolescence [Crone et al., 2009; Dumontheil et al., 2008, 2010; Wendelken et al., 2011].
There is evidence that functional selectivity of the left RLPFC for relational integration develops during adolescence [Crone et al., 2009; Dumontheil et al., 2010; Wendelken et al., 2011], and that task performance and structural changes can partly explain changes in left RLPFC activity from adolescence to adulthood [Dumontheil et al., 2010]. Here, we investigated whether connectivity changes between RLPFC and other regions co‐activated during relational processing may drive changes in RLPFC activity and task performance during development [Crone and Dahl, 2012].
Resting state functional connectivity magnetic resonance imaging (rs‐fcMRI) measures the slow, spontaneous fluctuations in the blood‐oxygenation level dependent (BOLD) that occur in the absence of an experimental task. The term “functional connectivity” refers to correlations between the time courses of the BOLD signal in different brain regions [Vogel et al., 2010]. These correlations appear to be strongest between functionally related regions, even when those regions do not possess direct anatomical connections [Vogel et al., 2010]. Recent developmental rs‐fcMRI research has shown that long‐range connections (e.g., frontoparietal) are strengthened, while short‐range connections (e.g., within the frontal cortex) are weakened during childhood and adolescence [Dosenbach et al., 2010; Fair et al., 2008, 2007; Supekar et al., 2009; Uddin et al., 2010; Vogel et al., 2010].
These changes have been proposed to reflect a progressive integration and segregation of functions across brain regions, leading to the maturation of cognitive abilities [Fair et al., 2007; Rubia, 2012]. Developmental segregation of regions in local networks may be partly related to synaptic pruning [Petanjek et al., 2011], which is thought to result in decreased gray matter volumes as observed with structural MRI scans [Paus et al., 2008]. In contrast, the integration of anatomically disparate regions may be assisted by the myelination of long distance cortical axon tracts that occurs during development and can be observed using structural MRI or diffusion tensor imaging (DTI) [Uddin et al., 2011]. However, this mapping between large‐scale structural changes and functional connectivity changes is not a perfect relationship [Supekar et al., 2010] and other theories suggest that increased rs‐fcMRI connectivity reflects an increased history of co‐activation [Vogel et al., 2010, for discussion].
Developmental rs‐fcMRI research has recently been criticized [Colonnese and Khazipov, 2012; Kelly et al., 2012]. The spontaneous brain activity in early development has certain features that resemble activity patterns observed in the mature brain in the absence of a cognitive task, or at rest [Colonnese and Khazipov, 2012]. However, spontaneous brain activity at rest in early development and adulthood could have different origins or function, such as circuit formation in children versus varying attentional states in adults [Colonnese and Khazipov, 2012]. Rs‐fcMRI data is also sensitive to participants' motion, which could be a confound when studying development [Power et al., 2012]. Moreover, the slow fluctuations of resting state activity may in part be explained by cardiovascular and respiratory processes. Inferences made based on group differences may therefore be confounded when the inferences concern factors, such as age, that have been shown to affect neurovascular coupling [Colonnese and Khazipov, 2012; Kelly et al., 2012].
Other groups have investigated functional connectivity changes between brain regions that co‐activate during cognitive tasks. Their results reveal distinct patterns of functional connectivity depending on the experimental task. Some studies have associated maturation of cognitive control abilities to increases in functional connectivity within frontal or frontoparietal networks and between frontoparietal and fronto‐subcortical networks during the transitions from early to mid‐adolescence and from adolescence to adulthood [Barbalat et al., 2013; Christakou et al., 2011; Neufang et al., 2008; Stevens et al., 2007]. In contrast, a decrease in frontoparietal connectivity has been suggested to underlie the maturation of social emotions [Burnett and Blakemore, 2009]. Functional connectivity methods rely on statistical dependencies or correlations between spatially segregated neuronal events, and do not create models of connectivity to infer causality within neuronal networks [Friston, 2003]. Effective connectivity is another way to quantify functional integration in neuronal systems, which rests on a mechanistic model of how the data were caused [Stephan and Friston, 2010], as it describes networks of directional effects of one neural element over another.
Very few studies have used effective connectivity measures to study cognitive development. Hwang et al. [2010] used Granger Causality Analysis (GCA) to show that changes in connectivity strength were associated with changes in functional activation with age during an inhibitory control task. GCA models temporal dependencies in the data without referencing the experimental input [Friston, 2009]; it is therefore an effective connectivity method that largely does not require the specification of a model including structural parameters. Dynamic Causal Modeling (DCM), which employs an explicit forward, or generative, model to explain the observed data from the experimental manipulations, may be more appropriate to study effective connectivity [Friston, 2003, 2009; Friston and Penny, 2011; Smith, 2012]. DCM models the hidden neuronal and biophysical states that generate the observed data, thus inferring the unobserved neuronal activity from the fMRI BOLD signal [Friston, 2009]. Two previous developmental studies used DCM: one found developmental changes in effective connectivity using a response inhibition task during transition from adolescence into adulthood [Stevens et al., 2007]; the other demonstrated an age‐dependent modulation of connectivity in the network of regions sensitive to faces between late childhood and adulthood [Cohen Kadosh et al., 2011].
In this study, we used DCM to re‐examine our previously collected data on relational reasoning development [Dumontheil et al., 2010; see Hillebrandt et al., 2013 for a similar approach]. Comparing relational integration to the processing of single relations across age groups (11–14 years, 14–18 years, and 22–30 years) had revealed activation in RLPFC, anterior insula (AI), dorsolateral prefrontal cortex (DLPFC), inferior parietal lobe (IPL), and medial superior frontal gyrus (mSFG). Here, we explored whether (1) there were age‐dependent changes in the strength of fixed and modulatory connections, we contrasted frontoparietal and frontoinsular connectivity, in line with the long‐ versus short‐range distinction observed in previous rs‐fcMRI research [Dosenbach et al., 2010; Fair et al., 2008], but also forward and backward connections [Friston 2002, 2005, 2012; Fuster, 2002, 2009; Miller and Cohen 2001; Salin and Bullier 1995]; (2) whether developmental changes in connectivity strength may be linked to structural changes; and finally (3) whether developmental changes seen in behavior could be accounted for by connectivity strength, in particular whether prefrontal cortex (PFC) connections showed increasing specificity for relational integration [Crone et al., 2009; Dumontheil et al., 2010; Wendelken et al., 2011]. Relational integration has been shown to be more specifically supported by the left RLPFC [Bunge et al., 2009], and developmental changes in this dataset were observed in the left PFC [Dumontheil et al., 2010]. As the mechanisms underlying interhemispheric integration remain poorly understood [Stephan et al., 2007], the DCM analyses presented here were limited to activations in the left hemisphere.
MATERIALS AND METHODS
Participants
Thirty‐seven right‐handed female participants, aged between 11 and 30 years old, took part in this study [Dumontheil et al., 2010]. Participants had no history of psychiatric or neurological disorders and all provided informed consent (or their legal guardian if younger than 18). This study was approved by the UCL National Hospital for Neurology and Neurosurgery Ethics Committee.
Participants were divided into three age groups: young adolescent [N = 11, age range 11.0–14.4 years, 12.8 ± 1.0 (mean ± SD)], mid adolescent (N = 13, 14.7–18.5 years, 16.0 ± 1.0), adults (N = 13, 22.5–30.4 years, 25.5 ± 2.8). General cognitive ability of the participants was assessed using the two subtests form of the WASI [Wechsler, 1999], and neither differed between age groups [F(2, 34) = 0.51, P = 0.60], nor varied as a function of age [r = 0.028, P = 0.87].
Behavioral Paradigm
Participants performed a relational reasoning task, which reliably activates RLPFC [Christoff et al., 2003; Smith et al., 2007]. The task has two conditions, during which two pairs of black and white items are presented on the screen (Fig. 1). In the Relational condition, participants were instructed to identify whether two pairs of items, which could vary in shape and/or texture, differed or changed along the same dimension. If both pairs showed either texture differences or shape difference, participants were asked to respond “yes” (match trial). Alternatively, if one pair of items differed in texture while the other pair differed in shape, the participants were asked to respond “no” (no‐match trial). In the Control condition, the bottom pair was always identical in shape and texture, and the participants had to identify whether one of the items in the top pair had the same shape (or texture) as the bottom pair (Fig. 1). Relational trials required participants to integrate information from two relations, while the Control condition required participants to identify single relations.
Figure 1.
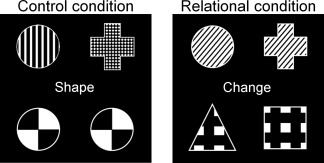
Stimuli of the experimental paradigm. In the Control condition (left), participants were asked whether one of the items in the first pair of items (top row) had the same shape (or texture) as the second pair of items (bottom row). In this example, the top left item has the same shape (circle) as the bottom items, thus the answer is yes. In the Relational condition (on the right), participants were asked whether the two pairs changed along the same dimension (shape or texture). Here both pairs change along the shape dimension, so the answer is yes.
Participants performed two sessions of the task, each consisting of five 32 s Relational blocks, five 32 s Control blocks and four 20 s Fixation blocks. Blocks started with an instruction (1.2 s) asking participants to “Match Change,” “Match Shape,” or “Match Texture,” which was followed by eight trials of the same condition. Stimuli were presented for 3.5 s, followed by a blank screen for 500 ms. Block order was counterbalanced within and between participants [see Dumontheil et al., 2010, for more details].
MRI Data Acquisition
A 1.5 Tesla Siemens Avanto MRI scanner was used to acquire both 3D T1‐weighted fast‐field echo structural images and multi‐slice T 2*‐weighted echo‐planar volumes with BOLD contrast (TR = 3 s; TE = 50 ms; TA = 2.9143 s), and 140 volumes comprising 35 axial slices with a resolution of 3 × 3 × 3 mm covering the whole brain were acquired in two 7 min functional scanning sessions.
Voxel‐Based Morphometry
Gray matter volumes were extracted using voxel‐based morphometry (VBM) [Ashburner and Friston, 2000] from the five ROIs on all participants using SPM5 VBM5 toolbox (v1.15 http://dbm.neuro.uni-jena.de/vbm). Each participant's structural T1 image was normalized to the standard T1 Montreal Neurological Institute (MNI) template. Structural scans were segmented into cerebrospinal fluid, gray and white matter; modulation for non‐linear warping only was performed using the Jacobian determinants. This method approximates a proportional adjustment of volumes for overall head size [O'Brien et al., 2006]. Images were resampled into 1.5 × 1.5 × 1.5 mm voxels and smoothed with an isotropic 12 mm, full‐width half‐maximum Gaussian kernel. The MarsBaR toolbox for SPM5 was used to calculate mean gray matter adjusted volumes for each ROI.
FMRI Analyses
FMRI data were analyzed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm). In the preprocessing step, the volumes were realigned and corrected for differences in slice acquisition times. Then the structural image was co‐registered to the mean realigned functional image, and was subsequently segmented and spatially normalized (embedded in the segmentation step) to the gray and white matter templates based on the MNI reference brain. Finally, the data was spatially smoothed with an isotropic 8 mm, full‐width half‐maximum Gaussian kernel. For all participants, head movement was less than 3 mm within each scanning session.
Mean translations and rotations from the estimates obtained from the realignment processing step were calculated for each participants. Statistical tests performed on the 33 participants included in the DCM analyses indicated there was no difference in mean movement between the age groups [Fs(2,30) > 2.6, Ps > 0.74]. There was also no correlation between movement and age as a continuous measure [translation: Pearson r = −0.162, P = 0.37; rotation: r = −0.028, P = 0.88].
The volumes acquired during the two sessions were concatenated in a single time course (for DCM analyses) and the variance in the BOLD signal was decomposed with a set of regressors in a general linear model (GLM) [Friston et al., 1995]. Three boxcar regressors representing the instructions, Relational and Control blocks, one regressor representing all error trials, and an additional regressor representing the session effect were convolved with a canonical hemodynamic response function, and, together with regressors representing residual movement‐related artefacts corresponded to the full model of the data. Due to poor accuracy, data from the second session of one participant was excluded from analysis. The data were high‐pass filtered to a cut‐off of 1/128 Hz. Fixation blocks were modeled implicitly.
This first level GLM was used to compute the least‐squares parameter estimates of the height of the best‐fitting response function of the model regressors at each voxel. Parameter estimates were combined to produce a Relational > Control contrast image for each participant. These contrast images were entered in a second level of analysis to perform a group‐level one‐sample t‐test. The resulting activation map was used to define the regions‐of‐interest for the effective connectivity analysis. All the analyses in the current study were done on the left hemisphere clusters due to stronger developmental functional changes in this hemisphere [Dumontheil et al., 2010; Wendelken et al., 2011].
Dynamic Causal Modeling
DCM is a Bayesian framework for modeling and inferring the directed connectivity among hidden (unobserved) neuronal states from measurements of brain activity, in this case BOLD activity. It can be used to analyze task or set‐dependent effective connectivity, i.e., changes in coupling strength, providing information about the changes in directed influence of one area over another in certain psychological contexts. The constructed models are elaborated based on a model that quantifies how synaptic activity translates into hemodynamic responses; coupling parameters are then estimated based on the observed fMRI signal [Friston, 2009; Stephan and Friston, 2010]. Using bilinear differential equations, DCM models how the activity in a given brain area causes [causality here is considered in the context of control theory; Marreiros et al., 2008] changes in the neural dynamics of other brain regions [Friston et al., 2003]. Given the observed data, the likelihood that the model accurately represents the true neural dynamics is then estimated within a Bayesian framework [Friston, 2009].
Three types of coupling parameters are estimated in DCM. The first parameters (DCM.A) estimate fixed connections between brain regions [also referred to as endogeneous, direct, intrinsic, or average connectivity; Friston et al., 2003], i.e., the effect that one brain region has upon another, in a baseline condition. The second parameter (DCM.B) estimates the modulation of the fixed connections between brain regions as a result of a particular task condition, i.e., the impact of the task on the connectivity between brain regions, rather than the effect that the task has on specific brain regions. Finally, the third parameter (DCM.C) indexes the driving input to the model. The extrinsic driving input usually consist of a sensory contrast that sets the system in motion, as opposed to modulatory contrasts, which are of more attentional nature and affect the coupling between brain regions [Stephan et al., 2010].
Definition of the regions of interest (ROIs)
ROIs were defined from the 2nd‐level Relational > Control contrast across all participants. Anatomical templates from the Automated Anatomical Labeling (AAL) repository [Tzourio‐Mazoyer et al., 2002] were used to identify the clusters. We used a significance threshold of P < 0.001 uncorrected for multiple comparisons to define clusters in the AI, mSFG, and RLPFC and a family wise error (FWE) corrected threshold of P < 0.05 to define clusters in the IPL and DLPFC. Different statistical thresholds were used to permit the definition of ROIs representative of anatomical regions corresponding to activations seen in previous literature. An uncorrected threshold was used for those regions with smaller extant to permit the coverage of the region of interest, e.g. the RLPFC, across participants [e.g., Smith et al., 2007 for individual variability in localization of RLPFC activations]. In addition, ensuring the clusters were of similar size enabled us to approximate the approach used in other studies of building ROIs from spheres of a fixed diameter [Bitan et al., 2009] and ensured some consistency in terms of the numbers of data points informing the extracted timecourse data in each ROI. The MarsBaR toolbox (http://marsbar.sourceforge.net) was used to split the lateral frontal activation cluster into RLPFC and DLPFC regions, using a cut‐off of y = 40 mm [Wendelken et al., 2011].
These five functionally defined ROIs were then used to extract the time series for each participant. This approach of using clusters which are functionally defined and anatomically constrained to identify individual participant's volumes of interest (VOIs) was favored to using spheres of arbitrary size around activation peaks [Stephan et al., 2010].
Extraction of VOI time series
We extracted VOIs in each participant by obtaining the principle eigenvariate of the group of voxels showing greater activation in Relational versus Control within each of the five ROIs in the left hemisphere (see previous section on the use of anatomical templates to define ROIs). A threshold of P < 0.05 uncorrected for multiple comparisons was used for the extraction [Hyde et al., 2006]. This procedure was done on concatenated data from the two sessions and the time series were adjusted for the effects of interest (i.e., variance explained by the regressors of interest, namely the Relational and Control regressors). VOIs were extracted using the SPM8 Eigenvariate toolbox. Due to lack of AI activation we were unable to extract VOIs for four participants from the Relational > Control contrast. Therefore, all the subsequent DCM analyses were conducted on the 33 remaining participants (young adolescents N = 10, mid‐adolescents N = 13, adults N = 10).
DCM specification
To model our experimental input (Relational and Control conditions) and the modulatory effect (Relational condition), a new GLM was generated for each subject. This model only differed from the previously described GLM model in that Relational and Control conditions were modeled using a first regressor combining both conditions (this represented the presence of a visual input), and a second regressor modeling Relational blocks only (modulatory effect of relational integration).
A DCM model including the five VOIs was then constructed and estimated for each participant using DCM in SPM12a (DCM12). This model was a “full” model in a sense that it incorporated all reciprocal fixed connections between and within the five regions, while the effect of the modulatory input (Relational condition) was modeled on all reciprocal connections between the five regions, excluding the self‐connections for simplicity [see Hillebrandt et al., 2013, for a similar approach]. The visual input, i.e., the contrast of Relational and Control task blocks (comprising the presentation of four black and white shapes), versus fixation blocks (comprising a small central fixation cross), was set to the most posterior region, the IPL. In other words, we would expect this region to show sensitivity to presentation of the visual stimuli prior to prefrontal regions. All DCMs were deterministic (i.e., did not model noise), two‐state models (i.e., activity in one brain region is modeled so that is has both inhibitory and excitatory neuronal populations, and a positivity constraint is introduced to allow influences of one area on another to be excitatory only [i.e., glutamatergic], making the model more realistic [Marreiros et al., 2008]), bilinear (i.e. an input‐dependent change in connectivity was modeled as a second‐order interaction between the input and activity in a source region) [Stephan and Friston, 2010], and included mean‐centered inputs (see release notes for SPM8 (r4010): http://www.fil.ion.ucl.ac.uk/spm/software/spm8/SPM8_Release_Notes_r4010.pdf).
In order to improve the fit of the DCM model, the second session was removed for three of the participants for whom the original DCM full model failed to explain any of the variance. The fit improved for two of the participants.
Post‐hoc Bayesian model selection
Originally, the model selection step in DCM involved a hypothesis driven procedure in which each model was fitted to the data and subsequently compared with other neurobiologically relevant models. However, here we used a novel method to explore very large numbers of models (i.e., model spaces with >16 free parameters) using a “post‐hoc” procedure in which only the full model is inverted (estimated) and the model evidence for any reduced model is then obtained using a greedy search procedure [Friston and Penny, 2011; Friston et al., 2011; Hillebrandt et al., 2013; Rosa et al., 2012] to select the winning model. This greedy search takes a subset of parameters with the least evidence and searches over all reduced models within that subset (in a reduced model some connections are “turned off”) and hence removes those with redundant parameters to find the winning model. This stage ends once all model parameters have been examined [Friston and Penny, 2011; Rosa et al., 2012]. The resulting optimal parameter estimates allow inferences on whether connections are differentially modulated across participants, by analysis of individual changes in connection strengths. Critically, post‐hoc routines and the conventional variational free energy approach have been shown to yield very similar results [Rosa et al., 2012].
Analysis of parameters in the optimal model
Parameter estimates of the optimal model were investigated for age effects and association with brain structure and performance. To reduce the number of tests performed, the 20 connections were grouped using two approaches. First, we organized the connections according to a well‐established anatomical and functional asymmetry between forward and backward connections [Fox and Friston, 2012; Friston 2002, 2005, 2012]. This forward versus backward distinction is based on a hierarchical organization of brain function and structure that has accumulated considerable evidence, particularly in the context of executive prefrontal function [Badre, 2008; Badre and D'Esposito, 2007, 2009; Fuster, 2002, 2009; Koechlin and Summerfield, 2007; Miller and Cohen, 2001], predictive coding [Friston, 2002, 2005, 2012; Friston and Kiebel, 2009; Kilner et al., 2007] but also basic visual perception [Salin and Bullier, 1995]. Second, on the basis of rs‐fMRI data [Dosenbach et al., 2010; Fair et al., 2008; Rubia et al., 2012], we grouped the connections according to their length, contrasting frontoparietal long‐range connections (to and from the IPL) and frontoinsular short‐range connections (within the frontal cortex and between the frontal cortex and the insula).
Mixed repeated measures ANOVA including age group as a between‐subject factor and Connection direction (forward/backward) or Connection length (short/long) were performed to test for changes in connectivity with age. These analyses were run on both fixed and modulatory parameter estimates of effective connectivity. Correlations and multiple regressions were further used to investigate potential associations between effective connectivity measures, age, and brain structure measures.
RESULTS
Behavioral Results
Analyses were conducted on the 33 participants included in the DCM analyses (young adolescents N = 10, mid adolescents N = 13, adults N = 10), a slightly smaller total sample than described in Dumontheil et al. [2010]. Results from a 2 (Condition: Relational, Control) × 3 (Age group: young adolescence, mid adolescence, and adulthood) mixed repeated measures ANOVA performed on accuracy revealed a main effect of Condition [F(1,30) = 26.62, P < 0.001] with lower accuracy in Relational (88.8% ± 8.5) compared to Control (96.4% ± 3.2) trials. The main effect of Age group and the Condition × Age group interaction were not significant (Ps > 0.2). However, using age as a continuous variable reveals a marginal decrease with age of the difference in accuracy between Relational and Control trials [F(1,31) = 3.42, P = 0.07, β = −0.315].
Analysis of reaction times (RT) showed a main effect of Condition [F(1,30) = 211.14, P < 0.001], with slower RT in Relational (2018 ms ± 393) compared to Control trials (1234 ms ± 200). There was no main effect of Age group (P = 0.33), but the Condition × Age group interaction was significant [F(2,30) = 3.46, P = 0.045] and predominantly explained by faster response to Relational trials in mid adolescents compared to young adolescents [Dumontheil et al., 2010]. Using age as a continuous variable revealed no linear change with age in the difference in RT between Relational and Control trials [F(1,31) = 0.03, P > 0.8, β = −0.033].
Thus although performance was high overall, there was some evidence of an improvement in accuracy and RT in Relational versus Control trials with age.
Main Effect on the Relational > Control Contrast
The main effect of the experimental condition (Relational > Control) revealed bilateral activations in the RLPFC, AI, DLPFC, IPL, mSFG, and occipital regions across all participants (Table 1) [Dumontheil et al., 2010].
Table 1.
Whole‐brain analyses (FWE, P < 0.05) of the main effect of experimental condition (Relational > Control) averaged across the three age groups
| Label | Peak voxel | Cluster size | Z | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Frontal lobe | ||||||
| Right | Dorsolateral PFC | 51 | 32 | 28 | 459 | 7.52 |
| Rostrolateral PFC | 36 | 62 | 1 | 5.80 | ||
| Anterior insula | 36 | 23 | −5 | 33 | 5.82 | |
| Left | Dorsolateral PFC | −48 | 26 | 31 | 525 | 7.10 |
| Rostrolateral PFC | −48 | 47 | 4 | 6.83 | ||
| Anterior insula | −36 | 20 | −5 | 2 | 4.78 | |
| Medial | Medial superior frontal gyrus | 0 | 17 | 52 | 268 | 7.10 |
| Parietal lobe | ||||||
| Right | Inferior parietal lobule | 48 | −40 | 52 | 693 | 7.51 |
| Left | Inferior parietal lobule | −33 | −58 | 49 | 494 | 6.81 |
| Temporal lobe | ||||||
| Right | Inferior temporal gyrus/fusiform | 36 | −61 | −14 | 1033 | 7.16 |
| Occipital lobe | ||||||
| Right | Middle occipital gyrus | 36 | −85 | 7 | (within temporal cluster) | 6.95 |
| Left | Middle occipital gyrus | −33 | −85 | 7 | 594 | 6.36 |
PFC, prefrontal cortex; Z, z‐score [Dumontheil et al., 2010]
DCM analyses were performed on activations from five regions in the left hemisphere (RLPFC, DLPFC, mSFG, AI, and IPL; Fig. 2).
Figure 2.
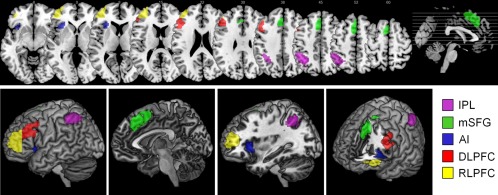
Main effect of Relational > Control. The main effect of the experimental condition (Relational > Control) revealed activation in the RLPFC, DLPFC, AI, mSFG, and IPL across all participants. Only activations in the left hemisphere used in the DCM analyses are shown here. Top row: horizontal slices ranging from z = −8 to z = 60. Bottom row: lateral and medial view, lateral view with a cut‐off at x = −32, lateral frontal view with a cut‐off at y = 22, z = 2. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Post‐hoc Selection of DCM
Posterior probabilities extracted from the DCM post‐hoc analysis show whether a parameter exists or not (e.g., a connection between two brain regions). Comparing the model evidence for all possible models showed that the full DCM had the highest posterior probability, (0.56; Fig. 3A). Across participants, the full DCM explained the most 0–26% (mean 10.61% ± 5.9) of the variance in our data. Bayesian parameter average estimates (BPA matrix) provided strong evidence for the presence of the reciprocal fixed connectivity between all VOIs, and the modulation of all those connections by the relational integration demands (Relational blocks; the Bayesian parameter average posterior probabilities for the a and b matrices were 1). A similar procedure was used for each of the three age groups separately and the comparison of model evidence for all possible models showed that the full DCM had the highest posterior probability in all three groups.
Figure 3.
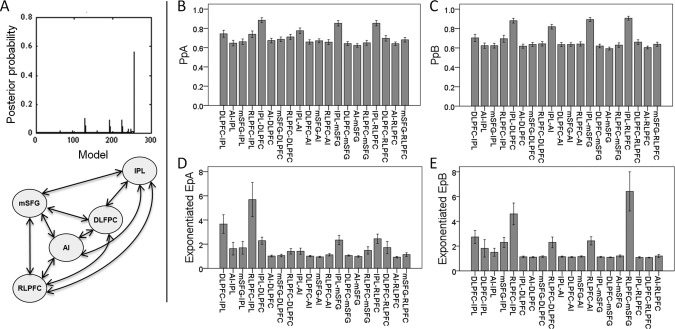
Results of the post‐hoc model search. (A) The full DCM, as illustrated here, had the highest probability compared with all other possible models. (B and C) Mean (± SE) connection probabilities for the fixed (PpA) and modulatory (PpB) connections. Probabilities were above chance (>50%) for all connections. (D and E) Mean (± SE) exponentiated parameter estimates for fixed (EpA) and modulatory (EpB) connections are also shown. Probability and parameter estimate values for self‐connections are not included for simplicity.
Connections probabilities for all individual fixed and modulatory connections were above chance (>50%; Fig. 3B,C). Note that all the parameter estimates were scale parameters that were exponentiated prior to plotting to ensure positivity as per convention for two state DCMs [Marreiros et al., 2008] (Fig. 3D,E), while all the statistical analyses were conducted on non‐exponentiated data.
To summarize, the DCM analyses indicated that the full model had the highest evidence. Therefore, the statistical analyses below were conducted on parameter estimates from the full model estimated in each participant, to look at quantitative differences in fixed or modulatory connection strength.
Developmental Changes in Connection Strength
To limit the number of statistical tests performed, we grouped the full DCM model parameter estimates of the 20 connections in two ways. We first organized the connections according to a well‐established anatomical and functional asymmetry between forward and backward connections. Mixed repeated measures ANOVA including Age group as a between‐subject factor and Connection direction (forward vs. backward) as a within‐subject factor showed no evidence of an interaction between Connection direction and Age group for fixed [EpA; F(2,30) = 0.72, P > 0.4], or modulatory (EpB) parameter estimates [F(2,30) = 0.38, P > 0.6]. This grouping was thus not pursued further.
Second, we grouped the connections according to their length, contrasting frontoparietal long‐range (i.e., reciprocal connections between the IPL and the four frontal clusters), and frontoinsular short‐range connections (i.e., reciprocal connections within the frontal lobe). Mixed repeated measures ANOVA including Age group as a between‐subject factor and Connection length (frontoparietal vs. frontoinsular) as a within‐subject factor were performed on the fixed (EpA) and modulatory (EpB) parameter estimates.
Analysis of the fixed connections showed a main effect of Connection length [F(1,30) = 68.91, P < 0.001], with stronger frontoparietal than frontoinsular connections, no main effect of age group [P > 0.9] but a significant interaction between Connection length and Age group [F(2,30) = 3.59, P = 0.04; Fig. 4A]. Follow‐up tests showed a main effect of Age group on the frontoinsular connections [F(2,30) = 4.54, P = 0.019] with greater connectivity strength in young adolescents than mid adolescents [P = 0.039], and adults [P = 0.006]. There was no effect of Age group on frontoparietal connections [F(2,30) = 1.05, P > 0.3]. Note that using Age as a continuous measure in the repeated measures ANOVA revealed a marginally significant interaction between Connection length and Age [F(1,31) = 3.96, P = 0.055].
Figure 4.
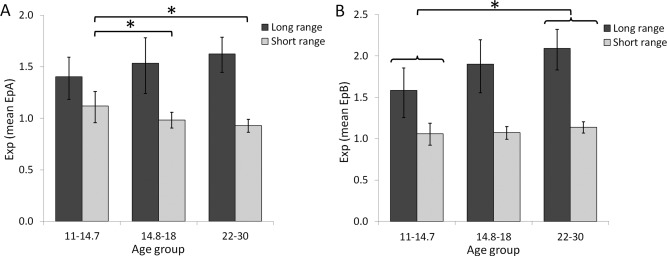
Parameter estimates of the fixed and modulatory short‐range and long‐range connections plotted for each age group. (A) Exponentiated mean and 95% confidence interval (CI) of the parameter estimates of fixed connections (EpA). Long‐range connections were stronger than short‐range connections, and there was a significant interaction between Connection length and Age group. Short‐range connections were stronger in young adolescents than mid adolescents and adults (indicated by asterisk), while long‐range connections did not differ between age groups. (B) Exponentiated mean and 95% CI of the parameter estimates of the modulatory connections. Long‐range connections were again stronger than short‐range connections. Connectivity strength was also greater in adults than in young adolescents (indicated by asterisk).
Analysis of the modulatory connections also showed a main effect of Connection length [F(1,30) = 130.84, P < 0.001], and a main effect of Age group [F(2,30) = 3.64, P = 0.038], with greater connectivity strength in adults than young adolescents [P = 0.012; Fig. 4B]. The other paired comparisons were not significant (Ps > 0.1). Moreover, there was no interaction between Connection length and Age group [F(2,30) = 1.85, P > 0.17].
As rs‐fcMRI data has been shown to be sensitive to participants' motion [Power et al., 2012], we tested whether fixed and modulatory parameter estimates were correlated with mean translation and mean rotation movement estimates across participants. There was a trend for a negative correlation between mean translation and frontoparietal modulatory connection strength [Pearson r = −0.313, P = 0.076]. No other associations were observed [all Ps > 0.22]. Although mean translation did not vary significantly with age (see “Materials and Methods” section), we tested whether the association observed here may account for the increase in frontoparietal modulatory connection strength with age. A second mixed repeated measures ANOVA was performed, including mean translation as a covariate. The results showed that the main effect of age group became marginal [F(2,29) = 3.20, P = 0.055], however the difference between young adolescents and adults remained significant [P = 0.017].
To summarize, analysis of the parameter estimates from the full model showed that there was no difference in development of forward versus backward connections. However, there were differential developmental changes of long versus short‐range fixed connections, with a decrease in frontoinsular short‐range fixed connections strength with age and stable frontoparietal long‐range fixed connections strength. Modulatory connections showed an increase in strength with age overall, with no distinction between frontoinsular and frontoparietal connections.
Analysis of Structural Data
Gray matter volumes in all five ROIs decreased with age [β range −0.78 to −0.50, all Ps < 0.001; Fig. 5A]. A multiple regression analysis was conducted to investigate whether gray matter volumes, extracted from our five ROIs, could predict frontoinsular short‐range fixed connectivity strength, which was found to decrease with age (previous section). All five gray matter volume measures were entered simultaneously in one regression to test for region‐specific effects. Results showed that greater gray matter volumes in the left RLPFC predicted greater fixed frontoinsular connections strength [β =1.24, P = 0.045; Table 2] independently of gray matter volumes in the other ROIs.
Figure 5.
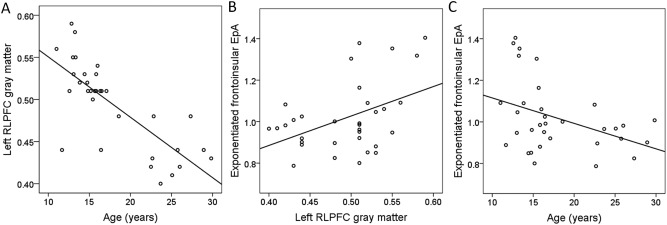
Scatter plots of left RLPFC adjusted gray matter volume as a function of age, and the parameter estimates of frontal short‐range fixed connections (EpA) as a function of age and RLPFC structure. (A) Left RLPFC adjusted gray matter volume plotted as a function of age. (B) Frontal short‐range exponentiated EpA plotted as a function of adjusted gray matter volume in the left RLPFC. (C) Frontal short‐range exponentiated EpA plotted as a function of age. Both measures (i.e., RLPFC structure and age) predicted fixed short‐range connection strength, but no significant mediation was observed. Note that statistics were performed on non‐exponentiated data and the fit line is shown here for illustration purposes.
Table 2.
Fixed frontal short‐range connections predicted by gray matter volumes in the five ROIs
| Dependent variable: Short‐range EpA | Unstandardized coefficient B | Standardized β | t |
|---|---|---|---|
| (Constant) | −0.640 | −2.47 | |
| AI GM | −0.969 | −0.358 | −0.95 |
| DLPFC GM | −1.710 | −0.597 | −1.28 |
| IPL GM | 0.647 | 0.281 | 0.98 |
| mSFG GM | −0.377 | −0.182 | −0.51 |
| RLPFC GM | 3.786 | 1.243 | 2.10a |
This table presents results of a multiple regression analysis entering mean adjusted gray matter volumes in the five ROIs of the DCM in a single analysis [F(5,27) = 1.90, P = 0.13, R 2 = 0.26]. Greater gray matter volumes in the left RLPFC predicted greater fixed short‐range connections strength (EpA) independently of gray matter volumes in the other ROIs.
GM, Gray matter.
P < 0.05.
To test for a possible mediation of the developmental change in short‐range fixed connectivity by left RLPFC structure [using the approach proposed by Baron and Kenny, 1986], we included age and left RLPFC gray matter volume in a multiple regression, with frontoinsular fixed connectivity as the dependent variable. This model was compared to the earlier regressions showing that age and RLPFC gray matter volume, when entered separately, both significantly predicted frontoinsular fixed connectivity, and that age predicts RLPFC gray matter volume. Although the multiple regression model including both age and RLPFC gray matter structure together significantly accounted for variance in frontoinsular fixed connectivity [R2 = 0.19, F(2,30) = 3.41, P = 0.046], neither regressor was significant [Ps > 0.27]. These results provide therefore no evidence that left RLPFC structure may have mediated the effect of age on frontoinsular fixed connectivity.
Further analysis indicated that left RLPFC gray matter volume also positively predicted Relational versus Control accuracy [R2 = 0.12, β = 0.35, P = 0.046].
To summarize (Fig. 5B,C), age and gray matter volume in the left RLPFC both predicted fixed frontoinsular short‐range connection strength, but neither age nor RLPFC provided additional predictive power, given the other. Gray matter volume in the left RLPFC further predicted Relational versus Control accuracy.
Connectivity Changes Predict Performance on the Relational Reasoning Task
Regression analyses were performed on the four types of connections (fixed and modulatory, short and long‐range) to test whether connectivity strength could predict the difference in accuracy between Relational and Control trials. The results indicate that Relational versus Control accuracy was significantly predicted by the strength of fixed and modulatory frontoinsular short‐range connections [R2 = 0.12, β = 0.35, P = 0.047 and R 2 = 0.16, β = −0.40, P = 0.019, respectively]. Weaker fixed connections and stronger modulatory connections predicted a smaller difference between Relational and Control accuracy, i.e., a relatively better performance in Relational trials (Fig. 6A,B). No association between long‐range connectivity and accuracy was observed.
Figure 6.
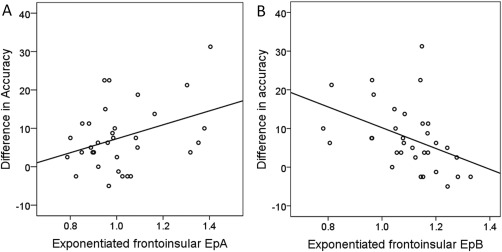
Scatter plot of Relational versus Control accuracy as a function of frontal fixed (EpA) and modulatory (EpB) short‐range connections. (A) The difference in accuracy between Control and Relational trials (a greater positive value means poorer performance in Relational than Control trials) was positively predicted by the strength of fixed connections. (B) The difference in accuracy between Control and Relational trials was negatively predicted by the strength of modulatory connections. Thus, overall weaker fixed connections and stronger modulatory connections predicted a smaller difference between Relational and Control accuracy, i.e. a relatively better performance in Relational trials. Note that statistics were performed on non‐exponentiated data and the fit line is shown here for illustration purposes.
The difference in accuracy between Relational and Control trials showed a trend decrease with age. We therefore performed further regressions including age as a second regressor to test whether the prediction of performance by frontoinsular connection strength may be independent of the effect of age on performance. A multiple regression model including fixed frontoinsular connection strength and age as regressors did not significantly account for Relational versus Control accuracy [R 2 = 0.16, P = 0.07]; neither regressor was significant [fixed connectivity β = 0.27, P = 0.15; age β = −0.21, P = .25]. However, a model including modulatory frontoinsular connection strength and age significantly predicted 11.3% more variance in accuracy [R 2 = 0.21, P = 0.03; modulatory connectivity β = −0.35, P = 0.046, age β = −0.23, P = .18] than a model including age alone as a single regressor.
Based on the opposite relationship between fixed and modulatory frontoinsular connectivity and accuracy, we further explored whether those participants that had greater modulatory frontoinsular connections also showed weaker fixed frontoinsular connections. Indeed, a correlation analysis showed revealed a significant negative correlation between the strength of fixed and modulatory frontoinsular short‐range connections [Pearson Correlation r = −0.41, P = 0.018]. This association remained significant when age was covaried in a partial correlation [r = −0.35, P =0.049].
Similar regression analyses were performed for reaction times. None of the four types of connections predicted the difference in RT between Relational and Control trials [all Ps > 0.3]. Note that the results were similar when accuracy and RT in Relational trials, rather than the difference in accuracy and RT between Relational and Control trials, were entered in the analyses.
To summarize (Fig. 6A,B), fixed and modulatory frontoinsular short‐range connections showed opposite patterns of development and association with accuracy in Relational versus Control trials. On one hand, fixed frontoinsular connectivity decreased with age and was negatively correlated with performance overall, but not when age was covaried. On the other hand, modulatory frontoinsular connectivity did not change with age and was positively correlated with performance, independently of age.
DISCUSSION
Here we used DCM to investigate the development of relational reasoning through changes in effective connectivity during the transition from adolescence to adulthood. We re‐examined our previously collected data on relational reasoning development [Dumontheil et al., 2010] that revealed a main effect of the experimental condition (Relational–Control) in the RLPFC, AI, DLPFC, IPL, mSFG across three age groups (11–14.7 years, 14.8–18.5 years, and 22.5–30 years) and condition × age group effects in left RLPFC, left AI and mSFG. Our aim was to focus on the left hemisphere and see whether there were age‐dependent changes in the strength of connections, when grouped into long‐ versus short‐range (i.e., frontoparietal vs. frontoinsular) or into forward and backward connections in line with previous research [Fair et al., 2008; Friston, 2002, 2005, 2012; Fuster, 2002, 2009]. Second, we examined whether developmental changes in the strength of connectivity may be linked to structural changes with age. Finally, we tested whether behavioral changes seen in our relational reasoning task could be predicted by connectivity strength, in particular, whether PFC connections showed increasing developmental specificity for relational integration [Crone et al., 2009; Dumontheil et al., 2010; Wendelken et al., 2011].
The PFC shows a complex pattern of functional activation in response to cognitive tasks during development, with reports of both increases and decreases of task‐related activations with age [Luna et al., 2010 for review]. These changes may partly reflect structural changes [Dumontheil et al., 2010], but also connectivity changes between the brain regions co‐activated by specific cognitive processes [Crone and Dahl, 2012]. Indeed, task‐based functional connectivity research has shown distinct connectivity changes with age depending on experimental paradigm used [Barbalat et al., 2012; Christakou et al., 2011; Neufang, et al., 2008; Stevens et al., 2007]. Resting state fc‐MRI studies have shown that the cognitive control networks are already online during rest in early‐adolescence, and it is mainly the connectivity strength within such networks that undergoes maturational changes with age [Fair et al., 2008; Jolles et al., 2011]. Our DCM results from the full model, provides further evidence of broadly similar network of connections, including all reciprocal fixed and modulatory connections within the relational reasoning network (reciprocal connections between RLPFC, DLPFC, AI, mSFG, and IPL; Fig. 3), during young adolescence, mid adolescence, and adulthood.
Next, to examine whether there were age‐dependent changes in the strength of fixed and modulatory connections, we grouped the connections according to their direction (i.e., forward vs. backward connections) or their length (long‐ vs. short‐range), factors that have been distinguished in prior research [Fair et al., 2008; Friston, 2002, 2005, 2012]. We did not find differential age effects on the connection strengths when the grouping was done according to direction. Thus this grouping was not pursued further. In contrast, our results revealed a significant interaction between age group and connection length on the strength of fixed connections across the three age groups. This age‐related difference was driven by a significant decrease in the strength of fixed frontoinsular (short‐range) connections, strongest between young (age 11–14) and mid adolescence (age 14–17), while the fixed frontoparietal (long‐range) connections remained stable across the age groups (Fig. 4A). Modulatory connections showed an increase in strength with age overall between young adolescence and adulthood, with no distinction between frontoinsular and frontoparietal connections, although the increase in connectivity with age was qualitatively more pronounced for frontoparietal long‐range connections (Fig. 4B).
This differential pattern of developmental changes in the strength of frontoinsular and frontoparietal connections is broadly consistent with the results of developmental rs‐fcMRI studies, which have found a decrease in short‐range connectivity and increase in long‐range connectivity with age [Dosenbach et al., 2010; Fair et al. 2008; Uddin et al., 2010; Vogel et al., 2010]. In the current study, the decrease in short‐range connectivity was limited to the fixed connectivity, while the increased long‐range connectivity was more pronounced in the modulatory connectivity measures. Rs‐fMRI studies, which are limited by the unconstrained nature of the task and the sensitivity to group differences in movement, do not make a distinction between fixed and modulatory connectivity strengths. Our results suggest that more complex models using task‐based designs, such as DCMs, are necessary to study connectivity changes during development in more details. Further work using both rs‐fMRI and DCMs will be needed to better understand the relationship between these two types of connectivity measurement.
The current study further showed an association between frontoinsular short‐range effective connectivity and relational integration accuracy (as contrasted to accuracy during the manipulation of single relations). Fixed and modulatory frontoinsular connectivity strengths showed an opposite relationship with accuracy (Fig. 6A,B) such that greater relational integration accuracy was associated with weaker fixed and stronger modulatory frontoinsular connectivity. Only the latter association remained significant when age was included in the model. Indeed, modulatory frontoinsular connectivity strength per se did not increase over age (but overall modulatory connections increased between young adolescence and adulthood; Fig. 4B); however, fixed frontoinsular connectivity strength decreased with age (Figs. 4A and 5C). Moreover, those participants who had greater modulatory frontoinsular connections also showed weaker fixed frontoinsular connections. Overall, these relationships suggest increasing specificity of frontoinsular connections for relational integration with age, associated with higher relational integration accuracy.
Note that RT decreased during adolescence only rather than over the whole age range, and no correlation between RT and connectivity measures were observed. It is possible that such associations may be observed during adolescence specifically, when RT shows improvements. However, the sample size in the current study was considered too small to repeat the correlation analyses within the adolescent group only.
This pattern of developmental changes in the control condition (here corresponding to the fixed connectivity) but not in the relational condition is similar to that observed in terms of BOLD activation by Wendelken et al. [2011]. In a very similar experimental paradigm, left RLPFC activation in the control condition decreased with age, while activation in the relational integration condition remained stable with age. Therefore, the results of our effective connectivity lend further support for an increasing specialization of frontal cortex function for relational integration during development, in line with previous fMRI data [Crone et al., 2009; Dumontheil et al., 2010; Wendelken et al., 2011].
It is thought that cognitive development requires both integration and segregation of information [Johnson 2001]; thus as developmental strengthening of long‐range connections may represent the “integration” of information across broader cognitive networks over time, weakening of the short‐range connections may represent the segregation of connected regions into separate networks [Fair et al., 2007; Vogel et al., 2010]. Therefore, the weakening of fixed short‐range frontoinsular connections and strengthening of modulatory long‐range frontoparietal connections in our data may reflect segregation of closely linked prefrontal regions and integration of distantly located frontoparietal regions during the transition from adolescence into adulthood.
Changes in the strength of connectivity may in part reflect the macro‐level structural changes during development. Strengthening of long‐range functional connections may be linked to increased myelination and signal transmission efficiency, which could facilitate the integration of information between functionally linked but distant regions [Bunge and Wright, 2007; Vogel et al., 2010]. In contrast, the weakening of short‐range connections may reflect selective synaptic pruning between brain regions, which continues well into adolescence [Petanjek et al., 2011]. DTI data was not collected in this study, preventing us to test the association between underlying white matter changes and effective connectivity. However, using VBM, we tested whether gray matter volumes would predict connectivity strength. Our results showed that gray matter volume in the left RLPFC, along with age, predicted changes in the strength of fixed short‐range frontoinsular connections. This effect was specific to this region as it remained when all five ROIs were included in the regression as predictors. Thus, the present data suggest that continued synaptic pruning with age in RLPFC could lead to a pattern of segregation of already connected regions within the frontal lobe and insula that is specific to relational integration.
Cognitive control gradually develops throughout adolescence [Luna et al., 2010], and many fMRI studies have shown that the co‐activation of frontal and parietal regions is necessary for this cognitive improvement [Miller and Cohen, 2001]. As described above, our results showed that the frontal (short‐range) connections predicted relational integration performance. We observed no such association between frontoparietal (long‐range) connections and performance, despite the fact that the strength of modulatory frontoparietal connections increased between the early adolescent and adult groups (Fig. 4B). Therefore, while prior research suggests that the maturation of long‐range connections may underlie aspects of integrative cognitive controls that are developing during adolescence [Crone and Dahl, 2012, for review], in the current study, we found that performance was more directly associated with frontal connectivity, and RLPFC structure, which may be more directly relevant for the development of relational integration. In a study using a similar paradigm, Wendelken et al. [2011] observed that cortical thickness in the IPL decreased with age and predicted RLPFC and IPL activation during the manipulation of single relations, but not RLPFC activation (and less strongly IPL activation) during relational integration. This pattern suggests that maturation of IPL structure and function may be more directly associated with the manipulation of single relations, while (left) RLPFC may more specifically support relational integration, as proposed by neuroimaging studies in adults [Bunge et al., 2009; Christoff et al., 2001; Kroger et al., 2002; Wendelken et al., 2008].
This is the first study to investigate the development of relational reasoning through changes in effective connectivity with age. In line with previous rs‐fcMRI results, we showed distinct developmental trajectories in the long‐range frontoparietal and short‐range frontoinsular connections and demonstrate how changes in connectivity may be linked to structural changes. We also showed how changes in connectivity strength could explain behavioral changes in response to the relational reasoning task, and how the frontal lobe and insula shows increasing selectivity for relational reasoning. One limitation of this study may be that the relational reasoning paradigm we used followed a block‐design. Thus, specific neural processes through which the modulation of relational integration occurs cannot be identified precisely within the experimental trials. Second, motion‐related artifacts are thought to affect functional connectivity time course data in rs‐fcMRI studies [Power et al., 2012], which have lengthy “rest” periods. Movement could potentially affect the connectivity changes in task based fMRI studies as well; however, a task based fMRI paradigm is more constrained than rs‐fcMRI, with shorter duration and structured repetition of experimental blocks (here blocks lasted 20–32 s), which reduces the likelihood that the group differences seen in our results could be substantially influenced by movement. Further, there was no age difference in mean movement amplitude in the present study, and parameter estimates were not correlated with mean movement amplitude across participants. Third, the optimal DCM only partially explained the variance in the data and the range across participants was quite large (0–26%). We used a novel procedure, which selected the full connectivity model as optimal. Bayesian model selection considers a trade‐off between accuracy and model complexity and may not necessarily represent the actual neural system engaged by the cognitive task [Stephan et al., 2010]. In addition, the proportion of variance explained in DCMs may increase if methodological advances improve the modeling of interhemispheric connectivity [Stephan et al., 2007] and permit DCMs including regions in both hemispheres. Finally, because of the large number of regions involved in the Relational versus Control comparisons, we grouped the connections according to connection direction or connection length. A more stringent contrast, possibly using an event‐related design, may identify a smaller network of brain regions specific to relational integration and enable the study of individual connections during development.
CONCLUSION
Our earlier investigation of functional, behavioral and structural changes associated with relational reasoning in adolescence concluded that performance and structural changes could partly account for the changes in RLPFC activity with age [Dumontheil et al., 2010]. Results from the current study, give a better understanding of how the connectivity changes within PFC could further contribute to such complex pattern of functional activation during development. Studying the typical development of connectivity can inform future research investigating the atypical developmental trajectory of common neurologic and psychiatric illnesses, such as autism, attention deficit hyperactivity disorder (ADHD), or Tourette syndrome, which have all be found to show atypical patterns of resting state functional connectivity [Uddin et al., 2010 and Vogel et al., 2010 for reviews]. Here, we show that the study of effective connectivity during an experimental paradigm can more specifically inform how particular cognitive processes develop. Combining behavioural, structural, functional, and connectivity data will therefore be critical to further our understanding of the developmental mechanisms underlying the maturation of higher cognitive functions.
REFERENCES
- Ashburner J, Friston KJ (2000): Voxel‐based morphometry: The methods. Neuroimage 11:805–821. [DOI] [PubMed] [Google Scholar]
- Badre D (2008): Cognitive control, hierarchy, and the rostro‐caudal organization of the frontal lobes. Trends Cogn Sci 12:193–200. [DOI] [PubMed] [Google Scholar]
- Badre D, D'Esposito M (2007): Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. J Cogn Neurosci 19:2082–2099. [DOI] [PubMed] [Google Scholar]
- Badre D, D'Esposito M (2009): Is the rostro‐caudal axis of the frontal lobe hierarchical? Nat Rev Neurosci 10:659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbalat G, Bazargani N, Blakemore SJ (2013): The influence of prior expectations on emotional face perception in adolescence. Cereb Cortex 23:1542–1551. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA (1986): The moderator‐mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol 51:1173–1182. [DOI] [PubMed] [Google Scholar]
- Bitan T, Cheon J, Lu D, Burman DD, Booth JR (2009): Developmental increase in top‐down and bottom‐up processing in a phonological task: An effective connectivity, fMRI study. J Cog Neurosci 21:1135–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Helskog EH, Wendelken C (2009): Left, but not right, rostrolateral prefrontal cortex meets a stringent test of the relational integration hypothesis. Neuroimage 46:338–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Wright SB (2007): Neurodevelopmental changes in working memory and cognitive control. Curr Opin Neurobiol 17:243–250. [DOI] [PubMed] [Google Scholar]
- Burnett S, Blakemore SJ (2009): Functional connectivity during a social emotion task in adolescents and in adults. Eur J Neurosci 29:1294–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett S, Sebastian C, Cohen Kadosh K, Blakemore SJ (2011): The social brain in adolescence: Evidence from functional magnetic resonance imaging and behavioural studies. Neurosci Biobehav Rev 35:1654–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakou A, Brammer M, Rubia K (2011): Maturation of limbic corticostriatal activation and connectivity associated with developmental changes in temporal discounting. Neuroimage 54:1344–1354. [DOI] [PubMed] [Google Scholar]
- Christoff K, Prabhakaran V, Dorfman J, Zhao Z, Kroger JK, Holyoak KJ, Gabrieli JD (2001): Rostrolateral prefrontal cortex involvement in relational integration during reasoning. Neuroimage 14:1136–1149. [DOI] [PubMed] [Google Scholar]
- Christoff K, Ream JM, Geddes LP, Gabrieli JD (2003): Evaluating self‐generated information: Anterior prefrontal contributions to human cognition. Behav Neurosci 117:1161–1168. [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh K, Cohen Kadosh R, Dick F, Johnson MH (2011): Developmental changes in effective connectivity in the emerging core face network. Cereb Cortex 21:1389–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonnese M, Khazipov R (2012): Spontaneous activity in developing sensory circuits: Implications for resting state fMRI. Neuroimage 62:2212–2221. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE (2012): Understanding adolescence as a period of social‐affective engagement and goal flexibility. Nat Rev Neurosci 13:636–650. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, van Leijenhorst L, Honomichl RD, Christoff K, Bunge SA (2009): Neurocognitive development of relational reasoning. Dev Sci 12:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, Wig GS, Vogel AC, Lessov‐Schlaggar CN, Barnes KA, Dubis JW, Feczko E, Coalson RS, Pruett JR Jr, Barch DM, Petersen SE, Schlaggar BL (2010): Prediction of individual brain maturity using fMRI. Science 329:1358–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontheil I, Burgess PW, Blakemore SJ (2008): Development of rostral prefrontal cortex and cognitive and behavioural disorders. Dev Med Child Neurol 50:168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontheil I, Houlton R, Christoff K, Blakemore SJ (2010): Development of relational reasoning during adolescence. Dev Sci 13:F15–F24. [DOI] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL (2008): The maturing architecture of the brain's default network. Proc Natl Acad Sci USA 105:4028–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL (2007): Development of distinct control networks through segregation and integration. Proc Natl Acad Sci USA 104:13507–13512. 17679691 [Google Scholar]
- Ferrer E, O'Hare ED, Bunge SA (2009): Fluid reasoning and the developing brain. Front Neurosci 3:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Friston KJ (2012): Distributed processing; Distributed functions? Neuroimage 61:407–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K (2002): Functional integration and inference in the brain. Prog Neurobiol 68:113–143. [DOI] [PubMed] [Google Scholar]
- Friston K (2003): Learning and inference in the brain. Neural Netw 16:1325–1352. [DOI] [PubMed] [Google Scholar]
- Friston K (2005): A theory of cortical responses. Philos Trans R Soc Lond B Biol Sci 360:815–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K (2009): Causal modelling and brain connectivity in functional magnetic resonance imaging. PLoS Biol 7:0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K (2012): Prediction, perception and agency. Int J Psychophysiol 83:248–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W (2003): Dynamic causal modelling. Neuroimage 19:1273–1302. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R (1995): Analysis of fMRI time‐series revisited. Neuroimage 2:45–53. [DOI] [PubMed] [Google Scholar]
- Friston K, Kiebel S (2009): Predictive coding under the free‐energy principle. Philos Trans R Soc Lond B Biol Sci 364:1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Li B, Daunizeau J, Stephan KE (2011): Network discovery with DCM. Neuroimage 56:1202–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Penny W (2011): Post hoc Bayesian model selection. Neuroimage 56:2089–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM (2002): Frontal lobe and cognitive development. J Neurocytol 31:373–385. [DOI] [PubMed] [Google Scholar]
- Fuster JM (2009): Cortex and memory: Emergence of a new paradigm. J Cogn Neurosci 21:2047–2072. [DOI] [PubMed] [Google Scholar]
- Hillebrandt HF, Dumontheil I, Blakemore S‐J, Roiser JP (2013): Dynamic causal modelling of effective connectivity during perspective taking in a communicative task. Neuroimage 76:116–124. [DOI] [PubMed] [Google Scholar]
- Hwang K, Velanova K, Luna B (2010): Strengthening of top‐down frontal cognitive control networks underlying the development of inhibitory control: A functional magnetic resonance imaging effective connectivity study. J Neurosci 30:15535–15545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde KL, Zatorre RJ, Griffiths TD, Lerch JP, Peretz I (2006): Morphometry of the amusic brain: A two‐site study. Brain 129:2562–2570. [DOI] [PubMed] [Google Scholar]
- Johnson MH (2001): Functional brain development in humans. Nat Rev Neurosci 2:475–483. [DOI] [PubMed] [Google Scholar]
- Jolles DD, van Buchem MA, Crone EA, Rombouts SA (2011): Functional brain connectivity at rest changes after working memory training. Hum Brain Mapp 21:385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, Biswal BB, Craddock RC, Castellanos FX, Milham MP (2012): Characterizing variation in the functional connectome: Promise and pitfalls. Trends Cogn Sci 16:181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Friston KJ, Frith CD (2007): The mirror‐neuron system: A Bayesian perspective. Neuroreport 18:619–623. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Summerfield C (2007): An information theoretical approach to prefrontal executive function. Trends Cogn Sci 11:229–235. [DOI] [PubMed] [Google Scholar]
- Kroger JK, Sabb FW, Fales CL, Bookheimer SY, Cohen MS, Holyoak KJ (2002): Recruitment of anterior dorsolateral prefrontal cortex in human reasoning: A parametric study of relational complexity. Cereb Cortex 12:477–485. [DOI] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O'Hearn K (2010): What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn 72:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marreiros AC, Kiebel SJ, Friston KJ (2008): Dynamic causal modelling for fMRI: A two‐state model. Neuroimage 39:269–278. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD (2001): An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24:167–202. [DOI] [PubMed] [Google Scholar]
- Neufang S, Fink GR, Herpertz‐Dahlmann B, Willmes K, Konrad K (2008): Developmental changes in neural activation and psychophysiological interaction patterns of brain regions associated with interference control and time perception. Neuroimage 43:399–409. [DOI] [PubMed] [Google Scholar]
- O'Brien LM, Ziegler DA, Deutsch CK, Kennedy DN, Goldstein JM, Seidman LJ, Hodge S, Makris N, Caviness V, Frazier JA, Herbert MR (2006): Adjustment for whole brain and cranial size in volumetric brain studies: A review of common adjustment factors and statistical methods. Harv Rev Psychiatry 14:141–151. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN (2008): Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci 9:947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB, Rakic P, Kostovic I (2011): Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA 108:13281–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012): Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa MJ, Friston K, Penny W (2012): Post‐hoc selection of dynamic causal models. J Neurosci Methods 208:66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K (2012): Functional brain imaging across development. Eur Child Adolesc Psy [epub ahead of print, DOI: 10.1007/s00787-012-0291-8]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin P, Bullier J (1995): Corticocortical connections in the visual system: Structure and function. Physiol Rev 75:107–154. [DOI] [PubMed] [Google Scholar]
- Smith R, Keramatian K, Christoff K (2007): Localizing the rostrolateral prefrontal cortex at the individual level. Neuroimage 36:1387–1396. [DOI] [PubMed] [Google Scholar]
- Smith SM (2012): The future of FMRI connectivity. Neuroimage 62:1257–1266. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ (2010): Analyzing effective connectivity with fMRI. WIREs Cogn Sci 1:446–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Marshall JC, Penny WD, Friston KJ, Fink GR (2007): Interhemispheric integration of visual processing during task‐driven lateralization. J Neurosci 27:3512–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Penny WD, Moran RJ, den Ouden HE, Daunizeau J, Friston KJ (2010): Ten simple rules for dynamic causal modeling. Neuroimage 49:3099–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD (2007): Functional neural networks underlying response inhibition in adolescents and adults. Behav Brain Res 181:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Musen M, Menon V (2009): Development of large‐scale functional brain networks in children. PLoS Biol 7:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Uddin LQ, Prater K, Amin H, Greicius MD, Menon V (2010): Development of functional and structural connectivity within the default mode network in young children. Neuroimage 52:290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V (2010): Typical and atypical development of functional human brain networks: Insights from resting‐state FMRI. Front Syst Neurosci 4:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar KS, Ryali S, Menon V (2011): Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J Neurosci 31:18578–18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel AC, Power JD, Petersen SE, Schlaggar BL (2010): Development of the brain's functional network architecture. Neuropsychol Rev 20:362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1999): Wechsler Abbreviated Scale of Intelligence (WASI). San Antonio, TX: Harcourt Assessment. [Google Scholar]
- Wendelken C, Nakhabenko D, Donohue SE, Carter CS, Bunge SA (2008): "Brain is to thought as stomach is to??": Investigating the role of rostrolateral prefrontal cortex in relational reasoning. J Cogn Neurosci 20:682–693. [DOI] [PubMed] [Google Scholar]
- Wendelken C, O'Hare ED, Whitaker KJ, Ferrer E, Bunge SA (2011): Increased functional selectivity over development in rostrolateral prefrontal cortex. J Neurosci 31:17260–17268. [DOI] [PMC free article] [PubMed] [Google Scholar]


